Abstract
Purpose of Review
Obesity is a significant public health problem and a major risk factor for the development and progression of atherosclerosis and its cardiovascular manifestations. Lower extremity peripheral artery disease (PAD) affects 3%–10% of the Western population and, if left untreated, can lead to devastating outcomes with both an increased risk of morbidity and mortality. Interestingly, the association between obesity and PAD remains debatable. Whereas it is well known that PAD and obesity frequently overlap in the same patients, many studies have demonstrated a negative association between obesity and PAD and a protective effect of obesity on disease development and progression, a phenomenon described as the “obesity paradox.” Possible mechanisms for this paradox may include genetic background, as assessed by mendelian randomization studies, adipose tissue dysfunction, and body fat distribution rather than adiposity, while other factors, such as sex, ethnicity, sarcopenia in the elderly population, or aggressive treatment of co-existing metabolic conditions in individuals with obesity compared to those with normal weight, could have some impact as well.
Recent Rindings
Few reviews and meta-analyses examining systematically the relationship between obesity and PAD exist. The impact of PAD development due to the presence of obesity remains largely controversial. However, the most current evidence, backed by a recent meta-analysis, suggests a potential protective role of a higher body mass index on PAD-related complications and mortality.
Summary
In this review, we discuss the association between obesity and PAD development, progression, and management, and the potential pathophysiologic mechanisms linking the two diseases.
Keywords: Adiposity, Body mass index, Cardiovascular disease, Obesity, Obesity paradox, Peripheral artery disease
Introduction
Obesity, defined as a body mass index (BMI) of 30 kg/m2 or higher in Caucasians, is a major public health crisis, with more than 700 million children and adults affected worldwide and a constantly increasing prevalence [1–4]. It is well established that obesity is an independent risk factor and predisposes to many comorbidities, including insulin-resistant states (i.e., metabolic syndrome, type 2 diabetes mellitus (T2DM)), various types of cancer, the, severity of COVID-19, and a wide spectrum of cardiovascular diseases (CVD), including coronary artery disease (CAD) and potentially peripheral artery disease (PAD) [2, 5–12].
PAD is a vascular disease caused mostly by atherosclerosis (other causes include connective tissue disease and vasculitis), defined as partial or complete stenosis of one or more arteries [13]. The prevalence of PAD ranges between 3%-10%, but it can be as high as 15%-20% among elderly patients, based on older data while the prevalence is higher now. More than 200 million people suffer from PAD worldwide, with symptoms varying from subclinical to severe lifestyle limitations [14, 15]. There are various classification systems, but the clinical presentation of PAD is usually classified by the Rutherford scale based on the patient's symptoms [16]. Asymptomatic PAD is defined as Rutherford Stage 0, while patients presenting with mild or moderate claudication, or walking-induced leg muscle pain relieved by rest, are classified as Stages 1 and 2, respectively. Stage 3 patients cannot complete standard treadmill exercise, while stage patients exhibit foot pain at rest, and at Stages 5 and 6 ulcers and/or gangrene [17–19]. PAD does not only affect patient’s quality of life but is associated with a 3‐ to 6‐fold increase in cardiovascular morbidity and mortality risk [13].
While associations of PAD with coronary or cerebrovascular atherosclerosis, hypertension, T2DM, smoking, Black race, and advanced age are well established, [15, 20, 21], the relationship with obesity is yet to be clarified. More than 65% of adults with PAD have also overweight (BMI 25-30 kg/m2) or obesity [22]. High fat mass is associated with declining of the ambulatory status and vascular health in patients with PAD and claudication [23]. However, studies in patients with overweight and mild obesity have identified better CVD related prognosis, compared to those within lean and underweight BMI range (18.5 to 25 and <18.5 kg/m2 accordingly) [4, 24]. This observation is known as the “obesity paradox”[24, 25, 26•]. Understanding the real meaning - beyond biases due to confounding variables – of this association can be particularly useful in further designing treatment strategies for patients with PAD [27, 28].
The purpose of this review is to present the current evidence for the association between obesity and PAD in terms of prevalence, pathophysiology, clinical phenotypes, and overall outcomes. Very few reviews and meta-analyses have been conducted regarding the association between obesity and PAD, and the current review offers a systematic presentation of all the major studies examining obesity and the risk of developing PAD and its implications for PAD outcomes.
Effect of Weight on Peripheral Artery Disease: Epidemiological Evidence and Controversy
A plethora of studies have examined the association between obesity and PAD [13] (Table 1). One of the major studies supporting a connection between obesity and PAD is a large cohort from Israel of over 10,000 men, which identified that those with new-onset intermittent claudication (IC) had a higher BMI (0.5 kg/m2) compared to men without IC [29]. Intriguingly, traditional indices of upper or lower body fat accumulation did not show any differences between groups [29]. In another study, Vogt et al. showed that upper body obesity, as assessed by the waist-to-hip ratio (WHR), and not BMI, was associated with an increased risk of developing PAD [30]. WHR was also shown to correlate with PAD in a group of non-insulin-dependent diabetic patients, while a Taiwanese study on patients with end-stage renal disease found that abdominal obesity was also independently associated with PAD [31, 32]. These findings could potentially be explained by the body fat distribution and the well-established detrimental effects of abdominal and visceral fat [33]. In a recent large-scale study, high BMI was associated with an increased risk of PAD only for women, suggesting a potential sex-specific underlying mechanism [34•]. In the same study, patients with overweight but not obesity (BMI 25–29.9 kg/m2) were shown to have the lowest prevalence of PAD compared to other BMI groups [34•].
Table 1.
Main findings from studies examining BMI and/or other indices of obesity in relationship to PAD development risk
| Study-Year | Purpose of study | Number of Participants | Follow-up period | PAD definition | Main Findings |
|---|---|---|---|---|---|
| Studies showing a positive relationship | |||||
| Yeboah et al. (2016) [62] | Association of obesity and PAD in individuals without known CVD | 623 | n/a - case control | ABI ≤ 0.9 | Abdominal obesity and BMI ≥ 30 kg/m2 were associated with PAD |
| Hung et al. (2013) [32] | Association between abdominal obesity and PAD | 204 | n/a - cross sectional study | ABI of <0.9 | Abdominal obesity was associated with PAD |
| Jakovljevic et al. (2011) [61] | Association of obesity and body fat distribution with PAD | 102 | n/a – case control | Fontaine Stage II - IV | Overweight, body fat > 26.5% and WHR > 1.02 were associated with PAD |
| Planas et al. (2001) [60] | Association of BMI and WHR with PAD | 699 | n/a - cross sectional study | ABI<0.9 | Abdominal obesity was associated with PAD |
| Katsilambros et al. (1996) [31] | Risk factors for atherosclerosis in individuals withT2DM | 193 | n/a – cross sectional study | ABI < 0.89 and/or IC | WHR significantly related to PAD |
| Vogt et al. (1993) [30] | Prevalence of PAD ( women ≥ 65 years) | 1,601 | n/a | ABI <0.9 | Abdominal obesity adjusted for BMI was associated with PAD, |
| Kannel et al. (1985) [35] | PAD predisposing factors | 295 | 26 years | Development of IC | Inverse relationship between IC and weight in men, quadratic relationship in women |
| Studies with no relationship or mixed results in subgroups | |||||
| Heffron et al. (2020) [34•] | Association between PAD and BMI | 20,000 | n/a | ABI<0.9 |
BMI <25 and ≥ 40 kg/m2 were associated with modestly increased odds of PAD in males. In women, the odds of PAD were similar in underweight and class II obesity and a markedly elevated in class III obesity |
| Zhang et al. (2019) [43] | Association between BMI and vascular complications (individuals with T2DM) | 3,224 | 5 years | ABI < 0.9 | U-shaped relationship between BMI (<21 and > 27 kg/m2) PAD. |
| Ix et al. (2011) [131] |
Association of BMI with prevalence and incidence of PAD ( ≥65 years) |
5,419 | 13.2 years | ABI < 0.90 or clinical diagnosis of PAD. |
The crude prevalence of PAD was lower among individuals with a higher BMI. Individuals who had never smoked and had higher BMI trended towards a higher PAD prevalence (not statistically significant). No association of BMI with incident clinical PAD in total group Individuals who had never smoked were associated with an approximately 30% greater risk of PAD incidence. |
| Skilton et al. (2011) [132] | Impact of metabolic risk factors on ABI and PAD incidence | 3,682 | 9 years | ABI <0.90 | Overweight and obesity are not associated with PAD |
| Subramaniam et al. (2011) [133] | Prevalence of ABI in a multi-ethnic Asian population and association of risk factors with PAD | 4,132 | 6 years | ABI ≤ 0.9 or > 1.4 | BMI not associated with PAD |
| Carbayo et al. (2007) [134] | Prevalence of ABI and relationship of risk factors in ( > 40 years) | 1,322 | 5 years | ABI < 0.9 | BMI was not associated with PAD. |
| Allison et al. (2011) [40] | Association of CVD risk factors and PAD among various ethnicities | 6,814 | 2 years | ABI <0.9 | BMI did not differ among groups with low or normal ABI |
| Fujiwara et al. (2004) [135] |
Prevalence of asymptomatic PAD and associated risk factors |
1,398 | 2 years | ABI <0.9 | BMI was not significantly associated |
| Murabito et al. (2002) [47] | PAD prevalence and associated risk factors | 3,313 | 3 years | ABI<0.9 | BMI was significantly associated with PAD only in women |
| Hooi et al. (2001) [44] |
Incidence of asymptomatic and symptomatic PAD and the association of risk factors |
2,327 | 7 – 9 years | asymptomatic and symptomatic PAD / ABI < 0.95 | BMI was not associated with PAD. |
| Meijer et al. (2000) [48] | Etiology of PAD and impact of risk factors | 6,450 | 5 years | ABI < 0.90 | BMI was not associated with PAD. |
| Ness et al. (2000) [45] |
Association of risk factors with the prevalence of symptomatic PAD |
1,911 | 1,5 year | PAD defined clinically or from the history of related operations. | BMI was not associated with PAD. |
| Ingolfsson et al. (1994) [46] | Trends in IC and the impact of potential risk factors on CVD and total mortality | 9,141 | 8 years | IC | BMI and body weight did not show an association. |
| Bowlin et al. (1994) [29] | Prevalence, incidence, and risk factors of IC in men | 10,059 | 5 years | IC | BMI (Quetelet’s index) was associated with baseline and new-onset IC. There was no association between central (subscapular skinfold thickness) or peripheral (triceps skinfold thickness) obesity. |
| Bainton et al. (1994) [136] | Prevalence, incidence, and risk factors of IC in men | 2,348 | 10 years | IC | No association between BMI and the development of IC |
| Newman et al. (1993) [41] | Risk factors for CVD events (> 65 years) | 5,201 | 1 year | ABI <0.9 | BMI showed no association with PAD |
| Fowkes et al. (1992) [36] | Relation of various risk factors and PAD | 1,592 | n/a - cross sectional | ABI <0.9 | BMI showed no significant association |
| Studies showing protective relationship | |||||
| Cantú-Brito et al. (2020) [137] | Prevalence of PAD, risk factors, and six-month outcomes in high-risk stable ACS patients | 830 | 6 months | ABI <0.9 | Low ABI was inversely associated with BMI >27 |
| Sritara et al. (2007) [42] |
Prevalence and risk factors of PAD |
2,359 | n/a | ABI< 0.9 | Overweight was negatively associated with PAD. |
| Criqui et al. (2005) [39] |
Association between PAD and ethnicity and CVD risk factors |
2,343 | n/a - cross-sectional | ABI <0.90 or an abnormal Doppler or previous revascularization for PAD. | A higher BMI was negatively associated with PAD |
| Tseng et al. (2003) [138] |
Prevalence and risk factors of PAD (individuals with T2DM) |
610 | 1 year | ABI < 0.9 | A lower BMI was associated with PAD |
| Curb et al. (1996) [38] |
Relationships of ABI and risk factors (Japanese American men > 70 years) |
3,450 | 5-years | ABI <0.9 | A higher BMI was negatively associated with lower ABI |
| Beks et al. (1995) [37] | Prevalence of PAD and association with risk factors | 631 | n/a | Vascular surgery on the lower part of the aorta, the iliac, or the lower extremity arteries, an ABI < 0.90, or a monophasic or absent Doppler flow curve | A higher BMI was negatively associated with PAD |
ABI Ankle Branch Index, BMI Body Mass Index, CKD Chronic Kidney Disease, CLI Chronic Intermittent Claudication. IC Intermittent Claudication, N/A Not Available, OR Odds Ratio, PAD Peripheral Artery Disease, WHR Waist-to-hip Ratio
Similarly interesting are other studies suggesting that a high BMI might be a protective factor against PAD [13, 35]. In the Framingham study, BMI was weakly associated with the development of IC in men, but weight showed an inverse relationship with the presence of PAD [35]. In women, an increased risk was recorded for the extremes (either low or high weights), while the overweight individuals in that study were found to have a lower risk as described before [34•]. A significant inverse association between BMI and ankle brachial pressure index (ABI) was shown in an analysis of the Edinburgh Artery Study [36]. A study from the Netherlands (Hoorn Study) and studies from the US in various ethnic populations have also shown a protective role of higher BMI against PAD [37–41]. Taiwanese subjects with a higher BMI were also shown to be at a significantly lower risk of PAD in a study of patients with diabetes mellitus [42]. More recently, a U-shaped relationship, i.e., a higher risk for PAD at the extremities of the BMI scale, was depicted in another study [43].
Finally, a considerable proportion of studies did not document any significant, positive, or negative associations between either claudication or PAD and obesity [13, 44, 45]. In the Reykjavik study, a prospective population study, weight and BMI did not have an association with IC [46]. In the Framingham Offspring Study, BMI was associated with ABI level only for females, while no association between BMI and PAD was observed among elderly patients in two large Dutch studies [44, 47, 48].
Pathophysiological Links Between Obesity and Peripheral Artery Disease
Obesity is a diverse, multifaceted chronic disease that is linked to a plethora of comorbidities in still unclear ways, which could partially be attributed to genetic background. [10] To investigate the association between obesity and numerous conditions and possible causality, several genome-wide association studies (GWAS) have been performed, unveiling more than 300 single-nucleotide polymorphisms (SNPs). associated with various indices of obesity, including BMI, WHR, and other adiposity features [49]. Notably, in a Mendelian randomization study, exploring SNPs associated with higher BMI in 11,477 individuals of Chinese origin has shown that the presence of 14 SNPs associated with higher BMI may predispose to a higher risk of PAD, which implicates a genetic link and a causal association, at least in the examined population [50].
Excessive accumulation of white adipose tissue (WAT) is a hallmark of obesity [33]. However, a major driver of obesity-related comorbidities and health impacts is adiposopathy, defined as WAT dysfunction rather than just fat mass accumulation [10, 33, 51, 52]. WAT dysfunction includes adipocyte hypertrophy, impairments in lipid metabolism (reduced capacity to buffer the daily influx of dietary lipids), thereby contributing to ectopic fat accumulation, decreased adipose tissue blood flow, mitochondrial dysfunction, altered oxygenation, and a state of chronic low-grade inflammation [33, 53–55] (Fig. 1). Body fat distribution also plays a pivotal role in whole-body metabolism, CVD risk, and development [56]. Upper body fat (abdominal subcutaneous and visceral) accumulation has been linked to unfavorable effects, while gluteofemoral (lower body) fat has been shown to possess opposing, protective properties [33, 52, 57–59].
Fig. 1.
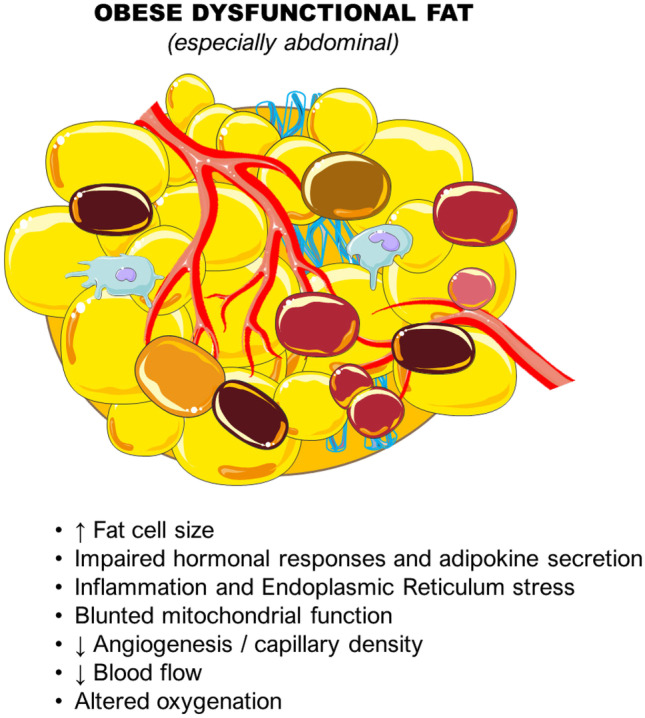
Characteristics of dysfunctional white adipose tissue, a major driver of the pathophysiological implications of obesity. (All images are originated from the free medical site http://smart.servier.com/ (accessed on February 8, 2023) by Servier licensed under a Creative Commons Attribution 3.0 Unported License)
Numerous studies have indicated that abdominal fat deposition is associated with PAD [31, 60–62]. The presence of WAT inflammation in abdominal obesity is well established, and a pro-inflammatory phenotype of obese WAT could promote CVD development [63–68]. Hypertrophic WAT has an impaired capacity to store meal-derived fatty acids [69] and results in ectopic fat accumulation. The storage of excess lipids in non-adipose tissues in obesity is strongly associated with insulin resistance and atheromatous plaque formation [65, 70, 71]. Furthermore, hypertrophic adipocytes are characterized by a pro-inflammatory adipocytokine phenotype, which may further aggravate disease development [66, 72]. WAT inflammation is also determined by the infiltration of various populations of pro-inflammatory immune cells [73, 74] including macrophages [75–79]. All these cell populations and adipocytokine dysregulation could lead to further systemic vascular dysfunction and damage. A typical example of adipocytokine dysregulation in obesity is hypoadiponectinemia [80]. Plasma levels of adiponectin were significantly lower among patients with evidence of symptomatic PAD (intermittent claudication) than among those without [81]. These observations have driven the hypothesis that the pathophysiology of obesity and its complications, including its effect on PAD, is driven by WAT dysfunction rather than an increase in WAT mass alone and possibly relates to depot-specific fat accumulation, as reflected clinically by the WHR [33, 54, 64, 71, 82].
It is postulated that lower body WAT consists of cells with more anti-inflammatory properties [52]. In line with that hypothesis, interleukin-6 (IL-6) release (quantified by an arterio–venous difference technique model) showed a lower IL-6 release rate from femoral adipose tissue than from the corresponding abdominal tissue [57]. Perivascular fat could also play a role in disease development. For example, periaortic fat deposition is associated with PAD [83]. Based on the local differences in pro-inflammatory cytokine production and the influence of perivascular fat on disease development, there may be a beneficial effect of femoral adiposity and potentially a protective role for the arteries of the lower limbs [10, 49, 50]. Finally, observations from studies showing an increased risk of PAD for women [34•, 35, 47] could be attributed partially to the cardiometabolic transition occurring during and after the menopause, resulting in a re-distribution of the WAT and potentially a loss of the beneficial characteristics of the gluteofemoral fat [52, 84].
Obesity Paradox or Sarcopenia and Frailty-related Symptoms and Outcomes in Underweight Elderly?
The “obesity paradox” is based on observations in patients with overweight or obesity and known PAD who had a better prognosis and fewer symptoms compared to their peers without overweight or obesity [85–87]. A partial explanation for this may be that a significant percentage of the underweight elderly patients suffered from frailty and sarcopenia. Sarcopenia is defined as an unintentional loss of lean muscle mass, frequently associated with aging [88]. Patients with sarcopenia and normal weight and with sarcopenia and obesity are two distinct sarcopenia groups [88, 89]. The preservation of muscle mass in collaboration with myokines and circulating hormones influences the progression of PAD [90]. Almost 25% of patients with intermittent claudication have sarcopenia, and those patients tend to have worse walking performance compared to their non-sarcopenic peers [91]. Mechanistic studies comparing elderly individuals with or without obesity and PAD identified that obesity decreased the time to claudication and delayed post-exercise hemodynamic recovery, suggesting that muscle metabolic demand, not the total workload, is responsible for the start of claudication and maximal exercise tolerance. Moreover, claudication duration might be responsible for the time needed to complete hemodynamic recovery after exercise [92]. Sarcopenia increases platelet dysfunction, promotes hypercoagulable states, and impairs wound healing through the expression of atherosclerotic cytokines [93, 94]. This pathophysiologic cascade can lead to amputation, major adverse cardiovascular events, major adverse limb events, and mortality [85, 95]. Other factors involved in the obesity paradox, but not linked directly to adiposity, could be attributed to the variation in vascular disease and atheromatous plaque morphology in coronary artery disease (CAD) versus PAD [96]. Thrombosis has a significant role in the pathogenesis of PAD as opposed to CAD, where weight, atherosclerosis is the predominant factor [97]. Another potential explanation is that patients with obesity and PAD receive more aggressive medical treatment and have better outcomes in some of the studies in comparison to patients with normal weight who might be less aggressively treated because of their overall lower risk factor profile [98–100]. Other cardinal pathophysiologic mechanisms related to obesity that could explain this phenomenon include higher energy reserves, inflammatory preconditioning, an anti-inflammatory immune profile, endotoxin neutralization, adrenal steroid synthesis, renin-angiotensin system activation, cardioprotective metabolic effects, and prevention of muscle wasting [25]. A summary of the potential factors explaining the complex relationship between obesity and PAD is depicted in Fig. 2.
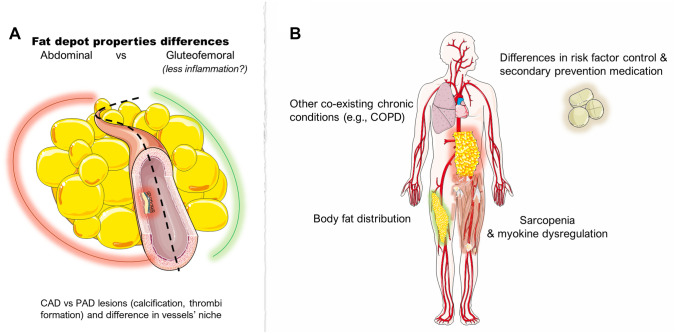
Fig. 2.
Postulated pathophysiological mechanisms explaining the obesity paradox and discrepancies among studies. Panel A shows parameters within various adipose tissue depots and differences in histopathological level. Panel B presents potential cofounders that partially could explain the BMI subgroup differences regarding the association of obesity and PAD and clinical outcomes. BMI: body mass index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; PAD: peripheral artery disease. (All images are originated from the free medical site http://smart.servier.com/ (accessed on February 8, 2023) by Servier licensed under a Creative Commons Attribution 3.0 Unported License)
Finally, the potential pathophysiologic mechanisms and observations leading to the conclusion of an existing “obesity paradox” should be further examined, taking into account the methodological limitations of studies, such as the retrospective design, the potential selection bias (e.g., due to ethnicity, age, or sex), the use of BMI as an obesity index (which could lead to an underestimation of the impact of body composition and body fat distribution), and the inadequate adjustment for confounding variables [11, 25].
Impact of Obesity or Underweight Status on Disease Progression, Management, and Mortality in Patients With PAD
Various studies have examined the role of obesity in PAD progression. (Table 2). Therapeutic options for PAD include pharmacological management, lifestyle interventions (nutritional and exercise), and revascularization for cases that do not improve with standard management or for chronic limb-threatening ischemia (CLTI) [19].
Table 2.
Main findings from studies examining the impact of obesity on disease progression and mortality in patients with PAD
| Study-Year | Purpose of study | Number of Patients | Follow-up period | Group definition / categories (PAD/CLI/Rutherford x) | Main Findings |
|---|---|---|---|---|---|
| Studies showing poorer disease progression | |||||
| Gardner and Montgomery (2010) [109] |
Compare resting energy expenditure of patients with IC and CLI. |
140 | n/a | Fontaine stage II or stage III; ABI ≤ 0.90 for patients with IC; a history of rest pain and an ABI ≤ 0.40 for patients with CLI | Patients with CLI had a lower resting energy expenditure, higher body fat percentage, and higher fat mass than patients with IC |
| Giugliano et al. (2010) [108] |
Prognostic impact of obesity (BMI and WC) |
190 | ~ 2.5 years | ABI < 0.90 | General adiposity, BMI, and abdominal obesity (WC) were significantly associated with increased CVD risk |
| Gardner and Montgomery (2008) [107] | Effects of metabolic syndrome components on IC and other parameters | 423 | 2 years | Fontaine stage II PAD |
More metabolic syndrome components had worsened IC physical function, health-related quality of life, and peripheral circulation. Abdominal obesity was the predictor of exercise performance during the treadmill and 6-minute walk tests. |
| Golledge et al. (2007) [106] | Association of obesity and metabolic syndrome to IC severity and prognosis | 60 | 2 years | IC and stenosis greater than 50% in the aortoiliac, infrainguinal, or both arteries in CTA | Obesity was associated with the severity of PAD More outcome events in patients with metabolic syndrome or obesity. WC independently predicted the likelihood of outcome events. |
| McDermott et al. (2006) [105] | Associations between baseline weight and weight change and functional decline | 389 | ~ 2 - 4 years | ABI < 0.90 | Baseline BMI < 30 kg/m2 was associated with greater average annual functional decline (6-minute walk performance, usual-paced 4-m walking velocity, and fast-paced 4-m walking velocity). |
| Studies with no relationship or mixed results | |||||
| Zierfuss et al. (2020) [128] |
Predictive power of general and visceral obesity with cardiovascular and overall mortality |
367 | 5 years | Stable PAD (Fontaine I - II) | General and visceral adiposity, did not predict mortality |
| Doshi et al. 2017 [127] |
Assessing in-hospital outcomes of percutaneous revascularization in patients with PAD |
62,445 | n/a | ICD-9-CM diagnostic codes 440.0, 440.2, 440.3, 440.8, 440.9, 444.0, 444.8, 444.9, 447.8, and 447.9 for patients with PAD. Procedure codes 39.90, 00.55, 17.56, and 39.50 for interventional procedures specific for PAD | Obesity was not associated with in-hospital mortality, but increased length of stay and median cost of hospitalization |
| Studies showing protective relationship | |||||
| Ko et al. (2020) [139] | Clarify patient characteristics and prognosis according to different treatment locations; risk factors for each treatment location. | 2,230 | 2 years | IC / CLI | BMI < 18 was a risk factor for major adverse limb events and predictor of overall survival |
| Arinz et al. (2020) [140] | Perioperative outcomes after LEB and PVI in patients with severe obesity | 29,138 | 30 days | IC / CLI | Severe obesity was not significantly associated with increased perioperative CVD complications, return to the operating room, or mortality. |
| Januszek et al. (2019) [125] | Relationship between BMI and the long-term results of endovascular treatment from retrograde access in patients with PAD. | 405 | ~ 3 years | Patients that underwent retrograde ER regarding the CTO of the infra-inguinal lower limb arteries | Mortality rate was higher in the group of patients with BMI < 25 kg/m2. |
| Keller et al. (2019) [119•] | Association of adverse in-hospital outcomes in PAD patients relative to underweight and obesity | 5,611,484 | n/a | ICD-code I70.2 of the German nationwide database | Patients with obesity received less treatment with major amputation, had a lower mortality rate. Patients with underweight had higher in-hospital mortality |
| Ludhwani and Wu (2019) [120] | Retrospective study to verify the existence of the obesity paradox in patients with PAD | 248,288 | n/a | Primary discharge diagnosis of PAD | Obesity was associated with a better short-term prognosis (lower odds of amputation, need for intervention, and in-hospital mortality). |
| Pacha et al. (2019) [122] | Association of in-hospital mortality and adverse CVD outcomes with underweight status in PAD patients undergoing EVT | 685 | n/a | Patients with severe PAD that underwent EVT | Patients with low-BMI and PAD have worse in-hospital survival and more adverse outcomes after EVT (higher incidence of in-hospital mortality, MACE, open bypass surgery, and infection |
| Higashitani et al. (2018) [141] | Evaluate preoperative clinical symptoms for EVT and relationship to post-EVT death and CVD prognosis in patients with PAD, including ALI | 2,238 | ~ 10 months | IC / CLI | BMI < 18 was strongly associated with all-cause mortality and MACE |
| Shean et al. (2018) [142] | Evaluate the relationship between BMI and postoperative complications following lower extremity EI | 3,246 | 30 days | IC / CLI / ALI | Few major complications occurred in patients with obesity |
| Tanaka et al. (2018) [126] | Short and long-term effects and potential challenges of metabolic syndrome | 154 | 6 to 8 years | Patients with aortoiliac occlusive disease who underwent open bypass surgery | Patients without obesity had a greater risk of long-term mortality. |
| Senda et al. (2018) [143] | To evaluate whether underweight status predicted a poor prognosis in patients with claudication | 441 | ~ 3,5 years | IC | All-cause and CVD mortality were significantly higher in the underweight group |
| Murata et al. (2015) [123] | Relationship between BMI and long-term outcomes in patients with CLI following EVT | 1,088 | ~1.5 years | CLI that underwent EVT of isolated infrapopliteal artery lesions | Low BMI was associated with a poor prognosis. Normal weight patients had a small but significant increase in mortality compared with overweight/obesity group |
| Soga et al. (2014) [144] | Identify prognostic factors of 2-year mortality in CLI patients | 995 | ~ 2 years | CLI | Underweight was among the prognostic factors associated with death |
| Iida et al. (2012) [145] | Risk-stratified EVT midterm outcomes: (overall survival, limb salvage, and re-intervention) in patients with CLI | 406 | ~1.5 year | CLI (Rutherford 5 and 6) | Patients with obesity or overweight showed a better survival rate |
| Badheka et al. (2011) [146] |
Risk factors for development of critical PAD in patients with stable CAD and impact of PAD on CVD outcomes |
8,290 | 4.7 years | Development of critical PAD (needing angioplasty, bypass grafting, or aneurysm repair) | Inverse relationship between BMI and critical PAD |
| Kanhai et al. (2012) [118] |
Impact of general and abdominal adiposity on occurrence of vascular events and mortality |
4,206 | 4.5 years |
Recent clinical diagnosis of PAD (Fontaine stage II, III, IV). |
General adiposity (BMI) was associated with a decreased risk for vascular and all-cause mortality. |
| Kuijk et al. (2011) [147] | Prevalence of obesity and association on long-term survival; medical treatment at time of discharge and obesity and long-term survival | 2,933 | 6 years | ABI < 0.9 or a history of IC with a previous intervention | Underweight was an independent predictor of mortality, while overweight protected for all-cause mortality. |
| Abbas et al. (2010) [124] | Predictors of poor outcomes in female patients undergoing EI for symptomatic PAD. | 292 | ~ 2 years | Patients who underwent EI | Low BMI predicted poor outcomes (subsequent EI and TVR, limb loss, and vascular surgery, death) |
| Kumakura et al. (2010) [117] | Long-term survival in patients with PAD and severity of symptoms and association to CKD, and BMI | 652 | 15 years | IC or CLI | Low BMI was a significant risk factor for mortality |
| Galal et al. (2008)[116] |
Influence of COPD on the relationship between BMI and mortality in patients with PAD |
2,392 | 4.37 years | Vascular surgical interventions (abdominal aortic surgery, carotid endarterectomy), or lower limb arterial revascularization procedures. | Patients classified as underweight were at increased risk for mortality. Potential cofounder overrepresentation of individuals with moderate-to-severe COPD |
ABI Ankle Branch Index, ALI Acute Limb Ischemia, BMI Body Mass Index, CAD Coronary Artery Disease, CAT Computed Tomographic Angiogram, CKD Chronic Kidney Disease, CLI Chronic Intermittent Claudication, COPD Chronic Obstructive Pulmonary Disease, CTO Chronic Total Occlusions, CVD cErebrovascular Disease, EI Endovascular Intervention, EVT Endovascular Therapy, ER Endovascular Recanalization, IC Intermittent Claudication ICD-9-CM, LEB Lower Extremity Bypass, MACE Major Adverse Cardiovascular Events, MWD Maximum Walking Distance, N/A Not Available, PAD Peripheral Artery Disease, PVI Peripheral Vascular Interventions, WC Waist Circumference, WHR Waist-to-hip Ratio
Weight loss effectively slows the course of atherosclerosis by improving risk factors such as diabetes, hyperlipidemia, hypertension, and adiposopathy [101]. Farah et al. showed that the walking capacity of over 100 patients with PAD fluctuated according to their comorbidities, with diabetes and CAD being independent predictors of decreased functional capacity [102]. Another study identified that patients who intentionally lost more than 5 pounds had a lower yearly drop in 6-minute walk distance, despite losing greater muscle in the calf area, compared to patients who gained weight [103]. A recent meta-analysis of weight-reduction studies including patients with established PAD showed that every 1kg loss of fat mass predicted a 0.01 m/s improvement in walking speed with the appropriate adherence to calorie restriction and physical activity [104]. All these implicate the benefit of weight loss interventions on the functional capacity of patients.
Few studies examined the association between obesity and functional decline in individuals with PAD. This was assessed with 6-minute walk performance; graded treadmills measured maximum walking distance (MWD) and initial claudication distance [105, 106]. A small study of 60 subjects showed that obesity and waist circumference were predictors of disease progression and were associated with the likelihood of major cardiovascular or revascularization events [106]. Another study that examined combinations of metabolic syndrome components found that abdominal obesity was predictive of unfavorable outcomes, including worsening IC symptoms and overall quality of life [32, 107]. Giugliano et al. showed that abdominal and, to a lesser degree, general obesity were associated with worse prognosis (CVD events), while Gardner et al. showed that patients with CLTI had a lower resting expenditure, a higher body fat percentage, and, compared to patients with IC only, a higher fat mass [108, 109].
The “obesity paradox” has potential implications for CLTI as well. Untreated or inadequately treated PAD may lead to CLTI or even amputation [110]. CLTI patients may have a much higher risk of amputation and mortality compared to those with claudication [88]. Although the one-year risk of limb loss is excessively high at 30% and the five-year all-cause mortality is 50% in patients with CLTI [111–113], higher BMI is associated with lower rates of mortality in patients with lower extremity ulcers [114].
Association of Obesity and Mortality in Patients With PAD
The GetABI study included 1,400 patients with established PAD, showing no correlation between obesity (BMI > 30 kg/m2) and mortality [115]. However, other studies did show a significant inverse correlation, with a Dutch and a Japanese study documenting that all-cause mortality was lower in patients with obesity compared to underweight individuals, although the overrepresentation of patients with chronic obstructive pulmonary disease and malnutrition, respectively, in the underweight group could have affected the results [116, 117]. Another study that assessed various manifestations of vascular disease, including CAD and PAD, showed that general adiposity was associated with an increased risk of vascular mortality for CAD, but the risk was lower in patients with PAD [118]. Two other large studies showed that obesity was associated with improved in-hospital outcomes, including mortality, compared to individuals without obesity [119• 120]. Again, this could have been confounded by the increased rates of frailty and sarcopenia in the group without obesity.
Impact of Obesity on Post-revascularization Outcomes
Revascularization is frequently performed in patients with ischemic ulcers or rest discomfort to avoid amputation [121]. Interestingly, Pacha et al. showed that patients with PAD and low BMI have worse in-hospital mortality (4.8% vs. 1.2%) and more adverse cardiovascular events (7.9% vs. 4.1%) after revascularization compared to their peers with normal BMI [122]. Another study that examined the long-term outcomes of over 1,000 CLTI patients after endovascular revascularization found that the 3-year overall survival rates were 33.3%, 61.2%, and 69.8% in the underweight, normal weight, and overweight/obesity groups, respectively. [123]. Those findings have been replicated by other studies [122–126] but there were also studies that failed to find a difference between the two groups [127, 128]. Finally, in a recent meta-analysis examining 12 studies, mortality among patients with PAD was higher in those who were underweight and lower in those with concomitant obesity [129••]. Interestingly, the authors of this meta-analysis concluded that obesity-related mortality was lower in individuals with PAD who had concurrent CAD than in those who did not have CAD [129••]. However, further research is necessary to validate these results and determine their long-term implications for individuals with CAD. This includes observing if increased monitoring and vigorous treatments in this patient population lead to a higher prevention of complications or if other physiological factors may play a role in mediating protection.
Conclusion and Future Perspectives
While there are a plethora of studies examining the detrimental effect of obesity on cardiovascular outcomes and in patients with PAD, there is also a significant body of literature suggesting improved outcomes following interventional treatments (revascularization) in patients with obesity and PAD compared to their peers without obesity. However, those studies identified the benefit in elderly patients, with COPD, frailty, and sarcopenia probably contributing to worse outcomes in the population without obesity. Larger prospective studies with a longer follow-up period examining the complex associations and adjusting for all those covariates, including ethnicity, sex, age, body fat distribution, and the overall degree of treatment of concurrent diseases, are needed to elucidate the complex association between obesity and PAD. Finally, further studies examining the impact of bariatric surgery [130] or novel medicinal treatments, like the dual incretin analogues [99], on PAD progression are warranted.
Funding
The authors received no funding for this study or the preparation of the manuscript.
Compliance with Ethical Standards
Ethics Approval and Consent to Participate
No ethics approval was necessary since it is a review manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria Dalamaga and Damianos Kokkinidis contributed equally to this work.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Collaborators TGO. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 3.González-Muniesa P, Mártinez-González M-A, Hu FB, et al. Obesity. Nature Reviews Disease Primers. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 4.Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol. 2018;15(1):45–56. doi: 10.1038/nrcardio.2017.108. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;00253-er.02017-00253. 10.1210/er.2017-00253. [DOI] [PMC free article] [PubMed]
- 7.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/s0140-6736(08)60269-x. [DOI] [PubMed] [Google Scholar]
- 8.Sheree DM, Sean LM. Metabolic reprogramming in type 2 diabetes and the development of breast cancer. J Endocrinol. 2018;237(2):R35–R46. doi: 10.1530/JOE-18-0037. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 10.Lempesis IG, Tsilingiris D, Liu J, Dalamaga M. Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies. Metabol Open. 2022;15:100208. doi: 10.1016/j.metop.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalamaga M, Christodoulatos GS, Karampela I, Vallianou N, Apovian CM. Understanding the co-epidemic of obesity and covid-19: current evidence, comparison with previous epidemics, mechanisms, and preventive and therapeutic perspectives. Curr Obes Rep. 2021;10(3):214–243. doi: 10.1007/s13679-021-00436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lempesis IG, Karlafti E, Papalexis P, et al. COVID-19 and liver injury in individuals with obesity. World J Gastroenterol. 2023;29(6):908–916. doi: 10.3748/wjg.v29.i6.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 14.Nosova EV, Conte MS, Grenon SM. Advancing beyond the "heart-healthy diet" for peripheral arterial disease. J Vasc Surg. 2015;61(1):265–274. doi: 10.1016/j.jvs.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet (London, England) 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 16.Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R. Overview of classification systems in peripheral artery disease. In: Seminars in interventional radiology. Vol 31. Thieme Medical Publishers, pp 378-388. 2014. [DOI] [PMC free article] [PubMed]
- 17.Hossain P, Kokkinidis DG, Armstrong EJ. How To Assess a Claudication and When To Intervene. Curr Cardiol Rep. 2019;21(12):138. doi: 10.1007/s11886-019-1227-4. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JL, Halperin JL, Albert NM, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 accf/aha guideline recommendations): a report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;127(13):1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 19.Kullo IJ, Rooke TW. Peripheral artery disease. N Engl J Med. 2016;374(9):861–871. doi: 10.1056/NEJMcp1507631. [DOI] [PubMed] [Google Scholar]
- 20.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308(16):1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 22.Thomas J, Delaney C, Suen J, Miller M. Nutritional status of patients admitted to a metropolitan tertiary care vascular surgery unit. Asia Pac J Clin Nutr. 2019;28(1):64–71. doi: 10.6133/apjcn.201903_28(1).0010. [DOI] [PubMed] [Google Scholar]
- 23.Gardner AW, Bright BC, Ort KA, Montgomery PS. Dietary intake of participants with peripheral artery disease and claudication. Angiology. 2011;62(3):270–275. doi: 10.1177/0003319710384395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63(14):1345–1354. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Karampela I, Chrysanthopoulou E, Christodoulatos GS, Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Curr Obes Rep. 2020;9(3):231–244. doi: 10.1007/s13679-020-00394-x. [DOI] [PubMed] [Google Scholar]
- 26.Simati S, Kokkinos A, Dalamaga M, Argyrakopoulou G. Obesity Paradox: Fact or Fiction? Curr Obes Rep. 2023 doi: 10.1007/s13679-023-00497-1. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 28.Unkart JT, Allison MA, Criqui MH, et al. Life's simple 7 and peripheral artery disease: the multi-ethnic study of atherosclerosis. Am J Prev Med. 2019;56(2):262–270. doi: 10.1016/j.amepre.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowlin SJ, Medalie JH, Flocke SA, Zyzanski SJ, Goldbourt U. Epidemiology of intermittent claudication in middle-aged men. Am J Epidemiol. 1994;140(5):418–430. doi: 10.1093/oxfordjournals.aje.a117264. [DOI] [PubMed] [Google Scholar]
- 30.Vogt MT, Cauley JA, Kuller LH, Hulley SB. Prevalence and correlates of lower extremity arterial disease in elderly women. Am J Epidemiol. 1993;137(5):559–568. doi: 10.1093/oxfordjournals.aje.a116709. [DOI] [PubMed] [Google Scholar]
- 31.Katsilambros NL, Tsapogas PC, Arvanitis MP, Tritos NA, Alexiou ZP, Rigas KL. Risk factors for lower extremity arterial disease in non-insulin-dependent diabetic persons. Diabet Med. 1996;13(3):243–6. 10.1002/(SICI)1096-9136(199603)13:3<243::AID-DIA69>3.0.CO;2-U. [DOI] [PubMed]
- 32.Hung P-H, Tsai H-B, Lin C-H, Hung K-Y. Abdominal obesity is associated with peripheral artery disease in hemodialysis patients. Plos One. 2013;8(6):e67555. doi: 10.1371/journal.pone.0067555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lempesis IG, van Meijel RLJ, Manolopoulos KN, Goossens GH. Oxygenation of adipose tissue: a human perspective. Acta Physiologica. 2020;228(1):e13298. doi: 10.1111/apha.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heffron SP, Dwivedi A, Rockman CB, et al. Body mass index and peripheral artery disease. Atherosclerosis. 2020;292:31–36. doi: 10.1016/j.atherosclerosis.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the framingham study. J Am Geriatr Soc. 1985;33(1):13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerald R, Fowkes F, Housley E, Riemersma RA, et al. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the edinburgh artery study. Am J Epidemiol. 1992;135(4):331–340. doi: 10.1093/oxfordjournals.aje.a116294. [DOI] [PubMed] [Google Scholar]
- 37.Beks PJ, Mackaay AJC, de Neeling JND, de Vries H, Bouter LM, Heine RJ. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn Study. Diabetologia. 1995;38(1):86–96. doi: 10.1007/BF02369357. [DOI] [PubMed] [Google Scholar]
- 38.Curb JD, Masaki K, Rodriguez BL, et al. Peripheral artery disease and cardiovascular risk factors in the elderly. Arterioscler Thromb Vasc Biol. 1996;16(12):1495–1500. doi: 10.1161/01.ATV.16.12.1495. [DOI] [PubMed] [Google Scholar]
- 39.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and Peripheral Arterial Disease. Circulation. 2005;112(17):2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 40.Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the multi-ethnic study of atherosclerosis (mesa) J Am Coll Cardiol. 2006;48(6):1190–1197. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 41.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Cardiovascular heart study (chs) collaborative research group. Circulation. 1993;88(3):837–845. doi: 10.1161/01.CIR.88.3.837. [DOI] [PubMed] [Google Scholar]
- 42.Sritara P, Sritara C, Woodward M, et al. Prevalence and Risk Factors of Peripheral Arterial Disease in a Selected Thai Population. Angiology. 2007;58(5):572–578. doi: 10.1177/0003319707303652. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Guo Y, Shen X, Zhao F, Yan S. Lower body mass index is not of more benefit for diabetic complications. J Diabetes Invest. 2019;10(5):1307–1317. doi: 10.1111/jdi.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooi JD, Kester ADM, Stoffers HEJH, Overdijk MM, van Ree JW, Knottnerus JA. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153(7):666–672. doi: 10.1093/aje/153.7.666. [DOI] [PubMed] [Google Scholar]
- 45.Ness J, Aronow WS, Ahn C. Risk factors for symptomatic peripheral arterial disease in older persons in an academic hospital-based geriatrics practice. J Am Geriatr Soc. 2000;48(3):312–314. doi: 10.1111/j.1532-5415.2000.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 46.Ingolfsson IÖ, Sigurdsson G, Sigvaldason H, Thorgeirsson G, Sigfusson N. A marked decline in the prevalence and incidence of intermittent claudication in icelandic men 1968–1986: A strong relationship to smoking and serum cholesterol—The Reykjavik study. J Clin Epidemiol. 1994;47(11):1237–1243. doi: 10.1016/0895-4356(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 47.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PWF. Prevalence and clinical correlates of peripheral arterial disease in the framingham offspring study. Am Heart J. 2002;143(6):961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 48.Meijer WT, Grobbee DE, Hunink MGM, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: the rotterdam study. Arch Intern Med. 2000;160(19):2934–2938. doi: 10.1001/archinte.160.19.2934. [DOI] [PubMed] [Google Scholar]
- 49.Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6(3):223–236. doi: 10.1016/s2213-8587(17)30200-0. [DOI] [PubMed] [Google Scholar]
- 50.Huang Y, Xu M, Xie L, et al. Obesity and peripheral arterial disease: A Mendelian Randomization analysis. Atherosclerosis. 2016;247:218–224. doi: 10.1016/j.atherosclerosis.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 51.Bays HE. Adiposopathy: Is “Sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57(25):2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 52.Lempesis IG, Hoebers N, Essers Y, et al. Physiological oxygen levels differentially regulate adipokine production in abdominal and femoral adipocytes from individuals with obesity versus normal weight. Cells. 2022;11(22):3532. doi: 10.3390/cells11223532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 54.Goossens GH, Blaak EE. Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Front Endocrinol. 2015;6(55). 10.3389/fendo.2015.00055. [DOI] [PMC free article] [PubMed]
- 55.Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond) 2014;38(8):1019–1026. doi: 10.1038/ijo.2013.200. [DOI] [PubMed] [Google Scholar]
- 56.Sagris M, Theofilis P, Antonopoulos AS, et al. Inflammatory mechanisms in covid-19 and atherosclerosis: current pharmaceutical perspectives. Int J Mol Sci. 2021;22(12). 10.3390/ijms22126607. [DOI] [PMC free article] [PubMed]
- 57.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11(2):90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 58.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34(6):949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 59.Lempesis IG, Goossens GH, Manolopoulos KN. Measurement of human abdominal and femoral intravascular adipose tissue blood flow using percutaneous Doppler ultrasound. Adipocyte. 2021;10(1):119–123. doi: 10.1080/21623945.2021.1888471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Planas A, Clara A, Pou J, et al. Relationship of obesity distribution and peripheral arterial occlusive disease in elderly men. Int J Obes. 2001;25(7):1068–1070. doi: 10.1038/sj.ijo.0801638. [DOI] [PubMed] [Google Scholar]
- 61.Jakovljević B, Stojanov V, Lović D, Paunović K, Radosavljević V, Tutić I. Obesity and fat distribution as predictors of aortoiliac peripheral arterial disease in middle-aged men. Eur J Intern Med. 2011;22(1):84–88. doi: 10.1016/j.ejim.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 62.Yeboah K, Puplampu P, Yorke E, Antwi DA, Gyan B, Amoah AGB. Body composition and ankle-brachial index in Ghanaians with asymptomatic peripheral arterial disease in a tertiary hospital. BMC Obes. 2016;3(1):27. doi: 10.1186/s40608-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 64.Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Prac Res Clin Endocrinol Metabol. 2013;27(2):163–177. doi: 10.1016/j.beem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32(1):261–286. doi: 10.1146/annurev-nutr-071811-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hroussalas G, Kassi E, Dalamaga M, Delimaris I, Zachari A, Dionyssiou-Asteriou A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas. 2008;59(4):339–349. doi: 10.1016/j.maturitas.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Marouga A, Dalamaga M, Kastania AN, et al. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin Lab. 2013;59(9–10):1121–1128. doi: 10.7754/clin.lab.2012.121112. [DOI] [PubMed] [Google Scholar]
- 69.Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes. 2013;62(5):1417–1425. doi: 10.2337/db12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haczeyni F, Bell-Anderson KS, Farrell GC. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes Rev. 2018;19(3):406–420. doi: 10.1111/obr.12646. [DOI] [PubMed] [Google Scholar]
- 71.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94(2):206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 72.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metabol. 2007;92(3):1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 73.Lee B-C, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim et Biophys Acta (BBA) - Mol Basis Dis. 2014;1842(3):446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 75.O'Rourke RW, White AE, Metcalf MD, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54(6):1480–1490. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127(1):5–13. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McNelis Joanne C, Olefsky Jerrold M. Macrophages, Immunity, and Metabolic Disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 79.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762. 10.1038/nature01705. https://www.nature.com/articles/nature01705#supplementary-information. [DOI] [PubMed]
- 81.Lim PS, Hu CY, Wu MY, Wu TK, Chang HC. Plasma adiponectin is associated with ankle-brachial index in patients on haemodialysis. Nephrology. 2007;12(6):546–552. doi: 10.1111/j.1440-1797.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 82.Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10(3):207–215. doi: 10.1159/000471488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox CS, Massaro JM, Schlett CL, et al. Periaortic fat deposition is associated with peripheral arterial disease. Circ Cardiovasc Imaging. 2010;3(5):515–519. doi: 10.1161/CIRCIMAGING.110.958884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17(1):47–66. doi: 10.1038/s41574-020-00431-8. [DOI] [PubMed] [Google Scholar]
- 85.Miller M, Delaney C, Penna D, et al. A 3-year follow-up study of inpatients with lower limb ulcers: evidence of an obesity paradox? J Multidiscip Health. 2012;5:181–186. doi: 10.2147/JMDH.S33625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karampela I, Christodoulatos GS, Dalamaga M. The role of adipose tissue and adipokines in sepsis: inflammatory and metabolic considerations, and the obesity paradox. Curr Obes Rep. 2019;8(4):434–457. doi: 10.1007/s13679-019-00360-2. [DOI] [PubMed] [Google Scholar]
- 88.Sagris M, Kokkinidis DG, Lempesis IG, et al. Nutrition, dietary habits, and weight management to prevent and treat patients with peripheral artery disease. Rev Cardiovasc Med. 2020;21(4):565–575. doi: 10.31083/j.rcm.2020.04.202. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Tsilingiris D, Dalamaga M. The non-linear relationship between muscle mass and BMI calls into question the use of BMI as a major criterion for eligibility for bariatric surgery. Metabol Open. 2022;13:100164. doi: 10.1016/j.metop.2022.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Addison O, Prior SJ, Kundi R, et al. Sarcopenia in peripheral arterial disease: prevalence and effect on functional status. Arch Phys Med Rehabil. 2018;99(4):623–628. doi: 10.1016/j.apmr.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kokkinidis DG, Armstrong EJ, Giri J. Balancing Weight Loss and Sarcopenia in Elderly Patients With Peripheral Artery Disease. J Am Heart Assoc. 2019;8(13):e013200. doi: 10.1161/jaha.119.013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ritti Dias RM, Forjaz CLM, Cucato GG, et al. Obesity decreases time to claudication and delays post-exercise hemodynamic recovery in elderly peripheral arterial disease patients. Gerontology. 2009;55(1):21–26. doi: 10.1159/000155219. [DOI] [PubMed] [Google Scholar]
- 93.Hicks CW, Yang C, Ndumele CE, et al. Associations of Obesity With Incident Hospitalization Related to Peripheral Artery Disease and Critical Limb Ischemia in the ARIC Study. J Am Heart Assoc. 2018;7(16):e008644. doi: 10.1161/JAHA.118.008644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sugai T, Watanabe T, Otaki Y, et al. Decreased psoas muscle computed tomography value predicts poor outcome in peripheral artery disease. Circ J Offic J Japan Circ Soc. 2018;82(12):3069–3075. doi: 10.1253/circj.CJ-18-0726. [DOI] [PubMed] [Google Scholar]
- 95.Kokkinidis DG, Strobel A, Jawaid O, et al. Development and validation of a predictive score for anterograde crossing of infrapopliteal chronic total occlusions: (The Infrapop-CTO Score) Catheter Cardiovasc Interv. 2020;95(4):748–755. doi: 10.1002/ccd.28693. [DOI] [PubMed] [Google Scholar]
- 96.Narula N, Olin JW, Narula N. Pathologic disparities between peripheral artery disease and coronary artery disease. Arterioscler Thromb Vasc Biol. 2020;40(9):1982–1989. doi: 10.1161/ATVBAHA.119.312864. [DOI] [PubMed] [Google Scholar]
- 97.Yin D, Matsumura M, Rundback J, et al. Comparison of plaque morphology between peripheral and coronary artery disease (from the CLARITY and ADAPT-DES IVUS substudies) Coron Artery Dis. 2017;28(5):369–375. doi: 10.1097/MCA.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 98.Hira RS, Kataruka A, Akeroyd JM, et al. Association of body mass index with risk factor optimization and guideline-directed medical therapy in us veterans with cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2019;12(1):e004817. doi: 10.1161/CIRCOUTCOMES.118.004817. [DOI] [PubMed] [Google Scholar]
- 99.Lempesis IG, Liu J, Dalamaga M. The catcher in the gut: tirzepatide, a dual incretin analog for the treatment of type 2 diabetes mellitus and obesity. Metabol Open. 2022;100220. 10.1016/j.metop.2022.100220. [DOI] [PMC free article] [PubMed]
- 100.Vallianou NG, Tsilingiris D, Kounatidis D, Lempesis IG, Karampela I, Dalamaga M. Sodium‑glucose cotransporter‑2 inhibitors in obesity and associated cardiometabolic disorders: where do we stand? Pol Arch Intern Med. 2022;132(10). 10.20452/pamw.16342. [DOI] [PubMed]
- 101.Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187–194. doi: 10.1007/s13679-017-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farah BQ, Souza Barbosa JP, Cucato GG, et al. Predictors of walking capacity in peripheral arterial disease patients. Clinics (Sao Paulo) 2013;68(4):537–541. doi: 10.6061/clinics/2013(04)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nayak P, Guralnik JM, Polonsky TS, et al. Association of six-minute walk distance with subsequent lower extremity events in peripheral artery disease. Vasc Med. 2020;25(4):319–327. doi: 10.1177/1358863X20901599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sagris M, Giannopoulos S, Giannopoulos S, et al. Transcervical carotid artery revascularization: a systematic review and meta-analysis of outcomes. J Vasc Surg. 2021 doi: 10.1016/j.jvs.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 105.McDermott MM, Criqui MH, Ferrucci L, et al. Obesity, weight change, and functional decline in peripheral arterial disease. J Vasc Surg. 2006;43(6):1198–1204. doi: 10.1016/j.jvs.2006.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45(1):40–46. doi: 10.1016/j.jvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Gardner AW, Montgomery PS. The effect of metabolic syndrome components on exercise performance in patients with intermittent claudication. J Vasc Surg. 2008;47(6):1251–1258. doi: 10.1016/j.jvs.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giugliano G, Brevetti G, Laurenzano E, Brevetti L, Luciano R, Chiariello M. The prognostic impact of general and abdominal obesity in peripheral arterial disease. Int J Obes. 2010;34(2):280–286. doi: 10.1038/ijo.2009.244. [DOI] [PubMed] [Google Scholar]
- 109.Gardner AW, Montgomery PS. Resting energy expenditure in patients with intermittent claudication and critical limb ischemia. J Vasc Surg. 2010;51(6):1436–1441. doi: 10.1016/j.jvs.2009.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25(6):1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 111.Kokkinidis DG, Giannopoulos S, Haider M, et al. Active smoking is associated with higher rates of incomplete wound healing after endovascular treatment of critical limb ischemia. Vasc Med. 2020;25(5):427–435. doi: 10.1177/1358863x20916526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kokkinidis DG, Armstrong EJ. Current developments in endovascular therapy of peripheral vascular disease. J Thorac Dis. 2020;12(4):1681–1694. doi: 10.21037/jtd.2019.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(SupplS):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 114.Kinlay S. Management of Critical Limb Ischemia. Circ Cardiovasc Interv. 2016;9(2):e001946. doi: 10.1161/CIRCINTERVENTIONS.115.001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120(21):2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 116.Galal W, van Gestel YR, Hoeks SE, et al. The obesity paradox in patients with peripheral arterial disease. Chest. 2008;134(5):925–930. doi: 10.1378/chest.08-0418. [DOI] [PubMed] [Google Scholar]
- 117.Kumakura H, Kanai H, Aizaki M, et al. The influence of the obesity paradox and chronic kidney disease on long-term survival in a Japanese cohort with peripheral arterial disease. J Vasc Surg. 2010;52(1):110–117. doi: 10.1016/j.jvs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 118.Kanhai DA, Kappelle LJ, Van der Graaf Y, Uiterwaal CS, Visseren FL. The risk of general and abdominal adiposity in the occurrence of new vascular events and mortality in patients with various manifestations of vascular disease. Int J Obes. 2012;36(5):695–702. doi: 10.1038/ijo.2011.115. [DOI] [PubMed] [Google Scholar]
- 119.Keller K, Hobohm L, Geyer M, et al. Obesity paradox in peripheral artery disease. Clin Nutr. 2019;38(5):2269–2276. doi: 10.1016/j.clnu.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 120.Ludhwani D, Wu J. Obesity paradox in peripheral arterial disease: results of a propensity match analysis from the national inpatient sample. Cureus. 2019;11(5):e4704. doi: 10.7759/cureus.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salomon du Mont L, Leclerc B, Morgant MC, et al. Impact of Nutritional State on Critical Limb Ischemia Early Outcomes (DENUCRITICC Study) Ann Vasc Surg. 2017;45:10–15. doi: 10.1016/j.avsg.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 122.Moussa Pacha H, Al-khadra Y, Darmoch F, et al. Association between underweight body mass index and in-hospital outcome in patients undergoing endovascular interventions for peripheral artery disease: a propensity score matching analysis. J Endovasc Ther. 2019;26(3):411–417. doi: 10.1177/1526602819839046. [DOI] [PubMed] [Google Scholar]
- 123.Murata N, Soga Y, Iida O, et al. Complex relationship of body mass index with mortality in patients with critical limb ischemia undergoing endovascular treatment. Eur J Vasc Endovasc Surg. 2015;49(3):297–305. doi: 10.1016/j.ejvs.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 124.Abbas AE, Goodman LM, Timmis R, Boura J. Predictors of poor outcome in female patients undergoing endovascular intervention. J Interv Cardiol. 2010;23(4):401–410. doi: 10.1111/j.1540-8183.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 125.Januszek R, Ruzsa Z, Nyerges A, et al. Body mass index and long-term outcomes in patients with chronic total occlusions undergoing retrograde endovascular revascularization of the infra-inguinal lower limb arteries. Cardiol J. 2019;0(0). 10.5603/CJ.a2019.0097. [DOI] [PMC free article] [PubMed]
- 126.Tanaka A, Perlick A, Miller CC, III, et al. Metabolic syndrome but not obesity adversely affects outcomes after open aortoiliac bypass surgery. Ann Vasc Surg. 2018;46:155–161. doi: 10.1016/j.avsg.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 127.Doshi R, Rao G, Shlofmitz E, Donnelly J, Meraj P. Comparison of In-hospital outcomes after percutaneous revascularization for peripheral arterial disease in patients with a body mass index of kg2 versus kg/m2 (from the national inpatient sample) Am J Cardiol. 2017;120(9):1648–1652. doi: 10.1016/j.amjcard.2017.07.065. [DOI] [PubMed] [Google Scholar]
- 128.Zierfuss B, Höbaus C, Herz CT, Pesau G, Koppensteiner R, Schernthaner G-H. Predictive power of novel and established obesity indices for outcome in PAD during a five-year follow-up. Nutr Metabol Cardiovasc Dis. 2020;30(7):1179–1187. doi: 10.1016/j.numecd.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 129.Lin DS-H, Lo H-Y, Yu A-L, Lee J-K, Chien K-L. Mortality risk in patients with underweight or obesity with peripheral artery disease: a meta-analysis including 5,735,578 individuals. Int J Obes. 2022;46(8):1425–1434. doi: 10.1038/s41366-022-01143-x. [DOI] [PubMed] [Google Scholar]
- 130.Moussa O, Ardissino M, Muttoni S, et al. Long-term incidence and outcomes of obesity-related peripheral vascular disease after bariatric surgery. Langenbecks Arch Surg. 2021;406(4):1029–1036. doi: 10.1007/s00423-020-02066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ix JH, Biggs ML, Kizer JR, et al. Association of body mass index with peripheral arterial disease in older adults: the cardiovascular health study. Am J Epidemiol. 2011;174(9):1036–1043. doi: 10.1093/aje/kwr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skilton MR, Chin-Dusting JP, Dart AM, et al. Metabolic health, obesity and 9-year incidence of peripheral arterial disease: the DESIR study. Atherosclerosis. 2011;216(2):471–476. doi: 10.1016/j.atherosclerosis.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 133.Subramaniam T, Nang EEK, Su Chi L, et al. Distribution of ankle—brachial index and the risk factors of peripheral artery disease in a multi-ethnic Asian population. Vasc Med. 2011;16(2):87–95. doi: 10.1177/1358863X11400781. [DOI] [PubMed] [Google Scholar]
- 134.Carbayo JA, Divisón JA, Escribano J, et al. Using ankle-brachial index to detect peripheral arterial disease: Prevalence and associated risk factors in a random population sample. Nutr Metabol Cardiovasc Dis. 2007;17(1):41–49. doi: 10.1016/j.numecd.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 135.Fujiwara T, Saitoh S, Takagi S, et al. Prevalence of asymptomatic arteriosclerosis obliterans and its relationship with risk factors in inhabitants of rural communities in Japan: Tanno-Sobetsu study. Atherosclerosis. 2004;177(1):83–88. doi: 10.1016/j.atherosclerosis.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 136.Bainton D, Sweetnam P, Baker I, Elwood P. Peripheral vascular disease: consequence for survival and association with risk factors in the Speedwell prospective heart disease study. Br Heart J. 1994;72(2):128–132. doi: 10.1136/hrt.72.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cantú-Brito C, Chiquete E, Antezana-Castro JF, Toapanta-Yanchapaxi L, Ochoa-Guzmán A, Ruiz-Sandoval JL. Peripheral artery disease in outpatients with a recent history of acute coronary syndrome or at high atherothrombotic risk. Vascular. 2020;1708538120938921. 10.1177/1708538120938921. [DOI] [PubMed]
- 138.Tseng C-H. Prevalence and risk factors of peripheral arterial obstructive disease in taiwanese type 2 diabetic patients. Angiology. 2003;54(3):331–338. doi: 10.1177/000331970305400309. [DOI] [PubMed] [Google Scholar]
- 139.Ko T, Higashitani M, Uemura Y, et al. Clinical outcome and diverse risk factors for different therapeutic target locations of peripheral artery disease. J Atheroscler Thromb. 2020;27(8):769–779. doi: 10.5551/jat.52647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arinze N, Farber A, Levin SR, et al. Perioperative outcomes after lower extremity bypass and peripheral vascular interventions in patients with morbid obesity and superobesity. J Vasc Surg. 2020;71(2):567–574.e564. doi: 10.1016/j.jvs.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 141.Higashitani M, Uemura Y, Mizuno A, et al. Cardiovascular outcome and mortality in patients undergoing endovascular treatment for symptomatic peripheral artery disease- short-term results of the toma-code registry. Circ J Offic J Japan Circ Soc. 2018;82(7):1917–1925. doi: 10.1253/circj.CJ-18-0105. [DOI] [PubMed] [Google Scholar]
- 142.Shean KE, Zettervall SL, Deery SE, et al. Fewer complications in the obese following lower extremity endovascular interventions. Ann Vasc Surg. 2018;49:17–23. doi: 10.1016/j.avsg.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Senda K, Miura T, Minamisawa M, et al. Predictive value of underweight status for patients with peripheral artery disease with claudication. Angiology. 2018;69(6):513–522. doi: 10.1177/0003319717736627. [DOI] [PubMed] [Google Scholar]
- 144.Soga Y, Iida O, Takahara M, et al. Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv. 2014;7(12):1444–1449. doi: 10.1016/j.jcin.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 145.Iida O, Soga Y, Hirano K, et al. Midterm outcomes and risk stratification after endovascular therapy for patients with critical limb ischaemia due to isolated below-the-knee lesions. Eur J Vasc Endovasc Surg. 2012;43(3):313–321. doi: 10.1016/j.ejvs.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 146.Badheka AO, Rathod AD, Bharadwaj AS, et al. Outcomes and risk prediction model for peripheral arterial disease in patients with stable coronary artery disease. Angiology. 2011;62(6):473–479. doi: 10.1177/0003319711398650. [DOI] [PubMed] [Google Scholar]
- 147.van Kuijk J-P, Flu W-J, Galal W, et al. The influence of polyvascular disease on the obesity paradox in vascular surgery patients. J Vasc Surg. 2011;53(2):399–406. doi: 10.1016/j.jvs.2010.08.048. [DOI] [PubMed] [Google Scholar]