Abstract
Compounds containing 6-azabicyclo[3.2.1]octane scaffold have many applications in the chemical and pharmaceutical industry. However, their synthesis is challenging and mainly limited to chemical synthesis. Herein we report that ene reductases (EREDs) can facilitate an unprecedented intramolecular β-C–H functionalization reaction for the synthesis of bridged bicyclic nitrogen heterocycles containing the 6-azabicyclo[3.2.1]octane scaffold. To streamline the synthesis of these privileged motifs, we developed a gram scale one-pot chemoenzymatic cascade by combining iridium photocatalysis with EREDs using readily available N-phenylglycines and cyclohexenones that can be obtained from biomass. Further derivatization using enzymatic or chemical methods can convert 6-azabicyclo[3.2.1]octan-3-one to 6-azabicyclo[3.2.1]octan-3α-ols, which can be potentially utilized for the synthesis of azaprophen and its analogues for drug discovery. Mechanistic studies revealed the reaction requires oxygen presumably to produce oxidized flavin which can selectively dehydrogenate the 3-substituted cyclohexanone derivatives to form the α,β-unsaturated ketone, which subsequently undergoes spontaneous intramolecular Aza-Michael addition under basic conditions.
Keywords: enzyme catalysis, β-C–H functionalization, bridged bicyclic nitrogen scaffolds, heterocycles, photocatalysis
Graphical Abstract

An unprecedented β-C–H functionalization reaction that is enabled by ene reductases is reported here. When the reaction is coupled with photocatalysis, various 6-azabicyclo[3.2.1]octan-3-ones can be easily synthesized using readily available cyclohexenones and N-phenylglycines. This chemoenzymatic reaction can be scaled up to gram-scale and the product can be further selectively derivatized.
Introduction
Carbonyl compounds are vital to chemical and pharmaceutical industry. One key strategy to access the carbonyl compounds and their derivatives is C(sp3)-H functionalization.[1] While direct C-H functionalization of α-positions of carbonyl compounds is well-studied, the more challenging direct β-C-H functionalization is under-explored due to the significantly less acidicity of the β-C–H bonds.[2] Traditional approaches to obtain β-functionalized carbonyl compounds mainly capitalize on the conjugative addition of a nucleophile to an α,β-unsaturated carbonyl compound. Most of these approaches require extra step(s) to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated ones (Scheme 1a),[2] which are less efficient than the direct introduction of functional groups at β-positions of saturated carbonyl compounds. Although multiple innovative methods have been successfully developed for direct β-functionalization of carbonyl compounds, most of them require expensive transition-metal catalysts and additives which limit their broad applications (Scheme 1b).[2–3] Hence, developing an economical strategy for efficient β-C–H functionalization of carbonyl compounds is highly desirable. On the other hand, distinct from organo- and metal catalysts, enzymes, as biodegradable catalysts, possess unparalleled regioselectivity and can promote challenging chemical transformations with high efficiency under the mild conditions.[4] Therefore, exploration of enzymes for novel direct β-C-H functionalization reactions can provide a green and sustainable approach to access chemically and pharmaceutically important carbonyl compounds.
Scheme 1.
A. The traditional approaches for accessing β-functionalized carbonyl compounds. B. Recently innovative approaches for direct β-functionalization of carbonyl compounds. C. Representative examples of naturally occurring and synthetic small molecules containing the 6-azabicyclo[3.2.1]octane ring system. D. Reported synthetic routes for 6-azabicyclo[3.2.1]octan-3-one. E. Novel direct β-C–H functionalization strategy in this study.
Bridged bicyclic nitrogen scaffolds play an important role for drug discovery which can be reflected from the high retail sales of the related drugs such as tiotropium bromide and varenicline.[5] As one important class of bridged bicyclic nitrogen scaffolds, the 6-azabicyclo[3.2.1]octane ring system is frequently observed in a myriad of natural occurring and synthetic small molecules, and it is considered as a privileged scaffold for drug discovery (Scheme 1c).[6] Although a number of synthetic strategies have been developed for synthesizing molecules containing this type of ring system,[7] only four synthetic approaches[7g, 7l, 7q, 7r] have been developed to access 6-azabicyclo[3.2.1]octan-3-ones which can serve as the synthetic intermediates for accessing bioactive natural products and drug candidates including azaprophen[8], actinobolamine[7e], and cocaine analogues[6] (Scheme 1c). Despite being elegantly designed, these chemical synthetic methods suffer from either multi-step synthesis with low efficiency or complicated starting materials, and limited substrate scope (Scheme 1d).[7g, 7l, 7q, 7r] Therefore, development of efficient approaches for synthesizing compounds with 6-azabicyclo[3.2.1]octan-3-one skeleton will be of great importance to the chemical and pharmaceutical industry.
Herein we report an unprecedented cofactor-free enzymatic dehydrocyclization (EDC) reaction that is enabled by ene reductases (EREDs) via intramolecular β-C-H functionalization of 3-substituted cyclohexanone derivatives (Scheme 1e). When coupled with photocatalysis, it allowed easy access to various 6-azabicyclo[3.2.1]octan-3-ones using readily available N-phenylglycines and cyclohexenones that can be converted from biomass-derived biofuel,[9] which provides a promising way for upgrading biomass to value-added chemicals. Besides, the chemoenzymatic reactions could be performed at gram-scale, and the products could be further selectively converted to the synthetic intermediates of drugs like azaprophen by both chemical and enzymatical methods. The underlying mechanism of this new-to-nature EDC reaction was investigated.
Results and Discussion
During our effort in exploring new-to-nature reactivity of EREDs, we discovered the thermophilic Old Yellow Enzyme[10] (TOYE) from Thermoanaerobacter pseudethanolicus E39 could efficiently convert substrate 1a to product 2a at room temperature without supplementing any cofactor (Table 1, entry 1). In the control experiment, when the TOYE was replaced by flavin mononucleotide (FMN) sodium salt, the product could not be detected, indicating that the reaction was catalyzed by the enzyme (Table 1, entry 2). We further screened several other EREDs for this cyclization reaction and found they all could promote the cyclization reaction but with a relatively lower efficiency (Table 1, entries 3–5). Among them, 12-oxophytodienoic acid reductase 3 (OPR3) from the tomato (Solanum lycopersicum)[11] (Table 1, entry 3) showed the second highest efficiency for the enzymatic transformation while the Old Yellow Enzymes OYE1[12] (Table 1, entry 4) and OYE3[13] (Table 1, entry 5) from Saccharomyces cerevisiae had low conversion efficiency. Further evaluation of the reaction conditions demonstrated that the pH of the reaction buffer plays the most important role in this reaction (Table 1, entries 6–8). Only trace amount of conversion could be detected when the reaction buffer was at a lower pH of 6.0 (Table 1, entry 6). Very low level of cyclized product could be observed at pH 7.0 (Table 1, entry 7) while a high pH at 9.0 (Table 1, entry 1) led to the highest conversion rate. Too high pHs such as 10 and 11 can cause lowered conversion efficiency (Table 1, entries 9–10). The enzymatic cyclization can reach up to 4200 total turnover number (TTN) at a conversion rate of 42.6% when 0.1 mol% TOYE was utilized for the reaction (Table 1, entry 9). Further increase of enzyme loading led to a higher conversion rate but a lowered TTN (Table 1, entries 1 and 10). The percentage of co-solvent dimethyl sulfoxide (DMSO) utilized in the reaction is also important for the reaction (Table 1, entries 11 and 12). When 5% DMSO (Table 1, entry 11) used in the reaction, the conversion rate was dramatically reduced compared to the standard condition with 20% DMSO (Table 1, entry 1). Furthermore, the conversion rate was dropped to 78.0% when the incubation time was reduced to 22 hours (Table 1, entry 13). Interestingly, no cyclized product could be observed (Table 1, entry 14) when the nicotinamide adenine dinucleotide phosphate (NADPH) regeneration system was added into the reaction, suggesting that oxidative catalysis is operative.
Table 1.
Evaluation of the reaction parameters.[a]

| |||
|---|---|---|---|
| Entry | Modification from standard conditions | Conversion [%][b] | TTN[c] |
|
| |||
| 1 | None | 90.3 | 1807 |
| 2 | FMN-Na (2 mol%) instead of TOYE | 0 | 0 |
| 3 | OPR3 instead of TOYE | 44.6 | 893 |
| 4 | OYE1 instead of TOYE | 18.0 (21.9)[d] | 359 |
| 5 | OYE3 instead of TOYE | 19.9 (21.7)[d] | 398 |
| 6 | pH 6.0 | 2.2 | 45 |
| 7 | pH 7.0 | 24.7 | 493 |
| 8 | pH 8.0 | 77.8 | 1557 |
| 9 | pH 10.0 | 73.2 | 1464 |
| 10 | pH 11.0 | 21.1 | 422 |
| 11 | 0.1 mol‰ TOYE | 42.6 | 4263 |
| 12 | 0.25 mol‰ TOYE | 70.0 | 2798 |
| 13 | 5% DMSO | 69.9 | 1398 |
| 14 | 10% DMSO | 81.6 | 1632 |
| 15 | 22 h | 78.0 | 1560 |
| 16 | NADPH (20 mM) | 7.2 | 144 |
| 17 | NADPH (10 mM) | 14.1 | 282 |
Reactions performed using 1a (10 mM) and TOYE (0.5 mol‰) in a mixture of 0.8 mL sodium phosphate buffer (50 mM, pH 9.0) and 0.2 mL DMSO at 24 ºC for 44 h.
Percent conversion [%] determined by GC-MS as described in the Supporting Information.
Total turnover number (TTN) determined by [product]/[enzyme].
Substrate assumption rate [%] determined by GC-MS as described in the Supporting Information.
Since substrate 1a can be conveniently synthesized by photocatalysis with readily available 2-cyclohexen-1-one (3a) and N-phenylglycine (4a),[14] we combined photocatalysis with the enzymatic catalysis to achieve readily operated one-pot reaction. In addition, because it is time-consuming, labor-intensive, and expensive to purify the enzyme, we decided to use the wet E. coli whole cells containing TOYE as an alternative of the purified enzyme for the reaction. We first employed the previously reported photocatalytic reaction[14] to perform the Giese reaction in DMSO with 3a and 4a to generate substrate 1a. We then added the reaction buffer and wet E. coli whole cells containing TOYE to the same reaction vial to perform the second step reaction. This readily operated one-pot, two-step reaction resulted in 84% isolated yield of 1a at 0.1 mmol scale. In the control experiment, E. coli cells without functional TOYE could not perform the dehydrocyclization reaction.
With this convenient photobiocatalytic approach, we successfully synthesized a series of structurally diverse 6-azabicyclo[3.2.1]octan-3-ones with readily available cyclohexenones and N-phenylglycines (Scheme 2 and Figure S1). This substrate scope study indicates that the reaction could tolerate both electron-withdrawing (2b-2j) and electron-donating groups (2k-2n) at para- and meta- positions on the phenyl ring of substrate 4. The substitutions at meta positions (2c, 2f, 2h, and 2n) of the substrate aromatic ring have relatively higher yields than those at para positions (2b, 2e, 2g, and 2m). However, only small substitution like fluoro (2d) at the ortho position could be accepted. Decent yields could be obtained when the alpha position of amino acid derivatives is substituted by different functional groups (2o-2q). As to the olefin substrates, the 2, 4, and 6 positions of the cyclohexanone ring could allow different substitutions but with relatively low efficiency (2r-2x) and only product 2t has a decent yield of 53%. When the carbon atom at 4 position of the cyclohexanone ring is replaced with oxygen atom (2y), the reaction can also happen to form an oxa-analogue with 40% yield. For those cyclized products with low yields, we determined the product yields of the photobiocatalytic step which can demonstrate that the enzymatic step plays a major role for their low overall yields (Table S1). Furthermore, we scaled up the stepwise one-pot photobiocatalytic process to 7.5 mmol scale (Figure S2). Encouragingly, this gram-scale enzymatic synthesis allowed us to successfully obtain 1.1 g of 2a in 75% isolation yield. This demonstrates the great potential of our photobiocatalytic approach for further larger scale production of 6-azabicyclo[3.2.1]octane containing heterocycles.
Scheme 2.
Substrate scope of one-pot two-step chemoenzymatic reaction. Reaction conditions for step 1 to generate 1: 3 (0.1 mmol, 1 equiv), 4 (0.1 mmol, 1 equiv), Li2CO3 (20 mol %), [Ir(dtbbpy)(ppy)2]PF6 (1 mol %), degassed DMSO (2 mL), N2, blue light-emitting diodes (LEDs), at room temperature, 22 h. Reaction conditions for step 2: 2 mL reaction mixture of step 1, 8 mL sodium phosphate buffer (50 mM, pH 9.0), 250 mg E. coli BL21 TOYE wet cells, flavin mononucleotide (FMN) sodium salt (1 mg/mL), room temperature for 44 h. a: no external FMN-Na in the reaction.
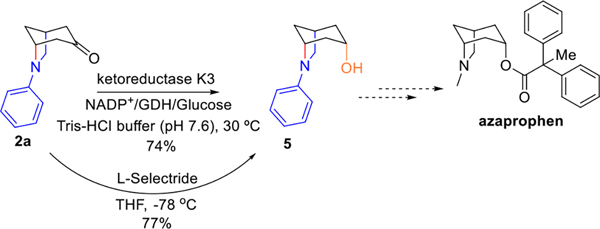
To demonstrate the practical application of the above-mentioned photobiocatalytic process, we selected the key building block of azaprophen as a target compound for synthesis. Azaprophen is a drug candidate that has been frequently studied (Scheme 3).[7r, 8, 15] It was reported to be 50 times more potent than atropine as an antimuscarinic agent as measured by the inhibition of acetylcholine-induced contraction of guinea pig ileum. [15a] The most essential parts of this racemic drug candidate for the potent bioactivity are the subunits derived from 6-azabicyclo[3.2.1]octan-3α-ols. We therefore tested both chemical and enzymatic strategies to convert the cyclized product 2a to 6-phenyl-6-azabicyclo[3.2.1]octan-3α-ols. We first employed L-selectride for the selective reduction of 6-phenyl-6-azabicyclo[3.2.1]octan-3-one and successfully obtained the 6-phenyl-6-azabicyclo[3.2.1]octan-3α-ols, which can serve as key components for synthesizing azaprophen and its analogues (Scheme 3).[7r, 15j, 15k] However, the chemical derivatization suffers from harsh reaction conditions and the generation of hazardous chemical wastes.[7r] We therefore attempted to develop an enzymatic method for the derivatization. Encouragingly, when we utilized our recently engineered ketoreductase K3[4d] for the reduction reaction, it could go on smoothly at 30 °C with good conversion rate (74%) and only 6-phenyl-6-azabicyclo[3.2.1]octan-3α-ols were generated (Scheme 3). Considering this enzymatic derivatization method is environmental-friendly, it could be coupled with our photobiocatalytic approach to access numerous 6-azabicyclo[3.2.1]octan-3α-ols. Notably, the ketoreductase K3 may also be a good candidate to selectively convert other bulky ketones with bicyclic bridged backbones such as atropine, which is worthy of future exploration.
Scheme 3.
Cyclized product derivatization.
After exploration of racemic substrates for the enzymatic cyclization reaction, we further explored optically pure substrates. We obtained (S)-1a and (R)-1a by chiral separation of 1a using a chiral HPLC column. When we employed (S)-1a in the reaction, product (1S, 5R)-2a could be obtained with 96% conversion rate and over 99% ee (Scheme 4A). The over 99% conversion to (1R, 5S)-2a with over 99% ee could be achieved when (R)-1a was utilized as a substrate for the reaction and the absolute configuration of the product was determined by X-ray crystallography (Scheme 4A and Figure S3).[16] The combined result suggests that the enzymatic reaction is stereoretentive for the optically pure substrates. Further development of chemical or enzymatic method(s) to synthesize the enantiopure substrates will allow the easy access to enantiopure cyclized products when combined with this enzymatic cyclization reaction.
Scheme 4.
The influence of oxygen and substrates to the enzymatic dehydrocyclization reactions. A) Enzymatic specificity to optical pure substrates. B) The influence of oxygen to the reaction. C) Spontaneous cyclization reaction of the dehydrogenated substrate.
Next, we explored the mechanism of the enzymatic cyclization reaction. Based upon the result that the reaction is strongly inhibited by reductive conditions with NADPH, we surmised the enzyme could facilitate the oxidative dehydrogenation of the 3-substituted cyclohexanone 1a. As no external oxidizing reagents are added, we anticipated that dioxygen would serve as the oxidant. Performing the reaction of 1a under anaerobic conditions resulted in no reaction in contrast to the control experiments performed in air where the substrate could be easily converted to the cyclized product (Scheme 4B and Figure S4). This demonstrates that the enzymatic cyclization reaction requires dioxygen. We then deduced that the reaction might involve enzymatic dehydrogenation of the substrate to have α,β-desaturation at position C5-C6 and then undergo either spontaneous or ERED promoted cyclization. Our attempt to obtain such an intermediate with substrate 1a was failed as the intermediate likely cyclized immediately after it was generated. Indeed, our failed attempt of using a chemical method[9c] to dehydrogenate substrate 1a to synthesize the potential alkene intermediate indicates that it is challenging to dehydrogenate this type of substrates. Fortunately, we observed the alkene intermediate during the enzymatic cyclization reaction for product 2s. We isolated intermediate 1s′ and found it underwent cyclization reaction without the addition of TOYE, which proves that the cyclization reaction is a spontaneous reaction (Scheme 4C and Figure S5). The generation of carbon-carbon double bond selectively at C5-C6 position of the 3-substituted cyclohexanones at room temperature is extremely difficult for chemical catalysis due to several major challenges. Firstly, the regioselectivity of the dehydrogenation of 3-substituted cyclohexanones is difficult to be controlled by the traditional chemical catalysis methods. Both C2-C3 and C5-C6 dehydrogenated products are possibly generated during the catalytic process.[9b, 9c] Secondly, the potential overoxidation of the substrate to phenolic compounds can reduce the reaction efficiency.[9b, 9c] Thirdly, the α,β-desaturation of cyclohexanones is an endothermic process[17] which usually requires a relative high reaction temperature.[9b, 9c] Hence, these challenges make it a daunting task to chemically generate 3-substitued cyclohexenones with high regioselectivity in mild conditions. Of note, some recently developed direct β-C–H functionalization methods also combine the initial α,β-desaturation of carbonyl compounds with the subsequent conjugate addition. However, these methods all require costly transition-metals to facilitate the catalytic process.[2–3] Compared to these methods, our metal-free β-C–H functionalization method is more economic and readily operated.
The high regioselectivity of enzymatic dehydrogenation may be determined by the steric effects of substrate substitutions which influence the substrate binding with TOYE. In fact, this could be observed for 1a, 1r, and 1s (Figure S6). Compared to the unsubstituted 1a, the only differences between 1r and 1s are the methylated positions and they both showed lower levels of dehydrogenation. Although the one-pot reactions for 2r and 2s have similar product yields, the fact that 1s generated more uncyclized dehydrogenated product than 1r demonstrates that the 3, 6-substituted substrate is much more suitable for the enzymatic dehydrogenation than the 2, 3-substituted substrate. We also coupled 3j and 4a as substrates for the one-pot reaction but did not observe either the cyclized or the dehydrogenated products (Figure S6). However, the related Giese product (1z) could be observed from GC-MS spectra, which additionally corroborates the important substitution effects of the substrates.
The native function of EREDs is to reduce the α,β-unsaturated carbon−carbon double bond of Michael acceptors, especially those containing a carbonyl group.[18] The process involves several key amino acid residues including Asn/His or His/His which were reported as conserved residues to anchor substrates in the enzyme active site.[18] After the substrate is positioned suitably in the active site by these two amino acid residues, FMN can transfer the hydride from NADPH or other cosubstrates to the β position of the substrate while the α position could get a proton from a conserved tyrosine residue.[18–19]
The possible dehydrogenative mechanism could be the reverse pathway of the native function of EREDs when NADPH or other cosubstrates are not present in the reaction. It may go with the initial deprotonation of C6-H of the substrate at high pH (pH 9.0), then have the hydride transferred from the C5-H of the substrate to the N5 atom of FMN which can be supported by the dramatical UV spectral changes when 1a and TOYE are mixed (Figure S7). While the formed FMNH2 is oxidized by dioxygen to generate hydrogen peroxide, the dehydrogenated product undergoes the spontaneous cyclization to deliver the cyclized product (Scheme 5). Indeed, dimethyl sulfone, the oxidized product of cosolvent DMSO by hydrogen peroxide, was identified with increased concentration from the reaction mixture (Figure S8A–B). Moreover, the positive test result with peroxide test kit for the reaction mixtures (Figure S8C) and the dropped conversion efficiency at low pH (pH 6.0) also supports this hypothesis. In this proposed mechanism, two factors may influence this highly regioselective dehydrogenation process. One is the proper positioning of the C5-H of the substrate above the N5 atom of FMN to allow efficient hydride transfer. Another one is to have suitable pH to promote the deprotonation of the C6-H by the conserved tyrosine residue of EREDs. Indeed, the mutagenesis of this tyrosine residue to a phenylalanine residue can abolish or dramatically reduce the dehydrocyclization efficiency of EREDs (Table 2, entries 1–4).
Scheme 5.
Proposed mechanism for TOYE promoted dehydrocyclization reaction.
Table 2.
Evaluation of the mutants of ene reductases.[a]
| Entry | Enzyme | Conversion [%][b] | TTN[c] |
|---|---|---|---|
|
| |||
| 1 | TOYE-Y168F | 0 | 0 |
| 2 | OYE1-Y196F | 0 | 0 |
| 3 | OYE3-Y196F | 0 | 0 |
| 4 | OPR3-Y190F | 3.8 | 77 |
Reactions performed using 1a (0.01 mmol) and enzyme (0.5 mol‰) in a mixture of 0.8 mL sodium phosphate buffer (50 mM, pH 9.0) and 0.2 mL DMSO at 24 oC for 44 h.
Percent conversion [%] determined by GC-MS as described in the Supporting Information.
Total turnover number (TTN) determined by [product]/[enzyme].
Conclusion
In summary, we discovered that EREDs could promote one novel enzymatic dehydrocyclization reaction in the absence of cofactor NADPH. This type of metal-free direct β-C–H functionalization process has not been reported before. Combining photocatalysis with this enzymatic reaction allowed the efficient synthesis of a series of 6-azabicyclo[3.2.1]octan-3-ones. Using readily available starting materials in a one-pot, two-step reaction, gram scale syntheses of the 6-azabicyclo[3.2.1]octan-3-one derivatives were achieved. The reaction product could be further selectively converted to corresponding alcohols by both enzymatic and chemical catalysis methods. The mechanistic study of the enzymatic dehydrocyclization reaction reveals that the substrate first undergoes a highly regioselective enzymatic dehydrogenation reaction involving dioxygen followed by spontaneous intramolecular Aza-Michael addition to forge the bicyclic bridge. Mutagenesis studies demonstrate that a conserved tyrosine residue of EREDs plays an essential role in the catalysis of this reaction. The enzymatic dehydrocyclization reaction developed in this study could serve as a facile and green way to synthesize structurally diverse bicyclic nitrogen heterocycles. Further screening and engineering of EREDs may expand the substrate scope of the enzymatic dehydrocyclization reaction for synthesizing other heterocyclic ring systems that are widely present in bioactive natural products and drugs. Notably, the cyclohexenones used in the study can be easily converted from biomass-derived aromatic ethers.[9] Thus, when combined with current photocatalytic approach, it may allow us to develop a useful route for upgrading biomass to value-added and pharmaceutical relevant chemicals.
Supplementary Material
Acknowledgements
This work was supported by the US Department of Energy (no. DE-SC0018420). NMR data were collected at the Carl R. Woese Institute for Genomic Biology Core Facility, on a 600-MHz NMR funded by NIH grant no. S10-RR028833. We thank Dr. Maolin Li, Dr. Zhengyi Zhang, and Lawrence Lin for insightful discussions and technical help.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information for this article is given via a link at the end of the document.
References
- [1].Liu BX, Yang LY, Li PF, Wang F, Li XW, Org. Chem. Front. 2021, 8, 1085–1101. [Google Scholar]
- [2].Wang CP, Dong GB, ACS Catal. 2020, 10, 6058–6070. [Google Scholar]
- [3].a) Huang ZX, Dong GB, J. Am. Chem. Soc. 2013, 135, 17747–17750; [DOI] [PubMed] [Google Scholar]; b) Desai LV, Hull KL, Sanford MS, J. Am. Chem. Soc. 2004, 126, 9542–9543; [DOI] [PubMed] [Google Scholar]; c) Zhang FL, Hong K, Li TJ, Park H, Yu JQ, Science 2016, 351, 252–256; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pirnot MT, Rankic DA, Martin DBC, MacMillan DWC, Science 2013, 339, 1593–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Harrison W, Huang XQ, Zhao HM, Accounts Chem. Res. 2022, 55, 1087–1096; [DOI] [PubMed] [Google Scholar]; b) Bell EL, Finnigan W, France SP, Green AP, Hayes MA, Hepworth LJ, Lovelock SL, Niikura H, Osuna S, Romero E, Ryan KS, Turner NJ, Flitsch SL, Nature Reviews Methods Primers 2021, 1; [Google Scholar]; c) Huang XQ, Wang BJ, Wang YJ, Jiang GD, Feng JQ, Zhao HM, Nature 2020, 584, 69–74; [DOI] [PubMed] [Google Scholar]; d) Huang XQ, Feng JQ, Cui JW, Jiang GD, Harrison W, Zang X, Zhou JH, Wang BJ, Zhao HM, Nat. Catal. 2022, 5, 586–593; [Google Scholar]; e) Chen K, Arnold FH, Nat. Catal. 2020, 3, 203–213. [Google Scholar]
- [5].McGrath NA, Brichacek M, Njardarson JT, J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar]
- [6].Quirante J, Vila X, Bonjoch J, Kozikowski AP, Johnson KM, Bioorgan. Med. Chem. 2004, 12, 1383–1391. [DOI] [PubMed] [Google Scholar]
- [7].a) Biswas S, Van Steijvoort B, Waeterschoot M, Bheemireddy NR, Evano G, Maes BUW, Angew. Chem. Int. Ed. 2021, 60, 21988–21996; Angew. Chem. 2021, 133, 22159–22167; [DOI] [PubMed] [Google Scholar]; b) Xu Y, Sun Q, Tan TD, Yang MY, Yuan P, Wu SQ, Lu X, Hong X, Ye LW, Angew. Chem. Int. Ed. 2019, 58, 16252–16259; Angew. Chem. 2019, 131, 16398–16405; [DOI] [PubMed] [Google Scholar]; c) Yeh MCP, Chang YM, Lin HH, Adv. Synth. Catal. 2017, 359, 2196–2201; [Google Scholar]; d) Takeda M, Inoue H, Noguchi K, Honma Y, Kawamori M, Tsukamoto G, Yamawaki Y, Saito S, Chem. Pharm. Bull. 1976, 24, 1514–1526; [DOI] [PubMed] [Google Scholar]; e) Holmes AB, Kee A, Ladduwahetty T, Smith DF, J. Chem. Soc. Chem. Comm. 1990, 1412–1414; [Google Scholar]; f) Han G, LaPorte MG, Folmer JJ, Werner KM, Weinreb SM, J. Org. Chem. 2000, 65, 6293–6306; [DOI] [PubMed] [Google Scholar]; g) Kwak YS, Winkler JD, J. Am. Chem. Soc. 2001, 123, 7429–7430; [DOI] [PubMed] [Google Scholar]; h) Quirante J, Vila X, Escolano C, Bonjoch J, J. Org. Chem. 2002, 67, 2323–2328; [DOI] [PubMed] [Google Scholar]; i) Roberson CW, Woerpel KA, J. Am. Chem. Soc. 2002, 124, 11342–11348; [DOI] [PubMed] [Google Scholar]; j) Grainger RS, Welsh EJ, Angew. Chem. Int. Ed. 2007, 46, 5377–5380; Angew. Chem. 2007, 119, 5473–5476; [DOI] [PubMed] [Google Scholar]; k) Campbell CL, Hassler C, Ko SS, Voss ME, Guaciaro MA, Carter PH, Cherney RJ, J. Org. Chem. 2009, 74, 6368–6370; [DOI] [PubMed] [Google Scholar]; l) Hodgson DM, Shelton RE, Moss TA, Dekhane M, Org. Lett. 2010, 12, 2834–2837; [DOI] [PubMed] [Google Scholar]; m) Grainger RS, Betou M, Male, Pitak MB, Coles SJ, Org. Lett. 2012, 14, 2234–2237; [DOI] [PubMed] [Google Scholar]; n) Cormier M, Jean A, Blanchet J, Rouden J, Maddaluno J, De Paolis M, Tetrahedron Lett. 2014, 55, 5074–5077; [Google Scholar]; o) Diaba F, Montiel JA, Serban G, Bonjoch J, Org. Lett. 2015, 17, 3860–3863; [DOI] [PubMed] [Google Scholar]; p) Liu T, Mei TS, Yu JQ, J. Am. Chem. Soc. 2015, 137, 5871–5874; [DOI] [PMC free article] [PubMed] [Google Scholar]; q) Furstoss PTR, Waegell B, J. Chem. Soc., Chem. 1970, 6, 384–385; [Google Scholar]; r) Pitner JB, Carroll FI, J. Chem. Soc., Perkin Trans. 1 1991, 1375–1381. [Google Scholar]
- [8].Carroll FI, Abraham P, Mascarella SW, Singh P, Moreland CG, Sankar SS, Kwon YW, Triggle DJ, J. Med. Chem. 1991, 34, 1436–1440. [DOI] [PubMed] [Google Scholar]
- [9].a) Meng QL, Hou MQ, Liu HZ, Song JL, Han BX, Nat. Commun. 2017, 8, 14190; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Diao TN, Stahl SS, J. Am. Chem. Soc. 2011, 133, 14566–14569; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chen M, Dong GB, Angew. Chem. Int. Ed. 2021, 60, 7956–7961; Angew. Chem. 2021, 133, 8035–8040. [DOI] [PubMed] [Google Scholar]; d) Boot M, Frijters P, Luijten C, Somers B, Baert R, Donkerbroek A, Klein-Douwel RJH, Dam N, Energ. Fuel 2009, 23, 1808–1817. [Google Scholar]
- [10].Adalbjornsson BV, Toogood HS, Fryszkowska A, Pudney CR, Jowitt TA, Leys D, Scrutton NS, ChemBioChem 2010, 11, 197–207. [DOI] [PubMed] [Google Scholar]
- [11].Breithaupt C, Kurzbauer R, Lilie H, Schaller A, Strassner J, Huber R, Macheroux P, Clausen T, P. Natl. Acad. Sci. USA 2006, 103, 14337–14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Saito K, Thiele DJ, Davio M, Lockridge O, Massey V, J. Biol. Chem. 1991, 266, 20720–20724. [PubMed] [Google Scholar]
- [13].Niino YS, Chakraborty S, Brown BJ, Massey V, J. Biol. Chem. 1995, 270, 1983–1991. [DOI] [PubMed] [Google Scholar]
- [14].Millet A, Lefebvre Q, Rueping M, Chem. Eur. J. 2016, 22, 13464–13468. [DOI] [PubMed] [Google Scholar]
- [15].a) Carroll FI, Abraham P, Parham K, Griffith RC, Ahmad A, Richard MM, Padilla FN, Witkin JM, Chiang PK, J. Med. Chem. 1987, 30, 805–809; [DOI] [PubMed] [Google Scholar]; b) Witkin JM, Witkin KM, Chiang PK, Eur. J. Pharmacol. 1990, 183, 1944–1944; [Google Scholar]; c) Genovese RF, Elsmore TF, Pharmacol. Biochem. Be. 1989, 32, 495–498; [DOI] [PubMed] [Google Scholar]; d) Arias MS, Galvez E, Somoza A, J. Mol. Struct. 1990, 218, 87–92; [Google Scholar]; e) Gennings C, Carter WH, Harris LW, Carchman RA, Campbell ED, Boyle RM, Talbot BG, Solana RP, Fund. Appl. Toxicol. 1990, 14, 235–242; [DOI] [PubMed] [Google Scholar]; f) Genovese RF, Eur. J. Pharmacol 1990, 176, 271–279; [DOI] [PubMed] [Google Scholar]; g) Karle JM, Karle IL, Chiang PK, Acta. Crystallogr. B 1990, 46, 215–222; [DOI] [PubMed] [Google Scholar]; h) Philip A, Pitner JB, Carroll FI, Kwon YW, Triggle DJ, Abstr. Pap. Am. Chem. S. 1990, 200, 25; [Google Scholar]; i) Solana RP, Gennings, Carter WH, Anderson D, Lennox WJ, Carchman RA, Harris LW, J. Am. Coll. Toxicol. 1991, 10, 215–222; [Google Scholar]; j) Triggle DJ, Yong WK, Abraham P, Pitner JB, Mascarella SW, Carroll FI, J. Med. Chem. 1991, 34, 3164–3171; [DOI] [PubMed] [Google Scholar]; k) Philip A, Pitner JB, Joo YJ, Triggle DJ, Carroll FI, J. Chem. Soc., Chem. 1990, 984–985. [Google Scholar]
- [16].CCDC 2233284 ((1R, 5S)-2a contain the supplementary crystallographic data for this paper. These data are provided free of charge from the Cambridge Crystallographic Data Centre. [Google Scholar]
- [17].Rogers DW, Zhao YP, Traetteberg M, Hulce M, Liebman J, J. Chem. Thermodyn. 1998, 30, 1393–1400. [Google Scholar]
- [18].Toogood HS, Scrutton NS, ACS Catal. 2018, 8, 4333–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) Winkler CK, Clay D, Entner M, Plank M, Faber K, Chem. Eur. J. 2014, 20, 1403–1409; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lonsdale R, Reetz MT, J. Am. Chem. Soc. 2015, 137, 14733–14742; [DOI] [PubMed] [Google Scholar]; c) Abramovitz AS, Massey V, J. Biol. Chem. 1976, 251, 5327–5336; [PubMed] [Google Scholar]; d) Fox KM, Karplus PA, Structure 1994, 2, 1089–1105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.