Abstract
Umbelliferone (UB) is a phenylpropanoid-based pharmacologically active agent with promising anti-tumor activities. However, complete elucidation of its therapeutic efficacy remains challenging due to low solubility and bioavailability. The present study aimed to develop a liposomal delivery system for UB to enhance its therapeutic efficacy against Dalton’s ascites lymphoma tumor model. Umbelliferone-encapsulated nanoliposomes (nLUB) were prepared using the thin-film hydration method and performed a series of characterizations to confirm successful development. The nLUB showed a particle size of 116 ± 3.2 nm with a negative surface charge and encapsulation efficiency of 78%. In vitro study results showed that nLUB treatment significantly enhanced cellular uptake and apoptosis induction in lymphoma cells compared to free UB. nLUB treatment significantly stabilized body weight, reduced tumor growth, and improved the serum biochemical and hematological parameters of experimental animals, thereby improving their overall survivability compared to an free UB. Our result indicates that nanoencapsulation enhanced the therapeutic potential of UB, which may find its way to clinical application in the near future.
Keywords: Umbelliferone, Nanoliposomes, Lymphoma, Tumor mitigation
Introduction
Today, cancer remains one of the significant causes of morbidity and mortality, responsible for 10 million deaths globally in 2020 (Sung et al. 2021). Chemotherapy is the most predominant treatment method employed to treat cancer. It is used in concurrent or subsequent treatment, combined with radiotherapy, surgery, and immunotherapy (Wu et al. 2014). Chemotherapeutic agents are more effective on cancer cells but can also adversely affect normal cells. Several factors, such as non-specificity, poor patient tolerance, and rapid elimination, can hinder the therapeutic efficiency of chemotherapeutic agents. Moreover, with chemotherapeutic drug treatment, cancer patients suffer from side effects like nausea, diarrhea, constipation, thrombocytopenia, and alopecia (Kumar et al. 2017). Thus, it represents a significant challenge for the scientific community to search for new drug formulations that are safe and effective against this implacable disease of the modern world (Kang et al. 2012).
Umbelliferone (UB, 7-hydroxycoumarin) is a plant-derived polyphenolic compound that is widely distributed in the plant families of Apiaceae, Rutaceae, and Asteraceae. UB has received significant attention because of its pharmacological activities such as antioxidant, anti-hyperglycemic, anti-hyperlipidemic, anti-diabetic, anti-inflammatory, antinociceptive, anti-tumor, and immunomodulatory activities (Vasconcelos et al. 2009; Ramesh and Pugalendi 2006; Ramu et al. 2016; Rauf et al. 2014; Lopez-Gonzalez et al. 2004; Vijayalakshmi and Sindhu 2017). It exhibited an anti-cancer effect on liver, renal, oral, and colon cancer (Yu et al. 2015; Vijayalakshmi and Sindhu 2017; Muthu et al. 2013). UB is well tolerated by humans and is not toxic under the evaluated circumstances, demonstrating its immense potential for pharmacological use (Cruz et al. 2020). Despite its potential benefits in pre-clinical studies, the poor water solubility, low bioavailability, and short half-life of UB significantly limit its competent pharmacological applications (Singh and Rahman 2020). Thus, novel drug delivery approaches are required to deliver UB to the tumor site.
Nano-drug delivery systems have been used to deliver anti-cancer drugs with low solubility and less bioavailability to tumor regions (Singh et al. 2009). Previous studies have reported the use of different drug delivery approaches, such as PLG nanoparticles (Kumar et al. 2017), silica nanoparticles (Kundu et al. 2020), nanocomposites (Iglesias-Montes et al. 2021), and phospholipids-based matrix film (Telange et al. 2019), to overcome the limitations of UB. Liposomes have received significant attention as drug delivery systems due to their unique biocompatibility, biodegradability, size, nontoxic nature, and lack of immune response (Immordino et al. 2006; Zocchi et al. 2020). The nanoliposomal formulations have been shown to help the selective delivery of drugs to the tumor site (Kang et al. 2016). Subsequently improves treatment efficacy while evading toxicity in the normal cell by high selective accumulation in tumors via enhanced permeability and retention (EPR) effect and active cellular uptake, thereby avoiding off-target toxicity (Sun et al. 2016; Shi et al. 2020).
PEGylation on the surface of liposomal carriers has been shown to extend blood circulation time while reducing mononuclear phagocyte system uptake (Immordino et al. 2006). It can be used to avoid rapid clearance by the reticuloendothelial system, avoid aggregation between liposomal particles and immunogenicity and allow prolonged circulation time (Suk et al. 2016). In that way, nanoliposome encapsulation of UB will provide a prospect to increase the therapeutic effects of UB and therefore create a prominent opportunity to treat cancer (Singh and Rahman 2020). Thus, the present study has been designed to enhance the therapeutic potential of UB by PEGylated nanoencapsulation and investigate the effect of nLUB on the induction of apoptosis in lymphoma cells and in regulating experimental solid tumor progression.
Materials and methods
Materials
Umbelliferone, Hoechst, Triton X 100, and methyl thiazolyl diphenyltetrazolium bromide (MTT) were purchased from Sigma Aldrich (USA). Dulbecco's Modified Eagles Medium, Fetal Bovine Serum, and Trypan Blue were obtained from Euro clone (Italy). Cholesterol and Phosphatidylcholine were purchased from Avanti polar lipids (USA). All other reagents and chemicals used were of analytical grade.
Cell culture
In vitro and in vivo studies were performed using Dalton's lymphoma ascites cells (DLA). The cells were provided by Dr. Girija Kuttan (Amala Cancer Research Centre, Thrissur, Kerala, India). The cells were maintained in DMEM media supplemented with antibiotics (Penicillin and streptomycin), 10% FBS, and non-essential amino acids.
Preparation of nanoliposome encapsulated UB
UB-loaded nanoliposomes were prepared using the thin-film hydration method as described earlier (Abeesh et al. 2021). Briefly, Lipid thin film was prepared by mixing Phosphatidylcholine, cholesterol, PEG, and UB at a molar ratio of 2:1:0.1:0.5 respectively in an organic solvent (chloroform). Subsequently, the formed lipid film was hydrated with PBS, and the resulting solution was subjected to ultrasound sonication for size reduction using a sonicator. The prepared liposomal suspension is extruded through a polyether sulfonate membrane (Millipore, Germany) and stored at 4 °C.
Characterization of nanoliposome encapsulated UB
The nanoliposome's particle size, polydispersity index, and zeta potential were characterized through the Dynamic light scattering system (DLS) (Nanopartica, Horiba scientific apparatus, Japan). The samples were diluted with distilled water and analyzed using DLS with a scattering angle of 90° at 25 °C. Mean particle size, polydispersity index, and zeta potential of the nanoliposomal formulations were described as mean ± standard deviations. FTIR spectra of free UB, nLUB, and unencapsulated nanoliposomes were obtained using an FTIR spectrophotometer (Shimadzu, Japan). The sample analysis was carried out using the potassium bromide disc method. The morphology of liposomes was analyzed using a high-resolution transmission electron microscope (JEM2100: Jeol, Tokyo, Japan) by the negative staining method.
Drug encapsulation
UB’s encapsulation efficiency (EE) in the nLUB was determined via the dialysis method as described previously (Abeesh et al. 2021). Briefly, the unencapsulated UB was separated using a dialysis membrane. After dialysis, nLUB was digested using methanol and precipitated lipids were separated by ultracentrifugation (45,000 rpm, 1 h). The concentration of UB in the nanoliposomal formulation was determined by creating a standard curve at the wavelength of 339 nm. The encapsulation efficiency (EE) is determined following the equation,
The drug loading efficiency (DL) was calculated using the following formulae,
In vitro drug release study
The pH-dependent in vitro release kinetics of UB from nLUB was carried out using the previously described protocol with slight modification (Kundu et al. 2020). The released kinetics of UB from the nLUB were analyzed in two different buffer solutions (pH 6 and pH7.4) using the dialysis diffusion method. The samples were kept in a 50 ml beaker containing buffer solutions and allowed to stir (300 rpm, 37 °C). The release amount of UB from nLUB was checked at 339 nm, and UB’s release rate was estimated. All the experiments were carried out in triplicate.
Hemolysis study
A hemolysis study was performed according to the previously described method (Evans et al. 2013). Briefly, the erythrocytes solutions (2%) were prepared and treated with different concentrations of nLUB (0.1–100 µg/ml) and incubated at 37°c for 2 h. after incubation, cells were centrifuged (3500 rpm, 5 min) and separated the supernatant. The absorbance was measured using a microplate reader at 540 nm (Biotek, USA). The percentage of hemolysis was calculated using the following formulae,
where AbN, AbP, and AbS are the absorbance values of the negative control, positive control, and sample, respectively.
Cellular uptake study
In vitro cellular uptake of nLUB against DLA cells was determined qualitatively and quantitatively by fluorescent microscopy and fluorimeter. Briefly, lymphoma cells were seeded on 24 well plates and incubated for 1 h at 37 °C. The cells were treated with nLUB for 30 min, 1, 2, and 6 h, respectively. For qualitative analysis, after incubation, cells were fixed with 4% paraformaldehyde, co-stained with DAPI, and observed under a fluorescent microscope. After treatment, the cells were incubated with PBST (PBS containing 1% Triton X 100) for quantitative analysis. The cells were centrifuged (10,000 rpm for 5 min) and collected supernatant, and the concentration of UB was estimated using a fluorimeter (BioTek, USA.
MTT assay
The cytotoxicity of UB and nLUB were studied in DLA cells by MTT assay. Briefly, the lymphoma cells (5 × 103 cells/well) were seeded on 96 well plates in DMEM supplemented with FBS (10%) and incubated for 24 h and 48 h at 37 °C and 5% CO2. After incubation, cells were treated with different concentrations of either nLUB or UB (25, 50, 100, and 150 µg/ml) and incubated for 24 h. Following incubation, MTT (5 mg/ml) solution was added and incubated at 37 °C for 4 h. Subsequently, the dimethyl sulfoxide was used to solubilize the formed formazan crystals, and absorbance was taken using a microplate reader (BioTek, USA) at 570 nm, and the percentage of cytotoxicity was estimated.
Acridine orange/ethidium bromide (AO/EtBr) staining
The DLA cells were seeded (1 × 104) into flat bottom 96 well plates and incubated for 1 h. Subsequently, the cells were treated with either UB (50 µg/ml) or nLUB (50 µg/ml) for 6 h at 37°c. Following treatments, the cell was incubated with 0.01 ml AO/EtBr staining solution. The stained cells were observed and photographed using a fluorescence microscope with a 20 × objective (Olympus, Japan).
Experimental solid tumor mitigation study
The experimental solid tumors were established in 6-week-old male Balb/c mice purchased from Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), Kerala, India. The in vivo experiments were carried out after obtaining approval from Institutional Animal Ethics Committee, Regional Cancer Centre (Kerala, India). The animals were fed ad libitum with a standard diet, maintained in a controlled condition, and housed at the animal house facility of the Regional Cancer Centre.
Solid tumors were generated by injecting DLA cells (1 × 106) into the right hind limb of Balb/c mice (n = 12). Subsequently, tumor-induced experimental animals were randomly divided into three groups: tumor control, tumor + UB, and tumor + nLUB. Animals were injected i.p. with vehicle control, UB, and nLUB (30 mg/kg bwt) for ten days. Every fifth day, the tumor growth of the experimental animals was monitored by measuring tumor radius and calculating tumor volume by the following formula. V = 4/3π(r1)2r2, where r1 and r2 are different plane tumor radii. On the 30th day, experimental animals were sacrificed by cervical dislocation, and tumors were removed and fixed in formalin to be used for histopathology analysis. Blood samples collected by the cardiac puncture method from animals were subjected to hematology and serum biochemical analysis [Serum glutamic oxaloacetic transaminase (SGOT), glutamic pyruvate transaminase (SGPT), alkaline phosphatase (ALP), and Glutathione (GSH)]. Further, the remaining experimental animals (n = 6) were used for survival analysis.
Statistical analysis
All values were expressed as mean ± standard deviation. All experiments were performed in triplicate (n = 3). The statistical analysis was carried out using one-way analysis of variance followed by Dunnett's test using GraphPad InStat 5, (GraphPad software, USA). Log rank test for Kaplan–Meier survival analysis was performed using GraphPad prism (USA). P value < 0.05 was considered statistically significant.
Results
Preparation and characterization of nanoliposome encapsulated UB
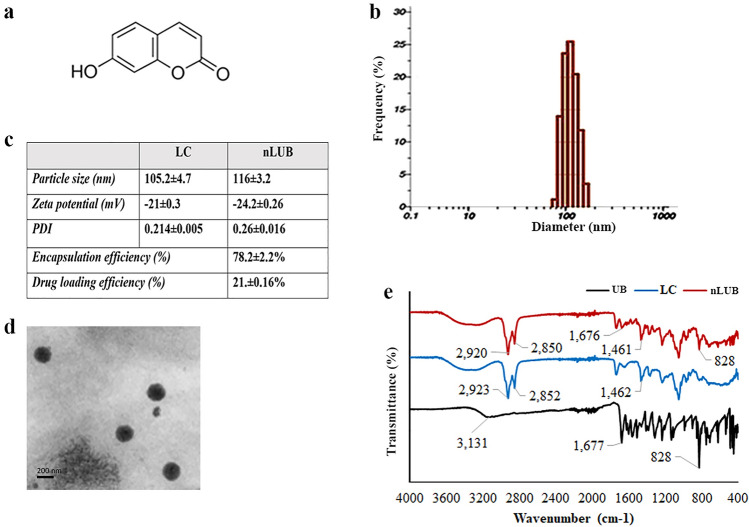
In this study, PEGylated nanoliposome formulations were developed by the thin-film hydration method to enhance the therapeutic effect of UB. The chemical structure of UB is shown in Fig. 1a. The UB encapsulated liposomes were prepared using the thin-film hydration method and characterized in terms of particle size, polydispersity index, and zeta potential using DLS. The particle size distribution of nLUB has been shown in Fig. 1b. The particle distribution results indicated a single peak with a narrow distribution. The physicochemical characteristics of the liposomal formulation are shown in Fig. 1c. The DLS analysis showed that the average particle size of the control liposome was 105.2 ± 4.7 nm and the polydispersity index was 0.214 ± 0.005. In contrast, the nLUB showed an average size of 116 ± 3.2 nm and a polydispersity index of 0.268 ± 0.016. The study results showed that nLUB has a PDI value of < 0.26, indicating the formation of narrow, homogenous liposomal particles. We obtained the negatively charged formulations with less than 200 nm particle size. The zeta potentials of empty and drug-loaded liposomes were − 21 ± 0.3 mV and − 24.2 ± 0.26 mV, respectively. Transmission electron microscope (TEM) analysis was performed to determine the morphology of the synthesized liposomes, and the results are shown in Fig. 1d. TEM micrograph of nLUB clearly showed the formation of a liposome size ranging from 100–120 nm and appeared to be a spherical shape. The morphology of liposomes was further confirmed by TEM, which confirmed the presence of round liposomes of < 125 nm. Further, FTIR analysis was performed to determine the molecular interactions of drugs and liposomal formulations. The signature peaks of UB showed at 3131 cm−1, 1677 cm−1, and 828 cm−1 (Fig. 1e). The characteristic peaks of pegylated nanoliposomes were observed at 2923 cm−1, 2852 cm−1, and 1462 cm−1. When UB was incorporated into the nanoliposomes, the peak location was similar to the phospholipids at 2920 cm−1 and 2850 cm−1. Meanwhile, nLUB exhibited 1676 cm−1 and 828 cm−1, which are the characteristic peaks of UB, indicating the existence of UB in the liposomes. The encapsulation efficiency of nLUB was 78 ± 2.2%, and the drug loading efficiency of UB in nLUB was found to be 21.3 ± 0.16%.
Fig. 1.
a Chemical structure of Umbelliferone. b Particle size distribution histogram of nLUB. c Particle size, zeta potential, Polydispersity index, and encapsulation efficiency of the nLUB formulations. d TEM micrographs of nLUB e FTIR spectra of liposome control, UB and nLUB formulations
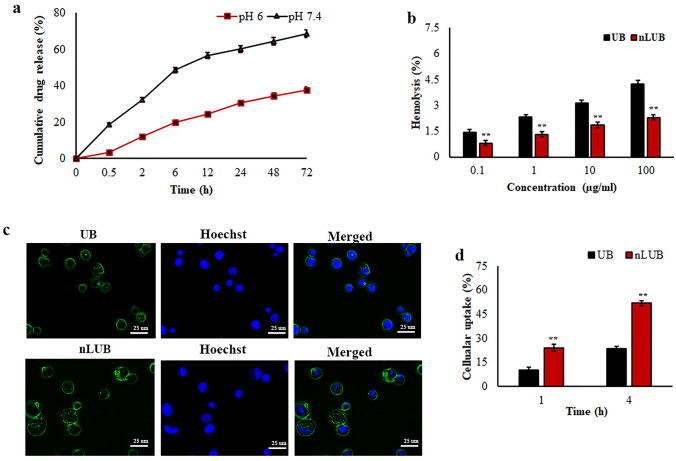
The results of the in vitro release of UB from liposomal formulation are presented in Fig. 2a. Two different pH solutions (pH 6 and 7.4) were taken as the release media. The results showed that the maximum UB release from nLUB was obtained at pH 7.4 after 24 h, while < 30% release was observed at pH 6. As shown in Fig. 2b, nLUB treatment had negligible hemolysis when interacting with erythrocytes. The lysis percentage of nLUB was found to be less than 4%. UB treatment showed significantly (p < 0.01) higher hemolysis compared to nLUB. Reduced hemolysis was contributed by nLUB when compared to the UB, which improves biocompatibility toward RBCs. A cellular internalization study of the nanoliposomal formulations was performed using lymphoma cells. As shown in Fig. 2c, lymphoma cells treated with nLUB showed stronger fluorescence than UB at 1 and 4 h, suggesting that liposome encapsulation could facilitate effective cellular uptake of UB. Moreover, the quantitative cellular uptake study results also revealed that nanoencapsulation of UB significantly (p < 0.01) improved the cellular uptake behavior of UB (Fig. 2d). The uptake efficiency of nLUB was found to be more in lymphoma cells when compared to the UB.
Fig. 2.
a In vitro drug release kinetics of nLUB in different pH conditions. b Hemolysis assay results of UB and nLUB. c Fluorescent micrographs of nLUB cellular internalization in lymphoma cells. d Quantitative cellular uptake analysis of nLUB in lymphoma cells
nLUB treatment decreased lymphoma cell proliferation
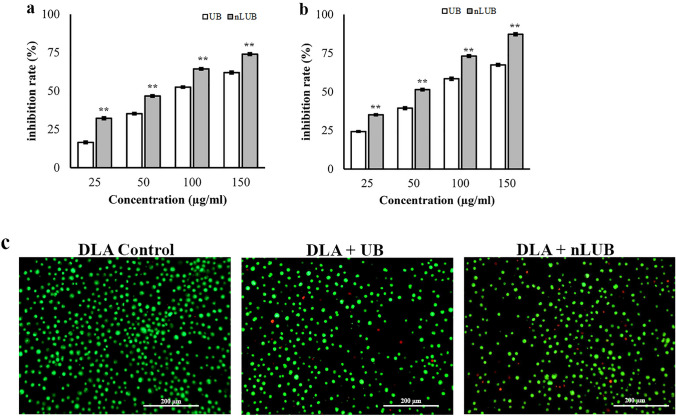
The effect of nLUB on the proliferation of DLA cells was evaluated using the MTT assay. In vitro studies were carried out to confirm the enhanced anti-cancer activity of nLUB compared with the UB in lymphoma cells at equivalent drug concentrations. Both free UB and nLUB was seen to be cytotoxic against DLA cell line when the cells were treated with escalating concertation of the UB or nLUB (25, 50, 100 and 150 µg/ml). After treatment of 24 h, the inhibition rate of nLUB to DLA cell growth was significantly higher than that of free UB. With the treatment time prolonged to 48 h, the inhibition rate of nLUB was higher than that of UB at even lower concentration of 25 µg/ml (p < 0.01). As shown in Fig. 3a, b, nanoencapsulation of UB showed a significant enhancement in the cytotoxicity of free UB in dose and time-dependent manner. IC50 values of UB and nLUB on Lymphoma cells were 98 μg/ml and 54 μg/ml, respectively. The results demonstrated that treatment with nLUB significantly (p < 0.01) improves the antiproliferative effect compared to UB in lymphoma cells. Empty liposomes did not affect cell proliferation of lymphoma cells (data not shown). Although UBs reduced the viability of tumor cells effectively, the extent of cell viability reduction in the case of nLUB was significantly (p < 0.01) higher than that of the UB.
Fig. 3.
a, b Cytotoxicity results of nLUB in lymphoma cells at 24 and 48 h, respectively. c AO/EtBr-stained micrographs after treatment with UB and nLUB in lymphoma cells
The in vitro apoptotic effect of nLUB was detected by acridine orange ethidium bromide fluorescent staining of DLA cells. As shown in Fig. 3c. The green color shows live cells, while the greenish-yellow shows early apoptotic cells. AO/EtBr results showed that treatment with nLUB improved apoptotic effects in lymphoma cells compared to UB. This result indicated that nanoencapsulation of UB decreased tumor cell proliferation and stimulated apoptosis in lymphoma cell lines. The results showed a significant (p < 0.01) enhancement after nLUB treatment in a dose-dependent manner compared to UB. Also, AO/EtBr staining results showed that nLUB administration improved the apoptotic induction effect in lymphoma cells compared to UB. All these observations support the improved induction of the apoptotic response of nLUB in the treated cell. Thus, nLUB could be a promising formulation to inhibit lymphoma cell proliferation.
nLUB treatment reduced murine tumor growth of experimental animal
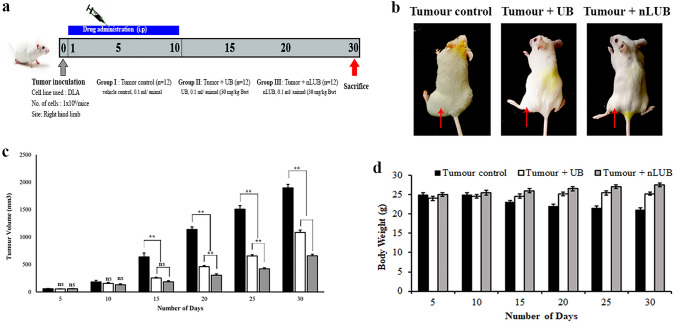
The schematic representation of the in vivo tumor study is shown in Fig. 4a. Representative photographs of DLA tumor-bearing animals from each group are shown in Fig. 4b. As shown in Fig. 4c, the mean tumor volume of the tumor control group was 1897 ± 64 mm3. The nLUB treatment group evidenced superior efficacy, with a mean tumor load of 659 ± 28 mm3 compared to 1085 ± 42 mm3 in the UB or tumor-alone group. The tumor inhibition rates of the free UB are lower than those of the nLUB at the same dose of 30 mg/kg. The in vivo experimental tumor study results showed that tumor inhibition for nLUB was higher and significant (p < 0.01) compared to tumor control or UB. This result indicated that nanoencapsulation of UB significantly (p < 0.01) improved efficacy in lymphoma solid tumor treatment in comparison to UB or tumor control groups. The body weight changes of the experimental animals are shown in Fig. 4d. in tumor control animals, body weight and tumor load were progressively increased. The results showed that nLUB administration significantly (p < 0.01) stabilized the body weight change of experimental animals compared to UB or Tumor control groups.
Fig. 4.
a Schematic representation of an in vivo solid tumor study. b Representative images of lymphoma-induced solid tumor-bearing animals from tumor control, free drug, and nLUB treated groups. c Effect of nLUB on the tumor volume of experimental animals up to 30th day. d Effect of nLUB on the Bodyweight of the lymphoma induced solid tumor-bearing animals during the experiment period
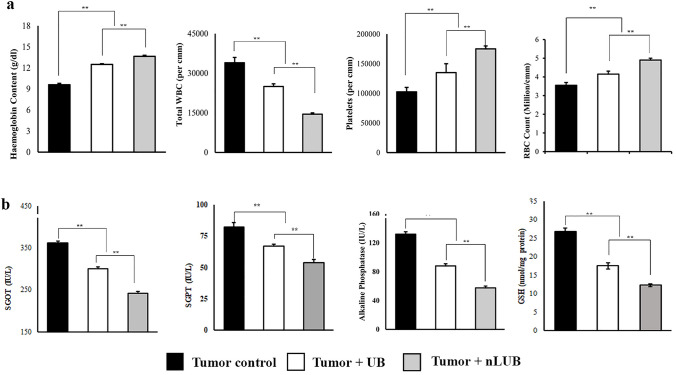
30th day, blood was collected from the experimental animal and used to analyze various hematological (Hb content, WBC count, RBC, and platelet count) and serum biochemical parameters (SGOT, SGPT, ALP, and GSH). The tumor development makes the experimental animals anemic by reducing their Hb, RBC, and platelet counts (Fig. 5a). The nLUB administration significantly (p < 0.01) improved the Hb (13.65 ± 0.15 g/dl), platelets (175,000 ± 5000 per mm3), and RBC (4.9 ± 0.1 million per mm3) content of the experimental animals when compared to tumor control (9.6 ± 0.2 g/dl, 102,500 per mm3, and 3.55 ± 0.13 million per mm3 respectively) or the UB group (12.5 ± 0.1 g/dl, 135,000 per mm3 and 4.15 ± 0.16 million per mm3 respectively). In addition, it was seen that nLUB treatment significantly (p < 0.01) reduced the elevated WBC count when compared to tumor control or UB (34,000 ± 2000 per mm3 and 25,000 ± 1100 per mm3, respectively). These results demonstrate that nLUB treatment effectively resists tumor-associated changes in the hematological parameters of the tumor-bearing animals compared to the UB.
Fig. 5.
a Effect of nLUB on the hematological parameters of experimental animals. b Effect of nLUB serum biochemical parameter of the experimental animals
The effect of nLUB treatment on various serum biochemical parameters such as SGOT, SGPT, ALP, and GSH of lymphoma tumor-bearing mice is shown in Fig. 5b. Tumor progression causes an elevation in the serum biochemical parameters of the experimental animals. When compared to the tumor alone (361 ± 5.8 IU/l, 82 ± 3.5 IU/l, 132 ± 3.4 IU/l, and 26. ± 0.85 nmol/mg protein, respectively) or the UB group (300 ± 4.9 IU/l, 67 ± 1.2 IU/l, 88 ± 3.0 IU/l, and 17.5 ± 0.78 nmol/mg protein, respectively), the nLUB treated group had significantly reduced levels of SGOT (242 ± 4.7 IU/l), SGPT (54 ± 2.3 IU/l), ALP (57.5 ± 2.5) and GSH (12.3 ± 0.41 nmol/mg protein). Thus, nLUB administration effectively protects the serum biochemical parameters of the tumor-bearing animals compared to UB treatment.
nLUB treatment improved survival rate of experimental animal
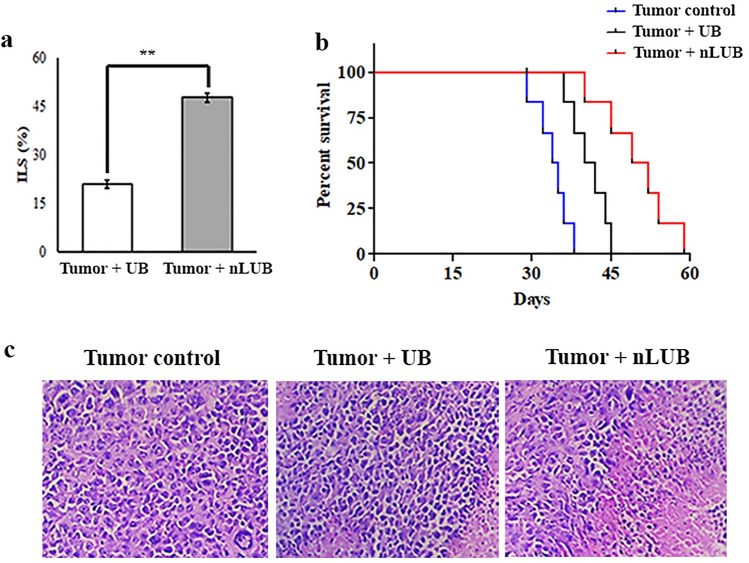
As shown in Fig. 6a, nLUB treatment showed a significant (p < 0.01) increase in life span (47.76 ± 1.3%) of the experimental animals when compared to the UB-treated groups (20.89 ± 1.25%). The mean survival of mice treated with nLUB was 49.5 days, 22.2% longer than that of UB-treated mice. As shown in Fig. 6b, nLUB administrated showed a significant (p < 0.001) increase in the experimental animals' life span compared to tumor control or UB-treated groups. This result indicated that the nLUB administration prolonged the survival of the lymphoma tumor-bearing animals. Figure 6c depicts a histopathological examination of lymphoma-induced solid tumor section of tumor control, UB, and nLUB. Tumor control groups exhibited a higher mitotic rate, and cells were arranged in sheets with infiltrating neoplasm. Treatment with nLUB reduced the number of mitotic rate and neoplastic cells compared to the tumor control or UB-treated group.
Fig. 6.
a Effect of nLUB on the lifespan of experimental animals. b Kaplan Meier survival plots of tumor control, UB and nLUB administrated mice. c Representative micrographs showing the histopathological changes in resected tumors at × 10 magnification
Discussion
Chemotherapy is one of the most frequently used treatment approaches for patients with cancer. However, most currently available chemotherapeutic drugs have several intrinsic limits, such as poor solubility, lack of specificity, and harmful side effects that directly affect the patient's quality of life. Increasing the bioavailability of the drug is the key to improving the efficiency of chemotherapy in cancer treatment (Lu et al. 2021). In this study, PEGylated nanoliposomal formulations were developed by the thin-film hydration method to enhance the therapeutic effect of UB with improved solubility and bioavailability. The physicochemical characteristics of liposomes, such as particle size, zeta potential, and polydispersity index, are important factors that aid in identifying the suitability of drug formulation for therapeutic purposes (Sarabandi et al. 2019; Damari et al. 2018). Previous studies have reported that the liposomal particles’ size and distribution are essential factors in their EE, cellular uptake, stability, and distribution (Wei et al. 2017; He et al. 2010). In clinical applications, liposomal drugs with an average diameter below 200 nm have been proven to be most beneficial (Allen and Cullis 2013).
DLS results demonstrated that nLUB had a suitable particle size for EPR-based tumor accumulation. For nanoliposome-based drug delivery, a PDI value below 0.3 is considered to be acceptable and indicates a homogenous population (Danaei et al. 2018). The study results showed that nLUB has a PDI value of < 0.3, indicating the formation of narrow, homogenous liposomal particles. Zeta potential is one of the essential parameters of nanoparticles that directly affect the stability of formulation and can be employed for understanding the surface charge of nanosuspension. Neutral or negatively charged liposomes with particle sizes less than 200 nm exhibited longer in vivo circulation time (Heurtault et al. 2003). We obtained the negatively charged formulations with less than 200 nm particle size. TEM analysis confirmed the presence of round liposomes of < 125 nm.
Umbelliferone has to exhibit pH-sensitive drug release. The present study results supported the pH-sensitive drug release of nLUB. It is well documented that pH-dependent drug delivery systems improve the therapeutic efficacy of the drug by reducing loss to blood circulation (Kundu et al. 2020). The pH-responsive drug release of nLUB executes targeted cell death, specifically in the tumor area. Biocompatibility is a critical factor in determining the quality of drug formulation for in vivo application(Ullah et al. 2016). Reduced hemolysis was contributed by nLUB when compared to the UB, which improves biocompatibility toward RBCs. Efficient cellular uptake is significant for drug delivery to the tumor site. The cellular uptake of nanoformulations is dependent on various factors, such as particle size, particle concentration, surface properties, and incubation time (Dong et al. 2015). Therefore, in the present study, we examined the cellular uptake behavior of nLUB using DLA cells. The uptake efficiency of nLUB was found to be more in DLA cells when compared to the UB. It is well documented that PEGylated liposomes provide a shealth effect to the formulations as it circulates within the body. To aid in delivery efficiency and to allow more circulation time for cargo molecules to reach tumor sites (Suk et al. 2016; Zucker et al. 2012). Considering this, PEGylation of nanoliposome resulted in sustained release and prolonged circulation apart from providing stability. Together these results demonstrate that the nLUB would meet the requirement for an adequate drug delivery system by improving biocompatibility and cellular uptake.
Furthermore, we examined the therapeutic efficacy of nLUB against in vitro and in vivo experimental tumor models. In vitro cytotoxicity studies were carried out to confirm the enhanced anti-cancer activity of nLUB compared with the UB in DLA cells at equivalent drug concentrations. The results showed a significant enhancement after nLUB treatment in a dose and time-dependent manner compared to UB. Further, AO/EtBr staining results showed that nLUB administration improved the apoptotic induction effect in DLA cells compared to UB. All these observations support the improved induction of the apoptotic response of nLUB in the treated cell. Thus, nLUB could be a promising formulation to inhibit DLA cell proliferation.
Further investigation was carried out to determine the therapeutic efficacy of nLUB in DLA-induced solid tumor models. The solid tumor was induced by intramuscular injection of DLA cells into the right hind limb of mice (Vishnu et al. 2021). The in vivo experimental tumor study results showed that tumor inhibition for nLUB was higher and found significant when compared to tumor control or UB. This result indicated that nanoencapsulation of UB improved efficacy in DLA solid tumor treatment compared to UB or tumor control groups. Previous studies have reported that a drug candidate's capability to decrease tumor burden, positively modulate hematological and biochemical parameters, stabilize body weight change, and prolong the life span of tumor-bearing animals are essential indicators of anti-cancer efficiency agents (Sakthivel et al. 2012; Dhamija et al. 2013). Considering this, the present study results have shown that nLUB administration in DLA solid tumor-bearing mice significantly reduced the tumor loads, stabilized body weights, and prolonged the lifespan of tumor-bearing mice compared to UB.
Serum glutamic oxaloacetic transaminase, glutamic pyruvate transaminase, and alkaline phosphatase levels have been raised during DLA tumor progression (Priya et al. 2011). Similarly, the present study indicated that DLA-bearing mice showed higher levels of SGOT, SGPT, and ALP than UB. However, it was significantly reduced by the nLUB treatment in experimental animals compared to UB. GSH is a nonprotein thiol essential for tumor cell proliferation. Tumor cells have elevated levels of GSH than the surrounding normal cells, characteristic of a higher cell proliferation rate and resistance to chemotherapy (Guruvayoorappan and Kuttan 2007; Sakthivel et al. 2012). Treatment with nLUB significantly reduced the GSH levels in experimental animals compared to UB. Thus, the overall results of this study highlight the enhanced protective effect of liposome-encapsulated UB against DLA-induced solid tumors when compared to UB. nLUB administration can significantly improve anti-cancer efficacy and overcome the therapeutic limitations of UB by nano-liposomal encapsulation. Thus, the nLUB would be an excellent therapeutic formulation for drug delivery to tumors.
Conclusion
In the present study, we successfully prepared and characterized Umbelliferone encapsulated nanoliposomal drug delivery system. In vitro characterization results indicated that nLUB had an improved sustained-release behavior than the free drug and facilitated cellular uptake of the liposome in a time-dependent manner. This formulation enhanced UB’s in vitro anticancer efficacy by increasing its solubility, and bioavailability. Furthermore, nLUB remarkedly improved in vivo antitumor efficacy by suppressing the tumor growth as well as prolonging the survival of experimental animals. Further studies will be required to determine the antitumor effectiveness of nLUB in different xenograft tumors to establish a sufficient basis for its wide clinical application in the treatment of cancers.
Acknowledgements
The authors acknowledge the Kerala Biotechnology Commission, Kerala State Council for Science Technology and Environment (KSCSTE) (no. 454/2020/KSCSTE)., and Department of Biotechnology (DBT) (DBT/JRF/BET-16/1/2016/Al/37-458) for providing financial assistance to the study. The authors thank Dr. S. Lakshmi, Head, Division of Cancer Research and Dr. Rekha A. Nair, Director, Regional Cancer Centre, for their valuable support.
Author contributions
PA: investigation, methodology, analysis, writing—original draft, editing, visualization, funding acquisition. CG: conceptualization, review—editing, supervision, project administration, funding acquisition.
Data availability
The data will be available on request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical approval
Animal experiments were approved by the Institutional Animal Ethics Committee, Regional Cancer Centre (Kerala, India).
Informed consent
Not applicable.
References
- Abeesh P, Vishnu WK, Guruvayoorappan C. Preparation and characterization of withaferin A loaded pegylated nanoliposomal formulation with high loading efficacy: in vitro and in vivo anti-tumour study. Mater Sci Eng C. 2021;128:112335. doi: 10.1016/j.msec.2021.112335. [DOI] [PubMed] [Google Scholar]
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Cruz LF, de Figueiredo GF, Pedro LP, Amorin YM, Andrade JT, Passos TF, Rodrigues FF, Souza ILA, Gonçalves TPR, dos Santos Lima LAR. Umbelliferone (7-hydroxycoumarin): a non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed Pharmacother. 2020;129:110432. doi: 10.1016/j.biopha.2020.110432. [DOI] [PubMed] [Google Scholar]
- Damari SP, Shamrakov D, Varenik M, Koren E, Nativ-Roth E, Barenholz Y, Regev O. Practical aspects in size and morphology characterization of drug-loaded nano-liposomes. Int J Pharm. 2018;547(1–2):648–655. doi: 10.1016/j.ijpharm.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamija I, Kumar N, Manjula S, Parihar V, Setty MM, Pai K. Preliminary evaluation of in vitro cytotoxicity and in vivo antitumor activity of Premna herbacea Roxb. in Ehrlich ascites carcinoma model and Dalton’s lymphoma ascites model. Exp Toxicol Pathol. 2013;65(3):235–242. doi: 10.1016/j.etp.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Dong H, Parekh HS, Xu ZP. Particle size-and number-dependent delivery to cells by layered double hydroxide nanoparticles. J Colloid Interface Sci. 2015;437:10–16. doi: 10.1016/j.jcis.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Evans BC, Nelson CE, Shann SY, Beavers KR, Kim AJ, Li H, Nelson HM, Giorgio TD, Duvall CL. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. JoVE. 2013;73:e50166. doi: 10.3791/50166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruvayoorappan C, Kuttan G. Immunomodulatory and antitumor activity of Biophytum sensitivum extract. Asian Pac J Cancer Prev. 2007;8(1):27. [PubMed] [Google Scholar]
- He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Heurtault B, Saulnier P, Pech B, Proust J-E, Benoit J-P. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24(23):4283–4300. doi: 10.1016/S0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- Iglesias-Montes ML, Luzi F, Dominici F, Torre L, Manfredi LB, Cyras VP, Puglia D. Migration and degradation in composting environment of active polylactic acid bilayer nanocomposites films: combined role of umbelliferone, lignin and cellulose nanostructures. Polymers. 2021;13(2):282. doi: 10.3390/polym13020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1(3):297. [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Mao C-P, He L, Tsai Y-C, Liu K, La V, Wu T-C, Hung C-F. Tumor-targeted delivery of IL-2 by NKG2D leads to accumulation of antigen-specific CD8+ T cells in the tumor loci and enhanced anti-tumor effects. PLoS ONE. 2012;7(4):e35141. doi: 10.1371/journal.pone.0035141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Sun Y, Zhu J, Li W, Zhang A, Kuang T, Xie J, Yang ZJCDM. Delivery of nanoparticles for treatment of brain tumor. Curr Drug Metab. 2016;17(8):745–754. doi: 10.2174/1389200217666160728152939. [DOI] [PubMed] [Google Scholar]
- Kumar V, Bhatt PC, Rahman M, Kaithwas G, Choudhry H, Al-Abbasi FA, Anwar F, Verma A. Fabrication, optimization, and characterization of umbelliferone β-d-galactopyranoside-loaded PLGA nanoparticles in treatment of hepatocellular carcinoma: in vitro and in vivo studies. Int J Nanomed. 2017;12:6747. doi: 10.2147/IJN.S136629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu M, Chatterjee S, Ghosh N, Manna P, Das J, Sil PC. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater Sci Eng C. 2020;116:111239. doi: 10.1016/j.msec.2020.111239. [DOI] [PubMed] [Google Scholar]
- Lopez-Gonzalez JS, Prado-Garcia H, Aguilar-Cazares D, Molina-Guarneros JA, Morales-Fuentes J, Mandoki JJ. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer. 2004;43(3):275–283. doi: 10.1016/j.lungcan.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Lu T, Haemmerich D, Liu H, Seynhaeve AL, van Rhoon GC, Houtsmuller AB, Ten Hagen TL. Externally triggered smart drug delivery system encapsulating idarubicin shows superior kinetics and enhances tumoral drug uptake and response. Theranostics. 2021;11(12):5700. doi: 10.7150/thno.55163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu R, Thangavel P, Selvaraj N, Ramalingam R, Vaiyapuri M. Synergistic and individual effects of umbelliferone with 5-flurouracil on the status of lipid peroxidation and antioxidant defense against 1, 2-dimethylhydrazine induced rat colon carcinogenesis. Biomed Prev Nutr. 2013;3(1):74–82. doi: 10.1016/j.bionut.2012.10.011. [DOI] [Google Scholar]
- Priya R, Ilavenil S, Kaleeswaran B, Srigopalram S, Ravikumar S. Effect of Lawsonia inermis on tumor expression induced by Dalton’s lymphoma ascites in Swiss albino mice. Saudi J Biol Sci. 2011;18(4):353–359. doi: 10.1016/j.sjbs.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh B, Pugalendi K. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9(4):562–566. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]
- Ramu R, S. Shirahatti P, Zameer F, Lakkappa Dhananjaya B, MN NP, Assessment of in vivo antidiabetic properties of umbelliferone and lupeol constituents of banana (Musa sp. Var. Nanjangud Rasa Bale) flower in hyperglycaemic rodent model. PLoS ONE. 2016;11(3):e0151135. doi: 10.1371/journal.pone.0151135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf A, Khan R, Khan H, Pervez S, Pirzada AS. In vivo antinociceptive and anti-inflammatory activities of umbelliferone isolated from Potentilla evestita. Nat Prod Res. 2014;28(17):1371–1374. doi: 10.1080/14786419.2014.901317. [DOI] [PubMed] [Google Scholar]
- Sakthivel K, Kannan N, Angeline A, Guruvayoorappan C. Anticancer activity of Acacia nilotica (L.) Wild. Ex. Delile subsp. indica against Dalton’s ascitic lymphoma induced solid and ascitic tumor model. Asian Pac J Cancer Prev. 2012;13(8):3989–3995. doi: 10.7314/APJCP.2012.13.8.3989. [DOI] [PubMed] [Google Scholar]
- Sarabandi K, Jafari SM, Mohammadi M, Akbarbaglu Z, Pezeshki A, Heshmati MK. Production of reconstitutable nanoliposomes loaded with flaxseed protein hydrolysates: stability and characterization. Food Hydrocolloids. 2019;96:442–450. doi: 10.1016/j.foodhyd.2019.05.047. [DOI] [Google Scholar]
- Shi Y, Van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10(17):7921. doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Lillard JWJE., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Rahman M. Umbelliferone loaded nanocarriers for healthcare applications. Curr Biochem Eng. 2020;6(1):25–33. doi: 10.2174/2212711906666190730100144. [DOI] [Google Scholar]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Kang C, Wang M, Zhu J, Jin L, Cheng X. Nanosized camptothecin conjugates for single and combined drug delivery. Eur J BioMed Res. 2016;2:8–16. doi: 10.18088/ejbmr.2.1.2016.pp8-14. [DOI] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Telange DR, Nirgulkar SB, Umekar MJ, Patil AT, Pethe AM, Bali NR. Enhanced transdermal permeation and anti-inflammatory potential of phospholipids complex-loaded matrix film of umbelliferone: formulation development, physico-chemical and functional characterization. Eur J Pharm Sci. 2019;131:23–38. doi: 10.1016/j.ejps.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Ullah S, Shah MR, Shoaib M, Imran M, Elhissi AM, Ahmad F, Ali I, Shah SWA. Development of a biocompatible creatinine-based niosomal delivery system for enhanced oral bioavailability of clarithromycin. Drug Deliv. 2016;23(9):3480–3491. doi: 10.1080/10717544.2016.1196768. [DOI] [PubMed] [Google Scholar]
- Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Agra MF, Nunes XP, Giulietti AM, Ribeiro-dos-Santos R, Soares MB. Effects of umbelliferone in a murine model of allergic airway inflammation. Eur J Pharmacol. 2009;609(1–3):126–131. doi: 10.1016/j.ejphar.2009.03.027. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi A, Sindhu G. Umbelliferone arrest cell cycle at G0/G1 phase and induces apoptosis in human oral carcinoma (KB) cells possibly via oxidative DNA damage. Biomed Pharmacother. 2017;92:661–671. doi: 10.1016/j.biopha.2017.05.128. [DOI] [PubMed] [Google Scholar]
- Vishnu WK, Abeesh P, Guruvayoorappan C. Pyrazole (1, 2-diazole) induce apoptosis in lymphoma cells by targeting BCL-2 and BCL-XL genes and mitigate murine solid tumour development by regulating cyclin-D1 and Ki-67 expression. Toxicol Appl Pharmacol. 2021;418:115491. doi: 10.1016/j.taap.2021.115491. [DOI] [PubMed] [Google Scholar]
- Wei Y, Liang J, Zheng X, Pi C, Liu H, Yang H, Zou Y, Ye Y, Zhao L. Lung-targeting drug delivery system of baicalin-loaded nanoliposomes: development, biodistribution in rabbits, and pharmacodynamics in nude mice bearing orthotopic human lung cancer. Int J Nanomed. 2017;12:251. doi: 10.2147/IJN.S119895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Gao Y, Qi Y, Chen L, Ma Y, Li Y. Peptide-based cancer therapy: opportunity and challenge. Cancer Lett. 2014;351(1):13–22. doi: 10.1016/j.canlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Yu SM, Hu DH, Zhang JJ. Umbelliferone exhibits anticancer activity via the induction of apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Mol Med Rep. 2015;12(3):3869–3873. doi: 10.3892/mmr.2015.3797. [DOI] [PubMed] [Google Scholar]
- Zocchi MR, Tosetti F, Benelli R, Poggi AJC (2020) Cancer nanomedicine special issue review anticancer drug delivery with nanoparticles: extracellular vesicles or synthetic nanobeads as therapeutic tools for conventional treatment or immunotherapy. Cancers 12(7):1886 [DOI] [PMC free article] [PubMed]
- Zucker D, Andriyanov AV, Steiner A, Raviv U, Barenholz Y. Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: relating structure and pharmacokinetics to therapeutic efficacy. J Control Release. 2012;160(2):281–289. doi: 10.1016/j.jconrel.2011.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available on request.