Abstract
Genome engineering via targeted nucleases, specifically CRISPR-Cas9, has revolutionized the field of gene therapy research, providing a potential treatment for diseases of the blood and immune system. While numerous genome editing techniques have been used, CRISPR-Cas9 homology-directed repair (HDR)-mediated editing represents a promising method for the site-specific insertion of large transgenes for gene knock-in or gene correction. Alternative methods, such as lentiviral/gammaretroviral gene addition, gene knock-out via non-homologous end joining (NHEJ)-mediated editing, and base or prime editing, have shown great promise for clinical applications, yet all possess significant drawbacks when applied in the treatment of patients suffering from inborn errors of immunity or blood system disorders. This review aims to highlight the transformational benefits of HDR-mediated gene therapy and possible solutions for the existing problems holding the methodology back. Together, we aim to help bring HDR-based gene therapy in CD34+ hematopoietic stem progenitor cells (HSPCs) from the lab bench to the bedside.
Keywords: HDR, CRISPR-Cas9, gene editing, gene therapy
1. Introduction
Genome editing via targeted nucleases has fundamentally changed life-science research since it provides the ability to introduce new genes or correct mutated genes at the chosen genomic locations in a multitude of different species [1,2,3,4,5,6,7,8]. By creating a double-strand break (DSB) at a specific target site in the genome, researchers have been able to induce desired changes by harnessing the cell’s endogenous DNA repair pathways, of which there are two major mechanisms: non-homologous end joining (NHEJ) and homology-directed repair (HDR). NHEJ is an error-prone mechanism that can introduce small insertions or deletions (INDELs), leading to frameshift mutations. It has been widely used for the inactivation or knock-out of target genes [9,10]. HDR, on the other hand, requires a template, such as the sister chromatid or homologous chromosome, to repair the DSB in an error-free way, making it more appropriate for treating genetic disorders. In the context of genome editing, this has allowed researchers to utilize an exogenous donor template to actuate gene knock-in or gene correction using homologous DNA sequences via the HDR repair pathway [11].
Targeted nucleases, such as zinc-finger nucleases (ZFNs) and transcription-activator-like-effector-nucleases (TALENs) [12,13,14], have been used in the past for genome-editing purposes; however, the advent of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-CRISPR-associated 9 (Cas9) technology has made gene-editing applications simpler and more widely accessible due to the versatility, flexibility, and precision of the technology [4,5,15]. More specifically, in 2005, researchers were able to integrate a corrective transgene into the IL2RG gene via HDR with a plasmid donor template after DSB introduction by ZFNs in the K562 cell line [16]. It took another two years to adapt the capability to HSPCs [17]. The requirement for custom-made nucleases for each genomic target constituted a major hurdle while using TALENs or ZFNs, consequently impeding progress in the successful translation to gene correction in HSPCs. Despite this, specific pre-clinical and clinical trials have still been effectively conducted [18,19]. Hence, once the CRISPR-Cas9 technology was introduced to the field of genome editing, it greatly facilitated the attempts to conduct gene editing in HSPCs, especially for the purpose of ex vivo gene therapy using patients’ own hematopoietic cells. In this process, the patient’s HSPCs or T lymphocytes are isolated from the blood, edited ex vivo, and then the modified cells are transplanted back into the patient. This technique simultaneously eliminates the need to search for a human leukocyte antigen (HLA)-matched donor, which can be difficult to find, while negating the risk of graft rejection or graft versus host disease (GvHD). Thus, such a method could provide an ideal alternative to the allogeneic bone marrow transplant—the gold-standard treatment of genetic diseases of the blood and immune system—and is well suited for correcting inborn errors of immunity and blood disorders, including β-globin-related diseases such as sickle-cell disease (SCD) and β-thalassemia [20,21,22,23,24,25,26,27,28], Severe Combined Immunodeficiency (SCID) [29,30], Polyendocrinopathy Enteropathy X-linked Syndrome (IPEX) [31], Wiskott-Aldrich syndrome (WAS) [32], X-linked hyper-immunoglobulin M (hyper-IgM) (XHIM) [33], and X-linked Chronic Granulomatous Disease (X-CGD) [34]—to name a few. Previous studies and trials utilized lentiviral (LV) or gammaretroviral (γRV) vectors for ex vivo genome editing to integrate a normal copy of the gene into the genome in a semi-random fashion and transfused the cells back into the patients [35,36,37,38]. Regretfully, a number of these cases resulted in a leukemic transformation due to the activation of proto-oncogenes in the patients upon insertional mutagenesis [39,40,41,42,43]. Although significant improvements to these vectors have been made, major safety concerns still exist for LV and γRV vector-based treatments. Despite this, LV-based gene therapy for numerous forms of SCID (RAG1, IL2RG, DCRLE1C, and ADA), WAS, and other inborn errors of immunity are currently undergoing clinical trials [44,45,46,47,48,49,50,51,52,53]. To that end, a targeted CRISPR-Cas9/HDR-based methodology for site-specific transgene integration could alleviate the concerns plaguing LV and γRV vectors -incomplete phenotypic correction, dysregulated hematopoiesis, and insertional mutagenesis related to the semi-random integration and constitutive expression of the transgene [54,55].
While opportunities to apply CRISPR-Cas9-based gene therapies are vast, the current safety and efficacy barriers are inhibiting it from becoming a widely accepted method in the clinic. These include genotoxicity, toxicity associated with the exogenous DNA delivery method, insufficient HDR, low engraftment efficiency, and poor survival of transplanted cells in mice models. In this review, we aim to summarize the different methods of inducing HDR in CD34+ HSPCs for the purpose of correcting inborn errors of the blood and immune system. Additionally, we aim to highlight the recent and ongoing studies utilizing genome-editing HDR in HSPCs and the pitfalls currently preventing HDR-based genome editing from becoming a widely accepted clinical application. Additionally, we offer potential ways to improve the efficiency of HDR, reduce DNA-donor-delivery-associated toxicity, improve engraftment efficiencies in murine models, and increase edited cell survival. Together, these improvements will help to advance new CRISPR-Cas9-based techniques toward being applied clinically in the near future.
2. Gene-Editing Nucleases
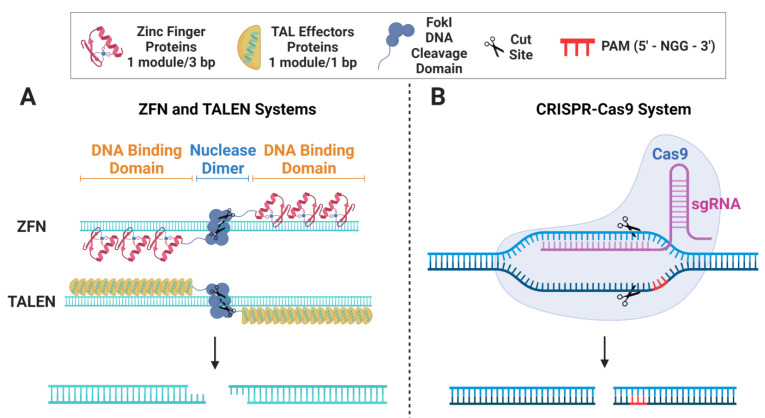
TALENs, ZFNs, and CRISPR-Cas9 are all gene-editing tools that have been developed to induce targeted changes to an organism’s DNA. However, they differ in their underlying mechanisms and overall efficiency. ZFNs were one of the first gene-editing tools to be developed, and they operate via modifying zinc-finger protein domains that are fused to the DNA cleavage domain of the FokI restriction enzyme to bind to specific DNA sequences, as shown in Figure 1A. Once localized to its target locus, the ZFN nucleases produce a DSB with sticky ends [12,13,14,56,57]. Similarly, the TALEN system utilizes the FokI enzyme bound to sequence-specific TAL effector proteins [58] to induce a sticky-ended DSB at the targeted site [12,56,57] (Figure 1A). While ZFNs and TALENs can be highly specific and accurate, they are more difficult to customize, design, and implement than CRISPR-Cas9. Contrary to ZFNs and TALENs that require the production of distinct enzymes for each DNA target locus, CRISPR-Cas9 operates with a customizable guide RNA (gRNA) and a uniform nuclease (Cas9), regardless of the target. After the gRNA and Cas9 enzyme form a ribonucleoprotein (RNP) complex, the gRNA scans the DNA for a sequence that is complementary to the 20 nt spacer region with an adjacent upstream protospacer adjacent motif (PAM) sequence (5′-NGG-3′ in S. pyogenes) [59]. After the PAM sequence is recognized, the gRNA undergoes seed nucleation to form an RNA:DNA hybrid duplex and the now-active Cas9 nuclease induces the DSB 3 nt upstream to the PAM sequence (Figure 1B). While this review focuses specifically on HDR-based gene editing, it is important to note that the underlying mechanisms, other applications, drawbacks, and possible considerations for the different gene-editing nucleases have been extensively reviewed in Carroll et al. [12]. Additionally, while all three gene-editing tools are effective at making targeted changes to an organism’s DNA, CRISPR-Cas9 is the most widely employed in gene therapy research due to its intrinsic simplicity and versatility, as well as the massive improvements in its specificity, efficiency, and tolerability in HSPCs [60,61,62].
Figure 1.
Genome-editing Nucleases. (A) Schematic depicting ZFN and TALEN genome-editing systems and the resulting DSB. (B) Schematic depicting the CRISPR-Cas9 genome-editing systems and the resulting DSB.
3. Mechanism of HDR
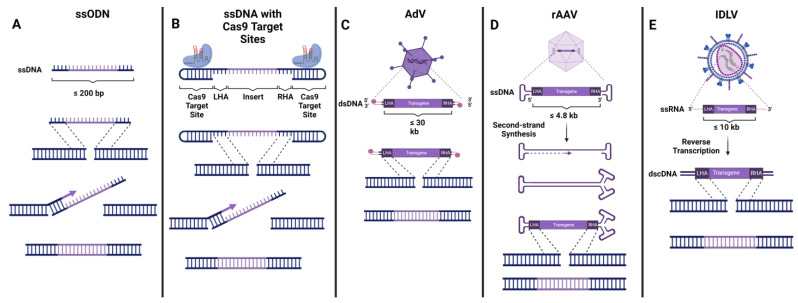
Since we will not be providing in-depth coverage of the mechanisms and different types of DNA repair involved in genome-editing applications in this review, readers may refer to Nambiar et al. [10] for a detailed analysis of this topic. Briefly, for context, once the genome-editing-induced DSB is created, a signalling cascade commences, and the cell’s DNA repair machinery is recruited to the break site. Depending on the type of HDR—canonical HDR, synthesis-dependent stranded annealing (SDSA), break-induced replication (BIR), single-stranded annealing (SSA), or single-stranded templated repair (SSTR)—the mechanism varies slightly [63,64]. In canonical HDR, the exposed DSB ends are trimmed and processed to create 3′ overhangs, while RAD51, along with other repair factors, searches for a homologous template in order to repair and refill the resected DNA. RAD51 is an ATPase that creates a nucleoprotein filament on single-stranded DNA and locates and infiltrates homologous DNA sequences for the purpose of facilitating precise DNA repair. In a native intracellular context, a homologous template comes in the form of a sister chromatid or the homologous chromosome, while in the context of gene-editing applications, it comes in the form of an exogenously introduced DNA donor template. After such a template is detected, the template undergoes strand invasion, and D-loop formation is established by annealing the overhanging tails to the homologous sequence. Once this occurs, the DNA polymerase begins to copy the sequence of the template onto the broken strand, creating a new, repaired strand of DNA. When the newly synthesized strand of DNA recognizes another homologous sequence, the elongation process is aborted, and the strand is ligated to the other exposed DSB end [65]. In gene-editing methodologies for therapeutic applications, the exogenous DNA donor template can be delivered in a number of ways including, naked single-stranded oligodeoxynucleotides (ssODNs) or longer single-stranded DNA (ssDNA), adenovirus 5/35 serotype (AdV), recombinant adeno-associated virus serotype 6 (AAV), and integration-deficient lentivirus (IDLV) (Figure 2). Since these DNA donor template platforms lack any integration machinery of their own, the DNA donors contain homology arm sequences flanking the transgene, enabling the complete integration of the entire sequence between the two homology arms into the nuclease-induced DSB. Each platform has its own benefits and drawbacks, which has led researchers to choose different methodologies for different applications, as outlined below (Table 1).
Figure 2.
Delivery Mechanisms for HDR. (A) Single-stranded oligodeoxynucleotides (ssODNs). Together with ssODNS, DSBs are processed by the RAD51-independent SSTR pathway, in which the ssDNA filament invades the homologous region to form a D-loop structure, enabling DNA synthesis by a polymerase and eventual resection of the DSB [66]. (B) ssDNA with dsDNA Cas9 target sequences on the ends. These donors have been shown to induce efficient HDR at donor lengths of up to ~3.5 kb. The NLS on the Cas9 allows for more efficient trafficking of the donor into the nucleus, enabling enhanced HDR efficiencies [67]. (C) Adenovirus 5/35 (AdV). AdVs are capable of carrying very large dsDNA payloads of ~30–35 kb. The 5′ termini of AdV genome bind covalently to virus-coded terminal proteins, enabling better stability and markedly reduced HDR-independent incorporation into the genome [68]. (D) Recombinant adeno-associated virus serotype 6 (AAV). AAV vectors carry an ssDNA payload with inverted terminal repeats that create dsDNA loops at both the 5′ and 3′ ends of the genome. After entering the cell, the genome is believed to undergo second-strand synthesis, providing a dsDNA intermediate to facilitate HDR and integration into the genome [69,70,71]. (E) Integration-deficient Lentivirus (IDLV). IDLVs carry single-stranded viral RNA along with other enzymes including reverse transcriptase. When the ssRNA payload is released into the cytoplasm it undergoes reverse transcription, producing a dsDNA product that can then be imported into the nucleus to serve as an HDR template [72].
Table 1.
Benefits and drawbacks of different DNA donor template delivery platforms.
| Delivery Platform | Benefits | Drawbacks |
|---|---|---|
| Non-viral delivery of naked DNA |
|
|
|
|
|
|
||
| AdV |
|
|
| ||
| AAV |
|
|
| ||
| ||
| IDLV |
|
|
| ||
| ||
|
4. Delivery of DNA Donor Template
4.1. Non-Viral Donor DNA Delivery
The delivery of the exogenous donor template via single-stranded oligodeoxynucleotides (ssODNs) provides a non-viral platform that enables the highly specific insertion of relatively short sequences (maximum 200 bp) [73] (Figure 2A). These viral-free vectors can be easily synthesized; however, since they are delivered as free-floating ssDNA, they degrade rapidly, reducing their efficiency [74]. ssODN integration into the genome is mediated by the RAD51-independent single-strand template repair (SSTR) pathway rather than via conventional HDR [75]. A higher HDR efficiency has been achieved by using asymmetrically designed ssODNs, namely with donor DNA that is complementary to the non-target strand [76]. ssODNs have been used to correct the HBB and CYBB genes, respectively, both in vitro (in isolated HSPCs) and in vivo (in mice) [22,26,34]. Interestingly, ssODN-mediated HDR frequencies in the HBB gene were found to be lower than those achieved with AAV in vitro; however, the ssODN-modified cells showed improved engraftment and survival rates when transplanted to immunodeficient mice [25,26]. A recent report demonstrated enhanced HDR in primary immune cells, including HSPCs, by designing longer ssDNA HDR templates flanked by short double-strand Cas9 target sequences that recruit CRISPR-Cas9 RNP complexes (Figure 2B). Shy et al. showed that the nuclear localization signal (NLS) on the Cas9 nuclease helped improve nuclear trafficking of the ssDNA/RNP complex, creating a favorable environment for the efficient repair of Cas9-mediated DSB by HDR using the single-strand part of the donor. In concert with HDR enhancers, they achieved the efficient correction of IL2RA and CTLA4 pathogenic mutations and established GMP manufacturing practices, paving the road for potential future clinical trials [67]. While ssODNs or other non-viral platforms for DNA donor delivery are excellent ways to deliver short transcripts, these vectors are limited in their size. Therefore, they do not enable the addition of reporter gene cassettes and/or the preservation of the gene’s endogenous regulatory elements in the delivered transgene.
4.2. AdV
Adeno Viruses (AdVs) are vectors that can deliver a 28–36 kb non-enveloped dsDNA molecule with both 5′ ends capped with covalently attached proteins. Additionally, a high-capacity variant (Hc-AdV) with an increased carrying capacity of ~37 kb has been developed to deliver even larger transgenes [77,78,79] (Figure 2C). Aside from the ability to carry large payloads, these vectors have shown a marked benefit in the form of reduced HDR-independent transgene integration to the break sites compared to other viral vectors that carry uncapped donor DNA [80]. However, their integration efficiency is limited, yielding only 2–5% HDR following editing with ZFNs [26].
4.3. AAV
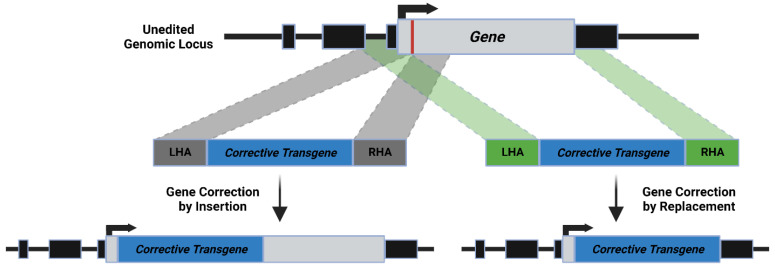
AAV vectors are known to be a highly efficient delivery mechanism of a donor DNA molecule to HSPCs and have been covered extensively in Dudek et al. [81]. However, these vectors have a limited carrying capacity of up to ~5 kb in size, making them applicable for the delivery of transgenes larger than ssODN, yet still unable to deliver entire cDNA for larger genes [82,83]. The ssDNA payload of the AAV donor contains inverted terminal repeats (ITRs) on both ends of the fragment that play a fundamental role in the life cycle of the virus [84]. After the ssDNA sheds its viral protein coat and enters the nucleus, it undergoes second-strand synthesis to dsDNA, with dsDNA serving as a predominant template for HDR-mediated gene targeting [69,85,86] (Figure 2D). AAV donor DNA sequences have been shown to induce high-quality HDR in CD34+ HSPCs with minimum homology arm lengths of ~300–400 bp, although, in certain cases, extending the homology arms to as long as 1.6–2.2 kb has shown marked improvement in HDR efficiency in HSPCs and several other primary cells [20,25,87,88]. The ease of use and early success in using AAV for transgene delivery in gene-therapy applications has boosted its popularity as a therapeutic vector. Pre-clinical studies using AAV vectors to develop potential treatments for SCD and β-thalassemia [20], SCID-X1 [30,89], WAS [32], IPEX [31], CGD [90,91,92], Hemophilia [93], XLA (X-Linked Agammaglobulinemia) [94], XMEN (X-linked Immunodeficiency with Magnesium Defect, Epstein–Barr Virus (EBV) Infection, and Neoplasia) [95], and most recently RAG2-SCID [29,87,96,97] have been conducted in CD34+ HSPCs, showing highly efficient frequencies of HDR. Interestingly, most AAV donor structures have the homology arms flanking the immediate genomic sequences 5′ and 3′ to the Cas9-induced DSB, leading to the insertion of the transgene into the break site. However, recent works have shown that distancing the 3’ homology arm from the DSB site allows for the replacement of up to several kilobases of genomic DNA with the transgene [20,87] (Figure 3). Allen et al. utilized this replacement strategy to replace the entire endogenous RAG2 coding sequence, allowing for transgenes to maintain critical endogenous regulatory elements while preserving the integrity and 3D architecture of the genomic locus as much as possible by limiting the introduction of kilobases of new DNA [87]. This replacement strategy is particularly useful for correcting tightly controlled genes, such as RAG2, that are regulated by spatiotemporal elements in the surrounding locus [98]. Additionally, this replacement strategy was used by Cromer et al. to replace the α-globin (HBA) gene with the β-globin (HBB) gene to restore the balance in β-thalassemia-patient-derived HSPCs [20]. While AAVs are non-pathogenic and elicit a low immune response, they are still capable of triggering a toxic DDR proportional to the amount of virus used or the multiplicity of infection (MOI) [99]. Thus, finding the delicate balance between inducing high frequencies of HDR while limiting the MOI used is critical. Unfortunately, a recent Phase 1/2 clinical study by Graphite Bio for the treatment of SCD was paused due to the development of pancytopenia in the first patient treated with their AAV-based therapy [100], highlighting the importance of finding this balance.
Figure 3.
HDR formulations: Insertion vs. Replacement. Both techniques utilize homology arms; however, whereas the insertion method (gray dotted lines) has homology arms centered at the Cas9 cut site (depicted as the red line downstream to the gene’s ATG), the replacement method (green dotted lines) has a left homology arm flanking the genomic DNA upstream to the cut site while the right homology arm is distanced to be downstream to the region being replaced, in the depicted case, downstream to the stop the codon of the gene’s open reading frame (ORF).
4.4. IDLV
To circumvent the limited carrying capacity of AAV vectors, some researchers have utilized co-delivery dual-vector approaches, namely splitting the transgene between two distinct AAV vectors that are transduced simultaneously [101,102]. IDLV vectors could provide a more straightforward alternative to AAV when larger payloads are desired. IDLVs are lentiviral vectors packaged with a catalytically inactive integrase, allowing for effective transduction while limiting genomic integration since the genomic payload remains episomal. These vectors carry the donor sequence in the form of RNA, which is converted into dsDNA with exposed ends via reverse transcription upon entry into the target cell. IDLVs have a significantly larger carrying capacity than AAVs (~10 kb) and alleviate the safety concerns regarding the semi-random integration of standard LVs since they are expressed transiently and lack the integration mechanisms of traditional LV vectors [72,103,104,105] (Figure 2E). The IDLV system could provide a valuable tool for the replacement of larger genes, potentially increasing the chance of a functional gene correction with minimal interference to the endogenous locus structure and spatiotemporal regulatory elements. To date, studies in HSPCs using IDLV-based HDR to correct SCID-X1- and SCD-causing mutations have shown encouraging results when paired with ZFN or CRISPR-Cas9 [17,19,26,103,106].
5. Existing Barriers to Efficient HDR-Based Therapies
5.1. Specificity
Despite the ability of genome-editing nucleases to induce site-specific targeted editing, undesired on- and off-target editing (and the effects associated with it) remain a significant issue [107,108]. In the case of CRISPR-Cas9, off-target editing occurs as a result of mismatched bases between the gRNA and DNA sequence being tolerated and still inducing a Cas9 DSB. Since the CRISPR-Cas9 system originated in bacteria, this flexibility is believed to be a beneficial evolutionary adaptation for immunity against previously encountered mutated bacteriophages [61]. Accompanying the induction of DSBs at off-target sites is the potential risk of inducing structural variations (translocations, inversions, insertions, and deletions) within edited sites, which can be particularly hazardous [109,110,111,112,113,114,115,116,117]. Numerous platforms and approaches have been developed for the purpose of detecting these off-target sites and structural variations [110,118,119,120]. Additionally, the unwanted integration of the exogenous HDR template into off-target edited sites has been reported [121]. While many studies have been conducted to both limit and quantify the off-target activity of the gene-editing nucleases [61], a recent study by Ferrari et al. showed that the use of IDLV showed less unwanted donor integration to off-target sites when compared to AAV vectors [121]. Additionally, genotoxicity stemming from the presence of unintended and unwanted genomic modifications at the on-target locus has been reported as an issue that, unlike the risk associated with off-target editing, cannot be alleviated via more specific DSB approaches. These genomic aberrations can vary from a few bp to the kilobase- or even megabase-scale [122].
5.2. Insufficient HDR/Bias towards NHEJ
Since HDR occurs only during the S/G2 phase of the cell cycle, in the slow-dividing quiescent HSPCs, there is often a bias towards the NHEJ repair mechanism [10,123]. While highly efficient HDR is a pre-requisite for any succsessful clinical application, depending on the disorder being treated, the threshold for the number of cells that need to have undergone successful HDR in the ex vivo editing process varies substantially [124]. For example, with SCID, a relatively small number of corrected cells (~1000 HSCs), if engrafted properly, could reconstitute the entire immune system [125,126,127]. Additionally, based on the reported cases of “naturally occurring gene therapy”, in which a revertant mutation in a single cell enabled T lymphocyte survival and the development of diverse TCR repertoires in a SCID patient [125,128,129,130], it is evident that a minuscule number of “corrected” cells can reconstitute the immune system and abrogate the SCID phenotype. Moreover, Dvorak et al. demonstrated that donor chimerism as low as 3% was sufficient to reconstitute T- and B-cell populations in SCID patients, while for diseases such as thalassemia or SCD, >20% is required [131]. For this reason, cases of suboptimal HDR editing efficiency can lead to the downfall of certain therapeutic applications. Thus, the development of methods to boost HDR frequency is critical. To address these challenges, researchers have experimented with Cas9/HDR template conjugates [61] and have also investigated the potential use of molecules, including i53 and/or DNA-PK inhibitors, to inhibit the NHEJ repair pathway and skew the repair mechanisms towards the HDR repair pathway. Through the use of these molecules, one can transiently inhibit the NHEJ repair pathway and control the cycling and quiescence of the edited cells. This has been shown to significantly increase HDR integration frequencies [67,75,132,133,134]. Additionally, recent studies have shown success in tipping the scale towards HDR by inhibiting DNA polymerase θ (Polθ), a key effector in the DSB repair pathway, by microhomology-mediated end-joining (MMEJ) [10,135,136]. While the stable knock-out or knock-down of DDR and repair proteins can produce irreversible and dangerous effects, utilizing these small molecules can have a transient knock-down effect in the short time window wherein gene editing and HDR occur. In addition to these techniques being efficient methods to enhance HDR frequencies, they can also contribute to reducing virus-associated toxicity by reducing viral load while maintaining highly efficient HDR. Additionally, Shin et al. demonstrated that HDR could be increased through controlled cycling and quiescence of the edited cells [106,137]. Lastly, when HDR efficiencies are low, extending the homology arms of the donor DNA to as long as 1.6–2.2 kb has shown a marked improvement in HDR frequencies via the use of AAV in HSPCs and several other primary cells [20,25,87,88].
5.3. Toxicity
In addition to genotoxicity associated with undesirable DNA repair outcomes resulting from Cas9-induced DSBs, HSPCs are very sensitive to DNA damage both at the on and off-target sites [10]. Additionally, HSPCs have innate immune cues and a plethora of antiviral factors and nucleic acid sensors that are activated in the process of exogenous gene delivery [138,139]. Since all current HDR-based gene-editing technologies expose HSPCs to some form of exogenous nucleic acid, the resulting immune response is of significant concern to researchers and clinicians alike. Additionally, AAV and IDLV vectors are both recognized by DDR proteins in HSPCs, triggering a DDR [99,124,140,141]. More specifically, the transduction of these viral vectors triggers a p53 response, leading to reduced HSPC proliferation, apoptosis, and poor transplantation to immunodeficient mice [106,141]. While the exact DDR mechanism has yet to be elucidated, Allen et al. showed that the level of the DDR was directly proportional to the MOI used [99]. Moreover, Iancu et al. showed that using high viral load led to prolonged viral presence in the cell population, decreased cell yield, and positive selection of unedited cells, leading to a consistent reduction of HDR frequencies over the course of T-cell differentiation in vitro. To alleviate this effect, Iancu and colleagues demonstrated that reducing the amount of viral vector enabled better HSPCs survival and T-cell differentiation of corrected SCID-patient cells in vitro [97]. Interestingly, transient p53 inhibition using a dominant negative p53 truncated form (GSE56) [142] has been shown to significantly preserve the transplanted HSPCs’ multilineage potential [106,141]. Unmitigated AAV-induced toxicity can explain, at least to some extent, the pancytopenia phenotype that occurred in the first AAV-mediated HDR-based clinical trial experiment for the correction of the HBB gene, which has since been halted [100]. Thus, fine-tuning viral-delivery systems to reduce toxicity by reducing the viral load uses and increasing HDR frequencies is critical. In contrast to AAV and IDLV vectors, ssODNs do not elicit p53 activation and are relatively well-tolerated by HSPCs [25,26]. It should be noted that a recent study by Ferrari et al. showed that, when comparing AAV and IDLV vectors directly, the latter induced both a less persistent DDR as well as lower unwanted donor integration, leading to improved clonogenic capacity and editing efficiency in long-term HSPCs [121]. While this would be a great step towards clinical translation in the form of HSPC gene therapy, further studies are required to confirm these results.
5.4. Poor Engraftment of Edited Cells
While gene-editing technologies and transgene delivery mechanisms have made a lot of progress in the past few years, poor engraftment frequencies of the LT-HSC-edited population in the bone marrow of immunodeficient mice [139] has been a recurring problem in proof-of-concept pre-clinical studies when the exogenous template being used for integration is delivered in a virus-dependent manner in CD34+ HSPCs [25,26,50,143]. In addition to the transgene-induced stress, HSPCs have been shown to lose their stemness and engraftment potential when exposed to extensive culturing protocols. This process is a product of poor cell fitness, impairing their ability to reach the bone marrow niche with subsequently reduced engraftment efficiencies [139,144,145,146,147]. Specifically, with the use of the AAV vector, high MOI is still required to reach clinically relevant levels of HDR. Since the toxic cellular responses mentioned above can account for the poor engraftment capabilities [139], the development of strategies to limit the viral dose while maintaining the HDR frequencies is crucial [75,132,133,137]. Additionally, Iancu et al. highlighted the possibility of enriching HDR-positive cells in order to limit the competition in vitro with HDR-negative cells that might prevail [97]. In that study, SCID patient cells were treated with CRISPR-Cas9 and AAV vectors carrying a corrective transgene, and both sorted HDR+ cells and unsorted cells were differentiated in vitro. While the unsorted corrected cells failed to efficiently differentiate and proliferate, the sorted population thrived and developed into CD3+ T cells with diverse T-cell receptor (TCR) repertoires [97]. While this was only performed in vitro, transplanting a homogenous enriched HDR-positive population could improve the engraftment frequencies in vivo, and follow-up studies in mice are required to elucidate this strategy further. Additionally, Poletto et al. showed that improved engraftment of AAV-edited CD34+ HSPCs in immunodeficient mice was possible via Busulfan-based myeloablation [148]. A study by Pavel-Dinu et al. also showed high human chimerism following the in vivo transplantation of cells edited with a WT cDNA cassette targeting the IL2RG locus [30]. This effect could be attributed to the substantial quantity of cells employed in this study, more than 2 × 106 cells per mouse, which may have been enough to offset the toxicity-related cell death and still result in the functional engraftment of the edited population. Albeit, while the engraftment was an overall success, the frequency of human cells was substantially reduced between 1° and 2° mice, which is indicative of reduced cell survival of the edited cells and/or competition with the unedited cells for the bone marrow niche. This issue can possibly be abrogated by (1) limiting the viral vector, and thus the viral-vector-induced toxicity; (2) increasing the input HDR, increasing the chances for the edited cells to compete for the bone-marrow niche; (3) using FACS enrichment of the edited cells before transplantation, limiting niche competition even further; (4) transplanting a greater number of cells, allowing for greater chances of overall engraftment; and/or (5) using ssODNs, which have shown better outcomes in vivo compared to the viral delivery of the transgene.
6. Conclusions
Ex vivo genome editing holds tremendous promise as a therapeutic strategy for monogenic disorders of the blood and immune system. HDR-based platforms offer a versatile approach to functional gene correction, allowing for the addition of curative transgenes to the endogenous locus. Additionally, strategies have been developed to completely remove mutated transcripts and replace them with corrective transgenes, which would allow for the conservation of important regulatory and spatiotemporal elements. Many of these targeted approaches are highly relevant for treating diseases in which possible disease-causing mutations are dispersed over the entirety of the gene, and a universal solution to treat any possible mutations in the given gene cannot be provided using NHEJ-based methodologies (Table 2). Such a HDR-mediated option could permit correcting all possible mutations in a given gene with a single corrective sequence, eliminating the need for patient-by-patient customization. While HDR-based gene therapies have yet to successfully reach the clinic and early results from clinical trials have had some challenges, the potential of these strategies is still tremendous. This review outlines several donor DNA delivery platforms available for HDR-mediated gene correction, including both viral-free and viral-dependent delivery systems, as well as the current drawbacks preventing each of them from being widely accepted in clinical applications. Additionally, we aim to highlight potential ways to alleviate these safety and efficacy concerns to help push these technologies toward a reliable and safe treatment. While research is currently being conducted to solve these major issues and the field of ex vivo genome editing continues to progress, the development of more precise and efficient editing tools will be key in translating HDR-based genome editing for the treatment of inborn errors of immunity, diseases of the blood system, and other genetic disorders.
Table 2.
HDR gene-editing studies for the correction of disorders of the blood and immune system, performed in HSPCs, using ZFNs, TALENs, and Cas9. In vitro refers to cell culture studies and in vivo refers to the transplantation of edited HSPCs to animal models.
| Targeted Gene | Nuclease | Donor Platform | Study | Reference | |
|---|---|---|---|---|---|
| SCID | WAS | Cas9 | AAV | in vivo | [32] |
| RAG2 | Cas9 | AAV | in vitro | [87,96,97] | |
| Cas9 | AAV | in vivo | [29] | ||
| IL2RG | ZFN | IDLV | in vitro | [17] | |
| ZFN | IDLV | in vivo | [103,134] | ||
| ZFN | AAV | in vivo | [134,141] | ||
| Cas9 | IDLV | in vivo | [134] | ||
| Cas9 | AAV | in vitro | [89] | ||
| Cas9 | AAV | in vivo | [30,89,134,141] | ||
| CGD | AAVS1 | Cas9 | AAV | in vivo | [90] |
| CYBB | Cas9 | ssODN | in vivo | [34] | |
| Cas9 | AAV | in vivo | [91] | ||
| NCF1 | ZFN | AAV | in vivo | [92] | |
| IPEX | FOXP3 | Cas9 | AAV | in vivo | [31] |
| SCD/ β-thalassemia |
HBB | ZFN | IDLV | in vivo | [19,26] |
| ZFN | ssODN | in vitro | [23] | ||
| ZFN | ssODN | in vivo | [19,26] | ||
| ZFN | AdV | in vivo | [26] | ||
| ZFN | AAV | in vivo | [26] | ||
| TALEN | ssODN | in vitro | [23] | ||
| Cas9 | IDLV | in vitro | [24] | ||
| Cas9 | IDLV | in vivo | [26] | ||
| Cas9 | ssODN | in vitro | [23] | ||
| Cas9 | ssODN | in vivo | [22,25,26,28] | ||
| Cas9 | AAV | in vivo | [20,21,25,26,27,88] | ||
| Cas9 | AdV | in vivo | [26,79] | ||
| Hemophilia | HBA | Cas9 | AAV | in vivo | [93] |
| XHIM | CD40L | TALEN | IDLV | in vivo | [33] |
| TALEN | AAV | in vivo | [33] | ||
| Cas9 | IDLV | in vivo | [33] | ||
| Cas9 | AAV | in vivo | [33] | ||
| XLA | BTK | Cas9 | AAV | in vivo | [94] |
| XMEN | MAGT1 | Cas9 | AAV | in vivo | [95] |
Acknowledgments
We would like to thank the members of the Hendel Lab for reading the manuscript and providing helpful feedback. Figure 1, Figure 2 and Figure 3 were created with BioRender.com.
Author Contributions
D.A., N.K. and A.H. wrote the review with assistance from M.R. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This study was funded in part by the European Research Council (ERC) under The European Union Horizon 2020 Research and Innovation Program (Grant No. 755758), The Israel Science Foundation (ISF)—Israel Precision Medicine Partnership (IPMP) (Grant No. 3115/19), and Israel Science Foundation (ISF)—Individual Research Grants (Grant No. 2031/19).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Soni S. Gene therapies for transfusion dependent β-thalassemia: Current status and critical criteria for success. Am. J. Hematol. 2020;95:1099–1112. doi: 10.1002/ajh.25909. [DOI] [PubMed] [Google Scholar]
- 2.Song R., Zhai Q., Sun L., Huang E., Zhang Y., Zhu Y., Guo Q., Tian Y., Zhao B., Lu H. CRISPR/Cas9 genome editing technology in filamentous fungi: Progress and perspective. Appl. Microbiol. Biotechnol. 2019;103:6919–6932. doi: 10.1007/s00253-019-10007-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz D.M., Cassiani P.J., Li L., Billy E., Korn J.M., Jones M.D., Golji J., Ruddy D.A., Yu K., McAllister G., et al. CRISPR Screens Provide a Comprehensive Assessment of Cancer Vulnerabilities but Generate False-Positive Hits for Highly Amplified Genomic Regions. Cancer Discov. 2016;6:900–913. doi: 10.1158/2159-8290.CD-16-0178. [DOI] [PubMed] [Google Scholar]
- 4.Wang H., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F., Jaenisch R. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sid H., Schusser B. Applications of Gene Editing in Chickens: A New Era Is on the Horizon. Front. Genet. 2018;9:456. doi: 10.3389/fgene.2018.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao X., Zhang M., Wang X., Ying W., Hu X., Dai P., Meng F., Shi L., Sun Y., Yao N., et al. Tild-CRISPR Allows for Efficient and Precise Gene Knockin in Mouse and Human Cells. Dev. Cell. 2018;45:526–536.e5. doi: 10.1016/j.devcel.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Ge Z., Zheng L., Zhao Y., Jiang J., Zhang E.J., Liu T., Gu H., Qu L. Engineered xCas9 and SpCas9-NG variants broaden PAM recognition sites to generate mutations in Arabidopsis plants. Plant Biotechnol. J. 2019;17:1865–1867. doi: 10.1111/pbi.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue Z., Wu M., Wen K., Ren M., Long L., Zhang X., Gao G. CRISPR/Cas9 Mediates Efficient Conditional Mutagenesis in Drosophila. G3 Genes|Genomes|Genetics. 2014;4:2167–2173. doi: 10.1534/g3.114.014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batzir N.A., Tovin A., Hendel A. Therapeutic Genome Editing and its Potential Enhancement through CRISPR Guide RNA and Cas9 Modifications. Pediatr. Endocrinol. Rev. 2017;14:353–363. doi: 10.17458/per.vol14.2017.BTH.Therapeu. [DOI] [PubMed] [Google Scholar]
- 10.Nambiar T.S., Baudrier L., Billon P., Ciccia A. CRISPR-based genome editing through the lens of DNA repair. Mol. Cell. 2022;82:348–388. doi: 10.1016/j.molcel.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porteus M. Genome Editing: A New Approach to Human Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2016;56:163–190. doi: 10.1146/annurev-pharmtox-010814-124454. [DOI] [PubMed] [Google Scholar]
- 12.Carroll D. Genome Engineering with Targetable Nucleases. Annu. Rev. Biochem. 2014;83:409–439. doi: 10.1146/annurev-biochem-060713-035418. [DOI] [PubMed] [Google Scholar]
- 13.Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188:773–782. doi: 10.1534/genetics.111.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porteus M.H., Carroll D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005;23:967–973. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- 15.Guo X., Li X.-J. Targeted genome editing in primate embryos. Cell Res. 2015;25:767–768. doi: 10.1038/cr.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urnov F.D., Miller J.C., Lee Y.-L., Beausejour C.M., Rock J.M., Augustus S., Jamieson A.C., Porteus M.H., Gregory P.D., Holmes M.C. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 17.Lombardo A.L., Genovese P., Beausejour C.M., Colleoni S., Lee Y.-L., Kim K.A., Ando D., Urnov F.D., Galli C., Gregory P., et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 18.Rai R., Thrasher A.J., Cavazza A. Gene Editing for the Treatment of Primary Immunodeficiency Diseases. Hum. Gene Ther. 2021;32:43–51. doi: 10.1089/hum.2020.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoban M.D., Cost G.J., Mendel M.C., Romero Z., Kaufman M.L., Joglekar A.V., Ho M., Lumaquin D., Gray D., Lill G.R., et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood. 2015;125:2597–2604. doi: 10.1182/blood-2014-12-615948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cromer M.K., Camarena J., Martin R.M., Lesch B.J., Vakulskas C.A., Bode N.M., Kurgan G., Collingwood M.A., Rettig G.R., Behlke M.A., et al. Gene replacement of α-globin with β-globin restores hemoglobin balance in β-thalassemia-derived hematopoietic stem and progenitor cells. Nat. Med. 2021;27:677–687. doi: 10.1038/s41591-021-01284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S., et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeWitt M.A., Magis W., Bray N.L., Wang T., Berman J.R., Urbinati F., Heo S.-J., Mitros T., Muñoz D.P., Boffelli D., et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci. Transl. Med. 2016;8:360ra134. doi: 10.1126/scitranslmed.aaf9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antony J.S., Latifi N., Haque A.K.M.A., Lamsfus-Calle A., Daniel-Moreno A., Graeter S., Baskaran P., Weinmann P., Mezger M., Handgretinger R., et al. Gene correction of HBB mutations in CD34+ hematopoietic stem cells using Cas9 mRNA and ssODN donors. Mol. Cell. Pediatr. 2018;5:9. doi: 10.1186/s40348-018-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoban M.D., Lumaquin D., Kuo C.Y., Romero Z., Long J., Ho M., Young C.S., Mojadidi M., Fitz-Gibbon S., Cooper A.R., et al. CRISPR/Cas9-Mediated Correction of the Sickle Mutation in Human CD34+ cells. Mol. Ther. 2016;24:1561–1569. doi: 10.1038/mt.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattabhi S., Lotti S.N., Berger M.P., Singh S., Lux C.T., Jacoby K., Lee C., Negre O., Scharenberg A.M., Rawlings D.J. In Vivo Outcome of Homology-Directed Repair at the HBB Gene in HSC Using Alternative Donor Template Delivery Methods. Mol. Ther. Nucleic Acids. 2019;17:277–288. doi: 10.1016/j.omtn.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero Z., Lomova A., Said S., Miggelbrink A., Kuo C.Y., Campo-Fernandez B., Hoban M.D., Masiuk K.E., Clark D.N., Long J., et al. Editing the Sickle Cell Disease Mutation in Human Hematopoietic Stem Cells: Comparison of Endonucleases and Homologous Donor Templates. Mol. Ther. 2019;27:1389–1406. doi: 10.1016/j.ymthe.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomova A., Clark D.N., Campo-Fernandez B., Flores-Bjurström C., Kaufman M.L., Fitz-Gibbon S., Wang X., Miyahira E.Y., Brown D., DeWitt M.A., et al. Improving Gene Editing Outcomes in Human Hematopoietic Stem and Progenitor Cells by Temporal Control of DNA Repair. Stem Cells. 2018;37:284–294. doi: 10.1002/stem.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S.H., Lee C., Dever D.P., Davis T.H., Camarena J., Srifa W., Zhang Y., Paikari A., Chang A.K., Porteus M.H., et al. Highly efficient editing of the β-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res. 2019;47:7955–7972. doi: 10.1093/nar/gkz475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavel-Dinu M., Gardner C.L., Nakauchi Y., Kawai T., Delmonte O.M., Palterer B., Bosticardo M., Pala F., Viel S., Malech H.L., et al. Genetically Corrected RAG2-SCID Human Hematopoietic Stem Cells Restore V(D)J-Recombinase and Rescue Lymphoid Deficiency. bioRxiv. 2022 doi: 10.1101/2022.07.12.499831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavel-Dinu M., Wiebking V., Dejene B.T., Srifa W., Mantri S., Nicolas C.E., Lee C., Bao G., Kildebeck E.J., Punjya N., et al. Gene correction for SCID-X1 in long-term hematopoietic stem cells. Nat. Commun. 2019;10:1634. doi: 10.1038/s41467-019-09614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin M., Lee E., Lakshmanan U., Shipp S., Froessl L., Barzaghi F., Passerini L., Narula M., Sheikali A., Lee C.M., et al. CRISPR-based gene editing enables FOXP3 gene repair in IPEX patient cells. Sci. Adv. 2020;6:eaaz0571. doi: 10.1126/sciadv.aaz0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rai R., Romito M., Rivers E., Turchiano G., Blattner G., Vetharoy W., Ladon D., Andrieux G., Zhang F., Zinicola M., et al. Targeted gene correction of human hematopoietic stem cells for the treatment of Wiskott - Aldrich Syndrome. Nat. Commun. 2020;11:4034. doi: 10.1038/s41467-020-17626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo C.Y., Long J.D., Campo-Fernandez B., de Oliveira S., Cooper A.R., Romero Z., Hoban M.D., Joglekar A.V., Lill G.R., Kaufman M.L., et al. Site-Specific Gene Editing of Human Hematopoietic Stem Cells for X-Linked Hyper-IgM Syndrome. Cell Rep. 2018;23:2606–2616. doi: 10.1016/j.celrep.2018.04.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Ravin S.S., Li L., Wu X., Choi U., Allen C., Koontz S., Lee J., Theobald-Whiting N., Chu J., Garofalo M., et al. CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci. Transl. Med. 2017;9:eaah3480. doi: 10.1126/scitranslmed.aah3480. [DOI] [PubMed] [Google Scholar]
- 35.Milone M.C., Odoherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loza L.I.M., Yuen E.C., McCray P.B. Lentiviral Vectors for the Treatment and Prevention of Cystic Fibrosis Lung Disease. Genes. 2019;10:218. doi: 10.3390/genes10030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Ravin S.S., Wu X., Moir S., Kardava L., Anaya-Obrien S., Kwatemaa N., Littel P., Theobald N., Choi U., Su L., et al. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016;8:335ra57. doi: 10.1126/scitranslmed.aad8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naldini L. Ex vivo gene transfer and correction for cell-based therapies. Nat. Rev. Genet. 2011;12:301–315. doi: 10.1038/nrg2985. [DOI] [PubMed] [Google Scholar]
- 39.Howe S.J., Mansour M., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M., Pike-Overzet K., Chatters S.J., De Ridder D., et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Investig. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang H.J., Bartholomae C.C., Paruzynski A., Arens A., Kim S., Yu S.S., Hong Y., Joo C.-W., Yoon N.-K., Rhim J.-W., et al. Retroviral Gene Therapy for X-linked Chronic Granulomatous Disease: Results from Phase I/II Trial. Mol. Ther. 2011;19:2092–2101. doi: 10.1038/mt.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moratto D., Giliani S., Bonfim C., Mazzolari E., Fischer A., Ochs H.D., Cant A.J., Thrasher A.J., Cowan M.J., Albert M.H., et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: An international collaborative study. Blood. 2011;118:1675–1684. doi: 10.1182/blood-2010-11-319376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Candotti F., Shaw K.L., Muul L., Carbonaro D., Sokolic R., Choi C., Schurman S., Garabedian E., Kesserwan C., Jagadeesh G.J., et al. Gene therapy for adenosine deaminase–deficient severe combined immune deficiency: Clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120:3635–3646. doi: 10.1182/blood-2012-02-400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nota Informativa Importante—Strimvelis®. [(accessed on 12 September 2022)]; Available online: https://www.aifa.gov.it/en/-/nota-informativa-importante-strimvelis-
- 44.Treatment of SCID Due to ADA Deficiency With Autologous Transplantation of Cord Blood or Hematopoietic CD 34+ Cells After Addition of a Normal Human ADA cDNA by the EFS-ADA Lentiviral Vector—Full Text View—ClinicalTrials.gov. [(accessed on 28 February 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02022696?term=lentiviral&cond=Ada-Scid&draw=2&rank=2.
- 45.Gene Therapy for Wiskott-Aldrich Syndrome (WAS)—Full Text View—ClinicalTrials.gov. [(accessed on 28 February 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01347242?term=lentiviral&cond=WAS&draw=2&rank=3.
- 46.Ferrua F., Cicalese M.P., Galimberti S., Giannelli S., Dionisio F., Barzaghi F., Migliavacca M., Bernardo M.E., Calbi V., Assanelli A.A., et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: Interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 2019;6:e239–e253. doi: 10.1016/S2352-3026(19)30021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cowan M.J., Yu J., Facchino J., Fraser-Browne C., Sanford U., Kawahara M., Dara J., Long-Boyle J., Oh J., Chan W., et al. Lentiviral Gene Therapy for Artemis-Deficient SCID. N. Engl. J. Med. 2022;387:2344–2355. doi: 10.1056/NEJMoa2206575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phase I/II Clinical Trial Stem Cell Gene Therapy in RAG1-Deficient SCID—Full Text View—ClinicalTrials.gov. [(accessed on 28 February 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04797260.
- 49.Lentiviral Gene Therapy for X-linked Severe Combined Immunodeficiency—Full Text View—ClinicalTrials.gov. [(accessed on 28 February 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03601286.
- 50.Perez L.G., van Eggermond M., van Roon L., Vloemans S.A., Cordes M., Schambach A., Rothe M., Berghuis D., Lagresle-Peyrou C., Cavazzana M., et al. Successful Preclinical Development of Gene Therapy for Recombinase-Activating Gene-1-Deficient SCID. Mol. Ther. Methods Clin. Dev. 2020;17:666–682. doi: 10.1016/j.omtm.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autologous Gene Therapy for Artemis-Deficient SCID—Full Text View—ClinicalTrials.gov. [(accessed on 28 February 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03538899?term=lentiviral&cond=artemis+scid&draw=2&rank=2.
- 52.Mamcarz E., Zhou S., Lockey T., Abdelsamed H., Cross S.J., Kang G., Ma Z., Condori J., Dowdy J., Triplett B., et al. Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1. N. Engl. J. Med. 2019;380:1525–1534. doi: 10.1056/NEJMoa1815408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohn D.B., Booth C., Shaw K.L., Xu-Bayford J., Garabedian E., Trevisan V., Carbonaro-Sarracino D.A., Soni K., Terrazas D., Snell K., et al. Autologous Ex Vivo Lentiviral Gene Therapy for Adenosine Deaminase Deficiency. N. Engl. J. Med. 2021;384:2002–2013. doi: 10.1056/NEJMoa2027675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marktel S., Scaramuzza S., Cicalese M.P., Giglio F., Galimberti S., Lidonnici M.R., Calbi V., Assanelli A., Bernardo M.E., Rossi C., et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ß-thalassemia. Nat. Med. 2019;25:234–241. doi: 10.1038/s41591-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 55.Brunetti-Pierri N. Safety and Efficacy of Gene-Based Therapeutics for Inherited Disorders. Springer; Berlin/Heidelberg, Germany: 2017. pp. 1–220. [DOI] [Google Scholar]
- 56.Yu N., Yang J., Mishina Y., Giannobile W. Genome Editing: A New Horizon for Oral and Craniofacial Research. J. Dent. Res. 2018;98:36–45. doi: 10.1177/0022034518805978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu X., Hulshoff M.S., Tan X., Zeisberg M., Zeisberg E.M. CRISPR/Cas Derivatives as Novel Gene Modulating Tools: Possibilities and In Vivo Applications. Int. J. Mol. Sci. 2020;21:3038. doi: 10.3390/ijms21093038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mak A.N.-S., Bradley P., Bogdanove A.J., Stoddard B.L. TAL effectors: Function, structure, engineering and applications. Curr. Opin. Struct. Biol. 2013;23:93–99. doi: 10.1016/j.sbi.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang F., Doudna J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017;46:505–529. doi: 10.1146/annurev-biophys-062215-010822. [DOI] [PubMed] [Google Scholar]
- 60.Hendel A., Bak R., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B., et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen D., Rosenberg M., Hendel A. Using Synthetically Engineered Guide RNAs to Enhance CRISPR Genome Editing Systems in Mammalian Cells. Front. Genome Ed. 2021;2:617910. doi: 10.3389/fgeed.2020.617910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro J., Iancu O., Jacobi A.M., McNeill M.S., Turk R., Rettig G.R., Amit I., Tovin-Recht A., Yakhini Z., Behlke M.A., et al. Increasing CRISPR Efficiency and Measuring Its Specificity in HSPCs Using a Clinically Relevant System. Mol. Ther. Methods Clin. Dev. 2020;17:1097–1107. doi: 10.1016/j.omtm.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devkota S. The road less traveled: Strategies to enhance the frequency of homology-directed repair (HDR) for increased efficiency of CRISPR/Cas-mediated transgenesis. BMB Rep. 2018;51:437–443. doi: 10.5483/BMBRep.2018.51.9.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeh C.D., Richardson C.D., Corn J.E. Advances in genome editing through control of DNA repair pathways. Nature. 2019;21:1468–1478. doi: 10.1038/s41556-019-0425-z. [DOI] [PubMed] [Google Scholar]
- 65.Azhagiri M.K.K., Babu P., Venkatesan V., Thangavel S. Homology-directed gene-editing approaches for hematopoietic stem and progenitor cell gene therapy. Stem Cell Res. Ther. 2021;12:500. doi: 10.1186/s13287-021-02565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salisbury-Ruf C.T., Larochelle A. Advances and Obstacles in Homology-Mediated Gene Editing of Hematopoietic Stem Cells. J. Clin. Med. 2021;10:513. doi: 10.3390/jcm10030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shy B.R., Vykunta V.S., Ha A., Talbot A., Roth T.L., Nguyen D.N., Pfeifer W.G., Chen Y.Y., Blaeschke F., Shifrut E., et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. 2022;41:521–531. doi: 10.1038/s41587-022-01418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee C.S., Bishop E.S., Zhang R., Yu X., Farina E.M., Yan S., Zhao C., Zeng Z., Shu Y., Wu X., et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4:43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarty D.M. Self-complementary AAV Vectors; Advances and Applications. Mol. Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 70.Mccarty D.M., Monahan P.E., Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 71.Kan Y., Ruis B., Lin S., Hendrickson E.A. The Mechanism of Gene Targeting in Human Somatic Cells. PLoS Genet. 2014;10:e1004251. doi: 10.1371/journal.pgen.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong W., Kantor B. Lentiviral Vectors for Delivery of Gene-Editing Systems Based on CRISPR/Cas: Current State and Perspectives. Viruses. 2021;13:1288. doi: 10.3390/v13071288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schubert M.S., Thommandru B., Woodley J., Turk R., Yan S., Kurgan G., McNeill M.S., Rettig G.R. Optimized design parameters for CRISPR Cas9 and Cas12a homology-directed repair. Sci. Rep. 2021;11:19482. doi: 10.1038/s41598-021-98965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen F., Pruett-Miller S.M., Davis G.D. Gene Editing Using ssODNs with Engineered Endonucleases. Methods Mol. Biol. 2014;1239:251–265. doi: 10.1007/978-1-4939-1862-1_14. [DOI] [PubMed] [Google Scholar]
- 75.Liu M., Rehman S., Tang X., Gu K., Fan Q., Chen D., Ma W. Methodologies for Improving HDR Efficiency. Front. Genet. 2019;9:691. doi: 10.3389/fgene.2018.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson C., Ray G., DeWitt M.A., Curie G.L., Corn J.E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 2016;34:339–344. doi: 10.1038/nbt.3481. [DOI] [PubMed] [Google Scholar]
- 77.Tasca F., Wang Q., Gonçalves M.A. Adenoviral Vectors Meet Gene Editing: A Rising Partnership for the Genomic Engineering of Human Stem Cells and Their Progeny. Cells. 2020;9:953. doi: 10.3390/cells9040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ricobaraza A., Gonzalez-Aparicio M., Mora-Jimenez L., Lumbreras S., Hernandez-Alcoceba R. High-Capacity Adenoviral Vectors: Expanding the Scope of Gene Therapy. Int. J. Mol. Sci. 2020;21:3643. doi: 10.3390/ijms21103643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li C., Psatha N., Gil S., Wang H., Papayannopoulou T., Lieber A. HDAd5/35++ Adenovirus Vector Expressing Anti-CRISPR Peptides Decreases CRISPR/Cas9 Toxicity in Human Hematopoietic Stem Cells. Mol. Ther.-Methods Clin. Dev. 2018;9:390–401. doi: 10.1016/j.omtm.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maggio I., Gonçalves M.A. Genome editing at the crossroads of delivery, specificity, and fidelity. Trends Biotechnol. 2015;33:280–291. doi: 10.1016/j.tibtech.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 81.Dudek A.M., Porteus M.H. Answered and Unanswered Questions in Early-Stage Viral Vector Transduction Biology and Innate Primary Cell Toxicity for Ex-Vivo Gene Editing. Front. Immunol. 2021;12:660302. doi: 10.3389/fimmu.2021.660302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen X., Gonçalves M.A.F.V. Engineered Viruses as Genome Editing Devices. Mol. Ther. 2016;24:447–457. doi: 10.1038/mt.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Earley L.F., Conatser L., Lue V., Dobbins M.A.L., Li C., Hirsch M.L., Samulski R.J. Adeno-Associated Virus Serotype-Specific Inverted Terminal Repeat Sequence Role in Vector Transgene Expression. Hum. Gene Ther. 2020;31:151–162. doi: 10.1089/hum.2019.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bijlani S., Pang K.M., Sivanandam V., Singh A., Chatterjee S. The Role of Recombinant AAV in Precise Genome Editing. Front. Genome Ed. 2022;3:799722. doi: 10.3389/fgeed.2021.799722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Z., Ma H.-I., Li J., Sun L., Zhang J., Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 87.Allen D., Knop O., Itkowitz B., Iancu O., Beider K., Lee Y.N., Nagler A., Somech R., Hendel A. CRISPR-Cas9 RAG2 Correction Via Coding Sequence Replacement to Preserve Endogenous Gene Regulation and Locus Structure. Research Square; Durham, NC, USA: 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bak R.O., Dever D.P., Porteus M.H. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 2018;13:358–376. doi: 10.1038/nprot.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brault J., Liu T., Liu S., Lawson A., Choi U., Kozhushko N., Bzhilyanskaya V., Pavel-Dinu M., Meis R.J., Eckhaus M.A., et al. CRISPR-Cas9-AAV versus lentivector transduction for genome modification of X-linked severe combined immunodeficiency hematopoietic stem cells. Front. Immunol. 2023;13:1067417. doi: 10.3389/fimmu.2022.1067417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Ravin S.S., Reik A., Liu P.-Q., Li L., Wu X., Su L., Raley C., Theobald N., Choi U., Song A.H., et al. Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat. Biotechnol. 2016;34:424–429. doi: 10.1038/nbt.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sweeney C.L., Pavel-Dinu M., Choi U., Brault J., Liu T., Koontz S., Li L., Theobald N., Lee J., Bello E.A., et al. Correction of X-CGD patient HSPCs by targeted CYBB cDNA insertion using CRISPR/Cas9 with 53BP1 inhibition for enhanced homology-directed repair. Gene Ther. 2021;28:373–390. doi: 10.1038/s41434-021-00251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merling R.K., Kuhns U.B., Sweeney C.L., Wu X., Burkett S., Chu J., Lee J., Koontz S., Di Pasquale G., Afione S.A., et al. Gene-edited pseudogene resurrection corrects p47phox-deficient chronic granulomatous disease. Blood Adv. 2016;1:270–278. doi: 10.1182/bloodadvances.2016001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pavani G., Laurent M., Fabiano A., Cantelli E., Sakkal A., Corre G., Lenting P.J., Concordet J.-P., Toueille M., Miccio A., et al. Ex vivo editing of human hematopoietic stem cells for erythroid expression of therapeutic proteins. Nat. Commun. 2020;11:3778. doi: 10.1038/s41467-020-17552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gray D.H., Villegas I., Long J., Santos J., Keir A., Abele A., Kuo C.Y., Kohn D.B. Optimizing Integration and Expression of Transgenic Bruton’s Tyrosine Kinase for CRISPR-Cas9-Mediated Gene Editing of X-Linked Agammaglobulinemia. CRISPR J. 2021;4:191–206. doi: 10.1089/crispr.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brault J., Liu T.Q., Bello E.A., Liu S., Sweeney C.L., Meis R.J., Koontz S., Corsino C., Choi U., Vayssiere G., et al. CRISPR-targeted MAGT1 insertion restores XMEN patient hematopoietic stem cells and lymphocytes. Blood. 2021;138:2768–2780. doi: 10.1182/blood.2021011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gardner C.L., Pavel-Dinu M., Dobbs K., Bosticardo M., Reardon P.K., Lack J., DeRavin S.S., Le K., Bello E., Pala F., et al. Gene Editing Rescues In vitro T Cell Development of RAG2-Deficient Induced Pluripotent Stem Cells in an Artificial Thymic Organoid System. J. Clin. Immunol. 2021;41:852–862. doi: 10.1007/s10875-021-00989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iancu O., Allen D., Knop O., Zehavi Y., Breier D., Arbiv A., Lev A., Lee Y.N., Beider K., Nagler A., et al. Multiplex HDR for disease and correction modeling of SCID by CRISPR genome editing in human HSPCs. Mol. Ther.-Nucleic Acids. 2022;31:105–121. doi: 10.1016/j.omtn.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyazaki K., Miyazaki M. The Interplay Between Chromatin Architecture and Lineage-Specific Transcription Factors and the Regulation of Rag Gene Expression. Front. Immunol. 2021;12:659761. doi: 10.3389/fimmu.2021.659761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen D., Weiss L.E., Saguy A., Rosenberg M., Iancu O., Matalon O., Lee C., Beider K., Nagler A., Shechtman Y., et al. High-Throughput Imaging of CRISPR- and Recombinant Adeno-Associated Virus–Induced DNA Damage Response in Human Hematopoietic Stem and Progenitor Cells. CRISPR J. 2022;5:80–94. doi: 10.1089/crispr.2021.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. [(accessed on 5 January 2023)]. Available online: https://ir.graphitebio.com/press-releases/detail/84/graphite-bio-announces-voluntary-pause-of-phase-12-cedar.
- 101.Chamberlain K., Riyad J.M., Weber T. Expressing Transgenes That Exceed the Packaging Capacity of Adeno-Associated Virus Capsids. Hum. Gene Ther. Methods. 2016;27:1–12. doi: 10.1089/hgtb.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marrone L., Marchi P.M., Azzouz M. Circumventing the packaging limit of AAV-mediated gene replacement therapy for neurological disorders. Expert Opin. Biol. Ther. 2022;22:1163–1176. doi: 10.1080/14712598.2022.2012148. [DOI] [PubMed] [Google Scholar]
- 103.Genovese P., Schiroli G., Escobar G., Di Tomaso T., Firrito C., Calabria A., Moi D., Mazzieri R., Bonini C., Holmes M.C., et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Banasik M.B., McCray P.B. Integrase-defective lentiviral vectors: Progress and applications. Gene Ther. 2009;17:150–157. doi: 10.1038/gt.2009.135. [DOI] [PubMed] [Google Scholar]
- 105.Ortinski P.I., O’donovan B., Dong X., Kantor B. Integrase-Deficient Lentiviral Vector as an All-in-One Platform for Highly Efficient CRISPR/Cas9-Mediated Gene Editing. Mol. Ther. Methods Clin. Dev. 2017;5:153–164. doi: 10.1016/j.omtm.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrari S., Jacob A., Beretta S., Unali G., Albano L., Vavassori V., Cittaro D., Lazarevic D., Brombin C., Cugnata F., et al. Efficient gene editing of human long-term hematopoietic stem cells validated by clonal tracking. Nat. Biotechnol. 2020;38:1298–1308. doi: 10.1038/s41587-020-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wienert B., Cromer M.K. CRISPR nuclease off-target activity and mitigation strategies. Front. Genome Ed. 2022;4:1050507. doi: 10.3389/fgeed.2022.1050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naseem A., Steinberg Z., Cavazza A. Genome editing for primary immunodeficiencies: A therapeutic perspective on Wiskott-Aldrich syndrome. Front. Immunol. 2022;13:4553. doi: 10.3389/fimmu.2022.966084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Höijer I., Emmanouilidou A., Östlund R., van Schendel R., Bozorgpana S., Tijsterman M., Feuk L., Gyllensten U., Hoed M.D., Ameur A. CRISPR-Cas9 induces large structural variants at on-target and off-target sites in vivo that segregate across generations. Nat. Commun. 2022;13:627. doi: 10.1038/s41467-022-28244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amit I., Iancu O., Levy-Jurgenson A., Kurgan G., McNeill M.S., Rettig G.R., Allen D., Breier D., Ben Haim N., Wang Y., et al. CRISPECTOR provides accurate estimation of genome editing translocation and off-target activity from comparative NGS data. Nat. Commun. 2021;12:3042. doi: 10.1038/s41467-021-22417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lattanzi A., Camarena J., Lahiri P., Segal H., Srifa W., Vakulskas C.A., Frock R.L., Kenrick J., Lee C., Talbott N., et al. Development of β-globin gene correction in human hematopoietic stem cells as a potential durable treatment for sickle cell disease. Sci. Transl. Med. 2021;13:eabf2444. doi: 10.1126/scitranslmed.abf2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boutin J., Rosier J., Cappellen D., Prat F., Toutain J., Pennamen P., Bouron J., Rooryck C., Merlio J.P., Lamrissi-Garcia I., et al. CRISPR-Cas9 globin editing can induce megabase-scale copy-neutral losses of heterozygosity in hematopoietic cells. Nat. Commun. 2021;12:4922. doi: 10.1038/s41467-021-25190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leibowitz M.L., Papathanasiou S., Doerfler P.A., Blaine L.J., Sun L., Yao Y., Zhang C.-Z., Weiss M.J., Pellman D. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. Nat. Genet. 2021;53:895–905. doi: 10.1038/s41588-021-00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adikusuma F., Piltz S., Corbett M.A., Turvey M., McColl S.R., Helbig K.J., Beard M.R., Hughes J., Pomerantz R.T., Thomas P.Q. Large deletions induced by Cas9 cleavage. Nature. 2018;560:E8–E9. doi: 10.1038/s41586-018-0380-z. [DOI] [PubMed] [Google Scholar]
- 115.Kosicki M., Tomberg K., Bradley A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018;36:765–771. doi: 10.1038/nbt.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turchiano G., Andrieux G., Klermund J., Blattner G., Pennucci V., el Gaz M., Monaco G., Poddar S., Mussolino C., Cornu T.I., et al. Quantitative evaluation of chromosomal rearrangements in gene-edited human stem cells by CAST-Seq. Cell Stem Cell. 2021;28:1136–1147.e5. doi: 10.1016/j.stem.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Nahmad A.D., Reuveni E., Goldschmidt E., Tenne T., Liberman M., Horovitz-Fried M., Khosravi R., Kobo H., Reinstein E., Madi A., et al. Frequent aneuploidy in primary human T cells after CRISPR–Cas9 cleavage. Nat. Biotechnol. 2022;40:1807–1813. doi: 10.1038/s41587-022-01377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsai S.Q., Zheng Z., Nguyen N.T., Liebers M., Topkar V.V., Thapar V., Wyvekens N., Khayter C., Iafrate A.J., Le L.P., et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2014;33:187–197. doi: 10.1038/nbt.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsai S.Q., Nguyen N.T., Malagon-Lopez J., Topkar V., Aryee M.J., Joung J.K. CIRCLE-seq: A highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat. Methods. 2017;14:607–614. doi: 10.1038/nmeth.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cameron P., Fuller C.K., Donohoue P.D., Jones B.N., Thompson M.S., Carter M.M., Gradia S., Vidal B., Garner E., Slorach E.M., et al. Mapping the genomic landscape of CRISPR–Cas9 cleavage. Nat. Methods. 2017;14:600–606. doi: 10.1038/nmeth.4284. [DOI] [PubMed] [Google Scholar]
- 121.Ferrari S., Jacob A., Cesana D., Laugel M., Beretta S., Varesi A., Unali G., Conti A., Canarutto D., Albano L., et al. Choice of template delivery mitigates the genotoxic risk and adverse impact of editing in human hematopoietic stem cells. Cell Stem Cell. 2022;29:1428–1444.e9. doi: 10.1016/j.stem.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boutin J., Cappellen D., Rosier J., Amintas S., Dabernat S., Bedel A., Moreau-Gaudry F. ON-Target Adverse Events of CRISPR-Cas9 Nuclease: More Chaotic than Expected. CRISPR J. 2022;5:19–30. doi: 10.1089/crispr.2021.0120. [DOI] [PubMed] [Google Scholar]
- 123.Yang H., Ren S., Yu S., Pan H., Li T., Ge S., Zhang J., Xia N. Methods Favoring Homology-Directed Repair Choice in Response to CRISPR/Cas9 Induced-Double Strand Breaks. Int. J. Mol. Sci. 2020;21:6461. doi: 10.3390/ijms21186461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferrari S., Vavassori V., Canarutto D., Jacob A., Castiello M.C., Javed A.O., Genovese P. Gene Editing of Hematopoietic Stem Cells: Hopes and Hurdles Toward Clinical Translation. Front. Genome Ed. 2021;3:618378. doi: 10.3389/fgeed.2021.618378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fischer A., Hacein-Bey-Abina S. Gene therapy for severe combined immunodeficiencies and beyond. J. Exp. Med. 2020;217:e20190607. doi: 10.1084/jem.20190607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hacein-Bey-Abina S., Hauer J., Lim A., Picard C., Wang G.P., Berry C.C., Martinache C., Rieux-Laucat F., Latour S., Belohradsky B.H., et al. Efficacy of Gene Therapy for X-Linked Severe Combined Immunodeficiency. N. Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clarke E.L., Connell J., Six E., Kadry N., Abbas A., Hwang Y., Everett J.K., Hofstaedter C., Marsh R., Armant M., et al. T cell dynamics and response of the microbiota after gene therapy to treat X-linked severe combined immunodeficiency. Genome Med. 2018;10:70. doi: 10.1186/s13073-018-0580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirschhorn R., Yang D.R., Puck J.M., Huie M.L., Jiang C.-K., Kurlandsky L.E. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nat. Genet. 1996;13:290–295. doi: 10.1038/ng0796-290. [DOI] [PubMed] [Google Scholar]
- 129.Stephan V., Wahn V., Le Deist F., Dirksen U., Bröker B., Müller-Fleckenstein I., Horneff G., Schroten H., Fischer A., de Saint Basile G. Atypical X-Linked Severe Combined Immunodeficiency Due to Possible Spontaneous Reversion of the Genetic Defect in T Cells. N. Engl. J. Med. 1996;335:1563–1567. doi: 10.1056/NEJM199611213352104. [DOI] [PubMed] [Google Scholar]
- 130.Speckmann C., Pannicke U., Wiech E., Schwarz K., Fisch P., Friedrich W., Niehues T., Gilmour K., Buiting K., Schlesier M., et al. Clinical and immunologic consequences of a somatic reversion in a patient with X-linked severe combined immunodeficiency. Blood. 2008;112:4090–4097. doi: 10.1182/blood-2008-04-153361. [DOI] [PubMed] [Google Scholar]
- 131.Dvorak C.C., Long-Boyle J., Dara J., Melton A., Shimano K.A., Huang J.N., Puck J.M., Dorsey M.J., Facchino J., Chang C.K., et al. Low Exposure Busulfan Conditioning to Achieve Sufficient Multilineage Chimerism in Patients with Severe Combined Immunodeficiency. Biol. Blood Marrow Transplant. 2019;25:1355–1362. doi: 10.1016/j.bbmt.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Canny M.D., Moatti N., Wan L.C.K., Fradet-Turcotte A., Krasner D., Mateos-Gomez P.A., Zimmermann M., Orthwein A., Juang Y.-C., Zhang W., et al. Inhibition of 53BP1 favors homology-dependent DNA repair and increases CRISPR–Cas9 genome-editing efficiency. Nat. Biotechnol. 2017;36:95–102. doi: 10.1038/nbt.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bischoff N., Wimberger S., Maresca M., Brakebusch C. Improving Precise CRISPR Genome Editing by Small Molecules: Is there a Magic Potion? Cells. 2020;9:1318. doi: 10.3390/cells9051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schiroli G., Ferrari S., Conway A., Jacob A., Capo V., Albano L., Plati T., Castiello M.C., Sanvito F., Gennery A.R., et al. Preclinical modeling highlights the therapeutic potential of hematopoietic stem cell gene editing for correction of SCID-X1. Sci. Transl. Med. 2017;9:eaan0820. doi: 10.1126/scitranslmed.aan0820. [DOI] [PubMed] [Google Scholar]
- 135.Wimberger S., Akrap N., Firth M., Brengdahl J., Engberg S., Schwinn M.K., Slater M.R., Lundin A., Hsieh P.P., Li S., et al. Simultaneous inhibition of DNA-PK and Polϴ improves integration efficiency and precision of genome editing. bioRxiv. 2022 doi: 10.1101/2022.12.15.520396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Belan O., Sebald M., Adamowicz M., Anand R., Vancevska A., Neves J., Grinkevich V., Hewitt G., Segura-Bayona S., Bellelli R., et al. POLQ seals post-replicative ssDNA gaps to maintain genome stability in BRCA-deficient cancer cells. Mol. Cell. 2022;82:4664–4680.e9. doi: 10.1016/j.molcel.2022.11.008. [DOI] [PubMed] [Google Scholar]
- 137.Shin J.J., Schröder M.S., Caiado F., Wyman S.K., Bray N.L., Bordi M., DeWitt M.A., Vu J.T., Kim W.-T., Hockemeyer D., et al. Controlled Cycling and Quiescence Enables Efficient HDR in Engraftment-Enriched Adult Hematopoietic Stem and Progenitor Cells. Cell Rep. 2020;32:108093. doi: 10.1016/j.celrep.2020.108093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mohrin M., Bourke E., Alexander D., Warr M.R., Barry-Holson K., Le Beau M.M., Morrison C.G., Passegué E. Hematopoietic Stem Cell Quiescence Promotes Error-Prone DNA Repair and Mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Piras F., Kajaste-Rudnitski A. Antiviral immunity and nucleic acid sensing in haematopoietic stem cell gene engineering. Gene Ther. 2020;28:16–28. doi: 10.1038/s41434-020-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piras F., Riba M., Petrillo C., Lazarevic D., Cuccovillo I., Bartolaccini S., Stupka E., Gentner B., Cittaro D., Naldini L., et al. Lentiviral vectors escape innate sensing but trigger p53 in human hematopoietic stem and progenitor cells. EMBO Mol. Med. 2017;9:1198–1211. doi: 10.15252/emmm.201707922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schiroli G., Conti A., Ferrari S., della Volpe L., Jacob A., Albano L., Beretta S., Calabria A., Vavassori V., Gasparini P., et al. Precise Gene Editing Preserves Hematopoietic Stem Cell Function following Transient p53-Mediated DNA Damage Response. Cell Stem Cell. 2019;24:551–565.e8. doi: 10.1016/j.stem.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Milyavsky M., Gan O.I., Trottier M., Komosa M., Tabach O., Notta F., Lechman E., Hermans K.G., Eppert K., Konovalova Z., et al. A Distinctive DNA Damage Response in Human Hematopoietic Stem Cells Reveals an Apoptosis-Independent Role for p53 in Self-Renewal. Cell Stem Cell. 2010;7:186–197. doi: 10.1016/j.stem.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 143.Frati G., Miccio A. Genome Editing for β-Hemoglobinopathies: Advances and Challenges. J. Clin. Med. 2021;10:482. doi: 10.3390/jcm10030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zonari E., Desantis G., Petrillo C., Boccalatte F.E., Lidonnici M.R., Kajaste-Rudnitski A., Aiuti A., Ferrari G., Naldini L., Gentner B. Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy. Stem Cell Rep. 2017;8:977–990. doi: 10.1016/j.stemcr.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Glimm H., Oh I.H., Eaves C.J. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0) Blood. 2000;96:4185–4193. doi: 10.1182/blood.V96.13.4185. [DOI] [PubMed] [Google Scholar]
- 146.Kallinikou K., Afonso F.D.A., Blundell M., Ings S.J., Watts M.J., Thrasher A., Linch D.C., Bonnet D., Yong K.L. Engraftment defect of cytokine-cultured adult human mobilized CD34(+) cells is related to reduced adhesion to bone marrow niche elements. Br. J. Haematol. 2012;158:778–787. doi: 10.1111/j.1365-2141.2012.09219.x. [DOI] [PubMed] [Google Scholar]
- 147.Larochelle A., Gillette J.M., Desmond R., Ichwan B., Cantilena A., Cerf A., Barrett A.J., Wayne A.S., Lippincott-Schwartz J., Dunbar C.E. Bone marrow homing and engraftment of human hematopoietic stem and progenitor cells is mediated by a polarized membrane domain. Blood. 2012;119:1848–1855. doi: 10.1182/blood-2011-08-371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Poletto E., Colella P., Vera L.N.P., Khan S., Tomatsu S., Baldo G., Gomez-Ospina N. Improved engraftment and therapeutic efficacy by human genome-edited hematopoietic stem cells with Busulfan-based myeloablation. Mol. Ther.-Methods Clin. Dev. 2022;25:392–409. doi: 10.1016/j.omtm.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study.