Abstract
Ginkgo biloba is a relict tree species showing high resistance to adverse biotic and abiotic environmental factors. Its fruits and leaves have high medicinal value due to the presence of flavonoids, terpene trilactones and phenolic compounds. However, ginkgo seeds contain toxic and allergenic alkylphenols. The publication revises the latest research results (mainly from 2018–2022) regarding the chemical composition of extracts obtained from this plant and provides information on the use of extracts or their selected ingredients in medicine and food production. A very important section of the publication is the part in which the results of the review of patents concerning the use of Ginkgo biloba and its selected ingredients in food production are presented. Despite the constantly growing number of studies on its toxicity and interactions with synthetic drugs, its health-promoting properties are the reason for the interest of scientists and motivation to create new food products.
Keywords: ginkgo biloba, patents, ginkgotoxin, pro-health properties, food industry
1. Introduction
The fossil remains of plants of the Ginkgoaceae family are well known to paleobotanists: representatives of this family lived 300 million years ago (in the Permian period), and they achieved the greatest importance in the Jurassic period (200 million years ago). Currently, only Ginkgo biloba L. (Figure 1) is a naturally occurring species in this group. This plant survived the mass extinctions in the Cretaceous and Paleogene periods and the Pleistocene glaciation, becoming a relict and endemic species in China [1].
Figure 1.
Ginkgo biloba stems and leaves (Rose Garden “Różanka”, Szczecin, 2021; fot. P. Biernacka).
Due to its high ornamental and medicinal value, ginkgo has been spread all over the world. It was favored by enormous adaptability to the environment, high resistance to air pollution and almost all pests and pathogens. The high viability of this species due to the duplication of genes responsible for resistance and stress reactions made it ideal for use in urban greenery arrangements. It is now commonly planted around the world in university campuses, parks and gardens, or along streets and sidewalks [1,2]. These trees are also a source of artistic and religious inspiration for the inhabitants of many continents [1]. Old specimens are commonly found in temples, old villages, or near streams in East Asia. Ginkgo is considered in China as a cultural symbol of hope and peace and is called the national tree of China [2].
Ginkgo biloba leaves are a popular herbal medicine registered in the Chinese Pharmacopeia (2015 edition) [3]. The preparations made from them are used, inter alia, in the treatment of cardiovascular and cerebrovascular diseases. The effectiveness in alleviating cardiovascular ailments was confirmed in the 1960s [4].
Ginkgo biloba leaf extract is one of the best-selling herbal remedies in the world and the most-sold herbal supplement in the US and Europe. It has great therapeutic potential, including scavenging free radicals, reducing oxidative stress, as well as reducing damage to the nervous system and reducing platelet aggregation. It also has anti-inflammatory, anti-cancer and anti-aging properties. Clinical studies also confirm its beneficial effects, among others, in the treatment of central nervous system disorders—Alzheimer’s disease and cognitive deficits [5].
The aim of the publication is to analyze the results of research mainly from 2015–2022 on the chemical composition of Ginkgo biloba and its biological activities, as well as toxicity and interactions with drugs. This publication contains a section devoted to the analysis of granted patents in the field of innovative possibilities of using Ginkgo biloba in the production of food and beverages.
2. Phytoconstituents of Plant
Ginkgo biloba contains many compounds with a unique structure that can be used in herbal medicine. These include, for example, terpene trilactones (ginkgolides), acylated flavonol glycosides (ginkgoghrelins), biflavones (ginkgetin), ginkgotides and ginkgolic acids [6].
Ginkgo extract contains over 60 bioactive ingredients, but the most important role is played by flavonoids and terpenoids. They usually constitute about 24% and 6% of the extract, respectively. Moreover, it contains organic acids, proanthocyanidins, tannins, sitosterols, carotenoids, polysaccharides, glucose and other ingredients (minerals and vitamins) [7].
2.1. Terpenoids
Plant secondary metabolites are a group of small-molecule organic compounds produced as a result of the secondary metabolic activity of plants. These substances are stored in certain organs or tissues of plants, are species-specific and are involved in the stress resistance of plants and the transmission of information. The main terpenoids present in ginkgo are bilobalides (sesquiterpene) and ginkgolides (diterpenes), which are the only ones to contain t-butyl [C17(CH3)3]. They are natural substances with functional groups that play an important role in the protection and treatment of cardiovascular and cerebrovascular diseases. Bilobalides and ginkgolides are present in all parts of the ginkgo seeds they contain, and the highest total terpenoid content was found in the embryo and endosperm [8].
So far, ten diterpenoid lactones have been discovered, named ginkgolides and labeled Q, P, N, M, L, K, J, C, B and A. The group of sesquiterpene lactones is bilobalide, and its isomers contain two lactone ring groups. In addition to these two groups of substances, Ginkgo biloba also contains nor-terpenoids, including three nor-sesquiterpenoids [9,10,11]. The terpenoid fraction of the extract consists mainly of ginkgolides A, B, C, J and M (about 2.8–3.4%) and bilobalides (2.6–3.2%) [12].
Ginkgolides have a high medicinal value. The terpene trilactones present in ginkgo, including ginkgolides A, B, C and bilobalide, correspond, inter alia, to its anti-epileptic effect on neurons within the brain’s hippocampus, improving memory and learning ability and ameliorating neuronal damage. Especially important is ginkgolide B. This terpenoid shows high biological activity due to its role as an antagonist of the platelet-activating factor receptor. This compound has antioxidant, anti-inflammatory and anti-apoptotic effects [13]. DeFeudis et al. (2003) [14] found that ginkgo extract, and especially ginkgolide B, inhibited the proliferation of the very aggressive human breast cancer cell line and xenografts of this cell line in mice. However, the inclusion of ginkgolide B in therapy may be associated with mild side effects, including headache, somnolence, hiccups, and general weakness. Ginkgolide C, on the other hand, has a different effect: its application may contribute to the reduction of lipid storage [7].
It is also important that the flavonoid and terpenoid fractions of ginkgo extracts can act in a complementary manner, inhibiting several processes related to carcinogenesis in the development of neoplastic diseases [14].
2.2. Flavonoids
Flavonoids are important natural bioactive compounds with a strong influence on the human body. Ginkgo biloba leaves contain a number of substances from this group, including flavonol glycosides, biflavones, proanthocyanidins and isoflavonoids. However, the majority are the multiform glycosides of quercetin, kaempferol, and isorhamnetin. Flavonoids are the main constituents of ginkgo leaf extract [8].
One hundred ten flavonoids belonging to seven classes have been identified in ginkgo extracts. The first class consists of 52 glycosides of flavonols and seven flavonols. Known aglycones of flavonol glycosides include quercetin, kaempferol and isorhamnetin. In addition, from the group of aglycons, there are also syringetin, myricetin, laricitrin, myricetin 3′,4′-dimethyl ether and patuletin. The second class consists of 14 flavone glycosides and five flavones. The third class included two flavanones and one flavanone glycoside, the fourth class—two isoflavones and one isoflavone glycoside, and the fifth class—four flavan-3-ole. The sixth class consisted of 13 biflavonoids, and the seventh consisted of nine biginkgosides [7].
Ginkgo flavonoids and their glycosides exhibit multidirectional biological activity, including antioxidant, anti-cancer, anti-bacterial, anti-viral, anti-inflammatory and neuroprotective properties. A strong therapeutic effect was shown by the combination of phenolic aglycones of quercetin, kaempferol or isorhamnetin [7,15].
Ginkgo biloba seedlings, up to 5 years old, contain more flavonoids and terpenoids than adult trees. Therefore, the young leaves are used to produce a standardized extract (EGb761). With the age of trees, the content of biologically active ingredients decreases, and, thus, the quality of extracts produced from them. In recent years, there has also been interest in increasing the content of flavonoids in Ginkgo biloba leaves, e.g., through foliar fertilization or alternative partial irrigation of the root zone [16].
2.3. Carboxylic Acids
Organic acids are common chemical components of plants that are characterized by high biological activity. Preparations made of Ginkgo biloba contain about 13% carboxylic acids, including quinic acid, chlorogenic acid, ascorbic acid, shikimic acid, gallic acid, protocatechuic acid, vanillic acid, isovanillic acid, coffee acid, sinapinic acid, ferulic acid, 6-hydroxybenzoic acid, p-coumaricic acid and p-hydroxybenzoic acid [7,11,17,18,19]. In addition, phenolic acids in Ginkgo biloba leaves also occur in glycosidic or covalently bonded forms [19]. In ginkgo leaves, quinic acid is the most occupied—2.26 g/100 g of dry weight. Shikimic acid is also present in large amounts—2.24 g/100 g dw. Malic acid was the least—0.58 g/100 g dw [20].
The organic acids present in Ginkgo biloba have a very strong free radical scavenging effect. Flavones and procyanidins are also characterized by the same activity [21]. Studies have shown that protocatechuic acid present in ginkgo has the ability to induce terminal kinase-dependent hepatocellular carcinoma cell death and increase the endogenous antioxidant potential of macrophages, and gallic acid exhibits antitumor activity [7].
2.4. Lignins
The richest part of ginkgo in lignin is the shells surrounding the seeds. The content of these substances can be as high as 40%. At the moment, 24 lignans and their isomers have been isolated from this plant [22].
Although lignins have interesting physicochemical properties and high biological activity, they are not often used due to the secondary metabolite of lignocellulosic biomass. Lignin consists mainly of three phenylpropane units: p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S). It increases the strength and rigidity of lignocellulose cell walls and provides a physical barrier against phytopathogen invasion and other environmental stresses. This means that lignin can be considered a bioactive macromolecule [11,22].
The lignins isolated from Ginkgo biloba include i.e. sesamin, ginkgool, pinoresinol, ginkgolide B, and lariciresinol. Their action is mainly based on antioxidant activity [9,10,11].
2.5. Proanthocyanidins
Proanthocyanidins are highly active, functional polyphenolic compounds. They are oligomers or polymers of a polyhydroxy flavan-3-alcohol [e.g., (+)—catechins and (-)—epicatechins)] and flavan-3,4-alcohol linked by a single C4-C8 or C4-C6 bond (type B) or by an additional C2-O-C7 or C2-O- bond C5 (type A) [23].
Proanthocyanidins constitute 4–12% of ginkgo leaves, and standardized extracts contain 7% of proanthocyanidins. Although studies on the composition of these compounds are still ongoing, it has already been shown that proanthocyanidins and flavan-3-oils have antioxidant activity and the ability to scavenge free radicals. Moreover, they alleviate ischemic-reperfusion damage conditions and exhibit antihypertensive, anti-atherosclerotic and anti-aggregating, immunomodulating, antiseptic and anti-inflammatory effects [17].
2.6. Polyprenols
Polyprenols consist of 12–20 cis- and two trans-isoprene units and one form of betulaprenol and are terminated in an isoprene unit (having a hydroxyl group). Polyprenols mainly occur as a mixture of homologs in the photosynthetic organs of plants and have a similar structure and composition to dolichols [24]. Ginkgo biloba leaf polyprenols are weakly polar unsaturated polyisoprenoid alcohols found in leaf lipids. There they occur mainly in the form of polyprene acetate [25]. Ginkgo leaf polyprenols exhibit antioxidant, immunomodulating, anti-bacterial, anti-viral, antitumor and hepatoprotective properties [24,25,26].
2.7. Polysaccharides
Among the many bioactive compounds found in ginkgo, there are also polysaccharides. Purified polysaccharides are obtained from ginkgo by extraction methods (hot water extraction, ultrasound-assisted and enzymatic extraction) and by purification methods (including ion exchange chromatography and gel filtration). Large amounts of structurally diverse polysaccharides, mainly in terms of monosaccharide composition, were isolated from both leaves, sarcotesta and seeds. However, most of them consist of rhamnose (Rha), galactose (Gal), mannose (Man), xylose (Xyl), arabinose (Ara), glucose (Glu) and fucose (Fuc) with different mole fractions of the individual components [11,27]. Ginkgo seeds are a rich source of mannose (Man), and the sarcotesta contains more galactose (Ga) and glucose (Glu) compared to leaves. Interestingly, the molecular weight of Ginkgo biloba polysaccharides shows a varied distribution ranging from 1.0 kDa to 5679 kDa [27].
Polysaccharides isolated from ginkgo have antioxidant properties, as well as anti-cancer, anti-inflammatory, hepatoprotective, antidepressant, immunostimulating and even anti-alopecia properties [28,29].
2.8. Alkylphenols and Alkylphenolic Acids
Alkylphenols occurring in the leaves of Ginkgo biloba can be divided into five groups: cardanols, α-hydroxycardanols, cardans, urushiols and isourushiols, and a group of alkylphenolic acids, which include ginkgolic acids. These compounds are among the toxic ingredients of Ginkgo biloba. Ginkgolic acid occupies a special place here, as it is considered to be toxic, mutagenic and sensitizing. However, despite their negative effect, a beneficial pharmacological effect on the human body was also shown for example, ginkgolic acid C17:1 in studies showed various antitumor effects [6].
2.9. Other
Sixty-eight chemical compounds have been identified in the composition of essential oils obtained from Ginkgo biloba leaves [30], among which the largest percentage was sesquiterpenes (42.11%) [7].
Ginkgo seeds and leaves are a source of vitamins B, C and E. Among the minerals, a relatively high content of zinc, iron, sodium, magnesium, potassium, calcium, carbon and nitrogen was found. According to research by Pereira et al. (2013) [20], among the macronutrients, carbohydrates (72.98 g/100 g dw) have the largest share in Ginkgo biloba leaves. The protein content is 12.27 g/100 g dw, the ash content is 12.27 g/100 g dw, and the fats have the smallest share—4.75 g/100 g dw. The content of free sugars such as fructose, glucose and sucrose was 1.42; 0.78; 0.23 g/100 g dw, respectively, a total of 2.43 g/100 g dw free sugars.
The approximate calorific value of Ginkgo biloba dried leaves is 287 kcal/g [31]. In turn, according to the study by Tomowa et al. (2021) [32], the protein content of Ginkgo biloba seeds was 5 g/100 g of raw seeds or about 11 g/100 g dw. The fat content in raw nuts was 1 g/100 g, and in dry matter, 2.04 g/100 g. The content of saturated acids was 19 g/100 g, polyunsaturated 40 g/100 g, and monounsaturated 41 g/100 g. Starch was isolated as seed after extraction with organic solvents and water. The average yield of the product after extraction was about 70 g/100 g dw of Ginkgo biloba seeds. Information on selected biologically active substances is presented in Table 1.
Table 1.
Selected biologically active substances contained in Ginkgo biloba (leaves, fruit, roots).
| Compound | Structure | Molecular Formula | Molecular Weight [g/mol] |
Main Toxicological and/or Pharmacological Effects | Source of Information |
|---|---|---|---|---|---|
| Diterpenes | |||||
| Ginkgolide A |

|
C20H24O9 | 408.399 | No toxicity Anti-inflammatory and immunostimulating effect |
[33,34] |
| Ginkgolide B |

|
C20H24O10 | 424.399 | No toxicity Beneficial effect on the functioning of the central nervous system |
[33,35] |
| Ginkgolide C |

|
C20H24O11 | 440.398 | No toxicity Reduces the accumulation of lipids, anti-cancer effect |
[33,36] |
| Ginkgolide M |
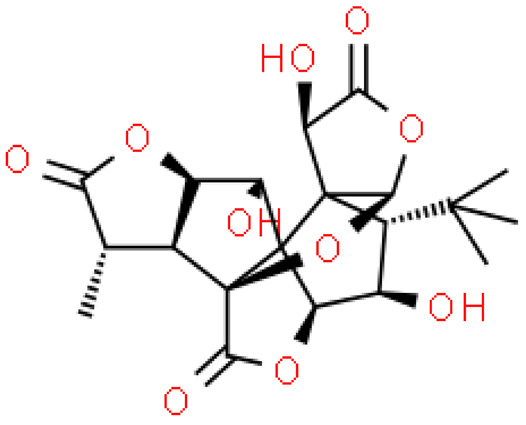
|
C20H24O10 | 424.399 | No toxicity Inhibitor of ligand-gated ion channels in the central nervous system |
[33,37] |
| Ginkgolide J |
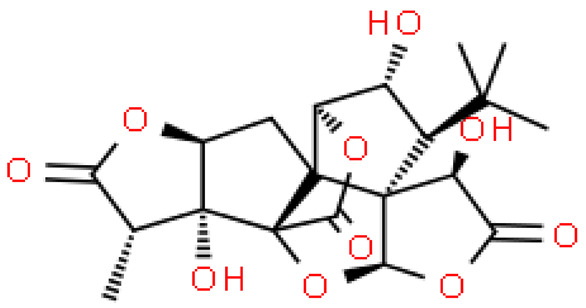
|
C20H24O10 | 424.399 | No toxicity Dementia treatment |
[33,38] |
| Ginkgolide P |
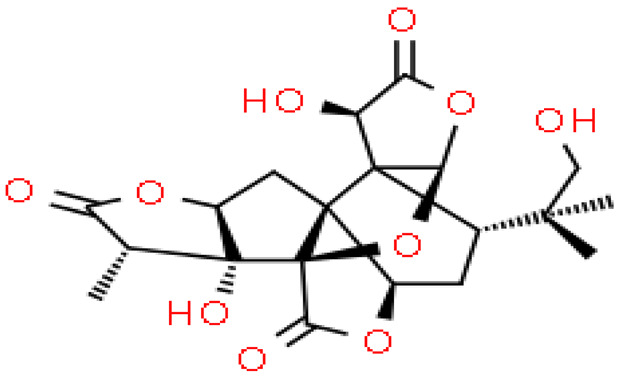
|
C20H24O10 | 424.399 | No data | [33] |
| Ginkgolide Q |

|
C20H24O11 | 463.126 | No data | [39] |
| Ginkgolide K |

|
C20H22O9 | 406.383 | No data Antioxidant, immunomodulatory and neuroprotective effects in ischemic stroke |
[33,40,41] |
| Ginkgolide L |
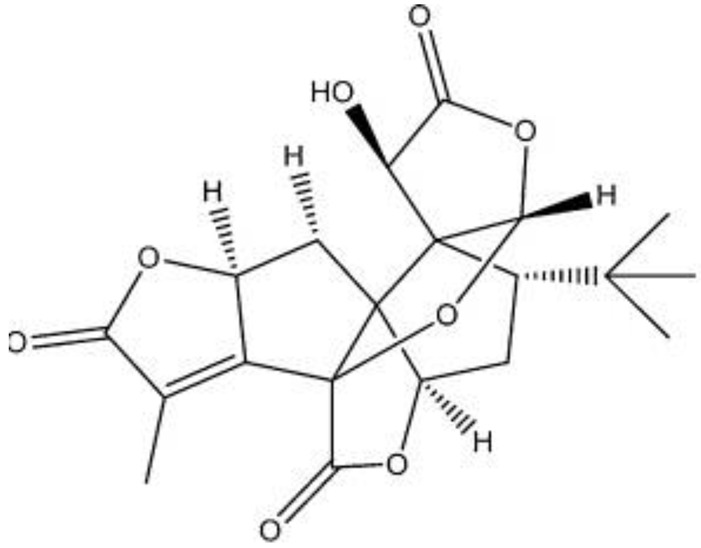
|
C20H22O8 | No date | No data | [42] |
| Ginkgolide N |

|
C20H24O11 | No data | No toxicity Protective effect on damaged PC12 cells induced by glutamate |
[43,44] |
| Sesquiterpenes | |||||
| Bilobalide |

|
C15H18O8 | 326.299 | May cause arrhythmia Neuroprotective, anti-inflammatory, antioxidant, anti-ischemic, protective effect on the circulatory system |
[33,45] |
| Bilobalide isomer |

|
No data | No data | No data | [46] |
| Flavonoids | |||||
| Quercetin |

|
C15H10O7 | 302.23 | Quercetin administration may cause cellular toxicity due to o-quinone/methide quinone side-production Anti-diabetic, anti-inflammatory, antioxidant, anti-microbial, anti-cancer effect, supporting the functioning of the circulatory and nervous systems |
[10,33,47,48] |
| Kaempferol |

|
C15H10O6 | 286.236 | Genotoxic and carcinogenic in vitro—no in vivo studies confirming this effect Antioxidant, anti-inflammatory, ability to scavenge free radicals |
[38,49,50] |
| Isorhamnetin |
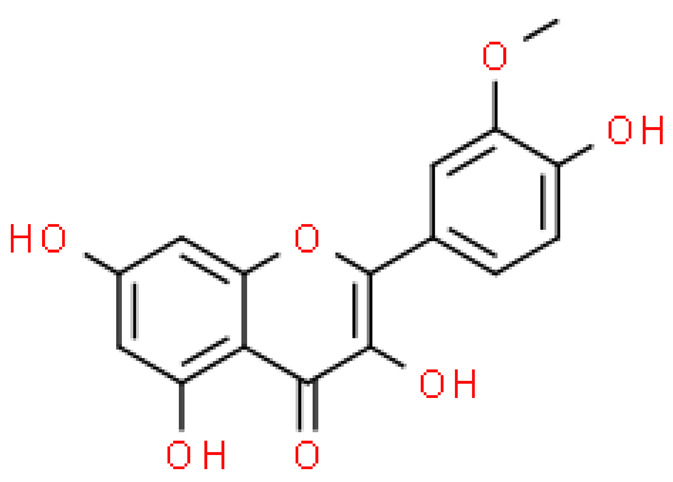
|
C16H12O7 | 316.262 | No toxicity Protective effect on the circulatory and nervous systems, anti-atherosclerotic, hypotensive, hypoglycemic, anti-cancer, anti-inflammatory effects |
[33,51] |
| Myricetin |

|
C15H10O8 | 318.235 | No toxicity Antioxidant, anti-inflammatory, anti-photoaging, anti-cancer, anti-platelet aggregation, anti-hypertensive, immunostimulating effect |
[33,52] |
| Apigenin |

|
C15H10O5 | 270.237 | No toxicity Anti-diabetic, anti-cancer, protective effect on the nervous system |
[33,53] |
| Luteolin |
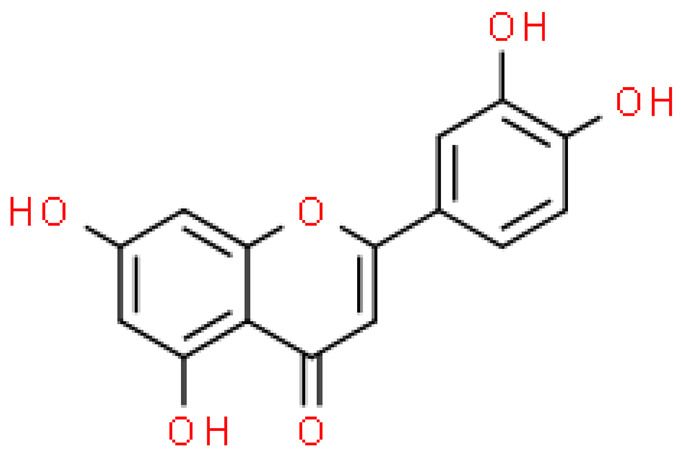
|
C15H10O6 | 286.236 | No toxicity Antioxidant, anti-inflammatory, anti-allergic and anti-cancer effect |
[33,54] |
| Genkwanin |

|
C16H12O5 | 284.263 | No toxicity Anti-inflammatory, immunomodulatory, anti-bacterial, anti-rheumatic effect |
[33,55] |
| Genistein |

|
C15H10O5 | 270.237 | A high dose of genistein has a strong teratogenic, endocrine-disrupting effect Anti-inflammatory effects, inhibition of nuclear factor Kappa-B, prostaglandins, pro-inflammatory cytokines, reactive oxygen species and free radical scavenging activity |
[33,56] |
| Epicatechin |

|
C15H14O6 | 290.268 | No toxicity Antioxidant, anti-inflammatory, anti-bacterial, anti-diabetic, anti-cancer effect |
[33,57] |
| Catechin |

|
C15H14O6 | 290.268 | Excessive dose may cause hepatitis Anti-cancer, anti-obesity, anti-diabetic, anti-inflammatory, anti-cardiovascular, anti-infective, hepatoprotective and neuroprotective |
[33,58] |
| Epigallocatechin |

|
C15H14O7 | 306.267 | Mild and acute health problems after using higher doses, i.e., skin irritation, hepatitis, hypoglycemia, dizziness—human and animal studies Anti-obesity, anti-microbial, anti-cancer, anti-inflammatory effect |
[33,59] |
| Gallocatechin |

|
C15H14O7 | 306.267 | May cause irritation of the respiratory tract (manifested by coughing and shortness of breath), skin and acute eye irritation Antioxidant and neuroprotective effect anti-diabetes, antivirus activities |
[33,60,61,62,63,64] |
| Amentoflavone |

|
C30H18O10 | 538.458 | It can be a strong inhibitor of some genes, e.g., CYP2C9 Anti-inflammatory, anti-microorganism, antioxidant, anti-angiogenesis, neuroprotective, musculoskeletal protection, radioprotection, metabolism regulation, anxiolytic/antidepressant, anti-cancer |
[33,65] |
| Bilobetin |
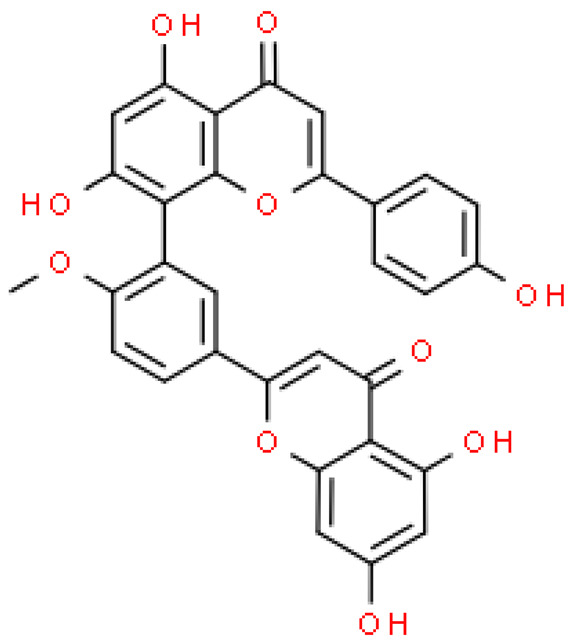
|
C31H20O10 | 552.484 | Extensive watery degeneration of hepatocytes Antifungal, anti-inflammatory, antioxidant, antihyperlipidemic and antiproliferative effects |
[33,66,67] |
| Sequoiaflavone |

|
C31H20O10 | 552.484 | LD toxicity in mice after oral and intraperitoneal administration at a dose above 3 gm/kg Anti-cancer activities |
[33,46,64,68] |
| Ginkgetin |

|
C32H22O10 | 566.511 | Extensive watery degeneration of hepatocytes Anti-cancer, anti-inflammatory, anti-microbial, anti-adipogenic and neuroprotective effect |
[33,66,69] |
| Alkylophenolic acid | |||||
| Ginkgolic acid (C13:0) |
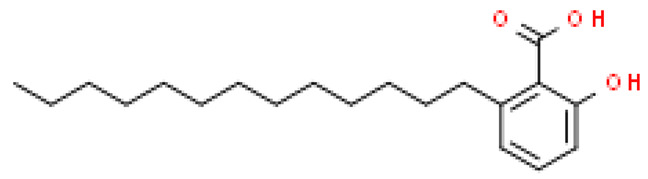
|
C22H32O3 | 320.466 | Cytotoxic, mutagenic, genotoxic, allergenic and neurotoxic in high doses Anti-inflammatory and anti-cancer, anti-diabetic, anti-fibrotic, anti-bacterial, anti-viral and reno/neuroprotective effects |
[6,33,70] |
| Ginkgolic acid (C15:1) |

|
C22H34O3 | 346.504 | Cytotoxic, mutagenic, genotoxic, allergenic and neurotoxic in high doses Anti-inflammatory and anti-cancer, anti-diabetic, anti-fibrotic, anti-bacterial, anti-viral and reno/neuroprotective effects |
[6,33,70] |
| Ginkgolic acid (C17:1) |

|
C24H38O3 | 374.600 | Cytotoxic, mutagenic, genotoxic, allergenic and neurotoxic in high doses Anti-inflammatory and anti-cancer, anti-diabetic, anti-fibrotic, anti-bacterial, anti-viral and reno/neuroprotective effects |
[6,33,70] |
| Ginkgolic acid (C17:2) |
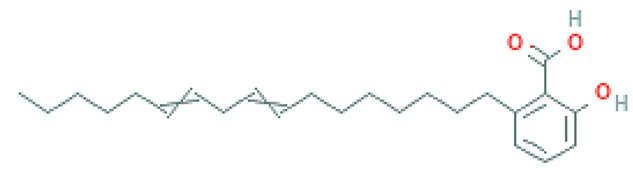
|
C24H36O3 | 372.500 | Cytotoxic, mutagenic, genotoxic, allergenic and neurotoxic in high doses Anti-inflammatory and anti-cancer |
[6,33,71] |
| Alkylphenols | |||||
| Cardanols (C15:0) |

|
C21H36O | 304.510 | No data | [33] |
| Cardanols (C15:1) |
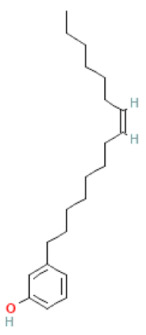
|
C21H34O | 302.500 | At high doses genotoxic effects Antioxidant, anti-cancer and antimutagenic effect. At low dose DNA damage repair |
[46,72,73] |
| Cardol (C15:0) |

|
C21H36O2 | 320.509 | Cytotoxic effect Anti-cancer effect—inhibits the proliferation of cancer cells and induces the death of cancer cells; antioxidant effect, neuroprotective effect |
[33,74,75,76,77] |
| Cardol (C15:1) |

|
C21H34O2 | 318.5000 | Cytotoxic effect No data |
[46,74] |
| Urushiol (C15:0) |

|
C21H36O2 | 320.509 | Allergenic effect (acute inflammation of the skin) Anti-bacterial effect, anti-cancer effect (cytotoxic against tumor cells) |
[33,78,79,80] |
| Proanthocyanidins | |||||
| Epicatechin-(4β→8)-catechin |

|
C30H26O12 | 578.520 | Anti-microbial activity and strong cytotoxicity against tumor cells | [33,81] |
| Gallocatechin-(4β→8)-catechin |
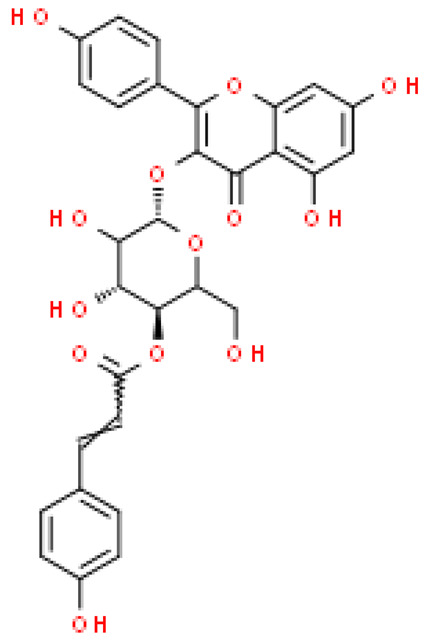
|
C30H26O13 | 594.520 | No data | [33] |
| Epiallocatechin-(4β→8)-gallocatechin |
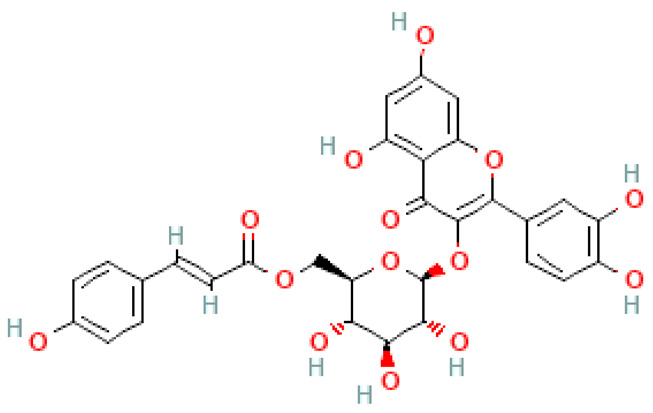
|
C30H26O14 | 610.500 | No data Changes in fat metabolism in hyperlipidemia |
[46,82] |
| Carboxylic acids | |||||
| Protocatechuic acid |

|
C7H6O4 | 154.120 | Cytotoxic, genotoxic, carcinogenic, hepatotoxic and nephrotoxic at high doses Antioxidant, anti-inflammatory, anti-diabetic, antihypertensive, anti-atherosclerotic, anti-aging, anti-cancer, neuroprotective, anti-bacterial, anti-viral effect and protective effect for organs |
[33,83] |
| p-hydroxybenzoic acid |

|
C7H6O3 | 138.120 | Possible reproductive risk and potential involvement in breast cancer Antioxidant, anti-bacterial, antimutagenic, anti-thrombotic and estrogenic activity |
[46,84,85,86,87,88,89] |
| Vanillic acid |

|
C8H8O4 | 168.147 | No toxicity Sedative, anti-depressant, antioxidant, anti-hypertensive, anti-nociceptive, anti-cancer, anti-fungal, reducing the severity of ulcerative colitis, hepatoprotective, wound healing |
[33,90,91] |
| Isovanillic acid |

|
C8H8O | 168.147 | No toxicity Anti-thrombotic and cytostatic activity |
[33,89,92,93] |
| Gallic acid |

|
C7H6O5 | 170.120 | At higher concentrations it can be toxic, e.g., cytotoxic effect. In vivo studies, the toxicity is relatively low Anti-inflammatory, antioxidant, anti-cancer, anti-bacterial, anti-diabetic, anti-obesity, anti-microbial, anti-myocardial ischemia |
[33,94] |
| p-coumaric acid |
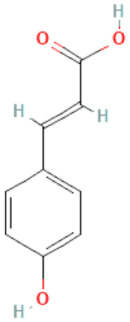
|
C9H8O3 | 164.160 | No toxicity Anti-mutagenic, anti-genotoxic, antioxidant, anti-microbial activity, inhibits cellular melanogenesis and plays a role in immune regulation in humans |
[46,95] |
| Caffeic acid |

|
C9H8O4 | 180.160 | It is anti-implantation during early pregnancy in mice at high doses Anti-inflammatory, antioxidant, anti-cancer, immunomodulatory and neuroprotective effect |
[46,96,97] |
| Sinapic acid |

|
C11H12O5 | 224.21 | May be cytotoxic at high doses Antioxidant, anti-inflammatory, anti-cancer, anti-hyperglycemic, anti-diabetic, anti-hypertensive, hepatoprotective, renoprotection, neuroprotective, anxiolytic, anti-bacterial effect |
[46,98] |
| Ferulic acid |

|
C10H10O4 | 194.180 | Weak toxicity, e.g., on platelets, white and red blood cells Antioxidant, anti-inflammatory, anti-fibrotic, anti-apoptotic, anti-platelet, anti-bacterial, protective effect on vascular endothelial cells |
[46,99] |
| Chlorogenic acid |

|
C16H18O9 | 354.310 | No toxicity Neuroprotective, anti-cancer, anti-bacterial, protective effect on the circulatory system, renoprotection, protective effect on the digestive system, hepatoprotection, support in the treatment of metabolic syndrome |
[46,100] |
| Ascorbic acid |

|
C6H8O6 | 176.124 | No toxicity It has an antioxidant effect, stimulates the production and activation of immune cells |
[33,101] |
| Quinic acid |

|
C7H12O6 | 192.167 | No toxicity Antioxidant, anti-diabetic, anti-cancer, anti-microbial, anti-viral, anti-aging, protective and analgesic effects |
[33,102] |
| Shikimic acid |

|
C7H10O5 | 174.151 | No toxicity Antioxidant, anti-inflammatory, anti-viral, antifungal, exfoliating, anti-acne, whitening, moisturizing, anti-aging, sebum-regulating, hair growth stimulating |
[33,103] |
| Lignans | |||||
| Sesamin |

|
C20H18O | 354.353 | In high doses, it can be a compound with a low and moderate degree of danger, e.g., it can cause loss of appetite, vomiting, diarrhea, hormone metabolism disorders Antioxidant and anti-inflammatory, anti-hypertensive, anti-atherosclerotic, lipolytic, anti-thrombotic, anti-diabetic and anti-obesity effects |
[33,104] |
| Ginkgool |

|
C20H24O7 | 376.000 | No data | [105] |
| Pinoresinol |

|
C20H22O6 | 358.385 | No toxicity Hypoglycemic effect, improving memory and learning ability, anti-cancer effect (stimulation of cancer cell apoptosis) |
[33,106,107,108,109] |
| Ginkgolide B |

|
C24H28O11 | 492.000 | No toxicity Anti-inflammatory and anti-aging effect |
[9,110] |
| p-hydroxyphenyl |

|
C6H6O2 | 110.111 | Oral administration causes acute poisoning (abdominal pain, vomiting, tachycardia, convulsions, convulsions and coma) or formation of neoplastic lesions; skin contact may cause irritation (discoloration or erythema) and allergic dermatitis Treatment of melasma and post-inflammatory hyperpigmentation of the skin (tyrosinase inhibitor) |
[33,111,112,113] |
3. Structure and Biosynthesis of Ginkgolides and Bilobalides
Ginkgolides have a similar molecular formula. Some have the notation C20H24O (A, B, C, M, J, P, Q, N), and some C20H22O (K, L) (Table 2) [34,40,43,44]. These compounds have a rare group in natural products. These are six 5-membered rings, including a spiro [4,4] carbocyclic nonane ring, three lactones, a tetrahydrofuran ring, and a tert-butyl moiety. All ginkgolides have a similar structure and differ in substituents R1, R2 and R3, which are permutations of H or OH [114].
Bilobalide was first isolated by a Major in 1967 [115]. It is characterized by a tendency to isomerization under mild acylation conditions to form diacyl derivatives of the spiro compound. Bilobalide, also belonging to the diterpenoids, also have a tert-butyl group.
Although the health-promoting properties of the terpenoids found in ginkgo have been thoroughly investigated, their biosynthesis is still not fully understood. One of the enzymes most often involved in ginkgolides biosynthesis is Gb LPS (levopimaradiene synthase), a diterpene synthase that catalyzes the synthesis of levopimaradiene (2)—possibly the precursor to all ginkgolides. Ginkgolides and bilobalides share a common three-step biosynthetic pathway. The first step involves the biosynthesis of two simple five-carbon units that build the isoprene skeleton of isopentyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP). In the second step, there is a repetitive condensation of IPP and DMAPP towards farnesyl precursors (FPP) and geranylgeranyl diphosphate (GGPP). This is followed by late cyclization and oxidation steps catalyzed by terpenoid synthases and cytochrome P450 (CYP-450) dependent monooxygenases, which define the specific carbon backbone and oxidation pattern of the product [114].
The cytosolic pathway of mevalonic acid in the cytosol, from 3-acetyl-CoA to IPP, is responsible for the synthesis of sesquiterpenoids and sterols, while for the formation of monoterpenoids, diterpenoids components—the plastid pathway of methylerythritol 4-phosphate producing IPP and dimethylallyl diphosphate from pyruvaldehyde-glyceraldehyde-3-glycerate [116]. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Table 2.
Research on the biological activity of Ginkgo biloba (2015–2022).
| Type of Activity | Substance | Result | Source |
|---|---|---|---|
| anti-inflammatory | Ginkgolides |
|
[54] |
| Extract |
|
[55] | |
| Ginkgolides |
|
[53] | |
| Leaf extract (EGb 761) |
|
[51] | |
| Bilobalide |
|
[52] | |
| Ethanol extract of flowers bilobetin isoginkgetin |
|
[50] | |
| Ginkgolide B |
|
[48] | |
| Amentoflavone |
|
[57] | |
| GBSP3a (water-soluble polysaccharide) |
|
[104] | |
| Ginkgolide A |
|
[47] | |
| Extract EGb 761 |
|
[10] | |
| Ginkgolide A |
|
[46] | |
| Leaf extract (IGbE-761®) |
|
[45] | |
| Ginkgo biloba leaf polysaccharides (PGBL) |
|
[44] | |
| Ginkgolide A |
|
[46] | |
| anti-bacterial | Leaf extract (GLE) |
|
[73] |
| Leaf extract (GLE) |
|
[63] | |
| Water extract Chloroform extract Methanol extract |
|
[62] | |
| Ethanol extract of leaves |
|
[64] | |
| Gelatin film with the addition of ginkgo extract (GBE) |
|
[67] | |
| Ginkgetin |
|
[117] | |
| Ginkgetin |
|
[58] | |
| Leaf extracts (GLE) |
|
[66] | |
| Amentoflavone |
|
[57] | |
| Ginkgolic acid (GA) C15:1 monomer |
|
[60] | |
| Polyprenol (GBP) |
|
[61] | |
| Antioxidant | supernatant obtained after mixing the fermented seed powder and saline |
|
[94] |
| hydroethanolic leaf extract and ingredients: flavone, ginkgolide, procyanidins, and organic acids |
|
[21] | |
| Leaf extract (EGb 761) |
|
[74] | |
| Ethanol extracts |
|
[64] | |
| Leaf extract (EGb 761) |
|
[51] | |
| Ethanol extract |
|
[73] | |
| extract (GBE) |
|
[67] | |
| Polysaccharides GBPS-2 and GBPS-3 |
|
[72] | |
| ginkgo biloba (10 mg/kg/day) |
|
[73] | |
| methanol extract from leaves ethanol (40%, 70% and 96% v/v) extracts from leaves |
|
[70] | |
| polysaccharide monomers |
|
[56] | |
| extract EGb 761 |
|
[69] | |
| Anti-cancer | bilobol isolated from fruit |
|
[87] |
| Ginkgetin (extract) |
|
[83] | |
| Methanol extract from kernel |
|
[82] | |
| Ginkgo biloba extract (EGb-761) |
|
[81] | |
Substances isolated from fresh male flowers:
|
|
[86] | |
| Ginkgolide B |
|
[86] | |
| Leaf extract IDN 5933 |
|
[80] | |
| Amentoflavone |
|
[57] | |
| Methanolic extract from leaves |
|
[79] | |
| Polysaccharide isolated from leaves (Se-GBLP) |
|
[13] | |
| Extract EGb 761 |
|
[78] | |
| Ginkgolide B (GB) |
|
[85] | |
| Extract EGb 761 |
|
[76] | |
| Extract EGb 761 |
|
[77] | |
| Ginkgolide B (GKB) |
|
[84] | |
| Extract EGb 761 |
|
[35] | |
| Anti-obesity, anti-atherogenic and anti-diabetic | Leave extract (GbE) |
|
[65] |
| Extract GbE |
|
[90] | |
| Extract (GbE) |
|
[55] | |
| Ginkgo biloba seeds (GBS) |
|
[91] | |
| vinegar obtained from fermented coats of ginkgo seeds |
|
[97] | |
| Extract GbE |
|
[96] | |
| Extract GbE |
|
[89] | |
| Ginkgo biloba leaves |
|
[93] | |
| Ginkgolide B |
|
[48] | |
| Ginkgolide C |
|
[95] | |
| Ginkgo biloba leaves |
|
[71] | |
| Ginkgolide B |
|
[74] | |
| Extract GbE |
|
[88] | |
| Neuroprotective and anti-neurodegenerative | Extract EGb |
|
[106] |
| Extract EGb 761 |
|
[103] | |
| Ginkgo biloba dropping pill (GBDP) Extract EGb 761 |
|
[104] | |
| Extract EGb 761 |
|
[74] | |
| Extracts (GB, EGb 761) Tablets |
|
[105] | |
| Extract EGb 761 |
|
[102] | |
| Ginkgolides |
|
[53] | |
| Extract EGb |
|
[3] | |
| Extract EGb 761 |
|
[101] | |
| Extract EGb 761 |
|
[98,100] | |
| Extract EGb 761 |
|
[99] | |
| Protection of sense organs | GBE capsule (120 mg: 27% flavone glycosides + 6.8% terpene lactones from ginkgo) |
|
[109] |
| Ginkgo leaf tablets |
|
[107] | |
| Extract EGb 761 |
|
[108] | |
| Cardiovascular protection | Ginkgolide B |
|
[48] |
4. Pharmacological Activities
Ginkgo biloba has been used for years as a herbal plant supporting memory processes. Initially, it was studied in terms of neuroprotective and anti-neurodegenerative effects. However, as a result of the analysis of the composition of the extracts obtained from the leaves and the influence of these extracts or their selected components, it was found that this plant has a wide multidirectional effect on the functioning of the organism. As a result of studies conducted on animals and on human tissue lines, it has been shown that ginkgo extracts and tablets and their selected ingredients exhibit anti-inflammatory, anti-bacterial, antioxidant, anti-cancer, anti-obesity, anti-diabetic, anti-atherogenic, cardioprotective and oto-protective effects (Table 2).
4.1. Anti-Inflammatory Effect
Extracts prepared in laboratories and their commercial formulas, such as individual ingredients: ginkgolides (A or B), bilobalide, amentoflavone, and water-soluble polysaccharides, were tested for anti-inflammatory properties. All studies have shown a positive damping effect on the developing inflammation. The most commonly observed reductions in nitric oxide, interferon, prostaglandin E2, TNF-α, IL-1, IL-4, IL-6, IL-12, and IL-1β were observed in inflamed tissues [118,119,120,121,122,123,124,125,126,127,128,129,130], as well as inter alia, changes in MAPK and NF-κB signaling pathways [116,122], caused, inter alia, by weaker translocation of the nuclear factor NF-κB [131,132]. In addition, there is also increased activation of AMPK protein kinase [122] and heme oxygenase [130].
4.2. Anti-Microbial Activity
The use of ginkgo for anti-bacterial purposes has been the subject of research for a long time. Initially, the effectiveness of extracts prepared from various parts of plants was analyzed—e.g., fruit, leaves and roots, as well as selected components of these extracts, e.g., ginkgo acids or free phenolic acids for the few taxa of bacteria. The most frequently tested microorganisms were Escherichia coli and Staphylococcus aureus. In these studies, inhibition of the activity of selected bacterial taxa was demonstrated, and the results became the basis for further research on anti-bacterial activity. In recent years, ginkgetin [129,133], amentofavone [132], ginkgolic acid C15:1 monomer [134] and polyprenol [135] and the effectiveness of leaf extracts obtained with the use of various solvents (water, ethanol, chloroform and methanol) [136,137,138,139]. The group of microorganisms has been significantly expanded, including taxa of gram-positive and gram-negative bacteria [125,126,134,136,138,140,141], intestinal microflora typical of the tested mammalian organisms [137,139], as well as human pathogenic fungi (e.g., Candida albicans used in the study by) [141]. In all the studies carried out, different effects on the activity of microorganisms were shown, and the strength of the effect depends on the tested pathogen and the dose/amount of the substance used. Moreover, higher efficiency of alcoholic extracts than water extracts was found [136].
4.3. Antioxidant Activity
The study of antioxidant activity was carried out on the supernatant obtained from fermented ginkgo seeds, leaf extracts obtained with the use of various solvents, as well as their individual components (including polysaccharides and their monomers, organic acids, procyanidins, flavone and ginkgolide). These analyzes were carried out using various methods (e.g., DPPH, ABTS, scavenging hydroxyl radicals or superoxide anion methods), which does not allow for a direct comparison of the results of all these studies. However, all studies showed the antioxidant activity of the tested substrates, often assessed as high or very high [21,126,131,138,141,142,143,144,145,146,147,148,149]. However, it was noted that this activity varies depending on the date of leaf harvest: it is the highest in the case of raw material harvested in autumn [68]. Both commercial EGb761 extracts [143], as well as methanol and ethanol extracts prepared under laboratory conditions, are highly active. In the latter case, extracts made in alcohol at a concentration of 40% and 70% are more effective compared to extracts made in the presence of alcohol with a concentration of 96% [144]. Among the tested extract components, procyanidins and flavones contained in ginkgo leaves showed the highest antioxidant activity [21].
4.4. Antitumor Activity
The research on antitumor activity was carried out using the tissue culture method, which consisted of treating selected tumor cell lines (Table 2) with selected substances. When analyzing the results of studies published mainly in 2015–2022, it can be concluded that work on the use of ginkgo extracts or their selected ingredients is widely conducted, but it should be intensified. In total, the effect of the extract and seven selected substances found in ginkgo was analyzed and tested on at least 22 cancer cell lines; however, usually, the effect of the selected substance on only 2–3 tumor cell lines was analyzed. Nevertheless, the discussed studies demonstrated cytotoxicity or inhibition of selected development phases of neoplastic cells that inhibit their proliferation, both when using extracts obtained from leaves [42,150,151,152,153,154,155,156,157], as well as selected components isolated from them: ginkgolide B [158,159,160] and polysaccharides isolated from leaves [13], bilobol isolated from the fruit [161] and chemical components isolated from fresh male flowers, incl. amentoflavone and its derivatives [134,160], bilobetin, isoginkgetin and sciadopitysin [160]. Very often, the effect of substance application was the improvement of parameters indicating the stage of disease development, which resulted in a slowdown or complete inhibition of tumor development (Table 2).
4.5. Anti-Obesity, Anti-Atherogenic and Anti-Diabetic
During the research, a positive, antiatherogenic, anti-obesity and anti-diabetic effect of ginkgo on the metabolism of mammalian organisms was observed. The effects of both leaf extracts [130,139,162,163,164] and seeds [165], vinegar obtained from fermenting seeds [166], leaves [148,167], well as ginkgolide B [123,168] and ginkgolide C [169] were investigated. During the research, positive changes in body weight (decrease) were observed [163,164,170], and a reduction in adipose tissue mass [167,168,169] was caused by the reduction of fat cells (adipocytes) [169,170]. Blood tests showed positive changes in the fat profile [145,164], including lowering cholesterol and triglycerides [167,168], and the regulation of lipid metabolism, glucose and insulin [166,167] had a positive effect on the condition and functioning of the kidneys [130]. Additionally, a study by Wang et al. (2022) [139] showed that the oral administration of Ginkgo biloba leaf extract is effective in relieving hypercholesterolemia, systemic inflammation and atherosclerosis, and these effects are associated with the modulation of the taxonomic composition of intestinal microbes, protection of the integrity of the intestinal mucosa, and improvement of microbial metabolic phenotypes (Figure 2).
Figure 2.
Selected health-promoting effects of Ginkgo biloba extract on the human body.
4.6. Neuroprotective and Anti-Neurodegenerative
In studies on the influence of ginkgo on the functioning of the nervous system, mainly ginkgo leaf extracts were used [3,140,171,172,173,174,175,176,177,178,179,180,181,182], less often tablets [176,177]. The use of all these types of preparations was conducive to the reduction of inflammatory processes within the nervous system [128,170], the number of nerve cells subject to damage and apoptosis [169,173] and the improvement of blood circulation within the cerebral vessels [171]. The therapies resulted in the improvement of memory, especially cognitive memory [3,168,169,170,171,172], which was of great importance for limiting and inhibiting neurodegenerative changes, especially in Alzheimer’s and Parkinson’s diseases. It is also important that these preparations enhance the effect of a drug traditionally used in the treatment of dementia symptoms occurring in neurodegenerative diseases—donepezil [140].
4.7. Protection of Other Organs
Research has shown that both EGb extract [179] and ginkgo leaf tablets [180,181] have a protective effect on the sensory organs. This is of great importance, especially for patients suffering from type 2 diabetes, because it allows to delay or completely inhibit damage to blood vessels in the eye’s retina leading to visual disturbances [179], as well as contributing to at least partial regeneration and improvement of the condition of these vessels [181]. In addition, the substances contained in the ginkgo leaf extract protect the hearing organ against damage caused by taking cisplatin during chemotherapy implemented during the treatment of neoplastic diseases [180]. One of the components found in ginkgo leaves, ginkgolide B, also has a protective effect on blood vessels, preventing the occurrence of atherosclerotic lesions [123].
5. Toxicity
Toxic components in Ginkgo biloba include alkylphenols. Their classification is presented in Section 5 (Phytoconstituents of the plant). These compounds are a mixture of several 2-hydroxy-6-alkylbenzoic acids. One of the toxic components is ginkgolic acid, designated as C13:0, C15:1 and C17:1 [182].
Ginkgo biloba standardized extract EGb761 is classified as a therapeutic agent for the treatment of the central system, the main one in the treatment of dementia, but also helpful in the treatment of Alzheimer’s and Parkinson’s. It is credited with relieving symptoms, memory functions and handling, dizziness, migraines, or tinnitus. To be able to attribute the pro-health effect of EGb761, it should contain 22–27% of flavonoids and 5–7% of terpenoids and less than 5 ppm ginkgo biloba acid (i.e., 0.0005% ginkgo biloba acid in the preparation) [6,32].
The seeds of this plant are used in Asian cuisine for the production of stuffing, soups, desserts, meat and vegetarian dishes, and the roasted seeds are a popular delicacy. While eating cooked ginkgo seeds is safer than eating them raw, they can be toxic if consumed in large amounts or over a long period of time, especially in children. Ginkgo seeds contain a toxic component of MPN (4-methoxypyridoxin) called ginkgotoxin, and in addition, ginkgo seed tests contain large amounts of alkylphenol (over 4% ginkgolic acid)—eating more than 10–20 nuts a day may pose a health risk [32]. However, it has been shown that the concentration of ginkgotoxin in the protein of ginkgo seeds increases during the growing season and reaches its maximum in early August, but then its content drops sharply. Canned and cooked seeds now contain only about 1% of ginkgotoxin present in raw seeds, which can be attributed to their water solubility. On the other hand, the content in roasted seeds is slightly lower than in raw seeds because the compound is thermally stable [6,183]. For this reason, when using Ginkgo biloba extract preparations, it is important to ensure the safety of patients.
According to the research of Gawron-Gzella et al. (2012) [184], the pharmacopoeial requirements for ginkgo leaf extract refer to the number of bilobalides and the sum of A, B and C ginkgolides, while the manufacturers of preparations indicate the content of total terpene lactones on the labels. Research shows that many manufacturers do not always keep the declared total of terpene lactones (6%), and the preparations do not always contain the correct portion of bilobalides and the sum of ginkgolides. The content of ginkgolic acid, with the applicable norm below 5 ppm, in dietary supplements was very often overstated, sometimes even 1600 times. As a result of consuming such a large amount of ginkgolic acid, problems with the digestive system (nausea, vomiting, diarrhea), headaches and dizziness, palpitations, anxiety, weakness or skin allergy. In the case of people with blood clotting problems and/or taking non-steroidal anti-inflammatory drugs, antiplatelet or anticoagulant medications, it can lead to internal hemorrhage [185]. Ginkgotoxin poisoning can also occur. The content of ginkgotoxin in seeds ranges from 170 to 404 μg/g. Concentrations above 170 μg/g cause toxicity and manifest as seizures, loss of consciousness and leg paralysis [186]. It is important that such poisoning can be prevented with vitamin B6: administration of a dose of 30 mg of pyridoxal 5′-phosphate (corresponding to 2 mg/kg of body weight) causes the symptoms of poisoning to cease [187].
The results of Boeteng & Yang (2021) [24] showed that the number of toxic compounds in fresh Ginkgo biloba seeds (ginkgotoxin, ginkgolic acid and cyanide) was significantly reduced during seed drying. The ginkgotoxin content was reduced by a factor of four, and the amounts of acrylamide, ginkgolic acid and cyanide in the dried seeds were reduced to a safe level (safety range). Of the four drying methods tested, radiant drying turned out to be the most effective: it lasted the shortest, and the obtained product showed the highest quality and content of bioactive compounds, as well as the strongest antioxidant activity.
Recently, the attention of scientists has been attracted by the possibility of using alkylphenols for medical purposes, which in appropriate doses, have beneficial effects, including anti-cancer and anti-bacterial properties [134].
A study by Borenstein et al. (2020) [187] has been shown to inhibit Herpes simplex type 1 virus multiplication, human cytomegalovirus genome replication and Zika virus infection. In addition, it inhibits the synthesis of all three classes of HIV, Ebola, Influenza A, and Epstein–Barr virus fusion proteins. The results also indicate that inhibition of virion entry by blocking the initial fusion event following ginkgolic acid administration post-infection suggests a possible secondary mechanism targeting protein and DNA synthesis. This is confirmed by the strong action of this acid, effective even after the infection process has taken place. The results also indicate the possibility of using it in the treatment of acute infections (e.g., caused by coronavirus, Ebola virus, Zika, influenza A and measles), as well as active local lesions (e.g., caused by HSV-1, HSV-2 and varicella viruses—VZV shingles).
The publication of Omidkhoda et al. (2019) [188] discusses the protective effect of using Ginkgo biloba leaf extract in case of poisoning caused by various factors: natural toxins (scorpion venom, lipopolysaccharides, aflatoxin B1, lysophosphatidylcholine, pentacyclic triterpenoids, cassava, cotton seed pigment called gossypol), chemical toxins (metals): aluminum, lead, cadmium, mercury; heavy metals contained in aqueous waste, fluorine, triethyltin, ethanol, carbon tetrachloride, pesticides, chemotherapeutic drugs, cigarette smoke, naphthalene or monosodium glutamate) and radiation. The beneficial effect of the extract on the poisoned organism is probably related to the high antioxidant activity of the extract (manifested by the reduction of lipid peroxidation and restoration of reduced dehydrogenases, glutathione peroxidase, superoxide dismutase and catalysis) and its anti-inflammatory effect.
According to the results of in vitro studies, biflavonoids (ginkgetin, isogingetin, amentoflavone, sciadopitysin and bilobetin) can also be toxic to the body. They were observed to be cytotoxic to human proximal tubular cells and to be less toxic to healthy human liver cells. In addition, activated apoptosis was associated with biflavonoid-induced nephrotoxicity. These data suggest that these biflavonoids exhibit potential hepatic and renal toxicity [24].
The supplement market should be more regulated so as not to lead to accidental poisoning. In addition, the production should be strictly regulated to ensure that such a supplement will not contain nutrients or be contaminated. Products, such as infusions, are a safe products as a kind of supplementation with a balanced diet. Despite the risks, special care should be taken by pregnant women and children.
6. Interactions of Ginkgo biloba Extracts with Drugs
Ginkgo biloba, as a raw material belonging to phytotherapeutic drugs, shows mainly antioxidant and neuroprotective properties. It contains pharmacologically active ingredients that may be useful in treating many diseases, but due to its antiplatelet effects, it may interact with other antiplatelet drugs (warfarin, aspirin) or herbal preparations with similar antiplatelet effects (garlic or ginseng) [189]. According to Kedzia and Alkiewicz (2006) [190], ginkgo preparations in combination with aspirin may cause hematomas in the anterior chamber of the eye and with paracetamol—subdural hematomas.
Research by Bogacz et al. (2016) [189] proved that extracts from this plant could modulate the expression of cytochrome P450 enzymes and, thus, influence transcription factors, thanks to which they can participate in the metabolism of xenobiotics (drugs, procarcinogens, vitamins and food components).
Ginkgo preparations may also accelerate the metabolism of omeprazole and esomeprazole, primarily by influencing the mechanism of CYP2C19 induction and consequently reducing the effectiveness of these drugs in preventing upper gastrointestinal bleeding. In addition, they increase the risk of bleeding while taking SSRIs or SNRIs [191]. Single cases of coma in humans have been shown to be caused by the concomitant intake of Ginkgo biloba preparations with trazodone, and cases of priapism have been observed as a result of an interaction between Ginkgo biloba and risperidone. It was also noted that the use of Ginkgo biloba may reduce the concentration and effectiveness of valproate and reduce the anxiolytic and hypnotic effects of benzodiazepines [192,193]. Woroń & Siwek (2018) [194] proved that the combination of ginkgo biloba with dormitive and/or anxiolytics or with fluoxetine caused side effects in the form of dizziness, somnolence and hypotension.
In the case of non-standardized extracts prepared in accordance with the European Pharmacopoeia, the effect of the extract remains uncertain. A significant drug interaction potential cannot be ruled out in the case of poorly standardized ginkgo leaf extracts used in many dietary supplements. A review of research to date shows that Ginkgo biloba extracts are very reactive. Therefore, patients should be checked for health prior to administration, and any possible signs of drug interactions should be carefully considered [6].
7. Patents
Ginkgo biloba has been used in Chinese medicine for centuries, but recently, the leaves and fruits of this plant have become objects of interest in the pharmaceutical, food and cosmetic industries. The greatest industrial interest in Ginkgo biloba leaves and fruits occurred at the end of the last century when several hundred patents were issued, mainly in Japan, China and the USA. However, at the beginning of the 21st century, more than a thousand patents were published each year, with the largest number of publications appearing in 2017 (over 7000); in the following years, the number decreased, with 391 patents published in 2021 and 275 in 2022 (state on 7 November 2022). Generally, referring to the Espacenet patent database (European Database Espacenet), during the last three decades, more than 29 thousand patents appeared all over the world. Most of them concern the application of ginkgo extracts in medicine, methods of extraction or preparation of tablets or pills.
Until 1990 of the 44 patents listed for the entry “Ginkgo biloba” in the Espacenet patent database (European Database Espacenet), many patents were related to cultivation and chemicals, as well as drugs, for example, anti-vomiting preparations or anti-inflammatory medicines, which contain ginkgo extract.
In the years 1990–2000, the content increases in the number of patents (458 patents) concerning mainly the extraction methods of valuable substances from Ginkgo biloba leaves [195,196,197,198,199,200,201] and the use of the extracts in medicine [179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202]. There have also been patented food products containing ginkgo biloba leaves, like tea mixtures [203] or drinks [204] and other products enriched with leaves extract, e.g., chewing gum [205], chocolate [206], and candies [207].
The new inventions concerned mainly extraction methods allowing to obtain extracts with a reduced content of toxic compounds, such as 4′-o-methyl pyridoxine and biflavones, alkyl polyphenols or ginkgolic acid. Extracts were usually obtained from ginkgo leaves using organic solvents, e.g., acetone or methanol, which were removed in the next steps of processing, and the resulting concentrate was dissolved in water, ethanol or other solvents, with the steps of purification and filtration to remove alkyl polyphenols [208]. The patented production process of extract EGb 761® involves extraction of a mixture of leaves from China, France, and the USA with 60% (m/m) aqueous acetone, acetone removal by evaporation, cooling with stirring the aqueous solution to precipitate chlorophylls, biflavones and most of the ginkgolic acid [23,202,209]. Extracts prepared using organic solvents are treated with lead compound (e.g., lead acetate) or insoluble polyamide than the filtrated solution is extracted with an aliphatic solvent, the aqueous-alcoholic solution is concentrated, ammonium sulfate is added, the solution is extracted with methylethylketone and ethanol. The concentrated, filtrated and dried extract contains less than 20 ppm, preferably less than 10 ppm and in particular less than 2 ppm 4′-O-methyl pyridoxine and/or less than 20 ppm, preferably less than 10 ppm and in particular less than 5 ppm biflavones [202,210].
Inventors from China in 2017 [211] patented a new method of obtaining flavonoids from ginkgo leaves by using fermentation with Aspergillus niger, which allows reducing the amount of undesired ginkgo acid in the extract. Moreover, the obtained extract is free of organic solvents. According to this procedure, cleaned and crushed leaves are mixed with water (40–60%) and sterilized. In the next step, the mixture is inoculated with Aspergillus niger, then enzymes are added, and the fermentation is carried out for 3–6 h at 25–30 °C. Subsequently, further protease is added, and the reaction lasts for the next 3–4 h, followed by 10 min long heat termination, centrifugation, extraction with ethanol, concentrating, and finally freeze-drying.
The raw material subjected to extraction was mainly Ginkgo biloba leaves, fresh or dried. They were also used to make mixtures for the production of infusions, e.g., a mixture of ginkgo leaves and other herbal materials [212,213] or beer [214].
In recent years, the subject of patents has also been products made from ginkgo nuts (also called ginkgo fruits or ginkgoes), e.g., shortbread [215], vinegar [216], beverages [217,218] or wines [219,220,221,222]. In some formulas, ginkgo powder obtained earlier from ginkgo nuts was used [223,224]. An important element was to obtain products with improved taste, devoid of bitterness. Due to the insufficient taste of the obtained products or the content of undesirable ingredients, there were searched formulas containing the addition of other valuable ingredients, e.g., wine or drink in which ginkgo fruits were used together with the other raw materials like sorghum, wheat, sugar, saffron etc. [225,226,227]. Reduction of bitterness was also caused by fermentation caused by yeast in wine or beer production, as well as Aspergillus niger or Lactobacillus strains [213,228,229,230,231].
Although the application of Ginkgo biloba in food was always motivated by its health-promoting properties, in recent years, this appeared to be the main reason for forming new products, and inventors evidenced their health-promoting activities [212,232,233,234].
Moreover, there have been patented many devices to facilitate the extraction, harvesting of ginkgo fruits and pre-treatment of ginkgo nuts.
8. Conclusions
Ginkgo biloba is a very popular raw material used not only in medicine but also in industrial technology. The content of ginkgolides, bilobalides, flavonoids and other bioactive ingredients contributes to its wide application. Currently, ginkgo is a herbal dietary supplement (EGb761); it is also used in complementary medicine and is an additive to cosmetics [198]. Products containing it are gaining popularity all over the world. Its primary action focuses on alleviating and/or preventing CNS dysfunction by regulating the level of cytokinins, antioxidant enzymes, kinases and receptors and modifying the activity of the PAF activating factor. Currently, more and more studies are carried out on the health-promoting properties of ginkgolic acids: their anti-cancer, neuroprotective and anti-bacterial activity is being tested. High hopes are associated with the possible medical use of ginkgolic acid. Although in 2000 years old traditional Chinese medicine, Ginkgo biloba seeds are used in the treatment of cough, asthma, tuberculosis, bladder infections, flatulence and diarrhea, however, such use of Ginkgo biloba, along with the development of knowledge about its active ingredients, brings a lot of concerns. The largest of these are the interactions between biologically active substances contained in Ginkgo biloba and drugs. Until now, not all the mechanisms by which the use of non-standardized extracts of Ginkgo biloba leaves can be used are known to cause excessive activity or inhibition of the drug’s action.
In conclusion, Ginkgo biloba is still an interesting research object for scientists dealing with, among others, medicine and food production. New products containing extracts or fractions of Ginkgo biloba fruit or leaves are being developed. So far, patented food products are not popular on the European food market, with some exceptions—products containing the addition of dried ginkgo leaves or Ginkgo biloba extracts, such as health beverages, are valued. Of great importance, there is the possibility of using the active substances contained in the leaves and seeds for the production of the so-called superfoods, other than dried leaf infusions. Focusing on the purification of Ginkgo biloba extracts can contribute to increasing the offer and improving the quality of dietary supplements and medicines. In addition, the development of a method for the isolation of specific Ginkgo biloba metabolites can provide compounds used for food enrichment.
It is worth noting that expanding the market with Ginkgo biloba products may have a positive impact on the development of Europe’s agricultural economy. This will result in no need to import the plant, e.g., from Asian countries, which seems to be a greener solution.
Author Contributions
All three authors are responsible for the concept of the work. P.B. is responsible for the chapters on the introduction, phytonutrients of ginkgo (including the table), structure and biosynthesis of ginkgolides and bilobalides, toxicity and interactions of extracts with drugs, as well as graphics and photos in the publication. I.A. is responsible for the part on pharmacological activities (including the table) and for the substantive control. K.F. is responsible for the patent part. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen Y., Fu C., Wu Z., Xu H., Liu H., Schneider H., Lin J. Ginkgo Biloba. Trends Genet. 2021;37:488–489. doi: 10.1016/j.tig.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Lin H.-Y., Li W.-H., Lin C.-F., Wu G.-R., Zhao Y.-P. International Biological Flora: Ginkgo biloba. J. Ecol. 2022;110:951–982. doi: 10.1111/1365-2745.13856. [DOI] [Google Scholar]
- 3.Liu H., Ye M., Guo H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharmacol. 2020;10:1688. doi: 10.3389/fphar.2019.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong Y., Wang S., Zhu B., Wang R., Cheng Y. A strategy for identifying effective and risk compounds of botanical drugs with LC-QTOF-MS and network analysis: A case study of Ginkgo biloba preparation. J. Pharm. Biomed. Anal. 2021;193:113759. doi: 10.1016/j.jpba.2020.113759. [DOI] [PubMed] [Google Scholar]
- 5.Toghueo R.M.K. Endophytes from Gingko biloba: The current status. Phytochem. Rev. 2020;19:743–759. doi: 10.1007/s11101-020-09679-4. [DOI] [Google Scholar]
- 6.Boateng I.D. A critical review of ginkgolic acids in Ginkgo biloba leaf extract (EGb): Toxicity and technologies to remove ginkgolic acids and their promising bioactivities. Food Funct. 2022;13:9226–9242. doi: 10.1039/D2FO01827F. [DOI] [PubMed] [Google Scholar]
- 7.Liu L., Wang Y., Zhang J., Wang S. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. Anal. 2021;193:113704. doi: 10.1016/j.jpba.2020.113704. [DOI] [PubMed] [Google Scholar]
- 8.Han S., Chio C., Ma T., Kognou A.L.M., Shrestha S., Chen F., Qin W. Extracting flavonoid from Ginkgo biloba using lignocellulolytic bacteria Paenarthrobacter sp. and optimized via response surface methodology. Biotechnol. Appl. Microbiol. 2021;15:867–878. [Google Scholar]
- 9.Shu P., Sun M., Li J., Zhang L., Xu H., Lou Y., Ju Z., Wei X., Wu W., Sun N. Chemical constituents from Ginkgo biloba leaves and their cytotoxicity activity. J. Nat. Med. 2020;74:269–274. doi: 10.1007/s11418-019-01359-8. [DOI] [PubMed] [Google Scholar]
- 10.Dong K.-L., Lin S., Wu Q.-L., Su R.-X., Wu Z.-L., Dong H.-Y., Li H.-L., Zhang W.-D. A new bilobalide isomer and two cis-coumaroylated flavonol glycosides from Ginkgo biloba leaves. Fitoterapia. 2020;142:104516. doi: 10.1016/j.fitote.2020.104516. [DOI] [PubMed] [Google Scholar]
- 11.Tabassum N.-E., Das R., Lami M.S., Chakraborty A.J., Mitra S., Tallei T.E., Idroes R., Mogamed A.A.-R., Hossain J., Dhama K., et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid.-Based Complement. Altern. Med. 2022;2022:8288818. doi: 10.1155/2022/8288818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalisz O., Wolski T., Gerkowicz M. Terapia zaburzeń krążenia obwodowego i mózgowego przy użyciu preparatów z miłorzębu dwuklapowego (Ginkgo biloba) Postępy Fitoter. 2005;3–4:91–97. [Google Scholar]
- 13.Chen D., Sun S., Cai D., Kong G. Induction of mitochondrial-dependent apoptosis in T24 cells by a selenium (Se)-containing polysaccharide from Ginkgo biloba L. leaves. Int. J. Biol. Macromol. 2017;101:126–130. doi: 10.1016/j.ijbiomac.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 14.DeFeudis F.V., Papadopoulos V., Drieu K. Ginkgo biloba extracts and cancer: A research area in its infancy. Fundam. Clin. Pharmacol. 2003;17:405–417. doi: 10.1046/j.1472-8206.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 15.Sun S., Li Y., Chu L., Kuanhg X., Song J., Sun C. Full-length sequencing of ginkgo transcriptomes for an in-depth understanding of flavonoid and terpenoid trilactone biosynthesis. Gene. 2020;758:144961. doi: 10.1016/j.gene.2020.144961. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z., Jiang B., Zhao B., Mau X., Lu J., Jin B., Wang L. Liquid profiling in plants: Identification and analysis of extracellular metabolites and miRNAs in pollination drops of Ginkgo biloba. Tress Physiol. 2020;40:1420–1436. doi: 10.1093/treephys/tpaa073. [DOI] [PubMed] [Google Scholar]
- 17.Cao J., Wang H., Zhang W., Cao F., Ma G., Su E. Tailor-Made Deep Eutectic Solvents for Simultaneous Extraction of Five Aromatic Acids from Ginkgo biloba Leaves. Molecules. 2018;23:3214. doi: 10.3390/molecules23123214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S., Gong X., Qu H. Near-infrared spectroscopy and HPLC combined with chemometrics for comprehensive evaluation of six organic acids in Ginkgo biloba leaf extract. J. Pharm. Pharmacol. 2022;74:1040–1050. doi: 10.1093/jpp/rgab177. [DOI] [PubMed] [Google Scholar]
- 19.Liu L.L., Ke Z., Xu W., Sun L., Ma A.-C. A strategy for quality control of ginkgo biloba preparations based on UPLC fingerprint analysis and multi-component separation combined with quantitative analysis. Chin. Med. 2022;17:72. doi: 10.1186/s13020-022-00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira E., Barros L., Ferreira I.C.F.R. Chemical characterization of Ginkgo biloba L. and antioxidant properties of its extracts and dietary supplements. Ind. Crops Prod. 2013;51:244–248. doi: 10.1016/j.indcrop.2013.09.011. [DOI] [Google Scholar]
- 21.Zhang L., Zhu C., Liu X., Su E., Cau F., Zhao L. Study on Synergistic Antioxidant Effect of Typical Functional Components of Hydroethanolic Leaf Extract from Ginkgo Biloba In Vitro. Molecules. 2022;27:439. doi: 10.3390/molecules27020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang B., Chen H., Zhao H., Wu W., Jin Y. Structural features and antioxidant behavior of lignins successively extracted from ginkgo shells (Ginkgo biloba L) Int. J. Biol. Macromol. 2020;163:694–701. doi: 10.1016/j.ijbiomac.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Kulić Ž., Ritter T., Röck B., Elsäßer J., Schneider H., Germen S. A Detailed View on the Proanthocyanidins in Ginkgo Extract EGb 761. Nat. Prod. Chem. Anal. Stud. 2022;88:398–404. doi: 10.1055/a-1379-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boateng I.D., Yang X.-M. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L. seed. J. Sci. Food Agric. 2021;101:3290–3297. doi: 10.1002/jsfa.10958. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C.H.-W., Li M.-F., Qi Z.W., Tao R., Ye J.-Z., Xue X.-Y., Wang C.H.-Z. The construction of a green and efficient system for the separation of polyprenols from Ginkgo biloba leaves. Process Biochem. 2021;100:252–259. doi: 10.1016/j.procbio.2020.10.013. [DOI] [Google Scholar]
- 26.Zhang C.H.-W., Wang C.H.-Z., Tao R., Ye J.-Z. Separation of polyprenols from Ginkgo biloba leaves by a nano silica-based adsorbent containing silver ions. J. Chromatogr. A. 2019;1590:58–64. doi: 10.1016/j.chroma.2019.01.047. [DOI] [PubMed] [Google Scholar]
- 27.Fang J., Wang Z., Wang P., Wang M. Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: A review. Int. J. Biol. Macromol. 2020;162:1897–1905. doi: 10.1016/j.ijbiomac.2020.08.141. [DOI] [PubMed] [Google Scholar]
- 28.Li H., Zhou G.-Y., Xu J.-P., Liu J.-A., Zhang H.-Y., Tan Y. Research progress on polysaccharides from Ginkgo biloba. J. Med. Plants Res. 2012;6:171–176. doi: 10.5897/JMPR11.1344. [DOI] [Google Scholar]
- 29.Li Y., Sheng Y., Liu J., Xu G., Yu W., Cui Q., Lu X., Du P., An L. Hair-growth promoting effect and anti-inflammatory mechanism of Ginkgo biloba polysaccharides. Carbohydr. Polym. 2022;278:118811. doi: 10.1016/j.carbpol.2021.118811. [DOI] [PubMed] [Google Scholar]
- 30.Tewari G., Mohan B., Kishor K., Tewari L.M., Nailwal T.K. Volatile constituents of Ginkgo biloba L. leaves from Kumaun: A source of (E)-nerolidol and phytol. J. Indian Chem. Soc. 2015;92:1583–1586. [Google Scholar]
- 31.Nwosu O., Okaka A.N.C., Ubaoji K.I. Evaluation of Nutritional and Anti-nutritional Compositions of Leaves of (Maiden Hair) Tree Found in Nigeria. J. Exp. Res. 2018;6:66–72. [Google Scholar]
- 32.Tomowa T., Slavova I., Tomov D., Kirova G., Argirova M.D. Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food. Horticulturae. 2021;7:218. doi: 10.3390/horticulturae7080218. [DOI] [Google Scholar]
- 33.ChemSpider Search and Share Chemistry. [(accessed on 14 March 2023)]. Available online: http://www.chemspider.com/
- 34.Sarkar C., Quispe C., Jamaddar S., Hossain R., Ray P., Mondal M., Mohamed Z.A., Sani M., Salehi B., Islam M.T., et al. Therapeutic promises of ginkgolide A: A literature-based review. Biomed. Pharmacother. 2020;132:110908. doi: 10.1016/j.biopha.2020.110908. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y., Xiong S., Liu P., Liu W., Wang Q., Liu Y., Tan H., Chen X., Shi X., Wang Q., et al. Polymeric Nanoparticles-Based Brain Delivery with Improved Therapeutic Efficacy of Ginkgolide B in Parkinson’s Disease. Int. J. Nanomed. 2020;15:10453–10467. doi: 10.2147/IJN.S272831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M.H., Baek S.H., Um J.-Y., Ahn K.S. Anti-neoplastic Effect of Ginkgolide C through Modulating c-Met Phosphorylation in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2020;21:8303. doi: 10.3390/ijms21218303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolshakov S., Dzyuba S.V., Decatur J., Nakanishi K. A Concise Synthesis of Ginkgolide M, a Minor Component of a Terpene Trilactone Fraction from Ginkgo biloba Roots. J. Nat. Prod. 2006;69:429–431. doi: 10.1021/np050403i. [DOI] [PubMed] [Google Scholar]
- 38.Vitolo O., Gong B., Cao Z., Ishii H., Jaracz S., Nakanishi K., Arancio O., Dzyuba S.V., Lefort R., Shelanski M. Protection against β-amyloid induced abnormal synaptic function and cell death by Ginkgolide J. Neurobiol. Aging. 2009;30:257–265. doi: 10.1016/j.neurobiolaging.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao H.-J., Zheng Y.-F., Li H.-Y., Peng G.-P. Two New Ginkgolides from the Leaves of Ginkgo biloba. Planta Med. 2011;77:1818–1821. doi: 10.1055/s-0030-1271153. [DOI] [PubMed] [Google Scholar]
- 40.Chen M., Zou W., Chen M., Cao L., Ding J., Xiao W., Hu G. Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur. J. Pharmacol. 2018;833:221–229. doi: 10.1016/j.ejphar.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Miao Q., Chai Z., Song L.-J., Wang Q., Song G.-B., Wang J., Yu J.-Z., Xiao B.-G., Ma C.G. The neuroprotective effects and transdifferentiation of astrocytes into dopaminergic neurons of Ginkgolide K on Parkinson’ disease mice. J. Neuroimmunol. 2022;364:577806. doi: 10.1016/j.jneuroim.2022.577806. [DOI] [PubMed] [Google Scholar]
- 42.Wang J.-X., Liu X.-G., Gan Z.-Y., Dong X., Lou F.-C., Li P., Yang H. Pharmacokinetics and tissue distribution study of ginkgolide L in rats by ultra-high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B. 2015;1006:30. doi: 10.1016/j.jchromb.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 43.Ma S., Liu X., Xu Q., Zhang X. Transport of ginkgolides with different lipophilicities based on an hCMEC/D3 cell monolayer as a blood–brain barrier cell model. Life Sci. 2014;114:93–101. doi: 10.1016/j.lfs.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Z.D., Zhang L.H., Zhang X.T., Fang C.S., Ma J.R. Protective Effects of Ginkgolide N Against Glutamate-Induced Injury in PC12 Cells. J. Chin. Med. Mater. 2015;38:1694–1698. [PubMed] [Google Scholar]
- 45.Lu J., Xie L., Liu K., Zhang X., Wang X., Dai X., Liang Y., Cao Y., Li X. Bilobalide: A review of its pharmacology, pharmacokinetics, toxicity, and safety. Phytother. Res. 2021;35:6114–6130. doi: 10.1002/ptr.7220. [DOI] [PubMed] [Google Scholar]
- 46.PubChem National Center for Biotechnology Information. USA: National Library of Medicine. [(accessed on 14 March 2023)]; Available online: https://pubchem.ncbi.nlm.nih.gov.
- 47.Salehi G., Machin L., Monzote L., Sharifi-Rad J., Ezzat S.M., Salem M.A., Merghany R.M., El Mahdy N.M., Kılıç C.S., Sytar O., et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega. 2020;5:11849–11872. doi: 10.1021/acsomega.0c01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyu Y.-L., Zhou H.-F., Yang J., Wang F.-X., Dun F., Li J.-Y. Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease. Mediat. Inflamm. 2022;2022:5665778. doi: 10.1155/2022/5665778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devi K.P., Malar D.S., Nabavi S.F., Sureda A., Xiao J., Nabavi S.M., Daglia M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015;99:1–10. doi: 10.1016/j.phrs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Alam W., Khan H., Shah M.A., Cauli O., Saso L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules. 2020;25:4073. doi: 10.3390/molecules25184073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong G., Guan Y.-Y., Zhang Z.-L., Ragman K., Wang S.-J., Zhou S., Luan X., Zhang H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020;128:110301. doi: 10.1016/j.biopha.2020.110301. [DOI] [PubMed] [Google Scholar]
- 52.Semwal D.K., Semwal R.B., Combrinck S., Viljoen A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutriens. 2016;8:90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E.B., Novellino E., et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Y., Ahi R., Wang X., Shen H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets. 2008;8:634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao Y., Sun Y.-W., Ji J., Gan L., Zhang C.-F., Wang C.-Z., Yuan C.-S. Genkwanin ameliorates adjuvant-induced arthritis in rats through inhibiting JAK/STAT and NF-κB signaling pathways. Phytomedicine. 2019;63:153036. doi: 10.1016/j.phymed.2019.153036. [DOI] [PubMed] [Google Scholar]
- 56.Goh Y.-X., Jalil J., Lam K.-W., Husain K., Premakumar C.M. Genistein: A Review on its Anti-Inflammatory Properties. Front. Pharmacol. 2022;13:1–23. doi: 10.3389/fphar.2022.820969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prakash M., Basavaraj B.V., Murthy K.N.C. Biological functions of epicatechin: Plant cell to human cell health. J. Funct. Foods. 2018;52:14–24. doi: 10.1016/j.jff.2018.10.021. [DOI] [Google Scholar]
- 58.Idemura M. Catechin in Human Health and Disease. Molecules. 2019;24:528. doi: 10.3390/molecules24030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehmood S., Maqsood M., Mahtab N., Khan M.I., Sahar A., Zaib S., Gul S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022;46:e14189. doi: 10.1111/jfbc.14189. [DOI] [PubMed] [Google Scholar]
- 60.Carloth. [(accessed on 14 March 2023)]. Available online: https://www.carlroth.com.
- 61.Park C.H., Park J.Y., Kang K.S., Hwang G.S. Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules. 2021;26:1387. doi: 10.3390/molecules26051387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun M.-Y., Shen Z., Zhou Q., Wang M.F. Identification of the antiglycative components of Hong Dou Shan (Taxus chinensis) leaf tea. Food Chem. 2019;297:124942. doi: 10.1016/j.foodchem.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Xiao T., Cui M., Zheng C., Zhang P., Ren S., Bao J., Gao D., Sun R., Wang M., Lin J., et al. Both baicalein and gallocatechin gallate effectively inhibit SARS-CoV-2 replication by targeting M pro and sepsis in mice. Inflammation. 2022;45:1076–1088. doi: 10.1007/s10753-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei Q., Li Q.-Z., Wang R.-L. Flavonoid Components, Distribution, and Biological Activities in Taxus: A review. Molecules. 2023;28:1713. doi: 10.3390/molecules28041713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiong X., Tang N., Lai X., Zhang J., Wen W., Li X., Li A., Wu Y., Liu Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021;12:768708. doi: 10.3389/fphar.2021.768708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y.-Y., Lu X.-Y., Sun J.-L., Wang Q.-Q., Zhang Y.-D., Zhang J.-B., Fan X.-H. Potential hepatic and renal toxicity induced by the biflavonoids from Ginkgo Biloba. Chin. J. Nat. Med. 2019;17:672–681. doi: 10.1016/S1875-5364(19)30081-0. [DOI] [PubMed] [Google Scholar]
- 67.Patel D.K. Biological Importance of a Biflavonoid ‘Bilobetin’ in the Medicine: Medicinal Importance, Pharmacological Activities and Analytical Aspects. Infect. Disord.-Drug Targets. 2022;22:22–30. doi: 10.2174/1871526522666220321152036. [DOI] [PubMed] [Google Scholar]
- 68.Yeh P.H., Shieh Y.D., Hsu L.C., Kuo L.M.Y., Lin J.H., Liaw C.C., Kuo Y.H. Naturally occurring cytotoxic [30→ 800-biflavonoidsfrom Podocarpus nakaii. J. Tradit. Compl. Med. 2012;2:220–226. doi: 10.1016/S2225-4110(16)30103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adan M., Rasul A., Hussain G., Shah M.A., Zahoor M.K., Anwar H., Sarfraz I., Riaz A., Manzoor M., Adem Ş., et al. Ginkgetin: A natural biflavone with versatile pharmacological activities. Food Chem. Toxicol. 2020;145:111642. doi: 10.1016/j.fct.2020.111642. [DOI] [PubMed] [Google Scholar]
- 70.Ding Y., Ding Z., Xu J., Li Y., Chen M. Pharmacological Activities of Ginkgolic Acids in Relation to Autophagy. Pharmaceuticals. 2022;15:1469. doi: 10.3390/ph15121469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shanmugam M.K., Garg M., Makhija P., Kumar A.P., Sharifi-Rad J., Zam W., Bishayee A. Ginkgolic Acids Confer Potential Anticancer Effects by Targeting Pro- Inflammatory and Oncogenic Signaling Molecules. Curr. Mol. Pharmacol. 2021;14:806–822. doi: 10.2174/1874467214666210126112413. [DOI] [PubMed] [Google Scholar]
- 72.De Sousa Leite A., Islam M.T., Paz M.F.C.J., Júnior A.L.G., da Silva Oliveira G.L., das Graças Lopes Cito A.M., de Carvalho Melo-Cavalcante A.A., Lopes J.A.D. Cytogenotoxic and mutagenic profiling of cashew nut shell liquids and cardanol. Clin. Phytoscience. 2019:5. doi: 10.1186/s40816-019-0136-9. [DOI] [Google Scholar]
- 73.Schneider B.U.C., Meza A., Beatriz A., Pesarini J.R., de Carvalho P.C., de Oliveira Mauro M., Karaziack C.B., Cunha-Laura A.L., Monreal A.C.D., Matuo R., et al. Cardanol: Toxicogenetic assessment and its effects when combined with cyclophosphamide. Genet. Mol. Biol. 2016;39:279–289. doi: 10.1590/1678-4685-gmb-2015-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satooka H., Kubo I. Prooxidative effect of cardols is involved in their cytotoxic activity against murine B16-F10 melanoma cells. Biochem. Biophys. Res. Commun. 2022;609:105–110. doi: 10.1016/j.bbrc.2022.04.033. [DOI] [PubMed] [Google Scholar]
- 75.Kustiawan P.M., Lirdprapamongkol K., Palaga T., Puthong S., Phuwapraisirisan P., Svasti J., Chanchao C. Molecular mechanism of cardol, isolated from Trigona incisa stingless bee propolis, induced apoptosis in the SW620 human colorectal cancer cell line. BMC Pharmacol. Toxicol. 2017;18:32. doi: 10.1186/s40360-017-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salehi B., Gültekin-Özgüven M., Kırkın C., Özçelik B., Morais-Braga M.F.B., Carneiro J.N.P., Bezerra C.F., da Silva T.G., Coutinho H.D.M., Amina B., et al. Anacardium Plants: Chemical, Nutritional Composition and Biotechnological Applications. Biomolecules. 2019;9:465. doi: 10.3390/biom9090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Almeida M.O., Bezerra T.T., Lima N.M.A., Sousa A.F., Trevisan M.T.S., Ribeiro V.G.P., Lomonaco D., Mazzetto S.E. Cardol-Derived Organophosphorothioates as Inhibitors of Acetylcholinesterase for Dengue Vector Control. J. Braz. Chem. Soc. 2019;30:2634–2641. [Google Scholar]
- 78.Liu B., Tai Y., Achanta S., Kaelberer M.M., Caceres A.I., Shao X., Fang J., Jordt S.-E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA. 2016;113:7572–7579. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Y., He X., Wang H., Wang H., Shi Z., Zhu S., Cui Z. Polyphenol-Enriched Extract of Lacquer Sap Used as a Dentine Primer with Benefits of Improving Collagen Cross-Linking and Antibacterial Functions. ACS Biomater. Sci. Eng. 2022;8:3741–3753. doi: 10.1021/acsbiomaterials.1c01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou H., Qi Z., Xue X., Wang C. Novel pH-Sensitive Urushiol-Loaded Polymeric Micelles for Enhanced Anticancer Activity. Int. J. Nanomed. 2020;15:3851–3868. doi: 10.2147/IJN.S250564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibrahim T.A., El Dib R.A., Al-Youssef H.M., Amina M. Chemical composition and antimicrobial and cytotoxic activities of Antidesm abunius L. Pak. J. Pharm. Sci. 2019;32:153–163. [PubMed] [Google Scholar]
- 82.Hui C.K., Majid N.I., Yusof H.M., Zainol K.M., Mohamad H., Zin Z.M. Catechin profile and hypolipidemic activity of Morinda citrifolia leaf water extract. Helion. 2020;6:e04337. doi: 10.1016/j.heliyon.2020.e04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song J., He Y., Luo C., Feng B., Ran F., Xu H., Ci Z., Xu R., Han L., Zhang D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020;161:105109. doi: 10.1016/j.phrs.2020.105109. [DOI] [PubMed] [Google Scholar]
- 84.Soni M.G., Carabin I.G., Burdock G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food Chem. Toxicol. 2005;43:985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 85.Lemini C., Silva G., Timossi C., Luque D., Valverde A., González Martínez M., Hernández A., Rubio Póo C., Chávez Lara B., Valenzuela F. Estrogenic effects of p-hydroxybenzoic acid in CD1 mice. Environ. Res. 1997;75:130–134. doi: 10.1006/enrs.1997.3782. [DOI] [PubMed] [Google Scholar]
- 86.Pugazhendhi D., Pope G.S., Darbre P.D. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005;25:301–309. doi: 10.1002/jat.1066. [DOI] [PubMed] [Google Scholar]
- 87.Oksana S., Marian B., Mahendra R., Hong Bo S. Plant phenolic compounds for food, pharmaceutical and cosmetics production. J. Med. Plants Res. 2012;6:2526–2539. [Google Scholar]
- 88.Chen J., Yang J., Ma L., Li J., Shahzad N., Kim C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020;10:2611. doi: 10.1038/s41598-020-59451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi J.-H., Kim S. In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur. Molecules. 2022;27:3496. doi: 10.3390/molecules27113496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mirza A.C., Panchal S.S. Safety Assessment of Vanillic Acid: Subacute Oral Toxicity Studies in Wistar Rats. Turk. J. Pharm. Sci. 2020;17:432–439. doi: 10.4274/tjps.galenos.2019.92678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ashwini S.I., Megha P.K., Aishwarya P.D., Shital M.K., Piyusha R.M., Vaishali D.T., Om R.L., Aniket P.N., Yash V.K., Shatrughna U.N., et al. A Review of the Pharmacological Characteristics of Vanillic Acid. J. Drug Deliv. Ther. 2021;11:200–204. [Google Scholar]
- 92.Istifli E.S., Sihoglu-Tepe A., Sarikurkcu C., Tepe B. Molecular interactions of some phenolics with 2019-nCoV and related pathway elements. Int. J. Second. Metab. 2021;8:246–271. doi: 10.21448/ijsm.958597. [DOI] [Google Scholar]
- 93.Garcia M.D., Ahumada M.C., Saenz M.T. Cytostatic Activity of Some Phenolic Acids of Scrophularia frutescens L. var. frutescens. Z. Für Nat. C. 1998;53:1093–1095. doi: 10.1515/znc-1998-11-1225. [DOI] [Google Scholar]
- 94.Bai J., Zhang Y., Tang C., Hou Y., Ai X., Chen X., Zhang Y., Wang X., Meng X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021;133:110985. doi: 10.1016/j.biopha.2020.110985. [DOI] [PubMed] [Google Scholar]
- 95.Kiliç I., Yeşiloğlu Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;115:719–724. doi: 10.1016/j.saa.2013.06.110. [DOI] [PubMed] [Google Scholar]
- 96.Alam M., Ahmed S., Elasbali A.M., Adnan M., Alam S., Hassan M.I., Pasupuleti V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022;12:860508. doi: 10.3389/fonc.2022.860508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y., Qiu S., Wang L., Zhang N., Shi Y., Zhou H., Liu X., Shao L., Liu X., Chen J., et al. Reproductive and developmental toxicity study of caffeic acid in mice. Food Chem. Toxicol. 2019;123:106–112. doi: 10.1016/j.fct.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 98.Pandi A., Kalappan V.M. Pharmacological and therapeutic applications of Sinapic acid—An updated review. Mol. Biol. Rep. 2021;48:3733–3747. doi: 10.1007/s11033-021-06367-0. [DOI] [PubMed] [Google Scholar]
- 99.Li D., Rui Y.-X., Guo S.D., Luan F., Liu R., Zeng N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021;284:119921. doi: 10.1016/j.lfs.2021.119921. [DOI] [PubMed] [Google Scholar]
- 100.Lu H., Tian Z., Cui Y., Liu Z., Ma X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020;19:3130–3158. doi: 10.1111/1541-4337.12620. [DOI] [PubMed] [Google Scholar]
- 101.Van Gorkom G.N.Y., Lookermans E.L., Van Elssen C.H.M.J., Bos G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients. 2019;11:977. doi: 10.3390/nu11050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benali T., Bakrim S., Ghchime R., Benkhaira N., El Omari N., Balahbib A., Hasan M.M., Bibi S., Bouyahya A. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022;19:1–30. doi: 10.1080/02648725.2022.2122303. [DOI] [PubMed] [Google Scholar]
- 103.Batory M., Rotsztejn H. Shikimic acid in the light of current knowledge. J. Cosmet. Dermatol. 2022;21:501–505. doi: 10.1111/jocd.14136. [DOI] [PubMed] [Google Scholar]
- 104.Dalibalta S., Majdalawieh A.F., Manjikian H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm. J. 2020;28:1276–1289. doi: 10.1016/j.jsps.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei X., Chen Y., Chen X., Liang J., Qu W. A new lignan from the roots of Ginkgo biloba. Chem. Nat. Compd. 2015;51:819–821. doi: 10.1007/s10600-015-1424-3. [DOI] [Google Scholar]
- 106.Yu J., Kwon H., Cho E., Jeon J., Kang R.H., Youn K., Jun M., Lee Y.-C., Ryu J.-H., Kim D.-H. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice. Food Chem. Toxicol. 2019;125:376–382. doi: 10.1016/j.fct.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 107.Ogungbe I.V., Crouch R.A., Demeritte T. (−) Arctigenin and (+) Pinoresinol Are Antagonists of the Human Thyroid Hormone Receptor β. J. Chem. Inf. Model. 2014;54:3051–3055. doi: 10.1021/ci500537e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fini L., Hotchkiss E., Fogliano V., Graziani G., Romano M., De Vol E.B., Qin H., Selgrad M. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis. 2014;29:139–146. doi: 10.1093/carcin/bgm255. [DOI] [PubMed] [Google Scholar]
- 109.Wikul A., Damsud T., Kataoka K., Phuwapraisirisan P. (+)-Pinoresinol is a putative hypoglycemic agent in defatted sesame (Sesamum indicum) seeds though inhibiting α-glucosidase. Bioorganic Med. Chem. Lett. 2012;22:5215–5217. doi: 10.1016/j.bmcl.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 110.Hoi Y.J., Alishir A., Jang T., Kang K.S., Lee S., Kim K.H. Antiskin Aging Effects of Indole Alkaloid N-Glycoside from Ginkgo Fruit (Ginkgo biloba fruit) on TNF-α-Exposed Human Dermal Fibroblasts. J. Agric. Food Chem. 2022;70:13651–13660. doi: 10.1021/acs.jafc.2c05769. [DOI] [PubMed] [Google Scholar]
- 111.World Health Organization . Hydroquinone Health and Safety Guide. Volume 101. World Health Organization; Geneva, Switzerland: 1996. [Google Scholar]
- 112.Chandra M., Levitt J., Pensabene C.A. Hydroquinone Therapy for Post-inflammatory Hyperpigmentation Secondary to Acne: Not Just Prescribable by Dermatologists. Acta Derm Venereol. 2012;92:232–235. doi: 10.2340/00015555-1225. [DOI] [PubMed] [Google Scholar]
- 113.Mpofana N., Chibi B., Visser T., Paulse M., Finlayson A.J., Ghuman S., Gqaleni N., Hussein A.A., Dlova N.C. Treatment of Melasma on Darker Skin Types: A Scoping Review. Cosmetics. 2023;10:25. doi: 10.3390/cosmetics10010025. [DOI] [Google Scholar]
- 114.Hébert M. Ph.D. Thesis. University of Ottawa; Ottawa, ON, Canada: 2022. Total Synthesis of (±)-Ginkgolide C and Formal Syntheses of (±)-Ginkgolide A and (±)-Ginkgolide B. [DOI] [PubMed] [Google Scholar]
- 115.Major R.T. The Ginkgo, the Most Ancient Living Tree: The resistance of Ginkgo biloba L. to pests accounts in part for the longevity of this species. Science. 1967;157:1270–1273. doi: 10.1126/science.157.3794.1270. [DOI] [PubMed] [Google Scholar]
- 116.Zeng Z., Zhu J., Chen L., Wen W., Yu R. Biosynthesis pathways of ginkgolides. Pharmacogn. Rev. 2013;7:47–52. doi: 10.4103/0973-7847.112848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li G., Wang G., Wang S., Deng Y.A. Ginkgetin in vitro and in vivo reduces Streptococcus suis virulence by inhibiting suilysin activity. J. Appl. Microbiol. 2019;127:1556–1563. doi: 10.1111/jam.14365. [DOI] [PubMed] [Google Scholar]
- 118.Zhang C., Lin L., Li G., Ma J., Han X., Fei R. PGBL inhibits the RAW 264.7 cells to express inflammatory factor. Bio-Med. Mater. Eng. 2015;26:2069–2075. doi: 10.3233/BME-151512. [DOI] [PubMed] [Google Scholar]
- 119.Mir M.A., Albaradie R.S. Immunomodulation of Inflammatory Markers in Activated Macrophages by Leaf Extracts of Gingko Biloba. Adv. Neuroimmune Biol. 2015;6:9–17. doi: 10.3233/NIB-150103. [DOI] [Google Scholar]
- 120.Zhao Q., Gao C., Cui Z. Ginkgolide A reduces inflammatory response in high-glucose-stimulated human umbilical vein endothelial cells through STAT3-mediated pathway. Int. Immunopharmacol. 2015;25:242–248. doi: 10.1016/j.intimp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 121.Zhaocheng J., Jinfeng L., Luchang Y., Yequan S., Feng L., Kai W. Ginkgolide A inhibits lipopolysaccharide-induced inflammatory response in human coronary artery endothelial cells via downregulation of TLR4-NF-κB signaling through PI3K/Akt pathway. Pharmazie. 2016;71:588–591. doi: 10.1691/ph.2016.6576. [DOI] [PubMed] [Google Scholar]
- 122.Li Y., Wu Y., Yao X., Hao F., Yu C., Bao Y., Wu Y., Song Z., Sun Y., Zheng L., et al. Ginkgolide A Ameliorates LPS-Induced Inflammatory Responses In Vitro and In Vivo. Int. J. Mol. Sci. 2017;18:794. doi: 10.3390/ijms18040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng Z., Yang X., Zhang L., Ansari I.A., Khan M.S., Han S., Feng Y. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating Lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother. Res. 2018;32:2417–2427. doi: 10.1002/ptr.6177. [DOI] [PubMed] [Google Scholar]
- 124.Yao Q.Q., Li L., Xu M.C., Hu H.H., Zhou H., Yu L.S., Zeng S. The metabolism and hepatotoxicity of ginkgolic acid (17:1) in vitro. Chin. J. Nat. Med. 2018;16:829–837. doi: 10.1016/S1875-5364(18)30124-9. [DOI] [PubMed] [Google Scholar]
- 125.Li M., Li B., Hou Y., Tian Y., Chen L., Liu S., Zhang N., Dong J. Anti-inflammatory effects of chemical components from Ginkgo biloba L. male flowers on lipopolysaccharide-stimulated RAW264.7 macrophages. Phytother. Res. 2019;33:989–997. doi: 10.1002/ptr.6292. [DOI] [PubMed] [Google Scholar]
- 126.de Souza G.A., de Marqui S.V., Matias J.N., Guiguer E.L., Barbalho S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Med. 2020;86:376–386. doi: 10.1055/a-1109-3405. [DOI] [PubMed] [Google Scholar]
- 127.Zhang H., Cao N., Yang Z., Fang X., Yang X., Li H., Hong Z., Ji Z. Bilobalide Alleviated Dextran Sulfate Sodium-Induced Experimental Colitis by Inhibiting M1 Macrophage Polarization Through the NF-kB Signaling Pathway. Front. Pharmacol. 2020;11:718. doi: 10.3389/fphar.2020.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li C., Liu K., Liu S., Aerqin Q., Wu X. Role of Ginkgolides in The Inflammatory Immune Response of Neurological Diseases: A Review of Current Literatures. Front. Syst. Neurosci. 2020;14:45. doi: 10.3389/fnsys.2020.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li C., Liu S., Aerqin Q., Shen D., Wu X., Liu K. The therapeutic effects of ginkgolides in Guillain-Barré syndrome and experimental autoimmune neuritis. J. Clin. Neurosci. 2021;87:44–49. doi: 10.1016/j.jocn.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 130.Chang T.-T., Chen Y.-A., Li S.-Y., Chen J.-W. Nrf-2 mediated heme oxygenase-1 activation contributes to the anti-inflammatory and renal protective effects of Ginkgo biloba extract in diabetic nephropathy. J. Ethnopharmacol. 2021;266:113474. doi: 10.1016/j.jep.2020.113474. [DOI] [PubMed] [Google Scholar]
- 131.Zhang C.-W., Wang C.-Z., Tao R. Characterization and antioxidant activities of polysaccharides extracted from enzymatic hydrolysate of Ginkgo biloba leaves. J. Food Biochem. 2017;41:e12352. doi: 10.1111/jfbc.12352. [DOI] [Google Scholar]
- 132.Shen X., Niu X., Li G., Deng X., Wang J. Amentoflavone ameliorates Streptococcus suis-induced infection in vitro and in vivo. Appl. Environ. Microbiol. 2018;84:e01804-18. doi: 10.1128/AEM.01804-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Q., Ye T., Long T., Peng X.B. Ginkgetin exerts anti-inflammatory effects on cerebral ischemia/reperfusion-induced injury in a rat model via the TLR4/NF-κB signaling pathway. Biosci. Biotechnol. Biochem. 2019;83:675–683. doi: 10.1080/09168451.2018.1553608. [DOI] [PubMed] [Google Scholar]
- 134.Hua Z., Wu C., Fan G., Tang Z., Cao F. The antibacterial activity and mechanism of ginkgolic acid C15:1. BMC Biotechnol. 2017;17:5. doi: 10.1186/s12896-016-0324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tao R., Wang C., Ye J., Zhou H., Chen H. Polyprenols of Ginkgo biloba Enhance Antibacterial Activity of Five Classes of Antibiotics. BioMed Res. Int. 2016;2016:4191938. doi: 10.1155/2016/4191938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karakaya F., Şahin B., Bülbül A.S., Ceylan Y., Kurt E., Tarakçı M.F. Investigation of antimicrobial and antibiofilm effects of Ginkgo biloba L. Res. J. Biol. Sci. 2020;13:28–36. [Google Scholar]
- 137.Kim J.-K., Choi M.S., Kim J.-Y., Yu J.S., Seo J.I., Yoo H.H., Kim D.-H. Ginkgo biloba leaf extract suppresses intestinal human breast cancer resistance protein expression in mice: Correlation with gut microbiota. Biomed. Pharmacother. 2021;140:111712. doi: 10.1016/j.biopha.2021.111712. [DOI] [PubMed] [Google Scholar]
- 138.Ražná K., Sawinska Z., Ivanišová E., Vukovic N., Terentjeva M., Stričík M., Kowalczewski P.Ł., Hlavačková L., Rovná K., Žiarovská J., et al. Properties of Ginkgo biloba L.: Antioxidant Characterization, Antimicrobial Activities, and Genomic MicroRNA Based Marker Fingerprints. Int. J. Mol. Sci. 2020;21:3087. doi: 10.3390/ijms21093087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y., Xu Y., Xu X., Wang H., Wang D., Yan W., Zhu J., Hao H., Wang G., Cao L., et al. Ginkgo biloba extract ameliorates atherosclerosis via rebalancing gut flora and microbial metabolism. Phytother. Res. 2022;36:2463–2480. doi: 10.1002/ptr.7439. [DOI] [PubMed] [Google Scholar]
- 140.Zhang N., Lan W., Wang Q., Sun X., Xie J. Antibacterial mechanism of Ginkgo biloba leaf extract when applied to Shewanella putrefaciens and Saprophytic staphylococcus. Aquac. Fish. 2018;3:163–169. doi: 10.1016/j.aaf.2018.05.005. [DOI] [Google Scholar]
- 141.Hu X., Yuan L., Han L., Li S., Song L. Characterization of antioxidant and antibacterial gelatin films incorporated with Ginkgo biloba extract. RSC Adv. 2019;9:27449. doi: 10.1039/C9RA05788A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sati P., Pandey A., Rawat S., Rani A. Phytochemicals and antioxidants in leaf extracts of Ginkgo biloba with reference to location, seasonal variation and solvent system. J. Pharm. Res. 2013;7:804–809. doi: 10.1016/j.jopr.2013.09.001. [DOI] [Google Scholar]
- 143.Zhou X., Qi Y., Chen T. Long-term pre-treatment of antioxidant Ginkgo biloba extract EGb-761 attenuates cerebral-ischemia-induced neuronal damage in aged mice. Biomed. Pharmacother. 2017;85:256–263. doi: 10.1016/j.biopha.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 144.Nowak A., Zielonka-Brzezicka J., Pechaiko D., Tkacz M., Klimowicz A. Ocena właściwości antyoksydacyjnych liści Ginkgo biloba L. po zakończeniu wegetacji [The evaluation of the antioxidant properties of Ginkgo biloba L. leaves after the end of the growing season] Pomeranian J. Life Sci. 2017;63:24–30. doi: 10.21164/pomjlifesci.222. [DOI] [Google Scholar]
- 145.Hussein A.A., Assad H.C., Rabeea I.S. Antihyperlipidemic, Antioxidant and Anti-Inflammatory Effects of Ginkgo Biloba in High Cholesterol Fed Rabbits. J. Pharm. Sci. Res. 2017;9:2163–2167. [Google Scholar]
- 146.Ren Q., Chen J., Ding Y., Cheng J., Yang S., Ding Z., Dai Q., Ding Z. In vitro antioxidant and immunostimulating activities of polysaccharides from Ginkgo biloba leaves. Int. J. Biol. Macromol. 2018;124:972–980. doi: 10.1016/j.ijbiomac.2018.11.276. [DOI] [PubMed] [Google Scholar]
- 147.Wang X., Gong X., Zhang H., Zhu W., Jiang Z., Shi Y., Li L. In vitro anti-aging activities of ginkgo biloba leaf extract and its chemical constituents. Food Sci. Technol. Camp. 2020;40:476–482. doi: 10.1590/fst.02219. [DOI] [Google Scholar]
- 148.Zhao J., Li K., Wang Y., Li D., Wang Q., Xie S., Wang J., Zuo Z. Enhanced anti-amnestic effect of donepezil by Ginkgo biloba extract (EGb 761) via further improvement in pro-cholinergic and antioxidative activities. J. Ethnopharmacol. 2021;269:113711. doi: 10.1016/j.jep.2020.113711. [DOI] [PubMed] [Google Scholar]
- 149.Zou M., Cao J., Zhang W., Tang C., Cao F., Su E. Improvement of quality of Ginkgo biloba seeds powder by solid-state fermentation with Eurotium cristatum for developing high-value ginkgo seeds products. J. Bioresour. Bioprod. 2022;7:135–144. doi: 10.1016/j.jobab.2021.10.002. [DOI] [Google Scholar]
- 150.Bai Y., Zhao F., Li Y., Wang L., Fang X.-J., Wang C.-Y. Ginkgo biloba extract induce cell apoptosis and G0/G1 cycle arrest in gastric cancer cells. Int. J. Clin. Exp. Med. 2015;8:20977–20982. [PMC free article] [PubMed] [Google Scholar]
- 151.Liu S.-Q., Xu C.-Y., Qin M.-B., Tan L., Zhuge C.-F., Mao Y.-B., Lai M.-Y., Huang J.-A. Ginkgo biloba extract enhances chemotherapy sensitivity and reverses chemoresistance through suppression of the KSR1-mediated ERK1/2 pathway in gastric cancer cells. Oncol. Rep. 2015;33:2871–2882. doi: 10.3892/or.2015.3923. [DOI] [PubMed] [Google Scholar]
- 152.Park Y.J., Ahn H.Y., Kim H.R., Chung K.H., Oh S.M. Ginkgo biloba extract EGb 761-mediated inhibition of aromatase for the treatment of hormone-dependent breast cancer. Food Chem. Toxicol. 2016;87:157–165. doi: 10.1016/j.fct.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 153.Ahmed H.H., Shousha W.G., El-Mezayen H.A., El-Toumy S.A., Sayed A.H., Ramadan A.R. Biochemical and molecular evidences for the antitumor potential of Ginkgo biloba leaves extract in rodents. Acta Biochim. Pol. 2017;64:25–33. doi: 10.18388/abp.2015_1200. [DOI] [PubMed] [Google Scholar]
- 154.Bonassi S., Prinzi G., Lamonaca P., Russo P., Paximadas I., Rasoni G., Rossi R., Ruggi M., Malandrino S., Sánchez-Flores M., et al. Clinical and genomic safety of treatment with Ginkgo biloba L. leaf extract (IDN 5933/Ginkgoselect®Plus) in elderly: A randomized placebo-controlled clinical trial [GiBiEx] BMC Complement. Altern. Med. 2018;18:22. doi: 10.1186/s12906-018-2080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fu Z., Lin L., Liu S., Qin M., He S., Zhu L., Huang J. Ginkgo Biloba Extract Inhibits Metastasis and ERK/Nuclear Factor kappa B (NF-kB) Signaling Pathway in Gastric Cancer. Med. Sci. Monit. 2019;25:6836–6845. doi: 10.12659/MSM.915146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fedorova Y., Tomova T., Minchev D., Turiyski V., Draganov M., Argirova M. Cytotoxic effect of Ginkgo biloba kernel extract on HCT116 and A2058 cancer cell lines. Heliyon. 2020;6:e04941. doi: 10.1016/j.heliyon.2020.e04941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu Q., Chen L., Yin W., Nie Y., Zeng P., Yang X. Anti-tumor effect of ginkgetin on human hepatocellular carcinoma cell lines by inducing cell cycle arrest and promoting cell apoptosis. Cell Cycle. 2022;21:74–85. doi: 10.1080/15384101.2021.1995684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun L., He Z., Ke J., Li S., Wu X., Lian L., He X., He X., Hu J., Zou Y., et al. PAF receptor antagonist Ginkgolide B inhibits tumourigenesis and angiogenesis in colitis-associated cancer. Int. J. Clin. Exp. Pathol. 2015;8:432–440. [PMC free article] [PubMed] [Google Scholar]
- 159.Zhi Y., Pan J., Shen W., He P., Zheng J., Zhou X., Lu G., Chen Z., Zhou Z. Ginkgolide B Inhibits Human Bladder Cancer Cell Migration and Invasion Through MicroRNA-223-3p. Cell. Physiol. Biochem. 2016;39:1787–1794. doi: 10.1159/000447878. [DOI] [PubMed] [Google Scholar]
- 160.Li M., Li B., Xia Z.-M., Tian Y., Zhang D., Rui W.-J., Dong J.-X., Xiao F.-J. Anticancer Effects of Five Biflavonoids from Ginkgo Biloba L. Male Flowers In Vitro. Molecules. 2019;24:1496. doi: 10.3390/molecules24081496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim S.-H., Yim S.-H. Effects of Bilobol from the Fruit Pulp of Ginkgo biloba on Cell Viability. Food Sci. Technol. 2022;42:e57522. doi: 10.1590/fst.57522. [DOI] [Google Scholar]
- 162.Hirata B.K.S., Banin R.M., Dornellas A.P.S., de Andrade I.S., Zemdegs J.C.S., Caperuto L.C., Oyama L.M., Ribeiro E.B., Telles M.M. Ginkgo biloba Extract Improves Insulin Signaling and Attenuates Inflammation in Retroperitoneal Adipose Tissue Depot of Obese Rats. Mediat. Inflamm. 2015;2015:419106. doi: 10.1155/2015/419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aziz T.A., Hussain S.A., Mahwi T.O., Ahmed Z.A., Rahman H.S., Rasedee A. The efficacy and safety of Ginkgo biloba extract as an adjuvant in type 2 diabetes mellitus patients ineffectively managed with metformin: A double-blind, randomized, placebo-controlled trial. Drug Des. Dev. Ther. 2018;12:735–742. doi: 10.2147/DDDT.S157113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Banin R.M., Machado M.M.F., de Andrade I.S., Carvalho L.O.T., Hirata B.K.S., de Andrade H.M., Júlio V.d.S., de Souza Figueiredo Borges Ribeiro J., Cerutti S.M., Oyama L.M., et al. Ginkgo biloba extract (GbE) attenuates obesity and anxious/depressive like behaviours induced by ovariectomy. Sci. Rep. 2021;11:44. doi: 10.1038/s41598-020-78528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jing F.-Y., Zhou Y.-Z., Wang H.-Y., Yin X.-L., Zhang Y.-Q. Enhancing antioxidant and anti-hyperglycaemic functions of gingko biloba L. seeds using thermal detoxification. J. Funct. Foods. 2021;87:104819. doi: 10.1016/j.jff.2021.104819. [DOI] [Google Scholar]
- 166.Hosoda S., Kawazoe Y., Shiba T., Numazawa S., Manabe A. Anti-Obesity Effect of Ginkgo Vinegar, a Fermented Product of Ginkgo Seed Coat, in Mice Fed a High-Fat Diet and 3T3-L1 Preadipocyte Cells. Nutrients. 2020;12:230. doi: 10.3390/nu12010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fan Y., Jin X., Man C., Gong D. Does Adjuvant Treatment With Ginkgo Biloba to Statins Have Additional Benefits in Patients With Dyslipidemia? Front. Pharmacol. 2018;9:659. doi: 10.3389/fphar.2018.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luo L., Li Y., Wang D., Zhao Y., Wang Y., Li F., Fang J., Chen H., Fan S., Huang C. Ginkgolide B lowers body weight and ameliorates hepatic steatosis in high-fat diet-induced obese mice correlated with pregnane X receptor activation. RSC Adv. 2017;7:37858–37866. doi: 10.1039/C7RA05621D. [DOI] [Google Scholar]
- 169.Huang W.-C., Chen Y.-L., Liu H.-C., Wu S.-J., Liou C.-J. Ginkgolide C reduced oleic acid-induced lipid accumulation in HepG2 cells. Saudi Pharm. J. 2018;26:1178–1184. doi: 10.1016/j.jsps.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hirata B.K.S., Cruz M.M., de Sá R.D.C.C., Farias T.S.M., Machado M.M.F., Bueno A.A., Alonso-Vale M.I.C., Telles M.M. Potential Anti-obesogenic Effects of Ginkgo biloba Observed in Epididymal White Adipose Tissue of Obese Rats. Front. Endocrinol. 2019;10:284. doi: 10.3389/fendo.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rojas C., Rojas-Castañeda J., Ruiz-Sánchez E., Montes P., Rojas P. Antioxidant properties of a Ginkgo biloba leaf extract (EGb 761) in animal models of Alzheimer’s and Parkinson’s diseases. Curr. Top. Nutraceutical Res. 2015;13:105–120. [Google Scholar]
- 172.Hashiguchi M., Ohta Y., Shimizu M., Maruyama J., Mochizuki M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J. Pharm. Health Care Sci. 2015;1:1–12. doi: 10.1186/s40780-015-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gargouri B., Carstensen J., Bhatia H.S., Huell M., Dietz G.P.H., Fiebich B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cell. Phytomedicine. 2018;44:45–55. doi: 10.1016/j.phymed.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 174.Meo F.D., Cuciniello R., Margarucci S., Bergamo P., Petillo O., Peluso G., Filosa S., Crispi S. Ginkgo biloba Prevents Oxidative Stress-Induced Apoptosis Blocking p53 Activation in Neuroblastoma Cells. Antioxidants. 2020;9:279. doi: 10.3390/antiox9040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nowak A., Kojder K., Zielonka-Brzezicka J., Wróbel J., Bosiacki M., Fabiańska M., Wróbel M., Sołek-Pastuszka J., Klimowicz A. The Use of Ginkgo Biloba L. as a Neuroprotective Agent in the Alzheimer’s Disease. Front. Pharmacol. 2021;1:775034. doi: 10.3389/fphar.2021.775034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yu D., Zhang P., Li J., Liu T., Zhang Y., Wang Q., Zhang J., Lu X., Fan X. Neuroprotective effects of Ginkgo biloba dropping pills in Parkinson’s disease. J. Pharm. Anal. 2021;11:220–231. doi: 10.1016/j.jpha.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Barbalho S.M., Direito R., Laurindo L.F., Marton L.T., Guiguer E.L., Goulart R.d.A., Tofano R.J., Carvalho A.C.A., Flato U.A.P., Capelluppi Tofano V.A., et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants. 2022;11:525. doi: 10.3390/antiox11030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sochocka M., Ochnik M., Sobczyński M., Gębura K., Zambrowicz A., Naporowski P., Leszek J. Ginkgo Biloba Leaf Extract Improves an Innate Immune Response of Peripheral Blood Leukocytes of Alzheimer’s Disease Patients. Nutrients. 2022;14:2022. doi: 10.3390/nu14102022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu Y.F. Clinical effect of pills of six ingredients with Rehmannia combined with Ginkgo biloba on prevention and treatment of early retinopathy in type 2 diabetes mellitus patients. Guoji Yanke Zazhi. 2017;17:1127–1129. [Google Scholar]
- 180.Dias M.A., Sampaio A.L.L., Venosa A.R., de Alencar Meneses E., Oliveira C.A.C.P. The chemopreventive effect of Ginkgo biloba extract 761 against cisplatin ototoxicity: A pilot study. Int. Tinnitus J. 2015;9:12–19. doi: 10.5935/0946-5448.20150003. [DOI] [PubMed] [Google Scholar]
- 181.Sabaner M.C., Dogan M., Altin S.S., Balaman C., Yilmaz C., Omur A., Zeybek I., Palaz M. Ginkgo Biloba affects microvascular morphology: A prospective optical coherence tomography angiography pilot study. Int. Ophthalmol. 2021;41:1053–1061. doi: 10.1007/s10792-020-01663-3. [DOI] [PubMed] [Google Scholar]
- 182.He X., Bernart M.W., Nolan G.S., Lin L., Lindenmaier M.P. High-performance liquid chromatography-electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba) J. Chromatogr. Sci. 2000;38:169–173. doi: 10.1093/chromsci/38.4.169. [DOI] [PubMed] [Google Scholar]
- 183.Stanković M.S. Biology and Ecology of Ginkgo biloba L. (GinkgoaceaeI) Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2016. [Google Scholar]
- 184.Gawron-Gzella A., Matławska I. Efficacy and safety of preparations from Ginkgo Biloba. Farm. Klin. 2012;1:37–47. [Google Scholar]
- 185.Nguyen T., Alzahrani T. Ginkgo Biloba 2022. [(accessed on 4 March 2023)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK541024/
- 186.Kajiyama Y., Fujii K., Takeuchi H., Manabe Y. Ginkgo Seed Poisoning. Prediatrics. 2002;109:325–327. doi: 10.1542/peds.109.2.325. [DOI] [PubMed] [Google Scholar]
- 187.Borenstein R., Hanson B.A., Markosyan R.M., Gallo E.S., Narasipura S.D., Bhutta M., Shechter O., Lurain N.S., Cohen F.S., Al-Harthi L., et al. Ginkgolic acid inhibits fusion of enveloped viruses. Sci. Rep. 2020;10:4746. doi: 10.1038/s41598-020-61700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Omindkhoda S.F., Razavi B.M., Hosseinzadeh H. Protective effects of Ginkgo biloba L. against natural toxins chemical tocicities, and radiation: A comprehensive review. Phytother. Res. 2019;33:2821–2840. doi: 10.1002/ptr.6469. [DOI] [PubMed] [Google Scholar]
- 189.Bogacz A., Karasiewicz M., Dziekan K., Procyk D., Górska-Paukszta M., Kowalska A., Mikołajczyk P.Ł., Ożarowski M., Czerny B. Impact of Panax ginseng and Ginkgo biloba extracts on expression level of transcriptional factors and xenobiotic-metabolizing cytochrome P450 enzymes. Herba Pol. 2016;61:42–54. doi: 10.1515/hepo-2016-0004. [DOI] [Google Scholar]
- 190.Kędzia B., Alkiewicz J. Interakcje pomiędzy lekami roślinnymi stosowanymi w inhalacjach a lekami syntetycznymi stosowanymi doustnie*. Postępy Fitoter. 2006;2:105. [Google Scholar]
- 191.Hansten P.D., Horn J.R. Top 100 Drug Interactions 2017. H&H Publications; Freeland, WA, USA: 2017. [Google Scholar]
- 192.Lit J.Z., Shear N.H. Drug Eruption & Reaction Manual. CRC Press; Boca Raton, FL, USA: 2017. [Google Scholar]
- 193.Braun L., Cohen M. Essential Herbs & Natural Supplements. Elsevier; Chatswood, Australia: 2017. [Google Scholar]
- 194.Woroń J., Siwek M. Unwanted effects of psychotropic drug interactions with medicinal products and diet supplements containing plant extracts. Psychiatr. Pol. 2018;52:983–996. doi: 10.12740/PP/OnlineFirst/80998. [DOI] [PubMed] [Google Scholar]
- 195.Ayroles G., Rossard R.-M., Cadiou M. Method for Obtaining an Extract or Ginkgo biloba Leaves. 4981688A. U.S. Patent. 1991 January 1;
- 196.Bombardelli E., Mustich G., Bertani M. New Extracts of ginkgo biloba and Their Methods of Preparation. EP0360556A1. 1993 April 21;
- 197.Matsumoto T. Production of Essence of Ginkgo. JPH02193907A. 1990 July 31;
- 198.Matsui K., Shinkawa Y., Tsuboi M., Kojima H., Ando Y. Simple Production of Extract with High Content of Flavonoid from Ginkgo Leaf. JPH03227985A. 1991 October 8;
- 199.Schwabe K.-P. Extracts from Ginkgo biloba Leaves-with High Content of Flavone Glycoside(s) and Ginkgolide(s) but with Low Alkyl Phenol(s) DE3940095A1. 1991 June 6;
- 200.Takane Y. Extraction of Active Component of Ginkgo Leaf and Production of Glycoside Extract of Active Component of Ginkgo Leaf. JPH0391490A. 1991 April 17;
- 201.O′Reilly J., Jaggy H. Active Component Concentrates and New Active Component Combinations from ginkgo biloba Leaves, Their Method of Preparation and Pharmaceuticals Containing the Active Component Concentrates or the Active Component Combinations. US5389370A. 1995 February 14;
- 202.Schwabe K.-P. Extract from Ginkgo biloba Leaves, Its Method of Preparation and Pharmaceuticals Containing the Extract. US5399348A. 1995 March 21;
- 203.Matsumoto T. Health Tea Containing Ginkgo Extract. JPH057368B2. 1993 January 28;
- 204.Matsumoto T. Health Drink Containing Ginkgo Extract. JPH0563147B2. 1993 September 9;
- 205.Matsumoto T., Matsumoto A. Ginkgo Extract-Containing Chewing Gum. JPH0231648A. 1990 February 1;
- 206.Matsumoto T., Matsumoto A. Ginkgo Extract-Containing Chocolate. JPH0231646A. 1990 February 1;
- 207.Matsumoto T. Candy Containing Ginkgo Leaf. JPH0550254B2. 1993 July 28;
- 208.Oschmann R., Waimer F., Hauer H. Preparation of Ginkgo biloba Extract Used for Treating e.g. Dementia or Cerebral Disorder, Involves Extracting Aqueous Alcoholic Solution of Ginkgo biloba Leaves with Heptane to Remove Alkyl Phenol Compounds. DE102006019863A1. 2006 November 16;
- 209.Erdelmeier C., Hauer H., Koch E., Lang F., Stumpf K.-H. Method for Preparing a Ginkgo Extract Having a Reduced Content of 4′-O-Methyl Pyridoxine and/or Biflavones. WO2006117171A1. 2006 November 9;
- 210.Erdelmeier C., Hauer H., Koch E., Lang F. Method for Preparing Ginkgo Extracts Having a Low Content of 4′-O-Methyl Pyridoxine and/or Biflavones. US8642099B2. 2014 February 4;
- 211.He F., Yuan Z., Chen X., Jiang Q., Zhao C., Zhang R. Fermentation Production Method for Efficiently Extracting Flavones from Ginkgo Leaves. CN107115367A. 2017 June 30;
- 212.Mo L., Guo C., Liu L. Compound Gingko Health-Care Tea and Preparation Method Thereof. CN114766573A. 2022 July 22;
- 213.Li F., Wang J., Fan Y., Zhao C., Li R. Ginkgo Beer Production Method. CN105316145A. 2016 February 3;
- 214.He X., Xuan S., Miao Z. Production Technology of Ginkgo Short Bread. CN108391689A. 2018 August 14;
- 215.Liu E. Ginkgo and Tartary Buckwheat Vinegar. CN103981077A. 2014 August 13;
- 216.Wang F., Cui X., Liu G., Lan X., He Y. Ginkgo Beverage and Preparation Method Thereof. CN104473273B. 2014 December 5;
- 217.Qian L. Preparation Method of Ginkgo Drink. CN104770812A. 2015 July 15;
- 218.Yunrong K. Method for Extracting Gingko Powder from Gingkoes and Preparing Gingko Healthy Wine. CN101597553A. 2009 December 9;
- 219.Lyu Q. Manufacturing Method of Ginkgo Health-Care Wine. CN103773660A. 2014 May 7;
- 220.Cao W. Brewing Method of Gingko Wine. CN113831990A. 2021 December 24;
- 221.Zhang Z., Zhang J. Gingko Wine and Production Method Thereof. CN113105986A. 2021 July 13;
- 222.Yang X. Process for Preparing Gingko Wine. CN114292718A. 2022 April 8;
- 223.Zhou W. Ginkgo Healthful Drink. CN103054116A. 2013 April 24;
- 224.Xu W., Zhao Q. Method for Preparing Gingko Powder from Fresh Gingko Fruits. CN114209048A. 2022 March 22;
- 225.Li C., Li T., Yue Y., Ma H., Zhang S. Ginkgo biloba Drink Containing Saffron Ingredient and Preparation Method Thereof. CN103584240A. 2014 February 19;
- 226.Zhuo M. Ginkgo Fruit Drink. CN106901108A. 2017 June 30;
- 227.Xu C., Xu R. Gingko Health Wine and Preparation Method Thereof. CN107974383A. 2018 May 1;
- 228.Liu M., Xu F. Preparation Method of Gingko Sauce. CN106962870A. 2017 July 21;
- 229.Tian W., Li Y., Yang C., Yang G., Liang J., Chen R. Gingko Herbal Extract, Fermented Yogurt and Preparation Method Thereof. CN106974286A. 2017 July 25;
- 230.Niu L. Ginkgo and Aloe Compound Functional Tea Drink and Preparation Method Thereof. CN114766574A. 2022 July 22;
- 231.Seo Y.C. Functional Foods Based on Ginkgo Nuts and the Making Method Thereof. KR20220015936A. 2022 February 8;
- 232.Qin J., Li C., Chen A.H., Cui Y., Dai X.J., Shao Y., Chen S.L., Wang N.X., Geng Z.H. Ginkgo Leaf and Agaricus Bisporus Compound Health Drink and Method of Making Same. CN102488280A. 2012 June 13;
- 233.Zhang G., Chen X., Ge X., Zhang S., Fang J., Gao W. Health-Promoting Functional Drink Suitable for People with Hypertension and Preparation Method Thereof. WO2016101319A1. 2016 June 30;
- 234.Zhang Z., Zhang J. Gingko Health-Care Wine and Processing Method Thereof. CN113249182A. 2021 August 13;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.




