Abstract
Background: Hypertension and oxidative stress are involved in the pathophysiological mechanism of stroke. We aimed to investigate the modification impact of the pro-oxidant–anti-oxidant balance (PAB) on the association between hypertension and stroke recurrence (SR). Methods: A cross-sectional design was conducted from December 2019 to December 2020 in 951 stroke patients in six hospitals across Vietnam. Hypertension was defined using antihypertensive medication or systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. PAB was estimated using weighting methods based on smoking, drinking, and overweight/obesity with pro-oxidant capacity, diet quality, fruit intake, vegetable intake, and physical activity with antioxidant capacity. The higher PAB scores indicated a beneficial balance shifting toward antioxidant dominance. SR was diagnosed by neurologists. Moreover, sociodemographic and health conditions were included as covariates. Multiple logistic regression analyses were used to explore the associations and interactions. Results: The hypertension and SR proportions were 72.8% and 17.5%, respectively. hypertension was associated with an increased SR likelihood (odds ratio (OR) = 1.93; p = 0.004), whereas a higher PAB score was associated with a lowered SR likelihood (OR = 0.87; p = 0.003). Moreover, hypertension interacting with every one-point increment of PAB was associated with a lowered SR likelihood (OR = 0.83; p = 0.022). Conclusions: The harmful impact of hypertension on SR could be alleviated by PAB. The interplay of health behaviors should be highlighted in the intervention strategies for stroke prevention.
Keywords: stroke recurrence, hypertension, oxidative stress, pro-oxidant–antioxidant balance, health behavior
1. Introduction
To date, stroke remains the most common cause of disability and mortality world-wide, which leads to a substantial global health burden [1]. The highest rate of stroke incidence was found in Southeast Asian countries, including Vietnam [2]. Although multilevel and multisectoral prevention strategies have been applied, the stroke recurrence (SR) rate has not changed over the years [3,4]. Moreover, the risk of SR increases over time [5], causing continuously increasing issues in societies and medical systems around the world [6], as well as in Vietnam [7]. Therefore, closing the gap in stroke prevention strategies is a high priority.
About 90% of strokes are caused by the presence of modifiable risk factors, and the regulation of core health behaviors (such as diet, physical activity, weight, and smoking) could avert about 75% of this burden [6,8]. Hypertension (HTN) is the most frequent danger for stroke and recurrent events [9,10]. Although hypertensive management is the most important primary and secondary prevention strategy for stroke, managing blood pressure in stroke patients is complex and challenging [11]. Moreover, evidence-based prevention strategies are not obviously improving modifiable risk factors and recurrent cardiovascular events (including stroke) [12]. Therefore, exploring factors that could be accessible for modifying the harmful impact of HTN on SR is necessary for prevention strategies.
Oxidative stress (OS) is characterized by the pro-oxidant–antioxidant balance (PAB) shifting toward the pro-oxidant predominance, resulting in molecular damage [13,14,15]. The existing evidence showed that the OS response could derive from endogenous (e.g., metabolic processes) and exogenous sources (e.g., core health behaviors), which are involved in the pathological mechanism of chronic diseases [16], including stroke [17,18,19]. In the endogenous aspect, OS is linked closely to chronic inflammatory disorders, which were identified as the major triggers for cardiovascular diseases (CVDs). The close interaction between OS and inflammatory response was reported regarding the overexpression of reactive oxygen species (ROS)-producing enzymes (e.g., NADPH NOXs) or aggravated inflammatory phenotypes in the absence of antioxidant defense proteins (e.g., glutathione peroxidases, heme oxygenase-1, and superoxide dismutase) [20]. Moreover, OS plays a critical role in the mechanism of CVDs through endothelial dysfunction, of which oxidative enzyme systems (e.g., xanthine oxidase and NADPH oxidase) contribute to the inactivation of nitric oxide—an endothelial regulator, leading to endothelial dysfunction [21]. In the exogenous aspect, potential diets and nutraceuticals could reduce OS and display antioxidant effects, which represents a therapeutic target in CVDs [21]. Additionally, regulating PAB could be a promising strategy for stroke therapeutics [22].
The PAB can be approached from pro-oxidative and antioxidative perspectives, and both can be considered in terms of their individual chemical constituents or their pooled effect [23]. Pro-oxidants can be from intracellular sources (such as NADPH NOXs, peroxisomes, and mitochondria) and external sources (such as pollutants, ultraviolet light, and ionizing) [24], whereas endogenous antioxidants involve the products of cellular metabolism (e.g., peroxiredoxin, catalase, and superoxide dismutase), and exogenous antioxidants involve diets and medications [24]. In the literature, the PAB has been directly estimated in saliva, urine, plasma, and serum [23], demonstrating the contribution of exogenous and endogenous agents. However, for example, serum PAB cannot reflect each agent’s specific pro-oxidant and antioxidant potentials and the complex interaction between the agents. Therefore, an indirect measurement tool based on lifestyle, nutrition, and medication factors was developed and validated to evaluate the individual PAB status and its impact on health outcomes [25,26,27]. However, the evidence regarding the indirect estimation of PAB based on health behaviors impacting SR is limited. Therefore, we aimed to examine the relationship between health behavior-based PAB and SR. Further, the modification role of PAB in the association between HTN and SR was explored.
2. Materials and Methods
2.1. Study Population
A cross-sectional study was launched from December 2019 to December 2020 to enroll patients with stroke in Vietnam. Due to the COVID-19 crisis, six invited hospitals agreed to participate in our study. Stroke patients were identified by neurologists using the 10th revision of the International Classification of Disease (ICD-10) coding I60–I69 [28], who were recruited from rehabilitation, neurology, and cardiovascular departments. Patients with stable stroke conditions (e.g., a Mini-Mental State Examination score of ≥22, vital signs within normal limits, recovery after the acute stroke and hospitalized at least one month), aged ≥ 18 years, and being able to reply to questions were eligible for selection. Moreover, we excluded patients with aphasia and impairment of vision and cognition. A minimum sample of 715 was estimated using SAS v9.4 software based on eight predictors in multiple logistic regression with a power of 0.8 and an alpha of 0.1. A satisfactory sample of 951 qualified patients was recruited for the final analysis. This study was reviewed and approved by the Institutional Ethical Review Committee of the Hanoi School of Public Health, Vietnam (IRB No. 498/2019/YTCC-HD3 and No. 312/2020/YTCC-HD3).
2.2. Data Collection and Measurement
2.2.1. Data Collection Procedure
A four-hour training session regarding data collection and infection control (e.g., wearing a mask, washing hands, and physical distancing) during the COVID-19 pandemic was provided for the interviewers (including medical students, doctors, and nurses) according to the guidelines provided by the World Health Organization (WHO) [29] and the Ministry of Health in Vietnam [30]. Then, patients were asked to sign an informed consent form before participating in a 30 min face-to-face survey at the bedside of each patient.
2.2.2. Assessment of Outcome
Stroke recurrence (SR) was identified by asking patients and their families whether the current hospitalization was the first or recurrent (including the number of times) stroke. Then, patients were regrouped into the first stroke vs. recurrent stroke to facilitate the analysis because of the limited sample size for the number of times of recurrent events.
2.2.3. Assessment of Hypertension
A standard clinical manual aneroid sphygmomanometer was used for three-time measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP). The average SBP and DBP were computed. Additionally, participants’ information about taking antihypertensive medication was obtained. Hypertension was identified as using any antihypertensive medication, SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg [31,32].
2.2.4. Assessment of Pro-oxidant–Antioxidant Balance
The PAB was indirectly established in the literature based on multiple components and comparable weighting methods (such as equal-weighted, study data-based, literature review-derived, and Bayesian methods) [33,34]. In the current study, the PAB components were defined based on core health behaviors related to stroke, including overweight/obesity, smoking, and drinking with pro-oxidant properties, physical activity, diet quality, fruit intake, and vegetable intake with antioxidant properties.
Drinking frequency and smoking status were self-reported. Body mass index (BMI, kg/m2) was computed and classified into under/normal weight, overweight, and obesity [35,36].
Diet quality was assessed using the Dietary Approaches to Stop Hypertension Quality (DASH-Q) questionnaire [37], and the 10-item DASH-Q questionnaire was validated for use in the Vietnamese context [38]. Patients were asked how many days they ate the food items over the previous seven days. A DASH-Q score was summed (ranging between 0 and 70) and categorized into tertiles, with greater tertiles indicating healthier diet quality.
Fruit intake and vegetable intake were assessed using the STEPwise questionnaire with four typical questions that patients were asked about their consumption of fruit and vegetables in terms of the number of days per week and the number of servings per day. The WHO STEPwise questionnaire was validated in the Vietnamese context [39]. According to WHO recommendations adopted in Asian countries (including Vietnam) [40], a minimum daily intake of five servings of fruit and vegetables (including two fruit and three vegetable servings) was proposed to prevent chronic diseases.
Physical activity was assessed using a short version of the International Physical Activity Questionnaire (IPAQ) [41], which was validated for use in the Vietnamese context [42,43]. Patients were asked how many minutes per day and days per week over the previous seven days they undertook sitting, walking, and moderate and vigorous activities. Then, the sum score of minutes per week for each of those four activities was multiplied by 1, 3.3, 4, and 8, respectively, to estimate the equivalent metabolic task scored in minutes per week (MET/min-wk) [44]. A higher MET score indicated a more intensive PA level. According to WHO [45] and the Physical Activity Guidelines Advisory Committee [46], a minimum PA level of 600 to 1200 MET/min-wk is recommended for adults with chronic conditions or disabilities.
All components were categorized into three levels and assigned 0, 1, or 2 values, accordingly. Then, the PAB score was calculated by summing all components after weighting by multiplying the values with −1 for pro-oxidant and +1 for antioxidant (Table 1). The PAB score ranged from −6 to 8, with higher PAB scores indicating a beneficial balance shifting toward antioxidant dominance.
Table 1.
Categories and weights of pro-oxidant–antioxidant balance components.
| Component | Categories | Definition | Weights |
|---|---|---|---|
| Pro-oxidants | |||
| Smoking | Never | Never smoked | 0 |
| Used to | Former smoker | −1 | |
| Current | Currently smokes | −2 | |
| Drinking | None | Never drink | 0 |
| Moderate | Drink 1–4 times/month on average | −1 | |
| Heavy | Drink daily or at least ≥ 3 times/week | −2 | |
| BMI (kg/m2) | Under/normal weight | BMI < 23 | 0 |
| Overweight/obesity | 23 ≤ BMI < 27.5 | −1 | |
| Obesity | BMI ≥ 27.5 | −2 | |
| Anti-oxidants | |||
| Fruit intake | Low | <2 servings/day | 0 |
| Moderate | 2 servings/day | +1 | |
| High | >2 servings/day | +2 | |
| Vegetable intake | Low | <3 servings/day | 0 |
| Moderate | 3 servings/day | +1 | |
| High | >3 servings/day | +2 | |
| Diet quality | Low | DASH-Q ≤ 24 | 0 |
| Moderate | 24 < DASH-Q ≤ 35 | +1 | |
| High | DASH-Q > 35 | +2 | |
| Physical activity (MET-min/wk) | Low | MET < 600 | 0 |
| Moderate | 600 ≤ MET ≤ 1200 | +1 | |
| High | MET > 1200 | +2 |
2.2.5. Assessment of Covariates
Socio-demographic factors (including age, gender, educational attainment, occupation, social status, marital status, and ability to pay for medication) were self-reported.
Stroke classification was categorized based on the ICD-10 into infraction (including cerebral infarction (I63)), hemorrhage stroke (including subarachnoid hemorrhage (I60), intracerebral hemorrhage (I61), other nontraumatic intracranial hemorrhage (I62)), and others (including stroke not specified as hemorrhage or infarction (I64); occlusion and stenosis of precerebral arteries, not resulting in cerebral infarction (I65); occlusion and stenosis of cerebral arteries, not resulting in cerebral infarction (I66); other cerebrovascular diseases (I67); cerebrovascular disorders in diseases classified elsewhere (I68); and sequelae of cerebrovascular disease (I69)).
Comorbidity was judged using the 16-item Charlson comorbidity index (CCI), which is validated and used widely in Vietnam [47,48]. In the current study, items of cerebrovascular disease or stroke and dementia were not included in the comorbid conditions due to the study participants and exclusion criteria, respectively. Additionally, the item of depression was not counted as a comorbidity to avoid the duplicated assessment. The remaining items were regrouped into none vs. one/more CCI to simplify the analysis.
Depressive symptoms were assessed using a two-item patient health questionnaire (PHQ-2), which was suggested for use in busy medical settings [49,50]. Patients were asked about the frequency of being affected by positive or depressed moods in the past two weeks and were rated on a three-point scale from 0 (not at all) to 3 (nearly every day). The PHQ-2 score ranged from 0 to 6, and a score ≥ 2 was suggested for clinicians to ensure that the few cases of depression were not overlooked [51].
Health literacy (HL) (including three domains of healthcare, disease prevention, and health promotion) was estimated using a 12-item short-form survey (HLS-SF12). The difficulty of performing each item was rated from 1 (very difficult) to 4 (very easy) based on a 4-point Likert scale. The HL index (the standardized HL indices) was calculated using the following formula:
in which mean displays the average of 12 items, while 1, 3, and 50 display the minimal possibility of the mean, the range of the mean, and the chosen maximum HL index score, respectively. The HL index varied from 0 (worse HL) to 50 (best HL).
2.3. Statistical Analysis
First, descriptive analyses were performed, and the independent sample T-test (or Kruskal–Wallis test) and chi-square test were used for continuous and categorical variables, respectively. Second, logistic regression analysis was used to examine the relationship between HTN and PAB with SR. The independent variables (IVs) with p < 0.2 in the bivariate analysis were selected for the multiple regression models. The multicollinearity among those IVs was controlled by examining their correlation using Spearman’s correlation. Because moderate correlations were found between age and occupation (rho = 0.34) and between gender and PAB (rho = −0.35) (Table S1), the representative variables were selected for multiple analysis models, including age, education, ability to pay for medication, stroke classification, depressive symptoms, and CCI. Third, interaction analysis was used to explore the combined impact of HTN and PAB on SR. Furthermore, the results of the interaction model were visualized via a simple slope analysis using PROCESS Macro of SPSS for moderation analysis. The slope plots were drawn using the estimated values of SR probability for two categories of HTN (yes vs. no) by three levels of PAB (one standard deviation below the mean (−1SD), the mean, and one standard deviation above the mean (+1SD)). All analyses were conducted using SPSS version 22 (IBM Corp., Armonk, NY, USA), and the p-value < 0.05 was defined as a statistically significant result.
3. Results
3.1. Characteristics of Stroke Patients
Out of 951 patients with stroke, the proportions of SR and HTN were 17.5% and 72.8%, respectively. The age ranged from 19 to 99 years, and the proportion of patients aged ≥65 was 53.7%. Compared to participants with the first stroke, those with SR had a higher percentage of age ≥ 65 (p = 0.011), were men (p = 0.042), were retired or infirm (p = 0.021), and had an infarction stroke (p = 0.012), HTN (p < 0.001), and CCI (p < 0.001) (Table 2).
Table 2.
Characteristics and stroke recurrence (n = 951).
| Variables | Total | First Stroke | Recurrent Stroke | |
|---|---|---|---|---|
| n (%) | (n = 785, 82.5%) | (n = 166, 17.5%) | p | |
| Socio-demographics | ||||
| Age (years) | 0.011 | |||
| <65 | 440 (46.3) | 378 (48.2) | 62 (37.3) | |
| ≥65 | 511 (53.7) | 407 (51.8) | 104 (62.7) | |
| Gender | 0.042 | |||
| Woman | 388 (40.8) | 332 (42.3) | 56 (33.7) | |
| Man | 563 (59.2) | 453 (57.7) | 110 (66.3) | |
| Occupation | 0.021 | |||
| Working | 518 (54.5) | 441 (56.2) | 77 (46.4) | |
| Retirement or infirmity | 433 (45.5) | 344 (43.8) | 89 (53.6) | |
| Education attainment | 0.363 | |||
| Illiterate or elementary | 216 (22.7) | 181 (23.1) | 34 (20.5) | |
| Junior high school | 257 (27.1) | 204 (26.0) | 53 (31.9) | |
| Senior high school | 251 (26.4) | 206 (26.3) | 45 (27.1) | |
| College/university or higher | 227 (23.9) | 193 (24.6) | 34 (20.5) | |
| Ability to pay for medication | 0.129 | |||
| Very or fairly difficult | 423 (44.5) | 358 (45.6) | 65 (39.2) | |
| Very or fairly easy | 528 (55.5) | 427 (54.4) | 101 (60.8) | |
| Marital status | 0.979 | |||
| Married | 837 (88.0) | 691 (88.0) | 146 (88.0) | |
| Single or Widowed/Divorced/Separated | 114 (12.0) | 94 (12.0) | 20 (12.0) | |
| Social status | 0.335 | |||
| Low | 111 (11.7) | 88 (11.2) | 23 (13.9) | |
| Middle or high | 840 (88.3) | 697 (88.8) | 143 (86.1) | |
| HL index (mean ± SD) | 23.4 ± 10.0 | 23.5 ± 10.0 | 22.8 ± 9.9 | 0.426 |
| Health conditions | ||||
| Stroke classification | 0.012 | |||
| Hemorrhage | 220 (23.2) | 196 (25.0) | 24 (14.5) | |
| Infraction | 637 (67.1) | 511 (65.3) | 126 (75.9) | |
| Others | 92 (9.7) | 76 (9.7) | 16 (9.6) | |
| Depressive symptoms | 0.145 | |||
| No | 461 (48.5) | 372 (47.4) | 89 (53.6) | |
| Yes | 490 (51.5) | 413 (52.6) | 77 (46.4) | |
| CCI | <0.001 | |||
| No | 476 (50.1) | 415 (52.9) | 61 (36.7) | |
| Yes | 475 (49.9) | 370 (47.1) | 105 (63.3) | |
| Hypertension | <0.001 | |||
| No | 259 (27.2) | 232 (29.6) | 27 (16.3) | |
| Yes | 692 (72.8) | 553 (70.4) | 139 (83.7) | |
| PAB (median, IQR) | 1.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 0.0 (−1.0, 2.0) | <0.001 |
Abbreviation: HL, health literacy; SD, standard deviation; CCI, Charlson comorbidity index; PAB, pro-oxidant–antioxidant balance; IQR, interquartile range.
3.2. Determinants of Stroke Recurrence
Among the investigated factors, the multiple analysis results (Table 3) showed that HTN (odds ratio, OR, 1.93; 95% confidence interval, 95%CI, 1.23, 3.04; p = 0.004) and CCI (OR, 1.55; 95%CI; 1.08, 2.23; p = 0.017) were associated with a higher likelihood of SR. Whereas PAB was associated with a lower likelihood of SR (OR, 0.87; 95%CI, 0.80, 0.95; p = 0.003). Moreover, hemorrhage stroke had a lower likelihood of recurrence compared to infarction stroke (OR, 0.55; 95%CI, 0.34, 0.90; p = 0.019).
Table 3.
Determinants of stroke recurrence (n = 951).
| Variables | Stroke Recurrence | |||||
|---|---|---|---|---|---|---|
| Crude Model | Adjusted Model | |||||
| OR * | 95%CI * | p * | OR ** | 95%CI ** | p ** | |
| Socio-demographics | ||||||
| Age (years) | ||||||
| <65 | 1.00 | 1.00 | ||||
| ≥65 | 1.55 | 1.10–2.19 | 0.012 | 1.28 | 0.88–1.85 | 0.191 |
| Gender | ||||||
| Woman | 1.00 | |||||
| Man | 1.44 | 1.01–2.04 | 0.042 | |||
| Occupation | ||||||
| Working | 1.00 | |||||
| Retirement or infirmity | 1.48 | 1.05–2.07 | 0.022 | |||
| Education attainment | ||||||
| Illiterate or elementary | 1.00 | 1.00 | ||||
| Junior high school | 1.38 | 0.86–2.22 | 0.181 | 1.22 | 0.74–2.01 | 0.420 |
| Senior high school | 1.16 | 0.71–1.89 | 0.544 | 1.17 | 0.70–1.95 | 0.546 |
| College/university or higher | 0.93 | 0.55–1.57 | 0.808 | 0.87 | 0.50–1.52 | 0.638 |
| Ability to pay for medication | ||||||
| Very or fairly difficult | 1.00 | 1.00 | ||||
| Very or fairly easy | 1.30 | 0.92–1.83 | 0.129 | 1.18 | 0.82–1.71 | 0.361 |
| Marital status | ||||||
| Married | 1.00 | |||||
| Single or Widowed/Divorced/Separated | 1.01 | 0.60–1.68 | 0.979 | |||
| Social status | ||||||
| Low | 1.00 | |||||
| Middle or high | 0.78 | 0.47–1.28 | 0.336 | |||
| HL index | 0.99 | 0.97–1.01 | 0.426 | |||
| Health conditions | ||||||
| Stroke classification | ||||||
| Hemorrhage | 1.00 | 1.00 | ||||
| Infraction | 2.01 | 1.26–3.21 | 0.003 | 1.79 | 1.10–2.91 | 0.019 |
| Others | 1.71 | 0.86–3.41 | 0.121 | 1.68 | 0.82–3.43 | 0.152 |
| Depressive symptoms | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.77 | 0.55–1.09 | 0.145 | 0.79 | 0.56–1.12 | 0.194 |
| CCI | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.93 | 1.36–2.72 | <0.001 | 1.55 | 1.08–2.23 | 0.017 |
| Hypertension | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.16 | 1.39–3.35 | 0.001 | 1.93 | 1.23–3.04 | 0.004 |
| PAB | 0.84 | 0.77–0.91 | <0.001 | 0.87 | 0.80–0.95 | 0.003 |
Abbreviation: OR, odds ratio; CI, confidence interval; HL, health literacy; CCI, Charlson comorbidity index; PAB, pro-oxidant–antioxidant balance. * Results of bivariate logistic regression analysis. ** Results of multivariate logistic regression analysis adjusted for age, education, ability to pay for medication, stroke classification, depressive symptoms, and CCI.
3.3. Modification Impact of PAB on the Association between HTN and Stroke Recurrence
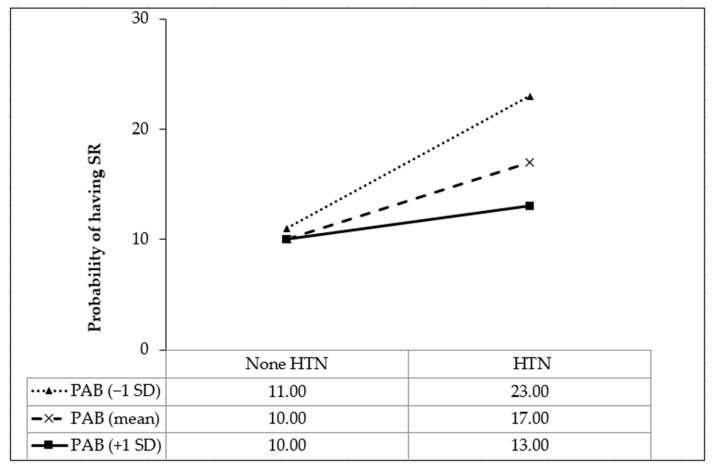
As shown in Table 4, compared to stroke patients without HTN and with the lowest PAB scores, those with HTN had an increased likelihood of SR (OR, 2.06; 95%CI, 1.28, 3.32; p = 0.003). However, the likelihood of SR decreased in stroke patients with HTN and with every one-point increment of PAB (OR, 0.83; 95%CI, 0.66, 0.94; p = 0.022). Moreover, the results of interaction were visualized in Figure 1. Simple slope analysis showed that the negative impact of HTN on SR was lowered by higher PAB values from −1SD (OR, 2.43; 95%CI, 1.29. 4.57; p = 0.005) to the mean (OR, 1.82; 95%CI, 1.16, 2.88; p = 0.008) and +1SD (OR, 1.36; 95%CI, 1.13, 2.58; p = 0.003).
Table 4.
Interaction of hypertension and pro-oxidant–antioxidant balance on stroke recurrence (n = 951).
| Interaction | Stroke Recurrence | |||||
|---|---|---|---|---|---|---|
| Crude Model | Adjusted Model | |||||
| OR * | 95%CI * | p * | OR ** | 95%CI ** | p ** | |
| Non-HTN × PAB (lowest score) | 1.00 | 1.00 | ||||
| HTN × PAB (lowest score) | 2.12 | 1.33–3.38 | 0.002 | 2.06 | 1.28–3.32 | 0.003 |
| Non-HTN × PAB (1-point increment) | 0.95 | 0.78–1.16 | 0.677 | 0.90 | 0.82–1.23 | 0.934 |
| HTN × PAB (1-point increment) | 0.86 | 0.69–0.97 | 0.012 | 0.83 | 0.66–0.94 | 0.022 |
Abbreviation: OR, odds ratio; CI, confidence interval; HTN, hypertension; PAB, pro-oxidant–antioxidant balance. * Results of bivariate logistic regression analysis. ** Results of multivariate logistic regression analysis, adjusted for age, education, ability to pay for medication, stroke classification, depressive symptoms, and comorbid conditions.
Figure 1.
Simple slope plot for the interaction between hypertension and pro-oxidant–antioxidant balance on stroke recurrence (n = 951). HTN, hypertension; PAB, pro-oxidant–antioxidant balance; SD, standard deviation.
4. Discussion
The current findings emphasize the positive impact of PAB on lowering SR, especially regarding the moderating role of PAB in mitigating the negative impact of HNT on SR.
Although OS is involved in the pathological mechanism of stroke, the study of OS in stroke patients is challenging to undertake because of the complexly interrelated processes. Further, OS is caused by numerous exogenous factors, and the combined (either addictive or synergistic) influences of those factors on the OS process should be considered. Therefore, a global estimation of the PAB score was established based on various dietary, lifestyle, and medication components [25]. In the literature, a low PAB score (reflecting excessive OS) was associated with poor health outcomes (such as chronic kidney diseases and all-cause mortality) [25], but little is known about PAB score and SR. Previous studies directly estimated the plasma PAB concentration and found heterogeneity associations between PAB and SR. Although the redox unbalances were greater in patients within one month of stroke onset than in their controls [52], the serum PAB concentration at the acute stroke phase was not associated with SR within five years [53]. To our knowledge, the current study was the first to find that a higher health behavior-based PAB score reflecting antioxidant properties could reduce the likelihood of SR.
The relationship between OS and inflammation is a vicious circle that links to HTN [54]. In stroke patients, persistent inflammatory responses are activated in those with HTN, which leads to an additional chain of OS procedures, and the HTN-OS correlation contributes to increasing SR [55]. That is why eliminating the free radical agents in the OS processes via “scavengers” is able to decrease the adverse consequences of stroke [55]. Previous studies showed that although the association between the antioxidative and anti-inflammatory capacities of a single dietary component with the hypotensive impact was not clear, adding such a component may become a new method for controlling the balance in OS–inflammation crosstalk [56]. Our findings supported the rationale that a greater PAB score could minimize the deleterious influence of HTN on SR probability. In the current study, the PAB score was estimated based on diet quality, fruit and vegetable intake, and physical activity in regard to anti-oxidative capacity. Therefore, the interplay of those components should be taken into consideration for the balanced regulation of OS and inflammation, as well as for preventing SR.
In recent decades, dietary nutrients have been used a novel intervention for building cardiovascular and neurological health due to their antioxidant properties [57]. However, the mechanism regarding the relationship between diet nutrients and neuro-cardiological diseases is not yet well defined, that metabolic control (including lipid, ROS, and energy metabolisms), immunological regulation (including astrocyte and microglial activation), and epigenetic modification (including non-coding RNA, DNA methylation, and histone modification) were assumed to be involved. Regarding dietary nutrients, the intervention programs focused on two forms of diet, including dietary supplements (such as vitamins and plant extracts) and dietary restriction (such as the DASH diet with decreasing cholesterol, total fat, and saturated fat and increasing fibers and minerals). Moreover, fruits and vegetables are rich sources of vitamins and plant extracts containing polyphenolic components, which are potent antioxidants and are reported to be beneficial to cardiovascular health [58]. However, the excessive intake of antioxidative dietary nutrients (e.g., iron and vitamin A) could lead to the side effects, such as cell death, because of ROS overproduction, causing OS. Additionally, there are anti-nutrient compounds contained in fruits and vegetables (such as tannins, oxalates, and lectins), which may threaten health [59]. Therefore, the approach of PAB needs to consider both the individual pro-oxidant and antioxidant components and their mutual effects.
The present study is the first to assess the potential role of PAB score in altering the relationship between HTN and SR. However, several limitations should be noted. First, the associations were recognized, but the causal inference could not be established in a cross-sectional study. Second, as the study participants were recruited in stable conditions of stroke, the findings could apply only to mild and moderate stroke patients but not to those with severe stroke conditions. However, the sample size was satisfactory for representativeness, with a power of evidence of up to 90%. Third, the questionnaires were responded to via self-reporting, and the “standard serving” size of food was not defined, which may lead to underestimation.
5. Conclusions
The current findings highlighted the critical contribution of PAB to prevent SR and mitigate the negative impact of hypertension on SR. Because multiple factors contribute to oxidative stress, we suggest that health promotion programs should include multiple dimensions to promote an overall healthy lifestyle to reduce oxidative stress. In the future research, the interplay of health behaviors should be considered in the strategic intervention of balancing the oxidative stress–inflammation relationship and preventing SR.
Acknowledgments
We sincerely thank the support of experts and researchers, as well as the patients from six hospitals participating in this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15102305/s1, Table S1: Spearman’s correlation (rho) among the studied variables (n = 951).
Author Contributions
Conceptualization, T.T.M.P., L.T.K.N., M.-T.V., K.M.P., M.H.N., T.C.L., B.N.D., L.T.H.L., N.H.D., T.T.P.N., H.P.L., C.Q.T., K.T.N., C.-J.H., C.-C.C., H.-C.H., C.-H.B. and T.V.D.; methodology, T.T.M.P., L.T.K.N., M.-T.V., K.M.P., M.H.N., T.C.L., B.N.D., L.T.H.L., N.H.D., T.T.P.N., H.P.L., C.Q.T., K.T.N., C.-J.H., C.-C.C., H.-C.H., C.-H.B. and T.V.D.; software, T.T.M.P., C.-H.B. and T.V.D.; formal analysis, T.T.M.P., C.-H.B. and T.V.D.; investigation, T.T.M.P., L.T.K.N., M.-T.V., K.M.P., M.H.N., T.C.L., B.N.D., L.T.H.L., N.H.D., T.T.P.N., H.P.L., C.Q.T., K.T.N. and T.V.D.; resources, C.-H.B. and T.V.D.; writing—original draft preparation, T.T.M.P., C.-H.B. and T.V.D.; writing—review and editing, T.T.M.P., L.T.K.N., M.-T.V., K.M.P., M.H.N., T.C.L., B.N.D., L.T.H.L., N.H.D., T.T.P.N., H.P.L., C.Q.T., K.T.N., C.-J.H., C.-C.C., H.-C.H., C.-H.B. and T.V.D.; supervision, C.-H.B. and T.V.D.; project administration, T.T.M.P., T.T.P.N., C.-H.B. and T.V.D.; funding acquisition, C.-H.B. and T.V.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethical Review Committee of Hanoi School of Public Health, Vietnam (IRB Nos. 498/2019/YTCC-HD3 and 312/2020/YTCC-HD3).
Informed Consent Statement
Written informed consent has been obtained from the stroke patients to publish this paper.
Data Availability Statement
The raw data supporting the findings of this article will be made available upon reasonable request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Science and Technology Council, Taiwan (grant number: MOST 111-2410-H-038-008-MY2).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Feigin V.L., Brainin M., Norrving B., Martins S., Sacco R.L., Hacke W., Fisher M., Pandian J., Lindsay P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke. 2022;17:18–29. doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flach C., Muruet W., Wolfe C.D.A., Bhalla A., Douiri A. Risk and Secondary Prevention of Stroke Recurrence: A Population-Base Cohort Study. Stroke. 2020;51:2435–2444. doi: 10.1161/STROKEAHA.120.028992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolmos M., Christoffersen L., Kruuse C. Recurrent Ischemic Stroke—A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2021;30:105935. doi: 10.1016/j.jstrokecerebrovasdis.2021.105935. [DOI] [PubMed] [Google Scholar]
- 5.Lin B., Zhang Z., Mei Y., Wang C., Xu H., Liu L., Wang W. Cumulative risk of stroke recurrence over the last 10 years: A systematic review and meta-analysis. Neurol. Sci. 2021;42:61–71. doi: 10.1007/s10072-020-04797-5. [DOI] [PubMed] [Google Scholar]
- 6.Feigin V.L., Roth G.A., Naghavi M., Parmar P., Krishnamurthi R., Chugh S., Mensah G.A., Norrving B., Shiue I., Ng M., et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 7.Pham T.L., Blizzard L., Srikanth V., Thrift A.G., Lien N.T., Thang N.H., Gall S.L. Case-fatality and functional status three months after first-ever stroke in Vietnam. J. Neurol. Sci. 2016;365:65–71. doi: 10.1016/j.jns.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 9.Pistoia F., Sacco S., Degan D., Tiseo C., Ornello R., Carolei A. Hypertension and Stroke: Epidemiological Aspects and Clinical Evaluation. High Blood Press. Cardiovasc. Prev. 2016;23:9–18. doi: 10.1007/s40292-015-0115-2. [DOI] [PubMed] [Google Scholar]
- 10.Wong Y.-S., Tsai C.-F., Ong C.-T. Risk factors for stroke recurrence in patients with hemorrhagic stroke. Sci. Rep. 2022;12:17151. doi: 10.1038/s41598-022-22090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wajngarten M., Silva G.S. Hypertension and Stroke: Update on Treatment. Eur. Cardiol. 2019;14:111–115. doi: 10.15420/ecr.2019.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridgwood B., Lager K.E., Mistri A.K., Khunti K., Wilson A.D., Modi P. Interventions for improving modifiable risk factor control in the secondary prevention of stroke. Cochrane Database Syst. Rev. 2018;5:Cd009103. doi: 10.1161/STROKEAHA.118.022213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niki E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016;595:19–24. doi: 10.1016/j.abb.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Sies H., Berndt C., Jones D.P. Oxidative Stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 15.Sies H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants. 2020;9:852. doi: 10.3390/antiox9090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orellana-Urzúa S., Rojas I., Líbano L., Rodrigo R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020;26:4246–4260. doi: 10.2174/1381612826666200708133912. [DOI] [PubMed] [Google Scholar]
- 18.Duan X., Wen Z., Shen H., Shen M., Chen G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid. Med. Cell. Longev. 2016;2016:1203285. doi: 10.1155/2016/1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignatelli P., Menichelli D., Pastori D., Violi F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018;76:713–722. doi: 10.5603/KP.a2018.0071. [DOI] [PubMed] [Google Scholar]
- 20.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M.T., Vujacic-Mirski K., Helmstädter J., Kröller-Schön S., Münzel T., et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019;2019:7092151. doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senoner T., Dichtl W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients. 2019;11:2090. doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., He Y., Chen S., Qi S., Shen J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020;158:104877. doi: 10.1016/j.phrs.2020.104877. [DOI] [PubMed] [Google Scholar]
- 23.Ialongo C. Preanalytic of total antioxidant capacity assays performed in serum, plasma, urine and saliva. Clin. Biochem. 2017;50:356–363. doi: 10.1016/j.clinbiochem.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Aranda-Rivera A.K., Cruz-Gregorio A., Arancibia-Hernández Y.L., Hernández-Cruz E.Y., Pedraza-Chaverri J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen. 2022;2:437–478. doi: 10.3390/oxygen2040030. [DOI] [Google Scholar]
- 25.Hernández-Ruiz Á., García-Villanova B., Guerra-Hernández E., Amiano P., Ruiz-Canela M., Molina-Montes E. A Review of A Priori Defined Oxidative Balance Scores Relative to Their Components and Impact on Health Outcomes. Nutrients. 2019;11:774. doi: 10.3390/nu11040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Ruiz Á., García-Villanova B., Guerra-Hernández E.J., Carrión-García C.J., Amiano P., Sánchez M.J., Molina-Montes E. Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS. Antioxidants. 2022;11:300. doi: 10.3390/antiox11020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Yuan H., Li Q., Geng S., Chen X., Zhu Y., Jiang H. Lifestyle-based oxidative balance score and its association with cardiometabolic health of the community-dwelling elderly: A cross-sectional secondary analysis. Front. Cardiovasc. Med. 2022;9:1000546. doi: 10.3389/fcvm.2022.1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization International Classification of Disease 10th Revision (ICD-10) [(accessed on 10 September 2021)]. Available online: https://icd.who.int/browse10/2019/en#I60-I69.
- 29.World Health Organization Country & Technical Guidance-Coronavirus Disease (COVID-19) [(accessed on 10 March 2020)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance.
- 30.Ministry of Health Coronavirus Disease (COVID-19) Outbreak in Vietnam. [(accessed on 5 May 2020)]; Available online: https://ncov.moh.gov.vn/
- 31.Van Minh H., Van Huy T., Long D.P.P., Tien H.A. Highlights of the 2022 Vietnamese Society of Hypertension guidelines for the diagnosis and treatment of arterial hypertension: The collaboration of the Vietnamese Society of Hypertension (VSH) task force with the contribution of the Vietnam National Heart Association (VNHA): The collaboration of the Vietnamese Society of Hypertension (VSH) task force with the contribution of the Vietnam National Heart Association (VNHA) J. Clin. Hypertens. 2022;24:1121–1138. doi: 10.1111/jch.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alwan A. Global Status Report on Noncommunicable Diseases 2010. World Health Organization; Geneva, Switzerland: 2011. p. 176. [Google Scholar]
- 33.Goodman M., Bostick R.M., Dash C., Terry P., Flanders W.D., Mandel J. A summary measure of pro- and anti-oxidant exposures and risk of incident, sporadic, colorectal adenomas. Cancer Causes Control. 2008;19:1051–1064. doi: 10.1007/s10552-008-9169-y. [DOI] [PubMed] [Google Scholar]
- 34.Mayne S.T., Wright M.E., Albanes D. Re: Hypothesis: Oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann. Epidemiol. 2007;17:930. doi: 10.1016/j.annepidem.2007.06.012. author reply 931. [DOI] [PubMed] [Google Scholar]
- 35.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 36.Pan W.H., Yeh W.T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008;17:370–374. [PubMed] [Google Scholar]
- 37.Warren-Findlow J., Reeve C.L., Racine E.F. Psychometric Validation of a Brief Self-report Measure of Diet Quality: The DASH-Q. J. Nutr. Educ. Behav. 2017;49:92–99.e91. doi: 10.1016/j.jneb.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen L.T.K., Do B.N., Vu D.N., Pham K.M., Vu M.T., Nguyen H.C., Tran T.V., Le H.P., Nguyen T.T.P., Nguyen Q.M., et al. Physical Activity and Diet Quality Modify the Association between Comorbidity and Disability among Stroke Patients. Nutrients. 2021;13:1641. doi: 10.3390/nu13051641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bui T.V., Blizzard C.L., Luong K.N., Truong Nle V., Tran B.Q., Otahal P., Srikanth V., Nelson M.R., Au T.B., Ha S.T., et al. Fruit and vegetable consumption in Vietnam, and the use of a ‘standard serving’ size to measure intake. Br. J. Nutr. 2016;116:149–157. doi: 10.1017/S0007114516001690. [DOI] [PubMed] [Google Scholar]
- 40.Kanungsukkasem U., Ng N., Van Minh H., Razzaque A., Ashraf A., Juvekar S., Masud Ahmed S., Huu Bich T. Fruit and vegetable consumption in rural adults population in INDEPTH HDSS sites in Asia. Glob. Health Action. 2009;2:1988. doi: 10.3402/gha.v2i0.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 42.Pham T., Bui L., Nguyen A., Nguyen B., Tran P., Vu P., Dang L. The prevalence of depression and associated risk factors among medical students: An untold story in Vietnam. PLoS ONE. 2019;14:e0221432. doi: 10.1371/journal.pone.0221432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen M.H., Pham T.T.M., Vu D.N., Do B.N., Nguyen H.C., Duong T.H., Pham K.M., Pham L.V., Nguyen T.T.P., Tran C.Q., et al. Single and Combinative Impacts of Healthy Eating Behavior and Physical Activity on COVID-19-like Symptoms among Outpatients: A Multi-Hospital and Health Center Survey. Nutrients. 2021;13:3258. doi: 10.3390/nu13093258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee P.H., Macfarlane D.J., Lam T.H., Stewart S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO) Global Recommendations on Physical Activity for Health. [(accessed on 26 December 2022)]. Available online: https://www.who.int/publications/i/item/9789241599979.
- 46.Piercy K.L., Troiano R.P., Ballard R.M., Carlson S.A., Fulton J.E., Galuska D.A., George S.M., Olson R.D. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P., Januel J.M., Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 48.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 49.Kroenke K., Spitzer R.L., Williams J.B. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med. Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 50.Espárrago Llorca G., Castilla-Guerra L., Fernández Moreno M.C., Ruiz Doblado S., Jiménez Hernández M.D. Post-stroke depression: An update. Neurologia. 2015;30:23–31. doi: 10.1016/j.nrl.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Manea L., Gilbody S., Hewitt C., North A., Plummer F., Richardson R., Thombs B.D., Williams B., McMillan D. Identifying depression with the PHQ-2: A diagnostic meta-analysis. J. Affect. Disord. 2016;203:382–395. doi: 10.1016/j.jad.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Ciancarelli I., Di Massimo C., De Amicis D., Carolei A., Tozzi Ciancarelli M.G. Evidence of redox unbalance in post-acute ischemic stroke patients. Curr. Neurovasc. Res. 2012;9:85–90. doi: 10.2174/156720212800410885. [DOI] [PubMed] [Google Scholar]
- 53.Mobarra N., Morovatdar N., Di Napoli M., Stranges S., Behrouz R., Amiri A., Farzadfard M.T., Hashemy S.I., Oskoii R., Khorram B., et al. The Association between Inflammatory Markers in the Acute Phase of Stroke and Long-Term Stroke Outcomes: Evidence from a Population-Based Study of Stroke. Neuroepidemiology. 2019;53:20–26. doi: 10.1159/000494685. [DOI] [PubMed] [Google Scholar]
- 54.Krzemińska J., Wronka M., Młynarska E., Franczyk B., Rysz J. Arterial Hypertension-Oxidative Stress and Inflammation. Antioxidants. 2022;11:172. doi: 10.3390/antiox11010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexandrova M.L., Bochev P.G. Oxidative stress during the chronic phase after stroke. Free Radic. Biol. Med. 2005;39:297–316. doi: 10.1016/j.freeradbiomed.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Ciancarelli I., Morone G., Iosa M., Cerasa A., Calabrò R.S., Iolascon G., Gimigliano F., Tonin P., Tozzi Ciancarelli M.G. Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome. Nutrients. 2023;15:108. doi: 10.3390/nu15010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao X.Y., Yin X.X., Guan Q.W., Xia Q.X., Yang N., Zhou H.H., Liu Z.Q., Jin W.L. Dietary nutrition for neurological disease therapy: Current status and future directions. Pharmacol. Ther. 2021;226:107861. doi: 10.1016/j.pharmthera.2021.107861. [DOI] [PubMed] [Google Scholar]
- 58.Barnard N.D., Goldman D.M., Loomis J.F., Kahleova H., Levin S.M., Neabore S., Batts T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients. 2019;11:130. doi: 10.3390/nu11010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petroski W., Minich D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020;12:2929. doi: 10.3390/nu12102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the findings of this article will be made available upon reasonable request to the corresponding authors.