Abstract
The lipid composition of membranes is a key determinant for cold tolerance, and enzymes that modify membrane structure seem to be important for low-temperature acclimation. We have characterized ALA1 (for aminophospholipid ATPase1), a novel P-type ATPase in Arabidopsis that belongs to the gene family ALA1 to ALA11. The deduced amino acid sequence of ALA1 is homologous with those of yeast DRS2 and bovine ATPase II, both of which are putative aminophospholipid translocases. ALA1 complements the deficiency in phosphatidylserine internalization into intact cells that is exhibited by the drs2 yeast mutant, and expression of ALA1 results in increased translocation of aminophospholipids in reconstituted yeast membrane vesicles. These lines of evidence suggest that ALA1 is involved in generating membrane lipid asymmetry and probably encodes an aminophospholipid translocase. ALA1 complements the cold sensitivity of the drs2 yeast mutant. Downregulation of ALA1 in Arabidopsis results in cold-affected plants that are much smaller than those of the wild type. These data suggest a link between regulation of transmembrane bilayer lipid asymmetry and the adaptation of plants to cold.
INTRODUCTION
Low temperature is one of the major environmental factors limiting plant growth. Cold-sensitive plants are injured and stunted in growth when temperatures are well below those for normal growth but still above the freezing point (reviewed in Nishida and Murata, 1996; Pearce, 1999; Thomashow, 1999). Many tropical or subtropical plant species, including a large number of crops such as tomato, rice, cotton, cucumber, and maize, are susceptible to chilling injury, and substantial losses in productivity result from the inability of such crops to withstand cold stress. Normal functioning of integral membrane proteins such as transporters and receptor proteins depends on the fluidity of the membrane, which is strongly influenced at a given temperature by its lipid composition (Squier et al., 1988; Gasser et al., 1990; reviewed in Hazel, 1995).
A major factor determining the fluidity of lipid membranes is the degree of unsaturation of membrane lipids. Thus, membranes with unsaturated acyl chains in phospholipids remain fluid at lower temperatures than do membranes with saturated lipids (Kates et al., 1984; Cevc, 1991; Cossins, 1994). One of the best-documented responses of plants to chilling stress is the increase in polyunsaturated acyl chains of membrane phospholipids, which allows membrane fluidity to be maintained (Hugly and Somerville, 1992; Nishida and Murata, 1996). This response indicates that after transfer to the cold, plants may increase the amount of unsaturated lipids by upregulating the activity of desaturase enzymes. Accordingly, expression in tobacco of a desaturase gene from a cyanobacterium results in increasing membrane lipid unsaturation in most membrane lipids concomitant with an increase in chilling tolerance (Ishizaki-Nishizawa et al., 1996).
The proportion of unsaturated fatty acids in the lipid acyl chains is particularly high in chloroplast membranes (Harwood, 1988). Several genetic loci (FAD2, FAD3, FAD4, FAD5, FAD6, FAD7, and FAD8) encoding enzymes involved in fatty acid desaturation of lipids in chloroplast or microsomal membranes have been identified (Browse and Somerville, 1991; Somerville and Browse, 1991). Although these mutants do not exhibit a visible phenotype when grown at 22°C, some of them, such as fad5 (defective in chloroplast Δ12 desaturase) and fad2 (defective in microsomal Δ12 desaturase), show diminished growth and partial chlorosis when grown at 5°C (Hugly and Somerville, 1992; Miquel et al., 1993). In addition, tobacco plants overexpressing the Arabidopsis FAD7 gene (coding for the chloroplast ω-3 desaturase) have enhanced cold tolerance (Kodama et al., 1994). Extensive unsaturation of phosphatidylglycerol, an abundant phospholipid in thylakoid membranes (Harwood, 1988), correlates with improved chilling tolerance of tobacco (Murata et al., 1982; Murata, 1983). Furthermore, overexpression of glycerol-3-phosphate acyltransferases in tobacco, which leads to decreased concentrations of saturated species of phosphatidylglycerol, results in plants that are more tolerant to chilling (Murata et al., 1992; Wolter et al., 1992; Moon et al., 1995).
For animal, bacterial, and viral systems, various membranes are known to have a characteristic asymmetry of fluidity (e.g., Cogan and Schachter, 1981; Seigneuret et al., 1984; Foley et al., 1986; Dudeja et al., 1991; Kitagawa et al., 1991, 1998; Julien et al., 1993; Müller et al., 1994; Schroeder et al., 1995; Igbavboa et al., 1996). Variation of lipid fluidity between individual hemileaflets may therefore be a general feature of biological membranes. Asymmetry in lipid fluidity of the two leaflets appears to be associated with an asymmetric phospholipid headgroup composition (reviewed in Hazel, 1995). For example, an increased content of phosphatidylcholine (PC) in the outer leaflet and of anionic phospholipids in the inner leaflet has often been associated with distinct lipid fluidity of individual leaflets of biological membranes. Interestingly, in several poikilothermic organisms, asymmetric alterations in membrane phospholipid headgroup composition are associated with low-temperature adaptation (Hazel, 1995; Miranda and Hazel, 1996). In plants, asymmetric transbilayer distribution of phospholipids has been documented (Cheesebrough and Moore, 1980; Rawyler and Siegenthaler, 1981; Dorne et al., 1985; Tavernier and Pugin, 1995; O'Brien et al., 1997), but the physiological significance of the phenomenon is not known.
Although a thorough understanding of the mechanisms generating membrane lipid asymmetry has not emerged, some of the enzymes that may play a major role in these processes have been identified in nonplant systems (Dolis et al., 1997). Yeast protein DRS2 (Tang et al., 1996) and bovine ATPase II (Tang et al., 1996; Ding et al., 2000) are putative aminophospholipid translocases and may play a role in lipid flipping. Both are members of a distinct subgroup of P-type ATPases, type IV (P4) ATPases (Axelsen and Palmgren, 1998), also referred to as third-type ATPases (Halleck et al., 1998). The drs2 mutant displays less capacity for internalization of aminophospholipids into intact cells (Tang et al., 1996), impairment in the assembly of the 40S ribosomal subunit (Ripmaster et al., 1993), hypersensitivity to Zn2+, Co2+, Mn2+, and Ni2+ but not to Ca2+ or Mg2+ (Siegmund et al., 1998), and inability to grow at temperatures colder than 23°C (Ripmaster et al., 1993). The latter phenotype suggests a role for DRS2 in cold tolerance of yeast.
Here, we have identified a gene family of P4 ATPases in Arabidopsis (ALA1 to ALA11; for aminophospholipid ATPase1 to 11). We provide genetic and biochemical evidence that ALA1 encodes an aminophospholipid translocase and that this gene is involved in cold tolerance of Arabidopsis.
RESULTS
Cloning an Arabidopsis P4-Type ATPase
Using database searches, we identified the presence of at least 11 P4 ATPases in Arabidopsis (GenBank accession numbers AB005245, AB005239, AB007258, AC022492, AC010926, AC005287, AB019229, AP000371, AC008075, AP001313, and AC007357), distributed over three different chromosomes (Table 1). The derived amino acid sequences all include the typical conserved domains of the P-type ATPase superfamily, including the DKTGTLT aspartyl phosphorylation motif (Axelsen and Palmgren, 1998). Moreover, they also contain variants of the SPDEx(A/S)(F/L)(V/L) and GxT(A/G)(I/V)ED(K/R)LQ motifs, which are believed to be specific to P4 ATPases (Axelsen and Palmgren, 1998; Halleck et al., 1998).
Table 1.
Overview of the ALA Gene Family in Arabidopsis
| GenBank Accession No. |
Swiss-Prot Accession No. |
Suggested Gene Name |
Chromosomal Location (Centimorgans)a |
Mol. Wt.b | Length (Amino Acids) |
pI |
|---|---|---|---|---|---|---|
| AB005245 | P98204 | ALA1 | 5.15 | 130,329 | 1158 | 5.9 |
| AB005239 | P98205 | ALA2 | 5.94 | 124,836 | 1107 | 5.9 |
| AC007258 | Q9XIE6 | ALA3 | 1.93 | 127,169 | 1123 | 7.7 |
| AC022492 | Q9LNQ4 | ALA4 | 1.23 | 138,189 | 1216 | 7.5 |
| AC010926 | Q9SGG3 | ALA5 | 1.115 | 139,341 | 1228 | 6.9 |
| AC005287 | Q9SLK6 | ALA6 | 1.83 | 140,682 | 1244 | 7.1 |
| AB019229 | Q9LVK9 | ALA7 | 3.20 | 140,928 | 1247 | 7.2 |
| AP000371 | Q9LK90 | ALA8 | 3.48 | 135,309 | 1189 | 6.2 |
| AC008075 | Q9SX33 | ALA9 | 1.104 | 136,045 | 1200 | 5.9 |
| AP001313 | Q9LI83 | ALA10 | 3.38 | 136,279 | 1202 | 5.7 |
| AC007357 | Q9SAF5 | ALA11 | 1.16 | 136,584 | 1203 | 6.5 |
The position in centimorgans is the position of the nearest known genetic marker according to the Arabidopsis Information Resource (http://www.arabidopsis.org).
Mol. Wt., calculated molecular weight.
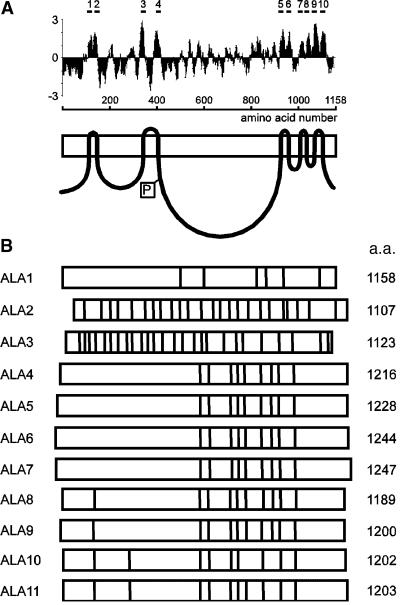
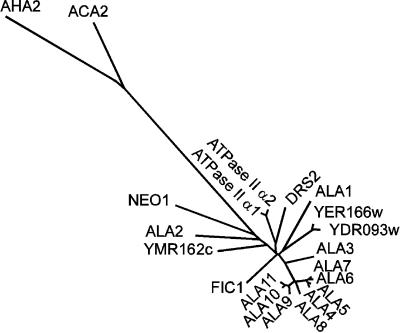
A full-length cDNA, ALA1, corresponding to a gene found on the genomic clone AB005245, was isolated (GenBank accession number AF175769) and found to encode a protein of 1158 amino acids with a predicted molecular mass of 130.3 kD. Hydrophobicity analysis of the ALA1 protein revealed a hydropathy pattern indicating 10 transmembrane helices (Figure 1A), a result very similar to those for the other predicted ALA proteins (data not shown). The exon structures of the different ALA genes are highly variable, but the close resemblance between genes ALA4 through ALA11 indicates that those pumps form a subfamily (Figure 1B). A phylogenetic tree based on a comparison of the 11 ALA1-like amino acid sequences and other selected P-type ATPases is depicted in Figure 2. ALA1, ALA2, and ALA3 are the most divergent members of the ALA family. The closest relatives to ALA1 are yeast DRS2 and bovine ATPase II, whereas ALA4 through ALA11 cluster together and form a separate group in the phylogenetic tree.
Figure 1.
Sequence Analysis of Members of the ALA Gene Family.
(A) Prediction of putative membrane-spanning domains of ALA1. Hydropathy plots were performed with a window of 17 amino acids (Kyte and Doolittle, 1982). Predicted membrane-spanning domains were confirmed by referring to the P-type ATPase database (http://biobase.dk/~axe/Patbase.html) and are marked at top as bars designated 1 to 10. At bottom is shown a simplistic model of the protein. The boxed P denotes the phosphorylation site.
(B) Distribution of introns and exons in the ALA gene family. a.a., amino acids. The positions of introns are shown by a vertical line within a box representing the coding sequence.
Figure 2.
Phylogenetic Tree of Conserved Sequence Segments of Selected P-Type ATPases.
The sequences are as follows. Type P4 ATPases: ALA1 to ALA11 (see Table 1 for accession numbers), Arabidopsis thaliana; YER166w (P32660), Saccharomyces cerevisiae; YDR093w (Q12675), S. cerevisiae; DRS2 (P39524), S. cerevisiae; ATPase II α1 (Q29449), Bos taurus; ATPase II α2 (Q9Y2Q0), Homo sapiens; FIC1 (O43520), H. sapiens; YMR162c (Q12674), S. cerevisiae; and NEO1 (P40527), S. cerevisiae. Type P2B ATPases: autoinhibited Ca2+-ATPase ACA2 (O81108), A. thaliana. Type P2A ATPases: endoplasmic reticulum–type Ca2+-ATPase (O04987), A. thaliana. Type P3A ATPases: plasma membrane H+-ATPase AHA2 (P19456), A. thaliana. The tree is based on the Fitch–Margoliash and least-squares method (PHYLIP; Felsenstein, 1989). The conserved segments used in the analysis are defined in Axelsen and Palmgren (1998).
Biochemical Properties of ALA1
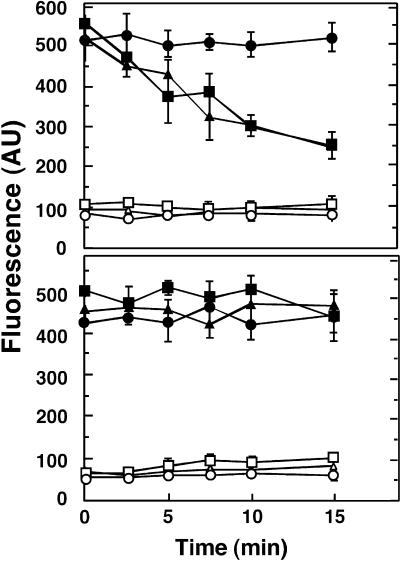
Lipid internalization assays were performed to identify a biochemical function for ALA1. The outer leaflet of the yeast plasma membrane was labeled with the fluorescent phosphatidylserine (PS) and PC derivatives 2-(6-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]-amino)hexanoyl (NBD)-PS and NBD-PC under conditions that block endocytosis, thus enabling measurements of the phospholipids being internalized by the cell (Tang et al., 1996). NBD-PS, but not NBD-PC, was rapidly transported from the yeast surface in wild-type cells, although this transport was abolished in drs2 cells (Tang et al., 1996) (Figure 3). However, as shown in Figure 3, the expression of ALA1 in drs2 cells restored internalization of NBD-PS from the outer leaflet of the yeast plasma membrane, a result indicating functional homology between the DRS2 and ALA1 proteins.
Figure 3.
Influence of ALA1 Expression on Aminophospholipid Flipping in Intact Yeast Cells.
(Top) 2-(6-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]-amino)hexanoyl (NBD)- phosphatidylserine (PS).
(Bottom) NBD-PC.
Internalization of NBD phospholipids by wild-type, drs2, and drs2-pYES2-ALA1 yeast cells was assayed. The outer leaflet of the yeast cell plasma membrane was labeled with fluorescent phospholipid analogs, and internalization was measured as a function of time. Fluorescence intensities are indicated in arbitrary units (AU). Values are means ±sd for three independent experiments, each with duplicate samples. Transport of NBD-PS (top) and NBD-PC (bottom) is shown for wild type (filled squares), drs2 (filled circles), and drs2-pYES2-ALA1 (filled triangles). Background (fatty acid–free BSA was omitted from the assay) supernatant values are as follows: wild-type (open squares), drs2 (open circles), and drs2-pYES-ALA1 (open triangles) strains.
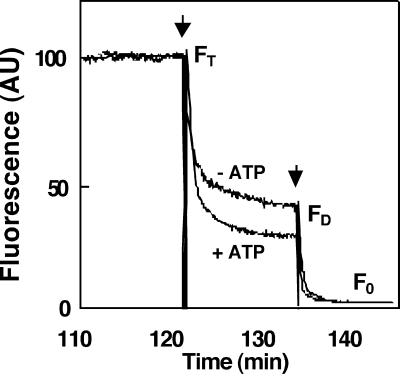
To assay the flippase activity more directly, we initially measured the transport of NBD phospholipids in reconstituted microsomes prepared from yeast cells expressing DRS2. The membrane-impermeable reagent sodium dithionite, which reduces the fluorescent NBD group of the NBD phospholipids into its nonfluorescent ABD form (McIntyre and Sleight, 1991), has been used to monitor transbilayer distribution of fluorescent lipids in vesicles (Suzuki et al., 1997). Using a similar approach, we measured the ATP-dependent changes in fluorescence of both the inner and outer leaflet of reconstituted NBD phospholipid–labeled yeast microsomes (Figure 4). Wild-type reconstituted yeast microsomal vesicles exhibited an ATP-dependent decrease in the amount of NBD-PS present in the inner leaflet, reflecting an outward-directed translocation of this phospholipid (Figure 4).
Figure 4.
Measurement of NBD-PS Translocase Activity in Reconstituted Yeast Microsomal Vesicles.
The assay was based on the reduction of the fluorescent aryl-nitro group of NBD phospholipids into a nonfluorescent aryl-amino group. Fluorescence intensity was recorded for 10 min (FT). The first arrow indicates addition of 30 μL of 1 M sodium dithionite in 1 M Tris-HCl, pH 10.0, to the reconstituted microsomes; the fluorescence was recorded after 10 min (FD). Membrane-impermeable sodium dithionite reduces the fluorescent aryl-nitro group of NBD phospholipids present in the outer leaflet of the vesicle membrane (Suzuki et al., 1997). The second arrow indicates addition of 100 μL of 30% Triton X-100 to disrupt the microsomal vesicles and allow sodium dithionite to quench fluorescence from the inner leaflet–resident NDB phospholipids; the fluorescence was recorded (F0). The fractions of NBD phospholipids present in the inner and outer leaflets were calculated from the following equations (Suzuki et al., 1997): 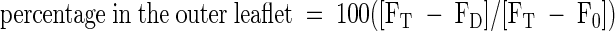 ;
; 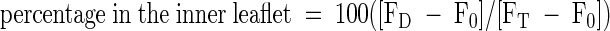 . −ATP, ATP was omitted from the assay; +ATP, ATP (3 mM) was included in the reaction mixture; AU, arbitrary units.
. −ATP, ATP was omitted from the assay; +ATP, ATP (3 mM) was included in the reaction mixture; AU, arbitrary units.
Several controls were performed to ensure that the lipid flipping in reconstituted yeast microsomes was dependent on the presence of the DRS2 protein (Table 2). NBD-PS transport was indeed markedly reduced in microsomal vesicles prepared from drs2 yeast cells. As expected, reconstitution in the absence of protein led to symmetrical labeling of the two leaflets of the membrane vesicles. No substantial lipid flipping was observed during the assay in the protein-free reconstituted system, indicating that lipid transport is protein mediated. Aminophospholipid flipping was inhibited by orthovanadate, an inhibitor of P-type ATPases. Furthermore, no lipid flipping was observed in the reconstituted system in the presence of affinity-purified histidine-tagged plasma membrane H+-ATPase (Lanfermeijer et al., 1998), indicating that lipid flipping is not an unspecific side activity of P-type ATPases in general.
Table 2.
NBD-PS Transport in Microsomal Vesicles Reconstituted in the Presence of Solubilized Membrane Proteins as Indicated
| Controls | −ATPa,b (%) | +ATPa,c (%) |
|---|---|---|
| Yeast DRS2 microsomes | 48 ± 5 | 29 ± 4 |
| Yeast drs2 microsomes | 51 ± 3 | 42 ± 6 |
| Yeast DRS2 microsomes + vanadate (200 μM) |
46 ± 4 | 45 ± 3 |
| No added protein | 49 ± 3 | 51 ± 3 |
| Plasma membrane H+-ATPase | 47 ± 3 | 46 ± 2 |
Values are given as inner leaflet NBD-PS (%) and are the means ±sd of three measurements from three independent reconstitution experiments. Protein-to-lipid ratios were 1:15.
ATP was omitted from the assay.
ATP (3 mM) was added to start the reaction.
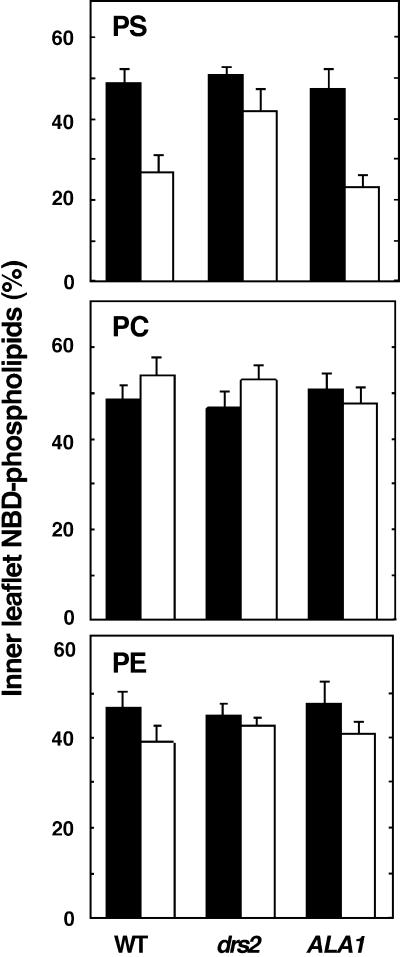
After establishing the lipid transport assay, we attempted to measure aminophospholipid translocation in vesicles containing the ALA1 protein. In microsomal vesicles from drs2 cells transformed with ALA1, the outward-directed translocation of NBD-PS was restored, indicating that PS translocation depends on ALA1 (Figure 5, top). No substantial ATP-dependent translocation of NBD-PC was observed with microsomes prepared from any of the three yeast strains used (Figure 5, middle), suggesting that ALA1 expression does not affect PC translocation.
Figure 5.
ATP-Dependent Translocation of NBD-Labeled Phospholipids by Reconstituted Microsomes Derived from Wild-Type, drs2, or drs2-pYES2-ALA1 Yeast Strains.
(Top) NBC-PS.
(Center) NBD-PC.
(Bottom) NBD-PE.
Paired bars at left show results in wild-type yeast (WT); at center, drs2 yeast; at right, drs2 yeast expressing ALA1. Solid black bars show results without ATP; open white bars, results with ATP. Error bars indicate standard errors of the mean ( ).
).
Very small but significant (Student's t test, P < 0.05) ATP-dependent outward-directed transport of NBD-phosphatidylethanolamine (NBD-PE) was detected in microsomal vesicles from wild-type yeast cells but not from microsomes prepared from drs2 cells (Figure 5, bottom). Moreover, expression of ALA1 in this strain restored ATP-dependent translocation of NBD-PE (Student's t test, P < 0.05), indicating that PE transport might also be affected by the ALA1 protein, albeit with a much lower efficiency than was PS.
ALA1 and Cold Tolerance of a Yeast drs2 Mutant
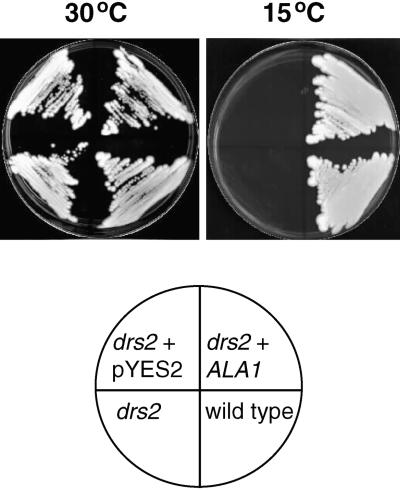
To learn more about the physiological function of ALA1, we tested the effect of expressing ALA1 in a drs2 strain grown at 15°C. The yeast drs2 mutant cannot grow below 23°C (Ripmaster et al., 1993). Figure 6 shows that ALA1 complements the cold-sensitive phenotype of the mutant, supporting the notion that ALA1 encodes a functional homolog of DRS2.
Figure 6.
Cold-Tolerant Phenotype of a drs2 Yeast Mutant Complemented by ALA1.
The drs2 null mutant (JWY2197; MATα ura3-52 drs2::TRP1 leu2-1 his3-11 lys2-801) was transformed with plasmid pYES2-ALA1 (ALA1) or with the empty pYES2 vector. The wild-type yeast strain (DS94; MATα ura3-52 trp1-1 leu2-3 his3-11 lys2-801) served as a control. Yeast was plated onto amino acid–supplemented synthetic minimal medium containing 2% galactose. Cells were grown for 3 days at 30°C or for 14 days at 15°C.
Cold Sensitivity in Transgenic Arabidopsis with Decreased ALA1
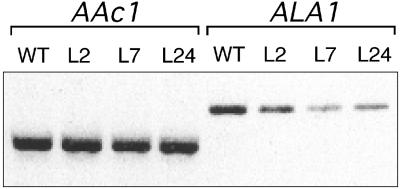
Because cold tolerance of yeast is influenced by the ALA1 protein, we investigated whether a similar function might exist for this polypeptide in Arabidopsis. Several homozygous lines of ALA1 antisense plants were generated. Among the 47 independent F4 lines produced, four (lines 2, 7, 12, and 24) were selected for further analysis. All showed that the steady state amount of ALA1 mRNA was decreased by 60 to 80% as judged by semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) (Figure 7).
Figure 7.
Decrease in Expression of ALA1 in ALA1 Antisense Arabidopsis.
ALA1-specific transcripts (ALA1) were amplified from total RNA derived from wild-type (WT) and different antisense plants (lines 2, 7, and 24 [L2, L7, and L24]) by RT-PCR. As an internal control, the actin AAc1 transcript was amplified by RT-PCR.
When grown at normal temperatures (20 and 25°C for night and day periods, respectively), antisense plants retained wild-type rosettes, stem size, and number of siliques (Figure 8A and Table 3). However, when grown at chilling temperatures (8 and 12°C for night and day, respectively), all four ALA1 antisense lines had smaller rosettes, smaller stems, and fewer siliques than did the wild-type plants (line 7 is shown in Figures 8B and 8C and Table 3). This phenotype was still true when the plants reached the end of their life cycle, demonstrating that the growth of antisense plants was impaired rather than delayed. The phenotypes of the ALA1 antisense plants strongly suggest that expression of this P4 ATPase is important for cold tolerance of Arabidopsis.
Figure 8.
Effect of Downregulation of ALA1 in Arabidopsis.
Growth of wild-type (left) and ALA1 antisense (line 7; right) plants at different temperatures.
(A) Normal temperature (20 to 25°C).
(B) Low temperature (8 to 12°C), lateral view.
(C) Low temperature (8 to 12°C), top view.
 .
.
Table 3.
Growth Phenotypes of Wild-Type and ALA1 Antisense Plants
| Plantsa | Growth Temperature °C |
Stem Length (cm) |
Rosette Diameter (cm) |
No. of Siliques |
|---|---|---|---|---|
| Wild type | 20–25 | 23.4 ± 3.9 | 7.2 ± 1.8 | NDb |
| 8–12 | 22.1 ± 3.2 | 7.4 ± 2.1 | 348 ± 53c | |
| Antisense line 7 | 20–25 | 21.8 ± 3.6 | 7.0 ± 1.6 | ND |
| 8–12 | 8.1 ± 2.7d | 2.8 ± 0.6d | 77 ± 26c |
Measurements are the mean ±sd (n = 10).
ND, not determined.
n = 5.
n = 12.
ALA1 Expression in Arabidopsis Tissues
To examine the expression of the ALA1 gene in Arabidopsis, we fused the ALA1 promoter to the β-glucuronidase (GUS) reporter gene. Analysis of the GUS activity in transgenic plants transformed with this construct suggested that ALA1 is expressed in a large number of tissues and cell types (Figure 9). In seedlings, the vascular tissue of the cotyledons, the root, and the cotyledonary node displayed high GUS activity (Figure 9A). Activity was low or absent in the emerging lateral roots, root hairs, and the hypocotyl. In the flower, GUS staining was apparent in stamens, particularly in the anthers (Figure 9B). In sepals, the reporter gene was expressed in vascular tissues, whereas expression was absent from petals.
Figure 9.
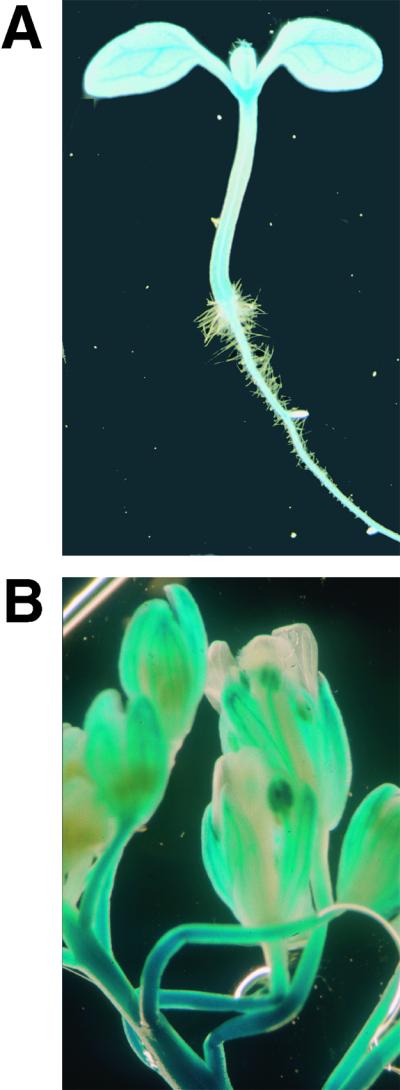
Activity of the GUS Reporter Gene in Transgenic Arabidopsis Containing the pALA1–GUS Fusion.
(A) GUS activity in a 6-day-old seedling.
(B) GUS expression pattern in flowers.
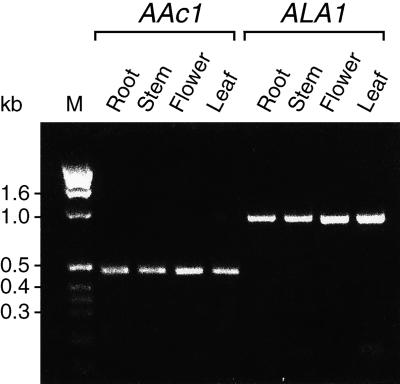
To confirm these data, we isolated total RNA from roots, stems, flowers, and rosette leaves. RT-PCR was used to generate cDNA and amplification products by using primers specific for ALA1 and, as a control, primers corresponding to the actin AAc1 cDNA. Using this approach, we found that ALA1 was expressed in all organs examined (Figure 10). Control experiments demonstrated that both the number of PCR cycles and the amount of RNA template used limited the amount of product obtained in a linear manner (data not shown). If reverse transcriptase was omitted during the RT step, no product was formed (data not shown), ruling out the possibility that a potential genomic DNA contamination in the RNA preparation influenced the results.
Figure 10.
ALA1 Expression in Various Organs of Arabidopsis.
ALA1-specific transcripts (ALA1) were amplified by RT-PCR from total RNA derived from different organs. The expected size of the resulting cDNA fragment was 839 bp. As an internal control, the actin Aac1 transcript was also amplified by RT-PCR. Sizes of the molecular length standards (M) run in parallel are indicated in kilobases (kb) at the left.
DISCUSSION
Identification of a Family of P4 ATPases in Arabidopsis
By searching for DRS2 homologs in the Arabidopsis genome, Axelsen and Palmgren (1998) identified a family (ALA) of relatively large membrane proteins with homology to P4 ATPases. This family consists of at least 11 members in Arabidopsis, with two related genes also present in rice (data not shown). The exon structures of the various Arabidopsis P4 ATPases suggest that both ancient gene duplications and more-recent duplication events have given rise to this novel family of putative transporters. Genome sequencing projects have also led to the identification of families of P4 ATPases in eukaryotes such as yeast, nematodes, mice, and humans, whereas no bacterial homologs have been identified (Axelsen and Palmgren, 1998).
ALA1 Protein: A Putative Aminophospholipid Translocase
Two lines of evidence suggest that ALA1 functions as an aminophospholipid translocase. First, ALA1 complements the deficiency in lipid internalization by intact cells exhibited by the drs2 yeast mutant. The complementation was assayed under conditions that limit endocytosis, suggesting that uptake is the result of transmembrane flipping. Second, in a reconstituted vesicle system, expression of ALA1 increases the capacity for aminophospholipid translocation across the membrane.
Increasing evidence suggests that several other P4 ATPases from animal and fungal systems are directly involved in aminophospholipid translocation. In animal cells, aminophospholipid translocases catalyze ATP-dependent aminophospholipid translocation and are sensitive to vanadate, an inhibitor of P-type ATPases (Seigneuret and Devaux, 1984; Daleke and Huestis, 1985; Zachowski et al., 1986). A candidate protein for this activity, the so-called ATPase II, has been purified from several sources, including chromaffin granules (Moriyama and Nelson, 1988), clathrin-coated vesicles (Xie et al., 1989), and the plasma membrane of erythrocytes (Morrot et al., 1990). The purified ATPase II is a PS-dependent and vanadate-sensitive ATPase, and membrane vesicles reconstituted with ATPase II purified from erythrocyte plasma membranes transport fluorescently labeled PS (Auland et al., 1994). Genes have been cloned encoding ATPase II of bovine chromaffin granules (Tang et al., 1996), bovine brain (Ding et al., 2000), and human tissues (Mouro et al., 1999). Several P-type ATPase consensus motifs have been identified in the sequences, hence identifying them as P4 ATPases (Axelsen and Palmgren, 1998). Four isoforms of bovine brain ATPase II have been produced in a baculovirus expression system, and dephosphorylation of the phosphorylated enzyme intermediate has been demonstrated to depend strictly on the presence of PS (Ding et al., 2000), as expected for a P-type ATPase transporting this substrate.
The sequences of ATPase II are homologous to DRS2, a yeast P-type ATPase. The drs2 mutant exhibits a decrease in the internalization of fluorescent-labeled PS supplied to the outer leaflet of the plasma membrane of intact cells (Figure 3) (Tang et al., 1996). Under certain conditions, drs2 mutants exhibit no measurable deficiencies in lipid translocation (Siegmund et al., 1998; Marx et al., 1999). These negative results may indicate that other aminophospholipid translocases are present in yeast and can functionally replace DRS2 (Siegmund et al., 1998; Marx et al., 1999). This notion is supported by the presence of four P4 ATPase genes (YER166w, NEO1, YMR16cv, and YDR093w; Swiss-Prot accession numbers P32660, P40527, Q12674, Q12675, respectively) in addition to DRS2 (Swiss-Prot accession number P39524) in the yeast genome (Figure 2). Also, in some cases, the activity of the DRS2 protein may have escaped detection, depending on the experimental conditions. In our hands, the following criteria were critical for the lipid internalization assay: (1) cells used for experimentation had to be harvested in early mid-log-phase (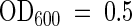 ). (2) The labeling of cells was optimal after 15 min; thus, the 1-min labeling time recommended by Tang et al. (1996) was insufficient. (3) The labeling had to be performed on ice; if labeling was performed at 30 or 23°C, temperatures that were used by others (Siegmund et al., 1998), it resulted in accumulation of the NBD lipid in drs2 cells, probably as a result of endocytosis. (4) Measurements had to be restricted to a 12- to 15-min period; longer exposure times, such as the 30 to 35 min used by Siegmund et al. (1998) and Marx et al. (1999), led to an internalization of NBD-PS also in the drs2 cells, albeit it occurred more slowly than it did in the wild type and without an immediate saturating uptake phase.
). (2) The labeling of cells was optimal after 15 min; thus, the 1-min labeling time recommended by Tang et al. (1996) was insufficient. (3) The labeling had to be performed on ice; if labeling was performed at 30 or 23°C, temperatures that were used by others (Siegmund et al., 1998), it resulted in accumulation of the NBD lipid in drs2 cells, probably as a result of endocytosis. (4) Measurements had to be restricted to a 12- to 15-min period; longer exposure times, such as the 30 to 35 min used by Siegmund et al. (1998) and Marx et al. (1999), led to an internalization of NBD-PS also in the drs2 cells, albeit it occurred more slowly than it did in the wild type and without an immediate saturating uptake phase.
Because we have not characterized the activity of purified, reconstituted ALA1 protein, this polypeptide could in principle be involved indirectly in lipid flipping by influencing the amount or activity of an unidentified aminophospholipid transport system. However, the close homology between the sequences of the ALA proteins and those of ATPase II and DRS2 suggests a direct role in aminophospholipid translocation. Given the ability of ALA1 to complement lipid internalization in the yeast drs2 mutant, we speculate that this protein is located in the plasma membrane. However, DRS2 is primarily localized to late Golgi vesicles in yeast (Chen et al., 1999), so an endomembrane localization of ALA1 cannot be ruled out. In this work, we have studied the function of ALA1 in more detail but have no evidence for the functions of ALA2 through ALA11. Perhaps specific expression of Arabidopsis P4 ATPase isoforms is a means of ensuring optimal expressions of various specificities at specific subcellular locations and at different times of development.
Involvement of ALA1 in Cold Tolerance of Arabidopsis
For investigating the physiological role of ALA1 in the plant, ALA1 antisense Arabidopsis plants were generated. The smaller size of the antisense plants in comparison with wild-type plants grown at 8 to 12°C indicates that ALA1 plays a role in tolerance to chilling temperatures. The fact that these plants do not exhibit such a phenotype when cultivated at normal, nonchilling temperatures supports this hypothesis.
An asymmetric distribution of PS may affect the fluidity of plant membranes directly. However, PS accounts for <2% of total phospholipid extracted from leaves of numerous plant species (Delhaize et al., 1999). Because PS is a minor phospholipid in plant membranes, we cannot rule out the possibility that PS may influence cold sensitivity indirectly, possibly by interfering with elements of signal transduction pathways involved in cold adaptation. In animals, PS activates the signal transducer protein kinase C (Nishizuka, 1992), whereas in plants, this lipid activates a calcium-dependent protein kinase (Szczegielniak et al., 2000). In animal and fungal plasma membranes, PS is normally distributed asymmetrically and is in general associated with the cytosolic face of the plasma membrane (Devaux, 1991; Schroit and Zwaal, 1991). The loss of this asymmetric distribution and the resulting appearance of PS at the cell surface allow phagocytes to identify cells that have undergone apoptosis (Fadok et al., 1992). Exposure of PS on the external face of the plasma membrane has also been associated with the apoptotic pathway in tobacco (O'Brien et al., 1998).
Whether the increased cold sensitivity of the ALA1 antisense plants is associated with alterations in transmembrane distribution of PE remains to be shown. Transbilayer redistribution of the aminophospholipid PE in response to cold has been reported before in poikilothermic animals (Miranda and Hazel, 1996). Several integral membrane enzyme activities, such as those of sarcoplasmic Ca2+-ATPase (Navarro et al., 1984) and the transduction enzyme protein kinase C (Stubb and Slater, 1996), are reported to be highly sensitive to the amount of bilayer-destabilizing lipid, such as PE, present in the membrane. Perturbation of membrane-associated enzymes in cold stress, due to failure to achieve a transbilayer redistribution of PE, could impair the growth of ALA1 antisense plants.
In conclusion, we have shown that ALA1, a putative aminophospholipid translocase, may play a role in cold tolerance in Arabidopsis. The actual mechanism by which ALA1 could participate in cold acclimation is not known. Differences in fatty acid compositions of particular membrane phospholipids, combined with transverse asymmetry, may result in differences in lateral diffusion and the local microviscosity of each hemileaflet of the membrane. Less-pronounced asymmetry would diminish this difference in fluidity of each leaflet. Local fluidity changes might affect the activity of important membrane proteins, and this in turn could influence the ability of the plant to adapt to cold. P4 ATPases in Arabidopsis may play an important role in this process.
METHODS
Cloning ALA1
The 5′ missing end of an Arabidopsis thaliana expressed sequence tag (N96084) with identity to the genomic clone AB005245 (GenBank accession number AB005245) was obtained by nested polymerase chain reaction (PCR) (Mundy et al., 1995), using the size-fractionated (3 to 6 kb) cDNA library CD4-16 as template (Kieber et al., 1993). The nucleotide sequence of the ALA1 cDNA has been deposited in GenBank (accession number AF175769). The PCR-generated cDNA was directionally cloned into the yeast/Escherichia coli shuttle vector pYES2 (Invitrogen) for expression of ALA1 under the control of the GAL1 promoter, generating plasmid pYES2-ALA1. The ALA1 cDNA from two independent PCR reactions was sequenced on both strands to ensure that no nucleotide substitutions had occurred during the PCR reaction.
Lipid Internalization by Intact Yeast Cells
Yeast (Saccharomyces cerevisiae) strains DS94 (wild type: MATα, ura3-52, trp1-1, leu2-3, his3-111, and lys2-801) (Tang et al., 1996) and JWY2197 (drs2 null mutant: MATα, ura3-52, drs2::TRP1, leu2-1, his3-11, and lys2-801) (Tang et al., 1996) were transformed with the empty pYES2 vector or pYES2-ALA1. The lipid internalization assay was performed as described by Tang et al. (1996) but with some modifications. Thus, cells used for experimentation were harvested in early mid-log-phase (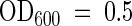 ; Shimadzu UV-160 spectrophotometer, Kyoto, Japan), cells were labeled for 15 min and on ice, and measurements were restricted to a 12- to 15-min period. 2-(6-[7-Nitrobenz-2-oxa-1,3-diazol-4-yl]-amino)hexanoyl (NBD)-labeled phospholipids were purchased from Avanti Polar lipids (Lipids, Inc., Alabaster, AL).
; Shimadzu UV-160 spectrophotometer, Kyoto, Japan), cells were labeled for 15 min and on ice, and measurements were restricted to a 12- to 15-min period. 2-(6-[7-Nitrobenz-2-oxa-1,3-diazol-4-yl]-amino)hexanoyl (NBD)-labeled phospholipids were purchased from Avanti Polar lipids (Lipids, Inc., Alabaster, AL).
Reconstitution of Yeast Microsomal Vesicles
Yeast cells were grown to an OD600 of 0.5 in minimal medium containing 2% galactose and supplemented with amino acids. Membranes obtained from homogenized cells were subjected to differential and sucrose step-gradient centrifugation as described previously (Regenberg et al., 1995). Microsomes enriched in plasma membrane vesicles were collected from the 43 to 53% (w/w) sucrose interface. Mixed phospholipids (soybean asolectin type II-S; Sigma) were dispersed by vortex-mixing under argon for 10 min in the reconstitution buffer (10 mM Mes-KOH, pH 6.5, 50 mM K2SO4, and 20% [v/v] glycerol) at a final concentration of 30 mg/mL. Yeast microsomes (155 μg) were mixed with 2216 μg of asolectin and 116 μg of NBD-phosphatidylcholine (PC), NBD-phosphatidylserine (PS), or NBD-phosphatidylethanolamine (PE) (dried overnight under vacuum) in a final volume of 208 μL (lipid/protein mass ratio of 15:1). The protein/lipid mixture was solubilized by adding 12 μL of 1 M octylglucoside (Boehringer Mannheim). Disposable 2-mL syringes fitted with glass wool at the bottom were filled with Sephadex G-50 gel (Pharmacia Biotechnology), equilibrated with reconstitution buffer, and centrifuged at 180g for 5 min at 4°C. The solubilized lipid/protein/detergent mixture (220 μL) was applied at the top of the column, and the column was centrifuged again for 7.5 min. This step removed the unincorporated NBD lipids and most of the detergent. Reconstituted membrane vesicles were then incubated for 30 min with 100 mg of Bio-beads (20 to 50 mesh; Bio-Rad) at room temperature in the presence of 1 mM phenylmethylsulfonyl fluoride and 10 μg/mL chymostatin to remove the remaining detergent.
Measurement of Phospholipid Flipping in Reconstituted Microsomal Vesicles
Reconstituted microsomes (30 μg of membrane protein) were incubated for 120 min at 25°C in a buffer containing 40 mM Tris-HCl, pH 7.4, 130 mM KCl, 4 mM MgCl2, and 15 μM verapamil (to inhibit ATP-binding cassette transporters). ATP (3 mM) was added to start the reaction. Fluorescence emission of NBD phospholipids in both outer and inner leaflets of the microsomal vesicles was monitored essentially as described by Suzuki et al. (1997). The spectrofluorometer (Cary-17; Varian, Inc., Palo Alto, CA) was equipped with a fluorescence accessory to redirect light from the monochromator to the phototube. The monochromator was set at 470 nm, and a 495-nm sharp-cutoff glass filter (model GG945; Schott Glass Technologies, Duryea, PA) was placed in front of the phototube. When indicated, 10 mM sodium dithionite was added to reduce NBD phospholipids present in the outer leaflet of the microsomes. After the reaction, the microsomes were solubilized by adding 1% (w/v) Triton X-100 to allow dithionite to reduce the inner leaflet-resident NBD phospholipids.
Construction of a pALA1–GUS Fusion and an Antisense ALA1 Construct
A promoter fragment of 2138 bp from the ALA1 genomic sequence (pALA1, positions −2135 to +3) was amplified by nested PCR from a genomic library of Arabidopsis ecotype Columbia. In the first PCR reaction, the forward primer used was the synthetic oligonucleotide 5′-AAGTGAAGACAGAGTATTTAGCAG-3′; the reverse primer was 5′-CAATGGTTGATTAAAGCGTTGGAC-3′. In the second PCR reaction, the forward primer was 5′-GCAACTGCAGGTTAAACAAACTTGTACGAAAGCG-3′ (an introduced PstI site is underlined); the reverse primer was 5′-GAACCATGACGGATACGCTTAGAT-3′. The resulting fragment was digested with PstI and BamHI and subcloned into the PstI-BglII sites of the binary vector p3301 in front of the E. coli GUS gene terminated by the nos polyadenylation signal.
For antisense expression of ALA1, a 1235-bp BamHI (made blunt-ended)-SacI cDNA fragment (bp 3 to 1238) was cloned into the binary vector pCROX18 (a kind gift of J. Mundy) in the antisense orientation, leaving expression of the antisense fragment under the control of the strong 35S promoter.
Plant Transformation
Constructs for expression of the pALA1–GUS fusion and antisense ALA1 were introduced into Agrobacterium tumefaciens strain pGV3101 by electroporation and selected for resistance to kanamycin (50 μg/mL). Arabidopsis ecotype Columbia was transformed by vacuum infiltration, and seeds were selected by spraying with glufosinate ammonium (BASTA, Frankfurt, Germany) (pALA1–GUS fusion vector) or by selection on plates of MS medium (Murashige and Skoog, 1962) with kanamycin (50 μg/mL) (ALA1 antisense vector). Individual transformed plants were selfed to select for homozygous plants.
Histochemical Localization of GUS Activity
Six-day-old seedlings grown under sterile conditions on an MS agar plate containing 1% sucrose (grown in 16 hr of light, 75% humidity, 100 μmol m−2 sec−1) or flowers of 5-week-old soil-grown plants (also grown in 16 hr of light, 75% humidity, 100 μmol m−2 sec−1) were rinsed with GUS buffer (100 mM sodium phosphate, pH 7.0, 1 mM EDTA, 1% Triton X-100, 3.5 mM K3Fe[CN]6, 3.5 mM K4Fe[CN]6, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-[d-glucuronide]) before vacuum infiltration in the GUS buffer for 5 min. Plants were incubated at 37°C for 16 hr. Chlorophylls were removed with 70% ethanol, and the plants were photographed with an Olympus SZH binocular microscope (Olympus Optical Co., Hamburg, Germany).
RNA Analysis
With the RNeasy Plant Kit (Qiagen), Arabidopsis total RNA was extracted from soil-grown plants or from seedlings grown for 8 days in liquid medium (MS basic salt mixture supplemented with 1% [w/v] sucrose, with constant light). The RNA preparations were treated with RNase-free DNase (Promega) after isolation. Actin (AAc1)- and ALA1-specific transcripts were detected with reverse transcription (RT)–PCR by using the rTth RNA-PCR kit (Perkin-Elmer) according to the manufacturer's instructions. The primer pairs 5′-GTGCTCGACTCTGGAGATGGTGTG-3′ and 5′-CGGCGATTCCAGGGAACA-TTGTGG-3′, and 5′-ACACTGCTTGGGCTTTAGG-3′ and 5′-TGC-TTGTTCACTAGGGGACTC-3′ were used to detect AAc1 and ALA1 transcripts, respectively, leading to amplification of 467-bp (AAc1) and 839-bp (ALA1) products from cDNA templates.
Acknowledgments
We thank P. Williamson for donating yeast strains DS94 and JWY2197, and T.H. Roberts for helpful comments on the manuscript. This work was supported by the European Union Biotechnology program, the Danish Biotech III program, and a French Foreign Office Lavoisier Grant to E.G.
References
- Auland, M.E., Roufogalis, B.D., Devaux, P.F., and Zachowski, A. (1994). Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc. Natl. Acad. Sci. USA 91 10938–10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen, K.B., and Palmgren, M.G. (1998). Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 46 84–101. [DOI] [PubMed] [Google Scholar]
- Browse, J., and Somerville, C. (1991). Glycerolipid synthesis: Biochemistry and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 467–506. [Google Scholar]
- Cevc, G. (1991). How membrane chain-melting phase-transition temperature is affected by the lipid chain asymmetry and degree of unsaturation: An effective chain-length model. Biochemistry 30 7186–7193. [DOI] [PubMed] [Google Scholar]
- Cheesebrough, T.M., and Moore, T.S. (1980). Transverse distribution of phospholipids in organelle membranes from Ricinus communis L. var. Hale endosperm. Plant Physiol. 65 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.Y., Ingram, M.F., Rosal, P.H., and Graham, T.R. (1999). Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 147 1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan, U., and Schachter, D. (1981). Asymmetry of lipid dynamics in human erythrocyte membranes studied with impermeant fluorophores. Biochemistry 20 6396–6403. [DOI] [PubMed] [Google Scholar]
- Cossins, A.R. (1994). Homeoviscous adaptation of biological membranes and its functional significance. In Temperature Adaptation of Biological Membranes, A.R. Cossins, ed (London: Portland Press), pp. 63–76.
- Daleke, D.L., and Huestis, W.H. (1985). Incorporation and translocation of aminophospholipids in human erythrocytes. Biochemistry 24 5406–5416. [DOI] [PubMed] [Google Scholar]
- Delhaize, E., Hebb, D.M., Richards, K.D., Lin, J.M., Ryan, P.R., and Gardner, R.C. (1999). Cloning and expression of a wheat (Triticum aestivum L.) phosphatidylserine synthase cDNA. Overexpression in plants alters the composition of phospholipids. J. Biol. Chem. 274 7082–7088. [DOI] [PubMed] [Google Scholar]
- Devaux, P.F. (1991). Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30 1163–1173. [DOI] [PubMed] [Google Scholar]
- Ding, J., Wu, Z., Crider, B.P., Ma, Y., Li, X., Slaughter, C., Gong, L., and Xie, X.-S. (2000). Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase: Effect of isoform variation on the ATPase activity and phospholipid specificity. J. Biol. Chem. 275 23378–23386. [DOI] [PubMed] [Google Scholar]
- Dolis, D., Moreau, C., Zachowski, A., and Devaux, P.F. (1997). Aminophospholipid translocase and proteins involved in transmembrane phospholipid traffic. Biophys. Chem. 68 221–231. [DOI] [PubMed] [Google Scholar]
- Dorne, A.J., Joyard, J., Block, M.A., and Douce, R. (1985). Localization of phosphatidylcholine in outer envelope of spinach chloroplast. J. Cell Biol. 100 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeja, P.K., Wali, R.K., Harig, J.M., and Brasitus, T.A. (1991). Characterization and modulation of rat small intestinal brush-border membrane transbilayer fluidity. Am. J. Physiol. 260 G586–G594. [DOI] [PubMed] [Google Scholar]
- Fadok, V.A., Voelker, D.R., Campbell, P.A., Cohen, J.J., Bratton, D.L., and Henson, P.M. (1992). Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148 2207–2216. [PubMed] [Google Scholar]
- Felsenstein, J. (1989). PHYLIP—Phylogeny inference package (version 32). Cladistics 5 164–166. [Google Scholar]
- Foley, M., MacGregor, A.N., Kusel, J.R., Garland, P.B., Downie, T., and Moore, I. (1986). The lateral diffusion of lipid probes in the surface membrane of Schistosoma mansoni. J. Cell Biol. 103 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser, K.W., Goldsmith, A., and Hopfer, U. (1990). Regulation of chloride transport in parotid secretory granules by membrane fluidity. Biochemistry 29 7282–7288. [DOI] [PubMed] [Google Scholar]
- Halleck, M.S., Pradhan, D., Christie, B., Berkes, C., Williamson, P., and Schegel, R.A. (1998). Multiple members of a third subfamily of P-type ATPases identified by genomic sequences and ESTs. Genome Res. 8 354–361. [DOI] [PubMed] [Google Scholar]
- Harwood, J.L. (1988). Fatty acid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39 101–138. [Google Scholar]
- Hazel, J.R. (1995). Thermal adaptation in biological membranes—Is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 75 19–42. [DOI] [PubMed] [Google Scholar]
- Hugly, S., and Somerville, C. (1992). A role for membrane polyunsaturation in chloroplast biogenesis at low temperature. Plant Physiol. 99 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbavboa, U., Avdulov, N.A., Schroeder, F., and Wood, W.G. (1996). Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J. Neurochem. 66 1717–1725. [DOI] [PubMed] [Google Scholar]
- Ishizaki-Nishizawa, O., Fujii, T., Azuma, M., Sekiguchi, K., Murata, N., Ohtani, T., and Toguri, T. (1996). Low-temperature resistance of higher plants is significantly enhanced by a nonspecific cyanobacterial desaturase. Nat. Biotechnol. 14 1003–1006. [DOI] [PubMed] [Google Scholar]
- Julien, M., Tournier, J.F., and Tocanne, J.F. (1993). Differences in the transbilayer and lateral motions of fluorescent analogs of phosphatidylcholine and phosphatidylethanolamine in the apical plasma membrane of bovine aortic endothelial cells. Exp. Cell Res. 208 387–397. [DOI] [PubMed] [Google Scholar]
- Kates, M., Pugh, E.L., and Ferrante, G. (1984). Regulation of membrane fluidity by lipid desaturases. Biomembranes 12 379–395. [Google Scholar]
- Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.A., and Ecker, J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72 427–441. [DOI] [PubMed] [Google Scholar]
- Kitagawa, S., Matsubayashi, M., Kotani, K., Usui, K., and Kametani, F. (1991). Asymmetry of membrane fluidity in the lipid bilayer of blood platelets: Fluorescence study with diphenylhexatriene and analogs. J. Membr. Biol. 119 221–227. [DOI] [PubMed] [Google Scholar]
- Kitagawa, S., Tachikawa, E., Kashimoto, T., Nagaoka, Y., Iida, A., and Fujita, T. (1998). Asymmetrical membrane fluidity of bovine adrenal chromaffin cells and granules and effect of trichosporin-B-VIa. Biochim. Biophys. Acta 1375 93–100. [DOI] [PubMed] [Google Scholar]
- Kodama, H., Hamada, T., Horiguchi, G., Nishimura, M., and Iba, K. (1994). Genetic enhancement of cold tolerance by expression of a gene for chloroplast ω-3 fatty-acid desaturase in transgenic tobacco. Plant Physiol. 105 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte, J., and Doolittle, R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157 105–132. [DOI] [PubMed] [Google Scholar]
- Lanfermeijer, F.C., Venema, K., and Palmgren, M.G. (1998). Purification of a histidine-tagged plant plasma membrane H+-ATPase expressed in yeast. Protein Expression Purif. 12 29–37. [DOI] [PubMed] [Google Scholar]
- Marx, U., Polakowski, T., Pomorski, T., Lang, C., Nelson, H., Nelson, N., and Herrmann, A. (1999). Rapid transbilayer movement of fluorescent phospholipid analogues in the plasma membrane of endocytosis-deficient yeast cells does not require the Drs2 protein. Eur. J. Biochem. 263 254–263. [DOI] [PubMed] [Google Scholar]
- McIntyre, J.C., and Sleight, R.G. (1991). Fluorescence assay for phospholipid membrane asymmetry. Biochemistry 30 11819–11827. [DOI] [PubMed] [Google Scholar]
- Miquel, M., James, D., Dooner, H., and Browse, J. (1993). Arabidopsis requires polyunsaturated fatty acids for low-temperature survival. Proc. Natl. Acad. Sci. USA 90 6208–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, E.J., and Hazel, J.R. (1996). Temperature-induced changes in the transbilayer distribution of phosphatidylethanol–amine in mitoplasts of rainbow trout (Oncorhynchus mykiss) liver. J. Exp. Zool. 274 23–32. [Google Scholar]
- Moon, B.Y., Higashi, S., Gombos, S., and Murata, N. (1995). Unsaturation of the membrane lipids of chloroplasts stabilizes the photosynthetic machinery against low-temperature photoinhibition in transgenic tobacco plants. Proc. Natl. Acad. Sci. USA 92 6219–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama, Y., and Nelson, N. (1988). Purification and properties of a vanadate- and N-ethylmaleimide-sensitive ATPase from chromaffin granule membranes. J. Biol. Chem. 263 8521–8527. [PubMed] [Google Scholar]
- Morrot, G., Zachowski, A., and Devaux, P.F. (1990). Partial purification and characterization of the human erythrocyte Mg2+-ATPase. FEBS Lett. 266 29–32. [DOI] [PubMed] [Google Scholar]
- Mouro, I., Halleck, S., Schlegel, B., Mattei, M.G., Williamson, P., Zachowski, A., Devaux, P., Cartron, J.-P., and Colin, Y. (1999). Cloning, expression, and chromosomal mapping of a human ATPase II gene, member of the third subfamily of P-type ATPases and orthologous to the presumed bovine and murine aminophospholipid translocase. Biochem. Biophys. Res. Commun. 257 333–339. [DOI] [PubMed] [Google Scholar]
- Müller, K., Labbe, C., and Zachowski, A. (1994). Phospholipid transverse asymmetry in trout spermatozoa plasma membrane. Biochim. Biophys. Acta 1192 21–26. [DOI] [PubMed] [Google Scholar]
- Mundy, J., Mayer, R., and Chua, N.-H. (1995). Cloning of genomic sequences using long-range PCR. Plant Mol. Biol. Rep. 13 156–163. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15 473–497. [Google Scholar]
- Murata, N. (1983). Molecular species composition of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Cell Physiol. 24 81–86. [Google Scholar]
- Murata, N., Sato, N., Takahashi, N., and Hamazaki, Y. (1982). Composition and positional distribution of fatty acids in phospholipids from leaves of chilling-sensitive and chilling-resistant plants. Plant Cell Physiol. 23 1071–1079. [Google Scholar]
- Murata, N., Ishizaki-Nishizawa, Q., Higashi, S., Hayashi, H., Tasaka, Y., and Nishida, I. (1992). Genetically engineered alterations in the chilling sensitivity of plants. Nature 356 710–713. [Google Scholar]
- Navarro, J., Toivio-Kinnucan, M., and Racker, E. (1984). Effect of lipid composition on the calcium/adenosine 5′-triphosphate coupling ratio of the Ca2+-ATPase of the sarcoplasmic reticulum. Biochemistry 23 130–135. [DOI] [PubMed] [Google Scholar]
- Nishida, I., and Murata, N. (1996). Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 541–568. [DOI] [PubMed] [Google Scholar]
- Nishizuka, Y. (1992). Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258 607–614. [DOI] [PubMed] [Google Scholar]
- O'Brien, I.E.W., Reutelingsperger, C.P.M., and Holdaway, K.M. (1997). Annexin-V and TUNEL use in monitoring the progression of apoptosis in plants. Cytometry 29 28–33. [PubMed] [Google Scholar]
- O'Brien, I.E., Baguley, B.C., Murray, B.G., Morris, B.A., and Ferguson, I.B. (1998). Early stages of the apoptotic pathway in plant cells are reversible. Plant J. 13 803–814. [Google Scholar]
- Pearce, R.S. (1999). Molecular analysis of acclimation to cold. Plant Growth Regul. 29 47–76. [Google Scholar]
- Rawyler, A., and Siegenthaler, P.A. (1981). Transmembrane distribution of phospholipids and their involvement in electron transport, as revealed by phospholipids A2 treatment of spinach thylakoids. Biochim. Biophys. Acta 635 348–358. [DOI] [PubMed] [Google Scholar]
- Regenberg, B., Villalba, J.M., Lanfermeijer, F.C., and Palmgren, M.G. (1995). Carboxy-terminal deletion analysis of plant plasma membrane H+-ATPase: Yeast as a model system for solute transport across the plant plasma membrane. Plant Cell 7 1655–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripmaster, T., Vaughn, G., and Woolford, J.L. (1993). DRS1 to DRS7, novel genes required for ribosome assembly and function in Saccharomyces cerevisiae. Mol. Cell. Biol. 13 7901–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, F., Woodford, J.K., Kavecansky, J., Wood, W.G., and Joiner, C. (1995). Cholesterol domains in biological membranes. Mol. Membr. Biol. 12 113–119. [DOI] [PubMed] [Google Scholar]
- Schroit, A.J., and Zwaal, R.F. (1991). Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim. Biophys. Acta 1071 313–329. [DOI] [PubMed] [Google Scholar]
- Seigneuret, M., and Devaux, P.F. (1984). ATP-dependent asymmetric distribution of spin-labelled phospholipids in the erythrocyte membrane: Relation to shape change. Proc. Natl. Acad. Sci. USA 81 3751–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret, M., Zachowski, A., Hermann, A., and Devaux, P.F. (1984). Asymmetric lipid fluidity in human erythrocyte membrane: New spin-label evidence. Biochemistry 23 4271–4275. [DOI] [PubMed] [Google Scholar]
- Siegmund, A., Grant, A., Angeletti, C., Malone, L., Nichols, J.W., and Rudolph, H.K. (1998). Loss of Drs2p does not abolish transfer of fluorescence-labeled phospholipids across the plasma membrane of Saccharomyces cerevisiae. J. Biol. Chem. 273 34399–34405. [DOI] [PubMed] [Google Scholar]
- Somerville, C., and Browse, J. (1991). Plant lipids: Metabolism, mutants and membranes. Science 252 80–87. [DOI] [PubMed] [Google Scholar]
- Squier, T.C., Bigelow, D.J., and Thomas, D.D. (1988). Lipid fluidity directly modulates the overall protein rotational mobility of the Ca2+-ATPase in the sarcoplasmic reticulum. J. Biol. Chem. 263 9178–9186. [PubMed] [Google Scholar]
- Stubb, C.D., and Slater, S.J. (1996). The effects of non-lamellar forming lipids on membrane protein–lipid interactions. Chem. Physiol. Lipids 81 185–195. [DOI] [PubMed] [Google Scholar]
- Suzuki, H., Kamakura, M., Morii, M., and Takeguchi, N. (1997). The phospholipid flippase activity of gastric vesicles. J. Biol. Chem. 272 10429–10434. [DOI] [PubMed] [Google Scholar]
- Szczegielniak, J., Liwosz, A., Jurkowski, I., Loog, M., Dobrowolska, G.Y., Ek, P., Harmon, A.C., and Muszynska, G. (2000). Calcium-dependent protein kinase from maize seedlings activated by phospholipids. Eur. J. Biochem. 267 3818–3827. [DOI] [PubMed] [Google Scholar]
- Tang, X., Halleck, M.S., Schlegel, R.A., and Williamson, P. (1996). A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 272 1495–1497. [DOI] [PubMed] [Google Scholar]
- Tavernier, E., and Pugin, A. (1995). Transbilayer distribution of phosphathidylcholine and phosphathidylethanolamine in the vacuolar membrane of Acer pseudoplatanus cells. Biochimie 77 174–181. [DOI] [PubMed] [Google Scholar]
- Thomashow, M.F. (1999). Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 571–599. [DOI] [PubMed] [Google Scholar]
- Wolter, F.-P., Schmidt, R., and Heinz, E. (1992). Chilling sensitivity of Arabidopsis thaliana with genetically engineered membrane lipids. EMBO J. 11 4685–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X.S., Stone, D.K., and Racker, E. (1989). Purification of a vanadate-sensitive ATPase from clathrin-coated vesicles of bovine brain. J. Biol. Chem. 264 1710–1714. [PubMed] [Google Scholar]
- Zachowski, A., Favre, E., Cribier, S., Hervè, P., and Deveaux, P.F. (1986). Outside-inside translocation of aminophospholipids in the human erythrocyte is mediated by a specific enzyme. Biochemistry 25 2585–2590. [DOI] [PubMed] [Google Scholar]