Abstract
Aim
Quantitative pupillometry is the guideline-recommended method for assessing pupillary light reflex for multimodal prognostication in comatose patients resuscitated from out-of-hospital cardiac arrest (OHCA). However, threshold values predicting an unfavorable outcome have been inconsistent across studies; therefore, we aimed to identify specific thresholds for all quantitative pupillometry parameters.
Methods
Comatose post-OHCA patients were consecutively admitted to the cardiac arrest center at Copenhagen University Hospital Rigshospitalet from April 2015 to June 2017. The parameters of quantitatively assessed pupillary light reflex (qPLR), Neurological Pupil index (NPi), average/max constriction velocity (CV/MCV), dilation velocity (DV), and latency of constriction (Lat) were recorded on the first three days after admission. We evaluated the prognostic performance and identified thresholds achieving zero percent false positive rate (0% PFR) for an unfavorable outcome of 90-day Cerebral Performance Category (CPC) 3–5. Treating physicians were blinded for pupillometry results.
Results
Of the 135 post-OHCA patients, the primary outcome occurred for 53 (39%) patients.
On any day during hospitalization, a qPLR < 4%, NPi < 2.45, CV < 0.1 mm/s, and an MCV < 0.335 mm/s predicted 90-day unfavorable neurological outcome with 0% FPR (95%CI: 0–0%), with sensitivities of 28% (17–40%), 9% (2–19%), 13% (6–23%), and 17% (8–26%), respectively on day 1.
Conclusion
We found that specific thresholds of all quantitative pupillometry parameters, measured at any time following hospital admission until day 3, predicted a 90-day unfavorable outcome with 0% FPR in comatose patients resuscitated from OHCA. However, at 0% FPR, thresholds resulted in low sensitivity. These findings should be further validated in larger multicenter clinical trials.
Keywords: Cardiac arrest, Post resuscitation care, Pupillometry, Prognostication
Introduction
Despite substantial achievements in post‑resuscitation care, out-of-hospital cardiac arrest (OHCA) survival remains low at approximately 10%.1 Even after successful resuscitation, the majority of patients die from anoxic brain injury, typically after withdrawal of life-sustaining treatment (WLST).2, 3 Thus, accurate neuroprognostication with prediction methods of high specificity (e.g., low false positive rate [FPR]) is crucial to identify patients with an unfavorable neurological outcome.
Since 2021, European resuscitation guidelines have recommended using quantitative pupillometry for assessing pupillary light reflex (PLR) as part of the current strategy for the multimodal neuroprognostication.1 Guidelines suggest that patients who remain comatose after cardiac arrest should have PLR assessed 72 hours after resuscitation. Contrarily, several studies confirm that quantitative pupillometry may yield outcome prediction with an 0% FPR before 72 hours.8, 9, 17, 18, 19, 20 However, the evidence level for using quantitative pupillometry is still low, and the greatest challenge in implementing quantitative pupillometry is agreement on specific standardized threshold values for outcome prediction.
Quantitative pupillometry yields not only quantitatively assessed PLR (qPLR; expressed as the percentage pupillary constriction to a calibrated light stimulus) and Neurological Pupil index (NPi) but several individual PLR parameters with the potential for high prognostic value in predicting unfavorable outcome in post-cardiac arrest patients.4
We aimed to assess the prognostic value of all the quantitative pupillometry parameters, with specific thresholds, in predicting a 90-day unfavorable neurological condition and mortality for comatose patients resuscitated from OHCA.
Methods
Study design
This retrospective observational diagnostic accuracy study included patients admitted to the cardiac arrest center at Copenhagen University Hospital Rigshospitalet from April 2015 to June 2017 with OHCA of a presumed cardiac cause.
Patients were assessed with quantitative pupillometry from admission until they died, regained consciousness, or otherwise were discharged. This study concept was approved as a quality assessment for clinical procedures and equipment by the ethics committee of the capital region of Denmark, which also waived the need for formal consent in accordance with Danish legislation. Hence, all quantitative pupillometry measurements were performed as part of the daily clinical practices by specialty staff nurses.
Data were collected in agreement with the Utstein-style guidelines by our emergency medical services. And the relevant OHCA elements concerning patients’ baseline characteristics, OHCA, and post-resuscitation were defined from their respective domains in the Utstein OHCA template definitions.5
Patients
Adult (≥18 years of age) patients admitted to this tertiary cardiac arrest center were eligible for the study when resuscitated from OHCA, with a sustained return of spontaneous circulation (ROSC) while unconscious (GCS < 9, not able to obey verbal commands), at the time of admission. Patients were excluded if they were admitted with other diagnoses than OHCA, were awake at admission, or if no assessment with quantitative pupillometry was performed.
Previous analysis has evaluated the prognostic value of NPi value for the outcome of 90-day mortality of this OHCA population.6
Postcardiac arrest care, prognostication, and WLST
Patients were treated according to the 2015 European Resuscitation Council (ERC) and European Society of Intensive Care Medicine (ESICM) post‑resuscitation care guidelines.7, 8 Treatment included vasoactive agents (norepinephrine and dopamine) for blood pressure targets, sedation with propofol and fentanyl to a Richmond agitation sedation scale of 4 or less, and targeted temperature management (TTM) to 36 °C. Patients were rewarmed (0.5 °C per hour) after 24 hours, and at 37 °C, sedation was gradually reduced.
In patients who were persistently unconscious when rewarmed and tapered off sedation, prognostication assessment using clinical examinations (including manual evaluation of the pupillary light reflex), neuroimaging, serial EEGs, and SSEPs, was commenced. The multimodal neuroprognostication based on mentioned predictors and the subsequent decision about continuation of care, or WLST, was made no earlier than 72 hours from ROSC. Quantitative pupillary was not a guideline-supported part of the prognostication algorithms during this study. Measurements were obtained for scientific purposes and were therefore not included in the later clinical decision of WLST.
Quantitative pupillometry
Serial measurements with quantitative pupillometry were performed with the NPi®-200 pupillometer (NeurOptics®, Irvine, CA, USA). The device produces a calibrated light stimulation of fixed intensity (1000 Lux) and duration (3.2 s), which archives a rapid measure (0.05 mm limit) of the pupil size and the PLR. The pupillometer measures human pupil size varying from 1 mm to 9 mm, with an accuracy of 0.03 mm.9 The PLR divides into several quantitative pupillary response parameters comprised of qPLR (%), average constriction velocity (CV, mm/s), maximum CV (MCV, mm/s), dilation velocity (DV, mm/s), and latency of constriction (Lat, s).9 Further, the NPi scalar value from 0 to 5 was obtained based on an algorithm derived from the quantitative pupillary response parameters.10 An NPi value ≥3 is considered a normal (“brisk”) response, <3 an abnormal (“sluggish”) response and a value of 0, a non-reactive (absent) response.
Each patient’s parameters were measured for both eyes with the same device and directly stored in an individual SmartGuard® data storage device (a single-use chin guard or spacer). This data was only available for the data analyst, who was not engaged in the daily activities or took part in evaluating the outcome. If patients were available for assessment (alive and unawake), measurements were performed on day 1, day 2, and day 3. For each time point, the lowest value of parameters between eyes was used in the analyses. Treating physicians were blinded for pupillometry results.
Outcome measures
The primary outcome was the 90-day neurological condition from hospital admission, assessed by the Cerebral Performance Categories (CPC) scale and obtained through chart review. CPC was dichotomized as favorable (CPC 1 = no symptoms or 2 = moderate disability) and unfavorable (CPC 3 = severe disability, 4 = coma or vegetative state, and 5 = death).11 The secondary outcome was 90-day mortality from any cause.
Statistical analysis
We assessed all variables with descriptive statistics and Kolmogorov–Smirnov test for normal distribution. We presented continuous variables as mean values (±standard deviation (SD)) or median (interquartile range (IQR)) and categorical variables as frequency (percentage). We calculated differences between groups with the Chi-squared test, Fisher exact test, or Wilcoxon test, as appropriate. We compared the differences in baseline characteristics to evaluate the available population at each time point. To analyze the progression of mean values over days and differences across outcome groups, we applied mixed models for repeated measures with the outcome group, time point, and the interaction term of the outcome group with time as fixed effects.
For each of the quantitative pupillary parameters, prognostic performance was analyzed by calculating specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), and Youden Index at optimal threshold values (those achieving 0% FPR and subsequent for 1–3% FPR, and thresholds maximizing the Youden Index), for each time point from admission.
Receiver operating characteristics (ROC) curves and calculation of area under the curve (AUC) displayed the prognostic value of the quantitative pupillometry parameters for predicting outcome, which was applied at all time points. As quantitative pupillometry was obtained before any competing prognostic test for outcome prediction was performed, only prehospital clinical predictors were identified and implemented in a multivariable model. These included age, sex, shockable primary heart rhythm at resuscitation, ST-segment elevation myocardial infarction (STEMI), time to ROSC, and bystander cardiopulmonary resuscitation (CPR) performed. The method described by De Long et al was used to calculate statistical differences between AUCs of clinical predictors alone and combined with quantitative pupillary response parameters.12
The association of both qPLR and NPi for the secondary outcome of 90-day mortality was assessed by Kaplan-Meier plots with the parameters stratified by quartiles.
R Studio, version 1.2.5001, was used for all analyses (RStudio Team [2020]. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA; URL: https://www.rstudio.com/).
Results
Characteristics of patients
Quantitative pupillometry was recorded for 248 unconscious patients at our cardiac arrest center at the time of this study. We excluded 15 patients participating in ongoing clinical trials, 12 due to erroneous social security numbers, and 86 admitted with cardiac diagnoses other than OHCA. Hence, 135 persistently comatose post-OHCA patients were available for analysis of quantitative pupillometry and therefore included in this study. Patients were predominantly males (n = 109, 81%) with a mean age of 61 ± 12 years (Supplementary Table 1).
All demographic and clinical characteristics of the patients, according to the two primary outcome subgroups, are reported in Table 1. Patients with an unfavorable outcome had a medical history with a significantly higher prevalence of atrial fibrillation and more elevated serum lactate at admission. They had a significantly lower rate of shockable primary rhythm, witnessed cardiac arrest, and had longer time-to-ROSC. Although we observed no difference in STEMI between the groups, the patients with an unfavorable outcome were less likely to undergo coronary angiography (CAG) and percutaneous coronary intervention (PCI). We found no significant differences in medical history, conditions of cardiac arrest, or findings and procedures on arrival at the hospital between the unconscious population available for quantitative pupillometry at any time point Supplementary Table 1.
Table 1.
Baseline characteristics.
| Outcome |
|||
|---|---|---|---|
| Favorable (CPC 1–2), n = 82 | Unfavorable (3–5), n = 53 | p-value | |
| Demographics | |||
| Male sex — no. (%) | 70 (85) | 39 (74) | 0.090 |
| Age — yr | 59 ± 10 | 62 ± 14 | 0.064 |
| Medical history — no. (%) | |||
| Hypertension, medically treated | 37 (45) | 32 (60) | 0.174 |
| Diabetes | 7 (9) | 12 (23) | 0.055 |
| Myocardial infarction | 7 (9) | 5 (9) | >0.999 |
| Atrial fibrillation | 4 (5) | 11 (21) | 0.012 |
| Heart failure | 8 (10) | 5 (9) | 0.726 |
| Asthma or Chronic obstructive pulmonary disease | 9 (11) | 4 (8) | 0.544 |
| Stroke | 5 (6) | 5 (9) | 0.635 |
| Chronic kidney disease | 3 (4) | 5 (9) | 0.348 |
| Conditions of cardiac arrest | |||
| Shockable rhythm — no. (%) | 78 (95) | 39 (74) | <0.001 |
| Witnessed arrest — no. (%) | 76 (93) | 43 (81) | 0.043 |
| First defibrillation by automated external defibrillator — no. (%) | 15 (18) | 4 (8) | 0.074 |
| Bystander cardiopulmonary resuscitation — no. (%) | 68 (83) | 38 (72) | 0.310 |
| Time to return of spontaneous circulation — min | 15 ± 10 | 26 ± 16 | <0.001 |
| Findings and procedures on arrival at hospital | |||
| ST-segment elevation myocardial infarction, no. (%) | 36 (44) | 22 (42) | 0.784 |
| Coronary angiogram obtained — no. (%) | 77 (99) | 41 (80) | <0.001 |
| Percutaneous coronary intervention performed — no. (%) | 41 (53) | 17 (35) | 0.049 |
| pH level | 7.2 ± 0.1 | 7.2 ± 0.2 | 0.664 |
| Lactate level — mmol/liter | 4.7 ± 3.9 | 8.2 ± 5.4 | <0.001 |
Plus–minus values are means ± SD.
CPC, Cerebral Performance Categories Scale (CPC 1 = no symptoms or 2 = moderate disability, CPC 3 = severe disability, 4 = coma or vegetative state, and 5 = death).
Outcome
A total of 82 (61%) patients had a favorable neurological outcome (CPC 1–2), and the primary outcome of unfavorable neurological outcome (CPC 3–5) occurred for 53 (39%) patients before 90 days.
From the total of 135 comatose patients at admission, 121 (90%) were comatose and available for pupillary assessment on day 2 and 75 (56%) on day 3. On day 2, 4 (3%) patients had died, and 9 (7%) had awakened (with a favorable outcome). On day 3, 11 (8%) patients had died, and 43 patients had awakened (with a favorable outcome). For 6 patients, pupillometry was not carried out on day 3 because patients were moved to other departments after the decision of WLST. In these specific and unusual cases, patients had a loss of gray–white matter discrimination and/or clear signs of herniation in CT, bilaterally absent N20 pathways with SSEP, and highly malignant EEGs; before 72 hours. All patients died within 4 days. A total of 52 (39%) died within 90 days. This population’s median time to death was 8 (IQR 6) days from admission.
Characteristics of quantitative pupillary response parameters
The median time from ROSC to the first quantitative pupillometry measurement was 15.2 hours (IQR 6.2–18.6 hours). All median values for the quantitative pupillometry parameters were (except for Lat) were significantly lower for patients with an unfavorable outcome than for those with a favorable outcome (Table 2). We found no statistical difference in the median values of Lat between the favorable and unfavorable outcome groups.
Table 2.
Median quantitative pupillometry values.
| Pupillometry parameter | Favorable outcome | Unfavorable outcome | p-value | |
|---|---|---|---|---|
| Day 1, n = 135 | n = 82 (61%) | n = 53 (39%) | ||
| qPLR (%) | 12.00 (9.0, 15.4) | 6.00 (3.5, 9.5) | <0.001 | |
| NPi | 4.30 (4.05, 4.50) | 3.75 (3.35, 4.15) | <0.001 | |
| CV (mm/s) | 0.58 (0.40, 0.68) | 0.32 (0.16, 0.44) | <0.001 | |
| MCV (mm/s) | 0.84 (0.69, 1.04) | 0.59 (0.42, 0.77) | <0.001 | |
| DV (mm/s) | 0.22 (0.18, 0.28) | 0.11 (0.06, 0.16) | <0.001 | |
| Lat (s) | 0.27 (0.25, 0.30) | 0.29 (0.25, 0.32) | 0.144 | |
| Day 2, n = 121 | n = 73 (60%) | n = 48 (40%) | ||
| qPLR (%) | 12.50 (11.0, 15.5) | 7.20 (5.0, 11.1) | <0.001 | |
| NPi | 4.35 (4.15, 4.50) | 3.75 (3.39, 4.21) | <0.001 | |
| CV (mm/s) | 0.68 (0.53, 0.83) | 0.40 (0.31, 0.60) | <0.001 | |
| MCV (mm/s) | 0.97 (0.84, 1.16) | 0.70 (0.55, 0.88) | <0.001 | |
| DV (mm/s) | 0.23 (0.18, 0.28) | 0.14 (0.07, 0.21) | <0.001 | |
| Lat (s) | 0.27 (0.25, 0.29) | 0.28 (0.23, 0.30) | 0.347 | |
| Day 3, n = 75 | n = 39 (52%) | n = 36 (48%) | ||
| qPLR (%) | 16.00 (12.0, 18.0) | 9.00 (5.0, 13.0) | <0.001 | |
| NPi | 4.45 (4.12, 4.62) | 3.60 (3.24, 4.06) | <0.001 | |
| CV (mm/s) | 0.87 (0.61, 1.00) | 0.56 (0.32, 0.77) | 0.002 | |
| MCV (mm/s) | 1.20 (0.94, 1.45) | 0.84 (0.57, 1.17) | 0.002 | |
| DV (mm/s) | 0.34 (0.21, 0.42) | 0.20 (0.08, 0.28) | <0.001 | |
| Lat (s) | 0.24 (0.22, 0.26) | 0.27 (0.23, 0.29) | 0.012 |
Median values (25%-75% quartiles) of qPLR and NPi, from admission (day 1) to day 3, according to Cerebral Performance Category (CPC) outcome within 90 days. CPC dichotomized as favorable (CPC 1–2) vs. unfavorable (CPC 3–5) outcome.
qPLR, quantitatively assessed pupillary light reflex; CV, average constriction velocity; MCV, maximum constriction velocity; DV, dilation velocity; Lat, Latency of constriction; NPi, neurological pupil index.
The progression of mean quantitative pupillometry values over admission days, between outcome groups (with p-values), are presented in Supplementary Fig. 1. Mean values between outcome groups were significantly different for all parameters at all time points. The mean values in the favorable outcome group did significantly increase (decrease for Lat) from day 1–3 for all parameters. All mean values, except for NPi, increased (decreased for Lat) significantly from day 1–3 in the unfavorable group. However, only the means of CV and MCV progressed significantly in both outcome groups from day 1–2.
Predictive value of quantitative pupillometry parameters
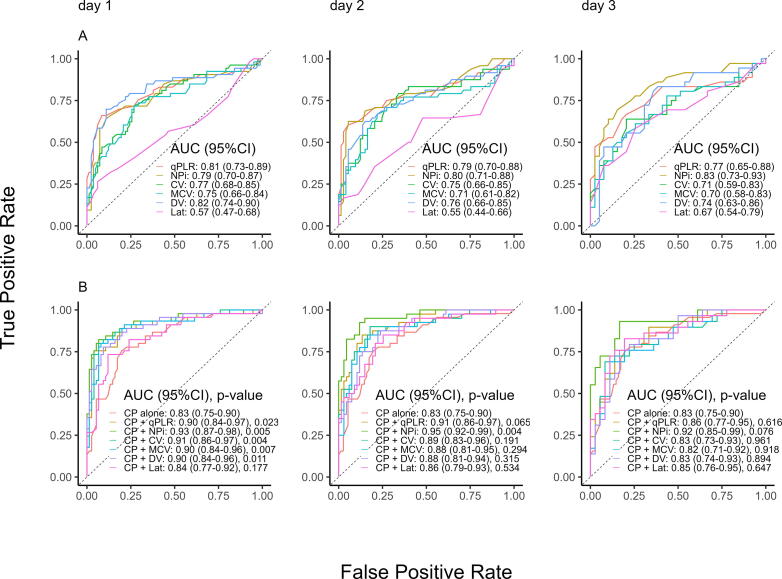
The prognostic value of all parameters, with AUCs (95% confidence interval) as indicators for the ability to predict an unfavorable neurological outcome, are depicted as ROC curves in Fig. 1.
Fig. 1.
Quantitative pupillometry parameters predicting unfavorable outcome. ROC curves of univariate (A) and multivariate (B) models predicting unfavorable outcome (Cerebral Performance Categories Scale 3–5) for all quantitative pupillometry parameters on day 1–3. Multivariable models adjusted for clinical predictors of age, sex, ST-elevation myocardial infarction, shockable primary rhythm, time to ROSC, and bystander cardiopulmonary resuscitation. ROC, receiver operating characteristic; AUC, area under the curve; qPLR, quantitatively assessed pupillary light reflex; CV, average constriction velocity; MCV, maximum constriction velocity; DV, dilation velocity; Lat, Latency of constriction; NPi, neurological pupil index; CP, prehospital clinical predictors.
We observed overall highest AUCs for qPLR, NPi, and DV (Fig. 1A). The best values for qPLR and DV were on day 1 with lesser results on the following days, but AUC for NPi was higher in the later days.
With the numerically highest AUC (0.82 [0.74–0.90] on day 1, DV performed significantly better than MCV (p = 0.010). On both day 1 and 2, qPLR significantly outperformed MCV (p = 0.049–0.034) with an AUC of 0.81 (0.73–0.89). NPi achieved a significantly higher AUC than CV (p = 0.027) and MCV (p = 0.028) on day 3.
In the multivariable model, qPLR, NPi, CV, MCV, and DV all achieved excellent performance with an AUC of 0.90–0.93, independent of the clinical predictors on day 1. However, NPi numerically outperformed the other parameters on all days (Fig. 1B). The AUC of NPi was significantly higher than qPLR (p = 0.005) on day 1, than of all other parameters (p = 0.007–0.032) on day 2, and on day 3 compared to CV (p = 0.050) and MCV (p = 0.041). NPi were further independent of the clinical predictors on both day 1 and 2 but not on day 3 (Fig. 1B).
When predicting the secondary outcome of 90-day mortality, the same tendency is observed as with the neurological outcome; qPLR and DV achieved the highest AUCs at day 1 (0.81 [0.73–0.89] and 0.82 [0.73–0.90]), and qPLR and NPi on day 2. The AUC of qPLR and DV were significantly higher than MCV (p = 0.017 and 0.016) on day 1, qPLR was higher than MCV (p = 0.026) on day 2, and NPi were higher than CV (p = 0.014), and MCV (p = 0.017) on day 3. All AUCs for the outcome of 90-day mortality are found in Supplementary Fig. 2.
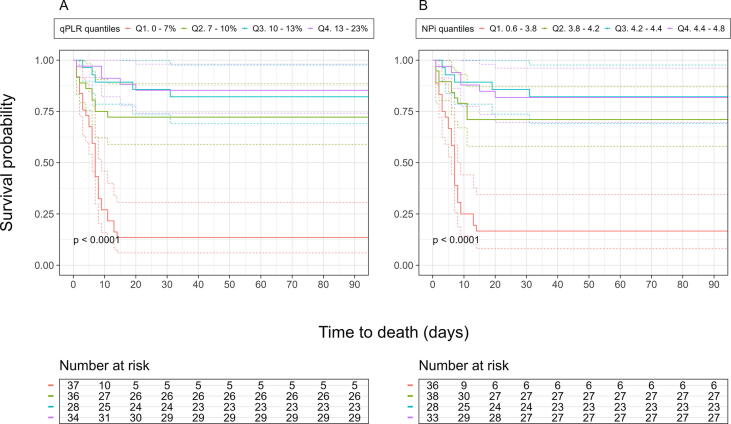
When stratified for quartile and presented with Kaplan-Meier statistics, patients with the lowest quartile (Q1) of both qPLR (0–7%) and NPi (0.60–3.81) had significantly (p < 0.001) worse probability of survival compared to the highest quartile (Q4) (Fig. 2). All parameters (except for Lat) had significantly (p < 0.001) worse outcome for Q1 compared to Q4 (Supplementary Fig. 3), and Q1 of qPLR, NPi, DV and CV (p < 0.001–0.045) had a significantly worse outcome than Q1 for Lat (Supplementary Fig. 4).
Fig. 2.
Survival probability of patients resuscitated from cardiac arrest by quartiles of qPLR and NPi. Kaplan Meier estimates of the probability of survival free of death from any cause until 90 days after admission stratified by quartiles of (A) qPLR and (B) NPi. Depicting p-value for difference between third and fourth quartile. OHCA, out-of-hospital cardiac arrest; qPLR, quantitatively assessed pupillary light reflex; NPi, neurological pupil index; Q1. first quartile; Q2. second quartile; Q3. third quartile; Q4. fourth quartile.
Optimal threshold values for quantitative pupillometry
On any day during hospitalization, a qPLR < 4%, NPi < 2.45, CV < 0.1 mm/s, and MCV < 0.335 mm/s had no false positive predictions of unfavorable outcome (0% FPR [0–0%]), with sensitivities of 28% (17–40%), 9% (2–19%), 13% (6–23%), and 17% (8–26%), respectively on day 1. A DV ≤ 0.04 mm/s achieved 0% FPR on day 1 (sensitivity of 23% [11–34%]) and 2 (sensitivity of 12% [4–23%]) and could only reach a 5% FPR (0–13%) on day 3 (sensitivity of 39% [25–56%]). All threshold values reached 0% FPR and were equal to or higher on day 2 and 3, compared to day 1.
We present threshold values for all quantitative pupillometry parameters, predicting an unfavorable neurological outcome, from day 1 to day 3 with the lowest FPR in Table 3, with 1–3% FPR in Supplementary Table 2, and those maximizing Youden Index (sensitivity + specificity − 100%) in Supplementary Table 3.
Table 3.
Prognostic performance of quantitative pupillometry parameters.
| Pupillometry parameter | Threshold | Sample size (n/N) | FPR (%) | Sensitivity (%) | PPV (%) | NPV (%) | Youden Index (%) | |
|---|---|---|---|---|---|---|---|---|
| Day 1, N = 135 | qPLR (%) | 3.99 | 15/135 | 0 (0–0) | 28 (17–40) | 100 (100–100) | 68 (65–73) | 28 (17–42) |
| NPi | 2.44 | 6/135 | 0 (0–0) | 9 (2–19) | 100 (100–100) | 63 (61–66) | 9 (2–19) | |
| CV (mm/s) | 0.09 | 7/135 | 0 (0–0) | 13 (6–23) | 100 (100–100) | 64 (62–67) | 13 (4–23) | |
| MCV (mm/s) | 0.33 | 9/135 | 0 (0–0) | 17 (8–26) | 100 (100–100) | 65 (63–68) | 17 (8–28) | |
| DV (mm/s) | 0.04 | 12/135 | 0 (0–0) | 23 (11–34) | 100 (100–100) | 67 (64–70) | 23 (11–34) | |
| Lat (s)* | 0.35 | 10/135 | 0 (0–0) | 11 (4–21) | 100 (100–100) | 64 (62–66) | 11 (4–21) | |
| Day 2, N = 121 | qPLR (%) | 4.49 | 9/121 | 0 (0–0) | 19 (8–29) | 100 (100–100) | 65 (62–68) | 19 (8–31) |
| NPi | 2.44 | 3/121 | 0 (0–0) | 6 (0–15) | 100 (100–100) | 62 (60–63) | 6 (0–15) | |
| CV (mm/s) | 0.13 | 7/121 | 0 (0–0) | 15 (6–25) | 100 (100–100) | 64 (62–67) | 15 (6–25) | |
| MCV (mm/s) | 0.41 | 9/121 | 0 (0–0) | 19 (8–31) | 100 (100–100) | 65 (62–68) | 19 (8–31) | |
| DV (mm/s) | 0.05 | 6/121 | 0 (0–0) | 12 (4–23) | 100 (100–100) | 63 (61–66) | 12 (4–23) | |
| Lat (s)* | 0.35 | 7/121 | 0 (0–0) | 12 (4–23) | 100 (100–100) | 63 (61–66) | 12 (4–23) | |
| Day 3, N = 75 | qPLR (%) | 5.00 | 10/75 | 0 (0–0) | 28 (14–44) | 100 (100–100) | 60 (56–65) | 28 (14–44) |
| NPi | 3.14 | 6/75 | 0 (0–0) | 17 (6–31) | 100 (100–100) | 57 (53–61) | 0.17 (6–28) | |
| CV (mm/s) | 0.23 | 7/75 | 0 (0–0) | 19 (8–33) | 100 (100–100) | 57 (54–62) | 19 (8–33) | |
| MCV (mm/s) | 0.41 | 4/75 | 0 (0–0) | 11 (3–22) | 100 (100–100) | 55 (53–58) | 11 (3–22) | |
| DV (mm/s) | 0.13 | 16/75 | 5 (0–13) | 39 (25–56) | 88 (71–100) | 63 (57–70) | 34 (17–50) | |
| Lat (s)* | 0.31 | 4/75 | 0 (0–0) | 11 (3–22) | 100 (100–100) | 55 (53–58) | 11 (3–22) |
Analysis of all quantitative pupillometry parameters. Thresholds are the highest value yielding predicting unfavorable neurological outcome (90-days CPC 3–5) with the lowest FPR achievable. Youden Index (sensitivity + specificity − 100%).
FPR, false positive rate; PPV, positive predictive value; NPV, negative predictive value; qPLR, quantitatively assessed pupillary light reflex; CV, average constriction velocity; MCV, maximum constriction velocity; DV, dilation velocity; Lat, Latency of constriction; NPi, neurological pupil index.
The threshold for Lat denotes the lowest values resulting in the presented FPR.
Discussion
In a large contemporary population of comatose patients resuscitated from OHCA with a presumed cardiac cause, we present extensive data on prognostic performance and specific threshold values of all quantitative pupillary parameters, predicting 90-day unfavorable neurological outcome and 90-day mortality.
We found that the median values of qPLR and NPi were consistently lower in the patients with an unfavorable outcome than in those with favorable neurologic outcomes, confirming previous findings.13, 14, 15, 16, 17, 18 Although sparsely investigated,4, 15 we observed the same tendency for CV, MCV, and DV. However, the novelty of this study is that a qPLR < 4%, an NPi < 2.45, a CV < 0.1 mm/s, and an MCV < 0.335 mm/s, predicted 90-day unfavorable neurological outcome with 0% FPR. All except Lat provided independent prediction on day 1. The AUC and median values suggest that Lat had no relation to outcome.
We observed that median values of the parameters of both outcome groups (not including NPi) increased progressively from admission through time points, as seen with other populations.14, 15, 16 Probably caused by the transient brain stem dysfunction, reflecting the natural recovery from hypoxic-ischemic brain injury and interference from sedatives and opioids.19, 20 NPi is a composite score of the parameters compared to a normative model, and it has been argued that NPi is only minimally influenced by medications.10, 21, 22, 23, 24
The balance of losing correctly or falsely predicted outcomes to patient deaths and awakenings between time points will affect the predictive ability across days. Most pupillometry parameters declined in prognostic performance (AUC) from day 1 to 3, except for NPi, which improved AUC across days (Fig. 1). These results are consistent with previous studies.4, 16
Because an absent PLR (by a manual evaluation) only achieves 0% FPR for predicting an unfavorable outcome in comatose post-OHCA patients after 96 hours from ROSC,25, 26 and because of the effect of sedation on PLR,27 guidelines still advise that assessments of PLR should not be evaluated earlier than ≥72 hours.28 However, in the current guidelines, quantitative pupillometry is recommended for assessments,28 and several studies have achieved 0% FPR in early outcome prediction.6, 14, 15, 16, 17, 18 Hence, the authors proposed that future studies should identify consistent threshold values for qPLR and NPi for 0% FPR.26
The collected results of our and other studies argue for the further adaptation of quantitative pupillometry in the early detection of both patients demanding further prognostication and possible WLST consideration; and those with the potential for intensified neuroprotective therapies.
The resulting sensitivity is low when aiming for 0% FPR in predictions. Thus, future studies should further explore the combined performance of multiple predictors (at higher thresholds) to increase sensitivity while keeping a low FPR.
When defining the threshold of quantitative pupillometry parameters predicting an unfavorable outcome, we have used the lowest value of the two eyes, if different, to ensure the most conservative that benefits the patients. The use of the higher value would potentially increase the threshold value associated with an unfavorable outcome, thus risking the over-decision of WLST when using the thresholds in the clinical setting. However, it should be emphasized that when using the proposed thresholds for neuroprognostication and subsequently deciding on WLST, the highest value should always be used as the representative to avoid falsely pessimistic predictions.
Limitations
As this study was retrospective, no control of exposure or outcome assessment could be made. Further, specific levels and timing of the withdrawal of sedation for the individual patients were not available in this registry. Anesthesia and opioids could potentially confound measurements of pupillary reflexes.21, 29, 30 However, previous studies have found similar results with or without sedation, with the NPi algorithm unaffected by the sedatives/analgesics.10, 21, 22, 23, 24 Intra- and interobserver reproducibility and repeatability were previously validated with low variability in measurements of quantitative pupillometry under the same clinical settings as this study.31
Prognostic parameters are generally subjects of self-fulfilling prophecy bias in outcome prediction. However, as quantitative pupillometry was not included in the clinical neuroprognostication, treating physicians and outcome assessors were blinded to the results. The staff performing the manual assessment of the PLR for the clinical prognostication was the same that obtained the quantitative pupillometry measurements. Thus, before the manual evaluation of the PLR, the assessor could potentially have known the quantitative pupillometry result. However, the assessors did not take part in the outcome assessment, and the final prognostication also included evaluations of EEG, neuroimaging, and SSEP.
Due to limited data and differences in the timing of measurements (day 1–3 vs. after day 3) and sample (all patients vs. only patients with low expectations for survival), this study does not offer data on the additional prognostic value of quantitative pupillometry, or validate it with independent prognostic value, compared to the other predictors (e.g., SSEP, EEG, and NSE). Even though quantitative pupillometry have previously been reported to increase the sensitivity of SSEP in predicting poor outcome in OHCA patients,16 it could be argued that it has qualities that differentiate it from other predictors (easy-to-use bedside prognostication tool with high early prognostic value); we strongly recommend that future studies validate the significant contribution of quantitative pupillometry to other predictors.
Conclusion
In this study, we identified specific rule-out thresholds for all quantitative pupillometry parameters, predicting 90-day unfavorable neurological outcome with 0% FPR in comatose post-OHCA patients at any time during the first three days of hospitalization. These results are essential for further improving neuroprognostication in post-cardiac arrest care.
CRediT authorship contribution statement
Benjamin Nyholm: Conceptualization, Methodology, Writing – review & editing, Visualization. Laust Emil Roelsgaard Obling: . Christian Hassager: Conceptualization, Methodology. Johannes Grand: . Jacob Eifer Møller: Conceptualization, Methodology. Marwan H. Othman: . Daniel Kondziella: . Jesper Kjaergaard: Conceptualization, Methodology, Writing – review & editing, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
Dr. Kjaergaard and Dr. Nyholm were supported by unrestricted grants from the Novo Nordisk Foundation [grant number NNF20OC0064043]. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.resplu.2023.100399.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
References
- 1.Nolan J.P., Sandroni C., Böttiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation. 2021;161:220–269. doi: 10.1016/j.resuscitation.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Laver S., Farrow C., Turner D., Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 3.Witten L., Gardner R., Holmberg M.J., et al. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation. 2019;136:93–99. doi: 10.1016/j.resuscitation.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura T., Namiki J., Sugawara Y., et al. Early outcome prediction with quantitative pupillary response parameters after out-of-hospital cardiac arrest: A multicenter prospective observational study. PloS One. 2020;15:e0228224. doi: 10.1371/journal.pone.0228224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins G.D., Jacobs I.G., Nadkarni V.M., et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: A Statement for Healthcare Professionals From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2015;1:328–340. doi: 10.1016/j.resuscitation.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Obling L., Hassager C., Illum C., et al. Prognostic value of automated pupillometry: an unselected cohort from a cardiac intensive care unit. Eur Heart J Acute Cardiovasc Care. 2019 doi: 10.1177/2048872619842004. [DOI] [PubMed] [Google Scholar]
- 7.Sandroni C., Cariou A., Cavallaro F., et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1816–1831. doi: 10.1007/s00134-014-3470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan J.P., Soar J., Cariou A., et al. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41:2039–2056. doi: 10.1007/s00134-015-4051-3. [DOI] [PubMed] [Google Scholar]
- 9.NeurOptics® NPI®-200 Pupillometer System—Instructions for Use © 2021 [Internet]. NeurOptics, Inc; Available from: https://neuroptics.com/wp-content/uploads/2018/09/NPi-200-Instructions-for-Use.pdf.
- 10.Chen J.W., Gombart Z.J., Rogers S., Gardiner S.K., Cecil S., Bullock R.M. Pupillary reactivity as an early indicator of increased intracranial pressure: The introduction of the Neurological Pupil index. Surg Neurol Int. 2011;2:82. doi: 10.4103/2152-7806.82248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossetti A.O., Rabinstein A.A., Oddo M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 2016;15:597–609. doi: 10.1016/S1474-4422(16)00015-6. [DOI] [PubMed] [Google Scholar]
- 12.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 13.Suys T., Bouzat P., Marques-Vidal P., et al. Automated quantitative pupillometry for the prognostication of coma after cardiac arrest. Neurocrit Care. 2014;21:300–308. doi: 10.1007/s12028-014-9981-z. [DOI] [PubMed] [Google Scholar]
- 14.Solari D., Rossetti A.O., Carteron L., et al. Early prediction of coma recovery after cardiac arrest with blinded pupillometry. Ann Neurol. 2017;81:804–810. doi: 10.1002/ana.24943. [DOI] [PubMed] [Google Scholar]
- 15.Heimburger D., Durand M., Gaide-Chevronnay L., et al. Quantitative pupillometry and transcranial Doppler measurements in patients treated with hypothermia after cardiac arrest. Resuscitation. 2016;103:88–93. doi: 10.1016/j.resuscitation.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Oddo M., Sandroni C., Citerio G., et al. Quantitative versus standard pupillary light reflex for early prognostication in comatose cardiac arrest patients: an international prospective multicenter double-blinded study. Intensive Care Med. 2018;44:2102–2111. doi: 10.1007/s00134-018-5448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riker R.R., Sawyer M.E., Fischman V.G., et al. Neurological Pupil Index and Pupillary Light Reflex by Pupillometry Predict Outcome Early After Cardiac Arrest. Neurocrit Care. 2020;32:152–161. doi: 10.1007/s12028-019-00717-4. [DOI] [PubMed] [Google Scholar]
- 18.Tamura T., Namiki J., Sugawara Y., et al. Quantitative assessment of pupillary light reflex for early prediction of outcomes after out-of-hospital cardiac arrest: A multicentre prospective observational study. Resuscitation. 2018;131:108–113. doi: 10.1016/j.resuscitation.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Jørgensen E.O. Course of neurological recovery and cerebral prognostic signs during cardio-pulmonary resuscitation. Resuscitation. 1997;35:9–16. doi: 10.1016/s0300-9572(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 20.Nolan J.P., Neumar R.W., Adrie C., et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication: A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Rollins M.D., Feiner J.R., Lee J.M., Shah S., Larson M. Pupillary effects of high-dose opioid quantified with infrared pupillometry. Anesthesiology. 2014;121:1037–1044. doi: 10.1097/ALN.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 22.Shoyombo I., Aiyagari V., Stutzman S.E., et al. Understanding the Relationship Between the Neurologic Pupil Index and Constriction Velocity Values. Sci Rep. 2018;8:6992. doi: 10.1038/s41598-018-25477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J.W., Vakil-Gilani K., Williamson K.L., Cecil S. Infrared pupillometry, the Neurological Pupil index and unilateral pupillary dilation after traumatic brain injury: implications for treatment paradigms. 2014;3:548. doi: 10.1186/2193-1801-3-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couret D., Boumaza D., Grisotto C., et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care Lond Engl. 2016;13:99. doi: 10.1186/s13054-016-1239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C.H., Wu C.Y., Liu C.C.Y., et al. Neuroprognostic Accuracy of Quantitative Versus Standard Pupillary Light Reflex for Adult Postcardiac Arrest Patients: A Systematic Review and Meta-Analysis. Crit Care Med. 2021;49:1790–1799. doi: 10.1097/CCM.0000000000005045. [DOI] [PubMed] [Google Scholar]
- 26.Sandroni C., D’Arrigo S., Cacciola S., et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46:1803–1851. doi: 10.1007/s00134-020-06198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soar J., Böttiger B.W., Carli P., et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Nolan J.P., Sandroni C., Böttiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47:369–421. doi: 10.1007/s00134-021-06368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opic P., Rüegg S., Marsch S., Gut S.S., Sutter R. Automated Quantitative Pupillometry in the Critically Ill: A Systematic Review of the Literature. Neurology. 2021;97:e629–e642. doi: 10.1212/WNL.0000000000012295. [DOI] [PubMed] [Google Scholar]
- 30.Olsen M.H., Jensen H.R., Ebdrup S.R., et al. Automated pupillometry and the FOUR score - what is the diagnostic benefit in neurointensive care? Acta Neurochir (Wien) 2020;162:1639–1645. doi: 10.1007/s00701-020-04381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyholm B., Obling L., Hassager C., et al. Superior reproducibility and repeatability in automated quantitative pupillometry compared to standard manual assessment, and quantitative pupillary response parameters present high reliability in critically ill cardiac patients. PloS One. 2022;17:e0272303. doi: 10.1371/journal.pone.0272303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.