Abstract
The S‐layer or surface layer protein (SLP) is the most ancient biological envelope, highly conserved in several Bacteria and Archaea. In lactic acid bacteria (LAB), SLP is only found in species belonging to the Lactobacillaceae family, many of them considered probiotic microorganisms. New reclassification of members within the Lactobacillaceae family (International Journal of Systematic and Evolutionary Microbiology, 2020, 70, 2782) and newly sequenced genomes demands an updated revision on SLP genes and domain organization. There is growing information concerning SLP occurrence, molecular biology, biophysical properties, and applications. Here, we focus on the prediction of slp genes within the Lactobacillaceae family, and specifically, on the neat interconnection between the two different modular SLP domain organizations and the new reclassified genera. We summarize the results in a concise tabulated manner to review the present knowledge on SLPs and discuss the most relevant and updated concepts regarding SLP sequence clustering. Our assessment is based on sequence alignments considering the new genera classification and protein domain definition with post‐translational modifications. We analyse the difficulties encountered to resolve the SLPs 3D structure, describing the need for structure prediction approaches and the relation between protein structure and its anchorage mechanism to the cell wall. Finally, we enumerate new SLP applications regarding heterologous display, pathogen exclusion, immunostimulation, and metal binding.
Manuscript is focus on the prediction of slp genes within the Lactobacillaceae family and interconnection between the two different modular SLP domain organizations of the new reclassified genera. SLP applications are reviewed regarding heterologous display, pathogen exclusion, immunostimulation, and metal binding.
INTRODUCTION
Lactic acid bacteria (LAB) are a varied group of Gram‐positive, low GC, microaerophilic, acid‐tolerant, non‐spore‐forming bacteria comprising five families: Lactobacillaceae, Streptococcaceae, Enterococcaceae, Carnobacteriaceae, and Aerococcaceae, belonging to the Phylum Firmicutes, Class Bacilli, Order Lactobacillales (Zheng et al., 2020). Coming from various plant and animal niches, some members of these families are used in food fermentation, lactic acid being the primary fermentation product (Oberg et al., 2022). Moreover, within the Lactobacillaceae family (https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=33958), some members are generally recognized as safe (GRAS) due to their abundant appearance in food (Carasi et al., 2021; Lebeer et al., 2018; Oberg et al., 2022; Salvetti & O’Toole, 2017; Stefanovic & McAuliffe, 2019; Sun et al., 2015; Zheng et al., 2020).
Bacterial S‐layer, found in several Bacteria and Archaea, consists of a two‐dimensional self‐assembling crystalline array of proteins or glycoproteins subunits, determining a semi‐porous proteinaceous and the outermost component of the cellular envelope. The SLP coat exhibits a thickness between 5 and 25 nm and pore size from 2 to 8 nm, covering the rigid cell wall matrix. In Gram‐positive bacteria, SLP binds non‐covalently to the cell wall components, primarily peptidoglycan and other components, such as proteins, teichoic and lipoteichoic acids, and other acidic or neutral polysaccharides known as secondary cell wall polymers (SCWP) (Sleytr et al., 2014; Zhu et al., 2016). As it is the outer layer of the cell wall, SLP participates in the maintenance of the bacterial cellular shape and host interaction with the external environment, as well as acting as a molecular sieve in the exchange of nutrients and metabolites (Gerbino, Carasi, Mobili, et al., 2015; Hynönen & Palva, 2013). Here, we present updated knowledge on the SLPs found in species belonging to the Lactobacillaceae family.
PREDICTION OF SURFACE LAYER PROTEIN GENE
SLP from LAB has only been identified in species belonging to the Lactobacillaceae family, particularly in the Lactobacillus genus. Noteworthy, this genus has been recently split into more than 20 new genera (Zheng et al., 2020) and includes a variety of probiotic strains. Figure 1 shows the search results within the Prokaryotic Synteny & Taxonomy Explorer, SyntTax (https://archaea.i2bc.paris‐saclay.fr/SyntTax/Default.aspx) (Oberto, 2013), in which the query protein is translated in the six frames using the TBLASTN algorithm and matched against the selected chromosomes of completely sequenced NCBI reference genomes available for Lactobacillaceae family. The predominant SLPs were selected as query proteins, such as the SlpA protein sequences from Lactobacillus acidophilus (Accession YP_193101.1), Lentilactobacillus kefiri (Accession SCA78670.1), and Levilactobacillus brevis (Accession ARW51672). We were able to observe a correlation between gene synteny and taxonomy (Figure 1). When phylogenetic trees were compared, based on the alignment of the 16S rRNA gene sequences and the SLP sequences, we could verify this correlation in concordance with the new classification described by Zheng et al. (2020).
FIGURE 1.
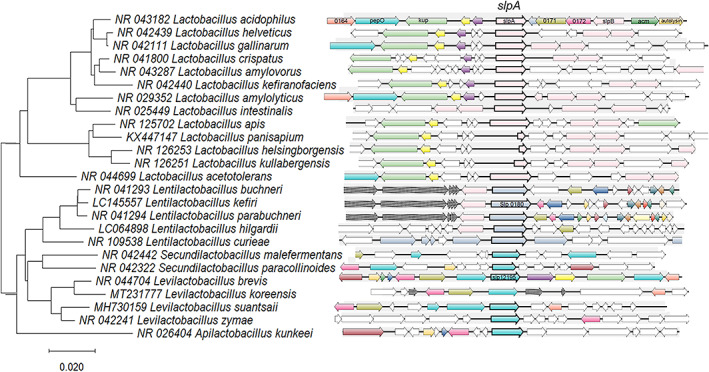
Phylogenetic alignment of the 16S rRNA and synteny. Left panel: Neighbour‐joining phylogenetic tree based on concatenated alignments of 16S rRNA genes from different species, obtained using MEGA X software (Kumar et al., 2018). The tree is drawn to scale. Branch length units are the same as those of the evolutionary distances used to infer the phylogenetic relationship, and the scale bar indicates the phylogenetic distances. NCBI Accession numbers are detailed for each protein and species. Right panel: SyntTax web service results for each species. The query protein (bolded arrow) is matched against the selected chromosomes, translated in the six frames using the TBLASTN algorithm. The DNA 15,000 bp sequence segment is centred on the TBLASTN hit and translated to all the open reading frames according to GenBank annotations. Paralogues are indicated by an identical colour.
Using this approach, we were able to re‐define gene sequences annotated with hypothetical functions that according to synteny, are potential S‐layer coding genes in Lactobacillus (Lb.), Lentilactobacillus (Len.), and Levilactobacillus (Lev.) species. Although automatic annotation has evolved, there are still hypothetical functions that include S‐layer‐associated protein (SLAP) domain predictions. Specifically, annotation as a hypothetical protein was found in the case of Lb. acetotolerans LA749 (LA749_00955), Lb. amylolyticus L6 (B1745_00825), Lb. helsingborgensis ESL0183 (DLD54_07660), Lb. kullabergensis ESL0186 (DKL58_08285), and Lb. panisapium ESL0416 (GYM71_09165), or SLAP domain‐containing proteins in Lb. intestinalis DSM 6629 (KBW87_00805) and Lb. kefiranofaciens 1207 (ICI50_01090). A high syntenic distribution could argue that those genes are, in fact, coding for SLP in those species. Synteny was found within the Lactobacillus genus in the following species: Lb. crispatus, Lb. helveticus, Lb. acidophilus, Lb. amylovorus, Lb. kefiranofaciens, Lb. gallinarum, Lb. panisapium, Lb. intestinalis, Lb. apis, Lb. acetotolerans, Lb. helsingborgensis, Lb. kullabergensis.
Secondary slp genes, slpB, and slpX, were also found in all analysed species, although not conserved (Table 1A and Figure 2). Complete genome analysis using SyntTax web service and TBLASTN normalized scores allowed to verify the presence of slpB and slpX genes. Since high sequence similarity can be found between SlpA and SlpB, a positive score was only inferred in those genomes where two different copies of the SLP protein coding gene were found, and duplicated results were discarded. According to synteny results, slpB and slpX were not predicted in all species using this approach (Table 1A). In Lactobacillus, we observed that slpX gene synteny was conserved for the species Lb. acidophilus, Lb. gallinarum, Lb. amylovorus, Lb. helveticus, Lb. intestinalis, Lb. crispatus, and Lb. kefiranofaciens (Table 1A and Figure 2). Nevertheless, slpB was found to a lesser extent.
TABLE 1.
Heatmap table for slp genes in (A) Lactobacillus sp. (B) Lentilactobacillus and Levilactobacillus using SyntTax web service.
| (A) | Percent normalized TBlastN score | ||
|---|---|---|---|
| Genome | slpA | slpB a | slpX |
| Query protein (Accession No.) | YP_193101.1 | CAA61561.1 | KHE30430.1 |
| Lactobacillus acidophilus La 14 | 80 | 88 | 79 |
| Lactobacillus gallinarum HFD4 | 52 | 0 | 15 |
| Lactobacillus amylovorus GRL1118 | 35 | 0 | 46 |
| Lactobacillus helveticus FAM22155 | 33 | 0 | 15 |
| Lactobacillus intestinalis DSM 6629 | 30 | 20 | 16 |
| Lactobacillus crispatus DC21 1 | 28 | 33 | 50 |
| Lactobacillus amylolyticus L6 | 27 | 0 | 0 |
| Lactobacillus kefiranofaciens 1207 | 26 | 0 | 60 |
| Lactobacillus panisapium ESL0416 | 16 | 0 | 0 |
| Lactobacillus helsingborgensis ESL0183 | 16 | 0 | 0 |
| Lactobacillus kullabergensis ESL0186 | 15 | 0 | 0 |
| Lactobacillus apis ESL0185 | 15 | 0 | 0 |
| Lactobacillus acetotolerans LA749 | 13 | 0 | 0 |
| (B) | Percent normalized TBlastN score | |||
|---|---|---|---|---|
| Genome | slpA Lentilactobacillus | slpB Lentilactobacillus | slpA Levilactobacillus | slpB Levilactobacillus |
| Query protein (Accession No.) | SCA78670.1 | QGV24204.1 | ANN49967.1 | AAK84948.1 |
| Lentilactobacillus kefiri DH5 | 97 | 84 | 24 | 20 |
| Lentilactobacillus buchneri ATCC_4005 | 84 | 71 | 25 | 21 |
| Lentilactobacillus parabuchneri KEM | 79 | 69 | 26 | 21 |
| Lentilactobacillus hilgardii LMG 07934 | 51 | 19 | 25 | 22 |
| Lentilactobacillus curieae CCTCC M 2011381 | 20 | 28 | 20 | 23 |
| Levilactobacillus brevis NPS QW 145 | 26 | 17 | 91 | 86 |
| Levilactobacillus zymae LZ395 | 29 | 16 | 33 | 18 |
| Levilactobacillus koreensis 26 25 | 21 | 17 | 25 | 17 |
| Levilactobacillus suantsaii CBA3634 | 21 | 16 | 27 | 18 |
Note: Green and yellow represent the percent normalized TBlastN score for specific genes in the reference genomes analysed. Red represents the absence of gene similarity. Scores below 20% are observed for gene annotation defined as S‐layer‐associated protein (SLAP) domain‐containing protein or hypothetical protein.
Only different loci were evaluated.
FIGURE 2.
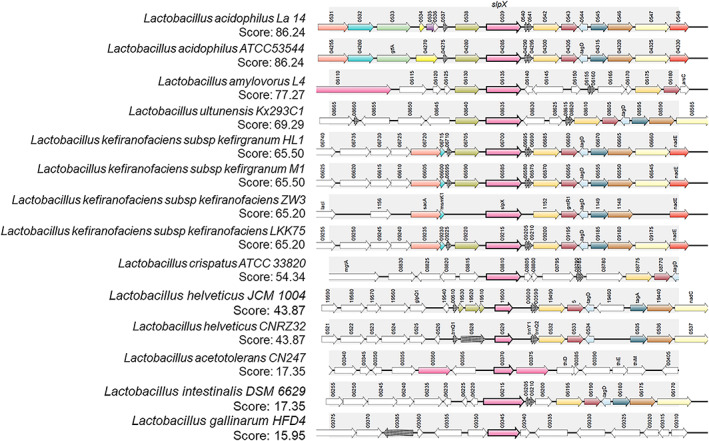
Synteny results for SlpX in Lactobacillus. The query protein SlpX (Lactobacillus acidophilus, Accession No. KHE30430.1, pink bolded arrow) is matched against the selected chromosomes translated in the six frames using the TBLASTN algorithm in SyntTax web service. The 15,000 bp DNA sequence segment is centred on the TBLASTN hit and translated to all the open reading frames according to GenBank annotations. Paralogues are indicated by identical colour. TBLASTN scores are shown.
In line with these findings, Johnson et al. (2016) reported that three genes can be found within members of the Lb. acidophilus homology group (including Lb. acidophilus, Lb. helveticus, Lb. crispatus, Lb. amylovorus, Lb. gallinarum): slpA, slpB, and slpX, being slpA and slpB in opposite orientation to each other. Interestingly, the presence or not of secondary slp genes in strains of Lb. helveticus showed high variability by comparative genomic analyses (Waśko et al., 2014) and consequently, they could be used for strain typing in dairy products (Moser et al., 2017). Also, Fontana et al. (2019) found an increased number of slp genes in isolates from natural whey cultures compared to other Lb. helveticus strains previously reported. Genomic instability could lead to chromosomal rearrangements, such as duplications, due mainly to the presence of mobile genetic elements, such as insertion sequences (IS) (Fontana et al., 2019). Regarding Lb. gallinarum strains, prior genomic research indicate that they have two genes encoding SLPs: one similar to that of the genus and another that is strain‐specific; however, each strain produces a single SLP, always encoded by the strain‐specific gene (Johnson et al., 2016). Although synteny between chromosomal regions was not complete among the compared strains (Lb. crispatus, Lb. acidophilus, Lb. amylovorus, and Lb. helveticus), genes were positioned in an overall syntenic organization, where the N‐acetylmuramidase and autolysin/amidase are directly downstream of the genes encoding the primary SLP, slpA and slpB (Palomino et al., 2015; Johnson et al., 2016). Although some new common features could be considered signature sequences (as discussed below), confirmation of the SLP presence still relies on electron microscopy due to the differences in the predicted protein sequences for each genus. Xing et al. (2017) found four SLPs in the Lb. kefiranofaciens ZW3 genome isolated from traditional functional fermentation product kefir. The larger number of SLPs in this strain suggests a higher advantage for adhesion in the gastrointestinal tract (GIT).
Lentilactobacillus and Levilactobacillus shared the same slpA gene distribution concerning genetic context (Figure 1). Synteny was found in the genus Lentilactobacillus in the following species: Len. buchneri, Len. kefiri, Len. parabuchneri, Len. curieae, Len. hilgardii; and in the Levilactobacillus genus for Lev. brevis, Lev. zymae, Lev. koreensis, Lev. suantsaii. The main slp genes are presented in Table 1B. The heterogeneity distribution of slp genes has been previously described in Len. buchneri DSM 20057 presenting at least four genes with different molecular weights (MW) as predicted products. Likewise, two complete genes and one truncated SLP gene have been identified by homology in Lev. brevis ATCC 367 (Makarova et al., 2006).
Although there may still be concealed sequences in the automatic gene annotation, many other genes will arise in the coming years. The genetic arrangement of the multiple SLP genes in lactobacilli is genus‐dependent at some degree, and there is no genetic organization based on a consensus synteny structure. Considering this approach and based on the similarity to SLPs from Len. kefiri and Lev. brevis, we were able to find three recently sequenced species encoding S‐layer genes: Secundilactobacillus malefermentans and Secundilactobacillus paracollinoides bacteria isolated from food fermentation or brewery environments (Jiang et al., 2022), and Apilactobacillus kunkeei an insect symbiont with similarity to Lev. brevis (Vergalito et al., 2020) isolated from an environmental source according to the GenBank record.
The following list of genomes do not have synteny for genes sequences that matched the SLPs used as queries: Acetilactobacillus jinshanensis, Amylolactobacillus amylophilus, Apilactobacillus bombintestini, Api. kunkeei, Bombilactobacillus bombi, Companilactobacillus alimentarius, Co. allii, Co. crustorum, Co. farciminis, Co. futsaii, Co. ginsenosidimutans, Co. heilongjiangensis, Co. pabuli, Co. paralimentarius, Co. zhachilii, Fructilactobacillus fructivorans, Fru. sanfranciscensis, Furfurilactobacillus rossiae, Lacticaseibacillus paracasei, Lcb. rhamnosus, Lactiplantibacillus plantarum, Lactobacillus delbrueckii subsp bulgaricus, Lb. gasseri, Lb. gasseri, Lb. iners, Lb. jensenii, Lb. johnsonii, Lb. paragasseri, Lb. taiwanensis, Lb. terrae, Lapidilactobacillus dextrinicus, Latilactobacillus curvatus, Lat. sakei, Ligilactobacillus animalis, Lig. ruminis, Lig. salivarius, Limosilactobacillus fermentum, Lim. mucosae, Lim. reuteri, Lim. vaginalis, Liquorilactobacillus mali, Loigolactobacillus coryniformis, Paucilactobacillus oligofermentans, Schleiferilactobacillus harbinensis.
SLP GENE EXPRESSION
SLPs can account for 10–15% of cell proteins. This high level of expression is a consequence of the presence of a strong slp gene promoter, but it also involves a high mRNA stability. While decay rates for mRNAs in Gram‐positive bacteria for about 80% of their genes had a half‐life of <7 min (Hambraeus et al., 2003), slp mRNA had a calculated half‐life of 15 min (Boot & Pouwels, 1996, Sun et al., 2013). 5′untranslated region (5′UTR) of slpA mRNA can influence translation efficiency by forming a stable stem‐loop structure that stabilizes the transcript and expose the ribosomal binding site. Actually, truncation of this sequence results in a significant reduction of expression efficiency (Boot & Pouwels, 1996; Antikainen et al., 2002; Sun et al., 2013). In order to develop a high‐level expression system for LAB, expression signals were identified upstream of the slpA gene of Lev. brevis and Lb. acidophilus and used in the low‐copy‐number vector pKTH2095 to express GusA and PepN reporter proteins in Lactococcus lactis, Lactiplantibacillus plantarum, and Lb. gasseri strains (Peterbauer et al., 2019). SLP encoding genes have been sequenced and cloned from species such as Lev. brevis, Lb. acidophilus, Lb. helveticus, and Lb. crispatus. SLP genes are preceded by more than one promoter, increasing the transcription efficiency and regulating the S‐layer gene expression in response to, for instance, growth stage or environmental conditions. As one of the most abundant cellular proteins, SLP expression takes place during all stages of the bacterial growth cycle. Bacterial cells need to synthesize, translocate to the surface, and incorporate this protein into the existing S‐layer lattice at high growth rates ‐around 500 subunits per second. At least 5 × 105 SLP subunits are needed during each cell generation when considering an average‐size cell (Hynönen & Palva, 2013). Studies on the upstream slpA sequence of Lev. brevis ATCC 8287 and Lb. acidophilus ATCC 4356 showed that there are two subsequent promoters’ sequences (P1 and P2) with no evidence of regulation during bacterial growth, at least under the conditions tested (Hynönen et al., 2014). Two promoters might offer a possibility to enhance and/or regulate gene expression. Notably, Klotz and Barrangou (2018) have recently reviewed the versatility of slp promoters for heterologous protein expression in various LAB hosts.
The transition from logarithmic to stationary phase produces notable SLP fluctuations (Klotz et al., 2017; Palomino et al., 2016) as well as other growth stage‐dependent cell surface characteristics, like cell wall integrity. SLPs are present during all growth stages, and genes are preferentially expressed in the stationary phase since this is the most similar condition to the GIT. The stationary phase cell needs to increase slp expression to maintain the integrity of the cell envelope structure. The fact that a differential peptidoglycan amount is found between SLP‐harbouring species and non‐SLP species could explain why S‐layer is required for growth in normal conditions. A decrease in peptidoglycan is observed in high salt conditions, and a consequent increase in fragility determines the need for the external highly compact S‐layer component (Palomino et al., 2016). Differential SLP expression profiles have been observed for Lb. acidophilus ATCC 4356 in the exponential or stationary growth phase at high salt concentrations. Interestingly, slp knock‐out mutants in Lb. acidophilus NCFM expressed slpA and slpX or slpB and slpX simultaneously with a differential SLAP expression pattern (Selle et al., 2017). Also, Hynönen and Palva (2013) postulated the impossibility of creating an entirely negative S‐layer mutant, indicating that at least one functional slp gene is essential for the growth of species carrying S‐layer. Therefore, since multiple simultaneous slp deletion mutants were proved to be non‐viable, it is difficult to assure that S‐layers are essential. In fact, the presence of S‐layer decreases susceptibility to mutanolysin (Valence & Lortal, 1995) and to extreme environmental conditions, including variations in pH, bile salts, proteases, and simulated gastrointestinal conditions (Eslami et al., 2013). Furthermore, removing the surface layer using lithium chloride drastically affects survival to osmotic stress or simulated gastric and intestinal conditions (Grosu‐Tudor et al., 2016; Khaleghi et al., 2010; Meng et al., 2014; Palomino et al., 2016). In Lb. acidophilus, slpA expression was increased in the presence of bile salts, acidic pH, and heat stress. The induction of slp expression may thus take part in a general strategy to adapt and survive the harsh conditions encountered in the environment and the digestive tract (Gerbino, Carasi, Mobili, et al., 2015). This opens interesting perspectives for using S‐layer as a protective coat for oral administration of unstable drug nanocarriers (Luo et al., 2019).
SLP DOMAINS ORGANIZATION
Lactobacillaceae SLPs are typically rich in basic and hydrophobic amino acids, exhibiting a generally high isoelectric point (pI) ‐between 9.4 and 10.4‐ and possess a molecular weight ranging from 35–71 kDa. In other Firmicutes, SLPs are larger and rich in acidic amino acids with a low pI. Although differences in SLP primary sequence are usually found, there are multiple similarities in terms of amino acid composition: high content of hydrophobic amino acids (31–39%), hydroxylated amino acids, serine, and threonine (23–33%), absence of cysteine residues, and the highest ratio of positively/negatively (Arg + Lys/Asp + Glu) charged residues, defining a high pI (Malamud, Bolla, et al., 2019).
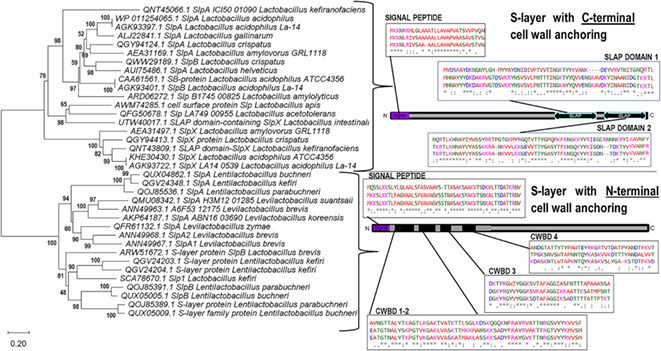
We were able to distinguish a clear clustering of structural characteristics in phylogenetic trees based on deduced SLP sequences (Figure 3) by selecting good integrity representative records from the NCBI database and predicted sequences in the reference genomes. As previous studies suggested, Lactobacillaceae SLPs comprise two‐modular regions with two essential domains: (1) the cell wall‐anchoring or attachment domain and (2) the self‐assembly domain (Prado‐Acosta et al., 2010; Smit et al., 2001, 2002). The disposition of these domains is also characteristic of different SLPs within the same species. This modular organization divides SLPs of Lentilactobacillus and Levilactobacillus species from those of Lactobacillus, in agreement with the new taxonomy classification (Figure 3). Although belonging to a different genus, SLP from Lentilactobacillus and Levilactobacillus shared similarity in primary sequence as deduced from BLAST scores in Table 1B. In Lactobacillus, the amino‐terminal (N‐terminal) region is involved in protein self‐assembly, and the carboxy‐terminal (C‐terminal) region in cell wall anchoring. On the contrary, in Levilactobacillus and Lentilactobacillus, the C‐terminal part is involved in self‐assembly and the N‐terminal part in cell wall anchoring, as detailed in Tables 2 and 3.
FIGURE 3.
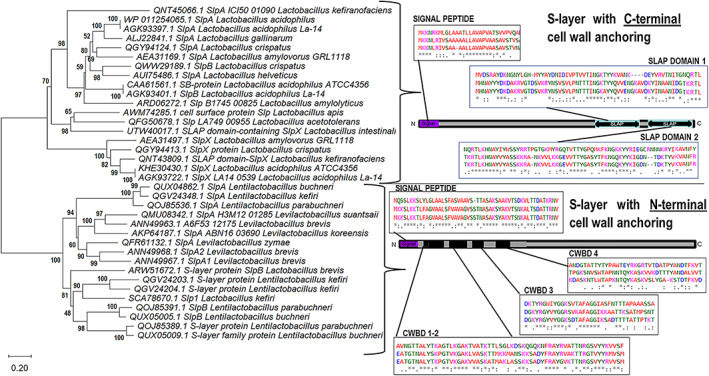
Neighbour‐joining phylogenetic tree based on concatenated alignments of S‐layer proteins. Neighbour‐joining phylogenetic tree based on concatenated alignments of S‐layer proteins from different Lactobacillaceae species using MEGA X software (Kumar et al., 2018). NCBI Accession numbers are detailed for each protein and species. The tree is drawn to scale. Branch length units are the same as those of the evolutionary distances used to infer the phylogenetic relationship. The scale bar indicates the phylogenetic distances expressed as the number of amino acid substitutions per sequence. Bootstrap values are indicated at the 500‐replicate nodes. Grouped proteins with similar domain dispositions (braces) are represented in the sketch from N‐terminal to C‐terminal. Signal peptide, S‐layer‐associated protein (SLAP) domains or cell wall‐binding domain (CWBD) are indicated in positions where the percentage of identical amino acids and conserved amino acids substitutions are higher than 50% from the sequence alignments (see Figure S1) ‘*’. indicates identical amino acid, ‘:’ indicates group similarity, ‘.’ indicates low group similarity.
TABLE 2.
Self‐assembly and adhesin regions in S‐layer proteins (SLPs) of Lactobacillaceae species.
| Strain | S‐layer protein | Location of Self‐assembly regions (residues/total residues) | Target molecule | Reference |
|---|---|---|---|---|
| Lactobacillus acidophilus ATCC 4356 | SlpA | N‐terminal (32–238/444) |
Fibronectin Collagen (I, IV) Mucin DC‐SIGN |
Martínez et al. (2012), Prado et al. (2016, 2019), Smit et al. (2001, 2002) |
| Lactobacillus acidophilus CICC 6074 | SlpA |
N‐terminal (32–55/444) N‐terminal (102–114, 170–174, 218–236/444) |
HT‐29 cells Membrane polar lipid liposomes |
Kong et al. (2022) |
|
Lactobacillus crispatus JCM 5810 |
CbsA |
N‐terminal (32–271/410) N‐terminal (1–287, 1–274, 31–287) |
Collagen (I, IV) Laminin |
Antikainen et al. (2002), Sillanpää et al. (2000) |
| Lactobacillus helveticus CNRZ 892 | SlpA | N‐terminal |
MIMLh5 TLR2 |
Sun et al. (2015) |
| Lactobacillus helveticus M92 | SLP | Not defined |
Fibronectin Laminin Collagen |
Uroić et al. (2016) |
| Leviactobacillus brevis D6 | SLP | Not defined |
Fibronectin Laminin Collagen |
Uroić et al. (2016) |
| Levilactobacillus brevis ATCC 8287 | SlpA | C‐terminal (179–435/435) |
Fibronectin Laminin |
Åvall‐Jääskeläinen et al. (2008), Pum et al. (2013) |
| Levilactobacillus brevis ATCC 367 | SlpA | C‐terminal (179–435/435) | Not defined | Rykov et al. (2018) |
| Lentiactobacillus kefiri CIDCA 8348 | SLP | Not defined |
Mincle SingR3 mLangerin DC‐SIGN |
Malamud et al. (2018) |
TABLE 3.
Interactions of S‐layer proteins (SLPs) of Lactobacillaceae species with the cell wall.
| Strain | S‐layer protein Accession No. | MW (kDa) pI Nº residues | Interaction site in S‐layer protein (CWBD residues numbers) | Cell wall receptor | Reference |
|---|---|---|---|---|---|
|
Lactobacillus acidophilus ATCC 4356 |
SlpA |
46.6 kDa pI: 9.59 444 aa |
C‐terminal 322–378 and 387–444 |
Wall teichoic acids Lipoteichoic acids |
Fina et al. (2019), Smit and Pouwels (2002) |
| Lactobacillus crispatus JCM 5810 |
CbsA |
46.7 kDa pI: 9.69 440 aa |
C‐terminal 251–410 288–410 |
Wall teichoic acids Lipoteichoic acids Negatively charged cell wall components |
Antikainen et al. (2002) |
| Lactobacillus crispatus K313 |
SlpB |
56 kDa pI: 9.48 533 aa |
C‐terminal 380–501 |
Teichoic acids | Sun et al. (2013) |
| Levilactobacillus brevis ATCC 8287 |
SlpA |
48.2 kDa pI: 9.45 435 aa |
N‐terminal 60–90 and 165–192 |
Probable neutral polysaccharides | Åvall‐Jääskeläinen et al. (2008) |
| Lentilactobacillus buchneri CD034 |
SlpB |
58.3 kDa pI: 9.87 558 aa |
N‐terminal | Lipoteichoic acids | Anzengruber et al. (2014), Bönisch et al. (2018) |
| Lentilactobacillus hilgardii B706 |
SlpLH1 |
41 kDa pI: 9.6 417 aa |
N‐terminal | Cell wall | Dohm et al. (2011) |
SLP contains a conserved N‐terminal signal peptide of 25–30 amino acids (Figure 3 and Figure S1A) (Cavallero et al., 2017; Malamud et al., 2017; Palomino et al., 2016), indicative of its ability to be secreted out of the cell via the Sec‐pathway, similar to other Gram‐positives bacteria (Fagan & Fairweather, 2014). The signal peptide can trigger the robust secretion of target molecules and includes the A–X–A typical motif that precedes the cleavage site for type I signal peptidases commonly found in Gram‐positive bacteria (van Roosmalen et al., 2004). The similarities observed in the SLP signal peptide from Lactobacillus, Lentilactobacillus, and Levilactobacillus (Figure 3 and Figure S1B), correlate with the phylogenetic relationship that exists among these genera and species and their two‐modular primary organization (Johnson et al., 2016; Sun et al., 2015).
The self‐assembly domain was mapped for some species (Table 2). After conducting a Clustal‐O alignment of Lactobacillus SLPs (Figure S1), we observed that the N‐terminal region involved in protein self‐assembly was highly variable in sequence. In contrast, the C‐terminal, involved in the cell wall binding, appeared conserved between the species of the genus. The anchoring domain or cell wall‐binding domain (CWBD) has been proposed for these species due to the high similarity found between the C‐terminal region (Table 3) (Hynönen et al., 2014; Waśko et al., 2014). When SLPs from Levilactobacillus and Lentilactobacillus were aligned (Figure 3), it was evidenced that the more conserved N‐terminal part of the protein was involved in the cell wall anchoring, while the C‐terminal variable region was associated with self‐assembly (Table 2). Therefore, it was possible to predict by similarity search the position of these two functional domains (self‐assembly or CWBD).
SLPs act as adhesins, mediating the binding of bacteria carrying them to specific components of the extracellular matrix (ECM). The self‐assembly domain coincides in location with domains responsible for ECM binding proteins, such as collagen, fibronectin, laminin, mucin, and even to cells (Table 2). The adhesion to different cell lines has been evaluated, including Caco‐2 and Intestine 407, the endothelial cell line EA‐hy926, and the urinary bladder cell line T24, avian and porcine intestinal epithelial cells, HeLa cells, and HT29. The location of adhesion regions has already been revealed at the N‐terminal for Lactobacillus (Antikainen et al., 2002; Fina et al., 2019) or C‐terminal for Lentilactobacillus and Levilactobacillus (Anzengruber et al., 2014; Åvall‐Jääskeläinen et al., 2008; de Leeuw et al., 2006; Vilen et al., 2009) (Table 2). This binding activity is specifically thought to mediate bacterial colonization of the gut, contributing to the probiotic's interaction with the host tissues and other factors, such as cell surface hydrophobicity, auto‐aggregation, mucin‐ and fibronectin‐binding proteins interplay. Different authors have proved that SLPs extracted from probiotic Lactobacillus have the ability to in vitro bind host cells and extracellular matrix proteins (Carasi et al., 2014; Prado et al., 2019; Prado‐Acosta et al., 2010; Waśko et al., 2014; Zhang et al., 2017; Zhu et al., 2016).
Cell surface hydrophobicity and cell aggregation could be attributed to the S‐layer. SLP removal negatively affects the adherence of Lev. brevis D6 and Lb. helveticus M92 to cells and their aggregation ability (Uroić et al., 2016). However, this was not the case for Lb. amylovorus (Hynönen et al., 2014). As evidenced, adhesion to epithelial cells or mucus may not be a universal feature of every SLP. Multiple S‐layer sequence alignment revealed high similarity (>70%) within the Lb. acidophilus group in the C‐terminal region (Figure S1). When compared with Lb. amylolyticus, an intermediate sequence similarity (approx. 40 %) was found in this region and low similarity in those presenting a N‐terminal anchoring domain. In fact, previous reports have correlated differences in sequences with different immunological properties (Suzuki et al., 2019).
SLPS CELL WALL ANCHOR
Gram‐positive bacteria, Bacillus and related genus, present S‐layer homologous (SLH) domains involved in SLP cell wall anchoring (Allievi et al., 2014; Blackler et al., 2018; Janesch et al., 2013; Sleytr et al., 2014; Suhr et al., 2016). In contrast, members of the Lactobacillaceae species lack such motifs, presenting instead a conserved CWBD as well as negatively charged cell wall carbohydrates. These negatively charged secondary cell wall polymers (SCWP) and the highly basic amino acids in the cell wall‐binding region interact via direct hydrogen bonds or electrostatic interactions, mediating the attachment to the cell wall. The interactions between SLPs and SCWP can be considered lectin‐like with some degree of specificity in target recognition (Fina et al., 2019).
Conserved carbohydrate‐binding motifs are detected in the cell wall‐anchoring domain, consisting of high pI peptides with positively charged regions. Previous reports have evaluated the ability of these motifs to bind the cell wall by creating truncated recombinant proteins spanning the N and C‐terminal regions of SlpA (Åvall‐Jääskeläinen et al., 2008; Fina et al., 2019). Two repeated amino acid sequences are predicted in the SlpA N‐terminal regions of Levilactobacillus and C‐terminal regions of Lactobacillus with homology to the tyrosine/phenylalanine carbohydrate‐binding motifs of clostridial toxins and streptococcal glucosyltransferases (von Eichel‐Streiber et al., 1992; Wren et al., 1991). These regions have also been found in the amino acid sequences of mature SlpA, positions 60–90 and 165–192 of the Lev. brevis N‐terminal region or 322–378 and 387–444 of the Lb. acidophilus C‐terminal region (Table 3) (Åvall‐Jääskeläinen et al., 2008; Fina et al., 2019).
The C‐terminal regions of SLP are particularly basic in the Lb. acidophilus group and related to the pfam03217 SLAP domain, recently included in the Conserved Protein Domain Family at NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=427201, created on 26‐April‐2021 and updated 29‐September‐2021). The domain is about 60 residues long and usually occurs in tandem pairs. SLAP domains are found in N‐acetylmuramidase, lysis, and autolysin amidases of other species involved in the recognition of cell envelope structures. Particularly for Lactobacillus, they are found in species presenting SLPs with cell wall‐anchoring motifs in the C‐terminal region: Lb. kefiranofaciens, Lb. crispatus, Lb. helveticus, Lb. amylolyticus, Lb. gallinarum, Lb. acidophilus, Lb. amylovorus and Lb. intestinalis. Although SLAP domains are similar in size to SLH domains, there is no sequence similarity between these two motifs.
It has been experimentally confirmed that these SLAP domains are functional and required for cell wall interaction in Lb. acidophilus ATCC 4356 (Fina et al., 2019). Moreover, the C‐terminal region of the Lb. acidophilus SLP has been used for heterologous display of proteins on the cell surface (Gordillo et al., 2020; Uriza et al., 2020).
The C‐terminal binding domain represents one‐third of the Lb. acidophilus SlpA protein (Table 3). This domain interacts with negatively charged SCWP and/or neutral polysaccharides (Fina et al., 2019; Sleytr et al., 2014). In Gram‐positive bacteria, lipoteichoic acid (LTA) and wall teichoic acid (WTA) coexist in the cell wall; however, certain bacterial species, such as Lacticaseibacillus casei and Lcb. rhamnosus, only present LTA (Allievi et al., 2019). The LTA structures of lactic acid bacteria show high diversity among species in the length of the glycerol‐phosphate chains, the degree of substitution, and the nature of the glycolipid anchor (Schneewind & Missiakas, 2014; Shiraishi et al., 2016). Additionally, glycosylation and/or D‐alanine substitutions of polyglycerol phosphate in LTA display noticeable differences. The glycosylation of LTA molecules has been demonstrated in several species but not yet in Lb. acidophilus (Sánchez Carballo et al., 2010; Shiraishi et al., 2016) and D‐alanine substitutions have only been found in Lcb. casei LTA (Allievi et al., 2019; Palomino et al., 2013). It is necessary to further investigate whether the LTA glycosylation is involved in the recognition and binding of the S‐layer to the cell. Fina et al. (2019) identified that the SLP C‐terminus binds to LTA in the cell wall of Lb. acidophilus ATCC 4356 stripped from S‐layer. Also, binding was inhibited in cells treated with LTA‐specific antibodies or LTA extracting compounds (SDS). The highly basic nature of the SLP C‐terminal portion resembles the behaviour of cationic peptides such as the well‐known teichoic acid or other LTA‐binding proteins (Rigden et al., 2003).
Two tandem repeats of SLAP domains are needed to interact with their substrate, offering multiple cooperative binding sites and strengthening carbohydrate attachment. The amino acid residues between 321 and 444 from the Lactobacillus SLP primary sequence are the minimum required for binding to glycoconjugates (Table 3). A structural model involving tyrosine residues in the interaction has already been predicted and experimentally verified (Fina et al., 2019), in agreement with consensus domain sequence in other species. Although the integrity of the sequence of the C‐terminal anchoring domain seems essential, the N‐terminal portion can influence the structure adopted by the mature protein and related function. As an example, Lb. acidophilus CP23 SlpA showed weaker immunomodulatory activity compared with Lb. acidophilus L‐92, and even though no difference was observed in the C‐terminal amino acid sequence, an insertion of an Ala–Val–Ala sequence was identified in the N‐terminus of the mature protein, resulting in the accumulation of misfolded SlpA in the culture supernatant of CP23 cells (Yanagihara et al., 2015). The differences between SLPs’ N‐terminal primary sequence produce different results also with regard to antimicrobial activity. Previous research showed that Lb. helveticus and Lb. acidophilus SLP display 74% identity and 83% similarity when compared, particularly in their C‐terminal region, differing mainly in the N‐terminal sequence. Consequently, treatment with Lb. helveticus SLP resulted in a reduced ability to antagonize Escherichia coli and Mycobacterium smegmatis (Prado et al., 2016).
Recent studies in Len. buchneri CD034 (Bonish et al., 2018) have elucidated for the first time the structure and binding force of LTA and the S‐layer O‐glycosylated protein using single‐molecule force microscopy and atomic force microscopy (AFM) over the protein's N‐terminal region (Table 3).
SLP POST‐TRANSLATIONAL MODIFICATIONS
Glycosylation is a frequent protein modification generally overlooked in bacteria. Both O‐ and N‐linked protein glycosylation examples have been shown in bacteria, as well as variations in the degree of glycosylation and glycan composition. Some organisms form complex S‐layer lattices consisting of different protein expression profiles depending on the species and growth conditions (Palomino et al., 2016). Glycosylation has been confirmed for Len. buchneri (Anzengruber et al., 2014), several Len. kefiri strains (Cavallero et al., 2017; Malamud et al., 2020), Lb. helveticus and Lb. acidophilus (Fina et al., 2019; Konstantinov et al., 2008). However, SLP glycan structures have only been elucidated for Len. buchneri (Anzengruber et al., 2014) and Len. kefiri (Cavallero et al., 2017; Malamud et al., 2020). Glycosylation as a post‐translational modification is usually indicated by the presence of two forms of SLP with mass differences (Rykov et al., 2018).
Anzengruber et al. (2014) propose a species‐wide SLP O‐glycosylation signature motif in Len. buchneri over a S‐A‐S‐S‐A‐S sequence. SlpB is the most abundant protein in this species, showing O‐glycosylation at four serine sites glycosylated with seven glucose (Glc) (α1‐6) residues ‐on average‐ within the sequence 152S‐A‐S‐S‐A‐S157. The SLP of Len. kefiri CIDCA 83111 is also O‐glycosylated in the signature motif S‐A‐S‐S‐A‐S, with 5–8 glucose units carrying galacturonic acid (Glc5‐8GalA), and another less abundant site at peptide 471T‐T‐T‐S‐A‐E476, substituted with a Glc5‐8GalA2 structure. As this protein is also N‐glycosylated, this was the first description of the structure of N‐glycans in S‐layer glycoproteins from the Lentilactobacillus species. Although there are 10 characteristic sequons (Asn‐X‐Ser/Thr) in the S‐layer amino acid sequence, only two different peptides are substituted with short structures containing neutral hexoses or deoxyhexoses and amino sugars (Cavallero et al., 2017; Malamud et al., 2020). This signature motif SASSAS is located in the first 150–160 amino acid residues. However, the signature motif SASSAS in SlpE, the primary SLP of Lev. brevis, is located at its end (Malamud et al., 2017; Rykov et al., 2018). Whether or not Lev. brevis S‐layer is glycosylated is still to be determined.
The glycosylation of the Lactobacillus acidophilus ATCC 4356 SLP was confirmed with a lectin transfer assay using Concanavalin A, a plant lectin that detects mannose residues linked to α‐linked N‐linked glycopeptides (Fina Martin et al., 2019). Results were consistent with previous reports in Lb. acidophilus and Lb. helveticus (Konstantinov et al., 2008). Nine possible N‐glycosylation sites (Asn‐X‐Ser/Thr) can be predicted from the SlpA primary sequence, eight in the N‐terminal and one in the C‐terminal region. Since most of these sites are present in the N‐terminal region, it suggests that N‐glycosylation of the mature SLP would not be involved in C‐terminal carbohydrate recognition; but this still needs to be confirmed.
Almost twice the amount of water is bound or coupled to a glycosylated S‐layer in comparison with the non‐glycosylated form (Schuster & Sleytr, 2015); however, little is known about the structure–function relationships of S‐layer glycan moieties involved in providing a hydration layer. Removal of the Lb. acidophilus S‐layer drastically affects survival to osmotic stress and modifies cell wall structure (Palomino et al., 2013, 2016). Lactobacilli are often exposed to changes in environmental osmolarity in both gastrointestinal tract and fermented foods that can compromise essential cell functions. Osmotic stress response triggers cell envelope modifications (Palomino et al., 2010, 2013; Piuri et al., 2003, 2005). In species that lack SLP, osmolarity response causes pleiotropic effects, including susceptibility to enzymatic lysis, increased sensitivity to cationic peptides, and increased capacity to form biofilm and transformation ability; these modifications result both from the changes in the peptidoglycan structure and the different zwitterion character of the lipoteichoic acid (LTA) molecule in high‐salt conditions (Palomino et al., 2010, 2013; Piuri et al., 2005). Modification in cell size and shape has also been reported affecting the genomic organization, especially DNA supercoiling affecting cell division and filamentation (Piuri et al., 2003, 2005). In species with SLP increase in its synthesis is observed in the stress condition as a way to counteract the fragility of the cell wall, due to a decrease in the cell wall thickness and envelope components (Palomino et al., 2016). Since changes in the osmolarity environment impact the cell turgor pressure, and consequently, the cell volume, a high degree of glycosylation might be protective against challenging environmental conditions as shown in Len. kefiri (Cavallero et al., 2017). In addition, glycosylation provided a negative surface charge that might stabilize proteins in high salt conditions, creating a favourable protein–water–salt hydration network, as proposed for other S‐layer glycoproteins (Schuster & Sleytr, 2015).
As demonstrated in Len. kefiri JCM 5818, glycosylation may also be critical for the crosstalk between bacteria carrying SLP and host cells (Prado Acosta et al., 2016). Len. kefiri S‐layer prevents binding of Lb. acidophilus to C‐type lectin receptors (CLR), such as the dendritic cells (DC)‐specific ICAM‐3‐grabbing non‐integrin (DC‐SIGN) receptor; therefore, it shows strong activity against infection of cells expressing DC‐SIGN. Deglycosylation by PNGase F remarkably reduces SLP activity, suggesting the presence of N‐glycosidic chains and their involvement in the adhesion process (Prado Acosta et al., 2016). Chemical oxidation of terminal glycans is used as well to understand the role of glycans in this type of process. Treatment with meta‐periodate of Len. kefiri CIDCA 8348 SLP results in a 50% reduction of binding to different CLR, including Mincle, SignR3, hDC‐SIGN, and mLangerin. Moreover, loss of glycan integrity decreases the adjuvant capacity and immunogenicity (Malamud et al., 2018). Malamud et al. (2020) described the glycosylation pattern of three Len. kefiri SLPs involved in immune activation via recognition of their glycans; this is also most likely replicated in other species (Prado et al., 2021; Prado & Lepenies, 2019).
SLP STRUCTURE STUDIES
Several methods have been used for purifying SLPs, all based on disturbing the non‐covalent association of SLP to the cell wall. Proteins bound to the bacterial surface are extracted using detergents through hydrogen bond breakdown with chaotropic agents (e.g., guanidine hydrochloride or urea) or by the replacement of cations (e.g., displacement of cations Na+, Li+, Ca2+) (do Carmo et al., 2018; Sahay et al., 2015; Sleytr et al., 2014). Given SLPs’ low water solubility related to their inherent self‐assembly property, simple methods can be used to purify and obtain large amounts of protein after dialysis. However, a two‐step procedure is needed to remove S‐layer‐associated proteins (Fina et al., 2019; Palomino et al., 2016), either associated with the SLP or anchored to the cell wall through non‐covalent interaction domains, as described by former proteomic studies (Johnson et al., 2013). Therefore, SLP also constitutes a framework for several proteins with different functions, including host interaction (Johnson et al., 2013, 2016, 2017; Waśko et al., 2014; Zhu et al., 2016).
To date, little is known regarding the atomic structure of full‐length lactobacilli SLPs. Specifically, there are several reasons why we lack three‐dimensional (3D) structural information. First, SLPs’ molecular weight ranges from 35 to 70 kDa, making it impossible to use nuclear magnetic resonance (NMR) structure analysis methods since they exclude molecules exceeding 30 kDa (Hynönen & Palva, 2013). Secondly, SLPs in solution form two‐dimensional (2D) crystals by self‐assembly instead of 3D, also presenting low solubility. Since 3D crystals are required for X‐ray structure analysis, all attempts to obtain nucleation so far have been unsuccessful in screening the appropriate conditions, constantly distressed by the self‐assembly of the 2D crystals (Pum & Sleytr, 2014; Sleytr et al., 2014). Finally, SLPs are usually glycosylated, influencing their secondary structure and even the stability and solubility of the whole protein (Solá & Griebenow, 2009; Szakonyi et al., 2006). SLPs secondary structure predictions are of limited value since the prediction algorithms are based on the available 3D structures of very dissimilar types of proteins. Even though no atomic resolution structure or 3D structure of any Lactobacillaceae full‐length SLP is yet available, the structure from Geobacillus stearothermophilus SLP, SbsB, was obtained, showing promising results by using specific nanobodies to stabilize the SLP (Baranova et al., 2012). Substantial efforts are currently being implemented to elucidate the 3D structure by cloning small peptides (<30 kDa) and resolving them by NMR and X‐ray diffraction to confirm predictions. In fact, recently (December 2022), 10 new 3D structures of Lactobacillus of cloned N‐ terminal or C‐terminal domains of the S‐layer protein from Lb. amylovorus and Lb. acidophilus were first resolved and released in Protein Data Bank (at https://www.rcsb.org/ with PDB Identifiers: 7QEH, 7QEC, 7QLH for Lb. amylovorus and 7QFG, 7QFK, 7QFL, 7QFI, 7QFJ, 7QLD, 7QLE for Lb. acidophilus). These very new findings will enable new insights about the residues involved in the self‐assembly and cell wall anchoring in a near future.
Bioinformatic modelling is an alternative method to predict information about protein structure and identify possible binding sites. In recent work, we built a homology‐based model of the SlpA C‐terminal region, using as a template, the N‐acetylmuramoyl‐L‐alanine amidase, Atl (Protein Data Bank (PDB) ID 4EPC) in the context of pfam03217 SLAP domain. Atl is the major murein hydrolase involved in cell separation in Staphylococcus and presents repeats able to bind to LTA as an anchor (Zoll et al., 2012). The structure model was presented (Fina et al., 2019), including the residues that are part of the most probable binding site (FPocket Druggability Score of 0.768) (Sosa et al., 2018) with a pocket volume of approximately 564 Å, enough to harbour two hexoses with individual volumes of 216 Å. Tyrosine fluorescence quenching was used to experimentally test the tyrosine involvement proposed to interact with carbohydrates (TYR361, TYR391, TYR393, TYR426, TYR437). Increasing concentrations of carbohydrates showed a decrease in fluorescence without changing the maximum emission and peak shape. The volume of the pocket (564 Å) supports the idea of interaction with sugar‐decorated macromolecules like LTA glycosylation or glycoproteins (Fina et al., 2019). Actually, Tyrosine residues are described as ligand interactors in PDB structures 7QEH and 7QFG, probably involving the binding to the bacterial cell through interaction with LTA (Eder et al., 2019)
Other strategies are being applied to unravel the molecular structure. Electron microscopy (EM), combined with freeze‐etching techniques and AFM, is employed to investigate the SLP presence in intact cells, as well as transmission electron microscopy (TEM) for the analysis of cell wall fragments. Regarding the determination of the secondary structure, most commonly used techniques like circular dichroism (CD) in the far‐UV region and Fourier transform infrared spectroscopy (FITR) have been used to provide the contents of α‐helix, β‐sheet, and random structures on extracted SLPs (Mobili et al., 2009; Eslami et al., 2013; Lighezan et al., 2016; Meng et al., 2014; Mobarak et al., 2017).
SLPS APPLICATIONS
Heterologous display
Over the last decades, there has been increasing interest in implementing SLPs for oral vaccine development. Due to their GRAS status, adjuvant properties, and the ability to display antigenic epitopes on bacterial surfaces, they have become excellent candidates to be used as antigen carriers. Furthermore, many lactobacilli strains are attractive vehicles for vaccine delivery since they can survive the hostile condition of the GIT (e.g., low pH, high bile concentration), colonize certain intestinal tissues, have an intrinsic adjuvant response and interact with cells of the immune system.
Recently, recombinant LAB, especially Lactococcus and Lactobacillus, have been used as a live vehicle for antigenic epitope display. Qin et al. (2014) constructed a food‐grade Lb. acidophilus SlpA‐based cell surface display vector and its feasibility was verified by the expression of green fluorescent protein (GFP) on Lbc. casei. Similarly, O’Flaherty and Klaenhammer (2016) could successfully express Clostridium botulinum and Bacillus anthracis antigens in the probiotic strain Lb. acidophilus NCFM; the engineered vaccine vector included the Lb. acidophilus SlpA signal peptide.
As mentioned earlier, the SLP C‐terminal portion interacts with the negatively charged SCWPs and/or with neutral polysaccharides. For this reason, it has been used as an anchor for heterologous surface display of various proteins in non‐genetically modified LAB. The C‐terminal portion of Lb. acidophilus ATCC 4356 was produced in E. coli fused to GFP. The purified fusion protein was then bound to the surface of Lb. acidophilus, Len. kefiri and Lb. helveticus previously stripped with 5M LiCl (Fina et al., 2019; Gordillo et al., 2020; Uriza et al., 2020). New strategies for SLPs remotion are currently being evaluated to obtain a more compatible process with an oral vaccine platform, such as pre‐growth in high salt concentration and subsequent stripping with NaCl. Moreover, protection also needs to be addressed in terms of binding stability when Lactobacillus cells decorated with the anchor protein are subjected to conditions mimicking the GIT (pH, high salt bile, pancreatin) (Gordillo et al., 2020). In addition, the C‐terminal portion of Lactobacillus crispatus K2‐4‐3.13 SLP ‐SlpB‐ was successfully evaluated. Hu et al. (2011) evaluated the capacity of SlpB to mediate surface display by exposing GFP and β‐galactosidase on the cell surface of Lactococcus lactis and several Lactobacillus species (Hu et al., 2011). Five years later, the carcinoembryonic antigen (CEA) was successfully displayed on Lc. lactis surface using this anchor protein. Upon oral administration, mice sera presented higher antigen‐specific secretory IgA levels (Zhang et al., 2016).
In a more recent study, SLAPs domains of Lactobacillus acidophilus ATCC 4356 SlpA have been used as a vaccine platform to display chimeric Shiga toxin‐producing Escherichia coli (STEC) antigens on the surface of Lactobacillus strains. Mice were immunized with Lactobacillus decorated with the fused SLAPs domains, and a STEC challenge infection was controlled efficiently (Uriza et al., 2020). Decoration of probiotic strains belonging to the Lactobacillaceae family, with heterologous proteins using SlpA C‐terminal as anchor domain, has turned into a promising strategy for developing of a universal platform for intestinal delivery of peptides or enzymes with therapeutic value.
Pathogen exclusion and immunostimulation
Probiotics have proven to be a good strategy for the modulation of the human intestinal and vaginal microbiota, as well as to stimulate the systemic and mucosal immune response to prevent and treat infectious diseases (De Boeck et al., 2021; Håkansson et al., 2019; Spacova et al., 2023). Several authors reported the SLP's role in antimicrobial properties and immune activation. Lb. acidophilus ATCC 4356 SLP C‐terminal region presented murein hydrolase activity by zymogram against the cell wall of Salmonella enterica serovar Newport (Prado et al., 2008) and E. coli (Meng et al., 2015). Indeed, Lb. acidophilus ATCC 4356 possesses an additional mechanism against Gram‐positive bacterial pathogens, such as Staphylococcus aureus and Bacillus cereus, provided by its SLP (Prado‐Acosta et al., 2010). Moreover, the murein hydrolase activity of SLPs was also verified by zymogram in Lev. brevis and Lb. helveticus (Palomino et al., 2016), offering an additional survival advantage to probiotic lactobacilli over the GIT's mixed microbiota. It was shown that the SLP inhibited bacterial infection through a blockage of the specific intercellular adhesion molecule DC‐SIGN (CD209) (Prado et al., 2016, 2019, 2021). Pre‐treatment of pathogen bacteria with different SLPs reduces bacteria viability and also prevents infection by enterobacteria (specifically Escherichia coli, Salmonella enterica serovar typhi, and Klebsiella pneumoniae) and from Mycobacterium smegmatis, a non‐pathogenic model for Mycobacterium infection. Pre‐treatment with lactobacilli SLP of eukaryotic cells expressing the DC‐SIGN receptor reduces their susceptibility to bacterial infections. Interestingly, glycosylation of the Len. kefiri S‐layer is essential for attachment to the receptor and thus the inhibition of infection (Palomino et al., 2016).
Other reports have shown that the Len. kefiri CIDCA 8348 SLP improves the response induced by lipopolysaccharides (LPS) in macrophages in a Ca2+‐dependent manner; the mechanism involves an interaction between the glycosylation of the protein and the macrophage inducible Ca2+‐dependent lectin receptor (Mincle), a member of the C‐type lectin family (Malamud, Carasi, et al., 2019). Immunostimulation is induced by SLP, evidenced by cytokine production of IFN‐β, IL‐12p70, and IL‐10 in dendritic cells (Malamud et al., 2020; Prado et al., 2021; Taverniti et al., 2019).
Furthermore, the antiviral properties of the purified SLP of Lb. acidophilus ATCC 4356 has been reported to prevent the infection of Junin, Semliki Forest, Chikungunya, Zika and dengue virus in 3T3 cells overexpressing the DC‐SIGN receptor (also a member of the C‐type lectin) (Martínez et al., 2012; Prado et al., 2019) and avian influenza virus H9N2 in dendritic cells (Gao et al., 2016). Additionally, SLP might suppress the inflammatory response with the inhibition of H9N2 virus infection. SLP treatment increased IL‐10 expression, which aided in the control of the exacerbated inflammation caused by H9N2 infection (Gao et al., 2016). In addition, it was suggested that Lb. acidophilus ATCC 4356 SLP is able to interact directly with herpes simplex type 1 (HSV‐1), human adenovirus type 5 (Adv‐5), and vesicular stomatitis virus (VSV) (Fina et al., 2019). Even though the protein did not show veridical activity, it presents an opportunity for capturing pathogens.
SLPs metal binding
Little is known about SLP's ability to bind metals. Basically, three function roles have been attributed to the SLP in bioadsorption: as a crystalline arrangement framework, as a protective component in hostile environments, and as an ion trap.
The participation of certain ions (for example, calcium, Ca2+) in the SLP self‐assembly has been demonstrated by treatment effect with chelating agents (Mobarak et al., 2017). The SLP‐ion interaction is unspecific and required in the specific assembly of the crystal lattice. Calcium neutralizes the SLP's carboxyl groups, reducing the protein's solubility and improving its packaging (electrostatic repulsion is avoided) (Liu et al., 2017). This lattice is essential in some physiological responses associated with the probiotic characteristic. Particularly, the immunomodulatory capacity of the Len. kefiri SLP, a key function in the probiotic characteristic of this strain, is affected by the presence of calcium. When exposing LPS‐stimulated macrophages to Len. kefiri SLP, all responses of cell surface markers and cytokines increased. After the addition of a bivalent ion chelator, this increase is lost, demonstrating the central role of calcium and glycosidic residues in macrophage receptors. Biotechnologically, these results support the development of adjuvants containing SLP in its calcium conformation to stimulate antigen‐presenting cells (Malamud et al., 2018; Malamud, Carasi, et al., 2019).
As already mentioned, the SLP is in direct contact with the extracellular medium, and hence, involved in bioadsorption processes. Particularly, SLP from GRAS and food‐grade microorganisms, along with other components in lactobacilli (for example, cell wall polysaccharides), can retain metals in high concentrations. To address this, biosorption studies have been performed with Len. kefiri S‐layer using FITR spectroscopy, showing that bivalent ions Cd2+, Zn2+, Pb2+, and Ni2+ interact with the carboxy group of the aspartic and glutamate side chains. This interaction introduces changes in the secondary structure of the SLP, including increased β‐sheet and lower α‐helix structures. The capacity of the SLP to bind these metals was quantified through Raman spectroscopy, and it was determined that Ni2+, Pb2+, and Cd2+ efficiently bind to the bacterial surface. Then, Len. kefiri SLP could be potentially employed to remove metals from consumption products (Gerbino, Carasi, Araujo‐Andrade, et al., 2015; Malamud, Bolla, et al., 2019). Once the ability of this species to capture lead (Pb2+) was demonstrated, research subsequently focused on the ability to remove this ion and the related SLP role. Pure SLP can retain lead with very high affinity, much higher than when attached to the corresponding bacteria encouraging its biotechnological use. The survival of Len. kefiri with or without SLP was studied in a medium supplemented with Pb2+, evidencing that the SLP plays a central role in cell protection rather than ion retention. Given this biosorption property, SLP isolated from Len. kefiri was employed to develop platinum or silver bionanocatalysts based on SLP/polymeric nanoparticles (Bolla et al., 2020; Huggias et al., 2020).
Generally unwanted, Len. hilgardii is predominantly isolated in wines with a high percentage of ethanol since it produces metabolites with disagreeable organoleptic characteristics. Due to the ability of this microorganism to live in a hostile environment with high ethyl concentration, low pH, phenolic compounds, and metal ions, the species SLP proved to be essential in survival. Interestingly, copper could be retained by the SLP and was excluded from inside the cells (Dohm et al., 2011).
CONCLUDING REMARKS
SLPs have great potential due to their abundant expression, surface location, self‐assembly capacity, immunostimulation, adhesion, and toxic remediation. In the same way, SLP GRAS status makes this purified protein an excellent candidate to be added to probiotic formulations, acting as a barrier for pathogens (do Carmo et al., 2018; Prado et al., 2016, 2019). Using the C‐terminal region as an anchor is a suitable strategy for surface antigen display without requiring genetic modification, including oral vaccine formulations. NaCl treatment of cells could also be used to maintain GRAS status of non‐genetically modified organisms (non‐GMO) (Gordillo et al., 2020; Sahay et al., 2015).
The difficulties encountered in the resolution of the SLPs 3D structure have delayed the structural characterization in Lactobacillaceae. This is essential for gaining a deeper insight into the beneficial properties of these proteins and for expanding the technological potential of new nano‐biotechnological tools in the life and non‐life sciences. A detailed glycosylation profile and the identification of cell wall interactors are also essential for advancing these developments. We expect soon to be able to obtain further knowledge on these pending facts.
AUTHOR CONTRIBUTIONS
M. Mercedes Palomino: Formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Mariana C. Allievi: Formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Tania B. Gordillo: Investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). Sabrina S. Bockor: Investigation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). Joaquina Fina Martin: Investigation (supporting); writing – original draft (supporting). Sandra M. Ruzal: Conceptualization (lead); formal analysis (lead); funding acquisition (equal); investigation (lead); methodology (lead); writing – original draft (lead); writing – review and editing (lead).
CONFLICT OF INTEREST
The authors declare that this review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
We would like to thank Dr. Estefanía Urdániz for her careful and critical reading of this manuscript. This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica, Buenos Aires, Argentina (PICT‐2020SERIEA‐01445, PICT‐2020SERIEA‐02170), CONICET (PIP11220200101411), and the Universidad de Buenos Aires (UBACyT 20020170100019BA, 20020190200136BA, 20020190200131BA) to SMR, MCA, and MMP. MMP, MCA, and SMR are members of the Research Career of CONICET. SSB and TBG are doctoral fellows, and JFM was a postdoc fellow of CONICET.
Palomino, M.M. , Allievi, M.C. , Gordillo, T.B. , Bockor, S.S. , Fina Martin, J. & Ruzal, S.M. (2023) Surface layer proteins in species of the family Lactobacillaceae . Microbial Biotechnology, 16, 1232–1249. Available from: 10.1111/1751-7915.14230
Maria M. Palomino and Mariana C. Allievi contributed equally to this work and shared first authorship.
REFERENCES
- Allievi, M.C. , Palomino, M.M. , Prado, A.M. , Lanati, L. , Ruzal, S.M. & Sánchez‐Rivas, C. (2014) Contribution of S‐layer proteins to the mosquitocidal activity of Lysinibacillus sphaericus . PLoS One, 9, e111114. Available from: 10.1371/journal.pone.0111114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allievi, M.C. , Ruzal, S.M. & Palomino, M.M. (2019) Modifications of Lactobacillus surface under environmental stress conditions. In: Ruzal, S.M. (Ed.) Lactobacillus genomics and metabolic engineering. Wymondham: Caister Academic Press, pp. 81–103. Available from: 10.21775/9781910190890.05 [DOI] [Google Scholar]
- Antikainen, J. , Anton, L. , Sillanpää, J. & Korhonen, T.K. (2002) Domains in the S‐layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self‐assembly. Molecular Microbiology, 46, 381–394. Available from: 10.1046/j.1365-2958.2002.03180.x [DOI] [PubMed] [Google Scholar]
- Anzengruber, J. , Pabst, M. , Neumann, L. , Sekot, G. , Heinl, S. , Grabherr, R. et al. (2014) Protein O‐glucosylation in Lactobacillus buchneri . Glycoconjugate Journal, 31, 117–131. Available from: 10.1007/s10719-013-9505-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åvall‐Jääskeläinen, S. , Hynönen, U. , Ilk, N. , Pum, D. , Sleytr, U.B. & Palva, A. (2008) Identification and characterization of domains responsible for self‐assembly and cell wall binding of the surface layer protein of Lactobacillus brevis ATCC 8287. BMC Microbiology, 8, 165. Available from: 10.1186/1471-2180-8-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova, E. , Fronzes, R. , Garcia‐Pino, A. , Van Gerven, N. , Papapostolou, D. , Péhau‐Arnaudet, G. et al. (2012) SbsB structure and lattice reconstruction unveil Ca2+ triggered S‐layer assembly. Nature, 487, 119–122. Available from: 10.1038/nature11155 [DOI] [PubMed] [Google Scholar]
- Blackler, R.J. , López‐guzmán, A. , Hager, F.F. , Janesch, B. , Martinz, G. , Gagnon, S.M.L. et al. (2018) Structural basis of cell wall anchoring by SLH domains in Paenibacillus alvei . Nature Communications, 9, 3120. Available from: 10.1038/s41467-018-05471-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla, P.A. , Huggias, S. , Serradell, M.A. , Ruggera, J.F. & Casella, M.L. (2020) Synthesis and catalytic application of silver nanoparticles supported on Lactobacillus kefiri S‐Layer proteins. Nanomaterials, 10, 2322. Available from: 10.3390/nano10112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot, H.J. , & Pouwels, P.H. (1996). Expression, secretion and antigenic variation of bacterial S‐layer proteins. Molecular Microbiology, 21(6), 1117–1123. Available from: 10.1046/j.1365-2958.1996.711442.x [DOI] [PubMed] [Google Scholar]
- Bönisch, E. , Oh, Y.J. , Anzengruber, J. , Hager, F.F. , López‐Guzmán, A. , Zayni, S. et al. (2018) Lipoteichoic acid mediates binding of a Lactobacillus S‐layer protein. Glycobiology, 28, 148–158. Available from: 10.1093/glycob/cwx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carasi, P. , Ambrosis, N.M. , De Antoni, G.L. , Bressollier, P. , Urdaci, M.C. & Serradell, M.A. (2014) Adhesion properties of potentially probiotic Lactobacillus kefiri to gastrointestinal mucus. Journal of Dairy Research, 81, 16–23. Available from: 10.1017/S0022029913000526 [DOI] [PubMed] [Google Scholar]
- Carasi, P. , Malamud, M. & Serradell, M.A. (2021) Potentiality of food‐isolated Lentilactobacillus kefiri strains as probiotics: state‐of‐art and perspectives. Current Microbiology, 79, 21. Available from: 10.1007/s00284-021-02728-x [DOI] [PubMed] [Google Scholar]
- Cavallero, G.J. , Malamud, M. , Casabuono, A.C. , Serradell, M.A. & Couto, A.S. (2017) A glycoproteomic approach reveals that the S‐layer glycoprotein of Lactobacillus kefiri CIDCA 83111 is O‐ and N‐glycosylated. Journal of Proteomics, 162, 20–29. Available from: 10.1016/j.jprot.2017.04.007 [DOI] [PubMed] [Google Scholar]
- De Boeck, I. , Spacova, I. , Vanderveken, O.M. & Lebeer, S. (2021) Lactic acid bacteria as probiotics for the nose? Microbial Biotechnology, 14, 859–869. Available from: 10.1111/1751-7915.13759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw, E. , Li, X. & Lu, W. (2006) Binding characteristics of the Lactobacillus brevis ATCC 8287 surface layer to extracellular matrix proteins. FEMS Microbiology Letters, 260, 210–215. Available from: 10.1111/j.1574-6968.2006.00313.x [DOI] [PubMed] [Google Scholar]
- do Carmo, F.L.R. , Rabah, H. , De Oliveira Carvalho, R.D. , Gaucher, F. , Cordeiro, B.F. , da Silva, S.H. et al. (2018) Extractable bacterial surface proteins in probiotic–host interaction. Frontiers in Microbiology, 9, 645. Available from: 10.3389/fmicb.2018.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm, N. , Petri, A. , Schlander, M. , Schlott, B. , König, H. & Claus, H. (2011) Molecular and biochemical properties of the S‐layer protein from the wine bacterium Lactobacillus hilgardii B706. Archives of Microbiology, 193, 251–261. Available from: 10.1007/s00203-010-0670-9 [DOI] [PubMed] [Google Scholar]
- Eder, M. , Dordic, A. , Sagmeister, T. , Damisch, E. , Vejzovic, D. , Berni, F. et al. (2019) Surface layer proteins of lactobacilli – determining the cell wall binding and their antibacterial effect. Acta Crystallographica. Section A, 75, e196. Available from: 10.1107/S2053273319093604 [DOI] [Google Scholar]
- Eslami, N. , Kermanshahi, R.K. & Erfan, M. (2013) Studying the stability of S‐layer protein of Lactobacillus acidophilus ATCC 4356 in simulated gastrointestinal fluids using SDS‐PAGE and circular dichroism. Iranian Journal of Pharmaceutical Research, 12, 45–54. [PMC free article] [PubMed] [Google Scholar]
- Fagan, R.P. & Fairweather, N.F. (2014) Biogenesis and functions of bacterial S‐layers. Nature Reviews Microbiology, 12(3), 211–222. Available from: 10.1038/nrmicro3213 [DOI] [PubMed] [Google Scholar]
- Fina, M.J. , Palomino, M.M. , Cutine, A.M. , Modenutti, C.P. , Fernández Do Porto, D.A. , Allievi, M.C. et al. (2019) Exploring lectin‐like activity of the S‐layer protein of Lactobacillus acidophilus ATCC 4356. Applied Microbiology and Biotechnology, 103, 4839–4857. Available from: 10.1007/s00253-019-09795-y [DOI] [PubMed] [Google Scholar]
- Fontana, A. , Falasconi, I. , Molinari, P. , Treu, L. , Basile, A. , Vezzi, A. et al. (2019) Genomic comparison of Lactobacillus helveticus strains highlights probiotic potential. Frontiers in Microbiology, 10, 1380. Available from: 10.3389/fmicb.2019.01380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X. , Huang, L. , Zhu, L. , Mou, C. , Hou, Q. & Yu, Q. (2016) Inhibition of H9N2 Virus invasion into dendritic cells by the S‐Layer protein from Lactobacillus acidophilus ATCC 4356. Frontiers in Cellular and Infection Microbiology, 6, 1–10. Available from: 10.3389/fcimb.2016.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbino, E. , Carasi, P. , Araujo‐Andrade, C. , Tymczyszyn, E.E. & Gómez‐Zavaglia, A. (2015) Role of S‐layer proteins in the biosorption capacity of lead by Lactobacillus kefir . World Journal of Microbiology and Biotechnology, 31, 583–592. Available from: 10.1007/s11274-015-1812-7 [DOI] [PubMed] [Google Scholar]
- Gerbino, E. , Carasi, P. , Mobili, P. , Serradell, M.A. & Gómez‐Zavaglia, A. (2015) Role of S‐layer proteins in bacteria. World Journal of Microbiology and Biotechnology, 31, 1877–1887. Available from: 10.1007/s11274-015-1952-9 [DOI] [PubMed] [Google Scholar]
- Gordillo, T.B. , Palumbo, M.C. , Allievi, M.C. , Fernández Do Porto, D.A. , Ruzal, S.M. & Palomino, M.M. (2020) Strategies to display heterologous proteins on the cell surface of lactic acid bacteria using as anchor the C‐terminal domain of Lactobacillus acidophilus SlpA. World Journal of Microbiology and Biotechnology, 36(11), 169. Available from: 10.1007/s11274-020-02945-9 [DOI] [PubMed] [Google Scholar]
- Grosu‐Tudor, S.S. , Brown, L. , Hebert, E.M. , Brezeanu, A. , Brinzan, A. , Fadda, S. et al. (2016) S‐layer production by Lactobacillus acidophilus IBB 801 under environmental stress conditions. Applied Microbiology and Biotechnology, 100(10), 4573–4583. Available from: 10.1007/s00253-016-7355-5 [DOI] [PubMed] [Google Scholar]
- Håkansson, Å. , Andrén, A.C. , Brundin, C. , Oscarsson, E. , Molin, G. & Agardh, D. (2019) Effects of Lactobacillus plantarum and Lactobacillus paracasei on the peripheral immune response in children with celiac disease autoimmunity: a randomized, double‐blind, placebo‐controlled clinical trial. Nutrients, 11, 1925. Available from: 10.3390/nu11081925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambraeus, G. , von Wachenfeldt, C. & Hederstedt, L. (2003) Genome‐wide survey of mRNA half‐lives in Bacillus subtilis identifies extremely stable mRNAs. Molecular Genetics and Genomics, 269, 706–714. Available from: 10.1007/s00438-003-0883-6 [DOI] [PubMed] [Google Scholar]
- Hu, S. , Kong, J. , Sun, Z. , Han, L. , Kong, W. & Yang, P. (2011) Heterologous protein display on the cell surface of lactic acid bacteria mediated by the s‐layer protein. Microbial Cell Factories, 10, 13. Available from: 10.1186/1475-2859-10-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggias, S. , Bolla, P.A. , Serradell, M.A. , Casella, M. & Peruzzo, P.J. (2020) Platinum nanoparticles obtained at mild conditions on S‐Layer protein/polymer particle supports. Langmuir, 36(5), 1201–1211. Available from: 10.1021/acs.langmuir.9b02868 [DOI] [PubMed] [Google Scholar]
- Hynönen, U. , Kant, R. , Lähteinen, T. , Pietilä, T.E. , Beganović, J. , Smidt, H. et al. (2014) Functional characterization of probiotic surface layer protein‐carrying Lactobacillus amylovorus strains. BMC Microbiology, 14, 199. Available from: 10.1186/1471-2180-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynönen, U. & Palva, A. (2013) Lactobacillus surface layer proteins: structure, function and applications. Applied Microbiology and Biotechnology, 97, 5225–5243. Available from: 10.1007/s00253-013-4962-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesch, B. , Messner, P. & Schäffer, C. (2013) Are the surface layer homology domains essential for cell surface display and glycosylation of the S‐layer protein from Paenibacillus alvei CCM 2051. Journal of Bacteriology, 195, 565–575. Available from: 10.1128/JB.01487-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L. , Xian, S. , Liu, X. , Shen, G. , Zhang, Z. , Hou, X. et al. (2022) Metagenomic study on Chinese homemade Paocai: the effects of raw materials and fermentation periods on the microbial ecology and volatile components. Food, 11, 62. Available from: 10.3390/foods11010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B.R. , Hymes, J. , Sanozky‐Dawes, R. , Henriksen, E.D.C. , Barrangou, R. & Klaenhammer, T.R. (2016) Conserved S‐layer‐associated proteins revealed by exoproteomic survey of S‐layer‐forming lactobacilli. Applied and Environmental Microbiology, 82, 134–145. Available from: 10.1128/AEM.01968-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B.R. , O’Flaherty, S. , Goh, Y.J. , Carroll, I. , Barrangou, R. & Klaenhammer, T.R. (2017) The S‐layer associated serine protease homolog PrtX impacts cell surface‐mediated microbe‐host interactions of Lactobacillus acidophilus NCFM. Frontiers in Microbiology, 8, 1185. Available from: 10.3389/fmicb.2017.01185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B.R. , Selle, K. , O’Flaherty, S. , Goh, Y.J. & Klaenhammer, T. (2013) Identification of extracellular surface‐layer associated proteins in Lactobacillus acidophilus NCFM. Microbiology, 159, 2269–2282. Available from: 10.1099/mic.0.070755-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleghi, M. , Kermanshahi, R.K. , Yaghoobi, M.M. , Zarkesh‐Esfahani, S.H. & Baghizadeh, A. (2010) Assessment of bile salt effects on S‐layer production, slp gene expression and some physicochemical properties of Lactobacillus acidophilus ATCC 4356. Journal of Microbiology, Biotechnology, 20(4), 749–756. [PubMed] [Google Scholar]
- Klotz, C. & Barrangou, R. (2018) Engineering components of the Lactobacillus S‐Layer for biotherapeutic applications. Frontiers in Microbiology, 9, 2264. Available from: 10.3389/fmicb.2018.02264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz, C. , O’Flaherty, S. , Goh, Y.J. & Barrangou, R. (2017) Investigating the effect of growth phase on the Surface‐Layer associated proteome of Lactobacillus acidophilus using quantitative proteomics. Frontiers in Microbiology, 8. Available from: 10.3389/fmicb.2017.02174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, W. , Gan, J. , Su, M. , Xiong, B. , Jiang, X. , Zhang, T. et al. (2022) Identification and characterization of domains responsible for cell wall binding, self‐assembly, and adhesion of S‐layer protein from Lactobacillus acidophilus CICC 6074. Journal of Agricultural and Food Chemistry, 70, 12982–12989. Available from: 10.1021/acs.jafc.2c03907 [DOI] [PubMed] [Google Scholar]
- Konstantinov, S.R. , Smidt, H. , de Vos, W.M. , Bruijns, S.C.M. , Singh, S.K. , Valence, F. et al. (2008) S‐layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proceedings of the National Academy of Sciences of the United States of America, 105, 19474–19479. Available from: 10.1073/pnas.0810305105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , Li, M. , Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. Available from: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer, S. , Bron, P.A. , Marco, M.L. , Van Pijkeren, J.P. , O’Connell, M.M. , Hill, C. et al. (2018) Identification of probiotic effector molecules: present state and future perspectives. Current Opinion in Biotechnology, 49, 217–223. Available from: 10.1016/j.copbio.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Lighezan, L. , Georgieva, R. & Neagu, A. (2016) The secondary structure and the thermal unfolding parameters of the S‐layer protein from Lactobacillus salivarius . European Biophysics Journal, 45, 491–509. Available from: 10.1007/s00249-016-1117-2 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Falke, S. , Drobot, B. , Oberthuer, D. , Kikhney, A. , Guenther, T. et al. (2017) Analysis of self‐assembly of S‐layer proteinSLPB53 from Lysinibacillus sphaericus . European Biophysics Journal, 46, 77–89. Available from: 10.1007/s00249-016-1139-9 [DOI] [PubMed] [Google Scholar]
- Luo, G. , Yang, Q. , Yao, B. , Tian, Y. , Hou, R. , Shao, A. et al. (2019) Slp‐coated liposomes for drug delivery and biomedical applications: potential and challenges. International Journal of Nanomedicine, 14, 1359–1383. Available from: 10.2147/IJN.S189935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova, K. , Slesarev, A. , Wolf, Y. , Sorokin, A. , Mirkin, B. , Koonin, E. et al. (2006) Comparative genomics of the lactic acid bacteria. Proceedings of the National Academy of Sciences of the United States of America, 103, 15611–15616. Available from: 10.1073/pnas.0607117103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud, M. , Bolla, P.A. , Carasi, P. , Gerbino, E. , Gómez‐Zavaglia, A. , Mobili, P. et al. (2019) S‐layer proteins from lactobacilli: biogenesis, structure, functionality, and biotechnological applications. In: Ruzal, S.M. (Ed.) Lactobacillus genomics and metabolic engineering. Wymondham: Caister Academic Press, pp. 105–130. Available from: 10.21775/9781910190890.06 [DOI] [Google Scholar]
- Malamud, M. , Carasi, P. , Assandri, M.H. , Freire, T. , Lepenies, B. & Serradell, M.A. (2019) S‐Layer glycoprotein from Lactobacillus kefiri exerts its immunostimulatory activity through glycan recognition by Mincle. Frontiers in Immunology, 10, 1422. Available from: 10.3389/fimmu.2019.01422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud, M. , Carasi, P. , Bronsoms, S. , Trejo, S.A. & Serradell, M.A. (2017) Lactobacillus kefiri shows inter‐strain variations in the amino acid sequence of the S‐layer proteins. Antonie Van Leeuwenhoek, 110, 515–530. Available from: 10.1007/s10482-016-0820-4 [DOI] [PubMed] [Google Scholar]
- Malamud, M. , Carasi, P. , Freire, T. & Serradell, M.A. (2018) S‐layer glycoprotein from Lactobacillus kefiri CIDCA 8348 enhances macrophages response to LPS in a Ca+2‐dependent manner. Biochemical and Biophysical Research Communications, 495, 1227–1232. Available from: 10.1016/j.bbrc.2017.11.127 [DOI] [PubMed] [Google Scholar]
- Malamud, M. , Cavallero, G.J. , Casabuono, A.C. , Lepenies, B. , Serradell, M.Á. & Couto, A.S. (2020) Immunostimulation by Lactobacillus kefiri S‐layer proteins with distinct glycosylation patterns requires different lectin partners. The Journal of Biological Chemistry, 295, 14430–14444. Available from: 10.1074/jbc.RA120.013934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, M.G. , Prado, A.M. , Candurra, N.A. & Ruzal, S.M. (2012) S‐layer proteins of Lactobacillus acidophilus inhibits JUNV infection. Biochemical and Biophysical Research Communications, 422, 590–595. Available from: 10.1016/j.bbrc.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, J. , Gao, S.‐M. , Zhang, Q.‐X. & Lu, R.R. (2015) Murein hydrolase activity of surface layer proteins from Lactobacillus acidophilus against Escherichia coli . International Journal of Biological Macromolecules, 79, 527–532. Available from: 10.1016/j.ijbiomac.2015.03.057 [DOI] [PubMed] [Google Scholar]
- Meng, J. , Zhu, X. , Gao, S.M. , Zhang, Q.X. , Sun, Z. & Lu, R.R. (2014) Characterization of surface layer proteins and its role in probiotic properties of three Lactobacillus strains. International Journal of Biological Macromolecules, 65, 110–114. Available from: 10.1016/j.ijbiomac.2014.01.024 [DOI] [PubMed] [Google Scholar]
- Mobarak, Q.E. , Kasra, K.R. , Erfan, M. , Ghadam, P. , Sardari, S. & Eslami, N. (2017) Characteristics of surface layer proteins from two new and native strains of Lactobacillus brevis . International Journal of Biological Macromolecules, 95, 1004–1010. Available from: 10.1016/j.ijbiomac.2016.10.089 [DOI] [PubMed] [Google Scholar]
- Mobili, P. , Londero, A. , Maria, T.M.R. , Eusébio, M.E.S. , De Antoni, G.L. , Fausto, R. et al. (2009) Characterization of S‐layer proteins of Lactobacillus by FTIR spectroscopy and differential scanning calorimetry. Vibrational Spectroscopy, 50, 68–77. Available from: 10.1016/j.vibspec.2008.07.016 [DOI] [Google Scholar]
- Moser, A. , Wüthrich, D. , Bruggmann, R. , Eugster‐Meier, E. , Meile, L. & Irmler, S. (2017) Amplicon sequencing of the slpH locus permits culture‐independent strain typing of Lactobacillus helveticus in dairy products. Frontiers in Microbiology, 8, 1380. Available from: 10.3389/fmicb.2017.01380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flaherty, S. & Klaenhammer, T.R. (2016) Multivalent chromosomal expression of the Clostridium botulinum serotype A neurotoxin heavy‐chain antigen and the the Bacillus anthracis protective antigen in Lactobacillus acidophilus . Applied and Environmental Microbiology, 82, 6091–6101. Available from: 10.1128/AEM.01533-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg, T.S. , McMahon, D.J. , Culumber, M.D. , McAuliffe, O. & Oberg, C.J. (2022) Review of taxonomic changes in dairy‐related lactobacilli. Journal of Dairy Science, 105, 2750–2770. Available from: 10.3168/jds.2021-21138 [DOI] [PubMed] [Google Scholar]
- Oberto, J. (2013) SyntTax: a web server linking synteny to prokaryotic taxonomy. BMC Bioinformatics, 14, 4. Available from: 10.1186/1471-2105-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino, M.M. , Allievi, M.C. , Gründling, A. , Sanchez‐Rivas, C. & Ruzal, S.M. (2013) Osmotic stress adaptation in Lactobacillus casei BL23 leads to structural changes in the cell wall polymer lipoteichoic acid. Microbiology, 159, 2416–2426. Available from: 10.1099/mic.0.070607-0 [DOI] [PubMed] [Google Scholar]
- Palomino, M.M. , Allievi, M.C. , Martin, J.F. , Waehner, P.M. , Prado, A.M. , Rivas, C.S. et al. (2015) Draft genome sequence of the probiotic strain Lactobacillus acidophilus ATCC 4356. Genome Announcements, 3, e01421. Available from: 10.1128/genomea.01421-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino, M.M. , Allievi, M.C. , Prado‐Acosta, M. , Sanchez‐Rivas, C. & Ruzal, S.M. (2010) New method for electroporation of Lactobacillus species grown in high salt. Journal of Microbiological Methods, 83(2), 164–167. Available from: 10.1016/j.mimet.2010.08.017 [DOI] [PubMed] [Google Scholar]
- Palomino, M.M. , Waehner, P.M. , Fina, M.J. , Ojeda, P. , Malone, L. , Sanchez, R.C. et al. (2016) Influence of osmotic stress on the profile and gene expression of surface layer proteins in Lactobacillus acidophilus ATCC 4356. Applied Microbiology and Biotechnology, 100, 8475–8484. Available from: 10.1007/s00253-016-7698-y [DOI] [PubMed] [Google Scholar]
- Peterbauer, C. , Heinl, S. , Berlec, A. & Grabherr, R. (2019) Recombinant gene expression in Lactobacilli: strategies and applications. In: Iin Ruzal, S.M. (Ed.) Lactobacillus Genomics and Metabolic Engineering. Wymondham: Caister Academic Press, pp. 169–186. Available from: 10.21775/9781910190890.9 [DOI] [Google Scholar]
- Piuri, M. , Sanchez‐Rivas, C. & Ruzal, S.M. (2003) Adaptation to high salt in Lactobacillus: Role of peptides and proteolytic enzymes. Journal of Applied Microbiology, 95(2), 372–379. Available from: 10.1046/j.1365-2672.2003.01971.x [DOI] [PubMed] [Google Scholar]
- Piuri, M. , Sanchez‐Rivas, C. & Ruzal, S.M. (2005) Cell wall modifications during osmotic stress in Lactobacillus casei. Journal of Applied Microbiology, 98(1), 84–95. Available from: 10.1111/j.1365-2672.2004.02428.x [DOI] [PubMed] [Google Scholar]
- Prado, A.M. , Geoghegan, E.M. , Lepenies, B. , Ruzal, S. , Kielian, M. & Martinez, M.G. (2019) Surface (S) layer proteins of Lactobacillus acidophilus block virus infection via DC‐SIGN interaction. Frontiers in Microbiology, 10, 810. Available from: 10.3389/fmicb.2019.00810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado, A.M. , Goyette‐Desjardins, G. , Scheffel, J. , Dudeck, A. , Ruland, J. & Lepenies, B. (2021) S‐Layer from Lactobacillus brevis modulates antigen‐presenting cell functions via the Mincle‐Syk‐Card9 Axis. Frontiers in Immunology, 12, 602067. Available from: 10.3389/fimmu.2021.602067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado, A.M. & Lepenies, B. (2019) Bacterial glycans and their interactions with lectins in the innate immune system. Biochemical Society Transactions, 47, 1569–1579. Available from: 10.1042/BST20170410 [DOI] [PubMed] [Google Scholar]
- Prado, A.M. , Palomino, M. , Allievi, M.C. , Sanchez, R.C. & Ruzal, S.M. (2008) Murein hydrolase activity in the surface layer of Lactobacillus acidophilus ATCC 4356. Applied and Environmental Microbiology, 74, 7824–7827. Available from: 10.1128/AEM.01712-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado, A.M. , Ruzal, S.M. & Cordo, S.M. (2016) S‐layer proteins from Lactobacillus sp. inhibit bacterial infection by blockage of DC‐SIGN cell receptor. International Journal of Biological Macromolecules, 92, 998–1005. Available from: 10.1016/j.ijbiomac.2016.07.096 [DOI] [PubMed] [Google Scholar]
- Prado‐Acosta, M. , Ruzal, S.M. , Allievi, M.C. , Palomino, M.M. & Rivas, C.S. (2010) Synergistic effects of the Lactobacillus acidophilus surface layer and nisin on bacterial growth. Applied and Environmental Microbiology, 76, 974–977. Available from: 10.1128/AEM.01427-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pum, D. & Sleytr, U.B. (2014) Reassembly of S‐layer proteins. Nanotechnology, 25, 312001. Available from: 10.1088/0957-4484/25/31/312001 [DOI] [PubMed] [Google Scholar]
- Pum, D. , Toca‐Herrera, J.L. & Sleytr, U.B. (2013) S‐layer protein self‐assembly. International Journal of Molecular Sciences, 14, 2484–2501. Available from: 10.3390/ijms14022484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, J. , Wang, X. , Kong, J. , Ma, C. & Xu, P. (2014) Construction of a food‐grade cell surface display system for Lactobacillus casei . Microbiological Research, 169, 733–740. Available from: 10.1016/j.micres.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden, D.J. , Galperin, M.Y. & Jedrzejas, M.J. (2003) Analysis of structure and function of putative surface‐exposed proteins encoded in the Streptococcus pneumoniae genome: A bioinformatics‐based approach to vaccine and drug design. Critical Reviews in Biochemistry and Molecular Biology, 38, 143–168. Available from: 10.1080/713609215 [DOI] [PubMed] [Google Scholar]
- Rykov, S.V. , Yegorov, Y.E. , Vishnyakova, H.S. & Berezina, O.V. (2018) Designing a cell surface display system of protein domains in lactobacilli based on S‐Layer proteins of Lactobacillus brevis ATCC 367. Russian Journal of Bioorganic Chemistry, 44, 199–209. Available from: 10.1134/s1068162018010156 [DOI] [Google Scholar]
- Sahay, B. , Ge, Y. , Colliou, N. , Zadeh, M. , Weiner, C. , Mila, A. et al. (2015) Advancing the use of Lactobacillus acidophilus surface layer protein A for the treatment of intestinal disorders in humans. Gut Microbes, 6, 392–397. Available from: 10.1080/19490976.2015.1107697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti, E. & O’Toole, P.W. (2017) When regulation challenges innovation: The case of the genus Lactobacillus . Trends in Food Science and Technology, 66, 187–194. Available from: 10.1016/j.tifs.2017.05.009 [DOI] [Google Scholar]
- Sánchez Carballo, P.M. , Vilen, H. , Palva, A. & Holst, O. (2010) Structural characterization of teichoic acids from Lactobacillus brevis . Carbohydrate Research, 345, 538–542. Available from: 10.1016/j.carres.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Schneewind, O. & Missiakas, D. (2014) Lipoteichoic acids, phosphate‐containing polymers in the envelope of gram‐positive bacteria. Journal of Bacteriology, 196, 1133–1142. Available from: 10.1128/JB.01155-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster, B. & Sleytr, U.B. (2015) Relevance of glycosylation of S‐layer proteins for cell surface properties. Acta Biomaterialia, 19, 149–157. Available from: 10.1016/j.actbio.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle, K. , Goh, Y.J. , Johnson, B.R. , O'Flaherty, S. , Andersen, J.M. , Barrangou, R. et al. (2017) Deletion of lipoteichoic acid synthase impacts expression of genes encoding cell surface proteins in Lactobacillus acidophilus . Frontiers in Microbiology, 8, 553. Available from: 10.3389/fmicb.2017.00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi, T. , Yokota, S.‐I. , Fukiya, S. & Yokota, A. (2016) Structural diversity and biological significance of lipoteichoic acid in Gram‐positive bacteria: focusing on beneficial probiotic lactic acid bacteria. Biosci Microbiota Food Health, 35, 147–161. Available from: 10.12938/bmfh.2016-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää, J. , Martínez, B. , Antikainen, J. , Toba, T. , Kalkkinen, N. , Tankka, S. et al. (2000) Characterization of the collagen‐binding S‐layer protein CbsA of Lactobacillus crispatus . Journal of Bacteriology, 182, 6440–6450. Available from: 10.1128/JB.182.22.6440-6450.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr, U.B. , Schuster, B. , Egelseer, E.‐M. & Pum, D. (2014) S‐layers: principles and applications. FEMS Microbiology Reviews, 38, 823–864. Available from: 10.1111/1574-6976.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit, E. , Jager, D. , Martinez, B. , Tielen, F.J. & Pouwels, P.H. (2002) Structural and functional analysis of the S‐layer protein crystallisation domain of Lactobacillus acidophilus ATCC 4356: Evidence for protein‐protein interaction of two subdomains. Journal of Molecular Biology, 324, 953–964. Available from: 10.1016/S0022-2836(02)01135-X [DOI] [PubMed] [Google Scholar]
- Smit, E. , Oling, F. , Demel, R. , Martinez, B. & Pouwels, P.H. (2001) The S‐layer protein of Lactobacillus acidophilus ATCC 4356: identification and characterisation of domains responsible for S‐protein assembly and cell wall binding. Journal of Molecular Biology, 305, 245–257. Available from: 10.1006/jmbi.2000.4258 [DOI] [PubMed] [Google Scholar]
- Smit, E. & Pouwels, P.H. (2002) One repeat of the cell wall binding domain is sufficient for anchoring the Lactobacillus acidophilus surface layer protein. Journal of Bacteriology, 184, 4617–4619. Available from: 10.1128/JB.184.16.4617-4619.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solá, R.J. & Griebenow, K. (2009) Effects of glycosylation on the stability of protein pharmaceuticals. Journal of Pharmaceutical Sciences, 98, 1223–1245. Available from: 10.1002/jps.21504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa, E.J. , Burguener, G. , Lanzarotti, E. , Defelipe, L. , Radusky, L. , Pardo, A.M. et al. (2018) Target‐Pathogen: a structural bioinformatic approach to prioritize drug targets in pathogens. Nucleic Acids Research, 46, D413–D418. Available from: 10.1093/nar/gkx1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacova, I. , De Boeck, I. , Cauwenberghs, E. , Delanghe, L. , Bron, P.A. , Henkens, T. et al. (2023) Development of a live biotherapeutic throat spray with lactobacilli targeting respiratory viral infections. Microbial Biotechnology, 16, 99–115. Available from: 10.1111/1751-7915.14189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic, E. & McAuliffe, O. (2019) A genomic perspective on niche adaptability in Lactobacillus . In: Ruzal, S.M. (Ed.) Lactobacillus genomics and metabolic engineering. Wymondham: Caister Academic Press, pp. 1–18. Available from: 10.21775/9781910190890.01 [DOI] [Google Scholar]
- Suhr, M. , Lederer, F.L. , Günther, T.J. & Raff, J. (2016) Characterization of three different unusual S‐layer proteins from Viridibacillus arvi JG‐B58 that exhibits two super‐imposed S‐layer proteins. PLoS One, 11, e0156785. Available from: 10.1371/journal.pone.0156785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Z. , Harris, H.M.B. , McCann, A. , Guo, C. , Argimón, S. , Zhang, W. et al. (2015) Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nature Communications, 6, 8322. Available from: 10.1038/ncomms9322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Z. , Kong, J. , Hu, S. , Kong, W. , Lu, W. & Liu, W. (2013) Characterization of a S‐layer protein from Lactobacillus crispatus K313 and the domains responsible for binding to cell wall and adherence to collagen. Applied Microbiology and Biotechnology, 97, 1941–1952. Available from: 10.1007/s00253-012-4044-x [DOI] [PubMed] [Google Scholar]
- Suzuki, S. , Yokota, K. , Igimi, S. & Kajikawa, A. (2019) Comparative analysis of immunological properties of S‐layer proteins isolated from Lactobacillus strains. Microbiology, 165, 188–196. Available from: 10.1099/mic.0.000766 [DOI] [PubMed] [Google Scholar]
- Szakonyi, G. , Klein, M.G. , Hannan, J.P. , Young, K.A. , Ma, R.Z. , Asokan, R. et al. (2006) Structure of the Epstein‐Barr virus major envelope glycoprotein. Nature Structural & Molecular Biology, 13, 996–1001. Available from: 10.1038/nsmb1161 [DOI] [PubMed] [Google Scholar]
- Taverniti, V. , Marengo, M. , Fuglsang, E. , Skovsted, H.M. , Arioli, S. , Mantegazza, G. et al. (2019) Surface layer of Lactobacillus helveticus MIMLh5 promotes endocytosis by dendritic cells. Applied and Environmental Microbiology, 85, e00138–e00119. Available from: 10.1128/AEM.00138-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriza, P.J. , Trautman, C. , Palomino, M.M. , Fina, M.J. , Ruzal, S.M. , Roset, M.S. et al. (2020) Development of an antigen delivery platform using Lactobacillus acidophilus decorated with heterologous proteins: a sheep in wolf's clothing story. Frontiers in Microbiology, 11, 509380. Available from: 10.3389/fmicb.2020.509380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uroić, K. , Novak, J. , Hynönen, U. , Pietilä, T.E. , Leboš, P.A. , Kant, R. et al. (2016) The role of S‐layer in adhesive and immunomodulating properties of probiotic starter culture Lactobacillus brevis D6 isolated from artisanal smoked fresh cheese. LWT‐ Food Science and Technology, 69, 623–632. Available from: 10.1016/j.lwt.2016.02.013 [DOI] [Google Scholar]
- Valence, F. & Lortal, S. (1995) Zymogram and preliminary characterization of Lactobacillus helveticus autolysins. Applied and Environmental Microbiology, 61(9), 3391–3399. Available from: 10.1128/aem.61.9.3391-3399.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roosmalen, M.L. , Geukens, N. , Jongbloed, J.D.H. , Tjalsma, H. , Dubois, J.‐Y.F. , Bron, S. et al. (2004) Type I signal peptidases of Gram‐positive bacteria. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1694(1–3), 279–297. Available from: 10.1016/j.bbamcr.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Vergalito, F. , Testa, B. , Cozzolino, A. , Letizia, F. , Succi, M. , Lombardi, S.J. et al. (2020) Potential application of Apilactobacillus kunkeei for human use: evaluation of probiotic and functional properties. Food, 9, 1535. Available from: 10.3390/foods9111535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilen, H. , Hynönen, U. , Badelt‐Lichtblau, H. , Ilk, N. , Jääskeläinen, P. , Torkkeli, M. et al. (2009) Surface location of individual residues of SlpA provides insight into the Lactobacillus brevis S‐layer. Journal of Bacteriology, 191, 3339–3349. Available from: 10.1128/JB.01782-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eichel‐Streiber, C. , Sauerborn, M. & Kuramitsu, H.K. (1992) Evidence for a modular structure of the homologous repetitive C‐terminal carbohydrate‐binding sites of toxins and Streptococcus mutans glucosyltransferases. Journal of Bacteriology, 174, 6707–6710. Available from: 10.1128/jb.174.20.6707-6710.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waśko, A. , Polak‐Berecka, M. , Kuzdraliński, A. & Skrzypek, T. (2014) Variability of S‐layer proteins in Lactobacillus helveticus strains. Anaerobe, 25, 53–60. Available from: 10.1016/j.anaerobe.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Wren, B.W. , Russell, R.R.B. & Tabaqchali, S. (1991) Antigenic cross‐reactivity and functional inhibition by antibodies to Clostridium difficile Toxin‐A, Streptococcus mutans glucan‐binding protein, and a synthetic peptide. Infection and Immunity, 59, 3151–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, Z. , Geng, W. , Li, C. , Sun, Y. & Wang, Y. (2017) Comparative genomics of Lactobacillus kefiranofaciens ZW3 and related members of Lactobacillus. spp reveal adaptations to dairy and gut environments. Scientific Reports, 7, 12827. Available from: 10.1038/s41598-017-12916-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara, S. , Kato, S. , Ashida, N. & Yamamoto, N. (2015) Lactobacillus acidophilus CP23 with weak immunomodulatory activity lacks anchoring structure for surface layer protein. Journal of Bioscience and Bioengineering, 119, 521–525. Available from: 10.1016/J.JBIOSC.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Gao, J. , Guo, Y. , Wu, Z. & Pan, D. (2017) Extraction of Lactobacillus acidophilus CICC 6074 S‐Layer proteins and their ability to inhibit enteropathogenic Escherichia coli . Current Microbiology, 74, 1123–1129. Available from: 10.1007/s00284-017-1291-1 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Hu, S. , Du, X. , Li, T. , Han, L. & Kong, J. (2016) Heterologous expression of carcinoembryonic antigen in Lactococcus lactis via LcsB‐mediated surface displaying system for oral vaccine development. Journal of Microbiology, Immunology, and Infection, 49, 851–858. Available from: 10.1016/J.JMII.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Wittouck, S. , Salvetti, E. , Franz, C. , Harris, H.M.B. , Mattarelli, P. et al. (2020) A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae . International Journal of Systematic and Evolutionary Microbiology, 70, 2782–2858. Available from: 10.1099/ijsem.0.004107 [DOI] [PubMed] [Google Scholar]
- Zhu, C. , Guo, G. , Ma, Q. , Zhang, F. , Ma, F. , Liu, J. et al. (2016) Diversity in S‐layers. Progress in Biophysics and Molecular Biology, 123, 1–15. Available from: 10.1016/j.pbiomolbio.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Zoll, S. , Schlag, M. , Shkumatov, A.V. , Rautenberg, M. , Svergun, D.I. , Götz, F. et al. (2012) Ligand‐binding properties and conformational dynamics of autolysin repeat domains in staphylococcal cell wall recognition. Journal of Bacteriology, 194, 3789–3802. Available from: 10.1128/JB.00331-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


