Abstract
The circadian clock is entrained to the daily cycle of day and night by light signals at dawn and dusk. Plants make use of both the phytochrome (phy) and cryptochrome (cry) families of photoreceptors in gathering information about the light environment for setting the clock. We demonstrate that the phytochromes phyA, phyB, phyD, and phyE act as photoreceptors in red light input to the clock and that phyA and the cryptochromes cry1 and cry2 act as photoreceptors in blue light input. phyA and phyB act additively in red light input to the clock, whereas cry1 and cry2 act redundantly in blue light input. In addition to the action of cry1 as a photoreceptor that mediates blue light input into the clock, we demonstrate a requirement of cry1 for phyA signaling to the clock in both red and blue light. Importantly, Arabidopsis cry1 cry2 double mutants still show robust rhythmicity, indicating that cryptochromes do not form a part of the central circadian oscillator in plants as they do in mammals.
INTRODUCTION
The circadian clock controls physiological and biochemical processes essential to the lives of all organisms. Possession of an endogenous oscillator allows an organism to anticipate dawn and dusk and to prepare for the marked environmental changes associated with these transitions (Dunlap, 1999). In mammals, the sleep/wake cycle and fluctuation in body temperature are both under the control of the circadian clock; in insects, processes such as larval eclosion can be timed to occur at the optimum time of day, giving an adaptive advantage important for survival; and in plants, the processing of the photosynthetic machinery begins just before dawn in preparation for the light-harvesting reactions.
Processes controlled by the circadian clock continue to oscillate with a period of ∼24 hr even in constant environmental conditions, thereby implicating the involvement of an endogenous oscillator. To be of use to the organism, however, this oscillator must first be synchronized to the environmental day/night cycle. The two most striking changes at dawn and dusk are in light and temperature, and both of these environmental factors are capable of entraining the circadian clock (Devlin and Kay, 2000a).
Recent studies have revealed much about the nature of the photoreceptors responsible for light input to the clock. In plants, light plays a crucial role in the regulation of development at every stage of the life history, and several plant photoreceptors have been well characterized (Johnson et al., 1994; Whitelam and Devlin, 1998). Of these, the phytochrome (phy) family, absorbing in the red region of the spectrum, and the cryptochrome (cry) family, absorbing in the blue, both mediate light input to the clock (Somers et al., 1998).
Phytochrome regulates a range of developmental processes in response to red and far-red light, including seedling establishment, shade avoidance, and transition to flowering. The phytochromes consist of a protein moiety of ∼124 kD with a covalently attached linear tetrapyrrole chromophore. The phytochrome molecule exists in two photointerconvertible forms—an inactive, red-absorbing form (Pr) and an active, far-red-absorbing form (Pfr) (Quail et al., 1995). Although the strongest absorption peak of phytochromes is in the red or far-red region of the spectrum, another important absorption peak occurs in the blue region; consequently, phytochromes have been implicated in mediating several responses to blue light as well (Whitelam et al., 1993; Casal and Mazzella, 1998; Neff and Chory, 1998).
Higher plants contain multiple phytochromes, the product of a multigene family. Arabidopsis has five phytochromes, phyA to phyE, and the function and modes of action of these distinct phytochrome species have been the subject of much recent investigation. Physiological studies of mutants deficient in one or more phytochromes have indicated both unique and overlapping roles for the various phytochrome family members (Whitelam et al., 1998).
Plant cryptochromes, which were discovered more recently (Ahmad and Cashmore, 1993; Hoffman et al., 1996), specifically mediate responses to blue light, showing a strong absorption peak in the blue region of the spectrum. However, because they also show a slight peak of absorption in the green region, cryptochromes have also been implicated in responses to green light (Lin et al., 1995; Whitelam, 1995).
The N termini of cryptochrome molecules show strong homology with the type II photolyase DNA repair enzymes. They possess two noncovalently linked chromophores, a light-harvesting pterin and a catalytic flavin (Cashmore et al., 1999). Arabidopsis contains two cryptochromes, cry1 and cry2, each with unique C-terminal extensions. Analysis of mutants deficient in one or both cryptochromes has revealed distinct roles for each (Cashmore et al., 1999).
The mode of action of the photoreceptors remains unclear, but several recent pieces of evidence have begun to shed light on it. Phytochrome and cryptochrome molecules linked to fluorescent protein tags have been used to study the subcellular localization of these molecules in living cells (Cashmore et al., 1999; Guo et al., 1999; Kircher et al., 1999; Yamaguchi et al., 1999). Both the phytochromes and the cryptochromes are localized in the cell nucleus; the phytochromes display a light-dependent nuclear localization (Kircher et al., 1999), whereas cryptochromes are constitutively nuclear (Cashmore et al., 1999; Guo et al., 1999; Mas et al., 2000). In addition, several molecules interacting with phyA and phyB have recently been identified by yeast two-hybrid screening. Phytochrome-interacting factor (PIF3; a basic helix-loop-helix transcription factor) (Ni et al., 1998), phytochrome kinase substrate (Fankhauser et al., 1999), and nucleoside diphosphate kinase (Choi et al., 1999) have all been identified as binding to phytochrome.
Interestingly, both cry1 and cry2 interact with phyA in vitro. Furthermore, in vivo evidence suggests that cry1 is phosphorylated in response to red light (Ahmad et al., 1998). The significance of this interaction has proved elusive, although missense mutations in cry1 have a slight, dominant negative effect on phyA signaling. Nonetheless, no decrease in phytochrome signaling has been demonstrated in a cry1 null mutant.
After their discovery in plants, cryptochromes were also discovered in insects and mammals (Cashmore et al., 1999). Cryptochrome mediates light input to the clock in Drosophila, exhibiting a light-dependent interaction with one of the molecules making up the clock mechanism (Ceriani et al., 1999). In contrast, the mammalian cryptochromes form part of a negative feedback loop that is itself the central oscillator (Kume et al., 1999). For example, mice deficient in both mCRY1 and mCRY2 are completely arrhythmic (van der Horst et al., 1999). Whether the mammalian cryptochromes also play any role in light-based resetting of the clock in mammals remains uncertain (Devlin and Kay, 1999, 2000a).
In Arabidopsis, as in other diurnal organisms, the length of the clock period decreases with increasing light intensity, a phenomenon known as Aschoff's rule (Aschoff, 1979). Using the CAB2::LUC construct to follow the circadian oscillation in CAB2 transcription, Millar et al. (1992)(1995) demonstrated that the period of oscillation decreases from a range of 30 to 36 hr in darkness to ∼24 hr in light. Furthermore, by generating fluence rate response curves to examine the effect of increasing intensities of red or blue light on period length in photoreceptor mutants, we demonstrated previously that phyA and phyB are involved in red light input to the clock and that phyA and cry1 are involved in blue light input to the clock (Somers et al., 1998).
Here, we have analyzed circadian photoperception in double and triple phytochrome and cryptochrome mutant combinations. We demonstrate that phyD and phyE also mediate red light input to the clock. We show that phyA and phyB act additively to regulate red light input to the clock, whereas cry1 and cry2 act redundantly in blue light input to the clock. Importantly, we demonstrate that cry1 and cry2 do not form part of the clock machinery itself in Arabidopsis, as they do in mammals. However, we demonstrate a novel role for cryptochrome in red light signaling downstream of phyA, specifically in light input to the clock, revealing a possible significance to the previously observed interaction between phyA and cryptochrome in vitro.
RESULTS
phyA, phyB, phyD, and phyE Act Additively in Red Light Input to the Clock
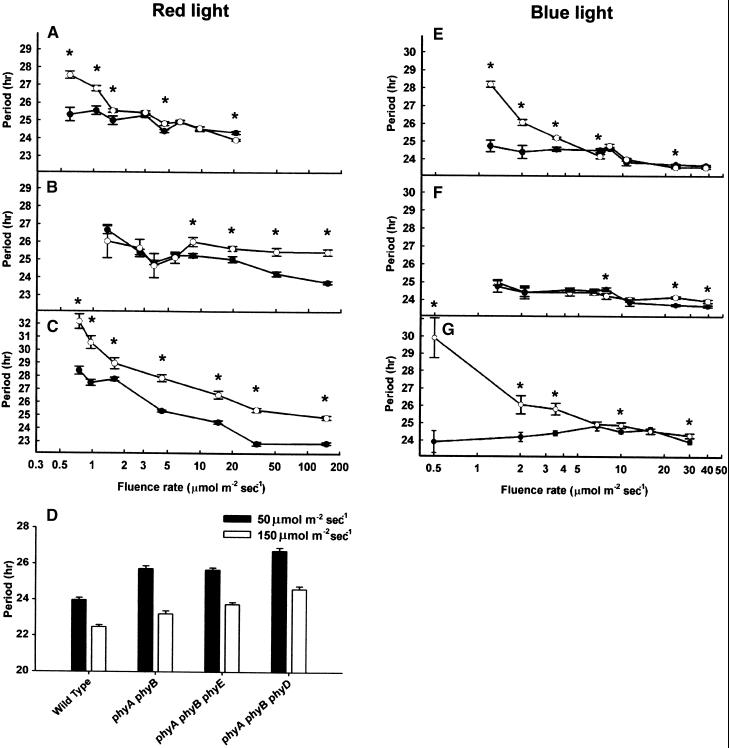
Wild-type and phytochrome mutant seedlings were entrained from germination for 6 days in 12-hr-white-light/12-hr-dark cycles, after which they were transferred to continuous red light at various different intensities. Circadian oscillation in the expression of CAB2::LUC was then monitored for 5 days more. As reported previously (Somers et al., 1998), in wild-type seedlings of Arabidopsis, the free running period of CAB2::LUC expression shortens with increasing intensity of red light. The phyA mutant, which is deficient in perception of low-fluence-rate red light, displays a longer than wild-type period under these conditions (Figure 1A). In contrast, the phyB mutant is deficient in perception of high fluence rates of red light (Figure 1B).
Figure 1.
Effect of Light Intensity on Period Length of the Circadian Rhythm of CAB2::LUC Bioluminescence in Wild-Type and Phytochrome-Deficient Seedlings.
Seedlings were germinated and grown in 12-hr-white-light/12-hr-dark cycles for 6 days and then transferred to constant red or blue light at the fluence rates indicated for 5 days. Values shown are means (±se) for wild type (closed circles) and mutants (open circles).
(A) Wild type and phyA mutant in red light.
(B) Wild type and phyB mutant in red light.
(C) Wild type and phyA phyB double mutant in red light.
(D) Mean period length for wild-type, phyA phyB, phyA phyB phyE, and phyA phyB phyD mutant seedlings in red light of 50 μmol m−2 sec−1 (solid bars) and 150 μmol m−2 sec−1 (open bars).
(E) Wild type and phyA mutant in blue light.
(F) Wild type and phyB mutant in blue light.
(G) Wild type and phyA phyB double mutant in blue light.
Asterisk, P < 0.01 (Student's two-tail heteroscedastic t test).
We examined the effect of fluence rate on length of period in phyA phyB double mutant seedlings. The phyA phyB double mutant displayed a long period across the whole range of red light fluence rates, indicating an additivity between phyA and phyB in red light control of period length (Figure 1C); that is, the effects attributable to the phyA and phyB mutations are combined in the double mutant.
The less abundant phytochromes phyD and phyE display a conditional redundancy with phyB (Devlin et al., 1998, 1999). Consistent with this, we observed a wild-type response to red light in the phyD and phyE monogenic mutants (data not shown). We then compared the roles of phyD and phyE in light input to the clock in the background of the phyA phyB double mutant. The highest two fluence rates of light were used for this comparison because too little CAB2 was expressed for detection in the triple mutants at low fluence rates. The phyA phyB phyD triple mutant displayed a slightly longer CAB2::LUC expression period than did the phyA phyB double mutant at high fluence rates of red light, indicating that phyD plays a role in the red light control of period length (Figure 1D). The phyA phyB phyE triple mutant was less responsive than the phyA phyB double mutant to red light at the highest light intensity used, which suggests a small but important role for phyE in control of period length by input of red light (Figure 1D). Both the phyA phyB phyD and phyA phyB phyE triple mutants retained a response to an increased fluence rate of red light, an indication that other phytochromes may also be active.
phyB Plays No Role in Blue Light Input to the Clock
The effect of fluence rate of blue light on the length of the free running period of the CAB2::LUC rhythm was examined in wild-type and phytochrome mutant seedlings. Seedlings were entrained in white light/dark cycles before transfer to blue light of different intensities for monitoring the circadian oscillation in CAB2::LUC expression. As reported previously (Somers et al., 1998), blue light shortens the free running period in wild-type seedlings from the dark period range of 30 to 36 hr (Millar et al., 1995). The phyA mutant was deficient in perception of low-fluence-rate blue light, displaying a longer than wild-type period length in those conditions. The phyB mutant, however, displayed a wild-type response to blue light for shortening the expression period (Figures 1E and 1F). The phyA phyB double mutant showed a lengthening of period at low fluence rates that was consistent with the loss of phyA but indicating no phyB function in blue light (Figure 1G).
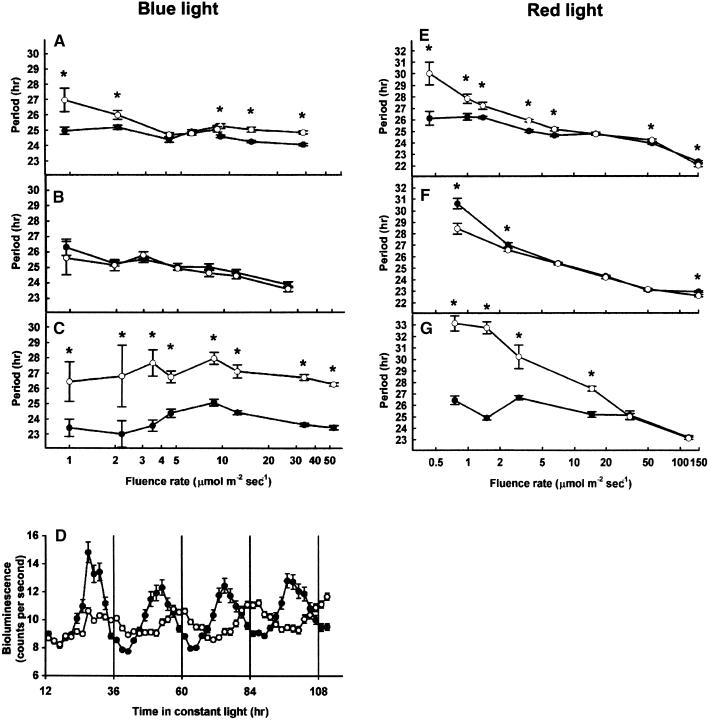
cry1 and cry2 Act Redundantly in Blue Light Input to the Clock and Are Not Essential for Circadian Rhythmicity
The length of the free running period of the CAB2 transcription rhythm was examined in cry1, cry2, and cry1 cry2 mutant seedlings in blue light. As previously observed (Somers et al., 1998), the cry1 mutant displayed a longer period than wild type at both low and high fluence rates of blue light but showed a wild-type period length at intermediate fluence rates of blue light. The cry2 mutant displayed a wild-type response across the whole range of blue light fluence rates, except for a slight decrease relative to wild type at the fluence rate of 2 μmol m−2 sec−1 (Figures 2A and 2B). The cry1 cry2 double mutant exhibited a long period of CAB2::LUC oscillation in all fluence rates of blue light, indicating a role for both cry1 and cry2 in perception of blue light in the control of the period length of the endogenous clock. cry1 and cry2 act with complete redundancy at intermediate fluence rates of blue light in the control of circadian period; that is, loss of both photoreceptors is required to see a change in phenotype over this range (Figure 2C). A role for cry2 at low and high fluence rates is also indicated because the cry1 cry2 double mutant shows a longer period than does the cry1 mutant at these fluence rates.
Figure 2.
Effect of Blue Light Intensity on Period Length of the Circadian Rhythm of CAB2::LUC Bioluminescence in Wild-Type and Cryptochrome-Deficient Seedlings.
Seedlings were germinated and grown in 12-hr-white-light/12-hr-dark cycles for 6 days and then transferred to constant blue or red light at the fluence rates indicated for 5 days. Values shown are means (±se) for wild type (closed circles) and mutants (open circles).
(A) Wild type and cry1 mutant in blue light.
(B) Wild type and cry2 mutant in blue light.
(C) Wild type and cry1 cry2 double mutant in blue light.
(D) Circadian rhythm of CAB2::LUC bioluminescence in wild-type and cry1 cry2 double mutant seedlings in blue light at 53 μmol m−2 sec−1.
(E) Wild type and cry1 mutant in red light.
(F) Wild type and cry2 mutant in red light.
(G) Wild type and cry1 cry2 double mutant in red light.
Asterisk, P < 0.01 (Student's two-tail heteroscedastic t test).
The oscillation of CAB2::LUC in the cry1 cry2 double mutant also has a slightly smaller amplitude at all fluence rates, consistent with a role for cryptochrome in the maintenance of a strong expression of CAB2 as well as in light input to the clock (Figure 2D).
Importantly, a robust rhythm of CAB2::LUC expression is still observed in the absence of both cry1 and cry2 (Figure 2D)—in sharp contrast to the situation in mammals, where loss of both cryptochromes results in arrhythmicity (van der Horst et al., 1999). This result, therefore, indicates a clear divergence in the organization of the mammalian and plant circadian machinery.
cry1 and cry2 Act in Red Light Input to the Clock
The period length of the rhythm of CAB2 transcription was examined in the cry1, cry2, and cry1 cry2 mutants in red light. The cry1 mutant displayed a deficiency in response to low-fluence-rate red light, showing a longer than wild-type period under these conditions, but demonstrated a wild-type period length in intermediate- and high-fluence-rate red light (Figure 2E). The cry2 mutant displayed a wild-type period length at all fluence rates of red light, except for a slightly shorter period at the lowest fluence rate examined (Figure 2F). The cry1 cry2 double mutant showed a long period of CAB2::LUC expression in both low and intermediate fluence rates of red light, and this effect extended to higher fluence rates than those at which cry1 deficiency alone is effective (Figure 2G). Furthermore, the effect of losing both cry1 and cry2 was a greater lengthening of the circadian period at low fluence rates than that seen with loss of cry1 alone (Figure 2G). This is consistent with a conditional redundancy in the action of cry1 and cry2 in red light signaling to the clock, the effect of cry2 deficiency being apparent only in a cry1 mutant background.
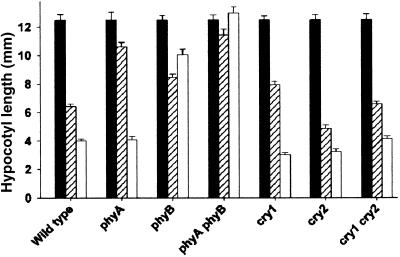
Because cryptochromes show no absorption peak in the red region of the spectrum (Lin et al., 1995), cry1 and cry2 may be acting as signal transduction components downstream of phytochrome, most notably in the range of fluence rates perceived by phyA. In fact, cryptochrome appears to be essential for phyA signaling to the clock. To test whether this is the case for other phytochrome-mediated responses to red light, we examined in phytochrome and cryptochrome mutants the effect of red light on inhibition of hypocotyl elongation during deetiolation and seedling establishment. One-day-old etiolated seedlings were grown in darkness or at one of several fluence rates of red light for 3 days, after which hypocotyl lengths were determined. Wild-type seedlings of Arabidopsis showed increasing inhibition of hypocotyl elongation with increasing fluence rate (Figure 3). Seedlings of the phyA mutant showed less inhibition of hypocotyl elongation at low fluence rates of red light, consistent with the range of fluence rates over which phyA acts in light input to the circadian clock (Figure 3). Seedlings of the phyB mutant, in contrast, showed decreased inhibition of hypocotyl elongation at high fluence rates of red light, consistent with the range of fluence rates over which phyB acts in light input to the circadian clock (Figure 3). The phyA phyB double mutant showed no noticeable response to red light at either low or high fluence rates, indicating that phyA and phyB account for the vast majority of the inhibition of hypocotyl elongation in red light (Figure 3). The cry1 and cry2 mutants behave very similarly to wild-type seedlings for inhibition of hypocotyl elongation at all fluence rates of red light (Figure 3). The cry1 mutant shows slightly longer hypocotyls than do wild-type seedlings in low fluence rates of red light, but this effect is much smaller than the effect observed in the absence of phyA. This indicates that cryptochrome is not essential for the phyA signaling in red light that controls inhibition of hypocotyl elongation (Figure 3).
Figure 3.
Effect of Red Light Intensity in Hypocotyl Length in Wild-Type and Photoreceptor-Deficient Seedlings.
One-day-old dark-grown wild-type and mutant seedlings were either maintained in darkness (solid bars) or transferred to low-fluence-rate red light (0.3 μmol m−2 sec−1; striped bars) or high-fluence-rate red light (30 μmol m−2 sec−1; open bars) for 3 days, after which hypocotyl lengths were measured. Mean (±se) hypocotyl lengths are normalized to dark-grown wild-type seedlings.
Mutations in phyA and cry1 Are Epistatic in Their Effects on Light Input to the Circadian Clock
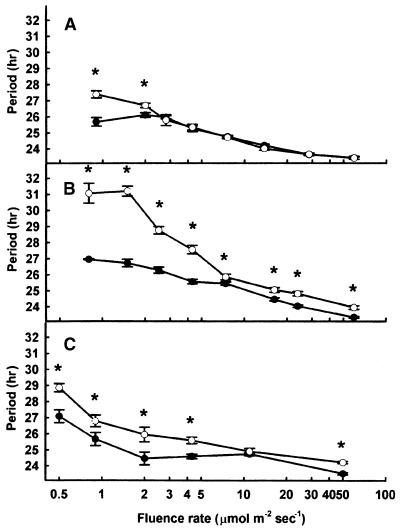
To test the hypothesis that cry1 may act downstream of phyA in light input to the clock in all wavelengths of light, as opposed to only in red light, we examined the fluence rate response curve for the length of the free running period of the CAB2::LUC rhythm in white light in phyA, cry1, and phyA cry1 mutants. Wild-type seedlings of Arabidopsis showed a decrease in period length in response to increasing the white light fluence rate (Figure 4A). The phyA and cry1 mutants were both deficient in response to low fluence rates of white light (Figures 4A and 4B), indicating that both phyA and cry1 are required for normal shortening of the period in response to low-fluence-rate white light, even though each alone is capable of acting as a photoreceptor under these conditions. At higher fluence rates of white light, the phyA mutant showed a wild-type period length consistent with the phenotype of phyA in monochromatic red or blue light. The cry1 mutant showed a deficiency in response to high-fluence-rate white light (Figure 4B), consistent with the decreased sensitivity to high-fluence-rate blue light in the cry1 mutant.
Figure 4.
Effect of White Light Intensity on Period Length of the Circadian Rhythm of CAB2::LUC Bioluminescence in Wild-Type and Phytochrome- and Cryptochrome-Deficient Seedlings.
Seedlings were germinated and grown in 12-hr-white-light/12-hr-dark cycles for 6 days and then transferred to constant red light at the fluence rates indicated for 5 days. Values shown are means (±se) for the wild type (closed circles) and mutants (open circles).
(A) Wild type and phyA mutant.
(B) Wild type and cry1 mutant.
(C) Wild type and phyA cry1 double mutant.
Asterisk, P < 0.01 (Student's two-tail heteroscedastic t test).
The phyA cry1 double mutant showed a deficiency in response to low-fluence-rate white light (Figure 4C). The phyA and cry1 mutations displayed epistasis at low fluence rates of white light; that is, no additivity was observed between the phyA and cry1 mutations. Curiously, the magnitude of the effect of loss of both phyA and cry1 at low fluence rates is less than that seen for the loss of cry1 alone. However, the lack of additivity between the phyA and cry1 mutations is consistent with the hypothesis that cry1 acts downstream of phyA in light signaling to the clock. The phyA cry1 double mutant showed a wild-type response to intermediate fluence rates of white light (10 μmol m−2 sec−1) but a deficient response to high-fluence-rate white light; this is consistent with a role of cry1 as a blue light photoreceptor in its own right at high fluence rates (Figure 4C).
Interestingly, the range of fluence rates over which cry1 acts to mediate both red and white light input to the clock extends farther than the range over which phyA acts (Figures 2E and 4B). In both red and white light, cry1 disrupts light input to the clock over some of the range of fluence rates in which phyB acts to mediate light to the clock. Thus, to some extent, cry1 may act downstream of phyB signaling, which may become important at the low end of the range of fluence rates over which phyB acts to mediate light input to the clock.
Loss of Phytochromes or Cryptochromes Does Not Affect the Circadian Clock in Darkness
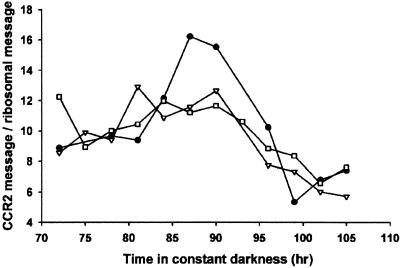
The length of the free running period of the circadian clock in wild-type, phyA phyB double mutant, and cry1 cry2 double mutant seedlings was examined in darkness. The circadian rhythm of CCR2 transcript abundance (Kreps and Simon, 1997) was used as a marker because the expression of CAB2 decreases rapidly in the absence of light. Seedlings were entrained in 12-hr-white-light/12-hr-dark cycles for 6 days and then transferred to constant darkness. Batches of seedlings were harvested after 72 hr in darkness and then at 3-hr intervals thereafter for another 33 hr. RNA was extracted from these seedlings, and after electrophoresis and transfer to nitrocellulose filters, samples were probed with a radiolabeled CCR2 probe. If the lengths of the circadian period in darkness were identical for wild-type and photoreceptor-mutant seedlings, the rhythms of CCR2 expression in both mutant and wild type would still be in phase over the course of the assay, despite the free running for 72 hr before sampling. Over the course of the 33-hr interval of the assay, the rhythm of CCR2 expression in wild type and in the phytochrome and cryptochrome double mutants coincided almost exactly, suggesting that there was no difference in period length between the different genotypes over the preceding 72 hr in darkness (Figure 5).
Figure 5.
Circadian Rhythm of CCR2 Expression in Wild-Type, phyA phyB, and cry1 cry2 Seedlings.
Seedlings were germinated and grown in 12-hr-white-light/12-hr-dark cycles for 6 days and then transferred to constant darkness. Tissue was harvested after 72 hr and then at 3-hr intervals thereafter for an additional 33 hr. Total RNA was extracted, and CCR2 RNA was quantified as described in Methods. Wild-type, closed circles; phyA phyB, open triangles; cry1 cry2, open squares.
DISCUSSION
Distinct and Overlapping Roles for Phytochromes and Cryptochromes in Light Input to the Circadian Clock
Analysis of Arabidopsis mutants deficient in multiple photoreceptors has revealed complex interactions between the various photoreceptors that mediate light input to the circadian clock. The action of phyA and phyB in red light is additive: phyA mediates low-fluence-rate red light input to the clock, whereas phyB mediates high-fluence-rate red light input to the clock. The phyA phyB double mutant behaves exactly as would be predicted from the combination of the two monogenic mutant phenotypes, pointing to a plasticity in the recruitment of these different photoreceptors for light input to the clock in different conditions. Roles for phyD and phyE in the perception of high-fluence-rate red light are also indicated. Importantly, the phyA phyB phyD and phyA phyB phyE triple mutants still show a strong response to an increase in the fluence rate, suggesting action by other phytochromes. Phytochrome-mediated responses have previously been observed in the phyA phyB phyD and phyA phyB phyE triple mutants in the shade avoidance response (Devlin et al., 1998, 1999). Generation of the phyA phyB phyD phyE quadruple mutant will be required to determine whether these four phytochromes alone can account for red light input to the clock or whether the remaining response in the triple mutants represents the action of phyC.
As was previously demonstrated (Somers et al., 1998), although the phyA mutant shows a deficiency in low-fluence-rate blue light input to the clock, the phyB mutant shows no defect in blue light input to the clock. Often a conditional redundancy is observed between phyA and phyB in which the action of one photoreceptor is able to compensate for the loss of another. In such cases, the effects of a mutation causing loss of one photoreceptor are apparent only in the absence of the other (Devlin et al., 1996). The response of the phyA phyB double mutant to blue light reveals no such conditional redundancy between phyA and phyB action in blue light, thus indicating that phyB is unlikely to play any role in blue light input to the clock.
The response of the cry1 cry2 double mutant reveals a conditional redundancy between cry1 and cry2 in blue light input to the clock. The action of cry2 in perception of intermediate fluence rates of blue light is apparent only in the absence of cry1, which otherwise compensates for loss of cry2 in the cry2 mutant.
Cryptochromes Are Not Required for Circadian Rhythmicity in Arabidopsis
The cry1 cry2 double mutant shows a strong circadian rhythm of CAB2::LUC expression in blue light. This is distinct from the phenotype of the mouse mCRY1−/− mCRY2−/− double mutant, which is arrhythmic in constant conditions (van der Horst et al., 1999). The mouse cryptochromes mCRY1 and mCRY2 form part of a transcriptional feedback loop that makes up the central circadian oscillator in mammals, and loss of both mCRY1 and mCRY2 stops the clock (Kume et al., 1999). The plant cryptochromes clearly do not act within the clock mechanism itself, which means that their role is distinct from that of the mammalian cryptochromes. This is consistent with a phylogenetic analysis of the animal and plant cryptochromes that suggests that cryptochromes arose independently in plants and animals (Cashmore et al., 1999). This analysis suggests that the plant cryptochromes diverged from the type II photolyases before the divergence of plants and animals, whereas the animal cryptochromes diverged more recently from the 6-4 photolyases. Animals are therefore presumed subsequently to have lost the cryptochrome sequences related to the type II photolyases (Cashmore et al., 1999). Intriguingly, however, despite their divergent origins and divergent roles, both animal and plant cryptochromes are associated with the circadian clock. Cryptochromes in insects are particularly interesting in that the role of the Drosophila cryptochromes is closer to that of the plant cryptochromes. Although dCRY interacts directly with the components of the central oscillator, it is not essential for the running of the clock, and its role is purely one of light input to the clock (Ceriani et al., 1999). Apparently, the recruitment of cryptochrome as the photoreceptor that mediates light input to the clock in insects and plants evidences considerable convergent evolution.
cry1 Is Required for phyA Signaling to the Clock
The response of the cry1 monogenic mutant and the cry1 cry2 double mutant to red light suggests roles for cry1 and cry2 as signal transduction components downstream of phyA and, to some extent, downstream of phyB in red light input to the clock.
Despite the fact that cryptochrome shows no peak of absorption in the red region of the spectrum (Lin et al., 1995), the cry1 mutant fails to perceive low fluence rates of red light that, in wild-type seedlings, mediate a shortening of the period of the clock. Furthermore, although the cry2 mutant shows a wild-type phenotype in red light, the cry1 cry2 double mutant displays a greater disruption of light signaling to the clock in low-fluence-rate red light than is seen in the cry1 monogenic mutant.
The effect of cryptochrome deficiency in red light input to the clock extends to higher fluence rates than the range over which phyA acts. Loss of the cryptochromes in red light also affects the lower end of the range of fluence rates over which phyB acts in light input to the clock. This suggests that some phyB signaling may also occur through cryptochrome. This effect is apparent only at the lower end of the range of fluence rates within which phyB acts, indicating that cryptochrome participation is not the major mechanism for phyB signaling; this finding may even represent some nonspecific action of phyB in the phyA signal transduction pathway. phyB can act in light input independently of cryptochrome, as evidenced by the normal response of the cryptochrome mutants at the higher end of the range of red light fluence rates over which phyB acts.
The fact that cry1 may be necessary for phyA signaling to the clock was further investigated. Both the phyA and cry1 monogenic mutants displayed a deficiency in the perception of low fluence rates of white light. Both phytochromes and cryptochromes act strongly as photoreceptors in white light (Koornneef et al., 1980; Johnson et al., 1994), and each is involved in light input to the clock under these conditions. The failure of each of these two photoreceptors to compensate for loss of the other suggests that they are not acting independently. Consistent with this, the phyA cry1 double mutant behaves like the cry1 mutant in that they both show a deficiency in response to white light over an almost identical range of fluence rates. The phyA cry1 double mutant shows, if anything, less of a deficiency in white light input to the clock than is seen in the cry1 mutant, although the reason for this remains unclear. Interestingly, the phyA cry1 double mutant demonstrates no additivity between the effects of the phyA and cry1 mutations, as might be expected for the combination of mutations within two components of a single pathway.
The cry1 mutant also showed a deficiency in perception of high-fluence-rate white light, consistent with the role of cry1 in perception of high fluence rates of blue light.
Ahmad et al. (1998) recently demonstrated that phyA directly interacts with cry1 and cry2 in vitro and that phyA mediates a red light–dependent phosphorylation of cry1. The action of cry1 and cry2 downstream of phyA provides the first evidence of a possible relevance for this interaction. phyA acts as a photoreceptor in this interaction, whereas cry1 and cry2 are acting purely as signal transduction components. That the cry1 allele used was null eliminates the possibility of any dominant negative effects by mutant cryptochrome on phyA signaling. The requirement for cryptochrome in phyA signaling in red light is independent of any light excitation of cry1 or cry2 because cryptochrome shows no substantial absorbance at red wavelengths (Lin et al., 1995). Cryptochromes have two chromophores, a pterin and a flavin (Cashmore et al., 1999), as do the photolyases. In type II photolyases, absorption of a photon of light by the light-harvesting pterin causes transfer of an electron to the catalytic flavin, which in turn donates this electron in a reaction that breaks any pyrimidine dimers that may have formed as a result of DNA damage (Cashmore et al., 1999). Cryptochrome has been proposed to make use of such redox signaling to trigger plant responses to light; perhaps the absorbance of light by phyA is also able to initiate this redox signal within the cryptochrome molecule. In addition, both phytochromes and cryptochromes are nuclear localized in light (Cashmore et al., 1999; Guo et al., 1999; Kircher et al., 1999; Yamaguchi et al., 1999; Mas et al., 2000), which suggests that the interaction between phyA and cryptochrome occurs in the nucleus. PhyA also interacts with the nuclear protein PIF3 (Ni et al., 1998), raising the possibility that phytochromes and cryptochromes may form part of larger signaling complexes in the nucleus.
This requirement for cryptochrome in red light signaling is not apparent in red light–mediated inhibition of hypocotyl elongation. Arabidopsis cry1 and cry1 cry2 mutant seedlings show a strong inhibition of hypocotyl elongation in both low and high fluence rates of red light. The strong inhibition of hypocotyl elongation seen in cryptochrome mutant seedlings in red light suggests that the requirement for cry1 in phyA signaling to the clock may be unique to the role of phyA in circadian photoperception.
Model for Circadian Photoperception in Arabidopsis
The circadian clock is of tremendous importance to plants. Not only does it allow them to prepare for the dawn, but it serves as a timekeeper to measure the length of day, a key determinant of flowering time in many species (Simpson et al., 1999; Devlin and Kay, 2000b). Plants show a plasticity in the recruitment of several different photoreceptors under different light conditions. In light input to the clock, all of the photoreceptors examined are capable of mediating the input under certain conditions. Circadian clocks in nature are synchronized to the day/night cycle on a daily basis by dawn and dusk signals: light pulses in the early morning cause phase advances, whereas light pulses in the evening cause phase delays (Daan and Pittendrigh, 1976; Aschoff, 1979). The shortening of periods by increasing light intensity, seen in our CAB2::LUC assay, is believed to be the net effect of phase advances and of delays occurring throughout the assay (Daan and Pittendrigh, 1976; Aschoff, 1979). A deficiency in the action of constant light in shortening periods in the various photoreceptor mutants should, therefore, reflect the involvement of these photoreceptors in entrainment of the clock. This is in agreement with our previous demonstration (Somers et al., 1998) that the deficiency in the perception of low-fluence-rate blue light in the phyA mutant affected both the period decrease in constant blue light and the ability to entrain to cycles of blue light and darkness. The involvement of all of the phytochromes and cryptochromes in this phase resetting allows correct entrainment of the circadian clock under a range of light conditions from direct sunlight to dense vegetative shade.
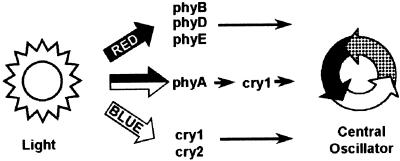
In summary, phyB, phyD, and phyE mediate high-fluence-rate red light input to the clock, and cry1 and cry2 mediate high-fluence-rate blue light input (Figure 6). Perception of both low-fluence-rate red light and low-fluence-rate blue light is mediated by phyA (Figure 6). This research also reveals a new role for plant cryptochrome as a component in the light signal transduction downstream of phytochrome (Figure 6). Although this light-independent role of cryptochrome appears to be limited to light input to the clock, it may, nonetheless, provide some more general clues in the search toward determining the mechanism of action of cryptochromes. Further research is also required to determine the nature of the central oscillator mechanism itself in plants. However, the characteristics of the circadian rhythm observed in the cry1 cry2 double mutant of Arabidopsis make clear that the mechanism of the circadian clock involves quite different molecules in plants and mammals.
Figure 6.
Elements Involved in Light Input to the Circadian Clock in Arabidopsis.
Both red light and blue light act in light input to the clock. The phytochromes phyB, phyD and phyE perceive high-fluence-rate red light signals, whereas the cryptochromes cry1 and cry2 perceive high-fluence-rate blue light signals. Low fluence rates of both red and blue light are perceived by phyA, with cry1 acting in a light-independent manner as a signal transduction component necessary for phyA action.
METHODS
Generation of Mutant Lines
Wild type, CAB2::LUC–expressing lines, and CAB2::LUC–expressing lines that were homozygous for phyA-201, phyB-1, cry1 (hy4-2.23N), or cry2-1 (Arabidopsis thaliana Columbia-4 ecotype) were those described previously (Somers et al., 1998). phyA phyB and phyA cry1 double mutant lines expressing CAB2::LUC were generated by crossing the respective monogenic mutants above and selecting for F2 plants homozygous for each mutation, using long hypocotyl in far-red light to screen for phyA, long hypocotyl in red light to select for phyB, and polymerase chain reaction to screen for cry1 according to the method described by Neff and Chory (1998). cry1 cry2 double mutants in the Landsberg erecta ecotype were selected from a cross of cry1 expressing CAB2::LUC with cry2 (fha1-1) by using polymerase chain reaction to select for cry1 (as above) and cry2 (with primers provided by D. Weigel, Salk Institute, La Jolla, CA; forward, 5′-GGTTTATCCTGGAAGAGCCTCAAGATG-3′; and reverse, 5′-CAAGAT-CGCTGAAATCGTGTTGT-3′), followed by digestion with BslI. This yielded fragments of 108 and 21 bp from the wild-type CRY2 gene and a single 129-bp fragment from the mutant cry2 (fha1-1) allele.
Light Sources
Broadband red light was obtained by filtering output from Sylvania (Danvers, MA) 20-W F20T12/2364 SR5965 red fluorescent tubes through one layer of medium red Roscolene plastic wrap No. 823 (Rosco, Stamford, CT). Broadband blue light was obtained by filtering output from Interelectric Corp. (Warren, PA) Biliblue 20-W F20T12/BBY fluorescent tubes through blue Plexiglas No. 2424 (Commercial Plastics, San Diego, CA). White light was provided by Philips (New York, NY) cool white 20-W F20T12/CW tubes. All light measurements were made with an LI-189 quantum radiometer (Li-Cor, Lincoln, NE).
Measurement of Period Length
Seeds of wild-type and photoreceptor mutant lines expressing a CAB2::LUC reporter construct (Millar et al., 1992) were sterilized and plated on solid Murashige and Skoog medium (Sigma) with 3% sucrose and kanamycin sulfate (50 μg mL−1). The seeds were stratified at 4°C in the dark for 4 days and then germinated and grown in 12-hr-white-light/12-hr-dark cycles for 6 days. Plastic supporting collars were placed over the seedlings (1.5-cm lengths of clear plastic drinking straws were sterilized by soaking in 70% ethanol; they were then dried and inserted into the agar medium) before transfer to constant red light (600 to 700 nm), blue light (400 to 500 nm), or white light at the intensities indicated. The rhythm of bioluminescence, representing CAB2 transcription, was monitored as described previously (Millar et al., 1995), and the period length was calculated by fitting a cosine wave function to the time series for each seedling (Millar et al., 1995; Somers et al., 1998). Each data point represents the mean (±se) for five to 18 seedlings. Each plot is representative of two to four independent experiments.
Measurement of Hypocotyl Length
Seeds of wild-type and photoreceptor-mutant lines were plated on solid Murashige and Skoog medium (Sigma), stratified at 4°C in the dark for 4 days, and then given a 2-hr white light pulse (50 μmol m−2 sec−1) at 22°C before being transferred to darkness, low-fluence-rate (0.3 μmol m−2 sec−1), or high-fluence-rate (30 μmol m−2 sec−1) red light. Hypocotyl length was measured after 3 days of treatments by using Scion Image software (Scion, Frederick, MD) to analyze digital images of seedlings laid out flat on agar plates. Data represent the mean (±se) of 30 seedlings for each treatment.
RNA Extraction and Gel Blotting
RNA extraction and detection of CCR2 message by RNA gel blotting were performed exactly as described by Somers et al. (2000).
Acknowledgments
This work was supported by a National Institutes of Health grant (No. GM 56006) to S.A.K. and by a European Molecular Biology Organization long-term fellowship (No. ALTF 720-1997) to P.F.D.
References
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 162–166. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., Jarillo, J.A., Smirnova, O., and Cashmore, A.R. (1998). The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1 939–948. [DOI] [PubMed] [Google Scholar]
- Aschoff, J. (1979). Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Z. Tierpsychol. 49 225–249. [DOI] [PubMed] [Google Scholar]
- Casal, J.J., and Mazzella, M.A. (1998). Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 118 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore, A., Jarillo, J.A., Wu, Y.J., and Liu, D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284 760–765. [DOI] [PubMed] [Google Scholar]
- Ceriani, M.F., Darlington, T.K., Staknis, D., Mas, P., Petti, A.A., Weitz, C.J., and Kay, S.A. (1999). Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science 285 553–556. [DOI] [PubMed] [Google Scholar]
- Choi, G., Yi, H., Lee, J., Kwon, Y.K., Soh, M.S., Shin, B., Luka, Z., Hahn, T.R., and Song, P.S. (1999). Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature 401 610–613. [DOI] [PubMed] [Google Scholar]
- Daan, S., and Pittendrigh, C.S. (1976). A functional analysis of circadian pacemakers in nocturnal rodents. III. Heavy water and constant light: Homeostasis of frequency? J. Comp. Physiol. 106 267–290. [Google Scholar]
- Devlin, P.F., and Kay, S.A. (1999). Blues news. Trends Cell Biol. 9 384. [DOI] [PubMed] [Google Scholar]
- Devlin, P.F., and Kay, S.A. (2000. a). Circadian photoperception. Annu. Rev. Physiol., in press. [DOI] [PubMed]
- Devlin, P.F., and Kay, S.A. (2000. b). Flower arranging in Arabidopsis. Science 288 1600–1602. [DOI] [PubMed] [Google Scholar]
- Devlin, P.F., Halliday, K.J., Harberd, N.P., and Whitelam, G.C. (1996). The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: Novel phytochromes control internode elongation and flowering time. Plant J. 10 1127–1134. [DOI] [PubMed] [Google Scholar]
- Devlin, P.F., Patel, S.R., and Whitelam, G.C. (1998). Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin, P.F., Robson, P.R., Patel, S.R., Goosey, L., Sharrock, R.A., and Whitelam, G.C. (1999). Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 119 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap, J.C. (1999). Molecular bases for circadian clocks. Cell 96 271–290. [DOI] [PubMed] [Google Scholar]
- Fankhauser, C., Yeh, K.C., Lagarias, J.C., Zhang, H., Elich, T.D., and Chory, J. (1999). PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284 1539–1541. [DOI] [PubMed] [Google Scholar]
- Guo, H., Duong, H., Ma, N., and Lin, C. (1999). The Arabidopsis blue light receptor cryptochrome 2 is a nuclear protein regulated by a blue light–dependent post-transcriptional mechanism. Plant J. 19 279–287. [DOI] [PubMed] [Google Scholar]
- Hoffman, P.D., Batschauer, A., and Hays, J.B. (1996). PHH1, a novel gene from Arabidopsis thaliana that encodes a protein similar to plant blue-light photoreceptors and microbial photolyases. Mol. Gen. Genet. 253 259–265. [DOI] [PubMed] [Google Scholar]
- Johnson, E., Bradley, M., Harberd, N.P., and Whitelam, G.C. (1994). Photoresponses of light-grown phyA mutants of Arabidopsis. Phytochrome A is required for the perception of daylength extensions. Plant Physiol. 105 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Kozma-Bognar, L., Kim, L., Adam, E., Harter, K., Schafer, E., and Nagy, F. (1999). Light quality–dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Rolf, E., and Spruit, C.J.P. (1980). Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z. Planzenphysiol. 100 147–160. [Google Scholar]
- Kreps, J.A., and Simon, A.E. (1997). Environmental and genetic effects on circadian clock-regulated gene expression in Arabidopsis. Plant Cell 9 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume, K., Zylka, M.J., Sriram, S., Shearman, L.P., Weaver, D.R., Jin, X., Maywood, E.S., Hastings, M.H., and Reppert, S.M. (1999). mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98 193–205. [DOI] [PubMed] [Google Scholar]
- Lin, C., Robertson, D.E., Ahmad, M., Raibekas, A.A., Jorns, M.S., Dutton, P.L., and Cashmore, A.R. (1995). Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 269 968–970. [DOI] [PubMed] [Google Scholar]
- Mas, P., Devlin, P.F., Panda, S., and Kay, S.A. (2000). Functional interaction of phytochrome B and cryptochrome 2. Nature 408 207–211. [DOI] [PubMed] [Google Scholar]
- Millar, A.J., Short, S.R., Chua, N.-H., and Kay, S.A. (1992). A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, A.J., Straume, M., Chory, J., Chua, N.-H., and Kay, S.A. (1995). The regulation of circadian period by phototransduction pathways in Arabidopsis. Science 267 1163–1166. [DOI] [PubMed] [Google Scholar]
- Neff, M.M., and Chory, J. (1998). Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 118 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95 657–667. [DOI] [PubMed] [Google Scholar]
- Quail, P.H., Boylan, M.T., Parks, B.M., Short, T.W., Xu, Y., and Wagner, D. (1995). Phytochromes: Photosensory perception and signal transduction. Science 268 675–680. [DOI] [PubMed] [Google Scholar]
- Simpson, G.G., Gendall, A.R., and Dean, C. (1999). When to switch to flowering. Annu. Rev. Cell Dev. Biol. 15 519–550. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Devlin, P.F., and Kay, S.A. (1998). Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282 1488–1490. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Schultz, T.F., Milnamow, M., and Kay, S.A. (2000). ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101 319–329. [DOI] [PubMed] [Google Scholar]
- van der Horst, G.T.J., et al. (1999). Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398 627–630. [DOI] [PubMed] [Google Scholar]
- Whitelam, G. (1995). Plant photomorphogenesis: A green light for cryptochrome research. Curr. Biol. 5 1351–1353. [DOI] [PubMed] [Google Scholar]
- Whitelam, G.C., and Devlin, P.F. (1998). Light signalling in Arabidopsis. Plant Physiol. Biochem. 36 125–133. [Google Scholar]
- Whitelam, G.C., Johnson, E., Peng, J., Carol, P., Anderson, M.L., Cowl, J.S., and Harberd, N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam, G.C., Patel, S., and Devlin, P.F. (1998). Phytochromes and photomorphogenesis in Arabidopsis. Philos. Trans. R. Soc. Lond. Ser. B 353 1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagatani, A. (1999). Light-dependent translocation of a phytochrome B–GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]