Abstract
Whilst there has been significant public health benefits associated with global use of COVID-19 spike protein vaccines, potential serious adverse events following immunization have been reported. Acute myocarditis is a rare complication of COVID19 vaccines and often it is self-limiting. We describe two cases experiencing recurrent myocarditis following mRNA COVID-19 vaccine despite a prior episode with full clinical recovery. Between September 2021-September 2022 we observed two male adolescents with recurrent myocarditis related to mRNA-based-COVID19 vaccine. During the first episode both patients presented with fever and chest pain few days after their second dose of BNT162b2 mRNA Covid-19 Vaccine (Comirnaty®). The blood exams showed increased cardiac enzymes. In addition, complete viral panel was run, showing HHV7 positivity in a single case. The left ventricular ejection fraction (LVEF) was normal at echocardiogram but cardiac magnetic resonance scanning (CMR) was consistent with myocarditis. They were treated with supportive treatment with full recovery. The 6 months follow-up demonstrated good clinical conditions with normal cardiological findings. The CMR showed persistent lesions in left ventricle ‘s wall with LGE. After some months the patients presented at emergency department with fever and chest pain and increased cardiac enzymes. No decreased LVEF was observed. The CMR showed new focal areas of edema in the first case report and stable lesions in the second one. They reached full recovery with normalization of cardiac enzymes after few days. These case reports outline the need of strict follow-up in patients with CMR consistent with myocarditis after mRNA-based-COVID19 vaccine. More efforts are necessary to depict the underlying mechanisms of myocarditis after SARS-CoV2 vaccination to understand the risk of relapsing and the long-term sequelae.
Keywords: Myocarditis, COVID-19, Adversomics, Vaccination, mRNA-vaccine, Side effects, Vaccinology, Vaccine safety
Introduction
Safe and effective COVID-19 vaccines are powerful tools for ensuring public health and controlling the SARS-CoV-2 infection.
Both BNT162b2 mRNA vaccine (Comirnaty®) and mRNA-1273 vaccine (Spikevax®) are licensed for use in children and adolescents by the age of 6 months.
Whilst there has been significant public health benefits associated with global use of COVID-19 spike protein vaccines, potential serious adverse events following immunization (AEFI) have been reported. Among them, pericarditis and myocarditis represent rare complications mostly reported in adolescents and young adults, especially in male gender following the diverse COVID vaccines available, but mostly in association with both mRNA vaccines: BNT162b2 and mRNA-1273 [1].
Although cardiologic symptoms are mostly self-limiting or controlled by nonsteroidal anti-inflammatory drugs (NSAID), it is not known whether patients with a prior history of myocarditis following a COVID-19 vaccine are at increased risk of recurrence of myocarditis. To date myocarditis following COVID-19 vaccination represent a self-limited episode and has been usually associated to a positive outcome without any history of relapse [2].
However, information about this new entity is rapidly evolving, and little is known about how this vaccine-mediated myocarditis differs from classic myocarditis.
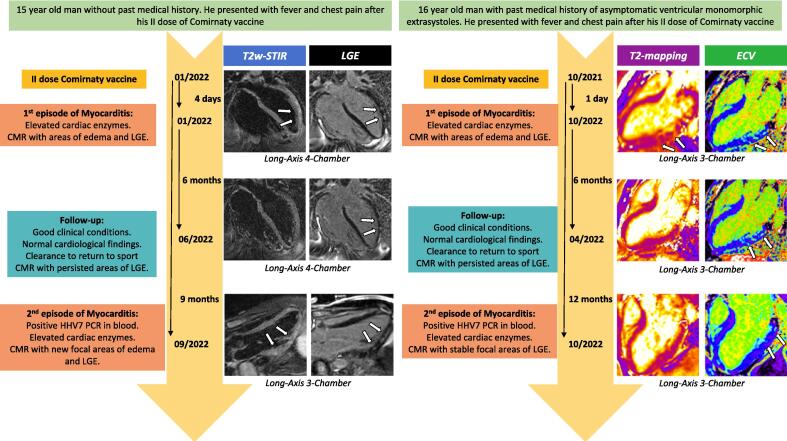
Here we firstly describe the case of two adolescents with relapsing myocarditis after full recovery from an initial episode of mRNA vaccine related myocarditis Fig. 1. Table 1 reports the patients’ demographic, clinic and laboratory characteristics.
Fig. 1.
Medical history and CMR images. Figure 1 displays a timeline with dates for acute myocarditis events along with most important clinical data and CMR findings. Case 1: CMR long-axis images in the 1st patient (left group) show the more traditional criteria for diagnosing acute myocarditis, using both T2w-STIR and late gadolinium enhancement (LGE) sequences to detect myocardial edema and necrosis/fibrosis respectively: 4-chamber views during the 1st episode of myocarditis (top row) display focal areas of non-ischemic subepicardial hyperintensity on both T2w-STIR and LGE sequences in the mid-apical antero-lateral segments of the left ventricle, consistent with acute myocarditis; analogous 4-chamber views during follow-up (middle row) document less conspicuous areas of LGE in the same segments without corresponding edema, in keeping with previous myocarditis; 3-chamber images during the 2nd episode of myocarditis (bottom row) show new areas of subepicardial edema and LGE in the infero-lateral segments of the left ventricle, consistent with relapsing acute myocarditis; areas of LGE in the mid-apical antero-lateral segments (not shown) were still visible. Case 2: CMR long-axis 3-chamber images in the 2nd patient (right group) illustrate the newer criteria for acute myocarditis, adding newer sequences including T1- and T2-mapping to make the diagnosis: views during the 1st episode of myocarditis (top row) show focal areas of non-ischemic subepicardial/intramyocardial increased T2-mapping and extracellular volume (ECV) -obtained from T1-mapping- in the mid-basal infero-lateral segments of the left ventricle, compatible with edema and necrosis respectively, due to acute myocarditis; images during follow-up (middle row) display persistent areas of increased ECV in the same segments without corresponding edema, suggestive of scarring due to previous myocarditis; images during the 2nd episode of myocarditis (bottom row) are characterized by less evident areas of increased ECV in the same segments but no edema, therefore without imaging evidence of recurring acute myocarditis. White arrows highlight the described findings.
Table 1.
Characteristics of patients with relapsing myocarditis after first episode following mRNA-SARS-CoV-2 vaccine.
| Case 1 |
Case 2 |
|||
|---|---|---|---|---|
|
Demographic and clinic characteristics |
1° episode | 2° episode | 1° episode | 2° episode |
| Age (years) | 15 | 15 | 16 | 17 |
| Sex | M | M | ||
| BMI | 18,29 | 31,48 | ||
| Onset of symptoms after vaccine (days) | 4 | 1 | ||
| Vaccination dose | 2nd | na | 2nd | na |
| Number of doses/interval (days) | 2/21 | 2/21 | ||
| Manufacurer - Lot number (1st dose/2nd dose) | Comirnaty - Pfizer – 33361 TB/FP8234 | Comirnaty - Pfizer - FF2382/FF2382 | ||
| Symptoms | Fever and chest pain | Fever and chest pain | Fever and chest pain | Fever, sore throat and chest pain |
| Treatment | Ibuprofen and mineralocorticoid receptor antagonists | IVIG and ACE inhibitors | Ibuprofen | IVIG, Ibuprofen and ACE inhibitors |
| Time to relapse (months) | na | 8 | na | 12 |
| Route of administratione/site of injections | Intramuscular/left deltoid | Intramuscular/left deltoid | ||
| Smoking status | Never smoker | Never smoker | ||
| Laboratory values | ||||
| CRP, mg/mL (NV < 0,5) | 2,03 | 4,84 | 3,62 | 7,55 |
| hsTnT, pg/mL (NV < 14) | 610 | 450 | 804 | 3310 |
| NT-proBNP, pg/mL (NV <1 3 5) | 1000 | 1170 | 1191 | 1563 |
| ECG | ||||
| ST segment elevation | No | Yes | No | No |
| ST segment depression | No | No | No | No |
| Non specific ST segment changes | Yes | No | No | No |
| T-wave inversion | No | No | No | No |
| Echocardiogram | ||||
| LV EF, % | 73,6 | 60 | 65,4 | 53,4 |
| Pericardial effusion | Yes | No | Yes | No |
| Pericardial hyperechogenicity | No | No | No | Yes |
| CMR | ||||
| LV EF, % | 64,6 | 61,4 | 73 | 62,5 |
| Regional hyperintensity on T2-weighted imaging | Yes | Yes | Yes | No |
| LGE | Yes | Yes | Yes | Yes |
Abbreviations: BMI: body mass index; na: not applicable; IVIG: intravenous immunoglobulin; ACE inhibitors: angiotensin-converting enzyme (ACE) inhibitors; CRP: c reactive protein; hs-TNT: high sensitivity troponin; NT-ptoBNP: N-terminal prohormone of brain natriuretic peptide; CMR: Cardiac magnetic resonance; NV: normal values; LV EF: left ventricular ejection fraction; LGE: late gadolinium enhancement.
Methods
We performed a prospective evaluation of all patients admitted at the pediatric emergency department of the Children’s Hospital “Bambino Gesù” between September 2021 and September 2022 with chest pain, shortness of breath, or palpitations after Covid-19 vaccines who met the CDC work case definition for myocarditis [3]. Only confirmed cases were enrolled in this study. The Institutional Ethical Committee approved the present study named “CACTUS” (2083_OPBG_2020 amendment). All patients’ parents or legal guardians enrolled in the study signed the informed consent.
Cardiac Magnetic Resonance imaging (cMRi) 1.5 Tesla was performed on admission upon pediatric cardiologist prescription and planned during outpatient follow-up. Markers of heart inflammation by cMRi were defined according to the Lake Louise criteria11 and included high signal intensity on T2-weighted imaging, increased T2 and T times denoting extracellular volume (ECV) fraction, and myocardial thickening attributable to edema as a sign of hyperemia. Measures of necrosis included contrast retention 10 to 15 min after injection of gadolinium (late gadolinium enhancement, LGE), indicating either acute myocarditis or scar formation with fibrosis. [4], [5]
Case 1
A 16-year-old male adolescent with no significant medical history presented to the emergency department in January 2022 with fever and severe chest pain four days after the second dose of Comirnaty® vaccine. Prior to vaccination, he experienced undiagnosed and asymptomatic SARS-CoV-2 infection as demonstrated by positive serology for anti-N and anti-S antibodies.
Initial vital signs were normal, except for fever (38 °C). Electrocardiography (ECG) showed abnormal changes in ventricular repolarization in the lateral leads. Transthoracic echocardiography revealed an ejection fraction (EF) of 58% and mild pericardial effusion. Serum High-Sensitivity Troponin T (hs-TnT) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP), 610 pg/ml (normal values NV < 14) and 1000 pg/ml (NV 〈1 3 5) respectively, were elevated. The C-Reactive Protein (CRP) was 2.03 mg/dl (NV < 0,5). Quantitative antigenic nasal swab for SARS-CoV-2 was negative as well as polymerase chain reaction (PCR) testing for influenza virus. We ran a complete viral panel including herpes viruses, enteroviruses, adenovirus and PB19 by PCR testing on blood which turned negative. Hence, the patient received conservative management, including oral ibuprofen and mineralocorticoid receptor antagonists. cMRi showed subepicardial areas of high signal intensity on T2 and post-contrast T1-weighted (late gadolinium enhancement, LGE) sequences in the antero-lateral wall of the left ventricle (LV), consistent with acute myocarditis. Moreover, we also looked for anti-heart muscle and anti-endothelial cell autoantibodies which resulted positive for anti-endothelial cells only. Clinical conditions rapidly improved and he was discharged 9 days after admission with normal hs-TnT and NT-proBNP levels. The patient returned after 6 months to normal sport practice following normal ergometer stress test. CMR at 6 months follow-up showed improving, but with persistent focal lesions with LGE in the LV wall.
Nine-months after the initial diagnosis of myocarditis, in September 2022 the patient presented to medical attention for fever and chest pain. ECG showed antero-lateral ST-elevation with normal findings at echocardiography. Cardiac enzymes, hs-TnT and NT-proBNP, were both elevated (450 pg/ml and 1170 pg/ml, respectively). Quantitative antigenic nasal swab for SAR-CoV2 resulted negative while the complete viral PCR evaluations, as previously described, showed residual copies for HHV7 (1124 copies/ml). On CMR new focal lesions along the LV wall with edema and LGE were observed. Altogether, these data were consistent with relapsing of acute myocarditis. Considering the myocarditis recurrence and HHV7 positivity, high dose intravenous immunoglobulins (IVIG) were administered along with angiotensin-converting enzyme (ACE) inhibitors with rapid improvement of clinical conditions and cardiac enzyme values.
The patient was discharged in good clinical conditions after 13 days from admission with normal hs-TnT and NT-proBNP values and proper indications concerning avoidance of strong physical activity for 6 months.
Case 2
A 16-year-old male presented to the emergency department in October 2021 with chest pain and fever for two days. He had received his second dose of BNT162b2 mRNA Covid-19 vaccine the day before the onset of chest pain. The patient was previously affected by asymptomatic ventricular monomorphic extrasystoles occasionally observed during a stress test and not requiring any medication.
On admission, ECG showed sinus tachycardia without any ST-T changes. Echocardiography revealed mild pericardial effusion. Quantitative antigenic nasal swab for SARS-CoV-2 was negative as well as respiratory viral pathogen panel PCR testing. Initial laboratory investigations revealed high CRP 3.62 mg/dl, while hs-TnT and NT-proBNP were 804 pg/ml and 1591 pg/ml, respectively.
The broad viral evaluations showed positivity for HHV7 (1750 copies/ml). A cMRi was performed 6 days after the symptoms’ onset and showed subepicardial/intramyocardial focal areas of signal intensity on T1- and T2-weighted sequences in the inferolateral wall of the LV, consistent with acute myocarditis. He received supportive care and ibuprofen and was discharged after 12 days of hospitalization. After discharge, regular cardiologic checks, blood exams, ergometer test (CPET) and Holter ECG examinations resulted normal. Six months after, due to the stability of cardiac findings during the follow-up visits the patient received clearance to return to sport.
In February 2022, he experienced SARS-CoV-2 infection with few symptoms.
CMR at six months follow-up showed diminished but persistent patchy areas of fibrosis in the same segments of the LV. Accordingly, treatment with mineralocorticoid receptor antagonists was started.
In October 2022, 8-months following the 1st episode, the patient was again admitted to the emergency department due to fever, acute chest pain and sore throat. ECG showed a ventricular ectopic beat with no alteration of ST-segment. On echocardiography, pericardial hyperechogenicity was documented with an LVEF of 53.4%. Blood tests demonstrated elevated CRP (7,55 mg/dl) and increased markers of myocardial damage, with hs-TnT of 3310 pg/ml and NT-proBNP 1563 pg/ml. Nasal swab for SARS-CoV2 was negative. Serological and PCR virological screening demonstrated few copies of HHV7 (<500 copies/ml).
No new focal areas with LGE or edema were observed in CMR. Due to chest pain and evidence of inversion of T wave to the subsequent ECG, treatment with Ibuprofen and ACE-inhibitor was started. In consideration of a plausible viral trigger, high dose IGIVs were administered. We also looked for anti-heart muscle and anti-endothelial cell autoantibodies detection which turned positive. Chest pain improved with NSAID treatment and supportive care, and he was discharged after 6 days of hospitalization with the indication to continue treatment with ACE-inhibitors and NSAID gradual tapering. At the time of discharge, cardiac enzymes were negative and ECG and echocardiogram findings were normal.
Discussion
Viral myocarditis unrelated to mRNA COVID-19 vaccination are life-threatening conditions and have been reported to lead to heart failure and cardiac transplantation, or death [3]. Conversely, current scientific evidence shows that myocarditis related to COVID-19 vaccination result in a more favorable outcome with quicker resolution of symptoms [1], [2], [6].
Long term outcome of myocarditis and pericarditis after mRNA COVID-19 vaccination is still a matter of debate since the recent onset of this condition and the need of a longer follow-up. CMR has been recently largely used for diagnosis and follow-up of myocarditis. Both cases described met the criteria for the diagnosis of acute myocarditis during their first presentation [3], [4], [5], [6]. On the other hand, only one patient met the criteria during the second episode. This is likely related to the diagnostic performance of CMR, which is known to yield great specificity compared to a less overwhelming sensitivity. Moreover, both cases had persistent LGE enhancement at follow-up evaluations. Similarly to other groups [2], [6], [7], [8], [9], [10], we recently described the persistence of LGE enhancement, suggestive of myocardial fibrosis, in patients with myopericarditis following mRNA COVID-19 vaccination [11].
Persistent cMRi findings, such as LGE, have been described in pediatric patients with myocarditis, but their actual role as predictor of AEFI during follow-up is uncertain [12], [13].
Indeed, the actual role of cMRi needs to be further clarified, since it is not clear if the suggestive findings of myocardial scarring could represent a risk factor for development of long term sequalae or a normal heart healing process.
Undoubtedly, cases hereby described highlight that children presenting with a pathological cMRi deserve a more stringent follow-up compared to those patients without any cMRi signs. The pathogenesis of COVID-19 mRNA-vaccination-related myocarditis still remains poorly understood. The three main mechanisms by which COVID-19 vaccines might induce myocarditis are i) immune reactivity related to higher levels of circulating spike protein [14], ii) antibodies to SARS-CoV-2 spike glycoproteins cross-reacting with myocardial contractile proteins [13], and iii) hormonal differences [13], [15]. All of these mechanisms can be influenced by immune–genetic background, age and sex [13]. The presence of anti-hearth antibodies (AHA) both reflects high intensity of the general immune inflammatory response and serves as one of the factors of the inflammatory heart injury. The detection of AHA in the cases described suggests their potential role in myocarditis pathogenesis opening the question if immune activation with inflammation (infection or immunization related) could trigger antibody mediated clinical relapse.
The immune system might detect the mRNA in the vaccine as an antigen, resulting in the activation of proinflammatory cascades and immunological pathways in the heart. Although nucleoside modifications of mRNA reduce their innate reactogenicity [16], the immune response to mRNA might still drive the activation of an aberrant innate and acquired immune response, which can explain the stronger response seen with mRNA vaccines compared to other types of COVID-19 vaccine. In line with this hypothesis Yonker L. et al recently reported that individuals who developed postvaccine myocarditis uniquely exhibit elevated levels of free spike protein in circulation, unbound by anti-spike antibodies, which appear to correlate with cardiac troponin T levels and innate immune activation with cytokine release [14].
Molecular mimicry between the spike protein of SARS-CoV-2 and cardiac self-antigens is another possible mechanism. Antibodies directed to SARS-CoV-2 spike glycoproteins might cross-react with structurally similar human protein sequences, including myocardial α-myosin heavy chain [17].
These autoantibodies might be innocent bystanders resulting from myocardial inflammation and injury, or might reflect a certain immune–genetic background that predisposes to developing hyperimmunity and myocarditis upon any trigger. However very recent findings in post mRNA vaccine myocarditis did not show evidence of cardiac targeted autoantibodies in these patients suggesting that this mechanism is unlikely to act as main pathogenic trigger [18].
Finally, given the increased incidence among male patients, differences in hormone signaling might be involved in the pathophysiology of COVID-19 mRNA-vaccination-related myocarditis. Testosterone can inhibit anti-inflammatory immune cells and promote a more aggressive T helper 1 cell-type immune response. By contrast, estrogen has inhibitory effects on pro-inflammatory T cells, resulting in a decrease in cell-mediated immune responses.
As noted in previous studies. viral infections could act as a trigger in a predisposed individual. Our cases show a new diagnosed HHV7 infection during the second episode and a persisting viral replication respectively with the second patient presenting symptoms compatible with an ongoing upper airway viral infection. HHV-7 has been recently described in association with severe myocarditis as potential causative agent [19]. The presumptive viral etiology of the second episode of myocarditis supported the choice to administer IVIG as suggested by international guidelines in association with supportive care [20], [21]. Since HHV-7 infection generally occurs during childhood and up to 95% of adults are seropositive [22], it can be considered an endemic virus and we cannot establish if HHV-7 detection is incidental or represent the causative agent of myocarditis. However, the rapid reduction of HHV-7 copies after their initial detection is more in favor of an incidental finding in this case.
Given the short follow-up time and the recent history of the disease, it is still not known whether patients with a prior history of myocarditis after mRNA based COVID-19 vaccine are at increased risk of recurrent myocarditis. Moreover, it would be important to perform genetic evaluation to clarify the presence of mutations in genes associated with cardiomyopathy. Such evaluations are still ongoing in our patients.
In the recent scientific literature, other case reports described recurrent myocarditis after mRNA-based-COVID19 vaccination in patients with a pre-existing episode of myocarditis not vaccine related [23], [24], [25], [26], [27]. To our knowledge this is the first report of relapsing myocarditis in previously healthy patients experiencing myocarditis after mRNA-based-COVID19 vaccine. Up to now the long term sequalae related to this kind of myocarditis are unknow. More efforts are needed to depict the underlying mechanisms beyond this phenomenon and to understand the risk of complications such as recurrence, potential evolution to dilated cardiomyopathy, and arrhythmias in the future.
Funding and support
We would like to thank the patients who participated in the study. We also thank Jennifer Faudella, Ilaria Pepponi and Giulia Neccia for their administrative assistance. This work was made possible thanks to the support from INCIPIT through an independent grant by “Fondazione Chiesi” to support the TELESCOPE project to PP, from Ministry of Health and IRCCS “Bambino Gesù” through 5x1000 grant to DA and “Current Research funds” to NC.
CRediT authorship contribution statement
Donato Amodio: Conceptualization, Data Curation, Formal Analysis, Funding acquisition, Methodology, Writing - original draft, Writing - review & editing. Emma Concetta Manno: Conceptualization, Data Curation, Writing - original draft, Writing - review & editing. Nicola Cotugno: Conceptualization, Data Curation, Funding acquisition, Writing - review & editing. Veronica Santilli: Data Curation, Writing - review & editing. Alessio Franceschini: Data Curation, Formal Analysis, Writing - review & editing. Marco Alfonso Perrone: Data Curation, Formal Analysis, Writing - review & editing. Marcello Chinali: Methodology, Writing - review & editing. Fabrizio Drago: Methodology, Writing - review & editing. Nicoletta Cantarutti: Data Curation, Writing - review & editing. Davide Curione: Data Curation, Formal Analysis, Methodology, Writing - original draft, Writing - review & editing. Renata Engler: Conceptualization, Data Curation, Supervision, Writing - review & editing. Aurelio Secinaro: Data Curation, Formal Analysis, Methodology, Writing - original draft, Writing - review & editing. Paolo Palma: Conceptualization, Data Curation, Formal Analysis, Funding acquisition, Methodology, Supervision, Writing - original draft, Writing - review & editing
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Paolo Palma reports financial support was provided by Chiesi Foundation.
Contributor Information
Donato Amodio, Email: donato.amodio@opbg.net.
Emma Concetta Manno, Email: emmaconcetta.manno@opbg.net.
Nicola Cotugno, Email: nicola.cotugno@opbg.net.
Veronica Santilli, Email: veronica.santilli@opbg.net.
Alessio Franceschini, Email: alessio.franceschini@opbg.net.
Marco Alfonso Perrone, Email: marcoalfonso.perrone@opbg.net.
Marcello Chinali, Email: marcello.chinali@opbg.net.
Fabrizio Drago, Email: fabrizio.drago@opbg.net.
Nicoletta Cantarutti, Email: nicoletta.cantarutti@opbg.net.
Davide Curione, Email: davide.curione@opbg.net.
Renata Engler, Email: renata.engler@gmail.com.
Paolo Palma, Email: paolo.palma@opbg.net.
Data availability
Data will be made available on request.
References
- 1.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kracalik I., Oster M.E., Broder K.R., Cortese M.M., Glover M., Shields K., et al. Outcomes at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults in the USA: a follow-up surveillance study. Lancet Child Adolesc Health. 2022;6(11):788–798. doi: 10.1016/S2352-4642(22)00244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law Y.M., Lal A.K., Chen S., et al. Diagnosis and Management of Myocarditis in Children: A Scientific Statement From the American Heart Association. Circulation. 2021 Aug 10;144(6):e123–e135. doi: 10.1161/CIR.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018 Dec 18;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 5.Mewton N., Liu C.Y., Croisille P., Bluemke D., Lima J.A.C. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaak A., Bischoff L.M., Faron A., Endler C., Mesropyan N., Sprinkart A.M., et al. Multiparametric cardiac magnetic resonance imaging in pediatric and adolescent patients with acute myocarditis. Pediatr Radiol. 2021;51(13):2470–2480. doi: 10.1007/s00247-021-05169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA. 2022;327(4):331. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puchalski M., Kamińska H., Bartoszek M., Brzewski M., Werner B. COVID-19-Vaccination-Induced Myocarditis in Teenagers: Case Series with Further Follow-Up. J Environ Res Public Health. 2022 Mar;19(6):3456. doi: 10.3390/ijerph19063456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amir G., Rotstein A., Razon Y., Beyersdorf G.B., Barak–Corren Y., Godfrey M.E., et al. CMR Imaging 6 Months After Myocarditis Associated with the BNT162b2 mRNA COVID-19 Vaccine. Pediatr Cardiol. 2022;43(7):1522–1529. doi: 10.1007/s00246-022-02878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubey S., Agarwal A., Nguyen S., Adebo D. Persistence of Late Gadolinium Enhancement on Follow-Up CMR Imaging in Children with Acute Myocarditis. Pediatr Cardiol. 2020 Dec;41(8):1777–1782. doi: 10.1007/s00246-020-02445-5. [DOI] [PubMed] [Google Scholar]
- 11.Manno E.C., Amodio D., Cotugno N., Rossetti C., Giancotta C., Santilli V., et al. Higher Troponin Levels on Admission are associated With Persistent Cardiac Magnetic Resonance Lesions in Children Developing Myocarditis After mRNA-Based COVID-19 Vaccination. Pediatr Infect Dis J. 2023;42(2):166–171. doi: 10.1097/INF.0000000000003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Małek Ł.A., Kamińska H., Barczuk-Falęcka M., et al. Children With Acute Myocarditis Often Have Persistent Subclinical Changesas Revealed by Cardiac Magnetic Resonance. Magn Reson Imaging. 2020 Aug;52(2):488–496. doi: 10.1002/jmri.27036. [DOI] [PubMed] [Google Scholar]
- 13.Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022 Feb;19(2):75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yonker L.M., Swank Z., Bartsch Y.C., Burns M.D., Kane A., Boribong B.P., et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023;147(11):867–876. doi: 10.1161/CIRCULATIONAHA.122.061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halsell J.R., Riddle J.R., Atwood J.E., et al. Myopericarditis Following Smallpox Vaccination Among Vaccinia-Naive US Military Personnel. JAMA. 2003;289(24):3283–3289. doi: 10.1001/jama.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 16.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005 Aug;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barmada A., Klein J., Ramaswamy A., Brodsky N.N., Jaycox J.R., Sheikha H., et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis. Sci Immunol. 2023;8(83) doi: 10.1126/sciimmunol.adh3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozdemir R., Kucuk M., Dibeklioglu S.E. Report of a Myocarditis Outbreak among Pediatric Patients: Human Herpesvirus 7 as a Causative Agent? Trop Pediatr. 2018 Dec 1;64(6):468–471. doi: 10.1093/tropej/fmx093. [DOI] [PubMed] [Google Scholar]
- 20.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015 Nov;12(11):670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 21.Ammirati E., Frigerio M., Adler E.D., Basso C., Birnie D.H., Brambatti M., et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13(11):e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark D.A., Freeland J.M.L., Mackie P.L.K., Jarrett R.F., Onions D.E. Prevalence of antibody to human herpesvirus 7 by age. J Infect Dis. 1993;168(1):251–252. doi: 10.1093/infdis/168.1.251. [DOI] [PubMed] [Google Scholar]
- 23.Umei T.C., Kishino Y., Watanabe K., Shiraishi Y., Inohara T., Yuasa S., Fukuda K. Recurrence of Myopericarditis Following mRNA COVID-19 Vaccination in a Male Adolescent. CJC Open. 2022;4(3):350–352. doi: 10.1016/j.cjco.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mapelli M., Amelotti N., Andreini D., et al. A case of myopericarditis recurrence after third dose of BNT162b2 vaccine against SARS-CoV-2 in a young subject: link or causality? Eur Heart J Suppl. 2022 doi: 10.1093/eurheartj/suac018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minocha P.K., Better D., Singh R.K., Hoque T. Recurrence of Acute Myocarditis Temporally Associated with Receipt of the mRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a Male Adolescent. J Pediatr. 2021 Nov;238:321–323. doi: 10.1016/j.jpeds.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasha M.A., Isaac S., Khan Z. Recurrent Myocarditis Following COVID-19 Infection and the mRNA Vaccine. Cureus. 2022 Jul 7;14(7):e26650. doi: 10.7759/cureus.26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Tano G., Moschini L., Calvaruso E.V., Danzi G.B. Recurrent Myocarditis after the First Dose of SARS-CoV-2 mRNA- 1273 Vaccine. Ann Clin Case Rep. 2021;6:2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Data will be made available on request.