Abstract
Wine represents a complex matrix in which microbial interactions can strongly impact the quality of the final product. Numerous studies have focused on optimizing microbial approaches for addressing new challenges to enhance quality, typicity, and food safety. However, few studies have investigated yeasts of different genera as resources for obtaining wines with new, specific traits. Currently, based on the continuous changes in consumer demand, yeast selection within conventional Saccharomyces cerevisiae and unconventional non-Saccharomyces yeasts represents a suitable opportunity. Wine fermentation driven by indigenous yeasts, in the various stages, has achieved promising results in producing wines with desired characteristics, such as a reduced content of ethanol, SO2, and toxins, as well as an increased aromatic complexity. Therefore, the increasing interest in organic, biodynamic, natural, or clean wine represents a new challenge for the wine sector. This review aims at exploring the main features of different oenological yeasts to obtain wines reflecting the needs of current consumers in a sustainability context, providing an overview, and pointing out the role of microorganisms as valuable sources and biological approaches to explore potential and future research opportunities.
Keywords: wine, Saccharomyces cerevisiae, non-Saccharomyces, fermentation, sustainability, ethanol reduction, wine aroma, functional wine, starter culture
1. Introduction
Wine is one of the earliest known fermented beverages, and it is strongly linked to a region and its people’s culture and customers. The needs of customers, even more concerned with climate change, sustainability, and health, have changed the way wine is produced in recent decades.
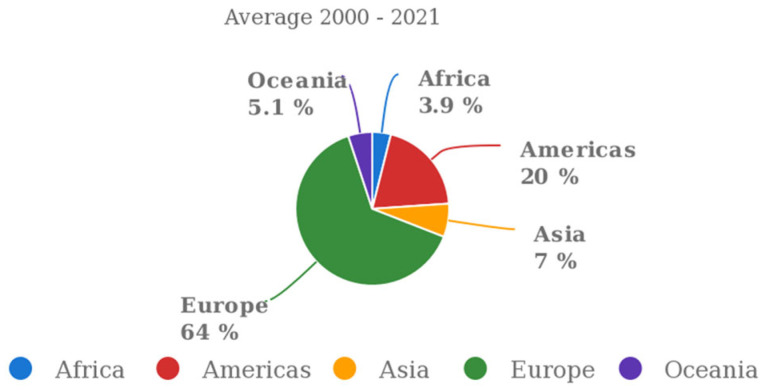
The world’s top wine producer is the European Union (Figure 1), with an estimated annual supply between 2016 and 2020 of 165 million hectoliters [1]. It accounted for 48% of consumption, 64% of output, and for 45% of wine-growing regions around the globe in 2020. Wine provided 7.6% of the value of all agri-food exports in the EU in 2020 [2]. From 2017 to 2022 (forecast updated to 31 October 2022), Italy is forecast to rank first with an average production of 48.8 Mio hl, followed by France, with 42.4 Million hL, and Spain, with 37.4 Million hL [3]. The wine industry has changed over time as consumers and producers have become more conscious of “green” issues pertaining to wine production, such as sustainability and carbon footprint. To satisfy niche market demands, many different wine subsets have been produced, such as organic, biodynamic, natural, or clean wine [4,5,6]. Moreover, starting in the 1990s, the Lifestyle of Health and Sustainability (LOHAS), a new way of living that prioritizes sustainability and health, has started to catch on all over the world. Customers show high levels of environmental consciousness, and one of the main objectives is to value quality over quantity. According to this philosophy of life, such consumers are willing to pay a superior price for intangible product qualities such as respect for environmental quality, human rights, and health [7]. In recent years, this mindset has had a substantial impact on consumer decisions in the agri-food industry, including the wine sector [8]. There is a strong environmental connotation in this context given the notable increase in consumer interest in purchasing wines from sustainable production on a global scale [9,10]. According to this trend, since 2013, organic wine consumption has doubled (976 million bottles produced) in contrast to traditional wine, which is seeing a decrease; organic wine is also currently one of the most important parts of the Italian food sector, with its 107,143 hectares of wine growing surface (+102% in 2019 vs. 2010) [11,12].
Figure 1.
Production share of wine by region [source: https://www.fao.org/faostat/en/, accessed on 18 May 2023].
Alcoholic fermentation (AF) and, more broadly, the biochemical or chemical transformation of must into wine are processes carried out by yeasts, which are mainly responsible for the final complex quality of wine [13]. The main two groups of oenological yeasts involved are the Saccharomyces genus, particularly Saccharomyces cerevisiae, with a key role in must AF, and non-Saccharomyces (NS) yeasts, a heterogeneous group that includes autochthonous strains, therefore related to a particular area, and which have recently been studied for their positive effects on wine fermentation [14,15].
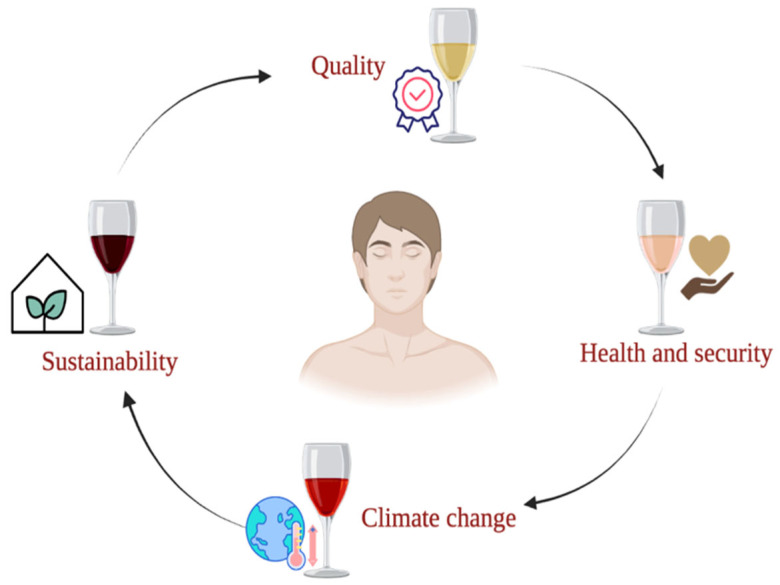
Currently, the increasing shift by consumers toward organic wines, perceived as more natural and healthier than traditional wines, has encouraged the bioprospecting of indigenous microorganisms (naturally occurring yeasts and bacteria) [16]. This change in consumer perception, as schematized in Figure 2, induces producers to select yeasts with distinctive technological traits. Strains of NS, therefore, can contribute to the satisfaction of customer needs by being able to: reduce production of SO2, acetaldehyde, H2S, and ethanol; control spontaneous and spoilage microbiota growth [17]; and improve the nutritional value or reduce detrimental molecule production, such as biogenic amines (BAs), mycotoxins (OTAs), and ethyl carbamate (EC) [18].
Figure 2.
Perception needs of current wine consumers.
The use of commercial starters of S. cerevisiae strains makes the production safer, simpler, and more stable from a microbiological point of view and consequently avoids economic losses at the expense of originality and typicality [19]. Nonetheless, the combination with native NS strains might boost wines with higher sensorial complexity than those produced using solely S. cerevisiae [20], conferring a distinctive volatile imprint of a particular geographical location, enhancing the production of healthy-value compounds, improving primary and secondary wine aromas, color stabilization, reducing ethanol content, and controlling microbial spoilage (by releasing killer toxins) [21,22,23,24].
Additionally, to obtain consciousness of interactions among microorganisms, omics approaches have been successfully applied at different fermentation stages. In detail, metagenomic, proteomic, transcriptomic, and metabolomic studies are effective in revealing the most technologically useful yeasts, although several limitations, such as cost, large amounts of data, the design of experiments, a lack of complete knowledge of genes, and the quality of the samples to be analyzed, still remain [25].
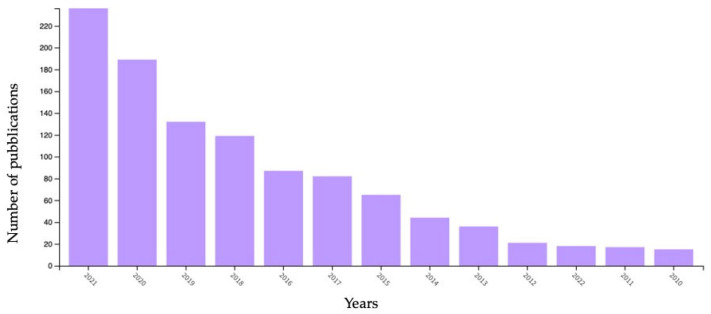
According to the current scientific evidence, it becomes even clearer how important it is to investigate in depth the dynamics of the involved microbial population. In the present study, a literature survey was carried out, taking into account a fixed timeline between 2010 and 2022, using the keyword “wine” and searching Science Direct. In this way, 45,089 publications were found, 3206 of which related to biotechnology and applied microbiology fields. Zooming in on the “wine sustainability” category, in the same years, 1064 publications were found, 192 of which regarded food science technology. Nevertheless, as reported in Figure 3, it is also noteworthy to observe the increase in the number of published scientific papers throughout the years, indicating the ever-increasing relevance of the sustainability topic [26].
Figure 3.
Records for “wine sustainability” found in the Web of Science between 2010 and 2021.
This review aims to report on the main challenges facing the wine industry, considering the increasing needs of consumers in terms of sustainability, health safety, and quality. In this context, the use of selected Saccharomyces and NS yeast strains in the production process represents a promising resource to meet the demands of both producers and consumers. The most commonly used oenological yeasts will be here described, taking into account both positive and negative traits, highlighting future perspectives in such an evolving sector, and pointing out the biotechnological progress in this field.
2. Microbiota of Vineyard and Fermenting Must
The microbiome presents in vineyards and involved in subsequent fermentation represents a complex and diverse system that includes the presence of different yeast species. In detail, the microbiota of grapes must originate mainly from grape skins and the winery environment. Yeasts are spatially distributed over grape berries and grape bunches, and their ecology varies according to the state of ripeness, as mature grapes present higher nutrient content, such as sugars [26,27]. As a matter of fact, at harvesting time, yeast populations on mature grapes are higher (103 to 106 CFU/g) than on immature grapes (10 to 103 CFU/g) [28].
As a microbial reservoir, soil may affect wine’s color, fragrance, taste, and quality, as well as crop production and metabolite synthesis. Gammaproteobacteria, which include Pseudomonas species, and Firmicutes, which include Bacillus spp., are the taxa mainly found in soil; additionally, the same S. cerevisiae genotypes have been found to be shared between soil and fruit niches [29]. As extensively reported, a plethora of yeasts have been isolated from many grape varieties [30], mainly affiliated with the following genera: Aureobasidium, Auriculibuller, Brettanomyces, Bulleromyces, Candida, Cryptococcus, Debaryomyces, Hanseniaspora, Issatchenkia, Kluyveromyces, Lipomyces, Metschnikowia, Pichia, Rhodosporidium, Rhodotorula, Saccharomyces, Sporidiobolus, Sporobolomyces, Torulaspora, Yarrowia, Zygoascus, and Zygosaccharomyces. From an ecological perspective, apiculate yeasts, such as Hanseniaspora spp., abound on grape surfaces and represent over 50% of the yeast population, while yeasts of the Candida, Kluyveromyces, Metschnikowia, Pichia, Starmerella, and Cryptococcus genera are found at lower densities [31]. Despite the extremely low occurrence of fermentative species of Saccharomyces on the surface of unharmed berries or even in vineyard soils, S. cerevisiae is the unquestioned dominating species in the winemaking fermentation process. In addition, it is well documented that the dynamics of the yeast population during wine fermentation follow a predictable pattern of growth, with NS yeasts initially present at higher concentrations (about 104 cells/mL) growing during the early stages (up to 4% v/v of ethanol), to be quickly replaced by strong fermentative S. cerevisiae strains [32]. In particular, during the initial stage of fermentation, microbiota is dominated by naturally occurring species with low ethanol tolerance, such as Hanseniaspora, Pichia, Metschnikowia, and Candida, as well as Torulaspora, Lachancea, Saccharomycodes, Zygosaccharomyces, Issatchenkia, Meyerozyma, Wickerhamomyces, Filobasidium, Sporobolomyces, Rhodosporidium, and Vishniacozyma, which are detected at lower densities. The diversity of the wine microbiota rapidly changes close to the conclusion of the fermentation process, with ethanol-sensitive yeasts, bacteria, and fungi largely replaced by S. cerevisiae [33]. To better understand the ecological background driving wine fermentation, it is relevant to predict the effects of environmental conditions on the metabolic pathway of yeasts that can switch from aerobic respiration to alcoholic fermentation. Under low O2 concentrations, yeasts conduct alcoholic fermentation as pyruvate is converted into ethanol and CO2, whereas at higher O2 concentrations, aerobic fermentation occurs and pyruvate is converted to acetyl CoA and CO2, with lower glucose consumption. Such a phenomenon, known as the “Pasteur effect”, is mainly reported for NS yeasts that prefer oxidative metabolism. In detail, this process is regulated by both pyruvate dehydrogenase (involved in respiration) and pyruvate decarboxylase (involved in fermentation) towards pyruvic acid in aerobic conditions and by ATP-mediated inhibition of the phosphofructokinase. In contrast, S. cerevisiae, in the presence of high sugar content (about 200 g/L), regardless of O2 presence, can drive alcoholic fermentation (a condition known as the “Crabtree effect”), which should be considered in the design of wine starters [34].
The application of PCR-based techniques, such as Single-Strand Conformational Polymorphism (SSCP), Denaturing Gradient Gel Electrophoresis (DGGE), Terminal Restriction Fragment Length Polymorphism (T-RFLP), and Automated rRNA Intergenic Spacer Analysis (ARISA), has upgraded the approach to studying wine microbiota, allowing to reveal microorganisms based on the presence of nucleic acids rather than their capacity to grow on specific media. The application of such techniques provides for the extraction of DNA to be used as a template for PCR directly from matrices, and it is generally quicker, more sensitive, and more accurate than culture-dependent techniques [35].
2.1. Saccharomyces cerevisiae
S. cerevisiae is the main yeast involved in wine fermentation and the most commonly employed microorganism for bread and beer production. Moreover, as a result of strong domestication, winemakers can select the best-performing S. cerevisiae strains to improve wine production in terms of flavor and fragrance from an enormous diversity of strains [36].
As previously reported, S. cerevisiae may be primarily found in soil and grape berries and at the initial stage of fermentation; however, it is not completely ruled out if it may be found on winery machinery, such as stemmer-crushers, pumps, pipes, or fermentation vessels, where it could contribute to enhancing biodiversity ecology [37].
From an enological point of view, S. cerevisiae is important for several traits, among which are tolerance to high ethanol concentrations and the production of killer factors.
As previously mentioned, thanks to the Crabtree effect, even with aerobic conditions, S. cerevisiae does not employ its respiratory system to metabolize saccharides and increase biomass growth, but instead, through pyruvate, it releases ethanol and other two-carbon molecules. As a result of this, S. cerevisiae creates and accumulates ethanol, which is deadly to most other microbial species capable of competing with it for sugar molecules and hence eliminates competition. After that, it continues to consume the produced ethanol, thus improving its own growth [38].
Focusing on must fermentation, higher alcohols (fusel alcohols), from a quantitative point of view, are the most important compounds produced by S. cerevisiae. Their biosynthesis is performed via amino acid catabolism, through the Ehrlich pathway [38]. Initially, transaminases, encoded by aro8, aro9, bat1, and bat2 genes, deaminate amino acids to the corresponding α-ketoacids. Secondly, α-ketoacids are converted into their corresponding aldehydes by enzymes encoded by one of the five decarboxylase genes (Pdc1p, Pdc5p, Pdc6p, Aro10p, and Thi3p) present in S. cerevisiae. Finally, the alcohol dehydrogenases catalyze the reduction of aldehydes into corresponding higher alcohols [39].
Recently, the selection of indigenous strains of S. cerevisiae has been widely applied, especially to improve the aroma profile and enhance the typicity of wines [40,41]. Inoculation of S. cerevisiae starters is a common practice, with dozens of different strains available on the market, selected for good fermentation kinetics (tolerance to osmotic and ethanol stresses and good nutritional efficiency in using available nitrogen sources). In addition, different starter cultures respond variably to the applied fermentation techniques and contribute differently to the development of sensory characteristics; as a result, yeast selection has become a key tool for developing and differentiating wines [42].
According to the International Organization of Vine and Wine (OIV), industrial starters can be marketed as: Active Dry Yeast (ADY), Active Frozen Yeast (AFY), Compressed Yeast (COY), Cream Yeast (CRY), Encapsulated (beads) or Immobilised Yeasts (ENY), and “levain de tirage” for sparkling wines [43].
The application of biotechnological tools, such as genetic engineering, in S. cerevisiae opens new possibilities for the development of novel, enhanced, or changed wine traits, qualities, tastes, aromas, or manufacturing methods [44]. In addition, more sophisticated genetic engineering techniques have gained increasing attention over the past ten years, mostly CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9), which uses the “adaptive immunity” mechanism naturally found in bacteria and archaea to construct a precise tool for genome editing [45,46]. In wine yeasts, this technique has been successfully applied to create engineered strains with lowered urea production [47], higher osmotic tolerance [48], or to improve wine aromatic complexity [46]. Moreover, a strain of S. cerevisiae, officially recognized by the Food and Drug Administration (FDA), has been engineered to perform malolactic fermentation [49].
2.2. Non-Saccharomyces Yeasts
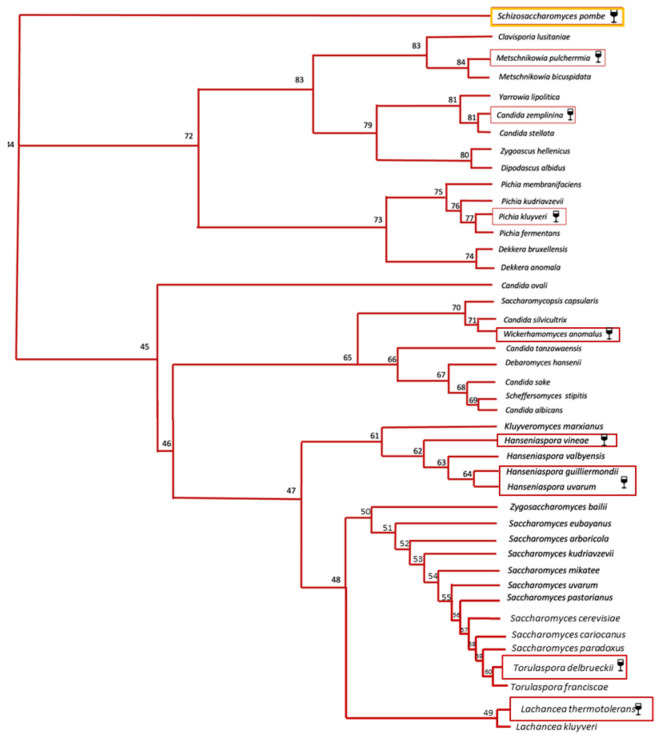
NS yeasts commonly occur in freshly crushed grape must, and although in the past they were supposed to be spoiling agents, only in the last few years has their role been reevaluated [23,50]. As shown in Figure 4 [51], these yeasts are distributed among Saccharomycetales.
Figure 4.
Phylogenetic tree showing the 41 yeast species of Saccharomycetales, obtained on the basis of the 18S ribosomal DNA sequence. Adapted from [51]. Schizosaccharomyces pombe (belonging to Schizosaccharomycetales) is represented as the outgroup species. Among the displayed species, the yeast species in the red boxes represent the NS yeasts of oenological interest described in the present review.
Nowadays, the interest in these unconventional native yeasts has greatly increased, leading large companies to market them as starters for industrial production [52]. Blends of S. cerevisiae and NS strains are also widely marketed and proposed to ensure both aromatic complexity and a proper fermentation process. An overview of the most widely used strains is given in Table 1.
In order to understand and characterize the most important technological features, over the years, the genome of non-Saccharomyces yeasts, such as the species Torulaspora delbrueckii, S. pombe, Debaryomyces hansenii, Lachancea kluyveri, Lachancea thermotolerans, Millerozyma arinose, Candida glabrata, and Zygosaccharomyces rouxii, has been fully sequenced [14]. More recently, the genome sequencing of Starmerella bacillaris type strains CBS 9494, FRI751, PAS13, PYCC 3044, and NP2 [53] revealed the important mechanism behind the high production of glycerol due to the presence of two copies of the GPP1 tandem array gene [54]. Important genomic findings also highlighted the aromatic profile of wine linked to metabolic aspects of yeasts, such as the production of esters due to overexpression and low expression of the EHT1 and EEB1 genes in T. delbrueckii [55]; the reduction of ethyl acetate in H. uvarum due to the disruption of the HuATF1 genes; and the role of the ARO8 and ARO9 genes in encoding aromatic amino acid aminotransferases in H. vineae [56,57,58].
3. Contribution of Selected Yeasts to Emerging Winemaking Demands
3.1. Enhancing the Aroma of Wine
The overall quality of a good wine is essentially determined by its aromatic and sensory profile, which is strongly linked to its typicity, origin region of production, and above all, by its ability to determine the consumer’s purchase choice [59].
In wines, more than 800 volatile organic compounds (VOCs) have been described and detected in concentrations ranging from ng/L to hundreds of g/L; however, only a few of them, at concentrations over their perception threshold, are odor-active compounds [60].
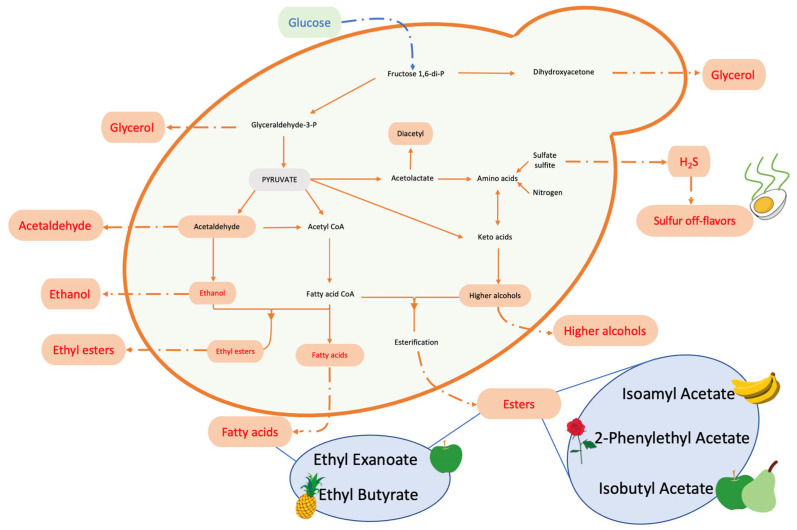
As recently reviewed by Romano et al. [57], the sensory complexity is mainly determined by three aromatic categories: primary aromas, characterized by terpenes, thiols, carotenoids, and norisoprenoids, originating from grape varieties that contribute significantly to wine typicity; secondary aromas, constituted mainly by higher alcohols, terpenoids, volatile fatty acids, esters, and phenols; carbonyl compounds and sulfur compounds, produced by yeasts during fermentation, which are involved in the “bouquet” of wine; and finally, tertiary aromas that are produced during wine aging (maturation and bottle aging), including volatile phenols, furanic compounds, and acetals. In particular, the main metabolisms involved in aromatic compound production are summarized in Figure 5.
Figure 5.
Main metabolic pathways in secondary aroma production performed by yeasts.
Each of these compounds is able to influence, in different ways, the aromatic complexity of wine by imparting a specific aroma and/or off-flavor. In detail, higher alcohols can affect the final bouquet in both positive and negative ways; excessive amounts can produce a strong, pungent smell and taste, while appropriate levels yield fruity traits [58]. Volatile phenols, derived from the precursors of hydroxycinnamic acid present in grape must, can enhance the fragrance of wine by conferring the characteristic aroma of spices, smoke, and leather, as well as by enhancing the bitterness and astringency of wine; however, when their levels exceed the threshold, they can induce off-flavors such as “band-aid”, “barnyard”, or “stable” [61]. In this context, Brettanomyces bruxellensis is strongly involved in phenolic off-flavors, primarily linked to 4-vinylphenol and 4-ethylphenol from p-coumaric acid and to 4-vinylguaiacol and 4-ethylguaiacol from ferulic acid. These compounds confer undesired flavors, described as “horse sweat”, “rancid cheese”, or “musky” [62]. Esters, which are produced by yeast during fermentation, can expressively affect fruity flavors. The most widespread ester found in wine is ethyl acetate, which shows a fruity/solvent-like aroma at a threshold level of 150 mg/L. However, many other esters, including isoamyl acetate (banana aroma), isobutyl acetate (apple/pear aroma), ethyl hexanoate (fruity, strawberry, green apple, anise aroma), and 2-phenylethyl acetate (honey, fruity, flowery aroma), have been detected [63]. Moreover, volatile thiols are another class of aromatic compounds found in wine. They are mostly produced during fermentation and can confer either a pleasant or negative aroma, such as furfuryl thiol, identified in Bordeaux red wines (Petite Manseng variety), that exhibits roasted coffee flavors [64]. Among carbonyl compounds, acetaldehyde is the most commonly detected in wine, at concentrations ranging from 10 mg/L to 75 mg/L and with a sensory threshold value of 100 mg/L. At low concentrations, it can give a fruity flavor; however, it can also provide off-flavors like “bruised apple” and “nutty”, which are also used as indicators of oxidation in wine. Sulfur compounds are regarded as undesirable molecules, producing negative sensory attributes such as rotten egg smell [65], and even if they commonly occur at relatively low concentrations, they exhibit a very low detection threshold.
It is already well established that the microbial population present in vineyards and in musts, which includes Saccharomyces and NS yeasts, has a decisive influence on the final aroma complexity of wines and, consequently, on consumer preferences [66]. In this context, “microbial terroir” has been recognized as the pool of native yeasts that are geographically unique and imprint a specific and distinct olfactory fingerprint on wine [67,68]. Therefore, secondary aromas have a greater influence among the main volatile compounds that define the overall quality of wine, for which the yeast inoculum and the fermentation methodology play an essential role [69]. Despite this, the importance of indigenous isolates of S. cerevisiae is also amply demonstrated during the production of wine, reflecting defined territorial characteristics [70,71]. Several studies reported that the use of native S. cerevisiae yeasts leads to wines with higher ester concentrations and sweet/fruity aromas [72]. In particular, Agarbati et al. [73] demonstrated that the inoculation of different S. cerevisiae biotypes isolated from a winery that had never used commercial starters resulted in wines with different sensory characteristics compared to wines produced with commercial starters. In detail, wines fermented with autochthonous yeasts were characterized by the presence of noticeable esters such as isoamyl acetate (banana scent) and ethyl octanoate (apple, pear, and peach aroma). Consistently, Chen et al. [74] demonstrated how the use of indigenous S. cerevisiae yeasts, as opposed to industrial starters, can lead to an increase in the aromatic composition regarding the production of esters like ethyl hexanoate or ethyl acetate, thus conferring an aromatic distinctive imprint linked to the geographical location [74].
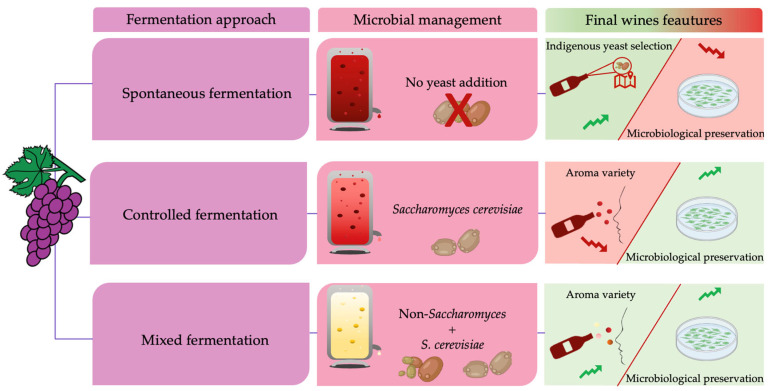
Among vineyard yeasts, the NS, when inoculated in must, produces a greater range of volatile metabolites compared to those obtained solely by S. cerevisiae [23]. In general, according to Carpena and co-workers [69] and as reported in Figure 6, three types of fermentations can be distinguished: spontaneous fermentations, in which selection of native population of NS and S. cerevisiae occurs, developing wines with high aroma complexity at the expense of less microbiological control; driven fermentations, in which inoculation with S. cerevisiae is performed inhibiting any spoilage growth or uncontrolled fermentation at the expense, however, of less aromatic complexity; and mixed fermentation, in which an initial inoculum with selected NS strains is usually performed to enhance the aromatic profile, followed by a sequential inoculum of selected S. cerevisiae starters to properly complete alcoholic fermentation; simultaneous inocula can also be performed by adding NS and S. cerevisiae at the same time.
Figure 6.
Wine features resulted from applying different fermentation approaches.
It has already been ascertained how the presence of beta-glucosidase enzymes within NS yeasts is linked to the aromatic production in wines by releasing aromatic monoterpenes [75].
Strains of Wickerhamomyces anomalus have been described for their high beta-glucosidase activities; in particular, in Muscat Bailey A must, the co-fermentation with W. anomalus, followed by S. cerevisiae, results in a significant increase in linalool, citronellol, and geraniol contents. In a sensory evaluation, the flavor, taste, and overall preference scores of co-fermented wines were higher than those of the control [76]. Additionally, the aroma diversity and taste characteristics (intensity, astringency, complexity, and persistence) of Cabernet Sauvignon wines were improved through co-inoculation of five W. anomalus strains with S. cerevisiae, and three of them showed interesting results in terms of aroma complexity. In detail, two of them exhibited higher concentrations of esters (acetate esters, ethyl esters, and other esters), as 2-phenylethyl acetate, while a third strain showed the highest contents of titratable acidity, total phenolic, total tannin, and total anthocyanin, but also a higher level of norisoprenoids, as ionone and damascenone (rose and honey flavor, respectively), carbonyl compounds, as 2-octanone and nonanal (soap and herbaceous green flavor, respectively), higher alcohols, as iso-pentanol (whiskey, malt flavor), and esters [77].
Furthermore, beta-glucosidase activity has been detected in other autochthonous wine species, as in Pichia fermentans, recently investigated in simultaneous and sequential fermentations with a commercial starter of S. cerevisiae, both in synthetic and grape juice medium. Specifically, co-inoculation showed a higher production of terpenes and C13-norisoprenoids related to fruity and floral traits, exploiting glycosidase activity present in grape juice medium, probably starting with glycoside precursors present in grapes [78]. Within the Pichia genus, Pichia kluyveri is the only species to be commercially available, and its oenological potential is mainly related to its metabolism, which is able to release volatile compounds such as esters and thiols, especially 3-sulfanylhexan-1-ol acetate [3_SHA] (passionfruit, box tree aroma) [79], although beta-glucosidase seems to be poorly released by yeasts [80]. Moreover, a strain of P. kluyveri isolated from pear showed a good production of esters, such as ethyl-acetate (banana aroma), even though their concentrations were found to be higher than their threshold (200 mg/L), thus conferring off-flavors [81]. Likewise, in Riesling Italico grape must P. kluyveri yield the highest concentrations of total esters, glycerol, and i-valeric acid [82]. Therefore, when a strain is selected as a starter, its potential production of both SO2 and isovaleric acid (associated with rancid cheese off-flavor) must be considered [79].
Regarding Torulaspora delbrueckii, Bely et al. [83] demonstrated the capacity of lowering the acetic acid (which confers an undesirable “vinegary” trait) under standard conditions. In detail, in co-culture with S. cerevisiae, at a 20:1 ratio, a lower production of volatile acidity (53%), and acetaldehyde (60%), has been observed compared to fermentation with a pure culture of S. cerevisiae. According to those results, in Chardonnay and Palomino wines obtained with sequential inocula of an industrial strain of T. delbrueckii, lower levels of both acetic acid and acetaldehyde were obtained, although the wines resulted in enhanced aroma intensity of fresh and tropical fruit traits [84]. In Riesling Italico grape must inoculated with single cultures of different NS strains, T. delbrueckii showed a higher production of aromatic compounds, such as isoamyl acetate (banana), hexyl acetate (pear and apple), phenylethyl acetate (roses and honey), and ethyl decanoate (floral), which enhanced the aroma traits and the sensory traits of wines [85]. On the same grape cultivar, Dutraive et al. [82] performed different sequential inocula with different native strains, among which T. delbrueckii exhibited very high concentrations of higher alcohols and low acetic acid content. Furthermore, the production of glycerol, related to mouthfeel sensation [23], by T. delbrueckii was also evaluated in Cabernet Sauvignon wine. The aromatic profile was explored in the red wines obtained using indigenous and commercial NS yeasts in sequential fermentation with S. cerevisiae D254 starter. In detail, the fermentation performed with the autochthonous T. delbrueckii was characterized by a higher amount of glycerol of about 9.73 g/L after malolactic fermentation, whereas fermentation with a respective commercial strain (Prelude™ Chr. Hansen) resulted in a higher content of isoamyl acetate and a lower content of acetic acid [86]. However, glycerol production could be affected by many variables, such as grape cultivar and nitrogen composition [84].
Among notable non-conventional yeasts, Lachancea thermotolerans is widely used for its high lactic acid and low acetic acid production; hence, it can produce wine without the addition of lactic acid bacteria (LAB) for malolactic fermentation [87]. Furthermore, in L. thermotolerans, the Crabtree effect is much weaker than in S. cerevisiae, thus contributing to lower ethanol production due to aerobic respiratory metabolism [88]. Moreover, promising results were obtained in evaluating its biocontrol attitude [89]. In Merlot musts, sequential inocula with L. thermotolerans resulted in “fresher” wines with reduced acidity and ethanol and increased ester content, mainly ethyl acetate and ethyl lactate, thanks to the presence of lactic acid precursor [90]. Nevertheless, the efficiency of L. thermotolerans in consuming acetic acid can be highly inhibited by high concentrations of sugars (such as glucose) in musts under aerobically limited conditions [87]. Furthermore, scientific evidence reports divergent results about wine aromatic profiles using L. thermotolerans, which generally shows a low amount of aromatic compounds compared to S. cerevisiae [91].
Frequently used simultaneously with L. thermotolerans, Schizosaccharomyces pombe exhibits important characteristics in wines. S. pombe, being able to perform malo-alcoholic fermentation, can affect the pH of wine and cause microbiological instability, releasing ethanol and CO2. Therefore, the availability of S. pombe strains on the market is based on their ability to bio-deacidificate wines [92]. Generally, from an aromatic point of view, fermentation with S. pombe produces a wine with a weak aromatic complexity, a higher concentration of acetaldehyde, propanol, and 2,3-butanediol, and a lower ester content; this aspect is particularly appealing for wines in which the varietal aroma of grapes is preferred above the fermentative aroma [50]. However, when compared to wines fermented with S. cerevisiae, no discernible change in flavor has been observed [23]. However, in contrast to L. thermotolerans, one of the main negative aspects concerning S. pombe is the high levels of acetic acid production [50]. It is also important to point out that the use of mixed cultures of L. thermotolerans and S. pombe can improve both the structure and color of wine thanks to the production of flavylium ions and polysaccharides [93].
Focusing on the aromatic potential of native yeasts, M. pulcherrima was shown to have a substantial impact on fermentation due to its high release of aromatic compounds, such as varietal thiols, and low production of acetate, ethanol, and acids. However, during the early stages of fermentation, M. pulcherrima can deplete the nitrogen reserve at the expense of S. cerevisiae, and thus the potential for nitrogen deficits must be carefully considered to avoid sluggish fermentation [94]. Aromatic complexity attributable to M. pulcherrima can be related to its high beta-glucosidase activity [76]. Moreover, when the inoculum of M. pulcherrima and Metschnikowia fructicola on pre-fermentative cold maceration in Sangiovese must was evaluated through solid-phase extraction and GC/MS, results revealed higher content of some terpenes and C13-norisoprenoids, nerol (citric aroma), geraniol (geranium), 8-hydroxy-linalool (cis), 3-oxo-α ionol, and some esters, isoamyl lactate (cream, nut) and ethyl isoamyl succinate, than those found in both controls and wines treated with pectic enzyme [95]. Promising results were obtained by Escribano-Viana et al. [96], which reported an overall positive aroma profile with a consistent concentration of higher alcohols, including 2-phenylethanol (rose-scented), and by Binati et al. [97], which confirmed a promoted yield of esters and reduced volatile phenols. Using commercially available strains, it was also reported how the sequential inocula of the industrial M. pulcherrima AWRI1149 and AWRI305 strains, together with S. cerevisiae, produced Shiraz wine with increased intensity of several desirable aromas and lower off-flavors [98]. Similar results were reported recently by Naselli et al. [99], who examined the effect of M. pulcherrima inocula on the aroma and sensory complexity of Catarratto wines. Specifically, they realized a noticeably increased aromatic richness of the inoculated wines, which was also attributed to the presence of esters, the most prevalent of which was ethyl decanoate.
Hanseniaspora, specifically H. guilliermondii, H. vinae, and H. uvarum, are also relevant species from an enological point of view thanks to their high production of aromatic compounds, such as acetate esters, 2-phenylethyl acetate, and ethyl acetate, and their high enzymatic activities, including beta-glucosidase and beta-xylosidase [100]. In Ecolly and Cabernet Sauvignon wines produced by using H. uvarum, specific varietal and fermentation volatile compounds were found. Particularly, sequential inocula enhanced the aromatic profile of the final product by notably boosting fruity and flowery aromas. Nevertheless, excessive levels of H. uvarum affected the fermentation kinetics, producing wines with a nail-polish-like aroma due to higher levels of acetate esters and volatile phenolics [101]. Moreover, strains of H. uvarum may produce an excessive amount (up to 1 g/L) of acetic acid, imparting the typical vinegary smell [102].
Lately, the use of Starmerella bacillaris (synonym Candida zemplina) has recently become widespread. Generally, this yeast is capable of resisting high concentrations of ethanol, and thus it is commonly present in the intermediate/final stages of must fermentation [103]. The most important peculiarities of this species are its high glycerol production (up to 14 g/L) [17] and its fructophilic character [104]. The mixed culture of S. bacillaris and S. cerevisiae seems to enhance the concentration of fatty acids, esters, and higher alcohols compared to the pure culture of S. cerevisiae [105]. High amounts of monoterpenes, such as linalool, geraniol, ocimene, and nerol, were also detected in Sauvignon Blanc wines [106], although the presence of these compounds is highly grape variety-dependent [50].
3.2. Reduction of Ethanol Content
Global warming has critically affected wine production as it has allowed an acceleration in the ripening of grapes and, thus, a higher accumulation of sugars, resulting in higher alcoholic wines [107]. Alcohol consumption is certainly the cause of many health and social problems [108], and consumer attention to health issues has become increasingly pronounced [109]. In this context, the wine industry is increasingly oriented toward promoting wines with reduced ethanol content. Several strategies, including agronomic and fermentation techniques, have been developed; nevertheless, the use of selected yeasts might be a suitable alternative with reduced pressure on wine aroma complexity and quality [110]. In particular, it would be possible to produce wine with lower ethanol by inoculum of Crabtree-negative yeasts (NS yeasts), which would preferentially consume sugars by respiration rather than fermentation. This would allow producers to take advantage of the standardization of partial aeration of must [111].
Among the NS yeasts, M. pulcherrima appears promising in producing ethanol-reduced wines thanks to its aerobic respiratory metabolisms that, in suitable aeration conditions, can aerobically metabolize more than 40% of sugars, thus significantly reducing the ethanol yield [112]. For example, Contreras et al. [113] demonstrated that M. pulcherrima, in sequential inoculum with S. cerevisiae, generated Shiraz wines with approximately 0.9% less ethanol than wines produced using S. cerevisiae in single cultures. A further study described the selection of a strain of M. pulcherrima for the production of lower-ethanol dry wines through a sequential inoculum with S. cerevisiae. In detail, based on the inoculation delay of M. pulcherrima, white wines (Chardonnay and Semillon blend must) contained between 0.6% and 1.2% less ethanol than wines fermented with S. cerevisiae in single culture [114]. Furthermore, a study carried out by Furlani et al. [115] described three strains of M. pulcherrima able to use more than 19 g/L of sugar to produce 1% (v/v) of ethanol in grape must. Additionally, M. pulcherrima and Starmerella bombicola are both promising wine yeasts to be used in immobilized forms in sequential fermentation to reduce ethanol content in Verdicchio must, with an ethanol reduction from 1.10 to 1.46% (v/v) and from 1.17 to 1.64% (v/v), respectively [116]. A recent study confirmed that a sequential fermentation with a native NS, M. pulcherrima CLI 68, and S. cerevisiae CLI 889 generated a reduction of alcohol content around 1.3% in Malvar wine with a higher glycerol content [117]. Furthermore, in Merlot grape juice, sequential inoculum of M. pulcherrima followed by S. cerevisiae resulted in about 11.7% v/v less alcohol production and a 12.3% reduction when observed using Meyerozyma guilliermondii. However, inoculation during fermentation with M. pulcherrima alone seems to negatively affect the overall aroma of the final product for producing ethyl acetate, which is lower if co-inoculated with S. uvarum [118]. Similar results were obtained by Rocker et al. [119] that showed how M. pulcherrima could be a promising yeast, reducing 3.8% ethanol in Riesling must at the expense of isovaleric acid.
It has also been shown that if M. pulcherrima can aerobically metabolize more than 40% of sugars, excessive aerobic conditions have an impact on ethyl acetate production. Canonico et al. [120] proved that, in Verdicchio must, the combined use of immobilized cells at a low level (5% (w/v) of beads) and at an aeration flow of 20 mL/L/min produced a relevant ethanol reduction (1.38% (v/v)), with the production of ethyl acetate still relevant but at an unacceptable level compared to those produced as free cells. Moreover, wines produced with M. pulcherrima and Z. bailii under 0.025 VVM (volume of air per volume of culture per minute) aeration resulted in a promising balance between ethanol reduction and volatile profile in Chardonnay must [121].
Moreover, an additional study using different species, namely T. delbrueckii and Z. bailii, showed the greatest potential for producing wines with reduced ethanol concentration under limited aerobic conditions and on defined media, with over 2% [v/v] reductions, depending on the aeration regime, compared to the anaerobic control [122].
Interesting results were also obtained using Pichia spp. In particular, P. kluyveri is able to produce about 0.36 g of ethanol per g of sugar, with a yield that is about 22% lower than that of S. cerevisiae [79]. Wines with reduced ethanol concentrations without excessive acetic acid levels were produced by sequentially inoculating P. kluyveri with S. cerevisiae in high-sugar Merlot grape musts [123].
However, S. cerevisiae must be used in conjunction with these NS strains since they usually exhibit a poor ability to drive the wine fermentation alone. Using only selected S. cerevisiae strains, Tronchoni et al. [124] reported low ethanol yield and less acetic acid production under aerobic conditions at laboratory scale, replacing NS yeasts as reported by Gonzalez et al. [111].
From an ethanol-reduction perspective, Gene Modification (GM) techniques have been applied to partially redirect carbon metabolism from ethanol production by shifting carbon to other endpoints [125]. In detail, the overexpression of GPD1 and/or GPD2 genes, encoding the glycerol-3-phosphate dehydrogenase isozymes, increases the synthesis of glycerol while decreasing the synthesis of ethanol [126]. In addition, Cuello et al. [127] developed a low-alcohol producer strain through a genetic modification of the S. cerevisiae Pdc2p transcription factor, encoded by the PDC2 gene, that is involved in the regulation of the availability of the PDC (pyruvate decarboxylase) isozymes, which catalyze the reaction of pyruvate to acetaldehyde in the ethanol biosynthetic pathway. Through homologous recombination, the strain can reduce the ethanol concentration up to 1.89% v/v without affecting the overall fermentation kinetics. More recently, it was shown that by mutating the general transcription factor Spt15p, the TATA-binding protein, a strain of S. cerevisiae (YS59-409) was developed with an ethanol reduction of about 34.9% [128]. Nevertheless, only a few efforts have been made in this direction, probably because of consumer uncertainty about GM technologies.
3.3. Reduction of Sulfur Dioxide
Sulfur dioxide (SO2) is widely applied in winemaking at specific stages since it can inhibit the Maillard process and regulate oxidative activities, such as polyphenol oxidase, that affect the nutritional and sensory qualities of wine [129,130]. SO2 is mainly used for its antibacterial properties against spoilage microorganisms, to reduce the growth of molds during the initial stages of fermentation or deleterious bacteria and yeasts throughout fermentation, minimizing undesirable secondary fermentations. Furthermore, when SO2 is added before bottling, it can prolong shelf life and reduce the chance of undesirable aroma production [131]. On the other hand, sulphite exposure has been related to bronchoconstriction, urticaria, headaches, dermatitis, diarrhea, or worsening of asthmatic symptoms [132,133]. Therefore, it is necessary to take into account the long-term effect on consumers. In Europe, the International Organization of Vine and Wine (OIV) recommends the following limit for SO2 to be added during winemaking: 150 mg/L for red wines, 200 mg/L for white wines, 300 mg/L for wines with a sugar content over 4 g/L, and 400 mg/L for sweet and special wines [134].
Bioprotection has been widely proposed as an effective approach to replacing SO2 in winemaking [135]. Specifically, selected yeast strains can inhibit the growth of unwanted microorganisms since they can overtake spoilage fungi for space and nutrients as well as synthesize biocontrol compounds as killer toxins [136]. Among wine spoilage microorganisms prevented by SO2, Brettanomyces bruxellensis is the most important since, as reported before, its presence is related to undesirable traits such as the typical “horse sweat” odor [62,137].
In this context, the use of NS yeasts as biocontrol has already been established, in particular by employing them both at harvest and during fermentation, limiting the development of spoilage microorganisms and preventing chemical and enzymatic oxidation [102].
The NS Torulaspora delbrueckii and M. pulcherrima species have been widely used to drive industrial fermentations without reduced-added sulfites. The ability of T. delbrueckii to bioprotect is attributable to the killer toxin TdKT, which exhibits a broad spectrum against wine spoilage yeasts without altering S. cerevisiae growth [138]. Windholtz et al. [139] showed that T. delbrueckii was found to have efficacy in bioprotection under sulfite-free conditions without altering the complex flavor profile of Merlot red wines. Similar results were obtained by Simonin et al. [140], although native yeasts are still less effective than sulphites at preventing oxidation of the must.
On the other hand, the antibacterial activity of M. pulcherrima seems to be related to the pulcherriminic acid (precursor of pulcherrimin), which immobilizes the iron in the growth medium; in this case, it has already been proven that M. pulcherrima is efficacious in inhibiting B. bruxellensis [141] as well as fungal species, such as Botrytis cinerea [142]. A recent study verified that, under experimental conditions, M. pulcherrima reduced the growth of spoilage microbiota in Pinot Noir grapes in pre-fermentation stages without any change in aroma profile [135]. However, due to the amensalism of M. pulcherrima to S. cerevisiae through iron depletion, adverse effects on fermentation kinetics could be observed when mixed cultures are used [143].
Among the non-conventional yeasts, the use of W. anomalus against Brettanomyces additionally stands out due to the presence of the killer toxin KTCf20 in some specific strains [144]. Similarly, Comitini et al. [145] demonstrated the efficiency of selected strains of W. anomalus, isolated from dairy environments, as a biocontrol agent against B. bruxellensis.
Furthermore, strains of Candida pyralidae showed production of two killer toxins, CpKT1 and CpKT2, capable of suppressing the proliferation of B. bruxellensis in similar winemaking conditions [146].
Likewise, in S. cerevisiae, the presence of a naturally synthesized biocide, saccharomycin, has recently been detected; in particular, it was evaluated that the S. cerevisiae CCMI 885, both in natural and synthetic must, prevented the growth of B. bruxellensis by greatly reducing SO2 addition [147]. Using selected strains of S. cerevisiae, Capece et al. [148] proposed a biotechnological approach to perform wine fermentation without SO2 addition, demonstrating the dominance of starter cultures over indigenous microbiota. In terms of sensory complexity, as previously reported by Miceli et al. [149], wines without SO2 (with or without bioprotection) were recently described as notably different from wines with SO2, mainly after 2 years of aging [150]. However, in the literature, scarce and divergent findings are reported on the sulphites’ impacts on the aromatic profile [135].
However, despite the encouraging findings, the preservation efficacy of SO2 in wines remains relevant, given that no other approach has been suggested as being able to totally replace SO2 [151]. The output of sulphite released by yeasts should also be considered since S. cerevisiae can produce an average of about 10 mg/L of SO2, which increases to 30 mg/L for some selected strains [152].
4. Enological Yeasts and Health-Related Compounds
4.1. Biogenic Amine Reduction Content
Biogenic amines (BAs) are low-molecular-weight compounds obtained from amino acid precursors, mainly by microbial decarboxylation. Several investigations have reported the presence of different BAs in any type of wine, such as histamine, tyramine, and putrescine [153]. BA levels in wine depend on many factors, including grape variety, climatic conditions, agricultural practices, vinification techniques, strains used in fermentation and aging, and wine parameters such as pH, alcohol content, and sulfur dioxide content [154]. Red wines showed higher levels of BAs, especially putrescine (PUT) and histamine (HIS), than white wines, with an average concentration of 7.30 and 2.45 mg/L, respectively [155]. It is relevant to highlight that, in sensitive consumers, exposure to high BA concentrations may result in health issues such as headache, anaphylaxis, nausea, hypertension, nervous symptoms, organ failure, and renal intoxication. Furthermore, ingestion of high levels of HIS is associated with the onset of histamine poisoning, characterized by hypertension, flushing, tachycardia, and gastrointestinal symptoms; ingestion of tyramine is associated with hypertensive crisis and cardiovascular symptoms [156,157]. Lactic acid bacteria (LAB) are considered the main cause of BA production, either during malolactic fermentation or due to contamination during various stages of winemaking [158]. Among wine yeasts, B. bruxellensis shows the highest amine production, while it appears to be a strain-dependent condition for S. cerevisiae [159]. Moreover, it has already been reported that some non-conventional yeasts, such as Debaryomyces hansenii, can reduce the BA contents in food even though the species is not suitable for winemaking due to its reduced ethanol tolerance and production of unpleasant aromas [160].
Therefore, exploring other NS yeasts that do not produce BAs as starters can reduce the concentrations of these compounds in wine. In this way, L. thermotolerans and Shizosaccharomyces pombe can be combined to simultaneously perform AF and malic acid degradation, thereby eliminating MLF in the traditional winemaking process and reducing the activity of LAB and, subsequently, the risk of BA production in wine [161,162,163]. Furthermore, it was similarly observed that the co-inoculum of T. delbrueckii TD20 with H. vineae HV6 significantly reduced tyramine level in Petit Manseng must [164], and it has been reported that the use of the H. uvarum FS35 strain, in sequential inoculum with commercial S. cerevisiae, produced a wine with lower level of putrescine and higher amounts of glycerol, lactic acid, acetic acid, phenylethyl alcohol, ethyl acetate and beta-phenylethyl acetate compared with the control fermentation; proving that H. uvarum FS35 strain is a promising strain to reduce BAs in wines through copper amine oxidase 1 (CuAO1) activity that lead to a coordinated response in the oxidative deamination of putrescine to 4-amino-butanal and subsequent dehydrogenation to 4-amino-butanoate [165].
4.2. Resveratrol-Increasing Content
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) belongs to the polyphenols’ stilbenoids group, possessing two phenol rings linked by an ethylene bridge. This natural polyphenol has been detected in more than 70 plant species, especially in grapes’ skin and seeds, and was found in discrete amounts in red wines and various foods [166]. Resveratrol has been associated with a wide range of pharmacological properties such as anti-inflammatory, anti-oxidative, anti-aging, anti-diabetic, cardioprotective, and neuroprotective attributed to its anti-oxidative, anti-inflammatory, and immuno-modulating effects [167]. Furthermore, the antimicrobial activity of this compound has been widely demonstrated [168]. However, due to the alcohol content, these beneficial effects cannot be achieved exclusively by consuming of wine [169]. In this context, finding biotechnological solutions for increasing the availability of resveratrol in wines could be necessary to achieve a healthy effect. The average concentration of total resveratrol in red wine is 7 mg/L, 2 mg/L in rosé wines, and 0.5 mg/L in white wine, but it ranges widely according to grape variety, geographical indication, and winemaking processes [170]. Despite the agronomic practices that can be adopted to increase resveratrol availability, the use of selected yeasts during winemaking could be a suitable alternative, especially those producing beta-glucosidase enzymes [171].
The use of genetically modified S. cerevisiae strains to increase resveratrol levels has already been amply demonstrated in previous years [172,173,174,175,176]. For instance, the industrial strain S. cerevisiae EC1118 could in vitro produce 8.249 and 3.317 mg/L of trans-resveratrol with or without antibiotic addition, respectively, by incorporating the coenzyme A ligase 4cl gene, lacking in S. cerevisiae and coming from Arabidopsis thaliana, and the resveratrol synthase gene from Vitis vinifera [177].
Alternatively, the use of selected native yeast strains is also a viable alternative; Gaensly et al. [178], isolated beta-glucosidase-producing strains of H. uvarum capable of increasing resveratrol content up to 102% during fermentation of Bordò must (V. labrusca) through hydrolysis of piceides. Lately, a study analyzed that the trans-resveratrol concentration in red Tempranillo wines inoculated with T. delbrueckii was three times higher than in wine obtained by using S. cerevisiae and 4.5 times higher when Z. bailii was inoculated [179,180]. Nevertheless, more and more studies increasingly focus on the reuse of wine wastes for bioactive compound recovery, such as resveratrol; in this perspective, Costa et al. [180] showed that the use of an engineered strain of S. cerevisiae, which is also capable of hydrolyzing xylose, on wine waste, such as musts, pruning residues, and wine lees, can lead to modest resveratrol production, representing a viable sustainable alternative in a circular economy context.
4.3. Probiotic Wine
Probiotics are dietary supplements that include viable microorganisms that can survive in (or briefly colonize) the human gastrointestinal tract and have a positive impact on consumer health. Exogenously added probiotics of a species or strain can temporarily colonize the intestinal tract and re-establish the microbiota when the normal native microbiota has been disrupted, restoring essential physiological functions [181].
Among Saccharomyces yeasts, Saccharomyces boulardii is the only species officially recognized to have significant probiotic effects on human health because of its immune-modulatory, antioxidant, antiviral, antibacterial, anti-inflammatory, and anticarcinogenic properties [182]. The advantageous effects of S. boulardii as a probiotic are supported by observable phenotypic features and physiological characteristics, including viability at low pH, ideal growth temperature, and tolerance to the gastrointestinal environment [183]. Furthermore, since it is naturally resistant to antibiotics, yeast cannot contribute to the spread of antimicrobial resistance [184]. Through investigations of synthetic must and in vitro testing, De Paula et al. [185] validated S. boulardii as potentially able to tolerate ethanol stress, offering new possibilities for the formulation of novel probiotic wines. Recently, the use of S. boulardii as a probiotic in winemaking has been reported for the first time [186] using the strain S. cerevisiae var. boulardii, CECT 1474, in the fermentation of a Monstrell must for the production of rosé wine; the results showed how the final product exhibited no significant differences from an aromatic point of view, compared to the control, and also preserved probiotic characteristics for about 6 months even during storage at 25 ± 0.5 °C and ~4 °C. On the other hand, among NS yeasts, several strains, isolated from different locations, with potential probiotic and healthy activities have been reported, especially belonging to the genera Debaryomyces, Kluyveromyces, Yarrowia, and Torulaspora, but also Candida, Pichia, Hanseniaspora, Metschnikowia, and species such as L. thermotolerans and Metschnikowia ziziphicola [187,188]. Within native yeasts, Kluyveromyces marxianus fragilis B0399 was the first to be reported as a probiotic, officially recognized to be used in both livestock feed and human consumption [189,190] thanks to its strong adherence to human enterocyte-like Caco-2 cells and its ability to modulate the immune response by inducing pro-inflammatory cytokines in peripheral blood mononuclear cells [190].
Probiotic characteristics have also been recently detected in other NS yeasts, and in a recent study, 15 strains belonging to the genus Pichia were selected for their potential probiotic activities like adhesion and resistance to gastrointestinal conditions, showing promising results compared to S. cerevisiae var. boulardii CNCM I-745 used as [191]. In Table 1 an overview on the non-Saccharomyces yeasts most commonly used in winemaking process, and their positive and negative effects on aroma compounds and biotechnological impacts is reported.
Table 1.
Main non-Saccharomyces yeasts with promising effects on aroma and the winemaking process. Their availability on the market, from the main industrial starter companies, is also reported. AFY (active frozen yeast); ADY (active dry yeast); CRY (cream yeast); FLY (fresh liquid yeast); ENY (encapsulated yeast).
| Yeast (Teleomorphic/ Anamorphic Form) |
Aroma and Quality Contribution |
Main Aromatic Metabolites |
Flavors [57] |
Biotechnological Impacts |
Negative Impacts |
Producer [52,58] |
Formula | References |
|---|---|---|---|---|---|---|---|---|
|
Pichia
kluvveri |
High production of thiols and ester | 3-sulfanylhexan-1-ol, acetate |
Passionfruit, box tree |
Low ethanol yield Potential probiotic activities |
Isovaleric acid and H2S production |
CHR Hansen | AFY | [66,67,68,69,70,175] |
| Hanseniaspora spp./Klockera spp. | High production of esters High production of β-glucosidase |
2-phenylethyl- acetate Ethyl acetate |
Floral, rose Fruity |
Potential resveratrol increasing Biogenic amine reducing |
Acetic acid production in H. uvarum |
Oenobrands (H. vinae) |
ADY | [88,89,90,152,166] |
|
Torulaspora
delbruekii/Candida colliculosa |
Low acetic acid High glycerol production High production of esters |
Isoamyl acetate Hexyl acetate Phenylethyl acetate |
Banana, pear Pear, apple Rose, honey |
Potential use as biocontrol in SO2-reduced wines |
Oxidation not fully controlled as sulphite addition | Lallemand CHR Hansen Laffort Agrovin Enartis Oeno Probiotec |
ADY AFY ADY ADY ADY/ CRY ADY FLY |
[70,71,72,73,126,127,128] |
|
Lachancea
thermotolerans |
High Lactic acid production Low acetic acid production Improvement of color and structure |
Ethyl acetate Ethyl lactate |
Fruity Fruity, buttery |
Low ethanol yield Potential biogenic amine reducing in co-inoculation with S. pombe Biocontrol agent |
Possible lack of aromatic complexity |
Lallemand CHR Hansen Enartis Lamothe-Abiet AEB Group Probiotec |
ADY AFY CRY ADY ADY FLY |
[75,76,77,78,79,149] |
|
Metschnikowia
pulcherrima/Candida pulcherrima |
High production of β -glucosidase High productions of thiols |
2- phenylethanol | Rose | Low ethanol yield Potential use as biocontrol in SO2-reduced wines |
High ethyl- acetate Amensalism over S. cerevisiae |
AEB group Lallemand |
ADY ADY |
[64,82,83,84,100,129] |
|
Starmerella
bacillaris/Candida zemplinina |
Fructophilic character High glycerol production Terpenes production |
Linalool Geraniol |
Rose Rose |
Low ethanol yield High resistance to ethanol |
- | BioEnologia | CRY | [16,91,92,93,94,104] |
| Wickerhamomyces anomalus/Candida pelliculosa | High production of β-glucosidase High productions of acetate esters |
2-phenylethyl acetate Isoamyl acetate Ethyl acetate |
Floral, rose Banana, pear Fruity |
Potential use as biocontrol in SO2-reduced wines |
- | Probiotec | FLY | [13,64,132] |
| Schizosaccharomyces pombe | Malic acid reduction Improvement of color and structure |
Propanol 2,3-butanediol |
Pungent, harsh Fruity, butter |
Potential biogenic amine reducing in co-inoculation with L. thermotolerans |
Acetic acid production | Proenol BioEnologia |
ENY CRY |
[50,80,81,150] |
5. Winemaking Sustainability and the Circular Economy
Climate change appears to pose more and more of a threat every year, which is why sustainable and environmentally friendly approaches must be adopted in industrial production. As has already been amply demonstrated, the wine sector, especially in Italy and generally in Europe, represents an unparalleled economic source given the high quantities of wine produced. As a result, the wine industry yields a considerable amount of waste products (like grape pomace and lees) but also chemicals derived from enological practices (sulphites), which emerge as environmentally unsustainable. Furthermore, energy costs resulting from the winemaking process must also be considered.
Vinification wastes contribute to ecological issues since the use of fermentative wastes, combined with other substances, poses a threat to both the environment and public health. Accordingly, it is crucial to take ecological precautions to safeguard the environment, and even more so considering the financial benefits of retrieving vineyard waste [192]. Considering the broad aspects described in this review, the development and use of commercial non-Saccharomyces yeast starters by companies appear promising for making wine in a more sustainable manner and in a circular economy context.
The use of specific yeast strains at different stages of the production process (pre- and post-fermentative stages) could represent an opportunity to improve energy savings, cut costs, and bring companies in a greener direction. The use of selected strains to ferment at desired temperatures could be a strategy to reduce heat dissipation and, therefore, energy saving [193]. Additionally, non-Saccharomyces yeasts can also be used as bio-protection in wines, thus avoiding the possible occurrence of spoilage and stuck fermentations (which would create further economic losses) and reducing the dispersion of chemical substances such as SO2 [92].
Regarding the treatment and management of vineyard waste, introducing the knowledge of biorefinery might have great outcomes regarding the sustainability of the environment as well as creating extra economic income [194,195]. Bioconversion can be employed to provide grape seed oil, ethanol, condensed tannins, tartaric acid, phenolic-rich extracts, bacterial cellulose, succinic acid, and, most importantly, bioactive compounds using grape pomace, stalks, and wine lees; in this way, a full valorization of winery wastes in a bioeconomy framework can be performed [196].
Grape pomace (GP) might be a valuable source of useful bioactive substances such as antioxidants, nutraceuticals, single-cell proteins, and volatile chemical compounds, with an emerging research interest in their therapeutic effects on human and animal health and their use as feed additives in livestock [197].
Wine lees (WL) are common winery wastes generated after the fermentation process at the bottom of wine containers, mainly composed of precipitated yeast, whose recovery has been widely reported in the literature in recent years [198], in particular for the extraction of bioactive compounds such as mannoproteins [199] and β-glucan [200], but also for the isolation of non-Saccharomyces species with remarkable antioxidant properties [201].
Alternatively, waste from the wine industry can be used as a substrate by oenological yeasts for the production of useful compounds, as reported by Williams et al. [202] regarding the use of Kluyveromyces marxianus strain Y885 for secreting pectinolytic enzyme. Thus, the data collected to date, already confirmed at the laboratory level, must be validated on a larger scale in order to establish ways to exploit these species at the industrial level. As a result, more research may lead to new perspectives and fascinating advances in the field of oenological sustainability.
6. Conclusions and Future Perspectives
The wine sector represents one of the most relevant market segments within the agribusiness economy; therefore, the challenges involved are highly variable and related to consumer requests. In the last century, the prominent role of S. cerevisiae in winemaking has been widely demonstrated and supported by producers. Furthermore, in recent years, “green” issues have become pivotal themes, and consumers are increasingly directed to consume safer products with a guaranteed provenance. Nowadays, with the introduction of advanced technologies, the revaluation of NS species has been greatly considered. The exploitation of native yeasts, isolated and selected directly from the production area, not only contributes to the qualitative and aromatic identity of a region but also helps in producing wines with reduced ethanol, SO2, and other substances that could compromise human health. Furthermore, the reduction of chemicals during the various stages of winemaking could be completely or partially replaced by a suitable and sustainable microbial approach in an environmentally friendly way. Moreover, industrial starter cultures can be set up by mixing selected strains exhibiting the most promising technological traits to obtain enhanced wines.
Acknowledgments
This study was conducted within a Ph.D. research program in Biotecnologie (XXXVII cycle) by Nunzio A. Fazio who received a grant from INPS (AY 2021–2022) in the frame of “Dottorati innovativi—Intersettoriali, vertenti sulle tematiche dell’iniziativa “Industria 4.0”, caratterizzati dal forte interesse industriale e dal coinvolgimento di imprese che svolgano attività industriali dirette alla produzione di beni o di servizi in coerenza con la “Strategia Nazionale di Specializzazione Intelligente 2014–2020” funded by the European Commission, titled “Risorse microbiche per la valorizzazione delle produzioni enologiche dell’Etna” carried out at the University of Catania, Department of Agriculture, Food, and Environment (Scientific Tutors: Cinzia Caggia and Cinzia L. Randazzo).
Author Contributions
Conceptualization, C.C. and C.L.R.; methodology, C.C.; software, A.P.; validation, N.R. and C.L.R.; investigation, N.A.F., N.R. and P.F.; resources, C.C.; data curation, A.P.; writing—original draft preparation, N.A.F.; writing—review and editing, C.C. and C.L.R.; visualization, N.R.; supervision, C.C. and C.L.R.; project administration, C.C.; funding acquisition, C.C. and C.L.R. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Faostat, Food and Agriculture Organization of the United Nations. [(accessed on 12 January 2023)]. Available online: https://www.fao.org/faostat/en/#data.
- 2.Europe Commission Support and Protection of EU Grape Growers, Wine Makers, Traders and Consumers through Policy, Legislation, Labelling, Trade Measures and Market Monitoring. [(accessed on 12 January 2023)]. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/wine_en.
- 3.Organizzazione Internazionale della Vigna e del Vino Prospettive Della Produzione Mondiale Di Vino. 2022. [(accessed on 12 January 2023)]. Available online: https://www.oiv.int/sites/default/files/documents/IT_Prospettive_della_produzione_mondiale_di_vino-Prime_stime_OIV_2022_1.pdf.
- 4.Martins A.A., Araújo A.R., Graça A., Caetano N.S., Mata T.M. Towards sustainable wine: Comparison of two Portuguese wines. J. Clean. Prod. 2018;183:662–676. doi: 10.1016/j.jclepro.2018.02.057. [DOI] [Google Scholar]
- 5.Maykish A., Rex R., Sikalidis A.K. Organic winemaking and its subsets; Biodynamic, natural, and clean wine in California. Foods. 2021;10:127. doi: 10.3390/foods10010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei R., Wang L., Ding Y., Zhang L., Gao F., Chen N., Song Y., Li H., Wang H. Natural and sustainable wine: A Review. Crit. Rev. Food Sci. Nutr. 2022:1–12. doi: 10.1080/10408398.2022.2055528. [DOI] [PubMed] [Google Scholar]
- 7.Lerro M., Yeh C.-H., Klink-Lehmann J., Vecchio R., Hartmann M., Cembalo L. The effect of moderating variables on consumer preferences for sustainable wines. Food Qual. Prefer. 2021;94:104336. doi: 10.1016/j.foodqual.2021.104336. [DOI] [Google Scholar]
- 8.Moscovici D., Gow J., Alonso Ugaglia A., Rezwanul R., Valenzuela L., Mihailescu R. Consumer preferences for organic wine—Global analysis of people and place. J. Clean. Prod. 2022;368:133215. doi: 10.1016/j.jclepro.2022.133215. [DOI] [Google Scholar]
- 9.Fabbrizzi S., Alampi Sottini V., Cipollaro M., Menghini S. Sustainability and natural wines: An exploratory analysis on consumers. Sustainability. 2021;13:7645. doi: 10.3390/su13147645. [DOI] [Google Scholar]
- 10.Pícha K., Navrátil J. The factors of lifestyle of health and sustainability influencing pro-environmental buying behaviour. J. Clean. Prod. 2019;234:233–241. doi: 10.1016/j.jclepro.2019.06.072. [DOI] [Google Scholar]
- 11.Varia F., Macaluso D., Agosta I., Spatafora F., Dara Guccione G. Transitioning towards organic farming: Perspectives for the future of the Italian organic wine sector. Sustainability. 2021;13:2815. doi: 10.3390/su13052815. [DOI] [Google Scholar]
- 12.SINAB La Filiera Vitivinicola Biologica. 2017. [(accessed on 12 January 2023)]. Available online: https://www.sinab.it/reportannuali/la-filiera-vitivinicola-biologica.
- 13.Berbegal C., Spano G., Tristezza M., Grieco F., Capozzi V. Microbial resources and innovation in the wine production sector. S. Afr. J. Enol. Vitic. 2017;38 doi: 10.21548/38-2-1333. [DOI] [Google Scholar]
- 14.Jolly N.P., Varela C., Pretorius I.S. Not Your Ordinary Yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014;14:215–237. doi: 10.1111/1567-1364.12111. [DOI] [PubMed] [Google Scholar]
- 15.Lappa I.K., Kachrimanidou V., Pateraki C., Koulougliotis D., Eriotou E., Kopsahelis N. Indigenous yeasts: Emerging trends and challenges in winemaking. Curr. Opin. Food Sci. 2020;32:133–143. doi: 10.1016/j.cofs.2020.04.004. [DOI] [Google Scholar]
- 16.Cravero M.C. Organic and biodynamic wines quality and characteristics: A Review. Food Chem. 2019;295:334–340. doi: 10.1016/j.foodchem.2019.05.149. [DOI] [PubMed] [Google Scholar]
- 17.Ivit N.N., Longo R., Kemp B. The Effect of non-Saccharomyces and Saccharomyces non-cerevisiae yeasts on ethanol and glycerol levels in wine. Fermentation. 2020;6:77. doi: 10.3390/fermentation6030077. [DOI] [Google Scholar]
- 18.Vilela A. Non-Saccharomyces yeasts and organic wines fermentation: Implications on human health. Fermentation. 2020;6:54. doi: 10.3390/fermentation6020054. [DOI] [Google Scholar]
- 19.Paul Lukacs Wine’s Homogenization: A Brief History. 2019. [(accessed on 12 January 2023)]. Available online: https://www.winereviewonline.com/Paul_Lukacs_on_Homogenization.cfm#.
- 20.Mas A., Portillo M.C. Strategies for microbiological control of the alcoholic fermentation in wines by exploiting the microbial terroir complexity: A Mini-Review. Int. J. Food Microbiol. 2022;367:109592. doi: 10.1016/j.ijfoodmicro.2022.109592. [DOI] [PubMed] [Google Scholar]
- 21.Padilla B., Gil J.V., Manzanares P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016;7:411. doi: 10.3389/fmicb.2016.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vejarano R. Non-Saccharomyces in winemaking: Source of mannoproteins, nitrogen, enzymes, and antimicrobial compounds. Fermentation. 2020;6:76. doi: 10.3390/fermentation6030076. [DOI] [Google Scholar]
- 23.Borren E., Tian B. The important contribution of non-Saccharomyces yeasts to the aroma complexity of wine: A Review. Foods. 2021;10:13. doi: 10.3390/foods10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mencher A., Morales P., Tronchoni J., Gonzalez R. Mechanisms involved in interspecific communication between wine yeasts. Foods. 2021;10:1734. doi: 10.3390/foods10081734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sirén K., Mak S.S.T., Fischer U., Hansen L.H., Gilbert M.T.P. Multi-omics and potential applications in wine production. Curr. Opin. Biotechnol. 2019;56:172–178. doi: 10.1016/j.copbio.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 26.WOS. [(accessed on 12 January 2023)]. Available online: https://wcs.webofknowledge.com.
- 27.Rosini G., Federici F., Martini A. Yeast flora of grape berries during ripening. Microb. Ecol. 1982;8:83–89. doi: 10.1007/BF02011464. [DOI] [PubMed] [Google Scholar]
- 28.Barata A., Malfeito-Ferreira M., Loureiro V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012;153:243–259. doi: 10.1016/j.ijfoodmicro.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Liu D., Zhang P., Chen D., Howell K. From the vineyard to the winery: How microbial ecology drives regional distinctiveness of wine. Front. Microbiol. 2019;10:2679. doi: 10.3389/fmicb.2019.02679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Rousseaux S., Tourdot-Maréchal R., Sadoudi M., Gougeon R., Schmitt-Kopplin P., Alexandre H. Wine microbiome: A dynamic world of microbial interactions. Crit. Rev. Food Sci. Nutr. 2017;57:856–873. doi: 10.1080/10408398.2014.983591. [DOI] [PubMed] [Google Scholar]
- 31.Ciani M., Comitini F. Yeasts in the Production of Wine. Springer; New York, NY, USA: 2019. Yeast ecology of wine production; pp. 1–42. [Google Scholar]
- 32.Albergaria H., Arneborg N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016;100:2035–2046. doi: 10.1007/s00253-015-7255-0. [DOI] [PubMed] [Google Scholar]
- 33.Conacher C.G., Luyt N.A., Naidoo-Blassoples R.K., Rossouw D., Bauer F.F. The ecology of wine fermentation: Model for the study of complex microbial ecosystems. Appl. Microbiol. Biotechnol. 2021;105:3027–3043. doi: 10.1007/s00253-021-11270-6. [DOI] [PubMed] [Google Scholar]
- 34.Romano P., Ciani M., Cocolin L. Microbiologia Della Vite e del Vino. CEA; Rozzano, Milano, Italy: 2022. [Google Scholar]
- 35.Morgan H.H., du Toit M., Setati M.E. The grapevine and wine microbiome: Insights from high-throughput amplicon sequencing. Front. Microbiol. 2017;8:820. doi: 10.3389/fmicb.2017.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortimer R.K. Evolution and variation of the yeast (Saccharomyces) genome. Genome Res. 2000;10:403–409. doi: 10.1101/gr.10.4.403. [DOI] [PubMed] [Google Scholar]
- 37.Mortimer R., Polsinelli M. On the origins of wine yeast. Res. Microbiol. 1999;150:199–204. doi: 10.1016/S0923-2508(99)80036-9. [DOI] [PubMed] [Google Scholar]
- 38.Parapouli M., Vasileiadi A., Afendra A.-S., Hatziloukas E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020;6:1–32. doi: 10.3934/microbiol.2020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hazelwood L.A., Daran J.-M., van Maris A.J.A., Pronk J.T., Dickinson J.R. The Ehrlich Pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008;74:2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furdíková K., Makyšová K., Špánik I. Effect of indigenous S. cerevisiae strains on higher alcohols, volatile acids and esters in wine. Czech J. Food Sci. 2017;35:131–142. doi: 10.17221/79/2016-CJFS. [DOI] [Google Scholar]
- 41.Garofalo C., Berbegal C., Grieco F., Tufariello M., Spano G., Capozzi V. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018;285:7–17. doi: 10.1016/j.ijfoodmicro.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez R., Morales P. Truth in wine yeast. Microb. Biotechnol. 2022;15:1339–1356. doi: 10.1111/1751-7915.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.OIV Monograph of Saccharomyces Yeast. [(accessed on 12 January 2023)]. Available online: https://www.oiv.int/public/medias/5370/oiv-oeno-576a-2017-en.pdf.
- 44.Molina-Espeja P. Next generation winemakers: Genetic engineering in Saccharomyces cerevisiae for trendy challenges. Bioengineering. 2020;7:128. doi: 10.3390/bioengineering7040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raschmanová H., Weninger A., Glieder A., Kovar K., Vogl T. Implementing CRISPR-Cas technologies in conventional and non-conventional yeasts: Current state and future prospects. Biotechnol. Adv. 2018;36:641–665. doi: 10.1016/j.biotechadv.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Vilela A. An overview of CRISPR-based technologies in wine yeasts to improve wine flavor and safety. Fermentation. 2021;7:5. doi: 10.3390/fermentation7010005. [DOI] [Google Scholar]
- 47.Vigentini I., Gebbia M., Belotti A., Foschino R., Roth F.P. CRISPR/Cas9 system as a valuable genome editing tool for wine yeasts with application to decrease urea production. Front. Microbiol. 2017;8:2194. doi: 10.3389/fmicb.2017.02194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muysson J., Miller L., Allie R., Inglis D.L. The use of CRISPR-Cas9 genome editing to determine the importance of glycerol uptake in wine yeast during icewine fermentation. Fermentation. 2019;5:93. doi: 10.3390/fermentation5040093. [DOI] [Google Scholar]
- 49.Husnik J.I., Volschenk H., Bauer J., Colavizza D., Luo Z., van Vuuren H.J.J. Metabolic engineering of malolactic wine yeast. Metab. Eng. 2006;8:315–323. doi: 10.1016/j.ymben.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Zilelidou E.A., Nisiotou A. Understanding wine through yeast interactions. Microorganisms. 2021;9:1620. doi: 10.3390/microorganisms9081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masneuf-Pomarede I., Bely M., Marullo P., Albertin W. The genetics of non-conventional wine yeasts: Current knowledge and future challenges. Front. Microbiol. 2016;6:1563. doi: 10.3389/fmicb.2015.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vejarano R., Gil-Calderón A. Commercially available non-Saccharomyces yeasts for winemaking: Current market, advantages over Saccharomyces, biocompatibility, and safety. Fermentation. 2021;7:171. doi: 10.3390/fermentation7030171. [DOI] [Google Scholar]
- 53.Raymond Eder M.L., Rosa A.L. Genetic, physiological, and industrial aspects of the fructophilic non-Saccharomyces yeast species, Starmerella bacillaris. Fermentation. 2021;7:87. doi: 10.3390/fermentation7020087. [DOI] [Google Scholar]
- 54.Lemos W.J.F., Jr., da Silva Duarte V., Treu L., Campanaro S., Nadai C., Giacomini A., Corich V. Whole genome comparison of two Starmerella bacillaris strains with other wine yeasts uncovers genes involved in modulating important winemaking traits. FEMS Yeast Res. 2018;18:foy069. doi: 10.1093/femsyr/foy069. [DOI] [PubMed] [Google Scholar]
- 55.Tondini F., Lang T., Chen L., Herderich M., Jiranek V. Linking gene expression and oenological traits: Comparison between Torulaspora delbrueckii and Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2019;294:42–49. doi: 10.1016/j.ijfoodmicro.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Comitini F., Agarbati A., Canonico L., Ciani M. Yeast interactions and molecular mechanisms in wine fermentation: A comprehensive review. Int. J. Mol. Sci. 2021;22:7754. doi: 10.3390/ijms22147754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romano P., Braschi G., Siesto G., Patrignani F., Lanciotti R. Role of yeasts on the sensory component of wines. Foods. 2022;11:1921. doi: 10.3390/foods11131921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morata A., Escott C., Bañuelos M., Loira I., del Fresno J., González C., Suárez-Lepe J. Contribution of non-Saccharomyces yeasts to wine freshness. A Review. Biomolecules. 2019;10:34. doi: 10.3390/biom10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Souza Gonzaga L., Capone D.L., Bastian S.E.P., Jeffery D.W. Defining wine typicity: Sensory characterisation and consumer perspectives. Aust. J. Grape Wine Res. 2021;27:246–256. doi: 10.1111/ajgw.12474. [DOI] [Google Scholar]
- 60.Pittari E., Moio L., Piombino P. Interactions between polyphenols and volatile compounds in wine: A Literature Review on physicochemical and sensory insights. Appl. Sci. 2021;11:1157. doi: 10.3390/app11031157. [DOI] [Google Scholar]
- 61.Kheir J., Salameh D., Strehaiano P., Brandam C., Lteif R. Impact of volatile phenols and their precursors on wine quality and control measures of Brettanomyces/Dekkera yeasts. Eur. Food Res. Technol. 2013;237:655–671. doi: 10.1007/s00217-013-2036-4. [DOI] [Google Scholar]
- 62.Cibrario A., Miot-Sertier C., Paulin M., Bullier B., Riquier L., Perello M.-C., de Revel G., Albertin W., Masneuf-Pomarède I., Ballestra P., et al. Brettanomyces bruxellensis phenotypic diversity, tolerance to wine stress and wine spoilage ability. Food Microbiol. 2020;87:103379. doi: 10.1016/j.fm.2019.103379. [DOI] [PubMed] [Google Scholar]
- 63.Sumby K.M., Grbin P.R., Jiranek V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010;121:1–16. doi: 10.1016/j.foodchem.2009.12.004. [DOI] [Google Scholar]
- 64.Blanchard L., Tominaga T., Dubourdieu D. Formation of furfurylthiol exhibiting a strong coffee aroma during oak barrel fermentation from furfural released by toasted staves. J. Agric. Food Chem. 2001;49:4833–4835. doi: 10.1021/jf010539w. [DOI] [PubMed] [Google Scholar]
- 65.Swiegers J.H., Bartowsky E.J., Henschke P.A., Pretorius I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005;11:139–173. doi: 10.1111/j.1755-0238.2005.tb00285.x. [DOI] [Google Scholar]
- 66.Belda I., Ruiz J., Esteban-Fernández A., Navascués E., Marquina D., Santos A., Moreno-Arribas M. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules. 2017;22:189. doi: 10.3390/molecules22020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bokulich N.A., Thorngate J.H., Richardson P.M., Mills D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA. 2014;111:E139–E148. doi: 10.1073/pnas.1317377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francesca N., Chiurazzi M., Romano R., Aponte M., Settanni L., Moschetti G. Indigenous yeast communities in the environment of “Rovello Bianco” grape variety and their use in commercial white wine fermentation. World J. Microbiol. Biotechnol. 2010;26:337–351. doi: 10.1007/s11274-009-0181-5. [DOI] [Google Scholar]
- 69.Carpena M., Fraga-Corral M., Otero P., Nogueira R.A., Garcia-Oliveira P., Prieto M.A., Simal-Gandara J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods. 2020;10:51. doi: 10.3390/foods10010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mercado L., Sturm M.E., Rojo M.C., Ciklic I., Martínez C., Combina M. Biodiversity of Saccharomyces cerevisiae populations in Malbec vineyards from the “Zona Alta Del Río Mendoza” region in Argentina. Int. J. Food Microbiol. 2011;151:319–326. doi: 10.1016/j.ijfoodmicro.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Torija M.J., Rozès N., Poblet M., Guillamón J.M., Mas A. Yeast population dynamics in apontaneous fermentations: Comparison between two different wine-producing areas over a period of three years. Antonie Van Leeuwenhoek. 2001;79:345–352. doi: 10.1023/A:1012027718701. [DOI] [PubMed] [Google Scholar]
- 72.García M., Esteve-Zarzoso B., Crespo J., Cabellos J.M., Arroyo T. Influence of native Saccharomyces cerevisiae strains from D.O. “Vinos de Madrid” in the volatile profile of white wines. Fermentation. 2019;5:94. doi: 10.3390/fermentation5040094. [DOI] [Google Scholar]
- 73.Agarbati A., Canonico L., Comitini F., Ciani M. Ecological distribution and oenological characterization of native Saccharomyces cerevisiae in an organic winery. Fermentation. 2022;8:224. doi: 10.3390/fermentation8050224. [DOI] [Google Scholar]
- 74.Chen Y., Jiang J., Song Y., Zang X., Wang G., Pei Y., Song Y., Qin Y., Liu Y. Yeast diversity during spontaneous fermentations and oenological characterisation of indigenous Saccharomyces cerevisiae for potential as wine starter cultures. Microorganisms. 2022;10:1455. doi: 10.3390/microorganisms10071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swangkeaw J., Vichitphan S., Butzke C.E., Vichitphan K. The characterisation of a novel Pichia anomala β-glucosidase with potentially aroma-enhancing capabilities in wine. Ann. Microbiol. 2009;59:335–343. doi: 10.1007/BF03178336. [DOI] [Google Scholar]
- 76.Lee S.-B., Park H.-D. Isolation and investigation of potential non-Saccharomyces yeasts to improve the volatile terpene compounds in Korean Muscat Bailey A wine. Microorganisms. 2020;8:1552. doi: 10.3390/microorganisms8101552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J., Yan J., Zhang W., Zhang Y., Dong Z., Luo H., Liu M., Su J. Comparison of potential Wickerhamomyces anomalus to improve the quality of Cabernet Sauvignon wines by mixed fermentation with Saccharomyces cerevisiae. LWT. 2023;173:114285. doi: 10.1016/j.lwt.2022.114285. [DOI] [Google Scholar]
- 78.Li N., Wang Q.-Q., Xu Y.-H., Li A.-H., Tao Y.-S. Increased glycosidase activities improved the production of wine varietal odorants in mixed fermentation of P. fermentans and high antagonistic S. cerevisiae. Food Chem. 2020;332:127426. doi: 10.1016/j.foodchem.2020.127426. [DOI] [PubMed] [Google Scholar]
- 79.Vicente J., Calderón F., Santos A., Marquina D., Benito S. High potential of Pichia kluyveri and other Pichia species in wine technology. Int. J. Mol. Sci. 2021;22:1196. doi: 10.3390/ijms22031196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Escribano R., González-Arenzana L., Garijo P., Berlanas C., López-Alfaro I., López R., Gutiérrez A.R., Santamaría P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017;54:1555–1564. doi: 10.1007/s13197-017-2587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai Y.T., Hsieh C.W., Lo Y.C., Liou B.K., Lin H.W., Hou C.Y., Cheng K.C. Isolation and identification of aroma-producing non-Saccharomyces yeast strains and the enological characteristic comparison in wine making. LWT. 2022;154:112653. doi: 10.1016/j.lwt.2021.112653. [DOI] [Google Scholar]
- 82.Dutraive O., Benito S., Fritsch S., Beisert B., Patz C.-D., Rauhut D. Effect of sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on riesling wine chemical composition. Fermentation. 2019;5:79. doi: 10.3390/fermentation5030079. [DOI] [Google Scholar]
- 83.Bely M., Stoeckle P., Masneuf-Pomarède I., Dubourdieu D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008;122:312–320. doi: 10.1016/j.ijfoodmicro.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 84.Puertas B., Jiménez M.J., Cantos-Villar E., Cantoral J.M., Rodríguez M.E. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of Palomino and Chardonnay wines in warm climates. J. Appl. Microbiol. 2017;122:733–746. doi: 10.1111/jam.13375. [DOI] [PubMed] [Google Scholar]
- 85.Quincozes L., Marcon Â.R., Spinelli F.R., Gabbardo M., Eckhardt D.P., Cunha W.M.d., Costa V.B., Jacques R.J.S., Schumacher R.L. Physicochemical, aromatic and sensory properties of the ‘Riesling Italico’ wines fermented with Saccharomyces and non-Saccharomyces yeasts. Ciência Rural. 2020;50 doi: 10.1590/0103-8478cr20190622. [DOI] [Google Scholar]
- 86.Zhang B., Ivanova-Petropulos V., Duan C., Yan G. Distinctive chemical and aromatic composition of red wines produced by Saccharomyces cerevisiae co-fermentation with indigenous and commercial non-Saccharomyces strains. Food Biosci. 2021;41:100925. doi: 10.1016/j.fbio.2021.100925. [DOI] [Google Scholar]
- 87.Vilela A. Lachancea thermotolerans, the non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation. 2018;4:56. doi: 10.3390/fermentation4030056. [DOI] [Google Scholar]
- 88.Vicente J., Navascués E., Calderón F., Santos A., Marquina D., Benito S. An integrative view of the role of Lachancea thermotolerans in wine technology. Foods. 2021;10:2878. doi: 10.3390/foods10112878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nally M.C., Ponsone M.L., Pesce V.M., Toro M.E., Vazquez F., Chulze S. Evaluation of behaviour of Lachancea thermotolerans biocontrol agents on grape fermentations. Lett. Appl. Microbiol. 2018;67:89–96. doi: 10.1111/lam.13001. [DOI] [PubMed] [Google Scholar]
- 90.Hranilovic A., Albertin W., Capone D.L., Gallo A., Grbin P.R., Danner L., Bastian S.E.P., Masneuf-Pomarede I., Coulon J., Bely M., et al. Impact of Lachancea thermotolerans on chemical composition and sensory profiles of Merlot wines. Food Chem. 2021;349:129015. doi: 10.1016/j.foodchem.2021.129015. [DOI] [PubMed] [Google Scholar]
- 91.Benito S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018;102:6775–6790. doi: 10.1007/s00253-018-9117-z. [DOI] [PubMed] [Google Scholar]
- 92.Loira I., Morata A., Palomero F., González C., Suárez-Lepe J. Schizosaccharomyces pombe: A promising biotechnology for modulating wine composition. Fermentation. 2018;4:70. doi: 10.3390/fermentation4030070. [DOI] [Google Scholar]
- 93.Benito Á., Calderón F., Benito S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation. 2019;5:54. doi: 10.3390/fermentation5030054. [DOI] [Google Scholar]
- 94.Seguinot P., Ortiz-Julien A., Camarasa C. Impact of nutrient availability on the fermentation and production of aroma compounds under sequential inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020;11:305. doi: 10.3389/fmicb.2020.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benucci I., Luziatelli F., Cerreti M., Liburdi K., Nardi T., Vagnoli P., Ruzzi M., Esti M. Pre-fermentative cold maceration in the presence of non-Saccharomyces strains: Effect on fermentation behaviour and volatile composition of a red wine. Aust. J. Grape Wine Res. 2018;24:267–274. doi: 10.1111/ajgw.12326. [DOI] [Google Scholar]
- 96.Escribano-Viana R., González-Arenzana L., Garijo P., López R., Santamaría P., Gutiérrez A.R. Selection process of a mixed inoculum of non-Saccharomyces yeasts isolated in the D.O.Ca. Rioja. Fermentation. 2021;7:148. doi: 10.3390/fermentation7030148. [DOI] [Google Scholar]
- 97.Binati R.L., Lemos Junior W.J.F., Luzzini G., Slaghenaufi D., Ugliano M., Torriani S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A Study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020;318:108470. doi: 10.1016/j.ijfoodmicro.2019.108470. [DOI] [PubMed] [Google Scholar]
- 98.Varela C., Bartel C., Espinase Nandorfy D., Bilogrevic E., Tran T., Heinrich A., Balzan T., Bindon K., Borneman A. Volatile aroma composition and sensory profile of Shiraz and Cabernet Sauvignon wines produced with novel Metschnikowia pulcherrima yeast starter cultures. Aust. J. Grape Wine Res. 2021;27:406–418. doi: 10.1111/ajgw.12484. [DOI] [Google Scholar]
- 99.Naselli V., Prestianni R., Badalamenti N., Matraxia M., Maggio A., Alfonzo A., Gaglio R., Vagnoli P., Settanni L., Bruno M., et al. Improving the aromatic profiles of Catarratto wines: Impact of Metschnikowia pulcherrima and glutathione-rich inactivated yeasts. Antioxidants. 2023;12:439. doi: 10.3390/antiox12020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin V., Valera M., Medina K., Boido E., Carrau F. Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—A Review. Fermentation. 2018;4:76. doi: 10.3390/fermentation4030076. [DOI] [Google Scholar]
- 101.Hu K., Jin G.-J., Xu Y.-H., Tao Y.-S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018;108:119–127. doi: 10.1016/j.foodres.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 102.Morata A., Loira I., González C., Escott C. Non-Saccharomyces as biotools to control the production of off-flavors in wines. Molecules. 2021;26:4571. doi: 10.3390/molecules26154571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Englezos V., Giacosa S., Rantsiou K., Rolle L., Cocolin L. Starmerella bacillaris in winemaking: Opportunities and risks. Curr. Opin. Food Sci. 2017;17:30–35. doi: 10.1016/j.cofs.2017.08.007. [DOI] [Google Scholar]
- 104.Englezos V., Torchio F., Cravero F., Marengo F., Giacosa S., Gerbi V., Rantsiou K., Rolle L., Cocolin L. Aroma profile and composition of Barbera wines obtained by mixed fermentations of Starmerella bacillaris (synonym Candida zemplinina) and Saccharomyces cerevisiae. LWT. 2016;73:567–575. doi: 10.1016/j.lwt.2016.06.063. [DOI] [Google Scholar]
- 105.Englezos V., Pollon M., Rantsiou K., Ortiz-Julien A., Botto R., Río Segade S., Giacosa S., Rolle L., Cocolin L. Saccharomyces cerevisiae-Starmerella bacillaris strains interaction modulates chemical and volatile profile in red wine mixed fermentations. Food Res. Int. 2019;122:392–401. doi: 10.1016/j.foodres.2019.03.072. [DOI] [PubMed] [Google Scholar]
- 106.Beckner Whitener M.E., Stanstrup J., Panzeri V., Carlin S., Divol B., du Toit M., Vrhovsek U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon Blanc co-fermented with seven different yeasts. Metabolomics. 2016;12:53. doi: 10.1007/s11306-016-0962-4. [DOI] [Google Scholar]
- 107.Jones G.V. Climate change and global wine quality. Clim. Change. 2005:319–343. doi: 10.1007/s10584-005-4704-2. [DOI] [Google Scholar]
- 108.Poznyak V., Rekve D. Global Status Report on Alcohol and Health. World Health Organization; Geneva, Switzerland: 2018. p. 450. [Google Scholar]
- 109.Deroover K., Siegrist M., Brain K., McIntyre J., Bucher T. A scoping review on consumer behaviour related to wine and health. Trends Food Sci. Technol. 2021;112:559–580. doi: 10.1016/j.tifs.2021.03.057. [DOI] [Google Scholar]
- 110.Varela C., Dry P.R., Kutyna D.R., Francis I.L., Henschke P.A., Curtin C.D., Chambers P.J. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015;21:670–679. doi: 10.1111/ajgw.12187. [DOI] [Google Scholar]
- 111.Gonzalez R., Quirós M., Morales P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Technol. 2013;29:55–61. doi: 10.1016/j.tifs.2012.06.015. [DOI] [Google Scholar]
- 112.Morata A., Loira I., Escott C., del Fresno J.M., Bañuelos M.A., Suárez-Lepe J.A. Applications of Metschnikowia pulcherrima in wine biotechnology. Fermentation. 2019;5:63. doi: 10.3390/fermentation5030063. [DOI] [Google Scholar]
- 113.Contreras A., Curtin C., Varela C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015;99:1885–1895. doi: 10.1007/s00253-014-6193-6. [DOI] [PubMed] [Google Scholar]
- 114.Hranilovic A., Gambetta J.M., Jeffery D.W., Grbin P.R., Jiranek V. Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: The effect of sequential inoculation timing. Int. J. Food Microbiol. 2020;329:108651. doi: 10.1016/j.ijfoodmicro.2020.108651. [DOI] [PubMed] [Google Scholar]
- 115.Mestre Furlani M.V., Maturano Y.P., Combina M., Mercado L.A., Toro M.E., Vazquez F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017;17:fox010. doi: 10.1093/femsyr/fox010. [DOI] [PubMed] [Google Scholar]
- 116.Canonico L., Comitini F., Oro L., Ciani M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016;7:278. doi: 10.3389/fmicb.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.García M., Esteve-Zarzoso B., Cabellos J.M., Arroyo T. Sequential non-Saccharomyces and Saccharomyces cerevisiae fermentations to reduce the alcohol content in wine. Fermentation. 2020;6:60. doi: 10.3390/fermentation6020060. [DOI] [Google Scholar]
- 118.Varela C., Sengler F., Solomon M., Curtin C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016;209:57–64. doi: 10.1016/j.foodchem.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 119.Röcker J., Strub S., Ebert K., Grossmann M. Usage of different aerobic non-Saccharomyces yeasts and experimental conditions as a tool for reducing the potential ethanol content in wines. Eur. Food Res. Technol. 2016;242:2051–2070. doi: 10.1007/s00217-016-2703-3. [DOI] [Google Scholar]
- 120.Canonico L., Comitini F., Ciani M. Metschnikowia pulcherrima selected strain for ethanol reduction in wine: Influence of cell immobilization and aeration condition. Foods. 2019;8:378. doi: 10.3390/foods8090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Canonico L., Solomon M., Comitini F., Ciani M., Varela C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019;84:103247. doi: 10.1016/j.fm.2019.103247. [DOI] [PubMed] [Google Scholar]
- 122.Contreras A., Hidalgo C., Schmidt S., Henschke P.A., Curtin C., Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 123.Aplin J.J., Edwards C.G. Impacts of non-Saccharomyces species and aeration on sequential inoculation with Saccharomyces cerevisiae to produce lower alcohol Merlot wines from Washington State. J. Sci. Food Agric. 2021;101:1715–1719. doi: 10.1002/jsfa.10769. [DOI] [PubMed] [Google Scholar]
- 124.Tronchoni J., Gonzalez R., Guindal A.M., Calleja E., Morales P. Exploring the Suitability of Saccharomyces cerevisiae strains for winemaking under aerobic conditions. Food Microbiol. 2022;101:103893. doi: 10.1016/j.fm.2021.103893. [DOI] [PubMed] [Google Scholar]
- 125.Varela J., Varela C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019;56:88–96. doi: 10.1016/j.copbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Lopes M. De B.; Ur-Rehman, A.; Gockowiak, H.; Heinrich, A.J.; Langridge, P.; Henschke, P.A. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae Glycerol 3-Phosphate Dehydrogenase Gene (GPD2) Aust. J. Grape Wine Res. 2000;6:208–215. doi: 10.1111/j.1755-0238.2000.tb00181.x. [DOI] [Google Scholar]
- 127.Cuello R.A., Flores Montero K.J., Mercado L.A., Combina M., Ciklic I.F. Construction of low-ethanol–wine yeasts through partial deletion of the Saccharomyces cerevisiae PDC2 gene. AMB Express. 2017;7:67. doi: 10.1186/s13568-017-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Du Q., Liu Y., Song Y., Qin Y. Creation of a low-alcohol-production yeast by a mutated SPT15 transcription regulator triggers transcriptional and metabolic changes during wine fermentation. Front. Microbiol. 2020;11:597828. doi: 10.3389/fmicb.2020.597828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guerrero R.F., Cantos-Villar E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A Parameter Review. Trends Food Sci. Technol. 2015;42:27–43. doi: 10.1016/j.tifs.2014.11.004. [DOI] [Google Scholar]
- 130.Oliveira C.M., Ferreira A.C.S., De Freitas V., Silva A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011;44:1115–1126. doi: 10.1016/j.foodres.2011.03.050. [DOI] [Google Scholar]
- 131.Du Toit M., Pretorius I.S. Microbial spoilage and preservation of wine: Using weapons from nature’s own arsenal—A Review. S. Afr. J. Enol. Vitic. 2019;21:74–96. doi: 10.21548/21-1-3559. [DOI] [Google Scholar]
- 132.Häberle M., Geier J., Mahler V. Contact allergy and intolerance to sulphite compounds: Clinical and occupational relevance. Allergo J. Int. 2017;26:53–66. doi: 10.1007/s40629-016-0003-x. [DOI] [Google Scholar]
- 133.Witkowski M., Grajeta H., Gomułka K. Hypersensitivity reactions to food additives—Preservatives, antioxidants, flavor enhancers. Int. J. Environ. Res. Public. Health. 2022;19:11493. doi: 10.3390/ijerph191811493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.OIV SO2 and Wine: A Review. OIV Collective Expertise Document. 2021. [(accessed on 12 January 2023)]. Available online: https://www.oiv.int/public/medias/7840/oiv-collective-expertise-document-so2-and-wine-a-review.pdf.
- 135.Simonin S., Roullier-Gall C., Ballester J., Schmitt-Kopplin P., Quintanilla-Casas B., Vichi S., Peyron D., Alexandre H., Tourdot-Maréchal R. Bio-protection as an alternative to sulphites: Impact on chemical and microbial characteristics of red wines. Front. Microbiol. 2020;11:1308. doi: 10.3389/fmicb.2020.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leyva Salas M., Mounier J., Valence F., Coton M., Thierry A., Coton E. Antifungal microbial agents for food Bbopreservation—A Review. Microorganisms. 2017;5:37. doi: 10.3390/microorganisms5030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pinto L., Baruzzi F., Cocolin L., Malfeito-Ferreira M. Emerging technologies to control Brettanomyces spp. in wine: Recent advances and future trends. Trends Food Sci. Technol. 2020;99:88–100. doi: 10.1016/j.tifs.2020.02.013. [DOI] [Google Scholar]
- 138.Villalba M.L., Susana Sáez J., del Monaco S., Lopes C.A., Sangorrín M.P. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016;217:94–100. doi: 10.1016/j.ijfoodmicro.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 139.Windholtz S., Redon P., Lacampagne S., Farris L., Lytra G., Cameleyre M., Barbe J.-C., Coulon J., Thibon C., Masneuf-Pomarède I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT. 2021;149:111781. doi: 10.1016/j.lwt.2021.111781. [DOI] [Google Scholar]
- 140.Simonin S., Alexandre H., Nikolantonaki M., Coelho C., Tourdot-Maréchal R. Inoculation of Torulaspora delbrueckii as a bio-protection agent in winemaking. Food Res. Int. 2018;107:451–461. doi: 10.1016/j.foodres.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 141.Oro L., Ciani M., Comitini F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014;116:1209–1217. doi: 10.1111/jam.12446. [DOI] [PubMed] [Google Scholar]
- 142.Sipiczki M. Metschnikowia Strains Isolated from Botrytized Grapes Antagonize Fungal and Bacterial Growth by Iron Depletion. Appl. Environ. Microbiol. 2006;72:6716–6724. doi: 10.1128/AEM.01275-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harlé O., Legrand J., Tesnière C., Pradal M., Mouret J.-R., Nidelet T. Correction: Investigations of the mechanisms of interactions between four non-conventional species with Saccharomyces cerevisiae in oenological conditions. PLoS ONE. 2021;15:e0233285. doi: 10.1371/journal.pone.0253680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fernández de Ullivarri M., Mendoza L.M., Raya R.R. Characterization of the killer toxin KTCf20 from Wickerhamomyces anomalus, a potential biocontrol agent against wine spoilage yeasts. Biol. Control. 2018;121:223–228. doi: 10.1016/j.biocontrol.2018.03.008. [DOI] [Google Scholar]
- 145.Comitini F., Agarbati A., Canonico L., Galli E., Ciani M. Purification and characterization of WA18, a new mycocin produced by Wickerhamomyces anomalus active in wine against Brettanomyces bruxellensis spoilage yeasts. Microorganisms. 2020;9:56. doi: 10.3390/microorganisms9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mehlomakulu N.N., Setati M.E., Divol B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food Microbiol. 2014;188:83–91. doi: 10.1016/j.ijfoodmicro.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 147.Branco P., Coutinho R., Malfeito-Ferreira M., Prista C., Albergaria H. Wine spoilage control: Impact of saccharomycin on Brettanomyces bruxellensis and its conjugated effect with sulfur dioxide. Microorganisms. 2021;9:2528. doi: 10.3390/microorganisms9122528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Capece A., Pietrafesa R., Siesto G., Romano P. Biotechnological approach based on selected Saccharomyces cerevisiae starters for reducing the use of sulfur dioxide in wine. Microorganisms. 2020;8:738. doi: 10.3390/microorganisms8050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miceli A., Negro C., Tommasi L., de Leo P. Polyphenols, resveratrol, antioxidant activity and achratoxin a contamination in red table wines, Controlled Denomination of Origin (DOC) wines and wines obtained from organic farming. J. Wine Res. 2003;14:115–120. doi: 10.1080/09571260410001678030. [DOI] [Google Scholar]
- 150.Pelonnier-Magimel E., Windhotz S., Masneuf Pomarède I., Barbe J.C. Sensory characterisation of wines without added sulfites via specific and adapted sensory profile. OENO One. 2020;54:671–685. doi: 10.20870/oeno-one.2020.54.4.3794. [DOI] [Google Scholar]
- 151.Santos M.C., Nunes C., Saraiva J.A., Coimbra M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012;234:1–12. doi: 10.1007/s00217-011-1614-6. [DOI] [Google Scholar]
- 152.Zara G., Nardi T. Yeast metabolism and its exploitation in emerging winemaking trends: From sulfite tolerance to sulfite reduction. Fermentation. 2021;7:57. doi: 10.3390/fermentation7020057. [DOI] [Google Scholar]
- 153.Costantini A., Vaudano E., Pulcini L., Carafa T., Garcia-Moruno E. An overview on biogenic amines in wine. Beverages. 2019;5:19. doi: 10.3390/beverages5010019. [DOI] [Google Scholar]
- 154.Stój A., Płotka-Wasylka J., Simeonov V., Kapłan M. The content of biogenic amines in Rondo and Zweigelt wines and correlations between selected wine parameters. Food Chem. 2022;371:131172. doi: 10.1016/j.foodchem.2021.131172. [DOI] [PubMed] [Google Scholar]
- 155.Esposito F., Montuori P., Schettino M., Velotto S., Stasi T., Romano R., Cirillo T. Level of biogenic amines in red and white wines, dietary exposure, and histamine-mediated symptoms upon wine ingestion. Molecules. 2019;24:3629. doi: 10.3390/molecules24193629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Medina M.Á., Urdiales J.L., Rodríguez-Caso C., Ramírez F.J., Sánchez-Jiménez F. Biogenic amines and polyamines: Similar biochemistry for different physiological missions and biomedical applications. Crit. Rev. Biochem. Mol. Biol. 2003;38:23–59. doi: 10.1080/713609209. [DOI] [PubMed] [Google Scholar]
- 157.Dasa F., Bejo W., Abdo T. Importance and toxicity of biogenic amines in fresh and processed foods. J. Food Technol. Nutr. Sci. 2022;147:24–26. doi: 10.47363/JFTNS/2022(4)147. [DOI] [Google Scholar]
- 158.Sumby K.M., Grbin P.R., Jiranek V. Implications of new research and technologies for malolactic fermentation in wine. Appl. Microbiol. Biotechnol. 2014;98:8111–8132. doi: 10.1007/s00253-014-5976-0. [DOI] [PubMed] [Google Scholar]
- 159.Caruso M., Fiore C., Contursi M., Salzano G., Paparella A., Romano P. Formation of biogenic amines as criteria for the selection of wine yeast. World J. Microbiol. Biotechnol. 2002;18:159–163. doi: 10.1023/A:1014451728868. [DOI] [Google Scholar]
- 160.Bäumlisberger M., Moellecken U., König H., Claus H. The potential of the yeast Debaryomyces hansenii H525 to degrade biogenic amines in food. Microorganisms. 2015;3:839–850. doi: 10.3390/microorganisms3040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Benito Á., Calderón F., Benito S. Combined use of S. pombe and L. thermotolerans in winemaking. Beneficial effects determined through the study of wines’ analytical characteristics. Molecules. 2016;21:1744. doi: 10.3390/molecules21121744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Benito S. Combined Use of Lachancea thermotolerans and Schizosaccharomyces pombe in winemaking: A Review. Microorganisms. 2020;8:655. doi: 10.3390/microorganisms8050655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang B., Tan F., Chu R., Li G., Li L., Yang T., Zhang M. The effect of non-Saccharomyces yeasts on biogenic amines in wine. Trends Food Sci. Technol. 2021;116:1029–1040. doi: 10.1016/j.tifs.2021.09.008. [DOI] [Google Scholar]
- 164.Zhang B., Liu H., Xue J., Tang C., Duan C., Yan G. Use of Torulaspora delbrueckii and Hanseniaspora vineae co-fermentation with Saccharomyces cerevisiae to improve aroma profiles and safety quality of Petit Manseng wines. LWT. 2022;161:113360. doi: 10.1016/j.lwt.2022.113360. [DOI] [Google Scholar]
- 165.Han B., Gao J., Han X., Deng H., Wu T., Li C., Zhan J., Huang W., You Y. Hanseniaspora uvarum FS35 degrades putrescine in wine through the direct oxidative deamination pathway of copper amine oxidase 1. Food Res. Int. 2022;162:111923. doi: 10.1016/j.foodres.2022.111923. [DOI] [PubMed] [Google Scholar]
- 166.Salehi B., Mishra A., Nigam M., Sener B., Kilic M., Sharifi-Rad M., Fokou P., Martins N., Sharifi-Rad J. Resveratrol: A double-edged sword in health benefits. Biomedicines. 2018;6:91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sharifi-Rad J., Quispe C., Durazzo A., Lucarini M., Souto E.B., Santini A., Imran M., Moussa A.Y., Mostafa N.M., El-Shazly M., et al. Resveratrol’ biotechnological applications: Enlightening its antimicrobial and antioxidant properties. J. Herb. Med. 2022;32:100550. doi: 10.1016/j.hermed.2022.100550. [DOI] [Google Scholar]
- 168.Kunová S., Felsöciová S., Tvrdá E., Ivanišová E., Kántor A., Žiarovská J., Terentjeva M., Kačániová M. Antimicrobial activity of resveratrol and grape pomace extract. Potravin. Slovak J. Food Sci. 2019;13:363–368. doi: 10.5219/1054. [DOI] [Google Scholar]
- 169.Mikolajková M., Ladicka N., Janusova M., Ondrova K., Mikulaskova H.K., Dordevic D. Resveratrol content in wine—resveratrol biochemical properties. Maso Int. J. Food Sci. Technol. 2021;11:31–38. doi: 10.2478/mjfst-2022-0005. [DOI] [Google Scholar]
- 170.Pastor R.F., Restani P., Di Lorenzo C., Orgiu F., Teissedre P.-L., Stockley C., Ruf J.C., Quini C.I., Garcìa Tejedor N., Gargantini R., et al. Resveratrol, human health and winemaking perspectives. Crit. Rev. Food Sci. Nutr. 2019;59:1237–1255. doi: 10.1080/10408398.2017.1400517. [DOI] [PubMed] [Google Scholar]
- 171.Tıraş Z.Ş.E., Okur H.H., Günay Z., Yıldırım H.K. Different approaches to enhance resveratrol content in wine. Ciência e Técnica Vitivinícola. 2022;37:13–28. doi: 10.1051/ctv/ctv20223701013. [DOI] [Google Scholar]
- 172.Shin S.-Y., Jung S.-M., Kim M.-D., Han N.S., Seo J.-H. Production of resveratrol from tyrosine in metabolically engineered Saccharomyces cerevisiae. Enzyme Microb. Technol. 2012;51:211–216. doi: 10.1016/j.enzmictec.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 173.Wang Y., Halls C., Zhang J., Matsuno M., Zhang Y., Yu O. Stepwise increase of resveratrol biosynthesis in yeast Saccharomyces cerevisiae by metabolic engineering. Metab. Eng. 2011;13:455–463. doi: 10.1016/j.ymben.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Wang Y., Yu O. Synthetic scaffolds increased resveratrol biosynthesis in engineered yeast cells. J. Biotechnol. 2012;157:258–260. doi: 10.1016/j.jbiotec.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 175.Li M., Kildegaard K.R., Chen Y., Rodriguez A., Borodina I., Nielsen J. De novo production of resveratrol from glucose or ethanol by engineered Saccharomyces cerevisiae. Metab. Eng. 2015;32:1–11. doi: 10.1016/j.ymben.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 176.Li M., Schneider K., Kristensen M., Borodina I., Nielsen J. Engineering yeast for high-level production of stilbenoid antioxidants. Sci. Rep. 2016;6:36827. doi: 10.1038/srep36827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sun P., Liang J.-L., Kang L.-Z., Huang X.-Y., Huang J.-J., Ye Z.-W., Guo L.-Q., Lin J.-F. Increased resveratrol production in wines using engineered wine strains Saccharomyces cerevisiae EC1118 and relaxed antibiotic or auxotrophic selection. Biotechnol. Prog. 2015;31:650–655. doi: 10.1002/btpr.2057. [DOI] [PubMed] [Google Scholar]
- 178.Gaensly F., Agustini B.C., da Silva G.A., Picheth G., Bonfim T.M.B. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis Labrusca grape must. J. Funct. Foods. 2015;19:288–295. doi: 10.1016/j.jff.2015.09.041. [DOI] [Google Scholar]
- 179.Escribano-Viana R., Portu J., Garijo P., López R., Santamaría P., López-Alfaro I., Gutiérrez A.R., González-Arenzana L. Effect of the sequential inoculation of non-Saccharomyces/Saccharomyces on the anthocyans and stilbenes composition of Tempranillo wines. Front. Microbiol. 2019;10:773. doi: 10.3389/fmicb.2019.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Costa C.E., Romaní A., Møller-Hansen I., Teixeira J.A., Borodina I., Domingues L. Valorisation of wine wastes by de Novo biosynthesis of resveratrol using a recombinant xylose-consuming industrial Saccharomyces cerevisiae strain. Green Chem. 2022;24:9128–9142. doi: 10.1039/D2GC02429B. [DOI] [Google Scholar]
- 181.Sharifi-Rad J., Rodrigues C.F., Stojanović-Radić Z., Dimitrijević M., Aleksić A., Neffe-Skocińska K., Zielińska D., Kołożyn-Krajewska D., Salehi B., Milton Prabu S., et al. Probiotics: Versatile bioactive components in promoting human health. Medicina. 2020;56:433. doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abid R., Waseem H., Ali J., Ghazanfar S., Muhammad Ali G., Elasbali A.M., Alharethi S.H. Probiotic yeast Saccharomyces: Back to nature to improve human health. J. Fungi. 2022;8:444. doi: 10.3390/jof8050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pais P., Almeida V., Yılmaz M., Teixeira M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi. 2020;6:78. doi: 10.3390/jof6020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kaźmierczak-Siedlecka K., Ruszkowski J., Fic M., Folwarski M., Makarewicz W. Saccharomyces boulardii CNCM I-745: A non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. 2020;77:1987–1996. doi: 10.1007/s00284-020-02053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Paula B.P., Chávez D.W.H., Lemos Junior W.J.F., Guerra A.F., Corrêa M.F.D., Pereira K.S., Coelho M.A.Z. Growth parameters and survivability of Saccharomyces boulardii for probiotic alcoholic beverages development. Front. Microbiol. 2019;10:2092. doi: 10.3389/fmicb.2019.02092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mulero-Cerezo J., Tuñón-Molina A., Cano-Vicent A., Pérez-Colomer L., Martí M., Serrano-Aroca Á. Probiotic rosé wine made with Saccharomyces cerevisiae var. boulardii. Biol. Biotechnol. 2022 doi: 10.20944/preprints202209.0333.v1. [DOI] [PubMed] [Google Scholar]
- 187.Vilela A., Cosme F., Inês A. Wine and non-dairy fermented beverages: A novel source of pro- and prebiotics. Fermentation. 2020;6:113. doi: 10.3390/fermentation6040113. [DOI] [Google Scholar]
- 188.Smith I.M., Baker A., Arneborg N., Jespersen L. Non-Saccharomyces yeasts protect against epithelial cell barrier disruption induced by Salmonella enterica subsp. enterica serovar Typhimurium. Lett. Appl. Microbiol. 2015;61:491–497. doi: 10.1111/lam.12481. [DOI] [PubMed] [Google Scholar]
- 189.Quarella S., Lovrovich P., Scalabrin S., Campedelli I., Backovic A., Gatto V., Cattonaro F., Turello A., Torriani S., Felis G.E. Draft genome sequence of the probiotic yeast Kluyveromyces marxianus fragilis B0399. Genome Announc. 2016;4:e00923-16. doi: 10.1128/genomeA.00923-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maccaferri S., Klinder A., Brigidi P., Cavina P., Costabile A. Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl. Environ. Microbiol. 2012;78:956–964. doi: 10.1128/AEM.06385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alvarez SC V., Alaniz MJ L., Furlani MV M., Vazquez F., Agresti P.M., Nally M.C., Maturano Y.P. Bioprospecting of the probiotic potential of yeasts isolated from a wine environment. Fungal Genet. Biol. 2023;164:103767. doi: 10.1016/j.fgb.2022.103767. [DOI] [PubMed] [Google Scholar]
- 192.Soceanu A., Dobrinas S., Sirbu A., Manea N., Popescu V. Economic aspects of waste recovery in the wine industry. A multidisciplinary approach. Sci. Total Environ. 2021;759:143543. doi: 10.1016/j.scitotenv.2020.143543. [DOI] [PubMed] [Google Scholar]
- 193.Nardi T. Microbial resources as a tool for enhancing sustainability in winemaking. Microorganisms. 2020;8:507. doi: 10.3390/microorganisms8040507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bharathiraja B., Iyyappan J., Jayamuthunagai J., Kumar R.P., Sirohi R., Gnansounou E., Pandey A. Critical Review on bioconversion of winery wastes into value-added products. Ind. Crops Prod. 2020;158:112954. doi: 10.1016/j.indcrop.2020.112954. [DOI] [Google Scholar]
- 195.Ahmad B., Yadav V., Yadav A., Rahman M.U., Yuan W.Z., Li Z., Wang X. Integrated biorefinery approach to valorize winery waste: A review from waste to energy perspectives. Sci. Total Environ. 2020;719:137315. doi: 10.1016/j.scitotenv.2020.137315. [DOI] [PubMed] [Google Scholar]
- 196.Filippi K., Papapostolou H., Alexandri M., Vlysidis A., Myrtsi E.D., Ladakis D., Pateraki C., Haroutounian S.A., Koutinas A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value-added fractions. Bioresour. Technol. 2022;343:125989. doi: 10.1016/j.biortech.2021.125989. [DOI] [PubMed] [Google Scholar]
- 197.Chowdhary P., Gupta A., Gnansounou E., Pandey A., Chaturvedi P. Current trends and possibilities for exploitation of grape pomace as a potential source for value addition. Environ. Pollut. 2021;278:116796. doi: 10.1016/j.envpol.2021.116796. [DOI] [PubMed] [Google Scholar]
- 198.De Iseppi A., Lomolino G., Marangon M., Curioni A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020;137:109352. doi: 10.1016/j.foodres.2020.109352. [DOI] [PubMed] [Google Scholar]
- 199.Beșliu A., Chiselița N., Chiselița O., Efremova N., Tofan E., Sprincean A., Daniliș M. Biochemical composition and antioxidant activity of the mannoprotein preparation obtained yeast biomass from wine industry waste. Not. Sci. Biol. 2022;14:11229. doi: 10.55779/nsb14211229. [DOI] [Google Scholar]
- 200.Varelas V., Tataridis P., Liouni M., Nerantzis E.T. Valorization of winery spent yeast waste biomass as a new source for the production of β-glucan. Waste Biomass Valorization. 2016;7:807–817. doi: 10.1007/s12649-016-9530-4. [DOI] [Google Scholar]
- 201.Voce S., Iacumin L., Comuzzo P. Characterization of Non-Saccharomyces Yeast strains isolated from grape juice and pomace: Production of polysaccharides and antioxidant molecules after growth and autolysis. Fermentation. 2022;8:450. doi: 10.3390/fermentation8090450. [DOI] [Google Scholar]
- 202.Williams D.L., Schückel J., Vivier M.A., Buffetto F., Zietsman A.J.J. Grape pomace fermentation and cell wall degradation by Kluyveromyces marxianus Y885. Biochem. Eng. J. 2019;150:107282. doi: 10.1016/j.bej.2019.107282. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.