Abstract
Objective
Randomized trials testing the effect of antibiotics for chronic low back pain (LBP) with vertebral bone marrow changes on MRI (Modic changes) report inconsistent results. A proposed explanation is subgroups with low grade discitis where antibiotics are effective, but there is currently no method to identify such subgroups. The objective of the present study was to evaluate whether distinct patterns of serum cytokine levels predict any treatment effect of oral amoxicillin at one-year follow-up in patients with chronic low back pain and Modic changes at the level of a previous lumbar disc herniation.
Design
We used data from an overpowered, randomized, placebo-controlled trial (the AIM study) that tested 100 days of oral 750 mg amoxicillin vs placebo three times daily in hospital outpatients with chronic (>6 months) LBP with pain intensity ≥5 on a 0–10 numerical rating scale and Modic changes type 1 (oedema type) or 2 (fatty type). We measured serum levels of 40 inflammatory cytokines at baseline and analysed six predefined potential predictors of treatment effect based on cytokine patterns in 78 randomized patients; three analyses with recursive partitioning, one based on cluster analysis and two based on principal component analyses. The primary outcome was the Roland–Morris Disability Questionnaire score at one-year follow-up in the intention to treat population. The methodology and overall results of the AIM study were published previously.
Results
The 78 patients were 25–62 years old and 47 (60%) were women. None of the three recursive partitioning analyses resulted in any suggested subgroups. Of all main analyses, the largest effect estimate (mean difference between antibiotic and placebo groups) was seen in a subgroup not predefined as of main interest (Cluster category 3+4; −2.0, 95% CI: −5.2–1.3, RMDQ points; p-value for interaction 0.54).
Conclusion
Patterns of inflammatory serum cytokine levels did not predict treatment effect of amoxicillin in patients with chronic LBP and Modic changes.
Clinical Trial Registration Number
ClinicalTrials.gov (identifier: NCT02323412).
Keywords: low back pain, Modic changes, infection, cytokines, inflammation
Plain Language Summary
A suggested subgroup of patients with chronic low back pain that could benefit from specific treatments comprises those with vertebral endplate changes visible on MRI (Modic changes). One theory of Modic changes suggests that the etiology is a low-grade discitis, but no biomarker exists that could identify patients with infection that would benefit from antibiotic treatment. This article is the first to assess multiple serum biomarkers linked to infection as predictors of antibiotic treatment effect in patients with chronic low back pain and Modic changes. These results could guide further research that seeks to identify patients with chronic low back pain and infection.
Introduction
A suggested subgroup of patients with chronic low back pain (cLBP) have signal changes in the vertebral bone marrow that extend from the endplate (Modic changes, MCs) on magnetic resonance imaging. These are classified as type 1 (oedema type), 2 (fatty type) and 3 (sclerotic type) based on standard T1- and T2-weighted sequences.1
There are three main theories on the etiology of MCs – mechanic, autoimmunity and infection – which all have support in basic scientific research.2–12 While all these theories could be true on a population level, a single main etiology would be more likely for the individual patient. The infection theory, mainly implicating C.acnes in the intervertebral disc adjacent to MCs, has been assessed in several studies of local tissue samples, with conflicting results.10,13,14 Possible explanations for these conflicting results include colonization, contamination (suggesting a false-positive finding) or microbiologically inadequate techniques (suggesting a false-negative finding).15 Recent studies with microbiological techniques better suited to rule out contamination (ie fluorescence in situ hybridization) suggest that C.acnes could be present in a smaller subset of patients.8,16 Hence, an effect of antibiotic treatment, if any, would only be expected to occur in a subset of patients with cLBP and MCs.
In 2013, a randomized controlled trial testing the effect of antibiotics in patients with cLBP and MC type 1 reported a large treatment effect.17 The AIM study, intending to replicate the 2013 trial while also including patients with type 2 (but not type 1) MCs, found a statistically significant, but not clinically important, effect of amoxicillin.18 Subgroup analyses suggested better effects in patients aged <40 years19 and in a small subgroup with abundant MC-related oedema on MRI.20 Due to the potential of antibiotics to improve back pain but also to increase antibiotic resistance, it is essential to avoid both under- and overtreatment. There is still no currently available clinical test or investigation for individual patients to establish an underlying cause of MCs or to guide treatment. However, in vitro transcriptomic analyses of bone marrow aspirates/biopsies in patients with MCs suggest that the local immunological response from C.acnes infection is different from autoinflammation.21 Immunological parameters might therefore act as predictors of antibiotic treatment effect in our population of interest.
Cytokines are crucial signal molecules of the immune system produced by various cells and tissues,22 including disc cells,23 and are involved in the pathophysiology of disc degeneration and MCs.24,25 They play a role in orchestrating the host response to infection and trauma as well as in immune-mediated diseases.26 We have previously reported an increase in serum inflammatory cytokine levels in patients with cLBP with MCs, but the underlying mechanism is uncertain.27 The relationship between disease pathophysiology and cytokines is often complex due to a cascade of downstream signalling events for many cytokines and dynamic immune responses that can be influenced by various internal and external factors.28 Single cytokines may consequently be unspecific, and the relationship between pathophysiological processes and cytokines may better be understood by looking at patterns of multiple cytokines. In other populations a distinct cytokine pattern or cytokine-associated gene signature may distinguish between infection, and other inflammatory etiologies in conditions like encephalitis,29–31 systemic lupus erythematosus32 and diabetes.33 Serum cytokine levels differentiated patients with vertebral osteomyelitis (6 out of 16 patients with low virulent bacteria) from patients with disc degeneration/erosive osteochondrosis.34 Further, cytokine levels in mononuclear cells in the peripheral blood may discriminate pathogenic from commensal C.acnes phylotypes in skin.35 Local infection with C.acnes can induce increased cytokine levels in the intervertebral disc6,36–38 and serum39–42 (see Table 1 in the statistical analysis plan (SAP))43 A possible infectious etiology of MCs could therefore be associated with increased levels of certain cytokines and/or a distinct serum cytokine pattern. There are to our knowledge no previous studies that have assessed cytokines as predictors of amoxicillin effect in patients with chronic low back pain.
Table 1.
Cytokines Analyzed in the Present Study
| Description | Cytokines with a Biological Rationale for a Possible Association with C.acnes Infection in Disc | Cytokines with No Biological Rationale for a Possible Association with C.acnes Infection in Disc | ||
|---|---|---|---|---|
| Cytokines | TNF IFN-γa IL-1β IL-6a IL-8 IL-10 CCL2 CCL3a |
IL-2a | CCL15a | CXCL1 |
| IL-4a | CCL17a | CXCL2 | ||
| IL-16a | CCL19a | CXCL5b | ||
| GM-CSF | CCL20a | CXCL6a | ||
| MIFa | CCL21a | CXCL9 | ||
| CCL1 | CCL22a | CXCL10 | ||
| CCL7a | CCL 23a | CXCL11 | ||
| CCL8 | CCL24 | CXCL12 | ||
| CCL11a | CCL25a | CXCL13a | ||
| CCL13a | CCL26a | CXCL16a | ||
| CCL27a | CX3CL1a | |||
Notes: aCytokines with levels that were significantly different from healthy controls. bCytokine excluded from further analyses due to levels below the limit of quantification for more than half of the samples.
Abbreviations: TNF, Tumour necrosis factor; IFN-γ, interferon gamma; IL, Interleukin; GM-CSF, Granulocyte-macrophage colony-stimulating factor; MIF, Macrophage migration inhibitor factor; CCL, C-C motif ligand; CXCL, Chemokine C-X-C motif ligand; CX3CL, Chemokine C-X3-C Motif Ligand.
The objective of the present study was to evaluate whether distinct patterns of serum cytokine levels predict any treatment effect of 100 days of oral amoxicillin at one-year follow-up in patients with cLBP and type 1 or type 2 MCs at the level of a previous lumbar disc herniation. Our hypothesis was that patients in the antibiotic group with a certain pattern of serum cytokine levels at baseline report a significantly lower RMDQ score at one-year follow-up than patients in the placebo group with the same cytokine pattern. If this hypothesis is true, the clinical significance would be that amoxicillin is a possible treatment option for a subgroup of patients with cLBP.
Methods
We used data from the AIM study that included 180 patients from six Norwegian hospital outpatient clinics.18 Patients were included by medical doctors from June 2015 to September 2017. The inclusion criteria were age 18–65 years, LBP for more than 6 months with a mean intensity ≥5 of three 0–10 numerical rating scales (NRS), lumbar disc herniation on MRI in the preceding 2 years, and type 1 or type 2 MCs (with height ≥10% of vertebral body height and diameter >5 millimeters) at the previously herniated disc level (defined as index level). Patients with any specific diagnosis that could explain the patient’s low back symptom were excluded. Further details of the eligibility criteria and trial methods were published previously.18 Patients were centrally randomized to receive oral amoxicillin 750 mg or placebo (maize starch) three times daily for 100 days. The study medication had identical encapsulation, labels and containers. Patients were classified as MC type 1 group if they had type 1 present (even if another type was more extensive) and as MC type 2 group if they had type 2 MCs, but not any type 1 MCs, at index level. Allocation was computer-generated, concealed and stratified by prior disc surgery and MC type (1:1:1:1 allocation and block sizes of four and six). Patients received a prescription from their care providers with an allocation number to be used at dedicated pharmacies. All patients, care providers, research staff and statisticians were blinded to treatment allocation during the trial.
The primary outcome in the AIM study was pain-related disability measured by the Norwegian validated version of the Roland–Morris Disability Questionnaire (RMDQ, score range 0–24) at one-year follow-up.44,45 The minimal clinically important between-group difference in mean RMDQ score was defined as 4 points. Pain-related disability measured by the Oswestry Disability Index (ODI, score range 0–100)46 and LBP intensity NRS (score range 0–10)47 were secondary outcomes. RMDQ, ODI and LBP intensity NRS are all part of the recommended core outcome measurement set for clinical trials in nonspecific low back pain due to their measurement properties.45
The Clinical Trial Unit at Oslo University Hospital monitored the trial. The AIM study and statistical analysis plan (SAP) of the present analyses are registered at ClinicalTrials.gov (identifier: NCT02323412). The trial was approved by the Regional Committees for Medical Research Ethics South East Norway (project 2014/158/REK sør-øst C) and was performed in accordance with the Helsinki Declaration.
Patients
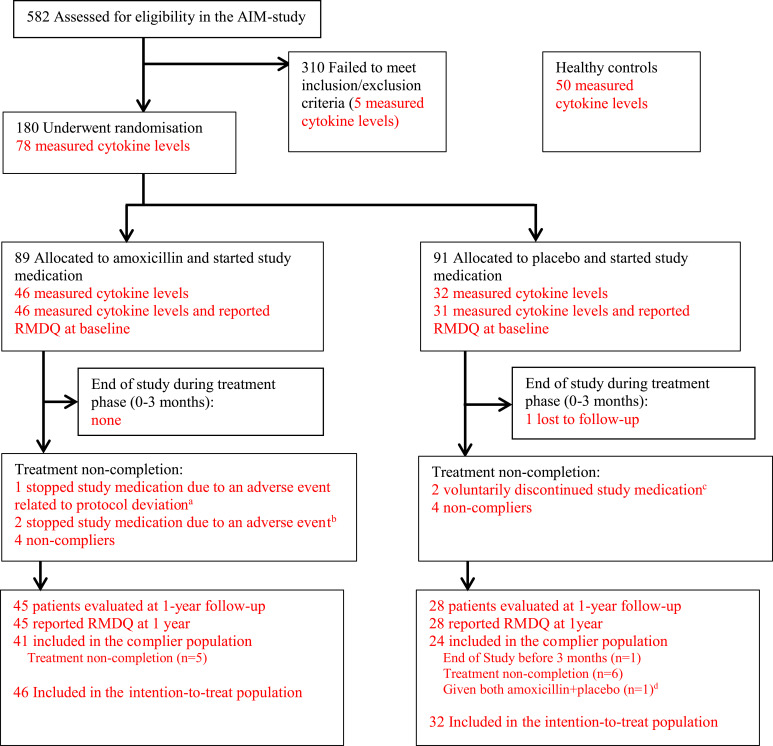
For the present study we measured serum cytokine levels in a selected group of patients in the AIM study with as “pure” MC type as possible; type 1 MCs had to be the most extensive MC type at two or more endplates at index level (MC1), or type 2 but not type 1 had to be present at the index level (MC2). The justification for this selection of patients was published previously.27 Out of 83 patients with measured cytokine levels at screening, 78 patients (43 MC1, 35 MC2) had been randomized and were included in the current analyses (Figure 1). All patients provided written informed consent.
Figure 1.
Flow chart (with numbers relevant for cytokine measurements in red). aOne patient in the amoxicillin group became pregnant (protocol deviation as all patients were instructed to use contraception), not included in the complier population. bTwo patients in the amoxicillin group stopped study medication due to adverse events and were included in the complier population. cTwo patients in the placebo group discontinued because they started three-month treatment with amoxicillin/clavulanic acid due to chronic low back pain. dDue to a mistake at pharmacy, the patient was given a mix of bottles containing amoxicillin and placebo.
Cytokine Measurements and Profiling
Blood was collected at screening, a median 40 (interquartile range, 34–42) days before randomization, by using BD vacutainer tubes with no additives. We stored the samples at room temperature for 45 minutes before centrifugation at 2000g for 10 minutes at room temperature. Serum was immediately withdrawn and stored at −80°C prior to cytokine analysis. We measured a standard panel of inflammatory cytokines (see Table 1) in serum using a Pro Human Chemokine multi-bead assay (Bio-Rad, Norway). Data was recorded with a Luminex IS 100 instrument (Bio-Rad, Hercules, CA, USA) and protein concentrations were finalized using recombinant standard curves. One cytokine (CXCL5) was not detected in the measurements of most patients and was excluded from the statistical analyses.
In order to identify subsets of subjects based on cytokine patterns, we performed cluster analyses that identified four categories of patients, from category 1 with the highest cytokine levels to category 4 with the lowest.27 These were further dichotomized into two categories (1+2 vs 3+4) in the present analyses. The cluster analysis was performed without post-randomization variables and done prior to any analyses in the present paper.
Statistical Analyses
We predefined six main analyses, each testing a potential predictor subgroup defined by patterns of cytokines (or using information from all or several cytokines), in accordance with the PROGRESS (PROGnosis RESearch Strategy) framework on statistical analyses of predictors of treatment effect.48 The rationale for each analysis (1A, 1B, 1C, 2A, 3A, 3B) is listed in Table 2. A detailed description of the algorithm parameters for analyses 1A, 1B and 1C are listed in Table S1 in the Supplementary Appendix. All six analyses used linear regression with RMDQ score at one year as the dependent variable and the interaction term (predictor*treatment group) as the independent variable adjusted for baseline RMDQ score, the potential predictor, the treatment group, the randomization stratification variables (MC type [1/2] and for former disc herniation surgery (yes/no]). The focus of our analyses was assessing the effect of assignment to amoxicillin treatment, and all analyses were of the intention-to-treat population. Missing values in RMDQ were imputed using multiple imputation. We assessed normality distribution of the residuals in these six linear regression analyses by QQ-plots and homogeneity of variance by Levene’s test. The subgroups were all based on data available at the time of randomization and the analyses were ranked from most to least likely predictors. We consider all analyses hypothesis-generating as they are not previously described in the literature and accordingly we do not adjust for multiple testing.
Table 2.
Main Analyses of Cytokine-Defined Predictors of Treatment Effect
| Hypothesis: Patients with a certain pattern of serum cytokine levels at baseline in the antibiotic group report a significantly lower RMDQ score at one-year follow-up than patients in the placebo group. | ||
| Variables and Subgroups | Analysis | Rationale |
| Post-defined subgroups Subgroups defined based on (predefined) data-mining statistical analyses with recursive partitioning (stage 1 of analysis 1). The three recursive partitioning models differ only in terms of variables included: |
Patterns of cytokines relevant for subgroups might be defined by combinations of individual cytokines. Recursive partitioning analyses search for subgroups by directly testing the interaction of combinations of individual cytokines and treatment group, with no assumption of cut-off values for cytokines. We used an adaptive SIDEScreen method as it screens for biomarker-by-treatment interactions in a more flexible way and also handles multiplicity in complex subgroup search problems.49 An infection of the intervertebral disc (discitis) could be associated with a distinct serum cytokine pattern that is different from the pattern in a patient without such infection. We have ranked the post-defined (data-driven) subgroup analysis first as there is limited knowledge about C.acnes intervertebral discitis and serum cytokine levels, and relevant classifications and cut-offs might go unnoticed in analysis of pre-defined subgroups.49 |
|
| i. Cytokines with a biological rationale for a possible association with C.acnes infection in disc (IL-1β, IL-8, TNF-α, IL‐6, IFN‐γ, IL-10, CCL3, CCL2) | 1 A | |
| ii. Cytokines with a biological rationale for a possible association with C.acnes infection in disc (IL-1β, IL-8, TNF-α, IL‐6, IFN‐γ, IL‐12, IL-10, CCL3, CCL2) + age and abundance of MC oedema (STIR composite variable)20 | 1 B | |
| iii. All 39 cytokines | 1 C | |
| Pre-defined subgroups Cytokine categories: Prespecified classification based on cluster analysis (Figure 2). To identify grouping of patients based on serum cytokine levels, we split the heatmap using k-means partitioning of patients and hierarchical clustering of cytokines. We used Pearson correlation for cytokines and complete linkage of Euclidean distance for individuals, giving four categories of patients. |
Patients with similar pathophysiology could arguably have more similar cytokine patterns than patients with different pathophysiology. Relevant subgroups might hence be defined by clustering patients based on how similar their cytokine patterns are. We used unsupervised clustering analysis as we wanted an analysis with minimal assumptions about the relationship within the data. We hypothesize that categories 1 and 2 are the most likely category of patients with treatment effect, due to increased levels of TNF-α, IFN‐γ and IL-10 (and correlated cytokines). |
|
| iv. Classification group dichotomized into category 1+2 (hypothesized group with treatment effect) vs category 3+4. | 2 A | |
| Principal component 1 (continuous variable, the one that explains the most variance) of principal component analyses calculated for the correlation matrix (Figure S1 in Supplementary Appendix) of: | The information from all cytokine levels that is relevant for predicting treatment effect might be better contained using a continuous variable, as suggested by the PROGRESS framework.48 We used principal component analyses as we wanted to preserve the maximum amount of information from the cytokines into one single variable. |
|
| v. Cytokines with levels that were known from previous literature to be significantly different from the healthy controls:27 (CCL11, CCL22, CCL21, IL4, CCL26, IL6, CXCL13, CX3CL1, CCL27, CXCL6, CCL20, IFNγ, CCL19, IL2, CCL17, IL16, CCL25, CCL7, CCL13, MIF, CCL3, CCL15, CCL23, CXCL16) | 3 A | |
| vi. All 39 cytokines | 3 B | |
Statistical analyses were performed in Stata 16 and RStudio (version 1.4.1717). More details on the analyses that defined the potential predictor subgroups, the imputation model and other statistical methods used are described in the SAP published at ClinicalTrials.gov.43
Sample Size and Power
The main trial (the AIM study) was overpowered (n=180) nearly three times as it was powered for both MC types (1 and 2) and power analyses suggested 66 analyzed patients were needed in each MC type group.18 We are unaware of methods to calculate power for our post-defined subgroups (analyses 1). For the cytokine categories (analysis 2) our sample size (n=78) will provide a power of 0.72 to detect an effect size of 6 RMDQ points in the interaction test (see details in the SAP).43 The number of participants in the subgroup of interest (cytokine category 1+2, n=24 vs n=13) will provide the power to detect a difference in mean RMDQ scores of 5 (α=0.05, β=0.2, SD=5) in the stratified analysis (assessing treatment effect within the subgroup category of interest, ie cytokine category 1+2).
Results
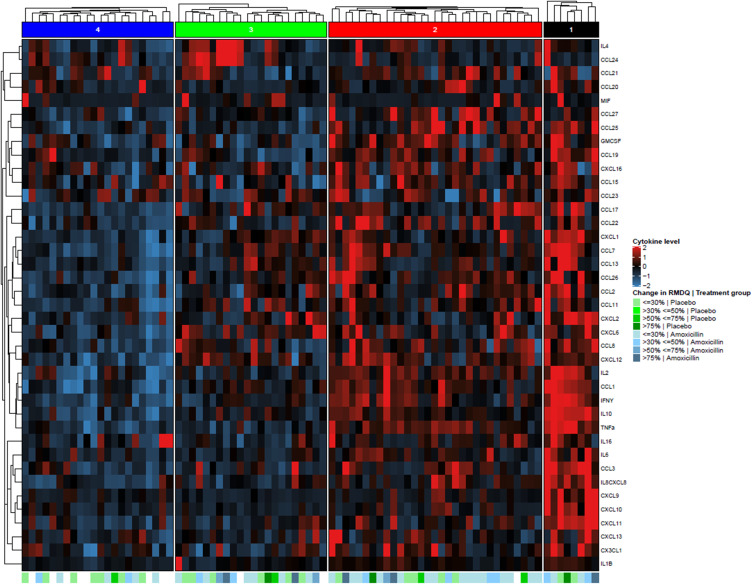
The 78 patients were 25–62 years old (mean 44 and standard deviation 8.9 years) and 47 (60%) were women. Forty-six patients were randomized to amoxicillin and 32 to placebo. The distribution of plate number used for cytokine analyses and baseline cytokine categories (based on cluster analysis) according to treatment group is presented in Table 3. Baseline clinical characteristics according to treatment group for each of the cytokine categories (category 1+2 and 3+4) are shown in Table S2 in the Supplementary Appendix. A visual summary of cytokine levels in each cytokine category, including information about treatment group and improvement of RMDQ, is presented in Figure 2.
Table 3.
Distribution of Baseline Cytokine Categories by Treatment Group
| Subgroups | Amoxicillin Group (N = 46) | Placebo Group (N = 32) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Kit no of cytokine analyses | ||||
| 1 | 6 | 19 | 8 | 17 |
| 2 | 7 | 22 | 7 | 15 |
| 3 | 4 | 13 | 10 | 22 |
| 4 | 6 | 19 | 8 | 17 |
| 5 | 4 | 13 | 10 | 22 |
| 6 | 5 | 16 | 3 | 7 |
| Cytokine categorya | ||||
| Category 1 | 5 | 11 | 3 | 9 |
| Category 2 | 19 | 41 | 10 | 31 |
| Category 3 | 13 | 28 | 7 | 22 |
| Category 4 | 9 | 20 | 12 | 38 |
| Sum | 46 | 100 | 32 | 100 |
Note: aCytokine categories based on cluster analysis as described in the main text, used for analysis 2.
Figure 2.
Heatmap showing serum cytokine levels of patients grouped by cluster analysis. Rows representing cytokine levels (log-transformed values) and columns representing individuals divided into four cytokine categories based on cluster analysis (analysis 2). Cells are color scaled according to cytokine concentration (blue=low, red=high).
Notes: At the bottom of the figure, green (placebo) and blue (amoxicillin) color shades represent change (improvement) between baseline and one-year follow-up (in percentage of baseline value), with darker color meaning more improvement. White represents missing values. A visual inspection of the heatmap does not provide any clear pattern of cytokines that seem to trend towards any treatment effect.
Abbreviation: RMDQ, Roland–Morris Disability Questionnaire (measure of patient-reported pain-related physical disability).
We found no evidence of clinically relevant or statistically significant interaction between subgroup and treatment groups in the main analyses (Table 4). None of the three recursive partitioning analyses resulted in any suggested subgroups that could be examined in analyses 1. For analyses 2, there were neither clinically relevant nor statistically significant differences between the treatment groups within each cytokine category (1+2 and 3+4). For analyses 3, neither of the principal component analyses resulted in any clinically relevant or statistically significant effect (Table 4 and Figure S1). Of all main analyses, the largest effect estimate (mean difference between antibiotic and placebo group) was seen in a subgroup not predefined as of main interest (cluster category 3+4; −2.0, 95% CI: −5.2 to 1.3, RMDQ points; p-value for interaction 0.54). Recursive partitioning with the secondary outcomes ODI and LBP intensity did not present in suggested subgroups. Sensitivity analyses (not predefined) using fractional polynomial interaction models for analysis 3 to assess the non-linear relationship of the interaction between principal components 1 and treatment group were negative (Figure S2A and S2B in the Appendix).
Table 4.
Predictors of Treatment Effect
| Subgroups | N | Predicted Mean Difference in RMDQ Between Treatment Groups (95% CI) | P-value | Interaction Between Treatment and Subgroup (95% CI) | P-value |
|---|---|---|---|---|---|
| Overall | 78 | −1.4 (−3.7 to 1.0) | 0.25 | – | – |
| Analysis 2 (subgroups based on cluster analysis) | |||||
| Cluster category 3+4 | 41 | −2.0 (−5.2 to 1.3) | 0.23 | – | – |
| Cluster category 1+2 | 37 | −0.5 (−3.9 to 2.9) | 0.77 | 1.4 (−3.2 to 6.1) | 0.54 |
| Analyses 3 (subgroups based on PCA) | |||||
| Principal component 1 (analysis 3A) | 78 | – | – | −0.1 (−1.0 to 1.1) | 0.89 |
| Principal component 1 (analysis 3B) | 78 | – | – | 0.0 (−0.8 to 0.8) | 0.97 |
Notes: Predicted mean difference in RMDQ between amoxicillin and placebo groups with 95% CI and estimated effects of interactions terms with 95% CI, both based on linear regression of the intention to treat group. Analyses 1 are omitted as the recursive partitioning analyses did not result in any suggested subgroup to be tested by linear regression analyses.
Abbreviations: N, number of patients included in analysis; RMDQ, Roland–Morris Disability Questionnaire (measure of physical function), score 0–24; PCA, principal component analysis.
Discussion
The present study found no evidence that serum cytokine levels using a panel of 40 proinflammatory cytokines predict antibiotic treatment response in patients with cLBP and Modic changes.
Interpretation
A possible explanation for our negative findings is that back pain related to MCs is not caused by bacterial disc infection and, hence, there is no treatment effect of oral amoxicillin in subgroups (or the whole population) of cLBP with MCs. Alternatively, serum cytokine levels could be too unspecific to identify patients with a local disc infection. Although our panel of cytokines are known to be increased in infection, it was selected based on the cytokines’ known association with inflammation, and their ability to distinguish infection from inflammation locally in discs is uncertain. Recent evidence suggests that for many cytokines (eg IL-1β, TNF, CCL20), but not all (eg IL-6), serum levels will not be correlated to disc level.50 A previous small study found no differences in serum cytokine levels in LBP patients with or without type 1 MCs, questioning the relevance of serum cytokines in patients with MCs.51
It has been suggested that only MCs type 1 (oedema type) are relevant when assessing predictors of antibiotic effects.52 However, our findings were also negative in the analyses that endorsed subgroups of patients with abundant MC oedema (analyses 1B).
Recent microbiological studies of tissue samples from lumbar disc herniations suggest that bacteria other than C.acnes might be present, which typically would not be sensitive to amoxicillin treatment.53 In the present study, the choice of antibiotic was based on the theory that MCs and LBP are related to a low grade discitis with C.acnes. We investigated whether patterns of serum cytokines would predict treatment effect of amoxicillin, and not whether patterns of serum cytokines are associated with any bacterial disc infection per se.
Limitations
A main limitation of our study might be insufficient power to detect minimally important differences in the interaction terms, ie small differences in treatment effects between those who did and those who did not belong to a subgroup. Thus, we cannot entirely exclude the possibility of subgroups based on cytokine patterns with small treatment effects. In particular, the recursive partitioning analyses would only be able to detect large effects.
However, we believe the power was acceptable in the stratified analyses (assessing treatment effect within the subgroup category of interest). Although the effect size in our power calculation (5 RMDQ points on a 0–24-point scale) was higher than the 4 RMDQ points we regarded as the minimally clinically important difference in the main trial, it is still less than 50% of the mean baseline value and small compared to the reported treatment effect (8.3 RMDQ points on a 0–23-point scale) in the 2013 trial. Also, the covariates we added probably increased power.54 For our cytokine categories of interest (1+2) the confidence interval of the treatment effect suggests that clinically relevant differences are unlikely. With no clinically relevant differences in the stratified analyses, it is arguably of little interest to test significance of the interaction terms. Furthermore, such small treatment effects would be unlikely given the theory that pain was due to an infection.
Another limitation is that we did not measure cytokines potentially relevant for discitis such as vascular endothelial growth factor (VEGF) and IL-12(p70).34 We did however measure a broad panel of cytokines which we believe covers the most important related to disc pathology.
A further limitation was due to two patients who guessed (correctly) that they were in the placebo group and decided to stop study medication. Both patients received antibiotics for their back pain from physicians outside the study but were still included in the placebo group for our intention to treat analysis.
The eligibility criteria of our trial, ie requiring previous disc herniation within two years or excluding specific diseases, might reduce the generalizability of our findings. Our results could possibly be different in other back pain populations, ie with sciatica. Minocycline (an antibiotic belonging to the tetracycline group) had a small, but not clinically relevant, effect on leg pain (1.5, CI: 0.2–2.8, on a 0–10 NRS) compared to placebo in a population with subacute lumbar radicular pain, but the study did not record whether the patients had MCs.55
Further Studies
Ideally, identification of individuals who could benefit from amoxicillin treatment would be by documenting evidence of C.acnes infection of the intervertebral disc or adjacent vertebral bone marrow. However, identifying C.acnes in such tissue is known to be challenging for several reasons, eg difficulties with collecting relevant and sufficient sample material and challenges with microbiological methods.56 Further studies intending to examine systemic biomarkers of C.acnes disc infection should be based on recognized microbiological methods that are specific to C.acnes virulence.
Conclusion
Patterns of serum inflammatory cytokine levels did not predict treatment effects of amoxicillin on back pain outcomes at one-year follow-up in patients with cLBP and type 1 or type 2 MCs at the level of a previous lumbar disc herniation.
Acknowledgments
The AIM study group: University Hospital North Norway, Tromsø (two patients): Terese Fors, Guro Kjos, Ida Beate Østhus (Department of Rehabilitation). Trondheim University Hospital, Trondheim (11 patients): Gunn Hege Marchand, Britt Elin Lurud, Fredrik Granvigen (Department of Physical Medicine and Rehabilitation), Hege Andersen (National Advisory Unit of Spinal Surgery), Øystein Petter Nygaard, Vidar Rao (Department of Neurosurgery). Haukeland University Hospital, Bergen (14 patients): Thomas Istvan Kadar and Siv Krüger Claussen (Department of Physical Medicine and Rehabilitation), Erling Andersen (Department of Clinical Engineering), Per Kristoffersen and Nils Vetti (Department of Radiology), Jörg Aßmus (Centre for Clinical Research). Vestre Viken Hospital, Drammen (16 patients): Sigrun Randen (Department of Physical Medicine and Rehabilitation), Hilde Presberg (Department of Neurology). Oslo University Hospital, Oslo (20 patients): Linda Margareth Pedersen, Bendik Slagsvold Winsvold (FORMI), Karianne Wiger Gammelsrud (Department of microbiology), Maria Dehli Vigeland, Benedicte Alexandra Lie, Siri Tennebø Flåm, Magnus Dehli Vigeland (Department of Medical Genetics). Østfold Hospital Trust (15 patients): Marianne Thorsø, Knut Morten Huneide, (Department of Physical Medicine and Rehabilitation), Veronica Sørensen (Department of Rheumatology). Thor Einar Holmgard (patient representative). We thank Helse Sør-Øst (grant no: 2015090) and Helse Vest (grant no: 911938 and 911891) for funding the AIM study, KLINBEFORSK (grant no: 2017201) and all patients participating in this study.
Funding Statement
Funding was granted by governmental organisations Helse Sør-Øst (grant No 2015090), Helse Vest (grant No 911938 and 911891) and KLINBEFORSK (grant no: 2017201), which had no part in the planning, performing, or reporting of the trial.
Data Sharing Statement
Requests to access data should be addressed to kjersti.storheim@medisin.uio.no. De-identified individual participant data (including data dictionary) will be available to medical researchers on request in accordance with local registration and ethical approval when the article is published until 1 July 2029. All proposals requesting data access will need to specify an analysis plan and will need approval of the scientific board before any data can be released.
Author Contributions
KG Analysis and interpretation of the data; AHP Interpretation and statistical expertise; ES and LMP Conception and design of study and acquisition of data; MW Acquisition of data, administrative, technical, and logistic support; HCA Acquisition of data and conception and design of study; EF, JSS, KKS, GLG, JIB, AE, JAZ, KS and LG Conception and design of study; JAZ, AE and KS Obtaining funding; LCHB Drafting of article and analysis and interpretation of data. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Lars Christian Haugli Bråten reports grants from Helse Sør-Øst, grants from KLINBEFORSK, during the conduct of the study. Dr Kaja Kristine Selmer reports grants from Norwegian Research Council, during the conduct of the study; personal fees from Roche, meeting sponsorship to institution (Oslo University Hospital) from Eisai AB, personal fees from OrionPharma, grants from Norwegian Research Council, grants from DAM foundation, grants from Novo Nordic Foundation, grants from NordForsk, grants from The National Advisory Unit on Rare Disorders in Norway, outside the submitted work. Dr Guro Goll reports personal fees from AbbVie, personal fees from Novartis, outside the submitted work. Prof. Dr. Ansgar Espeland reports grants from Helse Vest, during the conduct of the study. The authors report no conflicts of interest in this work.
References
- 1.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193. doi: 10.1148/radiology.166.1.3336678 [DOI] [PubMed] [Google Scholar]
- 2.Dudli S, Liebenberg E, Magnitsky S, Lu B, Lauricella M, Lotz JC. Modic type 1 change is an autoimmune response that requires a proinflammatory milieu provided by the “Modic disc”. Spine J. 2018;18(5):831–844. doi: 10.1016/j.spinee.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 3.Bobechko W, Hirsch C. Auto-immune response to nucleus pulposus in the rabbit. J Bone Joint Surg Br. 1965;47(3):574–580. doi: 10.1302/0301-620X.47B3.574 [DOI] [PubMed] [Google Scholar]
- 4.Geiss A, Larsson K, Junevik K, Rydevik B, Olmarker K. Autologous nucleus pulposus primes T cells to develop into interleukin‐4‐producing effector cells: an experimental study on the autoimmune properties of nucleus pulposus. J Orthopaed Res. 2009;27(1):97–103. doi: 10.1002/jor.20691 [DOI] [PubMed] [Google Scholar]
- 5.Gertzbein S, Tait J, Devlin S. The stimulation of lymphocytes by nucleus pulposus in patients with degenerative disk disease of the lumbar spine. Clin Orthop Relat Res. 1977;123:149–154. [PubMed] [Google Scholar]
- 6.Capoor MN, Konieczna A, McDowell A, et al. Pro-inflammatory and neurotrophic factor responses of cells derived from degenerative human intervertebral discs to the opportunistic pathogen Cutibacterium acnes. Int J Mol Sci. 2021;22(5):2347. doi: 10.3390/ijms22052347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25(13):1625–1636. doi: 10.1097/00007632-200007010-00005 [DOI] [PubMed] [Google Scholar]
- 8.Capoor M, Ruzicka F, Machackova T, et al. Prevalence of propionibacterium acnes in intervertebral discs of patients undergoing lumbar microdiscectomy: a prospective cross-sectional study. PLoS One. 2016;11(8):e0161676. doi: 10.1371/journal.pone.0161676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capoor MN, Ruzicka F, Schmitz JE, et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS One. 2017;12(4):e0174518. doi: 10.1371/journal.pone.0174518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Zheng Y, Yuan Y, et al. Modic changes and disc degeneration caused by inoculation of Propionibacterium acnes inside intervertebral discs of rabbits: a pilot study. Biomed Res Int. 2016;2016:9612437. doi: 10.1155/2016/9612437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudli S, Liebenberg E, Magnitsky S, Miller S, Demir-Deviren S, Lotz JC. Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J Orthopaed Res. 2016;34:1447–1455. doi: 10.1002/jor.23265 [DOI] [PubMed] [Google Scholar]
- 12.Rajasekaran S, Tangavel C, Aiyer SN, et al. Is infection the possible initiator of disc disease? An insight from proteomic analysis. Eur Spine J. 2017;26:1384–1400. doi: 10.1007/s00586-017-4972-3 [DOI] [PubMed] [Google Scholar]
- 13.Urquhart DM, Zheng Y, Cheng AC, et al. Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med. 2015;13:13. doi: 10.1186/s12916-015-0267-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Dong Z, Wu Y, et al. Association between lumbar disc degeneration and Propionibacterium acnes infection: clinical research and preliminary exploration of animal experiment. Spine. 2016;41(13):E764. doi: 10.1097/BRS.0000000000001383 [DOI] [PubMed] [Google Scholar]
- 15.Benoist M. The Michel Benoist and Robert Mulholland yearly European Spine Journal review: a survey of the “medical” articles in the European Spine Journal, 2018. Eur Spine J. 2019;28(1):10–20. doi: 10.1007/s00586-018-5857-9 [DOI] [PubMed] [Google Scholar]
- 16.Capoor MN, Birkenmaier C, Wang JC, et al. A review of microscopy-based evidence for the association of Propionibacterium acnes biofilms in degenerative disc disease and other diseased human tissue. Eur Spine J. 2019;28(12):2951–2971. doi: 10.1007/s00586-019-06086-y [DOI] [PubMed] [Google Scholar]
- 17.Albert HB, Sorensen JS, Christensen BS, Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J. 2013;22(4):697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bråten LCH, Rolfsen MP, Espeland A, et al. Efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM study): double blind, randomised, placebo controlled, multicentre trial. BMJ. 2019;367:l5654. doi: 10.1136/bmj.l5654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bråten LCH, Grøvle L, Espeland A, et al. Clinical effect modifiers of antibiotic treatment in patients with chronic low back pain and Modic changes-secondary analyses of a randomised, placebo-controlled trial (the AIM study). BMC Musculoskelet Disord. 2020;21(1):1–11. doi: 10.1186/s12891-020-03422-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristoffersen PM, Bråten LC, Vetti N, et al. Oedema on STIR modified the effect of amoxicillin as treatment for chronic low back pain with Modic changes—subgroup analysis of a randomized trial. Eur Radiol. 2021;31(6):4285–4297. doi: 10.1007/s00330-020-07542-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heggli I, Schüpbach R, Herger N, et al. OP0083 Infectious and Autoinflammatory Modic Type 1 Changes Have Different Pathomechanisms. BMJ Publishing Group Ltd; 2021. [Google Scholar]
- 22.Schirmer M, Kumar V, Netea MG, Xavier RJ. The causes and consequences of variation in human cytokine production in health. Curr Opin Immunol. 2018;54:50–58. doi: 10.1016/j.coi.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 23.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–3734. doi: 10.1007/s00586-016-4459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudli S, Miller S, Demir-Deviren S, Lotz JC. Inflammatory response of disc cells against Propionibacterium acnes depends on the presence of lumbar Modic changes. Eur Spine J. 2018;27(5):1013–1020. doi: 10.1007/s00586-017-5291-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA). 2014;1843(11):2563–2582. [DOI] [PubMed] [Google Scholar]
- 27.Gjefsen E, Gervin K, Goll G, et al. Macrophage migration inhibitory factor: a potential biomarker for chronic low back pain in patients with Modic changes. RMD Open. 2021;7(2):e001726. doi: 10.1136/rmdopen-2021-001726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altan-Bonnet G, Mukherjee R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat Rev Immunol. 2019;19(4):205–217. doi: 10.1038/s41577-019-0131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kothur K, Wienholt L, Mohammad SS, et al. Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating, anti-NMDAR and enteroviral encephalitis. PLoS One. 2016;11(8):e0161656. doi: 10.1371/journal.pone.0161656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michael BD, Griffiths MJ, Granerod J, et al. Characteristic cytokine and chemokine profiles in encephalitis of infectious, immune-mediated, and unknown aetiology. PLoS One. 2016;11(1):e0146288. doi: 10.1371/journal.pone.0146288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kothur K, Bandodkar S, Wienholt L, et al. Etiology is the key determinant of neuroinflammation in epilepsy: elevation of cerebrospinal fluid cytokines and chemokines in febrile infection‐related epilepsy syndrome and febrile status epilepticus. Epilepsia. 2019;60(8):1678–1688. doi: 10.1111/epi.16275 [DOI] [PubMed] [Google Scholar]
- 32.Kyogoku C, Smiljanovic B, Grün JR, et al. Cell-specific type I IFN signatures in autoimmunity and viral infection: what makes the difference? PLoS One. 2013;8(12):e83776. doi: 10.1371/journal.pone.0083776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeung W-CG, Al-Shabeeb A, Pang CNI, et al. Children with islet autoimmunity and enterovirus infection demonstrate a distinct cytokine profile. Diabetes. 2012;61(6):1500–1508. doi: 10.2337/db11-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinkmann J, Zeißler E-C, Scharrenberg JS, et al. The diagnostic value of cytokines for the discrimination of vertebral osteomyelitis and degenerative diseases of the spine. Cytokine. 2022;150:155782. doi: 10.1016/j.cyto.2021.155782 [DOI] [PubMed] [Google Scholar]
- 35.Yu Y, Champer J, Agak GW, Kao S, Modlin RL, Kim J. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Investig Dermatol. 2016;136(11):2221–2228. doi: 10.1016/j.jid.2016.06.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y, Chen Y, Zhou Z, et al. Association between chronic inflammation and latent infection of Propionibacterium acnes in non-pyogenic degenerated intervertebral discs: a pilot study. Eur Spine J. 2018;27(10):2506–2517. doi: 10.1007/s00586-017-5363-5 [DOI] [PubMed] [Google Scholar]
- 37.Jiao Y, Yuan Y, Lin Y, et al. Propionibacterium acnes induces discogenic low back pain via stimulating nucleus pulposus cells to secrete pro-algesic factor of IL-8/CINC-1 through TLR2–NF-κB p65 pathway. J Mol Med. 2019;97(1):25–35. doi: 10.1007/s00109-018-1712-z [DOI] [PubMed] [Google Scholar]
- 38.Schmid B, Hausmann O, Hitzl W, Achermann Y, Wuertz-Kozak K. The role of Cutibacterium acnes in intervertebral disc inflammation. Biomedicines. 2020;8(7):186. doi: 10.3390/biomedicines8070186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romics JL, Dolganiuc A, Velayudham A, et al. Toll‐like receptor 2 mediates inflammatory cytokine induction but not sensitization for liver injury by Propioni‐bacterium acnes. J Leukoc Biol. 2005;78(6):1255–1264. doi: 10.1189/jlb.0804448 [DOI] [PubMed] [Google Scholar]
- 40.Basal E, Jain A, Kaushal G. Antibody response to crude cell lysate of propionibacterium acnes and induction of pro-inflammatory cytokines in patients with acne and normal healthy subjects. J Microbiol. 2004;42(2):117–125. [PubMed] [Google Scholar]
- 41.Di Cesare PE, Chang E, Preston CF, Liu C-J. Serum interleukin-6 as a marker of periprosthetic infection following total Hip and knee arthroplasty. JBJS. 2005;87(9):1921–1927. doi: 10.2106/00004623-200509000-00003 [DOI] [PubMed] [Google Scholar]
- 42.Ugge H, Carlsson J, Söderquist B, Fall K, Andén O, Davidsson S. The influence of prostatic Cutibacterium acnes infection on serum levels of IL6 and CXCL8 in prostate cancer patients. Infect Agent Cancer. 2018;13(1):34. doi: 10.1186/s13027-018-0204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bråten LC, Gervin K, Pripp AH, et al. Statistical analysis plan for analysis of cytokines as prognostic factors and predictors of antibiotic treatment effect in the AIM trial. 2021.
- 44.Grotle M, Vøllestad M, Brox M. Cross-cultural adaptation of the Norwegian versions of the Roland-Morris Disability Questionnaire and the Oswestry Disability Index. J Rehabil Med. 2003;35(5):241–247. doi: 10.1080/16501970306094 [DOI] [PubMed] [Google Scholar]
- 45.Chiarotto A, Boers M, Deyo RA, et al. A core outcome measurement set for low back pain clinical trials. Orthopaed Proc. 2018;100-B(SUPP_2):29. [Google Scholar]
- 46.Fairbank JC, Pynsent PB. The Oswestry disability index. Spine. 2000;25(22):2940. doi: 10.1097/00007632-200011150-00017 [DOI] [PubMed] [Google Scholar]
- 47.Dworkin HR, Turk CD, Farrar TJ, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(12):9–19. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 48.Hingorani AD, van der Windt DA, Riley RD, et al. Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ. 2013;346:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipkovich I, Dmitrienko A, D’Agostino B. Tutorial in biostatistics: data‐driven subgroup identification and analysis in clinical trials. Stat Med. 2017;36(1):136–196. doi: 10.1002/sim.7064 [DOI] [PubMed] [Google Scholar]
- 50.Hiyama A, Suyama K, Sakai D, Tanaka M, Watanabe M. Correlational analysis of chemokine and inflammatory cytokine expression in the intervertebral disc and blood in patients with lumbar disc disease. J Orthopaed Res. 2022;40(5):1213–1222. doi: 10.1002/jor.25136 [DOI] [PubMed] [Google Scholar]
- 51.Boisson M, Borderie D, Henrotin Y, et al. Serum biomarkers in people with chronic low back pain and Modic 1 changes: a case-control study. Sci Rep. 2019;9(1):1–5. doi: 10.1038/s41598-019-46508-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilligan CJ, Cohen SP, Fischetti VA, Hirsch JA, Czaplewski LG. Chronic Low Back Pain, bacterial infection and treatment with antibiotics. Spine J. 2021;21:903–914. doi: 10.1016/j.spinee.2021.02.013 [DOI] [PubMed] [Google Scholar]
- 53.Ohrt‐Nissen S, Fritz BG, Walbom J, et al. Bacterial biofilms: a possible mechanism for chronic infection in patients with lumbar disc herniation–a prospective proof‐of‐concept study using fluorescence in situ hybridization. Apmis. 2018;126(5):440–447. doi: 10.1111/apm.12841 [DOI] [PubMed] [Google Scholar]
- 54.Lingsma H, Roozenbeek B, Steyerberg E. Covariate adjustment increases statistical power in randomized controlled trials. J Clin Epidemiol. 2010;63(12):1391. doi: 10.1016/j.jclinepi.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 55.Vanelderen P, Van Zundert J, Kozicz T, et al. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology. 2015;122(2):399–406. doi: 10.1097/ALN.0000000000000508 [DOI] [PubMed] [Google Scholar]
- 56.McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38(10):2021–2027. doi: 10.3174/ajnr.A5337 [DOI] [PMC free article] [PubMed] [Google Scholar]