Abstract
Thymic epithelial tumors (TETs), including thymoma, thymic carcinoma and neuroendocrine tumors, are uncommon tumors that originate from the epithelial cells of the thymus. Nevertheless, despite their rarity, they represent the most common tumor type located in the anterior mediastinum. Therapeutic choices based on staging and histology may include surgery with or without neoadjuvant or adjuvant therapy represented by chemotherapy, radiotherapy or chemo-radiotherapy. For patients with advanced or metastatic TETs, platinum-based chemotherapy remains the standard first-line treatment; however, some new drugs and combinations are currently under evaluation. In any case, proper management of patients with TETs requires a multidisciplinary team approach to personalize care for each patient.
Keywords: thymic epithelial tumors, thymoma, thymic carcinoma
1. Introduction
Thymic epithelial tumors (TETs), including thymoma, thymic carcinoma and neuroendocrine tumors (NETs) are uncommon tumors that originate from the epithelial cells of the thymus. The crude incidence rate of TETs in Europe is 1.7 cases per million per year, the incidence rates are similar in both genders, and the mean age at diagnosis is 50–60 years [1,2], as indicated in Table 1. Thymomas, which account for about half of anterior mediastinal tumors, are more frequent than thymic carcinomas and NETs, which represent 14–22% and 2–5% of thymic epithelial neoplasms, respectively [3].
Table 1.
Epidemiology of TETs (thymic epithelial tumors).
| Heterogeneous Group of Malignancies |
|---|
| Incidence: 1.7/1 million/year |
| Most common tumor type located in the anterior mediastinum |
| Any age (mean age at diagnosis 50–60 years), both genders (slight male prevalence) |
| No risk factors identified |
| Common association with autoimmune diseases (myasthenia gravis in 30% of the cases) |
| Prognosis: 5-year survival ~80% for thymoma, ~30% for thymic carcinoma |
Thymoma and thymic carcinoma act differently considering that in thymoma the cancer cells look like the normal cells of the thymus in many ways, grow slowly and rarely spread beyond the thymus, while in thymic carcinoma the cancer cells do not look like the normal cells of the thymus, grow quickly and metastases spread early. The treatment algorithm for resectable thymic tumors includes surgery followed by further treatments (radiotherapy or chemotherapy) depending on radicality, histotype and stage, while for patients with advanced or metastatic TETs, platinum-based chemotherapy remains the standard first-line treatment. However, as we shall see, several new drugs such as the multikinase inhibitors lenvatinib and regorafenib, the chemotherapeutic agent S-1 (tegafur) and immune checkpoint inhibitors (ICIs) including pembrolizumab, avelumab and nivolumab are currently under evaluation regarding metastatic disease. In any case, proper management of patients with TETs requires a multidisciplinary team approach to personalize care for each patient.
Prognosis depends on various factors such as histology, stage and surgical radicality, with an overall 5-year survival rate of 78% for thymoma and 30% for thymic carcinoma [4]. In clinical practice, cases of thymomas are often found incidentally during diagnostic work-ups for myasthenia gravis (MG). In fact, TETs, particularly thymomas, are often associated with paraneoplastic autoimmune diseases (ADs) such as MG, which is observed in about 30% of patients. Other, less-frequent paraneoplastic ADs include pure red cell aplasia, hypogammaglobulinemia, polymyositis, Cushing syndrome and systemic lupus erythematous [5]. A recent paper published by Singhal et al. investigated the possible existence of risk factors, including molecular abnormalities, for ADs in 48 patients with advanced TETs treated at Stanford University until 2020 [5]. In particular, 16/48 patients (33%) had an associated paraneoplastic AD, mainly represented, as expected, by MG (44%). In this retrospective study the only characteristic associated with an increased risk of AD was a histology of thymoma. Considering the close relationship between TETs and paraneoplastic ADs, the ThYmic MalignanciEs (TYME) Italian collaborative group evaluated the safety of 245 doses of SARS-CoV-2 mRNA vaccine administered to 126 patients with TETs (30% affected by ADs) [6]. The authors recorded no G3–G4 toxicities and a rate of G1–2 adverse events (AEs) comparable with that reported in the general population. In the last few years in the scientific community, the interest in these rare tumors has increased, as demonstrated by the creation of several working groups, including the International Thymic Malignancy Interest Group (ITMG), the Reseau Tumeurs et THYMques et Cancer (RYTHMIC) network and the TYME Italian collaborative group.
In this paper we report the current management of patients with TETs with a special focus on novel medical therapies for the advanced disease.
2. Diagnostic Work-Up
The diagnostic work-up should include a careful medical history, physical examination (with particular attention to neurological signs), laboratory tests, comprising measurement of inflammatory markers and immunological tests, radiological exams and histopathological assessment. The differential diagnosis should be made with other solid tumors such as lymphomas (Hodgkin and non-Hodgkin lymphoma), extragonadal germ cell tumors and metastatic carcinomas that can also affect the mediastinum. Regarding radiological evaluation, a chest X-ray is often the first exam that suggests the presence of a thymic mass. Chest computed tomography (CT) scanning represents the imaging modality of choice for TETs, while magnetic resonance imaging (MRI) may be useful to evaluate cystic lesions or areas of local invasion [7]. Interestingly, Japanese researchers performed a CT-based radiomics analysis in 61 patients with TETs to distinguish between thymic carcinoma and thymoma [8]. They concluded that two texture features, gray-level co-occurrence matrix (GLCM)-energy and solidity, were significant predictors of thymic carcinoma. Araujo-Filho et al. showed that a CT-based radiomics model could also be effective in the preoperative prediction of resectability in patients with TETs [9]. Recently, other researchers retrospectively reviewed the CT findings and the prognoses of 194 patients with TETs (32 low-risk thymomas, 52 high-risk thymomas and 110 thymic carcinomas) [10]. The authors demonstrated that CT signs of vessel invasion and pericardial mass in patients with thymic carcinoma and pericardial mass in those with high-risk thymoma are related to poorer outcomes.
Overall, fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scanning does not yet have an established role in the evaluation of TETs, considering that it can provide false-positive results in case of infection, thymic hyperplasia and other non-neoplastic processes [2]. However, PET/CT scanning may be considered in the case of thymic carcinoma due to its higher tumor metabolism or for the detection of occult metastases [2]. The definite diagnosis is the histological one performed with the analysis of tissue samples derived from a biopsy in the case of advanced disease or directly from surgical specimen in the case of upfront operable disease. The preferred procedures used to obtain appropriate histological samples are percutaneous core-needle biopsy or incisional surgical biopsy through mini-thoracotomy or mediastinotomy, while fine-needle aspiration biopsy (FNAB) is generally not recommended [2]. In any case a high level of expertise is required from the pathologists. For example, a systematic review of 467 cases discussed by the RYTHMIC tumor board from January 2012 to December 2016 showed a discordance rate of 39%, including a 6% rate of major discordance [11]. Recently, researchers of the Mayo Clinic (Rochester, MN, USA) used a panel of CD117, BAP1, mTAP and TdT in a series of 81 TETs including 44 thymomas and 37 thymic carcinomas and concluded that this panel could be useful to distinguish thymomas from thymic carcinomas [12].
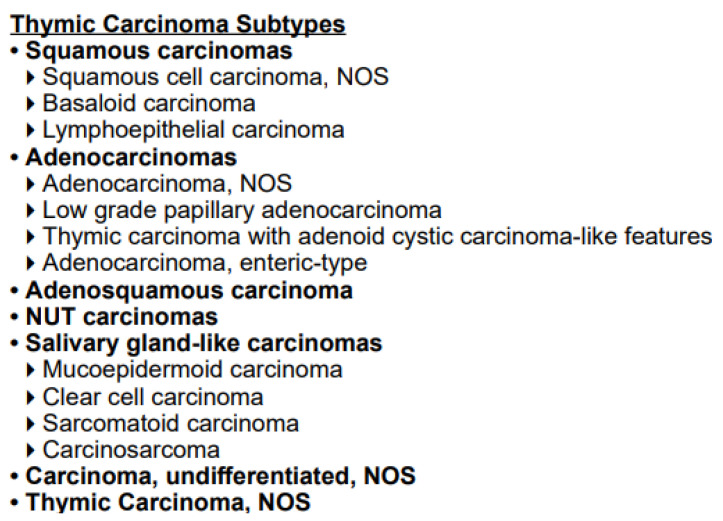
Table 2 and Figure 1 report the current available histologic classification for thymoma and thymic carcinoma according to the World Health Organization (WHO) [13]. In consonance with this classification, there are six entities represented by thymoma type A, AB, B1, B2, B3 and thymic carcinoma (type C). The subdivision into different subtypes is based on histologic characteristics including the content of non-neoplastic immature T-cells and neoplastic epithelial cells. Clinical aggressiveness increases in the following order: type A, AB, B1, B2, B3 and thymic carcinoma (type C). Thymic carcinoma includes several subtypes, the most common of which is squamous cell carcinoma.
Table 2.
Histological classification for thymoma according to the World Health Organization (WHO) [13].
| Thymoma Subtypes | Obligatory Criteria | Optional Criteria |
|---|---|---|
| Type A | Occurence of bland, spindle shaped epithelial cells; paucity or absence of immature T cells | Polygonal epithelial cells CD20+ Epithelial cells |
| Atypical type A variant | Criteria of type A, in addition comedo-type tumor necrosis; increased mitotic count, nuclear crowding | Polygonal epithelial cells CD20+ Epithelial cells |
| Type AB | Occurrence of bland, spindle shaped epithelial cells; abundance of immature T cells | Polygonal epithelial cells CD20+ Epithelial cells |
| Type B1 | Thymus-like architecture and cytology; abundance of immature T cells, areas of medullary differentiation; paucity of polygonal or dendritic epithelia cells without clustering | Hassall’s corpuscles; perivascular spaces |
| Type B2 | Increased numbers of single or clustered polygonal or dendritic epithelial cells intermingled with abundant immature T cells | Medullary islands; Hassall’s corpuscles; perivascular spaces |
| Type B3 | Sheets of polygonal slightly to moderately atypical epithelial cells; absent or rare intercellular bridges; paucity or absence of intermingled T cells | Hassall’s corpuscles; perivascular spaces |
| MNT (micronodular thymoma with lymphoid stroma) | Nodules of bland spindle or oval epithelial cells surrounded by an epithelial cell-free lymphoid strome | Lymphoid follicles; monoclonal B cells and/or plasma cells |
| Metaplastic thymoma | Biphasic tumor composed of solid areas of epithelial cells in a background of bland-looking spindle cells; absence of immature T cells | Pleomorphism of epithelial cells; actin, keratin, or EMA-positive spindle cells |
| Rare others (microscopic thymoma, sclerosing thymoma, lipofibroadenoma) |
Figure 1.
Histological classification for thymic carcinoma according to the World Health Organization (WHO) [13].
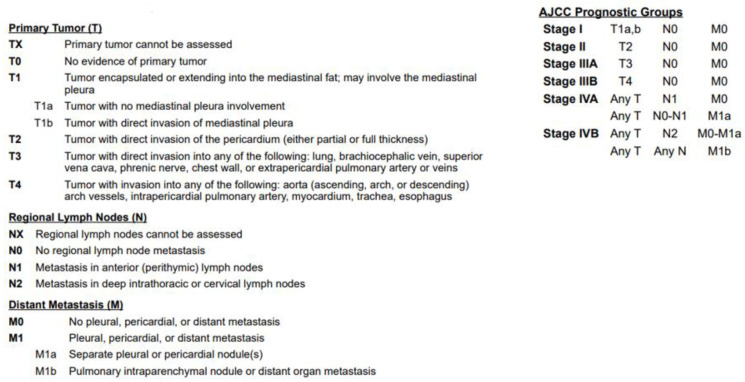
Figure 2 and Figure 3 describe the TNM 8th edition system and the Masaoka–Koga staging system, which are the two main systems usually used in the classification of TETs [14,15].
Figure 2.
TNM 8th edition staging system for TETs [14].
Figure 3.
Modified Masaoka clinical staging system for thymoma [15].
The Masaoka–Koga staging classification includes four stages that have the following characteristics: stage I: intact thymic capsule; stage II: microscopic transcapsular invasion (A)/macroscopic invasion into adjacent mediastinal fat but not through mediastinal pleura or pericardium (B); stage III: macroscopic invasion into adjacent organs without invasion of great vessels (A)/with invasion of great vessels (B); and stage IV: pleural or pericardial dissemination (A)/distant metastases (B) [15]. It should be remembered that Masaoka–Koga staging is a surgical pathology system and that the use of TNM staging could be preferable in the event of the presence of lymph nodal and distant metastases.
3. Treatment
First of all, it is strongly recommended that the management of patients with TETs should be discussed by a multidisciplinary tumor board (MTB) involving not only medical oncologists, radiation oncologists and thoracic surgeons but also radiologists, neurologists, immunologists and pathologists. According to the European Society for Medical Oncology (ESMO) guidelines, the treatment algorithm for resectable thymic tumor (Masaoka–Koga stage I–III, TNM stage I–IIIA) includes upfront surgery followed by further treatments (radiotherapy or chemotherapy) depending on radicality (R1 vs. RO), histotype and stage [2]. Complete thymectomy represents the preferred surgical approach; however, if the tumor is widely invasive (stage III–IVA), en bloc resection of all involved structures should be carried out. Video-assisted thoracoscopic surgery (VATS) is an option for stage I–II tumors, while is not recommended for stage III tumors [2].
Chao et al. compared the outcomes of 140 patients with stage I–II thymoma who had undergone VATS or median sternotomy (MST) [16]. This study showed no statistically significant differences in five-year survival between the two study groups; however, VATS was associated with better perioperative outcomes. Lymphadenectomy N1 and N2 is recommended in the case of thymic carcinoma, considering the high rate of lymph node metastases related to this histology. Recently, Pastorino et al. reported the experience of an Italian referral center on a series of 644 patients with rare thoracic cancers, including 212 thymoma patients, undergoing a surgical procedure [17]. The surgical procedure complexity was classified as high, intermediate or low according to the extent of surgical resection and kind of associated reconstruction. In particular, infiltration of mediastinal great vessels (superior vena cava and innominate veins), common in advanced thymic neoplasms, increases the technical complexity to achieve complete resection at the vascular site and requires an effective reconstructive strategy. In this retrospective study, overall survival (OS) in patients with thymoma was 96.2% at 1 year, 84.8% at 5 years and 64.6% at 10 years.
The treatment algorithm for potentially resectable thymic tumor (Masaoka–Koga stage III–IVA) has as its first step the execution of a biopsy followed by primary chemotherapy; if the tumor becomes resectable, surgery followed by postoperative radiotherapy (45–50 Gy) is used, while if the tumor remains unresectable, definitive radiotherapy (60 Gy) or concurrent chemoradiotherapy (60 Gy, cisplatin and etoposide) is used [2]. Other available options in the case of Masaoka–Koga stage IVB include definitive concomitant chemoradiotherapy and definitive chemotherapy [2]. Regarding chemotherapy, up to six cycles of platinum–anthracycline-based regimens as CAP (cyclophosphamide, doxorubicin, cisplatin), a combination of carboplatin and paclitaxel, and cisplatin and etoposide represent the most effective options. A pooled analysis including 15 studies (10 prospective and 5 retrospective) indicates both that platinum with anthracycline-based chemotherapy is superior to platinum with non-anthracycline-based chemotherapy in terms of overall response rate (ORR 69.4% vs. 37.8%) in advanced thymoma and that cisplatin-based chemotherapy is superior to carboplatin-based chemotherapy (ORR 53.6% vs. 32.8%) in advanced thymic carcinoma [18].
Chinese researchers published a retrospective comparison of first-line platinum-based chemotherapy between a group of 36 patients with type B3 thymoma and a group of 127 patients with thymic carcinoma (including 64.6% with squamous carcinoma) [19]. Among all patients, there were not significant differences between B3 thymoma and thymic carcinoma in terms of PFS (11.3 vs. 10.1 months, p = 0.118) and OS (58.3 vs. 35.1 months, p = 0.067). PFS (11.3 vs. 11.7 months, p = 0.161) and OS (58.3 vs. 40 months, p = 0.114) did not differ between patients with B3 thymoma and those with squamous carcinoma, while OS was different between patients with B3 thymoma and those with non-squamous carcinoma (58.3 vs. 30.6 months, p = 0.031). The use of different therapy regimens did not result in any differences in terms of survival. The author concluded that both B3 thymoma and thymic carcinoma (especially squamous carcinoma) can benefit from first-line platinum-based chemotherapy.
Although there are no further recognized standard lines of chemotherapy, interesting results have been reported in the literature regarding the combination of capecitabine plus gemcitabine (CAP-GEM) and the tyrosine kinase inhibitor sunitinib, while imatinib can be considered for thymic carcinoma with c-KIT mutation [20,21,22,23]. A recent retrospective analysis from the TYME Italian collaborative group examined data from 20 patients with platinum-resistant TETs receiving continuous daily dosing (CDD) of sunitinib at a dosage of 37.5 mg [24]. The authors concluded that the CDD schedule showed similar effectiveness but a better toxicity profile as compared with intermittent dosing historical data.
4. New Therapeutic Options for the Treatment of Patients with Advanced TETs
Several advances, concerning in particular the treatment of patients with aggressive disease (thymoma B2–B3 and thymic carcinoma) in progression after at least one line of standard platinum-based chemotherapy, have been reported in the literature in the last few years.
In the Japanese phase II REMORA trial, 42 patients with advanced or metastatic thymic carcinoma that progressed after at least 1 platinum-based chemotherapy treatment received the novel multikinase inhibitor lenvatinib at a dosage of 24 mg orally once daily until disease progression or the occurrence of unacceptable toxicity [25]. The ORR, the primary endpoint of the study, was 38% with a disease control rate (DCR: complete or partial response plus stable disease) of 57% and a median duration of response of 11.6 months. The most common treatment-related adverse events (TRAEs) were hypertension, decreased platelet count, palmar-plantar erythrodysesthesia syndrome, decreased platelet count and diarrhea. Seven (16.7%) patients terminated the study due to TRAEs including intestine perforation, pneumothorax, arthralgia, left ventricular dysfunction and pneumonitis, but there were no toxic deaths. All patients had at least one-step dose reduction. The authors concluded that lenvatinib could become a standard second-line treatment option for patients with thymic carcinoma. In the Italian Resound trial, 19 patients with advanced or recurrent B2–B3 thymoma and thymic carcinoma previously treated with platinum-containing chemotherapy received the oral multikinase inhibitor regorafenib administered at a dose of 160 mg daily for 3 weeks of every 4-week cycle until disease progression or the development of unacceptable toxicity [26]. The authors reported partial response (PR) in 13 patients (68.4%) and stable disease (SD) in 2 patients (10.5%), evaluated according to the Choi criteria, which are based on a combination of the values of tumor size and tumor density obtained via CT [27], a median progression free survival (PFS) of 9.6 months and a median OS of 33.8 months. However, grade 3–4 TRAEs were observed in 10 patients (52.6%).
Other Italian researchers evaluated the activity of everolimus in 51 patients with pretreated advanced thymoma (46% B2–B3) or thymic carcinoma [28]. They reported a DCR of 88%, a median PFS of 10.1 months and a median OS of 25.7 months. However, 14 patients (28%) had a serious TRAE, including liver toxicity, neutropenia and metabolic disorders, and 3 patients (6%) died due to pneumonitis.
Tsukita et al. evaluated S-1 (tegafur) in 40 previously treated patients with advanced thymoma and thymic carcinoma [29]. In this study the ORR was 17.5% (thymoma 10%; thymic carcinoma 25%), the median PFS was 7.0 months (thymoma 11.3 months; thymic carcinoma 5.4 months), and the median OS was 40.3 months (thymoma 58.5 months; thymic carcinoma 22.7 months). The main reported grade (G) 3–4 toxicities were anorexia (10%), neutropenia (7.5%) and pneumonitis (5%).
Another similar Japanese study with S-1 (tegafur) administered to 26 previously treated patients with thymic carcinoma showed an ORR of 30.8%, a DCR of 80.8%, a median PFS of 4.3 months and a median OS of 27.4 months [30]. Rajan et al. evaluated the anti-programmed cell death ligand 1 (PD-L1) antibody avelumab, which is currently approved for the treatment of urothelial carcinoma and Merkel cell carcinoma, in a phase I trial including seven patients with advanced thymoma and one patient with thymic carcinoma treated with at least one prior standard therapy [31]. In this study two out seven patients with thymoma (29%) had PR, while three out eight patients (including one with thymic carcinoma) had SD (37.5%). The authors reported G ≥ 3 immune-related adverse events (irAEs) in five out of eight patients (62%). Cho et al. conducted a phase II study with the anti-programmed cell death 1 (PD-1) antibody pembrolizumab in 33 pre-treated patients with TETs (26 thymic carcinoma, 7 thymoma) and with no autoimmune disease to evaluate its safety and efficacy [32]. They reported in patients with thymoma 2 (28.6%) PR results and 5 (71.6%) SD results, while in patients with thymic carcinoma they reported 5 (19.2%) PR results and 14 (53.8%) SD results; the median PFS (mPFS) was 6.1 months for both thymoma and thymic carcinoma, while the median OS (mOS) was 14.5 months for thymic carcinoma and was not reached for thymoma. Regarding toxicity, 5/7 (71.4%) patients with thymoma and 4/26 (15.4%) with thymic carcinoma reported G ≥ 3 irAEs, including myocarditis, hepatitis, myasthenia gravis, colitis, thyroiditis and glomerulonephritis. Giaccone et al. administered pembrolizumab to 40 patients with recurrent thymic carcinoma who had progressed after at least one line of chemotherapy [33]. In this phase II study, the ORR was 22.5%, and the DCR was 75%. In a post hoc analysis, the PFS and OS were longer in patients with high PD-L1 expression than in those with low or no PD-L1 expression. However, although only patients with thymic carcinoma and without history of autoimmune disease were enrolled, six patients (15%) developed serious irAEs. In a small Japanese phase II trial named the PRIMER study conducted in the same patient population, the anti-PD-1 nivolumab did not achieve the same efficacy previously observed with pembrolizumab [34]. In fact, the only result was stable disease (SD) observed in 11 out of 15 patients that, however, lasted ≥ 24 weeks in 5 out 11 patients. In the same study 2 out of 15 patients experienced serious irAEs (grade II adrenal insufficiency and grade III aspartate aminotransferase increase).
A Chinese retrospective study that compared ICIs with chemo-immunotherapy (CT+ICIs) in 77 patients affected by thymic carcinoma (previous chemotherapy: 50 patients (64.9%), PD-L1 status unknown: 62 patients (80.5%)), showed better results for the combination CT+ICIs in terms of PFS (mPFS 12.7 vs. 2.1) [35]. No differences were observed in terms of OS (mOS 35.4 vs. 26.7 months); however, the mOS values in patients receiving ICIs or CT+ICIs as the second or further line of treatment were 26.7 and 45.4 months, respectively. The authors observed a high incidence (15.6%) of G3–4 irAEs, as we have seen in other studies conducted in this clinical setting with immunotherapy.
In the CAVEATT study 32 pre-treated patients (27 thymic carcinoma, 3 thymoma B3, 2 mixed-type thymic carcinoma + thymoma B3) with at least 1 line of platinum-based chemotherapy received a combination of the PD-L1 inhibitor avelumab plus the antiangiogenic agent axitinib [36]. The study showed the following results: an ORR of 34%, a DCR of 91% and an mOS of 26.6 months, with a 24-month OS of 52.2%. The most common G3–4 TRAE was hypertension (19%), while 4/32 patients (12%) developed serious irAEs including interstitial pneumonitis and polymyositis. In a small Chinese phase II trial, 25 pre-treated patients (13 with ≥ 2 prior therapy lines), 15 (60%) with thymic carcinoma and 10 (40%) with thymoma, were treated with the new oral antiangiogenic agent apatinib [37]. In this study the ORR was 40% and the DCR 84%, while the median PFS and the median OS were 9 and 24 months, respectively. With respect to toxicity, no G4 or 5 TRAEs were recorded, and, as expected, the most common G3 toxicity was hypertension (32%).
A Korean phase II study evaluated the role of the cyclin-dependent kinase (CDK) 4/6 inhibitor palbociclib in 48 patients with TETs (24 thymoma, 23 thymic carcinoma) who failed at least 1 line of chemotherapy [38]. The study showed the following results: an ORR of 12.5%, an mPFS of 11 months and an mOS of 26.4 months. The most common TRAEs were neutropenia, anemia and thrombocytopenia. The authors concluded that palbociclib might represent a salvage treatment for patients with TETs.
5. Immune Checkpoint Inhibitors in the Management of TETs: Pros and Cons
Immunotherapy with ICIs may be a potential option for the treatment of advanced refractory TETs, considering the high frequency of PD-L1 expression particularly in type B3 thymoma and thymic carcinoma and the lack of recognized effective therapies other than platinum-based chemotherapy [39].
In 2016 Yang et al. reported a case of metastatic thymic carcinoma which responded to anti-PD-1 therapy with pembrolizumab dramatically [40]. Subsequently, as previously shown, anti-PD-1 and anti-PD-L1 antibody treatment has been evaluated in several clinical trials in pre-treated patients [31,32,33,34,35,36]. Overall, ICIs with anti-PD-1 or anti-PD-L1 antibodies administered after the failure of standard chemotherapy are effective, with a clinical response in approximately 20% of cases; however, the treatment is associated with an enhanced risk of severe irAES, especially in the case of thymoma (up to 70% of G ≥ 3 irAEs), despite the exclusion in these trials of patients with active autoimmune disease.
Reports of further cases of severe irAEs related to therapy with ICIs have been published recently. Liu et al. described 2 cases of severe immune myocarditis that occurred in patients with type B2 and B3 thymoma treated with gemcitabine, carboplatin and sintilimab (patient 1) or docetaxel, cisplatin and tislelizumab (patient 2) [41]. Patient 1 died within a few days. Mullenix et al. described three cases of ICIs inducing polymyalgia rheumatica (PMR)-like illness in patients with TETs enrolled in one of two clinical trials with avelumab or pembrolizumab (NCT 02146170, NCT 03076554) [42]. In contrast to myositis, which is characterized by elevated muscle enzymes and muscle weakness, PMR is characterized by morning stiffness and muscle pain. In addition, these patients should be monitored also for symptoms of giant cell arteritis including headache, double vision, flu-like symptoms and pain over the temples. Management of ICI-induced PMR-like illness required the use of corticosteroids and, in the case of sub-optimal response to steroids, anti-rheumatic drugs or anti-cytokine therapies such as tocilizumab. Jing et al. reported four cases of fatal toxicity caused by the anti-PD-1 inhibitors pembrolizumab and sintilimab regarding four patients with metastatic TETs who were ineligible for first-line platinum-based chemotherapy [43].
The combined use of anti-angiogenic therapy and ICIs could have synergistic activity, as reported by Conforti et al. in the CAVEATT study [36]. In fact, the authors reported interesting positive outcomes (an ORR of 34%, a DCR of 91%, a PFS of 7.5 months and a 24-month OS of 52.2%) with no drug-related deaths, although 4/32 patients (12%) developed serious irAEs.
The increased risk for irAEs in thymoma, as suggested by Ohm et al., could be explained by their immune microenvironment, including immature T cells that may provide the base for autoimmune reactions and therefore irAEs [44]. Thymic carcinoma and type B3 thymoma, which instead are characterized by an infiltration of mature T lymphocytes, consequently have lower risks of irAEs during ICI therapy and for this reason should be the two entities of choice regarding the use of these agents. He et al. analyzed the genomic profile of 10 patient samples (5 non-responders versus 5 responders) enrolled in their phase II study by using whole-transcriptome sequencing and whole-exome sequencing with the aim of identifying potential predictors of response to immunotherapy [45]. They found that alterations in genes that correlated with PD-L1 expression (BAP1 and CYLD) could be predictors for resistance response to immunotherapy. Recently, the NCCN guidelines added pembrolizumab as a possible therapeutic option for the treatment of refractory thymic carcinoma in view of its promising antitumor activity [46].
Table 3, which summarizes the main ongoing clinical trials in patients with advanced TETs, shows how immunotherapy administered alone or in combination with other agents is currently under study in several trials including patients with advanced B3 thymoma and thymic carcinoma which relapsed after at least one line of platinum-based chemotherapy. Interestingly, an ongoing Korean phase II study (NCT 03858582) will evaluate the efficacy and safety of neoadjuvant treatment with pembrolizumab at a dose of 200 mg plus chemotherapy (docetaxel 75 mg/m2 + cisplatin 75 mg/m2) for 3 cycles every 3 weeks. Patients with R0 resection will receive pembrolizumab at a dose of 200 mg for 32 cycles, while patients who had R1 or R2 resection will receive radiation therapy and pembrolizumab at a dose of 200 mg for 32 cycles.
Table 3.
Ongoing clinical trials in patients with TETs [47].
| Study Title | ClinicalTrials.gov Identifier | Patient Population |
Phase | Drug | Primary Endpoint |
Country |
|---|---|---|---|---|---|---|
| Nivolumab in patients with type B3 T and TC (NIVOTHYM) | NCT03134118 | Advanced B3 T and TC relapsed after at least one line of P-CHT | II | Nivolumab | PFS | Several European states |
| A pilot study to investigate the safety and clinical activity of avelumab in T and TC after progression on platinum-based chemotherapy | NCT03076554 | Advanced T and TC relapsed after at least one line of P-CHT | II | Avelumab | Safety ORR |
United States |
| Bintrafusp alfa (M7824) in subjects with T and TC | NCT04417660 | Advanced T and TC relapsed after at least one line of P-CHT | II | Bintrafusp alfa (M7824) | ORR | United States |
| Trial of sunitinib in patients with type B3 T or TC in second and further lines (Style Trial) | NCT03449173 | Advanced B3 T and TC relapsed after at least one line of P-CHT | II | Sunitinib | ORR | Italy |
| PT-112 in subjects with T and TC | NCT05104736 | Advanced T and TC relapsed after at least one line of P-CHT | II | PT-112 | ORR | United States |
| A study of KC1036 in patients with advanced TC | NCT05683886 | Advanced recurrent, unresectable and/or metastatic T | II | KC1036 | ORR | China |
| Pembrolizumab in treating participants with unresectable T or TC | NCT03295227 | Unresectable T or TC | I | Pembrolizumab | Safety | United States |
| Combination of pembrolizumab and lenvatinib in pre-treated TC patients (PECATI) | NCT04710628 | Advanced B3 T and TC relapsed after at least one line of P-CHT | II | Pembrolizumab Lenvatinib |
PFS | Several European states |
| A study of KN046 in patients with TC who failed ICIs | NCT04925947 | Advanced TC relapsed after P-CHT and at least one line of ICIs | II | KN046 | ORR | United States |
| KN046 in subjects with TC | NCT04469725 | Advanced TC relapsed after at least one line of P-CHT | II | KN046 | ORR | China |
| Pembrolizumab and sunitinib malate in treating participants with refractory metastatic or unresectable TC | NCT03463460 | Advanced TC relapsed after at least one line of P-CHT | II | Pembrolizumab Sunitinib |
ORR | United States |
| Carboplatin and paclitaxel with or without ramucirumab in treating patients with locally advanced, recurrent or metastatic TC | NCT03694002 | Advanced TC with no anti-cancer therapy for locally advanced or metastatic disease | II | Carboplatin Paclitaxel Ramucirumab | PFS | United States |
| Ramucirumab and carbo-paclitaxel for untreated thymic carcinoma/B3 thymoma with carcinoma RELEVENT Trial |
NCT03921671 | Chemotherapy-naïve patients with thymic carcinoma or B3 thymoma with areas of carcinoma | II | Carboplatin Paclitaxel Ramucirumab | ORR | Italy |
| A Phase II, neo-adjuvant pembrolizumab, docetaxel, cisplatin therapy followed by surgery and pembrolizumab consolidation therapy in locally advanced thymic epithelial tumor (TET) | NCT03858582 | Locally advanced thymic epithelial tumor (TET) | II | Pembrolizumab Docetaxel Cisplatin |
Major pathologic response rate | Korea |
Legend: TETs: thymic epithelial tumors; T: thymoma; TC: thymic carcinoma; P-CHT: platinum-based chemotherapy; ICIs: immune checkpoint inhibitors; PFS: progression-free survival; ORR: overall response rate.
6. Conclusions
TETs are rare and heterogeneous tumors. Their diagnosis is often incidental during a diagnostic work-up for MG because they, especially thymomas, are often associated with paraneoplastic Ads due to alterations in self-tolerance and the expression of novel antigens. The management of TETS requires a multidisciplinary approach in referral centers, considering the risk for the physicians to make a late or incorrect diagnosis and the need for the patients to start early the appropriate therapies. The creation of virtual MTB involving experts (pathologists, oncologists, radiation oncologists and thoracic surgeons) from geographically distant institutions might be a useful tool, as it is for the management of other rare cancers. For example, in France within the RYTHMIC network, the treatment of patients with TETs is discussed at a national MTB which is organized using a web-based system. Usually, surgery is the cornerstone of the treatment of these neoplasms when they are diagnosed in early stages, while platinum-based chemotherapy is the treatment of choice in case of metastatic disease. However, patients with metastatic TETs have limited treatment options beyond platinum-based chemotherapy due to the poor effectiveness showed by several other agents administered in subsequent lines of therapy, and for this reason new therapies have been explored in this clinical setting such as the antiangiogenic multikinase inhibitors lenvatinib and regorafenib, the mTOR inhibitor everolimus, the chemotherapeutic agent S-1 (tegafur) and ICIs. As we have previously reported, there are promising data on the use of anti-PD-1 and anti-PD-L1 antibodies including pembrolizumab, avelumab and nivolumab, but immune-related toxicity should be kept in mind. In fact, given the high incidence of autoimmunity, additional studies are needed to identify those who can benefit from ICIs without irAEs or with acceptable irAEs (<grade 3 toxicities). For these reasons, as reported in Table 3, ICIs are under evaluation in several ongoing studies including patients with thymic carcinoma and type B3 thymoma who have a lower risk of irAEs.
Author Contributions
Conceptualization, A.T.; Writing—original draft preparation, A.T., R.L., A.R.L. and M.T. Writing—review and editing, A.T. and M.T. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siesling S., van der Zwan J.M., Izarzugaza I., Jaal J., Treasure T., Foschi R., Ricardi U., Groen H., Tavilla A., Ardanaz E., et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur. J. Cancer. 2012;48:949–960. doi: 10.1016/j.ejca.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 2.Girard N., Ruffini E., Marx A., Faivre-Finn C., Peters S. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26((Suppl. S5)):v40–v55. doi: 10.1093/annonc/mdv277. [DOI] [PubMed] [Google Scholar]
- 3.Willner J., Zhou F., Moreira A.L. Diagnostic challenges in the cytology of thymic epithelial neoplasms. Cancers. 2022;14:2013. doi: 10.3390/cancers14082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden A.C., Yi E.S., Jenkins S.M., Edwards K.K., Donovan J.L., Cassivi S.D., Marks R.S., Garces Y.I., Aubry M.C. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J. Thorac. Oncol. 2015;10:691–700. doi: 10.1097/JTO.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 5.Singhal S., Hellyer J., Ouseph M.M., Wakelee H.A., Padda S.K. Autoimmune disease in patients with advanced thymic epithelial tumors. JTO Clin. Res. Rep. 2022;3:100323. doi: 10.1016/j.jtocrr.2022.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giugliano F., Zucali P.A., Galli G., Ballatore Z., Corti C., Aliaga P.T., Uliano J., Vivanet G., Curigliano G., Conforti F., et al. SARS-CoV-2 vaccine in patients with thymic epithelial tumours with and without active or pre-existing autoimmune disorders: Brief report of a TYME network safety analysis. Eur. J. Cancer. 2022;166:202–207. doi: 10.1016/j.ejca.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strange C.D., Ahuja J., Shroff G.S., Truong M.T., Marom E.M. Imaging evaluation of thymoma and thymic carcinoma. Front Oncol. 2022;11:810419. doi: 10.3389/fonc.2021.810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohira R., Yanagawa M., Suzuki Y., Hata A., Miyata T., Kikuchi N., Yoshida Y., Yamagata K., Doi S., Ninomiya K., et al. CT-based radiomics analysis for differentiation between thymoma and thymic carcinoma. J. Thorac. Dis. 2022;14:1342–1352. doi: 10.21037/jtd-21-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araujo-Filho J.A.B., Mayoral M., Zheng J., Tan K.S., Gibbs P., Shepherd A.F., Rimner A., Simone C.B., 2nd, Riely G., Huang J., et al. CT radiomic features for predicting resectability and TNM staging in thymic epithelial tumors. Ann. Thorac. Surg. 2022;113:957–965. doi: 10.1016/j.athoracsur.2021.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai H., Lan B., Li S., Huang Y., Jiang G., Tian J. Prognostic CT features in patients with untreated thymic epithelial tumors. Sci. Rep. 2023;13:2910. doi: 10.1038/s41598-023-30041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina T.J., Bluthgen M.V., Chalabreysse L., de Montpréville V.T., de Muret A., Dubois R., Hofman V., Lantuejoul S., Le Naoures C., Mansuet-Lupo A., et al. Impact of expert pathologic review of thymic epithelial tumours on diagnosis and management in a real-life setting: A RYTHMIC study. Eur. J. Cancer. 2021;143:158–167. doi: 10.1016/j.ejca.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Angirekula M., Chang S.Y., Jenkins S.M., Greipp P.T., Sukov W.R., Marks R.S., Olivier K.R., Cassivi S.D., Roden A.C. CD117, BAP1, MTAP, and TdT is a useful immunohistochemical panel to distinguish thymoma from thymic carcinoma. Cancers. 2022;14:2299. doi: 10.3390/cancers14092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . WHO Classification of Tumours Online, Thoracic Tumours-Tumours of the Thymus. 5th ed. World Heath Organization; Geneva, Switzerland: 2021. [(accessed on 7 January 2023)]. Available online: https://tumourclassification.iarc.who.int/chapters/35. [Google Scholar]
- 14.Brierley J.D., Gospodarowicz M.K., Wittekind C. TNM Classification of Malignant Tumours. 8th ed. Wiley Blackwell; Oxford UK: 2017. [Google Scholar]
- 15.Detterbeck F.C., Nicholson A.G., Kondo K., Van Schil P., Moran C. The Masaoka-Koga stage classification for thymic malignancies: Clarification and definition of terms. J. Thorac. Oncol. 2011;6((Suppl. S3)):S1710–S1716. doi: 10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 16.Chao Y.K., Liu Y.H., Hsieh M.J., Wu Y.C., Chen T.P., Lu M.S., Lu H.I., Liu H.P. Long-term outcomes after thoracoscopic resection of stage I and II thymoma: A propensity-matched study. Ann. Surg. Oncol. 2015;22:1371–1376. doi: 10.1245/s10434-014-4068-9. [DOI] [PubMed] [Google Scholar]
- 17.Pastorino U., Leuzzi G., Sabia F., Girotti P., Duranti L., Radaelli S., Fiore M., Stacchiotti S., Patrizia G., Salvioni R., et al. Long term outcome of complex surgical resection and reconstruction for rare thoracic cancers. Tumori J. 2023 16:3008916231154763. doi: 10.1177/03008916231154763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuma Y., Saito M., Hosomi Y., Sakuyama T., Okamura T. Key components of chemotherapy for thymic malignancies: A systematic review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based chemotherapy. J. Cancer. Res. Clin. Oncol. 2015;141:323–331. doi: 10.1007/s00432-014-1800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao Y., Si J., Jin J., Wei J., Xiang J., Xu C., Song Z. Comparison of efficacy and safety of platinum-based chemotherapy as first-line therapy between B3 thymoma and thymic carcinoma. Curr. Oncol. 2022;29:9452–9460. doi: 10.3390/curroncol29120743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmieri G., Buonerba C., Ottaviano M., Federico P., Calabrese F., Von Arx C., De Maio A.P., Marino M., Lalle M., Montella L., et al. Capecitabine plus gemcitabine in thymic epithelial tumors: Final analysis of a Phase II trial. Future Oncol. 2014;10:2141–2147. doi: 10.2217/fon.14.144. [DOI] [PubMed] [Google Scholar]
- 21.Thomas A., Rajan A., Berman A., Tomita Y., Brzezniak C., Lee M.J., Lee S., Ling A., Spittler A.J., Carter C.A., et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: An open-label phase 2 trial. Lancet Oncol. 2015;16:177–186. doi: 10.1016/S1470-2045(14)71181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remon J., Girard N., Mazieres J., Dansin E., Pichon E., Greillier L., Dubos C., Lindsay C.R., Besse B. Sunitinib in patients with advanced thymic malignancies: Cohort from the French RYTHMIC network. Lung Cancer. 2016;97:99–104. doi: 10.1016/j.lungcan.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Rossi V., Donini M., Sergio P., Passalacqua R., Rossi G., Buti S. When a thymic carcinoma “becomes” a GIST. Lung Cancer. 2013;80:106–108. doi: 10.1016/j.lungcan.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Antonarelli G., Corti C., Zucali P.A., Perrino M., Manglaviti S., Lo Russo G., Varano G.M., Salvini P., Curigliano G., Catania C., et al. Continuous sunitinib schedule in advanced platinum refractory thymic epithelial neoplasms: A retrospective analysis from the ThYmic MalignanciEs (TYME) Italian collaborative group. Eur. J. Cancer. 2022;174:31–36. doi: 10.1016/j.ejca.2022.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Sato J., Satouchi M., Itoh S., Okuma Y., Niho S., Mizugaki H., Murakami H., Fujisaka Y., Kozuki T., Nakamura K., et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): A multicentre, phase 2 trial. Lancet Oncol. 2020;21:843–850. doi: 10.1016/S1470-2045(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 26.Perrino M., De Pas T., Bozzarelli S., Giordano L., De Vincenzo F., Conforti F., Digiacomo N., Cordua N., D'Antonio F., Borea F., et al. Resound Trial: A phase 2 study of regorafenib in patients with thymoma (type B2-B3) and thymic carcinoma previously treated with chemotherapy. Cancer. 2022;128:719–726. doi: 10.1002/cncr.33990. [DOI] [PubMed] [Google Scholar]
- 27.Choi H., Charnsangavej C., Faria S.C., Macapinlac H.A., Burgess M.A., Patel S.R., Chen L.L., Podoloff D.A., Benjamin R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 28.Zucali P.A., De Pas T., Palmieri G., Favaretto A., Chella A., Tiseo M., Caruso M., Simonelli M., Perrino M., De Vincenzo F., et al. Phase II study of everolimus in patients with thymoma and thymic carcinoma previously treated with cisplatin-based chemotherapy. J. Clin. Oncol. 2018;36:342–349. doi: 10.1200/JCO.2017.74.4078. [DOI] [PubMed] [Google Scholar]
- 29.Tsukita Y., Inoue A., Sugawara S., Kuyama S., Nakagawa T., Harada D., Tanaka H., Watanabe K., Mori Y., Harada T., et al. Phase II study of S-1 in patients with previously-treated invasive thymoma and thymic carcinoma: North Japan lung cancer study group trial 1203. Lung Cancer. 2020;139:89–93. doi: 10.1016/j.lungcan.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Okuma Y., Goto Y., Ohyanagi F., Sunami K., Nakahara Y., Kitazono S., Kudo K., Tambo Y., Kanda S., Yanagitani N., et al. Phase II trial of S-1 treatment as palliative-intent chemotherapy for previously treated advanced thymic carcinoma. Cancer Med. 2020;9:7418–7427. doi: 10.1002/cam4.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajan A., Heery C.R., Thomas A., Mammen A.L., Perry S., O'Sullivan Coyne G., Guha U., Berman A., Szabo E., Madan R.A., et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. J. Immunother. Cancer. 2019;7:269. doi: 10.1186/s40425-019-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho J., Kim H.S., Ku B.M., Choi Y.L., Cristescu R., Han J., Sun J.M., Lee S.H., Ahn J.S., Park K., et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: An open-label phase II trial. J. Clin. Oncol. 2019;37:2162–2170. doi: 10.1200/JCO.2017.77.3184. [DOI] [PubMed] [Google Scholar]
- 33.Giaccone G., Kim C., Thompson J., McGuire C., Kallakury B., Chahine J.J., Manning M., Mogg R., Blumenschein W.M., Tan M.T., et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19:347–355. doi: 10.1016/S1470-2045(18)30062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsuya Y., Horinouchi H., Seto T., Umemura S., Hosomi Y., Satouchi M., Nishio M., Kozuki T., Hida T., Sukigara T., et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur. J. Cancer. 2019;113:78–86. doi: 10.1016/j.ejca.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang W., Lin G., Hao Y., Guan Y., Zhang Y., Xu C., Wang Q., Wang D., Jiang Z., Cai J., et al. Treatment outcomes and prognosis of immune checkpoint inhibitors therapy in patients with advanced thymic carcinoma: A multicentre retrospective study. Eur. J. Cancer. 2022;174:21–30. doi: 10.1016/j.ejca.2022.06.059. [DOI] [PubMed] [Google Scholar]
- 36.Conforti F., Zucali P.A., Pala L., Catania C., Bagnardi V., Sala I., Della Vigna P., Perrino M., Zagami P., Corti C., et al. Avelumab plus axitinib in unresectable or metastatic type B3 thymomas and thymic carcinomas (CAVEATT): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:1287–1296. doi: 10.1016/S1470-2045(22)00542-3. [DOI] [PubMed] [Google Scholar]
- 37.Song Z., Lou G., Wang Y., Yang Z., Wang W., Ji Y., Chen S., Xu C., Hu X., Zhang Y. Apatinib in patients with recurrent or metastatic thymic epithelial tumor: A single-arm, multicenter, open-label, phase II trial. BMC Med. 2022;20:154. doi: 10.1186/s12916-022-02361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung H.A., Kim M., Kim H.S., Kim J.H., Choi Y.H., Cho J., Park J.H., Park K.U., Ku B.M., Park S., et al. A Phase 2 study of palbociclib for recurrent or refractory advanced thymic epithelial tumors (KCSG LU17-21) J. Thorac. Oncol. 2023;18:223–231. doi: 10.1016/j.jtho.2022.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Padda S.K., Riess J.W., Schwartz E.J., Tian L., Kohrt H.E., Neal J.W., West R.B., Wakelee H.A. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J. Thorac. Oncol. 2015;10:500–508. doi: 10.1097/JTO.0000000000000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y., Ding L., Wang P. Dramatic response to anti-PD-1 therapy in a patient of squamous cell carcinoma of thymus with multiple lung metastases. J. Thorac. Dis. 2016;8:E535–E537. doi: 10.21037/jtd.2016.06.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S., Ma G., Wang H., Yu G., Chen J., Song W. Severe cardiotoxicity in 2 patients with thymoma receiving immune checkpoint inhibitor therapy: A case report. Medicine. 2022;101:e31873. doi: 10.1097/MD.0000000000031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullenix C., Ballman M., Chen H., Swift S., McAdams M.J., Tsai Y.T., Donahue R.N., Poretta T., Gupta S., Loehrer P.J., et al. Joint-predominant rheumatic complications of immune checkpoint inhibitor therapy in patients with thymic epithelial tumors. Oncologist. 2022;27:e353–e356. doi: 10.1093/oncolo/oyac026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing X., Zhu H., Li Y., Jia W., Zhai X., Li J., Yu J. Fatal toxicity induced by anti-PD-1 immune checkpoint inhibitor in thymic epithelial tumor. Immunotherapy. 2022;14:1097–1107. doi: 10.2217/imt-2021-0215. [DOI] [PubMed] [Google Scholar]
- 44.Ohm B., Jungraithmayr W. Balancing the risk of adverse events against the efficacy of immunotherapy in advanced thymic epithelial tumors. Cancers. 2022;15:289. doi: 10.3390/cancers15010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Y., Ramesh A., Gusev Y., Bhuvaneshwar K., Giaccone G. Molecular predictors of response to pembrolizumab in thymic carcinoma. Cell Rep. Med. 2021;2:100392. doi: 10.1016/j.xcrm.2021.100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NCCN Clinical Practice Guidelines in Oncology, Thymomas and Thymic Carcinomas Version 1.2023. [(accessed on 20 February 2023)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf.
- 47.Clinical Trials Home Page. [(accessed on 2 April 2023)]; Available online: https://clinicaltrials.gov.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.