Abstract
Purpose
This study aimed to evaluate the relationship between sleep, burnout, and psychomotor vigilance in residents working in the medical intensive care unit (ICU).
Methods
A prospective cohort study of residents was implemented during a consecutive 4-week. Residents were recruited to wear a sleep tracker for 2 weeks before and 2 weeks during their medical ICU rotation. Data collected included wearable-tracked sleep minutes, Oldenburg burnout inventory (OBI) score, Epworth sleepiness scale (ESS), psychomotor vigilance testing, and American Academy of Sleep Medicine sleep diary. The primary outcome was sleep duration tracked by the wearable. The secondary outcomes were burnout, psychomotor vigilance (PVT), and perceived sleepiness.
Results
A total of 40 residents completed the study. The age range was 26–34 years with 19 males. Total sleep minutes measured by the wearable decreased from 402 min (95% CI: 377–427) before ICU to 389 (95% CI: 360–418) during ICU (p < 0.05). Residents overestimated sleep, logging 464 min (95% CI: 452–476) before and 442 (95% CI: 430–454) during ICU. ESS scores increased from 5.93 (95% CI: 4.89, 7.07) to 8.33 (95% CI: 7.09,9.58) during ICU (p < 0.001). OBI scores increased from 34.5 (95% CI: 32.9–36.2) to 42.8 (95% CI: 40.7–45.0) (p < 0.001). PVT scores worsened with increased reaction time while on ICU rotation (348.5 ms pre-ICU, 370.9 ms post-ICU, p < 0.001).
Conclusions
Resident ICU rotations are associated with decreased objective sleep and self-reported sleep. Residents overestimate sleep duration. Burnout and sleepiness increase and associated PVT scores worsen while working in the ICU. Institutions should ensure resident sleep and wellness checks during ICU rotation.
Keywords: Residency training, Burnout, COVID-19, Intensive care unit, Sleepiness, Fatigue
Introduction
The COVID-19 pandemic had effects beyond the immediate threat of illness to patients infected by the virus. Resident physicians cared for high volumes of critically ill patients with little regard for their own levels of sleepiness or feelings of burnout [1, 2]. Burnout, as defined by the Agency for Healthcare Research and Quality, is a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment [3]. Burnout can threaten care quality, patient safety, and has had detrimental effects on the healthcare system [4, 5]. Resident burnout increased during COVID-19 with up to 45% of residents self-reporting poor sleep quality, increased workload, and sleep deprivation during the pandemic [1, 2, 6, 7]. As many as half of physicians had a self-reported sleep disorder or excessive sleepiness during this time [6, 8]. Concerns regarding personal protective equipment, their own health and safety, and treating critically ill patients—including their colleagues—contributed to these feelings of burnout [9].
Residents working in intensive care units (ICU) report sleep deprivation and high burnout levels [10]. During the pandemic, many hospitals increased their ICU capacities and relied on residents to meet increasing demands [11–13]. Residents are particularly at risk for burnout and sleep deprivation, which are associated with increased medical errors [14, 15]. However, the relationship between sleep, burnout, and psychomotor vigilance has not been studied in residents working in the medical ICU. Lack of sleep and sleep disorders have been shown to be associated with increased burnout, though in the studies of resident burnout during the pandemic, this association has been underassessed [16].
Resident sleep deprivation and fatigue have been extensively studied and judged to be major factors in medical errors [15]. Based on these observations, the Accreditation Council for Graduate Medical Education imposed mandatory work-hour limitations [17, 18]. In this prospective cohort study, we hypothesized that residents rotating through the medical ICU (MICU) during COVID-19 would experience higher sleep loss, burnout, and reduced reaction time as measured by a sleep wearable and psychomotor vigilance testing (PVT).
Methods
Participants, setting, and design
This study invited resident physicians who rotated through the MICU as part of their medical training at a tertiary care hospital in Morgantown, West Virginia. Participants consented and enrolled in this prospective study from August 2021 through May 2022. The West Virginia University Human Research Ethics Committee granted the study approval. Written informed consent was obtained from participants prior to enrollment.
Participants agreed to wear a sleep tracker (Fitbit Inspire 2) (San Francisco, CA) for four consecutive weeks: 2 weeks prior to their rotation in the MICU and 2 weeks during their MICU rotation. Exclusion criteria excluded residents who did not rotate in the MICU, those who wore their device for less than half of the study period, those who did not complete any of the accompanying surveys for data collection, and those who did not return the wearable device at the end of the study. Wearable sleep tracker devices offer cost-effective alternatives and have been shown to be reliable in tracking sleep compared to the gold standard actigraphy device [19].
MICU rotation dates were randomized based on a fixed annual schedule, pre-determined before the resident’s matriculation into the academic year. Residents ranged from their first to fourth year of post-graduate training. Specialties included internal medicine, transitional year, emergency medicine, anesthesia, and medicine/pediatrics combined residency. Shifts varied from day shift to night shift. Interns did not participate in night shift work in the MICU, whereas residents were required to work at least one span of three-night shifts in the MICU. The senior residents (second to fourth years) rotate for a duration of 4-week blocks, while the interns rotate for only 2-week blocks. Data collection methods included having residents complete the Oldenburg burnout inventory (OBI), Epworth sleepiness scale (ESS), a computer-based psychomotor vigilance test (PVT), American Academy of Sleep Medicine (AASM) sleep diary, and wearable sleep tracker data from Fitbit.
Data collection and analysis
Participants were instructed to complete the AASM sleep diary daily to record their self-estimated number of sleep minutes. This diary is frequently used across sleep centers for subjective sleep monitoring, and it asks questions such as time to fall asleep and total sleep minutes [20].
Participants were asked to complete the PVT every other day during the study period using a website-based software program. The test used milliseconds to measure the participant’s reaction time to see a red number randomly appear in a box on the screen (http://www.sleepdisordersflorida.com/pvt1.html). Participants were instructed to perform the PVT testing on the same days as their ESS. PVT assesses fatigue-related changes in alertness. PVT performance has been shown to worsen with time on task and has frequently been utilized in studies to test the impact of sleep loss or prolonged wakefulness [21, 22].
Participants were asked to complete the OBI at the end of the non-MICU block of time and the end of the MICU block. Published in 1998, this scale has shown good interrater reliability and convergence with other burnout scales in the literature [23, 24]. In this scale, burnout has different components, including sub-components that represent the participant’s exhaustion and disengagement [23–25].
Participants were asked to complete the ESS at the end of each work week during the study period. This validated scale is designed to assess daytime sleepiness; it is used clinically to assess patients’ daytime sleepiness [26, 27]. It asks participants to rate the likelihood of dozing in specific scenarios, ranging from sitting in traffic to sitting quietly after lunch without consuming alcohol.
Statistical analysis
Descriptive statistical analyses were performed to summarize the clinical outcome variables including the OBI, ESS, PVT, AASM sleep diary, and wearable sleep tracker-based sleep data. Mean and standard deviations were reported for continuous variables and proportions for categorical variables. A waterfall plot was used to explore the difference in clinical outcomes between pre-ICU and during ICU. Differences between groups and conditions were assessed by the Wilcoxon rank test for continuous variables and the Chi-square test or Fisher exact test as appropriate for categorical variables. Spearman’s correlation was estimated in the correlative analysis between two continuous outcomes. In the multivariate data analysis, a linear mixed-effects model was used to assess the clinical outcomes of the ESS and PVT, which can handle the multiple measurements from each participant over time and assess the independent variables associated with the outcome variables. In addition, a backward variable selection algorithm was performed to identify the independent predictors in the final model. Statistical analyses were performed with R software, version 3.6.3, and p-value of < 0.05 implied statistical significance in this study.
Results
A total of 50 residents were recruited for a randomized, consecutive 4-week period from August 2021 to May 2022, of which 40 (80%) completed the study. Participants’ characteristics are shown in Table 1.
Table 1.
Baseline demographic of study participants. Other: transitional year, emergency medicine, anesthesia, and medicine/pediatrics combined residency
| Baseline characteristics n(%) | |
|---|---|
| Age range: 26–34 years | |
| Gender | |
| F | 21 (53) |
| M | 19 (47) |
| Residency type | |
| IM | 24 (60) |
| Other | 16 (40) |
| Postgraduate year | |
| 1 | 23 (57) |
| 2 | 12 (30) |
| 3 | 3 (8) |
| 4 | 2 (5) |
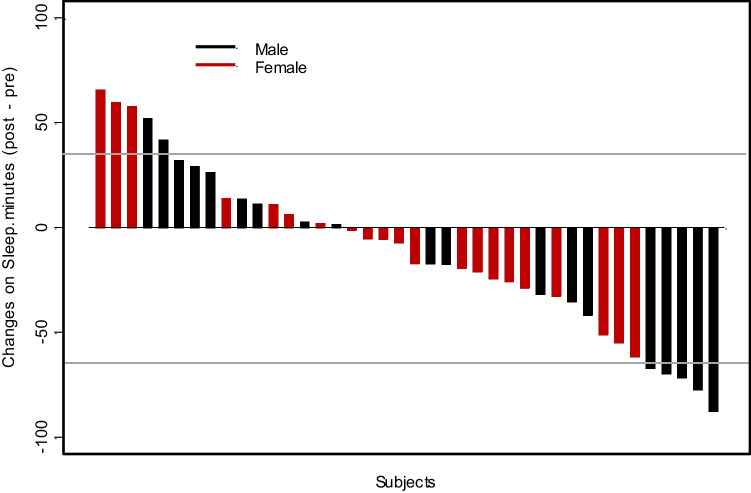
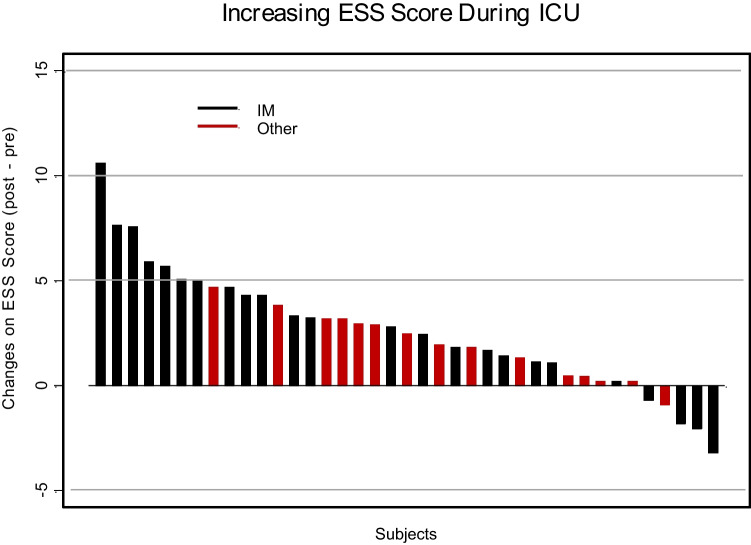
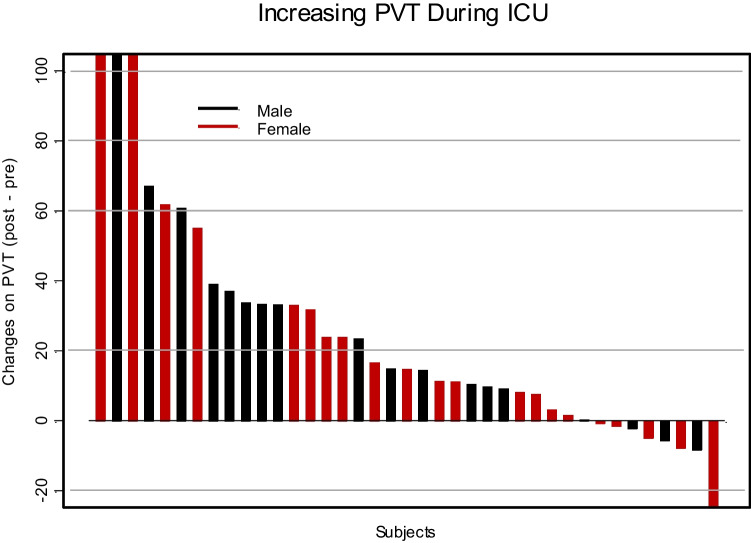
The mean resident sleep duration detected by a wearable sleep tracker before ICU (baseline) was 402 min (95% CI: 377–427), which significantly reduced to 389 min (95% CI: 360–418) during ICU (p < 0.05) (Fig. 1 and Table 2). In parallel, this period was assessed by residents’ sleep estimates as noted in the AASM sleep diary. Residents overestimated the amount of sleep they were getting according to their validated daily log by a mean of 62 min, with 464 min (95% CI: 452–476) before ICU and 442 (95% CI: 430–454) during ICU, which reflected a decrease in sleep of 22 min in their estimates pre- and during ICU (p < 0.02) (Table 2). This overestimation of sleep duration was significant—both at baseline (> 61 min, p < 0.001) and during ICU (> 53 min, p < 0.001) (Table 2). The ESS and OBI also increased significantly and so did the PVT (Table 2, Figs. 2 and 3).
Fig. 1.
Change in distribution of sleep for residents working in the ICU. Waterfall plot depicting distribution of sleep for participants pre-ICU and during ICU by gender
Table 2.
Results of Oldenburg Burnout Inventory including its subcomponents of disengagement and exhaustion, AASM sleep diary, Epworth sleepiness score, and psychomotor vigilance test before and during ICU rotation. Sleeping minutes represents sleep reported by the wearable device
| Measures | All subjects | Wilcoxon signed test | |||
|---|---|---|---|---|---|
| Pre-ICU | During ICU | ||||
| Mean | sd | Mean | sd | p-value | |
| Oldenburg score | 35 | 5 | 43 | 7 | < 0.001 |
| Disengagement | 17 | 3 | 20 | 4 | < 0.001 |
| Exhaustion | 18 | 3 | 23 | 3 | < 0.001 |
| AASM sleep diary | 464 | 39 | 442 | 38 | 0.002 |
| Epworth sleepiness score | 6 | 4 | 8 | 4 | < 0.001 |
| Psychomotor vigilance testing | 348 | 128 | 371 | 133 | < 0.001 |
| Sleeping minutes by wearable tracker | 402 | 81 | 389 | 96 | 0.019* |
*Mixed model
Fig. 2.
Change in distribution of Epworth sleepiness scores for residents working in the ICU
Fig. 3.
Change in distribution of psychomotor vigilance testing for residents in the ICU
On multivariate analysis of the impact on ESS, MICU rotation and mean sleep duration reduction based on the AASM sleep diary were independently inversely associated (coefficient − 0.021, p = 0.031). Increasing ESS was correlated with being in the MICU (coefficient 1.944, p < 0.001). ESS was also inversely associated with male gender (coefficient − 2.335, p = 0.034). An increase in the Oldenburg score was not independently associated with a reduced ESS (coefficient 0.365, p > 0.20). However, increased PVT was associated independently with exhaustion (coefficient of 4.56) (p = 0.009).
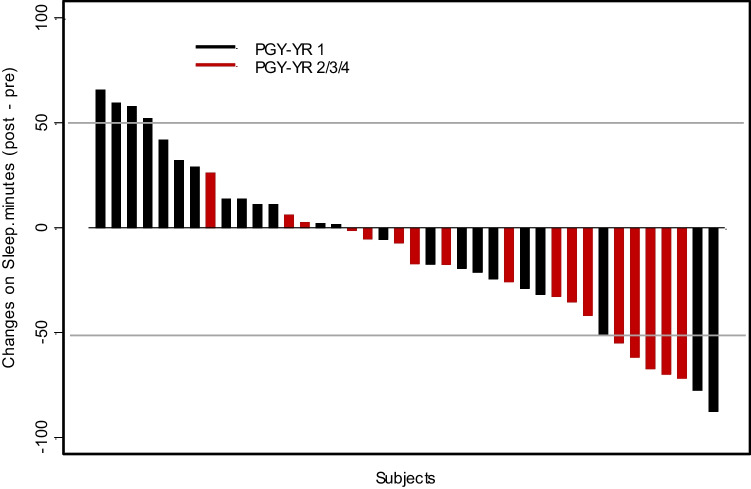
Interns had less reduction in the duration of total mean sleep during their MICU rotation compared to senior residents (< 26.5 min, p = 0.008) (Fig. 4). Other subset analyses for gender and residency type showed no significant differences (Figs. 1 and 4).
Fig. 4.
Change in distribution of sleep for residents working in the ICU. Waterfall plot depicting distribution of sleep for participants pre-ICU and during ICU by training year
Discussion
The study reveals that residents experienced significant sleep deprivation and burnout during MICU rotation compared to non-ICU rotations. Additionally, it was concerning that the PVT testing revealed that psychomotor reaction time increased during MICU rotation, suggesting a slower reaction time during MICU than pre-MICU. These factors may cumulatively significantly impact the well-being of the residents and consequently may also put patient care at risk. This may have been exacerbated due to the COVID-19 pandemic [1, 2, 6].
The mean sleep duration recorded by the wearable sleep tracker during the MICU rotation was 31 min less than the AASM recommended sleep duration of 7 or more hours per night [28]. The mean resident objective sleep duration recorded before ICU (baseline) reduced during the ICU rotation (13 min; p < 0.05). Although 13 min less sleep daily seems miniscule, this translates to a more profound sleep debt over the rotation. It has been shown that multi-task performance and subjective sleepiness are adversely affected by sleep debt [29, 30]. A similar study was conducted pre-pandemic on residents working in an ICU with actigraphy. The mean sleep duration was higher in their ICU group compared to our group (403 min versus 389 min), with decreased ESS scores concordant with our data suggesting working in the ICU has a negative effect on sleep [31].
Subjective sleep duration, the AASM sleep diary revealed a similar pattern of sleep reduction during MICU weeks and compared to pre-MICU. However, residents significantly overestimated their sleep, both pre-ICU and during ICU rotation, compared to the objective measurement the wearable sleep tracker provided. Consistent with previous studies indicating people tend to overestimate sleep time, residents significantly overestimated their sleep time (both pre-ICU and during ICU rotation) compared to objective measurement [32–34]. In fact, 35/40 (87%) of residents overestimated their sleep duration time. These data is concerning because it suggests poor insight regarding sleep duration estimation by residents. Based on this observation, it is recommended that the residents be encouraged to use a wearable sleep tracker during ICU rotation and objectively review their sleep duration.
The ESS scores for the participating resident group significantly increased from the pre-ICU period to the ICU rotation period. The number of residents showing subjective sleepiness (33%) nearly doubled during the ICU rotation period based on ESS. Decreased sleep has been shown to be associated with increased ESS [26, 35, 36]. Our study adds insight showing even small losses of sleep on a daily basis (13 min) can be associated with significant increases in ESS.
Also of concern are the PVT results showing significant deterioration in response time before and during MICU. The results may suggest blunting of cognition and decision-making in a fast-paced environment, which may negatively impact one’s ability to make effective decisions in patient care. However, PVT results have to be interpreted with caution, considering prior studies are conflicting in their correlation with objective sleepiness and PVT as measured by multiple sleep latency tests (MSLTs) [21, 37, 38].
Sleep deprivation was noted to be significantly less in interns compared to senior residents. This difference is most likely because interns do not take night calls during their ICU blocks as opposed to their senior residents but further studies will be required to further explain this phenomenon.
The strengths of the study are the prospective design of the study, robust participation, and low attrition rate. Unlike prior studies, we collected data over 2 weeks before and during MICU, which provided a sleep profile over time. Since there may be an overlap between sleep deprivation and burnout, we measured both elements. We chose to combine subjective methods (sleep diary) and objective methods (sleep wearable tracker) for measuring sleep duration, measuring subjective sleepiness by ESS, and adding PVT for assessing response time. This choice provided an additional dimension to the study.
While this study provides important insight into resident sleep/health, a few limitations should be noted. There is a lack of extensive commercial sleepwear testing and the possibility that the sleep-tracking devices may over- or underestimate sleep duration is a limitation of our work. However, studies have found them to be comparable to actigraphy [19]. PVT testing has shown mixed results with real-time outcomes and needs to be interpreted cautiously [21, 33, 34]. Sleep indices were collected up to 2 weeks into the MICU service, although in our program, senior residents rotate for a full 4 weeks. The rationale was to keep parity with interns who rotate in the MICU for only 2 weeks. This may have led to underestimating the sleep debt and subsequent outcomes in senior residents.
Conclusion
This study was conducted with participating residents rotating through the medical ICU during the COVID-19 pandemic. Our study highlights a significant escalation of sleepiness, burnout, and reduced reaction time. Residents notably overestimate their sleep duration during their MICU rotation. Monitoring sleep objectively and improving awareness of proper sleep and wellness programs before and during MICU may help mitigate some deleterious effects. Further research endeavors, including studying the effect of this proposed intervention, are recommended for preparing residents for future pandemics.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amanullah S, Shankar RR (20202) The impact of COVID-19 on physician burnout globally: a review. Healthc (Basel, Switzerland). 8(4). 10.3390/HEALTHCARE8040421 [DOI] [PMC free article] [PubMed]
- 2.Morgantini LA, Naha U, Wang H, et al. (2020) Factors contributing to healthcare professional burnout during the COVID-19 pandemic: a rapid turnaround global survey. PLoS One. 15(9 September). 10.1371/journal.pone.0238217 [DOI] [PMC free article] [PubMed]
- 3.(AHRQ) A for HR and Q. AHRQ works. Building bridges between research and practice: physician burnout. Published online 2017:1–3. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/ahrq-works/impact-burnout.pdf
- 4.Chuang CH, Tseng PC, Lin CY, Lin KH, Chen YY. Burnout in the intensive care unit professionals: a systematic review. Medicine (Baltimore) 2016;95(50):e5629. doi: 10.1097/MD.0000000000005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sriharan A, Ratnapalan S, Tricco AC, et al. Occupational stress, burnout, and depression in women in healthcare during COVID-19 pandemic: rapid scoping review. Front Glob women’s Heal. 2020;1:596690. doi: 10.3389/fgwh.2020.596690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa C, Teodoro M, Briguglio G, et al. Sleep quality and mood state in resident physicians during COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(15):8023. doi: 10.3390/IJERPH18158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chebib E, Chiesa-Estomba CM, Radulesco T, et al. Assessment of sleep features, mental health outcomes, and alcohol and tobacco consumption in residents and fellows in otolaryngology before and during the COVID-19 pandemic. JAMA Otolaryngol Neck Surg. 2022;148(8):719–723. doi: 10.1001/JAMAOTO.2022.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alnofaiey YH, Alshehri HA, Alosaimi MM, et al. Sleep disturbances among physicians during COVID-19 pandemic. BMC Res Notes. 2020;13(1):1–7. doi: 10.1186/S13104-020-05341-6/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoorob D, Shah S, La Saevig D, Murphy C, Aouthmany S, Brickman K. Insight into resident burnout, mental wellness, and coping mechanisms early in the COVID-19 pandemic. PLoS One. 2021;16(4):e0250104. doi: 10.1371/JOURNAL.PONE.0250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy R, Guntupalli K, Alapat P, Surani S, Casturi L, Subramanian S. Sleepiness in medical ICU residents. Chest. 2009;135(1):81–85. doi: 10.1378/chest.08-0821. [DOI] [PubMed] [Google Scholar]
- 11.Al-Dorzi HM, Aldawood AS, Almatrood A, et al. Managing critical care during COVID-19 pandemic: the experience of an ICU of a tertiary care hospital. J Infect Public Health. 2021;14(11):1635. doi: 10.1016/J.JIPH.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litton E, Huckson S, Chavan S, et al. Increasing ICU capacity to accommodate higher demand during the COVID-19 pandemic. Med J Aust. 2021;215(11):513–517. doi: 10.5694/MJA2.51318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litton E, Bucci T, Chavan S, et al. Surge capacity of intensive care units in case of acute increase in demand caused by COVID-19 in Australia. Med J Aust. 2020;212(10):463–467. doi: 10.5694/mja2.50596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veasey S, Rosen R, Barzansky B, Rosen I, Owens J (n.d) Sleep loss and fatigue in residency training a reappraisal. Accessed September 20, 2022. https://jamanetwork.com/ [DOI] [PubMed]
- 15.Owens JA, Veasey SC, Rosen RC. Physician, heal thyself: sleep, fatigue, and medical education. Sleep. 2001;24(5):493–495. doi: 10.1093/SLEEP/24.5.493. [DOI] [PubMed] [Google Scholar]
- 16.Weaver MD, Robbins R, Quan SF, et al. Association of sleep disorders with physician burnout. JAMA Netw Open. 2020;3(10):e2023256–e2023256. doi: 10.1001/JAMANETWORKOPEN.2020.23256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acgme. ACGME common program requirements section VI with background and intent.
- 18.ACGME resident/fellow education and training considerations related to coronavirus (COVID-19). Accessed September 20, 2022. https://www.acgme.org/newsroom/2020/3/acgme-residentfellow-education-and-training-considerations-related-to-coronavirus-covid-19/
- 19.Chinoy ED, Cuellar JA, Jameson JT, Markwald RR. Performance of four commercial wearable sleep-tracking devices tested under unrestricted conditions at home in healthy young adults. Nat Sci Sleep. 2022;14:493–516. doi: 10.2147/NSS.S348795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287. doi: 10.5665/SLEEP.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17(1):34–41. doi: 10.1111/J.1365-2869.2008.00635.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bihari S, Venkatapathy A, Prakash S, Everest E, McEvoy RD, Bersten A. ICU shift related effects on sleep, fatigue and alertness levels. Occup Med (Lond) 2020;70(2):107–112. doi: 10.1093/OCCMED/KQAA013. [DOI] [PubMed] [Google Scholar]
- 23.Demerouti E, Bakker AB, Vardakou I, Kantas A. The convergent validity of two burnout instruments: a multitrait-multimethod analysis. Eur J Psychol Assess. 2003;19(1):12–23. doi: 10.1027//1015-5759.19.1.12. [DOI] [Google Scholar]
- 24.Halbesleben JRB, Demerouti E. The construct validity of an alternative measure of burnout: investigating the English translation of the Oldenburg Burnout Inventory. Work & Stress. 2007;19(3):208–220. doi: 10.1080/02678370500340728. [DOI] [Google Scholar]
- 25.OanaTipa R, Tudose C, Pucarea VL. Measuring burnout among psychiatric residents using the Oldenburg Burnout Inventory (OLBI) instrument. J Med Life. 2019;12:354–360. doi: 10.25122/jml-2019-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17(8):703–710. doi: 10.1093/SLEEP/17.8.703. [DOI] [PubMed] [Google Scholar]
- 28.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. doi: 10.5665/SLEEP.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaki NFW, Spence DW, Subramanian P, et al. Basic chronobiology: what do sleep physicians need to know? Sleep Sci. 2020;13(4):256. doi: 10.5935/1984-0063.20200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenstein M. Chronobiology: stepping out of time. Nat. 2013;497(7450):10–12. doi: 10.1038/497S10a. [DOI] [PubMed] [Google Scholar]
- 31.Bordley J, Agustin AG, Ahmed MA, et al. Restoration of resident sleep and wellness with block scheduling. Med Educ. 2017;51(12):1241–1249. doi: 10.1111/MEDU.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taillard J, Sagaspe P, Philip P, Bioulac S (2021) Sleep timing, chronotype and social jetlag: impact on cognitive abilities and psychiatric disorders. Biochem Pharmacol 191. 10.1016/j.bcp.2021.114438 [DOI] [PubMed]
- 33.Dietch JR, Taylor DJ. Evaluation of the consensus sleep diary in a community sample: comparison with single-channel electroencephalography, actigraphy, and retrospective questionnaire. J Clin Sleep Med. 2021;17(7):1389–1399. doi: 10.5664/JCSM.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang ET, Huang CY, Lai HL. Differences between sleep logs and actigraphy combined with electroencephalography in adults with sleep disturbances. Biol Res Nurs. 2018;20(1):77–83. doi: 10.1177/1099800417725599. [DOI] [PubMed] [Google Scholar]
- 35.Rosen IM, Gimotty PA, Shea JA, Bellini LM. Evolution of sleep quantity, sleep deprivation, mood disturbances, empathy, and burnout among interns. Acad Med. 2006;81(1):82–85. doi: 10.1097/00001888-200601000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Perotta B, Arantes-Costa FM, Enns SC, et al. Sleepiness, sleep deprivation, quality of life, mental symptoms and perception of academic environment in medical students. BMC Med Educ. 2021;21(1). 10.1186/S12909-021-02544-8 [DOI] [PMC free article] [PubMed]
- 37.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. doi: 10.1093/SLEEP/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinges DF, Pack F, Williams K, et al. (1997) Cumulative sleepiness, mood disturbance and psychomotor vigilance performance decrements during aweek of sleep restricted to 4–5 hours per night. - PsycNET. Sleep J Sleep Res Sleep Med. 20(4):267–277. Accessed September 20, 2022. https://psycnet.apa.org/record/1997-06077-003 [PubMed]
- 39.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462–468. doi: 10.2188/JEA.JE20120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos RB, Giatti S, Aielo AN, et al. Prevalence and predictors of under or overestimation sleep duration in adults: the ELSA-Brasil study. Sleep Epidemiol. 2021;1:100013. doi: 10.1016/J.SLEEPE.2021.100013. [DOI] [Google Scholar]
- 41.Feehan LM, Geldman J, Sayre EC, et al. (2018) Accuracy of fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR mHealth uHealth. 6(8). 10.2196/10527 [DOI] [PMC free article] [PubMed]
- 42.Evenson KR, Goto MM, Furberg RD. (2015) Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 12(1). 10.1186/s12966-015-0314-1 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.