Abstract
The blood-sucking bug Triatoma infestans is the main Chagas disease vector in the Southern Cone of Latin America. Populations resistant to pyrethroid insecticides have been detected in the early 2000s and then expanded to the endemic area of northern Salta province, Argentina. In this context, the entomopathogenic fungus Beauveria bassiana has been shown to be pathogenic to pyrethroid-resistant T. infestans. In this study, both the bioinsecticidal activity and the residual effect of an alginate-based microencapsulation of a native strain of B. bassiana (Bb-C001) were tested under semi-field conditions against pyrethroid-resistant T. infestans nymphs. Fungal microencapsulated formulation caused higher nymph mortality than the unmicroencapsulated fungus and contributed to maintaining the conidial viability throughout the period evaluated under the tested conditions. These results suggest that alginate microencapsulation is an effective, simple, low-cost method that could be incorporated into the formulation of a bioinsecticide as a strategy to reduce the vector transmission of Chagas disease.
Keywords: Chagas disease, resistant triatomines, entomopathogenic fungi, microencapsulated, bioinsecticide
1. Introduction
The blood-sucking insect Triatoma infestans (Hemiptera: Reduviidae), the main Chagas disease vector in the Southern Cone of Latin America, has been the target of continuous control programs to reduce the risk of disease transmission. The control strategy used has been the indoor application of pyrethroid insecticides [1]. However, it is recognized that this strategy has limited efficacy, mainly in the Gran Chaco area shared by Argentina, Bolivia and Paraguay [2]. Moreover, in the last years, several foci of pyrethroid-resistant T. infestans have been documented in wide regions of Bolivia and Argentina [3,4,5]. These difficulties highlight the need to search for new tools to control this vector. The entomopathogenic fungus Beauveria bassiana (Ascomycota: Hypocreales) has proven to be useful in field trials, showing successful results after its application in houses infested with pyrethroid-resistant T. infestans [6,7], and is therefore a promising alternative for the vector control of Chagas disease in areas with a high degree of resistance to pyrethroids.
The success of a biopesticide of microbial origin lies in a suitable formulation, which depends on the characteristics of the microorganism and the target insect, its relationship with the formulation components, storage conditions and the surface or place of application [8]. In recent years, most research has been focused mainly on the selection of more virulent strains, mass production and field experimentation, leaving aside the search and development of more stable and effective formulations during storage and after application, respectively [9].
In this regard, microencapsulation with biopolymers is among the most innovative tools to improve biological formulations. Microbial microencapsulation is a process by which microorganisms are coated or entrapped within a matrix to protect them from adverse environmental conditions [10,11,12]. Alginate (an anionic polysaccharide extracted from some algae and bacteria) is one of the most natural polymers used in the encapsulation of microorganisms, since it can form gel matrices (in the presence of calcium) around the microorganism [13]. In the case of entomopathogenic fungi, microencapsulation allows them to withstand factors such as solar radiation, temperature and relative humidity that degrade their viability, reducing the dose and number of applications [14,15,16,17].
In this study, the bioinsecticide and residual effect of a microencapsulated formulation based on alginate and conidia of B. bassiana were tested on pyrethroid-resistant T. infestans under semi-field conditions. This contribution might help substantially improve biological formulations, prolonging their effect and optimizing their effectiveness under field conditions.
2. Materials and Methods
2.1. Fungus Source and Culture
Beauveria bassiana strain Bb-C001 was used in this study. The fungus was isolated from a T. infestans cadaver in the Gran Chaco region, Salta province, Argentina (22°16′52.78″ S; 62°42′5.60″ W) [18], and is kept in the Mycological Culture Collection of the School of Natural Sciences, National University of Salta, Argentina.
Aerial conidia were produced by fermentation in solid substrate, using white rice as substrate. Polypropylene bags containing 200 g of rice with 100 mL of distilled water (2:1) were prepared and sterilized in an autoclave at 121 °C at 1 atm of overpressure for 20 min. The bags were inoculated with 20 mL of a conidia suspension with a concentration of 1 × 108 conidia mL−1, incubated at 27 °C for a period of 10 to 15 days and dried in an oven at 25 °C for 3 days to reduce humidity to below 5%. Conidia were harvested using a 50-mesh analytical sieve (pore diameter = 297 µm). The concentration per g of material and the initial viability were determined by using a hemocytometer. For viability assay, 5 µL of a conidial suspension was punctually seeded into 15 plates with PDA medium and incubated at 27 °C. Twenty-four hours later, two different areas per plate were observed under a microscope (40×). Germination percentage was calculated as the number of germinated conidia/total number of conidia × 100 [19,20]. Finally, the conidia obtained were packed in 50 mL caramel-colored glass bottles and kept at −4 °C until use. The whole procedure (from sowing to obtaining conidia) was performed in a laminar flow cabinet to avoid contamination.
2.2. Fungal Microencapsulation
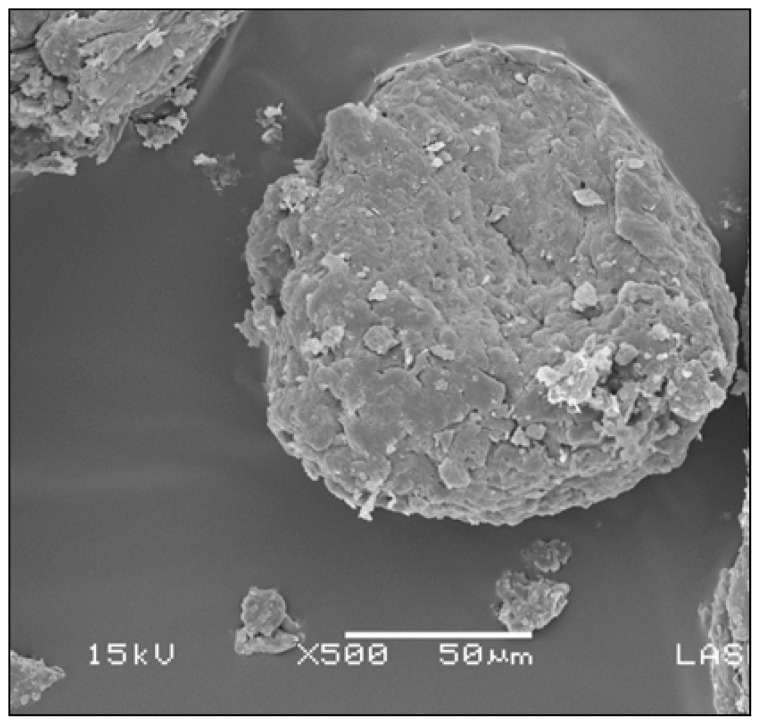
Conidial encapsulation was performed by ionic gelation, using sodium alginate as encapsulating matrix and 0.2 M calcium chloride (CaCl2), following the procedure described by Carrillo and Bashan with some modifications [21]. One gram of the active ingredient (1 × 1012 conidia/g) was mixed with 60 mL of 1% sodium alginate and then stirred up for 30 min. The mixture was sprayed in CaCl2 (300 mL) for the formation of microcapsules and allowed to consolidate under constant stirring for 1 h. The microcapsules were filtered and washed three times with sterile distilled water to remove CaCl2 residues. They were placed on sterile filter paper in a Petri dish and dried at 35 °C for 72 h. Subsequently, they were observed under scanning electron microscopy (SEM) to observe their structure (Figure 1) and stored in a hermetically sealed container with silica gel until use.
Figure 1.
Scanning electron micrograph (SEM) of an alginate microcapsule with B. bassiana conidia, scale 50 µm.
2.3. Insects
Fifth instar male nymphs of pyrethroid-resistant T. infestans were used. This insect colony comes from insects captured in Salvador Mazza locality, Salta province, Argentina (22°3′0″ S; 63°42′0″ W), and it is maintained in the Insectarium of the School of Natural Sciences, National University of Salta, at 30 °C, 50–60% relative humidity and a photoperiod of 12:12 h (light:dark). The insects were sexed following the procedure described by Brewer et al. [22] and fed with rat blood anesthetized with ketamine. All animal care and experimental laboratory protocols were carried out following the Regulations of the Institutional Committee for the Care and Use of Laboratory Animals and Field Studies (CICUALEC) of the Universidad Nacional de Salta.
2.4. Experimental Formulations
Two oil-based fungal formulations of 1 × 1012 conidia/g (initial viability of 99%) were prepared: one based on bare conidia (BbC) and the other based on microencapsulated conidia (MicBbC). An oily control without fungus was also prepared. Sunflower oil (Peng and Xia, 2011) was used as an oily vehicle [23], and the polymer Poloxamer 407 (P407) was incorporated into the mixture as a structured vehicle to obtain stable and easily redispersible formulations [24], and finally, diatomaceous earth (DE) was added as a thickener and adjuvant [25]. The proportions of each of the components in formulation are described in Table 1.
Table 1.
Proportion of components in formulation.
| Components | BbC 1 | MicBbC 2 | Control |
|---|---|---|---|
| Fungus (conidia/g) | 1 × 1012 | - | - |
| Microencapsulated (conidia/g) | - | 1 × 1012 | - |
| Sunflower oil (mL) | 30 | 30 | 30 |
| P407 (g) | 0.59 | 0.59 | 0.59 |
| DE (g) | 10 | 10 | 10 |
1 Fungal unmicroencapsulated formulation; 2 Fungal microencapsulated formulation.
2.5. Semi-Field Assays
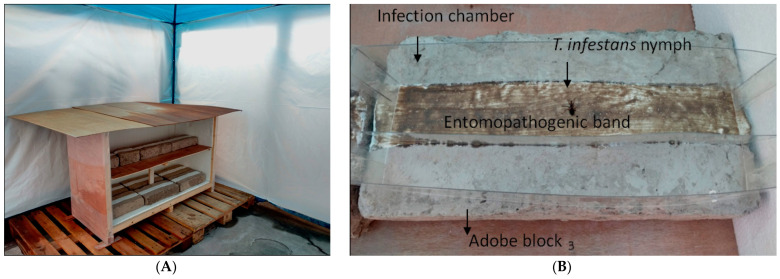
For the semi-field assays, adobe (sun-dried mudbricks) blocks of 8 × 38 × 19 cm (height, length and width, respectively) were used in order to simulate the interior walls of a house built with this material, typical of some native communities living in northern Salta province, Argentina. On the internal face of each adobe block, a surface area of 190 cm2 was waterproofed with liquid roofing membrane (SIKA®, Buenos Aires, Argentina) and allowed to dry for 24 h. Treatments were randomly assigned in triplicate. The formulations were applied with a brush on the membrane surface, constituting the “entomopathogenic band” (Figure 2B). The treated blocks were placed inside a gazebo for protection from rain, wind and UV radiation, which was located in a sector within the Laboratorio de Investigación y Producción de Biocontroladores (LIPBioc-MSPS, Ministerio de Salud Pública de la provincia de Salta, Argentina) facilities (Figure 2A). The tests were carried out at different times (day 0, 15, 30, 45 and 60) post-application of the entomopathogenic band.
Figure 2.
(A) Bioassay prototype for triatomine exposure to bioinsecticidal formulations. (B) Triatomines were exposed for 1 min on the entomopathogenic band for follow-up post-exposure.
To evaluate the entomopathogenic effect of the formulations, three replicates of 10 insects per treatment were used. The insects were placed on the entomopathogenic band and left in contact for 1 min; this procedure was performed with one insect at a time (Figure 2B). They were then transferred to plastic flasks and incubated at 27 °C, 50–60% RH and a photoperiod of 12:12 h, without feeding. Mortality was registered daily, and the mean mortality percentage and the time for 50% mortality (LT50) of insects treated with the formulations were calculated. Dead insects were put in a humid chamber to confirm that death was caused by fungal infection.
For viability and residual effect, samples of the different formulations were taken on the membrane band at the different times tested. Samples of the microencapsulated formulation (MicBbC) were placed in 10 mL of 0.2 M phosphate buffer (pH 7) with constant stirring for complete dissolution of the microcapsules, and the samples of the unmicroencapsulated fungus were placed in 10 mL of a 0.1% Tween 80 solution. Five aliquots (10 µL each) were taken from the suspension of each formulation and seeded in Petri dishes with selective medium (PDA mixed with copper oxychloride, cyproconazole, chloramphenicol and lactic acid to prevent the proliferation of other microorganisms) for triplicate and incubated at 27 °C for 24 h. Germination percentage was determined as described in Section 2.1.
2.6. Statistical Analyses
Statistical significance was used for mortality, which was assessed using ANOVA followed by Tukey’s multiple comparison test. LT50 was determined by constructing Kaplan and Meier survival curves. To find out if viability was affected by time, the data were arranged in a linear regression model. Graph Pad Prism v.8.0.1 GraphPad Software, San Diego, CA, USA) was used, with a significance level of 0.05 for statistical analyses.
3. Results
3.1. Entomopathogenic Effect of Both Bare and Microencapsulated Conidia on T. infestans Nymphs
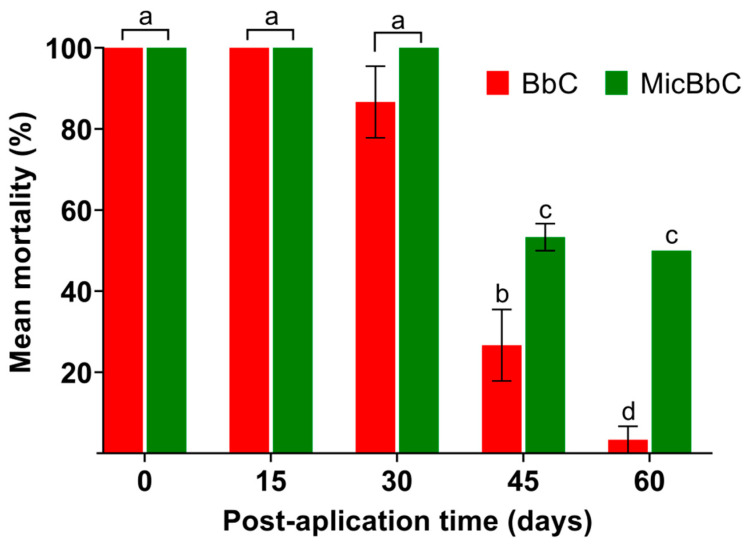
Statistically significant differences in mortality were observed between the evaluated formulations and the different post-application times (p < 0.0001). The MicBbC formulation caused, on average, higher nymph mortality than BbC at the end of the bioassays (Tukey, p < 0.05). During the first post-application month, high mortality was recorded for both BbC and MicBbC. From the second month onward, the mortality caused by BbC was significantly lower compared to MicBbC, which registered mortalities of about 50% (Tukey, p < 0.05) (Figure 3).
Figure 3.
Mean mortality (%) of the T. infestans nymphs treated with the B. bassiana formulations (BbC and MicBbC) at different post-application time periods. Different lowercase letters indicate significant differences between the different treatments at each time period and of each treatment over time (Tukey, p < 0.05). No mortality was observed in controls.
In relation to the LT50, statistically significant differences were observed according to the Kaplan and Meier tests between the formulations and the post-application times tested (p < 0.05). For the BbC formulation in the first month, the LT50 ranged between 5 and 10.5 days and were shorter than those observed for MicBbC which varied between 8.5 and 12.5 days. However, in the second month, the LT50 could only be calculated for the MicBbC formulation, and it varied between 26 and 28 days (Table 2).
Table 2.
Median lethal time (LT50) of the MicBbC and BbC formulations at different post-application time periods.
| Time (Days) 1 | BbC | MicBbC |
|---|---|---|
| LT50 (Days) | LT50 (Days) | |
| 0 2 | 5 | 8 |
| 15 | 10 | 11.5 |
| 30 | 10.5 | 12.5 |
| 45 | ND 3 | 26 |
| 60 | ND 3 | 28 |
1 Time post-application of the formulations on the membrane; 2 24 h after applying the membrane on the adobe blocks; 3 ND: not determined because it did not reach 50% mortality.
3.2. Viability and Residual Effect of Formulations
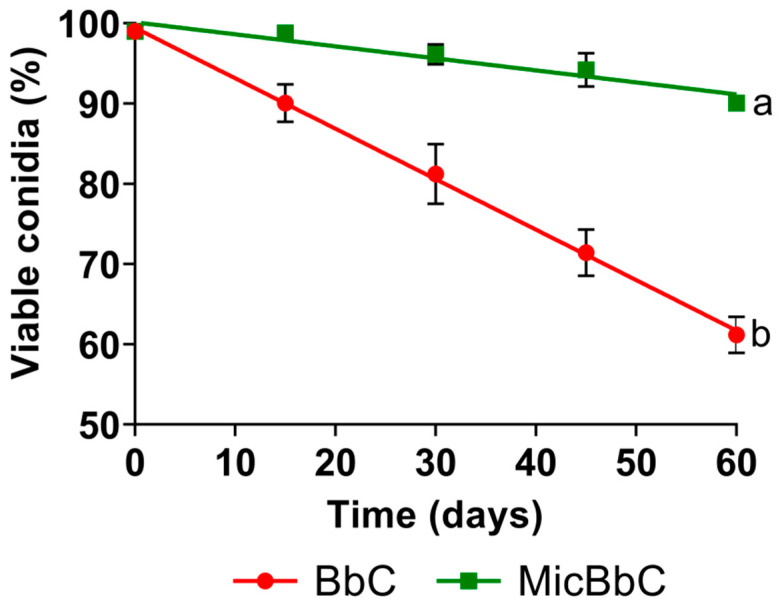
Conidia viability (calculated as germination percentage) showed significant differences between the formulations for the different post-application times evaluated (p < 0.0001). The differences were observed from day 15 post-application, and the highest viabilities were recorded for the formulated MicBbC, with values that ranged between 90 and 99%, while BbC showed a range between 61 and 90% for the different times tested. Regression analysis showed that there is a significant loss of viability over time that is different for each treatment, MicBbC (b = −0.15% viability day-1; p < 0.0001) and BbC (b = −0.63% viability day-1; p < 0.0001) (Figure 4).
Figure 4.
Viability of the experimental formulations (BbC and MicBbC) at different post-application times (0, 15, 30, 45 and 60 days). The negative slope indicates viability loss over time. Different letters indicate significant differences between the slopes of each line (p < 0.0001).
4. Discussion
The effectivity of the chemical control of the Chagas disease vector T. infestans based on indoor insecticide spraying as the sole tool is threatened by growing incidents of pyrethroid-resistant population detection [3,4,5]. A successful alternative for T. infestans indoor control is the use of the entomopathogenic fungus B. bassiana, which has shown good indoor biopesticide performance in field assays and has minimal risk to the environment and other organisms [6,7]. However, the adverse effects potentially caused by environmental conditions to which the fungus may be subjected as a living organism may be an inconvenience for its use in the long term. In this study, we formulated B. bassiana conidia in an alginate-based microencapsulation and tested its biopesticide activity against T. infestans nymphs on adobe blocks to simulate the interior walls of a typical house of this endemic area.
We found that the microencapsulated formulation (MicBbC) caused, on average, higher mortality in T. infestans nymphs than the unmicroencapsulated fungus (BbC) at all post-application times tested. During the first month, no differences were observed between both treatments, which caused high insect mortality. This might be due to that an active initial inoculum, with a high concentration and high viability (1 × 1012 conidia/g and 99%, respectively), was used in both treatments. It is well-known that the addition of oil to the fungal formulations as an adjuvant allows one to protect bare conidia, increasing stability and persistence in the conditions tested [23] without affecting their activity or interfering with the infection process [26,27] or the microencapsulation formation process. In the second month, there was an abrupt decrease in mortality in the nymphs treated with the BbC formulated compared to the MicBbC. Bare conidia (BbC formulation) were more exposed to adverse environmental conditions (low humidity and high temperatures), which caused a decrease in conidia survival in the short term and a loss of pathogenicity, in agreement with previous reports in other insects [28,29,30]. Conversely, in the MicBbC formulation, the conidia were protected by the gel matrix that contributed to maintaining their pathogenic capacity for longer periods. These results are also consistent with those reported by other authors [15,31,32].
It was also observed that the LT50 were higher for the MicBbC formulation throughout the evaluated period; however, this delay in the development of mycosis due to the slow and gradual release of the encapsulated microorganism did not affect total mortality, in concordance with previous reports [11,17]. On the contrary, the “slow-kill” bioinsecticide effect of the microencapsulated formulation may be advantageous for disease vector control, not only for the obvious extension of the useful life as a pathogen on the action surface but also by reducing the survival of the host without instant killing, which allows the fungus long-term control with lower possibilities of developing resistance [33].
Regarding viability, a greater loss was observed in the BbC compared with the MicBbC formulation for all the times tested. Other studies reported that the encapsulated conidia remain viable during storage, ensuring their persistence over time [16,34,35]. On the other hand, the incorporation of the pharmaceutical excipient P407 as a polymeric vehicle to improve the physical properties of the formulations allowed obtaining stable, homogeneous and redispersible formulations by simple agitation, facilitating its application as a bioinsecticide [36].
5. Conclusions
Microencapsulation with alginate proved to be an effective, simple and low-cost method that allows the biological control agent to carry out the infective process on the host more efficiently. These preliminary tests will serve to develop an optimal formulation, which allows maintaining the viability and virulence of B. bassiana conidia during storage and after application, which will contribute to a more effective infection of T. infestans in the field. Thus, the use of this microencapsulated entomopathogenic fungus in control programs is a promising alternative to reduce the vector transmission of Chagas disease.
Acknowledgments
We especially thank LIPBioc-MSPS for providing a space within their facilities to carry out semi-field assays.
Author Contributions
Investigation, analysis and interpretation of data, writing the manuscript, L.V.B.; Conceptualization, supervision, R.M.C. and N.P.; Critical review of the draft, R.M.C., N.P. and L.B.N.; Funding acquisition, L.B.N. and R.M.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Research Council of the National University of Salta (PA CI N° 2474 and 2353/0) and also supported by Ministerio de Salud Pública de Salta and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). N.P. is a member of the CONICET Researcher’s Career, Argentina.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Schofield C.J., Dias J.C.P. The southern cone initiative against Chagas disease. Adv. Parasitol. 1999;42:1–27. doi: 10.1016/s0065-308x(08)60147-5. [DOI] [PubMed] [Google Scholar]
- 2.Gurevitz J.M., Gaspe M.S., Enriquez G.F., Provecho Y.M., Kitron U., Gürtler R.E. Intensified surveillance and insecticide-based control of the Chagas disease vector Triatoma infestans in the Argentinean Chaco. PLoS Negl. Trop. Dis. 2013;7:2158. doi: 10.1371/journal.pntd.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lardeux F., Depickère S., Duchon S., Chavez T. Insecticide resistance of Triatoma infestans (Hemiptera, Reduviidae) vector of Chagas disease in Bolivia. Trop. Med. Int. Health. 2010;15:1037–1048. doi: 10.1111/j.1365-3156.2010.02573.x. [DOI] [PubMed] [Google Scholar]
- 4.Cardozo R.M., Panzera F., Gentile A.G., Segura M.A., Perez R., Diaz R.A., Basombrio M.A. Inheritance of resistance to pyrethroids in Triatoma infestans, the main Chagas disease vector in South America. Infect Genet Evol. 2010;10:1174–1178. doi: 10.1016/j.meegid.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Germano M.D., Acevedo G.R., Cueto G.M., Toloza A.C., Vassena C.V., Picollo M.I. New findings of insecticide resistance in Triatoma infestans (Heteroptera: Reduviidae) from the Gran Chaco. J. Med. Entomol. 2010;47:1077–1081. doi: 10.1603/ME10069. [DOI] [PubMed] [Google Scholar]
- 6.Pedrini N., Mijailovsky S.J., Girotti J.R., Stariolo R., Cardozo R.M., Gentile A., Juarez M.P. Control of pyrethroid-resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl. Trop. Dis. 2009;3:e434. doi: 10.1371/journal.pntd.0000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forlani L., Pedrini N., Girotti J.R., Mijailovsky S.J., Cardozo R.M., Gentile A.G., Juárez M.P. Biological control of the Chagas disease vector Triatoma infestans with the entomopathogenic fungus Beauveria bassiana combined with an aggregation cue: Field, laboratory and mathematical modeling assessment. PLoS Negl. Trop. Dis. 2015;9:e0003778. doi: 10.1371/journal.pntd.0003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanzini M.R., Batista S., Setten A., Toschi N. Compatibilidad de agentes tensoactivos con Beauveria bassiana y Metarhizium anisopliae. Manejo Integr. Plagas Costa Rica. 2001;59:15–18. [Google Scholar]
- 9.Lacey L.A., Grzywacz D., Shapiro-Ilan D.I., Frutos R., Brownbridge M., Goettel M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015;132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Park J.K., Chang H.N. Microencapsulation of microbial cells. Biotechnol. Adv. 2000;18:303–319. doi: 10.1016/S0734-9750(00)00040-9. [DOI] [PubMed] [Google Scholar]
- 11.Bashan Y., Hernández J.P., Leyva L.A., Bacilio M. Alginate microbeads as inoculant carriers for plant growth-promoting bacteria. Biol Fertil Soils. 2002;35:359–368. doi: 10.1007/s00374-002-0481-5. [DOI] [Google Scholar]
- 12.Yabur R., Bashan Y., Hernández-Carmona G. Alginate from the macroalgae Sargassum sinicola as a novel source for microbial immobilization material in wastewater treatment and plant growth promotion. J. Appl. Phycol. 2006;19:43–53. doi: 10.1007/s10811-006-9109-8. [DOI] [Google Scholar]
- 13.Gombotz W.R., Wee S. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998;31:267–285. doi: 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen G.R., Johnson J.B., Eschen D.J. Alginate Pellet Formulation of a Beauveria bassiana (Fungi: Hyphomycetes): Isolate Pathogenic to Cereal Aphids. J. Econ. Entomol. 1990;83:2225–2228. doi: 10.1093/jee/83.6.2225. [DOI] [Google Scholar]
- 15.Pereira R.M., Roberts D.W. Alginate and Cornstarch Mycelial Formulations of Entomopathogenic Fungi, Beauveria bassiana and Metarhizium anisopliae. J. Econ. Entomol. 1991;84:1657–1661. doi: 10.1093/jee/84.6.1657. [DOI] [Google Scholar]
- 16.Gerding-González M., France A., Sepulveda M.E., Campos J. Use of chitin to improve a Beauveria bassiana alginate-pellet formulation. Biocontrol Sci. Technol. 2007;17:105–110. doi: 10.1080/09583150600937717. [DOI] [Google Scholar]
- 17.Vemmer M., Patel A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control. 2013;67:380–389. doi: 10.1016/j.biocontrol.2013.09.003. [DOI] [Google Scholar]
- 18.Baldiviezo L.V., Pedrini N., Santana M., Mannino M.C., Nieva L.B., Gentile A., Cardozo R.M. Isolation of Beauveria bassiana from the Chagas Disease Vector Triatoma infestans in the Gran Chaco Region of Argentina: Assessment of Gene Expression during Host–Pathogen Interaction. J. Fungi. 2020;6:219. doi: 10.3390/jof6040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monzón A. Producción, uso y control de calidad de hongos entomopatógenos en Nicaragua. Avances en el fomento de productos fitosanitarios no-sintéticos. Manejo Integr. Plagas. 2001;63:95–103. [Google Scholar]
- 20.Arzumanov T., Jenkins N., Roussos S. Effect of aeration and substrate moisture content on sporulation of Metarhizium anisopliae var. acridum. Process Biochem. 2005;40:1037–1042. doi: 10.1016/j.procbio.2004.03.013. [DOI] [Google Scholar]
- 21.Carrillo A., Bashan Y. Microencapsulation as a potential carrier for plant growth-promoting bacteria. In: Ogoshi A., Kobayashi K., Homma Y., Kodama F., Kondo N., Akino S., editors. Plant Growth-Promoting Rhizobacteria-Present Status and Future Prospects. Faculty of Agriculture, Hokkaido University; Sapporo, Japan: 1997. pp. 460–463. [Google Scholar]
- 22.Brewer M., Garay M., Gorla D., Murua F., Favot R. Caracterización de los estadios ninfales del genero Triatoma laporte 1833 y Triatoma infestans Klug 1834 (Hemiptera, Reduviidae) Rev. Soc. Entomológica Argent. 1981;40:91–102. [Google Scholar]
- 23.Peng G., Xia Y. The mechanism of the mycoinsecticide diluents on the efficacy of the oil formulation of insecticidal fungus. Biocontrol. 2011;53:893–902. doi: 10.1007/s10526-011-9360-z. [DOI] [Google Scholar]
- 24.Simonazzi A., Davies C., Cid A.G., Gonzo E., Parada L., Bermúdez J.M. Preparation and characterization of Poloxamer 407 solid dispersions as an alternative strategy to improve benznidazole bioperformance. J. Pharm. Sci. 2018;107:2829–2836. doi: 10.1016/j.xphs.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Lord J.C. Desiccant dusts synergize the effect of Beauveria bassiana (Hyphomycetes: Moniliales) on stored-grain beetles. J. Econ. Entomol. 2001;94:367–372. doi: 10.1603/0022-0493-94.2.367. [DOI] [PubMed] [Google Scholar]
- 26.Malsam O., Kilian M., Oerke E.C., Dehne H.W. Oils for increased efficacy of Metarhizium anisopliae to control whiteflies. Biocontrol Sci. Technol. 2002;12:337–348. doi: 10.1080/09583150220128121. [DOI] [Google Scholar]
- 27.Luz C., Batagin I. Potential of oil-based formulations of Beauveria bassiana to control Triatoma infestans. Mycopathol. 2005;160:51–62. doi: 10.1007/s11046-005-0210-3. [DOI] [PubMed] [Google Scholar]
- 28.Inglis D.G., Goettel M.S., Butt T.M., Strasser H. Use of hyphomycetes fungi for managing insect pests. In: Butt T.M., Jackson C., Magan N., editors. Fungi as Biocontrol Agents: Progress, Problems and Potential. CABI International; Wallingford, UK: 2001. pp. 26–69. [Google Scholar]
- 29.Roy H.E., Steinkraus D.C., Eilenberg J., Hajek A.E., Pell J.K. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Annu. Rev. Entomol. 2006;51:331–357. doi: 10.1146/annurev.ento.51.110104.150941. [DOI] [PubMed] [Google Scholar]
- 30.Jaronski S.T. Ecological factors in the inundative use of fungal entomopathogens. Biocontrol. 2010;55:159–185. doi: 10.1007/s10526-009-9248-3. [DOI] [Google Scholar]
- 31.Pozo C. Master’s Thesis. Center for the Development of Biotic Products of the National Polytechnic Institute; Mexico City, Mexico: 2012. Effectiveness of a Beauveria bassiana Microencapsulation on Metamasius spinolae; p. 76. [Google Scholar]
- 32.Chumtong A., Wiwattanapatapee R., Viernstein H., Pengnoo A., Kanjanamaneesathian M. Spay-dried powder Bacillus megaterium for control of rice sheath blight disease: Formulation protocol and efficacy testing in laboratory and greenhouse. Cereal Res. Commun. 2016;4:131–140. doi: 10.1556/0806.43.2015.034. [DOI] [Google Scholar]
- 33.Lynch P.A., Grimm U., Thomas M.B., Read A.F. Prospective malaria control using entomopathogenic fungi: Comparative evaluation of impact on transmission and selection for resistance. Malaria J. 2012;11:383. doi: 10.1186/1475-2875-11-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C.P., Liu S.D. Formulation and characterization of the microencapsulated entomopathogenic fungus Metarhizium anisopliae MA126. J. Microencapsul. 2009;26:377–384. doi: 10.1080/02652040802365455. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira-Lopes A.R., Locatelli G.O., Melo-Barbosa R., Lobo M.J., Moura-Mascarin G., Finkler C.L.L. Preparation, characteriztion and cell viability of encapsulated Trichoderma asperellum in alginate beads. J. Microencapsul. 2020;37:270–282. doi: 10.1080/02652048.2020.1729884. [DOI] [PubMed] [Google Scholar]
- 36.Simonazzi A., Baldiviezo V., Virgili V., Bermúdez J.M., Arnal P., Herrera C.A., Enriquez C., Gentile A.G., Cardozo R.M. Evaluation of oily formulations of Beauveria bassiana using Poloxamer 407 for the development of a powerful bioinsecticide for the control of the Chagas disease vector. [(accessed on 20 April 2023)];Res. Eng. Fac. NOA. 2015 :152–158. Available online: https://www.researchgate.net/publication/294089509_Evaluacion_de_formulaciones_oleosas_de_Beauveria_bassiana_utilizando_Poloxamer_407_para_el_desarrollo_de_un_potente_bioinsecticida_contra_el_vector_de_la_enfermedad_de_Chagas. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.