Abstract
The identification of a blood marker of brain pathology that is sensitive to substance-induced neurotoxicity and dynamically responds to longitudinal changes in substance intake would substantially improve clinical monitoring in the field of substance use and addiction. Here, we explored the hypothesis that plasma levels of neurofilament light chain (NfL), a promising marker of neuroaxonal pathology, are elevated in chronic cocaine users and longitudinally associated with changes in cocaine use. Plasma NfL levels were determined using single molecule array (SIMOA) technology at baseline and at a 4-month follow-up. Substance use was subjectively assessed with an extensive interview and objectively measured via toxicological analysis of urine and 4-month hair samples. In a generalized linear model corrected for sex, age, and body mass index, NfL plasma levels were elevated in cocaine users (n=35) compared to stimulant-naïve healthy controls (n=35). A positive correlation between cocaine hair concentration and NfL levels was also found. Changes in cocaine hair concentration (group analysis of increasers vs. decreasers) over the 4-month interval predicted NfL levels at follow-up, indicating a rise in NfL with increased cocaine use and a reduction with decreased use. No associations between use or change of use of other substances (including the neurotoxic cocaine adulterant levamisole) and NfL levels were found. Our findings demonstrate that NfL is a sensitive marker for assessing cocaine-related neuroaxonal pathology, supporting the utility of blood NfL analysis in addiction research but also suggesting the detailed assessment of substance use in neurological studies and diagnostics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12035-023-03327-6.
Keywords: Neurofilament light chain, Cocaine, Neuropsychopharmacology, Neurotoxicity, Biological psychiatry
Introduction
Chronic cocaine use has been consistently associated with a range of structural brain alterations, including volumetric changes in cortical and subcortical regions and disruption of white matter tracts [1–5]. Although some recent longitudinal studies have suggested that neuroanatomical alterations in fronto-cortical areas covary with changes of cocaine consumption [6, 7], most of the findings are limited to cross-sectional observations. Thus, the extent to which these structural impairments are directly related to cocaine-induced brain pathology (neurotoxicity) rather than preexisting traits (predisposition) remains unclear [8, 9]. Moreover, cocaine adulteration with neurotoxic compounds (e.g., the anthelmintic levamisole) [10, 11], concomitant use of other illicit substances or alcohol [12], and comorbidity with psychiatric disorders (e.g., depression) [12–15] might influence brain integrity in chronic cocaine users (CU) independently of cocaine intake itself. Considering the limitations of current diagnostic methods, the introduction of new markers of active brain pathology may help clarify crucial questions on substance-induced brain pathology and provide new monitoring tools for physicians working in addiction medicine [16].
Neurofilament light chain (NfL) is a cytoskeletal protein that is released in extracellular matrices during neuroaxonal damage [17]. Since the recent introduction of highly sensitive assay methods, NfL has emerged as the most promising blood marker of neuroaxonal pathologies for clinical use [18]. In particular, NfL levels have been shown to reflect active brain disease, dynamically respond to therapeutic interventions, and offer prognostic information in a number of neuropsychiatric disorders, such as multiple sclerosis, neurodegenerative disorders, traumatic brain injuries, and depression [14, 19, 20]. Our recent work also reported elevated NfL levels in patients with chronic ketamine addiction, demonstrating its sensitivity to substance-related brain pathology [13]. However, NfL levels in chronic MDMA (“ecstasy”) users did not differ from levels in healthy participants [21]. The lack of white matter pathology in MDMA users from the same sample was also confirmed by diffusion tensor imaging measures.
Although cocaine use has been associated with several markers of brain pathology, this is the first study to investigate its impact on NfL levels. We compared the NfL levels of a sample of 35 CU with 35 healthy controls (HC) at baseline. Additionally, we investigated the dose–response relationship between NfL concentrations and cocaine consumption at baseline. Finally, we evaluated longitudinal changes in NfL levels in relation to objectively confirmed changes in cocaine consumption across a 4-month follow-up period as determined by hair testing. Based on previous cross-sectional findings linking structural brain alterations with chronic cocaine use [1–4], we hypothesized that NfL levels would be elevated in CU at baseline in a dose-dependent manner. Moreover, we expected that NfL levels at follow-up would be associated with changes in cocaine consumption during the 4-month interval, as previously shown for anatomical and cognitive measures in cocaine user populations [7, 22, 23]. Finally, we expected that levamisole (a common adulterant of cocaine) would have an additional impact on NfL levels, given its proven neurotoxic effects on white and gray matter [10, 11, 24, 25].
The identification of a blood marker of active brain pathology that is sensitive to substance-induced neurotoxicity and dynamically responds to longitudinal changes in substance intake would substantially improve clinical monitoring in the field of substance use and addiction. Moreover, considering the increasing attention NfL analysis is receiving in neurology and psychiatry, the identification of variables that confound NfL levels is crucial for its use as a marker in clinical settings.
Methods
Participants
The data were collected in the context of the Social Stress Cocaine Study (SSCP) at the Psychiatric Hospital of the University of Zurich.[15, 26] The general exclusion criteria were a family history of genetically transmitted psychiatric disorders (h2>0.5, e.g., autism, schizophrenia, or bipolar disorder); any severe neurological disorder or brain injury; a current diagnosis of infectious disease or severe somatic disorder; a history of autoimmune, endocrine, or rheumatoid arthritis; intake of medication with potential action on the central nervous system or the physiological stress system during the previous 72h; participation in a large previous study conducted by our lab, the Zurich Cocaine Cognition Study [7, 10]; and (for women) being pregnant or breastfeeding. The criteria for inclusion of CU in the study were cocaine as the primary substance of use; a lifetime cumulative consumption of at least 100 g of cocaine, estimated by self-report; and a current abstinence duration of <6 months. We also excluded CU who regularly used illegal substances other than cocaine, such as heroin or other opioids (with the exception of irregular cannabis use); those with a polysubstance use pattern according to DSM-IV-TR; and those with a DSM-IV-TR axis I adult psychiatric disorder diagnosis other than cocaine, cannabis, or alcohol abuse or dependence, previous depressive episodes, and attention deficit hyperactivity disorder (ADHD). Stimulant-naïve HC were matched for sex. The exclusion criteria for HC were a DSM-IV-TR axis I adult psychiatric disorder or recurrent illegal substance use (>15 occasions in the lifetime, with the exception of irregular cannabis use).
For the current investigation, a sample of 37 CU and 39 HC was initially available. However, in accordance with our exclusion criteria, two CU were retrospectively excluded because of polytoxic substance use patterns and low cocaine consumption, confirmed by toxicological hair analysis. Four HC were also excluded because of positive hair toxicology for cocaine or other substances according to published thresholds [27], which is in line with recent prevalence rates among young adults in the Zurich area [28]. Therefore, a sample of 35 CU and 35 HC with baseline data was considered for the final analyses.
Assessments
Procedure
The test procedure was similar at baseline (T1) and follow-up (T2) visits. The interval between T1 and T2 was approximately 4 months (number of days between T1 and T2, mean±SD: HC=135±19; CU=169±71). Both visits included a clinical and substance-related assessment, blood sampling for NfL analysis, and toxicological analysis of urine and hair.
Clinical and Substance Use Assessment
The psychopathological assessment was carried out by trained psychologists using the Structured Clinical Interview I (SCID-I) in accordance with DSM-IV-TR [29]. Depressive symptoms were assessed using the Beck Depression Inventory (BDI) [30]. The Childhood Trauma Questionnaire (CTQ) was used to screen for traumatic childhood experiences [31]. Symptoms of ADHD were assessed using the ADHD self-rating scale (ADHD-SR) [32]. Self-reported substance use was assessed with the structured and standardized Interview for Psychotropic Drug Consumption (IPDC) [33].
Neurofilament Light Chain Analysis
Plasma NfL levels were measured using single-molecule array (SIMOA) technology at the University Hospital Basel. Blood was collected in EDTA tubes and directly centrifuged for 15 min at 1500 g at room temperature. All plasma samples were frozen and stored at −80°C. All intra-assay coefficients of variation of duplicate determinations obtained by SIMOA analysis were below 15%. The mean coefficient of variation was 4.78% in the CU group and 6.25% in the HC group. To minimize the possibility of technical issues or batch effects, T1 and T2 samples were analyzed together at the end of the study.
Urine and Hair Toxicological Analysis
Urine analyses using a semi-quantitative enzyme multiplied immunoassay method targeted the following substances: amphetamines, barbiturates, benzodiazepines, cocaine, methadone, morphine-related opiates, and delta-9-tetrahydrocannabinol. In addition, quantitative analysis of hair samples using liquid chromatography tandem mass spectrometry (LC-MS/MS) was conducted to investigate substance consumption over the previous 4 months, as represented in the proximal 4 cm-segment of the hair samples taken from the occiput. In total, 88 substances and substance metabolites were assessed [34]. The parameter cocainetotal (= Cocaine + Benzoylecgonine + Norcocaine) was calculated. Together with the corresponding metabolic ratios, cocainetotal offers a robust procedure for discriminating between incorporation and contamination of hair [35].
Statistical Analysis
Preliminary Analysis
All statistical analyses were computed with SPSS version 25 (IBM Corp., SPSS Inc., Chicago, IL, USA) and R-Studio (Version 3.6.1). Quantitative variables were tested for normal distribution using the Kolmogorov–Smirnov test. Between-group comparisons (CU/HC) of sociodemographic and clinical data and substance use variables were performed using Student’s t-tests (for normally distributed quantitative data), Mann–Whitney U tests (for non-normally distributed quantitative data), or Pearson’s chi-square test (for categorical data). The significance level was set at p<0.05 (two-tailed).
Effects of Cocaine Intake on NfL Levels at Baseline
As a first step, we performed a generalized linear model (GLM) with Gaussian distribution and log link function to test group effects on NfL levels. We included group (CU/HC) and sex (female/male) as fixed factors and age and BMI as further covariates. The model selection was based on lognormal distribution of NfL levels. Age was included due to the strong link between advancing age and increased NfL levels [36], and BMI was included due to its potential confounding effects on NfL levels [36]. Sex was included to correct for potential sex-specific effects of cocaine use on brain integrity [3]. As a second step, Spearman’s correlation coefficients were calculated to investigate the dose–response relationships between substance use and NfL levels. Hair concentrations of substances and self-reported substance use variables were used for the correlation analysis.
Longitudinal Effects of Changes in Cocaine Use on NfL Levels at Follow-up
At follow-up, CU were characterized as either increasers (participants who elevated their cocaine use between T1 and T2) or decreasers (participants who reduced their cocaine use in the same period). The assignment criteria were based on absolute changes in cocaine concentration in toxicological hair analysis between T1 and T2. The effect of changes in cocaine consumption on NfL levels at follow-up was analyzed using a GLM with Gaussian distribution and log link function. We included group (increasers-/decreasers) and sex (female/male) as fixed factors and age, BMI, and NfL levels at baseline as further covariates. As a second step, Spearman’s correlation coefficients were calculated to investigate dose–response effects of changes in substance use on changes in NfL levels. For this analysis, Δ values were calculated to define changes in objective variables of illicit substance use and self-reported alcohol intake (i.e., ΔCOCAINE=[cocaine concentration in hair at T2]–[cocaine concentration in hair at T1]) and NfL (ΔNfL=[NfL level at T2]–[NfL level at T1]). Extreme outliers were detected using the 3xIQR detection rule on ΔNfL values and excluded from the longitudinal analysis. We applied the 3xIQR detection rule, as it is a conservative and robust method for small samples with symmetric distributions [37]. Data from participants, who did not take part to the T2 visit, were excluded from longitudinal analysis.
Results
Sociodemographic and Clinical Data and Substance Use Patterns
The sociodemographic and clinical data of the 35 CU and 35 HC are summarized in Table 1. Age and BMI score were both higher in CU compared to HC and were therefore included as cofactors in the GLM analysis investigating group effect on NfL values. We found that CU had fewer years of school than HC, which is in line with findings in other cocaine user populations [38]. As expected, CU scored significantly higher in the BDI and ADHD-SR and had a higher frequency of major depressive disorder (MDD) diagnosis than HC (according to DSM criteria) [38]. Substance use patterns are summarized in the supplementary materials (Table S1). In CU, 4/35 subjects reported regular intake of antidepressants (SSRI=2, SNRI=2), 3/35 subjects reported regular intake of low-dose, antipsychotics for anxiety/agitation (quetiapine=2, chlorprothixene=1), and 2/35 subjects reported regular intake of ADHD medications (atomoxetine=1, methylphenidate=1). No use of psychoactive medications was reported in HC. All participants were asked to abstain from psychoactive medications 72h before the visits.
Table 1.
Sociodemographic, clinical characteristics, and NFL levels at baseline
| Variables | Cocaine users (N=35) | Healthy controls (N=35) | Test statistics | df | P value |
|---|---|---|---|---|---|
| Age, years | 33.3 ± 7.3 | 29.49 ± 7.1 | U=397.5 | - | 0.011 |
| Sex (f/m) | 11/24 | 13/22 | x2=0.25 | 1 | 0.802 |
| BMI, kg/m2 | 25.2 ± 4.0 | 23.3 ± 3.2 | t=2.20 | 68 | 0.032 |
| School education, years | 9.6 ± 1.1 | 10.5 ± 1.5 | U=450.5 | - | 0.031 |
| Family psychiatric history (yes/no) | 3/32 | 0/35 | x2=3.13 | 1 | 0.239 |
| ADHD_SR, score | 15.0 ± 10.5 | 9.6 ± 7.8 | U=417.5 | - | 0.022 |
| ADHD DSM (yes/no)a | 9/26 | 5/30 | x2=1.45 | 1 | 0.371 |
| History of MDD DSM (yes/no)b | 14/21 | 3/32 | x2=9.40 | 1 | 0.004 |
| CTQ, score | 53.0 ± 14.8 | 47.1 ± 9.4 | U=484.5 | - | 0.132 |
| BDI, score | 7.9 ± 6.8 | 4.1 ± 5.4 | U=365.0 | - | 0.003 |
Table reports counts or means ± standard deviations. Significant group differences are shown in bold. t = Student t-test; x2 = Pearson chi-square; U = Mann-Whitney test
aCut-off according to DSM-IV criteria as assessed by the ADHD-SR questionnaire. bCut-off according to DSM-IV criteria as assessed by SCID-I interview
Abbreviations: ADHD, attention deficit hyperactivity disorder; BDI, Beck Depression Inventory; BMI, body mass index; CTQ, Childhood Trauma Questionnaire; MDD, major depressive disorder
In accordance with the inclusion criteria, hair samples confirmed a clear dominance of cocaine use over all other illegal substances. The mean cocaine concentration in hair (mean±SD: cocainetotal=33,102±67,934 pg/mg) was also in line with previous findings in dependent CU obtained by our group [38]. In contrast, the mean concentration of levamisole (a neurotoxic cocaine adulterant) in hair (mean±SD: 2962±9604 pg/mg) and the mean levamisole–cocaine ratio in hair (0.17) were substantially lower than in our previous reports [10]. However, this finding is coherent with the drop in levamisole prevalence observed in Switzerland during the period of recruitment (2017–2018) [10].
Effects of Cocaine Intake on NfL Levels at Baseline
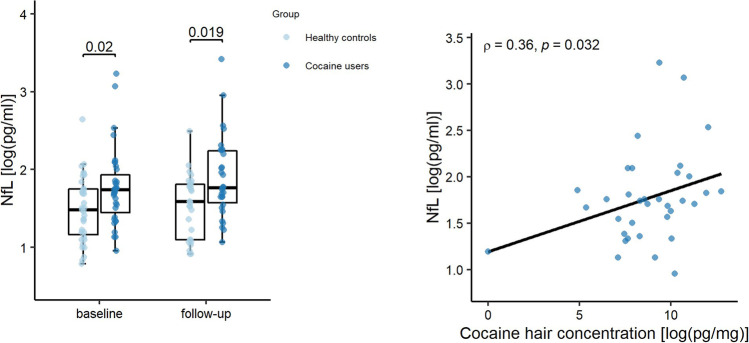
Unadjusted mean NfL levels in plasma were significantly higher in CU than in HC (mean/median [interquartile range (IQR)] in pg/ml: 6.75/5.70 [4.00–6.40] vs. 4.85/4.40 [3.05–5.75]; U=414.00, p=0.020), with medium effect size (Cohen’s d=0.51) (see Fig. 1). In the correlation analysis, a positive association of age with NfL levels (r(s)=0.510, p=0.002) and a negative association of BMI with NfL levels (r(s)=−0.430; p=0.010) were observed in HC, which is consistent with previous findings [36] (supplementary Table S2). In contrast, we found no significant correlation of any sociodemographic or clinical variable with NfL levels in CU (r(s)≤±0.33, p>0.05). Mean NfL levels did also not differ by sex in both HC and CU (HC: U=91.00, p=0.079; CU: U=93.50, p=0.174). In the GLM analysis accounting for age, sex, and BMI, a significant group effect confirmed higher NfL plasma levels in CU at baseline (see Table 2). Age was a significant covariate, while BMI showed a trend level negative association with NfL. Sex had no significant impact on the model. Exploratory GLMs including medication as between-subjects factor (considered singularly for each medication class, or pooled as present vs. absent) did not influence the significance of the group effect (all B>±0.31; all p<0.030). No medication class showed significant associations with NfL in the same analysis (all B<±0.64; all p>0.341). To further exclude the potential impact of demographic variables on elevated NfL levels in CU, we also compared NfL levels in 22 CU and 22 HC (from the same sample) who were matched for age (±2years) and did not differ in mean BMI. Again, we found significantly higher NfL levels in CU than in HC (Z=−2.22, p=0.026). At baseline, we found no association between NfL levels and subclinical symptoms of depression, ADHD, or childhood trauma (see Table S2). However, NfL was positively associated with hair concentration of cocainetotal (r(s)=0.364, p=0.032) (see Fig. 1). No significant association was found between NfL and levamisole, other illicit substances, or weekly alcohol intake in CU (see Table S3) (r(s)≤±0.27, p>0.05), in HC (r(s)≤±0.30, p>0.05) or in the whole sample (r(s)≤±0.17, p>0.05). Moreover, NfL was not associated with recent cocaine use assessed by urine toxicology in CU (r(s)=0.02, p=0.91) and with age of start of CU (r(s)=0.03, p=0.85).
Fig. 1.
NfL levels in healthy controls and chronic cocaine users. Left panel: Boxplots showing individual plasma NfL levels (log-transformed). Central horizontal lines indicate median values; boxes illustrate the ranges between lower and upper quartiles. Right panel: Scatterplot showing the relationship between hair concentration of cocainetotal and plasma NfL levels at baseline (both log-transformed). Cocainetotal (= Cocaine + Benzoylecgonine + Norcocaine) is, together with the corresponding metabolic ratios, a more robust procedure for discrimination between incorporation and contamination of hairs. Abbreviations: HC: Healthy Controls; CU: Cocaine Users; NfL: neurofilament light chain
Table 2.
Generalized linear model for sociodemographic and clinical characteristics predicting NfL levels at baseline
| Variables | Baseline (N=70) | |||
|---|---|---|---|---|
| Coefficient (B) | SE | Wald χ2 | P value | |
| Constant term | 1.85 | 0.57 | 10.45 | 0.001 |
| Group (HC/CU) | −0.28 | 0.14 | 4.03 | 0.045 |
| Age, years | 0.03 | 0.01 | 10.89 | 0.001 |
| Sex (f/m) | 0.22 | 0.15 | 2.04 | 0.153 |
| BMI, kg/m2 | −0.04 | 0.02 | 3.85 | 0.050 |
| Omnibus Test | LQ = 19.76 ; p = 0.001 | |||
Generalized linear model with normal distribution and log-link function. Dependent variable: NfL levels at baseline; factor: group (HC/CU); co-variables: BMI, age, sex. Significant effects are shown in bold.
Abbreviations: BMI, body mass index; CU, cocaine users; HC, healthy controls; LQ, likelihood quotient; NfL, neurofilament light chain
Longitudinal Effects of Increased/Decreased Cocaine Consumption on NfL Levels at Follow-up
At follow-up, 30 of 35 CU (females/males: 9/21) and 29 of 35 HC (females/males: 10/19) were available to be re-tested. The dropouts resulted from participants, who did not respond to the invitation for the T2 visit. Unadjusted mean NfL levels at follow-up were significantly higher in CU than in HC (mean/median [IQR] in pg/ml: 7.75/5.85 [3.42–8.28] vs. 5.03/4.90 [3.33–6.48]; U=280.50; p=0.019), with a large effect size (Cohen’s d=0.75) (see Fig. 1). In the GLM analysis of follow-up values, we observed a significant effect of group (CU/HC) on NfL at follow-up (B=−0.39, SE=4.73, p=0.030).
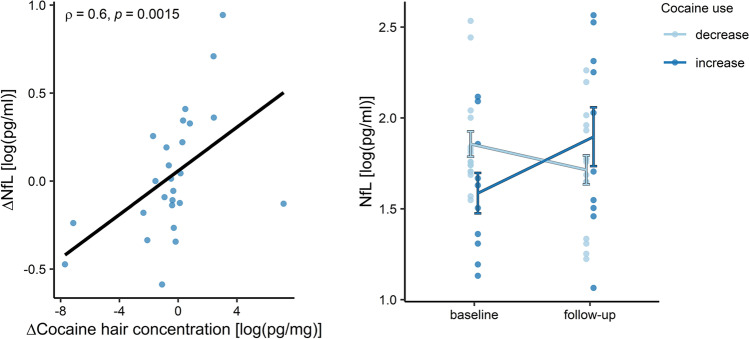
Regarding cocaine levels in hair, cocaine increasers (n=11) showed a mean increase of cocainetotal concentration of +10,296±13,633 pg/mg (mean change, percent: +441%; absolute change range: +475 pg/mg to +37,125 pg/mg), whereas cocaine decreasers (n=19) showed a mean decrease of cocainetotal of −31,280±60,877 pg/mg (mean change, percent: −56%; absolute change range: −570 pg/mg to −232,200 pg/mg). Four subjects were excluded from the longitudinal analysis, as they showed extreme variations in NfL values (above 3IQR) between T1 and T2. In the GLM adjusted for sex, age, BMI, and NfL levels at baseline, we found a significant group effect of change in cocaine use on NfL levels at follow-up (p<0.001) (see Fig. 2). In particular, ΔNfL ([NfL level at T2]–[NfL level at T1]) was significantly elevated in increasers (mean±SD: 2.29±2.50 pg/ml; T=2.90, p=0.018) and reduced in decreasers (mean±SD: −0.85±1.41 pg/ml; T=−2.407, p=0.029) but showed no significant change in HC (mean±SD: 0.09±1.25 pg/ml; T=0.385, p=0.703). In addition, NfL levels at baseline were positively associated with NfL levels at follow-up, while age, sex, and BMI had no significant impact on the model (see Table 3). Moreover, two CU reported changes of medication at follow-up: one started an antidepressant medication (fluoxetine), and one changed ADHD medication (from atomoxetine to methylphenidate). Excluding these participants from the GLM did not influence the significance of the group comparison (B=−0.46, p<0.001). Longitudinal dose–response effects of cocaine intake on NfL levels were demonstrated by a positive association of ΔCOCAINE in hair with ΔNfL (r(s)=0.58 p=0.002) (Fig. 2). In contrast, neither changes in hair concentration of other illegal substances or levamisole nor changes in alcohol were associated with changes in NfL values (see Table S4).
Fig. 2.
Longitudinal changes of NfL levels depending on variation of cocaine use. Left panel: Scatterplot showing the relationship between variations of hair concentration of cocainetotal and plasma NfL levels over the follow-up period. Delta values are calculated between timepoints (ΔX=[value X at T2]− [value X at T1]). Right panel: Plots showing longitudinal variations of NfL levels in chronic cocaine users with decreased vs. increased hair concentration of cocaine over the follow-up period. Mean values ± SD of NfL levels are indicated. Abbreviations: NfL: neurofilament light chain
Table 3.
Generalized linear model for sociodemographic and clinical characteristics predicting NFL levels at 4-month follow-up in cocaine users
| Variables | Cocaine users (N= 26) | |||
|---|---|---|---|---|
| Coefficient (B) | SE | Wald χ2 | P value | |
| Constant term | 1.63 | 0.59 | 7.67 | 0.006 |
| Group (increasers/decreasers) | -0.48 | 0.12 | 15.14 | >0.001 |
| NFL levels at baseline, pg/ml | 0.12 | 0.03 | 18.75 | >0.001 |
| Age, years | 0.00 | 0.01 | 0.16 | 0.686 |
| Sex (f/m) | -0.15 | 0.17 | 0.82 | 0.366 |
| BMI, kg/m2 | -0.01 | 0.02 | 0.37 | 0.541 |
| Omnibus Test | LQ =21.80; p = 0.001 | |||
Generalized linear model with normal distribution and log-link function. Dependent variable: NfL levels at follow-up; factor: group (increasers/decreasers); co-variables: NfL levels at baseline, BMI, age, sex. Significant effects are shown in bold.
Abbreviations: BMI, body mass index; LQ, likelihood quotient; NfL, neurofilament light chain
Discussion
The aim of the present study was to investigate a potential association between chronic cocaine use and NfL levels in blood using a longitudinal design to assess cocaine-related neurotoxicity. We found that plasma NfL levels were higher in CU than HC and were positively associated with cocaine use intensity, as confirmed by hair toxicology. Moreover, NfL levels at the 4-month follow-up were predicted by objectively verified changes in cocaine intake during the interval period, showing that increased cocaine use was associated with elevated NfL levels, while reduced use was associated with lower NfL concentrations.
The findings suggest that cocaine use may impact neuroaxonal structures. In particular, NfL elevation in CU was positively associated with increased cocaine intake, as confirmed by hair analysis, which makes it an objective, reliable, and effective way of discriminating between cocaine ingestion and external contamination [39]. In contrast, we found no association (cross-sectional or longitudinal) between NfL and intake of other illicit substances or alcohol in our sample. However, due to our preselection of primary cocaine users, we cannot rule out potential neurotoxic effects of other substances. Contrary to our expectations, NfL was not associated with the concentration of the adulterant levamisole, which has been linked to structural brain alterations in CU [10, 11, 24, 25]. The findings of previous studies by our group suggested that levamisole induces thinning of the prefrontal cortex and increases white matter pathology [10, 11]. However, in the current sample, the concentration of levamisole in hair was relatively low compared to our previous studies, which is in line with the observed reduction of levamisole concentration in cocaine samples in Switzerland during the recruitment period [10]. Similarly, we found no link between NfL levels and depressive symptoms, history of childhood trauma, or ADHD symptoms. Higher NfL levels have previously been described in patients with MDD, in patients with bipolar disorder, and in a sample of treatment-seeking ketamine users with a history of MDD [13, 14, 40]. An interaction between affective disorders and substance use in increasing the vulnerability to brain pathology has also been described, and disturbed activity of neurotrophic factors has been postulated as shared mechanism [41, 42]. However, in the present study, we specifically excluded subjects with history of bipolar disorder or current major depressive episodes. Thus, the lack of associations between NfL levels and depressive symptoms in the present study is consistent with the view that neuroaxonal pathology in MDD is rather stage-dependent [14]. Finally, NfL levels at follow-up were predicted by an interaction between baseline levels and changes in cocaine use over the interval period. This finding suggests that elevated NfL levels in CU may reflect direct substance-induced effects on neuroaxonal structures rather than a preexisting predisposition. This is consistent with evidence from patients with neuroinflammatory disorders, which demonstrated that NfL increases reflect active (stage-dependent) rather than preexisting (trait-dependent) brain pathology and that NfL levels may normalize with therapeutic interventions [43].
The elevation of NfL levels observed in CU is in line with clinical and preclinical evidence of cocaine-induced neurotoxicity [4, 44]. However, the lack of specificity of the NfL response does not allow us to make clear assumptions regarding the specific neuropathological processes involved in its elevation. In preclinical models, repeated cocaine exposure was shown to activate several neuropathological pathways (i.e., oxidative stress reaction, mitochondrial dysfunction, excitotoxicity, and autophagy), mostly mediated by long-term neurometabolic dysregulations [44, 45]. The effects of repeated cocaine administration included alterations of cytoskeletal structures (e.g., neurofilament proteins) and were linked to neuronal adaptations of dopaminergic circuits [46, 47]. Post-mortem investigations also suggested damage to dopaminergic neurons in the basal ganglia and white matter disruption in CU [4, 48]. Neuroimaging studies have found reductions in gray matter volumes, especially in the frontal and insular areas [1, 3, 5], and widespread alterations of major white matter tracts in CU [4]. These structural changes were linked to cognitive dysfunctions and seemed to be partially reversible after longer periods of abstinence or decreased cocaine use [2, 7]. In particular, recovery potential was observed for gray matter volume in the inferior frontal gyrus and the ventromedial prefrontal region [6] and for cortical thickness in the frontal lobe area [7]. Notably, previous studies have demonstrated the susceptibility of NfL levels to microstructural brain alterations that are relevant for cognitive performance have been already demonstrated in previous studies in patients with psychiatric disorders and even in healthy controls [14, 49]. Moreover, our findings are consistent with previous reports on the reversibility of neuroanatomical alterations in fronto-cortical brain areas [7]. Thus, repeated cocaine exposure may induce microstructural adaptations in fronto-cortical regions via neurometabolic dysregulations, resulting in peripheral NfL elevation and cognitive dysfunctions. A reduction of cocaine consumption might then partially restore neurometabolic homeostasis and allow structural recovery, specifically in frontal areas, resulting in normalization of NfL values. In contrast, increased cocaine consumption would likely result in continued neurometabolic dysregulation and induce further structural damage and NfL release. Potential interactions between cocaine use and neuroinflammation have been also reported in the past, but results have been rather inconsistent, and a clear role of inflammation in mediating cocaine-induced brain pathology has not been demonstrated [50].
Cocaine use has also been related to cerebrovascular disease via multiple pathways, including direct (vasospasm, cerebral vasculitis) and indirect (hypertensive surges, arrhythmias, induction of plaque growth, and enhanced platelet aggregation) mechanisms [51]. Potential acute manifestations, such as ischemic or hemorrhagic stroke, are accompanied by more subtle and chronic alterations, such as small vessels pathology with white matter hyperintensities [51]. Thus, NfL elevation in CU might also be (at least partially) caused by subclinical cerebrovascular pathology, as observed in patients with clinically silent small vessel disease [52]. However, the findings of a previous investigation by our group suggested that the extension of white matter hyperintensities may be associated more with cocaine adulteration with levamisole than with the level of cocaine intake [11]. Thus, considering the longitudinal response of NfL levels to changes in cocaine intake and the lack of association between NfL and levamisole, it is reasonable to assume that NfL may reflect cocaine-induced microstructural alterations rather than cerebrovascular disease.
Overall, the identification of a peripheral marker that is (1) minimally invasive, widely available, and methodically robust and (2) sensitive enough to detect substance-induced or at least substance-related neuroaxonal pathology and (3) respond to longitudinal variations in cocaine intake over a relatively short period (weeks to months), could be of significant benefit for the addiction field. For instance, it could be used to estimate the severity of neuropsychiatric sequelae of substance use, which could be useful for motivating patients to enter or intensify treatment. Not only could NfL analysis improve our understanding of substance-related brain damage, but it could also have relevant applications in clinical settings. Based on our findings, NfL appears to be generally suitable for monitoring the biological harm caused by different psychoactive substances and their adulterants. It may also be useful for identifying clinical factors involved in vulnerability or resilience to substance-related brain impairment, assessing the impact of therapeutic interventions on substance users, and facilitating personalized treatments. Moreover, considering the prevalence of cocaine use and the overall use of illicit stimulants in the general population, our study warrant consideration on illicit substance use as a potential confounding factor influencing NfL investigations in cohort studies with primary neurological or psychiatric diseases.
The present study has certain limitations that must be acknowledged. First, the sample size was moderate. However, by performing detailed assessments of substance use patterns and objective quantifications of substance intake by toxicological hair analysis, strictly excluding participants with neuropsychiatric comorbidities or severe polysubstance use, and adopting a longitudinal design, we were able to provide robust evidence of cocaine-induced effects on NfL levels. Second, the lack of neuroimaging data limits our speculations on the neuroanatomical correlates of NfL elevations and should be considered in future investigations. Third, we did not systematically assess blood pressure or physical activity (e.g., contact sports), or any marker of blood-brain-barrier permeability and inflammation, although their associations with NfL levels have been shown in previous works [36, 53]. Nonetheless, we excluded all participants with traumatic brain injury (including concussion or contusion), loss of consciousness, or a history of known neurological, cardiovascular, or autoimmune disorders. Moreover, alterations in the blood-brain-barrier permeability and in the inflammatory response have been mostly suggested after acute cocaine intake, but evidence of long-lasting changes of these markers in CU are still lacking [50, 54]. Importantly, NfL levels in our sample were associated with cocaine use in the last four months but not with use in the last three days (assessed by urine toxicology), which should be the case if NfL elevation was mostly driven by acute or subacute effects. Fourth, we cannot exclude that NfL levels may be affected by peripheral nerve pathology, as observed in vasculitic, hereditary, and inflammatory neuropathies [55, 56]. Future investigations should include neuroimaging data and eventually address other peripheral markers (e.g., alpha-internexin) which can inform on the specific involvement of central nervous system [57]. Nonetheless, the occurrence of peripheral nerve pathology due to cocaine use is considered unusual [58]. Fifth, we did not investigate peripheral inflammatory markers in our study and we cannot rule out potential effects of inflammation on NfL levels. However, associations between peripheral inflammatory markers and NfL levels in neuropsychiatric conditions have been shown to be limited [14, 59]. We also excluded all participants with infectious or autoimmune conditions to avoid indirect associations with NfL levels. Future studies including cerebrospinal fluid markers or animal models may clarify whether cocaine use is associated with brain pathology through neuroinflammatory alterations.
Taken together, our findings suggest a dose–response relationship of cocaine intake on NfL levels. The sensitivity, reliability, and low-invasivity of NfL make it an ideal candidate for longitudinal monitoring of active brain pathology in patients with chronic cocaine use. Accordingly, we also suggest considering illicit substance use, specifically of stimulants such as cocaine, as a significant confounding variable in investigations of NfL levels in all neurological and psychiatric studies and in its diagnostic application.
Supplementary Information
Acknowledgements
We are grateful to Monika Visentini, Monika Näf, Chantal Kunz, Marlon Nüscheler, Selina Maisch, Jocelyn Waser, Anna Burkert, Meret Speich, Maxine de Ven, Zoé Dolder, Zoe Hillmann, Jessica Grub, and Priska Cavegn for their excellent support with recruitment and assessment of the participants and to Dou Zhiwei and Torsten Hothorn for their much-appreciated advice on statistical methods.
Data Availability
Anonymized data will be shared by request with any qualified investigator with an institutional review board approval for the purposes of validation and/or replication using our center’s established procedures for sharing data.
Author Contributions
FB: conceptualization, formal analysis, writing—original draft; AKK, BKS, MRB: investigation, writing—review and editing; AM: investigation; ES: resources, supervision, writing—review and editing; JK: resources, methodology, writing—review and editing; BBQ: conceptualization, resources, supervision, writing—original draft, funding acquisition.
Funding
Open access funding provided by University of Zurich This study was supported by a grant of the Swiss National Science Foundation (SNSF, grant number: 105319_162639) to BBQ. BKS received a grant from the Coordination for the Improvement of Higher Education Personnel, CAPES, Brazil (grant number: 99999.001968/2015-07).
Declarations
Ethical Approval
This study was approved by the Research Ethics Committee of the Canton of Zurich (BASEC ID 2016-00278 and 2021-01853). Clinical and laboratory investigations were conducted in strict accordance with the principles of the Declaration of Helsinki.
Research Involving Human Participants and/or Animals
The research involves human samples.
Informed Consent
All participants provided written informed consent prior to their enrollment in the study and were financially compensated for their participation.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Informed consent for publication was obtained from all participants included in the study.
Competing Interests
The authors declare no competing interests.
Footnotes
The manuscript has been uploaded in the pre-print, open-access server MedRvix [60].
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hall MG, Alhassoon OM, Stern MJ, Wollman SC, Kimmel CL, Perez-Figueroa A, Radua J. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2015;41:290–299. doi: 10.3109/00952990.2015.1044607. [DOI] [PubMed] [Google Scholar]
- 2.He Q, Li D, Turel O, Bechara A, Hser Y-I. White matter integrity alternations associated with cocaine dependence and long-term abstinence: preliminary findings. Behav Brain Res. 2020;379:112388. doi: 10.1016/j.bbr.2019.112388. [DOI] [PubMed] [Google Scholar]
- 3.Rabin RA, Mackey S, Parvaz MA, Cousijn J, Li CS, Pearlson G, Schmaal L, Sinha R, et al. Common and gender-specific associations with cocaine use on gray matter volume: Data from the ENIGMA addiction working group. Hum Brain Mapp; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tondo LP, Viola TW, Fries GR, Kluwe-Schiavon B, Rothmann LM, Cupertino R, Ferreira P, Franco AR, et al. White matter deficits in cocaine use disorder: convergent evidence from in vivo diffusion tensor imaging and ex vivo proteomic analysis. Transl Psychiatry. 2021;11:252. doi: 10.1038/s41398-021-01367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Parvaz MA, Moeller SJ, D'Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, Goldstein RZ. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2017;22:1391–1401. doi: 10.1111/adb.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsiger S, Hänggi J, Germann J, Vonmoos M, Preller KH, Engeli EJE, Kirschner M, Reinhard C, et al. Longitudinal changes in cocaine intake and cognition are linked to cortical thickness adaptations in cocaine users. NeuroImage: Clin. 2019;21:101652. doi: 10.1016/j.nicl.2019.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jedema HP, Song X, Aizenstein HJ, Bonner AR, Stein EA, Yang Y, Bradberry CW. Long-term cocaine self-administration produces structural brain changes that correlate with altered cognition. Biol Psychiatry. 2021;89:376–385. doi: 10.1016/j.biopsych.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Kim BW, Blood AJ, et al. Cortical thickness abnormalities in cocaine addiction—a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008;60:174–188. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonmoos M, Hirsiger S, Preller KH, Hulka LM, Allemann D, Herdener M, Baumgartner MR, Quednow BB. Cognitive and neuroanatomical impairments associated with chronic exposure to levamisole-contaminated cocaine. Transl Psychiatry. 2018;8(1):235. doi: 10.1038/s41398-018-0279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad F, Hirsiger S, Winklhofer S, Baumgartner MR, Stämpfli P, Seifritz E, Wegener S, Quednow BB. Use of levamisole-adulterated cocaine is associated with increased load of white matter lesions. J Psychiatry Neurosci. 2021;46:E281–e291. doi: 10.1503/jpn.200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crunelle CL, Kaag AM, Van Wingen G, van den Munkhof HE, Homberg J, Reneman L, Van Den Brink W. Reduced frontal brain volume in non-treatment-seeking cocaine-dependent individuals: exploring the role of impulsivity, depression, and smoking. Front Human Neurosci. 2014;8:7. doi: 10.3389/fnhum.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y-L, Bavato F, Chung A-N, Liu T-H, Chen Y-L, Huang M-C, Quednow BB. Neurofilament light chain as novel blood biomarker of disturbed neuroaxonal integrity in patients with ketamine dependence. World J Biol Psychiatry. 2021;22(9):713–721. doi: 10.1080/15622975.2021.1907709. [DOI] [PubMed] [Google Scholar]
- 14.Bavato F, Cathomas F, Klaus F, Gütter K, Barro C, Maceski A, Seifritz E, Kuhle J, et al. Altered neuroaxonal integrity in schizophrenia and major depressive disorder assessed with neurofilament light chain in serum. J Psychiatric Res. 2021;140:141–148. doi: 10.1016/j.jpsychires.2021.05.072. [DOI] [PubMed] [Google Scholar]
- 15.Kluwe-Schiavon B, Kexel A, Manenti G, Cole DM, Baumgartner MR, Grassi-Oliveira R, Tobler PN, Quednow BB. Sensitivity to gains during risky decision-making differentiates chronic cocaine users from stimulant-naïve controls. Behav Brain Res. 2020;379:112386. doi: 10.1016/j.bbr.2019.112386. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Koob G, Baler R. Biomarkers in substance use disorders. ACS Chem Neurosci. 2015;6:522–525. doi: 10.1021/acschemneuro.5b00067. [DOI] [PubMed] [Google Scholar]
- 17.Yuan A, Rao MV, Veeranna, Nixon RA (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 9 [DOI] [PMC free article] [PubMed]
- 18.Leppert D, Kuhle J. Blood neurofilament light chain at the doorstep of clinical application. Neurol Neuroimmunol Neuroinflamm. 2019;6:e599. doi: 10.1212/NXI.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J. Neurofilaments as biomarkers in neurological disorders. Nature Reviews Neurology. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 20.Kuhle J, Kropshofer H, Haering DA, Kundu U, Meinert R, Barro C, Dahlke F, Tomic D, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92:e1007–e1015. doi: 10.1212/WNL.0000000000007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann J, Friedli N, Bavato F, Stämpfli P, Coray R, Baumgartner MR, Grandgirard D, Leib SL, et al. White matter alterations in chronic MDMA use: evidence from diffusion tensor imaging and neurofilament light chain blood levels. NeuroImage: Clin. 2022;36:103191. doi: 10.1016/j.nicl.2022.103191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonmoos M, Eisenegger C, Bosch OG, Preller KH, Hulka LM, Baumgartner M, Seifritz E, Quednow BB. Improvement of emotional empathy and cluster B personality disorder symptoms associated with decreased cocaine use severity. Front Psychiatry. 2019;10:213. doi: 10.3389/fpsyt.2019.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vonmoos M, Hulka LM, Preller KH, Minder F, Baumgartner MR, Quednow BB. Cognitive impairment in cocaine users is drug-induced but partially reversible: evidence from a longitudinal study. Neuropsychopharmacology. 2014;39:2200–2210. doi: 10.1038/npp.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu HM, Hsieh WJ, Yang CC, Wu VC, Wu KD. Leukoencephalopathy induced by levamisole alone for the treatment of recurrent aphthous ulcers. Neurology. 2006;67:1065–1067. doi: 10.1212/01.wnl.0000237344.06122.79. [DOI] [PubMed] [Google Scholar]
- 25.Vitt JR, Brown EG, Chow DS, Josephson SA. Confirmed case of levamisole-associated multifocal inflammatory leukoencephalopathy in a cocaine user. J Neuroimmunol. 2017;305:128–130. doi: 10.1016/j.jneuroim.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Kexel A-K, Kluwe-Schiavon B, Baumgartner MR, Engeli EJE, Visentini M, Kirschbaum C, Seifritz E, Ditzen B, Soravia LM, Quednow BB (2022) Cue-induced cocaine craving enhances psychosocial stress and vice versa in chronic cocaine users. medRxiv:2022.2001.2013.22268894 [DOI] [PMC free article] [PubMed]
- 27.Cooper GAA, Kronstrand R, Kintz P. Society of Hair Testing guidelines for drug testing in hair. Forensic Sci Int. 2012;218:20–24. doi: 10.1016/j.forsciint.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Quednow BB, Steinhoff A, Bechtiger L, Ribeaud D, Eisner M, Shanahan L (2021) High prevalence and early onsets: legal and illegal substance use in an urban cohort of young adults in Switzerland. Eur Addict Res 28:1–13 [DOI] [PubMed]
- 29.APA E (2000) Diagnostic and statistical manual of mental disorders, Text Revision (DSM-IV-TR). Washington, DC
- 30.Beck AT. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 32.Rösler M, Retz W, Retz-Junginger P, Thome J, Supprian T, Nissen T, Stieglitz RD, Blocher D, et al. Tools for the diagnosis of attention-deficit/hyperactivity disorder in adults. Self-rating behaviour questionnaire and diagnostic checklist. Nervenarzt. 2004;75:888–895. doi: 10.1007/s00115-003-1622-2. [DOI] [PubMed] [Google Scholar]
- 33.Quednow BB, Kühn K-U, Hoenig K, Maier W, Wagner M. Prepulse inhibition and habituation of acoustic startle response in male MDMA (‘ecstasy’) users, cannabis users, and healthy controls. Neuropsychopharmacology. 2004;29:982–990. doi: 10.1038/sj.npp.1300396. [DOI] [PubMed] [Google Scholar]
- 34.Scholz C, Cabalzar J, Kraemer T, Baumgartner MR (2020) A comprehensive multi-analyte method for hair analysis: substance-specific quantification ranges and tool for task-oriented data evaluation. J Anal Toxicol 45:701-712 [DOI] [PubMed]
- 35.Hoelzle C, Scheufler F, Uhl M, Sachs H, Thieme D. Application of discriminant analysis to differentiate between incorporation of cocaine and its congeners into hair and contamination. Forensic Sci Int. 2008;176:13–18. doi: 10.1016/j.forsciint.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Barro C, Chitnis T, Weiner HL. Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol. 2020;7:2508–2523. doi: 10.1002/acn3.51234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones PR. A note on detecting statistical outliers in psychophysical data. Atten Percept Psychophys. 2019;81:1189–1196. doi: 10.3758/s13414-019-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, Bolla KI, Quednow BB. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry. 2013;203:35–43. doi: 10.1192/bjp.bp.112.118091. [DOI] [PubMed] [Google Scholar]
- 39.Scholz C, Quednow BB, Herdener M, Kraemer T, Baumgartner MR. Cocaine hydroxy metabolites in hair: indicators for cocaine use versus external contamination☆. J Anal Toxicol. 2019;43:543–552. doi: 10.1093/jat/bkz022. [DOI] [PubMed] [Google Scholar]
- 40.Aggio V, Fabbella L, Finardi A, Mazza EB, Colombo C, Falini A, Benedetti F, Furlan R. Neurofilaments light: possible biomarker of brain modifications in bipolar disorder. J Affect Disord. 2022;300:243–248. doi: 10.1016/j.jad.2021.12.122. [DOI] [PubMed] [Google Scholar]
- 41.Bavato F, Stamatakos S, Ohki CMY, Seifritz E, Romualdi P, Grünblatt E, Quednow BB. Brain-derived neurotrophic factor protects serotonergic neurons against 3,4-methylenedioxymethamphetamine (“Ecstasy”) induced cytoskeletal damage. J Neural Transm. 2022;129(5-6):703–711. doi: 10.1007/s00702-022-02502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang M-C, Chen C-H, Liu T-H, Chung A-N, Liu Y-L, Quednow BB, Bavato F (2023) Comorbidity of ketamine dependence with major depressive disorder increases the vulnerability to neuroaxonal pathology. J Psychiatric Res 158:360-364 [DOI] [PubMed]
- 43.Cantó E, Barro C, Zhao C, Caillier SJ, Michalak Z, Bove R, Tomic D, Santaniello A, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76:1359. doi: 10.1001/jamaneurol.2019.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira RB, Andrade PB, Valentão P. A comprehensive view of the neurotoxicity mechanisms of cocaine and ethanol. Neurotox Res. 2015;28:253–267. doi: 10.1007/s12640-015-9536-x. [DOI] [PubMed] [Google Scholar]
- 45.Guha P, Harraz MM, Snyder SH. Cocaine elicits autophagic cytotoxicity via a nitric oxide-GAPDH signaling cascade. Proc Natl Acad Sci. 2016;113:1417–1422. doi: 10.1073/pnas.1524860113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beitner-Johnson D, Guitart X, Nestler E. Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. J Neurosci. 1992;12:2165–2176. doi: 10.1523/JNEUROSCI.12-06-02165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs K, Lajtha A, Sershen H. Effect of nicotine and cocaine on neurofilaments and receptors in whole brain tissue and synaptoneurosome preparations. Brain Res Bull. 2010;82:109–117. doi: 10.1016/j.brainresbull.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Little KY, Ramssen E, Welchko R, Volberg V, Roland CJ, Cassin B. Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 2009;168:173–180. doi: 10.1016/j.psychres.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 49.Beste C, Stock AK, Zink N, Ocklenburg S, Akgün K, Ziemssen T. How minimal variations in neuronal cytoskeletal integrity modulate cognitive control. Neuroimage. 2019;185:129–139. doi: 10.1016/j.neuroimage.2018.10.053. [DOI] [PubMed] [Google Scholar]
- 50.Ersche KD, Döffinger R. Inflammation and infection in human cocaine addiction. Curr Opin Behav Sci. 2017;13:203–209. doi: 10.1016/j.cobeha.2016.12.007. [DOI] [Google Scholar]
- 51.Treadwell SD, Robinson TG. Cocaine use and stroke. Postgrad Med J. 2007;83:389–394. doi: 10.1136/pgmj.2006.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, Pichler A, Barro C, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. 2017;89:2108–2114. doi: 10.1212/WNL.0000000000004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uher T, McComb M, Galkin S, Srpova B, Oechtering J, Barro C, Tyblova M, Bergsland N, et al. Neurofilament levels are associated with blood-brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult Scler. 2021;27:220–231. doi: 10.1177/1352458520912379. [DOI] [PubMed] [Google Scholar]
- 54.Barr JL, Brailoiu GC, Abood ME, Rawls SM, Unterwald EM, Brailoiu E. Acute cocaine administration alters permeability of blood-brain barrier in freely-moving rats— Evidence using miniaturized fluorescence microscopy. Drug Alcohol Depend. 2020;206:107637. doi: 10.1016/j.drugalcdep.2019.107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Körtvelyessy P, Kuhle J, Düzel E, Vielhaber S, Schmidt C, Heinius A, Leypoldt F, Schraven B, et al. Ratio and index of Neurofilament light chain indicate its origin in Guillain-Barré Syndrome. Ann Clin Translatl Neurol. 2020;7:2213–2220. doi: 10.1002/acn3.51207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Millere E, Rots D, Simrén J, Ashton NJ, Kupats E, Micule I, Priedite V, Kurjane N, et al. Plasma neurofilament light chain as a potential biomarker in Charcot-Marie-Tooth disease. Eur J Neurol. 2021;28:974–981. doi: 10.1111/ene.14689. [DOI] [PubMed] [Google Scholar]
- 57.Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna LRK, Eyer J, Peterson AC, et al. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci. 2006;26:10006–10019. doi: 10.1523/JNEUROSCI.2580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Souza A, Desai PK, de Souza RJ. Acute multifocal neuropathy following cocaine inhalation. J Clin Neurosci. 2017;36:134–136. doi: 10.1016/j.jocn.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 59.De Schaepdryver M, Lunetta C, Tarlarini C, Mosca L, Chio A, Van Damme P, Poesen K. Neurofilament light chain and C reactive protein explored as predictors of survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2020;91:436–437. doi: 10.1136/jnnp-2019-322309. [DOI] [PubMed] [Google Scholar]
- 60.Bavato F, Kexel AK, Kluwe-Schiavon B, Maceski A, Baumgartner MR, Seifritz E, Kuhle J, Quednow BB. A longitudinal investigation of blood neurofilament light chain levels in chronic cocaine users. In: Cold Spring Harbor Laboratory; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by request with any qualified investigator with an institutional review board approval for the purposes of validation and/or replication using our center’s established procedures for sharing data.