Abstract
Isolation and characterization of two severe alleles at the Stamina pistilloida (Stp) locus reveals that Stp is involved in a wide range of developmental processes in the garden pea. The most severe allele, stp-4, results in flowers consisting almost entirely of sepals and carpels. Production of ectopic secondary flowers in stp-4 plants suggests that Stp is involved in specifying floral meristem identity in pea. The stp mutations also reduce the complexity of the compound pea leaf, and primary inflorescences often terminate prematurely in an aberrant sepaloid flower. In addition, stp mutants were shorter than their wild-type siblings due to a reduction in cell number in their internodes. Fewer cells were also found in the epidermis of the leaf rachis of stp mutants. Examination of the effects of stp-4 in double mutant combinations with af, tl, det, and veg2-2—mutations known to influence leaf, inflorescence, and flower development in pea—suggests that Stp function is independent of these genes. A synergistic interaction between weak mutant alleles at Stp and Uni indicated that these two genes act together, possibly to regulate primordial growth. Molecular analysis revealed that Stp is the pea homolog of the Antirrhinum gene Fimbriata (Fim) and of UNUSUAL FLORAL ORGANS (UFO) from Arabidopsis. Differences between Fim/UFO and Stp mutant phenotypes and expression patterns suggest that expansion of Stp activity into the leaf was an important step during evolution of the compound leaf in the garden pea.
INTRODUCTION
The complex process of floral meristem identity and flower development has been partially dissected by studies of mutants in Antirrhinum and Arabidopsis. These studies have revealed the presence of an evolutionarily conserved hierarchy of genes regulating flower development. Floral meristem identity genes Floricaula (Flo) and Squamosa (Squa) from Antirrhinum, and their Arabidopsis orthologs LEAFY (LFY) and APETALA1 (AP1), are required during the transition to flowering (Coen et al., 1990; Huijser et al., 1992; Mandel et al., 1992; Weigel et al., 1992; Schultz and Haughn, 1993). Differentiation of the four floral organ types (sepals, petals, stamens, and carpels) requires overlapping activity of three classes of floral homeotic genes: A, B, and C (Schwarz-Sommer et al., 1990; Bowman et al., 1991; Coen and Meyerowitz, 1991). A activity alone produces sepals, A activity in combination with B activity produces petals, B activity in combination with C activity produces stamens, and C activity alone produces carpels (reviewed in Coen and Meyerowitz, 1991; Weigel and Meyerowitz, 1994; Yanofsky, 1995).
Identification of Fimbriata (Fim) from Antirrhinum and UNUSUAL FLORAL ORGANS (UFO) from Arabidopsis suggested a mechanism by which Flo and LFY may regulate activity of B- and C-class floral homeotic genes (Simon et al., 1994; Levin and Meyerowitz, 1995; Wilkinson and Haughn, 1995; Ingram et al., 1997; Lee et al., 1997). Mutations in Fim and UFO disrupt floral meristem identity and normal expression of B- and C-class genes. Overexpression of the class B gene APETALA3 (AP3) in a ufo mutant background is able to rescue floral organ defects in ufo mutants, demonstrating that AP3 acts downstream of UFO (Krizek and Meyerowitz, 1996). In Antirrhinum, Fim expression is dependent on early Flo expression (Simon et al., 1994), and regulation of class B activity by Flo may occur through Fim. However, in Arabidopsis, UFO does not act downstream of LFY because overexpression of UFO is unable to rescue floral defects in lfy mutants (Lee et al., 1997). Thus, LFY and UFO act as co-regulators specifying floral meristem identity. UFO may also possess functions independent of LFY because expression patterns of LFY and UFO do not overlap (Lee et al., 1997). However, UFO appears to partner with LFY to control floral meristem identity and class B gene activity in Arabidopsis.
The greatest departure from the paradigm of Flo orthologs playing a role solely in floral meristem identity occurs in the garden pea. The unifoliata (uni) mutant of pea is characterized by simplification of the compound leaf, and mutant plants bear flowers that consist entirely of sepals and carpels (Marx, 1987; Hofer et al., 1997). Molecular analysis has shown that Uni is the ortholog of Flo and LFY (Hofer et al., 1997), suggesting that activity of Uni has been recruited to compound leaf development during the evolution of the garden pea. Alternatively, common regulation of leaf and flower development by Uni may reflect an ancestral, pre-angiosperm function in leaves and leaf-like sporophylls of seed ferns (Hofer et al., 1997). This alternative was supported in part by identification of the Flo ortholog in tomato, Falsiflora (Fa), because the fa mutation reduces the number of small leaflets present on tomato compound leaves (Molinero-Rosales et al., 1999). The petunia ortholog of Flo, aberrant leaf and flower (alf), does not affect development of the simple leaves of petunia (Souer et al., 1998); however, it does have a similar effect on floral meristem identity as uni, flo, lfy, and fa mutations in pea, Antirrhinum, Arabidopsis, and tomato, respectively. Analysis of floral meristem identity genes in other species has been hampered by the lack of mutants.
Characterization of Stamina pistilloida (stp-1) mutant in the garden pea by Monti and Devreux (1969) suggests that Stp is required for normal development of petals and stamens. Mutant flowers contain petals with green sepaloid streaks, and two stamens show partial conversion to carpels (Monti and Devreux, 1969). This phenotype is characteristic of mutations in class B floral homeotic genes. Consistent with this, a more complete transformation of petals to sepals and stamens to carpels was found in a more severe mutant, stp-2 (Ferrandiz et al., 1999). However, stp-2 mutant flowers are also characterized by the production of ectopic flowers, a phenotype inconsistent with a strictly homeotic role, and it was suggested that Stp may play an important role in controlling growth of the common petal/stamen primordia in pea flowers (Ferrandiz et al., 1999). This article describes two additional alleles at Stp. Phenotypic analysis of these two mutants indicated that Stp, in addition to its role in the flower, is involved in leaf and inflorescence development. Many of the effects of stp on flower development in pea are also seen in fim mutants from Antirrhinum and ufo mutants from Arabidopsis (see Simon et al., 1994; Ingram et al., 1995, 1997; Levin and Meyerowitz, 1995; Wilkinson and Haughn, 1995). Molecular analysis reveals that Stp is the pea ortholog of Fim/UFO. Together, Stp and Uni define a developmental pathway that may regulate the growth pattern of all shoot-derived meristems in pea.
RESULTS
Morphology of Wild-Type Garden Pea
The wild-type pea is a caulescent model plant that bears zygomorphic flowers typical of the Papilionoideae. A schematic representation of a wild-type pea plant and secondary inflorescence is illustrated in Figure 1. Pea flowers are pentamerous, with five sepals fused at the base forming a cup. As illustrated in Figure 2A, the five petals are colored and differentiate into three forms. The standard petal is largest (25 to 30 mm across in domestic cultivars) and adaxial (uppermost); there are two wing petals laterally and two petals that fuse to form the keel (Figure 2A). Enclosed within the keel are 10 stamens—nine fused and one free—that surround a single central carpel (Makasheva, 1983; Tucker, 1989; Reid et al., 1996). The five petals and five of the 10 stamens are derived from four common primordia that arise soon after the adaxial sepal primordium appears. For a detailed description of floral ontogeny in pea, see Tucker (1989) and Ferrandiz et al. (1999).
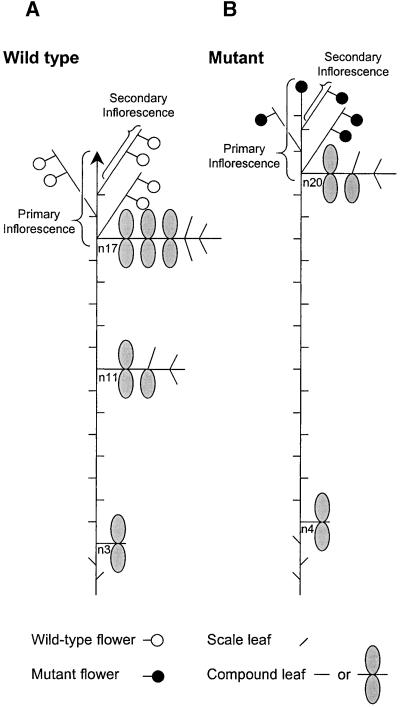
Figure 1.
Schematic Illustration of Heteroblastic Leaf Development and Inflorescence Morphology of Wild-Type and stp Mutant Plants.
(A) Wild-type plants always produce two scale-leaves before the first compound leaf, which bears two leaflets, at node 3 (n3). The pea leaf increases in complexity during ontogeny by the addition of tendrils and leaflets. After floral induction, the main apex is converted to an indeterminate primary inflorescence that bears secondary inflorescences in the leaf axils. Secondary inflorescences typically bear one or two flowers before terminating in a hairy stub.
(B) stp plants produce three scale-leaves and the first compound leaf at node 4 (n4). Heteroblastic development of the compound leaf is delayed, and leaves bearing more than two leaflets may not be produced. The primary inflorescence of stp plants often terminates in an aberrant flower, and the number of flowers borne on secondary inflorescences is reduced.
The nodes of leaflet changes and floral initiation are given as nx, where x denotes the node number. Typical values for wild-type and stp plants are given (see Table 1), and only significant changes in leaf form are shown. Each tick on the main axis represents a compound leaf–bearing node.
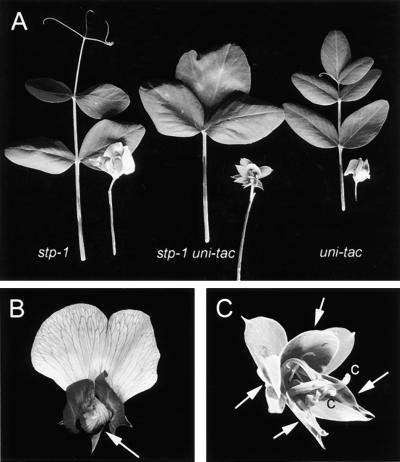
Figure 2.
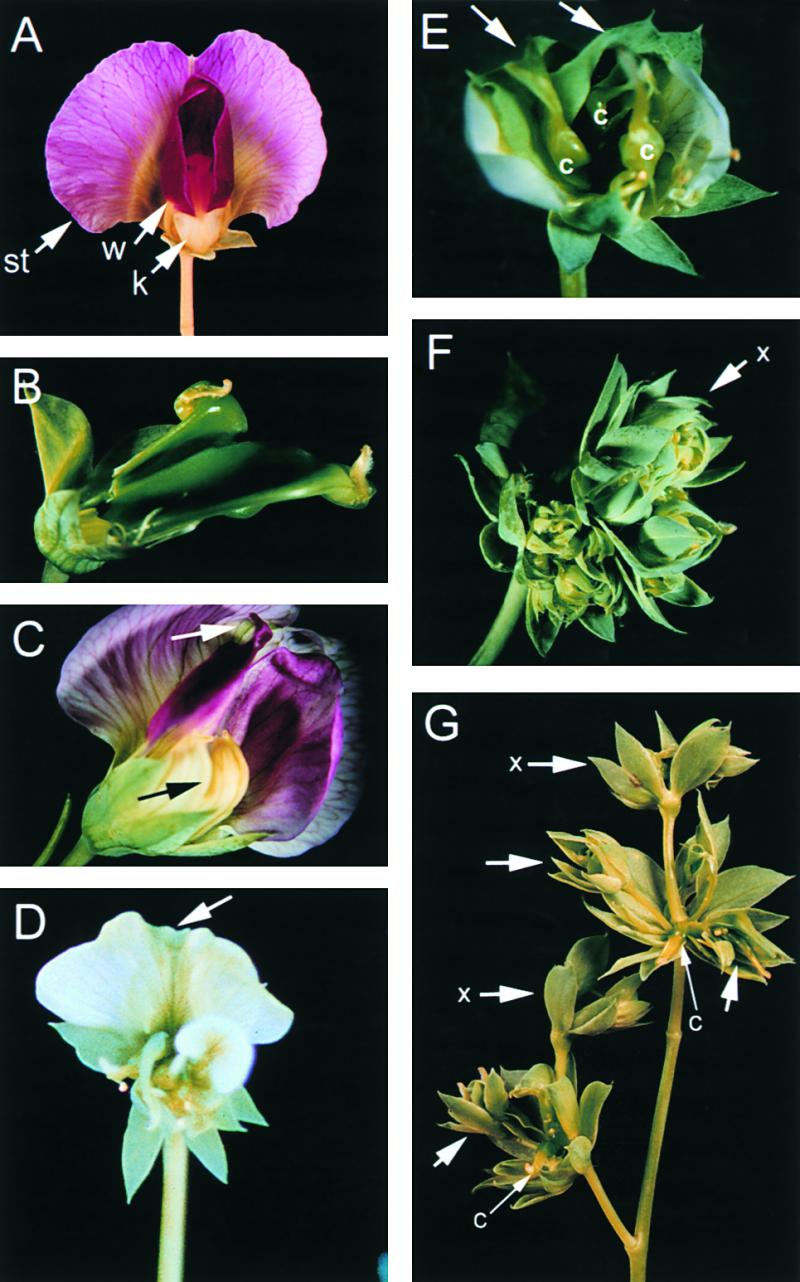
stp Allelic Series.
(A) Wild-type flower illustrating normal morphology of the standard (st) and wing (w) petals. The two fused keel petals (k) are just visible between the two wings. Within the keel are 10 stamens and a single central carpel.
(B) stp-1 flower with petals and stamens removed to show two fused carpels.
(C) stp-1 flower with green streaks through the standard petal (white arrow) and unfused keel petals (black arrow).
(D) Flowers from stp-3 plants showing a green streak through the standard petal (arrow).
(E) stp-3 flower illustrating homeotic transformation of petals to sepals (arrows) and stamens to carpels (c).
(F) stp-4 flower lacking petals and stamens, with the ectopic flower always present in stp-4 mutant flowers (x).
(G) stp-4 flower with the ectopic flower always present (x) and additional ectopic flowers on elongated pedicels (arrows) surrounding the central carpel (c).
Flowers are borne on leafless secondary inflorescences that arise in leaf axils on the primary inflorescence (Figure 1); in pea, flowering and the production of secondary inflorescences are synonymous. Primary and secondary inflorescences are morphologically distinct. Secondary inflorescences terminate in a hairy stub after producing one or two flowers (Tucker, 1989; Reid et al., 1996). The primary inflorescence bears leaves and, except for secondary inflorescence production, appears identical to the vegetative shoot from which it is derived. Pea leaves are compound and show regional differentiation; proximal pinnae are leaflets, distal ones tendrils; and two leafy stipules flank the petiole base (Makasheva, 1983; Marx, 1987). The terminal tendril appears to be a continuation of the leaf rachis but is indistinguishable from lateral tendrils. There is a consistent heteroblastic progression in the complexity of pea leaves; the first two nodes bear rudimentary scale leaves, and the first compound leaf occurs at node 3 (Figure 1). Leaf development occurs through the sequential addition of tendrils and leaflets at a steady rate until the most complex (adult) leaf form, typically with six leaflets, is produced (Makasheva, 1983; Wiltshire et al., 1994).
Origins and Characterization of the stp-3 and stp-4 Mutants
Two mutations with pronounced effects on floral morphology were isolated from an ethyl methanesulfonate mutagenesis program on cv Torsdag (HL107). Both mutants were characterized by partial or complete homeotic transformations of petals into sepals and the presence of additional carpels at the expense of stamens. A further distinguishing feature of the more severe mutant was the production of ectopic secondary floral meristems on elongated pedicels within the primary flower. Backcrosses to their initial line HL107 confirmed that both mutants showed single-gene recessive inheritance. Allelism tests between these mutants and the type line for stp (JI2163) revealed that these two new mutants represented more severe lesions at the previously identified Stp locus in pea (Monti and Devreux, 1969). Therefore, the new alleles were named stp-3 and stp-4, following the numbering system of Ferrandiz et al. (1999). The stp-2 allele described by Ferrandiz et al. (1999) was unavailable for this study.
A reinvestigation of the stp-1 mutant phenotype from the pure breeding type line JI2163 confirmed the analysis performed by Monti and Devreux (1969). The primary effect of stp-1 on flower development is a partial conversion of stamens on either side of the free adaxial stamen to carpels (Figure 2B). This homeosis ranged from slightly carpelloid stamens to fully formed carpels and was seen in all flowers examined. These carpelloid stamens were usually attached to the central carpel. In addition, wing, keel, and, more rarely, standard petals contained green sepal-like tissue in streaks through the center of the organ. Fusion of the two keel petals was often disrupted (Figure 2C).
The stp-3 mutant has a more severe phenotype than does stp-1. Green streaks were always found through the center of the petals (Figure 2D), keel petals failed to fuse, and in the most severe cases, all petals were converted to sepal-like organs (Figure 2E). Stamen-to-carpel transformations were also observed: the two adaxial stamens were converted to carpels or sepal/carpel chimeric organs. The free adaxial stamen was often completely converted to a carpel. Abaxial stamens formed groups of two or three connected by tube tissue that normally joins the nine fused stamens in wild-type flowers. The central carpel was unaffected and able to produce seed after pollination. The floral phenotype of stp-3 mutants was consistent within populations grown together but differed in severity among populations grown at different times (e.g., cf. Figures 2D and 2E). This may reflect an environmental effect on expression, as noted for stp-1 (Monti and Devreux, 1969). Severity of petal and stamen transformation was related such that plants with more normal petal development also possessed more normal stamen development. Lateral shoots produced late on stp-3 mutant plants bore flowers with a more severe phenotype that occasionally produced an ectopic flower on an elongated pedicel (not shown). These ectopic flowers were primarily composed of sepals and sepaloid carpels.
Primary flowers (those borne directly on secondary inflorescences) of the most severe mutant, stp-4, always contained five sepals in the first whorl and a central carpel. The second whorl was composed of four to eight sepals. Within these, and surrounding the central carpel, were a variable number of additional carpels, carpel-sepal chimeric organs, or sepals. There was always one ectopic (secondary) flower on an elongated pedicel in primary flowers of stp-4 mutants (Figures 2F and 2G). This was found above the central carpel of the primary flower and was contiguous to the carpel base (Figure 2G). Where additional ectopic flowers were found, they surrounded the central carpel. Ectopic flowers had one to eight sepals outermost, which were usually in a whorled or partially spiraled arrangement (Figure 2G). Within this whorl, the flower was disorganized. Although occasionally following the pattern of primary flowers, ectopic flowers often consisted of irregularly placed sepals, carpelloid sepals, and carpels. Petals, sepaloid petals, staminoid petals, and stamens (single or in pairs) were also found in secondary and tertiary ectopic flowers in varying proportions but were never seen in primary flowers (data not shown). If stamens and petals were found, they usually occurred together, although rare stamens and/or petals were found in ectopic flowers that consisted mostly of sepaloid and carpeloid organs. Secondary flowers consisting entirely of spirally arranged sepals were also occasionally observed.
Secondary flowers also often possessed their own ectopic (tertiary) flowers (not shown). By this stage, the whorled arrangement of organs was completely abandoned. Outer organs of tertiary flowers were generally sepaloid; inner organs were variable and could be sepal-, carpel-, petal-, or stamen-like in appearance, or chimeras of these. Sepaloidy was most prevalent.
Pleiotropic Effects of stp-3 and stp-4
In addition to their profound effects on flower development, stp-3 and stp-4 mutations delayed the node of flower initiation (the node bearing the first secondary inflorescence; Table 1 and Figure 1). This delay was more pronounced under short-day (non-inductive) conditions (not shown). In a HL107 background, wild-type plants typically produce two flowers on the first secondary inflorescence. In contrast, secondary inflorescences of stp-4 plants regularly bore only a single flower such that, on average, there were 0.4 fewer “flowers” on stp-4 inflorescences (Table 1), suggesting a role for Stp in secondary inflorescence development. Indeterminacy of primary inflorescences was also affected by stp mutations (Table 1). Growth of the primary inflorescences of stp-3 and stp-4 plants was occasionally arrested with an apparently terminal flower (Figure 3A). Under an 18-hr photoperiod, these terminal flowers were produced after as few as five (although usually more) reproductive nodes had formed and were often associated with a simplification of the leaf from compound to unifoliate in stp-4 mutant plants (Figure 3A). Wild-type plants typically produced only four or five reproductive nodes before undergoing monocarpic senescence and apical arrest, whereas stp-3 and stp-4 mutants not producing a terminal flower often produced >12 reproductive nodes and retained potential for further growth (Table 1). The stp-4 mutation was also associated with a release from dormancy of the two buds present in leaf axils on the primary inflorescence (Figure 3B). These buds are normally suppressed in the primary inflorescence of wild-type peas so that only secondary inflorescences develop.
Table 1.
Characteristics of stp-3 and stp-4 Mutant Plants and Their Wild-Type Siblingsa
| Characteristic | Stpb | stp-3 | tc | Stpb | stp-4 | t |
|---|---|---|---|---|---|---|
| Stem length (cm) | 49.44 ± 0.65 | 43.41 ± 0.98 | 5.13 | 50.44 ± 0.39 | 45.20 ± 0.59 | 7.41 |
| Node of first leaf with three or more leaflets | 11.27 ± 0.11 | 15.75 ± 0.38 | 11.27 | 11.48 ± 0.11 | 20.60 ± 0.51d | 17.48 |
| Node of flower initiation | 15.95 ± 0.06 | 18.73 ± 0.20 | 13.31 | 16.29 ± 0.08 | 20.09 ± 0.21 | 16.84 |
| Total nodes | 20.24 ± 0.14 | 31.18 ± 0.62 | 17.21 | 20.56 ± 0.14 | 31.73 ± 0.68 | 16.09 |
| Flowerse | NDf | ND | — | 1.69 ± 0.06 | 1.28 ± 0.09 | 3.82 |
Data shown are mean ±se. Student's t test was calculated between wild-type and mutant siblings (P < 0.001 in all cases).
Stp values were derived from wild-type siblings from populations segregating for either stp-3 or stp-4: wild type, 41 individuals; stp-3 or stp-4, 11 individuals.
t, Student's t value.
Only five stp-4 plants produced leaves bearing three or more leaflets; this value is from these five plants.
Number of flowers present on the first secondary inflorescence scored from wild-type and stp-4 segregants from two separate populations: wild type, 55 individuals; stp-4, 29 individuals.
ND, not determined.
Figure 3.
Characterization of the stp Mutant Phenotype.
(A) Primary inflorescence of an stp-4 plant apparently terminating in a sepaloid flower (arrowhead). The final leaf produced on this primary inflorescence is unifoliate.
(B) Primary inflorescence from an stp-4 plant that has released additional axillary shoots from suppression. Three axillary shoots are visible at each node. Arrowhead 1 indicates the secondary infloresence bearing a flower. Arrowhead 2 shows a lateral shoot bearing a leaf and a terminal sepaloid flower. Arrowhead 3 indicates the developing third axillary bud. A similar pattern is seen in all three nodes illustrated.
(C) Seedling phenotype of stp-4, wild type (WT), and uni-224. A scale-like leaf in stp-4 mutants (arrowheads) replaces the first compound leaf produced at node 3 on wild-type plants. In contrast, uni-224 seedlings possess unifoliate leaves at all early nodes (arrow).
The stp-3 and stp-4 mutants also showed pleiotropic effects on leaf development. Normal heteroblastic development of pea leaves occurs through an increase in the number of pinnae up to a typical maximum of six tendrils and six leaflets (Wiltshire et al., 1994). Transition from two to four leaflets per leaf and four to six leaflets per leaf appears to be genetically controlled and occurs at specific nodes (Wiltshire et al., 1994). stp-3 and stp-4 mutations delayed production of the first foliage leaf from node 3 to node 4; wild-type plants normally possess two scale leaves, with the first compound leaf at node 3 (Figures 1 and 3C). Infrequently, the first compound leaf on stp-4 mutant plants occurred at node 5, and four scale leaves were produced. In addition, both mutants significantly delayed the transition from two to four leaflets per leaf (Table 1 and Figure 1), and some stp-4 plants never produced leaves bearing more than two leaflets. Neither stp-4 nor stp-3 single mutant plants ever produced leaves bearing five or six leaflets. An example of the effect of stp-4 on wild-type leaf development can be seen in Figure 4A.
Figure 4.
Interactions between stp-4 and Mutations Affecting Leaf and Inflorescence Development in Pea.
In each case, the stp-4 mutant is at left and the corresponding Stp segregant is at right.
(A) Primary inflorescences from an stp-4 single mutant and its wild-type sibling illustrating the effect of stp-4 on leaf development at the onset of flowering. The leaf from the mutant possesses only two leaflets and four tendrils, whereas five leaflets and five tendrils are present on the corresponding wild-type leaf.
(B) The most complex leaves born on tl stp-4 and tl Stp siblings. The tl mutant converts tendrils to leaflets. Fewer leaflets were produced on the stp-4 tl leaves than on the Stp tl leaves.
(C) and (D) af stp-4 and af Stp (JI1195) adult leaves, respectively, showing the reduction in leaf complexity caused by stp-4 on the af phenotype.
(E) Leaves from similar nodes of the triple mutant stp-4 af tl. These also show a simplification of the leaf by the stp-4 mutation when compared with the pleiofila, Stp af tl leaf form. In addition to reducing the ramification of the af tl leaf, addition of the stp-4 allele also resulted in an increase in leaflet size.
(F) Mature, primary inflorescences from stp-4 det and Stp det siblings. The det mutation results in the conversion of the primary inflorescence into a secondary inflorescence bearing flowers, which produce pods. This phenotype is also seen in the stp-4 background. The converted inflorescence is indicated by an arrow in each case.
(G) Secondary inflorescences from stp-4 veg2-2 and Stp veg2-2 plants. The proliferation of the secondary inflorescence that results from the veg2-2 mutation is apparent in the stp-4 mutant background; however, the secondary inflorescences terminate in a sepaloid flower (arrow).
Measurements of internode lengths revealed that stp-3 and stp-4 plants were marginally but significantly (P < 0.001) shorter than their wild-type siblings (Table 1). In pea, shorter internodes may result from fewer cells per internode, from smaller cells, or from a combination of the two (Murfet, 1990). Measurements of epidermal cell length from internodes of stp-3, stp-4, and wild-type siblings suggested that the reduced internode length of stp-3 and stp-4 plants resulted from a reduction in cell number at each internode and not from reduced cell length (Table 2). A similar reduction in cell number was found in leaf petioles of stp-3 and stp-4 plants in comparison with their wild-type siblings (Table 2), indicating that the reduction in cell number is not specific to the main axis. These results suggest that Stp may play some role in regulating cell proliferation.
Table 2.
Epidermal Cell Lengths (μm) and Cell Numbers from Internode 7–8 and Leaf Petiole from Node 8 of stp or uni Mutants and Their Wild-Type Siblings (mean ±se)
| Internode
|
Leaf Petiole
|
|||
|---|---|---|---|---|
| Genotype and t Valuea | Cell Length | Cell Number | Cell Length | Cell Number |
| Stpb | 452.5 ± 15.0 | 240.6 ± 12.2 | 376.6 ± 8.0 | 170.4 ± 3.8 |
| stp-3 | 467.2 ± 9.0 | 187.3 ± 5.8 | 369.8 ± 8.0 | 139.0 ± 2.9 |
| t Value | 0.84 | 3.94c | 0.60 | 6.57d |
| Stpb | 468.2 ± 9.0 | 219.6 ± 5.0 | 372.5 ± 5.4 | 171.6 ± 5.3 |
| stp-4 | 453.7 ± 14.0 | 194.1 ± 8.0 | 361.3 ± 5.8 | 131.0 ± 4.0 |
| t Value | 0.87 | 2.69e | 1.41 | 6.10d |
| Unib | 522.5 ± 32.7 | 206.0 ± 18.9 | NDf | ND |
| uni-224 | 505.3 ± 72.1 | 158.1 ± 6.3 | ND | ND |
| t Value | 0.36 | 2.4e | — | — |
Student's t value from the mutant and their wild-type siblings.
Stp and Uni values were derived from wild-type siblings of populations segregating for stp-3, stp-4, or uni-224.
P < 0.01.
P < 0.001.
P < 0.05.
ND, not determined.
Observed pleiotropic phenotypes were found in both stp-3 and stp-4 plants, and were typically correlated with severity of floral phenotype. In addition, these pleiotropic traits cosegregated with the floral phenotype in all crosses examined. Thus, it was concluded that leaf simplification, reduction in internode length, and effects on inflorescence development were pleiotropic effects of the stp mutant alleles and did not result from a second, unrelated mutation. The relationship between the observed phenotypic effects of stp-3 and stp-4 and their effects on cell number in leaf rachis and internode is unknown.
Interaction between Leaf Homeotic Mutants and stp
To clarify the effect of stp mutants on leaf development, we examined the leaf phenotype of stp-4 in three genotypic backgrounds: tendril-less (tl), in which pinnae are all leaflets; afila (af), in which pinnae are all tendrils; and the double mutant pleiofila leaf form (af tl double mutant). The stp-4 tl double mutant plants possessed fewer pinnae than did their wild-type siblings (Figure 4B). Counts of leaflet production in stp-4 tl and Stp tl plants showed that stp-4 reduced the maximum number of leaflets borne on a tl leaf (data not shown). af stp-4 plants had fewer primary and secondary ramifications typical of the af single mutant such that double mutant leaves typically consisted of simple tendrils arising from the central rachis (Figure 4C). In contrast, afila (af Stp) leaves bear compound proximal tendrils and simple tendrils distally (Lu et al., 1996). However, effects of stp-4 on leaf development were most pronounced in the double mutant af tl (pleiofila) leaf form (Figure 4E). Complex pinnae normally seen in pleiofila leaves were severely reduced in the triple mutant, which produced leaves consisting of three similar units bearing leaflets smaller than those on wild-type plants but larger than those normally present on pleiofila leaves (Figure 4E). The additive phenotype of stp-4, tl, and af mutants suggests that Stp acts independently of genes controlling the final form of pea leaves.
Interactions between stp and Inflorescence Identity Genes
The effect of stp-4 on primary and secondary inflorescence development was examined more closely in two mutant backgrounds affecting inflorescence identity in pea: determinate (det; Marx, 1986a; Singer et al., 1990) and vegetative2-2 (veg2-2; Reid et al., 1996). The det mutant promotes a secondary inflorescence identity in the primary inflorescence, often converting the apical meristem into a terminal stub (Singer et al., 1990; Figure 4F). Conversely, veg2-2 mutants bear indeterminate secondary inflorescences that are primary inflorescence–like and produce compound leaves (Reid et al., 1996; Figure 4G). Thus, Det and Veg2 may be involved in the specification of primary and secondary inflorescences, respectively. The inflorescence structure characteristic of det mutant plants was also seen in stp-4 det double mutant plants (Figure 4F), and det appeared unable to suppress the development of ectopic flowers within stp-4 primary flowers (Figure 4F). The production of terminal flowers in stp-4 plants was unaffected by veg2-2, and the leaf-bearing proliferating secondary inflorescences of stp-4 veg2-2 plants often terminated in an aberrant flower (Figure 4G). The simple additive phenotypes of the stp-4 det and the stp-4 veg2-2 double mutants suggest that Stp functions independently of Det and Veg2 and that therefore Stp is not involved in primary or secondary inflorescence identity.
Interactions between stp and uni
The effects of stp-4 on both leaf and flower development are very similar to those seen in unifoliata (uni) mutants of pea (Marx, 1987; Hofer et al., 1997). Epidermal strips revealed that the severe uni-224 allele also affects cell number in a manner similar to stp-3 and stp-4 (Table 2). Crosses between plants carrying uni-224 and stp-4 indicated that the two genes are not allelic, which raised the possibility that Stp and Uni were involved in the same developmental pathway. Segregation of stp-4 and uni-224 phenotypes in F2 and dihybrid F3 populations was in accordance with a 9:3:4 ratio of wild-type/stp-4/uni-224 (214 wild type, 72 stp-4, and 89 uni-224; 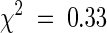 ; P > 0.8), indicating that uni-224 is completely epistatic to stp-4. The epistatic relationship of uni-224 to stp-4 encompassed effects of uni on leaf, flower, and inflorescence structure, including production of a small leaflet at node 2 (Figure 3C), which was present on all uni-224 segregants. The epistatic interaction and similar phenotype of the severe uni-224 and stp-4 mutants suggest that Uni and Stp act in the same developmental pathway. This possibility was further supported by the synergistic interaction between known weak alleles at Uni and Stp. The weakest mutant allele at Stp, stp-1, does not appear to affect leaf development, and its mutant phenotype is floral specific (Monti and Devreux, 1969; Figures 2B, 2C, and 5A). In contrast, the weakest described allele at Uni, uni-tac, primarily affects leaf development, although it can also affect flower development (Marx, 1986b; Figures 5A and 5B). An F2 population derived from the cross between the type lines carrying stp-1 and uni-tac segregated into four distinct classes: 65 wild type, 16 stp-1, 10 uni-tac, and 5 stp-1 uni-tac, corresponding to a 9:3:3:1 ratio (
; P > 0.8), indicating that uni-224 is completely epistatic to stp-4. The epistatic relationship of uni-224 to stp-4 encompassed effects of uni on leaf, flower, and inflorescence structure, including production of a small leaflet at node 2 (Figure 3C), which was present on all uni-224 segregants. The epistatic interaction and similar phenotype of the severe uni-224 and stp-4 mutants suggest that Uni and Stp act in the same developmental pathway. This possibility was further supported by the synergistic interaction between known weak alleles at Uni and Stp. The weakest mutant allele at Stp, stp-1, does not appear to affect leaf development, and its mutant phenotype is floral specific (Monti and Devreux, 1969; Figures 2B, 2C, and 5A). In contrast, the weakest described allele at Uni, uni-tac, primarily affects leaf development, although it can also affect flower development (Marx, 1986b; Figures 5A and 5B). An F2 population derived from the cross between the type lines carrying stp-1 and uni-tac segregated into four distinct classes: 65 wild type, 16 stp-1, 10 uni-tac, and 5 stp-1 uni-tac, corresponding to a 9:3:3:1 ratio (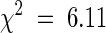 ; P > 0.1). Leaves of the double mutant plants were simplified and rarely possessed the subterminal tendrils characteristic of uni-tac (Figure 5A). The double mutant plants also had flowers consisting entirely of sepals and carpels (Figure 5C). Sepal/carpel mosaic organs were noted, and ectopic flowers were also seen. Leaf and flower phenotypes of the putative stp-1 uni-tac double mutant were confirmed in the F3 progeny from stp-1 segregants. The stp-1 and uni-tac single mutants segregating in the cross produced typical flower and leaf phenotypes (Figure 5B). The phenotype of stp-1 uni-tac double mutant flowers was reminiscent of that produced by severe stp and uni mutants, and the leaf phenotype was similar to those produced by uni mutants, suggesting that Stp and Uni act together to regulate plant development.
; P > 0.1). Leaves of the double mutant plants were simplified and rarely possessed the subterminal tendrils characteristic of uni-tac (Figure 5A). The double mutant plants also had flowers consisting entirely of sepals and carpels (Figure 5C). Sepal/carpel mosaic organs were noted, and ectopic flowers were also seen. Leaf and flower phenotypes of the putative stp-1 uni-tac double mutant were confirmed in the F3 progeny from stp-1 segregants. The stp-1 and uni-tac single mutants segregating in the cross produced typical flower and leaf phenotypes (Figure 5B). The phenotype of stp-1 uni-tac double mutant flowers was reminiscent of that produced by severe stp and uni mutants, and the leaf phenotype was similar to those produced by uni mutants, suggesting that Stp and Uni act together to regulate plant development.
Figure 5.
Interaction between Weak Alleles at stp and uni.
(A) Flowers and adult leaves from stp-1 Uni (stp-1), stp-1 uni-tac, and Stp uni-tac (uni-tac) siblings. stp-1 and uni-tac are known to be weak alleles; however, stp-1 uni-tac plants bore leaves that were simpler than those of either single mutant.
(B) uni-tac flower with unfused keel petals (arrow).
(C) stp-1 uni-tac double mutant flower. The arrows indicate petals converted to sepals. c, carpel.
The leaf and floral phenotype of the stp-1 uni-tac double mutant is highly reminiscent of the severe uni-224 mutant.
Molecular Characterization of Stp
The floral phenotypes of stp mutants and the genetic interactions between stp and uni mirror those described for fim in Antirrhinum and ufo in Arabidopsis (Simon et al., 1994; Levin and Meyerowitz, 1995; Wilkinson and Haughn, 1995; Ingram et al., 1997). This raised the possibility that Stp represented the pea ortholog of Fim and UFO. Homology between Uni and Flo/LFY (Hofer et al., 1997) and the role of Uni in leaf development also support homology between Stp and Fim/UFO. Furthermore, observed effects of stp-3 and stp-4 mutations on cell division (Table 2) are consistent with the proposed role of Fim and UFO in cell proliferation (Wilkinson and Haughn, 1995; Ingram et al., 1997; Meyerowitz, 1997). Therefore, the relationship between Stp and Fim/UFO was examined at a molecular level.
A cDNA clone containing a complete open reading frame and flanked by putative untranslated 5′ and 3′ regions and a poly(A) tail was isolated from a pea flowering-apex cDNA library probed with a polymerase chain reaction (PCR)–derived fragment from the conserved 3′ coding region of UFO. The complete nucleotide and deduced amino acid sequences of this clone are displayed in Figure 6. Database searches revealed significant similarity between the isolated cDNA and Antirrhinum and Arabidopsis Fim and UFO sequences, and to Imp-FIM from impatiens (Pouteau et al., 1997). Cross-hybridizing bands were not detected on DNA gel blots probed with peafim and washed at low stringency, indicating that this clone was present in the pea genome as a single copy. This cDNA clone was named peafim and is likely to represent the pea ortholog of Fim/UFO. Alignment of amino acid sequences, presented in Figure 7, revealed large areas of conservation between pea, Antirrhinum, and Arabidopsis sequences and high overall amino acid identity between peafim and Fim (63.7%), peafim and UFO (61.1%), and across the 179–amino acid Imp-FIM fragment (62.1%).
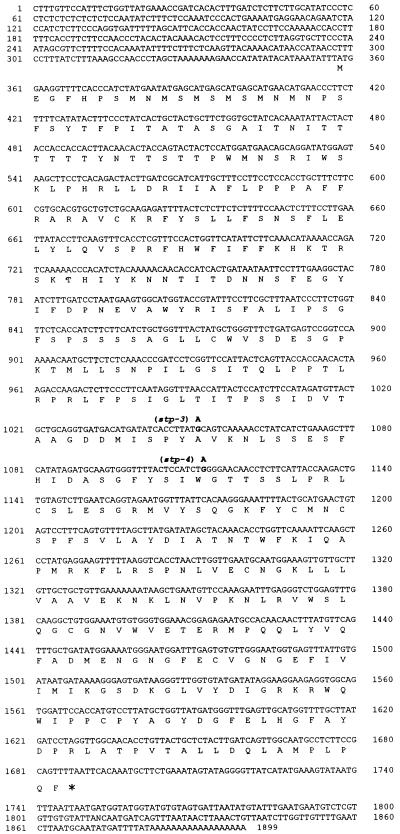
Figure 6.
Nucleotide and Predicted Amino Acid Sequence from the cDNA Clone peafim.
The positions of the point mutations present in the stp-3 and stp-4 mutant alleles are shown in boldface. The G-to-A substitution in stp-4 is predicted to result in premature termination of the protein. The stp-3 mutation is predicted to result in a nonconservative substitution of an alanine with a threonine residue. The asterisk denotes the stop codon.
Figure 7.
Alignment of the Deduced Amino Acid Sequences Encoded by Stp, Fim, UFO, and Imp-FIM (Impat).
Amino acids present in at least three sequences are shaded; hyphens indicate gaps due to alignment. The F-box motif is underlined.
To investigate the relationship between peafim and Stp, we first mapped the peafim clone onto a pea molecular map (see Hall et al., 1997). Peafim showed strong linkage to characters on pea linkage group VII, shown in Figure 8A, and fell between Cab1 (for chlorophyll a/b binding protein) and Rrn2 (for rRNA gene cluster2). Linkage analysis had previously placed stp in linkage group VII (Monti, 1970). This map location was confirmed using the more severe allele stp-4. Stp showed strong linkage (P < 0.0001) with both wa, a waxless mutant, and the isozyme locus Skdh with a map sequence of Skdh (10 centimorgans [cM]) stp (4 cM) wa (Figure 8C). Figure 8 shows an alignment of the classical and molecular maps, which indicates that peafim and Stp reside in the same region of linkage group VII in pea. Cosegregation analysis confirmed close linkage of Stp and peafim: a restriction fragment length polymorphism detected by the peafim cosegregated with 10 stp-4 mutant plants segregating in an F2 population of 50 individuals. Thus, the map position and cosegregation analysis supported the proposed relationship between Stp and peafim.
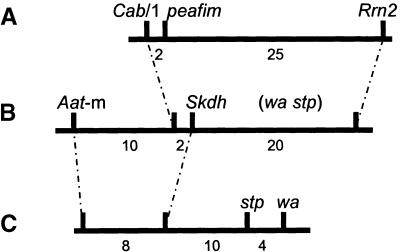
Figure 8.
Map Location of stp and peafim.
(A) Restriction fragment length polymorphism map position of peafim.
(B) Same region from the consensus pea map (Weeden et al., 1996), which has only an approximate position for stp and wa.
(C) Classical mapping of stp.
Dotted lines connect common markers, and only informative comparisons are shown. Map distance data for the classical map are approximate only.
To resolve the relationship between peafim and stp, we amplified peafim from genomic DNA of isogenic Stp (HL107), stp-3, and stp-4 lines. Sequence analysis of amplification products from stp-3 and stp-4 mutants revealed different single-base substitutions compared with Stp from HL107. The stp-4 mutant plants had a G-to-A nucleotide change at base 1111 that resulted in a stop codon at position 252 in the deduced amino acid sequence (Figure 6). The stp-3 sequence had a G-to-A nucleotide change at base 1051, which would result in a nonconservative alanine to threonine substitution at position 232 in the predicted amino acid sequence (Figure 6). This residue is conserved in all four sequences (Figure 7) and is therefore likely to be important for normal function of peafim protein. The nucleotide changes in peafim sequence from stp-3 and stp-4 plants and their predicted effects on the translated protein reflect the relative severity of the two mutant phenotypes.
Expression of peafim
Because peafim transcripts were undetectable by RNA gel blot analysis, we analyzed the spatial distribution of peafim expression by using in situ hybridization on serial sections of 15-day-old (vegetative) seedling apices and flowering apices of wild-type pea plants (Figure 9). Expression of peafim within floral meristems (Figures 9A and 9B) was comparable to that described for UFO in Arabidopsis and for Fim in Antirrhinum (Simon et al., 1994; Ingram et al., 1995, 1997; Lee et al., 1997) and occurred early during floral development. This expression preceded the appearance of the common petal/stamen primordia (defined as stage 5 by Ferrandiz et al. [1999]) and formed a ring around the central dome of floral primordia in the region of incipient common primordia (Figures 9A and 9B, f1). After initiation of these primordia, Stp expression formed a cup shape delineating presumptive petal primordia (Figures 9A and 9B, f2). Late expression of peafim in flowers, like that of Fim and UFO, was confined to the base of petal primordia. The similar expression patterns of peafim, UFO, and Fim in flowers are consistent with the similar floral phenotypes of ufo, fim, and stp mutants.
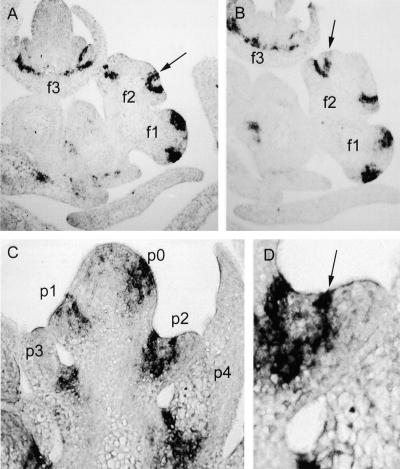
Figure 9.
In Situ Hybridization Analysis of Stp Expression in Wild-Type Pea Flowers and Seedlings.
(A) and (B) Serial sections through the same sample separated by 16 μm. Stp expression in the youngest flower (f1) occurs in a ring throughout the region of the incipient common primordia. This pattern appears as two discrete patches of expression in these adjacent sections. This expression becomes restricted to the boundary between sepal and common primordia (f2) and delineates each primordium into petal and stamen primordia (arrows). Later in flower development (f3), Stp expression becomes restricted to the base of the petals.
(C) Stp expression was also detected in the apical meristem and leaf axils of wild-type seedlings. Expression of Stp occurred throughout the incipient leaf primordium (p0) but became restricted to the leaf axil by p4. p0 to p4 identify leaf primordia initiated at plastochrons 1 to 4, respectively.
(D) Enlargement of leaf primordium p2 showing strong Stp expression within the leaf (arrow). This expression appears to delineate the developing leaflet primordia.
Expression of peafim was also found in the axils of leaf primordia (Figure 9C). Serial sections indicated that a semicircular wedge of expression existed in leaf axils up until at least plastochron 7 (P7), after which it became barely discernible (not shown). Expression was also observed within leaf primordia at P2, potentially representing a delineation of the first pair of leaflet primordia (Figure 9D). Weak expression was also detected within the apical meristem (Figure 9C). The presence of peafim transcript in flowers, apical meristem, leaf axils, and leaf primordia is consistent with the pleiotropic effects of the two stp mutations.
The map position of peafim and Stp, cosegregation analysis, and sequence analysis of the stp mutations support orthology between Stp, UFO, and Fim. Identification of such homologies provides an opportunity to compare pathways proposed to regulate development in pea, Arabidopsis, and Antirrhinum.
DISCUSSION
We have shown that Stp influences development of leaves, inflorescences, and flowers of pea. These pleiotropic effects are summarized in Figure 1. Analysis of a cDNA ortholog of Fim and UFO from the garden pea has provided strong evidence for orthology among Stp, Fim, and UFO. Although the floral phenotypes of stp, fim, and ufo mutants are very similar, the leaf phenotype associated with stp mutations is unique to pea. Expression of Stp within leaf primordia suggests that the leaf phenotype of stp-3 and stp-4 is indeed a consequence of those mutations. Stp acts synergistically with Uni but is genetically independent of homeotic genes controlling leaf and inflorescence development.
Stp and Flower Development
All four stp mutant alleles are characterized by homeotic changes within the flower (Monti and Devreux, 1969; Ferrandiz et al., 1999; this work). The phenotype of stp-1 consists entirely of floral homeotic changes characteristic of class B floral homeotic mutants (Ferrandiz et al., 1999; this work). Sepaloid tissue in petals of stp-1 flowers is also seen in weak Fim mutants (Simon et al., 1994). The severe floral phenotype of stp-4 mutants is also very similar to those of severe fim mutants. Both tend to produce flowers composed primarily of sepals, although petalloid and carpelloid tissues are also produced. The production of normal carpels in stp-4 mutants possibly reflects the early production of carpel primordia in pea (Tucker, 1989) or an inability of Stp to regulate C-class homeotic gene activity. The severe stp-4 mutant appeared less like severe ufo mutants of Arabidopsis because the filamentous structures and reduced or empty flowers were not produced on stp-4 mutant plants (Levin and Meyerowitz, 1995; Wilkinson and Haughn, 1995). However, homeotic changes within the flower are comparable, and termination of ufo mutant inflorescences by a carpelloid structure was also reflected in the determinancy of stp-3 and stp-4 primary inflorescences (Figure 3A). Furthermore, the delay in node of flower initiation caused by stp-3 and stp-4 mutations (Table 1) may be considered equivalent to the increase in coflorescence production in ufo mutants (Wilkinson and Haughn, 1995), because both represent a delay in production of the first flower. Whether the delay in flower initiation in stp mutants results from a homeotic conversion of early secondary inflorescences to vegetative shoots or from a delay in attaining reproductive competence is unclear.
The most significant difference among the floral phenotypes of Stp, UFO, and Fim mutants is the production of ectopic flowers within the primary flower (Figure 2). Ectopic flowers have not been described in ufo mutant flowers. Flowers of severe Fim mutants produce secondary flowers in the axils of sepals, and their overall phenotype is that of a proliferous inflorescence (Simon et al., 1994; Ingram et al., 1997). Flowers produced on stp-3 or stp-4 plants did not possess typical characteristics of pea inflorescences (e.g., a terminal stub), although the production of ectopic flowers may indicate some inflorescence identity. Placement of these ectopic flowers was consistent with their derivation from common petal/stamen primordia, as suggested by Ferrandiz et al. (1999). The position of the last common primordium to differentiate (e.g., see Tucker, 1989; Ferrandiz et al., 1999) corresponds to the position always occupied by an ectopic flower on stp-4 mutants and the position occasionally occupied by an ectopic flower on stp-3 mutants. This primordium, as the last to differentiate into individual petal and stamen primordia, appears to be more sensitive to loss of Stp activity. Presumably, the absence of normal Stp activity in stp mutants led to the common primordia reiterating an earlier program—that of the flower—rather than to dividing and differentiating into the petals and stamens. This possibility is supported by the presence of Stp transcripts within the common primordia (Figures 9A and 9B). The proposed role Stp plays in the differentiation of these primordia and the homeotic changes seen in stp mutant flowers is consistent with the proposed role Fim and UFO play in regulating class B gene activity.
Stp and Leaf Development
That Stp is essential for normal leaf development is seen clearly by a loss of the first foliar leaf at node 3, retarding of heteroblastic development, and simplification of the last leaves produced on stp mutants. These effects are most clearly seen in combination with the leaf homeotic mutants af and tl (Figures 4B, 4C, and 4E); however, the additive phenotypes of the double and triple mutants suggest that Stp acts independently of Af and Tl.
Although stp-4 mutants have a floral phenotype very similar to that seen in severe uni mutants, stp-4 plants always produce pinnate adult leaves. In contrast, severe uni mutants bear simple to trifoliate leaves (Hofer et al., 1997). Sequence analysis suggests that the stp-4 mutant protein would comprise only the N-terminal half, suggesting a severe disruption of protein function (by comparison, this would have a greater effect on the protein than ufo-2 and ufo-5 alleles, which have a strong mutant phenotype [Lee et al., 1997]). Therefore, rather than assume that stp-4 is insufficiently severe to simplify the leaves, we suggest that Stp is not an essential requirement for the difference between simple and compound leaves. Rather, Stp appears to increase complexity of an already compound leaf, an effect seen most clearly in the pleiofila (af tl double mutant) leaf form (Figure 4E). Either Uni is sufficient alone or additional genes must act with Uni to produce a pinnate leaf.
Expression of Stp in the base and axils of leaf primordia might explain differences between leaf phenotypes of uni and stp mutants. Stp is not expressed in distal regions of wild-type leaf primordia older than P1, and stp mutants do not affect the identity of the terminal structure, which retains tendril identity. In contrast, Uni transcripts are detected at the apex of wild-type leaf primordia until P4 (Hofer et al., 1997), and the terminal tendril is lost in uni mutants. Although Uni and Stp interact genetically (e.g., Figure 5A), their expression patterns do not completely overlap, particularly within older leaf primordia (Hofer et al., 1997; Figure 9C). It is possible that early overlapping expression of Stp and Uni within leaf primordia at P1 and P2 may be sufficient to coordinate their mutual control of compound leaf proliferation. Alternately, non-cell autonomy of the Flo protein (Carpenter and Coen, 1995; Hantke et al., 1995) suggests that Uni could exert its influence from a distance.
Despite the severe effect uni mutations have on leaf development in pea, differences between the leaves of Arabidopsis and pea appear to depend more on differences in gene expression between UFO and Stp rather than on differences between LFY and Uni. UFO transcripts are not detected in Arabidopsis leaves, and ufo mutations do not affect leaf development (Levin and Meyerowitz, 1995; Wilkinson and Haughn, 1995; Lee et al., 1997). Overexpression of UFO in Arabidopsis does result in a lobed leaf phenotype that is dependent on the presence of a wild-type LFY allele (Lee et al., 1997). However, overexpression of UFO does not produce a (pea-like) compound leaf in Arabidopsis, consistent with the proposal that expression of Stp in leaves is not sufficient to define the difference between a compound and a simple leaf but is only able to increase leaf complexity. Thus, the basic difference between a compound leaf and a simple leaf depends on alternate gene activities.
Fim homologs have only been characterized in simple-leaved plants and in pea, which has specialized compound leaves. In particular, a Fim homolog has not yet been identified in tomato, and it remains possible that mutants in tomato fim would not have a leaf phenotype, particularly because fa mutations have a limited effect on the tomato compound leaf (Molinero-Rosales et al., 1999). Thus, the role Fim homologs play in other compound-leafed plants, if any, is yet to be determined.
Biochemical Activity of the Fim Homologs
The predicted amino acid sequences of Stp, Fim, and UFO show a high degree of conservation around a motif known as the F-box (Bai et al., 1996; Figure 7). F-box proteins have been demonstrated to be integral components of the SCF complex (for Skp1, Cdc53, F-box proteins; reviewed in Krek, 1998; Patton et al., 1998; Galan and Peter, 1999). These act as ubiquitin protein ligases that, in combination with a ubiquitin conjugating enzyme, ubiquitylate specific phosphorylated proteins, targeting them for proteolysis. The F-box protein determines selectivity of SCF complexes, whereas Skp1 and Cdc53 are common components that assemble into various SCFs containing differing F-box protein partners. A dynamic equilibrium potentially exists between a pool of Skp and Cdc53 components and the various F-box proteins (Patton et al., 1998; Galan and Peter, 1999). Three fimbriata-associated proteins (FAPs) were isolated from Antirrhinum in a yeast two-hybrid screen using Fim as bait. These three FAP proteins share strong homology with Skp1 from yeast and p19skp1 from humans (Ingram et al., 1997). In a similar screen in Arabidopsis, two ARABIDOPSIS SKP-like (ASK) proteins interacting with UFO were isolated (Samach et al., 1999). ASK proteins also share homology to Skp1. These results suggest that Fim and UFO proteins, and by extrapolation the Stp protein, may participate in an SCF complex. Thus, it is possible that these plant F-box genes are involved in the targeting of proteins for ubiquitin-mediated proteolysis. F-box proteins were first identified through their role in regulating cell division in yeast and humans (Bai et al., 1996; Krek 1998), and the observed effect of stp mutants on cell division (Table 2) may suggest that Stp shares this function. However, F-box proteins are not restricted to forming SCF complexes, nor are they restricted to cell-cycle control, and other potential modes of action exist (Krek, 1998; Patton et al., 1998). Confirming the biochemical activity of Fim homologs and determining their potential target proteins remain a priority.
Fim and Flo Orthologs
The nature of the interaction between Fim orthologs (Stp, Fim, and UFO) and Flo orthologs (Uni, Flo, and LFY) is unclear. Flo orthologs potentially act as transcription factors, (Parcy et al., 1998), whereas Fim orthologs may control levels of specific proteins by targeting them for ubiquitin-mediated proteolysis. Despite this, results from pea, Antirrhinum, and Arabidopsis are consistent in suggesting that Fim orthologs function in concert with Flo orthologs to control plant development. Within the flower, Fim and Flo orthologs regulate expression of class B floral homeotic genes and influence development of the floral primordium. They may play a role in inflorescence development, a function seen most clearly by the delay in production of flowers, and in terminal flower production in mutants in Flo and Fim orthologs. In addition, Fim and Flo orthologs are central to compound leaf development in pea. Results from pea suggest that Uni and Stp may regulate development of meristems, irrespective of their eventual identity, and therefore play a central role in determining the final form of a pea plant.
METHODS
Sources of Mutant Lines
Two previously uncharacterized mutations affecting flower development were isolated from an ethyl methanesulfonate mutagenesis program on HL107 (Pisum sativum cv Torsdag) conducted by J.L. Weller (University of Tasmania, Hobart; for details, see Weller et al., 1997). The stp-4 mutant was identified from a single M2 population grown under an 8-hr photoperiod, and a single wild-type M2 sibling, found to be heterozygous for stp-4, was the progenitor of the mutant line used in these analyses. The stp-3 allele was originally isolated as a large-scale-leaf mutant from a screen of seedling phenotypes. This plant was found to possess floral homeotic defects and was rescued by crossing into the HL107 background. This mutagenesis program also provided an additional allele at Uni, uni-224. Allelism with uni was confirmed by crossing the mutant line with JI1396 (uni-tac); the two F1 plants possessed a uni-tac phenotype. Seed from these three populations were supplied by J.L. Weller. As mostly sterile mutants, stp-3, stp-4, and uni-224 alleles were maintained as heterozygous lines. Type lines for stp-1 (JI2163) and uni-tac (JI1396) were supplied by Mike Ambrose (John Innes Centre). All other lines used were from the pea germplasm collection at Hobart. Type lines segregating for stp-3 and stp-4 were deposited into the Hobart germplasm collection as lines HL289 and HL288, respectively.
Characterization and Genetic Interactions
Characterization of the two new stp mutant phenotypes was performed using mutant plants segregating from first and second backcrosses to the initial line pea cultivar Torsdag (HL107). Flower number per inflorescence (Table 1) was scored from two separate populations used in analyses of interaction between stp-4 and pim, and stp-4 and veg2-2. veg2-2 and pim segregants were not included in this analysis.
Crosses between homozygous stp-4 and heterozygous Uni/uni-244 plants were made to determine the relationship between uni and stp. Interaction between veg2-2 and stp-4 was examined in the cross between line Wt16123 (veg2-2) and homozygous stp-4 segregants from the original mutant line. Interactions among stp-4, af, and tl were examined in a single cross between HL117 (af tl) and an stp-4 plant from the first backcross. Interaction between stp-4 and det was examined in a cross between HL245 (r det veg1) and an stp-4 plant from the original mutant line. The det and r loci are tightly linked (Marx, 1986a), and only wrinkled (r) F2 seed were sown. The stp segregants in these populations were identified at the seedling stage by the loss of compound leaf at node 3 (e.g., Figure 3C), and af, tl, det, and veg2-2 segregants were clearly identifiable by their characteristic mutant phenotypes.
Linkage between stp and wa was examined in a cross between HL6 (Stp wa Sn) and a homozygous stp-4 plant (stp-4 Wa Sn) from the original population. Linkage was confirmed in a cross between an stp-4 wa Sn Aat3-F Skdh-F F3 recombinant and HL59 (Stp Wa sn Aat3-S Skdh-S). Recombination fractions were calculated using the product ratio method (Stephens, 1939), and joint segregation chi-squares were calculated using a 2 × 2 contingency table.
In Hobart, plants were grown one or two per 14-cm slimline pot in a 1:1 mix of vermiculite and dolerite chips topped with sand/peat potting mix. All plants were grown in a greenhouse under an 18-hr photoperiod consisting of natural daylight extended before dawn and after dusk, with a mixture of fluorescent and incandescent bulbs providing 25 μmols m−2 sec−1 at pot top. Plants used in the cosegregation analysis were grown at the John Innes Centre as described by Hofer et al. (1997).
Epidermal Strips
Epidermal strips (Table 2) were taken from internode 7–8; the leaf petiole (between the stem and first leaflet pair) was taken from node 8 from six stp-3 and stp-4 plants, and six of their wild-type siblings segregating in the second backcross to HL107. Samples were also taken from internode 7–8 from six uni-224 segregants and their wild-type siblings. Cell numbers were calculated for each plant by dividing internode or petiole length by the average of 10 cell lengths that were measured at random from that structure.
Isolation and Characterization of the peafim cDNA
Plaques (200,000) of a λ Zap cDNA library (Stratagene), prepared from RNA isolated from flowering shoot apices of JI813 (HL51y), were screened with a 580-bp polymerase chain reaction (PCR) fragment spanning nucleotides 244 to 824 of the Arabidopsis UFO gene (Ingram et al., 1995). This fragment was produced from plasmid PJAM 180, which contains a 3.4-kb genomic DNA insert containing the entire UFO coding region (kindly supplied by G. Ingram, John Innes Centre), using two primers specific to the Arabidopsis sequence: 5′ Fim oligo, TTCTCCAACACCTTCCTCGA; and 3′ Fim oligo, ACGCTAAAAGGGCTATAGTTCAT.
Plasmid and PCR-derived fragments were sequenced using a Perkin Elmer 9600 thermal cycler and dye terminator technology (Applied Biosystems International, Foster City, CA), according to the manufacturer's instructions. Sequences obtained were examined using Sequence Navigator (version 1.0; Applied Biosystems), SeqVu (version 1.0.1; Garvan Institute, Sydney, Australia), and Clustalw (version 1.5). The alignment in Figure 7 was produced using GCG10 localpileup and prettybox functions. Nucleotide and amino acid sequence data of the peafim clone have a GenBank accession number of AF004843.
For cosegregation analyses between stp-4 and peafim, total genomic DNA was isolated from HL107, HL111, and 50 F2 plants from a cross between HL111 and the original mutant line, following the method of Ellis (1994). For cDNA synthesis, total RNA was isolated from HL107 (Stp), JI2163 (stp-1), and stp-3 and stp-4 segregants from HL 289 and HL288, following the method outlined by Michael et al. (1996). Poly(A)+ RNA was purified with an mRNA isolation kit (Boehringer Mannheim) using biotin-labeled oligo(dT)20 and streptavidin magnetic particles, as described in the manufacturer's protocol. cDNA was synthesized from this poly(A)+ RNA, using Gibco BRL Superscript preamplification system (Life Technologies) for first-strand cDNA synthesis, following the manufacturer's instructions, except that 0.5 μM oligonucleotide SKTTT (5′-CGCTCTAGAACTAGTGGATCCTTTTTTTTTTTTTTTTT-3′) was used as a starting point for cDNA synthesis. Uni (XM 7175+) and uni (XM 7175−) cDNA was synthesized from total RNA using a T17 primer and M-MLV reverse transcriptase (Gibco BRL).
Oligonucleotides designed specifically to match regions of the 5′ and 3′ untranslated region of peafim sequence were used in PCR analysis of total genomic DNA and cDNA from mutant and wild-type lines: for peafimV.1, 5′-CTGAAAATGAGGAACAGAATCTACC-3′ (98 to 122); and peafimIII.1, 5′-CATATTAATCACTACACATACCATACC-3′ (1754 to 1780). The numbers in parentheses refer to nucleotide positions shown in Figure 6; the sequence of peafimIII.1 is the reverse complement of the sequence given in Figure 6. PCR products produced from genomic DNA from Stp, stp-3, and stp-4 plants were ligated into pGEM-T vector (Promega) and sequenced as described above. Wild-type and mutant sequences were confirmed in a second and third PCR product that were purified for direct sequencing using a QIAquick Gel extraction kit (Qiagen).
In Situ Hybridization
In situ hybridization was performed on sections of flowering and vegetative pea apices from HL107, as described previously (Hofer et al., 1997). The digoxygenin-labeled sense and antisense probes were made using a cloned wild-type genomic PCR product without the poly(A) tail present in the cDNA clone. Tissue was fixed in 4% formaldehyde overnight, dehydrated in an ethanol series, cleared in Histoclear, and embedded in Paramounat extra wax. Serial sections (8 μm) were attached to poly l-lysine–coated slides and probed with either sense or antisense digoxygenin-labeled peafim probes. Slides were developed using alkaline phosphatase–conjugated anti-digoxygenin antibodies, 5-bromo-4-chloro-3-indolyl phosphate, and nitroblue tetrazolium and left for 12 to 18 hr. No signal was detected using the sense probe (not shown).
Acknowledgments
The authors thank Jim Weller for supplying the original mutant seed; Diane Lester and Noel Ellis for assistance with the molecular analysis of Stp; and Ian Cummings, Tracey Jackson, Jodie van de Kamp, Sarah Scott, and Nadine Donovan for technical assistance. We also thank Noel Ellis, Trine Juul, Guy Wheeler, and Caroline Middlebrook for comments on the manuscript.
References
- Bai, C., Sen, P., Hofmann, K., Ma, L., Goebl, M., Harper, J.W., and Elledge, S.J. (1996). SKP1 connects cell cycle regulators to the ubiqitin proteolysis machinary through a novel motif, the F-box. Cell 86 263–274. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., Drews, G.N., and Meyerowitz, E.M. (1991). Genetic interactions among floral homeotic genes of Arabidopsis. Development 112 1–20. [DOI] [PubMed] [Google Scholar]
- Carpenter, R., and Coen, E.S. (1995). Transposon induced chimeras show that floricaula, a meristem identity gene, acts non-autonomously between cell layers. Development 121 19–26. [DOI] [PubMed] [Google Scholar]
- Coen, E.S., and Meyerowitz, E.M. (1991). The war of the whorls: Genetic interactions controlling flower development. Nature 353 31–36. [DOI] [PubMed] [Google Scholar]
- Coen, E.S., Romero, J.M., Doyle, S., Elliot, R., Murphy, G., and Carpenter, R. (1990). floricaula: A homeotic gene required for flower development in Antirrhinum majus. Cell 63 1311–1322. [DOI] [PubMed] [Google Scholar]
- Ellis, T.H.N. (1994). Approaches to the genetic mapping of pea. In Modern Methods of Plant Analysis, H.-F. Linskens and J.F. Jackson, eds (Berlin: Springer-Verlag), pp. 117–160.
- Ferrandiz, C., Navarro, C., Gomez, M.D., Canas, L.A., and Beltran, J.P. (1999). Flower development in Pisum sativum: From the war of the whorls to the battle of the common primordia. Dev. Genet. 25 280–290. [DOI] [PubMed] [Google Scholar]
- Galan, J.M., and Peter, M. (1999). Ubiquitin dependent deregulation of multiple F-box proteins by an autocatalytic mechanism. Cell Biol. 96 9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, K.J., Parker, J.S., Ellis, T.H.N., Turner, L., Knox, M.R., Hofer, J.M.I., Lu, J., Ferrandiz, C., Hunter, P.J., Taylor, J.D., and Baird, K. (1997). The relationship between genetic and cytogenetic maps of pea. II. Physical maps of linkage mapping populations. Genome 40 755–769. [DOI] [PubMed] [Google Scholar]
- Hantke, S.S., Carpenter, R., and Coen, E.S. (1995). Expression of floricaula in single cell layers of periclinal chimeras activates downstream homeotic genes in all layers of floral meristems. Development 121 27–35. [DOI] [PubMed] [Google Scholar]
- Hofer, J., Turner, L., Hellens, R., Ambrose, M., Matthews, P., Michael, A., and Ellis, N. (1997). Unifoliata regulates leaf and flower morphogenesis in pea. Curr. Biol. 7 581–587. [DOI] [PubMed] [Google Scholar]
- Huijser, P., Klein, J., Lönnig, W.E., Meijer, H., Saedler, H., and Sommer, H. (1992). Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS-box gene Squamosa in Antirrhinum majus. EMBO J. 11 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, G.C., Goodrich, J., Wilkinson, M.D., Simon, R., Haughn, G.W., and Coen, E.S. (1995). Parallels between UNUSUAL FLORAL ORGANS and Fimbriata, genes controlling flower development in Arabidopsis and Antirrhinum. Plant Cell 7 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, G.C., Doyle, S., Carpenter, R., Schultz, E.A., Simon, R., and Coen, E.S. (1997). Dual role for fimbriata in regulating floral homeotic genes and cell division in Antirrhinum. EMBO J. 16 6521–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek, W. (1998). Proteolysis and the G1-S transition: The SCF connection. Curr. Opin. Genet. Dev. 8 36–42. [DOI] [PubMed] [Google Scholar]
- Krizek, B.A., and Meyerowitz, E.M. (1996). The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122 11–22. [DOI] [PubMed] [Google Scholar]
- Lee, I., Wolfe, D.S., Nilsson, O., and Weigel, D. (1997). A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr. Biol. 7 95–104. [DOI] [PubMed] [Google Scholar]
- Levin, J.Z., and Meyerowitz, E.M. (1995). UFO: An Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 7 529–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, B., Villani, P.J., Watson, J.C., DeMason, D.A., and Cooke, T.J. (1996). The control of pinnae morphology in wildtype and mutant leaves of the garden pea (Pisum sativum L.). Int. J. Plant Sci. 157 659–673. [Google Scholar]
- Makasheva, R.K. (1983). The Pea. (New Delhi: Oxonian Press Pvt. Ltd.)
- Mandel, M.J., Gustafson-Brown, C., Savidge, B., and Yanofsky, M.F. (1992). Molecular characterisation of the Arabidopsis floral homeotic gene APETALA1. Nature 377 522–524. [DOI] [PubMed] [Google Scholar]
- Marx, G.A. (1986. a). Linkage relationship of Curl, Orc and ‘Det’ with markers on chromosome 7. Pisum Newslett. 18 45–48. [Google Scholar]
- Marx, G.A. (1986. b). Tendrilled acacia (tac) an allele at the uni locus. Pisum Newslett. 18 49–52. [Google Scholar]
- Marx, G.A. (1987). A suite of mutants that modify pattern formation in pea leaves. Plant Mol. Biol. Rep. 5 311–355. [Google Scholar]
- Meyerowitz, E.M. (1997). Genetic control of cell division patterns in developing plants. Cell 88 299–308. [DOI] [PubMed] [Google Scholar]
- Michael, A.J., Hofer, J.M.I., and Ellis, T.H.N. (1996). Isolation by PCR of a cDNA clone from pea petals with similarity to petunia and wheat zinc finger proteins. Plant Mol. Biol. 30 1051–1058. [DOI] [PubMed] [Google Scholar]
- Molinero-Rosales, N., Jamilena, M., Zurita, S., Gómez, P., Capel, J., and Lozano, R. (1999). FALSIFLORA, the tomato orthologue of FLORICAULA and LEAFY, controls flowering time and floral meristem identity. Plant J. 20 685–693. [DOI] [PubMed] [Google Scholar]
- Monti, L.M. (1970). Linkage studies on four induced mutants of pea. Pisum Newslett. 2 21–22. [Google Scholar]
- Monti, L.M., and Devreux, M. (1969). Stamina pistilloida: A new mutation induced in pea. Theor. Appl. Genet. 39 17–20. [DOI] [PubMed] [Google Scholar]
- Murfet, I.C. (1990). Internode length and anatomical changes in Pisum genotypes crys and cryc in response to extended daylength and applied gibberellin A1. Physiol. Plant. 79 497–505. [Google Scholar]
- Parcy, F., Nilsson, O., Busch, M., and Weigel, D. (1998). A genetic framework for floral patterning. Nature 395 561–566. [DOI] [PubMed] [Google Scholar]
- Patton, E.E., Willems, A.R., and Tyers, M. (1998). Combinatorial control in ubiquitin dependent proteolysis: Don't Skp the F-box hypothesis. Trends Genet. 14 236–243. [DOI] [PubMed] [Google Scholar]
- Pouteau, S., Nicolls, D., Tooke, F., Coen, E., and Battey, N. (1997). The induction and maintenance of flowering in Impatiens. Development 127 3343–3351. [DOI] [PubMed] [Google Scholar]
- Reid, J.B., Murfet, I.C., Singer, S.R., Weller, J.L., and Taylor, S.A (1996). Physiological-genetics of flowering in Pisum. Semin. Cell Dev. Biol. 7 455–463. [Google Scholar]
- Samach, A., Klenz, J.E., Kohalmi, S.E., Risseeuw, E., Haughn, G.W., and Crosby, W.L. (1999). The UNUSUAL FLORAL ORGANS gene of Arabidopsis thaliana is an F-box protein required for normal patterning and growth in the floral meristem. Plant J. 20 433–445. [DOI] [PubMed] [Google Scholar]
- Schultz, E.A., and Haughn, G.W. (1993). Genetic analysis of the floral initiation process (FLIP) in Arabidopsis. Development 119 745–765. [Google Scholar]
- Schwarz-Sommer, Z., Huijser, P., Nacken, W., Saedler, H., and Sommer, H. (1990). Genetic control of flower development by homeotic genes in Antirrhinum majus. Science 250 931–936. [DOI] [PubMed] [Google Scholar]
- Simon, R., Carpenter, R., Doyle, S., and Coen, E. (1994). Fimbriata controls flower development by mediating between meristem and organ identity genes. Cell 78 99–107. [DOI] [PubMed] [Google Scholar]
- Singer, S.R., Hsiung, L.P., and Huber, S.C. (1990). Determinate (det) mutant of Pisum sativum (Leguminosae: Papilionoideae) exhibits an indeterminate growth pattern. Am. J. Bot. 77 1330–1335. [Google Scholar]
- Souer, E., van der Krol, A., Kloos, D., Spelt, C., Bliek, M., Mol, J., and Koes, R. (1998). Genetic control of branching pattern and floral identity during Petunia inflorescence development. Development 125 733–742. [DOI] [PubMed] [Google Scholar]
- Stephens, W.L. (1939). Tables of the recombination fraction estimated from the product ratio. J. Genet. 39 171–180. [Google Scholar]
- Tucker, S.C. (1989). Overlapping organ initiation and common primordia in flowers of Pisum sativum (Leguminosae: Papilionoideae). Am. J. Bot. 76 714–729. [Google Scholar]
- Weeden, N.F., Swiecicki, W.K., Timmerman-Vaughan, G.M., Ellis, T.H.N., and Ambrose, M. (1996). The current pea linkage map. Pisum Genet. 28 1–4. [Google Scholar]
- Weigel, D., and Meyerowitz, E.M. (1994). The ABCs of floral homeotic genes. Cell 78 203–209. [DOI] [PubMed] [Google Scholar]
- Weigel, D., Alvarez, J., Smyth, D.R., Yanofsky, M.F., and Meyerowitz, E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69 843–859. [DOI] [PubMed] [Google Scholar]
- Weller, J.L., Murfet, I.C., and Reid, J.B. (1997). Pea mutants with reduced sensitivity to far-red light define an important role for phytochrome A in daylength detection. Plant Physiol. 114 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, M.D., and Haughn, G.W. (1995). UNUSUAL FLORAL ORGANS controls meristem identity and organ primordia fate in Arabidopsis. Plant Cell 7 1485–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire, R.J.E., Murfet, I.C., and Reid, J.B. (1994). The genetic control of heterochrony: Evidence from developmental mutants of Pisum sativum L. J. Evol. Biol. 7 447–465. [Google Scholar]
- Yanofsky, M.F. (1995). Floral meristems to floral organs: Genes controlling early events in Arabidopsis flower development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46 167–188. [Google Scholar]