Abstract
A recessive mutation of Arabidopsis designated sas1 (for sodium overaccumulation in shoot) that was mapped to the bottom of chromosome III resulted in a two- to sevenfold overaccumulation of Na+ in shoots compared with wild-type plants. sas1 is a pleiotropic mutation that also caused severe growth reduction. The impact of NaCl stress on growth was similar for sas1 and wild-type plants; however, with regard to survival, sas1 plants displayed increased sensitivity to NaCl and LiCl treatments compared with wild-type plants. sas1 mutants overaccumulated Na+ and its toxic structural analog Li+, but not K+, Mg2+, or Ca2+. Sodium accumulated preferentially over K+ in a similar manner for sas1 and wild-type plants. Sodium overaccumulation occurred in all of the aerial organs of intact sas1 plants but not in roots. Sodium-treated leaf fragments or calli displayed similar Na+ accumulation levels for sas1 and wild-type tissues. This suggested that the sas1 mutation impaired Na+ long-distance transport from roots to shoots. The transpiration stream was similar in sas1 and wild-type plants, whereas the Na+ concentration in the xylem sap of sas1 plants was 5.5-fold higher than that of wild-type plants. These results suggest that the sas1 mutation disrupts control of the radial transport of Na+ from the soil solution to the xylem vessels.
INTRODUCTION
Among abiotic stresses, salinity is one of the major causes of yield losses of crop plants (Boyer, 1982). Salt stress is a polymorphous stress that reduces yield through three direct effects: osmotic stress, nutritional stress, and ion toxicity. It is widely thought that breeding for salt tolerance will involve developing a pyramiding strategy for selecting favorable combinations of traits, each of which would improve one of the physiological adaptations to salt stress (Yeo and Flowers, 1986). However, it is not clear which traits are appropriate for use in breeding programs to improve salt tolerance. Many physiological and molecular responses to salinity have been described (Greenway and Munns, 1980; Munns, 1993; Yeo, 1998), but their effects on the improvement of salt tolerance have seldom been demonstrated. This observation made clear the necessity of developing genetic approaches to establish which responses are physiologically relevant to salt tolerance (Epstein et al., 1980).
The contributions of different physiological responses to salt tolerance were analyzed recently in genetic studies with a number of different variants (mutants or transgenic plants). The advantages of using mutants are that no prior knowledge of the molecular bases of the mechanism of interest is necessary and that mutants can reveal mechanisms previously unknown to be involved in salt tolerance. The identification and characterization of salt-tolerant and salt-hypersensitive mutants have drawn attention to several matters: (1) the control of Cl− transport from root to shoot (Abel, 1969); (2) the overaccumulation of proline (Kueh and Bright, 1982); (3) the overaccumulation of Na+ and K+ at the seed imbibition stage to decrease the osmotic potential of the embryo (Saleki et al., 1993); and (4) the maintenance of K+ nutrition (Zhu et al., 1998). Together with establishing the importance of the corresponding traits in tolerance, such approaches, when developed by mutant isolation, reveal the underlying mechanisms involved in physiological phenomena.
Despite physiological evidence that control of Na+ invasion of the tissues is a key determinant of salt tolerance (Yeo and Flowers, 1986; Niu et al., 1995), the mechanisms involved in this control are poorly understood. There is an ongoing debate regarding whether Na+ enters the cells by K+ transport systems and, if so, what kind of K+ transport systems could be involved (Rubio et al., 1995; Walker et al., 1996; Amtmann and Sanders, 1999). Based on our present knowledge, two kinds of transport systems are likely to play a major role in Na+ transport: transporters of the HKT1 family, with the Arabidopsis member suspected of transporting Na+ more efficiently than K+ (Uozumi et al., 2000), and the Na+/H+ tonoplastic antiporter, which is suspected to play a role in sequestering Na+ in the vacuole (Blumwald and Poole, 1985; Garbarino and DuPont, 1988). A recent report presenting the effects of the overexpression of a Na+/H+ tonoplastic antiporter in Arabidopsis has provided the first experimental evidence that control of Na+ transport within tissues has a great effect on salt tolerance (Apse et al., 1999). Thus, it is important to identify or construct other variants (mutants or transgenic plants) altered in functions involved in the control of Na+ transport and Na+ accumulation to evaluate the impact of these alterations on salt tolerance.
Arabidopsis is a glycophytic species that is sensitive to moderate levels of NaCl and accumulates a significant amount of Na+ in its shoots when exposed to salinity. We designed a screening procedure for selecting mutants altered in their capacity to accumulate Na+ in the leaves. This report describes the isolation and further characterization of a mutant overaccumulating Na+ in its shoots.
RESULTS
Isolation of a Na+-Overaccumulating Mutant
A total of 6625 M2 seedlings resulting from ∼800 M1 parents obtained after ethyl methanesulfonate mutagenesis were screened. Ten- to 12-day-old seedlings grown in vitro on standard medium were transferred to plates containing standard medium plus 35 mM NaCl. At the time of transfer, the seedlings generally displayed two or four leaves in addition to their cotyledons. Four to 6 days after transfer, one of the two oldest leaves of every plant submitted to the screening procedure was removed, and its Na+ concentration was determined.
Sodium concentration in leaves from individual plants ranged from 30 to 250 μmol·g−1 fresh weight, with an average value of 90 to 120 μmol·g−1 fresh weight and a standard deviation of 30 to 40 μmol·g−1 fresh weight, depending on the experiment considered. Seventy-six plants were selected that accumulated Na+ at a concentration exceeding the average Na+ concentration of the population plus three times its standard deviation. The leaf Na+ concentration of the selected plants was higher than twice the average Na+ concentration of the screened population. Only 1 of these 76 plants both survived salt stress and produced offspring displaying more than twofold overaccumulation of Na+ compared with the wild type. We named this putative mutant sas1 for sodium overaccumulation in shoot.
sas1 Plants Displayed a Small Stature
Mutant sas1 plants displayed a marked size reduction of both roots and aerial organs compared with wild-type plants when cultivated in the greenhouse. Seventeen days after sowing, the developmental status of both lines was identical, but sas1 rosettes were ∼50% smaller than wild-type rosettes (1.4 ± 0.5 and 2.6 ± 1.3 mg dry weight per plant, respectively; 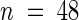 ). The difference increased as plants aged: the dry weight of wild-type plants was four times greater than that of sas1 plants during the 4th week of growth (data not shown) and 10 times greater at maturity (950 ± 230 mg dry weight per wild-type plant versus 90 ± 70 mg dry weight per sas1 plant;
). The difference increased as plants aged: the dry weight of wild-type plants was four times greater than that of sas1 plants during the 4th week of growth (data not shown) and 10 times greater at maturity (950 ± 230 mg dry weight per wild-type plant versus 90 ± 70 mg dry weight per sas1 plant; 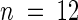 ). A wild-type plant produced 10 to 15 times more seed than a sas1 plant.
). A wild-type plant produced 10 to 15 times more seed than a sas1 plant.
Figure 1A shows that a difference in growth was also observed when plants were cultivated in Na+-free hydroponic culture conditions, indicating that the overall stature reduction of sas1 plants did not result from Na+ overaccumulation or toxicity.
Figure 1.
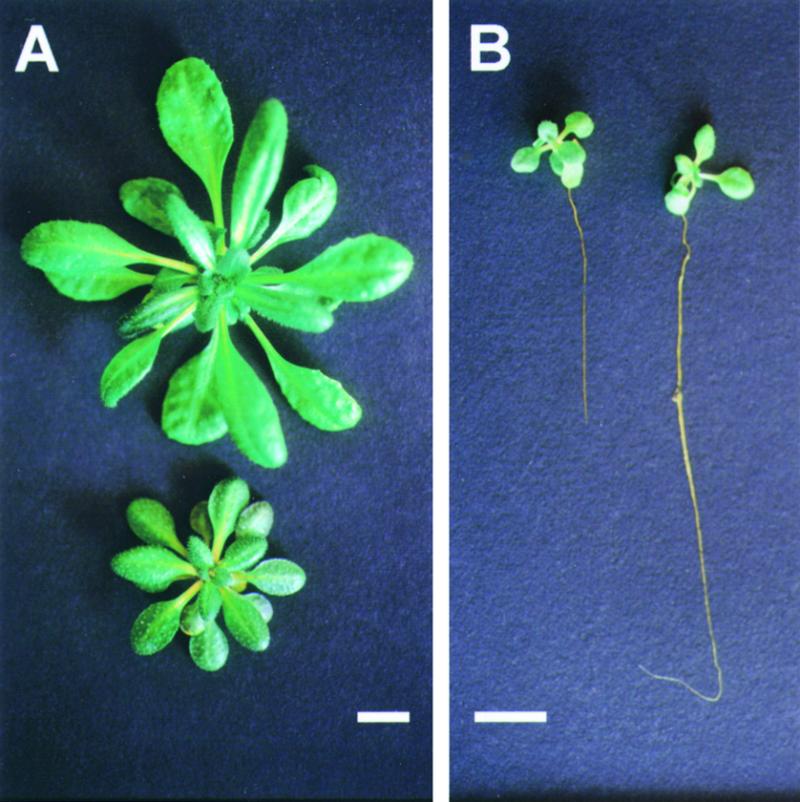
The Stature of sas1 Plants was Reduced.
(A) Top view of wild-type (top) and sas1 (bottom) rosettes of plants grown for 6 weeks in hydroponic culture on a Na+-free culture medium.
(B) sas1 (left) and wild-type (right) plants grown in vitro for 2 weeks.
Bars = 1 cm.
The overall growth reduction of sas1 plants was a particular feature of plants grown in the greenhouse or hydroponically. In contrast, when cultivated in vitro, sas1 plants still displayed reduced root length (between 50 and 85% root length reduction depending on the duration of the culture), but their rosettes were larger than those of wild-type plants (Figure 1B; see also Figure 3B). This result was also obtained when sucrose was withdrawn from the in vitro culture medium (data not shown). The mineral composition of the culture medium likely was not responsible for the conditional reduced size of sas1 shoots because the same culture medium, except for agar and sucrose, was used for hydroponic and in vitro cultures.
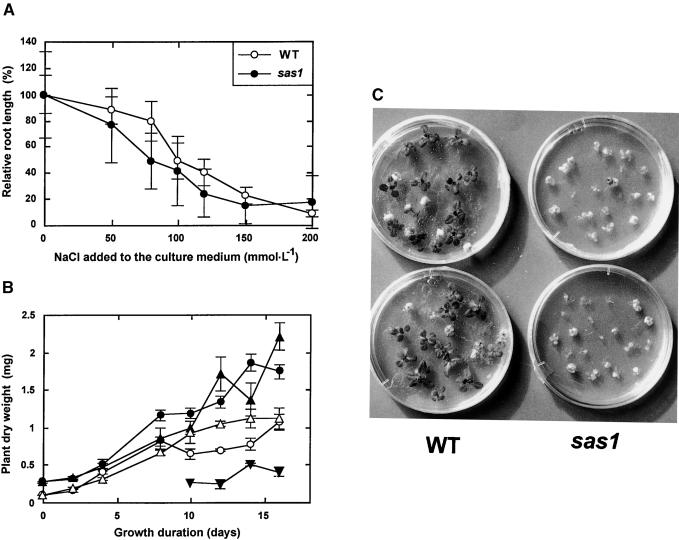
Figure 3.
Effect of NaCl Stress on Growth and Survival of Plants Grown in Vitro.
Eleven-day-old plants were exposed to NaCl stress in vitro.
(A) Root elongation that occurred during the 9 days after plant transfer on the NaCl-containing medium is expressed as percentage of the root elongation of plants of the same line that were transferred to and grown on medium in the absence of NaCl. Root elongation of control plants was 66.0 ± 9.6 mm for the wild type (WT) and 10.0 ± 3.3 mm for sas1. The error bars indicate standard deviation; n = 30.
(B) Dry weight of plants grown for up to 16 days after their transfer on the NaCl-containing medium. Filled circles and open circles, sas1 and wild-type plants, respectively, transferred to standard medium; open triangles, wild-type plants transferred to medium supplemented with 100 mM NaCl; filled triangles and filled inverted triangles, sas1 plants transferred to medium supplemented with 100 mM NaCl that were alive or dead, respectively, when harvested. The error bars indicate standard deviation; n = 9.
(C) Plants transferred to and grown for 4 weeks on standard medium supplemented with 120 mM NaCl.
Salt Sensitivity of sas1 Plants
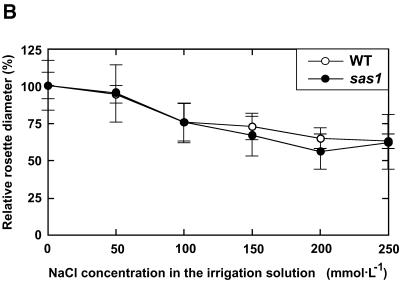
The salt sensitivity of sas1 plants was first evaluated with plants grown in the greenhouse in pots that were flooded once a week with solutions containing different NaCl concentrations. Figure 2 shows that increasing NaCl stress induced a strong growth reduction in both wild-type and sas1 plants. Nevertheless, the relative decrease in growth was identical for sas1 and wild-type plants: the rosette diameters were reduced proportionally by 15% as a result of an increase of 100 mmol·L−1 in NaCl concentration in the irrigation solution (Figure 2B). When salt stress was prolonged, particularly when there were dry, sunny days that increased the transpiration rate throughout the duration of the experiment, the sas1 plants died in conditions in which wild-type plants still survived and bolted. This is illustrated in Figure 2A for plants irrigated with 200 mM NaCl. However, we did not note any difference in survival rate between sas1 and wild-type plants in response to salt stress either when the experiment was performed in the greenhouse under a cloudy sky or when plants were grown in conditions in which they did not transpire much (data not shown).
Figure 2.
Effect of NaCl Stress on Growth and Survival of Plants Grown in the Greenhouse.
Three-week-old plants were flooded once a week during the subsequent 4 weeks with 0, 50, 100, 150, 200, and 250 mM NaCl.
(A) View of the plants 40 days after the first application of salt stress.
(B) Growth inhibition in response to the salt treatments. Rosette diameters are expressed as percentage of the rosette diameter of plants of the same line irrigated with tap water. The error bars indicate standard deviation; n = 20.
WT, wild type.
The effect of NaCl stress on vegetative growth was also evaluated in vitro by analyzing root elongation and whole-plant growth. Eleven-day-old plants cultivated in vitro on standard medium were transferred to medium supplemented with increasing concentrations of NaCl. Six to 11 days after transfer, both sas1 and wild-type roots showed growth reduction as a result of NaCl stress (Figure 3A). The sensitivity of root elongation to NaCl stress was slightly greater for sas1 plants than for wild-type plants. The whole-plant dry weight was measured from 0 to 16 days after transfer to medium containing NaCl at final concentrations of 0, 25, 50, 100, and 120 mmol·L−1. Increasing the NaCl concentration in the medium did not induce any growth reduction of the surviving plants of either line (see Figure 3B, which reports only the results of the 100 mM NaCl treatment). However, the highest NaCl concentrations (100 and 120 mmol·L−1) caused a significant number of the sas1 plants to die, a phenomenon that was not observed for wild-type plants (Figures 3B and 3C).
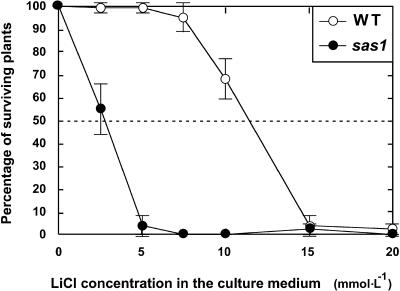
The salt sensitivity of sas1 plants was quantified by determining the LiCl concentration needed to kill 50% of the plants. LiCl was used instead of NaCl because preliminary experiments demonstrated that Li+ induced less ambiguous toxic effects on Arabidopsis plants than did Na+. Eleven-day-old sas1 and wild-type plants grown in vitro on standard medium were transferred to standard medium supplemented with LiCl at concentrations ranging from 0 to 20 mmol·L−1. Figure 4 shows that the survival curve of sas1 plants displayed the same shape as that of wild-type plants but that it was shifted down toward lower Li+ concentrations. Hence, the 50% lethal concentration for sas1 plants (2.8 mmol·L−1) was approximately one-quarter the value for wild-type plants (11.4 mmol·L−1).
Figure 4.
sas1 Plants Displayed LiCl Hypersensitivity.
Twenty 11-day-old in vitro–grown plants were transferred to standard medium supplemented with 0, 2.5, 5, 7.5, 10, 15, or 20 mmol·L−1 LiCl. Fourteen days later, plants displaying a green apical meristem were scored as surviving plants. The error bars indicate standard deviation; n = 5. WT, wild type.
In summary, these results show that the impact of salt stress on growth is comparable for sas1 and wild-type plants, whereas the impact of salt stress on survival discriminated sas1 plants from wild-type plants, sas1 plants being more sensitive to salt stress than were wild-type plants.
Relationship between Na+ Accumulation in Shoots and Growth
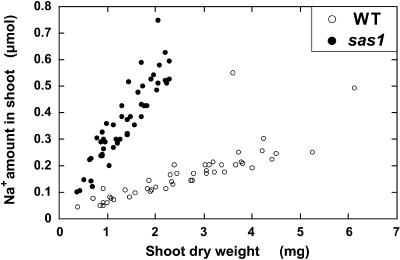
The dry weight of the entire shoot and the amount of Na+ accumulated in the shoot were determined 17 days after sowing for wild-type and sas1 plants cultivated in the greenhouse (Figure 5). The compost used to grow the plants contained ∼20 μmol Na+·g−1 dry weight, and the tap water used for irrigation contained Na+ at ∼1 mmol·L−1. Otherwise, no additional Na+ was added to the culture. Figure 5 shows that the amount of Na+ that accumulated in sas1 plants was four- to sixfold higher than that in wild-type plants of similar size. Thus, the Na+ overaccumulation in sas1 tissues was not the result of the size reduction of sas1 plants. Further analysis of the data presented in Figure 5 revealed that the sas1 plants accumulated between 171 and 324 μmol Na+·g−1 dry weight in shoots (average 275 ± 50), whereas wild-type plants accumulated ⩽152 μmol Na+·g−1 dry weight in shoots (average 65 ± 20). On average, sas1 plants accumulated fourfold more Na+ than did wild-type plants, and no overlap was observed between the values of shoot Na+ content for wild-type and sas1 plants. The difference between Na+ content in sas1 and wild-type shoots increased significantly as the plants aged (Table 1, section B).
Figure 5.
Relationship between Shoot Dry Weight and Na+ Amount in the Shoots.
Wild-type (WT) and mutant (sas1) plants were grown on compost in the greenhouse for 17 days. The shoot dry weight and the amount of Na+ accumulated in the shoots were measured for every plant (n = 48).
Table 1.
Na+ Accumulation in Calli, Detached Leaf Fragments, and Different Organs of Intact Plants
| Na+ Contentc
|
||||
|---|---|---|---|---|
| Tissuea | Number of Repeatsb | Wild Type | sas1 | Ratio of sas1 to Wild Type |
| A Calli | 5 | 265 ± 63 | 300 ± 100 | 1 |
| Leaf fragments | 6 | 1940 ± 190 | 1960 ± 220 | 1 |
| B Bud | 4 | 19.9 ± 4.5 | 187 ± 32 | 9.5 |
| Flower | 4 | 25.7 ± 5.1 | 325 ± 20 | 12.5 |
| Green silique | 4 | 21.0 ± 4.8 | 234 ± 37 | 11 |
| Stem | 4 | 90.8 ± 12.2 | 492 ± 48 | 5.5 |
| Senescing leaf | 4 | 71.7 ± 7.3 | 610 ± 48 | 8.5 |
| Expanding leaf | 4 | 14.2 ± 4.4 | 326 ± 24 | 23 |
| Hypocotyl | 4 | 66.4 ± 21.1 | 171 ± 37 | 2.5 |
| Root | 4 | 39.3 ± 6.1 | 45 ± 20 | 1 |
| C Xylem sap | 6 | 0.9 ± 0.25d | 5.1 ± 1.2d | 5.5 |
| D Shoot in hydroponic culture | 10 | 923 ± 75 | 2310 ± 330 | 2.5 |
| Root in hydroponic culture | 10 | 562 ± 22 | 186 ± 65 | 0.35 |
| E Shoot in vitro | 5 | 986 ± 67 | 2450 ± 140 | 2.5 |
| Root in vitro | 5 | 354 ± 31 | 262 ± 18 | 0.75 |
Six-week-old calli were cultivated for 6 days in liquid medium containing 35 mM NaCl. Leaf fragments collected from 4-week-old greenhouse-cultivated plants were incubated for 1 day in aerated 0.2 mM CaSO4 and 80 mM NaCl under light at 25°C. Plants cultivated in vitro or in hydroponic culture were grown for 12 or 16 days, respectively, on standard medium and then for an additional 6 days on standard medium supplemented with 35 mM NaCl. The other plant tissues and the xylem sap were collected from 6-week-old mature plants grown in the greenhouse.
Leaf fragments were analyzed individually. The other samples were collected from pools of at least six plants for greenhouse-cultivated plants and at least 20 plants for plants cultivated in vitro or hydroponically.
Na+ content expressed in μmol·g−1 dry weight ±sd.
Na+ concentration expressed in mmol·L−1.
Effect of the Transpiration Stream on Na+ Accumulation
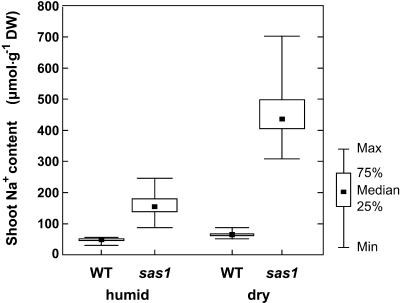
sas1 plants accumulated approximately twofold more Na+ in shoots than did wild-type plants when cultivated in vitro, whereas they accumulated fourfold more Na+ when cultivated in the greenhouse. To investigate whether this difference was due to different transpiration rates, we determined Na+ content in sas1 plants grown in the greenhouse under two different transpiring conditions: plants were cultivated for 3 weeks either in a renewed water-saturated atmosphere or in an atmosphere in which the humidity was maintained at an average of 45% (termed “dry atmosphere” below) (Figure 6). Both sas1 and wild-type plants accumulated more Na+ in shoots when cultivated in the dry atmosphere than when cultivated in the water-saturated atmosphere. However, wild-type plants accumulated only 40% more Na+ when cultivated in the dry atmosphere (67 ± 9 μmol·g−1 dry weight) than when cultivated in the water-saturated atmosphere (47 ± 7 μmol·g−1 dry weight), whereas sas1 plants accumulated nearly three times more Na+ when cultivated in the dry atmosphere (450 ± 90 μmol.g−1 dry weight) than when cultivated in the water-saturated atmosphere (160 ± 30 μmol·g−1 dry weight). sas1 plants markedly overaccumulated Na+ in shoots relative to wild-type plants regardless of the transpiring conditions, but the degree of the Na+ overaccumulation increased with the increase of the transpiration rates: sas1 plants accumulated 3.4-fold more Na+ than did wild-type plants when cultivated in the water-saturated atmosphere and 6.7-fold more Na+ than did wild-type plants when cultivated in the dry atmosphere.
Figure 6.
Effect of Humidity on Na+ Accumulation In Shoots.
sas1 and wild-type (WT) plants were grown in the greenhouse in either a water-saturated atmosphere (humid) or a 45% humidity atmosphere (dry) for 21 days. The shoot Na+ content of 30 plants was determined for each treatment. Data are presented in box plots: boxes encompass second and third quartiles of the population, bars represent minimum (Min) and maximum (Max) values, and filled squares represent median values. DW, dry weight.
The sas1 Mutation Specifically Increased Na+ Accumulation
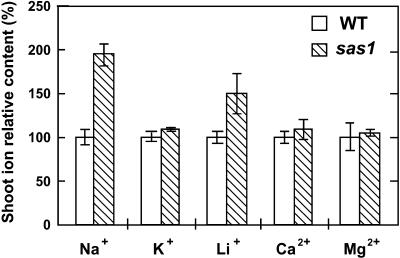
We investigated the overaccumulation of other cations in the shoots of sas1 plants in conditions in which the plants were cultivated on medium supplemented with these cations. Our study focused on the cationic macronutrients and on Li+, a toxic structural analog of Na+ (Mendoza et al., 1994). Plants were grown for 12 days on agar-solidified standard medium and then for an additional 6 days on standard medium supplemented with a chloride salt (KCl, NaCl, MgCl2, or CaCl2) at a final cation concentration of 35 mmol·L−1 or with LiCl at a final concentration of 2.5 mmol·L−1. For each treatment, the shoot content of the cation added to the culture medium was determined (Figure 7). sas1 overaccumulated Na+ and Li+ in shoots, whereas K+, Ca2+, and Mg2+ accumulated at similar levels in the two lines.
Figure 7.
Comparison of Na+, K+, Li+, Ca2+, and Mg2+ Accumulation in Shoots between sas1 and Wild Type.
Twelve-day-old plants grown in vitro were cultivated for 6 additional days on standard medium supplemented with NaCl (Na+), KCl (K+), CaCl2 (Ca2+), or MgCl2 (Mg2+) at a final concentration of 35 mmol·L−1 or with LiCl (Li+) at a final concentration of 2.5 mmol·L−1. For each treatment, the shoot content of the cation that was added to the standard culture medium was determined from a pool of 20 plants. Ion content in sas1 shoots was expressed as percentage of the wild-type value, which was standardized to 100. Wild-type ion contents were 1.6 ± 0.1 mmol·g−1 dry weight for Na+, 3.5 ± 0.3 mmol·g−1 dry weight for K+, 19.8 ± 1.2 μmol·g−1 dry weight for Li+, 0.7 ± 0.1 mmol·g−1 dry weight for Ca2+, and 0.8 ± 0.08 mmol·g−1 dry weight for Mg2+. The error bars indicate standard deviation; n = 6. WT, wild type.
To evaluate the influence of the counter anion on Na+ accumulation, Na+ shoot content was determined for plants cultivated on medium supplemented with various Na+ salts (NaCl, Na2SO4, NaNO3, or Na2CO3) at a final Na+ concentration of 35 mmol·L−1. The Na+ content in sas1 shoots was in the same range regardless of the accompanying anion of Na+, and it was always twofold higher than in wild-type shoots (data not shown).
Relationship between Na+ and K+ Accumulation in Shoots
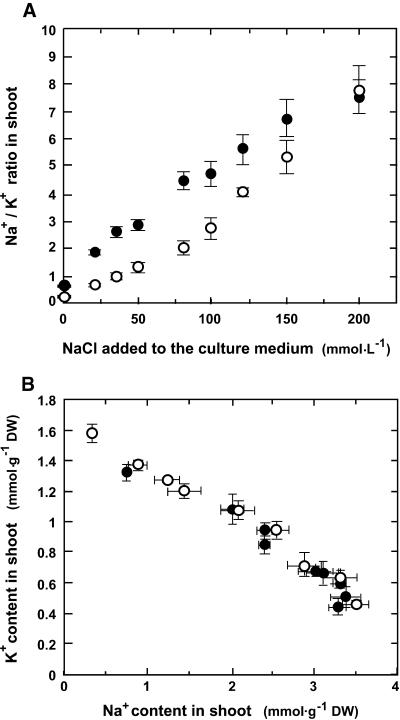
The relationship between Na+ and K+ contents in shoots was established for plants grown in vitro for 12 days on standard medium and for 6 additional days on the same medium supplemented with NaCl at 0, 20, 35, 50, 80, 100, 120, 150, or 200 mmol·L−1. Figure 8A shows that the Na+/K+ ratio in shoots depended on both the line considered and the Na+ concentration of the culture medium. The Na+/K+ ratio was always higher for sas1 plants than for wild-type plants, except when both lines were cultivated on the medium supplemented with the highest NaCl concentration (200 mmol·L−1). In the latter case, the Na+/K+ ratio was identical for both lines, which accumulated equivalent amounts of Na+ and K+ in their shoots. These results show that sas1 plants displayed a lesser degree of K+ versus Na+ selectivity at the whole-plant level.
Figure 8.
Relationship between Na+ and K+ Contents in Shoots.
(A) Ratios of shoot Na+ and K+ contents of sas1 and wild-type plants cultivated in vitro for 12 days on standard medium and for 6 additional days on standard medium supplemented with 0, 20, 35, 50, 80, 100, 120, 150, or 200 mM NaCl.
(B) Na+ and K+ contents in aerial tissues of sas1 and wild-type plants in the culture conditions described in (A). Each point corresponds to one of the NaCl treatments. DW, dry weight.
For each measurement, the Na+ and K+ contents were determined for the shoots of an average of 20 sas1 (filled circles) or wild-type (open circles) plants. The error bars indicate standard deviation; n = 6.
The data presented in Figure 8A were further analyzed to examine the relationship between Na+ and K+ contents in shoots (Figure 8B). This analysis showed that sas1 and wild-type plants accumulated Na+ and K+ roughly in the same ranges, and K+ and Na+ contents in aerial organs were not independent. Both lines adjusted their Na+ and K+ contents (expressed on a dry weight basis) according to a similar negative linear relationship: 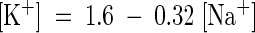 (
(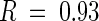 ), which demonstrated that there was a basic homeostatic relationship between Na+ and K+ such that the level of Na+ in the leaves apparently determined the amount of K+ that accumulated. The commonality of this relationship for both the mutant and the wild type shows that the sas1 mutation is not involved in control of the K+/Na+ balance in the aerial organs.
), which demonstrated that there was a basic homeostatic relationship between Na+ and K+ such that the level of Na+ in the leaves apparently determined the amount of K+ that accumulated. The commonality of this relationship for both the mutant and the wild type shows that the sas1 mutation is not involved in control of the K+/Na+ balance in the aerial organs.
Sodium Partitioning in the Plant
We investigated whether the Na+ overaccumulation observed in sas1 leaves was a cellular characteristic or the consequence of a perturbation in long-distance transport of Na+ in the plant. Calli induced from hypocotyls and leaf pieces collected from plants grown in the greenhouse were incubated in solutions containing NaCl at 0, 35, 50, or 80 mmol·L−1. Table 1, section A, reports Na+ content in calli and leaf fragments in one of the experiments performed with these materials. sas1 tissues accumulated Na+ at a level similar to that in the corresponding wild-type tissues over all durations and NaCl concentrations. These results suggest that sas1 is not impaired in a function involved in Na+ accumulation at the cellular level.
Sodium content was determined in different organs of mature 6-week-old plants cultivated on compost in the greenhouse and also in shoots and roots of plants cultivated in vitro or hydroponically (Table 1, sections B, D, and E). All aerial organs of sas1 plants accumulated significantly more Na+ than did their wild-type counterparts. In contrast and regardless of the culture conditions, there was no Na+ overaccumulation in the roots. Moreover, in both hydroponic and in vitro culture conditions that enabled harvest of complete and isolated root systems, Na+ contents in sas1 roots were 35 and 75%, respectively, of those in wild-type roots (Table 1, sections D and E). Thus, sas1 plants did not overaccumulate Na+ in all of their tissues, which further supports the hypothesis that the sas1 Na+ overaccumulation was not a cellular characteristic. Moreover, because leaf tissues overaccumulated Na+ only in intact plants and not in leaf fragments, we hypothesized that the sas1 mutation affected the long-distance transport of Na+.
Long-Distance Na+ Transport from the Roots to the Aerial Tissues
To determine whether Na+ overaccumulation in sas1 aerial organs could be the result of an alteration of the ascending Na+ stream, we first examined whether the transpiration stream was modified in sas1 plants. The transpiration stream value of sas1 plants expressed on a leaf area basis did not differ significantly between wild-type plants (49.5 ± 4.5 μL m−2·sec−1) and sas1 plants (45 ± 6 μL m−2·sec−1).
Interest was then focused on Na+ concentrations in the xylem sap of sas1 and wild-type plants. Table 1, section C, shows that the xylem sap of sas1 plants contained 5.5-fold more Na+ than the xylem sap of wild-type plants. The K+ concentration of the xylem sap of sas1 plants (13.9 ± 3.1 mmol·L−1) was slightly but not significantly lower (P > 0.1, 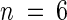 ) than that of wild-type plants (16.4 ± 2.1 mmol·L−1).
) than that of wild-type plants (16.4 ± 2.1 mmol·L−1).
Genetic Characterization and Map Position of sas1
We measured the leaf Na+ concentration of plants cultivated in the greenhouse to determine the phenotype of 176 plants of the selfed F2 population from a cross between sas1 and wild-type Landsberg erecta. The wild-type:mutant ratio (142:34) fitted a 3:1 segregation ratio (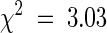 , P > 0.05), which indicated that the Sas− phenotype was caused by a single recessive nuclear mutation. The dwarf phenotype was independently checked among 255 plants of the same F2 population. The nondwarf:dwarf ratio (196:59) fitted a 3:1 segregation ratio (
, P > 0.05), which indicated that the Sas− phenotype was caused by a single recessive nuclear mutation. The dwarf phenotype was independently checked among 255 plants of the same F2 population. The nondwarf:dwarf ratio (196:59) fitted a 3:1 segregation ratio ( , P > 0.05), which indicated that the dwarf phenotype was caused by a single recessive nuclear mutation. To determine whether the dwarf phenotype cosegregates with the sas1 mutation, we analyzed 483 plants from the F2 population described above. All of the plants that displayed the Sas− phenotype also displayed the dwarf phenotype, which indicates that both characters are genetically colocalized. If the locus responsible for the dwarf phenotype is different from sas1, it is <0.4 centimorgans (cM) apart from sas1.
, P > 0.05), which indicated that the dwarf phenotype was caused by a single recessive nuclear mutation. To determine whether the dwarf phenotype cosegregates with the sas1 mutation, we analyzed 483 plants from the F2 population described above. All of the plants that displayed the Sas− phenotype also displayed the dwarf phenotype, which indicates that both characters are genetically colocalized. If the locus responsible for the dwarf phenotype is different from sas1, it is <0.4 centimorgans (cM) apart from sas1.
The map position of sas1 was determined by use of microsatellite (Bell and Ecker, 1994) and cleaved amplified polymorphic sequence (Konieczny and Ausubel, 1993) markers to analyze a selfed F2 population from the cross between a homozygous sas1 plant and a wild-type plant of ecotype Columbia. Forty-nine plants of the Sas− phenotype recovered from the cross were analyzed with 10 markers covering the entire genome. Linkage between sas1 and the markers GL1 and nga6 was established. No linkage was found with any of the other markers. Thus, sas1 was mapped to the bottom of chromosome III. The accurate map position of sas1 was determined by analyzing 78 plants of the Sas− phenotype recovered from the same cross with the markers GL1, PRC6(a), AFC1, and nga6, which cover the region at the bottom of chromosome III. sas1 mapped 2.6 cM north of nga6 and 0.6 cM south of AFC1.
DISCUSSION
sas1 Is Impaired in a Function That Controls Na+ Accumulation in Shoots
We report the isolation and characterization of a mutant (sas1) that is impaired in a function controlling Na+ accumulation in shoots. sas1 overaccumulated Na+ when cultivated on medium containing Na+ at concentrations ranging from 1 to 150 mmol·L−1. Because sas1 overaccumulated Na+ even when cultivated on medium containing millimolar concentrations of Na+, the process that controls Na+ accumulation, which is impaired in the sas1 mutant, is probably not induced by salt stress. sas1 mutants also overaccumulated Li+, a Na+ toxic structural analog, but did not overaccumulate K+, Ca2+, or Mg2+, indicating that the sas1 locus spe-cifically controls Na+ accumulation. These phenotypic characteristics identify sas1 as a new mutant type, particularly compared with the three salt overly sensitive (sos) mutants, which highlight the critical role for K+ nutrition in salt stress conditions (Wu et al., 1996; Liu and Zhu, 1997, 1998; Zhu et al., 1998).
sas1 Is a Pleiotropic Mutation That Also Alters Growth
sas1 plants displayed a small stature that was a conditional phenotype: sas1 shoots were markedly smaller than wild-type shoots when grown in the greenhouse or hydroponically, whereas they were slightly larger when grown in vitro. Surprisingly, this characteristic was observed whether NaCl was added to the culture medium or not. In contrast to shoots, sas1 roots displayed reduced growth compared with wild-type roots regardless of the culture conditions, which suggests that the reduction in root size is an intrinsic characteristic of sas1 plants. The small stature of sas1 plants was a result of neither salt stress nor Na+ overaccumulation, because sas1 plants grown in hydroponic culture on a Na+-free medium were still smaller than wild-type plants. Additional experiments are needed to identify the origin of the reduction in the size of sas1 plants and to determine the reason why this reduction is dependent on the culture conditions. Genetic analyses showed that the small stature of sas1 plants was determined by a single recessive locus that could not be separated from the sas1 locus. This phenotypic characteristic was then considered to be another feature of the sas1 mutation, which suggests that sas1 is a pleiotropic mutation.
Shoot Na+ Overaccumulation Is Due to an Alteration of the Root Radial Transport of Na+
The simplest hypothesis for why sas1 plants overaccumulate Na+ is that the increase of Na+ content in sas1 tissues is the result of a limited Na+ dilution by growth due to the size reduction of sas1 organs. However, this hypothesis cannot explain the Sas− phenotype, because sas1 plants grown in vitro were larger than wild-type plants and still overaccumulated Na+. Furthermore, greenhouse-cultivated sas1 and wild-type plants of similar size accumulated Na+ at a ratio of between 4:1 and 6:1 (Figure 5). Thus, Na+ overaccumulation is an intrinsic characteristic of sas1 plants. In this respect, sas1 mutants behave in a different manner than dwarf lines of rice (Yeo and Flowers, 1986; Yeo et al., 1990) or Glycine tomentella (Wilson et al., 1970), which overaccumulated Na+ and Cl−, respectively, compared with nondwarf or more vigorous lines.
Detailed analysis of Na+ accumulation in different organs of sas1 plants or under different culture conditions, including the culture of calli (Table 1), led to the conclusion that long-range Na+ transport was modified in sas1 mutants. Mass flow due to transpiration plays a major role in translocating Na+ to the aerial organs. One important feature of the sas1 mutant is the strong dependence of Na+ overaccumulation on air humidity (Figure 6) and hence on the transpiration stream. Also, sas1 plants accumulated up to sevenfold more Na+ than did wild-type plants in their shoots when cultivated on compost in the greenhouse, whereas they accumulated only two times more Na+ than did wild-type plants when cultivated in vitro. The transpiration stream of sas1 plants, however, was of a magnitude similar to that of wild-type plants. If we assume that this characteristic is valid during the entire life of the plant, then the Sas− phenotype cannot be attributed to an alteration of the transpiration stream in sas1 plants. The fact that Na+ was overaccumulated in the xylem sap of sas1 plants (Table 1, section C) leads us to conclude that the origin of the deregulation of the ascending stream of Na+ should be investigated as a root function that controls at least one step of the Na+ radial transport from the soil solution to the root xylem vessels.
From the data presented in Figure 5, one can calculate that the amount of Na+ accumulated in the shoot of a mutant plant was on average twice as high as the amount accumulated in the shoot of a wild-type plant during the same period. We estimated that the roots of sas1 plants cultivated on compost were five to 10 times smaller than wild-type roots, suggesting that both net Na+ uptake by the root cells and net Na+ xylem loading were significantly greater in sas1 plants than in wild-type plants. One cannot exclude the possibility that the sas1 mutation may have a direct impact on both net Na+ uptake into root cells and net Na+ xylem loading. However, our hypothesis is that the sas1 mutation directly causes an increase of net Na+ xylem loading, which in turn induces a general increase of Na+ transport across the root by a mechanism that remains to be elucidated and thus results in an increase of the net Na+ uptake by root cells. This hypothesis is supported by the fact that sas1 plants simultaneously accumulated less Na+ in their roots and more Na+ in their xylem sap than did wild-type plants (Table 1). Such a result is expected from alteration of a function that blocks or limits net Na+ xylem loading. Mechanisms involved in the control of Na+ xylem loading have been identified in other species. In wheat, a K+/Na+ discrimination factor limiting Na+ translocation to the shoot for the benefit of K+ loading operates at the xylem uptake step (Gorham et al., 1990). More detailed data are available on soybean, in which Na+ has been shown to be removed from the xylem vessels and exchanged for K+ at the xylem parenchyma (Läuchli, 1976; Lacan and Durand, 1996). Xylem loading has been shown to be a key step in the control of ion accumulation in the shoot, as shown for phosphate (Poirier et al., 1991) and potassium (Gaymard et al., 1998).
Sodium can also be transported from the soil solution to the xylem vessels wholly through the extracellular apoplasmic pathway (Pitman, 1982). Free extracellular diffusion of the soil solution to the xylem vessels is normally blocked by the Casparian Strip at the differentiated root endodermis. However, soil solution can reach the xylem vessels by the apoplasmic pathway at the root tip before the endodermis is fully differentiated or at the sites at which secondary root emergence causes temporary holes in the endodermal apoplasmic barrier (Pitman, 1982). Soil solution that flows to the xylem through the apoplasmic pathway contributes <1% of the xylem sap volume (Perry and Greenway, 1973; Hanson et al., 1985; Skinner and Radin, 1994), except for in rice, in which it can contribute up to 5.5% (Garcia et al., 1997). Nevertheless, the apoplasmic stream can contribute significantly to the ion concentration of the xylem sap because there is no barrier for the ions conducted in this stream. Two rice cultivars that accumulated different amounts of Na+ in shoots differed in the proportion of the soil solution that reached the xylem vessels by the apoplasmic pathway (Yeo et al., 1987; Yadav et al., 1996). Then, an alternative explanation for the overloading of Na+ in the xylem sap of the sas1 mutant could be an increase in the apoplasmic transport from the soil solution to the xylem vessels. This could be the result of gross anatomical changes in the root. In this case, a general increase of ion accumulation in sas1 shoots should be expected, particularly for Ca2+, which is supposed to be mainly transported across the root to the stele by the apoplasmic pathway (Harrison-Murray and Clarkson, 1973; Pitman, 1982). Our evidence does not favor this hypothesis because sas1 plants did not overaccumulate Ca2+ in the shoots (Figure 7).
The fine mechanisms by which control of net Na+ xylem loading is altered in sas1 plants remain to be elucidated.
sas1 Plants Display Increased Salt Sensitivity Compared with Wild-Type Plants
Surprisingly, the impact of NaCl stress on growth was similar for sas1 and wild-type plants (Figures 2B, 3A, and 3B), despite the great and contrasted differences in Na+ accumulation in shoots and roots between the two lines (Table 1). Among the glycophytic species, intervarietal salt tolerance, expressed as growth performance upon salt stress, is well correlated to the K+/Na+ ratio: the higher the leaf K+/Na+ ratio, the greater the plant salt tolerance (Volkmar et al., 1998). It is worth noting that in the experimental conditions we explored, sas1 plants displayed a significantly lower K+/Na+ ratio than did wild-type plants, but their relative growth reduction in response to salt stress was similar to that of wild-type plants. This result might be related to the fact that the sas1 mutation is not involved in control of the K+/Na+ balance in the shoot (Figure 8B).
In contrast, with regard to survival, sas1 plants displayed increased sensitivity to NaCl or LiCl treatments compared with wild-type plants (Figures 2A, 3C, and 4). Salt-induced leaf death is considered to be the consequence of salt accumulation in the cytoplasm or in the apoplast compartments (Munns, 1993); this accumulation occurs when the vacuolar capacity for Na+ storage is saturated or when the rate of Na+ export from the roots to the leaves exceeds the rate of Na+ delivery across the plasma membrane of leaf cells and/or Na+ compartmentation into vacuoles. sas1 hypersensitivity to salt stress could not be explained by saturation of the storage capacity for Na+ in sas1 leaves. Indeed, the shoot Na+ content of very healthy sas1 plants cultivated for 2 weeks on medium supplemented with 35 mM NaCl was 10 to 17% greater than the Na+ content of dying sas1 or wild-type plants cultivated for 6 days on medium supplemented with 200 mM NaCl (data not shown). Therefore, the kinetics of Na+ accumulation in sas1 aerial tissues, rather than the Na+ content itself, is likely to explain sas1 hypersensitivity to salt stress. This analysis is strengthened by the observation that the sas1 hypersensitivity to salt stress shown in Figure 2A was detectable only when plants transpired a lot (i.e., when more Na+ was imported in leaves in a given period). In conclusion, our results suggest that Arabidopsis possesses a mechanism of Na+ detoxification in leaves, such as Na+ compartmentation in the vacuole, that is efficient even when large amounts of Na+ are imported into the leaf but that this detoxification system can be overloaded when the rate of Na+ import into the leaf becomes too high.
Sodium transport from the leaf apoplast to the vacuole involves two transport events, first through the cell plasma membrane and then through the tonoplast. In Arabidopsis, Na+ transport across the tonoplast into the vacuole is a limiting step for salt tolerance (Apse et al., 1999); improving the efficiency of this transport had a positive impact on growth upon salt stress, as expected for a mechanism that decreases the cytoplasmic Na+ concentration. If Na+ transport across the tonoplast into the leaf vacuole was the step that limited the compartmentation of the excess Na+ translocated from the root in sas1 plants, we would expect that Na+ would be overaccumulated in the cytoplasmic compartment, which in turn would have a strong negative impact on growth. Because the impact on growth is similar for sas1 and wild-type plants in response to salt stress, we favor the hypothesis that the salt accumulation that causes leaf death in sas1 plants occurs in the apoplast. This suggests that, in terms of kinetics, the limiting step of Na+ transport from the apoplast to the vacuole in Arabidopsis leaves would be the net Na+ transport across the plasmalemma into leaf cells.
In conclusion, the sas1 mutant highlights a key point in the control mechanism of Na+ accumulation. This mutant is impaired in a root function that regulates Na+ radial transport in the root, most likely net Na+ xylem loading. Because this mutant displays some degree of salt sensitivity, it should serve as an invaluable tool for understanding salt tolerance mechanisms as well as the mechanisms that control Na+ invasion in tissues.
METHODS
Plant Materials
M2 seed derived from Arabidopsis thaliana ecotype Landsberg erecta after mutagenesis with ethyl methanesulfonate was purchased from Lehle Seeds (Round Rock, TX) under the reference M2E-4-2. Wild-type Arabidopsis seed of ecotypes Landsberg erecta and Columbia were obtained from the Nottingham Arabidopsis Stock Center (Nottingham, UK) under the references NW20 and N907, respectively.
Growing Conditions
For in vitro culture, seed was surface sterilized by soaking in a solution containing 1 to 4% (w/v) Bayrochlor (Indusco France, Gargenville, France) in 50% ethanol for 20 min under strong agitation, rinsed three times in 100% ethanol, and dried under a sterile air flow. Seed was then sown on standard 0.8% agar medium containing 5 mM KNO3, 5 mM Ca(NO3)2, 1 mM NaH2PO4, 1 mM MgSO4, 0.1 mM FeNaEDTA, 50 μM H3BO3, 50 μM MnSO4, 15 μM ZnSO4, 3 μM Na2MoO4, 2.5 μM KI, 50 nM CuSO4, 44 nM CoCl2, 3.5 mM Mes-KOH, pH 5.8, and 1% sucrose. When required, salt treatments were applied as follows: 12-day-old plants cultivated on agar-solidified standard medium were transferred for an additional 6 days to standard medium supplemented with NaCl, LiCl, or other salts at the appropriate concentration. The in vitro growth conditions were as follows: 16-hr photoperiod, 130 to 150 μE m−2·sec−1, and 21°C day/18°C night.
Calli were induced in vitro as described (Wu et al., 1996). For Na+ accumulation experiments, calli were transferred into liquid Murashige and Skoog (1962) medium with vitamins (reference M5519; Sigma), 2% (w/v) sucrose, 2.25 μmol·L−1 2,4-D, and 0.46 μmol·L−1 kinetin in flasks and shaken at 200 rpm at 25°C in the dark.
For hydroponic culture, seeds were sown on sand laid on a mesh floating on aerated deionized water. After cotyledons appeared, the culture system was transferred to the following Na+-free aerated solution: 1 mM KNO3, 0.5 mM Ca(NO3)2, 1 mM KH2PO4, 1 mM MgSO4, 50 μM Fe(NH4)EDTA, 50 μM H3BO3, 50 μM MnSO4, 15 μM ZnSO4, 2.5 μM KI, 0.4 μM (NH4)6Mo7O24, 50 nM CuSO4, and 44 nM CoCl2. NaCl was added when required. Growth-room culture conditions were as follows: 8-hr photoperiod, 21°C day/18°C night, and 60 to 80% humidity.
In the greenhouse, plants were grown on compost (Neuhaus Humin Substrat N2; Klasman-Deilmann GmbH, Geeste, Germany) and subirrigated with tap water. Greenhouse culture conditions were as follows: 16-hr photoperiod, 25°C day/21°C night, and sunlight power limited to 300 W·m−2. Humidity was not regulated in the greenhouse. For the experiment in which the humidity was controlled, plants were grown in transparent cabinets containing an air volume of 13.5 liters. An atmosphere in which the humidity was close to 100% was obtained in the first cabinet by injection of air that had bubbled in water, whereas air that had been fully desiccated on a silica gel column was injected into the second cabinet. In this cabinet, the combination of water evaporation from the compost and dry air flow led to an average humidity of 45%. In both cabinets, air was injected at a flow rate of ∼7 L·min−1.
Determination of Ion Contents
Plant material was weighed (for fresh weight determination) or dried for at least 24 hr at 80°C and then weighed (for dry weight determination). Plant material was then incubated in 0.1 N sulfuric acid at 50°C for 20 min for ion extraction. Cation concentrations in the extracts were determined by flame photometry (flame photometer 243 and aa/ae spectrophotometer 151; Instrumentation Laboratory Inc., Wilmington, MA).
Measurements of the Transpiration Stream
Plants were grown for 4 weeks on compost in the greenhouse. The entire plant was then carefully removed from the pot with the roots intact within the compost. The compost was rinsed away from the roots by placing them in water and shaking them gently. Plants were then transferred in plastic tubes filled with water. Parafilm was used to seal the tube around the hypocotyl to avoid direct water evaporation. The loss of water through transpiration was estimated by weighing the tube bearing the plant five times during a period of 3 hr and 20 min, which started at 30 min before noon. The leaf areas of the plants were determined by planimetry. The transpiration stream was expressed as liters of water per square meter of leaf per second.
Collection of Xylem Sap
Xylem sap was collected as root exudate after decapitation of the shoots of mature 6-week-old greenhouse-cultivated plants (Schurr, 1998). Plants were decapitated below the rosette. The first 2- to 3-μL drop was discarded to prevent contamination of the xylem sap with contents from damaged cells or phloem sap. The drops that emerged within the first couple of minutes were collected.
Genetic Analyses
The mutant was used as the male parent in the genetic crosses. For mapping analysis, we used the previously described cleaved amplified polymorphic sequence markers GapC, GL1, ASA1, NCC1, GPA1, DFR, and g8300 (Konieczny and Ausubel, 1993; Cherry et al., 1998) and the microsatellite markers nga6, nga8, and nga280 (Bell and Ecker, 1994). We also designed a new cleaved amplified polymorphic sequence marker for this analysis from the marker PRC6(a) (accession number X66825) located at the bottom of chromosome III between GL1 and nga6 (Camilleri et al., 1998). We amplified a 2-kb fragment with the primers GAAAAAGGTAAAAGAATGGCGAG and ACGGTAAGTTACACAATGAGA and digested it with SstI. The Landsberg erecta–amplified product remained undigested, whereas the Columbia-amplified product was cut into two fragments of 0.6 and 1.4 kb. Mapping data were analyzed with Mapmaker software (Lander et al., 1987), and genetic distances were determined using the Kosambi function.
Acknowledgments
We thank S. Joulié, C. Soleilhavoup, C. Boiteau, M.J. Battut, O. Specty, M. Salaün, L. Décousset, H. Baudot, and S. Gélin for technical help and J.C. Davidian and H. Sentenac for helpful discussions. A.N. and J.D. were supported financially by the French Ministry of Education and Research.
References
- Abel, G.H. (1969). Inheritance of the capacity for chloride inclusion and chloride exclusion by soybeans. Crop Sci. 9 697–698. [Google Scholar]
- Amtmann, A., and Sanders, D. (1999). Mechanisms of Na+ uptake by plant cells. Adv. Bot. Rev. 29 75–112. [Google Scholar]
- Apse, M.P., Aharon, G.S., Snedden, W.A., and Blumwald, E. (1999). Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285 1256–1258. [DOI] [PubMed] [Google Scholar]
- Bell, C.J., and Ecker, J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19 137–144. [DOI] [PubMed] [Google Scholar]
- Blumwald, E., and Poole, R.J. (1985). Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 78 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, J.S. (1982). Plant productivity and environment. Science 218 443–448. [DOI] [PubMed] [Google Scholar]
- Camilleri, C., Lafleuriel, J., Macadre, C., Varoquaux, F., Parmentier, Y., Picard, G., Caboche, M., and Bouchez, D. (1998). A YAC contig map of Arabidopsis thaliana chromosome 3. Plant J. 14 633–642. [DOI] [PubMed] [Google Scholar]
- Cherry, J.M., Flanders, D.J., Garcia-Hernandez, M., Huala, E., Rhee, S.Y., and Weng, S. (1998). AtDB: An Arabidopsis thaliana database. http://genome-www.stanford.edu/Arabidopsis/.
- Epstein, E., Norlyn, J.D., Rush, D.W., Kingsbury, R.W., Kelley, D.B., Cunningham, G.A., and Wrona, A.F. (1980). Saline culture of crops: A genetic approach. Science 210 399–404. [DOI] [PubMed] [Google Scholar]
- Garbarino, J., and DuPont, F.M. (1988). NaCl induces a Na+/H+ antiport in tonoplast vesicles from barley roots. Plant Physiol. 86 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, A., Rizzo, C.A., Ud-Din, J., Bartos, S.L., Senadhira, D., Flowers, T.J., and Yeo, A.R. (1997). Sodium and potassium transport to the xylem are inherited independently in rice, and the mechanism of sodium:potassium selectivity differs between rice and wheat. Plant Cell Environ. 20 1167–1174. [Google Scholar]
- Gaymard, F., Pilot, G., Lacombe, B., Bouchez, D., Bruneau, D., Boucherez, J., Michaux-Ferrière, N., Thibaud, J.-B., and Sentenac, H. (1998). Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94 647–655. [DOI] [PubMed] [Google Scholar]
- Gorham, J., Wyn Jones, R.G., and Bristol, A. (1990). Partial characterization of the trait for enhanced K+-Na+ discrimination in the D genome of wheat. Planta 180 590–597. [DOI] [PubMed] [Google Scholar]
- Greenway, H., and Munns, R. (1980). Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 31 149–190. [Google Scholar]
- Hanson, P.J., Sucoff, E.I., and Markhart III, A.H. (1985). Quantifying apoplastic flux through red pine root systems using trisodium, 3-hydroxy-5,8,10-pyrenetrisulfonate. Plant Physiol. 77 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Murray, R.S., and Clarkson, D.T. (1973). Relationships between structural development and the absorption of ions by the root system of Cucurbita pepo. Planta 114 1–16. [DOI] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F.M. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4 403–410. [DOI] [PubMed] [Google Scholar]
- Kueh, J.S.H., and Bright, S.W.J. (1982). Biochemical and genetical analysis of three proline-accumulating barley mutants. Plant Sci. Lett. 27 233–241. [Google Scholar]
- Lacan, D., and Durand, M. (1996). Na+-K+ exchange at the xylem/symplast boundary. Plant Physiol. 110 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander, E.S., Green, P., Abrahamson, J., Barlow, A., Daly, M.J., Lincoln, S.E., and Newburg, L. (1987). MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1 174–181. [DOI] [PubMed] [Google Scholar]
- Läuchli, A. (1976). Symplasmic transport and ion release to the xylem. In Transport and Transfer Processes in Plants, I.F. Wardlaw and J.B. Passioura, eds (New York: Academic Press), pp. 101–112.
- Liu, J., and Zhu, J.-K. (1997). An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl. Acad. Sci. USA 97 14960–14964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., and Zhu, J.-K. (1998). A calcium sensor homolog required for plant salt tolerance. Science 280 1943–1945. [DOI] [PubMed] [Google Scholar]
- Mendoza, I., Rubio, F., Rodriguez-Navarro, A., and Pardo, J.M. (1994). The protein phosphatase calcineurin is essential for NaCl tolerance to Saccharomyces cerevisiae. J. Biol. Chem. 269 8792–8796. [PubMed] [Google Scholar]
- Munns, R. (1993). Physiological processes limiting plant growth in saline soil: Some dogmas and hypotheses. Plant Cell Environ. 16 15–24. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15 473–497. [Google Scholar]
- Niu, X., Bressan, R.A., Hasegawa, P.M., and Pardo, J.M. (1995). Ion homeostasis in NaCl stress environments. Plant Physiol. 109 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, M.W., and Greenway, H. (1973). Permeation of uncharged organic molecules and water through tomato roots. Ann. Bot. 37 225–232. [Google Scholar]
- Pitman, M.G. (1982). Transport across plant roots. Q. Rev. Biophys. 15 481–554. [DOI] [PubMed] [Google Scholar]
- Poirier, Y., Thoma, S., Somerville, C., and Schiefelbein, J. (1991). A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 97 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio, F., Gassmann, W., and Schroeder, J.I. (1995). Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270 1660–1663. [DOI] [PubMed] [Google Scholar]
- Saleki, R., Young, P.G., and Lefebvre, D.D. (1993). Mutants of Arabidopsis thaliana capable of germination under saline conditions. Plant Physiol. 101 839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr, U. (1998). Xylem sap sampling: New approaches to an old topic. Trends Plant Sci. 3 293–298. [Google Scholar]
- Skinner, R.H., and Radin, J.W. (1994). The effect of phosphorus nutrition on water flow through the apoplastic bypass in cotton roots. J. Exp. Bot. 45 423–428. [Google Scholar]
- Uozumi, N., Kim, E.K., Rubio, F., Yamagushi, T., Muto, S., Tsuboi, A., Bakker, E.P., Nakamura, T., and Schroeder, J.I. (2000). The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 122 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar, K.M., Hu, Y., and Steppuhn, H. (1998). Physiological responses of plants to salinity: A review. Can. J. Plant Sci. 78 19–27. [Google Scholar]
- Walker, N.A., Sanders, D., and Maathuis, F.J.M. (1996). High affinity potassium uptake in plants. Science 273 977–979. [DOI] [PubMed] [Google Scholar]
- Wilson, J.R., Haydock, K.P., and Robins, M.F. (1970). The development in time of stress effects in two species of Glycine differing in sensitivity to salt. Aust. J. Biol. Sci. 23 537–551. [Google Scholar]
- Wu, S.J., Ding, L., and Zhu, J.K. (1996). SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, R., Flowers, T.J., and Yeo, A.R. (1996). The involvement of the transpirational bypass flow in sodium uptake by high- and low-sodium-transporting lines of rice developed through intravarietal selection. Plant Cell Environ. 19 329–336. [Google Scholar]
- Yeo, A. (1998). Molecular biology of salt tolerance in the context of whole-plant physiology. J. Exp. Bot. 49 915–929. [Google Scholar]
- Yeo, A.R., and Flowers, T.J. (1986). Salinity resistance in rice (Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils. Aust. J. Plant Physiol. 13 161–173. [Google Scholar]
- Yeo, A.R., Yeo, M.E., and Flowers, T.J. (1987). The contribution of an apoplastic pathway to sodium uptake by rice roots in saline condition. J. Exp. Bot. 38 1141–1153. [Google Scholar]
- Yeo, A.R., Yeo, M.E., Flowers, S.A., and Flowers, T.J. (1990). Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theor. Appl. Genet. 79 377–384. [DOI] [PubMed] [Google Scholar]
- Zhu, J.-K., Liu, J., and Xiong, L. (1998). Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell 10 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]