Abstract
Purpose of Review
Accumulating data on the consumption of plant-based diets and their impact on blood pressure indicate a consensus that plant-based diets are linked to reduced blood pressure. The suggested mechanisms of action are manifold, and, in this systematic review, we provide a summary of the most recent findings on plant-based diets and their impact on blood pressure, along with an analysis of the molecules accountable for the observed effects.
Recent Findings
The overwhelming majority of intervention studies demonstrate that plant-based diets result in lower blood pressure readings when compared to diets that are based on animal products. The various mechanisms of action are being clarified.
Summary
The data discussed in this systematic review allow us to conclude that plant-based diets are associated with lower blood pressure and overall better health outcomes (namely, on the cardiovascular system) when compared to animal-based diets. The mechanisms of action are being actively investigated and involve many macro- and micronutrients plentiful in plants and the dishes prepared with them.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11906-023-01243-7.
Keywords: Plant-based diets, Hypertension, Polyphenols, Cardiovascular disease, Vitamin C, Essential fatty acids
Introduction
Data concerning the intake of plant-based diets and their effects on blood pressure keep accumulating, and there is consensus that plant-based diets are associated with lower blood pressure. The proposed mechanisms of action are manifold, and, in this systematic review, we summarize the latest data on plant-based diets and blood pressure, with insights on the molecules responsible for the described effects.
We first want to briefly highlight two of the major cross-sectional studies available on this topic. On the one hand, the Epic-Oxford study included more than ten thousand British subjects and showed that vegans presented the lowest levels of hypertension and blood pressure (BP), while meat-eating individuals presented the highest ones [1••]. Likewise, in arbitrarily selected subjects from the Adventist Health Study-2 (AHS-2) cohort (comprising almost one hundred thousand Adventists in North America), vegans and vegetarians presented lower BP levels than the individuals eating meat [2]. Noteworthy, in both studies, adjustment for body mass index (BMI) reduced the magnitude of these findings.
The impact of dietary patterns on BP has also been explored in numerous prospective cohort investigations. In the context of the Coronary Artery Risk Development in Young Adults (CARDIA), a dose-dependent relationship was found between plant food intake (whole grains, fruits, vegetables, etc.) and reduced incidence of raised BP [3]. In addition, in the combined analysis of the Nurses’ Health Study (NHS) I and II and the Health Professionals Follow-up Study (HPFS), an association was found between hypertension and the consumption of meat and seafood [4]. Similar findings were reported in other smaller prospective studies [5-8].
Methods
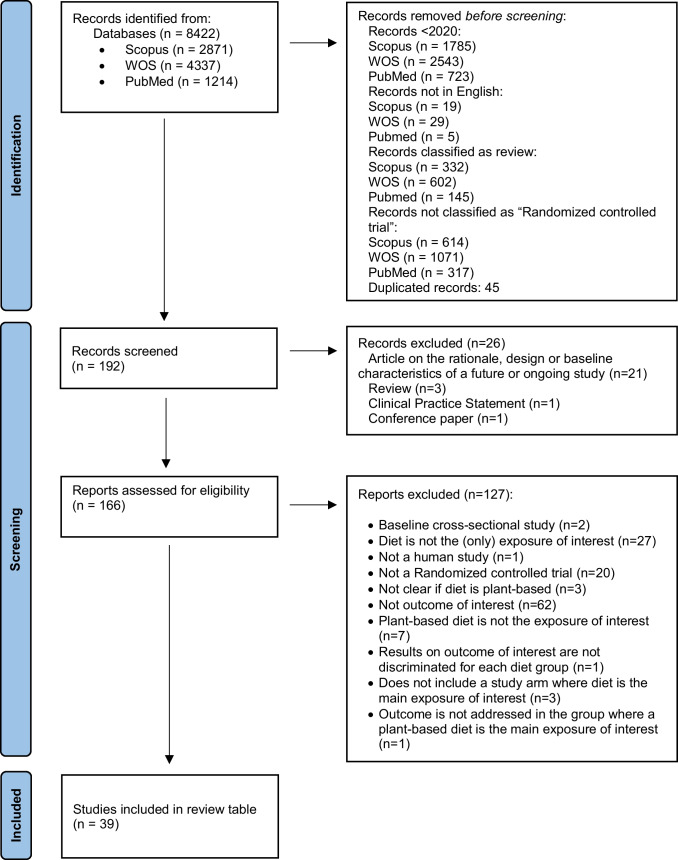
The search strategy — according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) [9] — is shown in Fig. 1. We searched PubMed, Web of Science, and Scopus. Literature search strategies used for each database and the syntax search terms are displayed in Supplementary Table 1. The search retrieved 8422 articles. After removing papers < 2020, duplicated records, reviews, non-human studies, etc., we review 39 papers, discussed in Table 1.
Fig. 1.
PRISMA 2020 flow diagram of plant-based diets studies (as related to blood pressure) retrieved
Table 1.
Recent studies on plant-based diets and blood pressure
|
Volunteers (n, age) |
Healthy/pathological context | Study arms/groups |
Study design Intervention period |
Main results on BP/hypertension | Ref |
|---|---|---|---|---|---|
| 62, 56.6 ± 10.9 y, group 1; 58.3 ± 8.4 y, group 2 | Overweight | (1) Med diet; (2) vegan diet. The Mediterranean diet followed the PREDIMED protocol. The low-fat vegan diet (∼75% of energy from carbohydrates, 15% protein, and 10% fat) consisted of vegetables, grains, legumes, and fruits. Participants were instructed to avoid animal products and added fats. Vitamin B12 was supplemented (500 µg/day) during the vegan phase of the study | Crossover. 16 weeks on diet 1 + 4 weeks on baseline diet + 16 weeks on diet 2 | SBP and DBP decreased when on the Med diet (− 9.3 and − 7.3 mmHg) and vegan diet (− 3.4 and 4.1 mmHg) (treatment effect + 5.9 [95% CI + 1.0 to + 10.9]; P = 0.02; and + 1.8 [95% CI − 4.6 to + 8.1]; P = 0.58, respectively) | [53] |
| 5373, 55–75 y | Overweight/obese with MetS | Control group: Med diet supplemented with EVOO and nuts, without caloric restriction or promotion of PA or weight loss goals. Intervention group: hypocaloric Med diet (with a caloric restriction of 30%) supplemented with EVOO and nuts, as well as an intensive lifestyle program with promotion of physical activity and weight loss goals including behavioral therapy | Parallel. 12 months | After 6 and 12 months, SBP and DBP decreased across successive quintiles of improvement in the carbohydrate quality index (categorized in quintiles and based on 4 criteria: total dietary fiber intake, glycemic index, whole grain/total grain ratio, and solid carbohydrate/total carbohydrate ratio) | [28] |
| 187, 49 ± 10 y | Overweight, moderate-to-severe obstructive sleep apnea | (1) Standard care group (SCG, n = 65), (2) Med diet group (MDG, n = 62), or (3) Med lifestyle group (MLG, n = 60). Intervention arms were subjected to a 6-month weight-loss behavioral intervention based on the Med diet | Parallel. 12 months | BP improved only in intervention arms and was significantly lower vs. SCG post-intervention. MLG had a lower relative risk of hypertension (MDG only showed a trend) compared to SCG | [31] |
| 2278, 59.6 ± 2.1 y (Q1); 74.2 ± 2.6 (Q4) | High CVD risk, but no CVD at enrolment | A traditional Med diet supplemented with either (1) complementary EVOO or (2) tree nuts, or (3) a control diet based on advice to follow a low-fat diet | Parallel. 3-year follow-up | SBP levels decreased in both quartiles (change was greater in the Q1). DBP decreased in Q4, contrary to Q1 (BP increased significantly) | [166] |
| 50, median (IQR): 62.8 (53.9, 70.8) | Rheumatoid arthritis | (1) Intervention diet group or (2) a control diet group. Intervention diet was rich in whole grain, fatty fish, nuts, vegetables, and fruit and supplemented with probiotics. The control diet resembled average nutritional intake in Sweden | Crossover. 10 weeks + a 4-month wash-out | SBP and DBP values showed a tendency for decreasing from baseline in both groups, but the difference between groups was not significant | [46] |
| 70, 61.1 ± 7.0y, PBDG; 62.8 ± 7.0 y, CG | Cardiometabolic risk | (1) Ad libitum whole-food plant-based diet group, consisting of vegetables, grains, legumes, and fruits and avoidance of animal products (PBDG, n = 36) or (2) control group (CG) which continued an omnivorous diet (n = 34) | Parallel. 8 weeks | Ambulatory BP monitoring was not significant between the groups, although PBDG showed more favorable effects for SBP and DBP vs controls | [55] |
| 60, median (range): 63.5 (51–75), A; 60.0 (44–74), B; 61.0 (34–74), C | T2DM | Investigational diet group (A) was compared with a food exchange system‐based diet, either in the form of ready meals provided to participants (group B) or not (group C). The investigational diet mimicked the average East Asian diet, but retained common characteristics of the Med and DASH diets | Parallel. 12 weeks | Reductions in SBP (− 1.90 ± 11.41 in group A vs 7.95 ± 11.92 in group C, P = 0.011) and DBP (− 1.60 ± 7.96 in group A vs 5.00 ± 9.85 in group C, P = 0.025) between baseline and week 12 were greater in A vs. C | [52] |
| 10371, 50–79 y | Normotensive postmenopausal women without prior history of CVD | (1) Intervention group (n = 4245), (2) comparison group (n = 6126). The dietary goals of the intervention group included a reduction in total fat intake to 20% of total energy, an increase in vegetable and fruit intake to 5 servings/d, and an increase in grain intake to 6 servings/d | Parallel. 8.3 years | Small reductions in BP were observed in the intervention group | [62] |
| 277, 48 ± 9 y | Moderate abdominal obesity or/and dyslipidemia | (1) Med/low carbohydrate diet (MED/LC, n = 80) or (2) low fat (LF, n = 79) iso-caloric weight loss intervention diet. The MED/LC diet consisted in high protein and fat intake, mostly from vegetarian sources, according to the Med diet; the LF diet was based on the AHA guidelines | Parallel. 18 months | At 6 months, a statistically significant reduction was found in both DBP and SBP in participants in the low RQ group consuming the MED/LC diet | [35] |
| 208, 48.8 ± 11.8 y | T2DM | Intervention group (IG, n = 104) or control group (n = 104). Both had usual medical care. IG also received extended nutritional advice, including instructions to eat meals with one-half of the plate consisting of non-starchy vegetables, one-quarter with lean protein, and one-quarter with low glycemic index carbohydrates such as whole grains | Parallel. 6 months | Significant decreases in BP from baseline were seen in both groups, but changes were significantly higher in the intervention group | [61] |
| 250 families (n = 720), 3-85y | Without major CVD | (1) Intervention group (127 families, n = 367) received educational sessions, cooking classes, written supporting material, and foods that form part of the Atlantic diet. (2) Control group (123 families, n = 353) followed their habitual lifestyle. The Atlantic diet has several characteristics in common with the Med diet | Parallel. 6 months | Non-significant intra- and inter-group differences in SBP and DBP | [49] |
| 40, 48.6 ± 7.6, controls; 51.9 ± 6.2, J-DASH 1; 50.0 ± 9.5, J-DASH 2 | Untreated high normal BP or stage 1 hypertension | J-DASH 1 and J-DASH 2 groups (n = 13 each) and the usual-diet group (n = 14). The J-DASH diet was in accordance with the nutritional composition of the DASH diet, but contained less total fat and saturated fatty acids | Parallel. 6 months: 2-month intervention + 4-month follow-up | J-DASH diets improved home measured BP and stabilized its variability compared to the usual diet | [51] |
| 235, 40–65 y | Moderate obesity | Secondary study of the DIRECT trial. (1) Low-fat, restricted-calorie diet (n = 85), (2) Med, restricted-calorie, diet (n = 76), or (3) low-carbohydrate, non–restricted-calorie diet (n = 74). Med diet and low-fat diet volunteers were counseled to consume low-fat grains, vegetables, fruits, and legumes and limit consumption of additional fats, sweets, and high-fat snacks. The low-carbohydrate, non–restricted-calorie diet was adapted from the Atkins diet | Parallel. 24 months | Significant reductions in SBP for all diet groups at both 6 months and 24 months (all P < 0.05 for within-group comparisons), and significant reductions in DBP for the Med diet group (P < 0.05 at both time points) | [36] |
| 44, mean (95%CI): 50.73 (48.65, 52.8) | MetS | (1) Med diet or (2) non-Med diet + nuts (50 g/day) | Crossover. 2 months + a 1-month wash-out | Non-significant intra- and inter-group differences in SBP and DBP | [30] |
| 71, 58 ± 8y (Fast + DASH) and 62 ± 8y (DASH) | CVD risk patients with MetS | (1) After a 5-day fasting patients were instructed to follow a modified DASH diet (Fast + DASH diet (n = 35)), with additional emphasis on plant-based and Med diet to optimize refeeding, or (2) DASH diet (n = 36) | Parallel. 12 weeks | DASH reduced office SBP; Fast + DASH led to a sustained reduction in 24 h ambulatory SBP and MABP. Fast + DASH and DASH reduced intake of antihypertensive medication in 43% and 17% of cases, respectively | [167] |
| 253, 38 ± 8, 1; 37 ± 9, 2; 38 ± 9, 3 | Prediabetes | 3 isocaloric-restricted diets: (1) Med diet, (2) a traditional Jiangnan diet high in plants, or (3) a control diet low in plants | Parallel. 6 months | SBP and DBP decreased significantly from baseline for all diets but differences between groups were non-significant | [40] |
| 43, 65 ± 15y | Chronic kidney disease | (1) Med diet, (2) vegetarian diet (very-low-protein diet or VLPD), (3) Med diet supplemented with one tablet of essential amino acids and ketoanalogs | Crossover. 6-month intervention + a 3-month washout | VLPD lowered both the SBP and DBP more effectively than Med diet or KA-supplemented Med diet (P < 0.05 in both cases) | [54] |
| 6636, 55–75 y (men) and 60–75 (women) | MetS | Intensive intervention group or control group. Intervention consisted of an energy-reduced Med diet with enhanced physical activity, behavioral support and weight loss compared to an energy-unrestricted Med diet (control) | Parallel. 1 year | A 1-SD increase in the DASH diet score was significantly associated with lower SBP (− 0.57 mmHg, P < 0.001) and DBP (− 0.15 mmHg, P = 0.03) | [50] |
| 108, 75 ± 6y | Apparently healthy | (1) Experimental group maintained a dietary pattern based on the traditional Cretan Med diet (i.e., vegetables, fruits, olive oil, legumes, fish, whole grain cereals, nuts and seeds and low consumption of processed foods, dairy products, red meat, and vegetable oils). (2) Control group maintained their customary lifestyle and diet | Parallel. 6 months | The Med diet resulted in improved SBP in comparison with the habitual diet. At 18 months (12 months after the end of the intervention study), this improvement was not sustained | [33] |
| 164, 59 ± 8.6, vegan group; 58 ± 11.7 y, vegetarian group | T2DM | (1) Vegan (n = 81) or (2) vegetarian (n = 83) diets. A low-carbohydrate vegan diet, high in canola oil and plant proteins, or a vegetarian therapeutic diet | Parallel. 3 months | No changes in BP medications and no treatment differences in BP. Both diets had significant reductions from baseline in SBP and DBP: − 4.12 and − 3.54 mmHg, respectively (vegan) and − 5.91 and − 4.13 mmHg, respectively (vegetarian) | [56] |
| 6932, 66–68y on average | Overweight/ obese with MetS | Four sub-groups of PREDIMED participants: (1) nonusers (n = 2188), and users of (2) one (n = 2401), (3) two (n = 1702), or (4) three (n = 641) hypertensive drugs | Parallel. 5 years | Nontreated participants on a Med diet had decreased need for antihypertensive drugs. Med diet decreased the need of escalating therapy in patients using 2 drugs at baseline and attenuated the association of drug use with the risk of incident cardiovascular events | [25] |
| 5800, 55-75y | Overweight/ obese with MetS | Control group: Med diet supplemented with EVOO and nuts, without caloric restriction or promotion of PA or weight loss goals. Intervention group: hypocaloric Med diet (with a caloric restriction of 30%) supplemented with EVOO and nuts, as well as an intensive lifestyle program with promotion of PA and weight loss goals including behavioral therapy | Parallel. 1 year | As nut consumption increased a significant decrease was found in SBP (P < 0.05) | [29] |
| 41, median (range): 58 (33–65), Med Diet; 58.5 (27–65), low-fat diet; 53 (25–67), Controls | Clinically stable heart or lung transplant recipients at least 6 months post-surgery | (1) Med diet, (2) low-fat diet. A Med diet supplemented with EVOO, or a low-fat diet based on modified British Heart Foundation dietary guidelines. The control group consisted of eligible transplant recipients who did not take part in the trial and remained clinically stable at points corresponding to trial baseline and end | Parallel. 12 months | SBP and DBP increased in the control group, whereas, compared with the Med group, the low-fat group showed a greater decline in these parameters. In the Med group, 4 patients moderately decreased their antihypertensive medication dose | [11] |
| 138 women, 47.2 ± 11.3 y | Obese | Participants were assigned to the almond-supplemented Med diet group (MDSA) or maintenance of usual diet (control) group in which no changes in dietary habits were advised | Parallel. 3 months | SBP and DBP decreased in MDSA and control groups, with no significant differences between groups | [37] |
| 60, 42.4 ± 6.8 y | Stable HIV infection | Diet1 group received dietary advice to reduce saturated fat intake to < 10% of energy intake. Diet2 group were supported to adopt the Med Portfolio Diet (Diet2) with additional cholesterol-lowering foods (nuts, stanols, soya, oats, beans) | Parallel. 6 months | Diet2 participants had a significantly lower SBP (mean difference adjusted for baseline − 7 mmHg, 95% CI − 2 to − 12, P = 0.008) compared to those in Diet1 | [45] |
| 21, 22 ± 4 y | Professional female handball players | (1) Free diet; (2) Med diet; or (3) High antioxidant diet | Parallel. 12 weeks | No significant differences were found in BP over time or between groups | [48] |
| 15, 36.1 ± 10.0 y | T1DM | Three isocaloric dietary patterns: (1) high-protein/low-carbohydrate diet with 20% of calories as carbohydrates, (2) Med/low glycemic index diet with 40% carbohydrates, (3) a reference diet with 50% carbohydrates | Crossover. 3 weeks, with 7-day washout periods | No significant differences were observed in BP changes between groups | [41] |
| 66, children (3–9 y), adolescents (10–19 y), adult (≥ 20y) | Living in a situation of extreme poverty | (1) Control group, (2) intervention group. More healthy food items such as fruits and vegetables, legumes, fish, nuts and seeds, and olive oil were consumed in the intervention group compared to the control group | Parallel. 10 weeks | BP did not improve in either the intervention or the control group | [63] |
| 70 adolescent women, 14 ± 1y | MetS | (1) Intervention group, (2) control group. The intervention group followed a prescribed Med diet, while participants in the control group received dietary advice according to the food pyramid | Parallel. 12 weeks | Significant reduction in SBP (− 8.4 ± 1.4 vs. 1.4 ± 1.4 mmHg, P < 0.001) in Med diet vs. control diet. No significant effect on DBP | [39] |
| 369, 64.57 ± 8.95, BALANCE; 64.11 ± 9.65, Control | CVD events in the previous 10 years | (1) BALANCE Program group, (2) control group. Control group: low-fat, low-energy, low-sodium, low-cholesterol diet; the BALANCE Program group: diet with a nutritional composition based on the Med and DASH diets | Parallel. 6 months | Decreases in SBP and DBP in BALANCE (mean (mmHg), − 6.0 and − 5.34, respectively)) and in control (− 8.4 and − 6.59, respectively) groups, with non-significant differences between groups | [38] |
| 366 women, 46.9 ± 11.1 y | BRCA1/2 mutations, with or w/o diagnosis of BC/OC and w/o metastases | Active dietary intervention group (IG) or control group (CG) | Parallel. 6 months | LOF variant carriers: decrease in SBP in IG and CG was equivalent (Δ = − 0.1 mmHg). In nonsynonymous variant carriers, SBP remained almost unaltered in IG, while an increase in CG (Δ = − 3.1 mmHg; P < 0.01) was observed | [47] |
| 96 children, 9 to 18 y | BMI > 95% age/sex predicted | (1) Plant-based diet consisting of only whole foods, including fruits, vegetables, beans, other legumes, and whole grains, limited salt, avocado and nuts, and no-added-fat. (2) AHA diet encourages eating plant-based whole foods, and low-sodium intake but permits some non-whole foods, low-fat dairy, selected plant oils, lean meat, and fish in moderation. (3) Med diet was similar to AHA (but with more emphasis given to fish and EVOO and/or nuts) | Parallel. 52 weeks, follow-up at weeks 4 and 52 | All diets were associated with similar statistically significant (P < 0.05 to < 0.001) improvements in SBP and DBP. Significant improvements in all groups at week 4 in SBP and DBP. Significant improvements at 52 weeks in both the AHA and the plant-based diet groups in SBP and DBP | [60] |
| 53, 52.8 ± 6.8y (fast + PBD) and 51.2 ± 11.5 (DGE diet) | Rheumatoid arthritis | (1) 7-day fast followed by an 11-week plant-based diet (fast + PBD) or (2) 12-week standard Deutsche Gesellschaft für Ernährung (DGE) diet | Parallel. 12 weeks | Neither dietary protocol lowered SBP, although there was a tendency to decrease DBP in the fast + plant-based diet group | [59] |
| 149, 39.63 ± 8.82 | Severely obese individuals (BMI ≥ 35.0 kg/m2) | (1) 52 mL/day of EVOO, (2) DieTBra, or 3) DieTBra + 52 mL/day of EVOO. Traditional Brazilian Diet (DieTBra): plenty of tropical fruits; raw and cooked vegetables and legumes (lunch and dinner); bread, coffee, and milk (breakfast); moderate consumption of dairy products; small portion of red meat; rare consumption of seafood and nuts; rice and beans (lunch and dinner); predominant use of soy oil | Parallel. 12 weeks | In the DieTBra + EVOO group, DBP (0.053) and SBP (P = 0.072) decreased without statistical significance. No differences between groups | [64] |
| 75, mean: 57.1 y | Low Med diet adherence, overweight, and at high CVD risk | (1) A community-based peer support intervention, (2) dietitian-led intervention, or 3) control group. (1) A community-based peer support intervention to encourage adherence to Med diet compared to a (2) dietitian-led intervention and a (3) minimal support intervention (control group) | Parallel. 12 months | No statistically significant differences between intervention groups. SBP and DBP were significantly different over time in the study population as a whole | [34] |
| 710, median (IQR): 57 (46, 63) | Overweight/obese and pre-diabetic | Available-case analysis where all participants were merged into 1 group to assess longitudinal associations of adherence to an overall plant-based diet and plant food intake with yearly changes. (1) high protein diet and high-intensity PA, (2) moderate protein diet and high-intensity PA, (3) high protein diet and moderate-intensity PA, (4) moderate-protein diet and moderate-intensity PA | Parallel. 8-week weight loss plus a 148-week intervention | Fruit and vegetable intake was inversely associated with increments in DBP. No associations between an overall plant-based diet and BP after adjustment for weight change | [168] |
| 63, 48.0 ± 10.6y | Risk of T2DM | Healthy US group (HUS, n = 21), Med group (Med, n = 22), vegetarian group (Veg, n = 20). Dietary patterns according to USDA dietary guidelines | Parallel. 12 weeks | SBP and DBP decreased in all groups (− 5.5 ± 2.7 mmHg HUS, − 3.2 ± 2.5 mmHg Med, − 2.4 ± 2.9 mmHg Veg; statistical significance only in HUS), yet changes were not significantly different between groups | [169] |
|
98, 51.1 (9.8) y (AHA group) and 49.2 (8.9) y (FLiO group) |
Overweight/obese subjects with NAFLD | (1) American Heart Association (AHA) dietary group or (2) the FLiO dietary group. The FLiO diet proposed a high adherence to the Med diet | Parallel. 2 years | DBP decreased significantly in both groups and SBP also decreased (statistical significance only in AAH diet). In both cases, values were not significantly different between groups | [44] |
| 228, 57.3 ± 9.28 y | T2DM | (1) Control group or (2) Med diet educational intervention group | Parallel. 6 months | SBP and DBP decreased significantly in comparison with the control group (P < 0.001 and P = 0.04, respectively) | [42] |
AHA American heart association, BALANCE Brazilian Cardioprotective Diet Program, BC/OC breast cancer and/or ovarian cancer, BMI body mass index, BP blood pressure, BRCA1/2 breast cancer gene 1/2, CI confidence interval, CVD cardiovascular disease, DASH Dietary Approaches to Stop Hypertension, DBP diastolic blood pressure, DieTBra traditional Brazilian diet, DIRECT diabetes remission clinical trial, EVOO extra virgin olive oil, FLiO fatty liver in obesity study, IQR interquartile range, J-DASH Japanese cuisine-based DASH, LOF loss-of-function, MABP mean arterial blood pressure, Med Mediterranean, MetS metabolic syndrome, NAFLD non-alcoholic fatty liver disease, PA physical activity, SBP systolic blood pressure, T1/2DM type 1/2 diabetes mellitus, USDA United States Department of Agriculture, VLPD very low protein diet, y years
Evidence on the Effects of Particular Plant-Based Diets
Many types of dietary patterns exist in the arena of essentially plant-based diets, including vegetarian diets and their variants, the Dietary Approach to Stop Hypertension (DASH) diet, the Mediterranean (Med) diet, the healthy Nordic diet, and many other high-fruit, vegetable, and whole grain diets.
Vegetarian Diets
Vegetarian diets usually do not include meat, poultry, or fish, although individual eating patterns do vary. Lacto-ovo-vegetarian diets are based on grains, vegetables, fruits, legumes, seeds, nuts, dairy products, and eggs, excluding meat, poultry, and fish. The lacto-vegetarian diet also excludes eggs besides meat, poultry, and fish. The vegan, or pure vegetarian diet not only excludes meat, poultry, or fish, but also dairy and additional animal products. A meta-analysis of 32 cross-sectional studies, including more than twenty-thousand individuals, indicated that a lower mean BP was related with the intake of vegetarian diets compared to omnivorous ones [10]. Accordingly, a recent meta-analysis, comprising a total of 187 participants from five controlled trials, showed a mean reduction in systolic blood pressure (SBP) (−5.47 mmHg (95% CI, −7.60, −3.34); P < 0.00001) and diastolic blood pressure (DBP) (−2.49 mmHg (−4.17, −0.80; P = 0.004) in subjects using a lacto-ovo vegetarian diet compared with the consumption of comparator diets, with a high certainty of the results for SBP and a moderate one for DBP [11]. Furthermore, a meta-analysis, which included 856 subjects from 15 randomized controlled trials (RCTs), showed that vegetarian diets significantly reduced SBP (−2.66 mmHg (−3.76, −1.55), P < 0.001) and DBP (−1.69 mmHg (−2.97, − 0.41), P < 0.001) compared to omnivorous diets [12]. In the subgroup analysis, a vegan diet showed higher decrease in SBP (−3.12 mmHg; (−4.54, −1.70), P < 0.001) compared to a lacto-ovo-vegetarian diet (−1.75 mmHg, (−5.38, 1.88), P = 0.05). The vegan diet showed a similar trend in terms of DBP reduction (−1.92 mmHg (−3.18, −0.66), P < 0.001), while those in a lacto-ovo-vegetarian diet did not. Conversely, another meta-analysis, including 677 individuals from nine controlled trials, showed that the consumption of vegan diets was not significantly associated with a mean reduction in SBP (−1.30 mmHg (−3.90, 1.29); P = 0.33) and DBP (− 0.81 mmHg (−2.91, 1.28); P = 0.45) compared with the consumption of comparator diets, although the reliability of results was low [13]. Moreover, the authors acknowledge that the use of BP-lowering comparator diets probably underrated the BP-lowering capability of vegan diets. Indeed, when the usual diet of volunteers was used as the comparator, there was a statistically significant overall effect estimate, although reliability remained low.
DASH Diet
The DASH randomized trial was designed to evaluate the effects on BP of a fundamentally plant-based diet, which included plant foods, low-fat dairy, and limited amounts of lean meat [14]. During this eight-week trial, volunteers consumed one of the following diets: (1) a “standard” American diet (control), (2) the DASH diet, or (3) a fruits and vegetables diet. Compared to the fruits and vegetables diet, the DASH diet had a reduced fat content and consisted in more daily servings of fruits and grains and fewer meat servings. The DASH diet reduced BP when compared with the control diet, especially in African Americans, and in already hypertensive subjects [15, 16]. The potential impact on BP reduction caused by a plant-based diet was verified in the DASH-sodium trial, which showed that restraining sodium led to further effects on BP reduction in combination with the DASH diet [17] and that higher BP levels at baseline were associated with an enhanced reduction of BP [18]. A recent meta-analysis comprising a total of 1400 participants from 11 controlled trials showed that SBP (−5.53 mmHg (95% CI −7.95, − 3.12); P < 0.00001) and DBP (–3.78 mmHg (–5.51 to –2.04); P < 0.0001) levels were reduced upon consumption of DASH diets compared with the consumption of comparator diets, with a high degree of certainty of the results [13].
Mediterranean Diet
The Mediterranean (Med) diet entails a moderate intake of poultry, eggs, seafood, and dairy products and a low consumption of red and processed meats. In this sense, the Med diet can be considered to a large extent to be a plant-based diet, as it comprises a high consumption of plant-based foods such as fruits, vegetables, legumes, grains, nuts, and olive oil [19]. In a meta-analysis of observational studies, including prospective and cross-sectional (four and eight, respectively) studies, representing over 30,000 individuals and 6342 cases of metabolic syndrome (MetS), BP showed an inverse significant association with high adherence to the Med diet (RR = 0.87; 95% CI: 0.77–0.97) [20]. Several RCTs have estimated the effects of the Med diet on BP, and at least three meta-analyses showed consistent results. The first one, which included six trials, comprising more than 7000 people, found that adopting a Med diet pattern for at least 1 year reduced both SBP (− 1.44 mmHg) and DBP (− 0.70 mmHg) levels [21]. Another meta-analysis included three RCTs focused on different healthy dietary patterns and reported an association between the Med diet and a reduction on SBP (− 3.02 mm Hg) and DBP (− 1.99 mm Hg) [22]. Finally, a more recent meta-analysis, comprising a total of 5276 participants from eight controlled trials, showed that SBP (− 0.95 mmHg; 95% CI, − 1.70, − 0.20); P < 0.00001) and DBP (− 0.69 mmHg (− 1.44, 0.06); P = 0.07) levels were lessened upon consumption of a Med diet compared with the consumption of comparator diets, with a moderate certainty of the results [13].
Nordic Diet
Like the DASH and Med diets, the healthy Nordic diet is essentially a plant-based dietary pattern, consisting of whole grains, fruits, vegetables, legumes, rapeseed oil, fatty fish (e.g., salmon), shellfish, seaweed, low-fat meat (e.g., poultry), and dairy, along with restraining salt and sugar-sweetened products [23]. A recent meta-analysis, comprising a total of 420 participants from three controlled trials, showed that the consumption of a healthy Nordic diet led to a reduction in SBP (− 4.47 mmHg; 95% CI, − 7.14, − 1.81); P < 0.001) and DBP (− 2.32 mmHg (− 3.83, − 0.82); P = 0.002) levels compared with the consumption of comparator diets, with a moderate certainty of the results [13].
Other High-Fruit/-Vegetable/-Fiber Diets
Diets not fitting in the previous categories, but that could still be contained in the plant-based diet arena include the ones characterized by increased consumption of fruit and vegetables and high-fiber diets focused on increasing whole-grain and legume consumption. In a meta-analysis including 140 subjects from two RCTs, high-fruit/high-vegetable diets were linked with reduction in SBP (− 0.57 mmHg (− 7.45, 6.32); P = 0.87) and DBP (− 0.96 mmHg (− 3.08, 1.15); P = 0.37) compared with the consumption of comparator diets [13]. Similarly, consumption of high-fiber diets resulted in a decrease in SBP (− 0.65 mmHg (− 1.83, 0.53); P = 0.28) and DBP (− 1.02 mmHg (− 3.86, 1.82); P = 0.48) compared with the consumption of comparator diets (meta-analysis with 316 subjects from two RCTs) [13]. However, in both cases, the certainty of evidence was extremely low.
In line with the results described above, Dinu and colleagues published the first umbrella review focusing on the effects of different popular diets on cardiometabolic risk factors [24•]. Regarding plant-based diets and BP outcomes, the review included meta-analyses of RCTs assessing the DASH (n = 6), Mediterranean (n = 11), vegetarian (n = 9), and Nordic (n = 2) diets, among others. Overall, the findings of this review support that balanced dietary patterns, favoring vegetables, fruits, whole grains, and plant-based protein, and limit sugar, sodium, and red and processed meat, are beneficial in adverse cardiometabolic scenarios. Notably, DASH and, especially, Med diets showed the most consistent findings regarding a reduction in BP accompanied by improvements in other parameters, whereas for the Nordic and vegetarian dietary patterns, only weak evidence of a beneficial effect in BP levels was found. Nevertheless, the authors emphasize that around 80% of the meta-analyses included in the study suffered from low methodological quality and weak strength of evidence and included, for many diets, a reduced number of clinical trials. Furthermore, the authors acknowledge the limitations concerning the understanding, meaning, and applicability of findings in clinical practice. For instance, meta-analyses, in many cases, included RCTs performed on individuals in a variety of pathological settings and the extrapolation of results to the general population is not straightforward.
Evidence Coming from Recent RCTs
In the last 3 years, evidence generated from RCTs on the effect of plant-based diets has been plentiful. Limiting this list to trials where at least one arm included a recognizable plant-based diet as the main exposure of interest and where BP and/or hypertension were outcomes of interest returned 39 papers (Table 1). Most studies concern the exposure of overweight/obese subjects at (high) risk of cardiometabolic disease, e.g., metabolic syndrome, type 2 diabetes mellitus (T2DM), and non-alcoholic fatty liver disease (NAFLD), to diets based on the Mediterranean or DASH dietary patterns, although vegan and vegetarian diets, among others, were also investigated. Healthy/pathological context scenarios other than the above mentioned include rheumatoid arthritis and chronic kidney disease, etc. The main results of these studies regarding BP values and/or hypertension reinforce the conclusions of the above-mentioned umbrella review and several other meta-analyses. Sub-studies from the PREDIMED and PREDIMED-Plus trials confirm that a high adherence to the Med diet is associated with lower BP values (vide infra). Of note, non-treated participants following a Med diet showed less need for the use of antihypertensive drugs [25]. Indeed, Med diet adherence was associated with decreased need of escalating antihypertensive therapy in patients who were using two drugs at baseline and attenuated the association of antihypertensive drug use with the risk of cardiovascular events [25]. As for the PREDIMED-Plus trial, at baseline, a tendency was found towards a lower Med diet adherence for participants with the highest validated MetS severity score, which (among many other parameters showing unfavorable readings) were characterized by high BP levels [26]. On the other hand, also at baseline, adherence to eight a priori high-quality dietary scores did not show inverse associations with hypertension [27]. Longitudinal analysis showed that the reductions in SBP and DBP were enhanced with higher carbohydrate quality index (categorized in quintiles and based on four criteria: total dietary fiber intake, glycemic index, whole grain/total grain ratio, and solid carbohydrate/total carbohydrate ratio) [28]. Decreases in SBP were significantly associated with increased nut consumption [29], although results from a 2-month crossover RCT showed non-significant intra- and inter-group differences in SBP and DBP in MetS subjects who consumed a Med diet or a non-Med diet plus nuts (50 g/day) [30]. Some studies propose that, alongside a higher adherence to Med diet, other factors, such as physical activity, low sedentary time, or low depression risk, could contribute to enhance the positive effects seen on hypertension (among other CVD risk factors) [31, 32].
Apart from the PREDIMED trial, in the last 3 to 4 years, many RCTs on the effects of Med diets have been published in different physio-pathological contexts. Adherence to Mediterranean-based dietary patterns was associated with favorable effects on BP in apparently healthy individuals [33], overweight/obese at high risk of CVD [34-38], MetS [39], prediabetic [40], T1DM [41], T2DM [42], NAFLD [43, 44], heart or lung transplant recipients [11], obstructive sleep apnea [31], HIV [45], rheumatoid arthritis [46], and cancer patients [47], among others. However, in general, the differences observed were not significant between the intervention groups on the Med diet and other groups. In some cases, neither intra-group nor inter-group significant differences were found, as was the case of individuals without major CVD who consumed the Atlantic diet, for 6 months. This diet has several characteristics in common with the Med diet such as high consumption of vegetables, fruits, whole grains, beans, and olive oil as a key fat source. It is also characterized by high intake of fish and seafood, starch-based products, nuts, milk, and cheese. In professional female handball players who consumed a free diet, a Med diet, or a high antioxidant diet, for 12 weeks, there were no significant differences in BP over time or between groups [48, 49].
Regarding the effects of DASH-based diets on BP, a longitudinal analysis was conducted with one-year data of changes in the DASH diet score and its association with cardiometabolic risk factors in PREDIMED-Plus trial [50]. After adjusting for several potential confounders, higher DASH diet scores were significantly associated with lower SBP (− 0.57 mmHg) and DBP (− 0.15 mmHg). Furthermore, in a RCT with individuals with untreated high normal BP or stage 1 hypertension, Japanese cuisine-based DASH (J-DASH) diets, which are in accordance with the nutritional composition of the DASH diet (but have less total fat and saturated fatty acids), improved home measured BP and stabilized its variability compared to a group who consumed their usual diet [51]. An investigational diet mimicking the average East Asian diet, but retaining common characteristics of the Med and DASH diets, also produced positive effects on BP, in subjects with T2DM [52]. Finally, in CVD risk patients with MetS, a modified DASH diet with additional emphasis on plant-based and Med diets to optimize refeeding also led to a reduction in SBP after 3 months. According to the results of this study, volunteers who had a 5-day fasting prior to following the modified DASH diet showed a sustained reduction both in 24-h ambulatory SBP and mean arterial BP (MABP). Indeed, subjects undergoing fasting reduced their intake of antihypertensive medication in 43% of cases, whereas on DASH alone this happened in 17% of cases.
In the context of RCTs aimed at assessing the effects of vegan and/or vegetarian diets where BP values were evaluated a few studies are worth discussing. In a crossover RCTwith overweight individuals, SBP and DBP values decreased (−9.3 and −7.3 mmHg, respectively) in subjects on a Med diet compared with a vegan diet (−3.4 and − 4.1 mmHg, respectively) [53]. In contrast, 6 months of a vegetarian diet (very-low-protein diet) significantly lowered SBP and DBP more effectively than a Med diet or a Med diet supplemented with essential amino acids and ketoanalogs (Med diet + KA) [54]. In another study, subjects with cardiometabolic risk factors were instructed to consume ad libitum whole-food plant-based diets, consisting of vegetables, grains, legumes, and fruits and avoidance of animal products. Compared to the control group, who consumed an omnivorous diet, there was a tendency for more favorable effects in SBP and DBP [55]. In another study, a low-carbohydrate vegan diet, high in canola oil and plant proteins, was compared to a vegetarian therapeutic diet in type 2 diabetics [56]. There were no changes in BP medications and no treatment differences in BP. However, within treatment, significant reductions in SBP and DBP were seen on the low-carbohydrate vegan (−4.12 and −3.54 mmHg, respectively) and on the vegetarian diet (−5.91 and −4.13 mmHg, respectively). Still on vegetarian diets, a plant-based diet built on the USDA healthy vegetarian meal plan, with modifications to exclude eggs and dairy products, was compared with the same diet except for eggs being permitted (2 eggs/d for 6 weeks while preserving an isocaloric condition) in individuals at risk of T2DM. SBP, DBP, and MABP decreased from baseline in both groups (statistical significance was only found for plant-based diet + eggs), yet changes were not significantly different between groups [57]. On the other hand, a plant-based diet with either 2 eggs/d or the equivalent amount of egg substitute showed no effect in SBP and DBP in MetS subjects [58]. A study carried out in prediabetic subjects who consumed one of three isocaloric-restricted diets: (1) Med diet, (2) a traditional Jiangnan diet high in plants, or (3) a control diet low in plants, for 6 months, showed significant decreases from baseline in SBP and DBP for all diets but differences between groups were non-significant [40]. In a another RCT, rheumatoid arthritis patients completed a 7-day fast followed by an 11-week plant-based diet or a 12-week standard Deutsche Gesellschaft für Ernährung (DGE) diet [59]. Neither dietary protocol lowered SBP, although there was a tendency to decrease DBP in the fasting + plant-based diet group. In another study, all 3 healthy eating patterns: (1) plant-based diet, (2) AHA diet, or (3) Med diet, were associated with similar statistically significant improvements in SBP and DBP, in children with BMI > 95% age/sex predicted [60].
In T2DM [61] and in normotensive women [62], increasing the amount of vegetable, fruit and whole grain dietary intake produced favorable effects in BP. On the other hand, BP did not improve in children, adolescents, and adults included in an intervention group instructed to eat more healthy food items, such as fruits and vegetables, legumes, fish, nuts and seeds, and olive oil, compared to the control group [63]. Furthermore, 12 weeks of consumption of a traditional Brazilian Diet (DieTBra), consisting of plenty of tropical fruits, rice and beans, raw and cooked vegetables and legumes, moderate consumption of dairy products, small portion of red meat, and predominant use of soy oil, did not produce a significant reduction in BP in severely obese individuals (BMI ≥ 35.0 kg/m2) [64].
Plant-Based Diet Components with Hypotensive Actions
Vitamin C
Fruits and vegetables are sources of vitamin C, whose effects on BP have been investigated both in clinical trials and in basic biochemistry experiments. Indeed, there is solid evidence from clinical studies that vitamin C treatment restores endothelial function in patients with coronary artery disease or coronary risk factors [65-67]. Of note, there are at least two meta-analyses [68, 69] that conclude that provision of vitamin C (usually 500 mg/d) significantly reduce SBP and DBP and that this effect is more pronounced in hypertensive patients. Yet, it is unfortunate that the near totality of internists or cardiologists do not prescribe ascorbic acid as supplement.
In terms of mechanisms of action, there is clear biochemical evidence that ascorbic acid stimulates endothelium-derived nitric oxide (EDNO) synthesis [70, 71] and that it does so mainly by increasing the intracellular levels of tetrahydrobiopterin, i.e., the most important cofactor for eNOS activity [72, 70]. Even though ascorbic acid is an important antioxidant [73, 74], it does not affect GSH levels in human aortic endothelial cells (HAEC), and it does not increase the GSH/GSSG ratio. Therefore, based on the literature [72, 71], it is unlikely that ascorbic acid directly improves the intracellular redox environment and, consequently, EDNO synthesis. The most conceivable mechanism of action involves the maintenance or even increase of tetrahydrobiopterin levels by reducing dihydro- or trihydrobiopterin back to tetrahydrobiopterin [75], as also shown by basic experiments in which ascorbic acid was found to stimulate purified eNOS activity directly, despite limiting concentrations of tetrahydrobiopterin and without the contribution of GSH [72]. One caveat is that data obtained from in vitro experiments should be interpreted with caution, as culture media are devoid of vitamin C. Indeed, cultured cells are in scorbutic states [76] and exogenous vitamin C first replenishes their stores and then, eventually, exerts biological effects [77].
A critical issue is that of the optimal dose of vitamin C that augments BP. The only complete pharmacokinetic study carried out with vitamin C is that of Levine et al. [78••], who concluded that vitamin C daily doses above 400 mg have no evident value. Also, a review of over 200 articles on vitamin C and health concluded that a daily intake of 100 mg of vitamin C is associated with lower incidence of heart disease, stroke, and cancer [79]. It should be noted that these amounts (1) are consistent with those reported by Levine et al. to saturate cells [78••]; (2) are consistent with the maximal velocity of the vitamin C transporter [80]; and (3) are achievable through a balanced diet [81]. Indeed, it is worth underscoring that, despite some propaganda on mega-doses, ascorbic acid’s concentrations are tightly regulated in the body [82, 83]. One way to overcome such limitations is to administer vitamin C intravenously [84] in order to reach millimolar plasma concentrations. However, the scant clinical trials of intravenous ascorbic acid for cancer treatment yielded negative results [85] and much more well-performed research on a large number of patients is needed to clarify this issue. However, pertinent to this review, Bruno and colleagues demonstrated that acute vitamin C infusion (3 g i.v. in 5 min) significantly lowers BP in hypertensive patients, but not in normotensive subjects [86]. These data confirm those of Heitzer et al. [87], who reported anti-hypertensive effects vitamin C infusion is smokers (whose endothelial function is notoriously impaired). Hence, there might be room for future high-quality trials in hypertensive patients, even though it might be quite impractical to perform ascorbic acid infusions in an outpatient setting.
In summary, ascorbic acid may play a key role in the plant-based diet beneficial effect on BP. These effects are likely not mediated by redox modulation and involve maintenance of cofactors such as tetrahydrobiopterin [88]. Based on the literature, we advocate the use of vitamin C by clinicians and patients as adjunct therapy of hypertension.
Potassium
Potassium is the most abundant intracellular mineral, and its role in the regulation of BP is well established [89], even if there is no agreement among authors [90]. Unfortunately, the contribution of potassium to the healthful effects of a plant-based diets is often overlooked [91] in favor of, e.g., (poly)phenols (vide infra). However, many vegetables such as green leafy vegetables and some fruits such as bananas are rich in potassium [92], which might at least in part explain [93] why vegetarian diets are associated with significant reductions in BP compared with omnivorous diets [12]. Historically, humans have been consuming quantities of potassium that were much higher than the current ones [94]. Life expectancy was much shorter, but current potassium intakes are often much lower than the recommended levels [95].
The first clinical evidence of the effects of potassium on BP is likely that of WL Addison [96], and some researchers have been investigating the mechanisms of actions that underlie potassium’s BP-lowering activities [97, 93]. One proposed mechanism involves the stimulatory effects of potassium on the sodium–potassium ATPase, which hyperpolarizes cells [98], hence decreasing cytosolic calcium concentrations and leading to augmented vasomotion. Also, potassium has been suggested to directly stimulate EDNO synthesis [99] and increase natriuresis [99, 91, 97] by inhibiting renal sodium reabsorption.
Finally, we propose an indirect effect of high potassium intake, i.e., that high consumption via a plant-based diet largely replaces that of sodium, either as a natural component of meats (especially smoked and cured meats), some comfort foods, e.g., pizza and savory snacks, or added as part of salt to enhance flavor and taste [100].
(Poly)phenols
As mentioned, nitric oxide synthesis can be modulated by dietary factors [101]. Many constituents of plants and, hence, plant-based diets are non-nutritional compounds that play key roles in plant physiology and interactions with the environment [102, 103]. The most actively studied minor components are (poly)phenols, which have been long investigated for their supposed antioxidant activities. Indeed, there is negative correlation between (poly)phenols intake and blood pressure [104]. Even though research has disproven their direct antioxidant actions, their effects on various enzyme activities appear to be even more interesting in terms of health protection: research in this area should be and, indeed, is further extensively promoted [105].
Among the various biological activities of (poly)phenols, their actions on endothelial function have been researched by various groups. Our own group reported the vasomodulating activities of wild plant extracts rich in (poly)phenols (namely wild artichoke and thyme) in vitro [106] and in vivo [107]. Others studied the vasodilator effects of olive extracts, rich in oleuropein, hydroxytyrosol [108, 109], and hypotensive peptides [110, 111] and used as decoction in folk medicine [112, 113].
As mentioned, direct antioxidant, free radical-scavenging activities of (poly)phenols have been ruled out due to their poor bioavailability and reactivity [114•, 115]. However, in biochemical terms, plasma membrane-associated eNOS is considered to be more constitutively active and extremely sensitive to agonist-induced intracellular Ca+2 fluxes. In addition to post-translational regulatory mechanisms dictating eNOS function, redox changes in the endothelium do indeed modify both enzyme activity as well as EDNO production, in turn adversely affecting vasomotion [116]. As discussed for ascorbic acid, eNOS synthetic activity is dependent on maintaining tetrahydrobiopterin in a reduced state. When the tetrahydrobiopterin/biopterin ratio is high eNOS produces NO, however, when that redox ratio declines, the internal electron transport chain of eNOS becomes uncoupled, which actually generates superoxide anion rather than NO [117]. Thus, in a pro-oxidative milieu [115], eNOS may not only become a target of oxidant species, but also may exacerbate oxidative stress and endothelial dysfunction, hence impairing vasomotion and leading to hypertension. In brief, even though (poly)phenols are not direct antioxidants, they contribute to maintain a proper endothelial redox status and contribute to the hypotensive actions of a fruit- and vegetable-rich diet.
In addition to epidemiological data (often related to the Mediterranean diet [118]) linking a plant-based diet with lower blood pressure [119], some human studies provided direct evidence of the (poly)phenol-induced increase in vasomotion. Three examples are worth mentioning. One is tea, whose intake (chronic or acute) increases flow-mediated dilation (FMD) in healthy subjects and hypertensive patients [120, 121]. The effects are highly likely due to catechin and its derivatives, given the juxtaposition of their plasma kinetics with FMD [122]. Another (poly)phenol-rich food that has been extensively studied is cocoa/chocolate [123, 124]; again, the most active component appears to be catechin, acting through mechanisms of actions that are only partly elucidated and likely fit with what we described above. Finally, recent research is addressing beetroot juice as functional food with remarkable hypotensive actions [125, 126]. In reason of its vasomodulating actions, beetroot juice is being used as a sport supplement [127] and attributed with ergogenic effects that need to be confirmed by human trials [127, 128].
In summary, (poly)phenols are a likely important contributor to the hypotensive actions of plant-based dietary regimens. Even though the extent and true nature of their biochemical actions has not been fully elucidated, it is very likely that a combination of indirect antioxidant activities (thereby maintaining eNOS function and avoiding its uncoupling), anti-inflammatory actions, direct eNOS hydroxylation, antagonism of L-type Ca2+ channels [129], etc. contributes to the augmented vasomotion associated with clinical trials and demonstrated by some human experiments.
Omega 3 Fatty Acids
Fatty acids of the omega 3 (or n-3) series, i.e., alfa-linolenic (18:3; ALA), eicosapentaenoic (20:5, EPA), and docosahexaenoic (22:6, DHA), are essential and must be derived from the diet [130]. Their cardiovascular effects have been the subject of much investigation [131] that — from a clinical point of view — led to mostly inconclusive results [132-135].
Among the potentially healthful actions of omega 3 fatty acids, modulation of endothelial function and, hence, of blood pressure has been proposed. The rationale behind the purported activities of omega 3 fatty acids on the endothelium and vasomotion is manifold. For example, omega 3 fatty acids are anti-inflammatory molecules [136] and inflammation greatly participates in the development of, e.g., arterial stiffness [117], impaired vasomotion [137], and hypertension [138]. Moreover, that are — paradoxically — anti- rather than pro-oxidant [139, 140], via molecular mechanisms that have been largely elucidated and include, e.g., inhibition of NADPH oxidase 4 (Nox 4), V sPLA2, and cyclooxygenases, as well as reduced superoxide production [140], thereby maintaining an adequate “peroxide tone” [141].
Human studies of the effects of omega 3 fatty acids on vascular tone and blood pressure yielded mixed results. For example, a recent publication by Bischoff-Ferrari et al. [142] reported that administration of 1 g/d of algal oil (330 mg of EPA and 660 mg DHA) had no significant effects in improving systolic and diastolic blood pressures of older adults. Yet, the uncertainty surrounding the hypotensive effects of omega 3 might depend on several factors such as the dose and the choice of patients. Accordingly, a recent dose–response metanalysis by Zhang et al. [143] reported a nonlinear dose–response relationship for systolic and diastolic blood pressure. The computed J-shaped curves implicate that dosages of EPA + DHA between 2 and 3 g/day (combined) were associated with the strongest effects. A slightly older metanalysis published by Guo and coworkers [144] concluded that provision of EPA and DHA leads to a significant reduction in systolic (EPA) and diastolic (DHA) blood pressure in subjects with dyslipidemia or with high C-reactive protein (CRP) concentrations, i.e., at high risk of cardiovascular disease. Whether such effects are due to a direct action of EPA and DHA on the endothelium or to the reduction of CRP concentrations, i.e., anti-inflammatory effects (or a combination of both mechanisms), remains to be elucidated. In this respect, Mori et al. [145] showed that omega 3 fatty acids, in particular DHA rather than EPA, improved vasodilator responses to endogenous and exogenous NO donors and attenuated vasoconstrictor response to norepinephrine in the forearm microcirculation of humans. The mechanisms appeared to be predominantly endothelium independent, based on enhanced vasodilatory responses after the co-infusion of acetylcholine with L-NMMA and the infusion of nitroprusside, both of which are endothelium independent.
As regards ALA (the vegetal omega 3 fatty acid), there are — to the best of our knowledge — no large RCTs exploring its effects on blood pressure, even when given at high doses [146]. However, the use of flaxseed products results in significant reductions of diastolic and systolic blood pressures [147], which might be at least in part due to its high content of ALA. The same applies to a nutraceutical providing Perilla Frutescens powder [148] and to high walnut use by elderly subjects [149]. Mechanistically, it is worth reminding that ALA is converted into oxylipins [150] with biological activities in the vessel wall. In short, epidemiological evidence is strongly suggestive of the cardioprotective actions of ALA [151], which might include modulation of blood pressure [152]. Clinical trials are scant and not concordant, calling for further research on this medium-chain fatty acid.
To summarize, a plant-based diet provides polyunsaturated fatty acids that, when consumed in high proportions, likely exert cardioprotective effects. Whether and to what extent such effects are modulated by augmented vasomotion and BP control is still unclear and requires more research.
Omega 6 Fatty Acids
With some exceptions, e.g., foods elaborated with coconut and palm oils, plant-based diets provide a proportionally high amount of omega 6 fatty acids, largely found in seed oils such as safflower, sunflower, and corn oils and in seeds and nuts, e.g., walnuts, almonds, hemp seeds, or cashews [153].
As opposed to their omega 3 counterparts, omega 6 fatty acids, e.g., linoleic acid (LA), cannot be studied as drugs or supplements because their levels of consumption are much higher than those of omega 3. Therefore, most of the data we have on omega 6 and human health come from observational studies. Further, LA is the precursor of arachidonic acid and, consequently, of pro-inflammatory eicosanoids. Hence, the hypothesis was formulated that we should reduce omega 6 consumption to avoid increasing systemic, low-grade inflammation, i.e., one of the major etiological agents in degenerative diseases (vide infra) [154].
This hypothesis has been disproven [155], and some clinical studies of low-to-modest quality did evaluate the effects of PUFA-rich diets on BP. Due to aforementioned difficulties inherent to omega 6 research, such studies should be interpreted as a “replacement” of one class of fatty acids with another one.
Some observational studies did not find any significant effect of higher intakes of omega 6 fatty acids on systolic and diastolic pressures [156-159]. One example is the International Study of Macro-Micronutrients and Blood Pressure, which enrolled 4680 subjects and analyzed the proportion of dietary LA. The authors reported a non-significant, inverse association between LA use and BP (systolic and diastolic) [160]. Yet, a sub-analysis of the control group, i.e., people who were free from cardiovascular diseases or diabetes, did not follow a special diet, and did not take food supplements or medicines, found that BP was modestly yet significantly reduced (− 1.4/ − 0.9 mmHg for each 3.8% more calories from LA) [160]. Other studies [161], nonetheless, did not confirm those data and a metanalysis reported no important effects of omega 6 fatty acids on either systolic or diastolic BP [162].
To summarize, omega 6 fatty acids, namely LA, do not appear to have relevant and clinically-important effects on BP. Therefore, the cardioprotective activities of appropriate omega 6 intakes [163, 164] are likely due to their cholesterol-lowering actions rather than to their effects on BP.
Conclusions
The data discussed in this systematic review allow us to conclude that plant-based diets are associated with lower BP and overall better health outcomes (namely, on the cardiovascular system) when compared with animal-based diets. The mechanisms of action are being actively investigated and involve many micronutrients plentiful in plants and the dishes prepared with them. We also stress the need to look at human health as closely intertwined with planetary health, as it is necessary to consider the overall “environmental pressure” of food production. In general, animal-based diets have a greater impact than plant-based ones; for this reason, the latter should be promoted in a “one health” framework [165].
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Data Availability
All data presented are contained within this manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.•• Appleby PN, Davey GK, Key TJ. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public health nutrition. 2002;5(5):645–54. 10.1079/phn2002332. Huge cohort study that started research on BP and diets. [DOI] [PubMed]
- 2.Pettersen BJ, Anousheh R, Fan J, Jaceldo-Siegl K, Fraser GE. Vegetarian diets and blood pressure among white subjects: results from the Adventist Health Study-2 (AHS-2) Public Health Nutr. 2012;15(10):1909–1916. doi: 10.1017/s1368980011003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, et al. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the coronary artery risk development in young adults (CARDIA) study. Am J Clin Nutr. 2005;82(6):1169–77; quiz 363–4. 10.1093/ajcn/82.6.1169. [DOI] [PubMed]
- 4.Borgi L, Curhan GC, Willett WC, Hu FB, Satija A, Forman JP. Long-term intake of animal flesh and risk of developing hypertension in three prospective cohort studies. J Hypertens. 2015;33(11):2231–2238. doi: 10.1097/hjh.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Manson JE, Buring JE, Sesso HD. Meat intake and the risk of hypertension in middle-aged and older women. J Hypertens. 2008;26(2):215–222. doi: 10.1097/HJH.0b013e3282f283dc. [DOI] [PubMed] [Google Scholar]
- 6.Miura K, Greenland P, Stamler J, Liu K, Daviglus ML, Nakagawa H. Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men: the Chicago Western Electric Study. Am J Epidemiol. 2004;159(6):572–580. doi: 10.1093/aje/kwh085. [DOI] [PubMed] [Google Scholar]
- 7.Golzarand M, Bahadoran Z, Mirmiran P, Sadeghian-Sharif S, Azizi F. Dietary phytochemical index is inversely associated with the occurrence of hypertension in adults: a 3-year follow-up (the Tehran Lipid and Glucose Study) Eur J Clin Nutr. 2015;69(3):392–398. doi: 10.1038/ejcn.2014.233. [DOI] [PubMed] [Google Scholar]
- 8.Chuang SY, Chiu TH, Lee CY, Liu TT, Tsao CK, Hsiung CA, et al. Vegetarian diet reduces the risk of hypertension independent of abdominal obesity and inflammation: a prospective study. J Hypertens. 2016;34(11):2164–2171. doi: 10.1097/hjh.0000000000001068. [DOI] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A et al. Vegetarian diets and blood pressure: a meta-analysis. JAMA Internal Medicine. 2014;174(4):577–87. 10.1001/jamainternmed.2013.14547. [DOI] [PubMed]
- 11.Entwistle TR, Miura K, Keevil BG, Morris J, Yonan N, Pohl M, et al. Modifying dietary patterns in cardiothoracic transplant patients to reduce cardiovascular risk: The AMEND-IT Trial. Clinical transplantation. 2021;35(2):e14186. 10.1111/ctr.14186. [DOI] [PubMed]
- 12.Lee KW, Loh HC, Ching SM, Devaraj NK, Hoo FK. Effects of vegetarian diets on blood pressure lowering: a systematic review with meta-analysis and trial sequential analysis. Nutrients. 2020;12(6). 10.3390/nu12061604. [DOI] [PMC free article] [PubMed]
- 13.Gibbs J, Gaskin E, Ji C, Miller MA, Cappuccio FP. The effect of plant-based dietary patterns on blood pressure: a systematic review and meta-analysis of controlled intervention trials. J Hypertens. 2021;39(1):23–37. doi: 10.1097/hjh.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 14.Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, et al. Descriptive characteristics of the dietary patterns used in the dietary approaches to stop hypertension trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8 Suppl):S19–27. 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed]
- 15.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–24. 10.1056/nejm199704173361601. [DOI] [PubMed]
- 16.Svetkey LP, Simons-Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the dietary approaches to stop hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159(3):285–293. doi: 10.1001/archinte.159.3.285. [DOI] [PubMed] [Google Scholar]
- 17.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. 10.1056/nejm200101043440101. [DOI] [PubMed]
- 18.Juraschek SP, Miller ER, 3rd, Weaver CM, Appel LJ. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70(23):2841–2848. doi: 10.1016/j.jacc.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M, Ros E. Benefits of the Mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis. 2015;58(1):50–60. doi: 10.1016/j.pcad.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Godos J, Zappalà G, Bernardini S, Giambini I, Bes-Rastrollo M, Martinez-Gonzalez M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: a meta-analysis of observational studies. Int J Food Sci Nutr. 2017;68(2):138–148. doi: 10.1080/09637486.2016.1221900. [DOI] [PubMed] [Google Scholar]
- 21.Nissensohn M, Román-Viñas B, Sánchez-Villegas A, Piscopo S, Serra-Majem L. The effect of the Mediterranean diet on hypertension: a systematic review and meta-analysis. J Nutr Educ Behav. 2016;48(1):42–53.e1. doi: 10.1016/j.jneb.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary patterns and blood pressure in adults: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2016;7(1):76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamsson V, Reumark A, Fredriksson IB, Hammarström E, Vessby B, Johansson G, et al. Effects of a healthy Nordic diet on cardiovascular risk factors in hypercholesterolaemic subjects: a randomized controlled trial (NORDIET) J Intern Med. 2011;269(2):150–159. doi: 10.1111/j.1365-2796.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 24.• Dinu M, Pagliai G, Angelino D, Rosi A, Dall'Asta M, Bresciani L, et al. Effects of popular diets on anthropometric and cardiometabolic parameters: an umbrella review of meta-analyses of randomized controlled trials. Adv Nutr. 2020;11(4):815–33. 10.1093/advances/nmaa006. An umbrella review of the most pupular healthy diets and their cardiovascular effects. [DOI] [PMC free article] [PubMed]
- 25.Ribó-Coll M, Lassale C, Sacanella E, Ros E, Toledo E, Sorlí JV, et al. Mediterranean diet and antihypertensive drug use: a randomized controlled trial. J Hypertens. 2021;39(6):1230–1237. doi: 10.1097/hjh.0000000000002765. [DOI] [PubMed] [Google Scholar]
- 26.Gallardo-Alfaro L, Bibiloni MDM, Mascaró CM, Montemayor S, Ruiz-Canela M, Salas-Salvadó J, et al. Leisure-time physical activity, sedentary behaviour and diet quality are associated with metabolic syndrome severity: the PREDIMED-Plus study. Nutrients. 2020;12(4). 10.3390/nu12041013. [DOI] [PMC free article] [PubMed]
- 27.Alvarez-Alvarez I, Toledo E, Lecea O, Salas-Salvadó J, Corella D, Buil-Cosiales P, et al. Adherence to a priori dietary indexes and baseline prevalence of cardiovascular risk factors in the PREDIMED-Plus randomised trial. Eur J Nutr. 2020;59(3):1219–1232. doi: 10.1007/s00394-019-01982-x. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-González MA, Fernandez-Lazaro CI, Toledo E, Díaz-López A, Corella D, Goday A, et al. Carbohydrate quality changes and concurrent changes in cardiovascular risk factors: a longitudinal analysis in the PREDIMED-Plus randomized trial. Am J Clin Nutr. 2020;111(2):291–306. doi: 10.1093/ajcn/nqz298. [DOI] [PubMed] [Google Scholar]
- 29.Julibert A, Del Mar BM, Gallardo-Alfaro L, Abbate M, Martínez-González M, Salas-Salvadó J, et al. Metabolic syndrome features and excess weight were inversely associated with nut consumption after 1-year follow-up in the PREDIMED-Plus study. J Nutr. 2020;150(12):3161–3170. doi: 10.1093/jn/nxaa289. [DOI] [PubMed] [Google Scholar]
- 30.Galié S, García-Gavilán J, Papandreou C, Camacho-Barcía L, Arcelin P, Palau-Galindo A, et al. Effects of Mediterranean diet on plasma metabolites and their relationship with insulin resistance and gut microbiota composition in a crossover randomized clinical trial. Clin Nutr. 2021;40(6):3798–3806. doi: 10.1016/j.clnu.2021.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Georgoulis M, Yiannakouris N, Kechribari I, Lamprou K, Perraki E, Vagiakis E, et al. Cardiometabolic benefits of a weight-loss mediterranean diet/lifestyle intervention in patients with obstructive sleep apnea: the “MIMOSA” randomized clinical trial. Nutrients. 2020;12(6). 10.3390/nu12061570. [DOI] [PMC free article] [PubMed]
- 32.Gallardo-Alfaro L, Bibiloni MDM, Bouzas C, Mascaró CM, Martínez-González M, Salas-Salvadó J, et al. Physical activity and metabolic syndrome severity among older adults at cardiovascular risk: 1-year trends. Nutr Metab Cardiovasc Dis. 2021;31(10):2870–2886. doi: 10.1016/j.numecd.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Murphy KJ, Dyer KA, Hyde B, Davis CR, Bracci EL, Woodman RJ et al. Long-term adherence to a Mediterranean diet 1-year after completion of the MedLey study. Nutrients. 2022;14(15). 10.3390/nu14153098. [DOI] [PMC free article] [PubMed]
- 34.McEvoy CT, Moore S, Erwin C, Kontogianni M, Wallace SM, Appleton KM, et al. Trial to Encourage Adoption and Maintenance of a MEditerranean Diet (TEAM-MED): a randomised pilot trial of a peer support intervention for dietary behaviour change in adults from a Northern European population at high CVD risk. Br J Nutr. 2022;128(7):1322–1334. doi: 10.1017/s0007114521003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenshluger A, Constantini K, Goldstein N, Shelef I, Schwarzfuchs D, Zelicha H, et al. Effect of dietary strategies on respiratory quotient and its association with clinical parameters and organ fat loss: a randomized controlled trial. Nutrients. 2021;13(7). 10.3390/nu13072230. [DOI] [PMC free article] [PubMed]
- 36.Yokose C, McCormick N, Rai SK, Lu N, Curhan G, Schwarzfuchs D, et al. Effects of low-fat, mediterranean, or low-carbohydrate weight loss diets on serum urate and cardiometabolic risk factors: a secondary analysis of the dietary intervention randomized controlled trial (DIRECT) Diabetes Care. 2020;43(11):2812–2820. doi: 10.2337/dc20-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osorio-Conles Ó, Olbeyra R, Moizé V, Ibarzabal A, Giró O, Viaplana J, et al. Positive effects of a Mediterranean diet supplemented with almonds on female adipose tissue biology in severe obesity. Nutrients. 2022;14(13). 10.3390/nu14132617. [DOI] [PMC free article] [PubMed]
- 38.Bersch-Ferreira AC, Hall WL, Santos RHN, Torreglosa CR, Sampaio G, Tereza da Silva J, et al. The effect of the a regional cardioprotective nutritional program on inflammatory biomarkers and metabolic risk factors in secondary prevention for cardiovascular disease, a randomised trial. Clin Nutr. 2021;40(6):3828–35. 10.1016/j.clnu.2021.04.035. [DOI] [PubMed]
- 39.Asoudeh F, Fallah M, Djafarian K, Shirzad N, Clark CCT, Esmaillzadeh A. The effect of Mediterranean diet on inflammatory biomarkers and components of metabolic syndrome in adolescent girls. J Endocrinol Invest. 2023 doi: 10.1007/s40618-023-02027-1. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y, Wang J, Sun L, Gu W, Zong G, Song B, et al. Isocaloric-restricted Mediterranean diet and Chinese diets high or low in plants in adults with prediabetes. J Clin Endocrinol Metab. 2022;107(8):2216–2227. doi: 10.1210/clinem/dgac303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimosthenopoulos C, Liatis S, Kourpas E, Athanasopoulou E, Driva S, Makrilakis K, et al. The beneficial short-term effects of a high-protein/low-carbohydrate diet on glycaemic control assessed by continuous glucose monitoring in patients with type 1 diabetes. Diabetes Obes Metab. 2021;23(8):1765–1774. doi: 10.1111/dom.14390. [DOI] [PubMed] [Google Scholar]
- 42.Zahedi M, Akhlagh SA, Aboomardani M, Alipoor R, Hosseini SA, Shahmirzadi AR. Efficacy of mediterranean diet on blood biochemical factors in type II diabetic patients: a randomized controlled trial. Gazi Medical Journal. 2021;31(4A):714–8. 10.12996/GMJ.2020.166.
- 43.Montemayor S, Mascaró CM, Ugarriza L, Casares M, Llompart I, Abete I, et al. Adherence to Mediterranean diet and NAFLD in patients with metabolic syndrome: the FLIPAN study. Nutrients. 2022;14(15). 10.3390/nu14153186. [DOI] [PMC free article] [PubMed]
- 44.Marin-Alejandre BA, Cantero I, Perez-Diaz-Del-Campo N, Monreal JI, Elorz M, Herrero JI, et al. Effects of two personalized dietary strategies during a 2-year intervention in subjects with nonalcoholic fatty liver disease: a randomized trial. Liver international: official journal of the International Association for the Study of the Liver. 2021;41(7):1532–1544. doi: 10.1111/liv.14818. [DOI] [PubMed] [Google Scholar]
- 45.Stradling C, Thomas GN, Hemming K, Taylor S, Taheri S. Randomized parallel-group pilot trial (best foods for your heart) comparing the effects of a Mediterranean Portfolio diet with a low saturated fat diet on HIV dyslipidemia. Clin Nutr. 2021;40(3):860–869. doi: 10.1016/j.clnu.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 46.Hulander E, Bärebring L, Turesson Wadell A, Gjertsson I, Calder PC, Winkvist A, et al. Diet intervention improves cardiovascular profile in patients with rheumatoid arthritis: results from the randomized controlled cross-over trial ADIRA. Nutr J. 2021;20(1):9. doi: 10.1186/s12937-021-00663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliverio A, Radice P, Colombo M, Paradiso A, Tommasi S, Daniele A, et al. The impact of Mediterranean dietary intervention on metabolic and hormonal parameters according to BRCA1/2 variant type. Frontiers in genetics. 2022;13:820878. 10.3389/fgene.2022.820878. [DOI] [PMC free article] [PubMed]
- 48.Miralles-Amorós L, Asencio-Mas N, Martínez-Olcina M, Vicente-Martínez M, Frutos JMG, Peñaranda-Moraga M, et al. Study the effect of relative energy deficiency on physiological and physical variables in professional women athletes: a randomized controlled trial. Metabolites. 2023;13(2). 10.3390/metabo13020168. [DOI] [PMC free article] [PubMed]
- 49.Calvo-Malvar M, Benítez-Estévez AJ, Sánchez-Castro J, Leis R, Gude F. Effects of a community-based behavioral intervention with a traditional Atlantic diet on cardiometabolic risk markers: a cluster randomized controlled trial (“the GALIAT study”). Nutrients. 2021;13(4). 10.3390/nu13041211. [DOI] [PMC free article] [PubMed]
- 50.Glenn AJ, Hernández-Alonso P, Kendall CWC, Martínez-González M, Corella D, Fitó M, et al. Longitudinal changes in adherence to the portfolio and DASH dietary patterns and cardiometabolic risk factors in the PREDIMED-Plus study. Clin Nutr. 2021;40(5):2825–2836. doi: 10.1016/j.clnu.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Umemoto S, Onaka U, Kawano R, Kawamura A, Motoi S, Honda N, et al. Effects of a Japanese cuisine-based antihypertensive diet and fish oil on blood pressure and its variability in participants with untreated normal high blood pressure or stage I hypertension: a feasibility randomized controlled study. J Atheroscler Thromb. 2022;29(2):152–173. doi: 10.5551/jat.57802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin SM, Ahn J, Park J, Hur KY, Kim JH, Lee MK. East Asian diet-mimicking diet plan based on the Mediterranean diet and the dietary approaches to stop hypertension diet in adults with type 2 diabetes: a randomized controlled trial. Journal of diabetes investigation. 2021;12(3):357–364. doi: 10.1111/jdi.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnard ND, Alwarith J, Rembert E, Brandon L, Nguyen M, Goergen A, et al. A Mediterranean diet and low-fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross-over trial. Journal of the American Nutrition Association. 2022;41(2):127–139. doi: 10.1080/07315724.2020.1869625. [DOI] [PubMed] [Google Scholar]
- 54.Rocchetti MT, Di Iorio BR, Vacca M, Cosola C, Marzocco S, di Bari I, et al. Ketoanalogs' Effects on intestinal microbiota modulation and uremic toxins serum levels in chronic kidney disease (Medika2 study). J Clin Med. 2021;10(4). 10.3390/jcm10040840. [DOI] [PMC free article] [PubMed]
- 55.Dressler J, Storz MA, Müller C, Kandil FI, Kessler CS, Michalsen A, et al. Does a plant-based diet stand out for its favorable composition for heart health? Dietary intake data from a randomized controlled trial. Nutrients. 2022;14(21). 10.3390/nu14214597. [DOI] [PMC free article] [PubMed]
- 56.Jenkins DJA, Jones PJH, Abdullah MMH, Lamarche B, Faulkner D, Patel D, et al. Low-carbohydrate vegan diets in diabetes for weight loss and sustainability: a randomized controlled trial. Am J Clin Nutr. 2022 doi: 10.1093/ajcn/nqac203. [DOI] [PubMed] [Google Scholar]
- 57.Njike VY, Treu JA, Kela GCM, Ayettey RG, Comerford BP, Siddiqui WT. Egg consumption in the context of plant-based diets and cardiometabolic risk factors in adults at risk of type 2 diabetes. J Nutr. 2021;151(12):3651–3660. doi: 10.1093/jn/nxab283. [DOI] [PubMed] [Google Scholar]
- 58.Thomas MS, Puglisi M, Malysheva O, Caudill MA, Sholola M, Cooperstone JL, et al. Eggs improve plasma biomarkers in patients with metabolic syndrome following a plant-based diet-a randomized crossover study. Nutrients. 2022;14(10). 10.3390/nu14102138. [DOI] [PMC free article] [PubMed]
- 59.Hartmann AM, Dell'Oro M, Spoo M, Fischer JM, Steckhan N, Jeitler M, et al. To eat or not to eat-an exploratory randomized controlled trial on fasting and plant-based diet in rheumatoid arthritis (NutriFast-Study) Front Nutr. 2022;9:1030380. doi: 10.3389/fnut.2022.1030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macknin M, Stegmeier N, Thomas A, Worley S, Li L, Hazen SL, et al. Three healthy eating patterns and cardiovascular disease risk markers in 9 to 18 year olds with body mass index >95%: a randomized trial. Clin Pediatr. 2021;60(11–12):474–484. doi: 10.1177/00099228211044841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashim SA, Mohd Yusof BN, Abu Saad H, Ismail S, Hamdy O, Mansour AA. Effectiveness of simplified diabetes nutrition education on glycemic control and other diabetes-related outcomes in patients with type 2 diabetes mellitus. Clinical nutrition ESPEN. 2021;45:141–149. doi: 10.1016/j.clnesp.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 62.Van Horn L, Aragaki AK, Howard BV, Allison MA, Isasi CR, Manson JE, et al. Eating pattern response to a low-fat diet intervention and cardiovascular outcomes in normotensive women: the Women’s Health Initiative. Curr Dev Nutr. 2020;4(3):nzaa021. 10.1093/cdn/nzaa021. [DOI] [PMC free article] [PubMed]
- 63.Miguel-Berges ML, Jimeno-Martínez A, Larruy-García A, Moreno LA, Rodríguez G, Iguacel I. The effect of food vouchers and an educational intervention on promoting healthy eating in vulnerable families: a pilot study. Nutrients. 2022;14(23). 10.3390/nu14234980. [DOI] [PMC free article] [PubMed]
- 64.Santos A, Rodrigues A, Rosa LPS, Noll M, Silveira EA. Traditional Brazilian diet and olive oil reduce cardiometabolic risk factors in severely obese individuals: a randomized trial. Nutrients. 2020;12(5). 10.3390/nu12051413. [DOI] [PMC free article] [PubMed]
- 65.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93(6):1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 66.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97(22):2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 67.Gokce N, Keaney JF, Jr, Frei B, Holbrook M, Olesiak M, Zachariah BJ, et al. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99(25):3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- 68.Juraschek SP, Guallar E, Appel LJ, Miller ER., 3rd Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95(5):1079–1088. doi: 10.3945/ajcn.111.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guan Y, Dai P, Wang H. Effects of vitamin C supplementation on essential hypertension: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(8):e19274. 10.1097/MD.0000000000019274. [DOI] [PMC free article] [PubMed]
- 70.Heller R, Munscher-Paulig F, Grabner R, Till U. L-Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem. 1999;274(12):8254–8260. doi: 10.1074/jbc.274.12.8254. [DOI] [PubMed] [Google Scholar]
- 71.Visioli F, Smith A, Zhang W, Keaney JF, Jr, Hagen T, Frei B. Lipoic acid and vitamin C potentiate nitric oxide synthesis in human aortic endothelial cells independently of cellular glutathione status. Redox Rep. 2002;7(4):223–227. doi: 10.1179/135100002125000604. [DOI] [PubMed] [Google Scholar]
- 72.Huang A, Vita JA, Venema RC, Keaney JF., Jr Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275(23):17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 73.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Visioli F, Keaney JF, Halliwell B. Antioxidants and cardiovascular disease; panaceas or tonics for tired sheep? Cardiovasc Res. 2000;47(3):409. doi: 10.1016/s0008-6363(00)00156-5. [DOI] [PubMed] [Google Scholar]
- 75.Carr AC, Zhu BZ, Frei B. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and alpha-tocopherol (vitamin E) Circ Res. 2000;87(5):349–354. doi: 10.1161/01.res.87.5.349. [DOI] [PubMed] [Google Scholar]
- 76.Smith AR, Visioli F, Hagen TM. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J. 2002;16(9):1102–1104. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- 77.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.•• Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci. U S A. 1996;93(8):3704–9. 10.1073/pnas.93.8.3704. The definitive human trial on vitamin C and its pharmacokinetics. [DOI] [PMC free article] [PubMed]
- 79.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69(6):1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 80.Michels AJ, Hagen TM, Frei B. A new twist on an old vitamin: human polymorphisms in the gene encoding the sodium-dependent vitamin C transporter 1. Am J Clin Nutr. 2010;92(2):271–272. doi: 10.3945/ajcn.2010.29979. [DOI] [PubMed] [Google Scholar]
- 81.Morelli MB, Gambardella J, Castellanos V, Trimarco V, Santulli G. Vitamin C and cardiovascular disease: an update. Antioxidants (Basel). 2020;9(12). 10.3390/antiox9121227. [DOI] [PMC free article] [PubMed]
- 82.Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22(6):463–493. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Visioli F, Reilly MM, Rimoldi M, Solari A, Pareyson D, for the C-T, et al. Vitamin C and Charcot-Marie-Tooth 1A: pharmacokinetic considerations. PharmaNutrition. 2013;1(1):10–2. 10.1016/j.phanu.2012.10.001. [DOI] [PMC free article] [PubMed]
- 84.Chen P, Reed G, Jiang J, Wang Y, Sunega J, Dong R, et al. Pharmacokinetic evaluation of intravenous vitamin C: a classic pharmacokinetic study. Clin Pharmacokinet. 2022;61(9):1237–1249. doi: 10.1007/s40262-022-01142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5(7):e11414. 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed]
- 86.Bruno RM, Daghini E, Ghiadoni L, Sudano I, Rugani I, Varanini M, et al. Effect of acute administration of vitamin C on muscle sympathetic activity, cardiac sympathovagal balance, and baroreflex sensitivity in hypertensive patients. Am J Clin Nutr. 2012;96(2):302–308. doi: 10.3945/ajcn.112.035022. [DOI] [PubMed] [Google Scholar]
- 87.Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94(1):6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 88.d'Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res. 2003;92(1):88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 89.Staruschenko A. Beneficial Effects of High Potassium: Contribution of Renal Basolateral K(+) Channels. Hypertension. 2018;71(6):1015–1022. doi: 10.1161/HYPERTENSIONAHA.118.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dickinson HO, Nicolson DJ, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006(3):CD004641. 10.1002/14651858.CD004641.pub2. [DOI] [PubMed]
- 91.Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep. 2011;13(4):309–317. doi: 10.1007/s11906-011-0197-8. [DOI] [PubMed] [Google Scholar]
- 92.US Department of Agriculture. https://fdc.nal.usda.gov. Accessed 17 January 2023.
- 93.Joshi S, Ettinger L, Liebman SE. Plant-based diets and hypertension. Am J Lifestyle Med. 2020;14(4):397–405. doi: 10.1177/1559827619875411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palmer BF, Clegg DJ. Achieving the benefits of a high-potassium, paleolithic diet, without the toxicity. Mayo Clin Proc. 2016;91(4):496–508. doi: 10.1016/j.mayocp.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Campbell S. Dietary reference intakes: water, potassium, sodium, chloride, and sulfate. Clin Nutr Insight. 2004;30:1–4. [Google Scholar]
- 96.Addison WL. The use of sodium chloride, potassium chloride, sodium bromide, and potassium bromide in cases of arterial hypertension which are amenable to potassium chloride. Can Med Assoc J. 1928;18(3):281–285. [PMC free article] [PubMed] [Google Scholar]
- 97.Murillo-de-Ozores AR, Gamba G, Castaneda-Bueno M. Molecular mechanisms for the regulation of blood pressure by potassium. Curr Top Membr. 2019;83:285–313. doi: 10.1016/bs.ctm.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R546–R552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- 99.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101(8):856–861. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 100.American Heart Association. Get the Scoop on Sodium and Salt. 2022. https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/sodium/sodium-and-salt. Accessed 17 January 2023.
- 101.Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002;22:61–86. doi: 10.1146/annurev.nutr.22.110901.145329. [DOI] [PubMed] [Google Scholar]
- 102.Bacchetta L, Visioli F, Cappelli G, Caruso E, Martin G, Nemeth E, et al. A manifesto for the valorization of wild edible plants. J Ethnopharmacol. 2016;191:180–187. doi: 10.1016/j.jep.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 103.Visioli F. Science and claims of the arena of food bioactives: comparison of drugs, nutrients, supplements, and nutraceuticals. Food Funct. 2022;13(24):12470–12474. doi: 10.1039/d2fo02593k. [DOI] [PubMed] [Google Scholar]
- 104.Medina-Remon A, Zamora-Ros R, Rotches-Ribalta M, Andres-Lacueva C, Martinez-Gonzalez MA, Covas MI, et al. Total polyphenol excretion and blood pressure in subjects at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2011;21(5):323–331. doi: 10.1016/j.numecd.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 105.Visioli F, Bogani P, Grande S, Galli C. Mediterranean food and health: building human evidence. J Physiol Pharmacol. 2005;56(Suppl 1):37–49. [PubMed] [Google Scholar]
- 106.Grande S, Bogani P, de Saizieu A, Schueler G, Galli C, Visioli F. Vasomodulating potential of mediterranean wild plant extracts. J Agric Food Chem. 2004;52(16):5021–5026. doi: 10.1021/jf049436e. [DOI] [PubMed] [Google Scholar]
- 107.Rossoni G, Grande S, Galli C, Visioli F. Wild artichoke prevents the age-associated loss of vasomotor function. J Agric Food Chem. 2005;53(26):10291–10296. doi: 10.1021/jf052499s. [DOI] [PubMed] [Google Scholar]
- 108.Zarzuelo A, Duarte J, Jimenez J, Gonzalez M, Utrilla MP. Vasodilator effect of olive leaf. Planta Med. 1991;57(5):417–419. doi: 10.1055/s-2006-960138. [DOI] [PubMed] [Google Scholar]
- 109.Romero M, Toral M, Gomez-Guzman M, Jimenez R, Galindo P, Sanchez M, et al. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016;7(1):584–593. doi: 10.1039/c5fo01101a. [DOI] [PubMed] [Google Scholar]
- 110.Alcaide-Hidalgo JM, Romero M, Duarte J, Lopez-Huertas E. Antihypertensive effects of virgin olive oil (unfiltered) low molecular weight peptides with ACE inhibitory activity in spontaneously hypertensive rats. Nutrients. 2020;12(1). 10.3390/nu12010271. [DOI] [PMC free article] [PubMed]
- 111.Alcaide-Hidalgo JM, Margalef M, Bravo FI, Muguerza B, Lopez-Huertas E. Virgin olive oil (unfiltered) extract contains peptides and possesses ACE inhibitory and antihypertensive activity. Clin Nutr. 2020;39(4):1242–1249. doi: 10.1016/j.clnu.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 112.Lasserre B, Kaiser R, Pham Huu C, Ifansyah N, Gleye J, Moulis C. Effects on rats of aqueous extracts of plants used in folk medicine as antihypertensive agents. Naturwissenschaften. 1983;70(2):95–96. doi: 10.1007/BF00365512. [DOI] [PubMed] [Google Scholar]
- 113.Razmpoosh E, Abdollahi S, Mousavirad M, Clark CCT, Soltani S. The effects of olive leaf extract on cardiovascular risk factors in the general adult population: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2022;14(1):151. doi: 10.1186/s13098-022-00920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.• Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41(12):1727–46. 10.1016/j.freeradbiomed.2006.04.033. This review questions the purported (and still believed) direct free radical-scevenging actions of (poly)phenols. [DOI] [PubMed]
- 115.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: the golden mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell. 2006;5(5):391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 117.Smith AR, Visioli F, Hagen TM. Plasma membrane-associated endothelial nitric oxide synthase and activity in aging rat aortic vascular endothelia markedly decline with age. Arch Biochem Biophys. 2006;454(1):100–105. doi: 10.1016/j.abb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 118.Medina-Remon A, Tresserra-Rimbau A, Pons A, Tur JA, Martorell M, Ros E, et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr Metab Cardiovasc Dis. 2015;25(1):60–7. 10.1016/j.numecd.2014.09.001. [DOI] [PubMed]
- 119.Gomez-Delgado F, Romero-Cabrera JL, Perez-Martinez P. Diet and vascular risk. Curr Opin Cardiol. 2022;37(4):343–349. doi: 10.1097/HCO.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 120.Duffy SJ, Keaney JF, Jr, Holbrook M, Gokce N, Swerdloff PL, Frei B, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104(2):151–156. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 121.Deka A, Vita JA. Tea and cardiovascular disease. Pharmacol Res. 2011;64(2):136–145. doi: 10.1016/j.phrs.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Widlansky ME, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG, et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr. 2007;26(2):95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46(2):398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 124.Ludovici V, Barthelmes J, Nagele MP, Enseleit F, Ferri C, Flammer AJ, et al. Cocoa, blood pressure, and vascular function. Front Nutr. 2017;4:36. doi: 10.3389/fnut.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borghi C, Tsioufis K, Agabiti-Rosei E, Burnier M, Cicero AFG, Clement D, et al. Nutraceuticals and blood pressure control: a European Society of Hypertension position document. J Hypertens. 2020;38(5):799–812. doi: 10.1097/HJH.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 126.Mirmiran P, Houshialsadat Z, Gaeini Z, Bahadoran Z, Azizi F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr Metab (Lond) 2020;17:3. doi: 10.1186/s12986-019-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olsson H, Al-Saadi J, Oehler D, Pergolizzi J, Jr., Magnusson P. Physiological effects of beetroot in athletes and patients. Cureus. 2019;11(12):e6355. 10.7759/cureus.6355. [DOI] [PMC free article] [PubMed]
- 128.Lopez-Torres O, Rodriguez-Longobardo C, Capel-Escoriza R, Fernandez-Elias VE. Ergogenic aids to improve physical performance in female athletes: a systematic review with meta-analysis. Nutrients. 2022;15(1). 10.3390/nu15010081. [DOI] [PMC free article] [PubMed]
- 129.Scheffler A, Rauwald HW, Kampa B, Mann U, Mohr FW, Dhein S. Olea europaea leaf extract exerts L-type Ca(2+) channel antagonistic effects. J Ethnopharmacol. 2008;120(2):233–240. doi: 10.1016/j.jep.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 130.Visioli F, Poli A. Fatty acids and cardiovascular risk. evidence, lack of evidence, and diligence. Nutrients. 2020;12(12). 10.3390/nu12123782. [DOI] [PMC free article] [PubMed]
- 131.Harris WS, Calder PC, Mozaffarian D, Serhan CN. Bang and Dyerberg’s omega-3 discovery turns fifty. Nature Food. 2021;2:303–305. doi: 10.1038/s43016-021-00289-7. [DOI] [PubMed] [Google Scholar]
- 132.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tadic M, Sala C, Grassi G, Mancia G, Taddei S, Rottbauer W, et al. Omega-3 fatty acids and coronary artery disease: more questions than answers. J Clin Med. 2021;10(11). 10.3390/jcm10112495. [DOI] [PMC free article] [PubMed]
- 134.Visioli F, Agostoni C. Omega 3 fatty acids and health: the little we know after all these years. Nutrients. 2022;14(2). 10.3390/nu14020239. [DOI] [PMC free article] [PubMed]
- 135.Sherratt SCR, Libby P, Budoff MJ, Bhatt DL, Mason RP. Role of omega-3 fatty acids in cardiovascular disease: the debate continues. Curr Atheroscler Rep. 2023;25(1):1–17. doi: 10.1007/s11883-022-01075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Calder PC, Deckelbaum RJ. Editorial: Omega-3 fatty acids: new studies, new data, new questions. Curr Opin Clin Nutr Metab Care. 2021;24(2):109–113. doi: 10.1097/MCO.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 137.Godo S, Takahashi J, Yasuda S, Shimokawa H. Role of inflammation in coronary epicardial and microvascular dysfunction. Eur Cardiol. 2021;16:e13. 10.15420/ecr.2020.47. [DOI] [PMC free article] [PubMed]
- 138.Ertuglu LA, Mutchler AP, Yu J, Kirabo A. Inflammation and oxidative stress in salt sensitive hypertension; the role of the NLRP3 inflammasome. Front Physiol. 2022;13:1096296. doi: 10.3389/fphys.2022.1096296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mori TA, Dunstan DW, Burke V, Croft KD, Rivera JH, Beilin LJ, et al. Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non-insulin-dependent diabetic patients. Metabolism. 1999;48(11):1402–1408. doi: 10.1016/s0026-0495(99)90150-6. [DOI] [PubMed] [Google Scholar]
- 140.Giordano E, Visioli F. Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fatty Acids. 2014;90(1):1–4. doi: 10.1016/j.plefa.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 141.Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17(2):174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 142.Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva JAP, Blauth M, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi: 10.1001/jama.2020.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang X, Ritonja JA, Zhou N, Chen BE, Li X. Omega-3 polyunsaturated fatty acids intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J Am Heart Assoc. 2022;11(11):e025071. 10.1161/JAHA.121.025071. [DOI] [PMC free article] [PubMed]
- 144.Guo XF, Li KL, Li JM, Li D. Effects of EPA and DHA on blood pressure and inflammatory factors: a meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;59(20):3380–3393. doi: 10.1080/10408398.2018.1492901. [DOI] [PubMed] [Google Scholar]
- 145.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102(1524–4539 (Electronic)):1264–9. 10.1161/01.cir.102.11.1264. [DOI] [PubMed]
- 146.Pieters DJ, Zock PL, Fuchs D, Mensink RP. Effect of alpha-linolenic acid on 24-h ambulatory blood pressure in untreated high-normal and stage I hypertensive subjects. Br J Nutr. 2019;121(2):155–163. doi: 10.1017/S0007114518003094. [DOI] [PubMed] [Google Scholar]
- 147.Ursoniu S, Sahebkar A, Andrica F, Serban C, Banach M, et al. Lipid and Blood Pressure Meta-analysis Collaboration. Effects of flaxseed supplements on blood pressure: a systematic review and meta-analysis of controlled clinical trial. Clin Nutr. 2016;35(3):615–25. 10.1016/j.clnu.2015.05.012. [DOI] [PubMed]
- 148.Hashimoto M, Tanabe Y, Hossain S, Matsuzaki K, Ohno M, Kato S, et al. Intake of alpha-linolenic acid-rich perilla frutescens leaf powder decreases home blood pressure and serum oxidized low-density lipoprotein in Japanese adults. Molecules. 2020;25(9). 10.3390/molecules25092099. [DOI] [PMC free article] [PubMed]
- 149.Domenech M, Serra-Mir M, Roth I, Freitas-Simoes T, Valls-Pedret C, Cofan M, et al. Effect of a walnut diet on office and 24-hour ambulatory blood pressure in elderly individuals. Hypertension. 2019;73(5):1049–57. 10.1161/HYPERTENSIONAHA.118.12766. [DOI] [PMC free article] [PubMed]
- 150.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in Our understanding of oxylipins derived from dietary PUFAs. Adv Nutr. 2015;6(5):513–540. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Naghshi S, Aune D, Beyene J, Mobarak S, Asadi M, Sadeghi O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies. BMJ. 2021;375:n2213. 10.1136/bmj.n2213. [DOI] [PMC free article] [PubMed]
- 152.Sala-Vila A, Fleming J, Kris-Etherton P, Ros E. Impact of alpha-linolenic acid, the vegetable omega-3 fatty acid, on cardiovascular disease and cognition. Adv Nutr. 2022;13(5):1584–1602. doi: 10.1093/advances/nmac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whelan J, Fritsche K. Linoleic acid. Adv Nutr. 2013;4(3):311–312. doi: 10.3945/an.113.003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Dore J, et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. doi: 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 155.Fritsche KL. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):173–175. doi: 10.1016/j.plefa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 156.Mutanen M, Kleemola P, Valsta LM, Mensink RP, Rasanen L. Lack of effect on blood pressure by polyunsaturated and monounsaturated fat diets. Eur J Clin Nutr. 1992;46(1):1–6. [PubMed] [Google Scholar]
- 157.Mensink RP, Janssen MC, Katan MB. Effect on blood pressure of two diets differing in total fat but not in saturated and polyunsaturated fatty acids in healthy volunteers. Am J Clin Nutr. 1988;47(6):976–980. doi: 10.1093/ajcn/47.6.976. [DOI] [PubMed] [Google Scholar]
- 158.Zock PL, Blijlevens RA, de Vries JH, Katan MB. Effects of stearic acid and trans fatty acids versus linoleic acid on blood pressure in normotensive women and men. Eur J Clin Nutr. 1993;47(6):437–444. [PubMed] [Google Scholar]
- 159.Aro A, Pietinen P, Valsta LM, Salminen I, Turpeinen AM, Virtanen M, et al. Lack of effect on blood pressure by low fat diets with different fatty acid compositions. J Hum Hypertens. 1998;12(6):383–389. doi: 10.1038/sj.jhh.1000610. [DOI] [PubMed] [Google Scholar]
- 160.Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, et al. Relationship of dietary linoleic acid to blood pressure. The International Study of Macro-Micronutrients and Blood Pressure Study [corrected]. Hypertension. 2008;52(2):408–14. 10.1161/HYPERTENSIONAHA.108.112383. [DOI] [PMC free article] [PubMed]
- 161.Wang L, Manson JE, Forman JP, Gaziano JM, Buring JE, Sesso HD. Dietary fatty acids and the risk of hypertension in middle-aged and older women. Hypertension. 2010;56(4):598–604. doi: 10.1161/HYPERTENSIONAHA.110.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hooper L, Al-Khudairy L, Abdelhamid AS, Rees K, Brainard JS, Brown TJ, et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11:CD011094. 10.1002/14651858.CD011094.pub4. [DOI] [PMC free article] [PubMed]
- 163.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119(6):902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 164.Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–1578. doi: 10.1161/CIRCULATIONAHA.114.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.One health framework. https://www.cdc.gov/onehealth/basics/index.html. Accessed 3 May 2023
- 166.Casas R, Ribó-Coll M, Ros E, Fitó M, Lamuela-Raventos RM, Salas-Salvadó J, et al. Change to a healthy diet in people over 70 years old: the PREDIMED experience. Eur J Nutr. 2022;61(3):1429–1444. doi: 10.1007/s00394-021-02741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maifeld A, Bartolomaeus H, Löber U, Avery EG, Steckhan N, Markó L, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 2021;12(1):1970. doi: 10.1038/s41467-021-22097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu R, Fogelholm M, Poppitt SD, Silvestre MP, Møller G, Huttunen-Lenz M, et al. Adherence to a plant-based diet and consumption of specific plant foods-associations with 3-year weight-loss maintenance and cardiometabolic risk factors: a secondary analysis of the PREVIEW intervention study. Nutrients. 2021;13(11). 10.3390/nu13113916. [DOI] [PMC free article] [PubMed]
- 169.Turner-McGrievy GM, Wilson MJ, Carswell J, Okpara N, Aydin H, Bailey S, et al. A 12-week randomized intervention comparing the healthy US, Mediterranean, and vegetarian dietary patterns of the US dietary guidelines for changes in body weight, hemoglobin A1c, blood pressure, and dietary quality among African American adults. J Nutr. 2023;153(2):579–587. doi: 10.1016/j.tjnut.2022.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented are contained within this manuscript.