Abstract
Vaccines against SARS-CoV-2 (COVID-19) proved beneficial for COVID-19 disease attenuation and preventing virus spreading. Cumulative reports of the rarity of antineutrophil cytoplasmic autoantibodies (ANCA)-associated vasculitis (AAV) raise concerns about its relationship with COVID-19 vaccination. Several case reports described ANCA-associated pauci-immune glomerulonephritis (ANCA-GN) following COVID-19 vaccination with some uniqueness. We systematically reviewed COVID-19 vaccine-induced ANCA-GN from PubMed, SCOPUS, and Cochrane library databases until 1 January 2023 according to PRISMA guidelines and presented our three cases. Twenty-six cases from 25 articles, including our 3 cases, were analyzed. Most cases were diagnosed following the second dose of the COVID-19 vaccine (59%) with a median (IQR) interval onset of 14 (16) days. The highest prevalence was related to the mRNA-type vaccine. Anti-myeloperoxidase (MPO) ANCA was far more common than the other ANCAs, with various positive autoantibodies. Fourteen cases (out of 29 cases, 48%) had extra-kidney AAV manifestation. Although severe kidney injury was observed in 10/29 (34%), remission was achieved in 89% (25/28) with no death. The mechanisms of the vaccine-inducing ANCA-GN were postulated here. Since ANCA-GN after the COVID-19 vaccine was rare, the benefit of the COVID-19 vaccine could outweigh the risk of ANCA-GN side effects in the pandemic era.
Keywords: SARS-CoV-2 vaccination, COVID-19 vaccine, ANCA-associated pauci-immune glomerulonephritis, ANCA-associated vasculitis
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the ongoing global pandemic of coronavirus disease 2019 (COVID-19), with a cumulative confirmed case of 765 million, including a 1% death rate [1]. Most COVID-19 vaccines are designed to elicit immune responses, ideally neutralizing antibodies against the SARS-CoV-2 spike protein, including mRNA (BNT162b2 and mRNA-1273), adenoviral-vectored (AZD1222, Ad26.COV2.S, and Gam-COVID-Vac), protein subunit (NVX-CoV2373), and whole-cell inactivated virus vaccines (CoronaVac, Sinopharm, BBIBP-CorV, and BBV152) [2]. COVID-19 vaccines prove their efficacy profiles on COVID-19 prevention and reduction in disease severity according to a recent meta-analysis of randomized controlled trials [3]. In addition, COVID-19 vaccine safety profiles were acceptable without increased risk of serious adverse events [3]. However, several de-novo and relapsing glomerulonephritis (GN) following COVID-19 vaccination, including minimal change disease (MCD), IgA nephropathy (IgAN), membranous nephropathy (MN), lupus nephritis (LN), anti-glomerular basement membrane (anti-GBM) disease, and ANCA-associated pauci-immune glomerulonephritis (ANCA-GN) have been documented [4,5,6]. ANCA-GN is a severe glomerular disease manifesting as systemic vasculitis involving glomeruli from ANCA-associated vasculitis (AAV) [7,8,9,10]. A recent systematic review of AAV following COVID-19 vaccines demonstrated a temporal relationship [11]. However, renal manifestation and outcomes of biopsy-proven ANCA-GN following the COVID-19 vaccine were limited. In this review, we presented our 3 cases and systematically reviewed biopsy-proven ANCA-GN following COVID-19 vaccines from the literature. We also discussed the disease’s uniqueness, possible mechanism, treatment, and outcomes.
2. Materials and Methods
2.1. Eligibility Criteria
The American College of Rheumatology/European Alliance of Associations for Rheumatology (ACR/EULAR) 2022 classified AAV into granulomatous polyangiitis (GPA), eosinophilic granulomatous polyangiitis (EGPA), microscopic polyangiitis (MPA), and renal-limited vasculitis (RLV) [12,13]. However, this is only a classification criterion for research purposes [12]. Therefore, in the systematic review, we included only kidney-biopsy-proven ANCA-GN following COVID-19 vaccination with or without extra-kidney manifestation of AAV.
2.2. Search Strategy and Study Selection
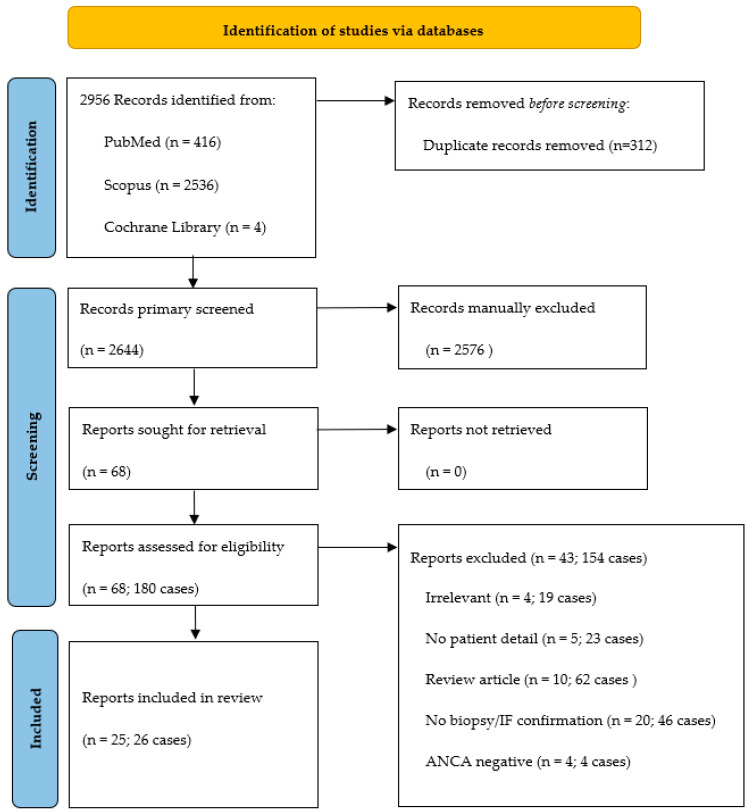
Two independent authors conducted systematic literature by searching relevant full-text articles in the PubMed, SCOPUS, and Cochrane library databases up to 1 January 2023. Keywords were (“COVID-19 vaccine”, OR “COVID-19”, OR “COVID-19 vaccination”, OR “SARS-CoV-2 vaccine”, OR “SARS-CoV-2”) AND (“ANCA”, OR “ANCA-associated glomerulonephritis”, OR “ANCA-associated vasculitis”, OR “glomerulonephritis”, OR “MPO-ANCA”, OR “PR3-ANCA”, OR “pauci-immune glomerulonephritis”, OR “anti-neutrophil cytoplasmic antibody”, OR “antineutrophil cytoplasmic antibody”). The eligible articles included the full-text report in the English language of biopsy-proven ANCA-GN with pauci-immune deposit by immunofluorescence (IF) study (defined as negative or weak [≤1+] staining of Ig and complement) or by electron microscopy (EM) (defined as no or faint electron-dense deposit [EDDs]) after COVID-19 vaccination. Patients’ age, vaccine types, and the disease onset from the vaccination were not restricted. The exclusion criteria were AAV without GN, ANCA-GN with a non-available IF/EM report, and AAV presenting or having disease flare before the onset of vaccination. We also included our 3 GN-ANCA cases diagnosed and treated at King Chulalongkorn Memorial Hospital, Bangkok, Thailand, from January 2022 to December 2022. Data extraction was anonymized under the General Data Protection Regulation [14], and informed consent, per the Declaration of Helsinki, were obtained from all three patients or their descendants (if unavailable). The PRISMA flow is shown in Figure 1.
Figure 1.
PRISMA flow diagram.
2.3. Data Extraction
Data included patient demographics, vaccine types, vaccination numbers, the interval between the last vaccination and onset of AAV, the clinical manifestation of ANCA-GN and AAV, serum creatinine (Scr) at baseline and peak, serologies (e.g., cytoplasmic [c] ANCA, perinuclear [p] ANCA, MPO-ANCA, PR3-ANCA, anti-nuclear antibody [ANA], Coombs antibodies, cryoglobulin, rheumatoid factor [RF]), kidney pathology finding, treatment, and outcomes after treatment.
2.4. Data Analysis and Definition
Severe acute kidney injury (AKI) was defined as dialysis requirement or peak Scr > 5.7 mg/dL. Outcomes were classified into remission with relapse, remission without relapse, and non-remission (refractory, dialysis-dependent, and death). Remission of ANCA-GN/AAV was defined according to the 2012 KDIGO Guidelines for Management of Glomerular Diseases [15] as the absence of erythrocyte in the urine, stable or reduced presence of proteins in the urine, and stable or improved glomerular filtration rate (GFR), together with the absence of symptoms indicating active disease in any extra-kidney organ system.
The continuous data were illustrated by using mean ± standard deviation (SD) for normal distribution data and median (interquartile range [IQR]) for non-normal distribution data. The categorical data were described as a ratio. Finally, the normally distributed variables were compared by t-test.
3. Results
3.1. Systematic Review
A total of 2956 relevant records were initially obtained from the database search. After removing 312 duplicated records, 2644 record titles and abstracts of articles were first screened according to the inclusion criteria. 68 articles were eligible for the second screening; 43 articles (154 cases) were excluded (4 with irrelevant to inclusion criteria, 5 no patients detail, 10 review articles, 20 without kidney-biopsy/IF confirmation; and 4 with negative ANCA); 26 cases from 25 articles, including our 3 cases were recruited for the systematic review (Figure 1). The details of the individual cases are shown in Supplementary Table S1.
The median (IQR) age was 70 (22) years, with female predominance (59%). Most cases presented ANCA-GN after receiving the second vaccination (59%) with a median interval gap of 14 (11–27) days. The mRNA COVID-19 vaccine was the most common (55%), followed by the viral vector vaccine (31%) and inactivated COVID-19 vaccine (14%). The top-three most common presentations were AKI, abnormal urine sediments, and constitutional symptoms. Extra-kidney manifestations included pulmonary involvement (diffuse alveolar hemorrhage and interstitial pneumonia) [8/29, 28%], neuromuscular involvement (muscle weakness, arthralgia/arthritis) [8/29, 28%], otologic/optic involvement [5/29, 17%], and Wallerian degeneration [1/29, 3%]. Severe AKI was observed in 34% (10/29). MPO-ANCA/pANCA was positive in 79% (23/29). A discrepancy of ANCA serologies was observed in one case (1/29 cases, cANCA + MPO-ANCA). A dual-positive ANCA (pANCA and cANCA) was detected in 3% (1/29). Autoantibodies were positive in 52% (13/25 cases), including ANA (64%, 7/11), Coombs antibody (100%, 3/3), cryoglobulin (33%, 1/3), and RF (25%, 2/8). The 2021 KDIGO Guidelines for Management of Glomerular Diseases [16] were adopted to treat most cases with ANCA-GN following COVID-19 vaccines. Plasmapheresis was prescribed in 21% (6/28), while rituximab was employed in 38% (11/29). Remission was achieved in 89% (25/28), with relapse in one of those. In non-remission, 11% (3/28) were dialysis-dependent, and no death occurred (Table 1).
Table 1.
Clinical spectrums and outcomes of ANCA-associated glomerulonephritis following SARS-CoV-2 vaccination by vaccine type.
| Parameters | Total (n = 29) |
mRNA Vaccine (n = 16) |
Viral Vector Vaccine (n = 9) |
Inactivated Vaccine (n = 4) |
p Value |
|---|---|---|---|---|---|
| Age, years | 70 (22) | 70 (25) | 72 (17) | 69 (33) | 0.63 |
| Female | 17/29 (59%) | 9/16 (56%) | 5/9 (56%) | 3/4 (75%) | 0.88 |
| Vaccination dose | 0.08 | ||||
| ● 1st dose | 9/29 (31%) | 3/16 (19%) | 5/9 (56%) | 1/4 (25%) | |
| ● 2nd dose | 17/29 (59%) | 12/16 (75%) | 2/9 (22%) | 3/4 (75%) | |
| ● 3rd dose or more | 3/29 (10%) | 1/16 (6%) | 2/9 (22%) | 0/4 (0%) | |
| Onset after last vaccination, days | 14 (16) | 14 (24) | 15 (8) | 20 (16) | 0.72 |
| Clinical manifestations | |||||
| ● AKI or RPGN | 14/29 (48%) | 8/16 (50%) | 5/9 (56%) | 1/4 (25%) | 0.76 |
| ● Sub-nephrotic proteinuria/NS | 9/29 (31%) | 3/16 (19%) | 4/9 (44%) | 2/4 (50%) | 0.30 |
| ● Abnormal urine sediments | 19/29 (66%) | 10/16 (62%) | 5/9 (56%) | 4/4 (100%) | 0.40 |
| ● Constitutional symptoms | 15/29 (52%) | 6/16 (38%) | 5/9 (56%) | 4/4 (100%) | 0.10 |
| ● Extra-kidney involvement | |||||
| - Lung (DAH, interstitial pneumonia, etc.) | 8/29 (28%) | 5/16 (31%) | 3/9 (33%) | 0/4 (0%) | 0.62 |
| - NM (muscle weakness, etc.) | 8/29 (28%) | 3/16 (19%) | 4/9 (44%) | 1/4 (25%) | 0.40 |
| - GI (N/V, diarrhea, etc.) | 4/29 (14%) | 1/16 (6%) | 1/9 (11%) | 2/4 (50%) | 0.10 |
| - ENT (hearing loss, tinnitus, etc.) | 3/29 (10%) | 1/16 (6%) | 2/9 (22%) | 0/4 (0%) | 0.70 |
| - Eyes (episcleritis, etc.) | 2/29 (7%) | 1/16 (6%) | 1/9 (11%) | 0/4 (0%) | 1.00 |
| ANCA serologies | |||||
| ● MPO-ANCA/pANCA | 23/29 (79%) | 13/16 (81%) | 7/9 (78%) | 3/4 (75%) | 1.00 |
| ● PR3-ANCA/cANCA | 5/29 (17%) | 3/16 (19%) | 1/9 (11%) | 1/4 (25%) | 1.00 |
| ● pANCA + cANCA | 1/29 (3%) | 0/16 (0%) | 1/5 (8%) | 0/4 (0%) | 1.00 |
| ● Discrepancy positive ANCA | 1/29 (3%) | 0/16 (0%) | 1/6 (17%) | 0/4 (0%) | 1.00 |
| Autoantibodies, n/N (%) | |||||
| ● ANA | 7/11 (64%) | 5/5 (100%) | 1/4 (20%) | 1/2 (50%) | 0.05 |
| ● Coombs/positive anti-globulin test | 3/3 (100%) | 1/1 (100%) | 2/2 (200%) | - | - |
| ● Cryoglobulin | 1/3 (33%) | 1/3 (33%) | - | - | - |
| ● RF | 2/8 (25%) | 0/4 (0%) | 2/3 (67%) | 0/1 (0%) | 0.21 |
| Baseline Scr, mg/dL | 0.9 (0.3) | 0.9 (0.2) | 1.0 (0.7) | 0.8 (0.7) | 0.14 |
| Peak Scr, mg/dL | 4.8 (3.5) | 3.5 (3.7) | 4.8 (2.7) | 5.9 (2.6) | 0.60 |
| De-novo GN | 26/29 (90%) | 15/16 (94%) | 8/9 (89%) | 4/4 (100%) | 1.00 |
| Treatment | |||||
| ● Corticosteroid | 29/29 (100%) | 16/16 (100%) | 9/9 (100%) | 4/4 (100%) | - |
| ● Rituximab | 11/29 (38%) | 9/16 (56%) | 2/9 (22%) | 0/4 (0%) | 0.09 |
| ● Plasmapheresis | 6/28 (21%) | 6/15 (40%) | 0/9 (0%) | 0/4 (0%) | 0.04 |
| ● Hemodialysis | 8/29 (28%) | 2/16 (12%) | 4/9 (44%) | 2/4 (50%) | 0.14 |
| Follow-up time, weeks | 8 (8) | 8 (7) | 14 (10) | 24 (40) | 0.48 |
| Outcomes | 0.65 | ||||
| ● Remission with relapse | 1/28 (3%) | 1/16 (6%) | 0/8 (0%) | 0/4 (0%) | |
| ● Remission without relapse | 24/28 (86%) | 14/16 (88%) | 6/8 (75%) | 4/4 (100%) | |
| ● Non-remission | 3/28 (11%) | 1/16 (6%) | 2/8 (25%) | 0/4 (0%) |
Remarks: Continuous variables were presented with median and interquartile range, while categorical variables were presented with frequency and percentage. Abbreviations: AKI, acute kidney injury; ANCA, antineutrophil cytoplasm antibody; ANA, anti-nuclear antibodies; cANCA, cytoplasmic ANCA; DAH, diffuse alveolar hemorrhage; ENT, ear nose throat; GI, gastrointestinal; GN, glomerulonephritis; MPO, anti-myeloperoxidase; NM, neuromuscular; N/V, nausea/vomiting; PR3, anti-proteinase 3; pANCA, perinuclear ANCA; RPGN; rapidly progressive glomerulonephritis; RF, rheumatoid factor.
3.2. Case Presentations
We described three cases of ANCA-GN following viral vectors (case #1) and mRNA (case #2 and #3) COVID-19 vaccines. Only one patient (case #2) was classified as microscopic polyangiitis (MPA) by ACR/EULAR 2022 [17].
Case #1
A 76-year-old female with type 2 diabetes mellitus, hypertension, and dyslipidemia presented with leg edema for 11 days prior to admission. She received the second COVID-19 vaccine (AZD1222) 3 months ago. A few weeks later, she developed chronic fever, weight loss, and productive cough. Several blood cultures were performed, yielding negative results. Non-contrast chest computed tomography (CT) revealed unusual interstitial pneumonia (UIP). Sputum cultures depicted normal flora. She denied a history of SARS-CoV-2 infection and had negative PCR for COVID-19. Her Scr was 1.5 mg/dL over the past year. Three-week before admission, her Scr increased to 1.7 mg/dL with 2+ proteinuria and erythrocyte of 10–20/HPF. A week later, she developed leg edema and foamy urine. Physical examination (PE) revealed temperature 38.9 °C, respiratory rate (RR) 20/min, blood pressure (BP) 165/91 mmHg, and bilateral 3+ leg pitting edema. Blood chemistries revealed hemoglobin 7.6 g/L, leukocyte count of 11.9 × 109/L with a neutrophil predominance (83%), Scr 3.5 mg/dL, and albumin 2.5 g/dL. Urinalysis demonstrated similar profiles with dysmorphic erythrocytes. The urine protein-creatinine ratio (UPCR) was 7.5 g/g creatinine. pANCA was positive with negative MPO-ANCA and PR3-ANCA. Serologies were positive for ANA (speckled pattern, 1:1280), RF 30 (0–20) IU/mL, and Coombs antibody (1+), while others were negative (e.g., anti-GBM antibody, viral hepatitis profiles, treponemal, and anti-HIV antibody). Complement levels were within normal values. The kidney biopsy disclosed 24 glomeruli with 7 global sclerosis (GS) and 7 cellular crescents. Fibrinoid necrosis of arteries and severe tubulointerstitial inflammation were observed. Interstitial fibrosis and tubular atrophy involved 30% of cortical tissue. IF revealed IgG (1+), C3 (1+), Kappa (1+), and Lambda (1+). Focal trivial subepithelial and intramembranous EDDs with diffuse thickening of GBM were observed on EM. Hence, ANCA-GN without extra-kidney organ involvement was diagnosed. Three-day of 1 gm/day pulse methylprednisolone and 500 mg pulse cyclophosphamide was started. Unfortunately, her kidney function remained deteriorated, requiring maintenance hemodialysis.
Case #2
A 69-year-old female presented with AKI while investigating anorexia, weight loss, and fatigue. She had hypertension for 2 decades and received 2 doses of AZD1222 and a COVID-19 vaccine booster with BNT162b2 (3 months ago). One month later, she developed anorexia, fatigue, a weight loss of 8 kg within 2 months, and established mixed conductive and sensorineural hearing loss. Chest X-ray and contrast computerized tomography (CT) revealed a subcentimeter pulmonary nodule. Two weeks after CT, her Scr increased from 0.7 to 2.4 mg/dL, and contrast-associated AKI was suspected. However, despite receiving an intravenous fluid infusion, she had a steadily increasing Scr. Her urinalysis revealed 1+ protein, leukocyte 50–100/HPF, and erythrocyte 3–5/HPF. Ciprofloxacin was prescribed on suspicion of having a urinary tract infection. PE revealed temperature 38.0 °C, BP 120/60 mmHg, marked pale conjunctivae, and no leg edema. Blood chemistries were notable for Scr 7.1 mg/dL, albumin 2.5 g/dL, hemoglobin 6.6 g/dL, leukocyte counts 12.1 × 109/L with neutrophils predominance (83%), and platelet counts 386 × 109/L. Urinalysis depicted trace protein, erythrocytes 3–5 cells/HPF, and UPCR 0.5 g/g creatinine. pANCA and MPO-ANCA were positive, with negative PR3-ANCA and cANCA. Serologies were positive, including ANA (speckled pattern, 1:80), cryoglobulin, and 1+ direct antiglobulin test, while the others were negative (e.g., anti-GBM antibody, RF, viral hepatitis profiles, treponemal, and anti-HIV antibody). Complement levels were within normal values. Serum protein electrophoresis and immunofixation demonstrated polyclonal gammopathy. No structural kidney abnormalities were detected by ultrasonography. The kidney biopsy disclosed 13 glomeruli with 2 GS and 10 cellular crescents. Fibrinoid necrosis was found on the vasculitic medium-sized artery. Mild tubulointerstitial fibrosis was observed. IF revealed non-specific staining for all immunoreactants. Hence, AAV with ANCA-GN was diagnosed. She received plasma exchange ×7 times, pulse methylprednisolone, and oral cyclophosphamide. One year later, she remained well without dialysis requirements and active AAV manifestation. Her serum creatinine 2.3 mg/dL and negative erythrocyturia.
Case #3
An 84-year-old female with underlying diseases of hypertension and dyslipidemia presented with a fever for one week prior to admission. She received complete primary series of AZD1222 and a booster dose of the COVID-19 vaccine with mRNA-1273 four months ago. One month later, she developed bilaterally severe sensorineural hearing loss and tinnitus. A few weeks before admission, she developed drowsiness, progressive dysphagia, decreased urine volume, and a weight loss of 8 kg. PE revealed BP 154/66 mmHg, temperature 38.5 °C, mildly pale conjunctivae, right eye episcleritis with afferent pupillary defect (RAPD), and 1+ leg edema. Blood chemistries were notable for Scr 5.1 mg/dL (baseline 0.9 mg/dL), albumin 2.7 g/dL, hemoglobin 9.6 g/dL, leukocyte counts 26.0 × 109/L with neutrophils predominance (80%), and platelet counts 209 × 109/L. Urinalysis depicted 2+ protein, leukocytes 30–50/HPF, erythrocytes 1–2/HPF, and UPCR 1.2 g/g creatinine. cANCA and MPO-ANCA were positive, with negative PR3-ANCA. Positive direct antiglobulin test at 1+ was detected, while others were negative (e.g., anti-GBM antibody, ANA, RF, viral hepatitis profiles, treponemal, and anti-HIV antibody). Complement levels were normal. Serum protein electrophoresis and immunofixation demonstrated polyclonal gammopathy. No structural kidney abnormalities were detected by ultrasonography. The kidney biopsy disclosed 20 glomeruli with 2 GS and 7 cellular crescents. Fibrinoid necrosis was noted in 6 glomeruli. Diffuse tubulointerstitial inflammation with abundant eosinophils and trivial tubulointerstitial fibrosis (<5%) were observed. IF revealed non-specific staining for all immunoreactants. ANCA-GN with eosinophilic interstitial nephritis was diagnosed. Due to the alteration of consciousness, magnetic resonance imaging (MRI) of the brain and orbits were performed, demonstrating Wallerian degeneration of the right corticospinal tracts and pons and bilateral optic neuritis. Intravenous pulse methylprednisolone and intravenous immunoglobulin were administered as per AAV. Temporary hemodialysis was initiated for 2 weeks. Subsequently, she remained well without AAV clinical flare with low-dose prednisolone and immunosuppressive agents; her Scr was 1.6 mg/dL with negative proteinuria and bland urine sediment.
4. Discussion
ANCA-GN secondary to COVID-19 vaccination was more prevalent in the patients with mRNA vaccinations (a median gap interval of 14 days), older age, and female gender. The top three most common manifestations were AKI, abnormal urine sediment, and constitutional symptoms. MPO-ANCA was distinctly dominant compared to the other ANCAs. Concomitant autoantibodies were documented in 52%, particularly Coombs antibody, ANA, and cryoglobulin. Although one-third (34%) presented with severe ANCA-GN, most cases had good outcomes, with a remission rate of 89%. No death occurred.
The higher immunogenicity and more common usage of the mRNA COVID-19 vaccine might explain its higher prevalence on ANCA-GN [18,19]. The median onset of ANCA-GN following COVID-19 vaccination was much shorter than other drug-induced ANCA-GN (14 days vs. 9 months) [20]. The difference might be related to the booster effect of the vaccine since most presented cases followed the second and third COVID-19 vaccinations. The booster dose of the COVID-19 vaccine increased the absolute risk of glomerular disease compared to naïve exposure [21]. The supporting evidence was noted in thioridazine-induced ANCA-AAV (leukocytoclastic vasculitis) that occurred 4 weeks after first exposure compared to a shorter duration of 24 h after rechallenging the dose [22] similar to minocycline-induced cutaneous lupus 1–2 days after repeated exposure compared to 23 months for the first exposure [23]. Moreover, one case series reported early onset of ANCA-AAV (12 days to 3 weeks) post-influenza vaccination [24].
Most AAVs have unknown etiology, known as primary AAVs; however, AAVs can be secondary to drugs (e.g., propylthiouracil, hydralazine, levamisole-contaminated cocaine) [9,25,26,27], vaccination (e.g., influenza and rabies vaccines) [28,29], and hematologic malignancies (e.g., leukemias, lymphomas) [26]. SARS-CoV-2 can infect host cells using the angiotensin-converting enzyme 2 (ACE2) receptor, expressed predominantly on endothelial cells’ surface, causing endotheliitis and hypercoagulable stage [30]. However, the vascular effects of COVID-19 vaccination have yet to be known. Irure-Ventura et al. [31] found that the incidence of new ANCA-positive patients had increased in 2021 compared to 2019 (2.4% vs. 1.2%).
AAV/ANCA-GN induced by vaccination has been well documented in post-influenza vaccination [28,32]. The authors performed an in-vitro experiment and found that the influenza vaccine (RNA vaccine) was able to induce PR3-ANCA production from peripheral blood mononuclear cells (PBMCs), while the decoy molecule, inactivated split virion influenza vaccine 2007, could not [33]. In addition, the AAV manifestations responded well to ribonuclease treatment in the vaccinated patients [33,34]. Unfortunately, the pathogenesis of ANCA-GN followed by the COVID-19 vaccine remains obscure [35].
COVID-19 vaccine induces ANCA-GN and AAV, possibly through molecular mimicry, stimulating productions of MPO-ANCA and PR3-ANCA via the adaptive immune system, likewise inducing antiviral neutralizing antibodies [36,37,38]. Then the ANCA ignites the inflammation cascade pathway by producing inflammatory cytokines (e.g., type 1 interferon, interleukin-6), activating Toll-like receptor (TLR), priming and activating neutrophils, causing neutrophils extracellular traps (NETs) formation, a mesh-like structure comprising DNA, histone, and neutrophil granules (e.g., MPO, PR3). Thus, MPO and PR3 on the NETs might further fuel the inflammatory fire and viciously start the ANCA production cascade [9,35,36,37,39]. mRNA vaccination in mice, monkeys, and humans activates TLR3, TLR4, and TLR7, causing robust cytotoxic T-lymphocyte activation [40,41,42]. Another possible mechanism of vaccine-inducing AVV involves monocytes upregulating human leukocyte antigen (HLA)-DR, which encodes cell-surface proteins responsible for regulating the immune system, in the patient at risk of AAV, having HLA-DR4 or DRB4 alleles [11,43]. Some HLA-DR alleles are connected with autoimmune disorders [43]. Our review found that concomitant positive autoantibodies were common (more than half) in COVID-19 vaccine-induced ANCA-GN/AAV patients. However, the interplaying mechanism between COVID-19 vaccine-induced ANCA-GN and autoantibodies needs further exploration.
Treatment of ANCA-GN following the COVID-19 vaccines in this systematic review mostly complied with the 2021 KDIGO Guidelines [16], whereby using a combination of glucocorticoid and cyclophosphamide or rituximab as the treatment backbone. Plasmapheresis was employed only in cases with severe AKI, double-positive ANCA, and anti-GBM antibody, or pulmonary hemorrhage [13,16]. Outcomes of ANCA-GN following COVID-19 were good, albeit severe manifestation, paradoxical to primary ANCA-GN with a remission rate of 89% compared to 39–48% [44]. However, this study has some limitations. First, the study included only articles in the English language. Secondly, this study pooled observational studies; thus, claiming a cause-effect relationship should be cautious.
5. Conclusions
ANCA-associated glomerulonephritis following the COVID-19 vaccine is rare, according to billions of vaccine doses that have been administered worldwide. The benefits of COVID-19 vaccination outweigh the risks of possible induced autoimmune diseases and ACNA-GN. Avoid unnecessary booster doses of the COVID-19 vaccine in high-risk patients (carrying HLA-DR4 or DRB4 alleles) should warrant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11050983/s1, Table S1: Clinical spectrums and outcomes of ANCA-associated glomerulonephritis following SARS-CoV-2 vaccination. Refs [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] mentioned in Supplementary Materials.
Author Contributions
Conceptualization, T.K.; Resources, T.K.; Writing—original draft preparation, T.T., A.B. and T.K.; Writing—review and editing, T.T., A.B., K.I., N.T. and T.K.; Supervision, N.T. and T.K.; Funding acquisition, T.T. and T.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Chulalongkorn University does not require ethical approval for reporting individual cases or case series.
Informed Consent Statement
Informed consents were obtained from all three patients or their descendants (if unavailable).
Data Availability Statement
Not applicable.
Conflicts of Interest
T.K. has received consultancy fees from VISTERRA, ELEDON, Otsuka OLE, and Otsuka VISIONARY as a country investigator is a current recipient of the National Research Council of Thailand and has received speaking honoraria from Astra Zeneca and Baxter Healthcare. The other authors declare that they have no conflict of interest.
Funding Statement
T.K. received funding support from the Thailand Science Research and Innovation Fund Chulalongkorn University (CU_FRB65_hea (19)_026_30_07) and Ratchadapisek Research Fund Chulalongkorn University (HEA663000115 and HEA663000116). T.T. received funding support from Naresuan University (No. MD2566C001).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO COVID-19 Dashboard. World Health Organization; Geneva, Switzerland: 2023. [(accessed on 2 May 2023)]. Available online: https://covid19.who.int/ [Google Scholar]
- 2.Li M., Wang H., Tian L., Pang Z., Yang Q., Huang T., Fan J., Song L., Tong Y., Fan H. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022;7:146. doi: 10.1038/s41392-022-00996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng H., Peng Z., Luo W., Si S., Mo M., Zhou H., Xin X., Liu H., Yu Y. Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines. 2021;9:582. doi: 10.3390/vaccines9060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thammathiwat T., Chompuk L., Worawichawong S., Boonpucknavig V., Sirilak S., Pongcharoen S., Pichitsiri W., Kanjanabuch T. Membranous Nephropathy following Full-Dose of Inactivated SARS-CoV-2 Virus Vaccination: A Case Report and Literature Review. Vaccines. 2023;11:80. doi: 10.3390/vaccines11010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klomjit N., Alexander M.P., Fervenza F.C., Zoghby Z., Garg A., Hogan M.C., Nasr S.H., Minshar M.A., Zand L. COVID-19 Vaccination and Glomerulonephritis. Kidney Int. Rep. 2021;6:2969–2978. doi: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caza T.N., Cassol C.A., Messias N., Hannoudi A., Haun R.S., Walker P.D., May R.M., Seipp R.M., Betchick E.J., Amin H., et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney360. 2021;2:1770–1780. doi: 10.34067/KID.0005372021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan-Chung C., Ong C.S., Chan L.L., Tan E.K. Eosinophilic granulomatosis with polyangiitis after COVID-19 vaccination. QJM. 2022;114:807–809. doi: 10.1093/qjmed/hcab273. [DOI] [PubMed] [Google Scholar]
- 8.Nakatani K., Sakata E., Fujihara M., Mizukawa K., Koyama T. Systemic Vasculitis Following SARS-CoV-2 mRNA Vaccination Demonstrated on FDG PET/CT. Clin. Nucl. Med. 2022;47:E403–E405. doi: 10.1097/RLU.0000000000004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuda S., Hirooka Y., Sugiyama M. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis after COVID-19 vaccination. Vaccines. 2021;9:842. doi: 10.3390/vaccines9080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirai T., Suzuki J., Kuniyoshi S., Tanno Y., Fujii H. Granulomatosis with Polyangiitis Following Pfizer-BioNTech COVID-19 Vaccination. Mod. Rheumatol. Case Rep. 2022;7:127–129. doi: 10.1093/mrcr/rxac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baier E., Olgemöller U., Biggemann L., Buck C., Tampe B. Dual-Positive MPO-and PR3-ANCA-Associated Vasculitis Following SARS-CoV-2 mRNA Booster Vaccination: A Case Report and Systematic Review. Vaccines. 2022;10:653. doi: 10.3390/vaccines10050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suppiah R., Robson J.C., Grayson P.C., Ponte C., Craven A., Khalid S., Judge A., Hutchings A., Merkel P.A., Luqmani R.A., et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Microscopic Polyangiitis. Arthritis Rheumatol. 2022;74:400–406. doi: 10.1002/art.41983. [DOI] [PubMed] [Google Scholar]
- 13.Jennette J.C., Nachman P.H. ANCA Glomerulonephritis and Vasculitis. Clin. J. Am. Soc. Nephrol. 2017;12:1680–1691. doi: 10.2215/CJN.02500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IT Governance Privacy Team . EU General Data Protection Regulation (GDPR), Third Edition: An Implementation and Compliance Guide. 3rd ed. IT Governance Publishing; Cambridgeshire, UK: 2019. [DOI] [Google Scholar]
- 15.Chapter 13: Pauci-immune focal and segmental necrotizing glomerulonephritis. Kidney Int. Suppl. 2012;2:233–239. doi: 10.1038/kisup.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rovin B.H., Adler S.G., Barratt J., Bridoux F., Burdge K.A., Chan T.M., Cook H.T., Fervenza F.C., Gibson K.L., Glassock R.J., et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:753–779. doi: 10.1016/j.kint.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Yazici H., Tascilar K., Yazici Y. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria sets for three types of antineutrophilic cytoplasmic antibody-associated vasculitis. Curr. Opin. Rheumatol. 2023;35:1–5. doi: 10.1097/BOR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 18.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Boulougoura A., Endo Y., Tsokos G.C. Abnormalities of T cells in systemic lupus erythematosus: New insights in pathogenesis and therapeutic strategies. J. Autoimmun. 2022;132:102870. doi: 10.1016/j.jaut.2022.102870. [DOI] [PubMed] [Google Scholar]
- 20.Deshayes S., Dolladille C., Dumont A., Silva N.M., Chretien B., De Boysson H., Alexandre J., Aouba A. POS0118 A worldwide pharmacoepidemiological update of drug-associated anca-associated vasculitis at the time of targeted therapies. Ann. Rheum. Dis. 2021;80:270. doi: 10.1136/annrheumdis-2021-eular.1465. [DOI] [Google Scholar]
- 21.Kronbichler A., Anders H.J. mRNA COVID-19 Vaccines and Their Risk to Induce a Relapse of Glomerular Diseases. J. Am. Soc. Nephrol. 2022;33:2128–2131. doi: 10.1681/ASN.2022091078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenfield J.R., McGrath M., Kossard S., Charlesworth J.A., Campbell L.V. ANCA-positive vasculitis induced by thioridazine: Confirmed by rechallenge. Br. J. Dermatol. 2002;147:1265–1267. doi: 10.1046/j.1365-2133.2002.05000_2.x. [DOI] [PubMed] [Google Scholar]
- 23.Lawson T.M., Amos N., Bulgen D., Williams B.D. Minocycline-induced lupus: Clinical features and response to rechallenge. Rheumatology. 2001;40:329–335. doi: 10.1093/rheumatology/40.3.329. [DOI] [PubMed] [Google Scholar]
- 24.Birck R., Kaelsch I., Schnuelle P., Flores-Suárez L.F., Nowack R. ANCA-Associated Vasculitis Following Influenza Vaccination: Causal Association or Mere Coincidence? JCR J. Clin. Rheumatol. 2009;15:289–291. doi: 10.1097/RHU.0b013e3181b55fe4. [DOI] [PubMed] [Google Scholar]
- 25.McGrath M.M., Isakova T., Rennke H.G., Mottola A.M., Laliberte K.A., Niles J.L. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin. J. Am. Soc. Nephrol. 2011;6:2799–2805. doi: 10.2215/CJN.03440411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folci M., Ramponi G., Shiffer D., Zumbo A., Agosti M., Brunetta E. ANCA-Associated Vasculitides and Hematologic Malignancies: Lessons from the Past and Future Perspectives. J. Immunol. Res. 2019;2019:1732175. doi: 10.1155/2019/1732175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoriello D., Bomback A.S., Kudose S., Batal I., Stokes M.B., Canetta P.A., Radhakrishnan J., Appel G.B., D’Agati V.D., Markowitz G.S. Anti-neutrophil cytoplasmic antibody associated glomerulonephritis complicating treatment with hydralazine. Kidney Int. 2021;100:440–446. doi: 10.1016/j.kint.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T. Vasculitis Following Influenza Vaccination: A Review of the Literature. Curr. Rheumatol. Rev. 2017;13:188–196. doi: 10.2174/1573397113666170517155443. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J., Cao J., Ye Q. Renal Side Effects of COVID-19 Vaccination. Vaccines. 2022;10:1783. doi: 10.3390/vaccines10111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malkan U.Y., Haznedaroglu I.C. Hematological aspects of the COVID-19 syndrome. Eur. Rev. Med. Pharmacol. Sci. 2022;26:4463–4476. doi: 10.26355/eurrev_202206_29086. [DOI] [PubMed] [Google Scholar]
- 31.Irure-Ventura J., Belmar-Vega L., Fernández-Fresnedo G., González-López E., Castro-Hernández C., Rodrigo-Calabia E., Heras-Vicario M., Ruiz San Millán J.C., López-Hoyos M. Increased induction of de novo serum ANCA and ANCA-associated vasculitis after mass vaccination against SARS-CoV-2. iScience. 2022;25:104847. doi: 10.1016/j.isci.2022.104847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Timmeren M.M., Heeringa P., Kallenberg C.G. Infectious triggers for vasculitis. Curr. Opin. Rheumatol. 2014;26:416–423. doi: 10.1097/BOR.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 33.Jeffs L.S., Nitschke J., Tervaert J.W., Peh C.A., Hurtado P.R. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin. Rheumatol. 2016;35:943–951. doi: 10.1007/s10067-015-3073-0. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Rao M., Xu G. New-Onset Acute Kidney Disease Post COVID-19 Vaccination. Vaccines. 2022;10:742. doi: 10.3390/vaccines10050742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassanzadeh S., Djamali A., Mostafavi L., Pezeshgi A. Kidney complications following COVID-19 vaccination; a review of the literature. J. Nephropharmacol. 2022;11:e1. doi: 10.34172/npj.2022.01. [DOI] [Google Scholar]
- 36.Prema J., Muthukumaran A., Haridas N., Fernando E., Seshadri J., Kurien A.A. Two Cases of Double-Positive Antineutrophil Cytoplasmic Autoantibody and Antiglomerular Basement Membrane Disease After BBV152/Covaxin Vaccination. Kidney Int. Rep. 2021;6:3090–3091. doi: 10.1016/j.ekir.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Y., Huang T., Xu G. ANCA-associated vasculitis following the CoronaVac vaccination. Ther. Adv. Chronic Dis. 2022;13:20406223221125708. doi: 10.1177/20406223221125708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cano-Gámez T., Teco-Cortes J.A., Soto-Abraham M.V., Álvarez-Hernández E. SARS-CoV-2 Vaccination as a Trigger for Perinuclear Antineutrophil Cytoplasmic Antibodies (p-ANCA) Associated With Rapidly Progressive Glomerulonephritis. Cureus. 2022;14:e29924. doi: 10.7759/cureus.29924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izzedine H., Bonilla M., Jhaveri K.D. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: True association or circumstantial? Nephrol. Dial. Transplant. 2021;36:1565–1569. doi: 10.1093/ndt/gfab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar N., Admane N., Kumari A., Sood D., Grover S., Prajapati V.K., Chandra R., Grover A. Cytotoxic T-lymphocyte elicited vaccine against SARS-CoV-2 employing immunoinformatics framework. Sci. Rep. 2021;11:7653. doi: 10.1038/s41598-021-86986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchan J. A vaccine built from potential immunogenic pieces derived from the SARS-CoV-2 spike glycoprotein: A computational approximation. J. Immunol. Methods. 2022;502:113216. doi: 10.1016/j.jim.2022.113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cagigi A., Loré K. Immune Responses Induced by mRNA Vaccination in Mice, Monkeys and Humans. Vaccines. 2021;9:61. doi: 10.3390/vaccines9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loo H.T., Hsu C.H., Chen L.F. HLA-DR4 and DRB4: Potential risk alleles for COVID-19 vaccination-related ANCA-associated vasculitis. Ther. Apher. Dial. 2022;27:593–594. doi: 10.1111/1744-9987.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Specks U., Merkel P.A., Seo P., Spiera R., Langford C.A., Hoffman G.S., Kallenberg C.G., St Clair E.W., Fessler B.J., Ding L., et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N. Engl. J. Med. 2013;369:417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderegg M.A., Liu M., Saganas C., Montani M., Vogt B., Huynh-Do U., Fuster D.G. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100:474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C.C., Chen H.Y., Lu C.C., Lin S.H. Case Report: Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis With Acute Renal Failure and Pulmonary Hemorrhage May Occur After COVID-19 Vaccination. Front. Med. 2021;8:2195. doi: 10.3389/fmed.2021.765447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidovic T., Schimpf J., Sprenger-Mähr H., Abbassi-Nik A., Soleiman A., Zitt E., Lhotta K. De Novo and Relapsing Glomerulonephritis following SARS-CoV-2 mRNA Vaccination in Microscopic Polyangiitis. Case Rep. Nephrol. 2021;2021:8400842. doi: 10.1155/2021/8400842. [DOI] [Google Scholar]
- 48.Dube G.K., Benvenuto L.J., Batal I. Antineutrophil Cytoplasmic Autoantibody–Associated Glomerulonephritis Following the Pfizer-BioNTech COVID-19 Vaccine. Kidney Int. Rep. 2021;6:3087–3089. doi: 10.1016/j.ekir.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feghali E.J., Zafar M., Abid S., Santoriello D., Mehta S. De-novo Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Following the mRNA-1273 (Moderna) Vaccine for COVID-19. Cureus. 2021;13:e19616. doi: 10.7759/cureus.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakroush S., Tampe B. Case Report: ANCA-Associated Vasculitis Presenting with Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front. Immunol. 2021;12:762006. doi: 10.3389/fimmu.2021.762006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaubschlager T., Rajora N., Diep S., Kirtek T., Cai Q., Hendricks A.R., Shastri S., Zhou X.J., Saxena R. De novo or recurrent glomerulonephritis and acute tubulointerstitial nephritis after COVID-19 vaccination: A report of six cases from a single center. Clin. Nephrol. 2022;97:289–297. doi: 10.5414/CN110794. [DOI] [PubMed] [Google Scholar]
- 52.Sekar A., Campbell R., Tabbara J., Rastogi P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021;100:473–474. doi: 10.1016/j.kint.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakoor M.T., Birkenbach M.P., Lynch M. ANCA-Associated Vasculitis Following Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021;78:611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villa M., Díaz-Crespo F., Pérez de José A., Verdalles Ú., Verde E., Almeida Ruiz F., Acosta A., Mijaylova A., Goicoechea M. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: Casualty or causality? Kidney Int. 2021;100:937–938. doi: 10.1016/j.kint.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.David R., Hanna P., Lee K., Ritchie A. Relapsed ANCA associated vasculitis following Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination: A case series of two patients. Nephrology. 2022;27:109–110. doi: 10.1111/nep.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia D.S., Martins C., da Fonseca E.O., de Carvalho V.C.P., de Rezende R.P.V. Clinical Images: Severe proteinase 3 antineutrophil cytoplasmic antibody glomerulonephritis temporally associated with Sinovac Biotech’s inactivated SARS-CoV-2 vaccine. ACR Open Rheumatol. 2022;4:277–278. doi: 10.1002/acr2.11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Hasbani G., Uthman I. ANCA-Associated Vasculitis following the First Dose of Pfizer-BioNTech COVID-19 Vaccine. Nephron. 2022;147:103–107. doi: 10.1159/000525562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim B.C., Kim H.S., Han K.H., Han S.Y., Jo H.A. A Case Report of MPO-ANCA-Associated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination. J. Korean Med. Sci. 2022;37:e204. doi: 10.3346/jkms.2022.37.e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noel E., Rashid U., Rabbani R., Khan W.A., Benjamin Y.S., Lee I. Antineutrophil Cytoplasmic Autoantibody-Associated Glomerulonephritis as a Possible Side Effect of COVID-19 Vaccination. Cureus. 2022;14:e30565. doi: 10.7759/cureus.30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obata S., Hidaka S., Yamano M., Yanai M., Ishioka K., Kobayashi S. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination. Clin. Kidney J. 2022;15:357–359. doi: 10.1093/ckj/sfab181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prabhahar A., Naidu G.S.R.S.N.K., Chauhan P., Sekar A., Sharma A., Sharma A., Kumar A., Nada R., Rathi M., Kohli H.S., et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: Case-based review. Rheumatol. Int. 2022;42:749–758. doi: 10.1007/s00296-021-05069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramezanzade E., Ghanbari R., Yazdanipour T. Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Glomerulonephritis in a 15-year-old Patient After Receiving the Second Dose of the BBIBP-CorV (Sinopharm) COVID-19 Vaccine: A Case Report. Nephro-Urol. Mon. 2022;14 doi: 10.5812/numonthly-127124. [DOI] [Google Scholar]
- 63.So D., Min K.W., Jung W.Y., Han S.W., Yu M.Y. Microscopic Polyangiitis Following mRNA COVID-19 Vaccination: A Case Report. J. Korean Med. Sci. 2022;37:e154. doi: 10.3346/jkms.2022.37.e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki M., Sekiguchi Y., Sasaki M., Inaba S., Oyama S., Inoue Y., Warabi M., Ohashi K., Inoshita S. Antineutrophil Cytoplasmic Antibody-associated Vasculitis after COVID-19 Vaccination with Pfizer-BioNTech. Intern. Med. 2022;61:2925–2929. doi: 10.2169/internalmedicine.9807-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yadav R., Shah S., Chhetri S. ANCA-associated vasculitis following Johnson and Johnson COVID-19 vaccine. Ann. Med. Surg. 2022;79:104123. doi: 10.1016/j.amsu.2022.104123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zamoner W., Scardini J.B., De Dio B.J., Marques A.D.M., Silva V.D.S., Garcia A.L., dos Santos D.C., Viero R.M. ANCA-associated vasculitis following Oxford-AstraZeneca COVID-19 vaccine in Brazil: Is there a causal relationship? A case report. Front. Med. 2022;9:1003332. doi: 10.3389/fmed.2022.1003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bansal S.B., Rana A.S., Manhas N., Rana A. Post COVID Vaccination (COVAXIN™ -BB152 V) Pauci-immune Crescentic Glomerulonephritis. Indian J. Nephrol. 2022;32:495–497. doi: 10.4103/ijn.ijn_352_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.