Abstract
Background
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are the most frequently prescribed medications worldwide and are widely used for patients with low‐back pain. Selective COX‐2 inhibitors are currently available and used for patients with low‐back pain.
Objectives
The objective was to assess the effects of NSAIDs and COX‐2 inhibitors in the treatment of non‐specific low‐back pain and to assess which type of NSAID is most effective.
Search methods
We searched the MEDLINE and EMBASE databases and the Cochrane Central Register of Controlled Trials up to and including June 2007 if reported in English, Dutch or German. We also screened references given in relevant reviews and identified trials.
Selection criteria
Randomised trials and double‐blind controlled trials of NSAIDs in non‐specific low‐back pain with or without sciatica were included.
Data collection and analysis
Two review authors independently extracted data and assessed methodological quality. All studies were also assessed on clinical relevance, from which no further interpretations or conclusions were drawn. If data were considered clinically homogeneous, a meta‐analysis was performed. If data were lacking for clinically homogeneous trials, a qualitative analysis was performed using a rating system with four levels of evidence (strong, moderate, limited, no evidence).
Main results
In total, 65 trials (total number of patients = 11,237) were included in this review. Twenty‐eight trials (42%) were considered high quality. Statistically significant effects were found in favour of NSAIDs compared to placebo, but at the cost of statistically significant more side effects. There is moderate evidence that NSAIDs are not more effective than paracetamol for acute low‐back pain, but paracetamol had fewer side effects. There is moderate evidence that NSAIDs are not more effective than other drugs for acute low‐back pain. There is strong evidence that various types of NSAIDs, including COX‐2 NSAIDs, are equally effective for acute low‐back pain. COX‐2 NSAIDs had statistically significantly fewer side‐effects than traditional NSAIDs.
Authors' conclusions
The evidence from the 65 trials included in this review suggests that NSAIDs are effective for short‐term symptomatic relief in patients with acute and chronic low‐back pain without sciatica. However, effect sizes are small. Furthermore, there does not seem to be a specific type of NSAID which is clearly more effective than others. The selective COX‐2 inhibitors showed fewer side effects compared to traditional NSAIDs in the RCTs included in this review. However, recent studies have shown that COX‐2 inhibitors are associated with increased cardiovascular risks in specific patient populations.
Plain language summary
Non‐steroidal anti‐inflammatory drugs for low‐back pain
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are the most frequently prescribed medications worldwide and are commonly used for treating low‐back pain. This review found 65 studies (including over 11,000 patients) of mixed methodological quality that compared various NSAIDs with placebo (an inactive substance that has no treatment value), other drugs, other therapies and with other NSAIDs. The review authors conclude that NSAIDs are slightly effective for short‐term symptomatic relief in patients with acute and chronic low‐back pain without sciatica (pain and tingling radiating down the leg). In patients with acute sciatica, no difference in effect between NSAIDs and placebo was found.
The review authors also found that NSAIDs are not more effective than other drugs (paracetamol/acetaminophen, narcotic analgesics, and muscle relaxants). Placebo and paracetamol/acetaminophen had fewer side effects than NSAIDs, though the latter has fewer side effects than muscle relaxants and narcotic analgesics. The new COX‐2 NSAIDs do not seem to be more effective than traditional NSAIDs, but are associated with fewer side effects, particularly stomach ulcers. However, other literature has shown that some COX‐2 NSAIDs are associated with increased cardiovascular risk.
The review noted a number of limitations in the studies. Only 42% of the studies were considered to be of high quality. Many of the studies had small numbers of patients, which limits the ability to detect differences between the NSAID and the control group. There are few data on long term results and long‐term side effects.
Background
Low‐back pain is a major health problem among populations in western industrialized countries and a major cause of medical expenses, absenteeism and disablement (Deyo 1991; van Tulder 1995). Although low‐back pain is usually a benign and self‐limiting disease which tends to improve spontaneously over time (Waddell 1987), a wide variety of therapeutic interventions is available for treatment (Deyo 1987; Spitzer 1987; van Tulder 1997). However, the effectiveness of most of these interventions has not yet been demonstrated beyond doubt and consequently, the therapeutic management of low‐back pain varies widely. A major challenge for researchers is to provide evidence of which treatment, if any, is of most benefit for (sub‐groups of) patients with low‐back pain.
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are the most frequently prescribed medications worldwide and are widely used for patients with low‐back pain. The rationale for the treatment of low‐back pain with NSAIDs is based both on their analgesic potential and their anti‐inflammatory action.
Guidelines for the management of low‐back pain in primary care have been published in various countries around the world (Airaksinen 2006; Koes 2001; Koes 2006; van Tulder 2006). All these guidelines recommend the prescription of NSAIDs as one option for symptomatic relief in the management of low‐back pain. In most guidelines, NSAIDs are recommended as a treatment option after paracetamol has been tried. In addition to symptomatic relief, facilitating early return to normal activities is a goal of NSAID therapy.
In recent years, the selective cyclooxygenase‐2 (COX‐2) inhibiting NSAIDs have become available as an alternative to traditional NSAIDs. The putative advantage of the COX‐2 inhibitors is lower risk of gastro‐intestinal side effects as compared with the traditional NSAIDs. However, recently there has been substantial debate on their cardiovascular safety. A few randomised clinical trials have evaluated the efficacy of selective COX‐2 inhibitors for low‐back pain and will be included in this updated systematic review.
Objectives
The objective of this systematic review was to determine if NSAIDs are more efficacious than various comparison treatments for non‐specific low‐back pain and if so, which type of NSAID is most efficacious.
Comparisons of NSAIDs with reference treatments that were investigated are NSAIDs versus placebo, NSAIDs versus acetaminophen/paracetamol, NSAIDs versus other drugs (e.g. narcotic analgesics or muscle relaxants), NSAIDs versus NSAIDs (e.g. traditional NSAIDs versus selective COX‐2 inhibitors), NSAIDs versus NSAIDs plus muscle relaxant, NSAIDs versus NSAIDs plus B vitamins, and NSAIDs versus non‐drug treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials (double‐blind, single‐blind and open‐label) and double‐blind controlled trials were included.
Types of participants
Subjects age 18 years or older, treated for non‐specific low‐back pain with or without sciatica, were included. Subjects with specific low‐back pain caused by pathological entities such as infection, neoplasm, metastasis, osteoporosis, rheumatoid arthritis, or fractures were excluded. Both acute (12 weeks or less) and chronic (more than 12 weeks) low‐back pain patients were included.
Types of interventions
One or more types of NSAIDs were included. Additional interventions were allowed if there was a contrast for NSAIDs in the study. For example, studies comparing NSAIDs plus muscle relaxants versus muscle relaxants alone were included, while studies comparing NSAIDs plus muscle relaxants versus paracetamol were not.
Types of outcome measures
Primary outcome measures were (in hierarchical order): 1) pain intensity (e.g. Visual Analog Scale or Numerical Rating Scale), 2) global measure (e.g. overall improvement, proportion of patients recovered), 3) back pain‐specific functional status (e.g. Roland Disability Questionnaire, Oswestry Scale), 4) return to work (e.g. return to work status, number of days off work), and 5) side effects (proportion of patients experiencing side effects). Physiological outcomes (e.g. range of motion, spinal flexibility, degrees of straight leg raising or muscle strength) and generic functional status (e.g. SF‐36, Nottingham Health Profile, Sickness Impact Profile) were considered secondary outcomes. Other symptoms such as health care consumption were also considered.
Search methods for identification of studies
All relevant RCTs meeting our inclusion criteria were identified by: A) a computer‐aided search of the MEDLINE (from 1966 to June 2007) and EMBASE (from 1988 to June 2007) databases using the search strategy recommended by the Cochrane Back Review Group (van Tulder 2003). RCTs published in English, Dutch and German were included because the review authors who conducted the methodological quality assessment and data extraction were able to read papers published in these languages. B) a search in CENTRAL, the Cochrane Library 2007, issue 2. C) screening references given in relevant reviews and identified RCTs.
The intervention specific search contained MeSH‐headings (explode 'anti‐inflammatory agents, non‐steroidal') and textwords in title, keywords and abstracts ('nsaid', 'non‐steroidal anti‐inflammatory drug'). The complete search strategy is presented in Appendix 1; Appendix 2; and Appendix 3.
Data collection and analysis
Study selection
Two review authors independently selected the trials to be included in the systematic review according to the inclusion criteria. Consensus was used to resolve disagreements concerning inclusion of RCTs.
Methodologic quality assessment
The criteria list recommended by the Cochrane Back Review Group (Table 1) was used for assessing the methodological quality of the included studies (van Tulder 2003). All items were scored as positive or negative. Unclear scores were not included, because we had decided not to contact the authors for additional information.
1. Operationalisation Quality Assessment.
| Criteria | Operationalisation |
| A. Was the method of randomisation adequate? | A random (unpredictable) assignment sequence. Examples of adequate methods are computer‐generated random numbers table and use of sealed opaque envelopes. Methods of allocation using date of birth, date of admission, hospital numbers, or alternation should not be regarded as appropriate. |
| B. Was the treatment allocation concealed? | Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. |
| C. Were the groups similar at baseline regarding the most important prognostic indicators? | In order to receive a "yes," groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of patients with neurological symptoms, and value of main outcome measure(s). |
| D. Was the patient blinded to the intervention? | The review author determines if enough information about the blinding is given in order to score a "yes." |
| E. Was the care provider blinded to the intervention? | The review author determines if enough information about the blinding is given in order to score a "yes." |
| F. Was the outcome assessor blinded to the intervention? | The review author determines if enough information about the blinding is given in order to score a "yes." |
| G. Were co‐interventions avoided or similar? | Co‐interventions should either be avoided in the trial design or be similar between the index and control groups. |
| H. Was the compliance acceptable in all groups? | The review author determines if the compliance to the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). |
| I. Was the drop‐out rate described and acceptable? | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop‐outs does not exceed 20% for immediate and short‐term follow‐ups, 30% for intermediate and long‐term follow‐ups and does not lead to substantial bias a "yes" is scored. |
| J. Was the timing of the outcome assessment in all groups similar? | Timing of outcome assessment should be identical for all intervention groups and for all important outcome assessments. |
| K. Did the analysis include an intention‐to‐treat analysis? | All randomized patients are reported/analyzed in the group to which they were allocated by randomization for the most important moments of effect measurement (minus missing values), irrespective of noncompliance and co‐interventions. |
The methodologic quality of the RCTs was independently assessed by two review authors. Consensus was used to resolve disagreements and a third review author was consulted if disagreements persisted. If the article did not contain information on the methodologic criteria, these criteria were scored as 'negative'. Because 31 of the 64 studies included in this review were published before 1990, we decided not to contact the authors for additional information.
We defined high quality studies as RCTs which fulfilled six or more of the validity criteria, but also performed sensitivity analyses exploring the results when high quality was defined as fulfilling five or more or seven or more of the 11 validity criteria. We also explored the results if high quality was defined as having adequate concealment of treatment allocation. Analyses were performed separately for traditional NSAIDs and selective COX‐2 NSAIDs, (sub)acute low‐back pain (12 weeks or less) and chronic low‐back pain (more than 12 weeks), short‐term follow‐up (less than six months after randomization) and long‐term follow‐up (six months or more), and low‐back pain with and without sciatica.
Data extraction
Two review authors independently extracted the data (using a standardized form). Data were extracted on type and dose of NSAIDs, type of reference treatment, follow‐up, presence or absence of sciatica, duration of current symptoms, and the outcomes described above.
Data analysis
The quantitative analysis (statistical pooling) was limited to clinically homogeneous studies for which the study populations, interventions and outcomes were considered by the review authors to be similar (see comparisons). We included a test for homogeneity of the Relative Risk (RR) of the RCTs. If studies were clinically and statistically homogeneous, Mean Differences, RRs and 95% CI were presented using the fixed‐effect model. If studies were clinically homogeneous but statistically heterogeneous, the random‐effects model was used. For Mean Differences, the Weighted Mean Difference (WMD) was presented if all studies reported an identical scale for an outcome measure (e.g. 100 mm VAS for Pain Intensity); otherwise the Standardized Mean Difference (SMD) was presented (e.g. 100 mm VAS and 5‐point scale for Pain Intensity).
A qualitative analysis was performed if relevant data enabling statistical pooling were lacking. In the qualitative analysis, a rating system of levels of evidence was used to summarize the results of the studies in terms of strength of the scientific evidence. The rating system consisted of four levels of scientific evidence based on the quality and the outcome of the studies: ‐ Strong evidence ‐ consistent findings among multiple high quality RCTs ‐ Moderate evidence ‐ consistent findings among multiple low quality RCTs and/or one high quality RCT ‐ Limited evidence ‐ one low quality RCT ‐ Conflicting evidence ‐ inconsistent findings among multiple RCTs ‐ No evidence from trials ‐ no RCTs or CCTs
Clinical relevance
In order to give an indication for clinical relevance, all included studies were checked on the following criteria (Shekelle 1994):
Are the patients described in detail so that you can decide whether they are comparable to those that you see in your practice?
Are the interventions and treatment settings described well enough so that you can provide the same for your patients?
Were all clinically relevant outcomes measured and reported?
Is the size of the effect clinically important? (van der Roer 2006)
Are the likely treatment benefits worth the potential harms?
Note that when studies comparing two or more medications determined that the medications were equally effective, the effects were considered negative with respect to the effectiveness of the intervention medication. All scores were added to the Characteristics of included studies Table, but no further conclusions were drawn from these results.
Results
Description of studies
The updated search resulted in 125 additional references from MEDLINE, 566 from EMBASE and 55 from the Cochrane Central Register of Controlled Trials. After filtering for duplicates, 467 references were left for assessment. During the first selection based on titles, keywords and abstracts, the two review authors agreed that 23 articles should be included, they were uncertain about five studies and rejected 439 studies. Assessment of the 28 full papers resulted in four more studies being rejected. One study reported on two trials (Dreiser 2001) and therefore this reference is listed twice. Six studies that had been included earlier in the review were also rejected, as two of these papers only described the onset of pain relief (Katz 2004, Veenema 2000), and four papers did not meet language criteria (Arslan 1999; Hong 2006; Kittang 2001; Natour 2002). Three papers were considered to contain additional information for earlier reported studies (Lloyd 2004; Nadler 2003; Pallay 2004).
Consequently, 15 new studies were included.
The previous search had resulted in 53 selected studies for this review. Three of these studies were excluded, one of them because the efficacy of a muscle relaxant instead of an NSAID was evaluated (Berry 1988b), the second only investigated different dosage regimes of the same NSAID (Pownall 1985) and the third because the study population consisted of back and neck pain patients (Coletta 1988).
Finally, 65 studies were included in this update, 59 of which were published in English and six in German:
sixteen studies compared one or more types of NSAIDs with a placebo (Amlie 1987; Babej‐Dolle 1994; Basmajian 1989; Berry 1982; Birbara 2003; Coats 2004; Dreiser 2001; Dreiser 2003; Goldie 1968; Jacobs 1968; Katz 2003; Lacey 1984; Radin 1968; Szpalski 1994; Weber 1980; Weber 1993);
seven studies compared one or more types of NSAIDs with paracetamol (Evans 1980; Hickey 1982; Innes 1998; Milgrom 1993; Muckle 1986; Nadler 2002; Wiesel 1980);
nine studies compared one or more types of NSAIDs to other drugs (Basmajian 1989; Braun 1982; Brown 1986; Chrubasik 2003; Evans 1980; Ingpen 1969; Metscher 2001; Sweetman 1987; Videman 1984a);
four studies compared some type of NSAIDs with non‐drug treatment (Nadler 2002; Postacchini 1988; Szpalski 1990; Waterworth 1985);
33 studies compared different types of NSAIDs (Aghababian 1986; Agrifoglio 1994; Aoki 1983; Babej‐Dolle 1994; Bakshi 1994; Berry 1982; Blazek 1986; Colberg 1996; Davoli 1989; Dreiser 2001b; Dreiser 2003; Driessens 1994; Evans 1980; Famaey 1998; Hingorani 1970; Hingorani 1975; Hosie 1993; Jaffe 1974; Listrat 1990; Matsumo 1991; Orava 1986; Pena 1990; Pohjolainen 2000; Schattenkirchner2003; Siegmeth 1978; Stratz 1990; Videman 1984b; Waikakul 1995; Waikakul 1996; Wiesel 1980; Ximenes 2007; Yakhno 2006; Zerbini 2005);
three studies compared NSAIDs with NSAIDs plus muscle relaxants (Basmajian 1989; Berry 1988b; Borenstein 1990); and
three studies compared NSAIDs with NSAIDs plus B vitamins (Bruggemann 1990; Kuhlwein 1990; Vetter 1988).
Twenty‐five studies included a homogeneous population of low‐back pain patients without sciatica (Agrifoglio 1994; Amlie 1987; Birbara 2003; Borenstein 1990; Chrubasik 2003; Coats 2004; Colberg 1996; Dreiser 2003; Driessens 1994; Famaey 1998; Hosie 1993; Ingpen 1969; Innes 1998; Katz 2003; Metscher 2001; Milgrom 1993; Nadler 2002; Orava 1986; Pohjolainen 2000; Schattenkirchner2003; Sweetman 1987; Szpalski 1994; Waterworth 1985; Wiesel 1980; Zerbini 2005); six studies included a homogeneous population of low‐back pain patients with sciatica (Braun 1982; Dreiser 2001; Goldie 1968; Radin 1968; Weber 1980; Weber 1993), while the other studies either did not specify whether or not the patients had sciatica, or included a mixed population of patients with and without sciatica .
Thirty‐seven studies reported exclusively on acute low‐back pain (Aghababian 1986; Agrifoglio 1994; Amlie 1987; Bakshi 1994; Basmajian 1989; Blazek 1986; Borenstein 1990; Braun 1982; Brown 1986; Colberg 1996; Dreiser 2001; Dreiser 2003; Evans 1980; Goldie 1968; Hingorani 1970; Hingorani 1975; Hosie 1993; Innes 1998; Kuhlwein 1990; Lacey 1984; Metscher 2001; Milgrom 1993; Nadler 2002; Orava 1986; Pena 1990; Pohjolainen 2000; Schattenkirchner2003; Stratz 1990; Sweetman 1987; Szpalski 1990; Szpalski 1994; Videman 1984a; Waterworth 1985; Weber 1993; Wiesel 1980; Ximenes 2007; Yakhno 2006); nine studies reported exclusively on chronic low‐back pain (Berry 1982; Birbara 2003; Chrubasik 2003; Coats 2004; Driessens 1994; Hickey 1982; Katz 2003; Videman 1984b; Zerbini 2005), while the other studies reported either on a mixed population of (sub)acute and chronic low‐back pain, or did not adequately specify if patients with acute or chronic low‐back pain were included (e.g. by reporting the mean and standard deviation of the duration of back pain in the population). Studies from this last category were included in the analyses for acute or chronic low‐back pain if the authors stated somewhere in the article that it was about acute or chronic low‐back pain, even if they did not provide data on the duration (e.g. mean (SD) number of days of low‐back pain episode).
Eight studies evaluated the efficacy of selective COX‐2 inhibitors. Three versus placebo (Birbara 2003;Coats 2004; Katz 2003) and five versus traditional NSAIDs (Dreiser 2001b; Famaey 1998; Pohjolainen 2000; Ximenes 2007; Zerbini 2005).
Risk of bias in included studies
Twenty‐eight studies (43%) were considered high quality. (Babej‐Dolle 1994; Bakshi 1994; Berry 1982; Berry 1988b; Birbara 2003; Blazek 1986; Chrubasik 2003; Coats 2004; Dreiser 2001; Dreiser 2003; Goldie 1968; Hickey 1982; Innes 1998; Jaffe 1974; Katz 2003; Kuhlwein 1990; Matsumo 1991; Orava 1986; Pohjolainen 2000; Schattenkirchner2003; Szpalski 1994; Vetter 1988; Videman 1984a; Weber 1980; Weber 1993; Ximenes 2007; Yakhno 2006; Zerbini 2005). The most prevalent methodologic shortcomings, which were identified in more than half of the trials, were that the randomization procedure and concealment of treatment allocation were inadequate, that measures were not taken in the study design to avoid co‐interventions, that compliance was unsatisfactory and that the length of follow‐up was inadequate.
Effects of interventions
NSAIDs versus placebo
Acute low‐back pain
Eleven studies comparing NSAIDs with placebo for acute low‐back pain were included in our meta‐analysis. Seven of these studies were high quality (Babej‐Dolle 1994; Dreiser 2001; Dreiser 2003; Goldie 1968; Szpalski 1994; Weber 1980; Weber 1993) and four low quality (Amlie 1987; Basmajian 1989; Jacobs 1968; Lacey 1984). Three studies reported on low‐back pain without sciatica (Amlie 1987; Dreiser 2003; Szpalski 1994), four on sciatica (Dreiser 2001; Goldie 1968; Weber 1980; Weber 1993), and the other four on a mixed population.
Six of these eleven studies reported sufficient data on pain intensity to enable statistical pooling (Amlie 1987; Babej‐Dolle 1994; Dreiser 2001; Dreiser 2003; Szpalski 1994; Weber 1993). The Chi‐square value for homogeneity of the weighted mean difference (WMD) was 16.18 (P < 0.01), indicating statistical heterogeneity among these studies.
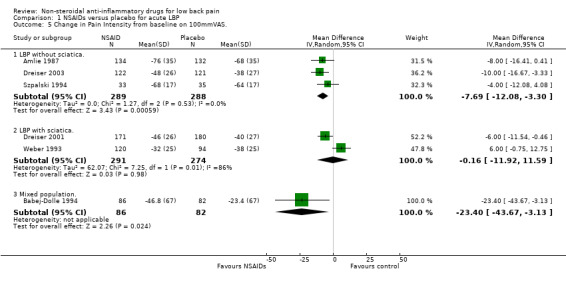
Potential explanations for this heterogeneity were explored. The meta‐analysis was split into studies reporting on low‐back pain without sciatica or a mixed population and studies reporting on low‐back pain with sciatica only. the studies that reported on non‐sciatic/mixed acute low‐back pain were statistically homogeneous (Chi‐square 3.47; P > 0.1), and using the fixed‐effect model, the pooled WMD was ‐8.39 (95% CI ‐12.68 to ‐4.10), indicating a statistically significant effect in favour of NSAIDs compared to placebo (graph 01.01). The sciatica‐only studies were still heterogeneous (Chi‐square 7.25; P < 0.01) and there was no statistical difference in effect between NSAIDs and placebo (WMD ‐0.16; 95% CI ‐11.92 to 11.52).
Seven of the eleven studies which compared NSAIDs with placebo for acute low‐back pain reported dichotomous data on global improvement (Basmajian 1989; Dreiser 2001; Dreiser 2003; Goldie 1968; Jacobs 1968; Szpalski 1994; Weber 1980). The Chi‐square value for homogeneity of the RR was 8.39 (P > 0.1), indicating statistical homogeneity among these studies. The pooled RR for global improvement after one week, using the fixed‐effect model, was 1.19 (95% CI 1.07 to 1.33), indicating a statistically significant effect in favour of NSAIDs compared to placebo (graph 01.02).
Ten of the eleven studies which compared NSAIDs with placebo for acute low‐back pain reported data on side effects. The Chi‐square value for homogeneity of the RR for side effects was 5.45 (P > 0.5), indicating homogeneity among the studies. Using the fixed‐effect model, the pooled RR for side effects was 1.35 (95% CI 1.09 to 1.68), indicating statistically significantly fewer side effects in the placebo group (graph 01.03).
Four of the eleven studies which compared NSAIDs with placebo for acute low‐back pain reported data on the need for additional analgesic use (Amlie 1987; Dreiser 2001; Weber 1980; Weber 1993). The Chi‐square value for homogeneity of the RR was 1.59 (df = 3; P > 0.5), indicating statistical homogeneity. Using the fixed‐effect model, the pooled RR for analgesic use was 0.80 (95% CI 0.71 to 0.91), indicating significantly less use of analgesics in the NSAIDs group (graph 01.04). Analgesics were not permitted in three trials (Goldie 1968; Jacobs 1968; Szpalski 1994).
Chronic low‐back pain
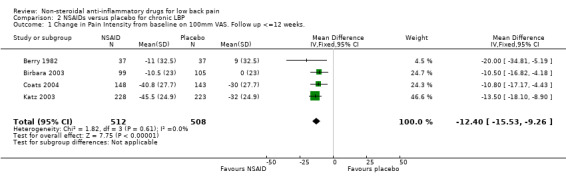
All four studies which compared NSAIDs with placebo for chronic low‐back pain reported sufficient data on pain intensity to enable statistical pooling. The Chi‐square value for homogeneity of the weighted mean difference (WMD) was 1.82 (P > 0.5), indicating statistical homogeneity among these studies. Using the fixed‐effect model, the pooled WMD was ‐12.40 (95% CI ‐15.53 to ‐9.26), indicating a statistically significant effect in favour of NSAIDs compared to placebo (graph 02.01).
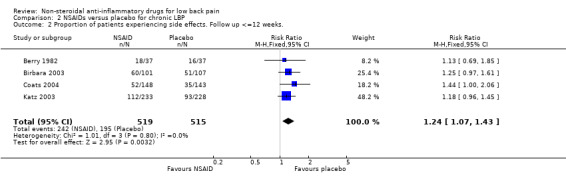
The four studies also reported data on side effects. The Chi‐square value for homogeneity of the RR for side effects was 1.01 (df = 3; P > 0.5), indicating homogeneity among the studies. Using the fixed‐effect model, the pooled RR for side effects was 1.24 (95% CI 1.07 to 1.43), indicating statistically significantly fewer side effects in the placebo group (graph 02.02).
NSAIDs versus paracetamol/acetaminophen
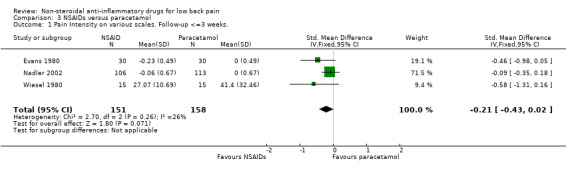
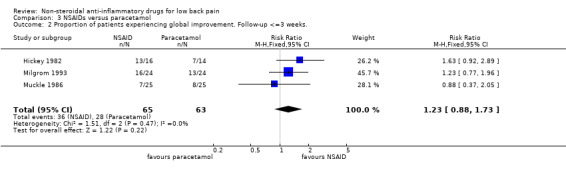
Six studies, one high quality study (Hickey 1982) and five low quality studies (Evans 1980; Milgrom 1993; Muckle 1986; Nadler 2002; Wiesel 1980), compared some type of NSAID with paracetamol or acetaminophen. There is moderate evidence that NSAIDs are equally effective for pain relief and global improvement compared with paracetamol for acute low‐back pain (Evans 1980; Milgrom 1993; Nadler 2002; Wiesel 1980); pooled SMD ‐0.21 (95% CI ‐0.43 to 0.02; N = 309); pooled RR 1.23 (95% CI 0.88 to 1.73; N = 128) (Hickey 1982, Milgrom 1993, Muckle 1986) (graphs 03.01 and 03.02). One low quality study in a mixed population of acute and chronic low‐back pain patients also found no differences (Muckle 1986). One high quality study (Hickey 1982) found limited evidence that NSAIDs are more effective for pain relief than paracetamol in patients with chronic low‐back pain.
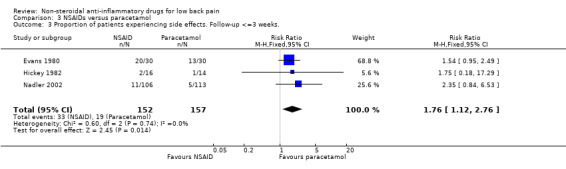
NSAIDs were associated with more side effects compared to paracetamol (RR 1.76; 95% CI 1.12 to 2.76, N = 309) (graph 03.03).
NSAIDs versus other drugs
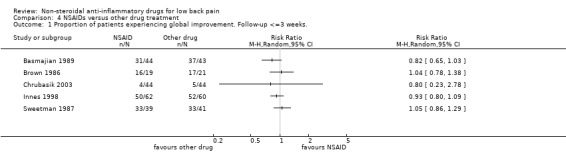
Nine studies (Basmajian 1989; Braun 1982; Brown 1986; Chrubasik 2003; Evans 1980; Ingpen 1969; Metscher 2001; Sweetman 1987; Videman 1984a) were identified that compared NSAIDs with some other kind of drug. Two of these studies were considered to be of high methodologic quality (Chrubasik 2003; Videman 1984a). Seven studies reported on acute low‐back pain, five of which, including one high quality study, did not find any statistical differences between NSAIDs and narcotic analgesics or muscle relaxants (Basmajian 1989; Braun 1982; Brown 1986; Sweetman 1987; Videman 1984a). Because of clinical heterogeneity, the pooled RR was not estimated (graph 04.01). There is moderate evidence that NSAIDs are not more effective than other drugs for acute low‐back pain.
For chronic low‐back pain, one high quality study (Chrubasik 2003) reported equal effectiveness for an NSAID (doloteffin) compared to a herbal medicine (Harpagophytum procumbens).
One study did not specify if patients with acute and/or chronic low‐back pain were included and data extraction was not possible from this study (Ingpen 1969).
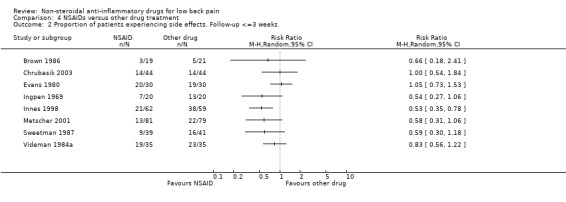
Side effects are plotted in Graph 04.02.
NSAIDs versus non‐drug treatments
Four low quality studies were identified comparing NSAIDs with non‐drug treatments such as spinal manipulation, physiotherapy and bed rest. There is conflicting evidence that NSAIDs are more effective than bed rest for acute low‐back pain, as one study (Szpalski 1990) reported a positive outcome and one study did not find a significant difference (Postacchini 1988). However, the latter study also showed a larger improvement in the NSAID group, and the small number in the bed rest group (N = 29) may have caused a lack of power to detect a statistically significant difference. Two studies found no differences between NSAIDs and physiotherapy or spinal manipulation in acute low‐back pain (Postacchini 1988; Waterworth 1985), therefore, there is moderate evidence that NSAIDs are not more effective than physiotherapy or spinal manipulation for acute low‐back pain. One study may have lacked power because of the small groups (Waterworth 1985). One low quality study comparing NSAIDs to a heat‐wrap for patients with acute low‐back pain, found a statistically significant difference in favour of the heat‐wrap. So, there is limited evidence that NSAIDs are less effective than a heat‐wrap for acute low‐back pain.
Comparison of different types of NSAIDs
Thirty‐three trials compared at least two or more different types of NSAIDs. Two trials compared two types of NSAIDs (diclofenac versus dipyrone and diclofenac versus etofenamat, respectively) administered by intramuscular injection (Babej‐Dolle 1994; Stratz 1990). One compared intramuscular diclofenac with intravenous meloxicam (Colberg 1996), one compared an intramuscular injection of tenoxicam with tenoxicam tablets (Listrat 1990), one compared ibuprofen tablets with felbinac foam (Hosie 1993), and one compared diclofenac gel with indomethacin plaster (Waikakul 1996).
One study reported on acute and chronic low‐back pain separately, and consequently a total of 20 studies included acute low‐back pain patients, four chronic low‐back pain patients, and eight an unspecified or mixed population of acute and chronic low‐back pain patients.
Six studies on acute low‐back pain reported differences between the NSAIDs and fifteen found no differences. Eleven studies on acute low‐back pain were of high quality (Babej‐Dolle 1994; Bakshi 1994; Blazek 1986; Dreiser 2001; Dreiser 2003; Jaffe 1974; Orava 1986; Pohjolainen 2000; Schattenkirchner2003; Ximenes 2007; Yakhno 2006). One of the high quality studies compared two types of NSAIDs administered by intramuscular injection and reported better results for dipyrone compared to diclofenac (Babej‐Dolle 1994). None of the other high quality studies evaluating NSAIDs capsules found any differences.
There seems to be no difference in the reported number and severity of side effects for the various types of NSAIDs. Only three low quality studies reported statistically significant differences in side effects (Agrifoglio 1994; Famaey 1998; Hingorani 1975).
Although 33 studies compared different types of NSAIDs, it was only possible to state that there is moderate evidence based on two trials that the relative effectiveness of meloxicam is equal to diclofenac for acute low‐back pain (Colberg 1996; Dreiser 2001b). None of the other studies compared the same two NSAIDs for acute or chronic low‐back pain.
COX‐2 NSAIDs versus traditional NSAIDs
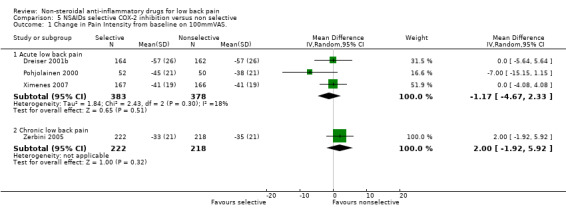
Five studies compared COX‐2 NSAIDs with traditional NSAIDs (meloxicam versus diclofenac (Dreiser 2001b); nimesulide versus diclofenac (Famaey 1998); nimesulide versus ibuprofen (Pohjolainen 2000); valdecoxib versus diclofenac (Ximenes 2007); etoricoxib versus diclofenac (Zerbini 2005)). Statistical pooling of three studies (Dreiser 2001b; Pohjolainen 2000; Ximenes 2007) found no statistically significant differences for pain relief for acute low‐back pain (graph 05.01). The fourth study showed similar results.
The fifth, a high quality study, found moderate evidence that there were no differences in pain relief between COX‐2 and traditional NSAIDs for chronic low‐back pain (Zerbini 2005).
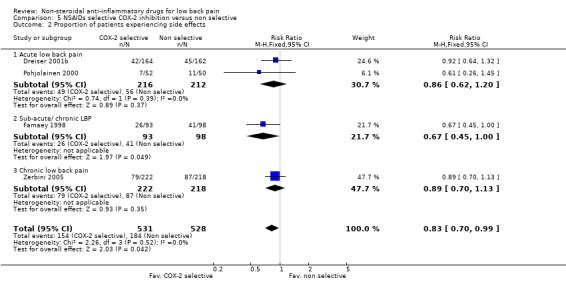
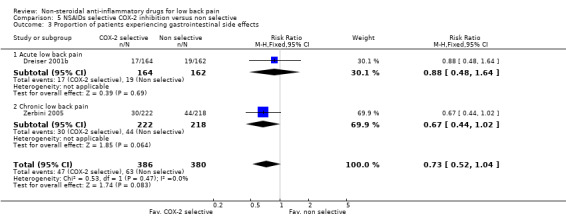
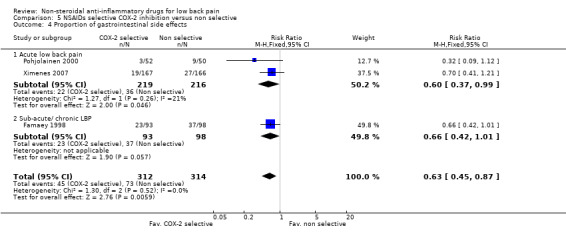
COX‐2 NSAIDs had statistically significantly fewer side‐effects (RR 0.83 ; 95% CI 0.70 to 0.99) (graphs 05.02, 05.03, 05.04).
NSAIDs versus NSAIDs plus muscle relaxants
Three studies, one high quality and two low quality RCTs, compared some type of NSAID with NSAIDs plus muscle relaxants for acute low‐back pain. In all three studies, the authors reported the combination of an NSAID with a muscle relaxant to be better than the NSAID alone, although there were no statistically significant differences. In two trials, side effects were more frequent in the combination groups (Berry 1988b; Borenstein 1990). The review authors concluded that no differences were shown in any of the three trials and therefore, there is moderate evidence that muscle relaxants do not provide any additional effect to NSAIDs alone for acute low‐back pain.
NSAIDs versus NSAIDs plus B vitamins
Three studies compared diclofenac with the addition of B vitamins for low‐back pain, two of which were high quality (Kuhlwein 1990; Vetter 1988). All three studies were published in German. Two studies included patients with acute low‐back pain (Bruggemann 1990; Kuhlwein 1990); one included patients with degenerative changes (Vetter 1988). The authors of all three studies reported positive results for the combination therapy, although there were no statistically significant differences reported in two of these (Bruggemann 1990; Vetter 1988). The review authors concluded that there is conflicting evidence that an NSAID (diclofenac) plus B vitamins are more effective than diclofenac alone for acute low‐back pain, and limited evidence that B vitamins do not provide additional effect to an NSAID for chronic degenerative low‐back pain.
Discussion
Efficacy
The results of the 65 RCTs which were included in this review suggest that NSAIDs are slightly effective for short‐term global improvement in patients with acute and chronic low‐back pain without sciatica.
The placebo‐controlled studies suggest that NSAIDs are effective in improving global improvement in patients with acute low‐back pain, but effects are only small. Quantitative analysis, in which the results of individual RCTs were statistically pooled, indicate statistically significant effects in favour of NSAIDs compared to placebo for populations with acute and chronic low‐back pain without sciatica. Besides the effectiveness of NSAIDs compared to placebo, the NSAIDs group with acute low‐back pain used less additional analgesics, but there was a statistically significantly higher number of patients with side‐effects in the NSAIDs group.
The studies regarding chronic low‐back pain also showed statistically significantly more side‐effects in the NSAIDs group compared with placebo. In addition, in all newly added studies assessing NSAIDs for chronic low‐back pain, a so called "flare design" was used, in which patients who were already responding well to NSAIDs are only included when they show a large worsening in low‐back pain complaints during a wash‐out period. This may have caused favourable results of the investigated NSAIDs, expressed in an overestimation of the effects and an underestimation of the side‐effects due to the selection of the study population, and certainly decreases the external validity for daily practice.
Whether NSAIDs are more effective than other drugs or non‐drug therapies for acute low‐back pain still remains unclear. There is conflicting evidence that NSAIDs are more effective than simple analgesics or bed rest, and moderate evidence that NSAIDs are not more effective than other drugs, physiotherapy or spinal manipulation for acute low‐back pain. NSAIDs in combination with muscle relaxants or B vitamins do not seem to provide more benefits than NSAIDs alone. However, except for the B vitamin studies, the group sizes in these studies were smaller than 65 and therefore, these studies simply may have lacked power to detect a statistically significant difference. Sample size calculations have shown that 65 patients are needed per group to detect a clinically relevant difference of 25% with a power of 0.80 and an alpha of 0.05. There is little evidence on the most effective way of administration, i.e. intramuscular injections, capsules or a combination of both, or gel, as only a few studies evaluated injections or gel.
There is strong evidence that various types of NSAIDs are equally effective for acute low‐back pain.
The efficacy of NSAIDS for patients with sciatica has not been shown in the studies included in this review. Even compared to placebo, the favourable effects of NSAIDs could not be demonstrated in this important subgroup of patients.
Side effects
There is no clear difference in the reported number or severity of side‐effects between the different types of NSAIDs in the studies included in this review. Numerous articles have reported on the side‐effects of NSAIDs, especially gastrointestinal events. In the studies presented in this review, side‐effects were also frequently reported, including abdominal pain, diarrhea, edema, dry mouth, rash, dizziness, headache, tiredness, etc. According to the authors of the studies, most side‐effects were considered to be mild to moderately severe. However, the sample sizes of most of the studies were relatively small and therefore, no clear conclusion can be drawn from these studies regarding the risks for gastrointestinal and other side effects of NSAIDs. Statistical pooling of all side effects of NSAIDs compared to placebo for acute low‐back pain indeed showed an increased RR, indicating the additional risk of using NSAIDs. The comparison of several COX‐2 selective NSAIDs with traditional NSAIDs showed a decreased RR of side‐effects associated with COX‐2 NSAIDs but more extended analyses of the separate risks of upper and lower gastrointestinal side‐effects and central nervous system side effects are needed. In this respect, the recent insights and debate on the increased risk for cardiovascular events associated with the use of selective COX‐2 inhibitors are of importance. These increased risks of COX‐2 NSAIDs (as compared with placebo) have been shown in large randomized clinical trials. These finding have lead to the removal of some selective COX‐2 inhibitors from the market. The current question is whether the increased risk holds for all selective COX‐2 inhibitors and perhaps for the traditional NSAIDs as well. But in order to validly answer this question, more valid data are needed from large epidemiologic studies of NSAID side effects. These data should also consider the dose, frequency and duration of the NSAID intake. It might well be, that in the majority of patients with low‐back pain, the intake is of short duration and might not reach the level associated with increased cardiovascular risks.
Henry (Henry 1996) reported the results of a meta‐analysis of controlled epidemiological studies on the relative risks of serious gastrointestinal complications due to NSAIDs. The authors concluded that ibuprofen was associated with the lowest relative risk of serious gastrointestinal complications. However, this was mainly attributable to the low doses of ibuprofen used in clinical practice. Because there are no important differences in efficacy between the different types of NSAIDs, Henry 1996 recommended using the lowest effective doses of drugs that seem to be associated with a comparatively low risk of serious gastrointestinal complications.
Methodological quality
This review shows that many RCTs examining the efficacy of NSAIDs in low‐back pain have methodological shortcomings. The randomization procedure was not described in most studies, making it impossible for the reader of the article to determine whether adequate procedures were used to exclude bias. In many studies, no measures were taken in the study design to avoid co‐interventions, so it remains unclear if a reported difference of effect was due to the NSAID alone, or to a co‐intervention (vice versa, if there was no difference in effect, this may be due to co‐interventions in the control group). Compliance was often not measured and reported or was not satisfactory, leaving it uncertain if the prescribed medication was actually taken and leaving it unclear if the effect (or lack of effect) was due to the prescribed regimen. The length of follow‐up was considered inadequate in more than half of the trials, because there was no long‐term follow‐up. Since international guidelines set prevention of disability as one of the main goals of early management and NSAIDs play a role in establishing this goal, one would be interested to know if NSAIDs also have any long‐term benefits. In post hoc sensitivity analyses, we repeated the meta‐analyses without the low quality studies, but these did not alter the direction of any of the conclusions.
Although not related to the internal validity or methodological quality of the RCTs, the small size of the study populations in many RCTs indicates that these studies may lack statistical power to detect clinically relevant differences in effects. Statistical pooling may be a way to overcome this problem, but as was shown in this review, pooling is not always feasible. Smaller sample sizes may also lead to an imbalance of important prognostic factors between the study groups after randomisation, which may lead to biased outcomes if, by chance, patients in one group had a more favourable prognosis.
The reported methodologic flaws are not unique for RCTs evaluating the efficacy of NSAIDs, but have been reported to be a general problem in low‐back pain trials (Koes 1995). On average, trials of drug therapy for low‐back pain even have a higher methodologic quality than non‐drug trials (van Tulder 1997). However, there is still a need to improve the development, conduct and reporting of RCTs in the field of back pain.
Limitations of the study
It is possible that the effectiveness of NSAIDs is overestimated in this review because of publication bias. The language criteria we used for including studies in this review might even have enlarged this risk, as positive results are more likely to be published in English. However we were not able to estimate the impact it might have had on our results, as the number of studies per comparison was small.
Authors' conclusions
Implications for practice.
The evidence from the 65 RCTs included in this review suggests that NSAIDs are effective for short‐term global improvement in patients with acute and chronic low‐back pain without sciatica, although the effects are small. It is still unclear if NSAIDs are more effective than simple analgesics and other drugs. Furthermore, there does not seem to be a specific type of NSAID which is clearly more effective than others, nor is there any evidence to recommend another route of administration besides oral capsules or tablets. NSAIDs plus muscle relaxants or B vitamins do not seem to be more effective than NSAIDs alone. For patients with sciatica, there is no evidence that NSAIDs are more effective than placebo.
Implications for research.
There is a need for RCTs, which meet the current high methodological standards, to evaluate the effectiveness of NSAIDs for treating patients with acute low‐back pain with sciatica and for treating patients with chronic low‐back pain with sciatica. It also seems useful to evaluate the most effective dose with the comparatively lowest risk of (serious) side effects.
Feedback
Inaccuracies in NSAIDs for low back pain review
Summary
please note that these comments refer to the 2002(2) version of the review. A substantial update is being published in The Cochrane Library 2008, issue 1 which should address the concerned outlined below (VEP, Managing Editor)
In the course of reviewing this review the commentator noted a number of problems. His intention was to feed them back to help improve the review.
According to the commentator, the problems were the following: 1. He stated that verifying the conclusions by looking directly at the results of the included studies was difficult. The actual results are helpfully given in the characteristics of included studies table. It would be helpful if this table could be sub‐divided according to the conditions (acute or chronic LBP) and comparator (placebo, other NSAID, other treatments) i.e. grouped according to the sub‐categories by which the review author report the results. 2. He indicated there are some discrepancies between the numerical results in the table of included characteristics and the Forest plots. 3. He also reports there are some instances where the results for the control have been entered for the experimental group and vice versa. 4. The "favours" labels at the bottom of the Forest plot for the outcome of numbers needing additional analgesia are the wrong way round. An OR > 1 favours the control for this outcome, not NSAID. 5. He feels that care needs to be taken in using the summary OR from the meta‐analysis to indicate the overall impact on pain and analgesic use in placebo controlled trials for acute LBP when only 3 of the 9 included studies contribute, without indicating that the other six did not measure these outcomes (which he doubts).
He states, "Basically I'm afraid that there needs to be a careful re‐run of the data abstraction and analysis. "
Reply
Review author has not responded to date
Contributors
2(2)Commentator ‐ Chris Hyde, Dept of Public Health & Epidemiology, University of Birmingham Criticism Editor ‐ Dr. Alf Nachemson
What's new
| Date | Event | Description |
|---|---|---|
| 19 January 2011 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 17 February 2010 | Amended | Contact details updated. |
| 9 September 2008 | Amended | ROB Tables edited |
| 11 June 2008 | Amended | Converted to new review format. |
| 17 October 2007 | New citation required and conclusions have changed | Substantive amendment |
| 30 June 2007 | New search has been performed | The literature search was updated from September 1998 to June 2007. Fifteen studies (number of patients = 5180) were added to the previous review. Of these, 12 studies (80%) were considered high quality. This brings the total to 65 included studies (total number of patients = 11,237). In addition, clinical relevance was assessed for all 65 included studies, but these results are not interpreted. In this update we could perform a meta‐analysis on placebo controlled chronic low back pain studies, and we compaired selective Cox‐2 inhibitors with traditional NSAIDs. |
| 15 May 2001 | Feedback has been incorporated | See feedback section |
Acknowledgements
Thanks to Rachel Couban, Trial Search Co‐ordinator, Cochrane Back Review Group for updating the literature search.
Appendices
Appendix 1. MEDLINE search strategy
(randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR "clinical trial" [tw] OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR "latin square" [tw] OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animal [mh] NOT human [mh]) Limits: Entrez Date from 1998/10/01
("low back pain"[MeSH Terms] OR low back pain[Text Word]) OR ("sciatica"[MeSH Terms] OR sciatica[Text Word]) Limits: Entrez Date from 1998/10/01
("non‐steroidal anti‐inflammatory agents"[Text Word] OR "anti‐inflammatory agents, non‐steroidal"[MeSH Terms] OR "anti‐inflammatory agents, non‐steroidal"[Pharmacological Action] OR Anti‐Inflammatory Agents, Non‐Steroidal[Text Word]) Limits: Entrez Date from 1998/10/01
acetylsalicyl* OR carbasalaatcalcium OR diflunisal OR aceclofenac OR alclofenac OR diclofenac OR indometacin OR sulindac OR meloxicam OR piroxicam OR dexibuprofen OR dexketoprofen OR fenoprofen OR flurbiprofen OR ibuprofen OR ketoprofen OR naproxen OR tiapro* OR metamizol OR phenylbutazone OR phenazone OR propyphenazone OR celecoxib OR etoricoxib OR nabumeton OR parecoxib Limits: Entrez Date from 1998/10/01
#3 OR #4 Limits: Entrez Date from 1998/10/1
#1 AND #2 AND #5 Limits: Entrez Date from 1998/10/1
Appendix 2. CENTRAL search strategy
(low back pain), from 1998 to 2007 in Clinical Trials
MeSH descriptor Low Back Pain explode all trees
(#1 OR #2), from 1998 to 2007 in Clinical Trials
nsaid*, from 1998 to 2007 in Clinical Trials
MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees
(#4 OR #5), from 1998 to 2007 in Clinical Trials
(#3 AND #6), from 1998 to 2007 in Clinical Trials
Appendix 3. EMBASE search strategy
'low back pain'/exp OR low‐back‐pain OR 'ischialgia'/exp OR ischialgia OR sciatica OR lumbago OR ('sacroiliac joint'/exp AND (pain/de OR backache/de)) AND ('nonsteroid antiinflammatory agent'/exp OR acetylsalicyl* OR carbasalaatcalcium OR diflunisal OR aceclofenac OR alclofenac OR diclofenac OR indometacin OR sulindac OR meloxicam OR piroxicam OR dexibuprofen OR dexketoprofen OR fenoprofen OR flurbiprofen OR ibuprofen OR ketoprofen OR naproxen OR tiapro* OR metamizol OR phenylbutazone OR phenazone OR propyphenazone OR celecoxib OR etoricoxib OR nabumeton OR parecoxib) AND 'clinical trial'/exp OR 'clinical study'/de OR 'prospective study'/de OR 'clinical article'/de OR 'controlled study'/exp OR 'major clinical study'/de OR 'double blind procedure'/de OR 'single blind procedure'/de OR 'crossover procedure'/de OR placebo/exp OR 'comparative study'/de OR randomization/exp OR ((singl* OR doubl* OR trebl*) AND (mask* OR blind*)) OR latin‐square OR placebo* OR random* OR control* OR prospectiv* OR volunteer* OR 'evaluation and follow up'/de AND [01‐10‐1998]/sd NOT ([animals]/lim NOT [humans]/lim)
Data and analyses
Comparison 1. NSAIDs versus placebo for acute LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Pain Intensity from baseline on 100mmVAS. Follow‐up <=3 weeks. | 4 | 745 | Mean Difference (IV, Fixed, 95% CI) | ‐8.39 [‐12.68, ‐4.10] |
| 2 Proportion of patients experiencing global improvement. Follow‐up <=3 weeks. | 7 | 954 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.07, 1.33] |
| 3 Proportion of patients experiencing side effects. Follow‐up <=3 weeks. | 10 | 1852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.09, 1.68] |
| 4 Additional analgesic use. Follow‐up <=3 weeks. | 4 | 898 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.91] |
| 5 Change in Pain Intensity from baseline on 100mmVAS. | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 LBP without sciatica. | 3 | 577 | Mean Difference (IV, Random, 95% CI) | ‐7.69 [‐12.08, ‐3.30] |
| 5.2 LBP with sciatica. | 2 | 565 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐11.92, 11.59] |
| 5.3 Mixed population. | 1 | 168 | Mean Difference (IV, Random, 95% CI) | ‐23.4 [‐43.67, ‐3.13] |
1.1. Analysis.
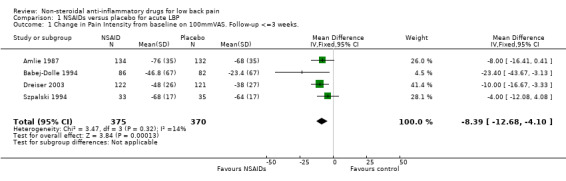
Comparison 1 NSAIDs versus placebo for acute LBP, Outcome 1 Change in Pain Intensity from baseline on 100mmVAS. Follow‐up <=3 weeks..
1.2. Analysis.
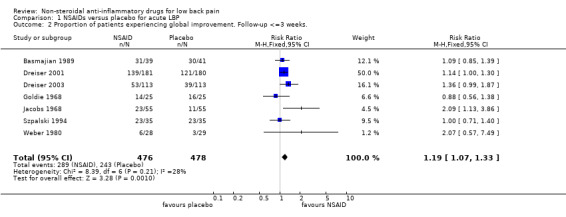
Comparison 1 NSAIDs versus placebo for acute LBP, Outcome 2 Proportion of patients experiencing global improvement. Follow‐up <=3 weeks..
1.3. Analysis.
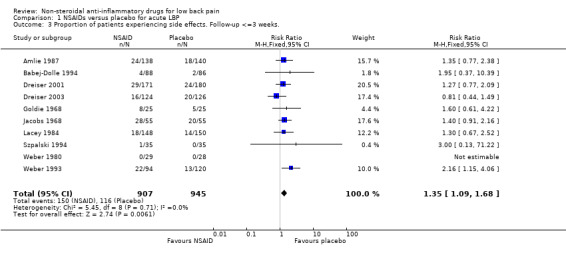
Comparison 1 NSAIDs versus placebo for acute LBP, Outcome 3 Proportion of patients experiencing side effects. Follow‐up <=3 weeks..
1.4. Analysis.
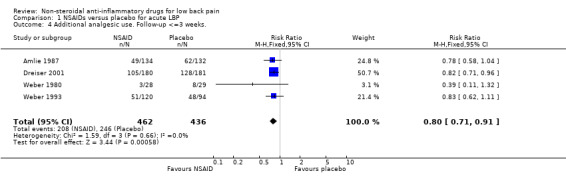
Comparison 1 NSAIDs versus placebo for acute LBP, Outcome 4 Additional analgesic use. Follow‐up <=3 weeks..
1.5. Analysis.
Comparison 1 NSAIDs versus placebo for acute LBP, Outcome 5 Change in Pain Intensity from baseline on 100mmVAS..
Comparison 2. NSAIDs versus placebo for chronic LBP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Pain Intensity from baseline on 100mm VAS. Follow up <=12 weeks. | 4 | 1020 | Mean Difference (IV, Fixed, 95% CI) | ‐12.40 [‐15.53, ‐9.26] |
| 2 Proportion of patients experiencing side effects. Follow up <=12 weeks. | 4 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.07, 1.43] |
2.1. Analysis.
Comparison 2 NSAIDs versus placebo for chronic LBP, Outcome 1 Change in Pain Intensity from baseline on 100mm VAS. Follow up <=12 weeks..
2.2. Analysis.
Comparison 2 NSAIDs versus placebo for chronic LBP, Outcome 2 Proportion of patients experiencing side effects. Follow up <=12 weeks..
Comparison 3. NSAIDs versus paracetamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Intensity on various scales. Follow‐up <=3 weeks. | 3 | 309 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.43, 0.02] |
| 2 Proportion of patients experiencing global improvement. Follow‐up <=3 weeks. | 3 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.88, 1.73] |
| 3 Proportion of patients experiencing side effects. Follow‐up <=3 weeks. | 3 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.12, 2.76] |
3.1. Analysis.
Comparison 3 NSAIDs versus paracetamol, Outcome 1 Pain Intensity on various scales. Follow‐up <=3 weeks..
3.2. Analysis.
Comparison 3 NSAIDs versus paracetamol, Outcome 2 Proportion of patients experiencing global improvement. Follow‐up <=3 weeks..
3.3. Analysis.
Comparison 3 NSAIDs versus paracetamol, Outcome 3 Proportion of patients experiencing side effects. Follow‐up <=3 weeks..
Comparison 4. NSAIDs versus other drug treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients experiencing global improvement. Follow‐up <=3 weeks. | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Proportion of patients experiencing side effects. Follow‐up <=3 weeks. | 8 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.1. Analysis.
Comparison 4 NSAIDs versus other drug treatment, Outcome 1 Proportion of patients experiencing global improvement. Follow‐up <=3 weeks..
4.2. Analysis.
Comparison 4 NSAIDs versus other drug treatment, Outcome 2 Proportion of patients experiencing side effects. Follow‐up <=3 weeks..
Comparison 5. NSAIDs selective COX‐2 inhibition versus non selective.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Pain Intensity from baseline on 100mmVAS. | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acute low back pain | 3 | 761 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐4.67, 2.33] |
| 1.2 Chronic low back pain | 1 | 440 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐1.92, 5.92] |
| 2 Proportion of patients experiencing side effects | 4 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.99] |
| 2.1 Acute low back pain | 2 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.20] |
| 2.2 Sub‐acute/ chronic LBP | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.45, 1.00] |
| 2.3 Chronic low back pain | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.13] |
| 3 Proportion of patients experiencing gastrointestinal side effects | 2 | 766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.52, 1.04] |
| 3.1 Acute low back pain | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.64] |
| 3.2 Chronic low back pain | 1 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.02] |
| 4 Proportion of gastrointestinal side effects | 3 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.87] |
| 4.1 Acute low back pain | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.37, 0.99] |
| 4.2 Sub‐acute/ chronic LBP | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.01] |
5.1. Analysis.
Comparison 5 NSAIDs selective COX‐2 inhibition versus non selective, Outcome 1 Change in Pain Intensity from baseline on 100mmVAS..
5.2. Analysis.
Comparison 5 NSAIDs selective COX‐2 inhibition versus non selective, Outcome 2 Proportion of patients experiencing side effects.
5.3. Analysis.
Comparison 5 NSAIDs selective COX‐2 inhibition versus non selective, Outcome 3 Proportion of patients experiencing gastrointestinal side effects.
5.4. Analysis.
Comparison 5 NSAIDs selective COX‐2 inhibition versus non selective, Outcome 4 Proportion of gastrointestinal side effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aghababian 1986.
| Methods | RCT, open‐label. Randomization procedure not described. | |
| Participants | 56 Patients. Inclusion criteria: mild to moderate acute LBP, 18 to 60 years of age. Exclusion criteria: taking analgesics, chronic back pain, pain longer than 72 hours, history of bleeding disorders, high blood pressure, heart, kidney, liver or ulcer disease, pregnant or breast feeding, allergic reactions to analgesics or NSAIDs. | |
| Interventions | NSAID (i): diflunisal capsules, 1000 mg initially, 500 mg every 8 to 12 hrs, 2 wks (N=16). NSAID (ii): naproxen capsules, 500 mg initially, 250 mg every 6 to 8 hrs, 2 wks (N=17). | |
| Outcomes | No. of patients (%) reporting no pain (on a ordinal 4‐point scale) after 2 weeks (i) 81% (ii) 41%. No significance tests reported. No adverse experiences were reported by the patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 1. Clinical relevance: patients description + intervention description + outcome measures + effect size + benefits/ harms + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Agrifoglio 1994.
| Methods | RCT; single‐blind. Randomization procedure not described. | |
| Participants | 100 Patients from 5 centers, 60 men and 40 women, aged 19 to 68 years. Inclusion criteria: acute lumbago, less than 48 hours, age 18 to 70 years, pain intensity at least 50 mm on VAS. Exclusion criteria: any disorder which might interfere with the study drug, anticoagulants or other drugs which may interfere with the assessment of treatment, pregnancy, breastfeeding or hormonal contraception. | |
| Interventions | NSAID (i): Aceclofenac 150 mg intramuscular b.i.d. for 2 days + 100 mg tablets b.i.d. for 5 days + 1 placebo tablet for 5 days (N=50). NSAID (ii): Diclofenac 75 mg intramuscular b.i.d. for 2 days + 50 mg tablets t.i.d. for 5 days (N=50). | |
| Outcomes | Mean improvement in pain intensity (VAS) after 8 days: (i) 65 (ii) 62. Global improvement good/very good in (i) 87% and (ii) 79% of patients. Side effects: (i) 1 (ii) 8 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 2. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Amlie 1987.
| Methods | RCT, double‐blind, placebo controlled. Randomization procedure not described. | |
| Participants | 282 Patients. Inclusion criteria: acute LBP lees than 48 hours, free from LBP for 3 months, age 18 to 60 years. Exclusion criteria: radicular symptoms, ankylosing spondylitis, rheumatoid arthritis, history of peptic ulcer or severe dyspepsia, hypersensitivity to aspirin or other NSAIDs, pregnancy or lactation, any other hematologic, hepatic, renal, pulmonary, cardiac or systemic disease. | |
| Interventions | NSAID (i): piroxicam 20 mg capsules, twice per day first two days, one per day next 5 days, 7 days (N=140). Reference treatment (ii): placebo capsules (N=142). | |
| Outcomes | (i) More pain relief than (ii) measured with visual analogue scale after 3 days. After 7 days no significant differences. Side effects similar (i) 18 (13%) (ii) 24 (17%). | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described |
Aoki 1983.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 237 Patients. Inclusion criteria: adults presenting at clinical centers with principal complaint LBP. Exclusion criteria: history of gastrointestinal, hepatic or renal disease, those with complications or requiring surgery, history of drug allergy, anticoagulants, abnormal baseline laboratory values, pregnancy, nursing mothers and women with childbearing potential. | |
| Interventions | NSAID (i): piroxicam 20 mg capsules, once per day, 14 days (N=116). NSAID (ii): indomethacin 25 mg capsules, three times per day, 14 days (N=114). | |
| Outcomes | No. of patients (%) who are (very much) improved after 1 and 2 weeks assessed by a physician (i) 49%, 70% (ii) 46%, 58%. Patients assessment after 2 weeks (very) good (i) 69% (ii) 62%. No significant differences. Side effects similar (i) 11% (ii) 13%. | |
| Notes | ‐Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described |
Babej‐Dolle 1994.
| Methods | RCT; observer‐blind, multicenter study. Randomization using a random number generator. Drugs were randomized and provided by pharmaceutical company in individualized patient's kits, which were assigned by the investigator according to the patients order of entry to the study. | |
| Participants | 260 patients aged over 18 years with lumbago or sciatic pain, 134 men, 126 women. Exclusion criteria: hypersensitivity to drugs or drug related malfunction of liver or kidney, polyneuropathy, previous disk surgery or vertebral fractures, psychiatric disease, alcohol and drug abuse, pregnancy or lactation. | |
| Interventions | NSAIDs (i): dipyrone intramuscular, 5 ml (= 2.5 G) 1 to 2 injections, 1 to 2 days (N=88). NSAIDs (ii): diclofenac intramuscular, 3 ml (= 75 mg), 1to 2 injections, 1‐2 days (N=86). Reference treatment (iii): placebo, 5 ml isotonic saline, 1to 2 injections, 1‐2 days (N=86). | |
| Outcomes | Mean (SD) pain intensity (VAS) at baseline and after 6 hours: (i) 80.2 (15.4), 33.4 (25.5); (ii) 79.2 (14.5), 41.7 (25.9); (iii) 78.2 (14.8), 54.8 (25.3). (i) significantly better than (ii) and (iii). No. (%) of patients recovered after 2 days (5‐point NRS): (i) 27 (32%); (ii) 10 (12%); (iii) 7 (9%). Mean (SD) finger‐toe distance at baseline and after 2 days: (i) 29.0 (15.3), 12.0 (13.3); (ii) 28.9 (15.2), 16.3 (14.3); (iii) 29.7 (15.7), 21.1 (14.7). Side effects: (i) 4 patients, (ii) 1 patient, (iii) 2 patients. | |
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance +; baseline similarity ‐; follow‐up ‐. Total score = 9. Clinical relevance: patients description ‐ intervention description + outcome measures + effect size + benefits/ harms + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Randomization using a random number generator. Drugs were randomized and provided by pharmaceutical company in individualized patient's kits, which were assigned by the investigator according to the patients order of entry to the study. |
Bakshi 1994.
| Methods | RCT; double‐blind, multicenter study. Randomization procedure not described. | |
| Participants | 132 patients, 62 men, 70 women. Inclusion criteria: acute back pain less than 1 week, moderate to severe pain (> 50 mm on VAS), at least 2 objective signs of lumbosacral pathology (tenderness, limited ROM or SLR < 75 degrees), LBP due to mechanical cause. Exclusion criteria: hypersensitivity to aspirin or NSAIDs, gastroduodenal ulcer, history of gastrointestinal bleeding, severe cardiac, hepatic or renal insufficiency, severe hypertension, history of maemopoietic or bleeding disorders, pregnancy, sensory or motor deficits in lower extremities, and infective, inflammatory, neoplastic, metabolic or structural cause. | |
| Interventions | NSAIDs (i): diclofenac 75 mg b.i.d., 14 days (N=66). NSAIDs (ii): piroxicam 20 mg b.i.d. 2 days plus once a day for 12 days (N=66). | |
| Outcomes | Mean pain intensity at rest (VAS) at baseline and after 4, 8 and 15 days: (i) 70.0, 43.3, 30.6, 22.7; (ii) 67.1, 44.5, 27.8, 21.0; mean finger tip to floor distance: (i) 28.6, 20.3, 17.8, 14.7; (ii) 29.4, 21.5, 16.9, 14.9. No. (%) of patients improved after 14 days: (i) 54 (82%), 58 (88%). Not significant. Side effects (i) 13 patients (1 withdrawal), (ii) 12 patients (4 withdrawals). | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Basmajian 1989.
| Methods | RCT; double‐blind, multicenter. Randomization procedure not described. | |
| Participants | 175 Patients from 18 clinics. Inclusion criteria: acute incidence of trauma or musculoskeletal strain, rigid criteria of clinical pain and spasm for the previous 7 days were required. | |
| Interventions | NSAID (i): diflunisal capsules 500 mg twice per day (N=44). NSAID plus muscle relaxant (ii): diflunisal capsules 500 mg + 5 mg cyclobenzaprine twice per day (N=43). Muscle relaxant (iii): cyclobenzaprine (flexeril) capsules 5 mg twice per day (N=43). Reference treatment (iv): placebo capsules twice per day (N=45). | |
| Outcomes | No. of patients reporting marked improvement after 2, 4 and 7 days: (i) 10, 14, 24 (ii) 10, 14, 31 (iii) 8, 18, 30 (iv) 10, 14, 20. Group (ii) significantly better after 4 days (based on total distribution). No other significant differences. No significant side effects were reported. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 5. Unclear presentation of results. Clinical relevance: patients description ‐ intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Berry 1982.
| Methods | RCT; double‐blind, cross‐over. Randomization procedure not described. | |
| Participants | 37 Patients, 24 women and 13 men, aged 32 to 79 years, median duration of back pain 3 years. Inclusion criteria: adult patients with chronic back pain (> 3 months) due to spondylosis, degenerative disease, sciatica, or nonspecific pain. Exclusion criteria: malignancy, infection, spondylolisthesis, abnormal alkaline phosphatase level, ESR > 25 mm/hr. | |
| Interventions | NSAID (i): naproxen sodium 275 mg capsules, 2 times 2 capsules per day, 14 days (N=37 in cross‐over design). NSAID (ii): diflunisal 250 mg capsules, 2 times 2 capsules per day, 14 days (N=37 in cross‐over design). Reference treatment (iii): placebo capsules (N=37 in cross‐over design). | |
| Outcomes | Decrease in pain by visual analogue scale. Group (i) reduction of pain (ii) no change (iii) increase of pain. Data in graphs. Group (i) significantly better than (iii) and somewhat better than (ii). Side effects similar in the three groups (i) 18 (ii) 18 (iii) 16. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size + benefits/ harms + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Berry 1988b.
| Methods | RCT; double‐blind, multicenter study. Randomization procedure not described. | |
| Participants | 105 patients, 58 men, 47 women, from seven general practitioners. Inclusion criteria: acute low back pain of at least moderate severity, recent onset, limitation of movement of the lumbar spine, age 18 to 65 years. Exclusion criteria: pregnancy or lactation, malignancy, osteoporosis, history of lumbar spine surgery, significant systemic disease, allergy or sensitivity to study drugs, rheumatic diseases. | |
| Interventions | NSAIDs (i): ibuprofen 400 mg plus placebo tid, 7 days (N=54). Reference treatment (ii): ibuprofen 400 mg plus tizanidine 4 mg tid, 7 days (N=51). | |
| Outcomes | Mean (SD) changes in pain at rest (VAS) between baseline and day 3 and baseline and day 7: (i) 16 (24.9), 33 (32.9); (ii) 18 (25.3), 29 (43.3). Number (%) of patient improved on functional status (restriction of movement) after 3 days: (i) 32 (60%), (ii) 32 (64%). No. (%) of patients improved according to physician after 3 and 7 days: (i) 36 (67%), 42 (81%); (ii) 39 (76%), 39 (85%). Not significant. Side effects: (i) 17 patients, (ii) 23 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Birbara 2003.
| Methods | RCT; "flare design"; double‐blind; double‐dummy; multicentre. Randomised according to computer generated random allocation table | |
| Participants | 314 outpatients, 190 women, 124 men from 46 centres in USA. Inclusion criteria: age 18 to 75 years; LBP >= 3 months, usage NSAID or Acetaminophen >= 30 days; pain without radiation to an extremity and without neurological signs or pain with radiation to an extremity, but not below the knee and without neurological signs; flare criteria after washout period. Exclusion criteria: secondary cause of LBP; surgery for LBP in previous 6 months; symptomatic depression; drug abuse in past 5 years; usage opioids > 4 days in previous month; corticosteroid injection in previous 3 months | |
| Interventions | NSAIDs (i): Etoricoxib 60 mg/ day, 12 weeks (N=101). NSAIDs (ii): Etoricoxib 90 mg/ day, 12 weeks (N=106). Reference treatment (iii): placebo, daily, 12 weeks (N=107). | |
| Outcomes | Mean difference (95% CI) pain intensity scale (100 mm VAS) at 12 weeks; (i vs iii) ‐10.45 (‐16.77 to ‐4.14); (ii vs iii) ‐7.5 (‐13.71 to ‐1.28) Mean difference (95% CI) LBP bothersomeness scale over 12 weeks (4‐pt Likert, 0= not at all, 4= extremely); (i vs iii) ‐0.38 (‐0.62 to ‐0.14); (ii vs iii) ‐0.33 (‐0.57 to ‐0.09) Mean difference (95% CI) RMDQ (0‐ 24 pt scale) over 12 weeks; (i vs iii) ‐2.42 (‐3.87 to ‐0.98); (ii vs iii) ‐2.06 (‐3.46 to ‐0.65) Side effects: (i) 60 patients (14 withdrew); (ii) 56 patients (17 withdrew); (iii) 51 patients (10 withdrew) | |
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up +. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Randomised according to computer generated random allocation table |
Blazek 1986.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 28 Patients, 20 women and 8 men. Inclusion criteria: lumbo‐ischialgia and femoralgia due to herniated disc. Exclusion criteria: gastrointestinal, hepatic or renal disease. | |
| Interventions | NSAID (i): diclofenac 25‐mg capsules, four times per day first 4 days and three times per day next 8 days, 12 days (N=14). NSAID (ii): biarison 300‐mg capsules, four times per day first 4 days and three times per day next 8 days, 12 days (N=14). | |
| Outcomes | Average improvement on ordinal 5‐points scale (0= no response, 4=very good response) during and after the intervention period of 12 days according to physician and patient (i) 2.6 and 2.8 (ii) 2.8 and 3. No significant differences in recovery rate. Side effects: mild side effects in three patients in each group. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Borenstein 1990.
| Methods | RCT; open‐label. Randomization procedure not described. | |
| Participants | 40 patients with acute low back pain referred to an orthopedist or rheumatologist, 28 men, 12 women. Inclusion criteria: acute muscle spasm with mild to moderate back pain, duration 10 days or less, age 18 to 60 years. Exclusion criteria: severe pain, hypersensitivity to study drugs, history of peptic ulcer or bleeding disorders, hypertension, hepatic, cardiovascular or renal disease, marked obesity, neurologic symptoms including sciatica history of back surgery, lactation or pregnancy. | |
| Interventions | NSAIDs (i): naproxen 500 mg initially plus 250 mg qid, 14 days (N=20). Reference treatment (ii): naproxen 500 mg initially plus 250 mg qid plus cyclobenzaprine 10 mg tid, 14 days (N=20). | |
| Outcomes | Physical examination (Schober), days to resolution of pain, days to sit without pain, functional capacity after 14 days: (i) 5, 12.5, 7, 15; (ii) 5, 8.5, 5, 9. Side effects: (i) 4 patients, (ii) 12 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat +; compliance +; baseline similarity +; follow‐up ‐. Total score = 5. Poor presentation of data. Only statistically significant results presented. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Braun 1982.
| Methods | RCT. Randomization procedure not described. | |
| Participants | 37 patients with acute lubo‐ischialgia. | |
| Interventions | NSAID (i): ketoprofen (Orudis) 200 mg intramuscular bid for 3 days plus 50 mg, 4 capsules plus 100 mg drink for 5 days (N=?). Reference treatment (ii): combination (Phenylbutazon‐Sodium, Carbamoylphenoxyacetose, Dexamethasone, Lydocaine‐ hydrochlorid, Cyanocobalamine) 1 intramuscular placebo injection, 3 days plus 3 tablets for 5 days plus 1 placebo drink for 5 days (N=?). | |
| Outcomes | Mean pain intensity at baseline and after 4 and 9 days: (i) 68, 56, 42 and (ii) 68, 52, 39; Laseque: (i) 48, 65, 74 and (ii) 51, 51, 60; fingertip‐to‐floor distance: (i) 28, 25, 17 and (ii) 36, 30, 26. No significant differences. No adverse effects. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 1. Clinical relevance: patients description ‐ intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Brown 1986.
| Methods | RCT; open‐label. Randomization procedure not described. | |
| Participants | 47 Patients. Inclusion criteria: initial or recurrent mild to moderate acute LBP, age 18 to 59 years. Exclusion criteria: pregnant or nursing women, allergy to aspirin or other NSAIDs, history of peptic ulcer, gastrointestinal bleeding or bleeding disorders, hypertension, cardiovascular, renal or hepatic disease, recurrent chronic pain, neurologic signs or symptoms, fracture. | |
| Interventions | NSAID (i): diflunisal (capsules), initial dose 1000 mg, 500 mg every 12 hrs, 15 days (N=19). Reference treatment (ii): acetaminophen 300 mg with codeine 50 mg, two capsules initially, one capsule every 4 hours, 15 days (N=21). | |
| Outcomes | Pain assessments by patient and investigator on 3‐point ordinal scale show similar improvement curves (data in graphs). No. of patients rating drugs as excellent or very good (i) 9 (ii) 9. No significant differences. Side‐effects: more side effects in (ii) 10 than in (i) 3. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 2. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Bruggemann 1990.
| Methods | RCT; double‐blind, multicenter study. Randomization procedure not described. | |
| Participants | 418 ambulant patients. Inclusion criteria: acute low back pain, age 18 years and older. Exclusion criteria: acute disc problems, intolerance of study drugs, disorders or drugs which could interfere with the study. | |
| Interventions | NSAID (i): Diclofenac sodium, 25 mg 2 capsules t.i.d., 7‐14 days (N=192). Reference treatment (ii): diclofenac sodium plus vitamin B1, B6, B12, 2 capsules t.i.d., 7 to 14 days (N=184). | |
| Outcomes | After 1 week 49% of (i) and 56,5% of (ii) no or weak pain and 25% in (i) and 29% in (ii) improved. Mean overall pain score at baseline and after 1 and 2 weeks: (i) 78.7, 37.7, 23.7, (ii) 83.7, 34.4, 23.6. Tolerability good or very good in 90% (i) and 88% (ii). Side effects: (i) 7 patients (ii) 12 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Chrubasik 2003.
| Methods | RCT; double‐blind; double‐dummy; single‐centre. Allocated according to a random number, exact procedure not described. | |
| Participants | 88 patients, 64 women, 24 men Inclusion criteria: age 45 to 75 years; LBP >= 6 months not attributable to a specific cause; exacerbation of complaints> 8 weeks, affecting rest and movement; pain>= 50 mm on 100 mm VAS; judged to require symptomatic treatment for 6 weeks Exclusion criteria: participation in other clinical study within 30 days; serious organic illness; drug/ alcohol abuse; requirement of psychotherapeutic agents; pregnancy/ lactation; allergies to medication; in patients over 50 constitutional symptoms. | |
| Interventions | NSAIDs (i): rofecoxib 12.5 mg/ day, 6 weeks (N=44). Reference treatment (ii): doloteffin 2400 mg/ day, 6 weeks (N=44). | |
| Outcomes | Number of patients pain free after 3 and 6 weeks: (i) 4, 7; (ii) 5, 10 Side effects: (i) 14 patients (1 withdrew); (ii) 14 patients (6 withdrew) | |
| Notes | Methodological quality: randomization ?; treatment allocation ?; withdrawals +; co‐interventions ?; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ?; baseline similarity +; follow‐up +. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear ‐ Allocated according to a random number, exact procedure not described. |
Coats 2004.
| Methods | RCT; "flare design"; double‐blind; multicentre. Randomised according to computer generated random allocation table, exact procedure not described. | |
| Participants | 293 patients, 166 women, 127 men from 42 centres in USA and Canada. Inclusion criteria: age >=18 years; LBP >= 3 months, regular usage of analgesic medication; pain without radiation to an extremity and without neurological signs or pain with radiation to an extremity, but not below the knee and without neurological signs; flare criteria after washout period. Exclusion criteria: secondary cause of LBP; surgery for LBP in previous 4 weeks; usage opioids or corticosteroids in previous 90 days; pending workers compensation claims; (possible) pregnancy or breastfeeding | |
| Interventions | NSAIDs (i): valdecoxib 40‐mg/ day, 4 weeks (N=148). Reference treatment (ii): placebo tablets, once daily, 4 weeks (N=143). | |
| Outcomes | Data in graphs;
Mean changed score on pain intensity scale (100 mm VAS) at 1 week and 4 weeks; (i) 29.2 and 41.9; (ii) 17.7 and 31.1; (i vs ii) all P< 0.001 Side effects: (i) 52 patients (1 withdrew); (ii) 35 patients (3 withdrew) |
|
| Notes | Methodological quality: randomization +; treatment allocation ?; withdrawals ‐; co‐interventions ?; patients blinded +; outcomes blinded +; care providers blinded ?; intention‐to‐treat ‐; compliance +; baseline similarity +; follow‐up +. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ Unclear presentation of results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear ‐ Allocated according to a random number, exact procedure not described. |
Colberg 1996.
| Methods | RCT; multicenter study (12 centers of orthopaedic surgeons, internal specialists and general practitioners). Randomization procedure not described. | |
| Participants | 183 patients, 103 men, 80 women. Inclusion criteria: age 18 years or older, acute lumbago, onset within 48 hours prior to treatment. Exclusion criteria: chronic or recurrent LBP, disk prolapse, trauma, history of gastrointestinal ulcer, coagulation or bleeding disorders, hypersensitivity to analgesics or NSAIDs, pregnant or breast‐feeding women or women without adequate contraception. | |
| Interventions | NSAIDs (i): meloxicam intravenous 1.5 ml (= 15 mg) 1 injection day 1 plus 15 mg tablets once a day, 7 days (N=92). NSAIDs (ii): diclofenac intramuscular 3 ml (= 75 mg) 1 injection day 1 plus 100 mg tablets once a day, 7 days (N=91). | |
| Outcomes | Percentage of patients recovered after 8 days on pain intensity, overall improvement, functional status and tolerance: (i) 91%, 89%, 67%, 96%; (ii) 88%, 91%, 54%, 98%. Side effects: (i) 6 patients (1 severe), (ii) 8 patients (1 severe). | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 3. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Davoli 1989.
| Methods | RCT. Randomization procedure not described. | |
| Participants | 30 patients, 26 women and 4 men, mean age 62 years, range 45 to 80 years. Inclusion criteria: acute or recurrent low back pain, with or without radiation, degree of pain at least 2 on a 5‐point scale. Exclusion criteria: pregnant or lactating women, gastroduodenal ulcer, depression, severe hepatic, renal and/or cardiovascular insufficiency, severe alterations of blood chemistry, hypersensitivity or intolerance to piroxicam, aspirin or other NSAIDS, use of NSAIDs or corticosteroids in previous 30 days. | |
| Interventions | NSAID (i): etodolac 200 mg bid, 7 days (N=15). NSAID (ii): piroxicam‐beta‐cyclodextrin one 20 mg tablet, 7 days (N=15). | |
| Outcomes | Mean degree of pain at baseline and after 7 days: (i) 3.1, 0.8, (ii) 3.13, 0.3. No significant difference. Side effects (i) 3 patients, (ii) 2 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 3. Very short‐term follow‐up, small sample size. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Dreiser 2001.
| Methods | RCT; double‐blind; double‐dummy; multicentre. Randomisation procedure not described. | |
| Participants | 532 outpatients with acute sciatica, age mean (SD), 47 (14); 44% male, 56% female. Inclusion criteria: age >= 18; common sciatica; onset of pain within 3 days; pain 100 mm VAS >= 50 mm; monoradiculagia (L5 or S1); positive straight‐leg‐raising test <=60 degrees; requirement of NSAID's. Exclusion criteria: treatment with any NSAID previous 3 days; adverse events due to NSAIDs; hypersensitivity to analgesics, antipyretics, or NSAIDs; concomitant treatment with anti‐coagulants, lithium, other NSAID, analgesic agents; previous or active peptic ulcer; former lumbar surgery; symptomatic sciatica during previous 6 months; cauda equina syndrome; paralysing sciatica; troncular sciatica; sciatica due to tumour; spindylodiscitis; known lumbar canal narrowing. | |
| Interventions | NSAID (i): Melocicam 7.5 mg/ day, 1 week (N=171). NSAID (ii): Melocicam 15 mg/ day, 1 week (N=181). Reference treatment (iii): placebo, daily, 1 week (N= 80). | |
| Outcomes | Pain mean changed score (SD), 100 mm VAS, after 7 days: (i) ‐46 (26); (ii) ‐45 (27); (iii) ‐40 (27); (i) vs (iii) and (ii) vs (iii) significantly lower (P< 0.05) Side effects: (i) 29 patients (2 withdrew); (ii) 35 patients (2 withdrew); (iii) 24 patients (3 withdrew) |
|
| Notes | Methodological quality: randomization ?; treatment allocation ?; withdrawals +; co‐interventions ?; patients blinded +; outcomes blinded +; care providers blinded ?; intention‐to‐treat +; compliance ?; baseline similarity +; follow‐up +. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear ‐ Randomization procedure not described. |
Dreiser 2001b.
| Methods | RCT; double‐blind; double‐dummy; multicentre. Randomisation procedure not described. | |
| Participants | 489 outpatients with acute sciatica, age mean (SD), 45 (14); 53% male, 47% female. Inclusion criteria: age >= 18; common sciatica; onset of pain within 3 days; pain 100 mm VAS >= 50 mm; monoradiculagia (L5 or S1); positive straight‐leg‐raising test <=60 degrees; requirement of NSAID's. Exclusion criteria: treatment with any NSAID previous 3 days; adverse events due to NSAIDs; hypersensitivity to analgesics, antipyretics, or NSAIDs; concomitant treatment with anti‐coagulants, lithium, other NSAID, analgesic agents; previous or active peptic ulcer; former lumbar surgery; symptomatic sciatica during previous 6 months; cauda equina syndrome; paralysing sciatica; troncular sciatica; sciatica due to tumour; spindylodiscitis; known lumbar canal narrowing. | |
| Interventions | NSAID (i): Meloxicam 7.5 mg/ day, 14 days (N=164). NSAID (ii): Meloxicam 15 mg/ day, 14 days (N=163). NSAID (iii): diclofenac, daily, 14 days (N=162). | |
| Outcomes | Pain mean changed score (SD), 100 mm VAS, after 14 days: (i) ‐57 (26); (ii) ‐56 (26); (iii) ‐57 (26); Side effects: (i) 42 patients (6 withdrew); (ii) 49 patients (6 withdrew); (iii) 45 patients (7 withdrew) |
|
| Notes | Methodological quality: randomization ?; treatment allocation ?; withdrawals +; co‐interventions ?; patients blinded +; outcomes blinded +; care providers blinded ?; intention‐to‐treat +; compliance ?; baseline similarity +; follow‐up +. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear ‐ Randomization procedure not described. |
Dreiser 2003.
| Methods | RCT; double‐blind; double‐dummy parallel group; multicentre. Drugs were randomised according to a random scheme in blocks of 3, provided by pharmaceutical company; the lowest available randomisation number was assigned to the patients at each site when entering the study. | |
| Participants | 369 GP patients, 187 female, 182 male Inclusion criteria: 18‐ 60 years, with untreated acute low back pain, onset within 2 days, pain>= 50 mm on 100 mm VAS, not due to an associated radicalagia; not radiating below gluteal fold, pain intermittent/ constant and aggravated by mechanical factors. Exclusion criteria: hypersensitivity to diclofenac/ ibuprofen/ paracetamol/ aspirin, current disease status that could interfere with safety or efficacy of study medication, concomitant treatments, pregnant or nursing, sensory or motor deficits in lower extremities, previous episode of LBP within 3 months, and infective, inflammatory, neoplastic, metabolic or structural cause. | |
| Interventions | NSAID (i): diclofenac‐K 12 mg, initial dose 2 tablets, 7 days flexible dose 4 to 6 tablets/ day (N=124) NSAID (ii): ibuprofen 200 mg, initial dose 2 tablets, days flexible dose 4 to 6 tablets/ day (N=122) Reverence treatment (iii): placebo, initial dose 2 tablets, 7 days flexible dose 4 to 6 tablets/day (N=126) | |
| Outcomes | Pain mean changed score (SD), 100 mm VAS, after 7 days: (i, N=122) ‐48 (26); (ii, N=119) ‐49 (24); (iii, N=121) ‐38 (27); (i) vs (iii) and (ii) vs (iii) significantly lower (P< 0.001)
Roland‐Morris Back Pain Questionnaire (0‐24, no‐ maximal disability) mean changed score (SD) after 7 days: (i, N=119) ‐8.6 (5.7), (ii, N=118) ‐8.1 (5.2), (iii, N=116) ‐5.7 (5.3); (i) vs (iii) and (ii) vs (iii) significantly lower (P< 0.001) Side effects: (i) 16 patients (4 withdrew); (ii) 14 patients (4 withdrew); (iii) 20 patients (8 withdrew) |
|
| Notes | Methodological quality: randomization ?; treatment allocation +; withdrawals +; co‐interventions ?; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance +; baseline similarity +; follow‐up +. Total score = 9. Clinical relevance: patients description + intervention description + outcome measures + effect size + benefits/ harms + |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Drugs were randomised according to a random scheme in blocks of 3, provided by pharmaceutical company; the lowest available randomisation number was assigned to the patients at each site when entering the study. |
Driessens 1994.
| Methods | RCT; double‐blind, double‐dummy, multiple‐dose. Randomization procedure not described. | |
| Participants | 62 patients from 5 rheumatology centers, 29 men, 33 women, mean age (SD) 52.6 (14.3). Inclusion criteria: hospital outpatients, chronic back pain for at least 4 weeks. Exclusion criteria: acute or chronic infections, neoplasm or metastases, other severe intercurrent systemic disease, sciatica, referred pain, pregnancy or lactation, contraindications for NSAID therapy. | |
| Interventions | NSAID (i): ibuN=rofen sustained‐release (SR) 800 mg bid plus placebo, 14 days (N=30). NSAID (ii): diclofenac SR 100 mg once per day plus placebo, 14 days (N=32). | |
| Outcomes | Mean (SD) improvement in pain intensity, global measure, physical examination (Schober), level of activity: (i) 2.0, 2.2 (1.5), 0.6 (0.6), 2.2 (ii) 2.3, 3.0 (1.7), 0.4 (0.6), 1.9. no significant differences. Side effects in (i) 4 patients, (ii) 16 patients. Significant. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Evans 1980.
| Methods | RCT; single‐blind, cross‐over. Allocation according to a random list. | |
| Participants | 60 Ambulant outpatients. Inclusion criteria: suffering from acute exacerbations of LBP of moderate intensity. Exclusion criteria: pregnancy, sensitive to any of the trial drugs, suffering from concomitant disease. | |
| Interventions | NSAID (i): aspirin 300 mg, 3 capsules 4 times per day, week (N=30). NSAID (ii): indomethacin 50 mg, 3 times per day, 1 week (N=30). NSAID (iii): mefenamic acid 250 mg, 2 capsules 3 times per day, 1 week (N=30). NSAID (iv): phenylbutazone 100 mg, 3 times per day, 1 week (N=30). Reference treatment (v): dextropropoxyphene 32.5 mg and paracetamol 325 mg capsules, 2 capsules 4 times per day, 1 week (N=30). Reference treatment (vi): paracetamol 500 mg capsules, 2 capsules 4 times per day, 1 week (N=30). | |
| Outcomes | Mean daily pain index during intervention period (on 4‐point ordinal scale) (i) 1.4 (ii) 1.5 (iii) 1.4 (iv) 1.4 (v) 1.7 (vi) 1.7. (iii) Significantly different from (v) and (vi); (i) significantly different from (v). Side effects: more side effects in (i) 20 (ii) 19 and (v) 19 than in (iii) 12 (vi) 13 and (iv) 4. | |
| Notes | Methodological quality: randomization +; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat +; compliance +; baseline similarity ‐; follow‐up ‐. Total score = 4. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Allocation according to a random list. |
Famaey 1998.
| Methods | Open label randomized trial, multicenter. Randomization procedure not described. | |
| Participants | 196 out‐patients, 123 female 73 male, subacute/ chronic low back pain patients without a specific diagnosis such as root entrapment syndromes, with at least severe pain at either rest, motion, standing or at night. Exclusion criteria: acute LBP; active/ previous history of gastric or duodenal ulcers, renal or hepatic diseases, history asprin/ NSAID intolerance; treatment with corticosteroids. | |
| Interventions | NSAIDs (i): nimesulide 100 mg, b.i.d., 4 weeks (N=95). Reference treatment (ii): diclofenac sodium 50 mg b.i.d., 4 weeks (N=101). | |
| Outcomes | Sum of patients with none, mild moderate/ sum of patients with severe, very severe pain at: rest, motion, night, standing. Data in graphs. No differences found between treatments. Side effects (i) 26, (ii) 41; (i) vs (ii) P=0.05. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up +. Total score = 4. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms + |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Goldie 1968.
| Methods | RCT; Neither doctor, nurse or patient were aware of which treatment was given as the bottles in which the capsules were dispensed only had a number on the label the code of which was known only to the manufacturer. | |
| Participants | 50 Patients; 26 men and 24 women, all but 5 patients were hospitalized. Inclusion criteria: sciatica, acute back pain less than 3 weeks. | |
| Interventions | NSAID (i): indomethacin 25 mg capsules, three per day, course of 50 capsules (N=25). Reference treatment (ii): placebo capsules (N=25). | |
| Outcomes | No. of patients with complete relief of pain after 7 and 14 days (i) 7, 14 (ii) 9, 16. No significant differences. Side effects comparable (i) 8 (ii) 5. | |
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 9. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ the bottles in which the capsules were dispensed only had a number on the label the code of which was known only to the manufacturer. |
Hickey 1982.
| Methods | RCT; double‐blind. Randomization was carried out prior to the trial by the firm supplying the drugs, which were code‐labelled and unknown to the investigator. | |
| Participants | 30 Patients, 26 women and 4 men. Inclusion criteria: chronic LBP. Exclusion criteria: pain from intervertebral disc prolapse, suspected neoplastic disease, neurological disease, pregnancy, peptic ulceration, gastrointestinal haemorrhage, current treatment with systemic corticosteroids or anticoagulants, liver or kidney disease, haemopoietic disorders and history of sensitivity to salicylates or paracetamol, psychiatric problems. | |
| Interventions | NSAID (i): diflunisal 500 mg capsules, twice per day, 4 weeks (N=16). Reference treatment (ii): paracetamol 1000 mg, four times per day, 4 weeks (N=13). | |
| Outcomes | No. of patients with none or mild low back pain after 2 and 4 weeks (i) 11, 13 (ii) 9, 7. Significantly more patients in (i) (10 out of 16) considered the therapy as good or excellent than in (ii) (4 out of 12). Side effects similar (i) 2 (ii) 1. | |
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size + benefits/ harms + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Randomization was carried out prior to the trial by the firm supplying the drugs, which were code‐labelled and unknown to the investigator. |
Hingorani 1970.
| Methods | CCT; double‐blind. Not randomized. | |
| Participants | 83 patients with acute low back pain warranting in‐patient treatment, 59 men, 24 women. Inclusion criteria: pain in the lower back of acute or chronic onset. Exclusion criteria: neoplasms, myeloma, Paget's disease, collagen disease, rheumatoid arthritis, ankylosing spondylitis and patients with contraindications to either drug. | |
| Interventions | NSAID (i): indomethacin 25 mg, qid, 7 days (N=40). NSAID (ii): oxyphenbutazone 100 mg, qid, 7 days (N=43). | |
| Outcomes | Total pain score (4‐points scale) at baseline and after 3 and 7 days: (i) 52, 48, 39, (ii) 60, 47, 44; total physical examination score (fingertip‐floor distance, inch): (i) 440, 384, 304, (ii) 530, 456, 380; total functional status score (movements; 4‐point scale): 113, 105, 77, (ii) 142, 117, 99; paracetamol use: after 3 and 7 days: (i) 7.4, 3.8, (ii) 7.7, 4.1. Only pain after 3 days significantly different. Side effects: (i) 5/40, (ii) 3/43. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Not randomized |
Hingorani 1975.
| Methods | RCT; double‐blind, cross‐over. Randomization procedure not described. | |
| Participants | 50 patients, 32 men, 18 women, mean age 48.4 years. Inclusion criteria: age 20 to 70 years, acute backache necessitating a stay in hospital of at least 3 weeks. Exclusion criteria: concomitant treatment with steroids, gold or mono‐amine oxidase inhibitors, systemic cause of backache, history of peptic ulcer, possibility of pregnancy. | |
| Interventions | NSAIDs (i): azapropazone 300 mg qid, 1 week (N=?). NSAIDs (ii): ketoprofen 50 mg qid, 1 week (N=?). | |
| Outcomes | No data presented. Side effects: (i) 3 patients (1 severe), (ii) 14 patients (10 severe). | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 5. Clinical relevance: patients description ‐ intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Hosie 1993.
| Methods | RCT; double‐blind, double‐dummy, multicenter. Randomization procedure not described. | |
| Participants | 287 patients, 151 men, 136 women, aged 18 to 63 years. Inclusion criteria: acute LBP less than 1 month. Exclusion criteria: sciatica, 2 or more episodes of LBP in the previous 6 months, nerve root pressure, previous vertebral fractures, spinal stenosis, spondylolisthesis, marked scoliosis, ankylosing spondylitis, osteoarthritis, Paget's disease, metabolic bone disease, systemic connective tissue disorders, malignancy, infection, referred pain from intra‐abdominal or intra‐pelvic disease, pregnancy, lactation, allergy to NSAIDs, history of bronchial asthma or peptic ulcer, gastrointestinal, renal, hepatic, cardiovascular, metabolic, haematological or dermatological disease. | |
| Interventions | NSAID (i): ibuprofen capsules 400 mg three times per day + placebo foam 3 times d, 14 days (N=147). NSAID (ii): felbinac (foam 3%) 3 times daily + placebo capsules 3 times d, 14 days (N=140). | |
| Outcomes | Patients (%) reporting none or mild severity after 1 and 2 weeks (i) 84, 92 (ii) 76, 88. No significant differences between the groups. No. of side effects (i) 22 (ii) 26. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Ingpen 1969.
| Methods | RCT; double‐blind, cross‐over. Paired bottles containing identical capsules were coded and randomized in groups of six. | |
| Participants | 20 patients with backache. Inclusion criteria: longstanding backache with X‐ray confirmation of degenerative changes. Exclusion criteria: sciatica. | |
| Interventions | NSAID (i): indomethacin 25 mg tid, 4 days (N=20). Reference treatment (ii): dextropropoxyphene HCL, 150 mg tid, 4 days (N=20). | |
| Outcomes | Data extraction not possible. Side effects: (i) 7 patients (ii) 13 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Paired bottles containing identical capsules were coded and randomized in groups of six. |
Innes 1998.
| Methods | RCT; double‐blind; multicentre. Randomised according to computer generated random allocation table, exact procedure not described. | |
| Participants | 122 emergency department patients with acute LBP, age mean (SD), 34.5 (10); 96 male, 26 female. Inclusion criteria: age 18 ‐ 60; weight >50 kg ;moderate/ severe LBP, well enough for discharge within 4 hours; onset of pain within 72 hours; requirement of oral analgesics. Exclusion criteria: treatment with investigational drug in previous 4 weeks; adverse events due to NSAIDs; hypersensitivity to analgesics, antipyretics, or NSAIDs; anti‐coagulants use within 4 weeks, concurrent treatment with other medications influencing pain intensity evaluations;active peptic ulcer within 6 months; conditions requiring treatment beyond analgesics; pregnancy or nursing; alcohol/ drug abuse; chronic LBP/ neurologic cause; interfering co‐existing injury/ illness. | |
| Interventions | NSAID (i): Dose 1 to 4 per day Ketrolac tromethamine 10 mg/ 4 to 6 hours as needed; dose 5, 6 per day acetaminophen 650 mg if needed; up to 7 days (N= 62). Reference treatment (ii): Dose 1 to 6 per day, acetaminophen 600 mg + codeine 60 mg/ 4 to 6 hours as needed; up to 7 days (N= 60). | |
| Outcomes | Pain mean changed score (SD), 100 mm VAS, after 6 hours: (i, N=55) ‐6 (17); (ii, N=58) ‐5 (18), no significant differences; % patients reporting 'a lot/ complete' pain relief after 4 days: (i) 53 %; (ii) 55%, no significant differences; % patients reporting 'no/ mild' impairment after 4 days: (i) 67 %; (ii) 62%, no significant differences. Side effects: (i) 21 patients (0 withdrew); (ii) 38 patients (7 withdrew) | |
| Notes | Methodological quality: randomization +; treatment allocation ?; withdrawals +; co‐interventions ?; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance +; baseline similarity +; follow‐up +. Total score = 8. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms + |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear ‐ exact procedure not described. |
Jacobs 1968.
| Methods | RCT; double‐blind. Patients were allotted the next serial number in their diagnostic category and given the appropriate numbered bottle. | |
| Participants | 110 patients with clinically diagnosed prolapsed intervertebral disc with or without radicular pain attending an outpatient clinic, maximum 60 years of age. Inclusion criteria: acute or chronic LBP, age < 60 years. Exclusion criteria: neoplastic, metabolic, or other bone disease, pregnancy, diabetes, epilepsy, peptic ulcer. | |
| Interventions | NSAID (i): indomethacin 25 mg capsules, 3 capsules per day for 2 days, 4 capsules per day next 5 days (N=25 with nerve root pain, and N=30 without nerve root pain). Reference treatment (ii): placebo capsules (N=25 with nerve root pain, and N=30 without nerve root pain). | |
| Outcomes | Group (i) significantly more effective than (ii) only in patients with nerve root pain (data in graphs). Number of side effects (i) 38 (ii) 23. | |
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Patients were allotted the next serial number in their diagnostic category and given the appropriate numbered bottle. |
Jaffe 1974.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 61 patients with low back pain with or without sciatica, 27 men, 34 women, 30 with acute onset and 31 with chronic pain. Exclusion criteria: peptic ulcer, renal or hepatic impairment, history of intolerance to study drugs, children. | |
| Interventions | NSAIDs (i): alclofenac 1 g tid, 7 days (N=15 acute; N=16 chronic). NSAIDs (ii): indomethacin 50 mg tid, 7 days (N=15 acute; N=15 chronic). | |
| Outcomes | Mean (SEM) change score in pain intensity (5‐point NRS), functional status (4‐point NRS) and Lasegue after 7 days for acute LBP: (i) 1.46 (0.28), 1.80 (0.03), 1.50 (0.30); (ii) 1.45 (0.27), 1.53 (0.23), 1.40 (0.23). Not significant. Mean (SEM) change score in pain intensity (5‐point NRS), functional status (4‐point NRS) and Lasegue after 7 days for chronic LBP: (i) 1.66 (0.62), 1.31 (0.63), 1.21 (0.52); (ii) 1.01 (0.71), 1.00 (0.58), 0.87 (0.42). Not significant. Side effects: (i) 5 patients, (ii) 3 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Katz 2003.
| Methods | RCT; "flare design"; double‐blind; parallel group; multicentre. Drugs were randomised according to a random allocation scheme; when entering the study, the lowest available randomisation numbers were assigned to the patients with pre‐study NSAID usage and the highest available numbers to patients that used other analgesics before the study. | |
| Participants | 690 patients from primary care practices or rheumatologists, 430 female, 260 male, 18 to 75 years, with chronic low back pain > 3 months, taking analgesics daily, flare criteria: pain > 40 mm and increase > 10 mm during washout period on 100 mm VAS, pain not radiating below the knee and without neurological signs. Exclusion criteria: CLBP due to known secondary causes, LBP surgery within 6 months, clinical depression within 2 years, drugs/ alcohol within 5 years, opioids > 4 days in previous month, corticosteroid injections previous 3 months. | |
| Interventions | NSAIDs (i): rofecoxib 25 mg/ day, 4 weeks (N= 233) NSAIDs (ii): rofecoxib 50 mg/ day, 4 weeks (N= 229) Reference treatment (iii): placebo, daily, 4 weeks (N= 228) | |
| Outcomes | Mean difference (95% CI) pain intensity scale (100 mm VAS) at 4 weeks; (i, N= 228 vs iii, N= 223) ‐13.5 (‐18.1 to ‐8.9); (ii, N= 224 vs iii) ‐13.8 (‐18.5 to ‐9.2)
Mean difference (95% CI) LBP bothersomeness scale over 4 weeks (4‐pt Likert, 0= not at all, 4= extremely); (i vs iii) ‐0.5 (‐0.6 to ‐0.3); (ii vs iii) ‐0.5 (‐0.7 to ‐0.3)
Mean difference (95% CI) RMDQ (0‐ 24 pt scale) over 4 weeks; (i vs iii) ‐2.2 (‐3.2 to ‐1.3); (ii vs iii) ‐2.3 (‐3.3 to ‐1.3) Side effects: (i) 112 patients (10 withdrew); (ii) 106 patients (10 withdrew); (iii) 93 patients (3 withdrew) |
|
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals +; co‐interventions ?; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ?; baseline similarity +; follow‐up +. Total score = 9. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate ‐ Drugs were randomised according to a random allocation scheme; when entering the study, the lowest available randomisation numbers were assigned to the patients with pre‐study NSAID usage and the highest available numbers to patients that used other analgesics before the study. |
Kuhlwein 1990.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 122 patients , aged 18‐65 years, 90 men, 32 women. Inclusion criteria: degenerative acute low back pain less than 3 days, strong functional limitations. Exclusion criteria: acute disc protrusion or disc prolapse, hypersensitivity for NSAIDs or vitamin B, peptic ulcer or gastrointestinal bleedings, malignancy, renal or hepatic disorders, anticoagulant treatment, pregnancy or lactation. | |
| Interventions | NSAIDs (i) diclofenac 25 mg t.i.d., 7 days (N=62). Reference treatment (ii): diclofenac 25 mg plus vitamins B1, B6, B12, t.i.d., 7 days (N=61). | |
| Outcomes | Mean (SD) pain intensity, physical examination (Laseque) at baseline and after 7 days: (i) 61.2 (10.8), 15.6 (13.2); 48.69 (10.8), 76.36 (11.3) and (ii) 40 (12.6), 3.6 (4.2); 49.43 (12.5), 82.80 (8.3). 35% in (i) and 78% in (ii) returned to work within 7 days (significant). 53% in (i) and 83% in (ii) success rate good or very good. Side effects: (i) 1 patient, (ii) 1 patient. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance +; baseline similarity +; follow‐up ‐. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Lacey 1984.
| Methods | RCT; double‐blind, placebo‐controlled. Randomization procedure not described. | |
| Participants | 337 patients with acute back or sacroiliac pain (< 72 hours) from over 200 general practitioners. Age and sex ratio unknown. | |
| Interventions | NSAID (i): piroxicam 10 mg capsules, four times per day first two days, two time per day next 12 days, 14 days (N=168). Reference treatment (ii): placebo capsules (N=169). | |
| Outcomes | Patient (%) improved after 1 week only in subgroups with initial moderate/severe pain (i) 82%/49% (ii) 53%/38%. No differences for subgroup with mild initial pain. Results after 2 weeks not reported (no data presented on side‐effects for subgroup with back pain; overall, including other musculoskeletal disorders, similar (i) 12 % (ii) 9%). | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 3. Clinical relevance: patients description ‐ intervention description + outcome measures + effect size + benefits/ harms + | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Listrat 1990.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 40 patients with low back pain, 22 men and 18 women, age unknown. Inclusion criteria: herniated or degenerative lumber disc with or without sciatica, pain on lateral bending > 40 mm on VAS. | |
| Interventions | NSAID (i): tenoxicam 20 mg intramuscular injection plus 1 placebo tablet, 4 days (N=20). NSAID (ii): tenoxicam 20 mg tablet plus intramuscular placebo injection (N=20). | |
| Outcomes | Mean (SD) pain intensity (VAS) before treatment and after 24 hours: (i) 55 (15), 34 (29), (ii) 50 (13), 37 (13). Side effects: (i) 5 patients, (ii) 5 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 3. Oral versus intramuscular administration, irrelevant timing of outcome assessment (24 hours). Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Matsumo 1991.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 155 outpatients and inpatients all of whom had complained of chronic lumbago for more than 1 month and had been diagnosed as having muscular pain, intervertebral disc disorders, spondylosis or sciatica, age and sex ratio unknown. | |
| Interventions | NSAID (i): ketoprofen 25 mg capsules, 150 mg per day, duration not given (N=77). NSAID (ii): diclofenac sodium 25 mg capsules, 75 mg per day, duration not given (N=78). | |
| Outcomes | No. of patients improved after 1, 2 weeks (i) 71%, 86% (ii) 62%, 79%. No significant differences. Side effects similar in both groups (i) 18% (ii) 21%. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Metscher 2001.
| Methods | RCT; double‐blind, multicentre. Randomization procedure not described. | |
| Participants | 193 patients, age 18‐ 70 years, acute low back pain; pain 100 mm VAS >= 50 mm; onset within 48 hours; not indicated for other than analgesic treatment | |
| Interventions | NSAID (i): dexketorofen‐trometamol 25 mg t.i.d., 7 days (N=97). NSAID (ii): tramadolhydrochlorid 50 mg t.i.d., 7 days (N=95). | |
| Outcomes | Pain difference mean changed score, 100 mm VAS, after 7 days: (i, N=81) vs (ii, N=79) ‐6 (4 (P< 0.05) Finger to floor distance mean changed score (+/‐ SEM klopt... zie boven), cm, after 7 days: (i) ‐22 (17); (ii) ‐18 (13); (i) vs (ii) significantly lower (P< 0.05) Side effects: (i) 13 patients; (ii) 22 patients | |
| Notes | Methodological quality: randomization ?; treatment allocation ?; withdrawals +; co‐interventions ‐; patients blinded ?; outcomes blinded ?; care providers blinded ?; intention‐to‐treat ‐; compliance ?; baseline similarity ?; follow‐up +. Total score = 2. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ Unclear presentation of results. (mean changed score pain was extracted from the graph) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear ‐ Randomization procedure not described. |
Milgrom 1993.
| Methods | RCT. Randomization according to their military identification numbers. | |
| Participants | 70 male infantry recruits with over exertion back pain, 32 with thoracic and 40 with lumbar pain, mean age 18 years. Inclusion criteria: no history of back pain, no back trauma, back pain exertionally related to carrying loads on the back, not present or markedly improved on nonexertional activities, no sciatica, no sudden onset. | |
| Interventions | NSAID (i): ibuprofen 800 mg tid, 7 days (N=24). Reference treatment (ii): paracetamol 1000 mg tid, 7 days (N=24). Reference treatment (iii): no drug treatment (N=22). | |
| Outcomes | % patients cured after 10 weeks: (i) 67%, (ii) 54%, (iii) 82%. No significant difference. Side effects not specified. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 3. Clinical relevance: patients description ‐ intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization according to their military identification numbers |
Muckle 1986.
| Methods | CCT; double‐blind. Not randomized. | |
| Participants | 50 hospitalized patients with lumbar disk syndrome, age and sex ratio unknown. Inclusion criteria: acute onset first time low back pain. Exclusion criteria: previous hospital admissions for back pain, marked changes in the lumbar spine on radiography. | |
| Interventions | NSAID (i): flurbiprofen 200 mg, 3 weeks (N=25). Reference treatment (ii): acetaminophen, 4 g, 3 weeks (N=25). | |
| Outcomes | No. of patients improved (physician's assessment of pain) after 1, 2, 3 and 6 weeks: (i) 13/23, 17/18, 7/8, 16/17, (ii) 8/22, 13/18, 8/9, 18/18. No significant difference. Side effects not specified. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 3. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Not randomized. |
Nadler 2002.
| Methods | RCT; single‐blind, multi‐center study. Stratified randomization, allocation procedure not described. | |
| Participants | 371 patients , age 18‐55 years, 216 women, 155 men. Inclusion criteria: acute nonspecific low back pain; pain intensity >=2 on 6 point scale; no LBP trauma within 48 hours; an answer of "yes" on the question "do the muscles in your low back hurt?"; Exclusion criteria: radiculopathy/ neurologic deficits; history of back surgery; fibromyalgia, diabetes mellitus; hypersensitivity for NSAIDs/ acetaminophen/ heat; peptic ulcer or gastrointestinal bleedings; renal or hepatic disorders; anticoagulant treatment; pregnancy. | |
| Interventions | NSAID (i): ibuprofen 200 mg, 2 tablets t.i.d. + 2 placebo tablets once a day , days (N= 106) Reverence treatment (ii): acetaminophen 500 mg, 2 tablets q.i.d., 4 days (N= 113) Reverence treatment (iii): heat wrap, 40 degrees centigrade, 8 hours/ day, 4 days (N= 113) Reverence treatment (iv): unheated wrap, 8 hours/ day, 4 days (N=19), no outcome measures presented Reverence treatment (V): placebo, 2 tablets q.i.d., 4 days (N= 20), no outcome measures presented | |
| Outcomes | Roland‐Morris Back Pain Questionnaire (0‐24, no‐ maximal disability) mean changed score after 4 days: (i, N=101) ‐2.7, (ii, N=104) ‐2.9, (iii, N=110) ‐4.9; (i) vs (ii) not presented; (i) vs (iii) significantly less effective (P< 0.001) Pain mean changed score, 0‐5 NRS, after 4 days: (i) ‐1.7; (ii) ‐2.0; (iii) ‐2.6; (i) vs (ii) not presented; (i) vs (iii) significantly less effective (P< 0.001); Side effects: no serious side effects occurred | |
| Notes | Methodological quality: randomization ?; treatment allocation ?; withdrawals +; co‐interventions ?; patients blinded ‐; outcomes blinded +; care providers blinded ‐; intention‐to‐treat ‐; compliance ?; baseline similarity +; follow‐up +. Total score = 4. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear ‐ Stratified randomization, allocation procedure not described. |
Orava 1986.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 133 patients. Inclusion criteria: acute lumbago less than 2 weeks after onset, bothersome LBP and considerable functional disability. Exclusion criteria: previous use of analgesics, antiinflammatory or muscle relaxing agents for this episode. | |
| Interventions | NSAID (i): diflunisal 500 mg capsules, twice daily, 7 days (N=66). NSAID (ii): indomethacin 50 mg capsules, three times daily, 7 days (N=67). | |
| Outcomes | Mean difference (95% CI) pain intensity scale (100 mm VAS) at 4 weeks; (i, N= 228 vs iii, N= 223) ‐13.5 (‐18.1 to ‐8.9); (ii, N= 224 vs iii) ‐13.8 (‐18.5 to ‐9.2) Mean difference (95% CI) LBP bothersomeness scale over 4 weeks (4‐pt Likert, 0= not at all, 4= extremely); (i vs iii) ‐0.5 (‐0.6 to ‐0.3); (ii vs iii) ‐0.5 (‐0.7 to ‐0.3) Mean difference (95% CI) RMDQ (0‐ 24 pt scale) over 4 weeks; (i vs iii) ‐2.2 (‐3.2 to ‐1.3); (ii vs iii) ‐2.3 (‐3.3 to ‐1.3) | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Pena 1990.
| Methods | Double‐blind study. Randomization procedure not described. | |
| Participants | 214 patients with acute low back pain, age and sex ratio unknown. | |
| Interventions | NSAID (i): etodolac 200 mg t.i.d., 5 days (N=?). NSAID (ii): diclofenac 50 mg t.i.d., 5 days (N=?). | |
| Outcomes | % patients showing improvement after 5 days on pain intensity, global measure, Laseque in (i) 50%, 65%, 66% and (ii) 55%, 60%, 74%. Side effects not specified. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 3. Clinical relevance: patients description ‐ intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Pohjolainen 2000.
| Methods | RCT; double‐blind; double‐dummy; multicentre. Randomised according to a list in permutation blocks, concealed allocation. | |
| Participants | 104 outpatients, 52 women, 52 men Inclusion criteria: age 18 to 65 years; acute LBP, onset within 30 days Exclusion criteria: chronic LBP, > 4 weeks; secondary cause of LBP; pain with radiation to an extremity below the knee;surgery for LBP; significant systematic disease; history of peptic ulceration; allergy to NSAIDs. | |
| Interventions | NSAID (i): nimesulide 100 mg b.i.d. + placebo once a day, 10 days (N= 52) NSAID (ii): ibuprofen 600 mg t.i.d., 10 days (N= 52) | |
| Outcomes | Oswestry LBP disability questionnaire pre‐mean (SD), post‐mean day 10 (SD): (i, N= 52) 35.8 (15,0), 10.0 (10.8); (ii, N=50) 35.1 (19.1), 16.5 (19.0), (i) vs (ii) significantly lower (P< 0.05)
Pain, 100 mm VAS, pre‐mean (SD), post‐mean day 10 (SD): (i, N= 52) 57.9 (20.6), 12.8 (15.4); (ii, N=50) 55.2 (21.4), 18.5 (19.9), (i) vs (ii) not significant
Schobers test , cm, pre‐mean (SD), post‐mean day 10 (SD): (i, N= 52) 5.2 (1.8), 6.9 (1.6); (ii, N=50) 5.4 (2.3), 6.3 (1.6); no significance test reported Side effects: (i) 7 patients (2 withdrew); (ii) 11 patients (1 withdrew) |
|
| Notes | Methodological quality: randomization ?; treatment allocation +; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ?; compliance +; baseline similarity +; follow‐up +. Total score = 9. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Randomised according to a list in permutation blocks, concealed allocation. |
Postacchini 1988.
| Methods | RCT. Randomization procedure not described. | |
| Participants | 459 patients with LBP, aged 17 to 58 years, sex ratio unknown. Exclusion criteria: neoplastic or infectious disease, pregnant or nursing women, serious general diseases, psychiatric disturbances or medico‐legal litigation. | |
| Interventions | NSAID (i): diclofenac 'full dosage', 10‐14 days (acute patients), 15‐20 days (chronic patients) (N=81). Reference treatment (ii): chiropractic manipulation (N=87). Reference treatment (iii): physiotherapy (N=78). Reference treatment (iv): bedrest (N=29). Reference treatment (v): back school (N=50). Reference treatment (vi): placebo (anti‐oedema gel) (N=73). | |
| Outcomes | Mean improvement on combined pain, disability, and spinal mobility score (5‐32) after 3 wks, 2 and 6 months. In subgroup with acute pain (i) 3.0, 10.7, 14.0 (ii) 7.5, 9.7, 12.3 (iii) 5.0, 8.4, 10.2 (iv) 5.4, 7.5, 7.3 (v) not included (vi) 1.8, 7.3, 11.0. Group (ii) significantly better than others after 3 wks; no other differences. In subgroup with chronic pain (i) 2.6, 2.2, 4.0 (ii) 2.2, 2.6, 4.3 (iii) 3.9, 4.2, 6.0 (iv) not included (v) 0.5, 4.6, 8.9 (vi) 0.7, 1.2, 2.0. Group (i) not significantly better. No data on side‐effects reported. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up +. Total score = 1. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Radin 1968.
| Methods | CCT; double‐blind trial. | |
| Participants | 25 patients admitted to hospital with prolapsed discs. Inclusion criteria: pain from the low back or buttock, radiating down the back of the leg to at least the heel, and either pain on straight‐leg‐raising referred to the contralateral thigh or back, muscular weakness, or sciatic nerve‐tenderness, aged 18 to 53 years. Exclusion criteria: X‐ray evidence of disease of the lumbosacral spine, history of prior back surgery, or upper gastrointestinal disease. | |
| Interventions | NSAID (i): phenylbutazone 100 mg capsules, six per day for two days, three per day next 8 days (N=6). Reference treatment (ii): placebo capsules (N=6). | |
| Outcomes | Group (i) improvement in motor weakness and painful straight leg raising. No results are reported for group (ii). Side effects reported in group (i) 3. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded ‐; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 4. Very small sample size, poor presentation of results. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Randomization procedure not described. |
Schattenkirchner2003.
| Methods | RCT; double‐blind; multicentre. Randomization procedure not described. | |
| Participants | 227 patients, mainly from general practices, 85 women, 142 men Inclusion criteria: age 20‐ 65 years; localised, uncomplicated acute LBP associated with degenerative spinal disorders; had pain without analgesic therapy during previous 24 hours and at rest at least a 100 mm VAS score of 60 Exclusion criteria: suspicion of serious underlying spinal condition, non‐specific back symptoms related to abdominal, pelvic or thoracic pathology; prior neurological deficits in lower extremities; surgery for LBP; lumbosacral facet syndrome; history of heamatological or bleeding disorders; severe cardiac, hepatic or renal insufficiency; severe hypertension; connective tissue diseases; gastroduodenal ulcer; history of GI bleeding; hypersensitivity to aspirin or NSAIDs; alcohol/ drug abuse; pregnant/ lactating. | |
| Interventions | NSAID (i): aceclofenac 100 mg b.i.d., 10 days (N=114) NSAID (ii): diclofenac 75 mg b.i.d., 10 days (N=113) | |
| Outcomes | Pain mean changed score (SD), 100 mm VAS, after 10 days: (i, N=114) ‐62 (25); (ii, N=113) ‐57 (23) ); QBPDS change form baseline (SD) after 4 days (i, N=100) 25.5 (14.8); (ii, N=105) 20.6 (12.1) Side effects: (i) 17 patients; (ii) 18 patients | |
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals +; co‐interventions +; patients blinded ?; outcomes blinded ?; care providers blinded +; intention‐to‐treat +; compliance +; baseline similarity ?; follow‐up +. Total score = 8. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | |
Siegmeth 1978.
| Methods | RCT; single‐blind. Clinician remaining unaware of the drug treatment of individual patients who were allocated to the trial by an independent third person. | |
| Participants | 30 patients with radiologically confirmed lumbar osteoarthritis, age and sex ration unknown. | |
| Interventions | NSAID (i): ibuprofen, 1200 mg per day, 14 days (N=15). NSAID (ii): diclofenac, 75 mg per day, 14 days (N=15). | |
| Outcomes | No. of patients reporting to be improved after 1, 3, 4 weeks (i) 5, 10, 6 (ii) 5, 12, 11. No significant differences. Side effects similar: one in each group. | |
| Notes | Methodological quality: randomization ‐; treatment allocation +; withdrawals +; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 5. Clinical relevance: patients description ‐ intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Clinician remaining unaware of the drug treatment of individual patients who were allocated to the trial by an independent third person. |
Stratz 1990.
| Methods | RCT; single‐blind, multi‐center study. Randomization procedure not described. | |
| Participants | 100 patients with acute low back pain, 50 men and 50 women, aged between 16 and 78 years. Inclusion criteria: acute or acute onset after a long symptom‐free period. Exclusion criteria: age less than 14 years, pregnancy or lactation, allergy, corticosteroid, other NSAID or anticoagulant treatment. | |
| Interventions | NSAID (i): diclofenac intramuscular 3 ml (= 75 mg), 1 to 3 injections, 1 to 3 days (N=50). NSAID (ii): etofenamat ml (= 1500 mg), 1 to 3 injections, 1to 3 days (N=50). | |
| Outcomes | Mean pain score (11‐point NRS) at baseline and after 3‐5 days: (i) 5.3, 2.7, (ii) 5.3, 2.4; physical examination (Schober): (i) 30, 52, (ii) 31, 59. No. of patients recovered after 3‐5 days;: (i) 27/47, (ii) 34/49. Not significant. Side effects: (i) 2, (ii) 0. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat +; compliance +; baseline similarity ‐; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Sweetman 1987.
| Methods | RCT. Randomization procedure not described. | |
| Participants | 122 Ambulant patients with acute LBP of 12 general practitioners, 65 men and 57 women, aged 15 to 72 years. Inclusion criteria: current episode longer than 24 hours and less than 28 days. Exclusion criteria: nerve root compression, arthritis, ankylosing spondylitis, infection, malignancy, renal or hepatic disease, peptic ulcers, pregnant or lactating women, sensitivity to test medications. | |
| Interventions | NSAID (i): mefenamic acid 500 mg three times per day + placebo twice daily (N=40). Reference treatment (ii): chlormezanone 100 mg and paracetamol 450 mg two capsules three times per day + placebo three times daily (N=42). Reference treatment (iii): ethoheptazine 75 mg and meprobamate 150 mg and aspirin 250 mg two capsules + placebo three times per day (N=40). | |
| Outcomes | No. of patients reporting no pain after 1 and 7 days (i) 7, 21 (ii) 12, 23 (iii) 10, 20. No. of patients with adverse events (i) 9 (ii) 10 (iii) 16. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals ‐; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 4. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Szpalski 1990.
| Methods | RCT. Use of randomization table. | |
| Participants | 110 patients with acute low back pain treated at an outpatient department. Inclusion criteria: pain present < 2 weeks, asymptomatic period of at least 4 months. Exclusion criteria: LBP related to industrial accident covered by insurance, specific pathology such as disc protrusion or trauma. | |
| Interventions | NSAID (i): tenoxicam 20 mg, 1 tablet daily, 14 days plus bed rest, 7 days strict, 7 days intermittent (N=49). Reference treatment (ii): bed rest, 7 days strict, 7 days intermittent (N=50). | |
| Outcomes | Mean % improvement (SD) between baseline and 14 days in ROM (I) 123 (24), (ii) 114 (23). Significant. 86% (i) versus 70% (ii) no need for further treatment. Side effects: 2 patients in (i). | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 2.
Physical examination only outcome. Outcome presented as percentages, baseline values not presented. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Use of randomization table |
Szpalski 1994.
| Methods | RCT; double‐blind, placebo‐controlled. Randomization procedure not described. | |
| Participants | 73 Patients with acute LBP, 47 men and 26 women, mean age about 38 years. Inclusion criteria: LBP less than 2 weeks, first presentation after an asymptomatic 6‐months period. Exclusion criteria: worker's compensation, herniated disc or spinal trauma, pregnancy or lactation, hypersensitivity to NSAIDs, history of gastrointestinal ulceration, current use of NSAIDs, anticoagulants, antidiabetics or lithium. | |
| Interventions | NSAID (i): tenoxicam 20 mg intramuscular injection on day one + 20 mg capsules 1 per day for day 2 to 14 (+ 7 days bedrest) (N=37). Reference treatment (ii): placebo injection + placebo capsules (+ 7 days bedrest) (N=36). | |
| Outcomes | Mean pain intensity on VAS on day 1, 8 and 15 (i) 7.4, 1.9, 0.6 (ii) 7.1, 2.8, 0.8. (i) significantly better on day 8. Side effects: one patient in group (i). | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Vetter 1988.
| Methods | RCT; double‐blind, multi‐center study. Block randomization for each center. | |
| Participants | 252 patients with acute degenerative low back disorders, 131 men and 107 women, aged between 19 and 82 years. Exclusion criteria: acute disk protrusion, malignancy, alcohol or drug abuse, pregnancy or lactation, gastrointestinal, hepatic or renal disease, hypersensitivity to study drugs. | |
| Interventions | NSAIDs (i): diclofenac 50 mg t.i.d., 14 days (N=126). Reference treatment (ii): diclofenac 50 mg plus vit. B1 50 mg, B6 50 mg, B12 0.25 mg, t.i.d., 14 days (N=126). | |
| Outcomes | Mean (SEM) pain intensity (VAS) at baseline and after 3, 7 and 14 days: (i) 64.8 (2.2), 54.2 (2.4), 45.0 (2.7), 33.8 (3.1); (ii) 66.9 (1.7), 52.9 (2.3), 39.3 (2.8), 30.5 (2.9). No. (%) of patients improved after 7 and 14 days: (i) 20 (19%), 27 (29%); (ii) 28 (29%), 27 (33%). Side effects: (i) 21 patients, (ii) 21 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance +; baseline similarity +; follow‐up ‐. Total score = 7. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Videman 1984a.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 70 Outpatients with acute LBP, duration from 1 day to 30 days, 41 men and 29 women, aged 20 to 64 years. Exclusion criteria: pregnant or nursing women, haematological, renal, hepatic, respiratory or circulatory disorders, history of peptic ulceration or gastrointestinal upset, sensitivity to narcotic analgesics or benzomorphan derivatives, weight less than 45 kg or more than 95 kg. | |
| Interventions | NSAID (i): diflunisal 250 mg capsule, 4 times per day, 3 weeks (N=35). Reference treatment (ii): meptazinol 200 mg capsule, 4 times per day, 3 weeks (N=35). | |
| Outcomes | Mean change in degree of pain on 100 mm visual analogue scale at three weeks (i) 45 (ii) 40. Similar improvement regarding capacity for daily tasks (data in graphs). No significant differences. Side effects similar (i) 19 (ii) 23 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Videman 1984b.
| Methods | RCT; double‐blind. Randomization procedure not described. | |
| Participants | 28 Outpatients with chronic severe LBP, 17 men and 11 women, aged 25 to 76 years. Exclusion criteria: pregnant or nursing women, compensation claims, haematological, renal or hepatic disease, peptic ulcer, intolerance to indomethacin. | |
| Interventions | NSAID (i): piroxicam 20 mg + placebo capsules, one per day + twice placebo, 6 weeks (N=14). NSAID (ii): indomethacin 25 mg capsules, three times per day, 6 weeks (N=14). | |
| Outcomes | Mean improvement on visual analogue scale (range 0‐31) after 6 weeks (i) 8 (ii) 9. Similar improvement rates (data in graphs). Side effects similar (i) 13 (ii) 15. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 5. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Waikakul 1995.
| Methods | RCT. Patients were randomly allocated to 2 groups according to the last 2 digits of their hospital numbers. | |
| Participants | 72 hospital patients with nonsurgical low back pain, 20 men, 52 women. Inclusion criteria: nonsurgical low back pain including disc syndrome, spondylosis, mild spondylolisthesis, spinal stenosis and postural back pain, aged 15 years and older. Exclusion criteria: presence of digestive, haematological, hepatic and renal disorders, hypersensitivity to proprionic acid derivatives, long‐term administration of steroids, indication for surgery. | |
| Interventions | NSAID (i): Loxoprofen 60 mg tid, 6 weeks (N=37). NSAID (ii): naproxen 250 mg tid, 6 weeks (N=35). | |
| Outcomes | Mean (SD) therapeutic results according to criteria of the Japanese orthopaedic Academic Society at baseline and after 1 and 6 weeks: (i) 16.86 (4.90), 22.27 (5.08), 27.44 (2.87) and (ii) 15.42 (5.03), 20.97 (6.05), 26.23 ( 3.11). Not significant. Side effects (i) 6 patients, (ii) 6 patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded ‐; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 3. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Inadequate ‐ allocated to 2 groups according to the last 2 digits of their hospital numbers. |
Waikakul 1996.
| Methods | RCT; single‐blind. Randomization procedure not described. | |
| Participants | 64 ambulant patients, 16 men, 48 women. Inclusion criteria: non‐surgical lumbago including disc syndrome, spinal stenosis, postural back pain. Exclusion criteria: need for surgery. | |
| Interventions | NSAID (i): indomethacin plaster twice a day, 4 weeks plus 1 mg oral vitamin B bid (N=30). NSAID (ii): diclofenac emulgel, 2 cm 4 times a day, 4 weeks plus 1 mg oral vitamin B bid (N=34). | |
| Outcomes | Mean (SD) score on 30‐point total rating scale at baseline and after 1, 2 and 3 weeks: (i) 18.13 (4.32), 21.43 (4.10), 24.27 (5.30), 25.87 (4.50), (ii) 18.65 (4.53), 20.46 (6.68), 24.30 (5.39), 26.33 (4.12). Not significant. % of patients with good improvement, safety and usefulness after 3 weeks: (i) 87%, 97%, 87%, (ii) 82%, 100%, 82%. Not significant. Side effects: (i) 3 patients, ii) none. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions ‐; patients blinded ‐; outcomes blinded ‐; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 4. Clinical relevance: patients description + intervention description ‐ outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Waterworth 1985.
| Methods | RCT. Randomization procedure not described. | |
| Participants | 112 patients presenting with acute LBP to their general practitioners, aged 18 to 50 years, 70 men and 38 women. Inclusion criteria: sudden onset of moderate to severe LBP with or without radiation, aggravated by sitting or physical activity, relieved by rest, present for less than 1 month, bothersome pain and a considerable degree of functional incapacity. Exclusion criteria: established spinal disorders, pregnant women, aspirin hypersensitivity, long‐term use of NSAIDs, steroids or anticoagulants, haematological, renal or hepatic disease, history of peptic ulcer. | |
| Interventions | NSAID (i): diflunisal 500 mg capsules, 1000 mg immediately, 500 mg twice daily, 10 days (N=36). Reference treatment (ii): physiotherapy: local heat, ultrasound and exercises (5 weekly sessions of 45 minutes (N=34). Reference treatment (iii): spinal manipulation and/or McKenzie therapy (5 sessions of 45 minutes weekly (N=38). | |
| Outcomes | Mean change in pain intensity on 4‐points scale after 4 and 12 days: (i) ‐0.9, ‐1.7 (ii) ‐0.9, ‐1.6 (iii) ‐1.1, ‐1.7. No significant differences in pain and mobility. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 4. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Weber 1980.
| Methods | CCT; double‐blind. Not randomized. | |
| Participants | 59 hospital patients with acute and subacute sciatica, 32 men and 27 women, aged 16 to 69 years. Exclusion criteria: indication for immediate surgical intervention, contra‐indications for NSAIDs. | |
| Interventions | NSAID (i): phenylbutazone 200 mg capsules, 2 capsules 3 times per day for 3 days, 1 capsule 3 times per day next 2 days, 5 days (N=28). Reference treatment (ii): placebo capsules (N=29). | |
| Outcomes | No. of patients reporting definite positive effect after intervention period (i) 14 (ii) 8. No significant differences. There were no side effects reported by the patients. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance ‐; baseline similarity ‐; follow‐up ‐. Total score = 6. Clinical relevance: patients description + intervention description + outcome measures ‐ effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Not randomized |
Weber 1993.
| Methods | RCT; double‐blind, placebo‐ controlled. Randomization procedure not described. | |
| Participants | 214 patients aged 18 to 75 years. Inclusion criteria: acute sciatica within 14 days of onset, radiating pain , L5 or S1 nerve root compression, with or without sensory and/or motor deficits, positive straight leg raising, reduced mobility of the lumbar spine in standing position, free from sciatica during past 6 months. Exclusion criteria: cauda equina syndrome, acute back, progressive paresis, suspected tumor or local infection, ankylosing spondylitis, rheumatoid arthritis, history of peptic ulcer or severe dyspepsia, hypersensitivity to aspirin or other NSAIDs, hematologic, hepatic, renal, pulmonary, cardiac, or systematic disease, severe psychiatric disease, drug addiction, alcoholism. | |
| Interventions | NSAID (i): piroxicam 20 mg capsules, 40 mg per day first two days, 20 mg per day next 12 days, 14 days (N=120). Reference treatment (ii): placebo capsules (N=94). | |
| Outcomes | Reduction of pain in back and leg measured by visual analogue scales after 4 weeks the same in the two groups (data in graphs). No significant differences. More side effects in (i) 22 than in (ii) 13. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance +; baseline similarity +; follow‐up ‐. Total score = 8. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Wiesel 1980.
| Methods | RCT. Randomization procedure not described. | |
| Participants | 45 patients admitted to a military hospital for bed rest, all men, aged 17 to 34 years. Inclusion criteria: no previous back pain, no radiating, normal neurologic and straight leg raising results, normal lumbar roentgenograms. Exclusion criteria: discovering entities such as spina bifida on the roentgenogram. | |
| Interventions | NSAID (i): aspirin 625 mg capsules, 4 times per day, 2 weeks (N=?). NSAID (ii): phenylbutazone 100 mg capsules, 4 times per day (first 5 days), no further information (N=?). Reference treatment (iii): acetaminophen (dosage not given), twice daily, 2 weeks (N=?). | |
| Outcomes | Mean no. of days before return to full activity (i) 5.7 (ii) 6.5 (iii) 5.7. No significant differences. No data on side‐effects given. | |
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded ‐; outcomes blinded ‐; care providers blinded ‐; intention‐to‐treat +; compliance ‐; baseline similarity +; follow‐up ‐. Total score = 4. Clinical relevance: patients description ‐ intervention description ‐ outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
Ximenes 2007.
| Methods | RCT; double‐blind; multicentre. Randomised according to computer generated random allocation table, stratified for pain intensity | |
| Participants | 340 patients from 31 centres, 173 female, 18 to 65 years, Patients with acute low back pain (<=72 hours), class 1a or 2a (Quebec Task Force), VAS Pain >50 mm | |
| Interventions | NSAIDs (i): valdecoxib 40 mg per day, second dose on day 1, 7 days (N=170) NSAIDs (ii): diclofenac 75 mg b.i.d., 7 days (N=170) | |
| Outcomes | Mean difference (95% CI) pain intensity scale (100 mm VAS) at 7 days; (i, N=167 vs ii, N=166) 0.26 (‐3.76 to 4.28) Mean difference (95% CI) OLBPDQ (0 to 24 pt scale) over 4 weeks; (i vs ii) 0.02% (‐3.21 to 3.16) | |
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance ‐; baseline similarity +; follow‐up +. Total score = 8. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Randomised according to computer generated random allocation table, stratified for pain intensity |
Yakhno 2006.
| Methods | RCT; double‐blind; multicentre. Randomised according to computer generated random allocation table | |
| Participants | 220 patients from 6 centres, 18‐55 years, Patients with acute low back pain (<5 days), 11‐point NRS Pain >5 | |
| Interventions | NSAIDs (i): lornoxicam on day 1: 16 mg once a day and 8 mg once a day; day 2 to 7: 8 mg b.i.d. (N=110) NSAIDs (ii): diclofenac on day 1: 150 mg once a day and 50 mg once a day; day 2 to 7: 50 mg b.i.d. (N=110) | |
| Outcomes | Sum of pain intensity differences from baseline on day 1‐6 (SE); (i, N=109) 4.2 (0.17); (ii, N=110) 3.8 (0.17); (i) significantly lower than (ii) P < 0.05 Side effects: (i) 27; (ii) 28 |
|
| Notes | Methodological quality: randomization +; treatment allocation +; withdrawals +; co‐interventions ‐; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat +; compliance +; baseline similarity +; follow‐up +. Total score = 10. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Adequate ‐ Randomised according to computer generated random allocation table |
Zerbini 2005.
| Methods | RCT; "flare design"; double‐blind, multicentre. Randomization procedure not described. | |
| Participants | 440 patients from 62 centres, 315 female, 125 male, 19 to 85 years, with chronic low back pain, regular usage of analgesic medication; pain without radiation to an extremity and without neurological signs or pain with radiation to an extremity, but not below the knee and without neurological signs; flare criteria after washout period; LBP intensity <= 80 on 100 mm VAS. | |
| Interventions | NSAIDs (i): etoricoxib 60 mg per day, 4 weeks (N=222) NSAIDs (ii): diclofenac 3 * 50 mg per day, 4 weeks (N=218) | |
| Outcomes | Mean difference (95% CI) pain intensity scale (100 mm VAS) at 4 weeks; (i, N= 222 vs ii, N= 218) 2.51 (‐1.50 to 6.51)
Mean difference (95% CI) RMDQ (0‐ 24 pt scale) over 4 weeks; (i vs ii) ‐0.23 (‐1.14 to 0.67)
Mean difference (95% CI) LBP bothersomeness scale over 4 weeks (4‐pt Likert, 0= not at all, 4= extremely); (i vs ii) ‐0.02 (‐0.17 to 0.13) Side effects: (i) 79 patients (15 withdrew); (ii) 87 patients (13 withdrew) |
|
| Notes | Methodological quality: randomization ‐; treatment allocation ‐; withdrawals +; co‐interventions +; patients blinded +; outcomes blinded +; care providers blinded +; intention‐to‐treat ‐; compliance +; baseline similarity +; follow‐up +. Total score = 8. Clinical relevance: patients description + intervention description + outcome measures + effect size ‐ benefits/ harms ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Randomization procedure not described. |
b.i.d. = twice a day t.i.d. = three times a day q.i.d. = four times a day NRS = numeric rating scale VAS = visual analogue scale SEM = standard error SD = standard deviation OLBPDQ ‐ Oswestry LBP Disability Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arslan 1999 | Language criteria. |
| Berry 1988a | No NSAID but muscle relaxant (tizanidine) evaluated. |
| Coletta 1988 | Not low back pain, but low back and neck pain. |
| Hong 2006 | Language criteria. |
| Katz 2004 | Only described the onset of pain relief. |
| Kittang 2001 | Language criteria. |
| Lloyd 2004 | Additional information to earlier reported study. |
| Nadler 2003 | Additional information to earlier reported study. |
| Natour 2002 | Language criteria |
| Pallay 2004 | Additional information to earlier reported study. |
| Pownall 1985 | Investigated different dosage regimes of the same NSAID. |
| Veenema 2000 | Only described the onset of pain relief. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN012605000036617.
| Trial name or title | A clinical trial of manipulative therapy and/or NSAIDs for significant acute low back pain |
| Methods | |
| Participants | 1) Primary complaint of pain extending in an area between the 12th rib and buttock crease. This may or may not be accompanied by leg pain. 2) New episode of low back pain. 3) Pain of less than 6 weeks duration (in accordance with the Cochrane Collaboration Back Review Group definition for acute pain). 4) Low back pain severe enough to cause moderate pain and moderate interference with normal work including work outside the home and housework (as measured by adaptations of items 7 and 8 of the SF‐36). |
| Interventions | Study group 1: NSAIDs (Diclofenac 50 mg bd) and spinal manipulative therapy for up to 4 weeks Study group 2: NSAIDs (Diclofenac 50 mg bd) and placebo spinal manipulative therapy for up to 4 weeks Study group 3: Placebo NSAIDs and spinal manipulative therapy for up to 4 weeks: Control group: Placebo NSAIDs and placebo spinal manipulative therapy for up to 4 weeks |
| Outcomes | The primary outcome is the number of days to recovery with recovery defined in two ways. Firstly recovery is defined as a pain score of 0 or 1 on a 0‐10 pain scale (numerical pain rating scale) that is maintained for seven consecutive days. Secondly recovery is defined as the first day that the patient has a pain score or 0 or 1 on a 0 to 10 pain scale. Pain measured at baseline, 1 week, 2 weeks, 4 weeks and 3 months. Disability measured at baseline, 1 week, 2 weeks, 4 weeks and 3 months. Global perceived effect and satisfaction/beliefs about treatment. |
| Starting date | 1/06/2005 |
| Contact information | Mr Mark Hancock email: M.Hancock@fhs.usyd.edu.au |
| Notes | NHMRC project grant , Australia exclusion criteria: 1) known or suspected serious spinal pathology (metastatic, inflammatory or infective diseases of the spine, cauda equina syndrome, spinal fracture). 2) nerve root compromise evidenced by at least two of the following (i) myotomal weakness, (ii) dermatomal or widespread sensory loss, (iii) hypo or hyperreflexia of the lower limb reflexes; 3) currently taking NSAIDs. 4) currently receiving SMT. 5) spinal surgery within the preceding 6 months; 6) history of peptic ulcer; 7) allergy to aspirin; 8) currently receiving anticoagulant therapy; 9) serious co‐morbidities preventing prescription of NSAIDs or paracetamol eg: cardiac, liver or renal failure |
NCT00393211.
| Trial name or title | Effect of Etoricoxib to Treat Chronic Low Back Pain |
| Methods | |
| Participants | Recurrent Low Back Pain |
| Interventions | Drug: MK0663, Etoricoxib / Duration of Treatment : 12 Weeks Drug: Comparator : placebo (unspecified) /Duration of Treatment : 12 Weeks |
| Outcomes | (i) Low back pain intensity scale (0‐100 mm VAS) over 4 weeks compared to placebo (ii) "Low back pain intensity scale (0‐100 mm VAS) over 12 weeks compared to placebo |
| Starting date | April 2000 (recruitment completed) |
| Contact information | Medical Monitor, Study Director, Merck |
| Notes | Inclusion Criteria : "Low back pain for previous 3 months and majority of days in last month "Regular use of acetaminophen or NSAID to treat low back pain in past month "Judged to be in otherwise good health Exclusion Criteria : "Low back pain that is due to secondary causes "Radicular/myelopathic pain "Surgery for low back pain within the past 6 months "Active lawsuit or claim pertaining to their low back pain "Disease that may confound the results of the study or pose risk to the patients "Corticosteroid use in past month "Previous participation in an MK0663 study |
Contributions of authors
Study selection: BW Koes and MW van Tulder original version MW van Tulder and PDDM Roelofs update
Methodologic quality assessment, data extraction and analyses: RJPM Scholten and MW van Tulder original version BW Koes and PDDM Roelofs update
Writing of the review protocol: RA Deyo, BW Koes, RJPM Scholten and MW van Tulder
All authors were involved in writing the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
Dutch Health Insurance Board, Not specified.
Declarations of interest
One of the authors (Maurits van Tulder) is co‐ordinating editor and one of the authors (Rick Deyo) is an editor with the Cochrane Back Review Group. Editors are required to conduct at least one Cochrane review. This requirement ensures that editors are aware of the processes and commitment needed to conduct reviews. This involvement does not seem to be a source of conflict of interest in the Cochrane Back Review Group. Any editor who is a review author is excluded from editorial decisions on the review in which they are contributors.
Edited (no change to conclusions)
References
References to studies included in this review
Aghababian 1986 {published data only}
- Aghababian RV, Volturo GA, Heifetz IN. Comparison of diflunisal and naproxen in the management of acute low back pain. Clin Ther 1986;9(Suppl. c):47‐51. [PubMed] [Google Scholar]
Agrifoglio 1994 {published data only}
- Agrifoglio E, Benvenutti M, Gatto P, Albanese L, Chrubino P, Marinoni EC, et al. Aceclofenac: a new NSAID in the treatment of acute lumbago. Multicentre single blind study vs. diclofenac. Acta Therapeutica 1994;20:33‐43. [Google Scholar]
Amlie 1987 {published data only}
- Amlie E, Weber H, Holme I. Treatment of acute low back pain with piroxicam: results of a double‐blind placebo‐controlled trial. Spine 1987;12:473‐6. [DOI] [PubMed] [Google Scholar]
Aoki 1983 {published data only}
- Aoki T, Kuroki Y, Kageyama T, Irimajiri S, Mizushima Y, Yamamoto K. Multicentre double‐blind comparison of piroxicam and indomethacin in the treatment of lumbar diseases. Eur J Rheum Inflam 1983;6:247‐52. [PubMed] [Google Scholar]
Babej‐Dolle 1994 {published data only}
- Babej‐Dolle R, Freytag S, Eckmeyer J, Zerle G, Schinzel S, Schmeider G, et al. Parenteral dipyrone versus diclofenac and placebo in patients with acute lumbago or sciatic pain: randomized observer‐blind multicenter study. Int J Clin Pharmacol Ther 1994;32:204‐9. [PubMed] [Google Scholar]
Bakshi 1994 {published data only}
- Bakshi R, Thumb N, Bröll H, Klein G, Mayrhofer F, Rainer F, et al. Treatment of acute lumbosacral back pain with diclofenac resinate: results of a double‐blind comparative trial versus piroxicam. Drug Invest 1994;8:288‐93. [Google Scholar]
Basmajian 1989 {published data only}
- Basmajian JV. Acute back pain and spasm: a controlled multicenter trial of combined analgesic and antispasm agents. Spine 1989;14:438‐9. [PubMed] [Google Scholar]
Berry 1982 {published data only}
- Berry H, Bloom B, Hamilton EBD, Swinson DR. Naproxen sodium, diflunisal, and placebo in the treatment of chronic back pain. Ann Rheum Dis 1982;41:129‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 1988b {published data only}
- Berry H, Hutchinson DR. Tizanidine and ibuprofen in acute low‐back pain: results of a double‐blind multicentre study in general practice. J Int Med Res 1988;16:83‐91. [DOI] [PubMed] [Google Scholar]
Birbara 2003 {published data only}
- Birbara CA, Puopolo AD, Munoz DR, Sheldon EA, Mangione A, Bohidar NR, et al. Treatment of chronic low back pain with etoricoxib, a new cyclo‐oxygenase‐2 selective inhibitor: improvement in pain and disability ‐ a randomized, placebo‐controlled, 3‐month trial. J Pain 2003;4(6):307‐15. [DOI] [PubMed] [Google Scholar]
Blazek 1986 {published data only}
- Blazek M, Keszthelyi B, Varhelyi M, Korosi O. Comparative study of biarison and voltaren in acute lumbar pain and lumbo‐ischialgia. Ther Hung 1986;34:163‐6. [PubMed] [Google Scholar]
Borenstein 1990 {published data only}
- Borenstein DG, Lacks S, Wiesel SW. Cyclobenzaprine and naproxen versus naproxen alone in the treatment of acute low back pain and muscle spasm. Clin Ther 1990;12:125‐31. [PubMed] [Google Scholar]
Braun 1982 {published data only}
- Braun H, Huberty R. Therapy of lumbar sciatica. A comparative clinical study of a corticoid‐free monosubstance and a corticoid containing combination drug. Medizinische Welt 1982;33:490‐1. [PubMed] [Google Scholar]
Brown 1986 {published data only}
- Brown FL, Bodison S, Dixon J, Davis W, Nowoslawski J. Comparison of diflunisal and acetaminophen with codeine in the treatment of initial or recurrent acute low back pain. Clin Ther 1986;9(Suppl. c):52‐58. [PubMed] [Google Scholar]
Bruggemann 1990 {published data only}
- Bruggemann G, Koehler CO, Koch EM. Results of a double‐blind study of diclofenac + vitamin B1, B6, B12 versus diclofenac in patients with acute pain of the lumbar vertebrae. A multicenter study. Klinische Wochenschrift 1990;68:116‐20. [DOI] [PubMed] [Google Scholar]
Chrubasik 2003 {published data only}
- Chrubasik S, Model A, Black A, Pollak S. A randomized double‐blind pilot study comparing Doloteffin and Vioxx in the treatment of low back pain. Rheumatology (Oxford) 2003;42(1):141‐8. [DOI] [PubMed] [Google Scholar]
Coats 2004 {published data only}
- Coats TL, Borenstein DG, Nangia NK, Brown MT. Effects of valdecoxib in the treatment of chronic low back pain: results of a randomized, placebo‐controlled trial. Clin Ther 2004;26(8):1249‐60. [DOI] [PubMed] [Google Scholar]
Colberg 1996 {published data only}
- Colberg K, Hettich M, Sigmund R, Degner FL. The efficacy and tolerability of an 8‐day administration of intravenous and oral meloxicam: a comparison with intramuscular and oral diclofenac in patients with acute lumbago. German Meloxicam Ampoule Study Group. Curr Med Res Opin 1996;13:363‐77. [DOI] [PubMed] [Google Scholar]
Davoli 1989 {published data only}
- Davoli L, Ciotti G, Biondi M, Passeri M. Piroxicam‐beta‐cyclodextrin in the treatment of low‐back pain. Controlled study vs etodolac. Curr Ther Res Clin Exp 1989;46:940‐7. [Google Scholar]
Dreiser 2001 {published data only}
- Dreiser RL, Parc JM, Velicitat P, Lleu PL. Oral meloxicam is effective in acute sciatica: two randomised, double‐blind trials versus placebo or diclofenac. Inflamm Res 2001;50(1):S17‐23. [DOI] [PubMed] [Google Scholar]
Dreiser 2001b {published data only}
- Dreiser RL, Parc JM, Velicitat P, Lleu PL. Oral meloxicam is effective in acute sciatica: two randomised, double‐blind trials versus placebo or diclofenac. Inflamm Res 2001;50(1):S17‐23. [DOI] [PubMed] [Google Scholar]
Dreiser 2003 {published data only}
- Dreiser RL, Marty M, Ionescu E, Gold M, Liu JH. Relief of acute low back pain with diclofenac‐K 12.5 mg tablets: a flexible dose, ibuprofen 200 mg and placebo‐controlled clinical trial. Int J Clin Pharmacol Ther 2003;41(9):375‐85. [DOI] [PubMed] [Google Scholar]
Driessens 1994 {published data only}
- Driessens M, Famaey JP, Orloff S, Chochrad I, Cleppe D, Brabanter G, et al. Efficacy and tolerability of sustained‐release ibuprofen in the treatment of patients with chronic back pain. Curr Ther Res Clin Exp 1994;55:1283‐92. [Google Scholar]
Evans 1980 {published data only}
- Evans DP, Burke MS, Newcombe RG. Medicines of choice in low back pain. Curr Med Res Opin 1980;6:540‐7. [DOI] [PubMed] [Google Scholar]
Famaey 1998 {published data only}
- Famaey JP, Bruhwyler J, Geczy J, Vandekerckhove K, Appelboom T. Open controlled randomized multicenter comparison of nimesulide and diclofenac in the treatment of subacute and chronic low back pain. Journal of Clinical Research 1998;1:219‐38. [Google Scholar]
Goldie 1968 {published data only}
- Goldie I. A clinical trial with indomethacin (Indomee) in low back pain and sciatica. Acta Orthop Scand 1968;39:117‐28. [DOI] [PubMed] [Google Scholar]
Hickey 1982 {published data only}
- Hickey RF. Chronic low back pain: a comparison of diflunisal with paracetamol. NZ Med J 1982;May:312‐4. [PubMed] [Google Scholar]
Hingorani 1970 {published data only}
- Hingorani K, Biswas AK. Double‐blind controlled trial comparing oxyphenbutazone and indomethacin in the treatment of acute low back pain. Br J Clin Pract 1970;24:120‐3. [PubMed] [Google Scholar]
Hingorani 1975 {published data only}
- Hingorani K, Templeton JS. A comparative trial of azapropazone and ketoprofen in the treatment of acute backache. Curr Med Res Opin 1975;3:407‐12. [DOI] [PubMed] [Google Scholar]
Hosie 1993 {published data only}
- Hosie GAC. The topical NSAID, felbinac, versus oral ibuprofen: a comparison of efficacy in the treatment of acute lower back injury. Br J Clin Res 1993;4:5‐17. [Google Scholar]
Ingpen 1969 {published data only}
- Ingpen ML. A controlled clinical trial of sustained‐action dextropropoxyphene hydrochloride. Br J Clin Pract 1969;23:113‐5. [PubMed] [Google Scholar]
Innes 1998 {published data only}
- Innes GD, Croskerry P, Worthington J, Beveridge R, Jones D. Ketorolac versus acetaminophen‐codeine in the emergency department treatment of acute low back pain. J Emerg Med 1998;16(4):549‐56. [DOI] [PubMed] [Google Scholar]
Jacobs 1968 {published data only}
- Jacobs JH, Grayson MF. Trial of anti‐inflammatory agent (indomethacin) in low back pain with and without redicular involvement. Br Med J 1968;3:158‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaffe 1974 {published data only}
- Jaffe G. A double‐blind, between‐patient comparison of alclofenac (' Prinalgin' ) and indomethacin in the treatment of low back pain and sciatica. Curr Med Res Opin 1974;2:424‐9. [DOI] [PubMed] [Google Scholar]
Katz 2003 {published data only}
- Katz N, Ju WD, Krupa DA, Sperling RS, Bozalis Rodgers D, Gertz BJ, et al. Efficacy and safety of rofecoxib in patients with chronic low back pain: results from two 4‐week, randomized, placebo‐controlled, parallel‐group, double‐blind trials. Spine 2003;28(9):851‐8. [DOI] [PubMed] [Google Scholar]
Kuhlwein 1990 {published data only}
- Kuhlwein A, Meyer HJ, Koehler CO. Reduced diclofenac administration by B vitamins: results of a randomized double‐blind study with reduced daily doses of diclofenac (75 mg diclofenac versus 75 mg diclofenac plus B vitamins) in acute lumbar vertebral syndromes. Klinische Wochenschrift 1990;68:107‐15. [DOI] [PubMed] [Google Scholar]
Lacey 1984 {published data only}
- Lacey PH, Dodd GD, Shannon DJ. A double‐blind placebo controlled study of piroxicam in the management of acute musculoskeletal disorders. Eur J Rheum Inflam 1984;7:95‐104. [PubMed] [Google Scholar]
Listrat 1990 {published data only}
- Listrat V, Dougados M, Chevalier X, Kramer F, Amor B. Comparison of the analgesic effect of tenoxicam after oral or intramuscular administration. Drug Invest 1990;2(Suppl 3):51‐2. [Google Scholar]
Matsumo 1991 {published data only}
- Matsumo S, Kaneda K, Nohara Y. Clinical evaluation of Ketoprofen (Orudis) in lumbago: a double blind comparison with diclofenac sodium. Br J Clin Pract 1991;35:266. [PubMed] [Google Scholar]
Metscher 2001 {published data only}
- Metscher, B. Kubler, U. Jahnel‐Kracht, H. Dexketoprofen‐trometamol and tramadol in acute lumbago [Dexketoprofen‐Trometamol und Tramadol bei akuter Lumbago]. Fortschr Med Orig 2001;118(4):147‐51. [PubMed] [Google Scholar]
Milgrom 1993 {published data only}
- Milgrom C, Finestone A, Lev B, Wiener M, Floman Y. Overexertional lumbar and thoracic back pain among recruits: a prospective study of risk factors and treatment regimens. J Spinal Dis 1993;6:187‐93. [DOI] [PubMed] [Google Scholar]
Muckle 1986 {published data only}
- Muckle DS. Flurbiprofen for the treatment of soft tissue trauma. Am J Med 1986;80:76‐80. [DOI] [PubMed] [Google Scholar]
Nadler 2002 {published data only}
- Nadler SF, Steiner DJ, Erasala GN, Hengehold DA, Hinkle RT, Beth Goodale M, et al. Continuous low‐level heat wrap therapy provides more efficacy than Ibuprofen and acetaminophen for acute low back pain. Spine 2002;27(10):1012‐7. [DOI] [PubMed] [Google Scholar]
Orava 1986 {published data only}
- Orava S. Medical treatment of acute low back pain. Diflunisal compared with indomethacin in acute lumbago. Int J Clin Res 1986;6:45‐51. [PubMed] [Google Scholar]
Pena 1990 {published data only}
- Pena M. Etodolac: analgesic effects in musculoskeletal and postoperative pain. Rheum Int 1990;10(Suppl):9‐16. [DOI] [PubMed] [Google Scholar]
Pohjolainen 2000 {published data only}
- Pohjolainen T, Jekunen A, Autio L, Vuorela H. Treatment of acute low back pain with the COX‐2‐selective anti‐inflammatory drug nimesulide: results of a randomized, double‐blind comparative trial versus ibuprofen. Spine 2000;25(12):1579‐85. [DOI] [PubMed] [Google Scholar]
Postacchini 1988 {published data only}
- Postacchini F, Facchini M, Palieri P. Efficacy of various forms of conservative treatment in low back pain: a comparative study. Neurol Orthop 1988;6:28‐35. [Google Scholar]
Radin 1968 {published data only}
- Radin EL, Bryan RS. Phenylbutazone for prolapsed discs?. Lancet 1968;Sept.:736. [DOI] [PubMed] [Google Scholar]
Schattenkirchner2003 {published data only}
- Schattenkirchner M, Milachowski KA. A double‐blind, multicentre, randomised clinical trial comparing the efficacy and tolerability of aceclofenac with diclofenac resinate in patients with acute low back pain. Clin Rheumatol 2003;22(2):127‐35. [DOI] [PubMed] [Google Scholar]
Siegmeth 1978 {published data only}
- Siegmeth W, Sieberer W. A comparison of the short‐term effects of ibuprofen and diclofenac in spondylosis. J Int Med Res 1978;6:369‐74. [DOI] [PubMed] [Google Scholar]
Stratz 1990 {published data only}
- Stratz T. Intramuscular etofenamate in the treatment of acute lumbago. Effectiveness and tolerance in comparison with intramuscular diclofenac‐Na. Fortschritte der Medizin 1990;108:264‐6. [PubMed] [Google Scholar]
Sweetman 1987 {published data only}
- Sweetman BJ, Baig A, Parsons DL. Mefenamic acid, chlormezanone‐paracetamol, ethoheptazine‐aaspirin‐meprobamate: a comparative study in acute low back pain. Br J Clin Pract 1987;41:619‐24. [PubMed] [Google Scholar]
Szpalski 1990 {published data only}
- Szpalski M, Poty S, Hayez JP, Debaize JP. Objective assessment of trunk function in patients with acute low back pain treated with Tenoxicam. A prospective controlled study. Neuro‐orthopedics 1990;10:41‐7. [Google Scholar]
Szpalski 1994 {published data only}
- Szpalski M, Hayez JP. Objective functional assessment of the efficacy of tenoxicam in the treatment of acute low back pain: a double blind placebo‐controlled study. Br J Rheumatol 1994;33:74‐78. [DOI] [PubMed] [Google Scholar]
Vetter 1988 {published data only}
- Vetter G, Bruggemann G, Lettko M, Schwieger G, Asbach H, Biermann W, et al. Shortening diclofenac therapy by B vitamins. Results of a randomized double‐blind study, diclofenac 50 mg versus diclofenac 50 mg plus B vitamins, in painful spinal diseases with degenerative changes. Zeitschrift fur Rheumatologie 1988;47:351‐62. [PubMed] [Google Scholar]
Videman 1984a {published data only}
- Videman T, Heikkila J, Partanen T. Double‐blind parallel study of meptazinol versus diflunisal in the treatment of lumbago. Curr Med Res Opin 1984;9:246‐52. [DOI] [PubMed] [Google Scholar]
Videman 1984b {published data only}
- Videman T, Osterman K. Double‐blind parallel study of piroxicam versus indomethacin in the treatment of low back pain. Ann Clin Res 1984;16:156‐60. [PubMed] [Google Scholar]
Waikakul 1995 {published data only}
- Waikakul S, Soparat K. Effectiveness and safety of loxoprofen compared with naproxen in nonsurgical low back pain: a parallel study. Clin Drug Invest 1995;10:59‐63. [Google Scholar]
Waikakul 1996 {published data only}
- Waikakul S, Danputipong P, Soparat K. Topical analgesics, indomethacin plaster and diclofenac emulgel for low back pain: a parallel study. J Med Assoc Thailand 1996;79:486‐90. [PubMed] [Google Scholar]
Waterworth 1985 {published data only}
- Waterworth RF, Hunter IA. An open study of diflunisal, conservative and manipulative therapy in the management of acute mechanical low back pain. NZ Med J 1985;May:372‐375. [PubMed] [Google Scholar]
Weber 1980 {published data only}
- Weber H, Aasand G. The effect of phenylbutazone on patients with acute lumbago‐sciatica: a double blind trial. J Oslo City Hosp 1980;30:69‐72. [PubMed] [Google Scholar]
Weber 1993 {published data only}
- Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double‐blind placebo‐controlled trial evaluating the effect of piroxicam. Spine 1993;18:1433‐8. [PubMed] [Google Scholar]
Wiesel 1980 {published data only}
- Wiesel SW, Cuckler JM, Deluca F, Jones F, Zeide MS, Rothman RH. Acute low back pain: an objective analysis of conservative therapy. Spine 1980;5:324‐30. [DOI] [PubMed] [Google Scholar]
Ximenes 2007 {published data only}
- Ximenes A, Robles M, Sands G, Vinueza R. Valdecoxib is as efficacious as diclofenac in the treatment of acute low back pain. Clinical Journal of Pain 2007;23(3):244‐50. [DOI] [PubMed] [Google Scholar]
Yakhno 2006 {published data only}
- Yakhno N, Guekht A, Skoromets A, Spirin N, Strachunskaya E, Ternavsky A, et al. Analgesic efficacy and safety of lornoxicam quick‐release formulation compared with diclofenac potassium: Randomised, double‐blind trial in acute low back pain. Clinical Drug Investigation 2006;26(5):267‐77. [DOI] [PubMed] [Google Scholar]
Zerbini 2005 {published data only}
- Zerbini C, Ozturk ZE, Grifka J, Maini M, Nilganuwong S, Morales R, et al. Efficacy of etoricoxib 60 mg/day and diclofenac 150 mg/day in reduction of pain and disability in patients with chronic low back pain: results of a 4‐week, multinational, randomized, double‐blind study. Curr Med Res Opin 2005;21(12):2037‐49. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arslan 1999 {published data only}
- Arslan S, Gokce Kutsal Y. Comparison of the efficacy of etofenamate and tenoxicam in the treatment of low back pain. Journal of Rheumatology and Medical Rehabilitation 1999;10(1):14‐8. [Google Scholar]
Berry 1988a {published data only}
- Berry H, Hutchinson DR. A multicentre placebo‐controlled study in general practice to evaluate the efficacy and safety of tizanidine in acute low‐back pain. J Int Med Res 1988;16:75‐82. [DOI] [PubMed] [Google Scholar]
Coletta 1988 {published data only}
- Coletta R, Maggiolo F, Tizio S. Etofenamate and transcutaneous electrical nerve stimulation treatment of painful spinal syndromes. Int J Clin Pharmacol Res 1988;8:295‐8. [PubMed] [Google Scholar]
Hong 2006 {published data only}
- Hong Y, Wu J, Wang B. The effect of moving cupping therapy on nonspecific low back pain. Chinese Journal of Rehabilitation Medicine 2006;21(4):340‐3. [Google Scholar]
Katz 2004 {published data only}
- Katz N, Rodgers DB, Krupa D, Reicin A. Onset of pain relief with rofecoxib in chronic low back pain: results of two four‐week, randomized, placebo‐controlled trials. Curr Med Res Opin 2004;20(5):651‐8. [DOI] [PubMed] [Google Scholar]
Kittang 2001 {published data only}
- Kittang G, Melvaer T, Baerheim A. Acupuncture versus antiflogistica by acute low back pain in general practice. Tidsskrift for den Norske Laegeforening 2001;121(10):1207‐10. [PubMed] [Google Scholar]
Lloyd 2004 {published data only}
- Lloyd A, Scott DA, Akehurst RL, Lurie‐Luke E, Jessen G. Cost‐effectiveness of low‐level heat wrap therapy for low back pain. Value Health 2004;7(4):413‐22. [DOI] [PubMed] [Google Scholar]
Nadler 2003 {published data only}
- Nadler SF, Steiner DJ, Petty SR, Erasala GN, Hengehold DA, Weingand KW. Overnight use of continuous low‐level heatwrap therapy for relief of low back pain. Arch Phys Med Rehabil 2003;84(3):335‐42. [DOI] [PubMed] [Google Scholar]
Natour 2002 {published data only}
- Natour J, Puertas EB, Radu AS, Freire M, Bonfiglioli R, Schincariol NRB, et al. Loxoprofen in the treatment of low back pain ‐ Clinical efficacy and safety in comparison to diclofenac. Revista Brasileira de Medicina 2002;59(3):161‐70. [Google Scholar]
Pallay 2004 {published data only}
- Pallay RM, Seger W, Adler JL, Ettlinger RE, Quaidoo EA, Lipetz R, et al. Etoricoxib reduced pain and disability and improved quality of life in patients with chronic low back pain: a 3 month, randomized, controlled trial. Scand J Rheumatol 2004;33(4):257‐66. [DOI] [PubMed] [Google Scholar]
Pownall 1985 {published data only}
- Pownall R, Pickvance NJ. Does treatment timing matter? A double blind crossover study of ibuprofen 2400 mg per day in different dosage schedules in treatment of chronic low back pain. Br J Clin Pract 1985;39:267‐75. [PubMed] [Google Scholar]
Veenema 2000 {published data only}
- Veenema KR, Leahey N, Schneider S. Ketorolac versus meperidine: ED treatment of severe musculoskeletal low back pain. Am J Emerg Med 2000;18(4):404‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Zippel 2007 {published data only}
- Zippel H, Wagenitz A. A multicentre, randomised, double‐blind study comparing the efficacy and tolerability of intramuscular dexketoprofen versus diclofenac in the symptomatic treatment of acute low back pain. Clin Drug Investig 2007;27(8):533‐543. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN012605000036617 {unpublished data only}
- Hancock MJ, Maher CG, Latimer J, McLachlan AJ, Cooper CW, Day RO, Spindler MF, McAuley JH. Manipulative therapy and/or NSAIDs for acute low back pain: design of a randomized controlled trial. BMC Musculoskelet Disord 2005; Vol. 6:57. [ACTRN012605000036617] [DOI] [PMC free article] [PubMed]
NCT00393211 {unpublished data only}
- Study Director: Medical Monitor, Merck. A Double‐Blind, Placebo‐Controlled, Parallel‐Group, 12‐Week Trial to Assess the Efficacy and Safety of MK0663 in Patients With Chronic Low Back Pain. ClinicalTrials.gov 2006. [NCT00393211] [Google Scholar]
Additional references
Airaksinen 2006
- Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber‐Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 2006;15 Suppl 2:S192‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deyo 1987
- Deyo RA, Tsui‐Wu Y‐J. Descriptive epidemiology of low‐back pain and its related medical care in the United States. Spine 1987;12:264‐8. [DOI] [PubMed] [Google Scholar]
Deyo 1991
- Deyo RA, Cherkin D, Conrad D, Volinn E. Cost, controversy, crisis: low back pain and the health of the public. Annu Rev Publ Health 1991;12:141‐56. [DOI] [PubMed] [Google Scholar]
Henry 1996
- Henry D, Lim LLY, Garcia Rodriguez LA, Perez Gutthann S, Carson JL, Griffin M, et al. Variability in risk of gastrointestinal complications with individual non‐steroidal anti‐inflammatory drugs: results of a collaborative meta‐analysis. BMJ 1996;312:1563‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koes 1995
- Koes BW, Bouter LM, Heijden GJMG. Methodological quality of randomized clinical trials on treatment efficacy in low back pain. Spine 1995;20:228‐35. [DOI] [PubMed] [Google Scholar]
Koes 2001
- Koes BW, Tulder MW, Ostelo R, Kim Burton A, Waddell G. Clinical guidelines for the management of low back pain in primary care: an international comparison. Spine 2001;26(22):2504‐13; discussion 2513‐4. [DOI] [PubMed] [Google Scholar]
Koes 2006
- Koes BW, Tulder MW, Thomas S. Diagnosis and treatment of low back pain. Bmj 2006;332(7555):1430‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shekelle 1994
- Shekelle PG, Andersson G, Bombardier C, Cherkin D, Deyo R, Keller R, et al. A brief introduction to the critical reading of the clinical literature. Spine 1994;19(18 Suppl):2028S‐2031S. [0362‐2436 (Print)] [DOI] [PubMed] [Google Scholar]
Spitzer 1987
- Spitzer WO, LeBlanc FE, Dupuis M (Eds). Scientific approach to the assessment and management of activity‐related spinal disorders. Spine 1987;7(Suppl):1‐59. [PubMed] [Google Scholar]
van der Roer 2006
- Roer N, Ostelo RW, Bekkering GE, Tulder MW, Vet HC. Minimal clinically important change for pain intensity, functional status, and general health status in patients with nonspecific low back pain. Spine 2006;31(5):578‐82. [1528‐1159 (Electronic)] [DOI] [PubMed] [Google Scholar]
van Tulder 1995
- Tulder MW, Koes BW, Bouter LM. A cost‐of‐illness study of back pain in the Netherlands. Pain 1995;62:233‐40. [DOI] [PubMed] [Google Scholar]
van Tulder 1997
- Tulder MW, Koes BW, Bouter LM. Conservative treatment of non‐specific low back pain: a systematic review of the most common therapeutic interventions for acute and chronic low back pain. Spine 1997;22:2128‐56. [DOI] [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, The Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
van Tulder 2006
- Tulder M, Becker A, Bekkering T, Breen A, Real MT, Hutchinson A, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J 2006;15 Suppl 2:S169‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waddell 1987
- Waddell G. A new clinical model for the treatment of low back pain. Spine 1987;12:632‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van Tulder 2000
- Tulder MW, Scholten RJPM, Koes BW, Deyo RA. Non‐steroidal anti‐inflammatory drugs for low‐back pain. Cochrane Database of Systematic Reviews 2000, Issue 2. [Art. No.: CD000396. DOI: 10.1002/14651858.CD000396] [DOI] [PubMed] [Google Scholar]


