Abstract
Extensive studies have been conducted on utilising natural fibres as reinforcement in composite production. All-polymer composites have attracted much attention because of their high strength, enhanced interfacial bonding and recyclability. Silks, as a group of natural animal fibres, possess superior properties, including biocompatibility, tunability and biodegradability. However, few review articles are found on all-silk composites, and they often lack comments on the tailoring of properties through controlling the volume fraction of the matrix. To better understand the fundamental basis of the formation of silk-based composites, this review will discuss the structure and properties of silk-based composites with a focus on employing the time–temperature superposition principle to reveal the corresponding kinetic requirements of the formation process. Additionally, a variety of applications derived from silk-based composites will be explored. The benefits and constraints of each application will be presented and discussed. This review paper will provide a useful overview of research on silk-based biomaterials.
Keywords: silk-based composites, time–temperature superposition, biomaterials
1. Introduction
In the production of composites materials, the increase in awareness of building a sustainable future has stimulated the idea of replacing non-renewable petroleum-based polymers with bio-based constituents that have low or zero carbon emissions [1]. For example, substantial research can be found on incorporating cellulosic fibres such as flax, hemp, kenaf and bamboo as reinforcement to produce natural-fibre-reinforced polymer composites (NFRPCs) for applications in the automotive industry [2,3,4]. Researchers believe that these NFRPCs have the potential to significantly reduce the weight of vehicles by 30% and cut down the overall manufacturing cost by 20% [5,6,7]. Comparatively, silks, a group of protein-based fibres that have evolved for millions of years and have properties that often exceed man-made materials, have had limited studies as a reinforcement for composites in engineering applications [8].
For the use of these natural-fibre composites in engineering applications, studies have pointed out that NFRPCs present a few drawbacks: (i) surface incompatibility between natural fibres and polymer matrices results in poor interfacial bonding between the fibre and matrix phases and negatively impacts the physical and mechanical performance of the composites; (ii) difficulty separating the constituents during the recycling process leads to degraded performance after recycling [5,9,10]. To overcome these concerns, considerable research has focused on exploring the potential of self-reinforced composites (SRCs), also referred to as single/all-polymer composites, where both the reinforcement and matrix are composed of the same polymer [11]. In 1975, Capiati and Porter first developed all-polyethylene (all-PE) composites through partially melting the crystals within the PE fibres, providing a gradual change in the morphology between fibre and matrix and resulting in a competitive interfacial shear strength comparable to conventional glass fibre reinforced with epoxy resin composites [12]. Following the success of all-PE composites, research on SRCs began around the world. The most-reported synthetic-fibre-based SRCs are fabricated using polyolefin-based (polyethylene and/or polypropylene), polyester-based (polylactic acid and/or polyethylene terephthalate) or polyamide-based (nylon) fibres or tapes. The techniques involved in fabricating SRCs include thermal processing [13], hot compaction [14,15,16,17], cool drawing [18], etc. [19].
Following the concept of SRCs and sustainable design of composite materials, all-natural fibre composites were first introduced by Nishino et al., leading to the design of all-cellulose composites (ACCs) [20]. There are generally two methods to fabricate ACCs: (i) an impregnation method (two-step approach), where fibres are impregnated by cellulose solution; and (ii) a surface-selective dissolution method, where surfaces of fibres are partially dissolved. Undissolved inner fibre cores serve as the reinforcement phase, while the dissolved fibres serve as the matrix phase upon coagulation [21,22,23]. Subsequently, research has been carried out utilising various sources of cellulose to fabricate ACCs, including ramie [24], cotton [25,26,27], hemp [28,29], flax [30], microcrystalline cellulose [21,31] and regenerated cellulose fibres such as Lyocell and Bocell [23,32,33]. Table 1 gives details of the mechanical properties of NFRPCs and SRCs, including synthetic-fibre-based and natural-fibre-based examples. It is shown that all-natural-fibre composites display mechanical properties comparable to those of NFRPCs.
Table 1.
Overview of the mechanical properties of NFRPCs and all-natural-fibre composites.
| Composite Type 1 | Tensile Strength (MPa) | Young’s Modulus (GPa) | Flexural Strength (MPa) | Flexural Modulus (GPa) | Reference |
|---|---|---|---|---|---|
| UD flax–epoxy | 284 | 26 | 218 | 18 | [34] |
| woven flax–epoxy | 68.3 | 5.9 | 101.6 | 2.1 | [35] |
| UD flax–PLA | 252.3 | 14.9 | - | - | [36] |
| Kenaf–HDPE | 32–52 | 3.0–6.5 | 58–75 | 3.7–4.1 | [37] |
| bamboo fibre–epoxy | 48–119 | - | 107–162 | - | [38] |
| wood pulp sheets–PLA | 121 | 10.5 | - | - | [39] |
| woven silk–epoxy | 100–175 | 5–8 | 125–250 | 5–7 | [40,41,42] |
| silk–PLA | 71 | 4 | 97 | 4 | [43] |
| all–PP | 60–135 | 2.7–3.9 | - | - | [44] |
| all–PLA | 58–83 | 2–3.4 | - | - | [45] |
| all–PLA | 58–83 | 2–3.4 | - | - | [45] |
| all–PET | 225–350 | 8–10 | - | - | [46] |
| all–PE | 160 | 4.2 | - | - | [47] |
| all–PA | 80–192 | - | - | - | [48] |
| all–cellulose | 250–910 | 9–26 | - | - | [49] |
| woven all-cellulose | 40–55 | 1.5–4 | - | - | [50] |
| all-silk | 83–151 | 2.8–3.1 | - | - | [51] |
1 UD: unidirectional; PLA: polylactic acid; HDPE: high-density polyethylene; PP: polypropylene; PET: polyethylene terephthalate; PE: polyethylene; PA: polyamide.
Great research effort has been put into exploiting natural-fibre-reinforced composites for biomedical applications, owing to their excellent mechanical properties, biocompatibility and tunability [52]. For example, owing to the specific hierarchical structure of silk protein, silk fibres exhibit superior mechanical, bio resorbable and biocompatible properties, resulting in their popular usages in biomaterial applications including tissue engineering, drug delivery and biodegradable medical devices [53,54,55,56,57,58,59,60,61]. A. Leal-Ega ña and T. Scheibel [62] reviewed silk-based materials for cell culture and tissue engineering, and they suggested that silk materials offer several advantages, including low toxicity, low immunoreactivity, wide pore-size distribution and elastic properties. They also pointed out that the mechanical properties of materials, including Young’s modulus, extensibility, strength and toughness, are crucial factors for both in vitro and in vivo biomedical applications, since these factors can greatly impact the cell behaviour, tissue regeneration and the integration of the graft into the body.
It is generally acknowledged that some key factors need to be considered when developing and selecting biocomposites for biomedical applications. For example, the chemical composition and surface biocompatibility of the chosen biocomposites must be able to provide a suitable chemical, biological and morphological environment for the host tissue. As addressed earlier, the chosen biocomposite’s mechanical properties need to be similar/comparable to the host tissue’s mechanical properties in order to prevent adverse reactions such as immunity response, allergic reactions and inflammation [8,63,64,65]. In order to optimise the properties of NFRCs for use in biomedical applications, researchers have conducted chemical and physical treatment of the natural fibres, while maintaining the requirements of biocompatibility, bioactivity and biostability. This has proven to be challenging because any additives comprised of different chemicals could potentially affect the biocompatibility and biodegradability of the resulting composite [52,66]. Hence, this opens up opportunities for exploring the potential for all-natural-fibre composites in biomedical applications, since homogeneity in the chemical-element composition of this type of composites can promote fibre/matrix adhesion and reduce complex synergistic immunoresponses.
Nevertheless, it is remarkable that, given the extraordinary physical and mechanical properties of silk proteins, more research investment has yet to be made with respect to the employment of silk fibres in producing silk-based biocomposites [42]. In this review, the structure of the fibrous proteins in silkworm silk and the properties of silk-based composites will be reviewed. Particularly, recent studies that employed several analysis methods to achieve the tunability of mechanical properties of silk biocomposites will be examined. Additionally, different applications of silk-based composites, their benefits and requirements, as well as a number of characterisation techniques will also be discussed. This is currently an evolving field with significant potential for producing silk-based composites with enhanced interfacial bonding, easier recyclability, and great tunability for future technological applications. It is believed that such an article would be of interest to the silk research world.
2. Silk Structure and Silk-Based Composites Properties
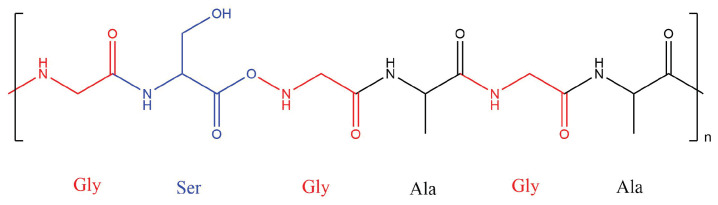
Silk is a common biological protein formed of a complex hierarchical structure with variable chemical compositions. The most common of these are the silk sericin and silk fibroin (SF) proteins. SF typically has a hexapeptide primary sequence dominated by glycine amino acid units, as seen in Figure 1 [67]. Raw fibre sheets of these biomaterials have inherent flaws compared to composites. Existing voids act as water channels to allow degradation by wetting, and hydrogen bonds can be broken by water molecules, which allows solvation and plasticisation [68]. This and other issues can be overcome by their inclusion in composites, which can improve material properties.
Figure 1.
Illustration of the common chemical structure and amino acid sequence of a silk fibroin protein with a hexapeptide sequence.
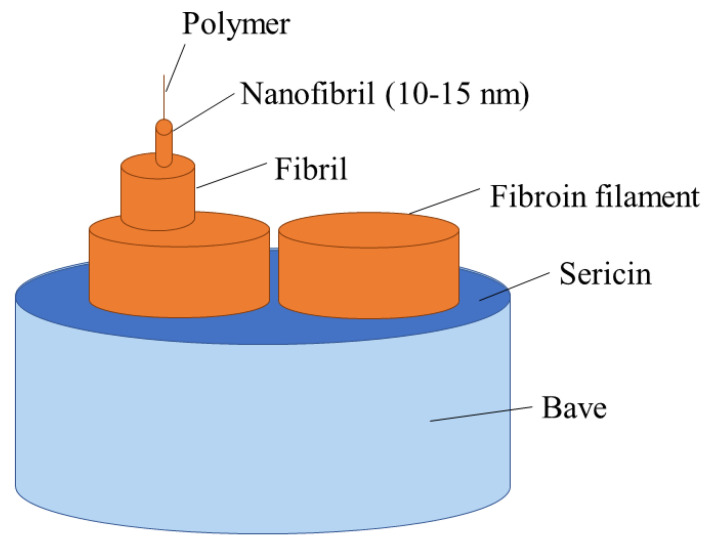
Silk forms a complex semi-crystalline hierarchical structure in its native state. The most common commercial silk is from the silk worm, Bombyx mori, but silk is also produced by spiders and other species [69]. It exists in heavy or light chains of 390 or 25 kDa, respectively, with chaperonin-like P25 proteins in a 6:6:1 ratio displaced along fibril axes [70,71]. Natural fibres of Bombyx mori are typically formed of two filaments of SF with a surrounding matrix of gummy silk sericin to maintain integrity. The continuous phase of silk sericin is removed by degumming using hot water, alkaline or acidic solutions, or other methods. The structure can be seen in Figure 2 [69]. In Bombyx mori, SF content is 66.5–73.5 wt%, while sericin content makes up 26.5–33.5 wt% [72].
Figure 2.
Illustration showing hierarchical structure of raw silk fibre with SF core and sericin coating.
The source of this silk can alter its properties with variations in strength, toughness and finish. Spiders alone can have seven different types of silk, controlled by the amino acid content of the protein. Dragline silk is rich in analine [73]. The precise effects of glycine and analine concentrations on SF crystallinity are disputed [69,73]. Shear and elongation stress also control conformation and crystallinity and, hence, material properties. Silk can reach a strength-to-weight ratio five times that of steel and three times that of Kevlar [73].
The high glycine content of SF allows for tight and stable packing of antiparallel -sheet crystallites in SF. These are associated with the mechanical strength of silk [69]. In its native state, Bombyx mori SF has a very complex structure with differing amino acid sequences promoting high- and low-order structures [74]. This typically consists of -sheet crystallites and -helix conformations, with the remaining molecules disordered [75]. SF can form silk I, II, or III. Silk I has an -helix or zigzag spatial conformation and is metastable. It often exists in phases within amorphous regions of semicrystalline SF and is shown to exhibit good swelling properties [69,76]. As with other crystal polymorphs, the structure has good chemical, thermal and enzymatic stability [76]. Silk II has the less soluble, stable -sheet crystal structure, which is a monoclinic system [69]. Silk III is an unstable polymorph only seen at air–water interfaces in regenerated silk [67]. High silk II content is often associated with preferable material properties such as strength and toughness.
3. Published Methods of Tailoring Mechanical Properties of Silk Composites
Mechanical properties of NFRPCs are one of the most important characteristics for their use in engineering and biomedical fields, and it is essential for composite materials to provide adequate mechanical behaviour (tensile, flexural, impact and hardness) based on their desired applications [77,78]. The mechanical properties of NFRPCs depend on various parameters, such as fibre alignment, fibre length, fibre orientation, volume fraction of fibres, aspect ratio of fibres and fibre–matrix adhesion [79,80,81,82,83]. Studies investigating mechanical performance of NFRPCs have focused on two major aspects: (i) influences of various treatments of fibres (physical, chemical and biological), fibre content, fabrication process and external coupling agents on mechanical properties; and (ii) incorporating experimental data and well-established models to predict mechanical behaviour [84,85,86,87,88,89,90,91,92,93].
Natural silk fibres from Bombyx mori have relatively high mechanical properties: 300–740 MPa (ultimate strength), 4–26% (breaking strain), 10–17 GPa (Young’s modulus) and 70–78 MJm (toughness). These properties often exceed those of synthetic fibres such as nylon, Kevlar and polypropylene [54,61,94,95,96,97,98]. The variation in mechanical properties of Bombyx mori silk fibres comes from several factors, such as the food, rearing conditions and health of silkworms [99], differences in the spinning process (natural spinning, forced spinning at a controlled drawing rate and modulated spinning in an electric field) [54,100,101] and genetic modification of the silk sequence [102,103]. For the purpose of broadening silk-based applications, silk fibres generally undergo the process of degumming, dissolution and regeneration and subsequently form the formats of sponges, hydrogels, films, mats and composites for versatile applications. Research suggests that these SF materials show good biocompatibility with a series of cell types and also promote the characteristics of adhesion, proliferation, growth and functionality [104].
In particular, research on tailoring the mechanical properties of silk-based composites also follows the two major aspects addressed earlier: (i) applying experimental treatments to tune the mechanical properties in order to achieve the desired structure–property–application relationship, as summarised in Table 2; and (ii) using analytical models to predict mechanical performance for further facilitation of composite material design and optimisation. For example, Jiang et al. [105] fabricated ultrathin multilayer SF films using a spin-assisted layer-by-layer assembly method with 8% w/v aqueous SF solution. These films exhibited outstanding tensile strength (∼100 MPa), Young’s modulus (6–8 GPa) and toughness (328 kJ m), comparable to the toughness of many conventional polymer composites (<100 kJ m). Burger et al. [106] fabricated SF/cellulose composites in hydrogel form, with the cellulose content varying between 0–17%. They reported that the compression properties of the hydrogels can be altered by the content of cellulose, with the compressive modulus varying between 53.3 and 70.5 kPa and compressive strength between 11.7 and 17 kPa. The biological properties of this composite hydrogel were investigated and showed good cell adhesion, cell growth and osteocyte differentiation of MC3T3-E1 cells. As a result, they proposed this composite hydrogel as a suitable scaffold for use in bone-tissue engineering. More recently, Yu et al. [107] found that the mechanical properties and drug-release behaviour of silk hydrogels can be tuned through ethylene glycol diglycidyl ether-assisted crosslinking of SF with carboxymethyl chitosan (CMCS). They investigated the SF/CMCS composite hydrogel at various mass ratios (1:0, 1:1, 1:2 and 0:1). The compressive fracture stress and strain values achieved were greatest at a 1:1 ratio (SF:CMCS): 140 kPa and 50%, respectively. The fracture energy reached a maximum value of 11.07 kJ m, with a compressive modulus of 22.24 kPa. They proposed that the addition of CMCS increases the molecular weight of SF and potentially induces the conformational transition of SF to increase the content of -sheet structure, thus enhancing the strength and elasticity of the composite hydrogel. They further tested the sustained release rate of the SF/CMCS hydrogel using trypan blue as the model drug and noticed the rate was almost 35% higher than that of the SF hydrogel [107]. This composite hydrogel is suggested to be used as a biomedical carrier for sustained drug release.
Table 2.
Summary of the tailored mechanical properties of silk-based composites through various experimental treatments.
| Formats of Silk-Based Composites | Experimental Treatments | Mechanical Properties | Biological Properties | Proposed Application | Reference |
|---|---|---|---|---|---|
| SF films | Spin-assisted layer-by-layer assembly method with 8% w/v aqueous SF solution | Tensile strength ∼100 MPa, Young’s modulus 6–8 GPa, Toughness 328 kJ m | - | Microscale biodevices, synthetic coatings for artificial skin | [105] |
| SF/cellulose composite hydrogel | Varying the content of cellulose between 0–17% | Compressive modulus 53.3–70.5 kPa, compressive strength 11.7–17 kPa | Good cell adhesion, cell growth and osteocyte differentiation of MC3T3-E1 cells | A suitable scaffold for use in bone-tissue engineering | [106] |
| SF/CMCS composite hydrogel | Ethylene glycol diglycidyl ether-assisted crosslinking of SF with CMCS and various mass ratios of SF/CMCS (1:0, 1:1, 1:2, 0:1) | When SF:CMCS is 1:1, compressive fracture stress and strain is 140 kPa, 50%, fracture energy 11.07 kJ m, compressive modulus 22.24 kPa | High sustained release rate when using trypan blue as the model drug | Biomedical carrier for sustained drug release | [107] |
SF: silk fibroin; CMCS: carboxymethyl chitosan; MC3T3-E1 cells: osteoblastic cell line derived from mouse calvaria.
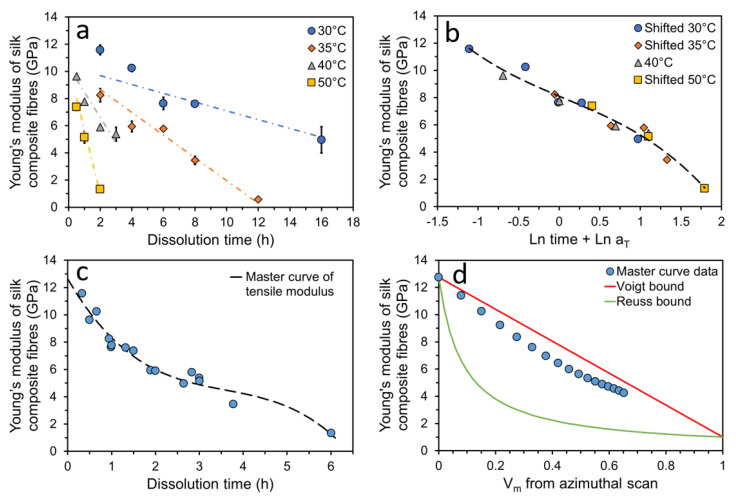
Studies also focus on exploiting analytical models to predict and tailor the mechanical properties of silk-based composites. It is acknowledged that modelling can reveal the structure–property relationship within composites with little time and cost compared to obtaining results from a range of experiments [108]. Lin et al. [109] constructed SF and collagen type II (col-II) composites in the membrane format with varied SF:col-II ratios (5:5, 7:3, 9:1). They employed a nonlinear viscoelastic constitutive model to predict the creep behaviour and tensile properties of these composite membranes and compared it with the corresponding experimental data. From their obtained stress–strain curves, it was observed that the Young’s modulus values increased with decreasing col-II and, conversely, the ultimate strain and tensile strength decreased with decreasing col-II. Importantly, both the experimentally observed creep strain and tensile stress–strain curves were in good agreement with predictions. Additionally, a live/dead cell double-staining and cell proliferation assay demonstrated that these composite membranes display good cell proliferation ability and biocompatibility and further indicated that the SF/col-II composite membrane is a potential scaffold material for cartilage repair. Guan et al. [110] focused on utilising a finite element analysis (FEA) model to investigate the importance of fibre properties and inter-fibre bonding strength on the resulting mechanical properties of silk-based composites. They simulated the inter-fibre bonding strength through constructing a fibre network with fixed short beams of sericin, and strength was varied by modifying the cross-sectional area and length. By combining conclusions from microstructural images of the cocoon samples after tensile failure with simulation results, they proposed that good mechanical properties of fibres and effective inter-fibre bonding are crucial for making a tough and strong fibre composite. In recent studies, all-silk composites (ASCs) were fabricated using a surface-selective dissolution method by Zhang et al. [111]. They reported that individual Bombyx mori silk threads can be partially dissolved in the chosen solvent (1-ethyl-3-methylimidazolium acetate) for various times and at various temperatures and can be further coagulated in a methanol bath. Upon doing so, the undissolved inner thread core acts as the reinforcement phase, while the dissolved outer layers of silk thread coagulated and formed a matrix phase surrounding the inner fibre core. Through use of wide-angle X-ray azimuthal scans, the fraction of preferred oriented crystalline and randomly oriented crystalline within the ASCs fabricated for different times and at different temperatures was compared, and the average crystalline orientation () values were calculated. They further noticed that the values were linearly related to the coagulated volume fraction of the matrix (). After conducting tensile tests on ASCs fabricated for various times and at various temperatures, they found that the Young’s modulus values decreased linearly with increases in dissolution time and more rapidly at increased dissolution temperature (Figure 3a). They proposed that the change in the Young’s modulus values followed a time–temperature superposition principle and further confirmed it through the employment of a shifting method in log space (Figure 3b), leading to the construction of a master curve for the Young’s modulus values at a chosen reference temperature (Figure 3c). Furthermore, they applied the well-established rule of mixture models to investigate the influence of reinforced fibre volume on the resulting Young’s modulus of the composite. Though some discrepancies between experimental and theoretical results existed, the experimental data suggested that the fabricated ASCs showed effective stress transfer between fibre–matrix up to a matrix fraction of ∼70% (Figure 3d). Consequently, they demonstrated the potential of ASCs and the possibility of altering their mechanical properties with controlled fabrication processes, dissolution times and temperatures.
Figure 3.
Tensile properties of all-silk composites in silk-thread format: (a) Young’s modulus of ASCs for various dissolution times and temperatures; (b) shifting each temperature dataset to a reference temperature dataset, 40 C, in log space; (c) master curve of Young’s modulus represented at 40 C; (d) Rule of mixture models predicting effectiveness of stress transfer between fibre–matrix. Reprinted (adapted) with permission from [111]. Copyright 2021 American Chemical Society.
4. Applications of Silk Composites
Although the fundamental research of silk composites is interesting due to the inherent complexity of the natural system and preparation conditions, they are often researched with intended applications in mind. Hence, research can either be approached as bottom-up, fundamental, blue-sky research or challenge-driven top-down research [112].
Silks have been primarily used for biomedical applications as they are perceived to be biocompatible, biodegradable and non-toxic [113]. It is of note that biocompatibility is not universal and must be specific to tissue and wound to encourage the correct immune response during healing [112]. Current uses of silk include sutures, surgical meshes and medical fabrics. Coating silk fibres with regenerated SF for use as sutures is one of the first examples of ASC use in medicine. Some future applications still being developed include tissue engineering and wound healing [112]. Microneedles also offer an exciting new development in transdermal vaccine delivery. SF microneedles offer a solution for controlled-release drug delivery with minimally invasive techniques [114,115]. As shown in works by Tsioris et al. and Stinson et al., SF microneedles provide favourable mechanical properties, biocompatibility, biodegradability, benign processing conditions and the ability to maintain the activity of biological compounds in its matrix [114,115]. This biomaterial could offer a new application for engineered biocomposites of SF in which the techniques mentioned above may confer improved toughness over simpler SF microneedle arrays.
Scaffolds of biomimetic materials are common forms of biosynergistic composites and function as host environments for cell and tissue growth and proliferation [116]. In order to be biomimetic, these engineered tissues must regulate healing phases by imitation of immunoresponse signals [117]. When preparing a composite for tissue engineering, it must provide [118,119,120,121]:
Appropriate permeability and absorption [119];
Correct release behaviour [119];
Structural stability [118];
This complexity requires careful preparation of aero- or hydrogels to meet these requirements. This, then, requires engineered tissues to mimic highly variable native or artificial tissue mechanical (shear, tensile or compressive) moduli [122,123,124]:
Articular cartilage—0.4–1.6 MPa [122];
Native femoral artery—≈9.0 MPa [122];
Human medial meniscus—≈1.0 MPa [122];
Fixation plates—≈700 MPa [123];
Cancellous bone—0.05–5 GPa [124].
SF is confirmed to promote cellular adhesion and proliferation of fibroblasts and keratinocytes [121,125,126]. Another benefit for biomedical uses and tissue engineering is that these materials can degrade in vivo and trigger minimal inflammatory response. They also aid in cell growth due to the intrinsic biocompatibility of SF materials [127,128]. This eliminates the need for implant-removal surgery but requires understanding of the degradation process to ensure it does not compromise efficiency [69,127,129]. Silk-based composites have also been prepared with drug retention and release capabilities that show some promise for targeted-drug-release applications [130]. Utilising ASCs could provide samples with high homogeneity, good material properties and tunable biological interactions for continued use in these applications. It is essential in biomedical applications of these composites to mimic the natural environment: for example, in the proliferation of fibroblasts on the surface of breast implants [126,131].
Wahab et al. utilised silk’s heavy-metal-adsorption properties in conjunction with low-cost bentonite clay to produce a composite capable of adsorbing lethal heavy-metal ions from aqueous solution [132]. They successfully impregnated SF with bentonite clay and achieved high monolayer adsorption values for Cd(II), Pb(II), Hg(II) and Cr(VI) [132]. Silk composites could offer future solutions in water sanitation aids as an environmentally sustainable tool.
Spider silk is seen to be of such high tensile quality, even compared to Bombyx mori silk [112], that composites reinforced with spider silk components have been thought of for use in aerospace engineering [133]. Mayank et al. prepared a transparent epoxy/spider-silk composite with material strength within the safety margins of typical standard acrylic aviation windowpanes [133]. They also showed improved impact deflection and stress loading. As a weight-reduced non-magnetic material, silk composites could offer an innovative material option for the aerospace industry [133]. It is of note that improved interfacial interactions of ASCs are seen due to chemically identical components of the matrix and the reinforcing fibres [50]. This is due to a favourable energetic interaction developed in identical biopolymers compared to phase-separated interfaces of polymer blends [134]. This interface is least influential in miscible polymer blends with favourable interactions, such as those between cellulose and silk fibroin [134]. ASCs could, therefore, reduce the likelihood of the primary failure mechanism of composites: failure at the interface of the reinforcement and the matrix. This implies the viability of ASCs in the uses mentioned throughout this article
Lastly, silk composites have also been used for decorative and restorative challenges, in line with their historic use. Cianci et al. produced a hybrid SF/cellulose solution to support and recover aged silk fabrics [71]. This forms a silk composite in which the cellulose improves silk crystallinity and material properties, allowing the sustainment of aged and degraded silk fabrics [71]. Untreated pristine silk and the same fabric treated with 1.35/1.35 wt% SF/cellulose nanocrystals dispersed in water then dried gave axial forces at breaking of ≈30 N and ≈35 N, respectively. This was a marked improvement over silk treated with single-component dispersions [71]. Other hybrid material composites have been produced for structural applications. Kimura and Aoki reinforced plywood and medium-density fibreboard with silk fabric using a polybutylene succinate matrix to create decorative laminates with improved flexibility and impact resistance [135]. ASCs could be used in a similar decorative capacity with dyed patterns from the fabric retained in the final product. As shown by Cianci et al., this could offer a use for waste silk with tangible benefits for the end product [71]. Though these improvements imply an impactful contribution from silk within these composites, it is essential to evaluate sustainability in the creation of these products. If overused, silk could contribute to environmental burdens rather than reducing them by increasing water usage and transport costs. We suggest that focusing on reusing old silk and effectively disposing of waste samples will be of huge value going forward.
5. Conclusions
With recent awareness of global environmental challenges, it has become of increasing importance to utilise sustainable biomaterials to replace non-renewable alternatives. In this role, silk possesses unique qualities of strength and biocompatibility that highlight it as a key contributor to future developments in NFRPCs. Through this review, we discussed the behaviours and uses of silk-based composites and reported on the enhancement of mechanical properties of silk-based composites. We separated enhancements into experimental (chemical and/or physical) treatments to achieve desired properties and the use of analytical models to predict the mechanical properties for further design and optimisation of the structure and properties. As an example of this, we reported on the modelling of the fabrication of ASCs using time–temperature superposition. This can be used to manipulate the mechanical properties and morphology of composites via changes to the dissolution time and temperature that alter the volume fraction of the matrix. This gives rise to clearly defined trends of material properties as a function of time, temperature or matrix fraction. ASCs can therefore be manipulated for desired purposes with good interfacial bonding between the fibre and the matrix due to chemical homogeneity. Finally, we discussed the applications of silk composites. This review focused on biomedical uses, with other examples in water sanitation, aeronautical engineering and decorative functional materials. This shows a future of applications in many industries with challenges highlighted in upscaling as well as achieving true sustainability with widespread use of these new materials.
Acknowledgments
We would like to give a huge thanks to my supervisor team, and the biopolymer dream team for all the support and fantastic ideas.
Abbreviations
The following abbreviations are used in this manuscript:
| NFRPC | Natural-fibre-reinforced polymer composite |
| SRC | Self-reinforced composite |
| PE | Polyethylene |
| HDPE | High-density polyethylene |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PET | Polyethylene terephthalate |
| PA | Polyamide |
| UD | Unidirectional |
| ASC | All-silk composite |
| ACC | All-cellulose composite |
| SF | Silk fibroin |
| CMCS | Carboxymethyl chitosan |
| Col-II | Collagen type II |
| FEA | Finite element analysis |
| Average crystalline orientation | |
| Volume fraction of matrix |
Author Contributions
Investigation, X.Z.; data curation, X.Z.; writing—original draft preparation, X.Z. and J.A.K.; writing—review and editing, M.E.R.; supervision, M.E.R.; funding acquisition, M.E.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data and sources available upon request from authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
James King was supported by the EPSRC CDT in Soft Matter for Formulation and Industrial Innovation, “SOFI2”, (EP/S023631/1).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Corbin A.C., Soulat D., Ferreira M., Labanieh A.R., Gabrion X., Malécot P., Placet V. Towards hemp fabrics for high-performance composites: Influence of weave pattern and features. Compos. Part B Eng. 2020;181:107582. doi: 10.1016/j.compositesb.2019.107582. [DOI] [Google Scholar]
- 2.Suddell B.C., Evans W.J. Natural Fibers, Biopolymers, and Biocomposites. CRC Press; Boca Raton, FL, USA: 2005. Natural fiber composites in automotive applications; pp. 253–282. [Google Scholar]
- 3.Mohanty A.K., Misra M., Drzal L.T. Natural Fibers, Biopolymers, and Biocomposites. CRC Press; Boca Raton, FL, USA: 2005. [Google Scholar]
- 4.Koronis G., Silva A., Fontul M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013;44:120–127. doi: 10.1016/j.compositesb.2012.07.004. [DOI] [Google Scholar]
- 5.Ahmad F., Choi H.S., Park M.K. A review: Natural fiber composites selection in view of mechanical, light weight, and economic properties. Macromol. Mater. Eng. 2015;300:10–24. doi: 10.1002/mame.201400089. [DOI] [Google Scholar]
- 6.Fantuzzi N., Bacciocchi M., Benedetti D., Agnelli J. The use of sustainable composites for the manufacturing of electric cars. Compos. Part C Open Access. 2021;4:100096. doi: 10.1016/j.jcomc.2020.100096. [DOI] [Google Scholar]
- 7.Naik V., Kumar M. A review on natural fiber composite material in automotive applications. Eng. Sci. 2021;18:1–10. doi: 10.30919/es8d589. [DOI] [Google Scholar]
- 8.Cheung H.Y., Ho M.P., Lau K.T., Cardona F., Hui D. Natural fibre-reinforced composites for bioengineering and environmental engineering applications. Compos. Part B Eng. 2009;40:655–663. doi: 10.1016/j.compositesb.2009.04.014. [DOI] [Google Scholar]
- 9.Xie Y., Hill C.A., Xiao Z., Militz H., Mai C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010;41:806–819. doi: 10.1016/j.compositesa.2010.03.005. [DOI] [Google Scholar]
- 10.Zhao X., Copenhaver K., Wang L., Korey M., Gardner D.J., Li K., Lamm M.E., Kishore V., Bhagia S., Tajvidi M., et al. Recycling of natural fiber composites: Challenges and opportunities. Resour. Conserv. Recycl. 2022;177:105962. doi: 10.1016/j.resconrec.2021.105962. [DOI] [Google Scholar]
- 11.Gao C., Yu L., Liu H., Chen L. Development of self-reinforced polymer composites. Prog. Polym. Sci. 2012;37:767–780. doi: 10.1016/j.progpolymsci.2011.09.005. [DOI] [Google Scholar]
- 12.Capiati N.J., Porter R.S. The concept of one polymer composites modelled with high density polyethylene. J. Mater. Sci. 1975;10:1671–1677. doi: 10.1007/BF00554928. [DOI] [Google Scholar]
- 13.Barkoula N.M., Peijs T., Schimanski T., Loos J. Processing of single polymer composites using the concept of constrained fibers. Polym. Compos. 2005;26:114–120. doi: 10.1002/pc.20082. [DOI] [Google Scholar]
- 14.Ward I., Hine P. Novel composites by hot compaction of fibers. Polym. Eng. Sci. 1997;37:1809–1814. doi: 10.1002/pen.11830. [DOI] [Google Scholar]
- 15.Ward I., Hine P. The science and technology of hot compaction. Polymer. 2004;45:1413–1427. doi: 10.1016/j.polymer.2003.11.050. [DOI] [Google Scholar]
- 16.Ward I. Developments in oriented polymers, 1970–2004. Plast. Rubber Compos. 2004;33:189–194. doi: 10.1179/174328904X4864. [DOI] [Google Scholar]
- 17.Rasburn J., Hine P., Ward I., Olley R., Bassett D., Kabeel M. The hot compaction of polyethylene terephthalate. J. Mater. Sci. 1995;30:615–622. doi: 10.1007/BF00356319. [DOI] [Google Scholar]
- 18.Yu L., Liu H., Dean K., Chen L. Cold crystallization and postmelting crystallization of PLA plasticized by compressed carbon dioxide. J. Polym. Sci. Part Polym. Phys. 2008;46:2630–2636. doi: 10.1002/polb.21599. [DOI] [Google Scholar]
- 19.Ward I.M., Coates P.D., Dumoulin M.M. Solid Phase Processing of Polymers. Hanser Publishers; Munich, Germany: 2000. [Google Scholar]
- 20.Nishino T., Matsuda I., Hirao K. All-cellulose composite. Macromolecules. 2004;37:7683–7687. doi: 10.1021/ma049300h. [DOI] [Google Scholar]
- 21.Gindl W., Keckes J. All-cellulose nanocomposite. Polymer. 2005;46:10221–10225. doi: 10.1016/j.polymer.2005.08.040. [DOI] [Google Scholar]
- 22.Nishino T., Arimoto N. All-cellulose composite prepared by selective dissolving of fiber surface. Biomacromolecules. 2007;8:2712–2716. doi: 10.1021/bm0703416. [DOI] [PubMed] [Google Scholar]
- 23.Soykeabkaew N., Nishino T., Peijs T. All-cellulose composites of regenerated cellulose fibres by surface selective dissolution. Compos. Part A Appl. Sci. Manuf. 2009;40:321–328. doi: 10.1016/j.compositesa.2008.10.021. [DOI] [Google Scholar]
- 24.Qin C., Soykeabkaew N., Xiuyuan N., Peijs T. The effect of fibre volume fraction and mercerization on the properties of all-cellulose composites. Carbohydr. Polym. 2008;71:458–467. doi: 10.1016/j.carbpol.2007.06.019. [DOI] [Google Scholar]
- 25.Arévalo R., Picot O.T., Wilson R.M., Soykeabkaew N., Peijs T. All-cellulose composites by partial dissolution of cotton fibres. J. Biobased Mater. Bioenergy. 2010;4:129–138. doi: 10.1166/jbmb.2010.1077. [DOI] [Google Scholar]
- 26.Liang Y., Hawkins J.E., Ries M.E., Hine P.J. Dissolution of cotton by 1-ethyl-3-methylimidazolium acetate studied with time–temperature superposition for three different fibre arrangements. Cellulose. 2021;28:715–727. doi: 10.1007/s10570-020-03576-x. [DOI] [Google Scholar]
- 27.Shibata M., Teramoto N., Nakamura T., Saitoh Y. All-cellulose and all-wood composites by partial dissolution of cotton fabric and wood in ionic liquid. Carbohydr. Polym. 2013;98:1532–1539. doi: 10.1016/j.carbpol.2013.07.062. [DOI] [PubMed] [Google Scholar]
- 28.Ouajai S., Shanks R.A. Preparation, structure and mechanical properties of all-hemp cellulose biocomposites. Compos. Sci. Technol. 2009;69:2119–2126. doi: 10.1016/j.compscitech.2009.05.005. [DOI] [Google Scholar]
- 29.Chen K., Xu W., Ding Y., Xue P., Sheng P., Qiao H., He J. Hemp-based all-cellulose composites through ionic liquid promoted controllable dissolution and structural control. Carbohydr. Polym. 2020;235:116027. doi: 10.1016/j.carbpol.2020.116027. [DOI] [PubMed] [Google Scholar]
- 30.Hawkins J.E., Liang Y., Ries M.E., Hine P.J. Time temperature superposition of the dissolution of cellulose fibres by the ionic liquid 1-ethyl-3-methylimidazolium acetate with cosolvent dimethyl sulfoxide. Carbohydr. Polym. Technol. Appl. 2021;2:100021. doi: 10.1016/j.carpta.2020.100021. [DOI] [Google Scholar]
- 31.Abbott A., Bismarck A. Self-reinforced cellulose nanocomposites. Cellulose. 2010;17:779–791. doi: 10.1007/s10570-010-9427-5. [DOI] [Google Scholar]
- 32.Adak B., Mukhopadhyay S. A comparative study on lyocell-fabric based all-cellulose composite laminates produced by different processes. Cellulose. 2017;24:835–849. doi: 10.1007/s10570-016-1149-x. [DOI] [Google Scholar]
- 33.Gindl-Altmutter W., Keckes J., Plackner J., Liebner F., Englund K., Laborie M.P. All-cellulose composites prepared from flax and lyocell fibres compared to epoxy–matrix composites. Compos. Sci. Technol. 2012;72:1304–1309. doi: 10.1016/j.compscitech.2012.05.011. [DOI] [Google Scholar]
- 34.Van de Weyenberg I., Truong T.C., Vangrimde B., Verpoest I. Improving the properties of UD flax fibre reinforced composites by applying an alkaline fibre treatment. Compos. Part A Appl. Sci. Manuf. 2006;37:1368–1376. doi: 10.1016/j.compositesa.2005.08.016. [DOI] [Google Scholar]
- 35.Cerbu C. Mechanical characterization of the flax/epoxy composite material. Procedia Technol. 2015;19:268–275. doi: 10.1016/j.protcy.2015.02.039. [DOI] [Google Scholar]
- 36.Couture A., Lebrun G., Laperrière L. Mechanical properties of polylactic acid (PLA) composites reinforced with unidirectional flax and flax-paper layers. Compos. Struct. 2016;154:286–295. doi: 10.1016/j.compstruct.2016.07.069. [DOI] [Google Scholar]
- 37.Wang Q., Jones J., Lu N., Johnson R., Ning H., Pillay S. Development and characterization of high-performance kenaf fiber–HDPE composites. J. Reinf. Plast. Compos. 2018;37:191–200. doi: 10.1177/0731684417739127. [DOI] [Google Scholar]
- 38.Chin S.C., Tee K.F., Tong F.S., Ong H.R., Gimbun J. Thermal and mechanical properties of bamboo fiber reinforced composites. Mater. Today Commun. 2020;23:100876. doi: 10.1016/j.mtcomm.2019.100876. [DOI] [Google Scholar]
- 39.Du Y., Wu T., Yan N., Kortschot M.T., Farnood R. Fabrication and characterization of fully biodegradable natural fiber-reinforced poly (lactic acid) composites. Compos. Part B Eng. 2014;56:717–723. doi: 10.1016/j.compositesb.2013.09.012. [DOI] [Google Scholar]
- 40.Yang K., Wu Z., Zhou C., Cai S., Wu Z., Tian W., Wu S., Ritchie R.O., Guan J. Comparison of toughening mechanisms in natural silk-reinforced composites with three epoxy resin matrices. Compos. Part A Appl. Sci. Manuf. 2022;154:106760. doi: 10.1016/j.compositesa.2021.106760. [DOI] [Google Scholar]
- 41.Hamidi Y.K., Yalcinkaya M.A., Guloglu G.E., Pishvar M., Amirkhosravi M., Altan M.C. Silk as a natural reinforcement: Processing and properties of silk/epoxy composite laminates. Materials. 2018;11:2135. doi: 10.3390/ma11112135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah D.U., Porter D., Vollrath F. Can silk become an effective reinforcing fibre? A property comparison with flax and glass reinforced composites. Compos. Sci. Technol. 2014;101:173–183. doi: 10.1016/j.compscitech.2014.07.015. [DOI] [Google Scholar]
- 43.Ho M.P., Lau K.T., Wang H., Bhattacharyya D. Characteristics of a silk fibre reinforced biodegradable plastic. Compos. Part B Eng. 2011;42:117–122. doi: 10.1016/j.compositesb.2010.10.007. [DOI] [Google Scholar]
- 44.Hine P., Ward I., Jordan N., Olley R., Bassett D. The hot compaction behaviour of woven oriented polypropylene fibres and tapes. I. Mechanical properties. Polymer. 2003;44:1117–1131. doi: 10.1016/S0032-3861(02)00809-1. [DOI] [Google Scholar]
- 45.Somord K., Suwantong O., Tawichai N., Peijs T., Soykeabkaew N. Self-reinforced poly (lactic acid) nanocomposites of high toughness. Polymer. 2016;103:347–352. doi: 10.1016/j.polymer.2016.09.080. [DOI] [Google Scholar]
- 46.Zhang J., Reynolds C., Peijs T. All-poly (ethylene terephthalate) composites by film stacking of oriented tapes. Compos. Part A Appl. Sci. Manuf. 2009;40:1747–1755. doi: 10.1016/j.compositesa.2009.08.008. [DOI] [Google Scholar]
- 47.Zhong F., Schwabe J., Hofmann D., Meier J., Thomann R., Enders M., Mülhaupt R. All-polyethylene composites reinforced via extended-chain UHMWPE nanostructure formation during melt processing. Polymer. 2018;140:107–116. doi: 10.1016/j.polymer.2018.02.027. [DOI] [Google Scholar]
- 48.Gong Y., Yang G. Manufacturing and physical properties of all-polyamide composites. J. Mater. Sci. 2009;44:4639–4644. doi: 10.1007/s10853-009-3708-0. [DOI] [Google Scholar]
- 49.Soykeabkaew N., Arimoto N., Nishino T., Peijs T. All-cellulose composites by surface selective dissolution of aligned ligno-cellulosic fibres. Compos. Sci. Technol. 2008;68:2201–2207. doi: 10.1016/j.compscitech.2008.03.023. [DOI] [Google Scholar]
- 50.Victoria A., Ries M.E., Hine P.J. Use of interleaved films to enhance the properties of all-cellulose composites. Compos. Part A Appl. Sci. Manuf. 2022;160:107062. doi: 10.1016/j.compositesa.2022.107062. [DOI] [Google Scholar]
- 51.Yuan Q., Yao J., Chen X., Huang L., Shao Z. The preparation of high performance silk fiber/fibroin composite. Polymer. 2010;51:4843–4849. doi: 10.1016/j.polymer.2010.08.042. [DOI] [Google Scholar]
- 52.Tavares T.D., Antunes J.C., Ferreira F., Felgueiras H.P. Biofunctionalization of natural fiber-reinforced biocomposites for biomedical applications. Biomolecules. 2020;10:148. doi: 10.3390/biom10010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keten S., Xu Z., Ihle B., Buehler M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 2010;9:359–367. doi: 10.1038/nmat2704. [DOI] [PubMed] [Google Scholar]
- 54.Shao Z., Vollrath F. Surprising strength of silkworm silk. Nature. 2002;418:741. doi: 10.1038/418741a. [DOI] [PubMed] [Google Scholar]
- 55.Paladini F., Pollini M. Novel Approaches and Biomaterials for Bone Tissue Engineering: A Focus on Silk Fibroin. Materials. 2022;15:6952. doi: 10.3390/ma15196952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altman G.H., Diaz F., Jakuba C., Calabro T., Horan R.L., Chen J., Lu H., Richmond J., Kaplan D.L. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 57.Kundu B., Kurland N.E., Bano S., Patra C., Engel F.B., Yadavalli V.K., Kundu S.C. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 2014;39:251–267. doi: 10.1016/j.progpolymsci.2013.09.002. [DOI] [Google Scholar]
- 58.Kundu B., Rajkhowa R., Kundu S.C., Wang X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013;65:457–470. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 59.Meinel L., Hofmann S., Karageorgiou V., Zichner L., Langer R., Kaplan D., Vunjak-Novakovic G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol. Bioeng. 2004;88:379–391. doi: 10.1002/bit.20252. [DOI] [PubMed] [Google Scholar]
- 60.Guo C., Li C., Mu X., Kaplan D.L. Engineering silk materials: From natural spinning to artificial processing. Appl. Phys. Rev. 2020;7:011313. doi: 10.1063/1.5091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koh L.D., Cheng Y., Teng C.P., Khin Y.W., Loh X.J., Tee S.Y., Low M., Ye E., Yu H.D., Zhang Y.W., et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015;46:86–110. doi: 10.1016/j.progpolymsci.2015.02.001. [DOI] [Google Scholar]
- 62.Leal-Egaña A., Scheibel T. Silk-based materials for biomedical applications. Biotechnol. Appl. Biochem. 2010;55:155–167. doi: 10.1042/BA20090229. [DOI] [PubMed] [Google Scholar]
- 63.Wang L., Wang C., Wu S., Fan Y., Li X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020;8:2714–2733. doi: 10.1039/D0BM00269K. [DOI] [PubMed] [Google Scholar]
- 64.Anderson J. 9.19 - Biocompatibility. In: Matyjaszewski K., Möller M., editors. Polymer Science: A Comprehensive Reference. Elsevier; Amsterdam, The Netherlands: 2012. pp. 363–383. [Google Scholar]
- 65.Omiyale B.O., Rasheed A.A., Akinnusi R.O., Olugbade T.O. Biotechnology—Biosensors, Biomaterials and Tissue Engineering Annual. Volume 2023. IntechOpen; Rijeka, Croatia: 2022. Influence of Mechanical Properties of Biomaterials on the Reconstruction of Biomedical Parts via Additive Manufacturing Techniques: An Overview. Chapter 1. [DOI] [Google Scholar]
- 66.Setyarini P.H., Cahyandari D. Proceedings of the IOP Conference Series: Materials Science and Engineering. Volume 494. IOP Publishing; Bristol, UK: 2019. Potential Natural Fiber-Reinforced Composite for Biomedical Application; p. 012018. [Google Scholar]
- 67.Love S.A., Hu X., Salas-de la Cruz D. Controlling the structure and properties of semi-crystalline cellulose/silk-fibroin biocomposites by ionic liquid type and hydrogen peroxide concentration. Carbohydr. Polym. Technol. Appl. 2022;3:100193. doi: 10.1016/j.carpta.2022.100193. [DOI] [Google Scholar]
- 68.Bai F., Dong T., Chen W., Wang J., Li X. Nanocellulose hybrid lignin complex reinforces cellulose to form a strong, water-stable lignin–cellulose composite usable as a plastic replacement. Nanomaterials. 2021;11:3426. doi: 10.3390/nano11123426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kostag M., Jedvert K., El Seoud O.A. Engineering of sustainable biomaterial composites from cellulose and silk fibroin: Fundamentals and applications. Int. J. Biol. Macromol. 2021;167:687–718. doi: 10.1016/j.ijbiomac.2020.11.151. [DOI] [PubMed] [Google Scholar]
- 70.Susanin A.I., Sashina E.S., Novoselov N.P., Zakharov V.V. Change of Silk Fibroin Molecular Mass During Dissolution in Ionic Liquids. Fibre Chem. 2020;52:208–213. doi: 10.1007/s10692-020-10182-x. [DOI] [Google Scholar]
- 71.Cianci C., Chelazzi D., Poggi G., Modi F., Giorgi R., Laurati M. Hybrid fibroin-nanocellulose composites for the consolidation of aged and historical silk. Colloids Surfaces Physicochem. Eng. Asp. 2022;634:127944. doi: 10.1016/j.colsurfa.2021.127944. [DOI] [Google Scholar]
- 72.Sashina E.S., Bochek A.M., Novoselov N.P., Kirichenko D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006;79:869–876. doi: 10.1134/S1070427206060012. [DOI] [Google Scholar]
- 73.Meirovitch S., Shtein Z., Ben-Shalom T., Lapidot S., Tamburu C., Hu X., Kluge J.A., Raviv U., Kaplan D.L., Shoseyov O. Spider silk-CBD-cellulose nanocrystal composites: Mechanism of assembly. Int. J. Mol. Sci. 2016;17:1573. doi: 10.3390/ijms17091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou W., He J., Du S., Cui S., Gao W. Electrospun Silk Fibroin/Cellulose Acetate Blend Nanofibres: Structure and Properties. Iran. Polym. J. 2011;20:389–397. [Google Scholar]
- 75.Sashina E.S., Golubikhin Y.A., Novoselov N.P. Thermochemical study on the dissolution and regeneration of fibroin from solutions in imidazole-based ionic liquids. Russ. J. Gen. Chem. 2012;82:1440–1443. doi: 10.1134/S107036321208018X. [DOI] [Google Scholar]
- 76.Zhao M., Qi Z., Tao X., Newkirk C., Hu X., Lu S. Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure. Int. J. Mol. Sci. 2021;22:4136. doi: 10.3390/ijms22084136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salernitano E., Migliaresi C. Composite materials for biomedical applications: A review. J. Appl. Biomater. Biomech. 2003;1:3–18. [PubMed] [Google Scholar]
- 78.Namvar F., Jawaid M., Tanir P.M., Mohamad R., Azizi S., Khodavandi A., Rahman H.S., Nayeri M.D. Potential use of plant fibres and their composites for biomedical applications. BioResources. 2014;9:5688–5706. [Google Scholar]
- 79.Elanchezhian C., Ramnath B.V., Ramakrishnan G., Rajendrakumar M., Naveenkumar V., Saravanakumar M. Review on mechanical properties of natural fiber composites. Mater. Today Proc. 2018;5:1785–1790. doi: 10.1016/j.matpr.2017.11.276. [DOI] [Google Scholar]
- 80.Sanjay M., Madhu P., Jawaid M., Senthamaraikannan P., Senthil S., Pradeep S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018;172:566–581. doi: 10.1016/j.jclepro.2017.10.101. [DOI] [Google Scholar]
- 81.Saheb D.N., Jog J.P. Natural fiber polymer composites: A review. Adv. Polym. Technol. J. Polym. Process. Inst. 1999;18:351–363. doi: 10.1002/(SICI)1098-2329(199924)18:4<351::AID-ADV6>3.0.CO;2-X. [DOI] [Google Scholar]
- 82.Khan M.Z., Srivastava S.K., Gupta M. Tensile and flexural properties of natural fiber reinforced polymer composites: A review. J. Reinf. Plast. Compos. 2018;37:1435–1455. doi: 10.1177/0731684418799528. [DOI] [Google Scholar]
- 83.Shah D.U., Porter D., Vollrath F. Opportunities for silk textiles in reinforced biocomposites: Studying through-thickness compaction behaviour. Compos. Part A Appl. Sci. Manuf. 2014;62:1–10. doi: 10.1016/j.compositesa.2014.03.008. [DOI] [Google Scholar]
- 84.Rong M.Z., Zhang M.Q., Liu Y., Yang G.C., Zeng H.M. The effect of fiber treatment on the mechanical properties of unidirectional sisal-reinforced epoxy composites. Compos. Sci. Technol. 2001;61:1437–1447. doi: 10.1016/S0266-3538(01)00046-X. [DOI] [Google Scholar]
- 85.Bledzki A., Mamun A., Lucka-Gabor M., Gutowski V. The effects of acetylation on properties of flax fibre and its polypropylene composites. Express Polym. Lett. 2008;2:413–422. doi: 10.3144/expresspolymlett.2008.50. [DOI] [Google Scholar]
- 86.Sawpan M.A., Pickering K.L., Fernyhough A. Improvement of mechanical performance of industrial hemp fibre reinforced polylactide biocomposites. Compos. Part A Appl. Sci. Manuf. 2011;42:310–319. doi: 10.1016/j.compositesa.2010.12.004. [DOI] [Google Scholar]
- 87.Boey J.Y., Lee C.K., Tay G.S. Factors Affecting Mechanical Properties of Reinforced Bioplastics: A Review. Polymers. 2022;14:3737. doi: 10.3390/polym14183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bahrami M., Abenojar J., Martínez M.Á. Recent progress in hybrid biocomposites: Mechanical properties, water absorption, and flame retardancy. Materials. 2020;13:5145. doi: 10.3390/ma13225145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chanda A., Sinha S.K., Datla N.V. The influence of fiber alignment, structure and concentration on mechanical behavior of carbon nanofiber/epoxy composites: Experimental and numerical study. Polymer Compos. 2021;42:1155–1173. doi: 10.1002/pc.25890. [DOI] [Google Scholar]
- 90.Garcia-Zetina F., Martinez E., Alvarez-Castillo A., Castano V. Numerical analysis of the experimental mechanical properties in polyester resins reinforced with natural fibers. J. Reinf. Plast. Compos. 1995;14:641–649. doi: 10.1177/073168449501400605. [DOI] [Google Scholar]
- 91.Tan P., Tong L., Steven G. Modelling for predicting the mechanical properties of textile composites—A review. Compos. Part A Appl. Sci. Manuf. 1997;28:903–922. doi: 10.1016/S1359-835X(97)00069-9. [DOI] [Google Scholar]
- 92.Pathan M., Ponnusami S., Pathan J., Pitisongsawat R., Erice B., Petrinic N., Tagarielli V. Predictions of the mechanical properties of unidirectional fibre composites by supervised machine learning. Sci. Rep. 2019;9:13964. doi: 10.1038/s41598-019-50144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulenga T.K., Ude A.U., Vivekanandhan C. Techniques for modelling and optimizing the mechanical properties of natural fiber composites: A review. Fibers. 2021;9:6. doi: 10.3390/fib9010006. [DOI] [Google Scholar]
- 94.Vepari C., Kaplan D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sirichaisit J., Brookes V.L., Young R.J., Vollrath F. Analysis of structure/property relationships in silkworm (Bombyx mori) and spider dragline (Nephila edulis) silks using Raman spectroscopy. Biomacromolecules. 2003;4:387–394. doi: 10.1021/bm0256956. [DOI] [PubMed] [Google Scholar]
- 96.Cunniff P.M., Fossey S.A., Auerbach M.A., Song J.W., Kaplan D.L., Adams W.W., Eby R.K., Mahoney D., Vezie D.L. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym. Adv. Technol. 1994;5:401–410. doi: 10.1002/pat.1994.220050801. [DOI] [Google Scholar]
- 97.Gosline J.M., Guerette P., Ortlepp C., Savage K. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999;202:3295–3303. doi: 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- 98.Kumar S., Doshi H., Srinivasarao M., Park J.O., Schiraldi D.A. Fibers from polypropylene/nano carbon fiber composites. Polymer. 2002;43:1701–1703. doi: 10.1016/S0032-3861(01)00744-3. [DOI] [Google Scholar]
- 99.Rahmathulla V. Management of climatic factors for successful silkworm (Bombyx mori L.) crop and higher silk production: A review. Psyche J. Entomol. 2012;2012:121234. [Google Scholar]
- 100.Jin H.J., Kaplan D.L. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 101.Servoli E., Maniglio D., Motta A., Migliaresi C. Folding and assembly of fibroin driven by an AC electric field: Effects on film properties. Macromol. Biosci. 2008;8:827–835. doi: 10.1002/mabi.200800057. [DOI] [PubMed] [Google Scholar]
- 102.Wen H., Lan X., Zhang Y., Zhao T., Wang Y., Kajiura Z., Nakagaki M. Transgenic silkworms (Bombyx mori) produce recombinant spider dragline silk in cocoons. Mol. Biol. Rep. 2010;37:1815–1821. doi: 10.1007/s11033-009-9615-2. [DOI] [PubMed] [Google Scholar]
- 103.Teulé F., Miao Y.G., Sohn B.H., Kim Y.S., Hull J.J., Fraser M.J., Jr., Lewis R.V., Jarvis D.L. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Natl. Acad. Sci. USA. 2012;109:923–928. doi: 10.1073/pnas.1109420109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hakimi O., Knight D.P., Vollrath F., Vadgama P. Spider and mulberry silkworm silks as compatible biomaterials. Compos. Part B Eng. 2007;38:324–337. doi: 10.1016/j.compositesb.2006.06.012. [DOI] [Google Scholar]
- 105.Jiang C., Wang X., Gunawidjaja R., Lin Y.H., Gupta M.K., Kaplan D.L., Naik R.R., Tsukruk V.V. Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 2007;17:2229–2237. doi: 10.1002/adfm.200601136. [DOI] [Google Scholar]
- 106.Burger D., Beaumont M., Rosenau T., Tamada Y. Porous silk fibroin/cellulose hydrogels for bone tissue engineering via a novel combined process based on sequential regeneration and porogen leaching. Molecules. 2020;25:5097. doi: 10.3390/molecules25215097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu X., Wang L., Xu B., Wang P., Zhou M., Yu Y., Yuan J. Conjugation of CMCS to silk fibroin for tuning mechanical and swelling behaviors of fibroin hydrogels. Eur. Polym. J. 2021;150:110411. doi: 10.1016/j.eurpolymj.2021.110411. [DOI] [Google Scholar]
- 108.Shinde S.S., Salve A., Kulkarni S. Theoretical modeling of mechanical properties of woven jutefiber reinforced polyurethanecomposites. Mater. Today Proc. 2017;4:1683–1690. doi: 10.1016/j.matpr.2017.02.008. [DOI] [Google Scholar]
- 109.Lin X.L., Gao L.L., Li R.X., Cheng W., Zhang C.Q., Zhang X.Z. Mechanical property and biocompatibility of silk fibroin–collagen type II composite membrane. Mater. Sci. Eng. C. 2019;105:110018. doi: 10.1016/j.msec.2019.110018. [DOI] [PubMed] [Google Scholar]
- 110.Guan J., Zhu W., Liu B., Yang K., Vollrath F., Xu J. Comparing the microstructure and mechanical properties of Bombyx mori and Antheraea pernyi cocoon composites. Acta Biomater. 2017;47:60–70. doi: 10.1016/j.actbio.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 111.Zhang X., Ries M.E., Hine P.J. Time–Temperature Superposition of the Dissolution of Silk Fibers in the Ionic Liquid 1-Ethyl-3-methylimidazolium Acetate. Biomacromolecules. 2021;22:1091–1101. doi: 10.1021/acs.biomac.0c01467. [DOI] [PubMed] [Google Scholar]
- 112.Holland C., Numata K., Rnjak-Kovacina J., Seib F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019;8:1800465. doi: 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- 113.El Seoud O.A., Jedvert K., Kostag M., Possidonio S. Cellulose, chitin and silk: The cornerstones of green composites. Emergent Mater. 2021;5:785–810. doi: 10.1007/s42247-021-00308-0. [DOI] [Google Scholar]
- 114.Tsioris K., Raja W.K., Pritchard E.M., Panilaitis B., Kaplan D.L., Omenetto F.G. Fabrication of silk microneedles for controlled-release drug delivery. Adv. Funct. Mater. 2012;22:330–335. doi: 10.1002/adfm.201102012. [DOI] [Google Scholar]
- 115.Stinson J.A., Raja W.K., Lee S., Kim H.B., Diwan I., Tutunjian S., Panilaitis B., Omenetto F.G., Tzipori S., Kaplan D.L. Silk Fibroin Microneedles for Transdermal Vaccine Delivery. ACS Biomater. Sci. Eng. 2017;3:360–369. doi: 10.1021/acsbiomaterials.6b00515. [DOI] [PubMed] [Google Scholar]
- 116.Pramanik R., Arockiarajan A. Mechanical and morphological characterization of a novel silk/cellulose-based soft composite. Mater. Lett. 2022;314:131871. doi: 10.1016/j.matlet.2022.131871. [DOI] [Google Scholar]
- 117.Tandon S., Kandasubramanian B., Ibrahim S.M. Silk-Based Composite Scaffolds for Tissue Engineering Applications. Ind. Eng. Chem. Res. 2020;59:17593–17611. doi: 10.1021/acs.iecr.0c02195. [DOI] [Google Scholar]
- 118.Chen Z.J., Zhang Y., Zheng L., Zhang H., Shi H.H., Zhang X.C., Liu B. Mineralized self-assembled silk fibroin/cellulose interpenetrating network aerogel for bone tissue engineering. Mater. Sci. Eng. C. 2021;134:112549. doi: 10.1016/j.msec.2021.112549. [DOI] [PubMed] [Google Scholar]
- 119.Ang-atikarnkul P., Watthanaphanit A., Rujiravanit R. Fabrication of cellulose nanofiber/chitin whisker/silk sericin bionanocomposite sponges and characterizations of their physical and biological properties. Compos. Sci. Technol. 2014;96:88–96. doi: 10.1016/j.compscitech.2014.03.006. [DOI] [Google Scholar]
- 120.Singh B.N., Pramanik K. Generation of bioactive nano-composite scaffold of nanobioglass/silk fibroin/carboxymethyl cellulose for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2018;29:2011–2034. doi: 10.1080/09205063.2018.1523525. [DOI] [PubMed] [Google Scholar]
- 121.Narayanan V., Sumathi S. Preparation, characterization and in vitro biological study of silk fiber/methylcellulose composite for bone tissue engineering applications. Polym. Bull. 2019;76:2777–2800. doi: 10.1007/s00289-018-2518-4. [DOI] [Google Scholar]
- 122.Dorishetty P., Balu R., Athukoralalage S.S., Greaves T.L., Mata J., de Campo L., Saha N., Zannettino A.C.W., Dutta N.K., Choudhury N.R. Tunable Biomimetic Hydrogels from Silk Fibroin and Nanocellulose. ACS Sustain. Chem. Eng. 2020;8:2375–2389. doi: 10.1021/acssuschemeng.9b05317. [DOI] [Google Scholar]
- 123.Lee J.M., Kim J.H., Lee O.J., Park C.H. The fixation effect of a silk fibroin-bacterial cellulose composite plate in segmental defects of the zygomatic arch: An experimental study. JAMA Otolaryngol. Head Neck Surg. 2013;139:629–635. doi: 10.1001/jamaoto.2013.3044. [DOI] [PubMed] [Google Scholar]
- 124.Chen J., Zhuang A., Shao H., Hu X., Zhang Y. Robust silk fibroin/bacterial cellulose nanoribbon composite scaffolds with radial lamellae and intercalation structure for bone regeneration. J. Mater. Chem. B. 2017;5:3640–3650. doi: 10.1039/C7TB00485K. [DOI] [PubMed] [Google Scholar]
- 125.Eivazzadeh-Keihan R., Khalili F., Khosropour N., Aliabadi H.A.M., Radinekiyan F., Sukhtezari S., Maleki A., Madanchi H., Hamblin M.R., Mahdavi M., et al. Hybrid Bionanocomposite Containing Magnesium Hydroxide Nanoparticles Embedded in a Carboxymethyl Cellulose Hydrogel plus Silk Fibroin as a Scaffold for Wound Dressing Applications. ACS Appl. Mater. Interfaces. 2021;13:33840–33849. doi: 10.1021/acsami.1c07285. [DOI] [PubMed] [Google Scholar]
- 126.Valencia–Lazcano A.A., Román–Doval R., De La Cruz–Burelo E., Millán–Casarrubias E.J., Rodríguez–Ortega A. Enhancing surface properties of breast implants by using electrospun silk fibroin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018;106:1655–1661. doi: 10.1002/jbm.b.33973. [DOI] [PubMed] [Google Scholar]
- 127.Carrasco-Torres G., Valdés-Madrigal M.A., Vásquez-Garzón V.R., Baltiérrez-Hoyos R., la Cruz-Burelo E.D.D., Román-Doval R., Valencia-Lazcano A.A. Effect of Silk Fibroin on cell viability in electrospun scaffolds of polyethylene oxide. Polymers. 2019;11:451. doi: 10.3390/polym11030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lan D., Liu Z., Zhou J., Xu M., Li Z., Dai F. Preparation and characterization of silk fibroin/polyethylene oxide nanofiber membranes with antibacterial activity. J. Biomed. Mater. Res. Part A. 2022;110:287–297. doi: 10.1002/jbm.a.37285. [DOI] [PubMed] [Google Scholar]
- 129.Feng Y., Li X., Li M., Ye D., Zhang Q., You R., Xu W. Facile Preparation of Biocompatible Silk Fibroin/Cellulose Nanocomposite Films with High Mechanical Performance. ACS Sustain. Chem. Eng. 2017;5:6227–6236. doi: 10.1021/acssuschemeng.7b01161. [DOI] [Google Scholar]
- 130.Park C.H., Jeong L., Cho D., Kwon O.H., Park W.H. Effect of methylcellulose on the formation and drug release behavior of silk fibroin hydrogel. Carbohydr. Polym. 2013;98:1179–1185. doi: 10.1016/j.carbpol.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 131.Lujerdean C., Baci G.M., Cucu A.A., Dezmirean D.S. The Contribution of Silk Fibroin in Biomedical Engineering. Insects. 2022;13:286. doi: 10.3390/insects13030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wahab N., Saeed M., Ibrahim M., Munir A., Saleem M., Zahra M., Waseem A. Synthesis, Characterization, and Applications of Silk/Bentonite Clay Composite for Heavy Metal Removal From Aqueous Solution. Front. Chem. 2019;7:654. doi: 10.3389/fchem.2019.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mayank , Bardenhagen A., Sethi V., Gudwani H. Spider-silk composite material for aerospace application. Acta Astronaut. 2022;193:704–709. doi: 10.1016/j.actaastro.2021.08.013. [DOI] [Google Scholar]
- 134.Hadadi A., Whittaker J.W., Verrill D.E., Hu X., Larini L., Cruz D.S.D.L. A Hierarchical Model to Understand the Processing of Polysaccharides/Protein-Based Films in Ionic Liquids. Biomacromolecules. 2018;19:3970–3982. doi: 10.1021/acs.biomac.8b00903. [DOI] [PubMed] [Google Scholar]
- 135.Kimura T., Aoki S. Application of silk composite to decorative laminate. Adv. Compos. Mater. Off. J. Jpn. Soc. Compos. Mater. 2007;16:349–360. doi: 10.1163/156855107782325212. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and sources available upon request from authors.