Abstract
Vaccination against mpox can control the outbreak by targeting high-risk groups such as the LGBTIQ+ community. The aim of the study was to evaluate the perceptions and intentions to get vaccinated against mpox among the LGBTIQ+ community in Peru. We conducted a cross-sectional study from 1 November 2022 to 17 January 2023 in Peru. We included individuals over 18 years old, belonging to the LGBTIQ+ community, and residing in the departments of Lima and Callao. To evaluate the factors associated with the intention to be vaccinated, we used Poisson regression with robust variance to create a multivariate model. The study comprised 373 individuals who self-identified as members of the LGBTIQ+ community. The participants had a mean age of 31 years (SD ± 9), with 85.0% males and 75.3% reporting to be homosexual men. The majority (88.5%) expressed their intention to receive the vaccine against mpox. Believing that the vaccine is safe was associated with a higher intention to be vaccinated (aPR: 1.24; 95% CI: 1.02 to 1.50; p = 0.028). Our study population showed a high level of mpox vaccination intent. Educational campaigns reinforcing the concept of vaccine safety should be conducted to increase the intention and possibly the vaccination rate in the LGBTIQ+ community.
Keywords: monkeypox, vaccination, public health, perception, intention, bisexuality, homosexuality, Peru
1. Introduction
Mpox, previously called monkeypox, is a reemerging zoonotic disease [1], endemic in the Congo basin countries of Africa, that was initially reported in humans in the 1970s. The first reported cases outside Africa occurred in 2003 in the United States [2]. As of 2018, cases were described in Israel, the UK, Singapore, and the USA [3]. In May 2022, there was a precipitous increase in the number of mpox cases reported in Europe and the Americas [3], for which it was declared a global health emergency by the World Health Organization (WHO) on 23 July 2022 [4]. In the current outbreak, according to Centers for Disease Control and Prevention (CDC) data through 29 March 2023, 86,746 cases and 112 deaths from mpox have been reported in 110 countries [5].
Vaccination against human smallpox has been considered a control measure against mpox because both viruses have antigenic similarity, and cross-immunization can be generated [6]. Currently, the WHO proposes the use of three different vaccines against mpox (MVA-BN, ACAM2000, and LC16) [7]; however, MVA-BN is the only vaccine approved by the Food and Drug Administration (FDA) for the prevention of mpox in adults older than 18 years at high risk of mpox infection [8]. In the current outbreak, the most affected group has been men who have sex with men (MSM), a population also at high risk of other sexually transmitted infections, such as HIV [9]. Among them, the risk of HIV infection is 26 times when compared to the general population [10]. Previous studies have reported that individuals with uncontrolled HIV had worse outcomes in the course of mpox [11,12,13]; in this regard, the case series by Mitjà et al. [13] showed that severe complications in mpox were more frequent in HIV patients with a CD4 cell count of <100/mm3, and all deaths occurred in HIV patients with a CD4 cell count of <200/mm3. Although the CDC suggests the use of tecovirimat for the treatment of mpox under the Expanded Access to Investigational New Drugs (EA-IND) protocol [14], there is currently no quality evidence on the efficacy and safety of the various drug treatments against mpox [15,16]. It has even been hypothesized that resistance to tecovirimat could develop in patients at risk of receiving a prolonged regimen, such as patients coinfected with HIV and mpox. [17]. Therefore, the development and implementation of prevention strategies against mpox in the MSM population are of high importance to avoid significant sequelae and fatal outcomes.
Furthermore, vaccination against mpox in the principal risk group such as gay, bisexual, and MSM has been one of the most important strategies to control the current mpox outbreak [18]. From 31 July to 1 October 2022, among vaccine-eligible men aged 18–49 years in 43 US jurisdictions, mpox incidence among unvaccinated individuals was estimated to be 10 times higher than that reported for fully vaccinated people and 7 times higher than the incidence described for those who received only the first vaccine dose [19]. Few studies have investigated the perception and acceptance of the vaccination against mpox in this high-risk group around the world. There is one study in the Netherlands in 2022 that revealed that 81.5% of gays, bisexuals, and MSM agreed with receiving the mpox vaccine [20], while another study conducted in China in the MSM population reported that 90.2% also agreed [21]. However, there are no studies in Latin America focusing on the acceptance of mpox vaccination in the MSM population, despite the high incidence reported in the Americas, making 87.8% of cases worldwide by February 2023. Globally, among the top 10 countries with the most cases of mpox infection, Peru (n = 3752) was in seventh position after Brazil, Colombia, and Mexico [22].
In Peru, the first case of mpox was described on 26 June 2022 [23]. Nationwide, 3785 confirmed cases and 20 deaths have been reported as of 19 March 2023 [24]. The vaccination process against this disease started in November 2022 in Peru, prioritizing people living with HIV in the first phase [25]. In the second phase, citizens at high risk of contracting sexually transmitted infections, such as MSM, transgender women, and sex workers, were included; in this phase, healthcare workers were also eligible for vaccination [25]. According to WHO, the 3’C model (confidence, complacency, and convenience) represents several factors that work together to influence vaccination decisions [26]. To determine which factors are important to improve, it is necessary to conduct studies on mpox vaccine uptake and whether these might have led to a change in vaccination intention in the population at high risk for mpox. Therefore, it is crucial to focus on evaluating the perceptions and mpox vaccination acceptance in the group at the highest risk such as the LGBTIQ+ community (L, lesbian; G, gay; B, bisexual; T, trans, I, intersexual; Q, queer; +, others) with the purpose of designing public health strategies to resolve doubts and fears about the vaccine. In this cross-sectional study, we evaluated the perception and intention to be vaccinated against mpox among the LGBTIQ+ community during the 2022 outbreak in Peru. We also determined the factors that are independently associated with vaccination intent.
2. Materials and Methods
2.1. Study Design, Setting, and Participants
We conducted a cross-sectional study from 1 November 2022 to 17 January 2023 in Peru. We adhered to the STROBE checklist for cross-sectional studies (Supplementary Material S1). At the start of data collection, there were 1898 reported cases of mpox in the country, which increased to 3728 by the end of the study period [24]. In response to the outbreak, the Peruvian Ministry of Health initiated free mpox vaccinations on 7 November 2022 [25]. Eligible participants for our study included individuals over 18 years old, self-identified as members of the LGBTIQ+ community, residing in the departments of Lima or Callao, not previously vaccinated against mpox, and not enrolled in an mpox clinical trial.
2.2. Sample and Diffusion
Due to the exploratory nature of the study, we determined the sample size based on a 50% intention to be vaccinated against mpox, a 95% confidence level, and a 5% margin of error. Using these parameters, the targeted sample size was 385 individuals. In addition, we considered 5% of possible surveys that did not meet the inclusion criteria or were incorrectly completed, so the minimum target number of completed surveys was 404. We used nonprobabilistic snowball sampling. We distributed the survey through various social media platforms, including Facebook, Twitter, Instagram, and WhatsApp. In order to recruit more participants, we used Grinder, which is a social networking and online dating application targeted toward members of the gay, bisexual, transgender, and queer community. In addition, we administered the survey in person to individuals who attended the clinics of three community-based organizations: “Voluntades Lima Norte”, “Diversex Lima Este”, and “Plan Camino Lima Centro” located in the department of Lima. These organizations focus on preventing sexually transmitted infections in the LGBTIQ+ community.
2.3. Questionnaire
The study utilized both online and printed surveys to gather data from participants (Supplementary Material S2). The online survey was created using Google Forms. Meanwhile, participants surveyed in person received a printed survey. The survey sections were as follows: (1) Informed consent, (2) Inclusion criteria, (3) Sociodemographic data, (4) Perceptions about the risk of mpox infection, and (5) Perceptions about the mpox vaccine. To measure perceptions, we employed similar surveys utilized in other countries to assess the intention of the LGBTIQ+ population to get vaccinated against mpox [27,28]. Additionally, we adapted the questions to the Peruvian reality and incorporated specific concepts of the current outbreak in the country. This survey was validated by experts in the field of infectious diseases from Peru and the United States of America.
2.4. Independent Variables
We considered the following independent variables: age, area of residence, healthcare worker status, education level, gender, sexual orientation, number of sexual partners in the last 3 months, HIV infection, sexually transmitted infection (other than HIV) in the last 3 months, knowledge of the current outbreak, history of mpox, perceptions toward the risk of infection with mpox, and perceptions regarding the mpox vaccine.
2.5. Dependent Variable
To define the variable “intention to be vaccinated against mpox”, we used the following question: “Do you plan to get vaccinated against mpox when the vaccine becomes available?” This question had five response options: 1. “I will get vaccinated,” 2. “It is likely that I will get vaccinated,” 3. “It is very unlikely that I will get vaccinated,” 4. “It is likely that I will not get vaccinated,” and 5. “I will not get vaccinated.” If the respondent answered option 1 or 2, they were considered to have the intention to get vaccinated. If they answered options 3, 4, or 5, they were considered to have no intention to get vaccinated.
2.6. Statistical Analysis
The statistical analysis was conducted using R software version 4.2.2. We described participant characteristics using frequencies for categorical variables and mean with standard deviation for numeric variables. In the bivariate analysis, we compared characteristics between the participants who had the intention to be vaccinated and those who did not. We used the Wilcoxon rank-sum test, Fisher’s exact test, and chi-square test of independence depending on the nature of the variable. Finally, we used Poisson regression with robust variance to create a multivariate model that included all variables with a p-value < 0.20 in the bivariate analysis. Variables with a p-value < 0.05 were considered statistically significant associated factors.
2.7. Ethical Aspects
The present study was approved by the institutional ethics committee of the Universidad Peruana Unión (Approval 2022-CE-FCS-UPeU-157) and was registered in the Proyectos de Investigación en Salud (PRISA, by its Spanish acronym) database of the Peruvian National Institute of Health. At the beginning of the survey, informed consent was requested from each participant, the survey was anonymous, and the data obtained were confidential.
3. Results
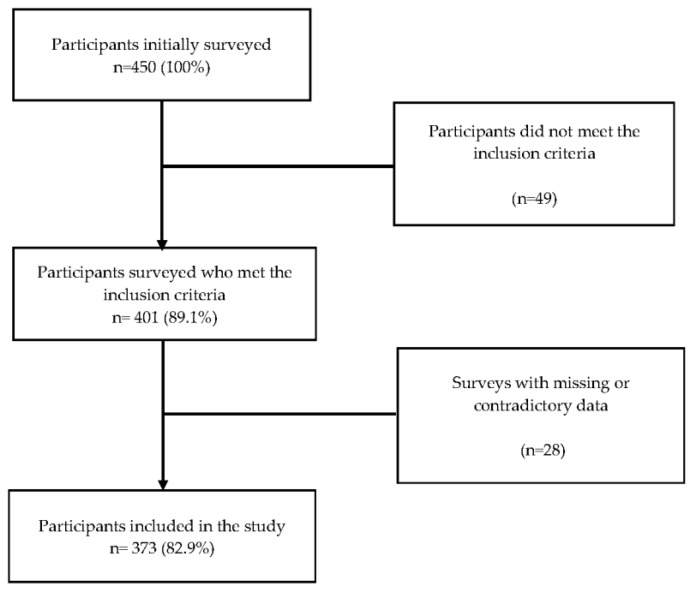
A survey was conducted among 450 participants (272 virtual surveys and 178 in-person surveys), of whom 49 individuals were excluded due to not meeting the inclusion criteria, and 28 participants had incomplete or conflicting survey data. Finally, our analysis was carried out with data collected from 373 (237 virtual surveys and 136 in-person surveys) participants who identified themselves as members of the LGBTIQ+ community (Figure 1).
Figure 1.
Participant selection flowchart.
The study sample comprised participants with an average age of 31 (SD: 9) years; most of them resided in central Lima (29.2%) and held university or higher education degrees (61.1%). In terms of gender, the majority self-identified as male (85.0%), while smaller proportions self-identified as female (6.2%), queer (3.2%), transexual (1.3%), transgender female (3.8%), and transgender male (0.5%). Regarding sexual orientation, the majority self-identified as homosexual men (75.3%), while smaller proportions self-identified as bisexual (14.7%), lesbian (3.2%), heterosexual (1.9%), pansexual (3.8%), and other (1.1%). In addition, 35.1% reported being infected with HIV, and 24.9% had a sexually transmitted infection other than HIV within the past 3 months (Table 1).
Table 1.
Comparison of characteristics between respondents with intention to be vaccinated and those who did not intend to be vaccinated (n = 373).
| Characteristic | n = 373 | No Intention to Be Vaccinated | Intention to Be Vaccinated | p-Value * |
|---|---|---|---|---|
| n = 43 | n = 330 | |||
| Age, mean (SD) | 31 (9) | 29 (8) | 31 (9) | 0.111 |
| Zone of residence | 0.029 | |||
| Callao | 28 (7.5%) | 5 (17.9%) | 23 (82.1%) | |
| Central Lima | 109 (29.2%) | 9 (8.3%) | 100 (91.7%) | |
| East Lima | 89 (23.9%) | 10 (11.2%) | 79 (88.8%) | |
| North Lima | 101 (27.1%) | 18 (17.8%) | 83 (82.2%) | |
| South Lima | 46 (12.3%) | 1 (2.2%) | 45 (97.8%) | |
| Healthcare worker | 0.838 | |||
| No | 326 (87.4%) | 38 (11.7%) | 288 (88.3%) | |
| Yes | 47 (12.6%) | 5 (10.6%) | 42 (89.4%) | |
| Education level | 0.022 | |||
| High school | 78 (20.9%) | 13 (16.7%) | 65 (83.3%) | |
| Technical | 56 (15.0%) | 9 (16.1%) | 47 (83.9%) | |
| College/university | 228 (61.1%) | 18 (7.9%) | 210 (92.1%) | |
| None of the above | 11 (2.9%) | 3 (27.3%) | 8 (72.7%) | |
| Gender | 0.053 | |||
| Male | 317 (85.0%) | 32 (10.1%) | 285 (89.9%) | |
| Female | 23 (6.2%) | 4 (17.4%) | 19 (82.6%) | |
| Queer | 12 (3.2%) | 1 (8.3%) | 11 (91.7%) | |
| Other | 21 (5.6%) | 6 (28.6%) | 15 (71.4%) | |
| Sexual Orientation | 0.340 | |||
| Homosexual men | 281 (75.3%) | 30 (10.7%) | 251 (89.3%) | |
| Bisexual | 55 (14.7%) | 6 (10.9%) | 49 (89.1%) | |
| Other | 37 (9.9%) | 7 (18.9%) | 30 (81.1%) | |
| Sexual partners in the last 3 months, mean (SD) | 6 (10) | 7 (8) | 6 (10) | 0.156 |
| HIV infection | 0.475 | |||
| No | 242 (64.9%) | 30 (12.4%) | 212 (87.6%) | |
| Yes | 131 (35.1%) | 13 (9.9%) | 118 (90.1%) | |
| Sexually transmitted infection in the last 3 months (other than HIV) | 0.048 | |||
| No | 280 (75.1%) | 27 (9.6%) | 253 (90.4%) | |
| Yes | 93 (24.9%) | 16 (17.2%) | 77 (82.8%) | |
| Knowledge about the current mpox outbreak | 0.999 | |||
| No | 30 (8.0%) | 3 (10.0%) | 27 (90.0%) | |
| Yes | 343 (92.0%) | 40 (11.7%) | 303 (88.3%) | |
| History of mpox disease | 0.141 | |||
| No | 329 (88.2%) | 35 (10.6%) | 294 (89.4%) | |
| Yes | 44 (11.8%) | 8 (18.2%) | 36 (81.8%) | |
| Perceptions about mpox | ||||
| Do you think mpox is a very contagious disease? | <0.001 | |||
| No | 49 (13.1%) | 15 (30.6%) | 34 (69.4%) | |
| Yes | 324 (86.9%) | 28 (8.6%) | 296 (91.4%) | |
| Mpox is transmitted by direct contact with the skin lesions of a sick person. | 0.103 | |||
| Agree | 335 (89.8%) | 35 (10.4%) | 300 (89.6%) | |
| Neither agree nor disagree | 30 (8.0%) | 7 (23.3%) | 23 (76.7%) | |
| Disagree | 8 (2.1%) | 1 (12.5%) | 7 (87.5%) | |
| Mpox can be transmitted by talking to a sick person. | 0.875 | |||
| Agree | 123 (33.0%) | 13 (10.6%) | 110 (89.4%) | |
| Neither agree nor disagree | 69 (18.5%) | 9 (13.0%) | 60 (87.0%) | |
| Disagree | 181 (48.5%) | 21 (11.6%) | 160 (88.4%) | |
| Mpox is transmitted by semen. | 0.734 | |||
| Agree | 194 (52.0%) | 20 (10.3%) | 174 (89.7%) | |
| Neither agree nor disagree | 114 (30.6%) | 15 (13.2%) | 99 (86.8%) | |
| Disagree | 65 (17.4%) | 8 (12.3%) | 57 (87.7%) | |
| Mpox is transmitted by saliva. | 0.141 | |||
| Agree | 199 (53.4%) | 17 (8.5%) | 182 (91.5%) | |
| Neither agree nor disagree | 101 (27.1%) | 16 (15.8%) | 85 (84.2%) | |
| Disagree | 73 (19.6%) | 10 (13.7%) | 63 (86.3%) | |
| Mpox is a disease that mainly affects gay or bisexual men. | 0.293 | |||
| Agree | 138 (37.0%) | 19 (13.8%) | 119 (86.2%) | |
| Neither agree nor disagree | 81 (21.7%) | 11 (13.6%) | 70 (86.4%) | |
| Disagree | 154 (41.3%) | 13 (8.4%) | 141 (91.6%) | |
| Fear of contracting mpox disease. | 0.002 | |||
| No | 107 (28.7%) | 21 (19.6%) | 86 (80.4%) | |
| Yes | 266 (71.3%) | 22 (8.3%) | 244 (91.7%) | |
| Do you think you are at risk of contracting mpox? | 0.011 | |||
| Agree | 211 (56.6%) | 18 (8.5%) | 193 (91.5%) | |
| Neither agree nor disagree | 99 (26.5%) | 11 (11.1%) | 88 (88.9%) | |
| Disagree | 63 (16.9%) | 14 (22.2%) | 49 (77.8%) | |
| Do you believe mpox is a serious or dangerous disease? | <0.001 | |||
| No | 125 (33.5%) | 24 (19.2%) | 101 (80.8%) | |
| Yes | 248 (66.5%) | 19 (7.7%) | 229 (92.3%) | |
| Condoms prevent the spread of mpox. | 0.164 | |||
| Agree | 116 (31.1%) | 17 (14.7%) | 99 (85.3%) | |
| Neither agree nor disagree | 94 (25.2%) | 13 (13.8%) | 81 (86.2%) | |
| Disagree | 163 (43.7%) | 13 (8.0%) | 150 (92.0%) | |
| Having many sexual partners increases the risk of contracting mpox. | 0.014 | |||
| Agree | 304 (81.5%) | 28 (9.2%) | 276 (90.8%) | |
| Neither agree nor disagree | 42 (11.3%) | 9 (21.4%) | 33 (78.6%) | |
| Disagree | 27 (7.2%) | 6 (22.2%) | 21 (77.8%) | |
| The Ministry of Health of Peru is adequately informing the population about mpox. | 0.975 | |||
| Agree | 115 (30.8%) | 13 (11.3%) | 102 (88.7%) | |
| Neither agree nor disagree | 99 (26.5%) | 11 (11.1%) | 88 (88.9%) | |
| Disagree | 159 (42.6%) | 19 (11.9%) | 140 (88.1%) | |
| Perceptions about the mpox vaccine | ||||
| Is there a vaccine to prevent mpox? | 0.115 | |||
| No | 20 (5.4%) | 4 (20.0%) | 16 (80.0%) | |
| I don’t know | 108 (29.0%) | 16 (14.8%) | 92 (85.2%) | |
| Yes | 245 (65.7%) | 23 (9.4%) | 222 (90.6%) | |
| Do you think that the vaccine against mpox would protect your health? | <0.001 | |||
| No | 51 (13.7%) | 18 (35.3%) | 33 (64.7%) | |
| Yes | 322 (86.3%) | 25 (7.8%) | 297 (92.2%) | |
| How safe do you think the mpox vaccine would be? | <0.001 | |||
| Not safe | 55 (14.7%) | 19 (34.5%) | 36 (65.5%) | |
| Safe | 318 (85.3%) | 24 (7.5%) | 294 (92.5%) | |
SD, standard deviation. * Statistically significant p-values are in bold.
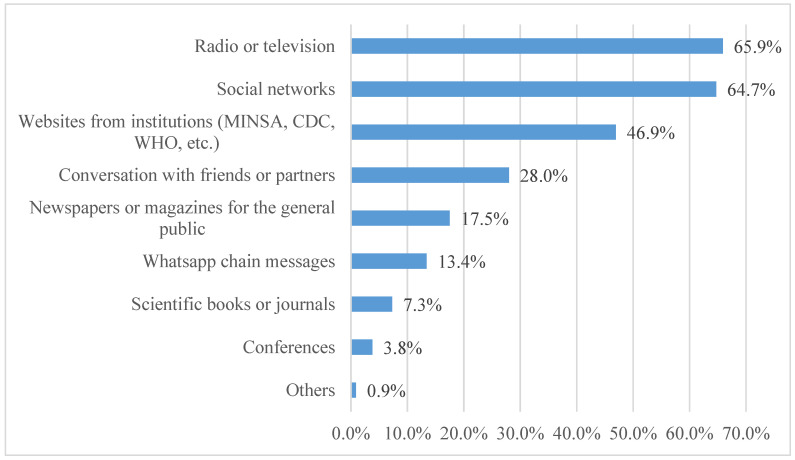
The majority (92.0%) were aware of the mpox outbreak, with 11.8% reporting having experienced the disease. In terms of sources of information about mpox, the most common sources were television or radio (65.9%), social networks (64.7%), websites from official health institutions (MINSA, CDC, WHO, etc.) (46.9%), and conversations with friends (28.0%) (Figure 2).
Figure 2.
Sources of information on mpox in the Peruvian LGBTIQ+ population (n = 343).
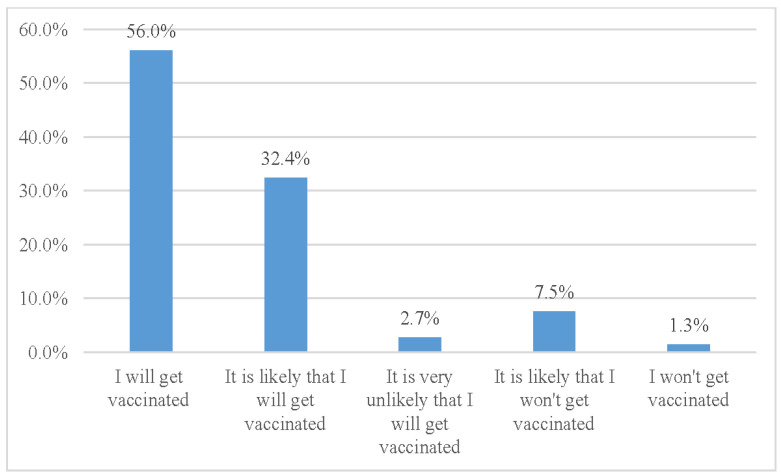
In terms of intention to be vaccinated against mpox, we found that 56.0% reported that they would get vaccinated, while an additional 32.4% reported that they would likely get vaccinated. A minority of participants reported that they would likely not get vaccinated or would not receive the vaccine (Figure 3).
Figure 3.
Intention of the LGBTIQ+ population to be vaccinated against mpox.
Furthermore, 54.4% of the participants reported that they would get vaccinated immediately. On the other hand, 32.4% indicated that they would wait to determine whether the vaccine was safe before being vaccinated. A smaller proportion (7.2%) expressed they would only consider being vaccinated if it were mandatory, while 0.8% reported that they would never get vaccinated. Additionally, 5.1% of the respondents reported that they had not yet decided on vaccination.
The intention to be vaccinated was significantly higher among participants who lived in South Lima (p = 0.029), had a university degree or higher education level (p = 0.022), did not report a history of sexually transmitted infection in the last 3 months (p = 0.048), believed that mpox was a highly contagious disease (p < 0.001), who were afraid of contracting mpox (p = 0.002), believed they were at risk of contracting mpox (p = 0.011), considered mpox a severe or dangerous disease (p < 0.001), thought that having many sexual partners increased the risk of acquiring mpox (p = 0.014), believed the vaccine would protect their health (p < 0.001), and believed the vaccine would be safe (p < 0.001) (Table 1).
In the multivariable model, we observed a positive association between the belief that the vaccine was safe and the intention to get vaccinated against mpox, which was statistically significant (aPR: 1.24; 95% CI: 1.02 to 1.50; p = 0.028) (Table 2). The rest of the variables included in the model did not achieve statistical significance.
Table 2.
Multivariable analysis of factors associated with the intention to get vaccinated.
| Characteristic | Multivariable Model | ||
|---|---|---|---|
| aPR | 95% CI | p-Value * | |
| Age, mean (SD) | 1.00 | 1.00–1.01 | 0.108 |
| Zone of residence in Lima | |||
| Callao | Ref. | ||
| Central | 1.01 | 0.86–1.18 | 0.910 |
| East | 1.01 | 0.86–1.19 | 0.888 |
| North | 0.96 | 0.81–1.13 | 0.614 |
| South | 1.10 | 0.94–1.27 | 0.240 |
| Education level | |||
| High school | Ref. | ||
| Technical | 0.97 | 0.84–1.11 | 0.647 |
| College/university | 1.02 | 0.93–1.12 | 0.676 |
| None of the above | 1.00 | 0.71–1.41 | 0.987 |
| Gender | |||
| Male | Ref. | ||
| Female | 0.97 | 0.82–1.15 | 0.723 |
| Queer | 0.89 | 0.71–1.11 | 0.289 |
| Other | 1.03 | 0.88–1.22 | 0.696 |
| Sexual partners in the last 3 months | 1.00 | 1.00–1.00 | 0.484 |
| Sexually transmitted infection in the last 3 months (other than HIV) | |||
| No | Ref. | ||
| Yes | 0.97 | 0.89–1.05 | 0.453 |
| History of mpox disease | |||
| No | Ref. | ||
| Yes | 0.94 | 0.83–1.07 | 0.363 |
| Do you think mpox is a very contagious disease? | |||
| No | Ref. | ||
| Yes | 1.13 | 0.95–1.34 | 0.179 |
| Mpox is transmitted by direct contact with the skin lesions of a sick person. | |||
| Agree | Ref. | ||
| Neither agree nor disagree | 0.98 | 0.80–1.19 | 0.807 |
| Disagree | 1.02 | 0.83–1.26 | 0.839 |
| Mpox is transmitted by saliva. | |||
| Agree | Ref. | ||
| Neither agree nor disagree | 0.98 | 0.89–1.08 | 0.726 |
| Disagree | 0.96 | 0.88–1.06 | 0.430 |
| Fear of contracting mpox disease. | |||
| No | Ref. | ||
| Yes | 1.01 | 0.92–1.12 | 0.805 |
| Do you think you are at risk of contracting mpox? | |||
| Agree | Ref. | ||
| Neither agree nor disagree | 1.04 | 0.95–1.14 | 0.423 |
| Disagree | 0.89 | 0.77–1.02 | 0.090 |
| Do you believe mpox is a serious or dangerous disease? | |||
| No | Ref. | ||
| Yes | 1.07 | 0.98–1.16 | 0.109 |
| Condoms prevent the spread of mpox. | |||
| Agree | Ref. | ||
| Neither agree nor disagree | 0.96 | 0.86–1.06 | 0.408 |
| Disagree | 0.98 | 0.90–1.07 | 0.632 |
| Having many sexual partners increases the risk of contracting mpox. | |||
| Agree | Ref. | ||
| Neither agree nor disagree | 0.97 | 0.81–1.16 | 0.746 |
| Disagree | 1.07 | 0.89–1.28 | 0.480 |
| Is there a vaccine to prevent mpox? | |||
| No | Ref. | ||
| I don’t know | 0.98 | 0.79–1.23 | 0.889 |
| Yes | 0.98 | 0.79–1.21 | 0.842 |
| Do you think that the vaccine against mpox would protect your health? | |||
| No | Ref. | ||
| Yes | 1.21 | 0.99–1.47 | 0.063 |
| How safe do you think the mpox vaccine would be? | |||
| Not safe | Ref. | ||
| Safe | 1.24 | 1.02–1.50 | 0.028 |
aPR, adjusted prevalence ratio; CI, confidence interval; Ref., reference. * Statistically significant p-values are in bold.
4. Discussion
This is the first study in Latin America that targeted the LGBTIQ+ community to determine perceptions about mpox and vaccination intent. The overall intention to get vaccinated against mpox in the LGBTIQ+ community in our study was 88.4%, which is one of the highest reported in the literature. One of the first studies on the LGBTIQ+ community was conducted by Wang et al. in the Netherlands, who reported a high intention to get vaccinated (70%). This study was conducted before the start of targeted mpox vaccination in the Netherlands and included 394 MSM [29]. These findings were in line with another Dutch study conducted around the early roll-out of pre-exposure mpox vaccination, which showed an 81.5% vaccination acceptance [20]. Furthermore, a large-scale European survey revealed an intention to get vaccinated of 85–90% in northern countries and 83–88% in western countries. This high vaccination acceptance was related to the perception of increased severity and transmission risk for mpox during this outbreak [30]. In addition, studies with a similar design were conducted in the United Kingdom [31] and China [21], which also reported a high rate of vaccination acceptance of 86% and 90.2%, respectively. According to these studies, the LGBTIQ+ community has a high intention to get vaccinated against mpox, which was also observed in our study.
Regarding the factors independently associated with the intention to be vaccinated against mpox in the LGBTIQ+ community, we found that 85.3% (n = 318) of the respondents considered the mpox vaccine to be safe. Furthermore, in adjusted multivariate analysis, believing that the vaccine was safe increased the intention of being vaccinated by 24%. There are few studies evaluating the association between the perception of safety and vaccination intent. In a study conducted in the Netherlands, it was reported that 45.1% of people eligible for pre-exposure vaccination (PPV) and 45% of people for nonpre-exposure vaccination (non-PPV) believed that the vaccine has no unpleasant adverse effects, and this factor increased the willingness to be vaccinated against mpox [20]. In a study conducted in Ghana on the general population, one of the main determinants of vaccine acceptance was confidence in the vaccine, which increased the odds of mpox vaccine acceptance (aOR: 2.45, 95% CI, 1.93–3.15, p < 0.001) [32]. Furthermore, in a study from China, one of the predictors of willingness to get the mpox vaccine among MSM living with HIV was to believe that the mpox vaccine was safe (aOR: 6.6, CI 95%: 2.7–16.4) [33]. It is worth mentioning that mpox vaccine safety has been demonstrated in prelicensure studies, such as a phase 3 study conducted in 2019 on the modified vaccinia Ankara vaccine [34]. Moreover, the CDC considers MVA-BN a safe vaccine, as it only generates mild adverse effects, such as pain, redness, and itching at the inoculation site. Severe allergic reactions are extremely rare [35]. Several authors have theorized that the high acceptance of the mpox vaccine may be due to the fact the population witnessed a worldwide decline in mortality during the COVID-19 pandemic following the implementation of COVID-19 vaccines [36,37]. We postulate that the good safety profile of COVID vaccines observed in many countries, including Peru, could have contributed to the perception of safety for the mpox vaccine in our study.
Regarding the place of residence, our study showed that the area with the highest intention of vaccination was South Lima. The reason behind that may have been that the vaccination campaign was initiated in East and South Lima, so people in those areas were probably better informed about the safety profile and efficacy of the vaccine [25,38]. Other studies have also reported differences in vaccination intent depending on the place of residence. Ahmed et al. [39] revealed that there were significant differences in knowledge and acceptance in the Kurdistan region of Iraq. Kurdistan is divided into four regions: Sulaymaniyah, Erbil, Duhok, and Halabja, where Sulaymaniyah demonstrated the highest level of knowledge, positive attitude, and concern toward mpox.
In the bivariate analysis of our study, we observed that mpox vaccination intent was significantly higher among participants who believed that mpox was a highly contagious disease (p < 0.001). This important finding has not been reported in the current literature and should be considered in future studies on mpox vaccine acceptance. Mpox is a contagious disease that is transmitted primarily through sexual contact [40]; however, transmission can also occur through other exposures, including nonsexual contact with active skin lesions and less commonly via direct contact (face to face) with saliva or upper respiratory secretions [41,42]. Of note, mpox is less contagious than smallpox and usually causes a less severe illness [43].
We also found a higher vaccination intent in participants who believed they were at higher risk of contracting mpox. A recent study performed in France showed that among MSM on pre-exposure prophylaxis (PrEP) or living with HIV, 54% of participants felt at risk of being infected with mpox. The authors also found that feeling at risk was an independent determinant of vaccine acceptance [44]. It is important to know that the current data have demonstrated that gay, bisexual, and other men who have sex with men (MSM) made up the majority of cases in the current mpox outbreak [18]. A case series across 16 countries identified several distinct features of the early 2022 mpox outbreak, in which 95% of the transmission was sexual contact and mostly in gay or bisexual men [45]. In Peru, 71.5% of mpox cases were mainly MSM and 55% living with HIV [24]. In addition, of all the patients hospitalized with mpox, 94% were MSM [46].
The present study also revealed that the intention to be vaccinated against mpox was higher in people who believed that the vaccine would protect their health (p < 0.001). These findings are similar to those reported by Ghazy et al. [32], who found that confidence was significantly associated with mpox vaccine acceptance in the Ghanaian population. In their study, they defined confidence as “trust in the vaccine’s dependability and effectiveness, in the health system, and the healthcare personnel” [32]. The vaccines proposed by WHO for mpox were initially designed for the prevention of smallpox [7], so evidence of their efficacy and effectiveness against mpox at the beginning of the current outbreak was sparse. However, observational studies on the effectiveness of the MVA-BN vaccine have been recently published. Payne et al. [47] analyzed weekly reports from 43 US jurisdictions and found that the incidence of mpox in unvaccinated adult men was 7,4-fold higher compared with adult men who received only one dose of MVA-BN vaccine and 9,6-fold higher compared with men who received two doses. Similarly, Wolff Sagy et al. [48] conducted a retrospective analysis in 2054 Israeli adults with risk factors for mpox infection, in which they found that vaccination with one dose of MVA-BN was associated with an 86% reduction in the risk of mpox. These real-world data demonstrate that the MVA-BN vaccine effectively protects against mpox infection.
Another important finding of our study was that mpox vaccination intent was higher among respondents who considered mpox a severe or dangerous disease (p < 0.001). Before the current mpox outbreak, WHO reported mpox mortality rates of up to 11% [49]. In addition, at the beginning of the current outbreak, different studies reported that the main affected group was MSM [50], who have a higher HIV prevalence compared to the general population [10] and were the predominant group of reported cases with mpox and HIV coinfection [45,51,52]. In response, in July 2022, the Peruvian government issued a guideline for the prevention and management of patients with mpox, which considered HIV+ and immunosuppressed patients at risk of developing more severe disease and mpox complications [53]. Likewise, the Ministry of Health of Peru organized multiple information campaigns on the prevention of mpox infection, mainly among vulnerable populations, such as people with HIV and MSM [54]. These data provided by the Ministry of Health during the current outbreak alerted the LGBTIQ+ community and may explain our results.
It is important to note that most participants in our study (92.0%) were aware of the mpox outbreak. The main source of information was television or radio (65.9%), followed by social networks (64.7%) and web pages of official health institutions (MINSA, CDC, WHO, etc.) (46.9%). The dissemination of truthful information with simple and easy-to-understand language by competent entities is key, as this factor probably contributed to the high acceptance of the mpox vaccine. However, when asked about the information provided by the Peruvian Ministry of Health, only 30.8% of participants claimed that this institution was adequately informing about mpox, which is concerning because it may lead to the development of conspiracy theories about the vaccine in the community [55]. This is clearly an aspect that the central government should improve—providing transparency and effective factual correction on the fake news spread through different media.
Limitations and Strengths
Our study has some limitations. Despite having a good sample size, it is not representative of the LGBTIQ+ population from Peru because we used nonprobability snowball sampling. In addition, it is possible that there was a response bias, as respondents probably wanted to give a socially accepted answer [56] in the context of the disinformation campaigns carried out in Peru [57]. Finally, most of the surveys were online, where there was no face-to-face control of the completion, so different factors could have affected the veracity of the answers given, such as the search for information and the completion of the same survey by two or more people. Regarding strengths, to our knowledge, this study is the first one in Latin America to assess mpox vaccination intent among the LGBTIQ+ community during the 2022 mpox outbreak. In addition, the survey (beginning 1 November) was conducted at the beginning of the vaccination campaign in Peru on 7 November 2022 [25], which would probably reflect more accurately a defined opinion of the participants regarding their willingness to receive the mpox vaccine [58].
5. Conclusions
Our study showed a high intention to be vaccinated against mpox among the LGBTIQ+ community in the departments of Lima and Callao in Peru, a finding that is related to the perception of this population to be at higher risk of contracting mpox, which is believed to be a severe and very contagious disease according to the study participants. We found that the perception of safety for the mpox vaccine was the only factor independently associated with the intention to be vaccinated; thus, this factor should be considered and reinforced in educational campaigns, which could potentially lead to an increase in the rate of vaccination. Despite the high percentage of mpox vaccine awareness found in our study, there are still concerns about information adequacy provided by the central government, which is a problem that should be addressed to avoid the development of conspiracy theories and misconceptions about the vaccine, especially in areas where the acceptance has been lower, such as Callao and East Lima.
Acknowledgments
We are grateful to the following people for their support in the recruitment of study participants: Katty Chong-Chinchay, Luis O. Castro-Asencios, Alberto E. Sánchez-Delgado, Gabriel M. Castro Jiménez, Carlos Campos-Urteaga, and Cesar Grados-Casalino.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11051008/s1. Supplementary material S1-STROBE Statement-Checklist of items that should be included in reports of cross-sectional studies; Supplementary material S2-Survey to evaluate the perceptions and intention to get vaccinated against mpox.
Author Contributions
J.M.A.-S., B.O.-S. and J.A.G.-Z. participated in the conceptualization of the study. J.M.A.-S., B.O.-S., J.A. and J.A.G.-Z. designed the study. J.M.A.-S. and L.P.-R. participated in the recruitment of study participants and data collection. D.R.S.-M. and A.N.S.-M. performed the data analyses. J.M.A.-S., B.O.-S., L.P.-R., D.R.S.-M., J.A. and J.A.G.-Z. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional ethics committee of the Universidad Peruana Unión (Protocol code: 2022-CE-FCS-UPeU-157| Date: 18 October 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Research data are not available due to privacy.
Conflicts of Interest
The authors declare that they do not have any conflict of interest.
Funding Statement
The article processing charges were funded by the Universidad Peruana Unión.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lum F.-M., Torres-Ruesta A., Tay M.Z., Lin R.T.P., Lye D.C., Rénia L., Ng L.F.P. Monkeypox: Disease Epidemiology, Host Immunity and Clinical Interventions. Nat. Rev. Immunol. 2022;22:597–613. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., Damon I.K., Reynolds M.G., Kuehnert M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 3.Damaso C.R. Phasing out Monkeypox: Mpox Is the New Name for an Old Disease. Lancet Reg. Health Am. 2023;17:100424. doi: 10.1016/j.lana.2022.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. [(accessed on 2 April 2023)]; Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern.
- 5.Centers for Disease Control and Prevention 2022 Global Map & Case Count. [(accessed on 3 April 2023)]; Available online: https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html.
- 6.Kmiec D., Kirchhoff F. Monkeypox: A New Threat? IJMS. 2022;23:7866. doi: 10.3390/ijms23147866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Vaccines and Immunization for Monkeypox. Interim Guidance. [(accessed on 5 April 2023)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/361894/WHO-MPX-Immunization-2022.2-eng.pdf?sequence=1&isAllowed=y.
- 8.FDA Monkeypox Update: FDA Authorizes Emergency Use of JYNNEOS Vaccine to Increase Vaccine Supply. [(accessed on 5 April 2023)]; Available online: https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply.
- 9.Bragazzi N.L., Kong J.D., Mahroum N., Tsigalou C., Khamisy-Farah R., Converti M., Wu J. Epidemiological Trends and Clinical Features of the Ongoing Monkeypox Epidemic: A Preliminary Pooled Data Analysis and Literature Review. J. Med. Virol. 2023;95:e27931. doi: 10.1002/jmv.27931. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Men Who Have Sex with Men. [(accessed on 5 April 2023)]; Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/populations/men-who-have-sex-with-men.
- 11.Ogoina D., Iroezindu M., James H.I., Oladokun R., Yinka-Ogunleye A., Wakama P., Otike-odibi B., Usman L.M., Obazee E., Aruna O., et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 12.Curran K.G., Eberly K., Russell O.O., Snyder R.E., Phillips E.K., Tang E.C., Peters P.J., Sanchez M.A., Hsu L., Cohen S.E., et al. HIV and Sexually Transmitted Infections among Persons with Monkeypox—Eight U.S. Jurisdictions, May 17–July 22, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1141–1147. doi: 10.15585/mmwr.mm7136a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitjà O., Alemany A., Marks M., Lezama Mora J.I., Rodríguez-Aldama J.C., Torres Silva M.S., Corral Herrera E.A., Crabtree-Ramirez B., Blanco J.L., Girometti N., et al. Mpox in People with Advanced HIV Infection: A Global Case Series. Lancet. 2023;401:939–949. doi: 10.1016/S0140-6736(23)00273-8. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Expanded Access IND Protocol: Use of Tecovirimat (TPOXX®) for Treatment of Human Non-Variola Orthopoxvirus Infections in Adults and Children. IND No. 116,039. CDC IRB No. 6402. [(accessed on 2 April 2023)]; Available online: https://www.cdc.gov/poxvirus/mpox/pdf/Tecovirimat-IND-Protocol-CDC-IRB.pdf.
- 15.Ortiz-Saavedra B., León-Figueroa D.A., Montes-Madariaga E.S., Ricardo-Martínez A., Alva N., Cabanillas-Ramirez C., Barboza J.J., Siddiq A., Coaguila Cusicanqui L.A., Bonilla-Aldana D.K., et al. Antiviral Treatment against Monkeypox: A Scoping Review. Trop. Med. Infect. Dis. 2022;7:369. doi: 10.3390/tropicalmed7110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegrist E.A., Sassine J. Antivirals with Activity against Mpox: A Clinically Oriented Review. Clin. Infect. Dis. 2023;76:155–164. doi: 10.1093/cid/ciac622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez A.E., Frattaroli P., Vu C.A., Paniagua L., Mintz J., Bravo-Gonzalez A., Zamudio P., Barco A., Rampersad A., Lichtenberger P., et al. Successful Outcome after Treatment with Cidofovir, Vaccinia, and Extended Course of Tecovirimat in a Newly-Diagnosed HIV Patient with Severe Mpox: A Case Report. Vaccines. 2023;11:650. doi: 10.3390/vaccines11030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philpott D., Hughes C.M., Alroy K.A., Kerins J.L., Pavlick J., Asbel L., Crawley A., Newman A.P., Spencer H., Feldpausch A., et al. Epidemiologic and Clinical Characteristics of Monkeypox Cases—United States, May 17–July 22, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Rates of Mpox Cases by Vaccination Status. [(accessed on 30 March 2023)]; Available online: https://www.cdc.gov/poxvirus/mpox/cases-data/mpx-vaccine-effectiveness.html.
- 20.Dukers-Muijrers N.H.T.M., Evers Y., Widdershoven V., Davidovich U., Adam P.C.G., Op de Coul E.L.M., Zantkuijl P., Matser A., Prins M., de Vries H.J.C., et al. Mpox Vaccination Willingness, Determinants, and Communication Needs in Gay, Bisexual, and Other Men Who Have Sex with Men, in the Context of Limited Vaccine Availability in the Netherlands (Dutch Mpox-Survey) Front. Public Health. 2023;10:1058807. doi: 10.3389/fpubh.2022.1058807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng M., Qin C., Qian X., Yao Y., Liu J., Yuan Z., Ma L., Fan J., Tao R., Zhou F., et al. Knowledge and Vaccination Acceptance toward the Human Monkeypox among Men Who Have Sex with Men in China. Front. Public Health. 2022;10:997637. doi: 10.3389/fpubh.2022.997637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization Multi-Country Outbreak of Mpox, External Situation Report # 17—2 March 2023. [(accessed on 5 April 2023)]; Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report---17---2-march-2023.
- 23.Ministerio de Salud del Peru Minsa Confirma Primer Caso de la Viruela del Mono en el Perú. [(accessed on 5 April 2023)]; Available online: https://www.gob.pe/institucion/minsa/noticias/627040-minsa-confirma-primer-caso-de-la-viruela-del-mono-en-el-peru.
- 24.Centro Nacional de Epidemiologia Prevención y Control de Enfermedades Sala Situacional de la Monkeypox (Viruela Símica) [(accessed on 15 March 2023)]. Available online: https://www.dge.gob.pe/sala-monkeypox/
- 25.Ministerio de Salud del Peru Minsa: El Lunes 7 de Noviembre Inicia la Vacunación Contra la Viruela del Mono Para Personas Viviendo Con VIH. [(accessed on 28 March 2023)]; Available online: https://www.gob.pe/institucion/minsa/noticias/666981-minsa-el-lunes-7-de-noviembre-inicia-la-vacunacion-contra-la-viruela-del-mono-para-personas-viviendo-con-vih.
- 26.Betsch C., Schmid P., Heinemeier D., Korn L., Holtmann C., Böhm R. Beyond Confidence: Development of a Measure Assessing the 5C Psychological Antecedents of Vaccination. PLoS ONE. 2018;13:e0208601. doi: 10.1371/journal.pone.0208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EMORY Rollins School of Public Healthh American Men’s Internet Survey (AMIS) Monkeypox Supplement: Online HIV Behavioral Survey of Men Who Have Sex with Men. [(accessed on 15 March 2023)]. Available online: https://emoryamis.org/wp-content/uploads/2022/08/2022-Monkeypox-Survey.pdf.
- 28.Meo S.A., Al-Khlaiwi T., Aljofan Z.F., Alanazi A.I., Meo A.S. Public Perceptions of the Emerging Human Monkeypox Disease and Vaccination in Riyadh, Saudi Arabia: A Cross-Sectional Study. Vaccines. 2022;10:1534. doi: 10.3390/vaccines10091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., d’Abreu de Paulo K.J.I., Gültzow T., Zimmermann H.M.L., Jonas K.J. Monkeypox Self-Diagnosis Abilities, Determinants of Vaccination and Self-Isolation Intention after Diagnosis among MSM, The Netherlands, July 2022. Eurosurveillance. 2022;27:2200603. doi: 10.2807/1560-7917.ES.2022.27.33.2200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes-Urueña J., D’Ambrosio A., Croci R., Bluemel B., Cenciarelli O., Pharris A., Dukers-Muijrers N., Nutland W., Niaupari S., Badran J., et al. High Monkeypox Vaccine Acceptance among Male Users of Smartphone-Based Online Gay-Dating Apps in Europe, 30 July to 12 August 2022. Eurosurveillance. 2022;27:2200757. doi: 10.2807/1560-7917.ES.2022.27.42.2200757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paparini S., Whitacre R., Smuk M., Thornhill J., Mwendera C., Strachan S., Nutland W., Orkin C. Public Understanding and Awareness of and Response to Monkeypox Virus Outbreak: A Cross-sectional Survey of the Most Affected Communities in the United Kingdom during the 2022 Public Health Emergency. HIV Med. 2022:1–14. doi: 10.1111/hiv.13430. [DOI] [PubMed] [Google Scholar]
- 32.Ghazy R.M., Yazbek S., Gebreal A., Hussein M., Addai S.A., Mensah E., Sarfo M., Kofi A., AL-Ahdal T., Eshun G. Monkeypox Vaccine Acceptance among Ghanaians: A Call for Action. Vaccines. 2023;11:240. doi: 10.3390/vaccines11020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu L., Sun Y., Li Y., Wang B., Yang L., Tian T., Wu X., Peng X., Liu Q., Chen Y., et al. Perception of and Vaccine Readiness towards Mpox among Men Who Have Sex with Men Living with HIV in China: A Cross-Sectional Study. Vaccines. 2023;11:528. doi: 10.3390/vaccines11030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittman P.R., Hahn M., Lee H.S., Koca C., Samy N., Schmidt D., Hornung J., Weidenthaler H., Heery C.R., Meyer T.P.H., et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019;381:1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 35.Duffy J., Marquez P., Moro P., Weintraub E., Yu Y., Boersma P., Donahue J.G., Glanz J.M., Goddard K., Hambidge S.J., et al. Safety Monitoring of JYNNEOS Vaccine During the 2022 Mpox Outbreak—United States, May 22–October 21, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1555–1559. doi: 10.15585/mmwr.mm7149a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.López L., Portugal W., Huamán K., Obregón C. Efectividad de Vacunas COVID-19 y Riesgo de Mortalidad en Perú: Un Estudio Poblacional de Cohortes Pareadas. An. Fac. Med. 2022;83:87–94. doi: 10.15381/anales.v83i2.21531. [DOI] [Google Scholar]
- 37.Centers for Disease Control and Prevention Impact of Vaccination on Risk of COVID-19–Related Mortality. [(accessed on 31 March 2023)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/data-review/vaccines.html#print.
- 38.El Peruano. Cenares Distribuyó 6 454 Frascos de la Vacuna Contra la Viruela del Mono Hasta El Momento. [(accessed on 16 March 2023)]. Available online: http://www.elperuano.pe/noticia/204132-cenares-distribuyo-6-454-frascos-de-la-vacuna-contra-la-viruela-del-mono-hasta-el-momento#:~:text=En%20lo%20que%20va%20del,Cada%20frasco%20contiene%205%20dosis.
- 39.Ahmed S.K., Abdulqadir S.O., Omar R.M., Abdullah A.J., Rahman H.A., Hussein S.H., Mohammed Amin H.I., Chandran D., Sharma A.K., Dhama K., et al. Knowledge, Attitude and Worry in the Kurdistan Region of Iraq during the Mpox (Monkeypox) Outbreak in 2022: An Online Cross-Sectional Study. Vaccines. 2023;11:610. doi: 10.3390/vaccines11030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allan-Blitz L.-T., Gandhi M., Adamson P., Park I., Bolan G., Klausner J.D. A Position Statement on Mpox as a Sexually Transmitted Disease. Clin. Infect. Dis. 2022;76:1508–1512. doi: 10.1093/cid/ciac960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallée A., Farfour E., Zucman D. Monkeypox Virus: A Novel Sexually Transmitted Disease? A Case Report from France. Travel Med. Infect. Dis. 2022;49:102394. doi: 10.1016/j.tmaid.2022.102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention How It Spreads | Mpox. [(accessed on 16 March 2023)]; Available online: https://www.cdc.gov/poxvirus/mpox/if-sick/transmission.html.
- 43.World Health Organization Monkeypox. [(accessed on 16 March 2023)]; Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox.
- 44.Zucman D., Fourn E., Touche P., Majerholc C., Vallée A. Monkeypox Vaccine Hesitancy in French Men Having Sex with Men with PrEP or Living with HIV in France. Vaccines. 2022;10:1629. doi: 10.3390/vaccines10101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S., et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 46.Sihuincha Maldonado M., Lucchetti A.J., Paredes Pacheco R.A., Martínez Cevallos L.C., Zumaeta Saavedra E.U., Ponce Zapata L.R., Lizarbe Huayta F.A., Matos Prado E.D. Epidemiologic Characteristics and Clinical Features of Patients with Monkeypox Virus Infection from a Hospital in Peru between July and September 2022. Int. J. Infect. Dis. 2023;129:175–180. doi: 10.1016/j.ijid.2023.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne A.B., Ray L.C., Cole M.M., Canning M., Houck K., Shah H.J., Farrar J.L., Lewis N.M., Fothergill A., White E.B., et al. Reduced Risk for Mpox After Receipt of 1 or 2 Doses of JYNNEOS Vaccine Compared with Risk Among Unvaccinated Persons—43 U.S. Jurisdictions, July 31–October 1, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1560–1564. doi: 10.15585/mmwr.mm7149a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolff Sagy Y., Zucker R., Hammerman A., Markovits H., Arieh N.G., Abu Ahmad W., Battat E., Ramot N., Carmeli G., Mark-Amir A., et al. Real-World Effectiveness of a Single Dose of Mpox Vaccine in Males. Nat. Med. 2023;29:748–752. doi: 10.1038/s41591-023-02229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel L. Making Sense of Monkeypox Death Rates. CMAJ. 2022;194:E1097. doi: 10.1503/cmaj.1096012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suárez Rodríguez B., Guzmán Herrador B.R., Díaz Franco A., Sánchez-Seco Fariñas M.P., del Amo Valero J., Aginagalde Llorente A.H., de Agreda J.P.A.P., Malonda R.C., Castrillejo D., Chirlaque López M.D., et al. Epidemiologic Features and Control Measures during Monkeypox Outbreak, Spain, June 2022. Emerg. Infect. Dis. 2022;28:1847–1851. doi: 10.3201/eid2809.221051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong L., Gonzales-Zamora J.A., Beauchamps L., Zachary H., Lichtenberger P. Clinical Presentation of Monkeypox among People Living with HIV in South Florida: A Case Series. Infez. Med. 2022;30:610–618. doi: 10.53854/liim-3004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortiz-Saavedra B., Montes-Madariaga E.S., Cabanillas-Ramirez C., Alva N., Ricardo-Martínez A., León-Figueroa D.A., Barboza J.J., Mohanty A., Padhi B.K., Sah R. Epidemiologic Situation of HIV and Monkeypox Coinfection: A Systematic Review. Vaccines. 2023;11:246. doi: 10.3390/vaccines11020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ministerio de Salud del Peru Norma Tecnica de Salud Para la Prevención y Manejo de los Pacientes Afectados Por Viruela del Mono (Viruela Símica)—Monkeypox. [(accessed on 16 March 2023)]; Available online: https://cdn.www.gob.pe/uploads/document/file/3346360/Norma%20T%C3%A9cnica%20de%20Salud%20para%20la%20Prevenci%C3%B3n%20y%20Manejo%20de%20los%20Pacientes%20Afectados%20por%20Viruela%20del%20Mono.pdf?v=1656858737.
- 54.Ministerio de Salud del Peru Minsa Promueve Cartilla Informativa Sobre Prevención de Viruela del Mono Para Poblaciones Vulnerables. [(accessed on 16 March 2023)]; Available online: https://www.gob.pe/institucion/minsa/noticias/639234-minsa-promueve-cartilla-informativa-sobre-prevencion-de-viruela-del-mono-para-poblaciones-vulnerables.
- 55.Caycho-Rodríguez T., Gallegos M., Valencia P.D., Vilca L.W. ¿Cuánto apoyan los peruanos las creencias de conspiración sobre las vacunas contra la COVID-19? Atención Primaria. 2022;54:102318. doi: 10.1016/j.aprim.2022.102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith P.B. Response Bias(Es) In: Michalos A.C., editor. Encyclopedia of Quality of Life and Well-Being Research. Springer; Dordrecht, The Netherlands: 2014. pp. 5539–5540. [Google Scholar]
- 57.El Peruano. El Otro Reto: Vacunar Contra la Desinformación. [(accessed on 16 March 2023)]. Available online: https://elperuano.pe/noticia/192793-el-otro-reto-vacunar-contra-la-desinformacion.
- 58.Gonzales-Zamora J.A., Soriano-Moreno D.R., Soriano A.N., Ponce-Rosas L., De-Los-Rios-Pinto A., Murrieta-Ruiz V., Morocho-Alburqueque N., Caira-Chuquineyra B., Alave J. Percepciones e Intención de Los Padres de Vacunar a Sus Hijos Bajo 12 Años de Edad Contra la COVID-19: Estudio Transversal en Perú. Rev. Chil. Infectol. 2022;39:273–286. doi: 10.4067/s0716-10182022000200273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are not available due to privacy.