Abstract
Oxidative stress (OS), defined as redox imbalance in favor of oxidant burden, is one of the most significant biological events in cancer progression. Cancer cells generally represent a higher oxidant level, which suggests a dual therapeutic strategy by regulating redox status (i.e., pro-oxidant therapy and/or antioxidant therapy). Indeed, pro-oxidant therapy exhibits a great anti-cancer capability, attributing to a higher oxidant accumulation within cancer cells, whereas antioxidant therapy to restore redox homeostasis has been claimed to fail in several clinical practices. Targeting the redox vulnerability of cancer cells by pro-oxidants capable of generating excessive reactive oxygen species (ROS) has surfaced as an important anti-cancer strategy. However, multiple adverse effects caused by the indiscriminate attacks of uncontrolled drug-induced OS on normal tissues and the drug-tolerant capacity of some certain cancer cells greatly limit their further applications. Herein, we review several representative oxidative anti-cancer drugs and summarize their side effects on normal tissues and organs, emphasizing that seeking a balance between pro-oxidant therapy and oxidative damage is of great value in exploiting next-generation OS-based anti-cancer chemotherapeutics.
Keywords: Cancer treatments, Oxidative drugs, Drug-induced oxidative stress, Adverse effects, Precision medicine
1. Introduction
Redox homeostasis is essential for human health, and its disturbance is a significant cause of multiple major diseases, such as type 2 diabetes, atherosclerosis, chronic obstructive pulmonary disease, Alzheimer's disease, and cancer [1,2]. Redox imbalance in favor of oxidant burden is defined as oxidative stress (OS), which is frequently found in cancer cells and has become an emerging hallmark of various cancers [3,4]. Since OS promotes many malignant behaviors of cancer cells [5], antioxidant therapy may be an effective strategy to inhibit cancer initiation and progression. However, relevant clinical trials have always been reported to fail [[6], [7], [8]].
Inspiringly, OS can also be targeted as a vulnerability, in which cancer cells are more sensitive to pro-oxidant therapy (i.e., the treatments by triggering cytotoxic OS in cancer cells). Numerous pro-oxidant strategies are developed, some of which have become mainstream anti-cancer treatments in clinical practice, such as radiotherapy, chemotherapy [[9], [10], [11]]. In fact, studies have found that most chemotherapeutic drugs can kill cancer cells by directly or indirectly facilitating reactive oxygen species (ROS) accumulation, such as doxorubicin, cisplatin, 5-fluorouracil and arsenic trioxide (ATO) [12,13]. However, they also have many destructive effects on normal tissues and organs, which are even life-threatening [12]. Upon further studies of the mechanisms of their adverse effects, these drugs are frequently found to be hemotoxic because they cause sharp ROS accumulation and/or disrupt the endogenous antioxidant defense systems [14]. The chemicals that induce OS, either by generating ROS or by inhibiting antioxidant systems are collectively referred to as “pro-oxidants".
Although pro-oxidants can greatly benefit cancer patients, pro-oxidant therapy always faces a critical problem – severe adverse effects, largely limiting their wider applications [15,16]. Cancer patients generally experience over 40 different side effects after pro-oxidant therapy, such as nerve damage and bone marrow suppression, which are intimately related to the indiscriminate damage of excessive drug-induced OS [17]. Moreover, these side effects lead to reduced administration dosage and frequency of anti-cancer drugs, affecting therapeutic outcomes and patient survival [18]. Notably, precisely inducing OS has recently been recognized as a new strategy method in cancer treatments, which enhances the anti-cancer effects by elevating local OS levels in tumors or specific organelles and molecules of cancer cells, thus largely relieving the oxidative damage of the normal tissues [19,20]. However, the relevant technology is still in its infancy, and a large amount of basic research still needs to be implemented to prove the feasibility of the theory. Accordingly, pro-oxidants are promising in cancer treatment, but the problems of severe adverse effects and insufficient elimination remain resolved.
In this review, we systemically summarize the dual impacts of drug-induced OS in cancer therapy and propose some approaches aimed at seeking a balance between pro-oxidant therapy and oxidative damage. A comprehensive understanding of drug-induced OS will greatly help select scientific and rational therapeutic strategies and improve the outcome and survival of cancer patients.
2. An overview of oxidative stress in cancer treatments
Biological redox steady states are nonequilibria [21]. The dynamic regulation of intracellular redox status is essential for maintaining cellular homeostasis normal functions, and it has been phrased as “the golden mean of healthy living” [22,23].
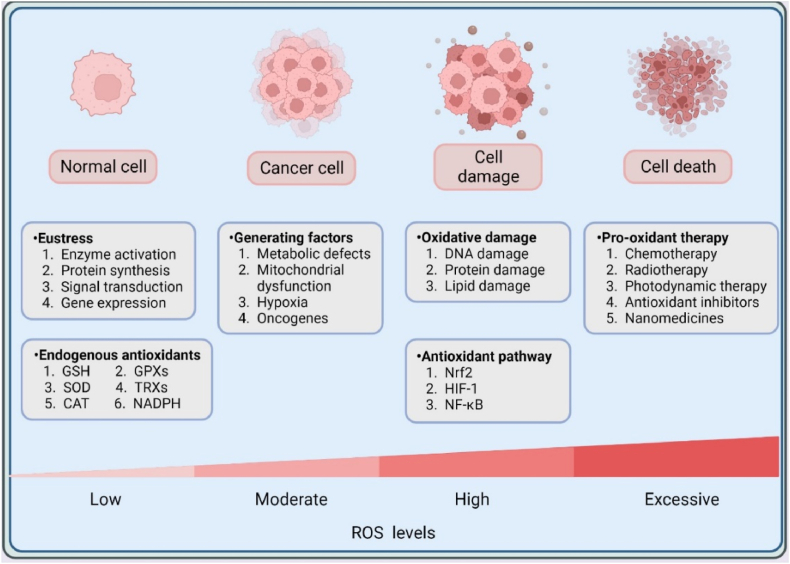
ROS are significant mediators of redox reactions, which are considered to be byproducts of cellular respiration [24]. ROS are primarily derived from numerous enzymatic and chemical reactions, such as cyclooxygenase, nicotinamide adenine dinucleotide phosphate NADPH oxidase (NOX), xanthine oxidase, lipogenesis, and iron-catalyzed Fenton reactions [25], occupying different forms like hydroxyl radicals (•OH), superoxide radicals (O2•−), and hydrogen peroxide (H2O2). In addition to ROS, redox molecules also consist of many other reactive substances, such as nitric oxide, hydrogen sulfide, and oxidized lipids [26,27]. Generally, low to moderate concentrations of ROS are beneficial for maintaining intracellular physiological activities and signaling pathways, while higher levels of ROS may lead to malignant transformation, cell damage and even death [28,29] (Fig. 1). For example, at a low physiological level of ROS in the nanomolar range, H2O2 is a significant factor in signal transduction through specific protein targets involved in the metabolic regulation and stress responses. This helps the cell adapt to constantly changing environments and stress [30]. However, excessive accumulation of H2O2 directly interrupts normal cellular functions.
Fig. 1.
The biological roles of ROS. Intracellular ROS levels mainly depend on the regulation of intracellular oxidative and antioxidant systems. In normal cells, endogenous antioxidants can effectively neutralize excessive ROS to maintain redox balance and cell homeostasis. However, higher ROS levels driven by different factors can lead to malignant transformation, which greatly promotes tumorigenesis and cancer progression. Furthermore, excessive ROS induced by dysregulated redox balance or exogenous pro-oxidant therapy will activate multiple antioxidant pathways and elicit oxidative damage to biomacromolecules and even cell death. Intracellular lipids, proteins, and DNA can be easily destroyed by ROS under OS, therefore carefully controlling the intracellular redox state is essential for human health.
In response to OS, cells have developed an antioxidant defense system to maintain cellular redox homeostasis and protect cells from damage. The antioxidant systems consist of endogenous and exogenous antioxidants. While exogenous antioxidants, such as N-acetyl cysteine, vitamin E (alpha-tocopherol), and vitamin C (or ascorbate), have shown significant antioxidant potential in preclinical studies, they still face challenges in clinical practice [31]. Endogenous antioxidants can be classified into antioxidant enzymes, catalytic antioxidant proteins, and small molecular scavengers based on their molecular weight. Specifically, antioxidant enzymes consist of superoxide dismutase (SOD), peroxiredoxin (PRX), and glutathione peroxidase (GPX); noncatalytic antioxidant proteins include thioredoxin (TRX), glutaredoxin, and metallothionein; small molecular weight antioxidants contain glutathione (GSH), bilirubin and urate [32].
Cancer cells present higher ROS levels than normal cells due to alterations in their metabolism, genomic instability, mitochondrial dysfunction, and tumor microenvironment modifications [33,34]. To avoid cell death, cancer cells also upregulate their antioxidant defense mechanisms to maintain ROS levels within a dynamic range [35]. Specifically, at the onset of cancer progression, cancer cells increase their NADPH production by activating antioxidant transcription factors and the pentose phosphate pathway (PPP), which enables them to survive at high ROS levels. During progression and metastasis, cancer cells respond to OS by increasing NADPH production in various ways, including activation of AMPK and PPP, reduced glutamine metabolism and folic acid metabolism [17]. In addition, appropriate levels of ROS often upregulate antioxidant transcription factors in cancer cells, such as nuclear factor erythroid 2-related factor 2 (Nrf2), to support cancer cell proliferation [36]. Notably, within this dynamic range, a certain amount of ROS is necessary for cancer development and normal metabolic of cancer cells [37,38]. For instance, OS can mediate inflammation-induced gene fusion formation to promote tumorigenesis [39], and some OS-regulated microRNAs, such as microRNA-210 and microRNA-128a, are involved in the cancer development [40]. In addition, some antioxidant pathways activated by OS, such as Nrf2, also significantly contribute to cancer progression [36]. Cancer cells maintain this “tense and fragile balance” by counteracting ROS accumulation to ensure cancer cell proliferation, metastasis, and other malignant phenotypes. From this perspective, reversing OS with antioxidants may be an effective way to treat cancer. However, most intracellular antioxidant defense is not provided exogenous or endogenous small molecules but antioxidant enzymes or proteins that utilize their specific substrates to reduce oxidants [5,41]. This may be the reason why small molecule antioxidants always fail in clinical trials [42,43]. A Mendelian randomization study explored the potential causal relationship between circulating vitamin C and the risk of five most common cancers. However, the results were negative and did not support the hypothesis that physiological levels of circulating vitamin C have a large influence on the risk of these cancers in European populations [44]. Similar outcomes were observed in studies conducted on breast, pancreatic, and prostate cancer patients (NCT00003639, NCT01080352, NCT00006392).
On the other side, persistent OS and intricate regulatory networks render cancer cells more susceptible to elevated ROS levels [45]. This high reactivity offers a breakthrough for many anti-cancer drugs and therapies [5,46]. Chemotherapeutic drugs with cytostatic activity eliminate cancer cells by generating high levels of intracellular ROS thereby triggering apoptosis, autophagy, and ferroptosis.
However, pro-oxidant therapy also has significant drawbacks. Many studies in the last two decades have shown that OS therapy can cause severe side effects [47]. For example, anthracyclines can inhibit nucleic acid synthesis and induce the accumulation of Fe2+ in cells, which can generate large amounts of O2− and OH− through the Fenton reaction and cause various forms of OS damage to cardiomyocytes [48,49]. Moreover, some cells, such as cancer stem cells (CSCs) and slow cycling cells, are less sensitive to OS and may survive the treatment and lead to cancer recurrence and metastasis [50]. These adverse reactions greatly limit the clinical application of pro-oxidant therapy, therefore it is urgent to identify the side effects before administering the drugs.
3. Representative drugs that induce oxidative stress in cancer treatments
Cancer cells are more vulnerable to OS-induced damage than normal cells [51]. Therefore, pro-oxidants have attracted increasing attention as potential chemotherapeutic agents [52,53]. Pro-oxidant therapy can be achieved by either directly generating ROS or targeting and inhibiting the endogenous antioxidant systems of cancer cells to indirectly elevate intracellular ROS concentrations. Various therapeutic approaches have been developed based on this principle, such as chemotherapeutic agents, small molecule compounds, phototherapy agents, drugs that modulate redox metabolites, and drugs that interfere with key signal transduction pathways in redox regulation [[54], [55], [56]]. However, in light of considerable side effects of pro-oxidant therapy, a comprehensive and detailed understanding of drug-induced OS is essential to enhancing anti-cancer effects and expanding clinical applications. In this section, we provide a list of several representative drugs that induce OS, whose pro-oxidant capacity and cancer-killing effects have been convincingly verified in both preclinical studies and clinical applications.
3.1. Direct ROS inducers
With the deepening of the understanding of cancer redox homeostasis in the medical community, cancer treatment strategies based on OS and oxidative damage have been proven effective. In fact, many FDA-approved anti-cancer drugs effectively eliminate cancer cells by increasing the production of ROS, which is supported by the enhanced antioxidant capacity decreasing cancer-killing effects of these drugs [57,58]. Chemotherapy drugs interfering with cell division (e.g., Vinca alkaloids and taxanes) or nucleic acid synthesis (e.g., 5-Fluorouracil, platinum complexes and anthracyclines) can trigger ROS production by disrupting mitochondrial functions [[59], [60], [61]]. Among these chemotherapy agents, anthracyclines as a class of anti-cancer antibiotics, are widely used in single or combined chemotherapy due to their strong ROS generation ability and anti-cancer effects. Evidence abounds that anthracyclines induce excess ROS in a variety of ways [15,62]. The widely accepted theories currently include DNA-intercalation and topoisomerase II poisoning [48]. For example, the specific chemical structure of anthracyclines decides that they can be reduced to the form of semiquinone. This process is catalyzed by NOX and nitric oxide synthase in the cytoplasm, as well as by mitochondrial electron transport chains (ETCs). These components can transfer electrons onto doxorubicin, forming a semiquinone complex. The latter is an unstable metabolite, that can be oxidized by oxygen in mitochondria, accompanied by the release of large amounts of ROS [63,64]. Another accepted mechanism of anthracycline-induced cytotoxicity is the formation of an anthracycline-iron complex that triggers lipid peroxidation, protein oxidation, and DNA damage by ROS production, leading to apoptosis and ferroptosis [65]. In addition, ATO is also a typical pro-oxidant chemotherapy agent [66]. In September 2000, FDA approved ATO (Trisenox™) for treating relapsed or refractory acute promyelocytic leukemia, and it since become the first-line therapy for acute promyelocytic leukemia [67]. The main source of ATO toxicity is its ability to induce OS through various pathways, such as disintegrating the mitochondrial membrane potential, deleting GSH and downregulating TRX-1 [[68], [69], [70]]; To extend the applications of ATO, numerous clinical trials have been conducted to investigate the role of ATO in solid tumors, but relevant results are disappointed (NCT01470248, NCT00024258). On the basis of this, the combined approaches have been proposed and gained exciting findings in preclinical studies. For instance, in intrahepatic cholangiocarcinoma (ICC), ATO and metformin synergistically prevented ICC proliferation by increasing intracellular ROS levels, cell apoptosis, and inducing G0/G1 cell cycle arrest in vitro and in vivo [71]. At present, many ongoing clinical trials are posted to assess the safety and efficacy of ATO coupling with other drugs in solid tumor therapy (NCT05721872, NCT04518501).
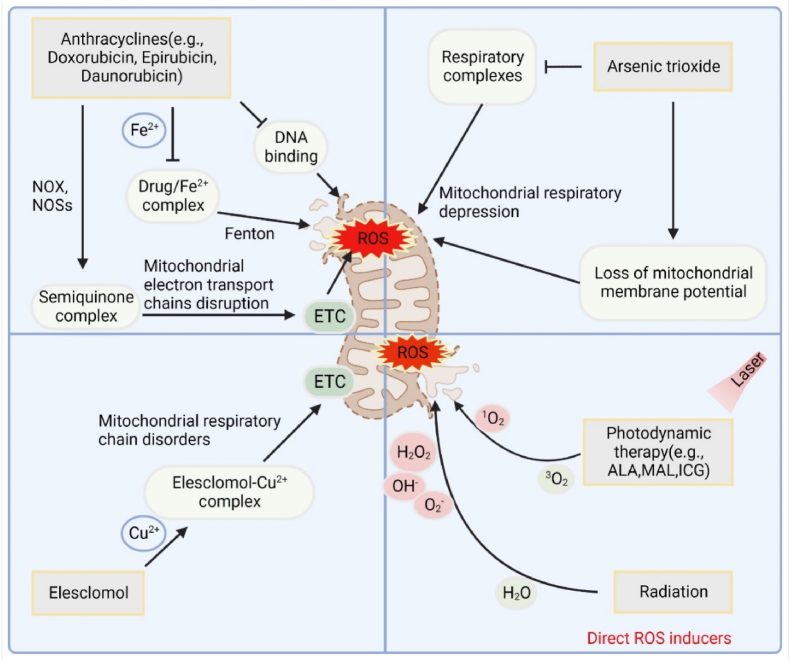
In recent years, some novel drugs (small molecule compounds) and repurposed traditional drugs have been reported to possess superior pro-oxidant properties and achieve remarkable therapeutic effects [72,73] (Fig. 2). For instance, elesclomol (STA-4783) is a synthetic small molecule compound that has exhibited anti-cancer activity in various cancers, such as melanoma and breast cancer [74]. Studies have shown that elesclomol can chelate copper ions and transport them into mitochondria, disrupting the mitochondrial respiratory chain and increasing cellular OS, ultimately leading to cancer cell apoptosis [75]. Several clinical trials (NCT00808418, NCT00088088, NCT00087997) have been conducted to assess the safety and efficacy of elesclomol, either alone or in combination with conventional chemotherapy agents, for the treatment of various cancers. Ivermectin is a broad-spectrum, high-efficiency, and low-toxicity antiparasitic drug that earned its discoverers the 2015 Nobel Prize in Physiology or Medicine. Dou et al. found that by blocking the PAK1/Akt axis in breast cancer, ivermectin exhibited an excellent pro-oxidant effect to induce autophagy in cancer cells via OS [76,77]. It is inspiring to note that ivermectin has entered clinical trials (NCT05318469, NCT02366884, NCT03305978), indicating its large potential for practical applications. In addition, photodynamic therapy (PDT) and sonodynamic therapy (SDT) are modern, noninvasive treatment modalities for the treatment of cancers and nonneoplastic diseases, which have exhibited superior antitumor activity. By exciting the photosensitizer molecules, PDT can utilize absorbed light to produce toxic ROS under NIR laser irradiation [78,79]. Likewise, SDT is a type of cancer treatment that uses ultrasound waves to activate a sonosensitizer drug, which then produces ROS and kills cancer cells [80,81]. Currently, a lot of clinical studies on PDT and CDT at different statuses have been carried out, and achieved some preliminary results (NCT03638622, NCT03344861, NCT03527225, NCT01012401).
Fig. 2.
Representative direct ROS inducers. A variety of anti-cancer drugs are capable of inducing OS, such as antibiotics (anthracyclines), antimetabolites (ATO), small molecule synthetic drugs (elesclomol) and photodynamic drugs, of which the mitochondria are the core functional organelles.
3.2. Indirect ROS inducers by blocking antioxidant systems
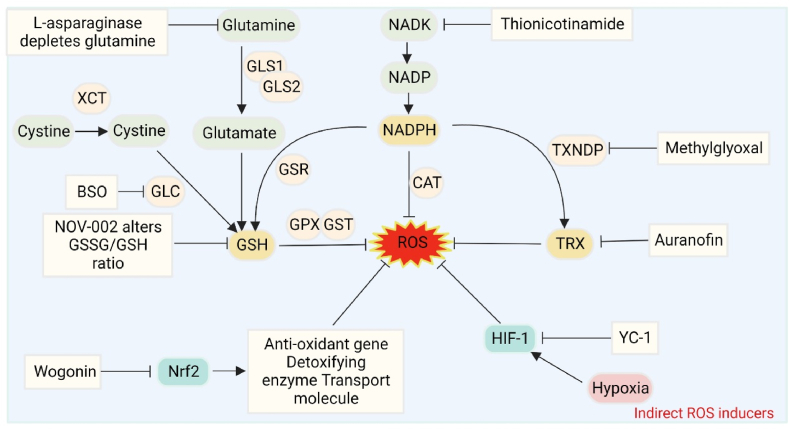
Except for direct ROS inducers, pro-oxidant strategies that target intracellular antioxidant systems and disrupt their balance can also elicit OS. The antioxidant process involves the neutralizing of ROS and the reversing of disulfide formation. This process relies on the regulation of two metabolic pathways: GSH and TRX [82]. Increasing studies have shown promise in molecules that modulate or interfere with redox signaling, which provide several viable therapeutic targets for many diseases, especially cancer [83] (Fig. 3).
Fig. 3.
Representative indirect ROS inducers. Representative indirect ROS inducers. Interventions by inhibiting the endogenous antioxidant systems, such as GSH, TRX, NADPH, Nrf2 and HIF-1 signaling pathways, can effectively augment the intracellular ROS levels and induce OS.
3.2.1. Drugs that affect GSH metabolism
GSH, a tripeptide composed of glycine, cysteine, and glutamate, is the most abundant and essential intracellular antioxidant. The reduced form of GSH is the predominant active state and form (approximately 95%) of GSH. When cells are exposed to ROS, the thiol group of the GSH cysteine can act as an electron donor to convert the reduced GSH to the oxidized form of GSH disulfide (GSSG) and protect cells from OS [84]. High levels of GSH are associated with resistance to chemotherapy and radiation independently [85,86]. Therefore, a promising strategy may be to selectively deplete GSH in cancer cells, which can increase intracellular ROS levels and induce OS-mediated cytotoxicity. Christine C. Gana et al. identified a novel small molecule modulator that induces OS by selectively enhancing the efflux of reduced GSH and abolishing the clonal capacity of cancer cells. Moreover, they showed that the toxicity could be rescued by the ROS scavenger N-acetylcysteine, suggesting that elevated GSH levels confer oxidative resistance in cancer cells, and that intracellular GSH depletion can overcome drug resistance and improve cancer therapy outcomes [87].
Furthermore, intervention in crucial steps in GSH synthesis is recommended as an effective way to decrease GSH levels. GSH synthesis depends on the catalysis of two ATP-dependent enzymes. First, γ -glutamic cysteine is synthesized from l-glutamic acid and cysteine. This conversion requires the linkage and modification of glutamate-cysteine ligase, which is the rate-limiting step for GSH synthesis. Second, glycine is added to the C-terminal of γ-glutamic cysteine with the catalysis of GSH synthase to eventually synthesizes GSH [88]. Buthionine sulfoximine (BSO), a γ-glutamic cysteine ligase inhibitor, has a potent inhibitory function on GSH synthesis. GSH deletion via BSO can sensitize breast cancer to radiotherapy in vitro and in vivo, suggesting that GSH inhibition can augment anticancer effects of pro-oxidant therapy [89]. Considering the capacity of powerful GSH elimination and reversing chemoradiotherapy resistance in preclinical studies, BSO has been used to reduce resistance to the chemotherapy of neuroblastoma in clinical trials, though the therapeutic outcomes are still unknown (NCT00005835, NCT00002730). Disulfiram, also known as antabuse, was discovered almost a century ago as an agent that inhibits acetaldehyde dehydrogenase, resulting in an immediate hangover when alcohol is ingested. In addition to its use in alcoholism treatment, disulfiram has been found to shift the ratio of GSH: oxidized glutathione (GSSG) to the oxidized state and induce OS. An in vitro and in vivo study has revealed that disulfiram can act as a potent radio-chemo sensitizer for head and neck squamous cell carcinoma, which results from the GSH exhaustion and ROS accumulation [90]. Several clinical trials are currently recruiting patients with various types of cancer to evaluate the therapeutic potential of disulfiram alone or in combination with chemotherapy drugs (NCT03323346, NCT02671890).
3.2.2. Drugs that regulate TRX metabolism
Aside from the GSH system, the TRX-PRX pathway is arguably the most crucial antioxidant system that maintains intracellular redox homeostasis by scavenging ROS, regulating redox enzymes, and cooperating with GSH redox systems [91]. It consists of TRX, TRX reductase (TRXR), and NADPH [92]. OS also occurs when the intracellular TRX system is inhibited. Inhibitors of the TRX system can suppress tumor development and progression by increasing OS, inducing cancer cell death [93]. Several natural and synthetic inhibitors of the TRX system have been developed as anticancer drugs, such as metal complexes, selenium compounds, penicillin derivatives and flavonoids [94,95]. The clinical efficacy and safety of TRX inhibitors remain to be evaluated, and relevant clinical trials are still in their infancy (NCT05187338, NCT01042746). Encouragingly, the TRX inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) has demonstrated excellent antitumor activity in both in vitro and in vivo experiments [96,97]. PX-12 has been shown to irreversibly thioalkylate the Cys 73 TRX residue, resulting in the inhibition of TRX redox activity. PX-12 is rapidly metabolized, producing two inactive metabolites, namely volatile 2-butanethiol and 2-mercaptoimidazole, which accompany TRX inhibition. As the first TRX inhibitor to enter the clinic, PX-12 has completed a clinical study to determine its efficacy and maximum tolerated dose in patients with advanced or metastatic cancer, though the results have not been posted (NCT00736372). Auranofin is an FDA-approved anti-rheumatic drug that can potentiate radiotherapy and chemotherapy by inhibiting the TRXR of cancer cells [98,99], and has been proven to have low toxicity and high selectivity in some tumor cells [[100], [101], [102]]. One study reported that auranofin could alleviate the intestinal toxicity of radiotherapy and chemotherapy by inducing reversible cell cycle arrest of normal intestinal cells via the p53/p21 pathway. Meanwhile, auranofin could enhance the cytotoxicity of radiotherapy and chemotherapy against colon cancer cells by inducing endoplasmic reticulum (ER) stress/unfolded protein response and apoptosis via proteasome inhibition [103]. Although auranofin has shown potential as an adjuvant drug for radiotherapy and chemotherapy, its differential anti-cancer effects in cancer and normal cells have not been well studied. A phase II clinical trial (NCT01737502) for patients with advanced or metastatic non-small cell lung cancer showed that the combination of auranofin and carboplatin/pemetrexed did not significantly improve the survival rate of patients or reduce toxic side effects. Another phase II clinical trial (NCT01730833) for patients with advanced or metastatic breast cancer showed that the combination of auranofin and paclitaxel prolonged the progression-free survival of some patients, but also increased toxic side effects. Currently, phase I/II clinical trials of auranofin have been conducted in canine osteosarcoma and several human cancer cells, showing promising results [104]. However, further basic and clinical research is necessary to validate and optimize its effectiveness.
3.2.3. Drugs that modulate NADPH metabolism
As previously reported, the regulation of high ROS levels depends on the content of GSH and TRX, both of which require the reductive production of NADPH [105]. GSH is converted to its oxidized form, GSSG, during the reduction of H2O2, and GSSG is reduced back to GSH by NADPH-dependent GSH reductase [106]. GSH is also involved as a substrate in the production of TRX-S2 and thioredoxin reductases 1 and 2 (TRXR1 and TRXR2) [90]. In addition, NADPH can maintain the antioxidant activity of catalase by binding to it [107,108]. Thus, reducing the ability of cancer cells to clear ROS by downregulating NADPH levels is expected to weaken their ROS buffering capacity and cause cell death [109]. NADK is a crucial enzyme in the cytoplasm that produces NADP, which can be rapidly converted to NADPH by reductase. Thionicotinamide is an NADK inhibitor prodrug that has been demonstrated to significantly downregulate NADPH levels by inhibiting NADK and glucose-6-phosphate dehydrogenase. This leads to increased ROS levels and subsequent apoptosis in colon cancer and lymphoma [110]. When used in combination with chemotherapy, thionicotinamide has been shown to have a better synergistic effect in eliciting tumor cytotoxicity [111]. Currently, no NADPH inhibitors have entered clinical trials. Although NADPH inhibitors have demonstrated potential for anti-tumor activity in vitro and in animal models, additional research is necessary to investigate their pharmacokinetic and toxicological properties in humans [93].
3.2.4. Drugs that interfere with redox signaling
Redox regulation involves a complex set of signal transduction regulatory pathways, similar to other body functions. Some of the canonical regulatory pathways include Nrf2-Keap 1 signaling [112], NF-κB signaling [113], and HIF-1 signaling [114], which are essential for maintaining redox homeostasis. Drugs that interfere with signal transduction pathways can also induce lethal OS for cancer therapy. PX-478 is a small molecule that has been shown to downregulate the hypoxia-induced expression of HIF-1α and HIF-1 transcription factor activity. It is a potent and selective inhibitor of HIF-1α, which is a key regulator of the cellular response to hypoxia. By inhibiting HIF-1α, PX-478 can reduce the expression of HIF-1 target genes, including VEGF and glucose transporter 1, which are involved in angiogenesis and glucose metabolism, respectively. PX-478 has been demonstrated anti-tumor activity in various cancer cell lines and animal models, and is currently undergoing clinical trials as a potential cancer treatment [115]. A phase I clinical study has evaluated safety and biological activity of PX-478 in advanced metastatic cancer (NCT00522652). Pharmacodynamic studies of PX-478 have revealed dose-proportional inhibition of HIF-1α levels. It means that as the dosage of PX-478 increases, the inhibition of HIF-1α levels also increases proportionally. This is important finding because it indicates that the drug is functioning as intended, and higher doses may potentially result in more significant therapeutic effects. The dose-proportional inhibition of HIF-1α levels has been observed in both preclinical studies and clinical trials of PX-478, supporting its potential as a promising cancer therapeutic agent. The NF-κB signaling pathway is a complex process that can be targeted at different stages, including Iκκ activation, IκB degradation, and NF-κB DNA binding. However, most of the research and efforts have been focused on developing Iκκb inhibitors. These inhibitors function by preventing the phosphorylation of IκB, which in turn prevents its degradation and keeps NF-κB in an inactive state in the cytoplasm. Though a lot of NF-κB signaling inhibitors have appeared, less or none enter the clinic. This may be attributed to the complexity and importance of NF-κB signaling in maintaining physiological homeostasis. Due to the pro-tumor activity of Nrf2, there is an increasing demand for negative modulators of this protein. Several small molecule compounds and natural compounds are identified to be Nrf2 inhibitors, such as ML385 and Brusatol, which exhibit significant antitumor activities in preclinical studies [116,117]. However, similar to NF-κB, there are currently no selective inhibitors available for Nrf2, and none are currently being evaluated in clinical trials.
4. The adverse effects of drug-induced oxidative stress in cancer treatments
As mentioned earlier, many drugs approved for clinical application have potent anti-cancer activity by inducing OS [118]. However, OS can also damage normal tissues that rapidly proliferate, such as gastrointestinal tract, bone marrow and hair follicles, leading to cardiac, hepatic, pulmonary, renal and gastrointestinal toxicities [119,120] (Table 1). Additionally, other adverse events reduce the efficacy of chemotherapy and promote cancer metastasis and recurrence, such as cancer cell adaptation to OS, cell cycle alterations by OS, or CSC escape from oxidative damage [[121], [122], [123]]. Therefore, pro-oxidant therapy has two major drawbacks in cancer treatment: side effects and drug resistance.
Table 1.
Side effects of the representative pro-oxidants.
| Drugs | Clinical applications | Major side effects | ROS sources | References |
|---|---|---|---|---|
| Anthracyclines (e.g., Doxorubicin, Epirubicin, Daunorubicin) | Breast, bile ducts, prostate, uterus, ovary, esophagus, stomach, liver, childhood solid cancers, osteosarcomas and soft tissue sarcomas, Kaposi's sarcoma, acute myeloblastic and lymphoblastic leukemia and Wilms Cancer | Neurologic disturbances, cumulative cardiotoxicity and bone marrow aplasia |
|
[49,124] |
| ||||
| Platinum complexes (e.g., cisplatin, carboplatin, oxaliplatin) | Testicular, ovarian, head and neck, bladder, lung, cervical cancer, melanoma, lymphomas. | Cisplatin: nephrotoxicity Carboplatin: myelosuppression Oxaliplatin: neurotoxicity |
|
[7,[125], [126]] |
| ||||
| 5-FU | Colorectal, breast, gastric, pancreatic, prostate, and bladder cancers | Cognitive disability cardiotoxicity |
|
[127,128] |
| ||||
| Gemcitabine | Colon, pancreatic, colon, ovarian, breast, head and neck and lung cancers | Myelosuppression, thrombocytopenia, anemia, granulocytopenia and neutropenia |
|
[129] |
| Arsenic trioxide | Acute promyelocytic leukemia | Cardiotoxicity, Hepatotoxicity |
|
[66,69] |
| ||||
| Cyclophosphamide | Leukemia, neuroblastoma, multiple myeloma, endometrial cancer, breast cancer, lung cancer, and organ transplant | Hemorrhagic cystitis, bone marrow suppression, cardiotoxicity, gonadal toxicity, and carcinogenesis | The metabolite, acrolein, increases lipid peroxidation. | [130,131] |
| Trastuzumab | HER2+ breast cancer | Cardiotoxicity |
|
[132,133] |
| ||||
| Paclitaxel | Ovarian, breast, and prostate cancer | Peripheral neuropathy | Impair the axonal transport of mitochondria and morphology and function. | [134] |
4.1. Oxidative stress contributes to drug-induced organ toxicity
Despite having favorable effects on cancer treatment, pro-oxidant therapy can also cause long-term organ damage and functional disabilities due to the non-targeted nature of oxidative damage. OS-induced organ damage can affect the design and completion of chemotherapy regimens, lower the quality of life, and shorten the lifetime of patients with cancer.
4.1.1. Cardiotoxicity
Cancer and heart disease are the two leading causes of human death. With the development of anti-cancer therapy, the survival rate of cancer patients has significantly improved. However, this also comes with a higher incidence of cardiovascular adverse events caused by cancer treatment, such as endothelial dysfunction, arrhythmia, and heart failure, which impair the quality of life of cancer patients [130,135]. A large body of literature evidence indicates that OS plays a vital role in the cardiotoxicity of different classes of anti-cancer drugs, including chemotherapy, targeted therapy, and immunotherapy [[136], [137], [138], [139]]. These adverse events can significantly impact the well-being and life quality of cancer patients, leading to dose modifications and interruption or discontinuation of anti-cancer treatments in severe cases.
Anthracyclines are one of the most prominent examples of chemotherapy-induced cardiac damage. One of the main causes of anthracycline-related cardiomyopathy is mitochondrial DNA injury due to OS exposure [140,141]. When the cumulative dose of doxorubicin exceeds 400–700 mg/m2 (adults) and 300 mg/m2 (children), it can induce OS and even congestive heart failure [142]. Anthracyclines can be reduced to semiquinones under the catalysis of enzymes and react with oxygen to generate superoxide anion (O2−), which is further converted by SOD to H2O2. H2O2 is a relatively stable and low-toxic species, but it can react with O2− in the iron-catalyzed Fenton reaction to generate highly reactive and toxic •OH [51,143] Iron also plays a crucial role in doxorubicin-induced cardiotoxicity. Doxorubicin can interact with iron to form reactive anthracycline-iron complexes through Fenton reactions, which disrupt normal iron homeostasis [144]. Excessive ROS production can damage multiple components near the mitochondria, interfere with normal mitochondrial function, and decrease energy production efficiency. The heart muscle consumes the most energy per gram of tissue and has mitochondria that account for 1/3 of the total cell volume. Therefore, mitochondrial dysfunction strongly affects the heart muscle, which depends on high metabolic demands and energy consumption. This may be the fundamental reason why cardiomyocytes are vulnerable to OS damage [145].
4.1.2. Neurotoxicity
The brain and nerves are also common areas of non-targeted OS damage. Like the myocardial tissue, cranial nerve cells are also non-regenerative cells, so oxidative brain injuries can seriously affect the patients' life quality and interfere with treatment regimens [146]. Pro-oxidants-induced OS may lead to “chemobrain” symptoms such as memory loss, distraction, and decreased executive function [147], as well as central or peripheral neuropathy symptoms, such as spontaneous pain and mechanical/cold allodynia in the extremities [148]. Compared to the multiple mechanisms by which chemotherapy drugs induce cardiac oxidative damage, the relevant mechanistic questions in the brain remain poorly understood [149]. Nowadays, it is known that the pathogenesis of this organ toxicity is more complex, in which OS plays a significant role [150]. Mitochondrial dysfunction, elevated levels of tumor necrosis factor-α (TNF-α), and the translational effects of the drug itself may be the sources of high ROS [151]. Previous studies have identified that ROS can downregulate and destroy the blood-brain barrier (BBB) by generating downstream cytokine-mediated signaling events, leading to OS in brain tissues, despite the existence of protective mechanisms at the BBB [152,153]. In addition, OS can damage to plasma proteins in the blood, which may be one of the mechanisms of oxidative damage that penetrates the BBB [154]. Several chemotherapeutic drugs have been demonstrated to be able to induce OS in the central or peripheral nervous system, suggesting that pro-oxidants can breach the BBB. In a rodent model of breast cancer, doxorubicin and cyclophosphamide increased protein oxidation and lipid peroxidation, respectively, in the mouse brain tissues [[155], [156], [157]]. Moreover, the commonly used anti-cancer drugs cisplatin and taxane can trigger OS in ganglion cells in rodent models by damaging mitochondrial structures, ultimately leading to cognitive impairment and peripheral neuropathy [158].
4.1.3. Hepatotoxicity
Drug-induced liver injury (DILI) is a major cause of drug discontinuation in both preclinical and clinical stages, posing a significant challenge for physicians, patients, and pharmaceutical companies [159,124]. The liver is the major site of metabolism for most chemotherapeutic drugs, which produces large amounts of ROS in the course of detoxifying xenobiotic and toxic substances, and accompanied OS has been shown to be linked to liver diseases, such as hepatotoxicity, and other liver pathological conditions [160,161]. Drugs, especially chemotherapeutic drugs, can also cause endogenous liver damage by interfering with specific cellular functions or producing toxic metabolites, which are dose-dependent and predictable. OS is also one of the essential mechanisms of DILI. Drugs-induced OS in the liver can result in lipid peroxidation, mitochondrial dysfunction, ER stress, and DNA damage, directly leading to liver damage [162,163]. Hepatotoxicity has been documented in patients receiving low-dose cisplatin [164]. Cisplatin accumulates in large amounts in liver cells, and induces hepatotoxicity by generating oxygen metabolites. It directly generates ROS (H2O2) through a cascade reaction, and simultaneously reduces the activity of most enzymes in the antioxidant defense system of hepatocytes, including SOD, catalase and GPX. In addition, cisplatin can bind to GSH to form a cisplatin-GSH conjugate, leading to GSH depletion and ROS accumulation [125,126]. Methotrexate (MTX) is a folic acid antagonist widely used in treating cancers such as osteosarcoma, acute lymphoblastic leukemia, breast cancer, and non-Hodgkin lymphoma [165]. However, it is also a representative drug that induces hepatotoxicity. Many models of hepatotoxicity have been established with MTX [166,167]. Previous reviews and studies have suggested that intraperitoneal injection of MTX can increase serum transaminases, bilirubin, and lipid peroxidation and reduce the activity of intracellular antioxidant enzymes in rat liver tissue, resulting in OS-mediated liver fibrosis, and intrinsic apoptosis [[168], [169], [170]].
4.1.4. Others
Because the damage of drug-induced OS is untargeted, the associated side effects can spread across multiple organs and systems in the body. Kidney damage caused by pro-oxidants is a growing concern. According to statistics, between 2007 and 2014, the incidence of acute kidney injury in patients receiving chemotherapy increased by three times (1.8%–5.2%) [171]. OS plays an important role in almost all cases of impaired kidney function. Pro-oxidants, such as cisplatin, cyclophosphamide, gemcitabine and MTX [172,129], generate ROS that contribute to the increased OS level in renal cells, which conduces to renal tissue damage, fibrosis, and abnormal renal function [173]. Myelosuppression is another common and severe side effect of cytotoxic drug use. Numazawa et al. suggested that 5-FU-induced OS was involved in myelosuppression [127]. In addition, a glycoprotein (ZPDC) isolated and purified from the fruit of Zanthoxylum piperitum was shown to protect against cyclophosphamide-induced OS and myelosuppression, indicating that drug-induced OS is a cause of chemotherapy-related myelosuppression [174]. Elevated OS can also lead to some degree of enterotoxicity. It is estimated that approximately 60%–100% of patients experience gastrointestinal toxicities during chemotherapy, including mucositis, diarrhea, and constipation [175]. Other commonly used cytotoxic drugs such as irinotecan and 5-FU have also been proven to interfere with gastrointestinal function by inducing OS [176,128]. In addition to these examples, the side effects of drug-induced OS on the body also include fatigue, aging, hair loss, and skeletal muscle weakness [[177], [178], [179]].
4.2. Drug-induced oxidative stress decreases anti-cancer efficacy
In recent decades, pleiotropic functions of OS have been gradually revealed, including inhibition of intracellular signal transduction, gene transcription, and DNA repair; delay or blockage of the cell cycle; induction of cell apoptosis; and the development of cancers [180]. Consequently, manipulating intracellular redox status of cancer cells seems to be an attractive and promising approach for cancer therapy. However, studies have shown that some pro-oxidant therapies also promote ROS-induced drug resistance [181,182]. Possible causes may include lipid peroxidation during OS induced by chemotherapy drugs. It produces plenty of electrophilic aldehydes, which can slow down the cell cycle of cancer cells, thereby decreasing the efficacy of drugs that rely on preventing cell replication cycles to kill cancers [183]. Secondly, heat shock protein (HSP) acts as a molecular chaperone and has a strong protective effect on cells. Studies have shown that during acute or chronic OS, intracellular activities like protein misfolding, protein aggregation, and destruction of regulatory complexes may lead to HSP overexpression and reduced apoptosis of cancer cells, which may be the causes of resistance to OS-based chemotherapy [184]. In addition to the above factors, the failure of pro-oxidant therapy caused by the adaptation and insensitivity to OS of a small population of cancer cells has gradually attracted attention, and it is also a major obstacle to pro-oxidant drug failure in clinical applications [185,186].
4.2.1. Oxidative stress activates endogenous antioxidant pathways
Over the past decade, it has been found that pro-oxidants, such as chemotherapeutics, can drive resistance phenotypes by continuously generating ROS and inducing OS. Indeed, intracellular ROS not only activates OS, but also promotes the resistance of cancer cells to OS by activating multiple antioxidative signaling pathways [187]. The effects of ROS depend on the severity and duration of exposure. Prolonged exposure to high doses of ROS can induce genetic mutations, destroy biomolecules, promote the transformation of normal cells into cancer cells, or induce cell death [188]. However, exposure to low concentrations of ROS seems to be involved in cell proliferation and differentiation. Several studies have indicated that cancer initiation and progression, malignant transformation, and chemoresistance may be promoted during the initial stages of ROS accumulation or when OS is weak. Correspondingly, cancer cells exhibit adaptations to OS that underlie their drug-resistant phenotype [188,189]. This view also supports the concept of metabolic reprogramming of cancer cells in response to stressful environments [190,191], that is, the adaptation of cancer cells to the stressful environment by reprogramming metabolic processes could serve as a leading cause of therapeutic resistance and cancer progression [192].
Cancer cells rely on a complex redox network to adapt to OS. Cancer cells rely on a complex redox network to adapt to Nrf2, which controls the expression of many ROS detoxification genes and contributes to drug resistance [193]. Nrf2 can be considered as the central node of the regulatory network. Previous studies have shown that Nrf2 can directly affect the synthesis and expression of redox-related substances such as GSH, mitochondrial antioxidant enzymes, NADPH, etc., under OS [[194], [195], [196], [197]]. In addition to directly affecting the functions of redox-related substances, Nrf2 also regulates other components involved in cancer drug resistance phenotypes [198,199]. For example, this transcription factor can enhance the efflux of chemotherapeutic drugs by inducing the overexpression of efflux pumps, resulting in a broad-spectrum drug resistance phenotype known as MDR [200]. Nrf2 has been reported to be upregulated in various OS cancer cell models and promoted chemoresistance. For example, Nrf2 is overexpressed in an H2O2-induced OS model of hepatocellular carcinoma, which can be effectively inhibited by the antioxidants tocopheryl acetate and S-adenosylmethionine [201]. Furthermore, Nrf2 was found to be significantly upregulated in chemo resistant cancer cells, and silencing Nrf2 was validated to promote cancer chemosensitivity to pro-oxidants, such as doxorubicin, cisplatin, and etoposide [202]. Nrf2 provides antioxidant protection to cancer cells by regulating the expression of antioxidant response elements and anti-apoptotic protein (Bcl-xL), ultimately facilitating cancer cell survival and drug resistance [203]. The mechanisms of chemoresistance involving Nrf2 inhibition of OS in various cancers have been extensively elucidated [[204], [205], [206], [207], [208], [209]]. Targeting the Nrf2 molecule is a potential therapeutic strategy to reverse the resistance of pro-oxidant therapy. An experimental report suggests that inhibition of the Nrf2-ARE pathway increases artesunate sensitivity and reverses OS resistance in head and neck cancer cells [210]. Chen et al. confirmed this notion by knocking down Nrf2 in the SKVO3 cell line increased cisplatin-induced ROS production and elevated p38/JNK phosphorylation levels, ultimately enhancing OS sensitivity to cisplatin [211]. The exploration of the regulation and function of Nrf2 expression deserves further investigation to identify new therapeutic targets beneficial for cancer therapy and overcoming drug resistance.
4.2.2. Oxidative stress induces phenotypic plasticity
CSCs are a subset of cancer cells with the capability of self-renewal and proliferation. They are considered to be the critical origin of cancer initiation [212,213]. Their presence can contribute to the resistance to multiple therapeutic modalities, which depends on the low intracellular ROS levels, superior DNA repair ability and powerful ROS scavenging systems. These properties enable CSCs to cope with environmental changes and resist therapy more effectively than normal cancer cells [214]. Studies have shown that DNA oxidation can transform some cancer cells into CSCs [215,216]. Moreover, ROS can also promote EMT, which gives cancer cells CSC-like traits, such as high self-renewal and drug/radiation resistance [217,218]. Additionally, CSCs have an enhanced ROS removal ability, manifested by enhanced mitochondrial respiration and GSH production, which helps them keep low ROS levels or even a quiescent (or dormant) state to escape injury during pro-oxidant therapy [214,[219], [220], [221]]. In conclusion, in the pro-oxidative therapy, a small group of cells is capable of escaping from oxidative damage, hence more precise methods of inducing destructive OS need to be conducted.
5. Strategies for enhancing antitumor effects of drug-induced oxidative stress while mitigating toxicity in cancer therapy
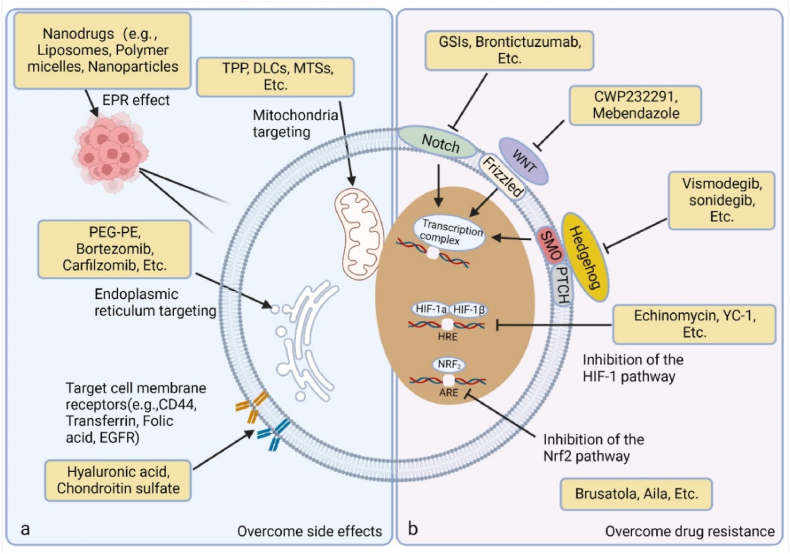
Globally, the cytotoxic effects of ROS achieved by manipulating redox equilibrium for cancer treatments have shown great potential in clinical translation [48,222]. However, due to the nonselective drawbacks of OS, pro-oxidant strategy inevitably leads to normal tissue damage and resistance to OS, which are the current focus of redox medicine and accounts for inconsistency between clinical and laboratory findings based on ROS. Further studies aimed at managing the adverse effects and therapy resistance induced by OS are needed to fully exert the therapeutic potential of pro-oxidant treatments. Therefore, the scientific community is trying to find a balance between the cytotoxic effect and the adverse effect to maximize the benefits of pro-oxidant therapy benefits, minimize adverse reactions, and improve survival [184,223] (Fig. 4).
Fig. 4.
Targeted strategies to maximize the efficacy and minimize the adverse effects of pro-oxidant anti-cancer therapy. a. To maximize the killing effect of OS and reduce the side effects related to oxidative damage, improving the targeting of drugs is a balanced approach. Recently, rapid progress has been made in encapsulating pro-oxidants with specific materials to make nanoscale particles. Due to the appropriate particle size or some special targeting recognition capabilities, nanoparticles can target and enrich cancer sites, or even subcellular structures of cancer cells such as cell membranes and mitochondria. b. For drug resistance considerations, targeting the phenotypic signal transduction pathways of CSCs is a wise choice to overcome resistance. The activation of some highly conserved signal transduction pathways may be involved in the phenotype of CSCs, including Notch, Hedgehog, and WNT pathways, and corresponding inhibitors have also successively shown the hope of overcoming the resistance of CSCs.
5.1. Precision targeting in cancer therapy: nanoparticle-based approaches
As we described above, one of the most important missions is to deliver anti-cancer drugs precisely to the target region to avoid severe nonselective injury of normal tissues. Several targeting strategies have achieved considerable progress and applications motivated by this consideration.
5.1.1. Targeting the primary lesion
To precisely deliver pro-oxidants to the primary lesion, many approaches have been developed and applied. For solid tumors, the enhanced permeability and retention effect provides a rationale and guidelines for tumor-targeted nanodrug cancer enrichment [224]. Specific modification of nanoscale particles can improve the biocompatibility and stability of the encapsulated drug and prolong the circulation time, thereby increasing its safety and effectiveness [225,226]. These findings have led to extensive research in nanomedicine, which has shown promising results in vitro and in animal models [227]. In the 1990s, the FDA approved the first nanodrug formulation, named liposomal doxorubicin (Doxil). As the first-generation nanodrug, Doxil improves the bioavailability and reduces its dose dependence, which officially opened the door for the nanomedicine field [228]. In addition to chemotherapeutic drugs, the combination of different redox-active compounds has also been described to contribute to pro-oxidant therapy. Fady N. and colleagues discovered two distinct oxidatively active species, azines and copper (II), that exhibit a high degree of synergy. They exploited biological differences between normal cells and cancer cells to target cancer cells and induce a high level of OS while avoiding damage to normal cells [229]. It is worth mentioning that targeted induction of OS remains the most crucial task in the nano platform [230]. In the past 30 years, researchers have been working on the development of a highly specific drug delivery system, which can reach the designated cancer microenvironment or cellular structures to improve the therapeutic efficacy and achieve cancer targeting. Many nanoparticles have been designed as drug delivery systems to target deeper subcellular units.
5.1.2. Targeting subcellular structures
In recent years, various theragnostic probes that target different subcellular organelles in cancer cells, including ER, lysosomes, and mitochondria, have been elucidated to be effective [231]. Triphenylphosphine is a lipophilic compound with mitochondrial targeting effect, that can easily pass through the biomembrane structure and bind to the mitochondrial membrane, precisely delivering drugs to mitochondria. For example, the chemical linking of mitochondria-targeting to transport DOX to mitochondria has successfully achieved the accurate induction of OS and apoptosis through the mitochondrial pathway in tumor cells [[232], [233], [234]]. Other approaches to target the mitochondrial compartment have focused on the protein channel (BAK), which is a pro-apoptotic effector protein at the outer mitochondrial membrane. Bcl-2 homology domain 3 mimetics, such as ABT199, which can activate the intrinsic apoptosis pathway of the BAK-BAX complex to introduce pores in the mitochondrial membrane, enhancing the activity of mitochondrial metabolism-targeted drugs, inducing mitochondrial dysfunction and OS [235]. Likewise, the ER, an essential intracellular organelle, is responsible for the synthesis, posttranslational modification, and delivery of biologically active proteins and participates in numerous enzymatic reactions, including redox balance, protein folding, intracellular calcium storage, and lipid biogenesis. Some FDA-approved drugs have shown the ability to target and induce ER stress, such as bortezomib [236], carfilzomib [237], and polyethylene glycol-phosphoethanolamine [238]. In addition, several drugs have been found to selectively target cancer cells and induce apoptosis by disrupting the cells’ ability to repair ER stress, exacerbating OS. These include the HSP 90 inhibitor (geldanamycin or 17-AAG) and the BiP inhibitor (versipelostatin) [239,240].
5.1.3. Targeting the specific molecules
Individualized molecular targeted drug therapy can selectively kill cancer cells with well-defined (or highly expressed) biomarkers of cancer driver genes, achieving precise drug delivery [241]. Cluster of differentiation-44 (CD44) is a glycoprotein that functions as a hyaluronic acid receptor and is widely expressed on mammalian cell surfaces. Overexpression of CD44 is a hallmark of various solid cancers [242]. Polysaccharide-based biomaterials, such as hyaluronic acid and chondroitin sulfate, are major CD44-targeting binding molecules that have good biocompatibility and selective CD44 targeting. Due to the unique functions of receptors and ligands in cancer cells, they have aroused great interest in drug delivery systems for cancer therapy [243]. Some nanoparticles thus acquire the homing ability of active targeting, which dramatically improves the precision of drug delivery [244,245]. For instance, in the study of Min Mu et al., HA was used to deliver drugs to overexpressed CD44 receptors on tumor surfaces, amplifying OS for tumor killing [246]. In addition to CD44, other promising active targets include transferrin [247], folic acid [248,249], and epidermal growth factor [250].
5.2. Antioxidants as effective allies in pro-oxidant therapy:a balanced strategy
Moreover, several novel strategies have emerged that offer a promising balance between the benefits and harms of pro-oxidant therapy. Unlike the simple use of antioxidants, some redox-active drugs can serve as adjuvants for cancer treatment, protecting normal tissues from OS damage while preserving or enhancing the efficacy of tumor radiotherapy and chemotherapy.
Antioxidant enzyme mimetics are compounds that mimic the function of antioxidant enzymes, protecting cells from OS. Several trials are underway or have been completed to evaluate the efficacy of antioxidant enzyme mimetics [[251], [252], [253], [254]]. For example, GC4419 is an investigational SOD mimetic drug that is being developed to protect normal tissues from radiation-induced oral mucositis and enhance the radiation therapy effect in patients with head and neck cancer (NCT03689712). It has demonstrated significant clinical benefit in a phase III trial and has been granted breakthrough therapy status by the FDA [255]. Ebselen is a GPX mimic that has been investigated as a radiomodulator for glioblastoma multiforme, a malignant brain tumor [256]. By increasing ROS levels and inducing DNA damage and apoptosis, ebselen may enhance the radiosensitivity of glioblastoma multiforme cells. Conversely, ebselen can protect normal cells from radiation-induced injury by reducing ROS levels and inflammation [257]. Ebselen has also demonstrated potential for preventing cisplatin-induced ototoxicity by mimicking GPX activity and modulating the p53 pathway [258].
Pharmacological ascorbate has been proposed as a potential anti-cancer agent when combined with radiation and chemotherapy, which can have therapeutic and adjuvant benefits for cancer treatment. It serves as a donor antioxidant in normal tissues, sparing them from the damage of radiotherapy and chemotherapy, while exploiting the fundamental differences in their oxidative metabolism, and potentiating the anticancer effects. Pharmacological ascorbate was used in a study to enhance the efficacy of radiation therapy and chemotherapy for advanced glioblastoma. The study found that pharmacological ascorbate enhanced the cytotoxicity of radiation therapy and temozolomide by altering the iron redox state within the tumor, while protecting normal tissues from damage [259]. In addition, a clinical study demonstrated that ascorbate selectively generates H2O2 in cancer cells by autoxidation, which interferes with the iron homeostasis in cancer cells and induces iron-mediated OS and cell death in NSCLC and GBM [260]. In line with this, other clinical studies have found that ascorbate reduced radiation-induced normal tissue toxicity in pancreatic or ovarian cancer and enhanced tumor radiosensitivity. They suggested that ascorbate could be used as a simple, nontoxic agent to improve the outcome of radiation therapy for pancreatic cancer [261,262]. Several clinical trials are currently underway to investigate the safety and efficacy of pharmacological ascorbate in various malignancies, such as pancreatic cancer, glioma and lung cancer [[263], [264], [265]].
6. Concluding remarks and future perspectives
Redox status has been shown to play a crucial role in determining cancer cell fate. While various drugs manipulating redox homeostasis have achieved promising outcomes in killing cancer cells, antioxidant therapy alone has been claimed to fail in some clinical trials. Cancer cells are more vulnerable to oxidative damage due to their higher basal level of ROS compared to normal cells, making pro-oxidant therapy a potential option for cancer treatment. However, the uncontrollable or indiscriminate OS can damage most cells, including both cancer cells and normal cells, leading to a poor outcome and affecting cancer patient survival. Additionally, pro-oxidant therapy can lead to the occurrence of a small population of cancer cells with stem cell properties, which can cause cancer recurrence and metastasis. Balancing the cancer-killing effects and oxidative tissue damage of pro-oxidant therapy remains an unresolved issue.
Inspiringly, the emergence of nanomedicine has made targeted strategies possible in cancer therapy. Specific properties of cancer cells have been utilized to deliver pro-oxidant agents to primary lesions and subcellular structures, resulting in significant success in killing cancer cells while sparing normal cells. Notably, though antioxidant therapy alone fails in clinical cancer treatment, combined strategies with chemoradiotherapy have exhibited superior effects in several clinical trials. Antioxidant agents can largely relieve oxidative damage and sensitize tumor tissues. Nonetheless, this approach still needs extensive experiments to evaluate the dosage of antioxidants and avoids excessive or insufficient neutralization of ROS toxic effects.
Given the complexity of redox mechanisms in different cancer patients, types of cancer, and stages of cancer progression, manipulating redox status arbitrarily may be inappropriate. Therefore, precisely regulating redox balance is of great significance and has become a key focus in developing next-generation redox medicine for cancer therapy. Several studies have suggested that specifically modulating antioxidant pathways shows great potential in decreasing adverse effects and overcoming treatment resistance. Though promising, a comprehensive understanding of redox mechanisms in cancer progression is still challenging and requires extensive experimental validation. Additionally, individual differences can lead to differential drug responses, making the development of individual-oriented precision medicine a necessity to advance redox medicine.
Author contributions
FG, JT, LL and CHH conceived the structure of the manuscript. HJ and JZ drafted the initial manuscript. FG, JT and LL prepared and revised the manuscript. HJ and JZ prepared the figures. BWL, RC, KJL, XHX and SJL are responsible for the sorting of the checksum format of writing. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from Guangdong Basic and Applied Basic Research Foundation (2019B030302012), National Natural Science Foundation of China (81821002), Natural Science Foundation of Ningbo (No. 2022J236), Wu Jieping Medical Foundation (320.6750.18492), Department of Education, Heilongjiang Province (SJGY20200447) and the Science and Technology Support Program of Sichuan Province (2021YFG0137). These figures were created by Biorender.
Contributor Information
Lin Liu, Email: 104797809@qq.com.
Jing Tang, Email: tangjing@wchscu.cn.
Feng Gao, Email: gf9777@126.com.
Data availability
The data that has been used is confidential.
References
- 1.Zuo J., Zhang Z., Luo M., Zhou L., Nice E., Zhang W., Wang C., Huang C.J.M. Redox signaling at the crossroads of human health and disease. Medcomm. 2022:e127. doi: 10.1002/mco2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennicke C., Cochemé H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell. 2021;81:3691–3707. doi: 10.1016/j.molcel.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Harris I.S., DeNicola G.M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30:440–451. doi: 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Forman H.J., Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngo B., Van Riper J.M., Cantley L.C., Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer. 2019;19:271–282. doi: 10.1038/s41568-019-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiel C., Le Gal K., Ibrahim M.X., Jahangir C.A., Kashif M., Yao H., Ziegler D.V., Xu X., Ghosh T., Mondal T., Kanduri C., Lindahl P., Sayin V.I., Bergo M.O. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178:330–345.e22. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Mangione C.M., Barry M.J., Nicholson W.K., Cabana M., Chelmow D., Coker T.R., Davis E.M., Donahue K.E., Doubeni C.A., Jaén C.R., Kubik M., Li L., Ogedegbe G., Pbert L., Ruiz J.M., Stevermer J., Wong J.B. Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US preventive services task force recommendation statement. JAMA. 2022;327:2326–2333. doi: 10.1001/jama.2022.8970. [DOI] [PubMed] [Google Scholar]
- 9.Lang X., Green M.D., Wang W., Yu J., Choi J.E., Jiang L., Liao P., Zhou J., Zhang Q., Dow A., Saripalli A.L., Kryczek I., Wei S., Szeliga W., Vatan L., Stone E.M., Georgiou G., Cieslik M., Wahl D.R., Morgan M.A., Chinnaiyan A.M., Lawrence T.S., Zou W. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei G., Zhuang L., Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer. 2022;22:381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian H., Zhang T., Qin S., Huang Z., Zhou L., Shen Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022;15:132. doi: 10.1186/s13045-022-01320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawat L., Hegde H., Hoti S.L., Nayak V. Piperlongumine induces ROS mediated cell death and synergizes paclitaxel in human intestinal cancer cells. Biomed. Pharmacother. Biomed. pharmacotherapie. 2020;128 doi: 10.1016/j.biopha.2020.110243. [DOI] [PubMed] [Google Scholar]
- 13.Zaidieh T., Smith J.R., Ball K.E., An Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer. 2019;19:1224. doi: 10.1186/s12885-019-6438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., Liang Y., Luo X., Hu Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 2020;11:291. doi: 10.1038/s41419-020-2488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace K.B., Sardão V.A., Oliveira P.J. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ. Res. 2020;126:926–941. doi: 10.1161/CIRCRESAHA.119.314681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C., Livingston M.J., Safirstein R., Dong Z. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat. Rev. Nephrol. 2023;19:53–72. doi: 10.1038/s41581-022-00631-7. [DOI] [PubMed] [Google Scholar]
- 17.Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volarevic V., Djokovic B., Jankovic M.G., Harrell C.R., Fellabaum C., Djonov V., Arsenijevic N. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019;26:25. doi: 10.1186/s12929-019-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020;17:474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin F., Bao Y.W., Wu F.G. Improving the phototherapeutic efficiencies of molecular and nanoscale materials by targeting mitochondria. Molecules. 2018;23 doi: 10.3390/molecules23113016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ursini F., Maiorino M., Forman H.J. Redox homeostasis: the Golden Mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 25.Tejero J., Shiva S., Gladwin M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019;99:311–379. doi: 10.1152/physrev.00036.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock J.T. Considerations of the importance of redox state for reactive nitrogen species action. J. Exp. Bot. 2019;70:4323–4331. doi: 10.1093/jxb/erz067. [DOI] [PubMed] [Google Scholar]
- 28.Hancock J.T., Veal D. Nitric oxide, other reactive signalling compounds, redox, and reductive stress. J. Exp. Bot. 2021;72:819–829. doi: 10.1093/jxb/eraa331. [DOI] [PubMed] [Google Scholar]
- 29.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 30.Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., Murphy M.P., Yamamoto M., Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022;23:499–515. doi: 10.1038/s41580-022-00456-z. [DOI] [PubMed] [Google Scholar]
- 31.Khalefa H.G., Shawki M.A., Aboelhassan R., El Wakeel L.M. Evaluation of the effect of N-acetylcysteine on the prevention and amelioration of paclitaxel-induced peripheral neuropathy in breast cancer patients: a randomized controlled study. Breast Cancer Res. Treat. 2020;183:117–125. doi: 10.1007/s10549-020-05762-8. [DOI] [PubMed] [Google Scholar]
- 32.Neha K., Haider M.R., Pathak A., Yar M.S. Medicinal prospects of antioxidants: a review. Eur. J. Med. Chem. 2019;178:687–704. doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Jones R.G., Thompson C.B. Tumor suppressors and cell metabolism: a recipe for cancer growth. Gene Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achille’' heel? Nat. Rev. Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura H., Takada K. Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 2021;112:3945–3952. doi: 10.1111/cas.15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss-Sadan T., Ge M., Hayashi M., Gohar M., Yao C.H., de Groot A., Harry S., Carlin A., Fischer H., Shi L., Wei T.Y., Adelmann C.H., Wolf K., Vornbäumen T., Dürr B.R., Takahashi M., Richter M., Zhang J., Yang T.Y., Vijay V., Fisher D.E., Hata A.N., Haigis M.C., Mostoslavsky R., Bardeesy N., Papagiannakopoulos T., Bar-Peled L. NRF2 activation induces NADH-reductive stress, providing a metabolic vulnerability in lung cancer. Cell Metabol. 2023;35:487–503.e7. doi: 10.1016/j.cmet.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J.H., Pyun W.Y., Park H.W. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9 doi: 10.3390/cells9102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Y., Feng Y., Huang Z., Zhang Y., Li X., Liu R., Li H., Wang T., Ding Y., Jia Z., Yang J. SOX15 transcriptionally increases the function of AOC1 to modulate ferroptosis and progression in prostate cancer. Cell Death Dis. 2022;13:673. doi: 10.1038/s41419-022-05108-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mani R.S., Amin M.A., Li X., Kalyana-Sundaram S., Veeneman B.A., Wang L., Ghosh A., Aslam A., Ramanand S.G., Rabquer B.J., Kimura W., Tran M., Cao X., Roychowdhury S., Dhanasekaran S.M., Palanisamy N., Sadek H.A., Kapur P., Koch A.E., Chinnaiyan A.M. Inflammation-induced oxidative stress mediates gene fusion formation in prostate cancer. Cell Rep. 2016;17:2620–2631. doi: 10.1016/j.celrep.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song R., Dasgupta C., Mulder C., Zhang L. MicroRNA-210 controls mitochondrial metabolism and protects heart function in myocardial infarction. Circulation. 2022;145:1140–1153. doi: 10.1161/CIRCULATIONAHA.121.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. : Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 42.Tanner M. USPSTF recommends against beta carotene or vitamin E supplements for preventing CVD or cancer in adults. Ann. Intern. Med. 2022;175:Jc110. doi: 10.7326/J22-0078. [DOI] [PubMed] [Google Scholar]
- 43.Xin J., Jiang X., Ben S., Yuan Q., Su L., Zhang Z., Christiani D.C., Du M., Wang M. Association between circulating vitamin E and ten common cancers: evidence from large-scale Mendelian randomization analysis and a longitudinal cohort study. BMC Med. 2022;20:168. doi: 10.1186/s12916-022-02366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y., Xu F., Jiang L., Miao Z., Liang X., Yang J., Larsson S.C., Zheng J.S. Circulating vitamin C concentration and risk of cancers: a Mendelian randomization study. BMC Med. 2021;19:171. doi: 10.1186/s12916-021-02041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Qi H., Liu Y., Duan C., Liu X., Xia T., Chen D., Piao H.L., Liu H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–4857. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y., Qi H., Liu Y., Duan C., Liu X., Xia T., Chen D., Piao H.L., Liu H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839–4857. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martins-Teixeira M.B., Carvalho I. Antitumour anthracyclines: progress and perspectives. ChemMedChem. 2020;15:933–948. doi: 10.1002/cmdc.202000131. [DOI] [PubMed] [Google Scholar]
- 49.Songbo M., Lang H., Xinyong C., Bin X., Ping Z., Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019;307:41–48. doi: 10.1016/j.toxlet.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Manda G., Isvoranu G., Comanescu M.V., Manea A., Debelec Butuner B., Korkmaz K.S. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol. 2015;5:347–357. doi: 10.1016/j.redox.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang J.Y., Ou-Yang F., Hou M.F., Huang H.W., Wang H.R., Li K.T., Fayyaz S., Shu C.W., Chang H.W. Oxidative stress-modulating drugs have preferential anticancer effects–- involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019;58:109–117. doi: 10.1016/j.semcancer.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Kim S.J., Kim H.S., Seo Y.R. Understanding of ROS-inducing strategy in anticancer therapy. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/5381692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang B., Wang Y., Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer lett. 2009;286:154–160. doi: 10.1016/j.canlet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 54.Verschoor M.L., Singh G. Ets-1 regulates intracellular glutathione levels: key target for resistant ovarian cancer. Mol. Cancer. 2013;12:138. doi: 10.1186/1476-4598-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Malley J., Kumar R., Inigo J., Yadava N., Chandra D. Mitochondrial stress response and cancer. Trends Cancer. 2020;6:688–701. doi: 10.1016/j.trecan.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei R., Zhao Y., Wang J., Yang X., Li S., Wang Y., Yang X., Fei J., Hao X., Zhao Y., Gui L., Ding X. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 2021;17:2703–2717. doi: 10.7150/ijbs.59404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niu B., Liao K., Zhou Y., Wen T., Quan G., Pan X., Wu C. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121110. [DOI] [PubMed] [Google Scholar]
- 58.Okuni N., Honma Y., Urano T., Tamura K. Romidepsin and tamoxifen cooperatively induce senescence of pancreatic cancer cells through downregulation of FOXM1 expression and induction of reactive oxygen species/lipid peroxidation. Mol. Biol. Rep. 2022;49:3519–3529. doi: 10.1007/s11033-022-07192-9. [DOI] [PubMed] [Google Scholar]
- 59.Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 60.Zhang C., Xu C., Gao X., Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022;12:2115–2132. doi: 10.7150/thno.69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu Z., Guo J., Hu M., Gao Y., Huang L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano. 2020;14:4816–4828. doi: 10.1021/acsnano.0c00708. [DOI] [PubMed] [Google Scholar]
- 62.Carrasco R., Ramirez M.C., Nes K., Schuster A., Aguayo R., Morales M., Ramos C., Hasson D., Sotomayor C.G., Henriquez P., Cortés I., Erazo M., Salas C., Gormaz J.G. Prevention of doxorubicin-induced Cardiotoxicity by pharmacological non-hypoxic myocardial preconditioning based on Docosahexaenoic Acid (DHA) and carvedilol direct antioxidant effects: study protocol for a pilot, randomized, double-blind, controlled trial (CarDHA trial) Trials. 2020;21:137. doi: 10.1186/s13063-019-3963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li, Y., Wang, J., Zhang, Y., Zhang, L., An, T., Ding, Y., Anthracycline-induced oxidative stress mediates cardiotoxicity via perturbation of ATP homeostasis and ER stress. Redox Biol., 36, 101661..
- 64.Ghigo A., Li M., Hirsch E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochim. Biophys. Acta. 2016;1863:1916–1925. doi: 10.1016/j.bbamcr.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 65.Renu K., V G.A., P B.T., Arunachalam S. Molecular mechanism of doxorubicin-induced cardiomyopathy–- an update. Eur. J. Pharmacol. 2018;818:241–253. doi: 10.1016/j.ejphar.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 66.Yedjou C., Tchounwou P., Jenkins J., McMurray R. Basic mechanisms of arsenic trioxide (ATO)-induced apoptosis in human leukemia (HL-60) cells. J. Hematol. Oncol. 2010;3:28. doi: 10.1186/1756-8722-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong C., Zhang N.J., Zhang L.J. Oxidative stress in leukemia and antioxidant treatment. Chin. Med. J. 2021;134:1897–1907. doi: 10.1097/CM9.0000000000001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar S., Yedjou C.G., Tchounwou P.B. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J. Exp. Clin. Cancer Res. : CRN. 2014;33:42. doi: 10.1186/1756-9966-33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang S., Wan X., Zou X., Sun S., Hao X., Liang C., Zhang Z., Zhang F., Sun B., Li H., Yu B. Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death Dis. 2021;12:88. doi: 10.1038/s41419-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J., Jin Z., Zhang S., Zhang X., Li P., Yang H., Ma Y. Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis. Cell. Mol. Immunol. 2023;20:51–64. doi: 10.1038/s41423-022-00956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ling S., Xie H., Yang F., Shan Q., Dai H., Zhuo J., Wei X., Song P., Zhou L., Xu X., Zheng S. Metformin potentiates the effect of arsenic trioxide suppressing intrahepatic cholangiocarcinoma: roles of p38 MAPK, ERK3, and mTORC1. J. Hematol. Oncol. 2017;10:59. doi: 10.1186/s13045-017-0424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z., Qin S., Chen Y., Zhou L., Yang M., Tang Y., Zuo J., Zhang J., Mizokami A., Nice E.C., Chen H.N., Huang C., Wei X. Inhibition of NPC1L1 disrupts adaptive responses of drug-tolerant persister cells to chemotherapy. EMBO Mol. Med. 2022;14 doi: 10.15252/emmm.202114903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Y., Chen H.N., Wang K., Zhang L., Huang Z., Liu J., Zhang Z., Luo M., Lei Y., Peng Y., Zhou Z.G., Wei Y., Huang C. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J. Hepatol. 2019;70:66–77. doi: 10.1016/j.jhep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 74.Soma S., Latimer A.J., Chun H., Vicary A.C., Timbalia S.A., Boulet A., Rahn J.J., Chan S.S.L., Leary S.C., Kim B.E., Gitlin J.D., Gohil V.M. Elesclomol restores mitochondrial function in genetic models of copper deficiency. Proc. Natl. Acad. Sci. U. S. A. 2018;115:8161–8166. doi: 10.1073/pnas.1806296115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y., Yang J., Zhang Q., Xu S., Sun W., Ge S., Xu X., Jager M.J., Jia R., Zhang J., Fan X. Copper ionophore elesclomol selectively targets GNAQ/11-mutant uveal melanoma. Oncogene. 2022;41:3539–3553. doi: 10.1038/s41388-022-02364-0. [DOI] [PubMed] [Google Scholar]
- 76.Dou Q., Chen H.N., Wang K., Yuan K., Lei Y., Li K., Lan J., Chen Y., Huang Z., Xie N., Zhang L., Xiang R., Nice E.C., Wei Y., Huang C. Ivermectin induces cytostatic autophagy by blocking the PAK1/akt Axis in breast cancer. Cancer Res. 2016;76:4457–4469. doi: 10.1158/0008-5472.CAN-15-2887. [DOI] [PubMed] [Google Scholar]
- 77.Wang K., Gao W., Dou Q., Chen H., Li Q., Nice E.C., Huang C. Ivermectin induces PAK1-mediated cytostatic autophagy in breast cancer. Autophagy. 2016;12:2498–2499. doi: 10.1080/15548627.2016.1231494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han H.H., Wang H.M., Jangili P., Li M., Wu L., Zang Y., Sedgwick A.C., Li J., He X.P., James T.D., Kim J.S. The design of small-molecule prodrugs and activatable phototherapeutics for cancer therapy. Chem. Soc. Rev. 2023;52:879–920. doi: 10.1039/d2cs00673a. [DOI] [PubMed] [Google Scholar]
- 79.Son S., Kim J.H., Wang X., Zhang C., Yoon S.A., Shin J., Sharma A., Lee M.H., Cheng L., Wu J., Kim J.S. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020;49:3244–3261. doi: 10.1039/c9cs00648f. [DOI] [PubMed] [Google Scholar]
- 80.Lin G., Nash G.T., Luo T., Ghosh I., Sohoni S., Christofferson A.J., Liu G., Engel G.S., Lin W. Deerfield Beach, Fla.; 2023. 2D Nano-Sonosensitizers Facilitate Energy Transfer to Enhance Sonodynamic Therapy. Advanced Materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Zhang X., Yang H., Yu L., Xu Y., Sharma A., Yin P., Li X., Kim J.S., Sun Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 2021;50:11227–11248. doi: 10.1039/d1cs00403d. [DOI] [PubMed] [Google Scholar]
- 82.Muri J., Kopf M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021;21:363–381. doi: 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- 83.Kirkpatrick D.L., Powis G. Clinically evaluated cancer drugs inhibiting redox signaling. Antioxidants Redox Signal. 2017;26:262–273. doi: 10.1089/ars.2016.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bansal A., Simon M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018;217:2291–2298. doi: 10.1083/jcb.201804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong Y., Xiao C., Li Z., Yang X. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem. Soc. Rev. 2021;50:6013–6041. doi: 10.1039/d0cs00718h. [DOI] [PubMed] [Google Scholar]
- 86.Mugoni V., Panella R., Cheloni G., Chen M., Pozdnyakova O., Stroopinsky D., Guarnerio J., Monteleone E., Lee J.D., Mendez L., Menon A.V., Aster J.C., Lane A.A., Stone R.M., Galinsky I., Zamora J.C., Lo-Coco F., Bhasin M.K., Avigan D., Longo L., Clohessy J.G., Pandolfi P.P. Vulnerabilities in mIDH2 AML confer sensitivity to APL-like targeted combination therapy. Cell Res. 2019;29:446–459. doi: 10.1038/s41422-019-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gana C.C., Hanssen K.M., Yu D.M.T., Flemming C.L., Wheatley M.S., Conseil G., Cole S.P.C., Norris M.D., Haber M., Fletcher J.I. MRP1 modulators synergize with buthionine sulfoximine to exploit collateral sensitivity and selectively kill MRP1-expressing cancer cells. Biochem. Pharmacol. 2019;168:237–248. doi: 10.1016/j.bcp.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Bin P., Huang R., Zhou X. Oxidation resistance of the sulfur amino acids: methionine and cysteine. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/9584932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miran T., Vogg A.T.J., Drude N., Mottaghy F.M., Morgenroth A. Modulation of glutathione promotes apoptosis in triple-negative breast cancer cells. Faseb. J. : Off. Pub. Fed. Am. Soc. Exp. Biol. 2018;32:2803–2813. doi: 10.1096/fj.201701157R. [DOI] [PubMed] [Google Scholar]
- 90.Yao W., Qian X., Ochsenreither S., Soldano F., DeLeo A.B., Sudhoff H., Oppel F., Kuppig A., Klinghammer K., Kaufmann A.M., Albers A.E. Disulfiram acts as a potent radio-chemo sensitizer in head and neck squamous cell carcinoma cell lines and transplanted xenografts. Cells. 2021;10 doi: 10.3390/cells10030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bjørklund G., Zou L., Wang J., Chasapis C.T., Peana M. Thioredoxin reductase as a pharmacological target. Pharmacol. Res. 2021;174 doi: 10.1016/j.phrs.2021.105854. [DOI] [PubMed] [Google Scholar]
- 92.Bian M., Fan R., Zhao S., Liu W. Targeting the thioredoxin system as a strategy for cancer therapy. J. Med. Chem. 2019;62:7309–7321. doi: 10.1021/acs.jmedchem.8b01595. [DOI] [PubMed] [Google Scholar]
- 93.Gencheva R., Arnér E.S.J. Thioredoxin reductase inhibition for cancer therapy. Annu. Rev. Pharmacol. Toxicol. 2022;62:177–196. doi: 10.1146/annurev-pharmtox-052220-102509. [DOI] [PubMed] [Google Scholar]
- 94.Bjørklund G., Zou L., Wang J., Chasapis C.T., Peana M. Thioredoxin reductase as a pharmacological target. Pharmacol. Res. 2021;174 doi: 10.1016/j.phrs.2021.105854. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J., Li X., Han X., Liu R., Fang J. Targeting the thioredoxin system for cancer therapy. Trends Pharmacol. Sci. 2017;38:794–808. doi: 10.1016/j.tips.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Kinoshita H., Shimozato O., Ishii T., Kamoda H., Hagiwara Y., Ohtori S., Yonemoto T. The thioredoxin-1 inhibitor, PX-12, suppresses local osteosarcoma progression. Anticancer Res. 2021;41:6013–6021. doi: 10.21873/anticanres.15420. [DOI] [PubMed] [Google Scholar]
- 97.Yu L., Guo Q., Luo Z., Wang Y., Weng J., Chen Y., Liang W., Li Y., Zhang Y., Chen K., Chen Z., Ding Y., Zhang Y. TXN inhibitor impedes radioresistance of colorectal cancer cells with decreased ALDH1L2 expression via TXN/NF-κB signaling pathway. Br. J. Cancer. 2022;127:637–648. doi: 10.1038/s41416-022-01835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Begum R., Thota S., Abdulkadir A., Kaur G., Bagam P., Batra S. NADPH oxidase family proteins: signaling dynamics to disease management. Cell. Mol. Immunol. 2022;19:660–686. doi: 10.1038/s41423-022-00858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Freire Boullosa L., Van Loenhout J., Flieswasser T., De Waele J., Hermans C., Lambrechts H., Cuypers B., Laukens K., Bartholomeus E., Siozopoulou V., De Vos W.H., Peeters M., Smits E.L.J., Deben C. Auranofin reveals therapeutic anticancer potential by triggering distinct molecular cell death mechanisms and innate immunity in mutant p53 non-small cell lung cancer. Redox Biol. 2021;42 doi: 10.1016/j.redox.2021.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui X.Y., Park S.H., Park W.H. Anti-cancer effects of auranofin in human lung cancer cells by increasing intracellular ROS levels and depleting GSH levels. Molecules. 2022;27 doi: 10.3390/molecules27165207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdalbari F.H., Telleria C.M. The gold complex auranofin: new perspectives for cancer therapy. Discover. Oncology. 2021;12:42. doi: 10.1007/s12672-021-00439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nag D., Bhanja P., Riha R., Sanchez-Guerrero G., Kimler B.F., Tsue T.T., Lominska C., Saha S. Auranofin protects intestine against radiation injury by modulating p53/p21 pathway and radiosensitizes human colon tumor. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2019;25:4791–4807. doi: 10.1158/1078-0432.CCR-18-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raninga P.V., Lee A.C., Sinha D., Shih Y.Y., Mittal D., Makhale A., Bain A.L., Nanayakarra D., Tonissen K.F., Kalimutho M., Khanna K.K. Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti-PD-L1 antibody for treatment of triple-negative breast cancer. Int. J. Cancer. 2020;146:123–136. doi: 10.1002/ijc.32410. [DOI] [PubMed] [Google Scholar]
- 104.Endo-Munoz L., Bennett T.C., Topkas E., Wu S.Y., Thamm D.H., Brockley L., Cooper M., Sommerville S., Thomson M., O'Connell K., Lane A., Bird G., Peaston A., Matigian N., Straw R.C., Saunders N.A. Auranofin improves overall survival when combined with standard of care in a pilot study involving dogs with osteosarcoma. Vet. Comp. Oncol. 2020;18:206–213. doi: 10.1111/vco.12533. [DOI] [PubMed] [Google Scholar]
- 105.Asantewaa G., Harris I.S. Glutathione and its precursors in cancer. Curr. Opin. Biotechnol. 2021;68:292–299. doi: 10.1016/j.copbio.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 106.Bela K., Riyazuddin R., Csiszár J. Plant glutathione peroxidases: non-heme peroxidases with large functional flexibility as a core component of ROS-processing mechanisms and signalling. Antioxidants. 2022;11 doi: 10.3390/antiox11081624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kirkman H.N., Gaetani G.F. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem. Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 108.Purohit V., Simeone D.M., Lyssiotis C.A. Metabolic regulation of redox balance in cancer. Cancers. 2019;11 doi: 10.3390/cancers11070955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreno-Sánchez R., Gallardo-Pérez J.C., Rodríguez-Enríquez S., Saavedra E., Marín-Hernández Á. Control of the NADPH supply for oxidative stress handling in cancer cells. Free Radical Biol. Med. 2017;112:149–161. doi: 10.1016/j.freeradbiomed.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 110.Tedeschi P.M., Bansal N., Kerrigan J.E., Abali E.E., Scotto K.W., Bertino J.R. NAD+ kinase as a therapeutic target in cancer. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2016;22:5189–5195. doi: 10.1158/1078-0432.CCR-16-1129. [DOI] [PubMed] [Google Scholar]
- 111.Tedeschi P.M., Lin H., Gounder M., Kerrigan J.E., Abali E.E., Scotto K., Bertino J.R. Suppression of cytosolic NADPH pool by thionicotinamide increases oxidative stress and synergizes with chemotherapy. Mol. Pharmacol. 2015;88:720–727. doi: 10.1124/mol.114.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kobayashi M., Yamamoto M. Molecular mechanisms activating the Nrf2-Keap 1 pathway of antioxidant gene regulation. Antioxidants Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 113.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin Z., Song J., Gao Y., Huang S., Dou R., Zhong P., Huang G., Han L., Zheng J., Zhang X., Wang S., Xiong B. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022;52 doi: 10.1016/j.redox.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee K., Kim H.M. A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch Pharm. Res. (Seoul) 2011;34:1583–1585. doi: 10.1007/s12272-011-1021-3. [DOI] [PubMed] [Google Scholar]
- 116.Ji L., Moghal N., Zou X., Fang Y., Hu S., Wang Y., Tsao M.S. The NRF2 antagonist ML385 inhibits PI3K-mTOR signaling and growth of lung squamous cell carcinoma cells. Cancer Med. 2023;12:5688–5702. doi: 10.1002/cam4.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang D.D., Chapman E. The role of natural products in revealing NRF2 function. Nat. Prod. Rep. 2020;37:797–826. doi: 10.1039/c9np00061e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rummel N.G., Chaiswing L., Bondada S., St Clair D.K., Butterfield D.A. Chemotherapy-induced cognitive impairment: focus on the intersection of oxidative stress and TNFα. Cell. Mol. Life Sci. : CM. 2021;78:6533–6540. doi: 10.1007/s00018-021-03925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shim H.S., Bae C., Wang J., Lee K.H., Hankerd K.M., Kim H.K., Chung J.M., La J.H. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain. 2019;15 doi: 10.1177/1744806919840098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh P., Singh A. Ocular adverse effects of anti-cancer chemotherapy. J. Cancer Therapeut. Res. 2012;1 [Google Scholar]
- 121.Shirato A., Kikugawa T., Miura N., Tanji N., Takemori N., Higashiyama S., Yokoyama M. Cisplatin resistance by induction of aldo-keto reductase family 1 member C2 in human bladder cancer cells. Oncol. Lett. 2014;7:674–678. doi: 10.3892/ol.2013.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu T., Pan C., Zhang T., Ni M., Wang W., Zhang S., Chen Y., Wang J., Fang Q. Nrf2 overexpression increases the resistance of acute myeloid leukemia to cytarabine by inhibiting replication factor C4. Cancer Gene Ther. 2022;29:1773–1790. doi: 10.1038/s41417-022-00501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agriesti F., Tataranni T., Pacelli C., Scrima R., Laurenzana I., Ruggieri V., Cela O., Mazzoccoli C., Salerno M., Sessa F., Sani G., Pomara C., Capitanio N., Piccoli C. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020;10:2287. doi: 10.1038/s41598-020-58871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kullak-Ublick G.A., Andrade R.J., Merz M., End P., Benesic A., Gerbes A.L., Aithal G.P. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66:1154–1164. doi: 10.1136/gutjnl-2016-313369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mirzaei S., Mohammadi A.T., Gholami M.H., Hashemi F., Zarrabi A., Zabolian A., Hushmandi K., Makvandi P., Samec M., Liskova A., Kubatka P., Nabavi N., Aref A.R., Ashrafizadeh M., Khan H., Najafi M. Nrf2 signaling pathway in cisplatin chemotherapy: potential involvement in organ protection and chemoresistance. Pharmacol. Res. 2021;167 doi: 10.1016/j.phrs.2021.105575. [DOI] [PubMed] [Google Scholar]
- 126.Mapuskar K.A., Wen H., Holanda D.G., Rastogi P., Steinbach E., Han R., Coleman M.C., Attanasio M., Riley D.P., Spitz D.R., Allen B.G., Zepeda-Orozco D. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019;20:98–106. doi: 10.1016/j.redox.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chuang C.H., Lin Y.C., Yang J., Chan S.T., Yeh S.L. Quercetin supplementation attenuates cisplatin induced myelosuppression in mice through regulation of hematopoietic growth factors and hematopoietic inhibitory factors. J. Nutr. Biochem. 2022;110 doi: 10.1016/j.jnutbio.2022.109149. [DOI] [PubMed] [Google Scholar]
- 128.Atiq A., Shal B., Naveed M., Khan A., Ali J., Zeeshan S., Al-Sharari S.D., Kim Y.S., Khan S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019;843:292–306. doi: 10.1016/j.ejphar.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 129.Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxidants Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coppola C., Rienzo A., Piscopo G., Barbieri A., Arra C., Maurea N. Management of QT prolongation induced by anti-cancer drugs: target therapy and old agents. Different algorithms for different drugs. Cancer Treat Rev. 2018;63:135–143. doi: 10.1016/j.ctrv.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 131.Ghobadi E., Moloudizargari M., Asghari M.H., Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expet Opin. Drug Metabol. Toxicol. 2017;13:525–536. doi: 10.1080/17425255.2017.1277205. [DOI] [PubMed] [Google Scholar]
- 132.Yousif N.G., Al-Amran F.G. Novel Toll-like receptor-4 deficiency attenuates trastuzumab (Herceptin) induced cardiac injury in mice. BMC Cardiovasc. Disord. 2011;11:62. doi: 10.1186/1471-2261-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dempsey N., Rosenthal A., Dabas N., Kropotova Y., Lippman M., Bishopric N.H. Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res. Treat. 2021;188:21–36. doi: 10.1007/s10549-021-06280-x. [DOI] [PubMed] [Google Scholar]
- 134.Klein I., Lehmann H.C. Pathomechanisms of paclitaxel-induced peripheral neuropathy. Toxics. 2021;9 doi: 10.3390/toxics9100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020;17:474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Najafi M., Hooshangi Shayesteh M.R., Mortezaee K., Farhood B., Haghi-Aminjan H. The role of melatonin on doxorubicin-induced cardiotoxicity: a systematic review. Life Sci. 2020;241 doi: 10.1016/j.lfs.2019.117173. [DOI] [PubMed] [Google Scholar]
- 137.Pantazi D., Tselepis A.D. Cardiovascular toxic effects of antitumor agents: pathogenetic mechanisms. Thromb. Res. 2022;213(Suppl 1):S95–s102. doi: 10.1016/j.thromres.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 138.Nemeth B.T., Varga Z.V., Wu W.J., Pacher P. Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br. J. Pharmacol. 2017;174:3727–3748. doi: 10.1111/bph.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Varricchi G., Marone G., Mercurio V., Galdiero M.R., Bonaduce D., Tocchetti C.G. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Curr. Med. Chem. 2018;25:1327–1339. doi: 10.2174/0929867324666170407125017. [DOI] [PubMed] [Google Scholar]
- 140.Fabiani I., Aimo A., Grigoratos C., Castiglione V., Gentile F., Saccaro L.F., Arzilli C., Cardinale D., Passino C., Emdin M. Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart Fail. Rev. 2021;26:881–890. doi: 10.1007/s10741-020-10063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levis B.E., Binkley P.F., Shapiro C.L. Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol. 2017;18:e445–e456. doi: 10.1016/S1470-2045(17)30535-1. [DOI] [PubMed] [Google Scholar]
- 142.Li D.L., Hill J.A. Cardiomyocyte autophagy and cancer chemotherapy. J. Mol. Cell. Cardiol. 2014;71:54–61. doi: 10.1016/j.yjmcc.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Russo M., Della Sala A., Tocchetti C.G., Porporato P.E., Ghigo A. Metabolic aspects of anthracycline cardiotoxicity. Curr. Treat. Options Oncol. 2021;22:18. doi: 10.1007/s11864-020-00812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu L., Wang L., Du Y., Zhang Y., Ren J. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol. Sci. 2023;44:34–49. doi: 10.1016/j.tips.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 145.Ali Pour P., Hosseinian S., Kheradvar A. Mitochondrial transplantation in cardiomyocytes: foundation, methods, and outcomes. Am. J. Physiol. Cell Physiol. 2021;321:C489–c503. doi: 10.1152/ajpcell.00152.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mounier N.M., Abdel-Maged A.E., Wahdan S.A., Gad A.M., Azab S.S. Chemotherapy-induced cognitive impairment (CICI): an overview of etiology and pathogenesis. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118071. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen L.D., Ehrlich B.E. Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases. EMBO Mol. Med. 2020;12 doi: 10.15252/emmm.202012075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Staff N.P., Grisold A., Grisold W., Windebank A.J. Chemotherapy-induced peripheral neuropathy: a current review. Ann. Neurol. 2017;81:772–781. doi: 10.1002/ana.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Holmes D. Trying to unravel the mysteries of chemobrain. Lancet Neurol. 2013;12:533–534. doi: 10.1016/S1474-4422(13)70087-5. [DOI] [PubMed] [Google Scholar]
- 150.McElroy T., Allen A.R. Vol. 9. Antioxidants; Basel, Switzerland: 2020. (A Bibliometric Review of Publications on Oxidative Stress and Chemobrain: 1990-2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ren X., Keeney J.T.R., Miriyala S., Noel T., Powell D.K., Chaiswing L., Bondada S., St Clair D.K., Butterfield D.A. The triangle of death of neurons: oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment ("chemobrain") involving TNF-α. Free Radical Biol. Med. 2019;134:1–8. doi: 10.1016/j.freeradbiomed.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lehner C., Gehwolf R., Tempfer H., Krizbai I., Hennig B., Bauer H.C., Bauer H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxidants Redox Signal. 2011;15:1305–1323. doi: 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Banks W.A., Rhea E.M. The blood-brain barrier, oxidative stress, and insulin resistance. Antioxidants. 2021;10 doi: 10.3390/antiox10111695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aluise C.D., Miriyala S., Noel T., Sultana R., Jungsuwadee P., Taylor T.J., Cai J., Pierce W.M., Vore M., Moscow J.A., St Clair D.K., Butterfield D.A. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radical Biol. Med. 2011;50:1630–1638. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 155.Nafees S., Rashid S., Ali N., Hasan S.K., Sultana S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFκB/MAPK pathway. Chem. Biol. Interact. 2015;231:98–107. doi: 10.1016/j.cbi.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 156.Keeney J.T.R., Ren X., Warrier G., Noel T., Powell D.K., Brelsfoard J.M., Sultana R., Saatman K.E., Clair D.K.S., Butterfield D.A. Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment ("chemobrain") Oncotarget. 2018;9:30324–30339. doi: 10.18632/oncotarget.25718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McAlpin B.R., Mahalingam R., Singh A.K., Dharmaraj S., Chrisikos T.T., Boukelmoune N., Kavelaars A., Heijnen C.J. HDAC6 inhibition reverses long-term doxorubicin-induced cognitive dysfunction by restoring microglia homeostasis and synaptic integrity. Theranostics. 2022;12:603–619. doi: 10.7150/thno.67410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chiu G.S., Maj M.A., Rizvi S., Dantzer R., Vichaya E.G., Laumet G., Kavelaars A., Heijnen C.J. Pifithrin-μ prevents cisplatin-induced chemobrain by preserving neuronal mitochondrial function. Cancer Res. 2017;77:742–752. doi: 10.1158/0008-5472.CAN-16-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Andrade R.J., Chalasani N., Björnsson E.S., Suzuki A., Kullak-Ublick G.A., Watkins P.B., Devarbhavi H., Merz M., Lucena M.I., Kaplowitz N., Aithal G.P. Drug-induced liver injury. Nat. Rev. Dis. Prim. 2019;5:58. doi: 10.1038/s41572-019-0105-0. [DOI] [PubMed] [Google Scholar]
- 160.Chen Z., Tian R., She Z., Cai J., Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 161.Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stephens C., Andrade R.J., Lucena M.I. Mechanisms of drug-induced liver injury. Curr. Opin. Allergy Clin. Immunol. 2014;14:286–292. doi: 10.1097/ACI.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 163.Iorga A., Dara L., Kaplowitz N. Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Man Q., Deng Y., Li P., Ma J., Yang Z., Yang X., Zhou Y., Yan X. Licorice ameliorates cisplatin-induced hepatotoxicity through antiapoptosis, antioxidative stress, anti-inflammation, and acceleration of metabolism. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.563750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Koźmiński P., Halik P.K., Chesori R., Gniazdowska E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taskin B., Erdoğan M.A., Yiğittürk G., Günenç D., Erbaş O. Antifibrotic effect of lactulose on a methotrexate-induced liver injury model. Gastroenterol. Res. Pract. 2017;2017 doi: 10.1155/2017/7942531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Norona L.M., Nguyen D.G., Gerber D.A., Presnell S.C., LeCluyse E.L. Editor's highlight: modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol. Sci. : Off. J. Soc. Toxicol. 2016;154:354–367. doi: 10.1093/toxsci/kfw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ebrahimi R., Sepand M.R., Seyednejad S.A., Omidi A., Akbariani M., Gholami M., Sabzevari O. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IĸBα/NFĸB in rats. Daru : J. Fac. Pharm. Tehran Univ. Med. Sci. 2019;27:721–733. doi: 10.1007/s40199-019-00309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hussein O.E., Hozayen W.G., Bin-Jumah M.N., Germoush M.O., Abd El-Twab S.M., Mahmoud A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ. Sci. Pollut. Res. Int. 2020;27:20725–20735. doi: 10.1007/s11356-020-08557-y. [DOI] [PubMed] [Google Scholar]
- 170.Mahmoud A.M., Hussein O.E., Hozayen W.G., Bin-Jumah M., Abd El-Twab S.M. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. Int. 2020;27:7910–7921. doi: 10.1007/s11356-019-07532-6. [DOI] [PubMed] [Google Scholar]
- 171.Nicolaysen A. Nephrotoxic chemotherapy agents: old and new. Adv. Chron. Kidney Dis. 2020;27:38–49. doi: 10.1053/j.ackd.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 172.Manohar S., Leung N. Cisplatin nephrotoxicity: a review of the literature. J. Nephrol. 2018;31:15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 173.Nezu M., Suzuki N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee J., Lim K.T. Protection against cyclophosphamide-induced myelosuppression by ZPDC glycoprotein (24 kDa) Immunol. Invest. 2013;42:61–80. doi: 10.3109/08820139.2012.732166. [DOI] [PubMed] [Google Scholar]
- 175.Akbarali H.I., Muchhala K.H., Jessup D.K., Cheatham S. Chemotherapy induced gastrointestinal toxicities. Adv. Cancer Res. 2022;155:131–166. doi: 10.1016/bs.acr.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhu H., Lu C., Gao F., Qian Z., Yin Y., Kan S., Chen D. Selenium-enriched Bifidobacterium longum DD98 attenuates irinotecan-induced intestinal and hepatic toxicity in vitro and in vivo. Biomed. Pharmacother. Biomed. pharmacotherapie. 2021;143 doi: 10.1016/j.biopha.2021.112192. [DOI] [PubMed] [Google Scholar]
- 177.Gilliam L.A., St Clair D.K. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxidants Redox Signal. 2011;15:2543–2563. doi: 10.1089/ars.2011.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bloniarz D., Adamczyk-Grochala J., Lewinska A., Wnuk M. The lack of functional DNMT2/TRDMT1 gene modulates cancer cell responses during drug-induced senescence. Aging. 2021;13:15833–15874. doi: 10.18632/aging.203203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bhoyrul B., Asfour L., Lutz G., Mitchell L., Jerjen R., Sinclair R.D., Holmes S., Chaudhry I.H., Harries M.J. Clinicopathologic characteristics and response to treatment of persistent chemotherapy-induced alopecia in breast cancer survivors. JAMA Dermatol. 2021;157:1335–1342. doi: 10.1001/jamadermatol.2021.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang R., Xue X., Wang Y., Zhao H., Zhang Y., Wang H., Miao D. BMI1 deficiency results in female infertility by activating p16/p19 signaling and increasing oxidative stress. Int. J. Biol. Sci. 2019;15:870–881. doi: 10.7150/ijbs.30488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liang W., He X., Bi J., Hu T., Sun Y. Role of reactive oxygen species in tumors based on the 'seed and soil' theory: a complex interaction (Review) Oncol. Rep. 2021;46 doi: 10.3892/or.2021.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xue D., Zhou X., Qiu J. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomed. Pharmacother. Biomed. pharmacotherapie. 2020;131 doi: 10.1016/j.biopha.2020.110676. [DOI] [PubMed] [Google Scholar]
- 183.Camarillo J.M., Rose K.L., Galligan J.J., Xu S., Marnett L.J. Covalent modification of CDK2 by 4-hydroxynonenal as a mechanism of inhibition of cell cycle progression. Chem. Res. Toxicol. 2016;29:323–332. doi: 10.1021/acs.chemrestox.5b00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jacob P., Hirt H., Bendahmane A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017;15:405–414. doi: 10.1111/pbi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui Q., Wang J.Q., Assaraf Y.G., Ren L., Gupta P., Wei L., Ashby C.R., Jr., Yang D.H., Chen Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates : Rev. Comment. Antimicrob. Anti Cancer Chemother. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 186.Wang J., Sun D., Huang L., Wang S., Jin Y. Targeting reactive oxygen species capacity of tumor cells with repurposed drug as an anticancer therapy. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/8532940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Victorino V.J., Pizzatti L., Michelletti P., Panis C. Oxidative stress, redox signaling and cancer chemoresistance: putting together the pieces of the puzzle. Curr. Med. Chem. 2014;21:3211–3226. doi: 10.2174/0929867321666140601164647. [DOI] [PubMed] [Google Scholar]
- 188.Mendes C., Serpa J. Metabolic remodelling: an accomplice for new therapeutic strategies to fight lung cancer. Antioxidants. 2019;8 doi: 10.3390/antiox8120603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Landriscina M., Maddalena F., Laudiero G., Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxidants Redox Signal. 2009;11:2701–2716. doi: 10.1089/ars.2009.2692. [DOI] [PubMed] [Google Scholar]
- 190.Chen X., Chen S., Yu D. Metabolic reprogramming of chemoresistant cancer cells and the potential significance of metabolic regulation in the reversal of cancer chemoresistance. Metabolites. 2020;10 doi: 10.3390/metabo10070289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dong S., Liang S., Cheng Z., Zhang X., Luo L., Li L., Zhang W., Li S., Xu Q., Zhong M., Zhu J., Zhang G., Hu S. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. : CRN. 2022;41:15. doi: 10.1186/s13046-021-02229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Santos R.M., Moreno C., Zhang W.C. Non-coding RNAs in lung tumor initiation and progression. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21082774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ryoo I.G., Kwak M.K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018;359:24–33. doi: 10.1016/j.taap.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 195.Wang C.Y., Xu Y., Wang X., Guo C., Wang T., Wang Z.Y. Dl-3-n-Butylphthalide inhibits NLRP3 inflammasome and mitigates alzheimer's-like pathology via nrf2-TXNIP-TrX Axis. Antioxidants Redox Signal. 2019;30:1411–1431. doi: 10.1089/ars.2017.7440. [DOI] [PubMed] [Google Scholar]
- 196.Friedmann Angeli J.P., Meierjohann S. NRF2-dependent stress defense in tumor antioxidant control and immune evasion. Pigm. Cell Melanoma Res. 2021;34:268–279. doi: 10.1111/pcmr.12946. [DOI] [PubMed] [Google Scholar]
- 197.Cai L., Jin X., Zhang J., Li L., Zhao J. Metformin suppresses Nrf2-mediated chemoresistance in hepatocellular carcinoma cells by increasing glycolysis. Aging. 2020;12:17582–17600. doi: 10.18632/aging.103777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang D., Hou Z., Aldrich K.E., Lockwood L., Odom A.L., Liby K.T. A novel Nrf2 pathway inhibitor sensitizes keap 1-mutant lung cancer cells to chemotherapy. Mol. Cancer Therapeut. 2021;20:1692–1701. doi: 10.1158/1535-7163.MCT-21-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.De Nuccio C., Bernardo A., Troiano C., Brignone M.S., Falchi M., Greco A., Rosini M., Basagni F., Lanni C., Serafini M.M., Minghetti L., Visentin S. NRF2 and PPAR-γ pathways in oligodendrocyte progenitors: focus on ROS protection, mitochondrial biogenesis and promotion of cell differentiation. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21197216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Z., Sun X., Feng Y., Wang Y., Zhang L., Wang Y., Fang Z., Azami N.L.B., Sun M., Li Q. Dihydromyricetin reverses MRP2-induced multidrug resistance by preventing NF-κB-Nrf2 signaling in colorectal cancer cell. Phytomedicine : Int. J. Phytother. Phytopharm. 2021;82 doi: 10.1016/j.phymed.2020.153414. [DOI] [PubMed] [Google Scholar]
- 201.Ma-On C., Sanpavat A., Whongsiri P., Suwannasin S., Hirankarn N., Tangkijvanich P., Boonla C. Oxidative stress indicated by elevated expression of Nrf2 and 8-OHdG promotes hepatocellular carcinoma progression. Med. Oncol. 2017;34:57. doi: 10.1007/s12032-017-0914-5. [DOI] [PubMed] [Google Scholar]
- 202.Cai Y., Zheng Y., Gu J., Wang S., Wang N., Yang B., Zhang F., Wang D., Fu W., Wang Z. Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78. Cell Death Dis. 2018;9:636. doi: 10.1038/s41419-018-0669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Niture S.K., Jaiswal A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radical Biol. Med. 2013;57:119–131. doi: 10.1016/j.freeradbiomed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhou Y., Zhou Y., Yang M., Wang K., Liu Y., Zhang M., Yang Y., Jin C., Wang R., Hu R. Digoxin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine via inhibiting Nrf2 signaling pathway. Redox Biol. 2019;22 doi: 10.1016/j.redox.2019.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pirpour Tazehkand A., Akbarzadeh M., Velaie K., Sadeghi M.R., Samadi N. The role of Her2-Nrf2 axis in induction of oxaliplatin resistance in colon cancer cells. Biomed. Pharmacother. Biomed. pharmacotherapie. 2018;103:755–766. doi: 10.1016/j.biopha.2018.04.105. [DOI] [PubMed] [Google Scholar]
- 206.Hayden A., Douglas J., Sommerlad M., Andrews L., Gould K., Hussain S., Thomas G.J., Packham G., Crabb S.J. The Nrf2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol. Oncol. 2014;32:806–814. doi: 10.1016/j.urolonc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 207.Cucci M.A., Grattarola M., Dianzani C., Damia G., Ricci F., Roetto A., Trotta F., Barrera G., Pizzimenti S. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free Radical Biol. Med. 2020;150:125–135. doi: 10.1016/j.freeradbiomed.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 208.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 209.Ge W., Zhao K., Wang X., Li H., Yu M., He M., Xue X., Zhu Y., Zhang C., Cheng Y., Jiang S., Hu Y. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap 1 binding. Cancer Cell. 2017;32:561–573.e6. doi: 10.1016/j.ccell.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 210.Roh J.L., Kim E.H., Jang H., Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen J., Solomides C., Simpkins F., Simpkins H. The role of Nrf2 and ATF2 in resistance to platinum-based chemotherapy. Cancer Chemother. Pharmacol. 2017;79:369–380. doi: 10.1007/s00280-016-3225-1. [DOI] [PubMed] [Google Scholar]
- 212.Srivastava A.K., Banerjee A., Cui T., Han C., Cai S., Liu L., Wu D., Cui R., Li Z., Zhang X., Xie G., Selvendiran K., Patnaik S., Karpf A.R., Liu J., Cohn D.E., Wang Q.E. Inhibition of miR-328-3p impairs cancer stem cell function and prevents metastasis in ovarian cancer. Cancer Res. 2019;79:2314–2326. doi: 10.1158/0008-5472.CAN-18-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fan C.W., Chen T., Shang Y.N., Gu Y.Z., Zhang S.L., Lu R., OuYang S.R., Zhou X., Li Y., Meng W.T., Hu J.K., Lu Y., Sun X.F., Bu H., Zhou Z.G., Mo X.M. Cancer-initiating cells derived from human rectal adenocarcinoma tissues carry mesenchymal phenotypes and resist drug therapies. Cell Death Dis. 2013;4:e828. doi: 10.1038/cddis.2013.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Prager B.C., Xie Q., Bao S., Rich J.N. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell. 2019;24:41–53. doi: 10.1016/j.stem.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Filipponi D., Emelyanov A., Muller J., Molina C., Nichols J., Bulavin D.V. DNA damage signaling-induced cancer cell reprogramming as a driver of tumor relapse. Mol. Cell. 2019;74:651–663.e8. doi: 10.1016/j.molcel.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 216.Ghanbari Movahed Z., Rastegari-Pouyani M., Mohammadi M.H., Mansouri K. Cancer cells change their glucose metabolism to overcome increased ROS: one step from cancer cell to cancer stem cell? Biomed. Pharmacother. Biomed. pharmacotherapie. 2019;112 doi: 10.1016/j.biopha.2019.108690. [DOI] [PubMed] [Google Scholar]
- 217.Lee S.Y., Jeong E.K., Ju M.K., Jeon H.M., Kim M.Y., Kim C.H., Park H.G., Han S.I., Kang H.S. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer. 2017;16:10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chatterjee R., Chatterjee J. ROS and oncogenesis with special reference to EMT and stemness. Eur. J. Cell Biol. 2020;99 doi: 10.1016/j.ejcb.2020.151073. [DOI] [PubMed] [Google Scholar]
- 219.Park H.K., Hong J.H., Oh Y.T., Kim S.S., Yin J., Lee A.J., Chae Y.C., Kim J.H., Park S.H., Park C.K., Park M.J., Park J.B., Kang B.H. Interplay between TRAP1 and sirtuin-3 modulates mitochondrial respiration and oxidative stress to maintain stemness of glioma stem cells. Cancer Res. 2019;79:1369–1382. doi: 10.1158/0008-5472.CAN-18-2558. [DOI] [PubMed] [Google Scholar]
- 220.Schieber M.S., Chandel N.S. ROS links glucose metabolism to breast cancer stem cell and EMT phenotype. Cancer Cell. 2013;23:265–267. doi: 10.1016/j.ccr.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 221.Diehn M., Cho R.W., Lobo N.A., Kalisky T., Dorie M.J., Kulp A.N., Qian D., Lam J.S., Ailles L.E., Wong M., Joshua B., Kaplan M.J., Wapnir I., Dirbas F.M., Somlo G., Garberoglio C., Paz B., Shen J., Lau S.K., Quake S.R., Brown J.M., Weissman I.L., Clarke M.F. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Moloney J.N., Cotter T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 223.Nicco C., Batteux F. ROS modulator molecules with therapeutic potential in cancers treatments. Molecules. 2017;23 doi: 10.3390/molecules23010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 225.Yang B., Chen Y., Shi J. Reactive oxygen species (ROS)-Based nanomedicine. Chem. Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 226.Pu Y., Yin H., Dong C., Xiang H., Wu W., Zhou B., Du D., Chen Y., Xu H. Sono-controllable and ROS-sensitive CRISPR-cas9 genome editing for augmented/synergistic ultrasound tumor nanotherapy. Adv. Mater. 2021;33 doi: 10.1002/adma.202104641. [DOI] [PubMed] [Google Scholar]
- 227.Guo B., Yang F., Zhang L., Zhao Q., Wang W., Yin L., Chen D., Wang M., Han S., Xiao H., Xing N. Cuproptosis induced by ROS responsive nanoparticles with elesclomol and copper combined with αpd-L1 for enhanced cancer immunotherapy. Adv. Mater. 2023 doi: 10.1002/adma.202212267. [DOI] [PubMed] [Google Scholar]
- 228.Gabizon A.A., Patil Y., La-Beck N.M. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist. Updates : Rev. Comment. Antimicrob. Anti Cancer Chemother. 2016;29:90–106. doi: 10.1016/j.drup.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 229.Akladios F.N., Andrew S.D., Parkinson C.J. Selective induction of oxidative stress in cancer cells via synergistic combinations of agents targeting redox homeostasis. Bioorg. Med. Chem. 2015;23:3097–3104. doi: 10.1016/j.bmc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 230.Xie J., Gong L., Zhu S., Yong Y., Gu Z., Zhao Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv. Mater. 2019;31 doi: 10.1002/adma.201802244. [DOI] [PubMed] [Google Scholar]
- 231.Li Z., Zou J., Chen X. In response to precision medicine: current subcellular targeting strategies for cancer therapy. Adv. Mater. 2022 doi: 10.1002/adma.202209529. [DOI] [PubMed] [Google Scholar]
- 232.Kalinovich A.V., Mattsson C.L., Youssef M.R., Petrovic N., Ost M., Skulachev V.P., Shabalina I.G. Mitochondria-targeted dodecyltriphenylphosphonium (C(12)TPP) combats high-fat-diet-induced obesity in mice. Int. J. Obes. 2016;40:1864–1874. doi: 10.1038/ijo.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Peng J., Hu X., Fan S., Zhou J., Ren S., Sun R., Chen Y., Shen X., Chen Y. Inhibition of mitochondrial biosynthesis using a "Right-Side-Out" membrane camouflaged micelle to facilitate the therapeutic effects of shikonin on triple-negative breast cancer. Adv. Healthc. Mater. 2022 doi: 10.1002/adhm.202200742. [DOI] [PubMed] [Google Scholar]
- 234.Lv B., Ma J., Wang Y., Qu X., Qiu J., Hua K. Mitochondria-targeted mesoporous organic silica nanoplatforms for overcoming cisplatin resistance by disturbing mitochondrial redox homeostasis. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.875818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 236.Nikesitch N., Lee J.M., Ling S., Roberts T.L. Endoplasmic reticulum stress in the development of multiple myeloma and drug resistance. Clin. Transl. Immunol. 2018;7 doi: 10.1002/cti2.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Oriol H. Goldschmidt, Rosiñol L., Straub J., Suvorov A., Araujo C., Rimashevskaya E., Pika T., Gaidano G., Weisel K., Goranova-Marinova V., Schwarer A., Minuk L., Masszi T., Karamanesht I., Offidani M., Hungria V., Spencer A., Orlowski R.Z., Gillenwater H.H., Mohamed N., Feng S., Chng W.J. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 238.Hadinoto K., Sundaresan A., Cheow W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur. J. Pharm. Biopharm. : Off. J. Arbeitsgemeinschaft Pharmazeutische Verfahrenstechnik e.V. 2013;85:427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 239.McLaughlin M., Vandenbroeck K. The endoplasmic reticulum protein folding factory and its chaperones: new targets for drug discovery? Br. J. Pharmacol. 2011;162:328–345. doi: 10.1111/j.1476-5381.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kalmar B., Lu C.H., Greensmith L. The role of heat shock proteins in Amyotrophic Lateral Sclerosis: the therapeutic potential of Arimoclomol. Pharmacol. Ther. 2014;141:40–54. doi: 10.1016/j.pharmthera.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 241.Deng Y., Wen H., Yang H., Zhu Z., Huang Q., Bi Y., Wang P., Zhou M., Guan J., Zhang W., Li M. Identification of PBK as a hub gene and potential therapeutic target for medulloblastoma. Oncol. Rep. 2022;48 doi: 10.3892/or.2022.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 2018;11:64. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li M., Sun J., Zhang W., Zhao Y., Zhang S., Zhang S. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydrate Polym. 2021;251 doi: 10.1016/j.carbpol.2020.117103. [DOI] [PubMed] [Google Scholar]
- 244.Lee H.S., Kang N.W., Kim H., Kim D.H., Chae J.W., Lee W., Song G.Y., Cho C.W., Kim D.D., Lee J.Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydrate Polym. 2021;253 doi: 10.1016/j.carbpol.2020.117187. [DOI] [PubMed] [Google Scholar]
- 245.Liu P., Chen N., Yan L., Gao F., Ji D., Zhang S., Zhang L., Li Y., Xiao Y. Preparation, characterisation and in vitro and in vivo evaluation of CD44-targeted chondroitin sulphate-conjugated doxorubicin PLGA nanoparticles. Carbohydrate Polym. 2019;213:17–26. doi: 10.1016/j.carbpol.2019.02.084. [DOI] [PubMed] [Google Scholar]
- 246.Mu M., Chen H., Fan R., Wang Y., Tang X., Mei L., Zhao N., Zou B., Tong A., Xu J., Han B., Guo G. A Tumor-Specific Ferric-Coordinated Epigallocatechin-3-gallate cascade nanoreactor for glioblastoma therapy. J. Adv. Res. 2021;34:29–41. doi: 10.1016/j.jare.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Johnsen K.B., Burkhart A., Thomsen L.B., Andresen T.L., Moos T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019;181 doi: 10.1016/j.pneurobio.2019.101665. [DOI] [PubMed] [Google Scholar]
- 248.Yi Q., Ma J., Kang K., Gu Z. Bioreducible nanocapsules for folic acid-assisted targeting and effective tumor-specific chemotherapy. Int. J. Nanomed. 2018;13:653–667. doi: 10.2147/IJN.S149458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Scaranti M., Cojocaru E., Banerjee S., Banerji U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020;17:349–359. doi: 10.1038/s41571-020-0339-5. [DOI] [PubMed] [Google Scholar]
- 250.Lyu H., Han A., Polsdofer E., Liu S., Liu B. Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm. Sin. B. 2018;8:503–510. doi: 10.1016/j.apsb.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.El-Mahdy M.A., Alzarie Y.A., Hemann C., Badary O.A., Nofal S., Zweier J.L. The novel SOD mimetic GC4419 increases cancer cell killing with sensitization to ionizing radiation while protecting normal cells. Free Radical Biol. Med. 2020;160:630–642. doi: 10.1016/j.freeradbiomed.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mapuskar K.A., Wen H., Holanda D.G., Rastogi P., Steinbach E., Han R., Coleman M.C., Attanasio M., Riley D.P., Spitz D.R., Allen B.G., Zepeda-Orozco D. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019;20:98–106. doi: 10.1016/j.redox.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Soares R.B., Manguinhas R., Costa J.G., Saraiva N., Gil N., Rosell R., Camões S.P., Batinic-Haberle I., Spasojevic I., Castro M., Miranda J.P., Amaro F., Pinto J., Fernandes A.S., Guedes de Pinho P., Oliveira N.G. MnTnHex-2-PyP(5+) displays anticancer properties and enhances cisplatin effects in non-small cell lung cancer cells. Antioxidants. 2022;11 doi: 10.3390/antiox11112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shin S.W., Choi C., Kim H., Kim Y., Park S., Kim S.Y., Batinic-Haberle I., Park W. MnTnHex-2-PyP(5+), coupled to radiation, suppresses metastasis of 4T1 and MDA-MB-231 breast cancer via AKT/snail/EMT pathways. Antioxidants. 2021;10 doi: 10.3390/antiox10111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Anderson C.M., Lee C.M., Saunders D.P., Curtis A., Dunlap N., Nangia C., Lee A.S., Gordon S.M., Kovoor P., Arevalo-Araujo R., Bar-Ad V., Peddada A., Colvett K., Miller D., Jain A.K., Wheeler J., Blakaj D., Bonomi M., Agarwala S.S., Garg M., Worden F., Holmlund J., Brill J.M., Downs M., Sonis S.T., Katz S., Buatti J.M. Phase IIb, randomized, double-blind trial of GC4419 versus placebo to reduce severe oral mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2019;37:3256–3265. doi: 10.1200/JCO.19.01507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Azad G.K., Tomar R.S. Ebselen, a promising antioxidant drug: mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014;41:4865–4879. doi: 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- 257.Tang Z., Dokic I., Knoll M., Ciamarone F., Schwager C., Klein C., Cebulla G., Hoffmann D.C., Schlegel J., Seidel P., Rutenberg C., Brons S., Herold-Mende C., Wick W., Debus J., Lemke D., Abdollahi A. Radioresistance and transcriptional reprograming of invasive glioblastoma cells. Int. J. Radiat. Oncol. Biol. Phys. 2022;112:499–513. doi: 10.1016/j.ijrobp.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 258.Benkafadar N., Menardo J., Bourien J., Nouvian R., François F., Decaudin D., Maiorano D., Puel J.L., Wang J. Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol. Med. 2017;9:7–26. doi: 10.15252/emmm.201606230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cushing C.M., Petronek M.S., Bodeker K.L., Vollstedt S., Brown H.A., Opat E., Hollenbeck N.J., Shanks T., Berg D.J., Smith B.J., Smith M.C., Monga V., Furqan M., Howard M.A., Greenlee J.D., Mapuskar K.A., St-Aubin J., Flynn R.T., Cullen J.J., Buettner G.R., Spitz D.R., Buatti J.M., Allen B.G., Magnotta V.A. Magnetic resonance imaging (MRI) of pharmacological ascorbate-induced iron redox state as a biomarker in subjects undergoing radio-chemotherapy. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schoenfeld J.D., Sibenaller Z.A., Mapuskar K.A., Wagner B.A., Cramer-Morales K.L., Furqan M., Sandhu S., Carlisle T.L., Smith M.C., Abu Hejleh T., Berg D.J., Zhang J., Keech J., Parekh K.R., Bhatia S., Monga V., Bodeker K.L., Ahmann L., Vollstedt S., Brown H., Shanahan Kauffman E.P., Schall M.E., Hohl R.J., Clamon G.H., Greenlee J.D., Howard M.A., Schultz M.K., Smith B.J., Riley D.P., Domann F.E., Cullen J.J., Buettner G.R., Buatti J.M., Spitz D.R., Allen B.G. O(2)(⋅-) and H(2)O(2)-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;31:487–500.e8. doi: 10.1016/j.ccell.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Alexander M.S., Wilkes J.G., Schroeder S.R., Buettner G.R., Wagner B.A., Du J., Gibson-Corley K., O'Leary B.R., Spitz D.R., Buatti J.M., Berg D.J., Bodeker K.L., Vollstedt S., Brown H.A., Allen B.G., Cullen J.J. Pharmacologic ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Res. 2018;78:6838–6851. doi: 10.1158/0008-5472.CAN-18-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ma Y., Chapman J., Levine M., Polireddy K., Drisko J., Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014;6:222ra18. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 263.Welsh J.L., Wagner B.A., van't Erve T.J., Zehr P.S., Berg D.J., Halfdanarson T.R., Yee N.S., Bodeker K.L., Du J., Roberts L.J., 2nd, Drisko J., Levine M., Buettner G.R., Cullen J.J. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Allen B.G., Bodeker K.L., Smith M.C., Monga V., Sandhu S., Hohl R., Carlisle T., Brown H., Hollenbeck N., Vollstedt S., Greenlee J.D., Howard M.A., Mapuskar K.A., Seyedin S.N., Caster J.M., Jones K.A., Cullen J.J., Berg D., Wagner B.A., Buettner G.R., TenNapel M.J., Smith B.J., Spitz D.R., Buatti J.M. First-in-Human phase I clinical trial of pharmacologic ascorbate combined with radiation and temozolomide for newly diagnosed glioblastoma. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2019;25:6590–6597. doi: 10.1158/1078-0432.CCR-19-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Furqan M., Abu-Hejleh T., Stephens L.M., Hartwig S.M., Mott S.L., Pulliam C.F., Petronek M., Henrich J.B., Fath M.A., Houtman J.C., Varga S.M., Bodeker K.L., Bossler A.D., Bellizzi A.M., Zhang J., Monga V., Mani H., Ivanovic M., Smith B.J., Byrne M.M., Zeitler W., Wagner B.A., Buettner G.R., Cullen J.J., Buatti J.M., Spitz D.R., Allen B.G. Pharmacological ascorbate improves the response to platinum-based chemotherapy in advanced stage non-small cell lung cancer. Redox Biol. 2022;53 doi: 10.1016/j.redox.2022.102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.