Abstract
Background
The results regarding the association between insulin-like growth factor binding protein 1 (IGFBP1) expression and cancer risk were controversial. We performed a meta-analysis to provide novel evidence on relationship between IGFBP1 expression and cancer risk.
Methods
PubMed, Embase, Cochrane library and Web of science were searched for relevant cohort and case-control studies exploring the relationship between IGFBP1 expression and cancer risk. Odds ratios (ORs) were pooled in this meta-analysis using random model. Subgroup analyses were performed based on ethnicity, tumor types, publication year, study type, Newcastle-Ottawa Scale (NOS) score and sex.
Results
A total of 27 studies including 16 cohort and 11 case-control studies were identified by literature search. No significant association was found between IGFBP1 expression and risk of various cancers [0.90, 95% confidence interval (CI): 0.79, 1.03]. The overall results showed that the pooled ORs were 0.71 (95% CI: 0.57, 0.88] for prostate cancer risk and 0.66 (95%CI: 0.44, 0.99) for colorectal cancer (CRC) risk. However, there is no significant association between IGFBP1 expression and risk for ovarian cancer (1.70, 95%CI: 0.41, 6.99), breast cancer (1.02, 95%CI: 0.85, 1.23), endometrial cancer (1.19, 95%CI: 0.64, 2.21), colorectal adenoma (0.93; 95%CI: 0.81, 1.07), lung cancer (0.81, 95%CI: 0.39, 1.68) or multiple myeloma (1.20, 95%CI: 0.98, 1.47).
Conclusion
In this study, compared with individuals at low IGFBP1 expression adjusted for age, smoking status, alcohol intake and so on, risk of the prostate cancer and CRC were decreased among individuals of high IGFBP1 expression. There needs further study to confirm this issue.
Keywords: Insulin-like growth factor binding protein 1(IGFBP1), Cancer risk, Systematic review, Meta-analysis
1. Introduction
Cancer has been recognized as a prickly global public problem widely and there were 24.5 million incident cancer cases and 9.6 cancer deaths worldwide. Especially lung cancer and colorectal cancer rank first and seconding the top 10 causes of death among various cancers respectively [1]. In the next 40 years the current epidemiological data show that the burden of malignant tumor is and will maintain a high proportion for a long time [2]. In consideration of this situation, a growing number of researchers attempted to seek risk factors of cancer to provide basis for cancer prevention. Moreover, numerous studies have indicated that several factors such as tobacco, alcohol, dietary habits and gene alterations are associated with high cancer risk [3,4]. Besides, tumor markers are also explored to predict prognosis, which provides a personalized treatment plan for cancer patients.
Insulin-like growth factor binding protein 1(IGFBP1) that contains 4 exons is located at chromosome 7q12.3 and its length is 5173bp, which belongs to IGF system [5]. The IGF system consists of several elements including two ligands, insulin-like growth factor 1(IGF-1) and insulin-like growth factor 2(IGF-2), two cell-membrane receptors, IGF-1 receptor and IGF-2 receptor, and six IGF-binding proteins, IGFBP1-IGFBP6 [6]. As a secreted protein, IGFBP1 is the most predominant IGFBP in amniotic fluid and expressed steadily and normally in the liver, endometrium and placenta among them [7]. But insulin that can inhibit IGFBP1 expression through the insulin receptor and growth hormone can regulate IGFBP1 expression [8]. In addition, the IGF-dependent mechanism is to be related with tumor behaviors including proliferation, migration, invasion and adhesion [5].
The relationship between IGFBP family (IGFBP1-6) and various cancers have been studied in increasing literatures. But study numbers of IGFBP4-6 were too few to perform the meta-analysis to assess cancer risk. For IGFBP1-3, their association with cancer risk was extensively studied. In 2022, we performed a meta-analysis, and showed that high IGFBP2 expression was significantly associated with worse prognosis in glioma and colorectal cancer patients [9]. IGFBP3 was studied widely, of which the associations with the risk of various cancers have been evaluated by several meta-analyses [10,11]. Several meta-analyses about the risks of IGFBP1 expression with single cancer type rather than various cancer types were reported. Chi and Rowlands et al. found that IGFBP1 expression was not significantly associated with prostate cancer and colorectal cancer risk [11,12], but Travis et al. discovered a significant correlation with prostate cancer risk [13]. What is more, several studies showed that the results of IGFBP1 expression correlated with risk of cancer were opposite or insignificant [[14], [15], [16], [17]], and none of these meta-analyses explored the relationship between IGFBP1 expression and breast cancer and endometrial cancer. To address these issues, we aimed to combine all available evidence to further comprehensively judge the relationship between IGFBP1 expression and various cancers risk in the present meta-analysis [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]].
2. Method
This systematic review and meta-analysis was conducted according to PRISMA Guidelines [41]. The protocol was developed with the International Prospective Register of Systematic Reviews–PROSPERO (Registration No. CRD42021245902).
2.1. Search strategy
Related studies were searched by PubMed, Embase, Web of science and Cochrane library databases up to April 2023. The search strategy was performed following Medical Subject Heading terms and the free keywords: (“Insulin-Like Growth Factor Binding Protein 1” OR “Insulin Like Growth Factor Binding Protein 1” OR “IGFBP-1” OR “IGF-Binding Protein 1” OR “IGF Binding Protein 1”) AND (“Neoplasms” OR “Tumor*” OR “Cancer*” OR “Malignancy*” OR “Malignant Neoplasm*” OR “Benign Neoplasm*”) AND (“Follow-Up Studies” OR “Case-Control Studies” OR “Prospective Studies”).
2.2. Inclusion and exclusion criteria
Two authors read each study and assessed whether it met inclusion criteria independently. The eligible study should meet the following criteria: (1) Cohort studies or Case-control studies; (2) Studies that described the association between IGFBP1 expression level and risk of cancer; (3) Studies that displayed the available HR, OR, RR data. Duplicated publications, letters to the editor, case studies, reviews, meta-analyses, conference proceedings and without available data were excluded.
2.3. Data extraction and quality assessment
Two reviewers independently extracted necessary data according to inclusion criteria. If any disagreement appeared, it should be settled through discussion. Following information were extracted from the included studies: name of the first author, year of study publication, country of participants, sex and age of participants, cancer type, number of case or control group, detection method, outcome measure, study type, NOS score, adjustment factors. Quality assessment of the included studies was performed by Newcastle-Ottawa Scale (NOS) scale [42]. A study which the NOS score is greater than or equal seven will be considered as high quality, otherwise it is low quality.
2.4. Statistical analysis
Extracted data were analyzed using Stata 12.0 software (Stata, College Station, College USA). The ORs and corresponding 95% CI were pooled using the Tierney’s method [43]. Cochrane Q and Higgins’s I2 statistics were used to evaluate the heterogeneity among the included studies. When I2 >50%, random effect model was used, otherwise fixed effect model. To explore the source of heterogeneity about study, the subgroup analysis based on ethnicity, cancer type, publication year, study type, NOS score and sex and meta-regression were performed. In addition, we performed a sensitivity analysis by omitting each study to evaluate the stability of the pooled results. The Begg’s regression test was used to assess if there is a publication bias in this meta-analysis [44].
3. Results
3.1. Literature selection and study characteristics
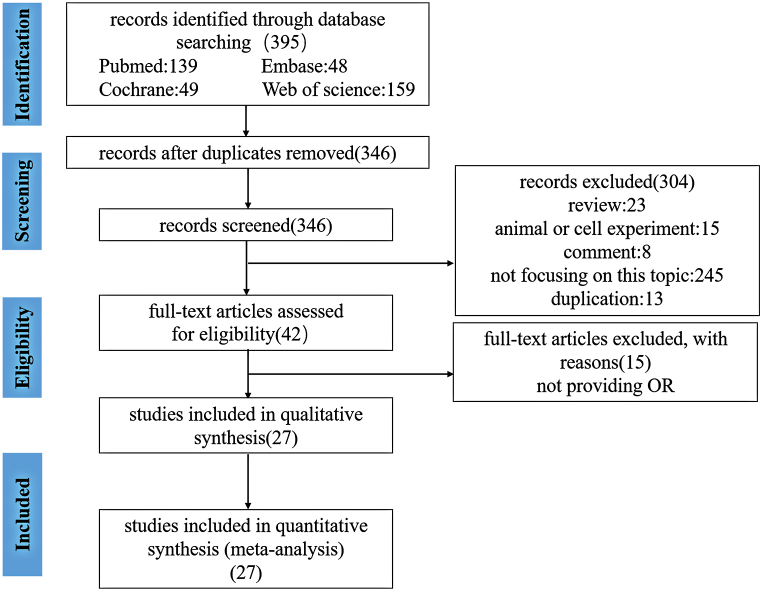
As shown in Fig. 1, we identified 395 articles using search strategy from PubMed, Embase, Cochrane library and Web of science. Due to duplicated articles, there were 346 articles after excluding 49 articles by EndNote X9. Then we further read title and abstract to evaluate the remaining articles. 304 articles were excluded owing to the following reasons: 23 articles were reviews; 15 articles were in relation to animal or cell experiment; 245 articles did not focus on association between IGFBP1 expression and various tumor risk; 8 articles were reported by comment; 13 were duplicated articles. In addition, 15 articles were removed by reading full-text article. Finally, 27 articles were included in this meta-analysis altogether.
Fig. 1.
Flow diagram of study selection.
The general characteristics of the included studies were summarized in Table 1. These studies published between 2000 and 2023 enrolled a total of 6899 cases and 18243 controls which were 20–82 at age from Italy, USA, China, Sweden, France, Swedish, Greece, UK, and covered over Brest cancer, prostate cancer, ovarian cancer, lung cancer, multiple myeloma, colorectal cancer, colorectal adenoma and endometrial cancer. 11 were case-control studies and 16 were cohort studies among the included studies. With regard to quality assessment, these studies mainly divided into high quality study with NOS score higher than or equal to 7 and study of low quality with NOS score lower than 7. Besides, the level of IGFBP1 expression was detected by Elisa, Immunoradiometric, RIA or Double-antibody. The pivotal outcome measure was ORs that were adjusted for age, education, parity, smoking status, BMI, physical activity, pack-years smoked, and alcohol intake as continuous variables, etc.
Table 1.
The main characteristic of included studies in this meta-analysis.
| Study | Year | Country | Cancer type | Case/control | Age | Sex | Detection method | Outcome | Study type | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Augustin | 2004 | Italy | Endometrial cancer | 73/108 | 61 | Female | Elisa | OR | Case-control | High |
| Birmann | 2012 | USA | Multiple myeloma | 354/709 | NA | Both | Elisa | OR | Cohort | Low |
| Cao | 2015 | USA | Prostate cancer | 957/1021 | 63.2 | Both | Elisa | OR | Cohort | Low |
| Chokkalingam | 2001 | China | Prostate cancer | 191/304 | NA | Female | Elisa | OR | Case-control | High |
| Chokkalingam | 2001 | China | Prostate cancer | 128/306 | 72 | Both | Elisa | OR | Case-control | High |
| Dalmaso | 2004 | Italy | Ovarian cancer | 59/108 | 55 | Female | Elisa | OR | Case-control | High |
| Jenab | 2007 | Western | Colorectal cancer | 180/213 | NA | Both | Immunoradiometric | OR | Cohort | High |
| Kaaks | 2002 | Sweden | Brest cancer | 513/987 | NA | Female | Immunoradiometric | OR | Cohort | High |
| Kaaks | 2000 | USA | Colorectal cancer | 102/200 | 35–65 | Female | Immunoradiometric | OR | Cohort | Low |
| Keinan-boker | 2003 | France | Brest cancer | 149/333 | 50–69 | Female | Immunoradiometric | OR | Cohort | Low |
| Krajcik | 2003 | USA | Brest cancer | 66/66 | 31–82 | Female | Immunoradiometric | OR | Case-control | High |
| Lacey | 2004 | USA | Endometrial cancer | 174/136 | 20–74 | Female | Elisa | OR | Case-control | High |
| Lukanoua | 2003 | USA | Ovarian cancer | 132/263 | 30–69 | Both | Immunoradiometric | OR | Cohort | High |
| LUKANOVA | 2001 | USA | Lung cancer | 93/186 | 32–70 | Female | Immunoradiometric | OR | Cohort | High |
| LUKANOVA | 2001 | USA | Endometrial cancer | 91/173 | NA | Female | Immunoradiometric | OR | Cohort | High |
| Muti | 2002 | Italy | Brest cancer | 69/265 | 35–69 | Female | Immunoradiometric | OR | Cohort | High |
| PALMQVIST | 2003 | Sweden | Colorectal cancer | 168/336 | NA | Female | Double-antibody | OR | Cohort | High |
| Schemhammer | 2004 | USA | Brest cancer | 178/272 | 60.5 | Female | Elisa | OR | Case-control | High |
| Schemhammer | 2004 | USA | Brest cancer | 78/157 | 45 | Female | Elisa | OR | Cohort | High |
| Vidal | 2012 | USA | Colorectal cancer | 190/448 | 56 | Both | Elisa | RR | Case-control | High |
| Wei | 2005 | USA | Colorectal adenoma | 182/350 | 30–55 | Female | Elisa | RR | Cohort | Low |
| Wei | 2006 | USA | Colorectal adenoma | 380/380 | 30–55 | Female | Elisa | OR | Cohort | Low |
| Weidrepass | 2003 | Swedish | Endometrial cancer | 288/392 | 50–74 | Female | RIA | OR | Case-control | High |
| Yamali | 2012 | Japan | Colorectal adenoma | 74/76 | 40–75 | Both | Elisa | OR | Case-control | Low |
| Pazaitou-panayiotou | 2007 | Greece | Brest cancer | 74/76 | NA | Female | Elisa | OR | Case-control | Low |
| Dong | 2019 | USA | Colorectal Adenoma | 1234/9172 | 55 | Female | Immunoradiometric | OR | Cohort | High |
| Eleanor | 2023 | UK | Prostate cancer | 722/1106 | 61 | Both | NA | OR | Cohort | High |
NOS: Newcastle-Ottawa Scale, OR: odds ratios, BMI: body mass index, IGF-I: Insulin-like growth factor I, USA: United States of America, NA: Not available.
3.2. Association IGFBP1 expression and various cancer risk
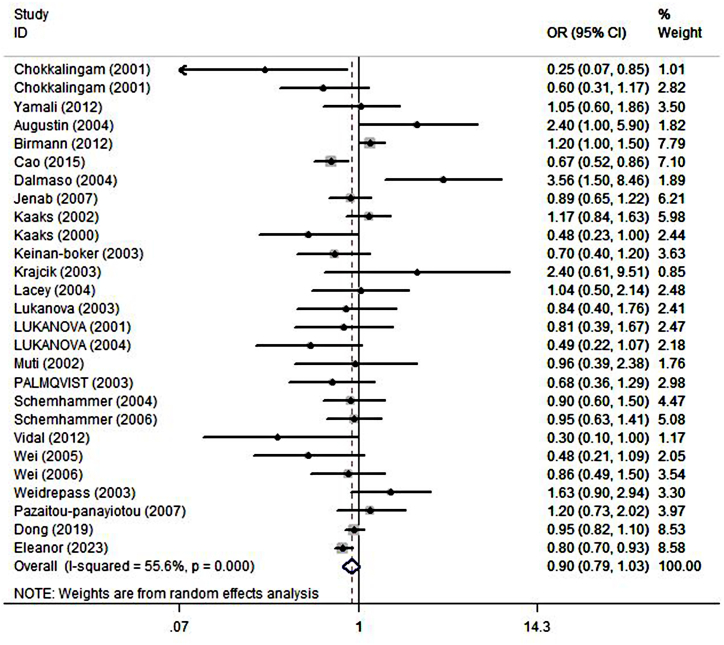
The combined effect estimation for OR using random model was illustrated in Fig. 2. After a series of screening, a total of 27 studies were included in this meta-analysis to assess the association between IGFBP1 expression and various cancers risk. The result showed that there was no significant association between IGFBP1 expression and various tumors risk (OR = 0.90; 95%CI: 0.79,1.03), with high heterogeneity (I2 = 55.6%, P < 0.000) (Fig. 2).
Fig. 2.
Forest plots showing the association between IGFBP1 expression and cancer risk. The squares and horizontal line crossing the square represent hazard ratios and 95% confidence interval respectively. The diamonds represent the pooled HR and 95%CI.
3.3. Subgroup analyses and meta-regression analysis
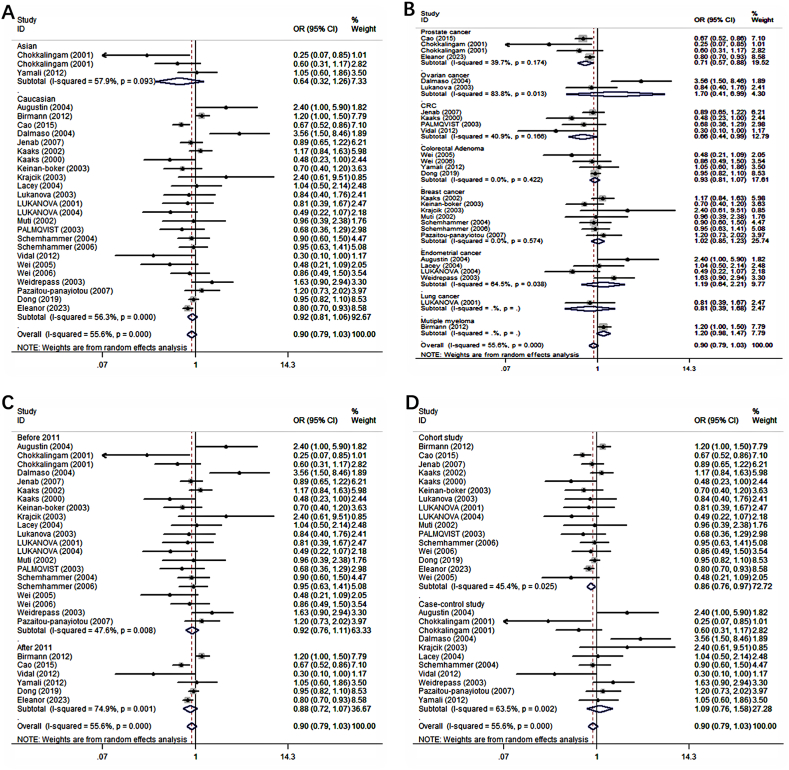
Subgroup analyses were performed to explore source of the heterogeneity based on ethnicity, cancer type, publication year, study type, NOS score and sex in Table 2. The significant association between IGFBP1 expression and cancer risk did not remain in Asian (OR = 0.64; 95%CI: 0.32, 1.26) and Caucasian (OR = 0.92; 95%CI: 0.81, 1.06) about the subgroup analyses of ethnicity (Fig. 3A). With regard to cancer type (Fig. 3B), high IGFBP1 expression could substantially reduce the risk of prostate cancer (OR = 0.71; 95%CI: 0.57, 0.88) and CRC (OR = 0.66; 95%CI: 0.44, 0.99). However, no significant association was found in ovarian cancer (OR = 1.70; 95%CI: 0.41, 6.99), breast cancer (OR = 1.02; 95%CI :0.85, 1.23), endometrial cancer (OR = 1.19; 95%CI: 0.64, 2.21), colorectal adenoma (OR = 0.93; 95%CI: 0.81, 1.07), lung cancer (OR = 0.81; 95%CI: 0.39, 1.68), or multiple myeloma (OR = 1.20; 95%CI: 0.98, 1.47). At the same time, significant association was observed in cohort study (OR = 0.86; 95%CI: 0.76, 0.97), but not case-control study (OR = 1.09; 95%CI: 0.76, 1.58) (Fig. 3D). In subgroup analyses concerning publication year (Fig. 3C), there was still no significant association between IGFBP1 expression and cancer risk about studies published before (OR = 0.92; 95%CI: 0.76, 1.11) and after 2011 (OR = 0.88; 95%CI: 0.72, 1.07). Meta-regression analysis showed that there were no variables which might account for heterogeneity between included studies in Table S1.
Table 2.
The result of subgroup analyses in this meta-analysis.
| Variables |
No of studies |
Model |
Pooled OR and 95%CI |
Heterogeneity |
|
|---|---|---|---|---|---|
| P value |
I2(%) |
||||
| Cancer type | |||||
| Prostate cancer | 4 | Fixed | 0.71 (0.57–0.88) | 0.174 | 39.7% |
| Ovarian cancer | 2 | Ran | 1.70 (0.41–6.99) | 0.013 | 83.8% |
| Colorectal cancer | 4 | Ran | 0.66 (0.44–0.99) | 0.166 | 40.9% |
| Brest cancer | 7 | Fixed | 1.02 (0.85–1.23) | 0.574 | 0% |
| Endometrial cancer | 4 | Ran | 1.19 (0.64–2.21) | 0.038 | 64.5% |
| Colorectal Adenoma | 4 | Fixed | 0.93 (0.81–1.07) | 0.422 | 0% |
| Lung cancer | 1 | – | 0.81 (0.39–1.68) | – | – |
| Multiple myeloma | 1 | – | 1.20 (0.98–1.47) | – | – |
| Ethnicity | |||||
| Asian | 3 | Ran | 0.64 (0.32–1.26) | 0.093 | 57.9% |
| Caucasian | 24 | Ran | 0.92 (0.81–1.06) | 0.000 | 56.3% |
| NOS score | |||||
| ≥7 | 19 | Ran | 0.94 (0.80–1.10) | 0.006 | 52.9% |
| <7 | 8 | Ran | 0.83 (0.64–1.09) | 0.005 | 65.6% |
| Publication year | |||||
| Before 2011 | 21 | Ran | 0.92 (0.76–1.11) | 0.008 | 47.6% |
| After 2011 | 6 | Ran | 0.88 (0.72–1.07) | 0.001 | 74.9% |
| Study type | |||||
| Cohort study | 16 | Ran | 0.86 (0.76–0.97) | 0.025 | 45.4% |
| Case-control study | 11 | Ran | 1.09 (0.72–1.58) | 0.002 | 63.5% |
| Sex | |||||
| Female | 19 | Ran | 0.97 (0.82–1.14) | 0.016 | 46.5% |
| Both | 8 | Ran | 0.84 (0.68–1.03) | 0.006 | 67.1% |
OR: odds ratio, Ran: Random.
Fig. 3.
Forest plots showing the odds ratios (OR) for the cancer risk by subgroups based on (A) ethnicity, (B) cancer type, (C) publication year, (D) study type. The squares and horizontal line crossing the square represent hazard ratios and 95% confidence interval respectively. The diamonds represent the pooled HR and 95%CI.
3.4. Sensitivity analysis and publication bias
Sensitivity analysis which removed each study in turn showed that the overall result was stable and no study could influence the combined OR in this meta-analysis (Supplementary Fig. 1). Publication bias were performed by funnel plot and Begg’s test. There was no obvious asymmetry from the shape of funnel plot in Supplementary Fig. 2A. Besides, Begg’s test demonstrated that there was no significant publication bias in this meta-analysis (Supplementary Fig. 2B).
4. Discussion
To the best of our knowledge, we failed to find significant association between IGFBP1 expression and risk of cancer. In addition, subgroup analyses were performed based on cancer types, ethnicity, publication year, study type, NOS score and sex. We found that there was no significant association between IGFBP1 expression and risk of ovarian cancer, breast cancer, endometrial cancer, colorectal adenoma, lung cancer or multiple myeloma, but a significant association between IGFBP1 expression and risk of prostate cancer and CRC.
The study provided by Travis et al. indicated that a significant association between circulating levels of IGF-I and prostate cancer risk was observed [45]. Besides, Wei et al. found high serum IGFBP1 level was significantly associated with lower risk of CRC in women [46,47]. This may be attributed to the fact that IGFBP1 may reduce the biological activity of IGF-I, resulting in a reduction in CRC risk [48]. But other studies showed a distinct different result, of which indicated that IGFBP1 expression had no association with risk of the CRC [31,49]. With regard to the relationship between IGFBP1 expression and breast cancer, many studies had shown that IGFBP1 was not associated with breast cancer risk in premenopausal or postmenopausal women, regardless of circulating IGFBP1 or single nucleotide polymorphisms and haplotype markers [26,33,47,[50], [51], [52], [53]]. Several studies showed IGFBP1 expression was significantly associated with risk of endometrial cancer [54], which is inconsistent with our finding. However, McGrath et al. found single nucleotide polymorphisms of IGFBP1 gene had no association with risk of endometrial cancer among Caucasian women [55]. In addition, there was no significant correlation between serum IGFBP1 level and lung cancer risk in women [56].
Latest progress showed that the role of IGFBP1 in cancer development remain controversial results. Although IGFBP1 was widely regarded as to exerts an inhibitory effect on cancer development, it has been proposed that IGFBP1 play a positive role in tumor growth or migration through IGF-independent pathway [57,58]. Bach et al. has demonstrated that the IGF system was involved in the normal process of biological metabolism, as well as in the development and progression of various cancers [58]. Several studies have shown that IGFBP1 might inhibit the proliferation of tumor cell [48,59,60]. IGFBP1 expression might be induced by ursolic acid through p38 mitogen activated kinase-like protein to inhibit the proliferation of hepatocellular carcinoma [61]. The overexpression of aldehyde dehydrogenase 1A1 and IGFBP1 could significantly reduce cell proliferation and invasiveness of SW480 cell line in vitro assays [25]. In addition, Luo et al. found IGFBP1 overexpression could inhibit the migration of BGC-823 cells. However, they also demonstrated that up-regulation of IGFBP1 can increase cell migration in gastric cancer cell line with H. pylori inflection [62].
There was a highly significant heterogeneity between the included studies in this meta-analysis. Therefore, we explore the potential possible source of the heterogeneity through subgroup analyses and meta-regression analysis. From the subgroup analyses, we found ethnicity and NOS score were unlikely to explain the reason of the high heterogeneity. In addition, publish year, study type and sex might be source of heterogeneity in this meta-analysis, but it should be interpreted with caution because of the low decline of the heterogeneity. Compared to publish year, study type and sex, cancer type was most likely to be the main source of heterogeneity. Besides, due to the result of p > 0.05, the meta-regression showed none of ethnicity, cancer type, publish year, study type, NOS score and sex were the source of the heterogeneity. Different adjusted factors with OR for each study might lead to existence of heterogeneity.
Unsimilar to previous meta-analyses, our study reported IGFBP1 expression was associated with prostate cancer and CRC risk and there was no significant correlation between IGFBP1 expression and ovarian cancer. Our study comprising of 1276 prostate cancer patients and 1631 controls used the same detection method (Elisa) to measure the level of IGFBP1. But previous meta-analysis included three literature, one of which was a brief communication, showed the patients and controls only were 1224 and 1780 respectively and the detection method measuring the level of IGFBP1 was different [11]. Although there was no significant association between IGFBP1 expression and CRC risk, the upper limit of corresponding 95%CI was 1.03 (OR = 0.85, 0.7–1.03) and p value was 0.101 [12]. Therefore, the result should be explained cautiously. Mean difference was used to estimate the strength of connection between level of IGFBP1 and ovarian cancer risk, which was different from the method of OR used in our meta-analysis [63].
There were some strengths in this meta-analysis. First, this meta-analysis consists of 27 studies with 25142 participants, which might increase statistical power of this meta-analysis. Second, there was no significant publication bias about included studies according to the result of Begg’s test. Third, the OR estimated outcome measure was used after adjusting for variables. Therefore, we infer that the results based on these studies were relatively reliable.
This meta-analysis also has several limitations. First, there were some confounding factors in the relationship between IGFBP1 expression and cancer risk. However, confounding factors could not be fully excluded because several studies were based on case-control studies, although adjusted ORs were adopted. Second, the significant high heterogeneity existed among included studies, though we conducted the pooled meta-analysis with random-effect model. However, the result which we performed subgroup analysis and meta-regression analysis to explore the sources of high heterogeneity could not explain heterogeneity. Third, the result of each cancer might be less powerful, because number pooled particular type of prostate tumor, ovarian cancer, endometrial cancer, lung cancer and multiple myeloma was ≤5. Finally, dose-response analysis was unable to be conducted to evaluate the relationship between grades of IGFBP1 level and cancer risk.
In conclusion, we found that high IGFBP1 expression was significantly associated with lower risk of prostate cancer and CRC. This study did not find significant association between IGFBP1 expression and risk of ovarian cancer, breast cancer, endometrial cancer, colorectal adenoma, lung cancer or multiple myeloma. Therefore, IGFBP1 may be a potential diagnostic and prognostic tumor marker in CRC and prostate cancer.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
All data are obtained in published article/supp. material/referenced during this in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16470.
Contributor Information
Yi-Wei Xu, Email: yiwei512@126.com.
Yu-Hui Peng, Email: pengyuhui666@163.com.
Fang-Cai Wu, Email: 280550109@qq.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Global Burden of Disease Cancer C., Fitzmaurice C., Abate D., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattiuzzi C., Lippi G. Current cancer epidemiology. J. Epidemiol. Glob. Health. 2019;9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund E., Dumeaux V. Systems epidemiology in cancer. Cancer Epidemiol. Biomarkers Prev. 2008;17:2954–2957. doi: 10.1158/1055-9965.EPI-08-0519. [DOI] [PubMed] [Google Scholar]
- 4.Rekkedal G. [WHO world health report 1997] Tidsskr. Sykepl. 1997;85:37–39. [PubMed] [Google Scholar]
- 5.Lin Y.W., Weng X.F., Huang B.L., et al. IGFBP-1 in cancer: expression, molecular mechanisms, and potential clinical implications. Am. J. Transl. Res. 2021;13:813–832. [PMC free article] [PubMed] [Google Scholar]
- 6.Furstenberger G., Senn H.J. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 7.Fagerberg L., Hallstrom B.M., Oksvold P., et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unterman T.G., Oehler D.T., Murphy L.J., Lacson R.G. Multihormonal regulation of insulin-like growth factor-binding protein-1 in rat H4IIE hepatoma cells: the dominant role of insulin. Endocrinology. 1991;128:2693–2701. doi: 10.1210/endo-128-6-2693. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B., Hong C.Q., Luo Y.H., et al. Prognostic value of IGFBP2 in various cancers: a systematic review and meta-analysis. Cancer Med. 2022;11:3035–3047. doi: 10.1002/cam4.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renehan A.G., Zwahlen M., Minder C., et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 11.Rowlands M.A., Gunnell D., Harris R., et al. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int. J. Cancer. 2009;124:2416–2429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi F., Wu R., Zeng Y.C., et al. Circulation insulin-like growth factor peptides and colorectal cancer risk: an updated systematic review and meta-analysis. Mol. Biol. Rep. 2013;40:3583–3590. doi: 10.1007/s11033-012-2432-z. [DOI] [PubMed] [Google Scholar]
- 13.Travis R.C., Appleby P.N., Martin R.M., et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016;76:2288–2300. doi: 10.1158/0008-5472.CAN-15-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaaks R., Lundin E., Manjer J., et al. Prospective study of IGF-I, IGF-binding proteins, and breast cancer risk, in northern and southern Sweden. Cancer Causes Control. 2002;13:307–316. doi: 10.1023/a:1015270324325. [DOI] [PubMed] [Google Scholar]
- 15.Keinan-Boker L., Bueno De Mesquita H.B., Kaaks R., et al. Circulating levels of insulin-like growth factor I, its binding proteins -1,-2, -3, C-peptide and risk of postmenopausal breast cancer. Int. J. Cancer. 2003;106:90–95. doi: 10.1002/ijc.11193. [DOI] [PubMed] [Google Scholar]
- 16.Augustin L.S., Dal Maso L., Franceschi S., et al. Association between components of the insulin-like growth factor system and endometrial cancer risk. Oncology. 2004;67:54–59. doi: 10.1159/000080286. [DOI] [PubMed] [Google Scholar]
- 17.Lukanova A., Zeleniuch-Jacquotte A., Lundin E., et al. Prediagnostic levels of C-peptide, IGF-I, IGFBP -1, -2 and -3 and risk of endometrial cancer. Int. J. Cancer. 2004;108:262–268. doi: 10.1002/ijc.11544. [DOI] [PubMed] [Google Scholar]
- 18.Birmann B.M., Neuhouser M.L., Rosner B., et al. Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the Multiple Myeloma Cohort Consortium. Blood. 2012;120:4929–4937. doi: 10.1182/blood-2012-03-417253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y., Nimptsch K., Shui I.M., et al. Prediagnostic plasma IGFBP-1, IGF-1 and risk of prostate cancer. Int. J. Cancer. 2015;136:2418–2426. doi: 10.1002/ijc.29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chokkalingam A.P., McGlynn K.A., Gao Y.T., et al. Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2001;61:4333–4336. [PubMed] [Google Scholar]
- 21.Chokkalingam A.P., Pollak M., Fillmore C.M., et al. Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer Epidemiol. Biomarkers Prev. 2001;10:421–427. [PubMed] [Google Scholar]
- 22.Dal Maso L., Augustin L.S.A., Franceschi S., et al. Association between components of the insulin-like growth factor system and epithelial ovarian cancer risk. Oncology. 2004;67:225–230. doi: 10.1159/000081322. [DOI] [PubMed] [Google Scholar]
- 23.Hang D., He X., Kvaerner A.S., et al. Plasma biomarkers of insulin and the insulin-like growth factor Axis, and risk of colorectal adenoma and serrated polyp. JNCI Cancer Spectr. 2019;3:pkz056. doi: 10.1093/jncics/pkz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenab M., Riboli E., Cleveland R.J., et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition. Int. J. Cancer. 2007;121:368–376. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 25.Kaaks R., Toniolo P., Akhmedkhanov A., et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J. Natl. Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 26.Krajcik R.A., Borofsky N.D., Massardo S., Orentreich N. Insulin-like growth factor I (IGF-I), IGF-binding proteins, and breast cancer. Cancer Epidemiol. Biomarkers Prev. 2002;11:1566–1573. [PubMed] [Google Scholar]
- 27.Lacey J.V., Jr., Potischman N., Madigan M.P., et al. Insulin-like growth factors, insulin-like growth factor-binding proteins, and endometrial cancer in postmenopausal women: results from a U.S. case-control study. Cancer Epidemiol. Biomarkers Prev. 2004;13:607–612. [PubMed] [Google Scholar]
- 28.Lukanova A., Lundin E., Micheli A., et al. Risk of ovarian cancer in relation to prediagnostic levels of C-peptide, insulin-like growth factor binding proteins-1 and -2 (USA, Sweden, Italy) Cancer Causes Control. 2003;14:285–292. doi: 10.1023/a:1023688603547. [DOI] [PubMed] [Google Scholar]
- 29.Lukanova A., Toniolo P., Akhmedkhanov A., et al. A prospective study of insulin-like growth factor-I, IGF-binding proteins-1, -2 and -3 and lung cancer risk in women. Int. J. Cancer. 2001;92:888–892. doi: 10.1002/ijc.1265. [DOI] [PubMed] [Google Scholar]
- 30.Muti P., Quattrin T., Grant B.J., et al. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol. Biomarkers Prev. 2002;11:1361–1368. [PubMed] [Google Scholar]
- 31.Palmqvist R., Stattin P., Rinaldi S., et al. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int. J. Cancer. 2003;107:89–93. doi: 10.1002/ijc.11362. [DOI] [PubMed] [Google Scholar]
- 32.Pazaitou-Pnaylotou K., Kelesidis T., Kelesidis I., et al. Growth hormone-binding protein is directly and IGFBP-3 is inverseley associated with risk of female breast cancer. Eur. J. Endocrinol. 2007;156:187–194. doi: 10.1530/EJE-06-0611. [DOI] [PubMed] [Google Scholar]
- 33.Schernhammer E.S., Holly J.M., Pollak M.N., Hankinson S.E. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2005;14:699–704. doi: 10.1158/1055-9965.EPI-04-0561. [DOI] [PubMed] [Google Scholar]
- 34.Schernhammer E.S., Tworoger S.S., Eliassen A.H., et al. Body shape throughout life and correlations with IGFs and GH. Endocr. Relat. Cancer. 2007;14:721–732. doi: 10.1677/ERC-06-0080. [DOI] [PubMed] [Google Scholar]
- 35.Vidal A.C., Lund P.K., Hoyo C., et al. Elevated C-peptide and insulin predict increased risk of colorectal adenomas in normal mucosa. BMC Cancer. 2012;12:389. doi: 10.1186/1471-2407-12-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watts E.L., Perez-Cornago A., Fensom G.K., et al. Circulating insulin-like growth factors and risks of overall, aggressive and early-onset prostate cancer: a collaborative analysis of 20 prospective studies and Mendelian randomization analysis. Int. J. Epidemiol. 2023;52:71–86. doi: 10.1093/ije/dyac124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei E.K., Ma J., Pollak M.N., et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol. Biomarkers Prev. 2005;14:850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 38.Wei E.K., Ma J., Pollak M.N., et al. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol. Biomarkers Prev. 2006;15:750–755. doi: 10.1158/1055-9965.EPI-05-0820. [DOI] [PubMed] [Google Scholar]
- 39.Weiderpass E., Brismar K., Bellocco R., et al. Serum levels of insulin-like growth factor-I, IGF-binding protein 1 and 3, and insulin and endometrial cancer risk. Br. J. Cancer. 2003;89:1697–1704. doi: 10.1038/sj.bjc.6601312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaji T., Iwasaki M., Sasazuki S., Tsugane S. Gender difference in the association of insulin and the insulin-like growth factor axis with colorectal neoplasia. Int. J. Obes. 2012;36:440–447. doi: 10.1038/ijo.2011.114. [DOI] [PubMed] [Google Scholar]
- 41.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Margulis A.V., Pladevall M., Riera-Guardia N., et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol. 2014;6:359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tierney J.F., Stewart L.A., Ghersi D., et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Travis R.C., Appleby P.N., Martin R.M., et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016;76:2288–2300. doi: 10.1158/0008-5472.CAN-15-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudolf Kaaks P.T., Akhmedkhanov Arslan, Lukanova Annekatrin, Carine Biessy HD., Rinaldi Sabina, Zeleniuch-Jacquotte Anne, Roy E Shore, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I,IGF-Binding proteins, and colorectal cancer risk in women. J. Natl. Cancer Inst. 2000;92:19. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 47.Wei E.K., Ma J., Pollak M.N., et al. A prospective study of C-peptide, insulin-like growth factor-1, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol. Biomark. Prev. 2005;14:850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 48.Pollak M.N., Schernhammer E.S., Hankinson S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 49.Jenab M., Riboli E., Cleveland R.J., et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition. Int. J. Cancer. 2007;121:368–376. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 50.Cheng I., Penney K.L., Stram D.O., et al. Haplotype-based association studies of IGFBP1 and IGFBP3 with prostate and breast cancer risk: the multiethnic cohort. Cancer Epidemiol. Biomark. Prev. 2006;15:1993–1997. doi: 10.1158/1055-9965.EPI-06-0361. [DOI] [PubMed] [Google Scholar]
- 51.Patel A.V., Cheng I., Canzian F., et al. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: findings from the Breast and Prostate Cancer Cohort Consortium (BPC3) PLoS One. 2008;3:e2578. doi: 10.1371/journal.pone.0002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudolf Kaaks1, Lundin2 Eva, Jm S.R., et al. Prospective study of IGF-I, IGF-binding proteins, and breast can_cer risk, in northern and southern Sweden. Cancer Causes Control. 2002;13:307–316. doi: 10.1023/a:1015270324325. [DOI] [PubMed] [Google Scholar]
- 53.Schemhammer E.S., Holly J.M., Hunter D.J., et al. Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in the Nurses Health Study II. Endocr. Relat. Cancer. 2006;13:583–592. doi: 10.1677/erc.1.01149. [DOI] [PubMed] [Google Scholar]
- 54.Lukanova A., Zeleniuch-Jacquotte A., Lundin E., et al. Prediagnostic levels of C-peptide, IGF-I, IGFBP-1,-2 and-3. Int. J. Cancer. 2004;108:262–268. doi: 10.1002/ijc.11544. [DOI] [PubMed] [Google Scholar]
- 55.McGrath M., Lee I.M., Buring J., De Vivo I. Common genetic variation within IGFI, IGFII, IGFBP-1, and IGFBP-3 and endometrial cancer risk. Gynecol. Oncol. 2011;120:174–178. doi: 10.1016/j.ygyno.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Annekatrin Lukanova1 * PT, 3, Arslan AKHMEDKHANOV2,3, Carine BIESSY1, Nancy J. HALEY4, Roy E. SHORE3, Elio RIBOLI 1 SRaRK A prospective study of insulin-like growth factor-I, IGF-binding proteins-1, -2 and -3 and lung cancer risk in women. Int. J. Cancer. 2001;92:888–892. doi: 10.1002/ijc.1265. [DOI] [PubMed] [Google Scholar]
- 57.Baxter R.C. IGF binding proteins in cancer: mechanistic and clinical insights. Nat. Rev. Cancer. 2014;14:329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 58.Bach L.A. IGF-binding proteins. J. Mol. Endocrinol. 2018;61:T11–T28. doi: 10.1530/JME-17-0254. [DOI] [PubMed] [Google Scholar]
- 59.Mohan S., Baylink D.J. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J. Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 60.Ngo T.H., Barnard R.J., Leung P.S., et al. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 61.Yang L.J., Tang Q., Wu J., et al. Inter-regulation of IGFBP1 and FOXO3a unveils novel mechanism in ursolic acid-inhibited growth of hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2016;35:59. doi: 10.1186/s13046-016-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo C., Sun F., Zhu H., et al. Insulin-like growth factor binding protein-1 (IGFBP-1) upregulated by Helicobacter pylori and is associated with gastric cancer cells migration. Pathol. Res. Pract. 2017;213:1029–1036. doi: 10.1016/j.prp.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Gianuzzi X., Palma-Ardiles G., Hernandez-Fernandez W., et al. Insulin growth factor (IGF) 1, IGF-binding proteins and ovarian cancer risk: a systematic review and meta-analysis. Maturitas. 2016;94:22–29. doi: 10.1016/j.maturitas.2016.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are obtained in published article/supp. material/referenced during this in article.