Abstract
We had previously isolated and characterized syringolin A, one of the molecular determinants secreted by Pseudomonas syringae pv syringae that is perceived by nonhost plant species such as rice. Here, we show that syringolin A is recognized by wheat and that it induces the accumulation of gene transcripts and increases protection against powdery mildew when applied before inoculation. Moreover, syringolin A essentially eradicates powdery mildew from infected wheat if applied after inoculation. This curative effect is accompanied by the induction of cell death and the reactivation of pathogenesis-related genes whose transcript levels initially accumulate after powdery mildew inoculation but then decline during the later course of infection. Because syringolin A has no fungicidal activity against a variety of fungi and its action on wheat cannot be mimicked by the fungicide cyprodinil, syringolin A is hypothesized to counteract the suppression of host defense reactions imposed by the pathogen on the colonized cells.
INTRODUCTION
Plants have evolved a variety of passive and active defense mechanisms against a plethora of potentially pathogenic microbes. A prominent form of an active defense response is the hypersensitive reaction, which is accompanied by localized cell death at the infection site and results in complete resistance. It has been demonstrated in many systems that these local events can induce systemic acquired resistance, that is, they cause the establishment of a physiological state in a plant that makes the plant more resistant to a variety of pathogens to which it normally would be susceptible (Hunt and Ryals, 1996; Sticher et al., 1997). A prerequisite for active defense reactions to occur is recognition of the attacking microbe by the plant. In a number of systems exhibiting race-specific resistance according to the gene-for-gene concept, genes governing the recognition process have been molecularly characterized (Hammond-Kosack and Jones, 1996; Baker et al., 1997; Ellis and Jones, 1998; Nürnberger, 1999). Phenotypically, active defense reactions evoked by nonhost pathogens are very similar to the ones observed in race-specific incompatible interactions. However, the molecular events underlying the recognition process between plants and nonhost pathogens have been less well characterized, mainly because genetic analysis was hampered by the apparent redundancy built into the system.
In rice, acquired resistance against the rice blast fungus Magnaporthe grisea can be induced by inoculation with the nonhost pathogen Pseudomonas syringae pv syringae (Smith and Métraux, 1991). We have recently isolated syringolin A, one of the bacterial determinants that are recognized by rice plants and cultured rice cells (Wäspi et al., 1998a; Hassa et al., 2000). Syringolin A (Figure 1) belongs to a family of small, structurally closely related peptides (syringolin A to F) that are synthesized nonribosomally and secreted by P. s. syringae (Wäspi et al., 1998a, 1999). Application of isolated syringolin A in micromolar concentrations onto rice plants evokes the activation of defense-related genes and induces resistance against the rice blast fungus (Wäspi et al., 1998a). However, syringolin A is redundant with respect to resistance induction because mutant P. s. syringae strains that do not synthesize syringolins are still recognized by rice plants and induce defense reactions and resistance (Reimmann et al., 1995; Wäspi et al., 1998a).
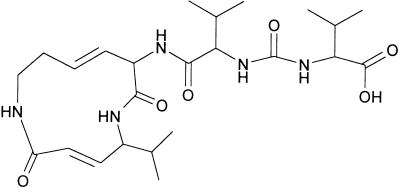
Figure 1.
Structure of Syringolin A.
The ring structure of syringolin A is formed by the two nonproteinogenic amino acids 5-methyl-4-amino-2-hexenoic acid and 3,4-dehydrolysine. The α-amino group of the latter is joined by a peptide bond to a valine residue, which is linked to another valine residue via a urea moiety.
Here, we report the effect of syringolin A on the interaction of powdery mildew with wheat. Syringolin A is perceived by wheat, whereas novel gene products appear after treatment. Syringolin A protects wheat to a moderate degree from a following infection with powdery mildew. However, it is also able to essentially eradicate powdery mildew from infected plants if applied after inoculation. This curative effect is accompanied by induction of cell death and the reactivation of pathogenesis-related genes whose transcript levels initially accumulate after powdery mildew inoculation but then decline during the later course of infection. Syringolin A is hypothesized to directly or indirectly reprogram colonized host cells to undergo cell death.
RESULTS
Syringolin Has Protective and Curative Effects against Powdery Mildew on Wheat
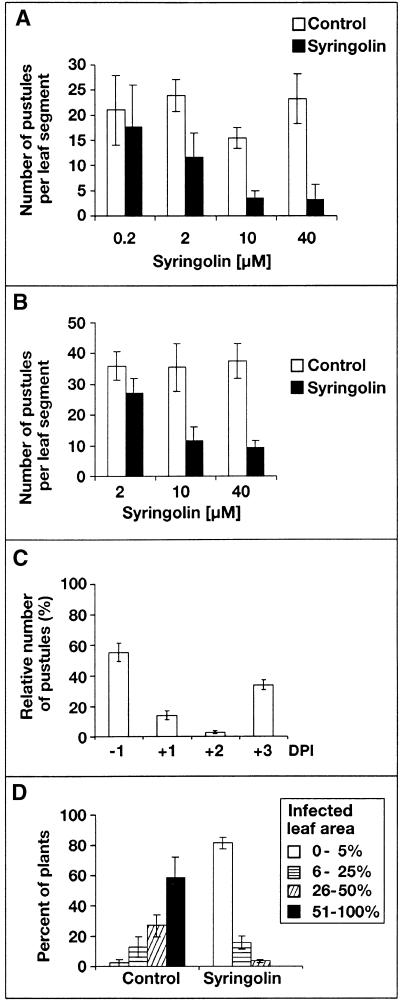
As shown in Figure 2A, syringolin A protected detached wheat leaf segments placed on agar plates from powdery mildew when applied 2 days before inoculation on the same (adaxial) leaf surface. The protective effect was translaminar, that is, it was also observed when syringolin A was applied onto the adaxial leaf surface and the inoculation occurred on the abaxial surface (Figure 2B). In addition to its protective effect, syringolin A proved to be an effective curative agent. Figure 2C shows the results of an experiment in which 40 μM syringolin A was sprayed on whole plants at different time points before and after inoculation with powdery mildew. Surprisingly, syringolin A appeared to be more effective when applied after rather than before the inoculation. Colonization of host tissue by powdery mildew was essentially stopped by syringolin A application at 2 days after the inoculation. This was also true if plants were more heavily inoculated, and the degree of infection was determined by estimating the relative infected leaf area (Figures 2D, 3A, and 3B).
Figure 2.
Protective and Curative Effects of Syringolin A.
(A) Detached leaf segments (4 cm long) were placed on agar plates, with the adaxial side facing up, and sprayed with solutions of syringolin A at the concentrations indicated. After 2 days, leaves were inoculated with powdery mildew spores; pustules were counted 5 days later. Means and standard deviations of a typical experiment (six leaf segments per treatment) are given. Control leaf segments were sprayed with 0.05% (v/v) Tween 20 in distilled water.
(B) Six 5-μL droplets of syringolin A solutions of the indicated concentrations or a control solution (0.05% Tween 20) were placed on the adaxial side of 4-cm-long leaf segments at regular space intervals. After drying, the leaf segments were put onto agar plates, with the adaxial side facing up. After 2 days, leaf segments were turned upside down, and the abaxial sides of the leaf segments were inoculated with powdery mildew spores; pustules were counted 5 days later. Means and standard deviations of a typical experiment (six leaf segments per treatment) are given.
(C) Pots with wheat plants were inoculated at a moderate spore density and sprayed with 40 μM syringolin A or a control solution (0.05% Tween 20) at different time points before or after inoculation. Pustules on primary leaves were counted 7 days after inoculation. The number of pustules on syringolin A–treated plants are expressed as the percentage of the number of pustules on plants treated with a control solution. Means and standard errors are given from three independent experiments, with a total of 36 to 107 plants per data point. DPI, days postinoculation.
(D) Wheat plants were inoculated at high spore densities and treated with 40 μM syringolin A or a control solution (0.05% Tween 20) 2 days later. Primary leaves were evaluated 7 days later by estimating the leaf area covered by pustules. The percentage of plants belonging to each of the four indicated infection classes is depicted. Mean values and standard errors of five independent experiments are given.
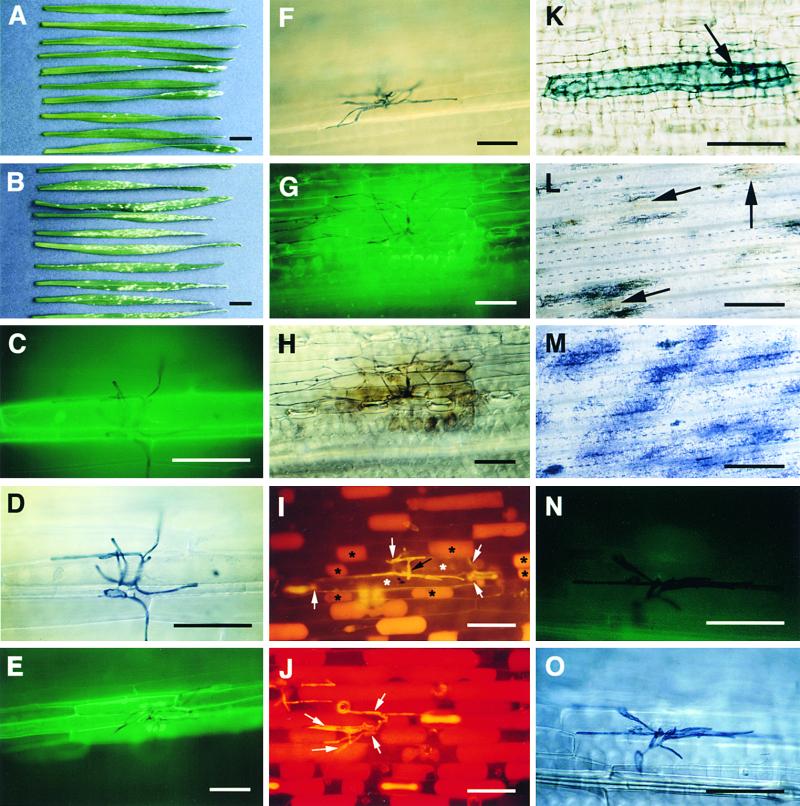
Figure 3.
Effect of Syringolin A on the Wheat–Powdery Mildew Interaction.
(A) and (B) Leaves of powdery mildew–inoculated plants treated with 40 μM syringolin A (A) or a control solution (B) at 2 days after inoculation. The photograph was taken 7 days after inoculation.
(C) to (H) Autofluorescence at infection sites on leaves treated with syringolin A at 2 days ([C] and [E]) or 3 days (G) after inoculation. The same infection sites under bright-field illumination are shown in (D), (F), and (H), respectively.
(I) and (J) Infection sites on leaves treated with 40 μM syringolin A (I) or a control solution (J) at 2 days after inoculation. Leaves were infiltrated with a hypertonic solution containing neutral red dye at 5 days (I) and 1 day (J) later, when fungal development was approximately equal. Live cells plasmolyze under these conditions and take up neutral red dye. The black arrow in (I) points to fungal structures (appearing yellow); black asterisks mark the protoplasts of live cells surrounding two dead cells, which are marked with white asterisks. The white arrows indicate the left and right borders of the two dead cells, which do not contain a protoplast. The white arrows in (J) point to fungal structures. All cells at the infection sites are plasmolyzed and stained red.
(K) Infected dead cell on a leaf treated with syringolin A at 2 days after inoculation and stained with trypan blue 5 days later. The arrow points to the haustorium. No other fungal structures are visible.
(L) and (M) Infections sites on a plant treated with syringolin A (L) or a control solution (M) at 4 days after inoculation. Leaves were photographed under bright-field illumination. The arrows in (L) point to examples of infection sites where tissue browning is visible.
(N) and (O) Infection site on a plant treated with 200 ppm (888 μM) cyprodinil and photographed under incident fluorescent light (N) or in the bright field (O). The image in (N) has been overexposed because of the absence of significant autofluorescence.
In (C) to (H) and (L) to (O), the leaves shown were stained with lactophenol/trypan blue for 1 hr before photography to stain fungal structures. In (A) to (I) and (K) to (O), the photographs shown were taken 7 days after inoculation.  ;
;  ;
;  .
.
These results raise the question of whether the observed effects of syringolin A were the result of its direct antifungal activity, its activation of plant defense reactions, or a combination of the two. This question could not be answered by in vitro tests with Blumeria graminis, which is an obligate biotrophic fungus. However, as reported earlier, syringolin A has no effect on the in vitro growth of the rice blast fungus M. grisea and the yeast Rhodotorula pilimanae up to the highest concentrations tested (250 μM) (Wäspi et al., 1998a). Further in vitro tests with a number of other fungi, including Fusarium graminearum and Trichoderma viride, indicated no inhibition of growth or germination by syringolin A at a concentration of 250 μM. No difference in the germination rate and rate of appressorium formation was observed 24 hr after powdery mildew inoculation on leaves sprayed with a control solution or 40 μM syringolin A at 24 hr before inoculation. Only 48 hr after inoculation, clear differences became apparent, as successful penetration, haustorium formation, and development of secondary hyphae were significantly suppressed on syringolin A–treated leaves (data not shown). In summary, these observations argue against a direct effect of syringolin A on early fungal development but do not rule out a fungitoxic activity affecting stages in the development of B. graminis f sp tritici that occur after appressorium formation.
Microscopic examination of infected leaves (Figures 3C to 3H) revealed that treatment with 40 μM syringolin A at various time points after inoculation triggered the accumulation of autofluorescing material in epidermal cells at nearly all infection sites (95.6% ± 2.8%), indicating that hypersensitive cell death was occurring (Koga et al., 1988, 1990; Carver et al., 1999). This interpretation is also supported by the observation, as exemplified in Figure 3I, that epidermal cells at infection sites on syringolin A–treated leaves were unable to undergo plasmolysis and to take up neutral red dye, indicating irreversible membrane damage (Koga et al., 1988; Görg et al., 1993). In contrast, infected cells on leaves treated with a control solution remained alive, as indicated by their ability to plasmolyze and accumulate neutral red dye (Figure 3J). That syringolin induces death of infected cells is also indicated by the fact that these cells can be stained with trypan blue (Figure 3K). When treatment with syringolin A occurred relatively late, that is, at 3 or 4 days after inoculation, autofluorescence was accompanied by tissue browning visible under the bright-field illumination (Figures 3H and 3L). None of these reactions occurred in syringolin A–treated uninfected leaves or in infected leaves treated with a control buffer solution (Figure 3M). The effect of syringolin A on infected leaves was compared with the action of the fungicide cyprodinil, which has good curative activity on powdery mildew–infected wheat (Knauf-Beiter et al., 1995). Treatment of infected wheat at 2 to 4 days after inoculation with a 200-ppm (888 μM) cyprodinil solution effectively stopped fungal growth, although no accumulation of autofluorescing material was visible at the majority of infection sites (80.0% ± 10.2%; Figures 3N and 3O).
Syringolin A Activates Host Genes
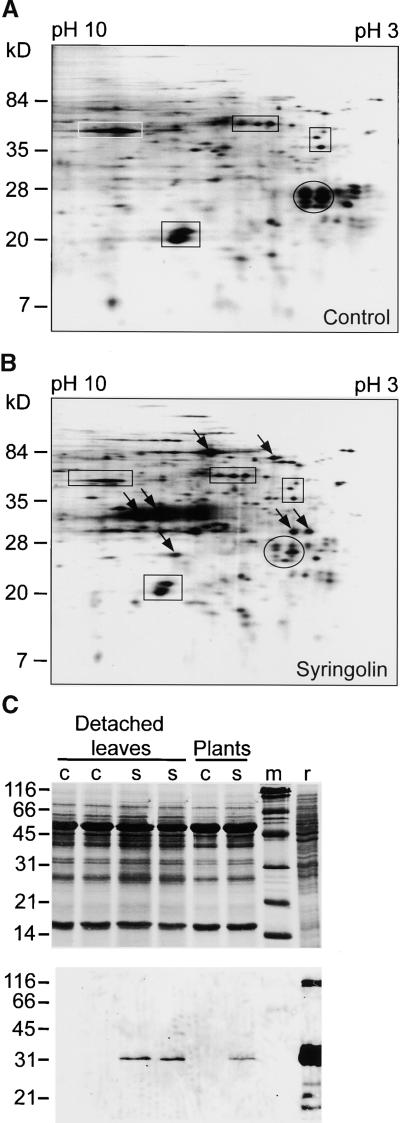
Because active host defense responses appeared to be involved in the action of syringolin A, we examined whether syringolin A treatment of uninfected plants induced gene expression. A number of putative defense gene transcripts have been cloned from wheat. Specific transcripts were reported to accumulate concomitantly with resistance induction triggered by either the nonhost pathogen B. graminis f sp hordei (WIR1 to WIR5; Schweizer et al., 1989; Dudler et al., 1991; Rebmann et al., 1991a, 1991b; Bull et al., 1992) or the chemical activator benzo(1,2,3)thiodiazole-7-carbothioic acid S-methyl ester (BTH) (WCI1 to WCI5; Görlach et al., 1996). RNA gel blot analysis of detached wheat leaves or whole plants revealed that none of these transcripts accumulated after spray application of 40 μM syringolin A (data not shown). To check for accumulation of transcripts that had not been cloned, we separated the 35S-methionine–labeled in vitro translation products of mRNA extracted at 10 or 24 hr after syringolin A or control treatment by using two-dimensional polyacrylamide gel electrophoresis and then visualized them with autoradiography. New products appeared reproducibly in the syringolin A–treated samples, as shown in the examples of autoradiograms in Figures 4A and 4B. In addition, protein gel blot analysis showed that a wheat homolog of the rice Pir7b esterase, which accumulates in rice plants upon syringolin A treatment (Wäspi et al., 1998b), was induced (Figure 4C). Thus, syringolin A activates host genes in wheat.
Figure 4.
Host Gene Activation Elicited by Syringolin A Treatment of Uninfected Plants.
(A) and (B) Autoradiography of 35S-labeled in vitro translation products of mRNA extracted from wheat leaves 10 hr after application of a control solution (A) or of 40 μM syringolin A (B), after separation by two-dimensional PAGE. For easier orientation, some spots whose intensity was reproducibly unaltered by syringolin A treatments have been enclosed in rectangles. Arrows in (B) indicate some spots whose intensity reproducibly increased when treated with syringolin A, whereas the intensity of the group of spots enclosed by an ellipsoid reproducibly decreased. The experiment was repeated three times independently, with RNA extracted at 10 and 24 hr after treatment with similar results. Size markers are indicated at left.
(C) Coomassie blue–stained gel (top) and corresponding protein gel blot (bottom) with proteins (50 μg per lane) extracted from detached leaves or plants at 72 hr after treatment with a control solution (c) and 40 μM syringolin A (s) as indicated. The protein gel blot was developed using a rabbit anti-Pir7b esterase antibody and a phosphatase-coupled secondary antibody. m, marker proteins whose size in kilodaltons is at left; r, protein extract (50 μg) of Escherichia coli, producing recombinant Pir7b esterase.
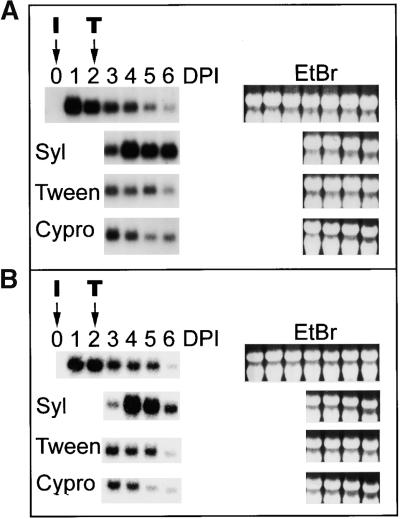
The WIR transcripts not only transiently accumulate upon the resistance-inducing inoculation with barley powdery mildew, a nonhost pathogen on wheat, but also after infection with wheat powdery mildew, although time course and level may be different, depending on transcript species and wheat cultivar (Kmecl et al., 1995). To check for any synergistic action syringolin A may have, we monitored WIR transcript levels in powdery mildew–infected wheat leaves after different treatments. As shown in Figure 5, WIR1 and WIR2 transcripts transiently accumulate after powdery mildew inoculation, reaching maximal levels after 1 day and declining thereafter over the next few days, although the fungus overgrows the leaf surface during this time and begins to sporulate after 5 or 6 days under the growth conditions employed. If powdery mildew–infected wheat was treated with 40 μM syringolin A at 2 days after inoculation, WIR1 and WIR2 transcript levels increased again on the following days (Figure 5). If infected wheat was cured by application of 888 μM cyprodinil, WIR1 and WIR2 transcript levels declined with the same time course as in untreated plants. Identical experiments using the WIR3 and WIR5 as well as the WCI cDNAs to probe gel blots revealed no increase of the corresponding transcript levels upon syringolin A application (data not shown). In summary, syringolin A has the capacity to re-induce the accumulation of a subset of transcripts that initially accumulate upon powdery mildew infection but then decline, although the fungus continues to grow and completes its development.
Figure 5.
Effect of Syringolin A on Host Transcript Accumulation in Infected Plants.
Plants were inoculated at day zero (I) and treated (T) with 40 μM syringolin A (Syl), a control solution (Tween), or 888 μM cyprodinil (Cypro) at 2 days after inoculation. RNA was extracted from leaves at the indicated time points and subjected to gel blot analysis. Autoradiography is shown at left; the corresponding ethidium bromide (EtBr)–stained gels are at right. DPI, days postinoculation.
(A) Gel blot probed with the WIR1 cDNA.
(B) Gel blot probed with the WIR2 cDNA.
DISCUSSION
Ectopic application of 40 μM syringolin A to wheat had both protective and curative effects with regard to powdery mildew infection. These properties are reminiscent for certain types of fungicides, as, for example, cyprodinil (Knauf-Beiter et al., 1995). However, syringolin A has no toxic activity against a range of fungal pathogens grown in vitro. Because B. graminis f sp tritici is an obligate biotroph, no antifungal test in vitro could be performed, and thus, a specific direct antifungal activity against this species cannot be excluded. However, syringolin A is an activator of plant defense responses. Applied onto rice plants, syringolin A was shown to protect rice from rice blast disease without having a direct toxic effect on the pathogen and to induce the accumulation of a set of defense-related transcripts (RIR1, PIR2, PIR3, and PIR7b) that were also induced by inoculation with the nonhost pathogen P. s. syringae, from which syringolin A was isolated (Wäspi et al., 1998a). In wheat, syringolin A also induces the accumulation of new transcripts (Figure 4). Thus, it appears likely that the observed protection of wheat by syringolin A was also the result of resistance induction. In this respect, it is interesting that the compatible SM strain of P. s. syringae that was originally isolated from wheat and that is pathogenic on this host does not produce syringolin A (Reimmann et al., 1995; Wäspi et al., 1998a).
In addition to its protective effect, syringolin A has a more powerful curative effect on powdery mildew–infected wheat (Figure 2). Syringolin A is able to essentially eradicate the fungus when applied 48 hr after inoculation. Its activity is associated with whole-cell autofluorescence and browning of epidermal cells at >95% of the infection sites, indicating that these cells died. Interestingly, WIR1 and WIR2 transcripts were now induced by syringolin A, in contrast to uninfected leaves, in which the elicitor did not affect the expression of these genes. As is evident from Figure 5, these two transcripts initially accumulate upon infection but then decline, although the fungus continues to overgrow the leaf. This could be explained by assuming that (1) the inducing stimulus is present only transiently, in spite of the continued fungal development; or (2) the host response system autonomously enters a refractory state (Felix et al., 1993); or (3) the host response becomes suppressed by the pathogen—or some combination of all three possibilities. In view of the continued colonization of the host by the pathogen, we consider the third hypothesis the most likely. Indeed, the observation at the cellular level that infection of barley coleoptile cells by B. graminis induced susceptibility of the same cells to a later attack by the nonhost pathogen Erysiphe pisi (Kuno et al., 1985a, 1985b) as well as by the same pathogen B. graminis (Kuno et al., 1991) is in agreement with this hypothesis. Moreover, induced accessibility to B. g. hordei of barley epidermal leaf cells that have previously been successfully infected by the same isolate has been reported recently (Lyngkjaer and Carver, 1999). Similar results were obtained in oat with B. graminis f sp avenae (Carver et al., 1999). Thus, it appears that in successfully colonized cells, defense reactions, including the occurrence of cell death (Carver et al., 1999), are suppressed.
The fact that syringolin A treatment of powdery mildew–infected wheat results in the re-induction of WIR1 and WIR2 transcript accumulation and the triggering of cell death associated with whole-cell autofluorescence indicates that syringolin A may relieve colonized host cells from suppression of defense reactions imposed by the pathogen. This results in the compatible interaction switching to an incompatible one associated with cell death reminiscent of the hypersensitive reaction observed in race-specific resistance following the gene-for-gene concept (Jørgensen, 1994; Hammond-Kosack and Jones, 1996; Baker et al., 1997; Ellis and Jones, 1998; Nürnberger, 1999). Significantly, our results suggest that wheat cells colonized by the powdery mildew fungus remain highly competent to undergo hypersensitive-like cell death. In principle, syringolin A may achieve this by directly acting on the host cell; for example, it may act as an elicitor that triggers, in synergy with fungal elicitors, those host defense reactions that are normally suppressed in colonized cells. Alternately, syringolin A may act indirectly through a fungal target by suppressing the suppression of host responses imposed by the colonizing fungus. Such a specific action of syringolin is in agreement with the fact that cyprodinil does not induce death of colonized cells on a large scale. In any case, elucidation of the mode of action of syringolin A promises to open up an exciting road to a novel strategy for powdery mildew control on cereals. Its mode of action is distinctly different from the one of the plant activator BTH, which has no significant curative activity on powdery mildew–infected wheat and induces a different set of genes (Görlach et al., 1996).
In addition, syringolin A may prove to be an excellent tool for studying the biochemical and molecular events underlying hypersensitive cell death, which it allows to induce on a large scale. Monitoring gene expression in host and pathogen after application of syringolin A provides one approach to achieve this. Such experiments will be possible in the near future using expressed sequence tag libraries and microarray hybridization techniques that are currently being developed.
METHODS
Growth of Plants, Infection with Powdery Mildew, and Microscopy
Seven-day-old seedlings of winter wheat (Triticum aestivum cv Fidel) were used for all experiments. Plants were grown in a growth chamber with 16-hr-light/8-hr-dark cycles at 26 and 12°C, respectively, and 60% relative humidity. For powdery mildew inoculations, a swiss field isolate of Blumeria graminis f sp tritici was used, which was maintained on wheat cultivar Kanzler by weekly transfer to fresh plants.
For inoculation of whole plants, spores from one to 12 heavily infected wheat leaves were blown into an inoculation chamber containing the test plants. Typically, this resulted in a spore density of between one and 20 conidiospores per mm2 leaf area. After inoculation, plants were again transferred to the growth chambers and kept under the conditions given above. For inoculation of wheat leaf segments, the apical 4 cm of primary leaves was cut and placed onto 0.5% (w/v) water-phytagar (Gibco BRL, Paisley, UK) containing 20 mg L−1 benzimidazole as senescence inhibitor in Petri dishes (5 cm in diameter) (Leath and Heun, 1990). The Petri dishes were placed inside the inoculation chamber and inoculated as described above. After inoculation, Petri dishes were incubated on the laboratory bench at room temperature.
For microscopic examination of infected wheat leaves, 3-cm-long leaf segments were stained by boiling in lactophenol/trypan blue/ethanol for 3 min. After incubation for 1 hr at room temperature, leaves were cleared in chloral hydrate for 1 hr as described (Kmecl et al., 1995). Leaf segments were then mounted on microscopic slides in 40% glycerol and analyzed by bright-field or blue light incident fluorescence microscopy (450- to 490-nm excitation filter; 515- to 565-nm emission filter). This procedure stained fungal structures but still allowed us to detect autofluorescence of hypersensitively reacting cells. To stain dead cells, leaf segments were incubated overnight in the lactophenol/trypan blue/ethanol solution at 37°C and destained in chloral hydrate for 24 hr.
Plasmolysis and Neutral Red Dye Staining Procedures
Plasmolysis of and neutral red dye uptake into epidermal cells were monitored essentially as described (Koga et al., 1988; Görg et al., 1993), except that the epidermis was not stripped. In short, infected leaf segments that were treated with 40 μM syringolin A at 2 days after inoculation were carefully vacuum-infiltrated at 7 days after inoculation with 0.1 M α-methyl-d-glucose in distilled water for 10 min. The infiltration was repeated sequentially with 0.2, 0.3, and 0.4 M α-methyl-d-glucose. The final incubation was performed in a buffer containing 0.5 M α-methyl-d-glucose, 25 mM sodium phosphate, pH 7.7, and 0.01% (w/v) neutral red dye (Fluka, Buchs, Switzerland) for 20 min. Examination using green light incident fluorescence microscopy (545-nm excitation filter; 610-nm long pass filter) was performed immediately after the final infiltration. Infected control leaves not sprayed with syringolin A were treated identically but examined 3 days after inoculation, when fungal colony size was approximately equal in the two samples.
Isolation and Application of Syringolin A
Conditioned medium of Pseudomonas syringae pv syringae strain B 301D-R (Xu and Gross, 1988) still cultures was prepared as described (Wäspi et al., 1999). For isolation of syringolin A, 1 liter of conditioned medium was lyophilized, redissolved in 100 mL of distilled water, and loaded on an Amberlite XAD 16 column (2.6 × 40 cm; Rohm and Haas, Philadelphia, PA) equilibrated with water. After washing with 300 mL of water and 300 mL of 20% (v/v) methanol, bound compounds were eluted with 400 mL of 70% (v/v) 2-propanol. The eluate was evaporated to dryness on a Rotavapor-R evaporator (Büchi, Flawil, Switzerland) at 50°C and redissolved in 25 mL of distilled water. After addition of trifluoroacetic acid to a final concentration of 0.06%, 5-mL aliquots were isocratically separated by reverse-phase HPLC on a Nucleosil 100 7 C18 250/10 column (Macherey-Nagel, Düren, Germany) with 20% (v/v) acetonitrile and 0.06% (v/v) trifluoroacetic acid in water, using a flow rate of 5 mL min−1. Absorption was monitored at 206 nm. The peak containing syringolin A eluted at 16 min (peak 1 as described by Wäspi et al. [1999]) was collected and lyophilized. Lyophilized eluate from five runs was dissolved in a total of 5 mL of 100 mM NaOH and run over a Superdex 30 16/60 gel filtration column (Pharmacia, Uppsala, Sweden) on an LCC-501 Plus FPLC system (Pharmacia) at a flow rate of 1 mL min−1 using distilled water as the running solvent. Absorption was monitored at 254 nm. The peak eluting between 50 and 75 min was collected and lyophilized. Lyophilized syringolin A was weighed and dissolved in an appropriate volume of distilled water (pH 7.0) containing 0.05% (v/v) Tween 20 to give a final concentration of 40 μM, if not otherwise indicated. This solution was sprayed onto plants or leaf segments placed on agar plates. To check for a translaminar effect of syringolin A, six 5-μL droplets of a syringolin A solution were placed onto the adaxial surface of detached leaf segments at regular distances. The segments were placed onto agar plates with the adaxial side facing up. The leaf segments were inoculated on the abaxial surface after being turned upside down.
Treatment with Cyprodinil
Cyprodinil (Novartis AG, Basel, Switzerland) was kindly provided by Dr. M. Oostendorp (Novartis Crop Protection AG, Basel, Switzerland). The active substance was dissolved in distilled water at concentrations of up to 20 ppm or in an 8:2 (v/v) water/acetone mixture for higher concentrations. The solutions were sprayed onto plants.
RNA Extraction, in Vitro Translation, and Gel Blot Analysis
Extraction of total RNA and RNA gel blot analysis were performed by standard procedures (Maniatis et al., 1982), using the 32P-labeled WIR (Kmecl et al., 1995) and WCI (Görlach et al., 1996) cDNA inserts as probes.
Poly(A)+ RNA isolation, translation in vitro using rabbit reticulocyte lysate (In Vitro Express Translation Kit; Stratagene, La Jolla, CA) in the presence of 35S-methionine and 35S-cysteine, and display of vitro translation products by two-dimensional polyacrylamide gel electrophoresis were performed as described (Schweizer et al., 1995).
Protein Extraction and Gel Blot Analysis
Proteins were extracted by grinding leaf tissue in 10 volumes of extraction buffer (5 mM 2-mercaptoethanol and 50 mM sodium phosphate, pH 7.0) with mortar and pestle at 4°C. The homogenates were centrifuged at 10,000g for 10 min. Protein concentrations in the supernatant were determined with the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA). Proteins were separated on 15% SDS–polyacrylamide gels and blotted onto nitrocellulose membranes using standard methods. Blots were incubated using a 1:1000 dilution of an antiserum obtained by immunizing rabbits with a recombinant Pir7b fusion protein (Wäspi et al., 1998b) in combination with a 1:2000 dilution of a secondary alkaline phosphatase-coupled anti-rabbit IgG antibody (Boehringer Mannheim). Blots were finally developed in a solution containing 3 mM 4-nitroblue tetrazolium chloride, 0.38 mM 5-bromo-4-chloro-3-indolylphosphate, 100 mM NaCl, and 100 mM Tris-HCl, pH 9.5.
Acknowledgments
We thank Dr. Michael Oostendorp of Novartis Crop Protection AG for the gift of cyprodinil. This work was supported by the Kommission für Technologie und Innovation (KTI Grant No. 3157.1), the Swiss National Science Foundation (Grant No. 31-58656.99), and the Kanton Zürich.
References
- Baker, B., Zambryski, P., Staskawicz, B., and Dinesh-Kumar, S.P. (1997). Signaling in plant–microbe interactions. Science 276 726–733. [DOI] [PubMed] [Google Scholar]
- Bull, J., Mauch, F., Hertig, C., Rebmann, G., and Dudler, R. (1992). Sequence of a wheat gene encoding a novel protein associated with pathogen-defense. Mol. Plant-Microbe Interact. 5 516–519. [DOI] [PubMed] [Google Scholar]
- Carver, T.L.W., Lyngkjaer, M.F., Neyron, L., and Strudwicke, C.C. (1999). Induction of cellular accessibility and inaccessibility and suppression and potentiation of cell death in oat attacked by Blumeria graminis f. sp. avenae. Physiol. Mol. Plant Pathol. 55 183–196. [Google Scholar]
- Dudler, R., Hertig, C., Rebmann, G., Bull, J., and Mauch, F. (1991). A pathogen-induced wheat gene encodes a protein homologous to glutathione-S-transferase. Mol. Plant-Microbe Interact. 4 14–18. [DOI] [PubMed] [Google Scholar]
- Ellis, J., and Jones, D. (1998). Structure and function of proteins controlling strain-specific pathogen resistance in plants. Curr. Opin. Plant Biol. 1 288–293. [DOI] [PubMed] [Google Scholar]
- Felix, G., Regenass, M., and Boller, T. (1993). Specific perception of subnanomolar concentrations of chitin fragments by tomato cells—Induction of extracellular alkalinization, changes in protein-phosphorylation, and establishment of a refractory state. Plant J. 4 307–316. [Google Scholar]
- Görg, R., Hollricher, K., and Schulze-Lefert, P. (1993). Functional analysis and RFLP-mediated mapping of the Mlg resistance locus in barley. Plant J. 3 857–866. [Google Scholar]
- Görlach, J., Volrath, S., Knauf Beiter, G., Hengy, G., Beckhove, U., Kogel, K.H., Oostendorp, M., Staub, T., Ward, E., Kessmann, H., and Ryals, J. (1996). Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack, K.E., and Jones, J.D.G. (1996). Resistance gene–dependent plant defense responses. Plant Cell 8 1773–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa, P., Granado, J., Freydl, E., Wäspi, U., and Dudler, R. (2000). Syringolin-mediated activation of the Pir7b esterase gene in rice cells is suppressed by phosphatase inhibitors. Mol. Plant-Microbe Interact. 13 342–346. [DOI] [PubMed] [Google Scholar]
- Hunt, M.D., and Ryals, J.A. (1996). Systemic acquired resistance signal transduction. Crit. Rev. Plant Sci. 15 583–606. [DOI] [PubMed] [Google Scholar]
- Jørgensen, J.H. (1994). Genetics of powdery mildew resistance in barley. Crit. Rev. Plant Sci. 13 97–119. [Google Scholar]
- Kmecl, A., Mauch, F., Winzeler, M., Winzeler, H., and Dudler, R. (1995). Quantitative field resistance of wheat to powdery mildew and defense reactions at the seedling stage: Identification of a potential marker. Physiol. Mol. Plant Pathol. 47 185–199. [Google Scholar]
- Knauf-Beiter, G., Dahmen, H., Heye, U., and Staub, T. (1995). Activity of cyprodinil—Optimal treatment timing and site of action. Plant Dis. 79 1098–1103. [Google Scholar]
- Koga, H., Zeyen, R.J., Bushnell, W.R., and Ahlstrand, G.G. (1988). Hypersensitive cell death, autofluorescence, and insoluble silicon accumulation in barley leaf epidermal cells under attack by Erysiphe graminis f. sp. hordei. Physiol. Mol. Plant Pathol. 32 395–409. [Google Scholar]
- Koga, H., Bushnell, W.R., and Zeyen, R.J. (1990). Specificity of cell type and timing of events associated with papilla formation and the hypersensitive reaction in leaves of Hordeum vulgare attacked by Erysiphe graminis f. sp. hordei. Can. J. Bot. 68 2344–2352. [Google Scholar]
- Kuno, H., Hayashimoto, A., Harui, M., and Ishizaki, H. (1985. a). Induced susceptibility and enhanced resistance at the cellular level in barley coleoptiles. I. The significance of timing of fungal invasion. Physiol. Plant Pathol. 27 43–54. [Google Scholar]
- Kuno, H., Kuroda, H., Hayashimoto, A., and Ishizaki, H. (1985. b). Induced susceptibility and enhanced resistance at the cellular level in barley coleoptiles. II. Timing and localization of induced susceptibility in a single coleptile cell and its transfer to an adjacent cell. Can. J. Bot. 64 889–895. [Google Scholar]
- Kuno, H., Kuroda, H., Toyoda, K., Yamaoka, N., and Kobayashi, I. (1991). Induced susceptibility and enhanced resistance at the cellular level in barley coleoptiles. IX. Accessibility induced by Erysiphe graminis which promotes the subsequent infection of challenging E. graminis. Ann. Phytopathol. Soc. Jpn. 57 57–60. [Google Scholar]
- Leath, S., and Heun, M. (1990). Identification of powdery mildew resistance genes in cultivars of soft red winter-wheat. Plant Dis. 74 747–752. [Google Scholar]
- Lyngkjaer, M.F., and Carver, T.L.W. (1999). Induced accessibility and inaccessibility to Blumeria graminis f. sp. hordei in barley epidermal cells attacked by a compatible isolate. Physiol. Mol. Plant Pathol. 55 151–162. [Google Scholar]
- Maniatis, T., Fritsch, E.F., and Sambrook, J. (1982). Molecular Cloning. A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Nürnberger, T. (1999). Signal perception in plant pathogen defense. Cell. Mol. Life Sci. 55 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebmann, G., Hertig, C., Bull, J., Mauch, F., and Dudler, R. (1991. a). Cloning and sequencing of complementary DNAs encoding a pathogen-induced putative peroxidase of wheat (Triticum aestivum L.). Plant Mol. Biol. 16 329–332. [DOI] [PubMed] [Google Scholar]
- Rebmann, G., Mauch, F., and Dudler, R. (1991. b). Sequence of a wheat cDNA encoding a pathogen-induced thaumatin-like protein. Plant Mol. Biol. 17 283–285. [DOI] [PubMed] [Google Scholar]
- Reimmann, C., Hofmann, C., Mauch, F., and Dudler, R. (1995). Characterization of a rice gene induced by Pseudomonas syringae pv. syringae: Requirement for the bacterial lemA gene function. Physiol. Mol. Plant Pathol. 46 71–81. [Google Scholar]
- Schweizer, P., Hunziker, W., and Mosinger, E. (1989). Complementary DNA cloning in vitro, transcription, and partial sequence analysis of messenger RNA from winter wheat Triticum aestivum L. with induced resistance to Erysiphe graminis f. sp. tritici. Plant Mol. Biol. 12 643–654. [DOI] [PubMed] [Google Scholar]
- Schweizer, P., Vallélian-Bindschedler, L., and Mösinger, E. (1995). Heat-induced resistance in barley to the powdery mildew fungus Erysiphe graminis f. sp. hordei. Physiol. Mol. Plant Pathol. 47 51–66. [Google Scholar]
- Smith, J.A., and Métraux, J.P. (1991). Pseudomonas syringae pathovar syringae induces systemic resistance to Pyricularia oryzae in rice. Physiol. Mol. Plant Pathol. 39 451–461. [Google Scholar]
- Sticher, L., Mauch-Mani, B., and Métraux, J.P. (1997). Systemic acquired resistance. Ann. Rev. Phytopathol. 35 235–270. [DOI] [PubMed] [Google Scholar]
- Wäspi, U., Blanc, D., Winkler, T., Ruedi, P., and Dudler, R. (1998. a). Syringolin, a novel peptide elicitor from Pseudomonas syringae pv. syringae that induces resistance to Pyricularia oryzae in rice. Mol. Plant-Microbe Interact. 11 727–733. [Google Scholar]
- Wäspi, U., Misteli, B., Hasslacher, M., Jandrositz, A., Kohlwein, S.D., Schwab, H., and Dudler, R. (1998. b). The defense-related rice gene Pir7b encodes an “alpha/beta hydrolase fold” protein exhibiting esterase activity towards naphthol AS-esters. Eur. J. Biochem. 254 32–37. [DOI] [PubMed] [Google Scholar]
- Wäspi, U., Hassa, P., Staempfli, A., Molleyres, L.-P., Winkler, T., and Dudler, R. (1999). Identification and structure of a family of syringolin variants: Unusual cyclic peptides from Pseudomonas syringae pv. syringae that elicit defense responses in rice. Microbiol. Res. 154 1–5. [Google Scholar]
- Xu, G.W., and Gross, D.C. (1988). Physical and functional analyses of the syrA and syrB genes involved in syringomycin production by Pseudomonas syringae pv. syringae. J. Bacteriol. 170 5680–5688. [DOI] [PMC free article] [PubMed] [Google Scholar]