Abstract
Purpose
Blindness is a well-known complication of filler injection in the glabellar region. Acute diplopia from filler injection without vision loss is a rare complication that typically results in clinical ophthalmoplegia which can have permanent sequelae. Here, we describe a patient who presented with acute diplopia with grossly intact full extraocular motility after glabella hyaluronic acid filler injection that resolved after 1 month.
Observations
A previously healthy 43-year-old woman underwent her first hyaluronic acid injection in the glabella and developed immediate binocular double vision with severe pain and skin mottling above her right eyebrow and central forehead. Hyaluronidase injections, nitroglycerin paste, and aspirin were immediately administered. On exam, there was significant skin mottling over the glabella, extending to the forehead and nose with a small incomitant horizontal and vertical misalignment. No changes to her vision were observed and extraocular motility was grossly full. The rest of her exam was unremarkable. Over the course of the following month, the patient's diplopia resolved, but she developed skin necrosis and scarring.
Conclusions
Importance: Proper knowledge of facial and periocular anatomy is critical for practitioners to safely perform filler injections and manage potential complications. Patients should be counseled about the potential rare risks of these elective procedures.
Keywords: Facial filler injection, Diplopia, Hyaluronic acid, Skin necrosis, Scarring
1. Introduction
Facial filler injections are currently the second most popular non-surgical aesthetic procedure after botulinum toxin injection, and include compounds such as hyaluronic acid derivatives, calcium hydroxyapatite, poly-l-lactic acid, and polymethylmethacrylate.1 Despite their popularity, complications from filler injections have been well documented, particularly injections of the glabella and nose that have included blindness.1,2 These complications can have long-term effects on visual function, sensation, cosmesis, and mental health.
The present report describes a patient who presented with acute transient diplopia after hyaluronic acid filler injection with evidence of filler migration. Previous reports often describe diplopia with clinically measurable ophthalmoplegia after filler injection and variable recovery of function.3, 4, 5 Our patient presented with transient diplopia and an incomitant strabismus. which resolved after 1 month. To our knowledge, this is the first case report of transient diplopia after filler injection with no clinically appreciable extraocular motility deficit and no vision loss.
2. Case report
A 43-year-old healthy woman underwent a glabellar injection of hyaluronic acid/lidocaine (Allergan plc, Dublin, Ireland) for effacement of superficial wrinkles. As the first syringe was being injected (0.45 mL at 17.5 mg/mL), she experienced severe pain above her right eyebrow and forehead. Upon opening her eyes, she immediately noticed double vision and developed nausea and vomiting, as well as skin mottling of her forehead. She was promptly injected with two doses of 300 units and a subsequent 150 units of hyaluronidase into the affected area and provided systemic therapy that included nitroglycerin paste, aspirin, and an empiric dose of Augmentin.
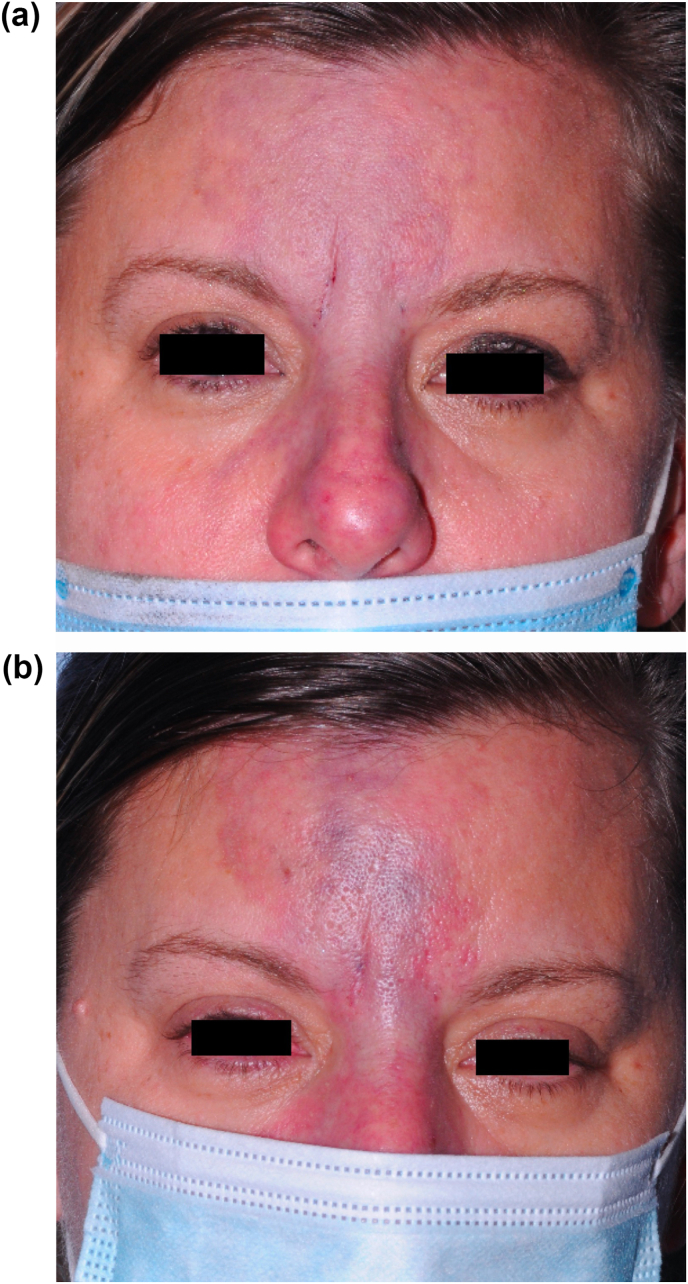
Within 2 hours after the injections, the patient presented to the ophthalmology clinic, where she reported pain localized to the forehead and binocular vertical diplopia that was worse with downgaze. She denied vision loss, eye pain, or pain that worsened with eye movements. External examination revealed significant skin mottling with dusky blue-red discoloration of the mid-upper forehead involving the glabella and extending to the tip of her nose but no ptosis (Fig. 1A). The affected area was numb to touch. Visual acuity was 20/20 in each eye and her pupils were equal and reactive to light with no afferent pupillary defect. Extraocular motility and visual fields were grossly full. Alignment exam was notable for 2 PD of esotropia that worse in left gaze as well as 2 PD of right hypertropia worse in inferior gaze. Slit lamp and funduscopic examination was otherwise unremarkable with no evidence of conjunctival injection, optic nerve pallor, vascular occlusion, retinal edema, or hemorrhage. Because the patient reported near resolution of her nausea and vomiting during her ophthalmology clinic evaluation with no evidence of any other neurological deficit, additional workup or imaging was not pursued.
Fig. 1.
External photographs at initial presentation (a) and 2-day follow-up (b) revealing worsening skin mottling involving the glabella and extending to the mid-tip of her nose.
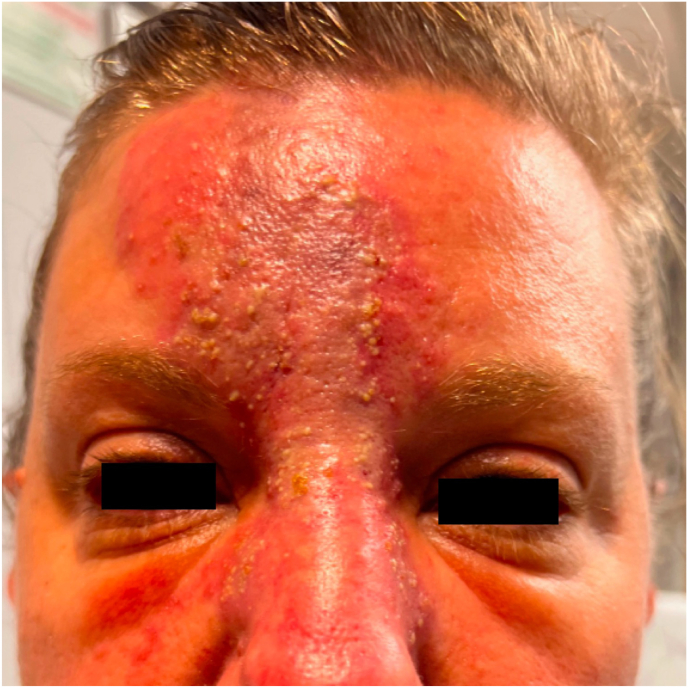
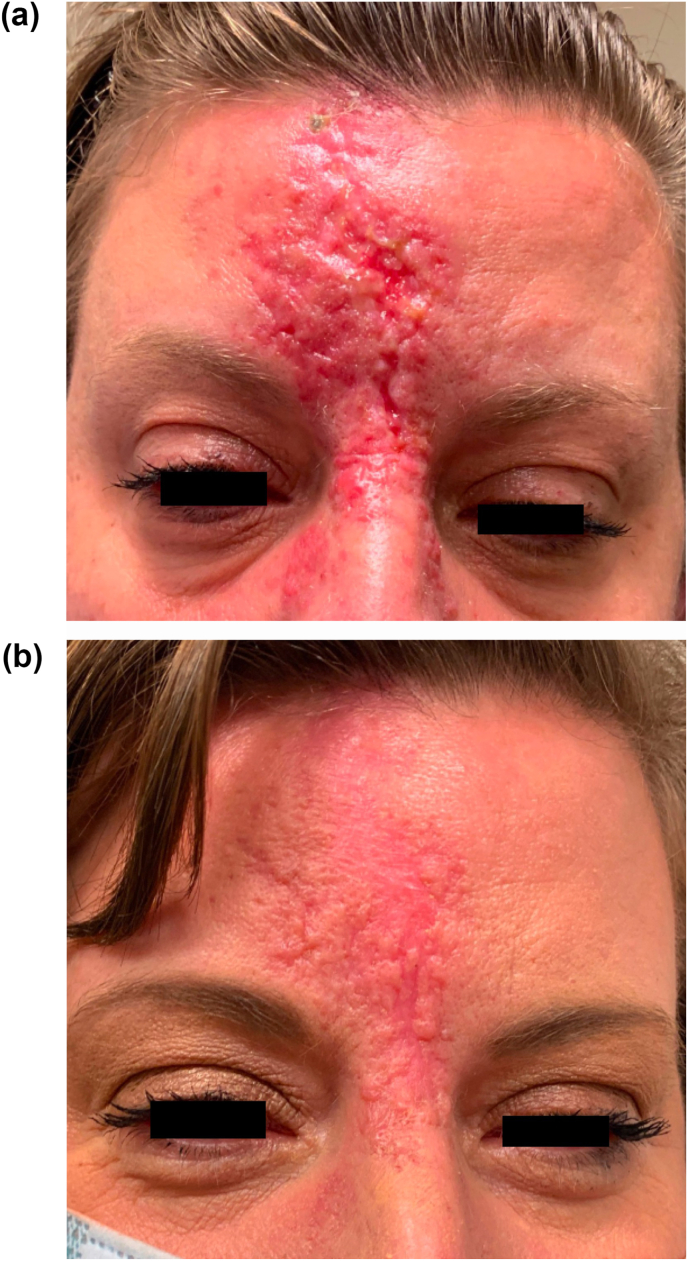
On follow up 2 days later, the patient reported continued improvement of her diplopia, with no symptoms in primary gaze, but still with intermittent horizontal binocular diplopia. On exam, there was increased erythema and mottling of the affected area of her forehead (Fig. 1B). However, on alignment testing, she was now noted to have 2–4 PD of exotropia worse on left gaze and persistent 1–3 PD of right hypertropia worse on downgaze. A week later, the patient presented to the emergency department after developing a severe pustular rash of the mottled area, consistent with clinical progression of her skin necrosis. She was empirically treated with 1 g Valtrex TID due to the severity of her findings and instructed to apply petroleum jelly and warm compresses to the affected area (Fig. 2). While the patient's diplopia completely resolved after 2 months, she developed hypertrophic scarring of her glabella and forehead (Fig. 3) for which she is scheduled to have scar revision after complete demarcation at 1 year.
Fig. 2.
External photograph at 1-week follow-up for which the patient presented with progression of skin necrosis; she was conservatively managed with empiric treatment for herpes viral superinfection.
Fig. 3.
External photographs at 2-weeks follow up (a) and 2-months follow-up (b) depicting hypertrophic scarring of her glabella and forehead, with plan for scar revision after 1 year.
3. Discussion
Cosmetic facial filler injections are commonly used to focally augment tissue volume to improve cosmesis.1,2 However, risks include ophthalmoplegia, skin necrosis, and blindness.3, 4, 5 This patient presented with transient diplopia with a small incomitant strabismus, but no clinically measurable ophthalmoplegia or vision loss after filler injection of the glabella. The glabella is a particularly vulnerable site for complication because of its vascular supply, which includes two significant branches of the ophthalmic artery, the supraorbital and supratrochlear arteries, the latter of which was likely occluded in the present case. Filler can be inadvertently injected into these branches, where it can migrate retrogradely towards the ophthalmic artery and its other branches.6 Possible mechanisms of diplopia include filler entering the arterial branches supplying the extraocular muscles and causing ischemia of the muscles, inflammation, or toxicity to the cranial nerves.3, 4, 5
Our patient had skin findings consistent with intravascular infiltration, as well as a small incomitant strabismus, but grossly full extraocular motility on multiple examinations. Iatrogenic ophthalmoplegia frequently occurs in the setting of occlusion of the ophthalmic artery and its branches and can resolve over time.5 Non-intravascular filler migration leading to ophthalmoplegia has also been reported in the literature. Suggested mechanisms of filler migration include a high volume of injected filler, facial muscle activity, and pressure-induced migration, which may lead to extravasation of filler into periorbital surrounding tissue.7 Filler extravasation into the periocular adnexa including the conjunctiva, muscles and further in the orbit may cause inflammatory sequalae and mechanical interference which may result in ophthalmoplegia.8 We hypothesize that in our patient, there may have been both an intravascular infiltration and extravascular migratory component to her diplopia given her presentation. Our patient initially presented with a small esotropia and right hypertropia, which resolved in primary gaze within a few hours. Two days later, examination revealed a small exotropia and hypertropia. It is feasible that her initial symptoms of nausea and vomiting were due to transient muscle ischemia and subsequent changes in her examination were due to the filler migrating and transiently affecting different recti muscles asymmetrically. It is also possible that all were affected simultaneously and simply resolved at different rates.
Treatment of vascular complications following cosmetic facial filler injection has evolved in recent years. In contrast to previously proposed daily hyaluronidase injections and adjunct topical nitroglycerin and hyperbaric oxygen,10,11 current recommendations for non-ophthalmic vascular complications utilize higher doses of hyaluronidase (without adjunctive therapies) to assure an effective concentration of hyaluronidase in the affected area to completely hydrolyze the filler.12, 13, 14 Notably, topical nitroglycerin is no longer recommended since resultant vasodilation may lead to further embolization of filler.15 It has been recommended that 500 units of hyaluronidase be injected into each affected area (e.g. lip, nose, forehead) hourly until skin color and capillary refill time have returned to normal.
Patients exhibiting vision loss can be managed similarly to patients with central retinal artery or vein occlusions,9,10 however there has yet to be a standardized protocol for the treatment of ocular complications following cosmetic facial filler injection. A systematic review and resultant treatment algorithm proposed by Aviv et al. suggests initially providing aspirin 300 mg, oxygen support, and periorbital hyaluronidase initially.14 Retrobulbar injection of hyaluronic acid has also been suggested.16 Other interventions, such as digital massage or administration of intraocular pressure lowering eye drops, are recommended with the goal of advancing the embolism and/or relieving ischemic burden. Additionally, methylprednisolone has been used to diminish retinal edema caused by ischemia and secondary damage to retinal ganglion cells.17
Our patient was given two doses of hyaluronidase after symptom onset and was fortunate to not have developed more severe ophthalmic complications or require vision-rescuing interventions. While the two doses may have aided in resolution of the patient's diplopia, it was insufficient in preventing skin necrosis of the affected area, which will require scar revision treatments.12 We herein describe a case of diplopia without gross motility deficits or vision changes following hyaluronic filler injection that eventually resolved. This case highlights the importance of understanding the orbitofacial anatomy and management of vascular complications when injecting facial filler by cosmeticians and physicians alike.
Patient consent
Written consent was obtained from the patient to prepare and publish this case report.
Funding
Yanoff Endowment Fund (VL). Research to Prevent Blindness unrestricted department award (VL and CAB).
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: CWC, EJS, CAB, VL.
Acknowledgements
None.
References
- 1.Lee W., Koh I.S., Oh W., et al. Ocular complications of soft tissue filler injections: a review of literature. J Cosmet Dermatol. 2020;19(4):772–781. doi: 10.1111/jocd.13213. [DOI] [PubMed] [Google Scholar]
- 2.Mortada H., Seraj H., Barasain O., et al. Ocular complications post-cosmetic periocular hyaluronic acid injections: a systematic review. Aesthetic Plast Surg. 2022;46:760–773. doi: 10.1007/s00266-021-02730-5. [DOI] [PubMed] [Google Scholar]
- 3.Downie E.M., Chen Y., Lucarelli M.J., et al. Isolated ophthalmoplegia following filler injections to the upper face. Ophthalmic Plast Reconstr Surg. 2020;36(6):152–154. doi: 10.1097/IOP.0000000000001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim A., Kim S.H., Kim H.J., et al. Ophthalmoplegia as a complication of cosmetic facial filler injection. Acta Ophthalmol. 2016;94(5):377–379. doi: 10.1111/aos.12893. [DOI] [PubMed] [Google Scholar]
- 5.Yang H.K., Lee Y., Woo S.J., et al. Natural course of ophthalmoplegia after iatrogenic ophthalmic artery occlusion caused by cosmetic filler injections. Plast Reconstr Surg. 2019;144(1):28–34. doi: 10.1097/PRS.0000000000005702. [DOI] [PubMed] [Google Scholar]
- 6.Hwang C.J., Chon B.H., Perry J.D. Blindness after filler injection: mechanism and treatment. Facial Plastic Surg Clin North America. 2021;29(2):359–367. doi: 10.1016/j.fsc.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Hamed-Azzam S., Burkat C., Mukari A., et al. Filler migration to the orbit. Aesthetic Surg J. 2021;41(6):559–566. doi: 10.1093/asj/sjaa264. [DOI] [PubMed] [Google Scholar]
- 8.Sung W., Tsai S., Chen L. Ocular complications following cosmetic filler injection. JAMA Ophthalmol. 2018;136(5) doi: 10.1001/jamaophthalmol.2018.0716. [DOI] [PubMed] [Google Scholar]
- 9.Park K.H., Kim Y.-K., Woo S.J., et al. Iatrogenic occlusion of the ophthalmic artery after cosmetic facial filler injections: a national survey by the Korean Retina Society. JAMA Ophthalmol. 2014;132(6):714–723. doi: 10.1001/jamaophthalmol.2013.8204. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J.L., Biesman B.S., Dayan S.H., et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthetic Surg J. 2015;35(7):844–849. doi: 10.1093/asj/sjv018. [DOI] [PubMed] [Google Scholar]
- 11.Graue G., Ochoa Araujo D.A., Plata Palazuelos C., et al. The M.A.STE.R.S algorithm for acute visual loss management after facial filler injection. J Cosmet Dermatol. 2020;19(11):2859–2866. doi: 10.1111/jocd.13393. [DOI] [PubMed] [Google Scholar]
- 12.DeLorenzi C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthetic Surg J. 2017;37(7):814–825. doi: 10.1093/asj/sjw251. [DOI] [PubMed] [Google Scholar]
- 13.Sansur S.A., Destang D. Use of the high-dose pulsed hyaluronidase protocol in the management of impending skin necrosis associated with hyaluronic acid fillers: a systematic review. Int J Oral Maxillofac Surg. 2023;52(1):79–87. doi: 10.1016/j.ijom.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Aviv U., Haik J., Weiss N., et al. Treatment algorithm for hyaluronic acid-related complication based on a systematic review of case reports, case series, and clinical experience. Craniomaxillofacial Trauma Reconstr. 2020;13(4):313–328. doi: 10.1177/1943387520952687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang C.J., Morgan P.V., Pimental A., Sayre J.W., Goldberg R.A., Duckwiler G. Rethinking the role of nitroglycerin ointment in ischemic vascular filler complications: an animal model with ICG imaging. Ophthalmic Plast Reconstr Surg. 2016;32(2):118–122. doi: 10.1097/IOP.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 16.Khan T.T., Colon-Acevedo B., Mettu P., DeLorenzi C., Woodward J.A. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss. Aesthetic Surg J. 2017;37(2):203–208. doi: 10.1093/asj/sjw132. [DOI] [PubMed] [Google Scholar]
- 17.Rumelt S., Dorenboim Y., Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128(6):733–738. doi: 10.1016/s0002-9394(99)00359-1. [DOI] [PubMed] [Google Scholar]