Abstract
Background
This retrospective, multicenter study evaluated the effect of pemafibrate treatment on liver function and fibrosis by liver function tests (LFTs) and various fibrotic biomarkers including FibroScan in non-alcoholic fatty liver disease (NAFLD) with hypertriglyceridemia.
Methods
A total of 138 NAFLD patients treated with pemafibrate at three hospitals between September 2018 and April 2021 were included. To evaluate the effect of pemafibrate treatment, FibroScan-aspartate aminotransferase (FAST) score, a novel index of steatohepatitis that can be calculated based on the aspartate aminotransferase (AST) value, controlled attenuation parameter (CAP), and liver stiffness measurement (LSM) was used.
Results
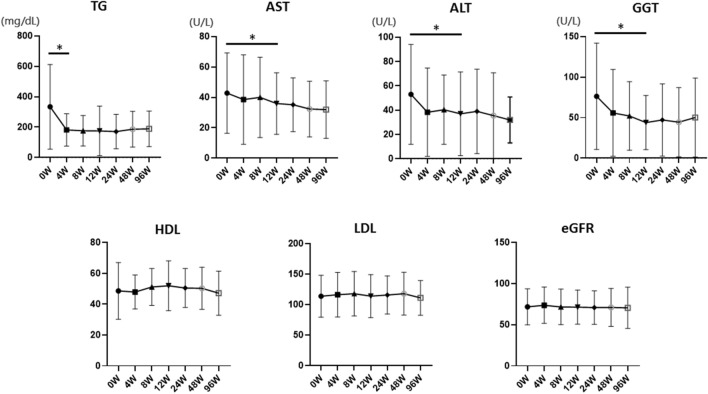
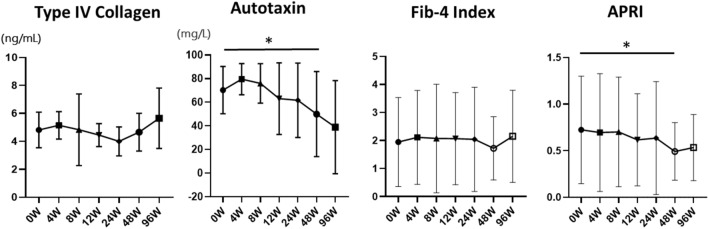
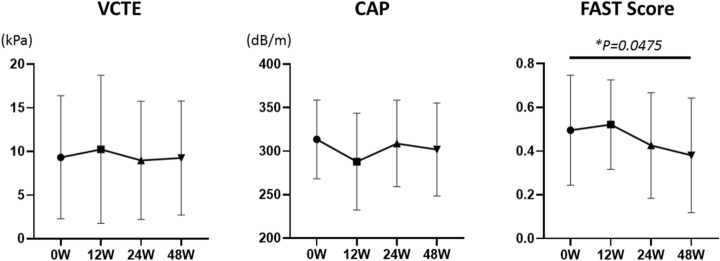
Serum TG levels were significantly decreased 4 weeks after pemafibrate treatment (p = 0.003). The levels of AST (p = 0.038), alanine aminotransferase (ALT) (p = 0.003), and gamma-glutamyl transferase (GGT) (p = 0.047) also significantly diminished 12 weeks after pemafibrate administration compared to before administration (p < 0.05). However, serum HDL-cholesterol (p = 0.193), LDL-cholesterol (p = 0.967), and eGFR (p = 0.909) levels were not significantly altered 12 weeks after pemafibrate administration. In addition, the fibrosis biomarkers’ Type IV collagen (p = 0.753) and FIB-4 index (p = 0.333) did not significantly differ, while Autotaxin (p = 0.006) and the AST-to-platelet ratio index (APRI) (p = 0.003) significantly decreased 48 weeks after pemafibrate administration. No significant reductions in LSM (p = 0.959) and CAP (p = 0.266) were detected using FibroScan 48 weeks after pemafibrate administration. FAST score was significantly improved (p = 0.0475).
Conclusion
Pemafibrate improved LFTs, including fibrotic biomarkers and FAST score, due to the hepatic anti-inflammatory effect, suggesting that pemafibrate may prevent disease progression in NAFLD patients with hypertriglyceridemia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12072-022-10453-1.
Keywords: NAFLD, Hypertriglyceridemia, Pemafibrate, Fibrotic biomarkers, FAST score
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become a common public health concern in recent years [1]. The global prevalence of NAFLD is approximately 25%, and has recently been increasing in the Asia–Pacific region [2]. NAFLD manifests in a broad spectrum of conditions, ranging from non-alcoholic fatty liver to non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma, making it critical to identify its pathogenesis and establish treatment methods [3–5]. To manage NAFLD, various guidelines have been recommended worldwide (European Association for the Study of the et al. 2016; [7, 8]). However, to date, there have been no recommendations on pharmacotherapy for the treatment of NAFLD/NASH.
Peroxisome proliferator-activated receptors (PPARs) include three types (α, δ, and γ) that form a subfamily of the nuclear receptor superfamily [9]. In particular, increased PPARγ expression is observed in patients with NAFLD, with increased triglyceride accumulation and de novo lipid formation in the liver [10]. On the other hand, PPARα is a heterogeneous molecular target that induces peroxisome proliferation [6] and is established as an important lipid regulator [11, 12]. Activated PPARα induces fatty acid uptake, utilization, and catabolism [13] and may improve NAFLD. Therefore, PPARα modulation is increasingly being considered as an important therapeutic molecules for NAFLD [14].
Pemafibrate (K-877; Palmodia® Tablets, Kowa Co., Nagoya, Japan), a selective PPARα modulator, is highly selective for PPARα. This drug was approved for the treatment of hyperlipidemia in July 2017 and launched in Japan in June 2018 [15]. Pemafibrate is characterized by high selectivity and can therefore be used in reduced doses. Ikeda et al. demonstrated that pemafibrate administration during short-time dramatically improves liver function tests (LFTs) for NAFLD patients with hypertriglyceridemia [16]. Although fibrates demonstrated worsening liver and kidney function test values, pemafibrate improved LFTs and did not augment blood creatinine or diminish the estimated glomerular filtration rate (eGFR), significantly. In addition, several reports have recently demonstrated that pemafibrate can recover liver dysfunction in NAFLD [17–21]. However, its efficacy in NAFLD has not yet fully been elucidated.
Therefore, we retrospectively evaluate the efficacy of pemafibrate on LFTs and non-invasive tests in NAFLD patients with hypertriglyceridemia in a multicenter study.
Materials and methods
Study design and protocols
The present study is a multicenter, retrospective, observational study enrolling 266 patients administered pemafibrate from 2018 to 2021 at Kagawa University Hospital, Kagawa Prefectural Central Hospital, or Mitoyo General Hospital. All patients selected for this study were diagnosed as a fatty liver using ultrasonography (US). Patients with chronic hepatitis due to other causes such as hepatitis B virus, hepatitis C virus, autoimmune hepatitis, and primary cholangitis were excluded. Hypertriglyceridemia was diagnosed based on an elevated blood concentration of fasting TG (≥ 150 mg/dL) or non-fasting TG (≥ 175 mg/dL). Patients were prescribed pemafibrate (oral, 0.1 mg, twice a day) and visited the outpatient clinic every 4–12 weeks. The patients also received a biochemical examination to investigate the lipid profile, liver function, and renal function every 4–12 weeks. We carried out transient elastography (FibroScan; ECHOSENS, Paris, France) to examine the liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) at pretreatment, at 12 weeks, 24 weeks, and 48 weeks, since, FibroScan-aspartate aminotransferase (FAST) score was reported to be improved after 48-week pemafibrate administration [19]. Patients who self-discontinued pemafibrate for any reason had a history of drinking (ethanol intake > 20 g/day for female and > 30 g/day for male), or had been taking pemafibrate for a short period (< 1 year) were excluded from the study.
According to the European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice guidelines for the management of non-alcoholic fatty liver disease (European Association for the Study of the et al. 2016), ultimately 138 NAFLD patients (88 male, 50 female) of high-risk fatty liver with metabolic syndrome and increased ALT were selected (Fig. 1). Patients with progressive NASH (bridging fibrosis and cirrhosis) were not confirmed by liver histology in most cases. Patient’s basic characteristics were examined, including sex, age, height, body weight, body mass index (BMI), and information about concomitant medications, which might be effective for NAFLD/NASH: dipeptidyl peptidase-4 (DPP4) inhibitor, metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitor, eicosapentaenoic acid (EPA), statin, ezetimibe, and ursodeoxycholic acid (UDCA). In addition, various fasting laboratory data, such as triglyceride (TG), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), HbA1c, fibrosis based on four factors (FIB-4) index [22], Shah et al. [23]; [24], AST-to-platelet ratio index (APRI) [25, 26], eGFR, Type IV collagen, and autotaxin were examined for this study. In addition, LSM, CAP, and FAST score were also evaluated using transient elastography [19]. Patients were prescribed pemafibrate (oral, 0.1 mg, twice a day) and visited the outpatient clinic every 2–8 weeks. The patients also received a biochemical examination to investigate the lipid profile, liver function, and renal function every 1–2 months. The Common Terminology Criteria for Adverse Events version 5.0 was used to evaluate adverse events (AEs) associated with pemafibrate.
Fig. 1.
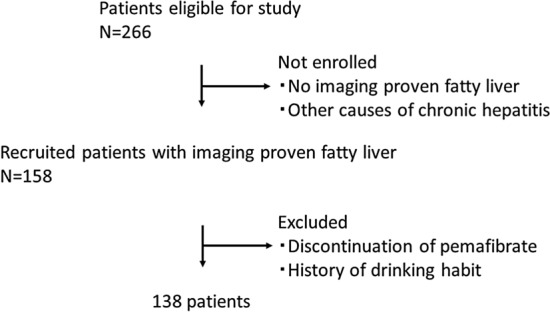
Flowchart of patient selection. Initially, there were 266 eligible patients. 108 patients were not enrolled, because they had not been proven to have fatty liver through imaging. Twenty patients with other causes of chronic hepatitis were also not enrolled. Patients who stopped pemafibrate for any reason and/or with a history of drinking and short duration of using pemafibrate were excluded from the study. Finally, 138 patients were enrolled for this study
Statistical analysis
GraphPad Prism version 8.4.2 (GraphPad Software, San Diego, USA) was used for statistical analyses. Data are presented as count (%), mean ± standard deviation, or median (25–75th). A comparison between the two treatment groups was performed by the chi-square test. The Student’s t test was used to compare numerical data for each group. A value of p < 0.05 was considered significant. Univariate analyses for continuous variables were undertaken using the Student’s t test, paired t test, and Mann–Whitney U test.
Results
Baseline characteristics of the patients
The median age of the patients was 59 years (range, 21–89 years; Table 1). Thirty-four patients had type 2 diabetes (T2DM) (24.6%). All patients were diagnosed as fatty liver using US. Six patients had been diagnosed with NASH by liver biopsy. Pre-pemafibrate treatment mean laboratory values were as follows: TG 387.3 ± 49.6 mg/dL, HDL-cholesterol 48.6 ± 1.6 mg/dL, LDL-cholesterol 113.7 ± 2.9 mg/dL, AST 42.8 ± 2.3 U/L, ALT 53.8 ± 3.6 U/L, GGT 96.2 ± 9.9 U/L, HbA1c 6.5 ± 0.1%, FIB-4 index 1.95 ± 0.18, APRI 0.72 ± 0.05, eGFR 71.8 ± 21.8, Type IV collagen 5.31 ± 2.6, and autotaxin 55.9 ± 33.5. DPP4 antagonist, metformin, SGLT2 inhibitor, EPA, statin, ezetimibe, and UDCA had been already prescribed in 12 (8.7%), 7 (5.1%), 9 (6.5%), 6 (4.3%), 15 (10.9%), 4 (2.9%), and 12 (8.7%) patients, respectively. Duration of pemafibrate administration was 120 (98–138) in 138 patients treated with pemafibrate (Table1) and 113 (101–128) in 60 patients treated with pemafibrate evaluated by Fibroscan (Table2). Pemafibrate was administered to all patients at 0.1 mg twice per day.
Table 1.
Characteristics of the 138 patients treated with pemafibrate
| Male/female | 88/50 | |
| Age (years) | 59 (21–89) | |
| Body height (m) | 163.2 (143–185) | |
| Pre-treatment body weight (kg) | 70 (42.5–109.5) | |
| Pre-treatment BMI (kg/m2) | 26.0 (18.3–40.6) | |
| Comorbidities | ||
| T2DM | 34 | 24.6% |
| Chronic hepatitis B* | 0 | 0% |
| CAD | 8 | 5.8% |
| IBD | 0 | 0% |
| Other | 24 | 17.4% |
| Biopsy-proven NASH | 6 | 4.3% |
| Pre-treatment laboratory values | ||
| TG (mg/dL) | 387.3±49.6 | |
| HDL-cholesterol (mg/dL) | 48.6±1.6 | |
| LDL-cholesterol (mg/dL) | 113.7±2.9 | |
| AST (U/L) | 42.8±2.3 | |
| ALT (U/L) | 53.8±3.6 | |
| GGT (U/L) | 96.2±9.9 | |
| HbA1c (%) | 6.5±0.1 | |
| FIB-4 index | 1.95±0.18 | |
| APRI | 0.72±0.05 | |
| eGFR | 71.8±21.8 | |
| Type IV collagen | 5.31±2.6 | |
| Autotaxin | 55.9±33.5 | |
| Concomitant medications | ||
| DPP4 antagonist | 12 | 8.7% |
| Metformin | 7 | 5.1% |
| SGLT2 inhibitor | 9 | 6.5% |
| EPA | 6 | 4.3% |
| Statin | 15 | 10.9% |
| Ezetimibe | 4 | 2.9% |
| UDCA | 12 | 8.7% |
| Duration of pemafibrate administration (weeks) | 120 (98–138) | |
APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; HbA1c, hemoglobin A1c; CAD, coronary artery disease; CT, computed tomography; DPP4, dipeptidyl peptidase-4; EPA, eicosapentaenoic acid; GERD, gastroesophageal reflux disease; GGT, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; IBD, inflammatory bowel disease; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; SGLT2, sodium-glucose cotransporter 2; T2DM, type 2 diabetes mellitus; TG, triglyceride; UDCA, ursodeoxycholic acid; US‚ ultrasonography
*HBV DNA is controlled under detection by nucleotide analog treatment. aHypertension. Data are expressed as median (range) or mean SEM. Numbers in parentheses refer to the percentage of patients
Table 2.
Characteristics of the 60 patients treated with pemafibrate evaluated by Fibroscan
| Male/female | 36/24 | |
| Age (years) | 57.1 (24–82) | |
| Body height (m) | 163.2 (143–171.8) | |
| Pre-treatment body weight (kg) | 73.5 (53–93.7) | |
| Pre-treatment BMI (kg/m2) | 27.8 (18.3–38.2) | |
| Comorbidities | ||
| T2DM | 23 | 38.3% |
| Chronic hepatitis B | 0 | 0% |
| CAD | 4 | 6.7% |
| IBD | 0 | 0% |
| Other | 10 | 16.7% |
| Biopsy-proven NASH | 2 | 3.3% |
| Pre-treatment laboratory values | ||
| TG (mg/dL) | 272.1±30.7 | |
| HDL-cholesterol (mg/dL) | 48.9±1.6 | |
| LDL-cholesterol (mg/dL) | 120.5±4.7 | |
| AST (U/L) | 52.7±3.9 | |
| ALT (U/L) | 74.3±6.1 | |
| GGT (U/L) | 98.7±11.4 | |
| HbA1c (%) | 6.5±0.2 | |
| FIB-4 index | 2.0±0.2 | |
| APRI | 0.7±0.07 | |
| eGFR (mL/min/1.73m2) | 66.1±14.5 | |
| Type IV collagen (ng/mL) | 4.64±0.9 | |
| Autotaxin (mg/L) | 78.3±11.5 | |
| LSM (kPa) | 9.33±71 | |
| CAP (dB/m) | 318.5±43.3 | |
| FAST Score | 0.45±0.22 | |
| Concomitant medications | ||
| DPP4 antagonist | 11 | 18.3% |
| Metformin | 7 | 11.7% |
| SGLT2 inhibitor | 9 | 15% |
| EPA | 5 | 8.3% |
| Statin | 11 | 18.3% |
| Ezetimibe | 17 | 28.3% |
| UDCA | 11 | 18.3% |
| Duration of pemafibrate administration (weeks) | 113 (101–128) | |
APRI, aspartate aminotransferase to platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; HbA1c, hemoglobin A1c; CAD, coronary artery disease; CT, computed tomography; DPP4, dipeptidyl peptidase-4; EPA, eicosapentaenoic acid; GERD, gastroesophageal reflux disease; GGT, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; IBD, inflammatory bowel disease; LDL, low-density lipoprotein; MRI, magnetic resonance imaging; SGLT2, sodium-glucose cotransporter 2; T2DM, type 2 diabetes mellitus; TG, triglyceride; UDCA, ursodeoxycholic acid; US, ultrasonography
Changes in LFTs and fibrosis markers
Serum TG levels were significantly decreased 4 weeks after pemafibrate treatment (p < 0.05) (Fig. 1). The levels of AST (p = 0.038), ALT (p = 0.003), and GGT (p = 0.047) also significantly diminished 12 weeks after pemafibrate administration (Fig. 2). However, serum HDL-cholesterol (p = 0.193), LDL-cholesterol (p = 0.967), and eGFR (p = 0.909) levels were not significantly altered by pemafibrate treatment (Fig. 1). In addition, as biomarkers of liver fibrosis, Type IV collagen (p = 0.753) and FIB-4 index (p = 0.333) did not significantly differ, while Autotaxin (p = 0.006) and APRI (p = 0.003) significantly decreased 48 weeks after pemafibrate administration (Fig. 3). BMI and HbA1c were not significantly altered by pemafibrate treatment during the follow-up period (Supplementary Fig. 1).
Fig. 2.
Pre- and post-laboratory data of pemafibrate treatment for 96 weeks. Triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and estimated glomerular filtration rate (eGFR) were shown. Data are expressed as mean with standard error of the mean (SEM). *p < 0.05
Fig. 3.
Pre- and post-laboratory data of fibrotic biomarkers on pemafibrate treatment. Type IV collagen, autotaxin, fibrosis based on four factors (FIB-4) index, and aspartate aminotransferase to platelet ratio index (APRI) were shown. Data are expressed as mean with standard error of the mean (SEM). *p < 0.05
Changes in liver steatosis and stiffness
Among the 138 patients, 60 who underwent FibroScan four times within a year were enrolled (Table 2). The median age of these patients was 57 years (range: 24–82 years). Twenty-three patients had type 2 diabetes (T2DM) (38.3%). Two patients were diagnosed with NASH by liver biopsy. The mean laboratory values before pemafibrate administration were as follows: TG 272.1 ± 30.7 mg/dL, HDL-cholesterol 48.9 ± 1.6 mg/dL, LDL-cholesterol 120.5 ± 4.7 mg/dL, AST 52.7 ± 3.9 U/L, ALT 74.3 ± 6.1 U/L, GGT 98.7 ± 11.4 U/L, HbA1c 6.5 ± 0.2%, FIB-4 index 2.0 ± 0.2, APRI 0.7 ± 0.07, eGFR 66.1 ± 14.5 (mL/min/1.73m2), Type IV collagen 4.64 ± 0.9 (ng/mL), Autotaxin 78.3 ± 11.5 (mg/L), LSM 9.33 ± 71 (kPa), CAP 318.5 ± 43.3 (dB/m), and FAST score 0.45 ± 0.22. DPP4 antagonist, metformin, SGLT2 inhibitor, EPA, statin, ezetimibe, and UDCA had been already prescribed in 11 (18.3%), 7 (11.7%), 9 (15%), 5 (8.3%), 11 (18.3%), 17 (28.3%), and 11 (18.3%) patients, respectively.
No significant reductions in LSM and CAP were detected 48 weeks after pemafibrate administration using FibroScan. In contrast, the FAST score was significantly recovered by pemafibrate treatment (p = 0.0475; Fig. 4).
Fig. 4.
Pre- and post-laboratory data of pemafibrate treatment for 48 weeks. Liver stiffness measurement (LSM) and controlled attenuation parameter (CAP), and FibroScan-aspartate aminotransferase (FAST) score were shown. Data are expressed as mean with standard error of the mean (SEM). *p < 0.05
Discussion
In the present study, pemafibrate significantly reduced TG levels in NAFLD with hypertriglyceridemia after 4 weeks of treatment and improved liver dysfunction after 12 weeks of treatment. Surprisingly, 48 weeks of treatment also significantly improved hepatic inflammation and fibrosis markers, as well as hepatic fibrosis in fibroscan. To our knowledge, this is the first multicenter report of a potential effect of pemafibrate on NAFLD patients.
To determine the effect of pemafibrate, we examined if hepatic dysfunction was improved and hepatic fibrosis was prevented by pemafibrate administration in NAFLD patients with hypertriglyceridemia. Previously, several studies have shown the effectiveness of pemafibrate on LFTs, supporting our results [17–21]. Several prospective studies reported that pemafibrate significantly reduced ALT, GGT, and TG levels and increased HDL-cholesterol levels in 20 NAFLD patients in a 12-week single-arm prospective study [21]. They also showed that BMI and insulin resistance were not related to changes in ALT levels. In contrast, a 3-month retrospective observational study of 38 NAFLD patients revealed that pemafibrate significantly decreased ALT, GGT, and TG levels and NAFLD fibrosis score [27] and increased HDL-cholesterol levels [17]. This supports our findings of lower TG levels after 4 weeks and significant reductions in ALT and GGT levels after 12 weeks. Furthermore, in ten biopsy-proven NASH patients treated with pemafibrate, LFTs were significantly improved, especially in NASH patients with high activity and advanced fibrosis [18]. Interestingly, 31 patients with NAFLD treated with pemafibrate and observed for 48 weeks demonstrated improved FAST scores [28] determined by the LSM obtained using vibration-controlled transient elastography (VCTE), estimation of the CAP obtained using a FibroScan device, and estimation of the AST level. The FAST score is expected to reduce unnecessary liver biopsies performed for patients unlikely to have significant disease [19, 28]. In the present study, LSM and CAP did not change significantly, but FAST scores decreased significantly before and 48 weeks after pemafibrate treatment; FAST scores were significantly lower before and 48 weeks after pemafibrate treatment, as measured by the FibroScan test, a comprehensive indicator of liver fibrosis in patients with NASH, which includes (1) liver stiffness, (2) CAP, and (3) AST, and, therefore, may have been the only significant difference. In our present study, FAST score, Autotaxin, and APRI were significantly diminished 48 weeks after pemafibrate administration (Fig. 3, 4). Lee et al. demonstrated that FAST score and APRI were significantly involved in the degree of fibrosis and steatosis [29]. This report supports our data that pemafibrate might improve liver function and fibrosis.
Another recent randomized trial [20] of 118 patients with MRI-proven NAFLD over 72 weeks demonstrated that pemafibrate treatment improved ALT, GGT, and ALP levels as well as cirrhosis. In this randomized controlled trial, there was no baseline statistical difference in liver fat mass; however, fat mass was reduced in the pemafibrate-treated group at 72 weeks. Reports of pemafibrate efficacy against hepatic lipidosis are inconsistent [30, 31]. However, based on the mechanism of pemafibrate, fat mass should be decreased by activating PPARα, and further investigation in humans is warranted. In the present study, Autotaxin and APRI were significantly reduced; APRI included the platelet count. A prospective study by Seko et al. [21] also showed a significant increase in platelet counts, which supports our data. Therefore, platelets might act as critical roles on hemostasis, wound-healing resolution of inflammation, the hepatitis process, and the progression from simple lipemia to NASH [32–34]. Therefore, increased platelet count may be the result of the disappearance of liver inflammation and also explains the significant reduction of APRI.
Autotaxin is highly expressed in the adipose tissue and has been implicated in diet-induced obesity and glucose homeostasis with multiple implications in metabolic disorders. In addition, lysophosphatidic acid (LPA) catalyzed by Autotaxin has been shown to influence stromal and immune cells [35]. Therefore, LPA participates in many processes that are intricately associated with the pathogenesis of different chronic inflammatory diseases. In addition, enhanced expression of Autotaxin has been detected systemically in patients with chronic inflammatory diseases, including chronic liver diseases [35]. Additionally, Autotaxin is degraded by hepatic sinusoidal endothelial cells [36]. Serum Autotaxin levels have been reported to increase in various diseases including certain types of cancer [37–40]. The main mechanism is thought to be the delayed degradation of serum Autotaxin due to the dysfunction of hepatic sinusoidal endothelial cells caused by liver fibrosis [41]. Recently, a correlation between serum Autotaxin concentration and histological severity has been reported in patients with advanced NAFLD [42]. Our data in the present study showed a significant decrease in Autotaxin levels after 48 weeks of pemafibrate treatment, which suggests amelioration of hepatic fibrosis. Further studies using histological analyses may reveal the efficacy of pemafibrate.
Our study was a retrospective, observational study. Selection bias could not be avoided owing to the enrollment method, which registered only patients diagnosed as NAFLD using imaging data. The lack of a control group is another limitation of current study. In addition, the severity of fibrosis which is the recommendation of giving pharmacological treatment to patient with NAFLD (European Association for the Study of the et al. 2016) was not assessed in most cases. This is also the limitation of this study. Liver biopsy is the best diagnostic method for detecting liver fibrosis. However, liver biopsy has several disadvantages including various complications [8]. Non-invasive tools, including scoring systems and various fibrotic biomarkers for assessing fibrosis, have recently been used instead of liver biopsy [43]. The results of the present study are supported by those of previous reports [17–19, 21].
DPP4 antagonists, metformin, and SGLT2 inhibitors, and thiazolidinediones have been demonstrated to have favorable effects on NAFLD in T2DM patients [44, 45]. In the present study, there were 11, 7, and 9 patients who had already taken DPP4 antagonists, metformin, and SGLT2 inhibitors before pemafibrate treatment. No patient had been prescribed thiazolidinediones in this study. No significant differences were detected in the LFTs considering DPP4 antagonists and SGLT2 inhibitors. The patients who had already been treated with DDP4 antagonists and SGLT2 inhibitors before pemafibrate treatment may not have shown differences in LFTs, as their diabetic status had already improved and stabilized. To determine this result, we also compared the pre- and post-HbA1c between with and without DPP4 antagonists or SGLT2 inhibitors. However, no differences were detected between pre-HbA1c (with vs. without DPP4 antagonists or SGLT-2 inhibitors) and post-HbA1c (with vs. without DPP4 antagonists or SGLT2 inhibitors) (data not shown).
As for the drugs for dyslipidemia, several studies have demonstrated the efficacy of β-Hydroxy β-methylglutaryl-CoA reductase inhibitors (statins) on NAFLD/NASH patients [46–49]. Recently, statins are recommended for NAFLD/NASH patients with hypercholesterolemia in the new guidelines of Japan [8]. Statins can ameliorate LFTs; however, consistent histological improvements are still controversial. Pemafibrate is the first fibrate that can be used safely in combination with a statin. In this study, six patients had been prescribed a statin. However, there were no significant differences in the LFTs considering drugs for dyslipidemia, including statins. UDCA is not recommended in the guidelines [8]. Eleven patients had taken UDCA; however, there were no significant differences in the LFTs considering UDCA administration. Furthermore, EPA and ezetimibe were concomitantly used in some participated patients. Previous studies demonstrated that these drugs possibly improve the NAFLD [30–32]. Therefore, their therapeutic efficacy might affect our present results. Accumulating evidences including our study have gradually revealed that pemafibrate can improve liver dysfunction and liver fibrosis in NAFLD patients with hypertriglyceridemia after pemafibrate treatment.
Conclusions
We demonstrated that pemafibrate improved liver dysfunction assessed by LFTs and liver stiffness evaluated by various fibrotic biomarkers including FibroScan in patients with NAFLD/NASH with hypertriglyceridemia. Therefore, pemafibrate might be a first standard medication for NAFLD.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Pre and post laboratory data of pemafibrate treatment for 96 weeks. Body Mass Index (BMI) and HbA1c were shown. Data are expressed as mean with standard error of the mean (SEM). *p < 0.05. (TIF 116 KB)
Acknowledgements
We thank Ms. Hirose and Ms. Kokado for assistance of data collection.
Author contributions
AM designed the concept of the present study. AM, KO, KT, MN, TT, KF, JT, ST, TH, MT, AM, TS, and AT performed data analyzes and AM wrote the draft of the manuscript, and KT, MO, and TM reviewed it. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Data availability
The data used in the present study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Asahiro Morishita, Kyoko Oura, Kei Takuma, Mai Nakahara, Tomoko Tadokoro, Koji Fujita, Joji Tani, Tingting Shi, Takashi Himoto, Miwa Tatsuta, Akio Moriya, Tomonori Senoo, Akemi Tsutsui, Takuya Nagano, Koichi Takaguchi, Masafumi Ono, and Tsutomu Masaki declare no conflict of interest.
Ethical approval
The present study conformed to the Clinical Research Guidelines and was approved by the ethical committee of Kagawa University, Faculty of Medicine (2019–269).
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marjot T, Moolla A, Cobbold JF, Hodson L, Tomlinson JW. Nonalcoholic fatty liver disease in adults: current concepts in etiology, outcomes, and management. Endocr Rev. 2020;41(1):66. doi: 10.1210/endrev/bnz009. [DOI] [PubMed] [Google Scholar]
- 2.Yip TC, Vilar-Gomez E, Petta S, Yilmaz Y, Wong GL, Adams LA, et al. Geographical similarity and differences in the burden and genetic predisposition of NAFLD. Hepatology. 2022 doi: 10.1002/hep.32774. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–157. doi: 10.1016/j.pharmthera.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):81–84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 8.Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol. 2021;56(11):951–963. doi: 10.1007/s00535-021-01796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 10.George J, Liddle C. Nonalcoholic fatty liver disease: pathogenesis and potential for nuclear receptors as therapeutic targets. Mol Pharm. 2008;5(1):49–59. doi: 10.1021/mp700110z. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Xiao G, Trujillo C, Chang V, Blanco L, Joseph SB, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) influences substrate utilization for hepatic glucose production. J Biol Chem. 2002;277(52):50237–50244. doi: 10.1074/jbc.M201208200. [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersten S. Integrated physiology and systems biology of PPARalpha. Mol Metab. 2014;3(4):354–371. doi: 10.1016/j.molmet.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita S, Masuda D, Matsuzawa Y. Clinical applications of a novel selective PPARalpha modulator, pemafibrate, in dyslipidemia and metabolic diseases. J Atheroscler Thromb. 2019;26(5):389–402. doi: 10.5551/jat.48918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda S, Sugihara T, Hoshino Y, Matsuki Y, Nagahara T, Okano JI, et al. Pemafibrate dramatically ameliorated the values of liver function tests and fibrosis marker in patients with non-alcoholic fatty liver disease. Yonago Acta Med. 2020;63(3):188–197. doi: 10.33160/yam.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinozaki S, Tahara T, Lefor AK, Ogura M. Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin Exp Hepatol. 2020;6(3):270–274. doi: 10.5114/ceh.2020.99528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatanaka T, Kakizaki S, Saito N, Nakano Y, Nakano S, Hazama Y, et al. Impact of pemafibrate in patients with hypertriglyceridemia and metabolic dysfunction-associated fatty liver disease pathologically diagnosed with non-alcoholic steatohepatitis: a retrospective. Single-Arm Study Intern Med. 2021;60(14):2167–2174. doi: 10.2169/internalmedicine.6574-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatanaka T, Kosone T, Saito N, Takakusagi S, Tojima H, Naganuma A, et al. Effect of 48-week pemafibrate on non-alcoholic fatty liver disease with hypertriglyceridemia, as evaluated by the FibroScan-aspartate aminotransferase score. JGH Open. 2021;5(10):1183–1189. doi: 10.1002/jgh3.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, et al. Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54(10):1263–1277. doi: 10.1111/apt.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seko Y, Yamaguchi K, Umemura A, Yano K, Takahashi A, Okishio S, et al. Effect of pemafibrate on fatty acid levels and liver enzymes in non-alcoholic fatty liver disease patients with dyslipidemia: a single-arm, pilot study. Hepatol Res. 2020;50(12):1328–1336. doi: 10.1111/hepr.13571. [DOI] [PubMed] [Google Scholar]
- 22.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 23.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, Nash Clinical Research N Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 25.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 26.Kruger FC, Daniels CR, Kidd M, Swart G, Brundyn K, van Rensburg C, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011;101(7):477–480. [PubMed] [Google Scholar]
- 27.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 28.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5(4):362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, et al. Comparison of FibroScan-aspartate aminotransferase (FAST) score and other non-invasive surrogates in predicting high-risk non-alcoholic steatohepatitis criteria. Front Med (Lausanne) 2022;9:869190. doi: 10.3389/fmed.2022.869190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda Y, Kessoku T, Ogawa Y, Tomeno W, Imajo K, Fujita K, et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep. 2017;7:42477. doi: 10.1038/srep42477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki Y, Asahiyama M, Tanaka T, Yamamoto S, Murakami K, Kamiya W, et al. Pemafibrate, a selective PPARalpha modulator, prevents non-alcoholic steatohepatitis development without reducing the hepatic triglyceride content. Sci Rep. 2020;10(1):7818. doi: 10.1038/s41598-020-64902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margraf A, Zarbock A. Platelets in Inflammation and Resolution. J Immunol. 2019;203(9):2357–2367. doi: 10.4049/jimmunol.1900899. [DOI] [PubMed] [Google Scholar]
- 33.Lisman T, Luyendyk JP. Platelets as modulators of liver diseases. Semin Thromb Hemost. 2018;44(2):114–125. doi: 10.1055/s-0037-1604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurokawa T, Ohkohchi N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol. 2017;23(18):3228–3239. doi: 10.3748/wjg.v23.i18.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magkrioti C, Galaris A, Kanellopoulou P, Stylianaki EA, Kaffe E, Aidinis V. Autotaxin and chronic inflammatory diseases. J Autoimmun. 2019;104:102327. doi: 10.1016/j.jaut.2019.102327. [DOI] [PubMed] [Google Scholar]
- 36.Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284(2):216–221. doi: 10.1016/j.canlet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, et al. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47(5):566–574. doi: 10.1165/rcmb.2012-0004OC. [DOI] [PubMed] [Google Scholar]
- 38.Masuda A, Fujii T, Iwasawa Y, Nakamura K, Ohkawa R, Igarashi K, et al. Serum autotaxin measurements in pregnant women: application for the differentiation of normal pregnancy and pregnancy-induced hypertension. Clin Chim Acta. 2011;412(21–22):1944–1950. doi: 10.1016/j.cca.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 39.Nakai Y, Ikeda H, Nakamura K, Kume Y, Fujishiro M, Sasahira N, et al. Specific increase in serum autotaxin activity in patients with pancreatic cancer. Clin Biochem. 2011;44(8–9):576–581. doi: 10.1016/j.clinbiochem.2011.03.128. [DOI] [PubMed] [Google Scholar]
- 40.Xu A, Ahsanul Kabir Khan M, Chen F, Zhong Z, Chen HC, Song Y. Overexpression of autotaxin is associated with human renal cell carcinoma and bladder carcinoma and their progression. Med Oncol. 2016;33(11):131. doi: 10.1007/s12032-016-0836-7. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda H, Yatomi Y. Autotaxin in liver fibrosis. Clin Chim Acta. 2012;413(23–24):1817–1821. doi: 10.1016/j.cca.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Fujimori N, Umemura T, Kimura T, Tanaka N, Sugiura A, Yamazaki T, et al. Serum autotaxin levels are correlated with hepatic fibrosis and ballooning in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2018;24(11):1239–1249. doi: 10.3748/wjg.v24.i11.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Younes R, Bugianesi E. NASH in Lean Individuals. Semin Liver Dis. 2019;39(1):86–95. doi: 10.1055/s-0038-1677517. [DOI] [PubMed] [Google Scholar]
- 44.Scheen AJ. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: a common comorbidity associated with severe complications. Diabetes Metab. 2019;45(3):213–223. doi: 10.1016/j.diabet.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Blazina I, Selph S. Diabetes drugs for nonalcoholic fatty liver disease: a systematic review. Syst Rev. 2019;8(1):295. doi: 10.1186/s13643-019-1200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyogo H, Ikegami T, Tokushige K, Hashimoto E, Inui K, Matsuzaki Y, et al. Efficacy of pitavastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatol Res. 2011;41(11):1057–1065. doi: 10.1111/j.1872-034X.2011.00849.x. [DOI] [PubMed] [Google Scholar]
- 47.Nakahara T, Hyogo H, Kimura Y, Ishitobi T, Arihiro K, Aikata H, et al. Efficacy of rosuvastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatol Res. 2012;42(11):1065–1072. doi: 10.1111/j.1872-034X.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 48.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Kargiotis K, Athyros VG, Giouleme O, Katsiki N, Katsiki E, Anagnostis P, et al. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21(25):7860–7868. doi: 10.3748/wjg.v21.i25.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Pre and post laboratory data of pemafibrate treatment for 96 weeks. Body Mass Index (BMI) and HbA1c were shown. Data are expressed as mean with standard error of the mean (SEM). *p < 0.05. (TIF 116 KB)
Data Availability Statement
The data used in the present study are available from the corresponding author upon reasonable request.