Abstract
Orthostatic intolerance and other autonomic dysfunction syndromes are emerging as distinct symptom clusters in Long Covid. Often accompanying these are common, multi-system constitutional features such as fatigue, malaise and skin rashes which can signify generalized immune dysregulation. At the same time, multiple autoantibodies are identified in both Covid-related autonomic disorders and non-Covid autonomic disorders, implying a possible underlying autoimmune pathology. The lack of specificity of these findings precludes direct interpretations of cause and association, but their prevalence with its supporting evidence is compelling.
Keywords: , autoimmunity, POTS, Long Covid, autoantibody, autoimmune
The autonomic nervous system and immune systems in acute and Long Covid—a complex relationship
The immune and autonomic nervous systems have a complex, reciprocal relationship which affects peripheral immune responses, particularly related to cytokines [1, 2]. Cells of the immune system express adrenergic and nicotinic receptors and can also release catecholamines [3, 4]. This relationship is evident in both acute Covid infection and later sequelae. The well-established cytokine release syndrome in acute Covid infection, clinically manifesting as acute respiratory distress syndrome (ARDS), is characterized by sympathetically mediated pro-inflammatory cytokine cascade (driven by IL-6) [5, 6]. It has been hypothesized that the chronically elevated sympatho-activation in existing conditions such as hypertension, heart disease and metabolic syndrome drives exaggerated sympathetic responses and poor outcomes [7]. The spike protein of the SARS-CoV-2 virus binds the angiotensin converting enzyme 2 (ACEII) receptor, resulting in increased angiotensin 2 activity and vasoconstriction [7].
On the other hand, the parasympathetically mediated cholinergic anti-inflammatory reflex regulates this sympathetically related inflammation by inhibiting cytokine production from macrophages thus reducing TNFα production [7–9]. This vagally mediated immune modulation is illustrated by a recent randomized controlled trial which found that transcutaneous auricular (non-invasive) vagal nerve stimulation reduces acute inflammation markers such as C-reactive protein in hospitalized Covid patients [10].
Further evidence of neuroimmune interactions is the finding that autonomic system autoantibodies which are present in both POTS and Long Covid [G-protein coupled receptor (GPCR) antibodies] [11, 12] are also present in acute Covid infection and correlate with disease severity [13].
The relationship between Long Covid, the autonomic nervous system and associated autoimmunity still needs full evaluation but unifying findings are emerging. In patients infected with Covid, a range of autoantibodies is seen against immune proteins such as cytokines, complement and cell surface proteins [14]. These autoantibodies may alter the immune response to acute infection, with correlations with inflammatory biomarkers such as C-reactive protein [14]. Anti-cardiac antibodies have also been identified in the acute severe phase of Covid infection, but without correlation with clinical outcome [15].
Patients with Long Covid have upregulated pro-inflammatory cytokines (IFNα, TNFα, IL6, IL17α, IL1β, IL14) [16] which persist for up to 9–12 months following infection [17] and are associated with symptoms at 12 months [18]. Autoantibodies are identified in both Covid-related autonomic disorders and non-Covid autonomic disorders, implying a possible underlying autoimmune pathology. The lack of specificity of these findings precludes direct interpretations of cause and association, but prevalence with its supporting evidence is compelling.
In this review, we discuss the role of the autonomic nervous and immune systems in Covid and Long Covid and their potential influence on symptoms and clinical practice. Additionally, overlap with non-Covid autonomic dysfunction is considered. Understanding these new disorders can inform both neuro-immunology and Long Covid management.
Long Covid—the new pandemic
Over 2% of the UK population now reports persistent symptoms following Covid infection [19], and post-Covid syndrome (or ‘Long Covid’) threatens physical health, workforce strength and societal wellbeing. The term ‘Long Covid’ encompasses ongoing symptomatic Covid (from 4 to 12 weeks) and post-Covid syndrome (symptoms lasting >12 weeks, not caused by an alternative diagnosis) [20]. Post-Covid syndrome is likely to be an ‘umbrella’ term for a wide range of undetermined conditions and physiological states. The definition from National Institute of Clinical Excellence emphasizes the heterogeneous multi-system nature of the syndrome, which can manifest as symptom clusters, often fluctuating over time [20]. Ambiguity about the condition (and lack of treatment options to date) can cause considerable uncertainty and anxiety for both clinicians and patients. An emerging but incompletely understood phenomenon increasingly seen in Long Covid and syncope clinics is cardiovascular autonomic dysfunction including postural orthostatic tachycardia syndrome (POTS) and orthostatic hypotension. Study of this apparent association with Long Covid may reveal underlying mechanisms and offer insight into non-Covid autonomic disorders with comprehension of autonomic nervous and neuroimmune system interaction.
Additionally, autoantibodies are increasingly being detected in Long Covid [18, 21–24]. Specifically, autoantibodies to inflammatory cytokines are present (such as IgG to IL-2, D8B, thyroglobulin and IFNδ) and these correlate with anti-SARS CoV2 IgG antibodies [25, 26]. Autoantibodies to antinuclear and extractable nuclear antigens are also elevated in individuals with Long Covid, and correlate with symptoms of fatigue and dyspnoea [18]. Notable autoantibodies include functionally active autoantibodies to the GPCRs (including α1- and β2-adrenoceptors, angiotensin receptor, nociception-like opioid receptor and muscarinic M2-receptor) [11]. This is significant for the overlap with POTS [12] and autonomic nervous system. Finally, the presence of GPCR autoantibodies in individuals with Long Covid correlates with impaired peripapillary vessel density in the eye, a biomarker for microcirculation health [27]. This partly validates theories of endothelial dysfunction and impaired coagulation.
A deep multi-omic investigation showed that some patients with Long Covid have autoantibodies at diagnosis, suggesting underlying subclinical autoimmunity [28]. Additionally, there are correlations with SARS-CoV-2 antibodies and symptoms of Long Covid, as well as a negative correlation between anti-SARS-CoV-2 IgG antibodies and autoantibodies [28].
Pro-inflammatory cytokines are associated with increased sympathetic activity which is also seen in non-Covid POTS [29]. This pro-inflammatory environment can stimulate microbiome-mediated autoantibody production, causing chronic immune activation or dysfunction [30]. Linking the above conditions are the common and non-specific symptoms of fatigue, cognitive symptoms and mental fatigue (so-called ‘brain fog’), gastrointestinal dysfunction and pain which are not unique to these diseases, but are non-specific sequelae of any immune dysregulation [31]. This is relevant in approach to therapeutics, which can either focus on underlying aetiology or consequential symptoms.
Orthostatic intolerance syndromes—and what Long Covid can tell us about non-Covid POTS
These non-specific immune symptoms described above often accompany orthostatic intolerance symptoms—such as dizziness, light-headedness, palpitations, chest pain and, also reduced exercise tolerance. Such conditions include POTS (defined as orthostatic symptoms, a sustained heart rate rise of >30 bpm [or >40 bpm in individuals under 19 years of age]) on standing, without a reduction in blood pressure [32]. Orthostatic intolerance refers to symptoms and related haemodynamic changes when upright.
Autonomic dysfunction in acute and Long Covid
Long Covid autonomic dysfunction
Autonomic dysfunction presents as an important but incompletely understood component of Long Covid. There have been many reports of clinical cases and series describing POTS, inappropriate sinus tachycardia and orthostatic hypotension (all impairments of the cardiovascular autonomic nervous system) following Covid infection [33–42]. Vasovagal syncope has also been described [37, 43], but it is not yet clear that this is due solely to the viral infection given its frequency in the general population. Indeed, this applies to all cardiovascular autonomic conditions given the lack of detailed autonomic data in this population prior to the onset of Long Covid.
The assessment of cardiovascular autonomic integrity involves some important tests and markers of autonomic dysfunction such as tilt testing, active standing test, Valsalva manoeuvre, deep breathing test, Holter ECG and 24-h ambulatory blood pressure monitoring [44]. Impaired autonomic function is seen to be an adverse prognostic marker in both acute and Long Covid. Heart rate variability (HRV) is the variation in time between successive heartbeats, and is a biomarker of autonomic health. It is usually derived from 24-h Holter ECG or shorter ECG registration [45], and is reduced following Covid infection [46, 47]. Even resting tachycardia during acute infection (a sign of sympathetic activity) is associated with a more severe disease course [48], independent of comorbidity and fever [49]. Increased HRV predicts survival in people with acute Covid over 70 years, and lower HRV increases risk of admission to critical care [50]. In young adults recovering from Covid, elevated sympathetic nervous system (SNS) activity is apparent despite normal heart rate and blood pressure readings, with exaggerated responses on standing [51, 52]. In survivors at 6 months following hospital discharge, HRV parameters correlate inversely with pulmonary fibrosis and diffusion restriction [53]. One group of post-Covid patients had higher sympathetic activity and lower parasympathetic activity than controls, but these changes were more marked in individuals who were obese or physically sedentary [52]. These individuals were not symptomatic, and so the direct clinical implications are less clear, but the findings of many of the studies reported above are nevertheless compelling as they highlight the dynamic nature of HRV and potential for modulation.
Mechanisms of orthostatic intolerance
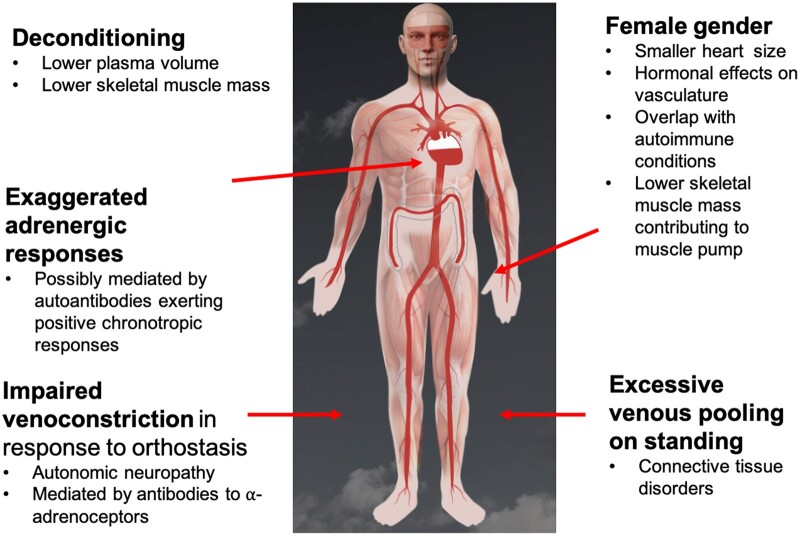
These symptoms and responses relate to the individual’s responses to venous pooling and subsequent haemodynamic responses (Fig. 1). In brief, when a healthy individual stands up, up to a third of their blood volume pools in their lower body vasculature (in the pelvis and legs) by gravitational forces [54]. The consequent reduction in cardiac venous return is recognized by stretch receptors in the carotid sinus and aortic arch, and mechanoceptors in the heart and lungs. These signals are processed in the brainstem via afferent fibres, and increased sympathetic noradrenergic output occurs via efferent sympathetic neurons. These result in vasoconstriction of lower body vasculature, and increased heart rate and inotropy to maintain cardiac output in healthy individuals. Simultaneously, the renin angiotensin system is activated and anti-diuretic hormone (ADH) is secreted, promoting volume expansion and water retention. However, these responses may be impaired in those with orthostatic intolerance syndromes who may have inadequate blood volume, excessive venous pooling in the lower body or impaired peripheral vasoconstriction (e.g. by antibodies to the adrenergic receptors in lower limb blood vessels) [33]. The elevated compensatory adrenergic and noradrenergic responses occur daily, extending into weeks and months, and portend chronic multi-system constitutional symptoms. The symptoms of venous pooling and reduced cardiac output may be dizziness, fatigue, weakness and these changes are considered to be important in the evolution of vasovagal syncope [55].
Figure 1.
Possible mechanisms contributing to POTS. The underlying mechanisms are likely related to excessive venous pooling on orthostasis which is caused by excessive venous distension. In response to this reduction in cardiac venous return, increased SNS output occurs, increasing venoconstriction which may be impaired if small fibre neuropathy is present. Additionally, autoantibody-mediated impairment of peripheral vasoconstriction may occur. Tachycardia occurs in response to reduced venous return, in order to maintain cardiac output. Deconditioning can result in smaller left ventricular mass, requiring higher heart rate to maintain cardiac output. Possible reasons for the higher prevalence in women could be smaller heart mass, smaller muscle pump and an overlap with autoimmunity. Figure adapted with permission from www.stopfainting.com
POTS ‘plus’ symptoms—and a link with constitutional immune symptoms
It is increasingly recognized that these haemodynamic abnormalities can be accompanied by multi-system constitutional symptoms which may have a major impact on symptom burden [56, 57]. Patients with POTS have a 5-fold higher symptom severity [58] than those without POTS—and while palpitations may be expected, less specific symptoms of fatigue and concentration difficulties are also prominent [58]. The activity restriction and morbidity associated with orthostatic intolerance syndromes is debilitating, and the non-specific fatigue, post-exertional malaise and cognitive symptoms (‘brain fog’) can be disproportionate to both the initial illness and the haemodynamic findings. Furthermore, those affected most commonly are in young and middle age, when they often have work and caring responsibilities, substantially reducing quality of life, sense of identity and employment.
While the association between these multi-system symptoms and POTS is notable, it is important to accept that there is no conclusive aetiological link identified. Ascribing such symptoms without a clear haemodynamic correlate carries risk of misdiagnosis, or worse, missing a potentially treatable other condition which mimics POTS [59]. This again highlights the need for a systematic clinical approach, and the need for defined research populations.
Support for an autoimmune mechanism in POTS and other forms of stress
There is a compelling association between POTS and autoimmunity: POTS is associated with a higher-than-expected frequency of defined autoimmune disorders. One cohort had positive anti-nuclear antibodies in 25% patients and a concurrent autoimmune diagnosis in 20% [43]. In a UK study, 4% of POTS patients had biopsy-confirmed coeliac disease, compared with 1% of controls, and 42% self-reported gluten sensitivity, compared with 19% of controls [61]. In a case series of 13 patients with confirmed Sjögrens Syndrome (SS), 8 fulfilled criteria for POTS [62] suggesting that if sought, other autoimmune conditions may be found to co-exist with POTS. As such, clinical suspicion and careful evaluation and work up are required. There is also a striking preponderance for POTS in women, with female:male ratios of 4–5:1 [63]. Potential mechanisms are the hormonal effects on vasculature, differences in heart and skeletal muscle size or the fact that oestrogen exposure and concomitant infection can synergistically trigger autoimmunity [60, 64]. Finally, inflammatory, immune and neuroendocrine protein biomarkers are altered in individuals with POTS compared with controls [65–67].
The response to standing in POTS is a form of orthostatic stress. However, other forms of stress can also increase autoimmunity risk: a large population- and sibling-matched retrospective cohort study in Sweden found that exposure to life stressors confers a 36% increased risk of autoimmune disease [68]. If the stress-related disorder is post-traumatic stress disorder, the risk is even higher [68]. As such, the underlying stressors of Covid infection, Long Covid and orthostatic stressors (along with psychosocial stressors related to chronic illness) may confound an association between POTS and autoimmunity. A further factor is the detrimental effect of stress in other immune-mediated conditions, such as cancer [69] and atherosclerosis [70], suggesting that it may be a common underlying factor in development of a wide range of diseases. There are no data examining pre-morbid levels of anxiety or psychological factors prior to onset of Long Covid and POTS but prevalence of anxiety and depression are high in these conditions [71, 72]. This includes altered emotional responsivity during orthostatic stress and increased vigilance towards symptoms [73, 74].
Auto-antibodies in POTS and Long Covid—significance and implications for future study
The association between POTS and autoantibodies is well recognized: auto-antibodies to the α and β receptors on GPCR antibodies, ganglionic acetylcholine receptor antibodies, angiotensin II receptor antibodies and antibodies to structural cardiac proteins have been identified in POTS [14, 27, 75–78]. The presence of autoantibodies can correlate with symptoms such as gastrointestinal disturbance [79], fatigue, muscle pain [80], exercise tolerance and standing time [81]. Antibodies to the α- and β- adrenoceptors may exert effects in POTS by either impaired vasoconstriction (mediated by α-adrenoceptors in the peripheral vasculature) resulting in venous pooling and reduced cardiac venous return) or positive chronotropic effects, exacerbating postural heart rate rise [11].
However, autoantibodies against GPCRs are not novel and they are described across a wide state of conditions in diseases ranging from SS to peripartum cardiomyopathy [82]. Specifically, GPCR antibodies have been found in disorders commonly associated with POTS, including inappropriate sinus tachycardia [83], complex regional pain syndrome [84] and chronic fatigue syndrome [85].
Although there is no causative link currently identified, the presence of autoantibodies is associated with a dysregulation of the immune response and, also, activation of transcription factors that may increase inflammatory responses. It is likely that more will be discovered in relation to both POTS and Long Covid. While the association between POTS and autoimmunity is compelling, understanding of specific mechanisms remains limited and as with many of the autoantibodies above, the specific mechanisms by which these autoantibodies exert effects, and their overall mechanisms, remain unclear. While their presence may reflect an underlying autoimmunity, it is essential to distinguish between pathogenic antibodies, and those antibodies suggesting mere autoimmunity but without a specific pathophysiological effect. This is an essential step in future research before treatment targets are developed.
There is also a need for standardization of antibody detection techniques. Currently, enzyme-linked immunosorbent assay (ELISA) is mostly used to detect human autoantibodies; however, non-specific binding is a well-recognized problem leading to frequent false positive results [86]. In a recent study, there was no difference in concentration of circulating antibodies against the most common cardiovascular GPCRs between POTS patients and controls using standard ELISA methodology [87]. This observation questions the use of ELISA for future explorative studies on POTS and Long Covid-associated dysautonomias and more studies are required for definitive conclusions. Non-specific binding sera contain increased concentrations of IgG and other inflammatory mediators, suggesting that this non-specific finding correlates with the IgG concentration and is therefore indicative of elevated IgG and inflammation [86].
Immunoprecipitation is also commonly used, but this has been difficult to develop for GPCRs. Equally, the requirement for radioisotopes limits its widespread application [88].
To summarize, multiple autoantibodies are identified in relation to both Long Covid and POTS, suggesting a possible link with autoimmunity, but the significance of these is as yet undetermined.
Associations with mast cell activation disorders
A prominent overlapping symptom cluster associated with both POTS [56, 57] and Long Covid [89, 90] includes a multi-system and often non-specific symptom cluster which is often referred to as ‘mast cell activation syndrome’ (and considered to be due to release of mast cell mediators) and includes gastrointestinal disturbances (nausea, vomiting, diarrhoea, cramps), asthma like symptoms, flushing, urticaria, angioedema and constitutional symptoms such as fatigue and fever [91]. Mast cells are found in multiple tissues across a wide range of organs, and are critical in neuro-immune responses, particularly to allergy and inflammation. They contain multiple key pro-inflammatory cytokines as well as allergic and inflammatory mediators such as histamine, heparin and tryptase, which are released on degranulation. Disorders can range from mast cell leukaemia and systemic mastocytosis (aggressive, life-limiting conditions) to the less-specific, more benign and much more common ‘idiopathic MCAS’ where symptoms of mediator release are present, and biochemically confirmed, but without a confirmed mutational or pathological underlying cause [91].
Rigorous characterization studies relating to the relationship between POTS and MCAS are lacking; moreover, there is still controversy around specific criteria for defining mast cell disorders. Different diagnostic consensus criteria exist, which are more [92, 93] and less [91, 94] restrictive. Criteria range from differentiating mutational, pathological or biochemical biomarkers, to more non-specific syndromes characterized by symptom clusters which can be attributed to other conditions. An argument for precise, biomarker-led diagnoses (comprising bone marrow mast cell morphology, mutational analysis and plasma and urinary mast cell mediator levels) ensures precise diagnosis and avoids overdiagnosis of non-specific symptoms clusters and missing important alternative diagnoses, but similarly misses an opportunity to treat empirically a common and debilitating condition [94].
In any case, the prominent co-existence of these symptoms in those with Long Covid and orthostatic intolerance syndromes is notable and should be the focus of rigorous, biomarker-led characterization studies. There is little convincing evidence to date. However, Shibao et al. [95] tested the hypothesis that plasma histamine can cause sympathetic overactivity via vasodilatation and compared POTS patients presenting flushing and raised urinary methylhistamine (a mast cell mediator metabolite) with patients reporting flushing but with normal urinary metabolites. These MCA/POTS patients had predominant flushing breathlessness, headache, lightheadedness, excessive diuresis, diarrhoea, nausea and vomiting, and had a marked hyperadrenergic orthostatic response, with higher noradrenaline levels and exaggerated blood pressure rise on standing compared with the POTS and control groups [95]. In another study of 69 patients with POTS, 64% reported additional non-orthostatic symptoms such as allergic complaints, skin rash or symptoms, and two-thirds of these had elevated prostaglandins and elevated histamine markers [57]. An observational study of individuals recovering from Long Covid found that 72% individuals who received histamine antagonists reported a symptomatic improvement [96]—however, placebo effects must be considered. Finally, emphasis on the lack of generally accepted and available as well as sensitive, and reliable tests for mast cell activation disorders is necessary, which hampers the explorative studies and comparisons between different patient cohorts.
An approach to management
Managing both Long Covid and POTS requires a multi-disciplinary holistic approach to understanding the wide-ranging symptoms and debilitating effects on daily life. A systematic multi-system approach, incorporating education and patient empowerment, as well as acknowledging the uncertainty around pathophysiology and the lack of evidence-based treatments, should be at the centre of the consultation, along with a tailored management plan incorporating established approaches and symptomatic treatments. This is summarized in Table 1.
Table 1.
General approach to management of orthostatic intolerance syndromes, POTS and Long Covid POTS
| Principle | Example |
|---|---|
| Multi-disciplinary team management | Primary and secondary care clinician, specialist nurse, psychologist, physiotherapist, occupational therapist, social worker, social prescriber |
| Patient education and health promotion |
|
| Holistic management incorporating principles of integrative care |
|
| Reducing lower body venous pooling |
|
| Managing reactive adrenergic response to orthostatic intolerance |
|
| Symptomatic management of symptoms suggestive of mast cell activation |
|
Managing orthostatic intolerance centres around reducing lower body venous pooling (expanding plasma volume, compression garments in lower body, especially lower abdomen, and isometric counter-pressure manoeuvres, α-agonist pharmacotherapy) [33], attenuating the reactive adrenergic response (e.g. β-blockers, breathing retraining), and slow cardiovascular and lower body reconditioning programmes, along with strong social and psychological support networks to facilitate managing a new and chronic condition. Pharmacotherapy may be needed to attenuate symptoms. However, there is insufficient evidence of underlying immune targets to consider immuno-therapeutics.
There have been case reports [97] and small open-label clinical studies [98] suggesting a beneficial effect of intravenous immunoglobulin therapy in POTS. While there are no controlled clinical trial data examining this, nor an established target to treat, it again supports a possible role for autoimmunity. However, as described above, the lack of distinct treatment targets and the specific role of autoantibodies being defined suggest that identifying an immunological target will take time to achieve [99].
Finally, in practice, we cautiously consider a trial of mast cell stabilizing medications for symptomatic benefit in patients significantly debilitated by symptoms—including H1 and H2 receptor antagonists and mast cell stabilizing treatment. Obtaining biomarker-led diagnoses including serum and urine metabolites is important, but not always pragmatic in an era of high volume, remote clinics, and we advocate a trial of such medications for a month if clinical suspicion is high. Our experience is that this approach is particularly helpful for individuals with nocturnal symptoms (including palpitations, shortness of breath and dizziness); a possible explanation is spontaneous release of mast cell mediators causing peripheral vasodilation and a reactive adrenergic response. Caution is required to balance benefits of symptomatic treatment with anticholinergic side effects, which can be harmful in older individuals or those with neurodegenerative disorders. Importantly, as before, placebo-effects must also be borne in mind.
Conclusion
The neuro-immune and autonomic nervous systems are increasingly recognized in autonomic dysfunction in Long Covid and cardiovascular autonomic disorders such as POTS. Studies are still in their infancy but as evidence accrues, further links may become apparent. The common associations with autoantibodies are compelling, but non-specific, and antibody tests are not entirely reliable and available. Similarly, many symptoms of immune dysregulation may accompany these disorders and reflect a final common pathway rather than focal or specific pathology—and clinically, these need to be managed pragmatically and sympathetically. Cardiovascular dysautonomic conditions are debilitating and the effects on society are significant. The widespread incidence and prevalence of Long Covid has highlighted the knowledge gaps in POTS research and a dual approach for answers is urgently needed.
Data availability
No data available.
Funding
No funding was required for this work.
Contributor Information
Fatema-Zahra El-Rhermoul, Department of Allergy and Clinical Immunology, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK.
Artur Fedorowski, Department of Cardiology, Karolinska University Hospital and Karolinska Institute, Stockholm 171 77, Sweden.
Philip Eardley, Imperial Syncope Unit, Imperial College Healthcare NHS Trust, London W12 0HS, UK.
Patricia Taraborrelli, Imperial Syncope Unit, Imperial College Healthcare NHS Trust, London W12 0HS, UK.
Dimitrios Panagopoulos, Imperial Syncope Unit, Imperial College Healthcare NHS Trust, London W12 0HS, UK.
Richard Sutton, National Heart and Lung Institute, Imperial College London, London SW3 6LY, UK.
Phang Boon Lim, Imperial Syncope Unit, Imperial College Healthcare NHS Trust, London W12 0HS, UK.
Melanie Dani, Imperial Syncope Unit, Imperial College Healthcare NHS Trust, London W12 0HS, UK; Cutrale Perioperative and Ageing Group, Department of Bioengineering, Imperial College London, London W12 0BZ, UK.
Author contributions
Fatema-Zahra El-Rherhmoul (Writing—original draft [equal]), Artur Fedorowski (Writing—review & editing [equal]), Philip Eardley (Writing—review & editing [supporting]), Patricia Taraborrelli (Writing—review & editing [supporting]), Dimitrios Panagopoulos (Writing—review & editing [supporting]), Richard Sutton (Writing—review & editing [equal]), Phang Boon Lim (Writing—review & editing [equal]), and Melanie Dani (Writing—original draft [equal])
Conflict of interest
The authors do not declare any conflicts of interest.
References
- 1. Kioussis D, Pachnis V.. Immune and nervous systems: more than just a superficial similarity? Immunity, 2009;31:705–10. 10.1016/j.immuni.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 2. Kenney MJ, Ganta CK.. Autonomic nervous system and immune system interactions. Compr Physiol 2014;4:1177–200. 10.1002/cphy.c130051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma D, David Farrar J.. Adrenergic regulation of immune cell function and inflammation. Semin Immunopathol 2020;42:709717. 10.1007/s00281-020-00829-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fujii T, Mashimo M, Moriwaki Y. et al. Expression and function of the cholinergic system in immune cells. Front Immunol 2017;8:1085. 10.3389/fimmu.2017.01085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konig MF, Powell M, Staedtke V. et al. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J Clin Investig 2020;130:3345–7. 10.1172/JCI139642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Staedtke V, Bai RY, Kim K. et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature 2018;564:273–7. 10.1038/s41586-018-0774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Porzionato A, Emmi A, Barbon S. et al. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J 2020;287:3681–8. 10.1111/febs.15481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldstein DS. The extended autonomic system, dyshomeostasis, and COVID-19. Clin Auton Res 2020;30:299–315. 10.1007/s10286-020-00714-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Rio R, Marcus NJ, Inestrosa NC.. Potential role of autonomic dysfunction in Covid-19 morbidity and mortality. Front Physiol 2020;11: 561749. 10.3389/fphys.2020.561749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tornero C, Pastor E, Garzando MDM. et al. Non-invasive vagus nerve stimulation for COVID-19: results from a randomized controlled trial (SAVIOR I). Front Neurol 2022;13:820864. 10.3389/fneur.2022.820864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallukat G, Hohberger B, Wenzel K. et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J Transl Autoimmun 2021;4:100100. 10.1016/j.jtauto.2021.100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gunning iii WT, Kvale H, Kramer PM. et al. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. J Am Heart Assoc 2019;8:e013602. 10.1161/JAHA.119.013602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cabral-Marques O, Halpert G, Schimke LF. et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nat Commun 2022;13:1220. 10.1038/s41467-022-28905-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang EY, Mao T, Klein J. et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021;595:283–8. 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- 15. Fagyas M, Nagy B, Ráduly AP. et al. The majority of severe COVID-19 patients develop anti-cardiac autoantibodies. Geroscience 2022, 10.1007/s11357-022-00649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Acosta-Ampudia Y, Monsalve DM, Rojas M. et al. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J Infect Dis 2022;225:2155–62. 10.1093/infdis/jiac017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phetsouphanh C, Darley DR, Wilson DB. et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022;23:210–6. 10.1038/s41590-021-01113-x [DOI] [PubMed] [Google Scholar]
- 18. Son K, Jamil R, Chowdhury A. et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long-COVID symptoms. Eur Respir J 2023;61:2200970. 10.1183/13993003.00970-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Office of National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 6 May 2022. Office of National Statistics, 2022.
- 20.NICE, RCGP, and SIGN. Covid-19 rapid guideline: managing the long-term effects of COVID-19; 2020. https://www./nice.org.uk/guidance/ng188. [PubMed]
- 21. Bertin D, Kaphan E, Weber S. et al. Persistent IgG anticardiolipin autoantibodies are associated with post-COVID syndrome. Int J Infect Dis 2021;113:23–5. 10.1016/j.ijid.2021.09.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lingel H, Meltendorf S, Billing U. et al. Unique autoantibody prevalence in long-term recovered SARS-CoV-2-infected individuals. J Autoimmun 2021;122:102682. 10.1016/j.jaut.2021.102682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pascolini S, Vannini A, Deleonardi G. et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin Transl Sci 2021;14:502–8. 10.1111/cts.12908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia X, Cao S, Lee AS. et al. Anti-nucleocapsid antibody levels and pulmonary comorbid conditions are linked to post-COVID-19 syndrome. JCI Insight 2022;7:e156713. 10.1172/jci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rojas M, Rodríguez Y, Acosta-Ampudia Y. et al. Autoimmunity is a hallmark of post-COVID syndrome. J Transl Med 2022;20:129. 10.1186/s12967-022-03328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peluso MJ, Mitchell A, Yu Wang C. et al. Low prevalence of interferon-α autoantibodies in people experiencing long COVID 1 symptoms 2. J Infect Dis 2023;227:246–50. 10.1093/infdis/jiac372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szewczykowski C, Mardin C, Lucio M. et al. Long COVID: association of functional autoantibodies against G-protein-coupled receptors with an impaired retinal microcirculation. Int J Mol Sci 2022;23:7209. 10.3390/ijms23137209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Su Y, Yuan D, Chen DG. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022;185:881–895.e20. 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shahabi S, Hassan ZM, Jazani NH. et al. Sympathetic nervous system plays an important role in the relationship between immune mediated diseases. Med Hypotheses 2006;67:900–3. [DOI] [PubMed] [Google Scholar]
- 30. Ortona E., Malorni W. Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur Respir J 2022;59:2102245. 10.1183/13993003.02245-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc 2012;87:1214–25. 10.1016/j.mayocp.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheldon RS, Grubb BP, Olshansky B. et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63. 10.1016/j.hrthm.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dani M, Dirksen A, Taraborrelli P. et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med 2021;21:E63–7. 10.7861/CLINMED.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miglis MG, Seliger J, Shaik R. et al. A case series of cutaneous phosphorylated α-synuclein in long-COVID POTS. Clin Auton Res 2022, 10.1007/s10286-022-00867-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miglis MG, Prieto T, Shaik R. et al. A case report of postural tachycardia syndrome after Covid-19. Clin Auton Res 2020;30:449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raj SR, Arnold AC, Barboi A. et al. Long-COVID postural tachycardia syndrome: an American autonomic society statement. Clin Auton Res 2021;31:365–8. 10.1007/s10286-021-00798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shouman K, Vanichkachorn G, Cheshire WP. et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res 2021;31:385–94. 10.1007/s10286-021-00803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blitshteyn S, Whitelaw S.. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res 2021;69:205–11. 10.1007/s12026-021-09185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drogalis-Kim D, Kramer C, Duran S.. Ongoing dizziness following acute COVID-19 infection: a single center pediatric case series. Pediatrics 2022;150:e2022056860. 10.1542/peds.2022-056860 [DOI] [PubMed] [Google Scholar]
- 40. Jamal SM, Landers DB, Hollenberg SM. et al. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. J Am Coll Cardiol 2022;79:2325–30. 10.1016/j.jacc.2022.03.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buoite Stella A, Furlanis G, Frezza NA. et al. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: a prospective multidomain observational study. J Neurol 2022;269:587–96. 10.1007/s00415-021-10735-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Campen CMC, Rowe PC, Visser FC.. Orthostatic symptoms and reductions in cerebral blood flow in long-haul covid-19 patients: similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Medicina (Lithuania )2022;58:28. 10.3390/medicina58010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus 2015;24:1364–9. 10.1177/0961203315587566 [DOI] [PubMed] [Google Scholar]
- 44. Sutton R. Diagnostic tools to assess dysfunction of autonomic control of the heart corresponding author. J Atr Fibrillation 2020;13:66–73. www.jafib.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaffer F, Ginsberg JP.. An overview of heart rate variability metrics and norms. Front Public Health 2017;5:258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shah B, Kunal S, Bansal A. et al. Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence. Indian Pacing Electrophysiol J 2022;22:70–6. 10.1016/j.ipej.2022.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kurtoğlu E, Afsin A, Aktaş İ. et al. Altered cardiac autonomic function after recovery from COVID-19. Ann Noninvasive Electrocardiol 2022;27:e12916. 10.1111/anec.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maloberti A, Ughi N, Bernasconi DP. et al. Heart rate in patients with SARS-CoV-2 infection: prevalence of high values at discharge and relationship with disease severity. J Clin Med 2021;10:5590. 10.3390/jcm10235590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vanoli J, Marro G, Dell’Oro R. et al. Elevated resting heart rate as independent in-hospital prognostic marker in COVID-19. Cardiol J 2022, 29(2):181-187. 10.5603/CJ.a2022.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mol MBA, Strous MTA, van Osch FHM. et al. Heart-rate-variability (HRV), predicts outcomes in COVID-19. PLoS ONE 2021;16:e0258841. 10.1371/journal.pone.0258841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stute NL, Stickford JL, Province VM. et al. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol 2021;599:4269–85. 10.1113/JP281888#support-information-section [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Freire APCF, Lira FS, von Ah Morano AE. et al. Role of body mass and physical activity in autonomic function modulation on post-COVID-19 condition: an observational subanalysis of fit-COVID study. Int J Environ Res Public Health 2022;19:2457. 10.3390/ijerph19042457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bai T, Zhou D, Yushanjiang F. et al. Alternation of the autonomic nervous system is associated with pulmonary sequelae in patients with COVID-19 after six months of discharge. Front Physiol 2022;12:805925. 10.3389/fphys.2021.805925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fedorowski A, Melander O.. Syndromes of orthostatic intolerance: a hidden danger. J Intern Med 2013;273:322–35. 10.1111/joim.12021 [DOI] [PubMed] [Google Scholar]
- 55. Jardine DL, Wieling W, Brignole M. et al. The pathophysiology of the vasovagal response. Heart Rhythm 2018;15:921–9. 10.1016/j.hrthm.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shaw BH, Stiles LE, Bourne K. et al. The face of postural tachycardia syndrome—insights from a large cross-sectional online community-based survey. J Intern Med 2019;286:438–48. 10.1111/joim.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kohno R, Cannom DS, Olshansky B. et al. Mast cell activation disorder and postural orthostatic tachycardia syndrome: a clinical association. J Am Heart Assoc 2021;10:e0211002. 10.1161/JAHA.121.021002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spahic JM, Hamrefors V, Johansson M. et al. Malmö POTS symptom score: assessing symptom burden in postural orthostatic tachycardia syndrome. J Intern Med 2023;293:91–99. 10.1111/joim.13566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olshansky B, Cannom D, Fedorowski A. et al. Postural orthostatic tachycardia syndrome (POTS): a critical assessment. Prog Cardiovasc Dis 2020;63:263–70. 10.1016/j.pcad.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vernino S, Bourne KM, Stiles LE et al. Postural orthostatic tachycardia syndrome (POTS): state of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Auton Neurosci 2021;235:102828. doi 10.1016/j.autneu.2021.102828 [DOI] [PMC free article] [PubMed]
- 61. Penny HA, Aziz I, Ferrar M. et al. Is there a relationship between gluten sensitivity and postural tachycardia syndrome? Eur J Gastroenterol Hepatol 2016:1383–87, 10.1097/MEG.0000000000000740 [DOI] [PubMed] [Google Scholar]
- 62. Goodman BP, Crepeau A, Dhawan PS. et al. Spectrum of autonomic nervous system impairment in Sjögren syndrome. Neurologist 2017;22:127–30. 10.1097/NRL.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 63. Thieben MJ, Sandroni P, Sletten DM. et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 2007;82:308–13. [DOI] [PubMed] [Google Scholar]
- 64. Molina V, Shoenfeld Y.. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity 2005;38:235–45. 10.1080/08916930500050277. [DOI] [PubMed] [Google Scholar]
- 65. Spahic JM, Ricci F, Aung N. et al. Proconvertase furin is down regulated in postural orthostatic tachycardia syndrome. Front Neurosci 2019;13:301. 10.3389/fnins.2019.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Medic Spahic J, Ricci F, Aung N. et al. Proteomic analysis reveals sex-specific biomarker signature in postural orthostatic tachycardia syndrome. BMC Cardiovasc Disord 2020;20:190. 10.1186/s12872-020-01465-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johansson M, Yan H, Welinder C. et al. Proteomic profiling in postural orthostatic tachycardia syndrome reveals new disease pathways. Sci Rep 2022;12:20051. 10.1038/s41598-022-24729-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Song H, Fang F, Tomasson G. et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA 2018;319:2388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kennedy B, Valdimarsdóttir U, Sundström K. et al. Loss of a parent and the risk of cancer in early life: a nationwide cohort study. Cancer Causes Control 2014;25:499–506. 10.1007/s10552-014-0352-z [DOI] [PubMed] [Google Scholar]
- 70. Steptoe A, Kivimäki M.. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70. 10.1038/nrcardio.2012.45 [DOI] [PubMed] [Google Scholar]
- 71. Premraj L, Kannapadi NV, Briggs J. et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci 2022;434:120162. 10.1016/j.jns.2022.120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Anderson JW, Lambert EA, Sari CI. et al. Cognitive function, health-related quality of life, and symptoms of depression and anxiety sensitivity are impaired in patients with the postural orthostatic tachycardia syndrome (POTS). Front Physiol 2014;5:230. 10.3389/fphys.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Owens AP, Low DA, Critchley HD. et al. Emotional orienting during interoceptive threat in orthostatic intolerance: dysautonomic contributions to psychological symptomatology in the postural tachycardia syndrome and vasovagal syncope. Auton Neurosci 2018;212:42–7. 10.1016/j.autneu.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 74. Owens AP, Low DA, Iodice V. et al. The genesis and presentation of anxiety in disorders of autonomic overexcitation. Auton Neurosci 2017;203:81–7. 10.1016/j.autneu.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 75. Fedorowski A, Li H, Yu X. et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 2017;19:1211–9. 10.1093/europace/euw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Watari M, Nakane S, Mukaino A. et al. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol 2018;5:486–92. 10.1002/acn3.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ruzieh M, Batizy L, Dasa O. et al. The role of autoantibodies in the syndromes of orthostatic intolerance: a systematic review. Scan Cardiovasc J 2017;51:243–7. [DOI] [PubMed] [Google Scholar]
- 78. Li H, Yu X, Liles C. et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc 2014;3:e000755. 10.1161/JAHA.113.000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sunami Y, Sugaya K, Miyakoshi N. et al. Association of autoantibodies to muscarinic acetylcholine receptors with gastrointestinal symptoms and disease severity in patients with postural orthostatic tachycardia syndrome. Immunol Res 2022;70:197–207. 10.1007/s12026-021-09256-7 [DOI] [PubMed] [Google Scholar]
- 80. Freitag H, Szklarski M, Lorenz S. et al. Autoantibodies to vasoregulative G-protein-coupled receptors correlate with symptom severity, autonomic dysfunction and disability in myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Med 2021;10:3675. 10.3390/jcm10163675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kharraziha I, Axelsson J, Ricci F. et al. Serum activity against G protein-coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J Am Heart Assoc 2020;9:e015989. 10.1161/JAHA.120.015989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vernino S, Stiles LE.. Autoimmunity in postural orthostatic tachycardia syndrome: current understanding. Auton Neurosci 2018;215:78–82. [DOI] [PubMed] [Google Scholar]
- 83. Chiale PA, Garro HA, Schmidberg J. et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta adrenergic receptors. Heart Rhythm 2006;3:1182–6. [DOI] [PubMed] [Google Scholar]
- 84. Kohr D, Singh P, Tschernatsch M. et al. Autoimmunity against the β-2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain 2011;152:2690–700. [DOI] [PubMed] [Google Scholar]
- 85. Freitag H, Szklarski M, Lorenz S. et al. Autoantibodies to vasoregulative G-protein-coupled receptors correlate with symptom severity, autonomic dysfunction and disability in myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Med 2021;10:3675. 10.3390/jcm10163675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Güven E, Duus K, Lydolph MC et al.. Non-specific binding in solid phase immunoassays for autoantibodies correlates with inflammation markers. J Immunol Methods 2014;403:26–36. 10.1016/j.jim.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 87. Hall J, Bourne KM, Vernino S. et al. Detection of G protein-coupled receptor autoantibodies in postural orthostatic tachycardia syndrome using standard methodology. Circulation 2022;146:613–22. 10.1161/CIRCULATIONAHA.122.059971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Burbelo PD, Lebovitz EE, Notkins AL.. Luciferase immunoprecipitation systems for measuring antibodies in autoimmune and infectious diseases. Transl Res 2015;165:325–35. 10.1016/j.trsl.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weinstock LB, Brook JB, Walters AS. et al. Mast cell activation symptoms are prevalent in long-COVID. Int J Infect Dis 2021;112:217–26. 10.1016/j.ijid.2021.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wechsler JB, Butuci M, Wong A. et al. Mast cell activation is associated with post-acute COVID-19 syndrome. Allergy: Eur J Allergy Clin Immunol 2022;77:1288–91. 10.1111/all.15188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Molderings GJ, Brettner S, Homann J. et al. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol 2011;4:10. 10.1186/1756-8722-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Akin C, Valent P, Metcalfe DD.. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol 2010;126:1099–103. 10.1016/j.jaci.2010.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Valent P, Akin C, Arock M. et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol 2012;157:215–25. 10.1159/000328760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Afrin LB, Ackerley MB, Bluestein LS. et al. Diagnosis of mast cell activation syndrome: a global “consensus-2.” Diagnosis 2021;8:137–52. 10.1515/dx-2020-0005 [DOI] [PubMed] [Google Scholar]
- 95. Shibao C, Arzubiaga C, Roberts LJ. et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005;45:385–90. 10.1161/01.HYP.0000158259.68614.40 [DOI] [PubMed] [Google Scholar]
- 96. Glynne P, Tahmasebi N, Gant V. et al. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med 2022;70:61–7. 10.1136/jim-2021-002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci 2020;21:100276. 10.1016/j.ensci.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Weinstock LB, Brook JB, Myers TL. et al. Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep 2018;2018:bcr2017221405. 10.1136/bcr-2017-221405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bornstein SR, Voit-Bak K, Donate T. et al. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: is there a role for extracorporeal apheresis? Mol Psychiatry 2022;27:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data available.