Abstract
Despite the successes achieved so far with the Global Programme to Eliminate Lymphatic Filariasis, there is still an appreciable number of lymphatic filarial patients who need alternative treatment and morbidity management strategies. The unresponsiveness of some cohorts to the drugs used in the mass drug administration program is currently raising a lot of questions and this needs urgent attention. Natural medicinal plants have a long‐standing history of being effective against most disease conditions. Countries such as India have been able to integrate their natural plant remedies into the treatment of lymphatic filarial conditions, and the results are overwhelmingly positive. Components of Azadirachta indica A. Juss, Parkia biglobosa, Adansonia digitata, and Ocimum spp have been shown to have anti‐inflammatory, anticancerous, and antimicrobial activities in animal models. Therefore, this review calls for attention toward the use of natural plant components as an alternate treatment against lymphatic filariasis to help reduce the World Health Organization's burden of providing drugs for people in need of treatment every year.
Keywords: antibiotics, anticancer, anti‐inflammatory, lymphatic filariasis, medicinal plants
1. INTRODUCTION
Lymphatic filariasis (LF) is a neglected tropical disease (NTD) that occurs when filarial parasites (Wuchereria bancrofti and Brugia spp) are transmitted to humans through mosquitoes (Figure 1). LF is a vector‐borne, long‐standing chronic disease which is the second leading cause of long‐term and permanent disability in the world. 1 This disease has been a public health concern, especially in Africa and South America. LF is caused by the lymph–dwelling nematode parasites; W. bancrofti, Brugia malayi, and Brugia timori. The filarial nematode W. bancrofti accounts for 91% of LF infections while B. malayi and B. timori are responsible for the remaining 10% in South and Southeast Asia. 2 , 3 , 4 The clinical manifestations of the LF include hydrocele and lymphedema (LE) (mainly known as elephantiasis).
Figure 1.
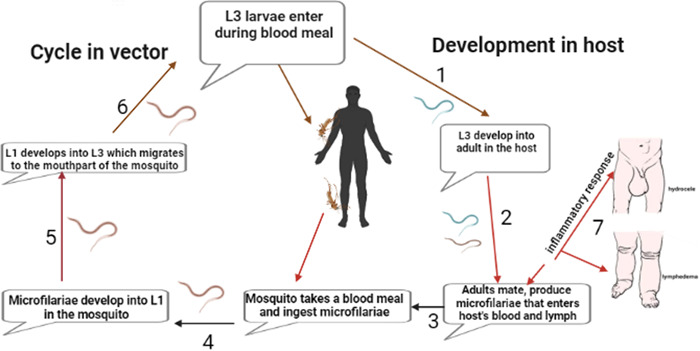
Lifecycle of filarial parasites, demonstrated with W. bancrofti. Both a vector and a mammalian host are required for the development of the nematode. (1) The infected vector transmits the third‐stage larvae (L3) into the human host during a blood meal. (2) The L3 mature into adult worms. (3) The parasites produce microfilariae (MF), which migrate to the lymphatics and blood for circulation. (4) The vector once again ingests the microfilariae during a blood meal from an infected host. (5) The microfilariae develop into the L1 stage. (6) The L1 larvae matures into L3 larvae which migrate to the vector's proboscis via the haemocel. (7) Clinical manifestations of LF due to inflammation mainly from worm antigen. Created with Biorender.com.
The development of modern medicines for NTDs from phytochemicals is intrinsically less likely to fail through problems of toxicity or other side effects when the phytochemical lead compounds originate from plant species, which are part of certificated herbal treatments. 5 Many have been monitored over a number of years/decades in clinical use with significant number of patients, for health conditions which are more serious than the NTD against which the phytochemicals could also shows promising activity. The current World Health Organization's (WHO) drugs of choice for its mass drug administration (MDA) program are ivermectin (IVM), albendazole (ALB), and diethylcarbamazine (DEC). 6 These drugs are effective in reducing microfilariae but not effective in killing the adult worms or relieving individuals with morbidity. 7 , 8 Furthermore, there is repopulation of microfilariae in the host system 6–12 months after treatment, hence the need to take the drugs annually. DEC has also been reported to cause severe side effects such as fever, gastrointestinal disturbance, headache, malaise, and skin rash that reduce patient's compliance. 9 The pathology is known to progress due to elevated inflammatory processes that unfolds in the presence of filarial antigen. 10 , 11 , 12
Data from field trials have shown potency of some antibiotics (doxycycline and minocycline) against LF. 7 , 13 , 14 , 15 , 16 This notwithstanding, these antibiotics have a longer regimen duration and do not support national treatment programs. Thus, there is still the need for more effective drugs with less adverse reaction, shorter regimen duration, and less cost to achieve WHO's aim of interrupting transmission and helping individuals with LF morbidity. Moreover, these antibiotics usually clear microfilaria with a delayed gradual effect on the early stages of pathology (hydrocele and LE).
The unavailability of vaccine and drugs for this condition increases the demand for cheap and orthodox methods which are antifilarial in nature. 17 , 18 Countries like India has been able to use conventional plant‐based methods in targeting the worms in LF. India has rich tradition of using medicinal plants or their products in treating different disease conditions through Ayurveda, Unani, and Siddha systems of medicine. 8 , 19 Several plant medicines have been developed and are being utilized in traditional therapeutics. 17 , 20 Natural products of plant origin with insecticidal properties have also been tried in the past for the control of a variety of insect pests and vectors. 2 , 17 , 19 , 21 Several antifilarial agents containing pentacyclic triterpene and oleanolic acid have also been discovered through research on medicinal plants used by local healers. 17 , 19 , 22 Natural plant products from Azadirachta indica A. Juss, Parkia biglobosa, Adansonia digitata, and Ocimum sp are known to have anti‐inflammatory, antibiotic, anticancer, antifungal, and wound healing effects. 23 , 24 , 25 , 26 These plants are enriched in Africa and known to have bioactive compounds that could be used in treating several conditions. The aim of this perspective is to share and ignite research interest toward the exploration of African medicinal plants as treatment options for individuals with LF and others living with NTDs (Figure 2). Focusing on isolated compounds, medicinal plants and folklore plants having antifilarial activity will help in reducing the burden of providing drugs for LF treatment.
Figure 2.
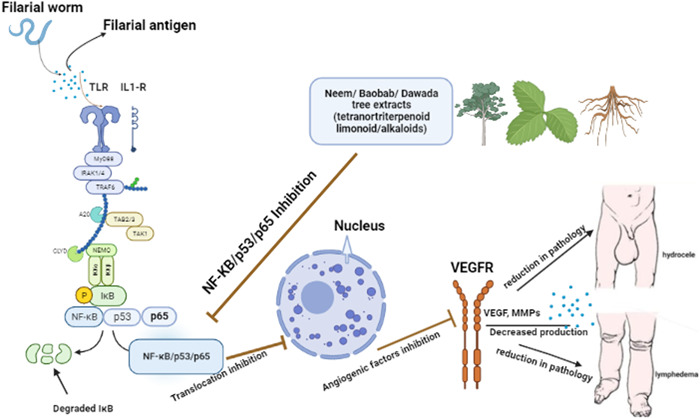
Possible anti‐inflammatory action of medicinal plants against lymphatic filariasis (LF). The pathogenesis of LF is proposed to be due mainly to elevated inflammatory cytokines (IL‐1, TNF‐⍺). Compounds from medicinal plants regulate this pathway by reducing inflammation thereby setting inflammation to levels that are not detrimental to one's health. Created with Biorender.com.
2. CONSTITUENTS OF NATURAL PLANT PRODUCTS AGAINST LF
Azadirachta indica A. Juss locally known as Neem tree (family meliaceae) is found mainly in the tropical regions, especially in West Africa and India. Animal model studies using Azadirachta indica A. Juss have shown that it plays an anti‐inflammatory role by reducing oedema through intramuscular and skin punch biopsy applications using the extracts. 27 , 28 Pathway analysis using in an animal model has established that the chief constituents (Azadirachtin, nimbolide, salannin, and quercetin) of the tree play pivotal roles in anticancer management through the modulation of various inflammatory pathways including p53, NF‐κB, and VEGF, but the exact molecular mechanism used by these constituents in the prevention of pathogenesis is not yet fully understood. Other studies have also confirmed its potency against filarial parasites and their vectors. 26 , 29 Neem is also known to have antimicrobial properties through its inhibitory effect on microbial growth by showing efficacies against Staphylococcus aureus and Methicillin‐resistant Staphylococcus aureus (MRSA) with greatest zones of inhibition noted at 100% concentration. 26 Tannins and flavonoids are the major polyphenols present in the bark of the trunks of P. biglobosa. These constituents have shown to be effective in the treatment of inflammatory diseases. 30
This approach could be explored in the treatment of inflammatory conditions such as is seen in LF pathologies. Various parts of P. biglobosa (family Fabaceae, locally called Dawadawa tree in Ghana) have good antimicrobial activities against some bacteria strains. 31 So far, there is still no clinical study conducted on the plants to investigate activity in most human diseases including LF. 31 With the inability of the filarial drugs to cure pathologies in LF, a look into this plant's constituents (flavonoids, saponins, tannins, and triterpenes) with its reported higher antimicrobial properties could reveal a higher activity against the Wolbachia bacteria which is known to have an endosymbiotic relationship with the filarial worm.
Adansonia digitata (Baobab) known locally as monkey bread tree is a forest tree, primarily in Africa and Asia, in the family Malvaceae. Fruit pulp extract of baobab possesses anti‐inflammatory, hepatoprotective, anticlastogenic, and other medicinal properties. 32 Baobab affords anti‐inflammatory compounds (campesterol, cholesterol, isofucosterol, β‐sitosterol, stigmasterol, and tocopherol) that can aid in several conditions, from injuries, aches, and pains to stomach upset and respiratory conditions. Polysaccharides from Adansonia digitata purified through permeation chromatography have proven to be a potential antioxidant and anti‐inflammatory food supplement. 33 It is paramount to reduce or regulate inflammation to levels that is tolerable for the body, therefore this plant and its components could help reduce elevated inflammation as seen in individuals with LF pathologies (Figure 3). This inspired a study reporting on the efficacious therapeutic activity of Azadirachtin against LF. It further indicated that a significant tetranortriterpenoid phytocompound found in Azadirachta indica, showed efficacies against the filarial parasite Setaria cervi in vitro. 34 Thus, remedies for LF pathology should be directed towards interventions that are known to invoke less or moderate inflammation. Data has shown that not only are individuals with LF pathologies faced with unfathomable societal stigma and discrimination, also they experience acute filarial attacks due to systemic inflammation. Exploration of the baobab tree to ascertain the exact inflammatory pathways targeted by the constituents of the plant could help ameliorate this occurrence which is mostly at its peak in the wet/rainy seasons. 35
Figure 3.
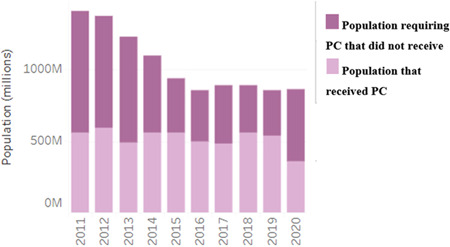
Preventive chemotherapy (PC) coverage per year for lymphatic filariasis.
Essential oils from three Ocimum spp from the family Lamiaceae (Ocimum gratissimum [OG], Ocimum tenuiflorum [OT,] and Hyptis suaveolens [HS]) have been shown to have repellent activities against vectors of malaria and LF. 36 This plant is locally known in Ghana as “Akoko mesa.” It may be worthwhile to reinvestigate the potential of essential oils derived from other Ocimum sp for possible production of mosquito coils, which could substantially be utilized in repelling blood‐seeking mosquitoes and break the vector‐human contacts (Table 1).
Table 1.
Plants and their constituents targeting various pathways for possible activity against LF.
| Plant species | Local name | Activities | Constituents | Pathways | Reference |
|---|---|---|---|---|---|
| 1. Azadirachta indica A. Juss | Neem tree | Anti‐inflammatory/antimicrobial | Azadirachtin, nimbolide, salannin, and quercetin | P53, NF‐κB, 100% zone of inhibition against MRSA | [26, 29] |
| 2. Parkia biglobosa | Dawadawa tree | Antimicrobial | Flavonoids, saponins, tannins, and triterpenes | Microbial RNA | [31] |
| 3. Adansonia digitata | Monkey tree | Anti‐inflammatory, hepatoprotective, anticlastogenic | Campesterol, cholesterol, isofucosterol, β‐sitosterol, stigmasterol, and tocopherol | Metabolic pathway/apoptotic pathway | [33] |
| 4. Ocimum sp | Akoko mesa | Repellent activity | Iron, magnesium silicates, plagioclase pyroxene. | Biocontrol (larviciding) | [53] |
3. PHARMACOKINETICS OF PLANT MEDICINES AGAINST LF
There is an urgent need to expand the available pharmaceutical repertoire. This must be preceded by experiments on the potencies, activity doses, and toxicity of potential medicinal plants. Studies on the antimicrobial activities of medicinal plants (neem oil extracts, P. biglobosa, etc) and phytochemicals using in‐vitro methods (broth dilution, disc or agar diffusion, and agar overlay assays) are used to determine the minimum inhibitory concentration and minimum bactericidal concentration of each treatment. 30 , 32 , 37 Alternatively, in vivo model studies have been implemented to reflect human infections and disease testing; these models include intraperitoneal or intravenous injection, or oral or gastric administration of plant extracts in mice, rats, guinea pigs, and rabbits. 38
While thousands of people die each year from supposedly “safe” over‐the‐counter remedies, deaths or hospitalizations due to medicinal plants are so rare to find. This notwithstanding, the appropriate volume of medicinal plants whether used as food or supplement is of great concern in both humans and animals. A study reported pathological neurological disorders in horses following a large intake of fresh Bambusa vulgaris leaves. 39 Surprisingly, the aqueous extract of this same plant is a popular antimalarial medicine in Ghana. 40 Dye exclusion and MTT assay showed the potency of azadirachtin against S. cervi with a median lethal dose (LC50) of 6.28 μg/mL for microfilariae (mf), and 9.55 μg/mL for adult parasites. A mouse model study on the toxicity of neem trees showed that clinical dosage should be less than 1600 mg/kg/day to prevent organ damage. 41 Elsewhere, it has been concluded on the toxicity of P. biglobosa that 1800 mg/kg and 1600 mg/kg were the intraperitoneal lethal dose 50 (LD50) for aqueous extract from roasted and fermented seeds, respectively. 42 This implies nontoxic dose should be less than the above doses. Moreover, after 60 min of oral administration of ethyl acetate extracts of Ocimum sp, mice with edeama experienced less inflammation. 43 Methanol extracts of Dipterocarpus zeylanicus (triterpene saponins) were investigated for macro and microfilaricidal activity through guided chromatography and shown to be potent against Setaria digitata. 44
However, most of these studies have not been replicated in humans to verify their efficacies, especially in LF. This calls for experts' attention on the effect of these plants to help counter the burden of NTDs on the African continents.
4. PROSPECTS OF MEDICINAL PLANTS AGAINST CHRONIC WOUNDS AMONG FILARIAL LE PATIENTS
LE manifestation in LF patients is mostly accompanied with the presence of lesions. The delayed healing of these lesions is mainly due to the impaired immunity and increased risk of infections associated with secondary LE, thereby the increased chronicity and difficulty in the management of filarial wounds. The crushed seeds of P. biglobosa has proven to have wound‐healing abilities toward snakebit among the Fulani tribes of northern Nigeria. 45 In LF there are several reports of sustained bacterial infection as cause of chronic wounds in LF pathologies, and this could be mitigated through treatment with P. biglobosa which has been shown to have wound healing activity. 46 Study has also shown that aqueous extracts of neem leaves have wound‐healing ability and this is an appropriate option, preferred for its naturality, ease of access, and is safe, with no known adverse effect. 47 Further investigations into the wound healing abilities of African medicinal plants as cure for LF wounds would bring relief to the debilitating conditions of LE patients with chronic wounds.
5. CURRENT CHALLENGES TO THE USE OF PLANT PRODUCTS IN LF
Although a large population of Africa rely on medicinal plants for primary healthcare, the scientific data on such plants remain limited. The safety profile of these medicinal plants is delineated especially for helminthic infections. Studies have found toxic concentrations of some plant extracts (Khaya senegalensis, Aframomum melegueta; IC50 of 61.1, 79.7, 61 μg/mL, respectively) used as treatment of helminthiasis. We mention here that although W. bancrofti infections account for higher proportion (91%) of total LF infections while B. malayi and B. timori are responsible for only 9%, data on medicinal plants against LF is highly scarce. 4 Specifically, research on medicinal plants against NTDs seems to be neglected. Thus, we advocate more works to be done on filaricidal plant extracts on human filarial infections.
6. PLANT PRODUCTS AS FUTURE REMEDIES AGAINST FILARIASIS
The availability and search for an effective drug against LF dates to the 1990s. This has been an endless battle with some successes; however, high throughput strategies and research needs to be done as WHO could not achieve its target in 2020 (Figure 3). 48 With the new target set for 2030, plant products should be explored as treatment options for individuals with LF especially in areas that have less MDA treatment coverage. Moreover, as the current mechanism of action of IVM, ALB, and DEC remain unable to clear the adult worm which sustains repopulation of the human host with microfilariae, this calls for more attention toward plants that have shown tremendous anti‐inflammatory, anticancerous and antimicrobial properties in animal models. 30 , 33 Data on the biomedical activity of these medicinal plants against LF in human populations is inadequate, thus research in this area seems neglected making the disease more neglected. With the influx of antimicrobial, anti‐inflammatory, and anticancer activity of some medicinal plants, it has necessitated an inquiry into the effect of these plants and their specific components on LF pathologies.
Another way by which these plants and their extracts can be used in the fight against LF is by using their components for vector control. 4 One of the strategies set by the WHO in eliminating LF is mosquito control. Mosquito control is a supplemental strategy supported by WHO. It is used to reduce transmission of LF and other mosquito‐borne infections. A study has proven the ability of these extracts as an effective vector control strategy. 4 The use of these plants' products for vector control could help in protecting our environment and reducing side effects of chemical‐based insecticides. This has also created gaps for exploring environmentally friendly methods in fighting diseases and their vectors. Furthermore, proper control of the filarial vector can be achieved via careful design of extraction and administration processes such as use of efficient bio‐chemical solvent extraction methods, preferably hydrophilic solvent, and logically controlled doses. 4
Expanding the coverage of food additives with medicinal benefits could help accelerate drug discovery efforts, especially in LF. P. biglobosa, which is usually used as local condiments in Africa, especially Ghana, has also been documented to have antimalarial activity during in vivo and in vitro studies. 24 , 49 The importance of P. biglobosa seed in African traditional medicine and food is undeniably wide and is of prehistoric origin. The growing interest of scientific focus on medicinal plants has contributed to the various experimental evidence including the anti‐inflammatory, antihypertensive, antidiabetes, antioxidant, and antimicrobial activities of these plants. Their activities are mainly linked to their phytochemical composition. Nonetheless, much work still needs to be done regarding the route of administration, toxicity, dosage, regimen, plant parts efficacies, and the medium of extraction.
7. PLANT ENDOPHYTIC FUNGI AS A SOURCE OF BIOACTIVE PLANT PRODUCTS AND FUNGAL PATHOGENS AS FACILE MODEL FOR EUKARYOTIC PATHOGENS
The development of low‐cost plant medicine‐based treatment of options ought to include the important endophytic fungi that are harbored by the key plants that serve as the leading treatment options. 50 An example is found with case of the Yew tree that is the sources of the vital cancer drug taxol. Several endophytic fungi isolated from this tree have been shown to also produce taxol. 51 While the cultivation of tree plants is subject to long generational time, that of endophytic fungi is short and amenable to the culturing in fermenters which can be controlled to produce desirable quantities of useful compounds.
Aside, endophytic fungal, fungal pathogens such as Candida albicans can be employed in large‐scale studies as general model for eukaryotic pathogens. This system offers a simple and low‐cost bioassay platform to test plant medicine preparations and all related samples and isolated compounds. Prescreening with fungal pathogens allows for large libraries of plant materials and compounds to be reduced to a small package of focused and enriched samples to be tested on filarial worms and their eggs. 52
8. CONCLUSION
Out of the 860 million and more people who required treatment for LF in 2020, the WHO progress dashboard indicates that only 360 million people received treatment. This is mainly due to the pressure mounted on WHO to supply these drugs (IVM, ALB, and DEC). This review presents possible insights on the efficacy of medicinal plants as direct filaricidal biomedicine and/or those employed as vector control agents. Methods used for biochemical extraction, screening procedures and structure elucidation of the bioactive compounds to validate the efficacy of plant extracts as another treatment option for LF should be investigated through further animal and human studies.
AUTHOR CONTRIBUTIONS
Fatima A. Fordjour: Conceptualization; resources; validation; visualization; writing—original draft; writing—review and editing. Priscilla Osei‐Poku: Writing—original draft. Afua K. A. Genfi: Conceptualization; Writing—review and editing. Kwaw G. Ainooson: Supervision; visualization; writing—review and editing. Kingsley Amponsah: Supervision; writing—review and editing. Patrick K. Arthur: Supervision; writing—review and editing. G. Richard Stephenson: Supervision; writing—review and editing. Alexander Kwarteng: Supervision; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Fatima Amponsah Fordjour affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
We would like to acknowledge the authors of the referenced studies.
Fordjour FA, Osei‐Poku P, Genfi AKA, et al. Use of medicinal plants as a remedy against lymphatic filariasis: Current status and future prospect. Health Sci Rep. 2023;6:e1295. 10.1002/hsr2.1295
DATA AVAILABILITY STATEMENT
The data are accessible via referenced articles. Any further data regarding the article can be made available upon reasonable request to the corresponding author.
REFERENCES
- 1. WHO . Progress Report 2000–2009 and Strategic Plan –2020 of the Global Programme to Eliminate Lymphatic Filariasis: Halfway Towards Eliminating Lymphatic Filariasis. WHO; 2010. [Google Scholar]
- 2. Ndjonka D, Rapado L, Silber A, Liebau E, Wrenger C. Natural products as a source for treating neglected parasitic diseases. Int J Mol Sci. 2013;14(12):3395‐3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moraga P, Cano J, Baggaley RF, et al. Modelling the distribution and transmission intensity of lymphatic filariasis in sub‐Saharan Africa prior to scaling up interventions: integrated use of geostatistical and mathematical modelling. Parasit Vectors. 2015;8(1):1‐16. 10.1186/s13071-015-1166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nazeh MA‐A, Nor ZM, Al‐Adhroey AH, Suhaimi A, Sivanandam S. Recent advances on the use of biochemical extracts as filaricidal agents. Evid Based Complement Altern Med. 2013;2013:986573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karimi A, Majlesi M, Rafieian‐Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4(1):27‐30. [PMC free article] [PubMed] [Google Scholar]
- 6. Gayen P, Nayak A, Saini P, et al. A double–blind controlled field trial of doxycycline and albendazole in combination for the treatment of bancroftian filariasis in India. Acta Trop. 2013;125:150‐156. [DOI] [PubMed] [Google Scholar]
- 7. Witt C, Ottesen EA. Lymphatic filariasis: an infection of childhood. Trop Med Int Health. 2001;6(8):582‐606. [DOI] [PubMed] [Google Scholar]
- 8. Sashidhara KV, Singh SP, Misra S, Gupta JSB, Misra‐Bhattacharya S. Galactolipids from Bauhinia racemosa as a new class of antifilarial agents against human lymphatic filarial parasite Brugia malayi. Eur J Med Chem. 2012;50:230‐235. [DOI] [PubMed] [Google Scholar]
- 9. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176(5):3248‐3256. [DOI] [PubMed] [Google Scholar]
- 10. Bennuru S, Klion AD, Kumaraswami V, Maldarelli G, Nutman TB. Elevated levels of plasma angiogenic factors are associated with human lymphatic filarial infections. Am J Trop Med Hyg. 2010;83(4):884‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debrah LB, Albers A, Debrah AY, et al. Single nucleotide polymorphisms in the angiogenic and lymphangiogenic pathways are associated with lymphedema caused by Wuchereria bancrofti. Hum Genomics. 2017;11(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fordjour FA, Asiedu E, Larbi A, Kwarteng A. The role of nuclear factor kappa B (NF‑κB) in filarial pathology. J Cell Commun Signal. 2021;4(6):e696. 10.1007/s12079-021-00607-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Debrah AY, Mand S, Toliat MR, et al. Plasma vascular endothelial growth factor‐A (VEGF‐A) and VEGF‐A gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg. 2007;77(4):601‐608. [PubMed] [Google Scholar]
- 14. Pfarr KM, Debrah AY, Specht S, Hoerauf A. Filariasis and lymphoedema. Parasite Immunol. 2009;31(11):664‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Debrah AY, Specht S, Klarmann‐schulz U, et al. Doxycycline leads to sterility and enhanced killing of female Onchocerca volvulus worms in an area with persistent microfilaridermia after repeated ivermectin treatment: a randomized, placebo‐controlled, double‐blind trial. Clin Infect Dis. 2015;61(4):517‐526. 10.1093/cid/civ363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Debrah AY, Mand S, Marfo‐debrekyei Y, et al. Macrofilaricidal activity in wuchereria bancrofti after 2 weeks treatment with a combination of rifampicin plus doxycycline. J Parasitol Res. 2011;2011:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta AK, Tandon N. Reviews on Indian medicinal plants. Indian Med plants. 2004.
- 18. Ranjan Behera D, Bhatnagar S. Filariasis: role of medicinal plant in lymphatic filariasis. Int J Herb Med. 2018;6(1):40‐46. http://www.florajournal.com/archives/2018/vol6issue1/PartA/6-6-32-438.pdf [Google Scholar]
- 19. Lakshmi V, Kumar R, Gupta P, et al. The antifilarial activity of a marine red alga, Botryocladia leptopoda, against experimental infections with animal and human filariae. Parasitol Res. 2004;93:468‐474. [DOI] [PubMed] [Google Scholar]
- 20. Sashidhara KV, Singh SP, Misra S, Gupta J, Misra‐Bhattacharya S. Galactolipids from Bauhinia racemosa as a new class of antifilarial agents against human lymphatic filarial parasite, Brugia malayi. Eur J Med Chem. 2012;50:230‐235. [DOI] [PubMed] [Google Scholar]
- 21. Das NG, Goswami D, Rabha B. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J Vector Borne Dis. 2007;44(2):145‐148. [PubMed] [Google Scholar]
- 22. Misra N, Sharma M, Raj K, Dangi A, Srivastava S, Misra‐Bhattacharya S. Chemical constituents and antifilarial activity of Lantana camara against human lymphatic filariid Brugia malayi and rodent filariid Acanthocheilonema viteae maintained in rodent hosts. Parasitol Res. 2007;100(3):439‐448. 10.1007/s00436-006-0312-y [DOI] [PubMed] [Google Scholar]
- 23. Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Altern Med. 2016;2016:7382506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Builders M, Wannang N, Aguiyi J. Antiplasmodial activities of Parkia biglobosa leaves: in vivo and in vitro studies. Ann Biol Res. 2011;4(2):8‐20. [Google Scholar]
- 25. Ntchapda F, Bonabe C, Atsamo AD, et al. Effect of aqueous extract of Adansonia digitata stem bark on the development of hypertension in L‐NAME‐induced hypertensive rat model. Evid Based Complement Altern Med. 2020;2020:3678469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamani HA, Pang EC, Mantri N, Deighton MA. Antimicrobial activity of Tulsi (Ocimum tenuiflorum) essential oil and their major constituents against three species of bacteria. Front Microbiol. 2016;7:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chattopadhyay R, Chattopadhyay R, Maitra SK. Possible mechanism of antiinflammatory activity of Azadirachta indica leaf extract. Indian J Pharmacol. 1993;25(2):99‐100. [Google Scholar]
- 28. Rao YV. Evaluation of topical anti‐inflam matory effect of Azadirachta indica leaf extract. Biology. 2012. [Google Scholar]
- 29. Al‐Rofaai A, Rahman WA, Sulaiman SF, Yahaya ZS. In vitro activity of neem (Azadirachta indica) and cassava (Manihot esculenta) on three pre‐parasitic stages of susceptible and resistant strains of Teladorsagia (Ostertagia) circumcincta. Vet Parasitol. 2012;188(1–2):85‐92. [DOI] [PubMed] [Google Scholar]
- 30. Ouedraogo N, Atchade C, Kadiatou T T, et al. Anti‐Inflammatory activity of extracts from Parkia biglobosa (Jacq.) R.Br. Ex G.Don. (Fabaceae‐Mimosoideae) trunk bark. J Pharmacol Toxicol. 2020;16(1):1‐8. [Google Scholar]
- 31. Saleh MSM, Jalil J, Zainalabidin S, Asmadi AY, Mustafa NH, Kamisah Y. Genus Parkia: phytochemical, medicinal uses, and pharmacological properties. Int J Mol Sci. 2021;22(2):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adegoke A, Gota V, Gupta S, Gbadegesin M, Odunola 1 O. Evaluation of antioxidant and anticancer activities of aqueous extract of the fruit pulp of Adansonia digitata Linn and its fractions. Afr J Med Med Sci. 2021;50:9‐17. [Google Scholar]
- 33. Ibrahim AY, Mahmoud G, Asker MMS. Anti‐inflammatory and antioxidant activities of polysaccharide from Adansonia digitata: an in vitro study. Int J Pharm Sci Rev Res. 2014;25(33):174‐182. [Google Scholar]
- 34. Mukherjee N, Joardar N, Sinha Babu SP. Antifilarial activity of azadirachtin fuelled through reactive oxygen species induced apoptosis: a thorough molecular study on Setaria cervi. J Helminthol. 2019;93(5):519‐528. [DOI] [PubMed] [Google Scholar]
- 35. Kwarteng A, Arthur YD, Yamba JK, et al. Influence of seasonal variation on reported filarial attacks among people living with lymphedema in Ghana. BMC Infect Dis. 2019;19(1):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malebo HM, Imeda C, Kitufe NA, et al. Repellence effectiveness of essential oils from some Tanzanian Ocimum and Hyptis plant species against afro‐tropical vectors of malaria and lymphatic filariasis. J Med Plants Res. 2012;7(11):653‐660. [Google Scholar]
- 37. Wylie MR, Merrell DS. The antimicrobial potential of the neem tree Azadirachta indica . Front Pharmacol. 2022;13:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. CDC . Antibiotic resistance [Internet]. Threats in the United States. 2019. Accessed November 20, 2022. http://www.cdc.gov/DrugResistance/Biggest-Threats.html
- 39. Barbosa JD, de Oliveira CMC, Duarte MD, Riet‐Correa G, Peixoto PVTC. Poisoning of horses by bamboo, Bambusa vulgaris . J Equine Vet Sci. 2006;26(9):393‐398. [Google Scholar]
- 40. Komlaga G, Cojean S, Dickson RA, et al. Antiplasmodial activity of selected medicinal plants used to treat malaria in Ghana. Parasitol Res. 2016;115(8):3185‐3195. [DOI] [PubMed] [Google Scholar]
- 41. Deng Y, Cao M, Shi D, et al. Toxicological evaluation of neem (Azadirachta indica) oil: acute and subacute toxicity. Environ Toxicol Pharmacol. 2013;35(2):240‐246. 10.1016/j.etap.2012.12.015 [DOI] [PubMed] [Google Scholar]
- 42. Ouédraogo S, Somé N, Ouattara S, et al. Acute toxicity and vascular properties of seed of Parkia biglobosa (JACQ) R. BR gift (Mimosaceae) on rat aorta. African J Tradit Complement Altern Med. 2012;9(2):260‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bawankule D, Kumar A, Agarwal K, et al. Pharmacological and phytochemical evaluation of Ocimum sanctum root extracts for its antiinflammatory, analgesic and antipyretic activities. Phcog Mag. 2015;11(42):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Senathilake KS, Karunanayake EH, Samarakoon SR, Tennekoon KH, de Silva ED, Adhikari A. Oleanolic acid from antifilarial triterpene saponins of Dipterocarpus zeylanicus induces oxidative stress and apoptosis in filarial parasite Setaria digitata in vitro. Exp Parasitol. 2017;177:13‐21. [DOI] [PubMed] [Google Scholar]
- 45. Balogun WG, Adebayo IA, Yusuf U, Seeni A. A review of the phytochemistry and medicinal activities of the popular African food additive: Parkia biglobosa seed. Orient Pharm Exp Med. 2018;18(4):271‐279. 10.1007/s13596-018-0337-7 [DOI] [Google Scholar]
- 46. Asiedu SO, Kini P, Aglomasa BC, et al. Bacterial diversity significantly reduces toward the late stages among filarial lymphedema patients in the Ahanta West District of Ghana: a cross‐sectional study. Health Sci Rep. 2022;5(4):e724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jayalakshmi M. Effect of neem leaves extract irrigation on the wound healing outcome in nurse managed diabetic foot ulcers. Nat J Physiol Pharm Pharmacol. 2020;11(1):1. [Google Scholar]
- 48. WHO . Accelerating work to overcome the global impact of NTDs: 2011–2020 Progress dashboard. WHO; 2022. [Google Scholar]
- 49. Modupe Iretiola Builders . World journal of pharmaceutical research seed extracts. World J Pharm Res. 2014;3(2):1672‐1682. [Google Scholar]
- 50. Blessie EJ, Wruck W, Abbey BA, et al. Transcriptomic analysis of marine endophytic fungi extract identifies highly enriched anti‐fungal fractions targeting cancer pathways in HepG2 cell lines. BMC Genom. 2020;21(1):265. 10.1186/s12864-020-6684- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vélëz H, Gauchan DP, García‐Gil MR. Taxol and β‐tubulins from endophytic fungi isolated from the Himalayan Yew, Taxus wallichiana Zucc. Front Microbiol. 2022;13:1‐14. 10.3389/fmicb.2022.956855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aboagye SY, Amarh V, Lartey PA, Arthur PK. Wood‐decaying fungi found in Southern Ghana: a potential source of new anti‐infective compounds. AAS Open Res. 2019;2:20. 10.12688/aasopenres.12957.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Adam AA, Ahmed Salma A, Mohamed Thana A, Azrag Rasha A, Mustfa Salma E, Hamdi Omer AA. Evaluation of repellent activities of the essential oil of Ocimum basilicum against Anopheles mosquito and formulation of mosquito repellent cream. Biomed Res Clin Pract. 2019;4(2):1‐5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are accessible via referenced articles. Any further data regarding the article can be made available upon reasonable request to the corresponding author.


