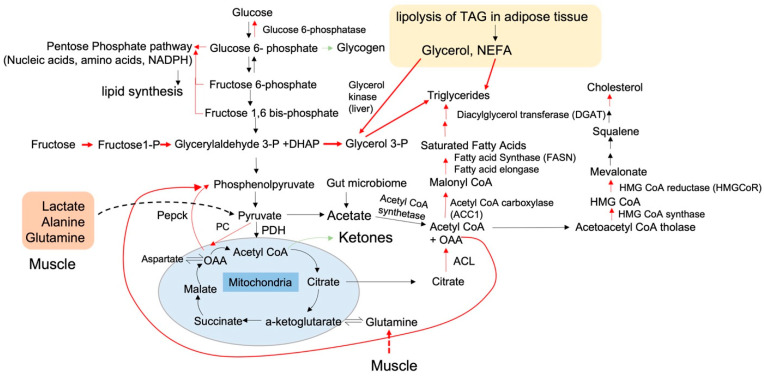
Figure 2.
A review of carbon flux through glycolysis, the Krebs cycle, and lipid, amino, and nucleic acid synthesis pathways in NAFLD. The glucose enters the hepatocytes through Glut2 transporters independently of insulin and undergoes phosphorylation by hexokinases, trapping glucose intracellularly. Glucose 6-phosphate can be metabolized in three ways: by being stored as glycogen typically after a meal, by being metabolized into nucleic acids, amino acids, and NADPH through the pentose phosphate pathway, or by being broken down into glyceraldehyde 3-phosphate (GAP) and DHAP (di-hydroxy acetone phosphate) in glycolysis. Other contributors to the GAP pool are fructose and glycerol, the latter being released as a byproduct of lipolysis from adipose tissue. Fructose is readily taken up by the liver and broken down by fructose 1-P aldolase into GAP. GAP is a major contributor to the pyruvate pool and serves as a backbone for triglycerides. The pyruvate pool receives major contributions from lactate, alanine, and glutamine released by the muscle. The pyruvate pool, in equilibrium with lactate, can be converted to acetate or contribute to acetyl coA or oxaloacetate (OAA). Acetyl CoA contributes to ketogenesis and acetate production or is cycled through the Kreb’s cycle. The acetyl-CoA combines with OAA to produce citrate, which can be shuttled out of mitochondria and reconverted to acetyl CoA and OAA. Acetyl CoA can generate ketones, especially during fasting, and/or serve as a precursor for triglycerides and cholesterol synthesis. The OAA is exported out through a malate shuttle and is converted to phosphoenol pyruvate (PEP) through the action of the gluconeogenic enzyme PEPCK (phosphor enol pyruvate carboxy kinase). Carbon flux through the various anabolic (lipid, glucose, nucleic, and protein synthesis) and catabolic (glycolysis, beta-oxidation, and Krebs–Electron transport chain) pathways is influenced by the overall nutritional load and the status of insulin sensitivity. Insulin sensitivity regulates the flux through lipid synthesis and glucose-producing pathways in the hepatocytes. The pathways highlighted in red are increased in NAFLD.