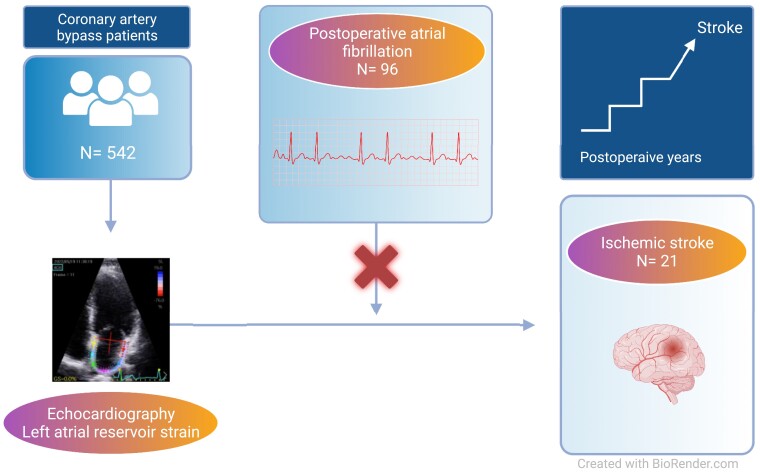
Abstract
Aims
Measures of left atrial (LA) function are known to predict both ischaemic stroke and atrial fibrillation in specific patient groups. The aim of this study was to investigate the value of LA reservoir strain for predicting ischaemic stroke in patients undergoing coronary artery bypass grafting (CABG) and investigate whether the presence of postoperative atrial fibrillation (POAF) modified this relationship.
Methods and results
Patients undergoing isolated CABG were included. The primary endpoint was ischaemic stroke. The association between LA reservoir strain and ischaemic stroke was investigated in uni- and multivariable Cox proportional hazards regression models including adjustment for POAF.
We included 542 patients (mean age 67.3±8.9 years, 16.4% female). During a median follow-up period of 3.9 years, 21 patients (3.9%) experienced an ischaemic stroke. In total, 96 patients (17.7%) developed POAF during the index hospitalization. In a multivariable-adjusted Cox proportional hazards regression model, LA reservoir strain was significantly associated with the development of ischaemic stroke [HR (hazard ratio) 1.09 (95% CI 1.02–1.17) per 1% decrease, P = 0.011]. The presence of POAF did not modify this association (p for interaction = 0.07). The predictive value of the LA reservoir strain persisted in multiple sensitivity analyses including restricting the analysis to patients with normal left atrial volumes (LAV<34 ml/m2), patients without POAF, patients without prior stroke, and when excluding patients who developed atrial fibrillation at any time during follow-up.
Conclusion
LA reservoir strain was independently associated with ischaemic stroke in CABG patients. The predictive value of LA reservoir strain was unaffected by the presence of POAF. Prospective studies are warranted to validate the potential usefulness of LA reservoir strain to predict postoperative ischaemic stroke in the setting of CABG.
Keywords: Echocardiography, Left atrial speckle tracking, ischaemic stroke, Postoperative atrial fibrillation, Coronary artery bypass grafting
Graphical Abstract
Graphical Abstract.
Introduction
Patients undergoing coronary artery bypass grafting (CABG) face a substantial risk of ischaemic stroke1—one of the leading causes of death and disability with more than 77 million yearly cases worldwide.2,3 In recent years, multiple studies have sought to investigate the aetiology of ischaemic stroke occurring in the absence of atrial fibrillation (AF).4–6 These contribute to the general evolving idea that cardiogenic thromboembolism is multifactorial and that other features of left atrial (LA) structure and function could play a central role. Among CABG patients, the echocardiographic measure of LA reservoir strain has been shown to predict postoperative atrial fibrillation (POAF), heart failure (HF), and cardiovascular (CV) death.7,8 LA reservoir strain has also been proven predictive of ischaemic stroke in other populations,9,10 but it is still unclear whether its predictive value is also present in CABG patients. Evidence is conflicting with regards to the clinical significance of POAF, where multiple studies have shown an association between POAF and ischaemic stroke in patients undergoing CABG,11,12 whereas other recent studies find no association between POAF and increased risk of thromboembolic events.3–5 In addition, the impact of POAF on the relation between LA reservoir strain and ischaemic stroke is unclear.
This study aimed to investigate the association between LA reservoir strain and long-term ischaemic stroke in patients undergoing CABG and to investigate whether the presence of POAF modified this relationship.
Methods
Patient population
We conducted a retrospective cohort study of patients undergoing isolated CABG surgery in the period of January 2006 through May 2011 at Gentofte University Hospital.
Before surgery, extensive echocardiography including two-dimensional speckle-tracking was performed. Only patients with available LA reservoir strain measures were included. Patients were excluded if CABG was performed as a rescue treatment. We also excluded patients with known atrial fibrillation and moderate to severe valvular disease.
Echocardiography
The echocardiographic examinations have performed a median of 14 (IQR [interquartile range]: 8–29) days before surgery using Vivid Dimension (GE Healthcare, Horten, Norway) ultrasound systems with 3.5 MHz transducers. All images were stored in a digital image archive. All conventional measures were later analysed using EchoPAC BT V.11.1.0, GE Healthcare, and LA strain using EchoPAC BT V.2.02 GE Healthcare, according to current guidelines by a single trained investigator blinded to follow-up data.16 Conventional echocardiographic parameters were obtained as previously described.17
LA reservoir strain
Since no specific software for LA strain measurements was available when the echocardiographic analyses were performed, the measures were obtained using left ventricular (LV) dedicated software using R-R gating. Using the views with the highest frame rate, six segments along the atrial endocardial wall were semi-automatically traced in the apical four-chamber and two-chamber views. Specific segments could be excluded or adjusted by the analyzer if deemed inaccurate. Average atrial strain values were used to calculate the LA strain parameters: LA reservoir strain, LA conduit strain, and LA contraction strain.
Covariates
Information about comorbidity, laboratory results, and the occurrence of POAF was obtained through a medical record review. POAF was defined as documented AF of any duration detected via inpatient telemetry or 12-lead electrocardiogram occurring after CABG and before discharge from the index hospitalization; the detection period lasted a median of 6 days (IQR = 5–14). To estimate the EuroSCORE II, the official calculator was used.18
Outcome
The primary endpoint was ischaemic stroke. Endpoint data was obtained from The Danish National Patient Registry.19
Statistics
Baseline characteristics were stratified according to the median LA reservoir strain value and presented and compared according to distribution. We used logistic regression to assess the relationship between LA reservoir strain and POAF. Uni- and multivariable Cox proportional hazards regression models were constructed to investigate the association between LA reservoir strain and the development of ischaemic stroke during follow-up. The multivariable model included adjustment for EuroSCORE II, left atrial volume index (LAVi), and prior stroke. To illustrate the continuous association between LA reservoir strain and the risk of ischaemic stroke, Poisson regression was used in a restricted cubic spline model. The number of knots was chosen according to the lowest Akaike information criterion. Cumulative incidence curves were constructed to display the risk of ischaemic stroke during the follow-up period according to the median LA reservoir strain value and the presence or absence of POAF. To assess the robustness of our results, multiple sensitivity analyses were performed. Stata/SE version 17.0 (StataCorp, College Station, TX, USA) was used to perform all the statistical analyses. P-values of ≤0.05 were deemed statistically significant.
Results
Baseline characteristics
The study population consisted of 542 patients. Of these, 89 patients (16.4%) were female and the mean age was 67.3 ± 8.9 years. The group with an LA reservoir strain below the median of 27.2% was older, more likely to have diabetes, and had a higher average EuroSCORE II. The baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics
| All (n = 542) | LA strain ≤ 27.2% (n = 271) | LA strain > 27.2% (n = 271) | P-value | |
|---|---|---|---|---|
| Clinical | ||||
| Age, mean (SD) | 67.3 (8.88) | 68.07 (9.08) | 66.53 (8.62) | 0.043 |
| Female sex, n (%) | 89 (16.4%) | 45 (16.6%) | 44 (16.2%) | 0.91 |
| EuroSCORE II, median (IQR) | 1.31 (0.93–1.96) | 1.49 (1.05–2.43) | 1.19 (0.88–1.67) | <0.001 |
| Hypertension, n (%) | 364 (67.2%) | 187 (69%) | 177 (65.3%) | 0.36 |
| Heart rate, mean (SD) | 69.59 (13.14) | 69.44 (13.67) | 69.73 (12.62) | 0.80 |
| Diabetes, n (%) | 133 (24.5%) | 78 (28.8%) | 55 (20.3%) | 0.022 |
| BMI, mean (SD) | 26.95 (3.74) | 27.08 (3.66) | 26.82 (3.82) | 0.42 |
| Smoking status, n (%) | 0.85 | |||
| Current | 117 (21.6%) | 56 (20.7%) | 61 (22.5%) | |
| Never | 267 (49.3%) | 134 (49.4%) | 133 (49.1%) | |
| Former | 158 (29.2%) | 81 (29.9%) | 77 (28.4%) | |
| Chronic obstructive lung disease, n (%) | 34 (6.3%) | 16 (5.9%) | 18 (6.6%) | 0.72 |
| Prior AMI, n (%) | 128 (23.7%) | 72 (26.6%) | 56 (20.7%) | 0.11 |
| Prior stroke, n (%) | 52 (9.6%) | 32 (11.8%) | 20 (7.4%) | 0.080 |
| CABG indication, n (%) | 0.096 | |||
| Stable angina pectoris | 271 (50%) | 123 (45.4%) | 148 (54.6%) | |
| Acute coronary syndrome | 271 (50%) | 148 (54.6%) | 123 (45.4%) | |
| Biochemistry | ||||
| C reactive protein (mg/L), median (IQR) | 4 (2–9) | 5 (2–10) | 2 (2–8) | 0.013 |
| eGFR (mL/min/1.73m2), mean (SD) | 71.86 (18.6) | 69.91 (18.61) | 73.82 (18.42) | 0.014 |
| Echocardiography | ||||
| GLS %, mean (SD) | −13.48 (3.96) | −12.51 (3.91) | −14.46 (3.78) | <0.001 |
| LVEF biplane, mean (SD) | 50.57 (11.02) | 47.78 (12.2) | 53.35 (8.9) | <0.001 |
| E/e’, median (IQR) | 10.07 (8.18–12.62) | 10.72 (8.8–13.88) | 9.45 (7.84–11.73) | <0.001 |
| LAVi (mL/m2), mean (SD) | 23.66 (8.32) | 25.9 (9.13) | 21.42 (6.74) | <0.001 |
BMI, body mass index; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; E/e´, ratio of early transmitral inflow velocity to early myocardial relaxation velocity; LAVi, left atrial volume index; SD, standard deviation.
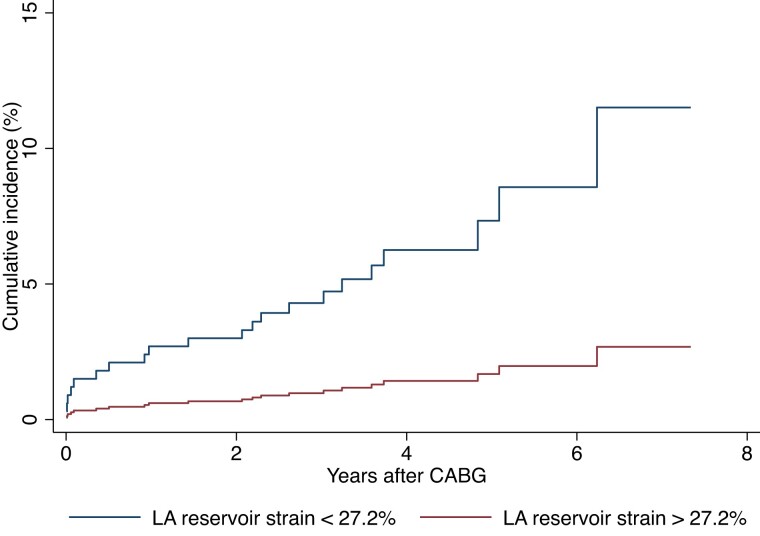
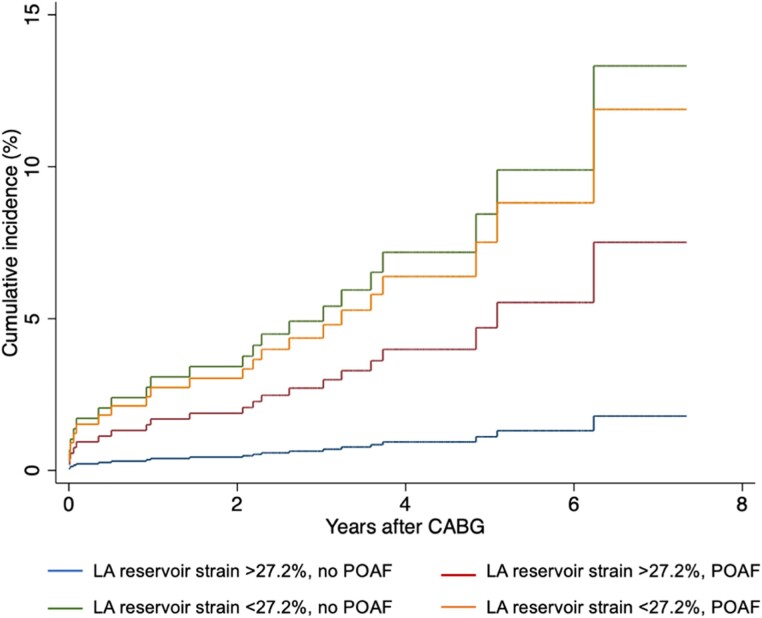
During a median follow-up period of 3.7 years (IQR 2.7–4.8 years), 21 (3.9%) patients developed ischaemic stroke and during the index hospitalization, 96 patients (17.7%) developed POAF. In univariable logistic regression, there was no association between LA reservoir strain and POAF [OR 1.00 (95% CI [confidence interval] 0.97–1.03) per 1% decrease]. The risk of stroke according to the median LA reservoir strain and the presence or absence of POAF is illustrated in Figures 1 and 2.
Figure 1.
Risk of ischaemic stroke according to median LA reservoir strain. Cumulative incidence curve illustrating the probability of ischaemic stroke during the follow-up period after CABG. HR and P-value are obtained from the univariable Cox proportional hazards regression model. LA, Left atrial; CABG, Coronary artery bypass grafting.
Figure 2.
Risk of ischaemic stroke according to median LA reservoir strain and POAF. Cumulative incidence curve displaying the risk of ischaemic stroke according to high and low LA strain and the presence or absence of POAF. LA, Left atrial; CABG, Coronary artery bypass grafting; POAF, Postoperative atrial fibrillation.
Usefulness of LA strain in risk stratification
LA reservoir strain was significantly associated with ischaemic stroke in univariable regression [HR 1.10 (95% CI 1.03–1.18, P = 0.003) per 1% decrease] and reservoir strain values below the median were associated with more than a four-fold increased risk of ischaemic stroke [HR 4.66 (95% CI 1.57–13.85, P = 0.006]. In a multivariable model adjusted for EuroSCORE II, LAVi, and prior stroke, LA reservoir strain remained a significant predictor of ischaemic stroke [HR 1.09 (95% CI 1.02–1.17, P = 0.011) per 1% absolute decrease]. Adding POAF as a covariate did not alter the significance of LA reservoir strain in the model, and no effect modification of POAF on the relationship between LA reservoir strain and ischaemic stroke was found (P for interaction = 0.07).
The prognostic value of the LA reservoir strain was also investigated according to different LA reservoir strain cutoffs (Table 2). Only a cutoff value of <23% was significant in multivariable regression.
Table 2.
Prognostic value of left atrial reservoir strain values in relation to ischaemic stroke
| Univariable | Multivariable | Multivariable + POAF | ||||
|---|---|---|---|---|---|---|
| HR | P-value | HR | P-value | HR | P-value | |
| LA reservoir strain < 23% | 4.41 | 0.001 | 3.89 | 0.004 | 4.01 | 0.004 |
| LA reservoir strain < 20% | 2.87 | 0.023 | 2.27 | 0.100 | 2.30 | 0.095 |
| LA reservoir strain < 18% | 2.23 | 0.150 | 1.68 | 0.389 | 1.72 | 0.366 |
| LA reservoir strain < 15% | 3.52 | 0.093 | 2.67 | 0.205 | 2.72 | 0.198 |
LA, left atrial.
Adding LA reservoir strain to a base model of EuroSCORE II, LAVi, and prior stroke increased the C-statistic compared to the base model (0.737 vs. 0.704). None of the abnormal conventional measures evaluated [LAVi > 34 mL/m2, septal-lateral E/e´ > 14, TR > 2,8 m/s, global longitudinal strain (GLS) < 16%, and left ventricular ejection fraction (LVEF) < 50%] were associated with ischaemic stroke in multivariable regression (Table 3).
Table 3.
Association between abnormal conventional echocardiographic measures and ischaemic stroke
| Univariable | Multivariable | Multivariable + POAF | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| LA reservoir strain < 23% | 4.41 | 0.001 | 3.89 | 0.004 | 4.01 | 0.004 |
| LAVi > 34 mL/m² | 0.85 | 0.826 | 0.34 | 0.302 | 0.34 | 0.298 |
| septal-lateral E/e´ > 14 | 2.93 | 0.017 | 2.29 | 0.093 | 2.37 | 0.082 |
| TR > 2,8 m/s | 1.57 | 0.301 | 1.55 | 0.321 | 1.53 | 0.333 |
| GLS < 16% | 7.77 | 0.045 | 6.59 | 0.067 | 6.61 | 0.067 |
| LVEF < 50% | 2.95 | 0.020 | 2.24 | 0.105 | 2.28 | 0.096 |
LA, left atrial; LAVi, left atrial volume index; E/e´, ratio of early transmitral inflow velocity to early myocardial relaxation velocity; TR, tricuspid regurgitation; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
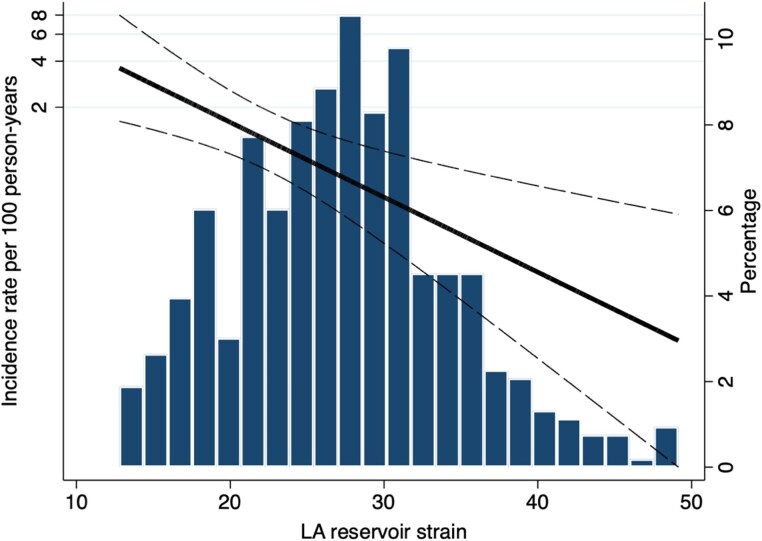
The continuous relationship between LA reservoir strain and the risk of ischaemic stroke is presented in restricted cubic spline modelling in Figure 3.
Figure 3.
Continuous relationship between LA reservoir strain and ischaemic stroke. LA, Left atrial.
Sensitivity analyses
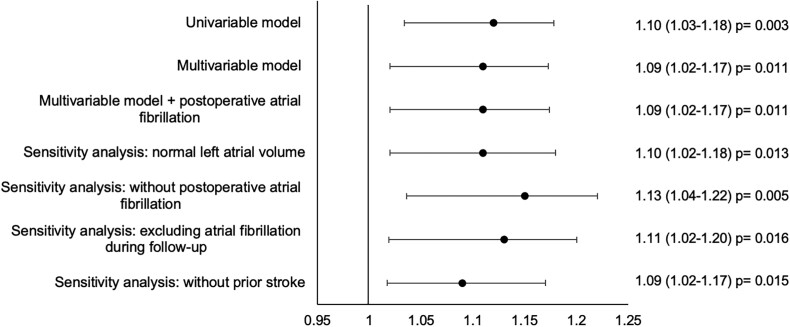
The association between LA reservoir strain and ischaemic stroke persisted in multiple sensitivity analyses including when restricting the analyses to patients with normal LAVi [adjusted HR per 1% decrease in LA reservoir strain: 1.09 (95% CI 1.02–1.18)], patients without POAF [adjusted HR per 1% decrease in LA reservoir strain: 1.13 (95% CI 1.04–1.22)], patients without new-onset AF during the follow-up period [adjusted HR per 1% decrease in LA reservoir strain 1.11 (95% CI 1.02–1.20)], and patients without prior stroke [adjusted HR per 1% decrease in LA reservoir strain: 1.09 (95% CI 1.02–1.17)] (Figure 4).
Figure 4.
Forest plot illustrating the association between LA reservoir strain and ischaemic stroke in uni- and multivariable Cox models. Hazard ratios per 1% decrease in left atrial reservoir strain. The multivariable model is adjusted for EuroSCOREII, LAVi, and prior stroke. Sensitivity analyses were performed using the multivariable model. LA, Left atrial.
Discussion
In this report, our two main findings were: (i) LA reservoir strain was an independent predictor of ischaemic stroke in patients undergoing CABG. (ii) Although POAF appeared in several patients, the presence of this phenomenon did not modify the abovementioned relationship.
Comparison to previous studies
The predictive value of echocardiographic measures with regard to cardiac events following CABG has been previously examined.20,21 LV hypertrophy has been linked to an increased risk of ischaemic stroke, while LA reservoir strain and LA contraction strain have been shown to predict HF and CV death.7
In previous studies, LAVi has been shown to be a significant predictor of stroke.22 However, we found that this measure was not associated with the development of ischaemic stroke in our study, whereas LA reservoir strain provided robust prognostic value independent of LAVi and in patients with normal LAVi.
How POAF affects the risk of subsequent stroke still seems to be unclear since conflicting results have been published in other studies. Several studies have found no association between POAF and risk of mortality and ischaemic stroke, even though it seems related to late-onset AF and treatable with oral anticoagulation.3–5 These arguments lean on that POAF may be seen as a prognostic confounder in a surgical population and are a consequence of underlying disease. At the same time, other studies find POAF to be an independent predictor of cerebrovascular accidents following isolated CABG because of the increased coagulation in relation to arrythmia and thereby higher occurrence of thromboembolic events.11,12 In our results, we saw no association between LA reservoir strain and POAF, which challenges the common point of view that LA mechanics and AF are closely linked.23 This suggests a persisting gap in knowledge of the association between LA mechanics and POAF such that the underlying pathophysiological mechanisms driving POAF may differ from other types of AF.
Pathophysiological mechanisms
LA reservoir strain is a measure reflecting the compliance and function of the LA. Several clinical conditions can cause changes in compliance and atrial remodelling leading to impaired LA reservoir strain.24 An explanation commonly used to describe the pathophysiological aetiology of stroke due to AF has previously relied on the decreased flow velocity of blood in the atrium. This would cause an extended stasis and thereby thrombus formation and embolism contributing to the development of an ischaemic stroke.25 However, AF potentially occurs as a co-phenomenon to other CV abnormalities causing embolism in some cases.
Today, the cause of stroke in patients with AF is partly considered to involve multiple thromboembolic factors. Rather than AF in itself being the only reason for atrial thromboembolism, other atrial abnormalities (structural/mechanical) could potentially be underlying mechanisms of both AF and clot formation, the latter of which also seems possible in the absence of evident atrial arrhythmia.26
Our results support this line of thought, as LA reservoir strain as a marker of atrial mechanical dysfunction was associated with the development of ischaemic stroke regardless of the presence of POAF and even in patients without evident new-onset AF during follow-up.
Additionally, non-cardioembolic subtypes of stroke are often related to risk factors also associated with AF, which could impose substantial diagnostic difficulties when distinguishing between the specific pathophysiological mechanisms.27
Clinical implications
Currently, the decision to initiate anticoagulation to reduce the risk of ischaemic stroke and other thromboembolic events following cardiac surgery primarily relies on the presence of evident AF.28 Our results indicate that the accuracy of this decision might be improved when also considering a preoperative assessment of LA mechanics, e.g. LA reservoir strain. Whether identifying and subsequently initiating long-term anticoagulation in a patient subgroup with impaired LA reservoir strain before cardiac surgery could improve outcomes would need to be investigated in a randomized controlled trial.
Secondary analyses of the NAVIGATE-ESUS randomized clinical trial, in which the effect of Rivaroxaban compared to Aspirin in patients with ‘embolic stroke of undetermined source’ were investigated, showed that patients with a LA diameter >4.6 cm could benefit from anticoagulation (doi:10.1001/jamaneurol.2019.0617).29 The same hypothesis is tested in the ARCADIA study, with the aim to test the hypothesis that Apixaban is superior to Aspirin in subjects with cryptogenic stroke and atrial cardiopathy for the prevention of recurrent stroke (doi:10.1177/1747493018799981).30 However, none of these studies have included decreased LA strain as an inclusion criterion.
Based on the findings of this retrospective post-hoc analysis, prospective studies are warranted to validate the potential usefulness of LA reservoir strain to predict postoperative ischaemic stroke and incidental AF in the setting of CABG.
Limitations
The potential presence of residual confounding factors cannot be excluded. We adjusted for multiple confounders to test the association between LA reservoir strain and ischaemic stroke with the risk of overfitting the Cox regression hazard models.31 At the same time, we had relatively few primary outcome events (n = 21) providing only limited statistical power to adjust for confounders, however, we adjusted for the EuroSCORE II, which contains information on several risk factors. The EuroSCORE II was developed to predict in-hospital mortality and not ischaemic stroke, but most risk factors are shared between the two outcomes. Since the study was performed specifically in patients undergoing isolated CABG, the findings cannot necessarily be extrapolated to patients undergoing other types of cardiac surgery.
A limitation of this study is its retrospective nature and that the cohort included patients who had CABG performed from January 2006 through May 2011 with a limited follow-up duration; however, with 542 patients included, this remains one of the largest studies in its field.
Unfortunately, our dataset did not allow us to investigate the association between LA strain and subsequent non-postoperative AF during long-term follow-up as a potential pathway to ischaemic stroke; however, the association between LA strain and incident AF has been demonstrated in several prior studies including in patients with ischaemic heart disease.32,33
An additional limitation was the lack of specific software for atrial two-dimensional speckle tracking at the time of analysis.
Conclusions
Left atrial reservoir strain was significantly associated with the development of ischaemic stroke in patients undergoing CABG. The presence of POAF did not alter this relationship. Based on the findings of this retrospective post-hoc analysis, prospective studies are warranted to validate the potential usefulness of LA reservoir strain to predict postoperative ischaemic stroke and incidental AF in the setting of CABG.
Contributor Information
Frederikke Vyff, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark.
Niklas Dyrby Johansen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark; Center for Translational Cardiology and Pragmatic Randomized Trials, Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Flemming J Olsen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark.
Lisa S Duus, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark.
Søren Lindberg, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark.
Thomas Fritz-Hansen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark.
Sune Pedersen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark.
Allan Iversen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark.
Søren Galatius, Department of Cardiology, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Rasmus Møgelvang, Department of Cardiology, Copenhagen University Hospital—Rigshospitalet, Copenhagen, Denmark.
Tor Biering-Sørensen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 8, 3.th., 2900 Hellerup, Copenhagen, Denmark; Center for Translational Cardiology and Pragmatic Randomized Trials, Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Lead author biography
 Frederikke Vyff, Medical Student at The University of Copenhagen, Denmark, Department of Cardiology at Center for Translational Cardiology and Pragmatic Randomized Trials (CTCPR), Cardiovascular Non-Invasive Imaging Research Laboratory (CIRL), Herlev and Gentofte Hospital, Denmark.
Frederikke Vyff, Medical Student at The University of Copenhagen, Denmark, Department of Cardiology at Center for Translational Cardiology and Pragmatic Randomized Trials (CTCPR), Cardiovascular Non-Invasive Imaging Research Laboratory (CIRL), Herlev and Gentofte Hospital, Denmark.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
None declared.
Conflict of interest: T.B.-S. has served as a steering committee member of the Amgen-financed GALACTIC-HF trial and the Boston Scientific-financed LUX-Dx TRENDS trial, served on advisory boards for Sanofi and Amgen, received speaker honoraria from Novartis and Sanofi Pasteur, and received research grants from GE Healthcare and Sanofi Pasteur.
All other authors declare no competing interests.
References
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, Tsao CW. Heart disease and stroke statistics—2021 update. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2. Sanna T, Diener H-C, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 3. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, Tsao CW; On behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2021 update: A report from the American heart association. Circulation 2021;143:266. [DOI] [PubMed] [Google Scholar]
- 4. Smietana J, Plitt A, Halperin JL. Thromboembolism in the absence of atrial fibrillation. Am J Cardiol 2019;124:303–311. [DOI] [PubMed] [Google Scholar]
- 5. Overvad TF, Nielsen PB, Larsen TB, Søgaard P. Left atrial size and risk of stroke in patients in sinus rhythm. Thromb Haemost 2016;116:206–219. [DOI] [PubMed] [Google Scholar]
- 6. Radu RA, Terecoasă EO, Băjenaru OA, Tiu C. Etiologic classification of ischemic stroke: where do we stand? Clin Neurol Neurosurg 2017;159:93–106. [DOI] [PubMed] [Google Scholar]
- 7. Duus LS, Lindberg S, Olsen FJ, Fritz-Hansen T, Pedersen S, Iversen A, Galatius S, Gislason G, Møgelvang R, Estépar RSJ, Biering-Sørensen T. Left atrial strain predicts heart failure and cardiovascular death in patients undergoing coronary artery bypass grafting. JACC Cardiovasc Imaging 2021;14:295–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Her A-Y, Kim J-Y, Kim YH, Choi E-Y, Min P-K, Yoon YW, Lee B-K, Hong B-K, Rim S-J, Kwon HM. Left atrial strain assessed by speckle tracking imaging is related to new-onset atrial fibrillation after coronary artery bypass grafting. Can J Cardiol 2013;29:377–383. [DOI] [PubMed] [Google Scholar]
- 9. Park J-H, Hwang I-C, Park JJ, Park J-B, Cho G-Y. Left atrial strain to predict stroke in patients with acute heart failure and Sinus rhythm. J Am Heart Assoc 2021;10:e020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao J-N, Chao T-F, Kuo J-Y, Sung K-T, Tsai J-P, Lo C-I, Lai Y-H, Su C-H, Hung C-L, Yeh H-I. Global left atrial longitudinal strain using 3-beat method improves risk prediction of stroke over conventional echocardiography in atrial fibrillation. Circ Cardiovasc Imaging 2020;13:e010287. [DOI] [PubMed] [Google Scholar]
- 11. Thorén E, Wernroth M-L, Christersson C, Grinnemo K-H, Jidéus L, Ståhle E. Compared with matched controls, patients with postoperative atrial fibrillation (POAF) have increased long-term AF after CABG, and POAF is further associated with increased ischemic stroke, heart failure and mortality even after adjustment for AF. Clin Res Cardiol 2020;109:1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitlock R, Healey JS, Connolly SJ, Wang J, Danter MR, Tu JV, Novick R, Fremes S, Teoh K, Khera V, Yusuf S. Predictors of early and late stroke following cardiac surgery. CMAJ Can Med Assoc J J Assoc Medicale Can 2014;186:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butt JH, Xian Y, Peterson ED, Olsen PS, Rørth R, Gundlund A, Olesen JB, Gislason GH, Torp-Pedersen C, Køber L, Fosbøl EL. Long-term thromboembolic risk in patients with postoperative atrial fibrillation after coronary artery bypass graft surgery and patients with nonvalvular atrial fibrillation. JAMA Cardiol 2018;3:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marazzato J, Masnaghetti S, De Ponti R, Verdecchia P, Blasi F, Ferrarese S, Trapasso M, Spanevello A, Angeli F. Long-Term survival in patients with post-operative atrial fibrillation after cardiac surgery: analysis from a prospective cohort study. J Cardiovasc Dev Dis 2021;8:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Melduni RM, Schaff HV, Bailey KR, Cha SS, Ammash NM, Seward JB, Gersh BJ. Implications of new-onset atrial fibrillation after cardiac surgery on long-term prognosis: a community-based study. Am Heart J 2015;170:659–668. [DOI] [PubMed] [Google Scholar]
- 16. Negishi K, Negishi T, Kurosawa K, Hristova K, Popescu BA, Vinereanu D, Yuda S, Marwick TH. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc Imaging 2015;8:489–492. [DOI] [PubMed] [Google Scholar]
- 17. Olsen FJ, Lindberg S, Pedersen S, Iversen A, Davidovski FS, Galatius S, Fritz-Hansen T, Gislason GH, Søgaard P, Møgelvang R, Biering-Sørensen T. Global longitudinal strain predicts cardiovascular events after coronary artery bypass grafting. Heart Br Card Soc 2021;107:814–821. [DOI] [PubMed] [Google Scholar]
- 18. Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–745. [DOI] [PubMed] [Google Scholar]
- 19. Wildenschild C, Mehnert F, Thomsen RW, Iversen HK, Vestergaard K, Ingeman A, Johnsen SP. Registration of acute stroke: validity in the danish stroke registry and the danish national registry of patients. Clin Epidemiol 2013;6:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kluck O, Berman M, Stamler A, Sahar G, Kogan A, Porat E, Sagie A. Value of echocardiography for stroke and mortality prediction following coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2006;6:30–34. [DOI] [PubMed] [Google Scholar]
- 21. Abdelrazek G, Mandour K, Osama M, Elkhashab K. Strain and strain rate echocardiographic imaging predict occurrence of atrial fibrillation in post-coronary artery bypass grafting patients. Egypt Heart J 2021;73:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan K, Yaghi S, Poppas A, Chang AD, Mac Grory B, Cutting S, Burton T, Jayaraman M, Tsivgoulis G, Sabeh MK, Merkler AE, Kamel H, Elkind MSV, Furie K, Song C. Left atrial volume Index is associated with cardioembolic stroke and atrial fibrillation detection after embolic stroke of undetermined source. Stroke 2019;50:1997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pellman J, Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr Physiol 2015;5:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasmussen SMA, Olsen FJ, Jørgensen PG, Fritz-Hansen T, Jespersen T, Gislason G, Biering-Sørensen T. Utility of left atrial strain for predicting atrial fibrillation following ischemic stroke. Int J Cardiovasc Imaging 2019;35:1605–1613. [DOI] [PubMed] [Google Scholar]
- 25. Goldman ME, Pearce LA, Hart RG, Zabalgoitia M, Asinger RW, Safford R, Halperin JL. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (the stroke prevention in atrial fibrillation [SPAF-III] study). J Am Soc Echocardiogr 1999;12:1080–1087. [DOI] [PubMed] [Google Scholar]
- 26. Kamel H, Okin PM, Elkind MSV, Iadecola C. Atrial fibrillation and mechanisms of stroke. Stroke 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katsi V, Georgiopoulos G, Skafida A, Oikonomou D, Klettas D, Vemmos K, Tousoulis D. Noncardioembolic stroke in patients with atrial fibrillation. Angiology 2019;70:299–304. [DOI] [PubMed] [Google Scholar]
- 28. de Ronsoni RM, Souza AZM, Leiria TLL, de Lima GG. Update on management of postoperative atrial fibrillation after cardiac surgery. Braz J Cardiovasc Surg 2020;35:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, Haeusler KG, Mikulik R, Kasner SE, Toni D, Arauz A, Ntaios G, Hankey GJ, Perera K, Pagola J, Shuaib A, Lutsep H, Yang X, Uchiyama S, Endres M, Coutts SB, Karliński M, Czlonkowska A, Molina CA, Santo G, Berkowitz SD, Hart RG, Connolly SJ. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol 2019;76:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamel H, Longstreth WT, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, Meinzer C, Dillon C, Ewing I, Spilker JA, Di Tullio MR, Hod EA, Soliman EZ, Chaturvedi S, Moy CS, Janis S, Elkind MSV. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke (ARCADIA) randomized trial: rationale and methods. Int J Stroke Off J Int Stroke Soc 2019;14:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per Variable in logistic and cox regression. Am J Epidemiol 2007;165:710–718. [DOI] [PubMed] [Google Scholar]
- 32. Svartstein A-SW, Lassen MH, Skaarup KG, Grove GL, Vyff F, Ravnkilde K, Pedersen S, Galatius S, Modin D, Biering-Sørensen T. Predictive value of left atrial strain in relation to atrial fibrillation following acute myocardial infarction. Int J Cardiol 2022;364:52–59. [DOI] [PubMed] [Google Scholar]
- 33. Hauser R, Nielsen AB, Skaarup KG, Lassen MCH, Duus LS, Johansen ND, Sengeløv M, Marott JL, Jensen G, Schnohr P, Søgaard P, Møgelvang R, Biering-Sørensen T. Left atrial strain predicts incident atrial fibrillation in the general population: the Copenhagen city heart study. Eur Heart J - Cardiovasc Imaging 2021;23:52–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.