Abstract
Purpose
There are approximately 150,000 women living with metastatic breast cancer (mBC) in the United States. Disparities in de novo mBC incidence and mortality exist across race/ethnicity, socioeconomic status (SES), and rurality. However, how SES and rurality independently impact mBC outcomes across different racial/ethnic groups is not fully understood. The purpose of this study was to determine the impact of SES and rurality on cancer-specific mortality among women with mBC by race/ethnicity.
Methods
We conducted a large, population-based retrospective cohort study in women aged 18 + years diagnosed with de novo mBC using the Surveillance, Epidemiology and End Results Census Tract-level SES and Rurality Database (2000–2015). Associations between SES/rurality and cancer-specific mortality were determined using Fine and Gray regression models. Subdistribution hazard ratios (SHR) and 95% confidence intervals (CI) by race/ethnicity and hormone receptor (HR) status were calculated.
Results
A cohort of 33,976 women were included with the majority being White (67%), 17% Black, 0.4% American Indian/Alaskan Native, 6% Asian/Pacific Islander, and 10% Latina/Hispanic. We observed the greatest increased risk of BC mortality among Black women with HR-negative mBC residing in neighborhoods with lower SES (lowest versus highest quintile: SHR 1.38, 95% CI 1.00–1.90) and in rural areas compared to urban areas (SHR 1.27, 95% CI 1.01–1.59).
Conclusion
Overall, BC-specific survival among women with de novo mBC differs by race/ethnicity, with the greatest adverse impacts of SES and rurality affecting Black women with HR-negative disease.
Keywords: De novo metastatic breast cancer, Cancer-specific survival, Racial disparity, Socioeconomic status, Rurality
Introduction
In the United States (US), there are approximately 150,000 women living with metastatic breast cancer (mBC), and approximately, 1 in 8 U.S. women will develop mBC in their lifetime [1]. Newly diagnosed primary mBC, or de novo mBC, is distinct from relapsing metastatic disease, which develops from a previously diagnosed early-stage breast cancer [1, 2]. The five-year relative survival rate among de novo mBC patients is approximately 27% [1]. While routine mammography screening has become more common, the incidence of de novo mBC has remained relatively steady for decades and the incidence of relapsing mBC has decreased [2, 3]. This evidence suggests that regular mammography screening does not eliminate the emergence of biologically aggressive mBC. Furthermore, studies have suggested that women with de novo mBC were disproportionately affected by social and structural determinants of health such as delayed access to care, low socioeconomic status (SES), and rural–urban residential area [2–4]. Although mBC makes up a smaller proportion of all advanced metastatic disease, it is important to understand the impact of mBC given that some of these cases could be prevented by early diagnosis to reduce morbidity and mortality [5].
Disparities exist in the incidence and survival of women diagnosed with de novo mBC, especially across racial/ethnic groups, geographic areas, and SES [4]. For example, Black women have higher incidence of de novo mBC compared to White women [5, 6]. Rurality is also associated with greater incidence of de novo mBC incidence [2, 6, 7]. Additionally, across studies measuring SES using various metrics, lower SES is consistently associated with a greater incidence of de novo mBC [4, 7]. Most studies investigating these health disparities have evaluated the effects of race/ethnicity, SES, and rurality on de novo mBC as separate (versus interacting or overlapping) associations [4, 5]. However, the association between SES and rurality on cancer-specific mortality across racial/ethnic groups is not well understood [2, 8]. Social determinants of health such as lack of access to care, distance to clinics, residential segregation by race, and lack of social support are known to be associated to BC outcomes [9]. We hypothesize that the independent impact of SES and rurality will differ across race/ethnicity due to the complex mechanisms determining racial disparities in breast cancer. Our objective was to determine the association between SES and rurality on cancer-specific mortality among women with de novo mBC across racial/ethnic groups using a competing risks approach. We hypothesized that neighborhood SES level, and living in a rural or urban area will have different levels of comorbidity, social determinants of health, and risks of other cause of death, and will differentially impact de novo mBC outcomes by race/ethnicity and hormone receptor (HR) status [1].
Methods
Database and participants
We conducted a large retrospective, population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Census Tract-Level SES and Rurality Database (2000–2015) between January 1, 2000 and December 31, 2015. The SEER database is a population-based cancer registry that collects data from cancer registries across 18 States and covers 48% of patients in the United States. Information from SEER database includes demographics, cancer characteristics, stage of disease, treatment, and outcomes [10]. This study was determined approved by the institutional review board of the University of Illinois Chicago.
We included women aged 18 years and older who were diagnosed with microscopically confirmed, first primary de novo mBC diagnosed between 2000 and 2015. Women who were diagnosed at autopsy, identified by death certificate data or with unknown race/ethnicity were excluded. Detailed information on de novo mBC cohort can be found in Supplemental Fig. 1.
Exposure
In the specialized Census Tract-level SES and Rurality Database, SES was estimated using the Yost index, a time-dependent composite score based on seven SES-related, social determinant of health variables: median household income, median house value, median rent, percent below 150% of poverty line, education index, percent working class, and percent unemployed. The development of the Yost index is grounded within a social determinants of health framework emphasizing aggregate measures of occupation, education, and income [11]. The Yost index was initially developed among BC patients using factor analysis and was later validated using the California cancer registry among BC patients [10, 11]. We analyzed associations with the Yost index categorized in quintiles with the lowest quintile (1st) representing areas with the lowest SES and the highest quintile (5th) representing areas with the highest SES.
Rurality was determined by the US Department of Agriculture’s Rural Urban Commuting Area (RUCA) codes with a binary classification: urban area commuting focused and nonurban area community focused [12]. The coding classification is based on population density, urbanization, and daily commuting and are based on census data from the American Community Survey. Urban area community designated included the following metropolitan areas and other areas (i.e., micropolitan, small town, and rural areas) with secondary flow 30–50% to an urbanized area. Nonurban community focused includes all other code designations. Patients with missing SES or rurality were excluded from the cohort.
Outcome and covariates
Cancer-specific death was identified using International Classification of Diseases (ICD) codes and identified through autopsy or death certification. Cause of death was determined based on SEER registry records. Patients were followed from the time of their de novo mBC diagnosis until death or the end of the study period. Patients with unknown information on vital status were excluded from the cohort. Demographic and clinical characteristics were collected at the time of diagnosis, including age (< 50, 50–64, 65–74, and 75 + years old), year of diagnosis (2000–2005, 2006–2010, 2011–2015), and marital status (not married, married). Available information on age was truncated at 85 years old and is summarized by medians and categorical age. Insurance status was collected starting 2007 and later where information was available; it was categorized as uninsured, Medicaid, insured, insured (not specified), and unknown. Joint HR status was derived from estrogen receptor (ER) and progesterone receptor (PR) status. HR-positive was defined as ER and/or PR positive or borderline, while HR-negative was defined as ER and PR negative. HER2 status was only available in the SEER registries among women diagnosed after 2010. Patients diagnosed prior to 2010 and patients diagnosed after 2010 with unknown HER2 status were assigned with an unknown HER2 status. Missing data on covariates were included as an independent unknown category for each variable. BC treatment including receipt of cancer-directed surgery, radiation therapy, and chemotherapy was also collected. Race and ethnicity were combined to create mutually exclusive race/ethnicity groups: non-Hispanic White, non-Hispanic Black, non-Hispanic American Indian/Alaska Native, non-Hispanic Asian/Pacific Islander, and Hispanic/Latina (any race) women.
Statistical analyses
Descriptive statistics of demographic and clinical characteristics were compared by race/ethnicity. Continuous and categorical variables were compared using the Wilcoxon rank test and Chi-square test, respectively. Overall and cancer-specific cumulative incidence functions were estimated following the approach of Gooley et al. [13] and compared using Fine and Gray models accounting for competing risks (i.e., other causes of death) [14]. Gray’s test was used to compare the equality of cumulative incidence functions [15].
Univariate and multivariable Fine and Gray competing risk regression models were fit to determine associations separately between race/ethnicity, SES, rurality, and BC-specific mortality. The main multivariable models for race/ethnicity, SES, and rurality were fit along with other characteristics including age, year of diagnosis, marital status, insurance status, HR status, HER2 status, and treatment. We also separately performed trend tests on the association between SES quintile on BC-specific mortality. We further evaluated the impact of SES and rurality independently by stratifying the multivariable model by race/ethnicity and HR status. Crude and adjusted subdistribution hazard ratios (SHR) and 95% confidence intervals (CI) with robust variance were estimated. To better understand the race/ethnicity difference in impacts of SES and rurality on cancer-specific mortality, we performed stratified analyses by race/ethnicity and by HR status. Several sensitivity analyses were performed to better understand the implication of missing values in HER2 status. First, we repeated our main analysis and restricted to women diagnosed after 2010. Second, we stratified our analysis by BC subtypes (Luminal A, Luminal B, HER2-enriched, Triple Negative) among women diagnosed after 2010 with joint HR and HER2 information available. All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
This retrospective cohort included 33,976 women diagnosed with de novo mBC between 2000 and 2015. The majority of women were White (67%), followed by Black (17%), Latina/Hispanic (10%), Asian/Pacific Islander (6%), and American Indian/Alaska Native (0.4%). Descriptive statistics by race/ethnicity are reported in Table 1. Median age at diagnosis was different across race/ethnicity and was higher among White women relative to Black, American Indian/Alaskan Native, Asian/Pacific Islander, and Hispanic/Latina women. Compared to White women, a higher proportion of Black women were not married (74% vs. 55%, P < 0.01), uninsured (5% vs. 2%, P < 0.01), and were diagnosed HR-negative mBC (29% vs. 19%, P < 0.01). Compared to White women, Asian/Pacific Islanders were more likely to be married (52% vs. 45%, P < 0.01), have Medicaid coverage (19% vs. 8%, P < 0.01), and have HER2-positive disease (15% vs.11%, P < 0.01), while Latina/Hispanic women were more likely to be uninsured (5% vs. 2%, P < 0.01), have HR-negative disease (22% vs. 19%, P < 0.01), and negative HER2 status (31% vs. 28%, P < 0.01). Receipt of surgery for de novo mBC did not differ significantly by race/ethnicity (P = 0.11). However, receipt of radiation (P = 0.04) and chemotherapy (P < 0.01) were significantly different across race/ethnicity.
Table 1.
Demographic and characteristics of women diagnosed with metastatic breast cancer by race/ethnicity in the SEER 18 registries, 2000–2015
| Total (N = 33,967) | White ((N = 22,693) | Black (N = 5657) | American Indian / Alaskan Native (N = 129 | Asian / Pacific Islander (N = 2149) | Latina/ Hispanic (N = 3348) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | P | |
|
| |||||||||||||
| Age, years | |||||||||||||
| Median (IQR) | 61 | 20 | 62 | 19 | 57 | 18 | 58 | 17 | 56 | 17 | 55 | 20 | |
| < 50 | 6990 | 20.57 | 3737 | 16.47 | 1464 | 25.88 | 37 | 28.68 | 600 | 27.92 | 1152 | 34.41 | < .0001 |
| 50–64 | 12,824 | 37.74 | 8269 | 36.44 | 2352 | 41.58 | 54 | 41.86 | 924 | 43.00 | 1225 | 36.59 | |
| 65–74 | 6999 | 20.60 | 5077 | 22.37 | 1015 | 17.94 | 24 | 18.60 | 344 | 16.01 | 539 | 16.10 | |
| 75 + | 7163 | 21.08 | 5610 | 24.72 | 826 | 14.60 | 14 | 10.85 | 281 | 13.08 | 432 | 12.90 | |
| Year of diagnosis | < .0001 | ||||||||||||
| 2000–2005 | 10,450 | 30.76 | 7321 | 32.26 | 1739 | 30.74 | 43 | 33.33 | 532 | 24.76 | 815 | 24.34 | |
| 2006–2010 | 10,845 | 31.92 | 7192 | 31.69 | 1785 | 31.55 | 46 | 35.66 | 708 | 32.95 | 1114 | 33.27 | |
| 2011–2015 | 12,681 | 37.32 | 8180 | 36.05 | 2133 | 37.71 | 40 | 31.01 | 909 | 42.30 | 1419 | 42.38 | |
| Marital status | < .0001 | ||||||||||||
| Not married | 19,482 | 57.34 | 12,363 | 54.48 | 4168 | 73.68 | 82 | 63.57 | 1032 | 48.02 | 1837 | 54.87 | |
| Married | 14,494 | 42.66 | 10,330 | 45.52 | 1489 | 26.32 | 47 | 36.43 | 1117 | 51.98 | 1511 | 45.13 | |
| Insurance status | < .0001 | ||||||||||||
| Uninsured | 957 | 2.82 | 456 | 2.01 | 279 | 4.93 | 2 | 1.55 | 58 | 2.70 | 162 | 4.84 | |
| Medicaid | 4260 | 12.54 | 1915 | 8.44 | 1050 | 18.56 | 31 | 24.03 | 405 | 18.85 | 859 | 25.66 | |
| Insured | 12,540 | 36.91 | 9030 | 39.79 | 1619 | 28.62 | 32 | 24.81 | 835 | 38.86 | 1024 | 30.59 | |
| Insured (not specified) | 3332 | 9.81 | 2341 | 10.32 | 536 | 9.47 | 12 | 9.30 | 160 | 7.45 | 283 | 8.45 | |
| Unknown | 12,887 | 37.93 | 8951 | 39.44 | 2173 | 38.41 | 52 | 40.31 | 691 | 32.15 | 1020 | 30.47 | |
| Hormone receptor status | < .0001 | ||||||||||||
| ER and/or PR positive or borderline | 22,418 | 65.98 | 15,474 | 68.19 | 225 | 57.01 | 80 | 62.02 | 1453 | 67.61 | 2186 | 65.29 | |
| ER and PR negative | 7148 | 21.04 | 4249 | 18.72 | 1622 | 28.67 | 30 | 23.26 | 498 | 23.17 | 749 | 22.37 | |
| Unknown | 4410 | 12.98 | 2970 | 13.09 | 810 | 14.32 | 19 | 14.73 | 198 | 9.21 | 413 | 12.34 | |
| HER2 status | |||||||||||||
| Positive or borderline | 3873 | 11.40 | 2393 | 10.55 | 667 | 11.79 | 13 | 10.08 | 313 | 14.56 | 487 | 14.55 | |
| Negative | 9723 | 28.62 | 6368 | 28.06 | 1627 | 28.76 | 34 | 26.36 | 673 | 31.32 | 1021 | 30.50 | |
| Unknown | 20,380 | 59.98 | 13,932 | 61.39 | 3363 | 59.45 | 82 | 63.57 | 1163 | 54.12 | 1840 | 54.96 | |
| Cancer-directed surgery | 0.1135 | ||||||||||||
| No | 15,268 | 44.94 | 10,202 | 44.96 | 2552 | 45.11 | 72 | 55.81 | 969 | 45.09 | 1473 | 44.00 | |
| Yes | 18,708 | 55.06 | 12,491 | 55.04 | 3105 | 54.89 | 57 | 44.19 | 1180 | 54.91 | 1875 | 56.00 | |
| Radiation | 0.0472 | ||||||||||||
| None/unknown | 22,710 | 66.84 | 15,110 | 66.58 | 3850 | 68.06 | 79 | 61.24 | 1404 | 65.33 | 2267 | 67.71 | |
| Yes | 11,266 | 33.16 | 7583 | 33.42 | 1807 | 31.94 | 50 | 38.76 | 745 | 34.67 | 1081 | 32.29 | |
| Chemotherapy | < .0001 | ||||||||||||
| None/unknown | 16,156 | 47.55 | 11,478 | 50.58 | 2440 | 43.13 | 56 | 43.41 | 877 | 40.81 | 1305 | 38.98 | |
| Yes | 17,820 | 52.45 | 11,215 | 49.42 | 3217 | 56.87 | 73 | 56.59 | 1272 | 59.19 | 2043 | 61.02 | |
| SES quintile | < .0001 | ||||||||||||
| 1st - lowest | 6861 | 20.19 | 2913 | 12.84 | 2786 | 49.25 | 34 | 26.36 | 194 | 9.03 | 934 | 27.90 | |
| 2nd | 6685 | 19.68 | 4351 | 19.17 | 1126 | 19.90 | 31 | 24.03 | 348 | 16.19 | 829 | 24.76 | |
| 3rd | 6773 | 19.93 | 4807 | 21.18 | 843 | 14.90 | 29 | 22.48 | 448 | 20.85 | 646 | 19.30 | |
| 4th | 6975 | 20.53 | 5275 | 23.25 | 574 | 10.15 | 21 | 16.28 | 543 | 25.27 | 562 | 16.79 | |
| 5th - highest | 6682 | 19.67 | 5347 | 23.56 | 328 | 5.80 | 14 | 10.85 | 616 | 28.66 | 377 | 11.26 | |
| Census urban area-based classification | < .0001 | ||||||||||||
| Urban | 30,864 | 90.86 | 20,164 | 88.86 | 5333 | 94.27 | 100 | 77.52 | 2062 | 95.95 | 3205 | 95.73 | |
| Rural | 3112 | 9.14 | 2529 | 11.14 | 324 | 5.73 | 29 | 22.48 | 87 | 4.05 | 143 | 4.27 | |
The distribution of area-level SES status differed significantly across all racial/ethnic groups (P < 0.01); almost half (47%) of White women resided in the neighborhoods with higher (4th or 5th) SES quintiles, while more than half of the Black women (68%), American Indian/Alaska Native women (50%), and Latina/Hispanic women (52%) lived in the neighborhoods with lower (1st or 2nd) SES quintile (Fig. 1). When compared to other race/ethnicity, American Indian/Alaskan Natives patient had the highest proportion of women lived in rural area (23%). Descriptive statistics stratified by the SES quintile and rurality are reported in supplemental material (Supplemental Tables 1 and 2).
Fig. 1.
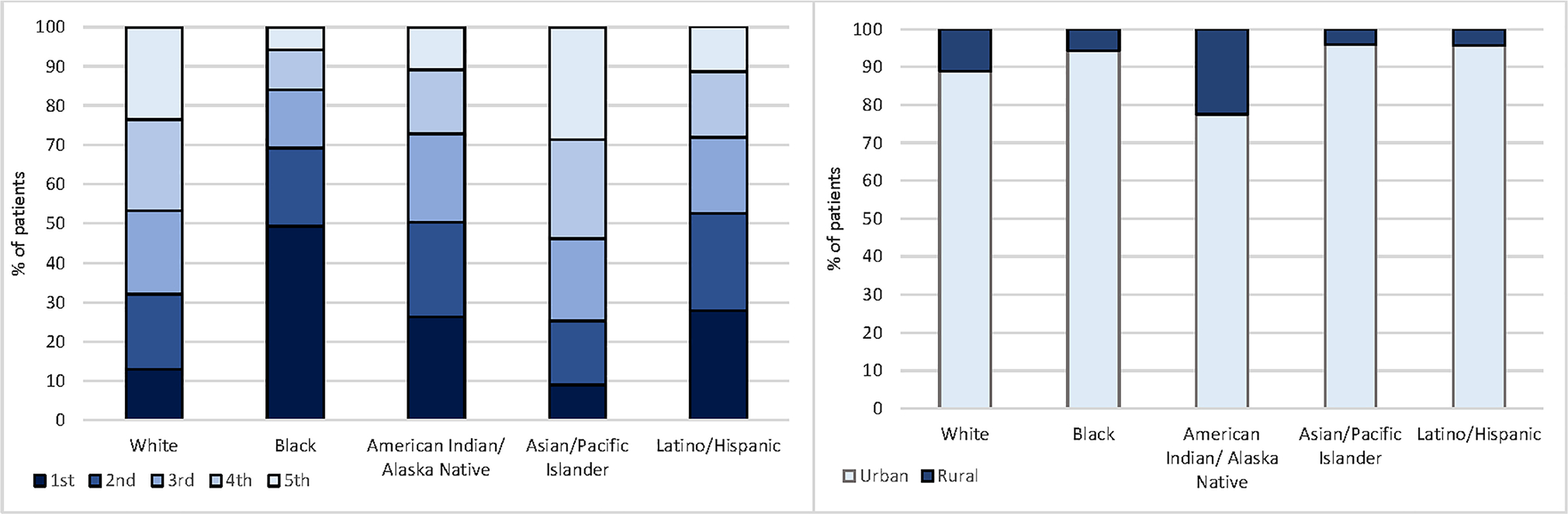
Distribution of SES quintile and rurality by race/ethnicity
Figure 2 reports overall and cancer-specific cumulative incidence by race/ethnicity, SES quintile, and rurality. After adjusting for competing risks, cancer-specific mortality was significantly higher compared to all other-cause mortality. Black women had the greatest (89%) five-year cumulative incidence of cancer-specific death, followed by Latina/Hispanic (83%), America Indian/Alaska Native (83%), White (81%), and Asian/Pacific Islander (81%) (Gray’s test, P < 0.01). Women residing in neighborhoods with the lowest SES quintile had the highest rate of five-year breast cancer-specific death (87%), while women who lived in the neighborhoods with the highest SES quintile had the lowest five-year incident BC death (81%) (Gray’s test, P < 0.01). The five-year cumulative incidences of cancer-specific death observed in women residing in rural (84%) versus urban (83%) areas were not significantly different (Gray’s test, P = 0.93).
Fig. 2.
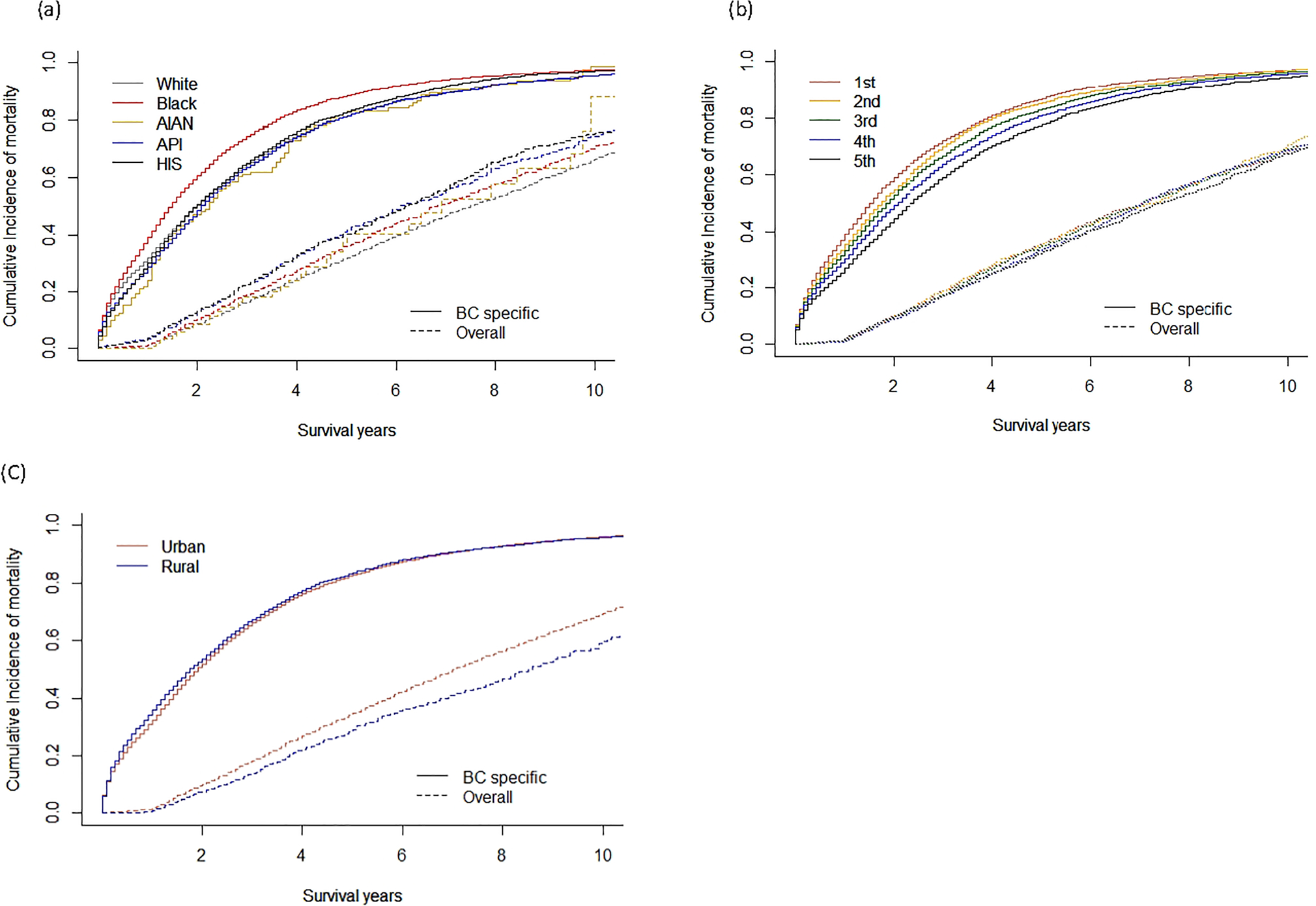
Cumulative incidence/hazard functions for BC mortality by a race/ethnicity, b SES quintiles, and c rurality
Overall, de novo mBC patients in the lowest SES quintile had a significantly increased risk of BC mortality compared to those in the highest SES quintile (SHR = 1.22, 95% CI 1.17–1.26). Compared to White women, Black women had 1.15 (95% CI 1.11–1.19) times higher cancer-specific mortality after adjusting for baseline demographics and clinical characteristics, while differences in American Indian/Alaska Native women (SHR = 0.96, 95% CI 0.82–1.12), Asian/Pacific Islander women (SHR = 0.97, 95% CI 0.93–1.01), and Latina/Hispanic women (SHR = 1.03, 95% CI 0.99 = 1.07) were not statistically significant. Women living in rural areas did not have an increased risk of BC-specific mortality (SHR = 1.00, 95% CI 0.96–1.05) compared to those living in urban areas (Table 2). The results from sensitivity analysis where we restricted to women diagnosed after 2010 with available HR and HER2 information were consistent with our main analysis (Supplemental Table 3).
Table 2.
Subdistribution hazard ratio (SHR) for overall breast cancer survival using Fine and Gray competing risk
| Crude SHR (95%CI) | Adjusted SHR (95%CI)a | |
|---|---|---|
|
| ||
| Race | ||
| White | Ref | Ref |
| Black | 1.26 (1.22, 1.30) | 1.15 (1.11, 1.19) |
| American Indian/ Alaska Native | 0.96 (0.82, 1.12) | 0.96 (0.82, 1.13) |
| Asian/ Pacific Islander | 0.97 (0.93, 1.01) | 1.00 (0.96, 1.05) |
| Latina/Hispanic | 1.03 (0.99, 1.07) | 1.00 (0.97, 1.04) |
| SES quintile | ||
| 1st | 1.35 (1.30, 1.39) | 1.22 (1.17, 1.26) |
| 2nd | 1.27 (1.22, 1.31) | 1.20 (1.15, 1.24) |
| 3rd | 1.18 (1.14, 1.22) | 1.13 (1.10, 1.18) |
| 4th | 1.10 (1.06, 1.13) | 1.08 (1.04, 1.11) |
| 5th | Ref | Ref |
| Trend (p-value) | < .0001 | < .0001 |
| Rurality | ||
| Urban | Ref | Ref |
| Rural | 1.03 (0.99, 1.07) | 1.00 (0.96, 1.05) |
Adjusted for race/ethnicity, SES quintile, rurality, age, year of diagnosis, ER/PR status, HER2 status, radiation, chemotherapy, surgery, marital status, and insurance status
Among women with HR-positive de novo mBC, residing in areas with lowest SES quintile was associated with increased risk of BC mortality among White (SHR = 1.19, 95% CI 1.12–1.26), Black (SHR = 1.21, 95% CI 1.05–1.39), and Hispanic (SHR = 1.18, 95% CI 1.02–1.37) women (Fig. 3). The effect of rurality was significantly associated with increased risk of BC mortality among White women (SHR = 1.09, 95% CI 1.03–1.15) but not significant among other race/ethnicity. Among women with HR-negative de novo mBC, we observed an increased risk of BC mortality among White (SHR = 1.26, 95% CI 1.12–1.40), and Black (SHR = 1.38, 95% CI 1.00–1.90) women. The effect of rurality was significantly associated with increased risk of BC mortality among HR-negative Black women (SHR = 1.27, 95% CI 1.01–1.59) but not significant among other HR-negative racial/ethnic groups. When we stratified results by BC subtypes, the results were consistent among White women. Findings vary across other race/ethnicity groups with smaller numbers of patients (Supplemental Table 4).
Fig. 3.
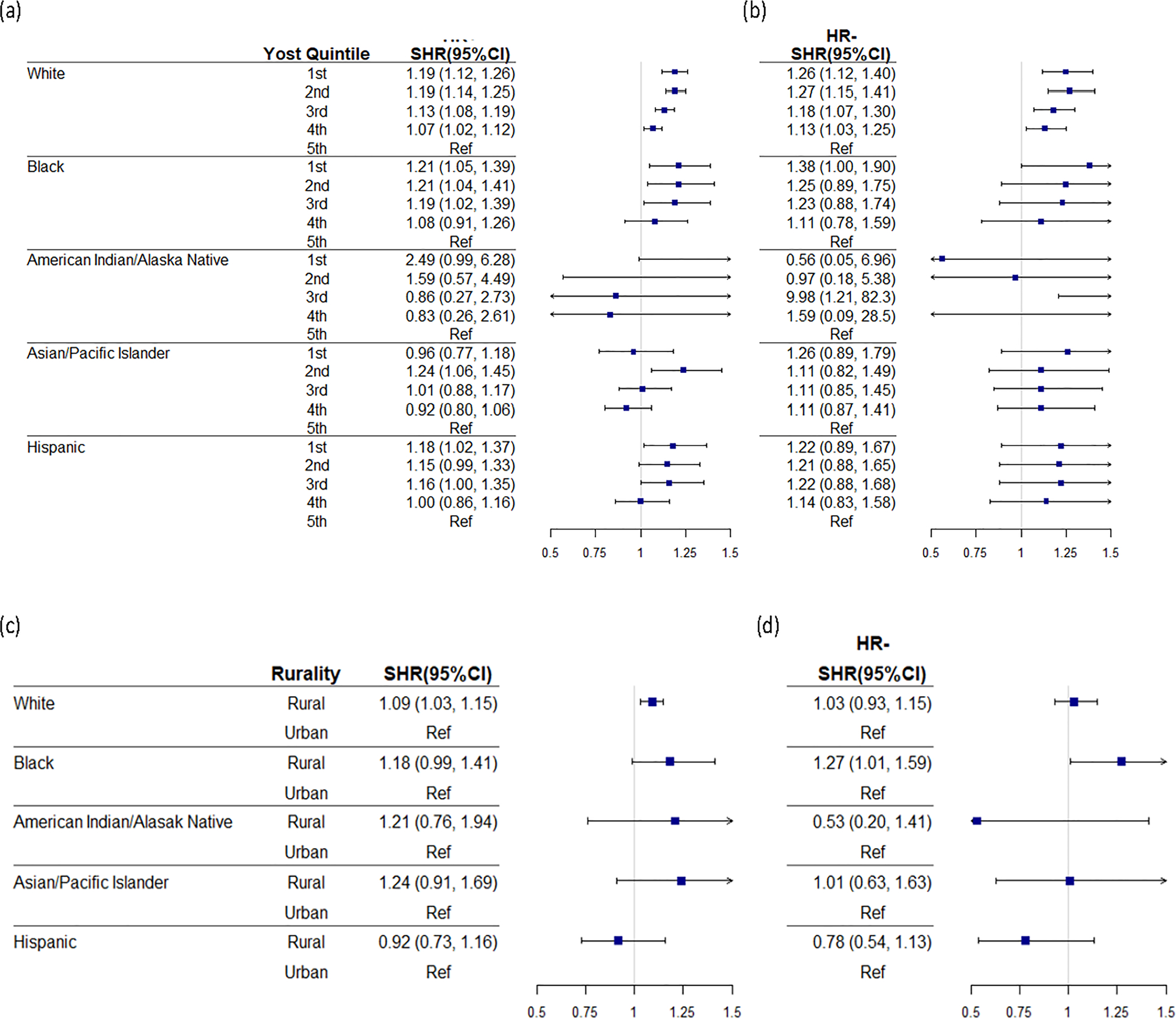
Forest plot for adjusted subdistribution hazard ratio (SHR) and 95% CI stratified by race/ethnicity and a socioeconomic status (SES) quintile among harmone receptor positive (HR +) women, b socioeconomic status (SES) quintile among harmone receptor negative (HR −) women
Discussion
In this population-based study, we found that among women diagnosed with de novo mBC, Black women and women living in neighborhoods of the lowest SES have greater risks of cancer-specific death. Specifically, the mortality of Black women with HR-negative de novo mBC was significantly influenced by SES and rurality. This study highlights the association of SES and rurality with race/ethnicity and HR status on de novo mBC mortality. Our results emphasize the importance of policies and prevention initiatives such as availability of early screening directed toward high-risk populations of women with higher incidence of de novo mBC facing both socioeconomic and structural disparities in health. Although mBC can be a distinctly aggressive disease in presentation, some mBC can likely be prevented by screening and can potentially help reduce BC mortality[5].
Previous literature describing disparities in BC incidence and survival outcomes across racial/ethnic groups, SES, and rural–urban residence often consider these factors in isolation [4, 5, 9]. However, the conclusion from this study is consistent with previous studies. A study conducted by Shariff-Marco et al. did not focus on de novo mBC and only evaluated patients within the San Francisco Bay area. However, they found lower neighborhood SES was associated with worse BC-specific survival among Asian Americans, [8]. Tao et al. utilized the California cancer registry and reported similar results that the lowest neighborhood SES quintile is associated with increased de novo metastatic breast cancer-specific mortality (HR:1.33, 95%CI 1.15–1.55). [4]. Additionally, MacKinnon et al. reported that low SES associated with higher incidence of de novo mBC among women living in severe or near poverty areas using block-level geographic data, but the authors focused on Florida residents and individual race was not included [5]. Limited studies outside of the United States have focused on association between SES, rurality, and mBC outcomes across different racial/ethnic groups. A review focused on European countries showed that lower SES is associated with worse mBC outcomes [16]. While our findings generally are aligned with previous literatures, most previous studies did not account for competing risks of death, which is a relevant concern given that de novo mBC women tend to be older, and risks of other cause of death also differ by SES and in rural versus urban areas [17]. Our research is distinguished from others in that it aimed to evaluate social determinates of health in tandem, utilized validated SES measures, and included an approach to account for competing risks of death among women with de novo mBC.
Rurality has been described as a risk factor for de novo mBC [2, 3]. The potential associations between rurality and BC outcomes are mediated by a number of factors such as distance to clinics and access to screening [9]. A meta-analysis including 21 studies has shown that women living in rural areas were 1.2 times more likely to be diagnosed with de novo mBC when compared to women living in urban areas [18]. MacKinnon et al. found that incidence of de novo mBC was six times higher among areas with the lowest mammography screening rate. Amey et al. utilized cancer registry data and assessed the interaction between race and residence on breast cancer mortality but did not focus specifically on de novo mBC, was limited to patients residing in Florida from 1981 to 1989, and thus did not account for recent trends in access to care, treatments, and competing risks [19]. Another retrospective study using cancer registry data showed that rurality was associated with lower survival among metastatic BC patients in Portugal [20]. One reason for these disparities may be access to care where health care providers are less accessible to women in rural areas [7, 19, 20]. Traveling greater distances to access cancer care for women in rural communities may be another contributing factor of being diagnosed with mBC [21]. Additionally, rurality and SES are highly correlated [2, 9, 22]. Kenzik et al. found differences in BC mortality across poverty level comparing women resided in rural to urban areas [22]. Furthermore, consistent with our findings, other studies have reported that rural Black women may be at particular disadvantage with respect to mBC outcomes. Multiple studies have reported that Black women residing in rural areas were 1.5 to 2 times more likely to be diagnosed with de novo mBC compared to Black women in urban areas. However, similar urban-rural disparities were not observed among White women [23, 24]. Finally, as we try to leverage these large real-world data sources, many studies mentioned above did not address American Indian/Alaska Native, Asian/Pacific Islander, and Hispanic/Latina women with de novo mBC, where a lack of data equity may be obscuring meaningful cancer health disparities.
There are several strengths to this study. First, we characterized associations with SES using the time-dependent Yost index, a measure incorporated in SEER’s specialized database. We recognize that multiple other SES measurements (e.g., Household prestige scale, The Cambridge scale) could be considered [25]; however, the Yost index is a validated measure that characterizes SES status through a factor analysis of seven different components representing social determinants of health [11] and preserves patient confidentiality by using Census tract-based composite measure [26]. Second, we applied a modeling approach to account for competing risks of death. This is important in studies of de novo mBC patients like ours that also indicated differences in other-cause death by race/ethnicity, SES, and rurality [24, 25]. Finally, to our knowledge, this is the first population-based mBC study to examine and jointly report on the impacts of SES and rurality, two correlated measures of social and structural determinants of health, on de novo mBC cancer-specific mortality within race/ethnicity. Previous studies have either focused on limited geography areas or have not focused on de novo mBC [8, 19].
Our study has limitations, including those inherent to the SEER database. Several variables were not available until later years of the study period. Insurance status was not available until after 2007 and HER2 status was not available until after 2010. However, results from our sensitivity analysis restricted to women with HER2 status were not substantively different from our main approach. We did not include people with missing geographic information necessary to determine their SES or rurality. Moreover, we also did not include patients with unknown information on dead or alive from SEER registry and we may have lost patients to follow up who moved to an area not covered by the SEER registry region [27]. For primary tumors, SEER provides information on initial stage at diagnosis and does not capture metastatic diagnoses that result from progression from early-stage BC. Known limitations on cancer treatment documentation in SEER include varying completeness, potential biases associated with unmeasured reasons for receipt of care, and the interpretation of sequence data noted in SEER guidelines [28]. According to guidelines, surgery is not the primary treatment for mBC, and radiotherapy may be given before or after the surgery to slow the growth of the cancer [29]. Thus, we do not have information on the reasons for BC treatments in SEER database. More studies on understanding patient and provider preferences in mBC treatment are important in contextualizing these findings. Specific information on other breast cancer treatments received (e.g., neoadjuvant chemotherapy, specific infused and oral agents used to treat mBC) was also not available. While SEER is a population-based database, depending on how rurality is defined, certain rural populations or areas may be underrepresented in the SEER database [30]. Finally, we used RUCA as our binary rurality measure (rural vs. urban), which may be an oversimplified representation of rurality as it does not include factors that may also impact de novo mBC mortality such as availability of cancer clinics or distance to hospitals [28].
In conclusion, we observed the highest increased risk of cancer-specific mortality among Black women with HR-negative de novo mBC and de novo mBC patients living in rural areas and neighborhoods with lower SES. Efforts in cancer prevention should reflect the complex associations relating the effects of race, SES, and rurality among women with mBC.
Supplementary Material
Funding
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Cancer Institute, Grant Numbers U54CA202995, U54CA202997, and U54CA203000 and National Center for Advancing Translational Sciences (KL2TR002002). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declarations
Conflict of interest Financial interests: Hsiao-Ching Huang is supported by the UIC/AbbVie Fellowship in Pharmacovigilance and patient safety. Huiwen Deng is supported by the UIC/AbbVie Fellowship in Health Economics and Outcomes Research. Colin C. Hubbard reports funding from Pfizer. Kent F Hoskins reports funding from Pfizer, Inc. Naomi Y Ko reports funding and advisory from Pfizer, Inc. Gregory S. Calip reports current employment with Flatiron Health, stock ownership in Roche and research grants from Pfizer unrelated to this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Non-financial interests: None.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10549-022-06603-6.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- 1.American Cancer Society (2019) Breast cancer facts & figures 2019–2020. American Cancer Society Inc, Atlanta [Google Scholar]
- 2.Daily K, Douglas E, Romitti PA, Thomas A (2021) Epidemiology of de novo metastatic breast cancer. Clin Breast Cancer. 10.1016/j.clbc.2021.01.017 [DOI] [PubMed] [Google Scholar]
- 3.Malmgren JA, C GS; Atwood MK; Mayer M; Kaplan HG, (2020) Metastatic breast cancer survival improvement restricted by regional disparity: surveillance, epidemiology, and end results and institutional analysis: 1990 to 2011. Cancer 126:390–399. 10.1002/cncr.32531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao L, Chu L, Wang LI, Moy L, Brammer M, Song C, Green M, Kurian AW, Gomez SL, Clarke CA (2016) Occurrence and outcome of de novo metastatic breast cancer by subtype in a large, diverse population. Cancer Causes Control 27:1127–1138. 10.1007/s10552-016-0791-9 [DOI] [PubMed] [Google Scholar]
- 5.MacKinnon JA, Duncan RC, Huang Y et al. (2007) Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiol Biomark Prev 16:756–762. 10.1158/1055-9965.EPI-06-0392 [DOI] [PubMed] [Google Scholar]
- 6.Heller DR, Chiu AS, Farrell K et al. (2019) Why has breast cancer screening failed to decrease the incidence of de novo stage IV disease? Cancers Basel. 10.3390/cancers11040500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams F, Thompson E (2016) Disparity in breast cancer late stage at diagnosis in missouri: does rural versus urban residence matter? J Racial Ethn Health Disparities 3:233–239. 10.1007/s40615-015-0132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shariff-Marco S, Yang J, John EM et al. (2014) Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomark Prev 23:793–811. 10.1158/1055-9965.EPI-13-0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coughlin SS (2019) Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat 177:537–548. 10.1007/s10549-019-05340-7 [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 sub (2000–2016) [Google Scholar]
- 11.Yost K, Perkins C, Cohen R et al. (2001) Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703–711. 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 12.United States Department of Agriculture (2020) Rural-urban commuting area codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/
- 13.Gooley TA, Leisenring W, Crowley J, Storer BE (1999) Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 18:695–706. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 15.Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154 [Google Scholar]
- 16.Vrdoljak E, Gligorov J, Wierinck L et al. (2021) Addressing disparities and challenges in underserved patient populations with metastatic breast cancer in Europe. Breast 55:79–90. 10.1016/j.breast.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh RR, Nadler MB, Desnoyers A et al. (2020) Influence of competing risks on estimates of recurrence risk and breast cancer-specific mortality in analyses of the early breast cancer trialists collaborative group. Sci Rep 10:4091. 10.1038/s41598-020-61093-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen-Pham S, Leung J, McLaughlin D (2014) Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol 24:228–235. 10.1016/j.annepidem.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 19.Amey CH, Miller MK, Albrecht SL (1997) The role of race and residence in determining stage at diagnosis of breast cancer. J Rural Health 13:99–108. 10.1111/j.1748-0361.1997.tb00939.x [DOI] [PubMed] [Google Scholar]
- 20.Gomes I, Aguiar P, Miranda A, Nunes C (2019) Overall survival of patients with locoregional and metastatic breast cancer: is the influence of baseline characteristics the same? Anticancer Res 39:5135–5142. 10.21873/anticanres.13708 [DOI] [PubMed] [Google Scholar]
- 21.Hahn KM, Bondy ML, Selvan M et al. (2007) Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. Am J Epidemiol 166:1035–1044. 10.1093/aje/kwm177 [DOI] [PubMed] [Google Scholar]
- 22.Kenzik KM, Rocque GB, Landier W, Bhatia S (2020) Urban versus rural residence and outcomes in older patients with breast cancer. Cancer Epidemiol Biomark Prev 29:1313–1320. 10.1158/1055-9965.EPI-19-1414 [DOI] [PubMed] [Google Scholar]
- 23.McLafferty S, Wang F, Luo L, Butler J (2011) Rural - urban inequalities in late-stage breast cancer: spatial and social dimensions of risk and access. Env Plann B Plann Des 38:726–740. 10.1068/b36145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell E, Kramer MR, Cooper HL et al. (2011) Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. J Urban Health 88:1117–1129. 10.1007/s11524-011-9612-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Measuring Socioeconomic Status. ESource Introd Soc Behav Sci Train Mater
- 26.Yu M, Tatalovich Z, Gibson JT, Cronin KA (2014) Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control 25:81–92. 10.1007/s10552-013-0310-1 [DOI] [PubMed] [Google Scholar]
- 27.Yu JB, Smith BD (2009) NCI SEER public-use data: applications and limitations in oncology research. Oncology 23 [PubMed] [Google Scholar]
- 28.SEER Acknowledgment of Treatment Data Limitations. https://seer.cancer.gov/data/sample-treatment-limitations.html [Google Scholar]
- 29.NCCN (2020) NCCN guidelines for patients metastatic breast cancer. 78 [Google Scholar]
- 30.Zahnd WE, Askelson N, Vanderpool RC et al. (2019) Challenges of using nationally representative, population-based surveys to assess rural cancer disparities. Prev Med 129S:105812. 10.1016/j.ypmed.2019.105812 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


