Highlights
-
•
Functional feed with olive extract improves plasma fatty acid profile in dairy cows.
-
•
Phenolic compounds in PhenoFeed Dry are hydroxytyrosol, tyrosol and verbascoside.
-
•
High total polyphenols content and antioxidant activity in the functional feed.
-
•
Close relationship between the diet fatty acid composition and plasma lipid content.
-
•
PUFA are the fatty acids most influenced by the phenolic composition of cows’ diet.
Keywords: Antioxidant activity, Dairy cows, Fatty acids, Functional feed, Holstein, Olea Europaea L. polyphenols
Abstract
This study evaluated the effects of supplementing with natural functional feed on the plasma fatty acid profile of lactating Italian Holstein-Friesian dairy cows. Thirty cows in mid-lactation received the natural olive extract PHENOFEED DRY (500 mg/cow/day) which mainly comprises hydroxytyrosol, tyrosol and verbascoside. The total content of polyphenols and the antioxidant power of standard feed, enriched feed and pure extract was evaluated respectively by Folin-Ciocalteu and DPPH assay, and a characterization in HPLC-UV (High-Performance Liquid Chromatography-Ultraviolet) of bioactive molecules present in the extract PHENOFEED DRY was performed. PHENOFEED DRY was provided for 60 days, and the plasma profile of fatty acids was determined by Gas Chromatography. The administration of enriched feed resulted in an increase in the ratio of Omega-6 to Omega-3 polyunsaturated fatty acids from 3:1 to 4:1 (p<0.001). This was not influenced by the calving order. The addition of polyphenols helped to keep monounsaturated (MUFA) and saturated (SFA) levels constant and results in a significant increase in polyunsaturated (PUFA) fatty acid after 15 days of administration. The Omega-6/Omega-3 ratio was in the optimal range. The findings show that inclusion of natural functional food such as plant polyphenols helps to maintain a healthy blood fatty acid profile in lactating dairy cows.
1. Introduction
Production performance and animal welfare in livestock are mainly influenced by genetics, environment, and nutrition. Therefore, the chemical-nutritional and functional aspects of the diet play an important role (D'Alessandro et al., 2017). According to Pauletto et al. (2020) and Serra et al. (2021), the inclusion of natural antioxidants such as polyphenols from plants in the diet of cattle helps to achieve a positive general welfare state. Diets supplemented with natural phenolic antioxidants improve production performance, immune status and general health, and reduce the risk of disease, in livestock (Alagawany and Abd El-Hack, 2015; Parrillo et al., 2017). In pasture-based grazing systems, access to polyphytic pastures, with high plant biodiversity, ensures an appropriate polyphenol intake (Avondo et al., 2013). In intensive systems, it is beneficial to supplement the diet with polyphenol-rich feed supplements (Fraisse et al., 2007; Maxin et al., 2020). Several studies where the management strategy included diets supplemented with natural antioxidants showed an improvement of bovine milk (Castillo et al., 2013) and goat meat (Adeyemi et al., 2016) quality, the improvement of animal health, and a strengthening of the immune response with a consequent reduction in the use of antibiotics and hormones (Cimmino et al., 2018). Polyphenols represent a wide class of plant substances with antioxidant, anti-inflammatory and anticancer properties (Gupta et al., 2014). Several studies have shown that the inclusion of polyphenols in the diet of farm animals, through plant extracts, induces positive effects on the quality of animal products with beneficial effects on animal welfare and human health (Castillo et al., 2013; Gupta et al., 2014; Adeyemi et al., 2016; Cimmino et al., 2018; Maxin et al., 2020). Alagawany and Abd El-Hack (2015) reported that the administration of rosemary extract, rich in phenolic compounds, to laying hens resulted in an increase in blood concentrations of IgA and IgM and in superoxide dismutase activity resulting in an increase in competence immunity and antioxidant capacity. Even the by-products of olive oil extraction containing polyphenols and used in the diet of farmed shrimps (Astacus leptodactylus) have determined benefits not only for their growth but also in immune and antioxidant capacity (Parrillo et al., 2017). Furthermore, the use of olive oil by-products influenced inflammatory and apoptotic pathways in rabbit livers by inhibiting pro-inflammatory enzymes or acting as antioxidants (Cappelli et al., 2021; Maranesi et al., 2021). Recently some studies have examined the effect of bioactive molecules on the qualitative and quantitative aspects of milk and meat production in dairy cows (Ianni et al., 2019; Ianni &Martino, 2020). However, only a few studies have evaluated the effects of polyphenols on the plasma fatty acid profile of livestock production animals. Some beneficial effects of polyphenols have been reported to involve changes in the relative amounts of different fatty acids. Indeed, dairy ewes fed a diet supplemented with phenolic tannins from chestnut or quebracho showed an increase in blood unsaturated fatty acids (vaccenic acid), a decrease in saturated fatty acids (stearic acid), and an increase in linoleic acid. conjugated in the rumen (rumenic acid) (Buccioni et al., 2017). Chestnut tannins have been previously shown to alter the rumen microbiome (Buccioni et al., 2015a) and fatty acids (FAs) profile of milk (Buccioni et al., 2015b) in dairy ewes. Phenolic compounds can therefore alter the status of fatty acids in blood and milk by modifying rumen function. Dairy cows undergo marked changes in metabolic balance and energy demand when transitioning from pregnancy to early lactation, and then through to peak lactation (Abdel-Raheem et al., 2010; Loften et al., 2014). Fatty acids are a source of energy and the inclusion of fats in the diet of dairy cows can help meet the demand for energy (Bionaz et al., 2020). An alternative approach to feeding fats, alluded to above, is to supplement the diet of dairy cows with natural polyphenols that influence rumen function and promote the endogenous production of favourable FAs. Rapeseed contains polyphenols (Pohl et al., 2016) and Holstein dairy cows fed rapeseed pomace from day 80 of lactation showed an increase in milk polyunsaturated fatty acids (PUFAs) (Musayeva et al., 2021). The by-products of olive oil extraction are rich in polyphenols and have great antioxidant potential for application in dairy cows (Di Nunzio et al., 2018; Tapia-Quirós et al., 2022). Consistent with the shift to natural livestock systems, the present study aimed to examine the blood FA profiles in lactating Holstein cows supplemented with by-products and products of the olive-oil supply chain which are rich in polyphenols (Romani et al., 2019). However, the influence of the addition of Olea europaea L. phenolic compounds and physiological factors, such as lactation phase and breed, on the composition of plasma fatty acids still remains a scarcely discussed topic. Only few authors describe the variation of the plasma fatty acid profile as a function of the lactation phase. Abdel-Raheem et al. (2010) show that at the beginning lactation in dairy cows (Simmental cows), when negative energy balance (NEB) and lipomobilization have their maximum expression, monounsaturated fatty acids (MUFAs) concentrations in plasma were higher than during the dry period or the other lactation phases (middle and late) during which concentrations decrease. Due to the limited data regarding the influence of natural bioactive extracts, supplemented in the diet of mid-lactation cows, on the plasma fatty acid profile, the aim of the present work was to administer an enriched feed with polyphenolic extract (PHENOFEED DRY) of Olea europaea L. to evaluate if this supplementation induces changes on plasma fatty acid composition in lactating Holstein cows in intensive breeding and with a high genetic merit.
2. Materials and methods
The procedure of this study was reviewed and approved by the Ethical Animal Care and Use Committee of the University of Naples “Federico II” (Protocol No. 99607-2017).
2.1. Animals and experimental design
The study was conducted at the dairy farm "Fratelli Mirra" located in Francolise (CE) (Italy) (41°07′56′'N 14°03′43′'E; 10 m above sea level) on thirty Italian Holstein-Friesian cows in mid-lactation phase (90 to 210 days). Cows were divided into three groups according to parity (Primiparous (n = 10), Secondiparous (n = 10) and Pluriparous (n = 10)) and cows in each group were fed individually. Cows were on average 180 days open and underwent artificial insemination during the study.Cows were maintained in loose housing and had direct access to feed and water, and received a standard feed ration supplemented with phenolic extract of O. europaea (PHENOFEED DRY) twice daily. For the trial, the analysis of plasma fatty acids composition of dairy cows fed enriched feed at different administration times was assessed. Samples were taken at time 0 (control, T0), before the administration of functional feed, and subsequently, during supplementation at 15 (T1), 30 (T2), 45 (T3), and 60 (T4) days and at 45 days after the trial endend (follow-up, T5).
2.2. Diet composition
Cows were fed, during study period (T0-T5), twice daily (morning and evening) with a total mixed ration (TMR) containing concentrate, maize silage, triticale, and oat hay. Refusals were recorded and then removed. Individual feedstuff and refusals from each animal category were sampled weekly and analyzed according to AOAC method (AOAC, 1990). The amount and composition of the diet is shown in Tables 1 and 2. The diet during the study was formulated by nutritionists of the company "Mangimi Liverini S.p.A." (Telese Terme - Benevento - Italy) and Mix 6350 PC flour was used. The chemical-nutritional composition of the diet was evaluated with AOAC methods (AOAC, 1990) and compared with Near Infrared (NIR) System analysis (Table 2).
Table 1.
Ingredients (% dry matter (DM)) and vitamins (UI/kg) of Mix 6350 PC flour.
| Ingredients | % DM |
|---|---|
| Maize | 36.84 |
| Soy Protein 46% | 24.00 |
| Sunflower 36% | 19.18 |
| Cotton Seed | 8.50 |
| Soybean Hulls | 1.88 |
| Molasses Cane | 1.68 |
| Roasted Soybean | 1.40 |
| Hydrogenated Palm Fat | 1.11 |
| Salt | 0.65 |
| Calcium | 1.09 |
| Phosphorus | 0.56 |
| Magnesium | 0.48 |
| Sulfur | 0.23 |
| Potassium | 1.05 |
| Sodium | 0.82 |
| Chlorine | 0.53 |
| VITAMINS | UI/kg |
| Vitamin A | 21988 |
| Vitamin B1 | 5324 |
| Vitamin B12 | 217 |
| Vitamin B2 | 2124 |
| Vitamin E | 105379 |
Table 2.
Chemical composition of standard diet (Mix 6350 PC flour (a); silage (b); hay(c)) on fresh matter (F.M.) and dry matter (D.M.).
| a. Flour Mix 6350 PC | ||
|---|---|---|
| Parameters | DM [%] | |
| Moisture | 11.32 | |
| Dry matter | 221.71 | |
| Crude protein | 14.21 | |
| Ash | 8.89 | |
| NDF1 | 17.98 | |
| ADF2 | 11.74 | |
| Starch | 27.90 | |
| Sugars | 5.66 | |
| b. Silage corn and triticale | ||
|---|---|---|
| Parameters | F.M. [%] | D.M. [%] |
| Humidity | 51.20 | |
| Dry matter | 48.80 | |
| Crude protein | 6.20 | |
| Raw fiber | 32.40 | |
| Ashes | 9.91 | |
| NDF1 | 58.40 | |
| ADF2 | 36.30 | |
| c. Oat hay | ||
|---|---|---|
| Parameters | F.M. [%] | D.M. [%] |
| Humidity | 9.50 | |
| Dry matter | 90.50 | |
| Crude protein | 8.37 | 9.25 |
| Raw fiber | 34.00 | 37.57 |
| Ashes | 10.80 | 11.93 |
| NDF1 | 61.30 | 67.73 |
| ADF2 | 40.60 | 44.86 |
NDF: neutral detergent fiber;
ADF: acid detergent fiber.
2.3. Preparation and composition of the feed supplemented with PHENOFEED DRY
TMR (MIX 6350 PC) was supplemented with PHENOFEED DRY phenolic extract, during the period from T1–T4, to provide phenolic extract 500 mg/cow/day, while at time T0 and T5 the cows received only TMR. The evaluation of the total polyphenols content and antioxidant activity of MIX 6350 PC and PHENOFEED DRY phenolic extract were carried out by Folin-Ciocalteu assay and DPPH test, while the qualitative-quantitative evaluation of phenolic extract was carried out by HPLC chromatographic analysis. Cows were fed for 60 days a water-soluble natural olive extract powder PHENOFEED DRY (PhenoFarm, 02038 Scandriglia, Rieti, Italy). The polyphenolic content of Phenofeed® Dry is ≥ 25 mg GAE (Gallic Acid Equivalent)/g (PhenoFarm technical data). Each cow received 500 mg PHENOFEED DRY daily in 15 kg Mix 6350 PC flour.
2.4. Microwave assisted extraction (MAE) of standard and enriched feed
To analyze the phenolic content, antioxidant activity, and phenolic composition of MIX 6350 PC and feed enriched with PHENOFEED DRY, phenolic compounds were extracted by microwave-assisted extraction (MAE) using a domestic microwave oven system (Samsung GE872T). One gram of feed was placed in contact with water as a solvent (leaching) for five minutes before irradiation (Bhuyan et al., 2015). Extraction was performed at 600 W for 1 min and then the extract was centrifuged at 4500 g at 20°C for 20 min and filtered through a 45 μm filter.
2.5. Total phenolic content (TPC)
Total phenolic content (TPC) in standard feed, PHENOFEED DRY, and enriched feed, was determined by the Folin-Ciocalteu method (Singleton and Rossi, 1965), modified according to Picariello et al. (2016). Gallic acid (GA) (Sigma, St. Louis, MO, USA) was used as a standard to construct the calibration curves. Results were expressed as mg gallic acid equivalent (GAE)/g dry matter of the raw material. Absorbance was monitored at 765 nm with a Biomate 3-Thermo Spectronic spectrophotometer (Thermo Fisher, Waltham, Massachusetts, USA). The assay was performed in triplicate.
2.6. HPLC-UV analysis
High performance liquid chromatography (HPLC) was used to characterize the PHENOFEED DRY extract. For HPLC-UV analysis, a Shimadzu LCMS-2010EV mass spectrometer was used, connected to an LC unit consisting of two LC-10AD VP pumps with an SCL-10A VP interface, on which a Supelco Discovery HS C18 column (5 µm, 150 x 2.1 mm id) was placed. In addition, the system was equipped with a diode array detector (SPD-10 M20A). The column temperature was set at 30 °C. For HPLC injection, phenolic extracts in water were filtered through a 0.22 µm cellulose acetate syringe filter.
The chromatographic conditions used were:
-
•
eluent phases: (A) water/acetic acid (97.5:2.5) (Sigma-Aldrich St. Louis, MO, USA), (B) acetonitrile (Carlo Erba Reagents) at a flow rate of 0.8 mL/min;
-
•
the linear gradient, according to the method proposed by Benavente-Garcia et al. (2000) with some modifications, is initiated from 95% (A) and 5% (B) to 75% (A) and 25% (B) in 20 min; it changes to 50% (A) and (B) in 15 min (35 min; total time); in 5 min it is changed to 20% (A) and 80% (B) (40 min; total time); after rebalancing in 5 min to the initial condition from 95% (A) and 5% (B) (45 min; total time). The UV spectrum was acquired at 280 nm.
Key phenolic compounds were identified by comparing retention times and absorption spectra with those of pure standards. The compounds were quantified using calibration curves of tyrosol (≥ 98%) and hydroxytyrosol (≥ 98%) standards (Sigma, St. Louis, MO, USA) and the regression equations and correlation coefficient (r2) were calculated. The content of the phenolic compounds was expressed in mg/g dry matter.
2.7. Antioxidant activity
The Antioxidant activity (DPPH assay) was used for the evaluation of the antioxidant capacity of plant extracts to scavenge free radicals generated by the DPPH reagent (Assimopoulou et al., 2005).
The radical scavenging ability of PHENOFEED DRY extract, and standard and enriched feed, was determined using the DPPH method with some modifications as reported in Siano et al. (2016). Briefly, 50 μL of extract was added to the radical solution (DPPH diluted in 0.004% methanol) (Cayman Chemical Co, USA). An equal volume (50 μL) of the solvent was used as a control. Absorbance was measured at 517 nm with a Biomate 3 spectrophotometer. Antiradical activity was expressed as the percent inhibition (I%) of the sample (As) relative to the initial concentration of DPPH (Ac) according to the equation I% = [(Ac–As) / Ac] × 100, where Ac is the absorbance of the control reaction (containing all reagents except the tested compound), and As is the absorbance of the tested compound. All determinations were performed in triplicate.
2.8. Blood samples
Blood samples were taken from all dairy cows during the study period (T0–T5). The experimental times (to T1 at T4) were compared with T0 and T5, in order to evaluate the fatty acids profiles before, during, and after the experimental phase (T1–T4). Blood sampling at time T5 was also performed to evaluate possible long-term effects of the treatment. The blood sampling was carried out in the pre- and post-milking phase, at 4:30 AM and 6:00 AM respectively.Blood was obtained from the external jugular vein into EDTA vacutainer tubes and stored at 4 °C until centrifuged.
2.9. Fatty acids analysis
To obtain fatty acid composition, blood and feed were extracted with methylene chloride and the organic phase was dried under a slight flow of nitrogen. Each sample was dried for 1 h over P2O5 and then treated for 20 min at 60 °C with a solution of boron trifluoride/methanol 10% (1.3 M, 0.5 mL) and 100 µL of dimethoxypropane (Crescenzo et al., 2015). Finally, each solution was extracted twice with hexane and the organic phase was dried. The fatty acid methyl esters were redissolved in hexane, filtered on a millex, and injected into a gas chromatograph. Gas chromatography analyses were obtained on an Shimadzu model GC2010 instrument equipped with a SP52–60 capillary column (Sigma-Aldrich, St Louis, MO, USA; 100 m x 0.25 inside diameter x 0.20 film thickness); flow rate 1.0 mL/min; injector temperature: 275 °C; splitting ratio: 1:20; detector temperature: 275 °C; carrier gas: helium for chromatography at a pressure of 1.8 psi; auxiliary gas: hydrogen for chromatography, under a pressure of 18 psi; air chromatography at a pressure of 22 psi; sensitivity of instrument: 4 to 16 times the minimum attenuation; amount of sample injected: 1.0 µL. Analyses were performed with the following temperature program: 175 °C for 10 min, 175–220 °C at 1.8 °C/min, and 220°C for 20 min. Fatty acid methyl esters were identified by comparing their retention times with those of 22 commercial fatty acid standards purchased from Supelco (Sigma-Aldrich Group, St. Louis, MO, USA), with the limit of quantitation of 14 ppb.
2.10. Statistical analysis
Statistical significance was determined by Two-way Anova analysis comparing individual fatty acids with each other and comparing the total of saturated, monounsaturated, and polyunsaturated fatty acids and specifically Omega-3 and Omega-6. The statistical significance of the fatty acids was evaluated to establish significant differences between the means (Tukey's test) at a 95% confidence level, where p<0.05 is considered significant. The variance was displayed as a standard error mean (SEM). All analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). The experimental results (T1–T4) were compared with controls (T0, T5) according to the "vertical" control mode, to evaluate the evolution of the lipid profile before, during and after the experimental phase.
3. Results
3.1. Total phenolic content (TPC) and antioxidant activity
The results for the total phenolic content were calculated in mg of gallic acid equivalents/g of dry weight (GAE/g DW).
Table 3 shows the total phenolic content. The polyphenolic amount of the feed enriched with PHENOFEED DRY (19.31 ± 0.02) was greater (p<0.05) than the standard feed (MIX 6350 PC) (9.24 ± 0.04). Hence, the daily ration of 15 kg of MIX 6350 PC feed was supplemented with about 10 mg GAE.
Table 3.
Total polyphenol content (mg gallic acid equivalents/g of dry matter) and antioxidant activity (% DPPH inhibition) in phenolic extracts. Data are expressed as mean ± SD, (n=3)1.
| Phenolic Extracts | TPC (mg GAE/g) | Inhibition % |
|---|---|---|
| Feed MIX 6350 PC | 9.24 ± 0.55 | 31.40 ± 1.14 |
| Enriched feed | 19.31 ± 0.63 | 50.30 ± 2.08 |
| PHENOFEED DRY | 36.81 ± 0.69 | 70.26 ± 1.32 |
Two way Anova analysis between TPC and inhibition % in three phenolic extracts. TPC vs % Inhibition in MIX 6350 PC, Enriched feed, PHENOFEED DRY *** p<0.001.
Antioxidant capacity of the different diets were: MIX 6350 PC, 31.40 ± 0.02%; PHENOFEED DRY, 70.26 ± 0.10%; and enriched feed (MIX 6350PC + PHENOFEED DRY), 50.30 ± 0.05%. These results were consistent with the total phenolic content.
3.2. HPLC-UV analysis
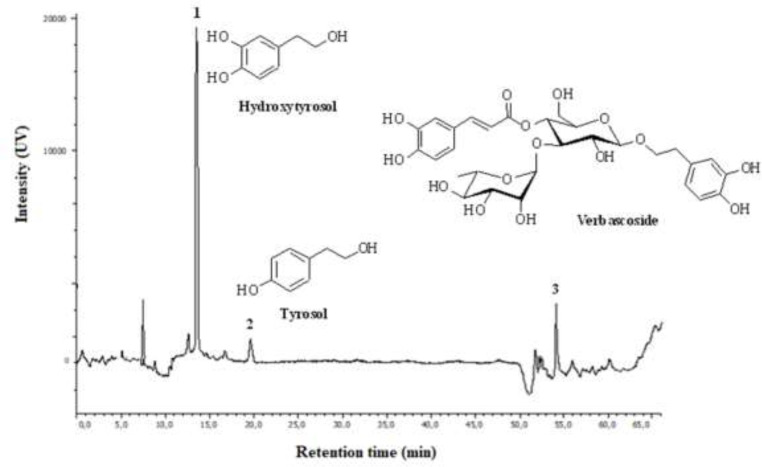
The main compounds present in PHENOFEED DRY were hydroxytyrosol, tyrosol and verbascoside (Fig. 1). Hydroxytyrosol showed the highest relative concentration. The relative concentrations of phenolic compounds was determined by comparing retention times and absorption spectra with those of pure standards as shown in Table 4.
Fig. 1.
Chromatographic profile of PHENOFEED DRY Extract.
Table 4.
Phenolic acids contents (mg/g of dry extract) of PHENOFEED DRY with retention time, standard curve and r2. Values are mean ± SD (n = 3).
| No. peaks |
Compounds | λ (nm) |
RT (min) |
Standard curve | r2 | Phenolic compounds concentration (mg/g) |
|---|---|---|---|---|---|---|
| 1 | Hydroxytyrosol | 280 | 14 | Y = 4E + 06x | 0.97 | 0.180 ± 0.05 |
| 2 | Tyrosol | 280 | 20 | Y = 7E + 07x | 0.99 | 0.028 ± 0.01 |
| 3 | Verbascoside | 280 | 54 | Y = 7E + 07x | 0.99 | 0.033 ± 0.10 |
3.3. Dietary fatty acid profile and polyphenols
Table 5 shows the content of individual fatty acids in MIX 6350 PC feed, PHENOFEED DRY extract and enriched feed. In order to understand the amount of fatty acids integrated in the diet, the total amount due to the addition of 500 mg per day of PHENOFEED DRY and the total content in 15 kg of enriched feed given to cows per day were calculated.
Table 5.
Fatty acids profile of the standard feed, PHENOFEED DRY and enriched feed1.
| N. | Fatty acid | Items | Standard Feed (g/100 g) | PHENOFEED DRY (g/100 g) | Enriched Feed (g/100g) | SEM | Total intake due to 500 mg/day of PHENOFEED DRY | Total content (g) in 15 kg of enriched feed administered cow/day |
|---|---|---|---|---|---|---|---|---|
| 1 | Myristic | C14:0 | 3.838 | 2.983 | 3.839 | 0.10 | 0.015 | 575.70 |
| 2 | Palmitic | C16:0 | 13.999*** | 12.971*** | 13.011*** | 0.29 | 0.065 | 2099.85 |
| 3 | Trans-palmitoleic | C16:1n 7t | 3.756 | 4.946 | 3.701 | 0.09 | 0.025 | 563.40 |
| 4 | Palmitoleic | C16:1n 7 | 2.896 | 1.843 | 2.897 | 0.03 | 0.009 | 434.40 |
| 5 | Stearic | C18:0 | 6.386 | 6.918 | 6.387 | 0.10 | 0.035 | 957.90 |
| 6 | Oleic | C18:1 n9 | 19.111*** | 16.945*** | 19.111*** | 0.50 | 0.085 | 2866.65 |
| 7 | Linolelaidic | C18:2 n6t | 5.243 | 5.213 | 6.242 | 0.09 | 0.026 | 786.45 |
| 8 | Linoleic | C18:2 n6 | 19.5*** | 15.339*** | 19.529*** | 0.36 | 0.077 | 2925.00 |
| 9 | Gamma-linoleic | C18:3 n6 | 0.126 | 2.189 | 0.126 | 0.18 | 0.011 | 18.90 |
| 10 | Eicosenoic | C20:1 n9 | 0.244 | 2.507 | 0.244 | 0.03 | 0.013 | 36.60 |
| 11 | Alpha-linolenic | C18:3 n3 | 3.136 | 3.158 | 3.136 | 0.05 | 0.016 | 470.40 |
| 12 | Eicosadienoic | C20:2 n6 | 0.146 | 1.621 | 0.146 | 0.03 | 0.008 | 21.90 |
| 13 | Dihomo-γ-linolenic | C20:3 n6 | 0.396 | 3.118 | 0.403 | 0.05 | 0.016 | 59.40 |
| 14 | Arachidonic | C20:4 n6 | 6.881 | 5.995 | 6.882 | 0.09 | 0.030 | 1032.15 |
| 15 | Lignoceric | C24:0 | 0.194 | 1.179 | 0.194 | 0.10 | 0.006 | 29.10 |
| 16 | Eicosapentaenoic | C20:5 n3 | 2.782 | 2.333 | 2.783 | 0.05 | 0.012 | 417.30 |
| (EPA) | ||||||||
| 17 | Nervonic | C24:1 n9 | 3.673 | 3.511 | 3.675 | 0.05 | 0.018 | 550.95 |
| 18 | Docosahexaenoic | C22:6 n3 | 7.693 | 7.231 | 7.694 | 0.14 | 0.036 | 1153.95 |
| (DHA) | ||||||||
| Total | ||||||||
| SFA | 24.417 | 24.051 | 23.431 | 0.58 | ||||
| MUFA | 29.68 | 29.752 | 29.628 | 0.69 | ||||
| PUFA | 45.903* | 46.197* | 46.941* | 1.04 |
Two-way Anova Analysis in standard feed, PHENOFEED DRY and enriched feed C16:0 vs C14:0 and C18:0 *** (p<0.001), C18:1 n9 vs C16:1 n7 *** (p<0.001), C18:2 n6 vs other PUFAs *** (p<0.001), PUFA vs MUFA and SFA * (p<0.05). SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids. SEM: standard error of the mean.
The results showed that the content of PUFAs compared to MUFA and SFA is higher (p<0.05) in standard feed, in enriched feed and in PHENOFEED DRY, but the amount of fatty acids added to the diet did not significantly influence the fatty acid composition of the feed. Indeed, the addition of PHENOFEED DRY did not significantly influence the fatty acid composition of feed, but it affects the phenolic content. The phenolic compounds contained in one gram of PHENOFEED DRY were four times higher than those contained in one gram of MIX 6350 PC feed (TPC 36.81 vs 9.24 mg/g).
3.4. Plasma fatty acid profile
The plasma profile of individual fatty acids from T0 to T5 is shown in Table 6. In primiparous cows, there was an increase in linoleic acid (8) (p<0.001) at T5 compared to T0 and at different administration times. Linoleic acid, belonging to the PUFA family, was higher than other fatty acids, such as palmitic acid (2), oleic acid (6), α-linolenic acid (11), DHA (18), EPA (16) and arachidonic acid (14) (p<0.001) and stearic acid (5) (p<0.01).
Table 6.
Fatty acid composition in plasma of lactating Holstein-Friesian cows (mid-lactation) fed with a standard diet (T0) and diet supplemented with PHENOFEED DRY1.
| Fatty Acid (%) | Experimental Time | SEM | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | ||||||||||||||
| P | S | PL | P | S | PL | P | S | PL | P | S | PL | P | S | PL | P | S | PL | ||
| C14:0 | 0.17 | 0.18 | 0.23 | 0.30 | 0.21 | 0.16 | 0.12 | 0.10 | 0.19 | 0.15 | 0.17 | 0.15 | 0.18 | 0.42 | 0.57 | 1.12 | 1.17 | 1.97 | 0.06 |
| C16:0 | 12.80 | 13.29 | 12.05 | 11.21 | 10.50 | 10.54 | 10.38 | 8.73 | 9.98 | 9.25 | 10.29 | 10.03 | 9.31 | 9.59 | 10.12 | 12.80 | 14.65 | 14.58 | 0.33 |
| C16:1 n7t | 0.44 | 0.40 | 0.55 | 0.64 | 0.25 | 0.43 | 0.12 | 0.45 | 0.18 | 0.19 | 0.34 | 0.84 | 0.11 | 0.55 | 0.57 | 0.09 | 0.06 | 0.02 | 0.06 |
| C16:1 n7 | 0.30 | 0.47 | 0.58 | 0.74 | 0.61 | 0.94 | 0.69 | 0.67 | 0.36 | 1.02 | 1.23 | 1.19 | 0.87 | 0.99 | 0.89 | 0.03 | 0.01 | 0.07 | 0.12 |
| C18:0 | 18.55 | 18.77 | 19.85 | 16.98 | 21.20 | 19.34 | 16.40 | 13.08 | 12.92 | 15.56 | 13.24 | 16.58 | 13.20 | 15.18 | 14.72 | 21.91 | 23.69 | 24.90 | 0.68 |
| C18:1 n9 | 10.01 | 11.70 | 11.56 | 12.55 | 13.64 | 13.16 | 14.44 | 14.85 | 19.59 | 14.30 | 19.09 | 17.15 | 16.63 | 16.77 | 17.58 | 22.43*** | 17.49 | 18.99 | 0.50 |
| *** | |||||||||||||||||||
| C18:2 n6t | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C18:2 n6 | 24.92 | 24.88 | 22.88 | 29.54 | 25.70 | 24.96 | 30.91 | 34.81 | 29.00** | 26.56 | 23.18 | 22.98 | 28.61 | 26.16 | 24.94 | 36.66*** | 37.77*** | 34.58 | 0.71 |
| C18:3 n6 | 0.48 | 0.47 | 0.35 | 0.77 | 0.46 | 0.34 | 0.75 | 0.78 | 0.38 | 0.71 | 0.46 | 0.57 | 0.65 | 0.36 | 0.37 | 0.31 | 0.22 | 0.20 | 0.06 |
| C20:1 n9 | 0.86 | 0.44 | 0.50 | 1.42 | 1.00 | 1.00 | 1.31 | 1.20 | 1.19 | 1.87 | 1.93 | 1.44 | 1.24 | 0.59 | 0.29 | 0.53 | 0.31 | 0.12 | 0.14 |
| C18:3 n3 | 0.48 | 0.45 | 0.65 | 0.35 | 0.26 | 0.32 | 0.33 | 0.29 | 0.22 | 0.54 | 0.49 | 0.35 | 0.38 | 0.32 | 0.36 | 0.25 | 0.29 | 0.31 | 0.07 |
| C20:2 n6 | 0.20 | 0.16 | 0.10 | 0.10 | 0.04 | 0.13 | 0.16 | 0.36 | 0.75 | 0.97 | 0.42 | 0.04 | 0.56 | 0.77 | 0.20 | 0.19 | 0.28 | 0.11 | 0.05 |
| C20:3 n6 | 3.66 | 3.49 | 3.96 | 4.13 | 4.70 | 5.21 | 4.21 | 4.73 | 4.55 | 6.50 | 6.67 | 5.51 | 6.10 | 4.41 | 5.20 | 0.41 | 0.42 | 0.82 | 0.21 |
| C20:4 n6 | 4.45 | 4.85 | 4.72 | 6.10 | 5.98 | 6.90 | 5.39 | 5.00 | 5.82 | 6.85 | 6.28 | 6.08 | 5.50 | 5.77 | 6.06 | 1.85 | 1.97 | 1.82 | 0.15 |
| C24:0 | 0.83 | 0.82 | 0.99 | 0.22 | 0.20 | 0.55 | 0.26 | 0.06 | 0.09 | 0.23 | 0.09 | 0.10 | 0.09 | 0.15 | 0.17 | 0.26 | 0.27 | 0.13 | 0.05 |
| C20:5 n3 | 0.48 | 0.37 | 0.40 | 0.75 | 0.72 | 0.53 | 0.77 | 0.80 | 0.80 | 0.90 | 0.90 | 0.61 | 0.85 | 0.75 | 0.27 | 0.31 | 0.19 | 0.20 | 0.09 |
| C24:1 n9 | 8.49 | 5.91 | 7.42 | 1.09 | 1.00 | 1.94 | 0.73 | 0.99 | 0.64 | 0.75 | 0.68 | 0.68 | 0.69 | 0.34 | 1.08 | 0.39 | 0.55 | 0.53 | 0.17 |
| C22:6 n3 | 12.88 | 13.35 | 13.21 | 13.11 | 13.53 | 13.55 | 13.03 | 13.10 | 13.34 | 13.65 | 14.54 | 15.70 | 15.03 | 16.88 | 16.61 | 0.46 | 0.66 | 0.65 | 0.47 |
| Total | |||||||||||||||||||
| SFA | 32.35 | 33.06 | 33.12 | 28.71 | 32.11 | 30.59 | 27.16 | 21.97 | 23.18 | 25.19 | 23.79 | 26.86 | 22.78 | 25.34 | 25.58 | 36.09 | 39.78 | 41.58 | 1.12 |
| ** | ** | ** | ** | ** | ** | ||||||||||||||
| MUFA | 20.10 | 18.92 | 20.61 | 16.44 | 16.5 | 17.47 | 17.29 | 18.16 | 21.96 | 18.13 | 23.27 | 21.3 | 19.54 | 19.24 | 20.41 | 23.47 | 18.42 | 19.73 | 0.98 |
| PUFA | 47.55 | 48.02 | 46.27 | 54.85 | 51.39 | 51.94 | 55.55 | 59.87 | 54.86 | 56.68 | 52.94 | 51.84 | 57.68 | 55.42 | 54.01 | 40.44 | 41.8 | 38.69 | 1.81 |
| ** | *** | *** | ** | *** | *** | ** | *** | *** | ** | *** | *** | ** | *** | *** | ** | *** | *** | ||
P-Primiparous, S-Secondiparous and PL-Pluriparous. T0-control diet; T1,T2,T3,T4-Experimental times, T5-45 days after the last administration of the enriched feed (end lactation). SEM: standard error of the mean. Analysis Two-way Anova P and S C18:2 n6 T5 vs T0,T1,T2,T3,T4 (p<0.001)***, P C18:1 n9 T5 vs T0,T1,T2,T3,T4 (p<0.001)***, PL C18:1 n9 T0 vs T2 (p<0.001)***; C18:2 n6 T0 vs T2 (p<0.01)**. P (PUFA vs MUFA T0, T1,T2,T3,T4,T5 p<0.01 **), S (PUFA vs MUFA T0,T1,T2,T3,T4,T5 p<0.001 ***), PL (SFA vs MUFA T0, T1,T2,T3,T4,T5 p<0.01**; PUFA vs MUFA T0,T1,T3,T3,T4,T5 p<0.001***).
Secondiparous cows showed the same trend as primiparous cows with higher levels of linoleic acid (8) compared to the other fatty acids (p<0.01), while among the SFA there was an increasing trend of stearic acid (5) compared to palmitic acid (2). In pluriparous cows, stearic acid (5) levels were higher than palmitic acid (2) levels, and linoleic acid (8) levels were always higher than the other fatty acids. In the latter cows, palmitic acid (2) and stearic acid (5) do not show significant variations, while oleic acid (6) and linoleic acid (8) showed variation between time T0 and T2 (p<0.001 and p<0.01, respectively) and linoleic acid (8) and DHA (18) between T0 and T5 (p<0.001).
Linoleic acid (8) at time T5 showed an increase of approximately 75% in all parity groups compared to T0. PUFAs showed an increase across T1 to T4 during administration of the enriched diet, and at T5 were the same as T0. At T5 there is an increase in the Omega-6 fraction and a decrease in Omega-3 with a consequent decrease in the total PUFAs content. After 60 days of administration of the enriched diet, the plasma fatty acid profile of cows showed a variation in the Omega-6/Omega-3 ratio in favor of Omega-6 (4:1 ratio).
Table 6 shows the total plasma fatty acid content of Holstein-Friesian dairy cows. There are significant differences between MUFA and PUFA for S and PL (p<0.001) compared to P (p<0.01), while for PL there are also significant differences between SFA and PUFA (p<0.01). PUFA content was higher (p<0.001) than SFA and MUFA during the experimental period (T0 to T4). At T5, the total PUFA levels had returned to T0 values, whereas SFA and MUFA are higher than at T0. At T5, the fatty acids still affected by the enriched diet were mainly MUFAs and SFAs since PUFAs were reduced due to the decrease of Omega-3.
Regarding calving order, PUFA content, in P, S, PL increases significantly (p<0.001) reaching higher levels in PL at T2 (p<0.001), while SFA levels remain constant, with higher levels of stearic acid (5) and palmitic acid (2) compared to the other SFAs (data shown in Supplementary Materials).
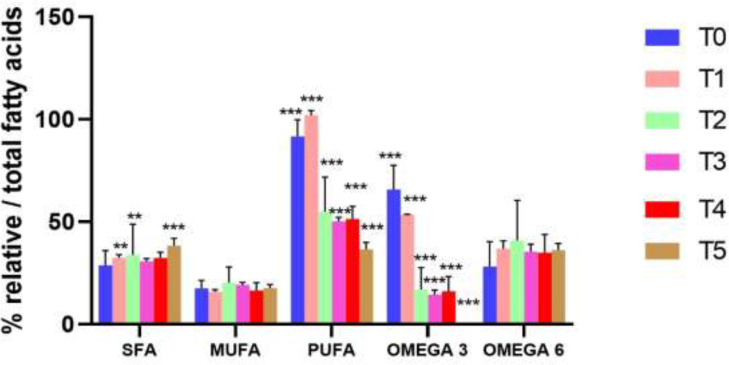
Fig. 2 shows the plasma profile of the different fatty acid groups analyzed at the different experimental times.
Fig. 2.
Plasma fatty acids profile in T0, T1, T2, T3, T4 and T5 f-up. Data are reported as mean ± SD. Two-way Analysis of Variance (ANOVA). (SFA T0-T4 vs PUFA T0-T4 and Omega-3 T0,T1,T5; p<0.001***), (SFA T1,T2 vs MUFA T1,T4; p<0.01**), (SFA T5 vs MUFA T0-T5 and PUFA T0-T2 and Omega-3 T0-T5; p<0.001***), (MUFA T0-T5 vs PUFA T0-T5 and Omega-3 T0, T1 and Omega-6 T0-T5; p<0.001***), (PUFA T0 vs PUFA T2-T5; p<0.001***), (Omega-3 T0-T5 vs Omega-3 T2-T5 and Omega-6 T0-T5; p<0.001***).
At T0, the plasma fatty acids profile showed a higher concentration of PUFAs than SFAs (p<0.05) and MUFAs (p<0.001). PUFAs were characterized by higher Omega-3 than Omega-6 (p<0.001) with minor differences between parity orders. There were no apparent differences between groups for SFAs and MUFAs.
At T1, total SFA, MUFA and PUFA did not differ to T0. There was a decrease in Omega-3 and an increase in Omega-6 (p<0.001) with no difference between parity groups.
At T2, SFA levels increased in pluriparous cows (p<0.05) and remained constant in primiparous and secondiparous cows. MUFA levels remained constant in primiparous and secondiparous cows and showed a slight increase in pluriparous cows. Total PUFA showed a decrease (p<0.001) in primiparous and secondiparous cows and slight increase in pluriparous cows. The acid composition of PUFA was lower (p<0.001) in Omega-3 in all treated groups. The Omega-6 content did not differ to T0 and T1 in primiparous and secondiparous cows and was increased (p<0.001) in pluriparous cows. Pluriparous cows showed an increase (p<0.001) in Omega-6 compared to Omega-3.
At T3, total PUFA was similar among the different parity groups and all groups showed an increase in the Omega-6/Omega-3 ratio (p<0.001). Omega-3 showed significant decrease compared to T0 and T1 (p<0.001). MUFA and SFA levels remained unchanged compared to T0, T1, and T2.
At T4, levels of SFA, MUFA and PUFA were similar to T0, T1, T2 and T3. The Omega-6/Omega-3 ratio was unchanged from T3.
At T5, Omega-3 was almost undetectable compared with times from T0 to T4 (p<0.001) and there was a statistically significant increase in SFA in all three groups of cows (p<0.001).
4. Discussion
This study demonstrated the effects of dietary polyphenols (PHENOFEED DRY) of the olive plant (O. europaea L.) on the plasma fatty acid composition in Holstein- Friesian dairy cows in mid-lactation. The findings showed that addition of PHENOFEED DRY to the diet was associated with an increase in PUFA, no change in SFA and MUFA, and a decrease in Omega-3 PUFA levels. Douglas et al. (2007) and Tessari et al. (2020) regarding the hypothesis that plasma concentrations of polyunsaturated fatty acids (PUFAs) of the Omega-6 lipid class are higher in the middle lactation phase since free fatty acids such as linoleic acid (8), γ-linolenic acid (9), ecosadienoic acid (12), and dihomo-γ-linolenic acid (13) increased. Brzozowska et al. (2018) also showed that the content of linoleic acid (8) and dihomo-γ-linolenic acid (13) was lower at the beginning of lactation and increased in the middle phase. Furthermore, our results show that in the three groups fed a diet supplemented with polyphenols from O. europaea, plasma Omega-6 levels did not change significantly, maintaining an Omega-6/Omega-3 ratio in the optimal values (3:1-4:1).
Abdel-Raheem et al. (2010) reported a decrease in plasma Omega-3 in early lactation in dairy cows due to greater utilization by the mammary gland when NEB is at its maximum negative peak, and then an increase in mid- to late-lactation. In the present study, Omega-3 was decreased in the mid- and late- lactation phase regardless of parity order; this finding could be explained by the effect of polyphenols added to the diet that may modulate the ruminal bio-hydrogenation by cellulosolytic bacteria as suggested by Vasta et al. (2019). Indeed, during lactation the composition and content of plasma fatty acids changed (Buccioni et al., 2015b); but it is also known that plant polyphenols administered during lactation act on the fatty acid bio-hydrogenation process, methane production, nitrogen utilization, and protein and fiber digestion, as well as on the ruminal microbial population (Buccioni et al., 2015b; Vasta et al., 2019).
Other studies show that, the inclusion of polyphenols in the diet induces an inhibitory effect on the bio-hydrogenation of PUFAs into SFAs, due to their influence on microbial activity and diversity. Indeed, a polyphenol-enriched diet can lead to a reduction in SFA and a reduction in Butyrivibrio bacterial by increasing vaccenic acid (trans-11 C18:1) levels. It has been shown that the addition of bioactive compounds (i.e. chestnut and quebracho tannins) can increase the duodenal flux of fatty acids such as vaccenic acid and, consequently, improve the nutritional value of milk fat through desaturation in rumenic acid (cis-9,cis-12 C18:2) in the mammary gland and other tissues (Buccioni et al., 2015a; Buccioni et al., 2015b).
Our results showed that during the administration of the feed enriched with polyphenols plasma levels of PUFAwere elevated compared with SFA in all three parity groups. It could be assumed that the action of polyphenols may have influenced, in particular, the amount of Omega-6 PUFAs compared with SFAs because of the inhibitory effect exerted by polyphenols on biohydrogenation. This was supported by the higher levels of SFAs and lower levels of PUFAs at T5, probably due to the absence of an inhibitory effect of polyphenols on the PUFA biohydrogenation process.
The main factors influencing biohydrogenation pathways in the rumen can vary based on species, diet (e.g. amount and type of forage, amount of starch and its degradability in the rumen), ruminal pH, amount and type of fat supplementation, and specific ingredients able to interact with ruminal bacteria (Buccioni et al., 2015a; Buccioni et al., 2015b). In addition, unsaturated fatty acids are able to interact with fiber particles resulting in the formation of a lipid film, reduction of their digestibility, prevention of bacterial attack and activity of cellulases present in the ruminal fluid, while saturated fatty acids bypass the rumen without interacting with either the ruminal micropopulation or other substances within it. It is also known that an excess of PUFAs in the rumen can be toxic to the ruminal biomass and therefore the process of bio-hydrogenation is useful in preserving the ruminal bacteria integrity (Vasta et al., 2019).
In dairy cows fed a diet enriched with canola oil, the fatty acid composition of the diet influenced the plasma fatty acid content in cows at mid-lactation phase (Loor et al., 2002). In the latter study, the diet administered, rich in linoleic and oleic acid, resulted in an increase of the same fatty acids in the plasma of cows even after 45 days from the end of treatment especially in primiparous and secondiparous cows. Plasma concentrations of vaccenic acid and linoleic acid are higher when dairy cows are fed soybean and flaxseed, while plasma concentrations of saturated fatty acids were not affected by supplementation (Liu et al., 2008). According to Liu et al. (2008) from the results obtained in the present study, it is possible to highlight that the composition of the diet influenced more the plasma content of unsaturated fatty acids (UFAs) and less the SFAs.
Regarding the Omega-6/Omega-3 ratio, the daily diet supplemented with polyphenols maintained this ratio in the range of 3:1-4:1. Feed rations for lactating cows usually have a high content of concentrates and corn silage resulting in a Omega-6/Omega-3 ratio often close to 15:1–18:1 (500 g Omega-6/34 g Omega-3) (Borreani et al., 2013). Altered levels between Omega-6 and Omega-3 not only reduce milk production and the lipid and protein content of the milk, but also induce a negative effect on antioxidant activity and immune function (Greco et al., 2015). These conditions induce the activation of a pro-inflammatory metabolic cascade with production of free radicals and negative effects on production and fertility (Greco et al., 2015; Greco et al., 2018). Omega-3 and Omega-6 PUFAs are essential for improving milk composition, metabolic and anti-inflammatory status and reproductive performance of lactating dairy cows (Greco et al., 2018; Dirandeh et al., 2013). It has shown that diet supplementation with products and by-products of the olive-oil chain is able to provide beneficial effects not only on the plasma fatty acid profile of cows, as analysed in the present study, but also to positively influence the quality of milk and dairy products (Molina-Alcaide and Yanez-Ruiz, 2008). Vargas-Bello-Pérez et al. (2018) show the influence of supplementation with unrefined olive oil on the milk quality of Friesian cows, in particular they show an increase in milk production and an improvement in the lipid profile of the cheese. Moreover, Molina-Alcaide and Yáñez-Ruiz (2008) show that the quantity and composition of the milk fatty acid profile is significantly influenced by the diet fed to the animals, particularly in ruminants. Specifically, they show that the lipid profile of milk from animals fed with olive leaves has a higher amount of oleic and linoleic acids (244 and 18.9 g/kg vs. 212 and 16.3 g/kg, respectively) and less saturated myristic and palmitic fatty acids (112 and 251 g/kg vs. 121 and 279 g/kg, respectively) than from animals fed with alfalfa hay. Therefore, the use of olive by-products appears to increase the monounsaturated fatty acid content and decrease the saturated fatty acid content in milk. It is also shown in other ruminants (goats) that supplementation with these by-products and co-products is able to improve not only the nutritional quality but also the functional quality of the milk in terms of phenolic composition (Ianni et al., 2021). In this regard, Ianni et al. (2021) show that supplementation with olive leaves (10%) increases the antioxidant power in goat milk and leads to a significant reduction in lipolytic events. Furthermore, Chiofalo et al. (2020), show that, in addition to an increase in unsaturated fatty acids (oleic acid, vaccenic acid and CLA) and a decrease in saturated fatty acids, there is also an improvement in the sensory profile (appearance, texture, taste and smell) in cheese derived from cows treated with dried olive cakes. The feed enriched with phenolic compounds present in the products and by-products of the olive-oil chain is therefore strongly influenced by the concentration and composition in antioxidant molecules (Molina-Alcaide and Yanez-Ruiz, 2008; Chiofalo et al., 2020). In addition, the use of extracts derived from this chain, in particular the PhenoFeed Dry extract used in this study, as stated in the product data sheet, does not present factors with potential toxic effects; in general, it is a good practice to use products and by-products obtained from crops that are not chemically treated (organic farming). In this regard, Molina-Alcaide and Yáñez-Ruiz (2008) show that the Cu content in olive leaves depends on the number of chemical treatments applied and weather conditions (wind, rain). In any case, it is always recommended to perform multi-residual chemical analysis before using extracts from plant matrices in the formulation of functional feeds.
5. Conclusions
In conclusion, our results indicate that the diet supplemented with phenolic compounds of Olea europaea L. influences the plasma fatty acid composition (SFA, MUFA, PUFA) in lactating Holstein-Friesian cows, independent of the calving order. In particular, a close relationship between the fatty acid composition of the diet and the plasma lipid content of lactating cows with significant impact on PUFA with an increase of Omega-6 versus Omega-3 during treatment was shown. We can hypothesize that the PUFA fraction, compared to MUFA and SFA, is the one most influenced by the phenolic composition of the diet. Therefore, enrichment of the diet with high-functional extracts could contribute to improving the metabolic performance of cows to sustain high levels of milk production in high-performance cows (Italian Holstein-Friesian breed), provided that dosages and timing of phenolic supplementation are established to avoid adverse effects. In this work, a new approach was adopted with regard to the timing of sampling (every 15 days) in order to avoid additional causes of stress to the animal and with regard to the sampling method of the control sampling carried out on the same animals studied (vertical control method) in order to remove possible errors in the assessment of results due to individual metabolic or other variables.
The use of natural bioactive molecules extracted by-products of the olive-oil supply chain for the production of functional feeds may represent a possible source of circular economy in view of environmental sustainability.
Fig. S1: Plasma fatty acid profile during the experimental period (T0-T5) of Primiparous cows, Fig. S2: Plasma fatty acid profile during the experimental period (T0-T5) of Secondiparous cows, Fig. S3: Plasma fatty acid profile during the experimental period (T0-T5) of Pluriparous cows.
Funding
This work was supported by the project “SALUTE: Gestione aziendale, benessere animale e metaboliti funzionali del latte”, supported by grant, project ID: 31 (year 2019), Ministero delle Politiche Agricole Alimentari e Forestali MIPAAF (Italy) and was supported by the project "O.Ri.delSannio: Organizzazione e riposizionamento della filiera lattiero-casearia ovina dell'Appennino del Sannio" - PSR Campania 2014/2020 Misura 16.1 - Azione 2 - CUP B88H19005350008.
Ethical statement
The procedure of this study was reviewed and approved by the Ethical Animal Care and Use Committee of the University of Naples “Federico II” (Protocol No. 99607-2017).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was carried out within the framework of the PhD in Industrial Characterization, POR-FSE 2014/2020 Campania Region (Italy), in Science and Technology for Environment and Health. The authors would like to thank the company “Mangimi Liverini” for formulating and producing the feed, the farm "Fratelli Mirra" for providing the animals for the experiments and the company “PhenoFarm” for providing the phenolic extract added to the cows' diet.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2023.100298.
Appendix. Supplementary materials
References
- Abdel-Raheem S., Schreiner M., Iben C. Lactational and seasonal variations in plasma fatty acids profiles in dairy cows. Wiener Tierärztliche Monatsschrift. 2010;97(5):149–156. [Google Scholar]
- Adeyemi K.D., Shittu R.M., Sabow A.B., Ebrahimi M., Sazili A.Q. Influence of diet and postmortem ageing on oxidative stability of lipids, myoglobin and myofibrillar proteins and quality attributes of gluteus medius muscle in goats. PLOS One. 2016;11(5) doi: 10.1371/journal.pone.0154603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M.E. The effect of rosemary herb as a dietary supplement on performance, egg quality, serum biochemical parameters, and oxidative status in laying hens. Journal of Animal and Feed Sciences. 2015;24(4):341–347. doi: 10.22358/jafs/65617/2015. [DOI] [Google Scholar]
- AOAC . Association of the official analytical chemists. 15th ed. AOAC; Washington DC: 1990. Official methods of analysis. [Google Scholar]
- Assimopoulou A.N., Sinakos Z., Papageorgiou V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytotherapy Research. 2005;11:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- Avondo M., Secchiari P., Battaglini L.M., Bonanno A., Pulina G. Soil, pasture and animal product quality. Italian Journal of Agronomy. 2013;8(3) doi: 10.4081/ija.2013.e19. e19–e19. [DOI] [Google Scholar]
- Benavente-Garcia O., Castillo J., Lorente J., Ortuno A., Del Rio J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chemistry. 2000;68:457–462. doi: 10.1016/S0308-8146(99)00221-6. [DOI] [Google Scholar]
- Bhuyan D.J., Vuong Q.V., Chalmers A.C., van Altena I.A., Bowyer M.C., Scarlett C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Industrial Crops and Products. 2015;69:290–299. doi: 10.1016/j.indcrop.2015.02.044. [DOI] [Google Scholar]
- Bionaz M., Vargas-Bello-Pérez E., Busato S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. Journal of Animal Science and Biotechnology. 2020;11(1):1–36. doi: 10.1186/s40104-020-00512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borreani G., Coppa M., Revello-Chion A., Comino L., Giaccone D., Ferlay A., Tabacco E. Effect of different feeding strategies in intensive dairy farming systems on milk fatty acid profiles, and implications on feeding costs in Italy. Journal of Dairy Science. 2013;96(11):6840–6855. doi: 10.3168/jds.2013-6710. [DOI] [PubMed] [Google Scholar]
- Brzozowska A.M., Lukaszewicz M., Oprzadek J.M. Energy-protein supplementation and lactation affect fatty acid profile of liver and adipose tissue of dairy cows. Molecules. 2018;23(3):618. doi: 10.3390/molecules23030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccioni A., Serra A., Minieri S., Mannelli F., Cappucci A., Benvenuti D., Rapaccini S., Conte G., Mele M. Milk production, composition, and milk fatty acid profile from grazing sheep fed diets supplemented with chestnut tannin extract and extruded linseed. Small Ruminant Research. 2015;130:200–207. doi: 10.1016/j.smallrumres.2015.07.021. [DOI] [Google Scholar]
- Buccioni A., Pauselli M., Viti C., Minieri S., Pallara G., Roscini, Rapaccini S., Trabalza Marinucci S., Lupi P., Conte G., Mele M. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. Journal of Dairy Science. 2015;98(2):1145–1156. doi: 10.3168/jds.2014-8651. [DOI] [PubMed] [Google Scholar]
- Buccioni A., Pauselli M., Minieri S., Roscini V., Mannelli F., Rapaccini S., Lupi P., Conte G., Serra A., Cappucci A., Brufani L., Ciucci F., Mele M. Chestnut or quebracho tannins in the diet of grazing ewes supplemented with soybean oil: Effects on animal performances, blood parameters and fatty acid composition of plasma and milk lipids. Small Ruminant Research. 2017;153:23–30. doi: 10.1016/j.smallrumres.2017.05.006. [DOI] [Google Scholar]
- Cappelli K., Ferlisi F., Mecocci S., Maranesi M., Trabalza-Marinucci M., Zerani M., Dal Bosco A., Acuti G. Dietary supplementation of olive mill waste water polyphenols in rabbits: Evaluation of the potential effects on hepatic apoptosis, inflammation and metabolism through RT-qPCR approach. Animals. 2021;11(10):2932. doi: 10.3390/ani11102932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo C., Pereira V., Abuelo Á., Hernández J. Effect of supplementation with antioxidants on the quality of bovine milk and meat production. The Scientific World Journal. 2013;13:2013. doi: 10.1155/2013/616098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiofalo B., Di Rosa A.R., Lo Presti V., Chiofalo V., Liotta L. Effect of supplementation of herd diet with olive cake on the composition profile of milk and on the composition, quality and sensory profile of cheeses made therefrom. Animals. 2020;10(6):977. doi: 10.3390/ani10060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino R., Barone C.M.A., Claps S., Varricchio E., Rufrano D., Caroprese M., Albenzio M., De Palo P., Campanile G., Neglia G. Effects of dietary supplementation with polyphenols on meat quality in Saanen goat kids. BMC Veterinary Research. 2018;14(1):181. doi: 10.1186/s12917-018-1513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzo R., Bianco F., Mazzoli A., Giacco A., Cancelliere R., Di Fabio G., Zarrelli A., Liverini G., Iossa S. Fat quality influences the obesogenic effect of high fat diets. Nutrients. 2015;7:9475–9491. doi: 10.3390/nu7115480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro A.G., Vizzarri F., Palazzo M., Martemucci G. Dietary verbascoside supplementation in donkeys: Effects on milk fatty acid profile during lactation, and serum biochemical parameters and oxidative markers. Animals. 2017;11(9):1505–1512. doi: 10.1017/S1751731117000441. [DOI] [PubMed] [Google Scholar]
- Di Nunzio M., Picone G., Pasini F., Caboni M.F., Gianotti A., Bordoni A., Capozzi F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Research International. 2018;113:392–400. doi: 10.1016/j.foodres.2018.07.025. [DOI] [PubMed] [Google Scholar]
- Dirandeh E., Towhidi A., Zeinoaldini S., Ganjkhanlou M., Ansari Pirsaraei Z., Fouladi-Nashta A. Effects of different polyunsaturated fatty acid supplementations during the postpartum periods of early lactating dairy cows on milk yield, metabolic responses, and reproductive performances. Journal of Animal Science. 2013;91(2):713–721. doi: 10.2527/jas.2012-5359. [DOI] [PubMed] [Google Scholar]
- Douglas G.N., Rehage J., Beaulieu A.D., Bahaa A.O., Drackley J.K. Prepartum nutrition alters fatty acid composition in plasma, adipose tissue, and liver lipids of periparturient dairy cows. Journal of Dairy Science. 2007;90:2941–2959. doi: 10.3168/jds.2006-225. [DOI] [PubMed] [Google Scholar]
- Fraisse D., Carnat A., Viala D., Pradel P., Besle J.M., Coulon J.B., Felgines C., Lamaison J.L. Polyphenolic composition of a permanent pasture: Variations related to the period of harvesting. Journal of the Science of Food and Agriculture. 2007;87:2427–2435. doi: 10.1002/jsfa.2918. [DOI] [Google Scholar]
- Greco L.F., Neves Neto J.T., Pedrico A., Ferrazza R.A., Lima F.S., Bisinotto R.S., Martinez N, Garcia M., Ribeiro E.S., Gomes G.C., Shin J.H., Ballou M.A., Thatcher W.W., Staples C.R., Santos J.E. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on performance and inflammatory responses to a lipopolysaccharide challenge in lactating Holstein cows. Journal of Dairy Science. 2015;98(1):602–617. doi: 10.3168/jds.2014-8805. [DOI] [PubMed] [Google Scholar]
- Greco L.F., Neves Neto J.T., Pedrico A., Lima F.S., Bisinotto R.S., Martinez N., Ribeiro E.S., Thatcher W.W., Staples C.R., Santos J.E.P. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on spontaneous luteolysis in lactating dairy cows. Journal of Dairy Science. 2018;101(11):10536–10556. doi: 10.3168/jds.2018-15065. [DOI] [PubMed] [Google Scholar]
- Gupta P.K., Di Pette D.J., Supowit S.C. Protective effect of resveratrol against pressure overload-induced heart failure. Food Science & Nutrition. 2014;2(3):218–229. doi: 10.1002/fsn3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianni A., Di Luca A., Martino C., Bennato F., Marone E., Grotta L., Cichelli A., Martino G. Dietary supplementation of dried grape pomace increases the amount of linoleic acid in beef, reduces the lipid oxidation and modifies the volatile profile. Animals. 2019;9(8):578. doi: 10.3390/ani9080578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianni A., Martino G. Dietary grape pomace supplementation in dairy cows: Effect on nutritional quality of milk and its derived dairy products. Foods. 2020;9(2):168. doi: 10.3390/foods9020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianni A., Innosa D., Oliva E., Bennato F., Grotta L., Saletti M.A., Pomilio F., Sergi M., Martino G. Effect of olive leaves feeding on phenolic composition and lipolytic volatile profile in goat milk. Journal of Dairy Science. 2021;104(8):8835–8845. doi: 10.3168/jds.2021-20211. [DOI] [PubMed] [Google Scholar]
- Liu Z.L., Yang D.P., Chen P., Lin S.B., Jiang X.Y., Zhao W.S., Li J.M., Dong W.X. Effect of dietary sources of roasted oilseeds on blood parameters and milk fatty acid composition. Czech Journal of Animal Science. 2008;53(5):219–226. doi: 10.17221/309-CJAS. [DOI] [Google Scholar]
- Loften J.R., Linn J.G., Drackley J.K., Jenkins T.C., Soderholm C.G., Kertz A.F. Invited review: Palmitic and stearic acid metabolism in lactating dairy cows. Journal of Dairy Science. 2014;97(8):4661–4674. doi: 10.3168/jds.2014-7919. [DOI] [PubMed] [Google Scholar]
- Loor J.J., Herbein J.H., Jenkins T.C. Nutrient digestion, biohydrogenation, and fatty acid profiles in blood plasma and milk fat from lactating Holstein cows fed canola oil or canolamide. Animal Feed Science and Technology. 2002;97(1-2):65–82. doi: 10.1016/S0377-8401(01)00356-X. [DOI] [Google Scholar]
- Maranesi M., Dall'Aglio C., Acuti G., Cappelli K., Trabalza Marinucci M., Galarini R, Suvieri C., Zerani M. Effects of dietary polyphenols from olive mill waste waters on inflammatory and apoptotic effectors in rabbit ovary. Animals. 2021;11(6):1727. doi: 10.3390/ani11061727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxin G., Cornu A., Andueza D., Laverroux S., Graulet B. Carotenoid, tocopherol, and phenolic compound content and composition in cover crops used as forage. Journal of Agricultural and Food Chemistry. 2020;68(23):6286–6296. doi: 10.1021/acs.jafc.0c01144. [DOI] [PubMed] [Google Scholar]
- Molina-Alcaide E., Yáñez-Ruiz D.R. Potential use of olive by-products in ruminant feeding: A review. Animal Feed Science and Technology. 2008;147(1-3):247–264. doi: 10.1016/j.anifeedsci.2007.09.021. [DOI] [Google Scholar]
- Musayeva K., Sederevičius A., Monkevičienė I., Žymantienė J., Oberauskas V., Kerzienė S., Baltušnikien A., Černauskienė J., Želvytė R. Milk fatty acid profile in cows as influenced by diet supplementation with rapeseed pomace and extruded full-fat soya in different feeding periods. Acta Veterinaria Brno. 2021;90(1):27–34. doi: 10.2754/avb202190010027. [DOI] [Google Scholar]
- Parrillo L., Coccia E., Volpe M.G., Siano F., Pagliarulo C., Scioscia E., Varricchio E., Safari O., Eroldogan T., Paolucci M. Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture. 2017;473:161–168. doi: 10.1016/j.aquaculture.2017.02.013. [DOI] [Google Scholar]
- Pauletto M., Elgendy R., Ianni A., Marone E., Giantin M., Grotta L., Ramazzotti S., Bennato F., Dacasto M., Martino G. Nutrigenomic effects of long-term grape pomace supplementation in dairy cows. Animals. 2020;10(4):714. doi: 10.3390/ani10040714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picariello G., De Vito V., Ferranti P., Paolucci M., Volpe M.G. Species- and cultivar-dependent traits of Prunus avium and Prunus cerasus polyphenols. Journal of Food Composition and Analysis. 2016;45:50–57. doi: 10.1016/j.jfca.2015.10.002. [DOI] [Google Scholar]
- Pohl F., Goua M., Bermano G., Russell W.R., Maciel P., Kong Thoo Lin P. Study into the polyphenol content and antioxidant activity of rapeseed pomace extracts. Proceedings of the Nutrition Society. 2016;75:E59. doi: 10.1017/S0029665116000495. (OCE2) [DOI] [Google Scholar]
- Romani A., Ieri F., Urciuoli S., Noce A., Marrone G., Nediani C., Bernini R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients. 2019;11(8):1776. doi: 10.3390/nu11081776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V., Salvatori G., Pastorelli G. Dietary polyphenol supplementation in food producing animals: Effects on the quality of derived products. Animals. 2021;11:401. doi: 10.3390/ani11020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siano F., Straccia M.C., Paolucci M., Fasulo G., Boscaino F., Volpe M.G. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. Journal of the Science of Food and Agriculture. 2016;96(5):1730–1735. doi: 10.1002/jsfa.7279. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- Tapia-Quirós P., Montenegro-Landívar M.F., Reig M., Vecino X., Cortina J.L., Saurina J., Granados M. Recovery of polyphenols from agri-food by-products: The olive oil and winery industries cases. Foods. 2022;11(3):362. doi: 10.3390/foods11030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari R., Berlanda M., Morgante M., Badon T., Gianesella M., Mazzotta E., Contiero B., Fiore E. Changes of plasma fatty acids in four lipid classes to understand energy metabolism at different levels of non-esterified fatty acid (NEFA) in dairy cows. Animals. 2020;10:1410. doi: 10.3390/ani10081410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Bello-Pérez E., Geldsetzer-Mendoza C., Morales M.S., Toro-Mujica P., Fellenberg M.A., Ibáñez R.A., Gómez-Cortés P., Garnsworthy P.C. Effect of olive oil in dairy cow diets on the fatty acid profile and sensory characteristics of cheese. International Dairy Journal. 2018;85:8–15. doi: 10.1016/j.idairyj.2018.04.006. [DOI] [Google Scholar]
- Vasta V., Daghio M., Cappucci A., Buccioni A., Serra A., Viti C., Mele M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. Journal of Dairy Science. 2019;102(5):3781–3804. doi: 10.3168/jds.2018-14985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.