ABSTRACT
Background and Aims:
Mechanical ventilation is an essential but limited resource worldwide. Appropriate perioperative utilisation of such useful resource demands in time prediction where literature does not have enough data. High C-reactive protein (CRP) and low albumin both represent a state of exaggerated inflammation and poor nutrition, the combination of which might represent the sick surgical patients. Therefore, we tried to evaluate the performance of ratio between preoperative CRP and albumin (CAR) for the prediction of postoperative mechanical ventilation.
Methods:
After approval from the ethics committee and trial registration, the study was carried out over 2 years. It included 580 adults undergoing non-cardiac surgeries under general anaesthesia. Blood samples were collected for estimation of CRP and albumin, and all were followed up for the need of mechanical ventilation in the postoperative period till hospital discharge.
Results:
Sixty-six of the analysed 569 patients (11.6%) required postoperative mechanical ventilation in whom the median CAR was higher {0.38 (0.10, 1.45)} than those who did not require the same {0.20 (0.07, 0.65)}, although not statistically significant. A ROC curve analysis found that there is a 58% chance that a CAR will distinguish between the patients requiring postoperative mechanical ventilation from those who do not (AUC = 0.58), which is statistically significant (P value = 0.024). Logistic regression did not result in a significant odds of mechanical ventilation with higher ratio {Odds ratio = 1.06 (0.98, 1.16)}.
Conclusions:
High CRP–albumin ratio was found to be associated with higher need for mechanical ventilation in patients undergoing surgery under general anaesthesia, but failed to predict the need for mechanical ventilation.
Key words: C-reactive protein, mechanical ventilation, postoperative period, serum albumin
INTRODUCTION
Mechanical ventilation in Intensive Care Unit (ICU) is an essential, expensive and a limited resource worldwide. Mechanical ventilation in the postoperative period adds to the morbidity and mortality of surgical patients. In addition, it incurs extra stress on the patient, family and economic burden on the hospital.[1] Hence, preoperative prediction of the same contributes to adequate optimisation and effective utilisation of the available resources. Unfortunately, the literature only provides us with predictors for mechanical ventilation in a very limited set of patients[2,3] leading to difficulty in the majority of other group of surgical patients.
Peri-operative inflammation is a state of high demand. Low albumin is a sign of poor nutritional status. Additionally, serum albumin being a negative acute phase reactant, the level is usually low in patients with heightened inflammation.[4] C-reactive protein (CRP) being a positive acute phase reactant, its level is usually raised in cases of any inflammation or any infections.[5] CRP–albumin ratio (CAR) has been widely proven as a prognostic indicator and mortality predictor in patients with various malignancies and severe sepsis.[6]
To the current date, we do not have a single simple preoperative predictor for postoperative need of mechanical ventilation. CAR, being a composite but simple parameter found useful in different conditions with high inflammation rate, we evaluated the utility of higher preoperative CAR as a predictor for the requirement of mechanical ventilation postoperatively (HICARV study).
METHODS
This study was carried out in the operation theatre complex of a tertiary care centre with 1000 beds from February 2018 to October 2019. The study was approved by the Institutional Ethics Committee followed by study registration with the Clinical Trials Registry of India (CTRI). The data analysis and statistical plan was written and posted on a publicly accessible server (http://ctri.nic.in) before data were accessed. This manuscript adheres to the applicable Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) guidelines.
This was a prospective observational study conducted in all adult patients undergoing elective and emergency non-cardiac surgeries at a tertiary healthcare institute under general anaesthesia with endotracheal intubation. Patients already on mechanical ventilation before surgery were excluded.
Initially, a detailed participant information sheet was given to all the patients containing information regarding the various elements of the study protocol. This was followed by obtaining written informed consent. In the preoperative area, one venous blood sample of 3 ml was collected from all patients for estimation of CRP and albumin. Thereafter, they underwent the respective surgery under standard general anaesthesia with endotracheal intubation. All patients were followed for the need of mechanical ventilation in the postoperative period till hospital discharge.
A sample size of 569 was required to perform a logistic regression to find a relationship between the CAR and the odds of a patient requiring mechanical ventilation in the postoperative period. The odds ratio was fixed as 2, and the baseline proportion of patients requiring ventilation was taken as 0.04 as collected from our own hospital ICU records. This achieves a power of 90%, and the alpha error allowed was 5%.
The normality of the distribution of data was assessed using Shapiro–Wilk test. To find association with mechanical ventilation, Mann–Whitney U-test was used for CRP–albumin ratio; CRP, albumin, age, body mass index (BMI), fluids, blood loss, duration of surgery and Chi-square test were used for area of surgery, malignancy, American Society of Anaesthesiologists (ASA) grading, Mallampati grading and vasopressor usage, and Fisher exact test was used for the type of surgery.
P value <0.05 was considered to be statistically significant. Univariate and multivariate logistic regression analysis was done to find the association of all and each of the significant factors with the outcome.
RESULTS
A total of 569 subjects were analysed [Figure 1]. The median age of the males in the study was 45 years (interquartile range/IQR: 33, 58) while that of females was 40 years (IQR: 30, 50). Majority of the study participants fell into less than 40 years (46.2%) age category followed by 40–60 years (40.2%) and 60 years or above age group (13.5%). Gender distribution of the study participants showed a higher proportion of males (53.4%) compared to females (46.6%).
Figure 1.
STROBE diagram depicting the flow of patients into the study
Median body mass index (BMI) of the male subjects was 22.3 kg/m2 (IQR: 19.1, 24.8) compared to 22.0 kg/m2 (IQR: 19.5, 25.0) for females. Table 1 depicts the other patient characteristics like region-wise distribution of disease for which surgery was planned, presence of malignancy, American Society of Anaesthesiologists physical status classification (ASA), and difficult airway as detected in the preanaesthetic clinic evaluation, and the nature of surgery.
Table 1.
Patient baseline characteristics and postoperative mechanical ventilation
| Variables | No mechanical ventilation n-503 | Mechanical ventilation n-66 | P |
|---|---|---|---|
| Age in years* [Median (IQR)] | 42.0 (31.0, 54.0) | 45.0 (35.7, 57.0) | 0.08 |
| Gender | |||
| Male | 261 (85.9%) | 43 (14.1%) | 0.04‡ |
| Female | 242 (91.3%) | 23 (8.7%) | |
| BMI* in kg/m2 [Median (IQR)] | 22.2 (19.5, 25.0) | 22.0 (19.0, 23.9) | 0.13 |
| Surgery | |||
| Head and neck | 148 (77.1%) | 44 (22.9%) | <0.001‡ |
| Thorax | 59 (93.7%) | 4 (6.3%) | |
| Abdomen and pelvis | 229 (92.7%) | 18 (7.3%) | |
| Others | 67 (100.0%) | 0 (0%) | |
| Malignancy | |||
| Absent | 346 (92.0%) | 30 (8.0%) | <0.001‡ |
| Present | 157 (81.3%) | 36 (18.7%) | |
| ASA§ Grade | |||
| I | 378 (88.3%) | 50 (11.7%) | 0.91 |
| II | 125 (88.7%) | 16 (11.3%) | |
| Difficult airway (MPG§ ≥3) | |||
| Absent | 412 (90.4%) | 44 (9.6%) | 0.00‡ |
| Present | 91 (80.5%) | 22 (19.5%) | |
| Nature of surgery | |||
| Elective | 479 (88.9%) | 60 (11.1%) | 0.14† |
| Emergent | 24 (80.0%) | 6 (20.0%) | |
| ICU admission | |||
| No | 415 (100%) | 0 (0%) | <0.001 |
| Yes | 88 (57.1%) | 66 (42.9%) | |
| Vasopressor requirement | |||
| No | 445 (94.1%) | 28 (5.9%) | <0.001 |
| Yes | 58 (60.4%) | 38 (39.6%) | |
| Intraoperative fluids (ml)* | 1200 (1000, 1700) | 2500 (2000, 3000) | <0.001 |
| Blood loss (ml)* | 150 (70, 250) | 500 (350, 800) | <0.001 |
| Duration of surgery (mins)* | 150 (110, 200) | 352.5 (267.5, 430) | <0.001 |
*Body mass index (BMI); median with inter-quartile range (IQR) after Mann-Whitney U-test. †Fisher exact test was used to calculate P, rest all were calculated using Chi-squared test. ‡Refers to P found statistically significant (P≤0.05). §ASA – American Society of Anaesthesiologists; MPG – modified Mallampati grading
ICU admission was required for 27.1% of the patients for various reasons. Vasopressors were required in only 17.0% of the patients. Postoperative mechanical ventilation was required in 66 patients (11.6%). Amongst those, 43 were males and 23 were females. Mean duration of the mechanical ventilation was 213.7 ± 1032.4 minutes. Gender, organ involvement, malignancy, difficult airway, vasopressors, intraoperative fluid administration, blood loss, ICU admission and duration of surgery were significantly different in those patients who required mechanical ventilation, whereas parameters like BMI, ASA class, nature of surgery were found to be insignificant [Table 1]. Evaluation of laboratory parameters suggested that median CRP value was higher in those patients who required mechanical ventilation but was not statistically significant. We found a statistically significant difference in the median levels of albumin and CAR in mechanical ventilation group as compared to those who did not [Table 2].
Table 2.
Comparison of blood parameters with mechanical ventilation
| Variables | No mechanical ventilation | Mechanical ventilation | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| Median | IQR (Q1, Q3) | Median | IQR (Q1, Q3) | ||
| CRP1 (mg/dl) | 0.68 | 0.21, 2.06 | 0.96 | 0.29, 3.86 | 0.074 |
| Albumin (g/dl) | 3.5 | 2.5, 4.0 | 3.25 | 2.15, 3.70 | 0.004* |
| CAR2 | 0.20 | 0.07, 0.65 | 0.38 | 0.10, 1.45 | 0.024* |
*Refers to P found statistically significant (P≤0.05). CRP – C-reactive protein, CAR – CRP–albumin ratio
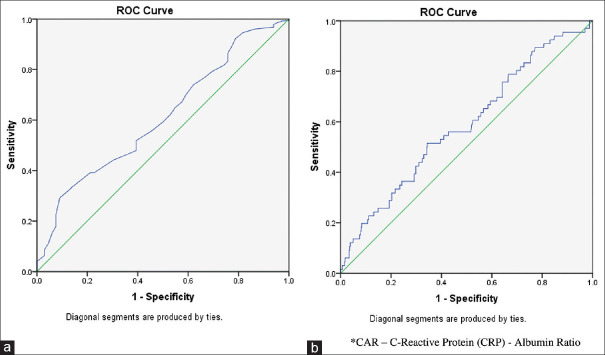
Since albumin and CAR were significantly different in patients requiring mechanical ventilation, we applied ROC curve to find out whether both the laboratory parameter discriminate between the requirement of mechanical ventilation or not. Figure 2 shows the ROC curve for albumin and CAR along with the need for mechanical ventilation, respectively. The area under the curve (AUC) for albumin was 0.61 (P = 0.004) and 0.58 for CAR (P value = 0.02) which were statistically significant. In the logistic regression analysis, after adjusting all the other variables, duration of surgery remained significantly associated with mechanical ventilation requirement [Table 3].
Figure 2.
Receiver operating characteristics curve for albumin levels and CRP-albumin ratio with mechanical ventilation requirement after surgery. (a) For albumin levels, (b) For CRP-albumin ratio
Table 3.
Logistic regression analysis
| Variables | Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
|
| ||||
| Lower limit | Upper limit | |||
| Age | 1.00 | 0.97 | 1.04 | 0.75 |
| Male Gender | 0.80 | 0.32 | 2.05 | 0.65 |
| BMI1 (Kg/m2) | 0.97 | 0.87 | 1.09 | 0.63 |
| Malignancy (reference non-malignancy) | 1.24 | 0.46 | 3.32 | 0.08 |
| Difficult airway (absent as reference) | 1.22 | 0.32 | 2.23 | 0.74 |
| Type of surgery (elective) | 2.92 | 0.44 | 19.51 | 0.27 |
| IV fluid | 1.00 | 0.99 | 1.00 | 0.86 |
| Blood loss | 1.00 | 1.00 | 1.00 | 0.18 |
| Vasopressor (absent as reference) | 1.51 | 0.60 | 3.77 | 0.38 |
| Duration of surgery | 1.01 | 1.00 | 1.01 | 0.001* |
| CRP2 (mg/L) | 0.98 | 0.92 | 1.03 | 0.41 |
| Albumin (g/dl) | 0.73 | 0.43 | 1.25 | 0.25 |
| CAR3 | 1.06 | 0.98 | 1.16 | 0.16 |
*Refers to P found statistically significant (P≤0.05). BMI – Body mass index, CRP – C-reactive protein, CAR – CRP–albumin ratio
A subgroup analysis of the CAR and its association with need for mechanical ventilation in patients with different malignancies was done. This revealed no significant association between CAR and need for ventilation even though the ratio was higher in patients requiring postoperative mechanical ventilation [Table 4].
Table 4.
Association of CAR1 with the need for mechanical ventilation amongst patients with malignancy
| n (%) | CAR [Median (IQR)] | P | |
|---|---|---|---|
| No mechanical ventilation | 157 (81.3) | 0.22 (0.09, 0.62) | 0.09 |
| Mechanical ventilation | 36 (18.6) | 0.40 (0.11, 0.99) |
CAR – CRP-albumin ratio
DISCUSSION
We observed CAR was significantly higher in patients requiring mechanical ventilation postoperatively as compared to those who did not require it, but it did not predict the same.
The CAR has been used in critically ill and malignant populations to assess varying outcomes. It has been found to be a predictor of long-term morbidity and mortality in critically ill[7-9] and a predictor of overall survival, cancer-free survival, recurrence-free survival in malignant patients.[6,10-14] To our knowledge, this ratio has never been studied as a marker to predict postoperative mechanical ventilation.
Surgical patients with different diseases have a combination of high CRP and low albumin resulting in a high CAR. CRP is a positive acute phase reactant; hence, its levels often increase secondary to inflammation, whereas serum albumin drops as it is a negative acute phase reactant.[15] In addition, undernutrition is often an accompaniment of different disease states further aggravating hypoalbuminaemia.[16] Therefore, the chances of such patients requiring mechanical ventilation support after surgery are expected to be higher.
A higher ratio with insignificant odds for predicting need of mechanical ventilation probably is a reflection of heterogenous groups of study participants. Additionally, the CRP value in itself varies during different time course of any disease in a given patient, who may present to the hospital during varying time periods of their disease course.[17] This would have affected the estimation of CRP and hence the ratio. Further, need for mechanical ventilation for a patient depends upon multiple factors like blood loss, blood transfusion and crystalloid transfusion.[18]
To the best of our knowledge, most of the published studies evaluating CAR and outcomes are retrospective in nature.[8,14,19,20] In contrast, the present study has the strength of a prospectively planned study and to follow outcome till the end.
CAR was found to be the predictor of overall survival and cancer-free survival in patients with malignancy.[6,10-14] Jabs et al.[21] demonstrated an enhanced expression of CRP by the cancer cells in renal cell carcinoma (RCC). Similarly, Sim et al.[22] and Steffens et al.[23] found an elevated CRP was associated with poorer survival in RCC patients. Szkandera, et al.[24] reviewed 304 patients and found that elevated CRP level is associated with poor survival in patients with soft tissue sarcoma. This conclusion was also corroborated by a meta-analysis done by Li et al.[25] In contrast, we found that the CAR did not predict the need of postoperative mechanical ventilation in the subset of our malignant patients. The reasons for such findings might be indicative of different behaviour of malignancy at different stages of the disease. Additionally, the primary outcome parameter in question probably matters. Although both mechanical ventilation and survival are valid outcome parameters with their own importance, the former one is a short-term outcome, whereas the latter is a long-term outcome. Hence, CAR may predict survival but not ventilation.
Preoperative hypoalbuminaemia is associated with higher mortality in patients who underwent surgery for RCC.[26] Liang et al.[27] found significant relationship amongst CRP, albumin and survival in soft tissue sarcoma patients. We found serum albumin was significantly lower in patients who required postoperative mechanical ventilation.
Guo et al. found low CAR to be associated with the early stages of RCC, whereas high CAR presents in the advanced or metastatic RCC suggesting that CAR could be a new prognostic indicator related to its progression.[11] More importantly, they also found that CAR is a predictor for recurrence of localised RCC who underwent full resection. Similarly, Shibutani et al.[28] concluded CAR to be a useful prognostic marker in patients with colorectal cancer who undergoing curative surgery. Other researchers found that increased CAR was significantly associated with larger tumour size, higher tumour grade and more advanced stages in soft tissue sarcoma patients,[27] pancreatic duct all cancer,[29] and laryngeal cancer.[30] Although not statistically significant, we observed a higher CAR in patients who required postoperative mechanical ventilation than in those who did not.
A few limitations of our study are inclusion of heterogeneous surgical patients and trying to predict an outcome with a single preoperative CAR. In spite of these, our study findings suggest higher CAR defines a possible need for postoperative mechanical ventilation. But this value alone cannot be used as a predictor for the same. CAR should be used only as a tool in conjunction with the clinical judgement till some future studies are exploring it further especially in different sub-groups like malignancy, ASA III or more, and those undergoing different categories of surgeries.
CONCLUSION
In summary, the CAR was found to be a valuable predictor of outcome in both critically ill and cancer patients similar to our findings. Although the results were statistically insignificant, an observation of higher CAR associated with higher need for mechanical ventilation is getting reflected sans prediction. Additionally, preoperative CAR will help us to triage the need of postoperative mechanical ventilation. To conclude, our study results indicate a higher preoperative CAR is associated with a need for postoperative mechanical ventilation in patients undergoing surgery under general endotracheal anaesthesia. This shall add to the optimisation of available limited resources.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Inglis R, Ayebale E, Schultz MJ. Optimizing respiratory management in resource-limited settings. Curr Opin Crit Care. 2019;25:45–53. doi: 10.1097/MCC.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campos JH. Prediction of postoperative mechanical ventilation after thymectomy in patients with myasthenia gravis:A myth or reality. J Cardiothorac Vasc Anesth. 2018;32:331–3. doi: 10.1053/j.jvca.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Ohkura Y, Miyata H, Konno H, Udagawa H, Ueno M, Shindoh J, et al. Development of a model predicting the risk of eight major postoperative complications after esophagectomy based on 10 826 cases in the Japan National Clinical Database. J Surg Oncol. 2020;121:313–21. doi: 10.1002/jso.25800. [DOI] [PubMed] [Google Scholar]
- 4.Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals:A systematic review. Am J Med. 2015;128:1023.e1–22. doi: 10.1016/j.amjmed.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation:conformational changes affect function. Biol Chem. 2015;396:1181–97. doi: 10.1515/hsz-2015-0149. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Jin K, Guo M, Long J, Liu L, Liu C, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. 2017;24:561–8. doi: 10.1245/s10434-016-5579-3. [DOI] [PubMed] [Google Scholar]
- 7.Ge X, Cao Y, Wang H, Ding C, Tian H, Zhang X, et al. Diagnostic accuracy of the postoperative ratio of C-reactive protein to albumin for complications after colorectal surgery. World J Surg Oncol. 2017;15:15. doi: 10.1186/s12957-016-1092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otowa Y, Nakamura T, Yamamoto M, Kanaji S, Matsuda Y, Matsuda T, et al. C-reactive protein to albumin ratio is a prognostic factor for patients with cStage II/III esophageal squamous cell cancer. Dis Esophagus. 2017;30:1–5. doi: 10.1093/dote/dox107. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Chen S, Zheng C, Ding M, Zhang L, Wang L, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17:1–8. doi: 10.1186/s12885-017-3220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803–10. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 11.Guo S, He X, Chen Q, Yang G, Yao K, Dong P, et al. The C-reactive protein/albumin ratio, a validated prognostic score, predicts outcome of surgical renal cell carcinoma patients. BMC Cancer. 2017;17:171. doi: 10.1186/s12885-017-3119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JE, Chung KS, Song JH, Kim SY, Kim EY, Jung JY, et al. The C-Reactive protein/albumin ratio as a predictor of mortality in critically ill patients. J Clin Med. 2018;7:333. doi: 10.3390/jcm7100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basile-Filho A, Lago AF, Menegueti MG, Nicolini EA, Rodrigues LAB, Nunes RS, et al. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients:A retrospective cohort study. Medicine (Baltimore) 2019;98:e16204. doi: 10.1097/MD.0000000000016204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh TK, Song IA, Lee JH. Clinical usefulness of C-reactive protein to albumin ratio in predicting 30-day mortality in critically ill patients:A retrospective analysis. Sci Rep. 2018;8:14977. doi: 10.1038/s41598-018-33361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watt DG, McSorley ST, Horgan PG, McMillan DC. Enhanced recovery after surgery:Which components, if any, impact on the systemic inflammatory response following colorectal surgery?:A systematic review. Medicine (Baltimore) 2015;94:e1286. doi: 10.1097/MD.0000000000001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosquera C, Koutlas NJ, Edwards KC, Strickland A, Vohra NA, Zervos EE, et al. Impact of malnutrition on gastrointestinal surgical patients. J Surg Res. 2016;205:95–101. doi: 10.1016/j.jss.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Sproston NR, Ashworth JJ. Role of C-Reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurajala I, Ramachandran G, Iyengar R, Durga P. The preoperative and intraoperative risk factors for early postoperative mechanical ventilation after scoliosis surgery:A retrospective study. Indian J Anaesth. 2013;57:14–8. doi: 10.4103/0019-5049.108554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao J, Agizamhan S, Zhao X, Jiang B, Qin H, Chen M, et al. Preoperative C-reactive protein/albumin ratio predicts outcome of surgical papillary renal cell carcinoma. Future Oncol. 2019;15:1459–68. doi: 10.2217/fon-2018-0611. [DOI] [PubMed] [Google Scholar]
- 20.Nakao Y, Yamashita YI, Arima K, Miyata T, Itoyama R, Yusa T, et al. Clinical usefulness of perioperative c-reactive protein/albumin ratio in patients with intrahepatic cholangiocarcinoma:A retrospective single institutional study. Anticancer Res. 2019;39:2641–6. doi: 10.21873/anticanres.13388. [DOI] [PubMed] [Google Scholar]
- 21.Jabs WJ, Busse M, Krüger S, Jocham D, Steinhoff J, Doehn C. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int. 2005;68:2103–10. doi: 10.1111/j.1523-1755.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 22.Sim SH, Messenger MP, Gregory WM, Wind TC, Vasudev NS, Cartledge J, et al. Prognostic utility of pre-operative circulating osteopontin, carbonic anhydrase IX and CRP in renal cell carcinoma. Br J Cancer. 2012;107:1131–7. doi: 10.1038/bjc.2012.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steffens S, Köhler A, Rudolph R, Eggers H, Seidel C, Janssen M, et al. Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC Cancer. 2012;12:399. doi: 10.1186/1471-2407-12-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Samonigg H, et al. Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br J Cancer. 2013;109:2316–22. doi: 10.1038/bjc.2013.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Liu X, Zhang J, Yao W. Prognostic role of elevated preoperative systemic inflammatory markers in localized soft tissue sarcoma. Cancer Biomark. 2016;16:333–42. doi: 10.3233/CBM-160571. [DOI] [PubMed] [Google Scholar]
- 26.Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59:923–8. doi: 10.1016/j.eururo.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Y, Xiao W, Guan YX, Wang W, Chen HY, Fang C, et al. Prognostic value of the C-reactive protein/Albumin Ratio (CAR) in patients with operable soft tissue sarcoma. Oncotarget. 2017;8:98135–47. doi: 10.18632/oncotarget.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res. 2016;36:995–1001. [PubMed] [Google Scholar]
- 29.Arima K, Yamashita YI, Hashimoto D, Nakagawa S, Umezaki N, Yamao T, et al. Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am J Surg. 2018;216:111–5. doi: 10.1016/j.amjsurg.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Kuboki A, Kanaya H, Nakayama T, Konno W, Goto K, Nakajima I, et al. Prognostic value of C-reactive protein/albumin ratio for patients with hypopharyngeal and laryngeal cancer undergoing invasive surgery involving laryngectomy. Head Neck. 2019;41:1342–50. doi: 10.1002/hed.25565. [DOI] [PubMed] [Google Scholar]