Abstract
Purpose
The main objective of this opinion paper was to bring to light and enhance our understanding of the amount of double-strand DNA breaks in sperm and whether there is a threshold of no return when considering repair by the oocyte/embryo.
Methods
A brief review of literature related to the theories proposed for the appearance of double-strand breaks in human spermatozoa. Further commentary regarding their detection, how oocytes or embryos may deal with them, and what are the consequences if they are not repaired. Finally, a strategy for dealing with patients who have higher levels of double-strand DNA breaks in sperm is proposed by reviewing and presenting data using testicular extracted sperm.
Results
We propose a theory that a threshold may exist in the oocyte that allows either complete or partial DNA repair of impaired sperm. The closer that an embryo is exposed to the threshold, the more the effect on the ensuing embryo will fail to reach various milestones, including blastocyst stage, implantation, pregnancy loss, an adverse delivery outcome, or offspring health. We also present a summary of the role that testicular sperm extraction may play in improving outcomes for couples in which the male has a high double-strand DNA break level in his sperm.
Conclusions
Double-strand DNA breaks in sperm provide a greater stress on repair mechanisms and challenge the threshold of repair in oocytes. It is therefore imperative that we improve our understanding and diagnostic ability of sperm DNA, and in particular, how double-strand DNA breaks originate and how an oocyte or embryo is able to deal with them.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02748-5.
Keywords: Double strand DNA breaks, Testicular sperm, DNA repair, Sperm DNA
The sperm DNA story
It is now more than 40 years since Evenson et al. [1] published a landmark study showing a relationship between mammalian sperm chromatin heterogeneity and fertility in a number of mammalian species, including humans. Ensuing studies have focused on the relationship between sperm chromatin/DNA damage and reproductive outcomes. Many of these studies have shown that men with high levels of sperm chromatin/DNA damage in their ejaculated sperm have a reduced chance of conceiving [2–6] and a higher incidence of fathering a pregnancy that could lead to a miscarriage [7–9]. Concurrently, the DNA sperm integrity tests available have still failed to convince many that they have clinical utility [10–12].
Although the predictive value of sperm chromatin/DNA fragmentation tests continues to be controversial, some recently introduced tests appear to significantly increase their clinical utility. Particularly those tests that measure double-strand breaks (DSB) in sperm, including COMET tests at neutral pH, which use specific software that makes test results more accurate, precise, and reproducible. The main objective of this opinion paper is to highlight the existence of DSB in sperm and postulate how they may arise and what their significance could be on the success of an IVF cycle.
The sensitivity to detect DSB is of paramount importance because, although DSB in sperm DNA are less frequent than single-strand breaks (SSB), they are highly deleterious, leading to genetic instability and chromosomal rearrangements [13]. Loss of control of DSB repair has gained increasing relevance since DSB repair plays a central role in the development of many human diseases [14]. Regarding embryo development, some studies suggest that the zygote responds to DSB in sperm DNA through mechanisms that delay the replication of paternal DNA, ultimately leading to embryo arrest [15, 16]. In particular, Casanovas et al. [16] showed that in patients with high levels of DSB, cleavage delay as assessed by morphokinetics is present throughout preimplantation development.
DNA double-strand breaks are repaired by means of two main mechanisms: nonhomologous end joining (NHEJ) and homologous recombination (HR) [17–19]. Both mechanisms operate in all eukaryotic cells that have been examined, but the relative contribution of each mechanism varies. For example, most mammalian cells seem to favor nonhomologous end joining. DSB restoration in the zygote occurs using NHEJ and HR repair pathways. These pathways are not equally important during the cell cycle. The choice of which repair pathway depends on the developmental stage of the embryo and the cell cycle. NHEJ works throughout the cell cycle, while HR functions during the S/G2 stage. DSB repair is obtained by stopping replication, and these breaks are preferably restored using HR [20]. In general, utilization of the DSB repair pathways during spermatogenesis from spermatogonia to sperm cells see the use of HR-based pathways in spermatogonia and spermatocytes, and the classical NHEJ pathway in spermatogonia, spermatocytes, round spermatids, and sperm cells. The alternative end joining (aEJ) pathway is utilized during spermatogenesis from spermatogonia to sperm cells except for spermatocytes (reviewed by [19]). It is believed that at the zygotic stage, NHEJ plays an essential role in the restoration of sperm DSBs [21]. Although some initial research has indicated the ability of the mammalian oocyte/embryo to repair DSB in sperm, little is understood of the consequences and impact it may have on the ensuing embryo and fetus. In particular, enhancing our understanding of the amount of DSB in sperm and whether there is a threshold of no return when considering repair by the oocyte/embryo is an area where more research should be targeted.
Why should we fear DSB in comparison to SSB and how do they originate?
There are several mechanisms of generation of DSB at the intra-testicular level: (i) during mitosis in spermatogenesis, DNA repair systems fail to repair DSB [22], (ii) during meiosis-I, ATM kinase fails to phosphorylate H2A histone at nuclear foci of DSB, enabling their identification and repair [23], and (iii) during spermiogenesis, topoisomerases play a dual role of DNA nucleases and ligases in order to provide relief of the torsional stress produced during the displacement of histones by protamines. If these breaks are not repaired by the ligase activity of topoisomerases, this will result in the generation of DSB followed by irreversible DNA degradation by a nuclease [15, 24–26]. It has been postulated that an intricate partnership also exists between topoisomerase integrated in the sperm chromatin and SUMOulation [27]. It would be thought that DSBs related to meiosis and mitosis would be targeted for clearance by apoptosis or by the Sertoli cells [22, 28–31]; however, the possible escape of sperm carrying these abnormalities is highly feasible. DSBs generated by anomalies in the final stages of histone-protamine replacement during spermiogenesis would less likely be policed by clearance mechanisms and could find their way into the ejaculated sperm population.
The aim of spermatogenesis is to create a highly organized and condensed chromatin sperm nucleus. This leads to an almost inert chromatin and shuts down gene transcription. The intricate folding needed to create this highly inert chromatin involves specific regions involving protamine and DNA toroids, which are thought to be connected to the nuclear matrix by toroid linker regions (see review by [32]). It has been postulated that these toroid regions in fully condensed sperm chromatin provide vulnerable, nuclease-sensitive regions that can be digested by external nucleases, leading to DNA strand breaks [32].
A further occurrence of DSB could manifest at the post-testicular level. We have proposed the occurrence of DSB during migration of spermatozoa through the epididymis via activation of endonucleases present in the nucleus of mature spermatozoa. Since oxygen radicals have been shown to activate sperm endonucleases [28], and it is well known that the levels of oxygen radicals in the epididymis can be relatively high [30, 33, 34], we postulate that DSB could also be generated during migration of spermatozoa through the epididymis. In support of this hypothesis, we can examine whether there is a difference in sperm quality and reproductive outcomes between sperm that is recovered in the testes versus that recovered in the ejaculate.
Outcomes in patients treated with testicular sperm
Numerous publications have shown that males who have increased levels of DNA strand breaks in their ejaculated sperm show lower levels of DNA strand breaks in their sperm retrieved from the testes (Table 1, Supplemental Table 1). A group of studies have also shown improvement when reverting to the use of testicular sperm after poor outcomes when using ejaculated sperm (Table 1). These studies have in general been retrospective in nature, comparing previous failed attempts with ejaculated sperm to those using testicular sperm (Table 1). The live birth outcomes in these selected patients are encouraging, and we would argue warrant a randomized trial to substantiate the growing data. The improvement in sperm characteristics is most convincing when examining the presence of DSB using various sperm DNA assessment techniques (Table 1, Supplemental Table 1). Furthermore, other parameters show improvement when using testicular sperm, including an improvement in blastocyst development [35] and a decrease in miscarriage rates. One of the more intriguing data sets supporting the induction of post-testicular DSB by activation of human sperm endonucleases in the epididymis is the improvement in pregnancy outcome observed in couples with repeated idiopathic IVF failure and embryo cleavage arrest using ejaculated sperm when compared to the use of testicular sperm in TESE-ICSI cycles [36]. Overall, the drawback of the majority of studies published examining this question is that they are comparing previous failed cycles and making the association with high DNA strand break levels in the ejaculated sperm. The direct improvement when using testicular sperm, which possess lower levels of DNA strand breaks, must be verified in more robust studies.
Table 1.
A summary of selected studies comparing fertilization, embryo development, and ongoing or live birth outcomes in patients using ejaculated and testicular sperm in relation to previous DNA fragmentation measurements or comparison of similar groups where male factors with low sperm counts were treated with testicular sperm. All cases utilized ICSI. Studies in grey indicate when the same patients had transfers using ejaculated and testicular sperm. Numbers highlighted in bold indicate a significant difference reported in the study
| Author | Ref. | Type of sperm sample | Number of embryo transfers | Etiology of patients studied | Sperm DNA fragmentation method (%) | MII (mean) | Fertilization Rate 2PN/MII (%) | Embryo cleavage rate (%) | Day 5 blastocysts per day 2 embryos (%) | Clinical pregnancy (%) | Implantation rate (%) | Miscarriage after clinical pregnancy (%) | Ongoing pregnancy Per transfer (%) |
Live birth Per transfer (%) |
No. of live born (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Sousa (N = 127) |
[35] | Ejaculate | 47 | RIF | None | 7.3 | 63.6 | 91.0 | 47.1 | 10.3 | 5.6 | 50.0 | 5.1 | 5.1 | 5.1 | |
| Testicular | 80 | 7.1 | 72.6 | 94.8 | 62.2 | 39.1 | 26.6 | 25.9 | 27.5 | 27.5 | 31.9 | |||||
|
Kahraman (1996) (N = 24) |
[53] | Ejaculate | 10 | AT | None | - | 54.5 | 94.4 | 20.0 | 100.0 | 0.0 | |||||
| Testicular | 14 | AT | - | 53.5 | 96.3 | 57.1 | 25.0 | 42.9 | ||||||||
|
Greco (2005) (N = 36) |
[54] | Ejaculate | 18 | RIF + hSDF |
TUNEL (23.6%) |
10.3 | 70.8 | 94.7 | - | 5.6 | 1.8 | 5.6 | 0.0 | 0.0 | 0.0 | |
| Testicular | 18 |
TUNEL (4.8%) |
10.4 | 74.9 | 95 | - | 44.4 | 20.7 | 0.0 | 44.4 | 44.4 | 66.7 | ||||
|
Hauser (2011) (N = 93) |
[55] |
Ejaculate Crypto-zoospermia |
34 | Crypto-zoospermia | None | 9.0 | 38.2 | 54.5 | 14.3 | 5.1 | 14.3 | 14.3 | 11.8 | |||
| Testicular | 59 | 9.1 | 48.4 | 54.3 | 31 | 12.1 | 27.9 | 27.9 | 18.6 | |||||||
|
Ben-Ami (2013) (N = 116) |
[56] | Ejaculate | 68 | Cryptoz + RIF | None | 8.1 | 38 | 69.7 | 15.1 | 5.7 | 2.9 | 9.4 | ||||
| Testicular | 48 | None | 10.2 | 46.7 | 78.4 | 42.5 | 20.7 | 12.5 | 27.5 | |||||||
|
Arafa (2018) (N = 72) |
[57] | Ejaculate | 36 | hSDF + RIF |
SCD (56.4%) |
- | 46.58 | 97.7 | - | 13.5 | 5.6 | 13.5 | 8.3 | 8.3 | ||
| Testicular | 36 |
SCD (15.3%) |
47 | 95.6 | 38.9 | 0.0 | 38.0 | 36.1 | 47.2 | |||||||
|
Herrero (2019) (N = 145) |
[58] | Ejaculate | 68 | RIF + RPL |
TUNEL (27.3%) SCSA (20.9%) |
6.5 | 63.6 | 10 | 41.7 | 11.4 | ||||||
| Testicular | 77 |
TUNEL (32.2%) SCSA (28.8%) |
6.5 | 62.7 | 27.9 | 25.0 | 23.4 | |||||||||
For abbreviations and explanations, see Supplemental Table 1key
Other approaches to limit DSBs in sperm prior to ICSI
Infertile males will routinely present with both SSB and DSB in their sperm population [7, 37]. The origin of these separate populations is speculative. While DSB may originate from earlier meiotic or chromatin reorganization, SSB have been largely linked with reactive oxygen species [38]. These reactive species also affect sperm motility by affecting the mitochondrial membrane. For this reason, any sperm selection system that selects progressively motile sperm (or eliminates immotile sperm) will be efficient in reducing single-stranded DNA-affected sperm [39]. On the contrary, DNA DSB could be present in spermatozoa with good motility and morphology. A number of other techniques have been proposed to eliminate sperm-carrying DSBs. In these cases, some microfluidic selection systems have proven to be effective in reducing these spermatozoa [40, 41]. Density gradient centrifugation has also been reported to have some efficiencies in removing sperm possessing DSB [42]. Unfortunately, the data examining the utility of microfluidic systems is still minimal. Finally, the interest in sperm selection techniques is growing rapidly, as evidenced by a number of recent reviews [43–45] that have examined simple solutions such as short abstinence [46] to more complex artificial intelligence-based technologies [47].
How can DSBs in sperm be the bad guy?
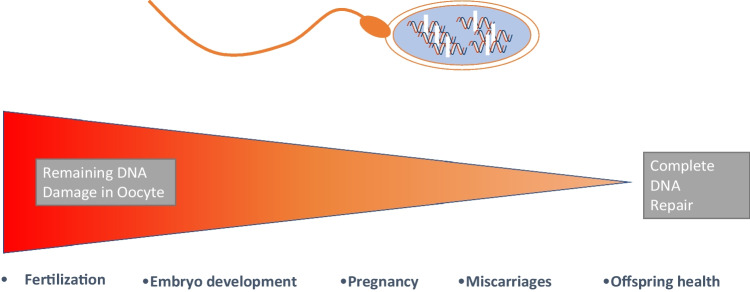
We have known for many years that the oocyte and developing preimplantation embryo depend on a multitude of highly orchestrated and synchronized events to develop and reach their final goal of a healthy live birth. Sperm are implicated in many of the major early hurdles in development, including activation of maternal mRNA stores in the first few days of development, embryonic genome activation, metabolic switches, compaction, and differentiation of cell lineages in the blastocyst, to name a few of the critical events that must occur to ensure viability [48, 49]. Stress from any of these events can have long-term consequences. Fertilization from a sperm possessing either SSB or DSB will incur stress on the oocyte during fertilization and the developing embryo. We have already seen the human embryo is armed with a plasticity that can cope with adverse events [50]. The best-emerging example is that human blastocysts that have been clinically diagnosed as mosaic aneuploid (displaying an abnormal copy number of chromosomes) can lead to healthy births, suggesting the presence of an in vivo mechanism to eliminate aneuploidy [51, 52]. As controversial as this may be, it does indicate that mechanisms also exist in the oocyte or embryo to repair both SSB and delivered by a fertilizing sperm. We propose that a threshold may exist that allows either complete or partial DNA repair. The closer that an embryo is exposed to the threshold, or if it cannot maintain the threshold, then depending on the level, the ensuing embryo will fail to reach various milestones, including blastocyst stage, implantation, pregnancy loss, an adverse delivery outcome, or offspring health (Fig. 1). Sperm DSB would obviously provide greater stress on repair mechanisms and challenge the threshold more often. It is therefore imperative that we improve our understanding and diagnostic ability of sperm DNA, and in particular, how DSB originate and how an oocyte or embryo is able to deal with them.
Fig. 1.
Consequences of fertilization by a sperm carrying double-strand breaks (DSB) in relation to the threshold of repair by the oocyte during fertilization. High levels of remaining DNA damage in the oocyte will lead to failure at earlier stages of development (fertilization or embryo), while lower levels of remaining DNA damage may manifest themselves later in development
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juan G. Alvarez, Email: jalvarez@androgen.es
Agustin García-Peiró, Email: agus7peiro@hotmail.com.
Alberto Barros, Email: abarros@med.up.pt.
Luís Ferraz, Email: ferrasluis@gmail.com.
Mário Sousa, Email: msousa@icbas.up.pt.
Denny Sakkas, Email: dsakkas@bostonivf.com.
References
- 1.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science. 1980;210:1131–3. doi: 10.1126/science.7444440. [DOI] [PubMed] [Google Scholar]
- 2.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 3.Frydman N, Prisant N, Hesters L, Frydman R, Tachdjian G, Cohen-Bacrie P, et al. Adequate ovarian follicular status does not prevent the decrease in pregnancy rates associated with high sperm DNA fragmentation. Fertil Steril. 2008;89:92–7. doi: 10.1016/j.fertnstert.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 5.Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl. 2011;13:69–75. doi: 10.1038/aja.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oleszczuk K, Giwercman A, Bungum M. Sperm chromatin structure assay in prediction of in vitro fertilization outcome. Andrology. 2016;4:290–6. doi: 10.1111/andr.12153. [DOI] [PubMed] [Google Scholar]
- 7.Ribas-Maynou J, García-Peiró A, Fernandez-Encinas A, Amengual MJ, Prada E, Cortés P, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One. 2012;7:e44679. doi: 10.1371/journal.pone.0044679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 9.Ruixue W, Hongli Z, Zhihong Z, Rulin D, Dongfeng G, Ruizhi L. The impact of semen quality, occupational exposure to environmental factors and lifestyle on recurrent pregnancy loss. J Assist Reprod Genet. 2013;30:1513–8. doi: 10.1007/s10815-013-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 11.Simon L, Liu L, Murphy K, Ge S, Hotaling J, Aston KI, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod. 2014;29:904–17. doi: 10.1093/humrep/deu040. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol. 2016;28:164–71. doi: 10.1097/GCO.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 13.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci U S A. 2003;100:12871–6. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Gawecka JE, Marh J, Ortega M, Yamauchi Y, Ward MA, Ward WS. Mouse zygotes respond to severe sperm DNA damage by delaying paternal DNA replication and embryonic development. PLoS One. 2013;8:e56385. doi: 10.1371/journal.pone.0056385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casanovas A, Ribas-Maynou J, Lara-Cerrillo S, Jimenez-Macedo AR, Hortal O, Benet J, et al. Double-stranded sperm DNA damage is a cause of delay in embryo development and can impair implantation rates. Fertil Steril. 2019;111(699–707):e1. doi: 10.1016/j.fertnstert.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Khokhlova EV, Fesenko ZS, Sopova JV, Leonova EI. Features of DNA repair in the early stages of mammalian embryonic development. Genes (Basel) 2020;11. [DOI] [PMC free article] [PubMed]
- 18.Ali A, Xiao W, Babar ME, Bi Y. Double-stranded break repair in mammalian cells and precise genome editing. Genes (Basel) 2022;13. [DOI] [PMC free article] [PubMed]
- 19.Talibova G, Bilmez Y, Ozturk S. DNA double-strand break repair in male germ cells during spermatogenesis and its association with male infertility development. DNA Repair (Amst) 2022;118:103386. doi: 10.1016/j.dnarep.2022.103386. [DOI] [PubMed] [Google Scholar]
- 20.Rothkamm K, Krüger I, Thompson LH, Löbrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–15. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17:1922–37. doi: 10.1093/hmg/ddn090. [DOI] [PubMed] [Google Scholar]
- 22.Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31:309–19. doi: 10.1016/j.rbmo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Stracker TH, Roig I, Knobel PA, Marjanović M. The ATM signaling network in development and disease. Front Genet. 2013;4:37. doi: 10.3389/fgene.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPherson S, Longo FJ. Chromatin structure-function alterations during mammalian spermatogenesis: DNA nicking and repair in elongating spermatids. Eur J Histochem. 1993;37:109–28. [PubMed] [Google Scholar]
- 25.McPherson SM, Longo FJ. Nicking of rat spermatid and spermatozoa DNA: possible involvement of DNA topoisomerase II. Dev Biol. 1993;158:122–30. doi: 10.1006/dbio.1993.1173. [DOI] [PubMed] [Google Scholar]
- 26.Marcon L, Boissonneault G. Transient DNA strand breaks during mouse and human spermiogenesis new insights in stage specificity and link to chromatin remodeling. Biol Reprod. 2004;70:910–8. doi: 10.1095/biolreprod.103.022541. [DOI] [PubMed] [Google Scholar]
- 27.Aitken RJ, Lewis SEM. DNA damage in testicular germ cells and spermatozoa. When and how is it induced? How should we measure it? What does it mean? Andrology 2023. [DOI] [PubMed]
- 28.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacheco S, Marcet-Ortega M, Lange J, Jasin M, Keeney S, Roig I. The ATM signaling cascade promotes recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 2015;11:e1005017. doi: 10.1371/journal.pgen.1005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–36. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 31.Vasileva A, Hopkins KM, Wang X, Weisbach MM, Friedman RA, Wolgemuth DJ, et al. The DNA damage checkpoint protein RAD9A is essential for male meiosis in the mouse. J Cell Sci. 2013;126:3927–38. doi: 10.1242/jcs.126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribas-Maynou J, Nguyen H, Wu H, Ward WS. Functional aspects of sperm chromatin organization. Results Probl Cell Differ. 2022;70:295–311. doi: 10.1007/978-3-031-06573-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ollero M, Gil-Guzman E, Lopez MC, Sharma RK, Agarwal A, Larson K, et al. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod. 2001;16:1912–21. doi: 10.1093/humrep/16.9.1912. [DOI] [PubMed] [Google Scholar]
- 34.Drevet JR. The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Mol Cell Endocrinol. 2006;250:70–9. doi: 10.1016/j.mce.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Sousa M, Cunha M, Pereira M, Silva J, Gonçalves A, Viana P et al. Clinical outcomes of 127 patients with recurrent implantation failure treated with testicular sperm aspiration (TESA) In: European Society of Human Reproduction, 2022:i218.
- 36.Esteves SC, Roque M, Bradley CK, Garrido N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril. 2017;108(456–67):e1. doi: 10.1016/j.fertnstert.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Ribas-Maynou J, García-Peiró A, Abad C, Amengual MJ, Navarro J, Benet J. Alkaline and neutral Comet assay profiles of sperm DNA damage in clinical groups. Hum Reprod. 2012;27:652–8. doi: 10.1093/humrep/der461. [DOI] [PubMed] [Google Scholar]
- 38.Drevet JR, Hallak J, Nasr-Esfahani MH, Aitken RJ. Reactive oxygen species and their consequences on the structure and function of mammalian spermatozoa. Antioxid Redox Signal. 2022;37:481–500. doi: 10.1089/ars.2021.0235. [DOI] [PubMed] [Google Scholar]
- 39.Lara-Cerrillo S, Ribas-Maynou J, Rosado-Iglesias C, Lacruz-Ruiz T, Benet J, García-Peiró A. Sperm selection during ICSI treatments reduces single- but not double-strand DNA break values compared to the semen sample. J Assist Reprod Genet. 2021;38:1187–96. doi: 10.1007/s10815-021-02129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parrella A, Keating D, Cheung S, Xie P, Stewart JD, Rosenwaks Z, et al. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J Assist Reprod Genet. 2019;36:2057–66. doi: 10.1007/s10815-019-01543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kocur OM, Xie P, Cheung S, Souness S, McKnight M, Rosenwaks Z et al. Can a sperm selection technique improve embryo ploidy? Andrology 2022. [DOI] [PMC free article] [PubMed]
- 42.Enciso M, Iglesias M, Galán I, Sarasa J, Gosálvez A, Gosálvez J. The ability of sperm selection techniques to remove single- or double-strand DNA damage. Asian J Androl. 2011;13:764–8. doi: 10.1038/aja.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lara-Cerrillo S, Urda Muñoz C, de la Casa Heras M, Camacho Fernández-Pacheco S, Gijón de la Santa J, Lacruz-Ruiz T et al. Microfluidic sperm sorting improves ICSI outcomes in patients with increased values of double-strand breaks in sperm DNA. Rev Int Androl 2022. [DOI] [PubMed]
- 44.Sakkas D, Ramalingam M, Garrido N, Barratt CL. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum Reprod Update. 2015;21:711–26. doi: 10.1093/humupd/dmv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaughan DA, Sakkas D. Sperm selection methods in the 21st century. Biol Reprod. 2019;101:1076–82. doi: 10.1093/biolre/ioz032. [DOI] [PubMed] [Google Scholar]
- 46.Barbagallo F, Cannarella R, Crafa A, Manna C, La Vignera S, Condorelli RA et al. The impact of a very short abstinence period on conventional sperm parameters and sperm DNA fragmentation: a systematic review and meta-analysis. J Clin Med 2022;11. [DOI] [PMC free article] [PubMed]
- 47.Dimitriadis I, Zaninovic N, Badiola AC, Bormann CL. Artificial intelligence in the embryology laboratory: a review. Reprod Biomed Online. 2022;44:435–48. doi: 10.1016/j.rbmo.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Tarozzi N, Nadalini M, Coticchio G, Zacà C, Lagalla C, Borini A. The paternal toolbox for embryo development and health. Mol Hum Reprod 2021;27. [DOI] [PubMed]
- 49.Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35:1953–68. doi: 10.1007/s10815-018-1304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coticchio G, Barrie A, Lagalla C, Borini A, Fishel S, Griffin D, et al. Plasticity of the human preimplantation embryo: developmental dogmas, variations on themes and self-correction. Hum Reprod Update. 2021;27:848–65. doi: 10.1093/humupd/dmab016. [DOI] [PubMed] [Google Scholar]
- 51.Barad DH, Albertini DF, Molinari E, Gleicher N. IVF outcomes of embryos with abnormal PGT-A biopsy previously refused transfer: a prospective cohort study. Hum Reprod. 2022;37:1194–206. doi: 10.1093/humrep/deac063. [DOI] [PubMed] [Google Scholar]
- 52.Yang M, Rito T, Metzger J, Naftaly J, Soman R, Hu J, et al. Depletion of aneuploid cells in human embryos and gastruloids. Nat Cell Biol. 2021;23:314–21. doi: 10.1038/s41556-021-00660-7. [DOI] [PubMed] [Google Scholar]
- 53.Kahraman S, Tasdemir M, Tasdemir I, Vicdan K, Ozgur S, Polat G, et al. Pregnancies achieved with testicular and ejaculated spermatozoa in combination with intracytoplasmic sperm injection in men with totally or initially immotile spermatozoa in the ejaculate. Hum Reprod. 1996;11:1343–6. doi: 10.1093/oxfordjournals.humrep.a019384. [DOI] [PubMed] [Google Scholar]
- 54.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–30. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 55.Hauser R, Bibi G, Yogev L, Carmon A, Azem F, Botchan A, et al. Virtual azoospermia and cryptozoospermia–fresh/frozen testicular or ejaculate sperm for better IVF outcome? J Androl. 2011;32:484–90. doi: 10.2164/jandrol.110.011353. [DOI] [PubMed] [Google Scholar]
- 56.Ben-Ami I, Raziel A, Strassburger D, Komarovsky D, Ron-El R, Friedler S. Intracytoplasmic sperm injection outcome of ejaculated versus extracted testicular spermatozoa in cryptozoospermic men. Fertil Steril. 2013;99:1867–71. doi: 10.1016/j.fertnstert.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 57.Arafa M, AlMalki A, AlBadr M, Burjaq H, Majzoub A, AlSaid S et al. ICSI outcome in patients with high DNA fragmentation: testicular versus ejaculated spermatozoa. Andrologia 2018;50. [DOI] [PubMed]
- 58.Herrero MB, Lusignan MF, Son WY, Sabbah M, Buckett W, Chan P. ICSI outcomes using testicular spermatozoa in non-azoospermic couples with recurrent ICSI failure and no previous live births. Andrology. 2019;7:281–7. doi: 10.1111/andr.12591. [DOI] [PubMed] [Google Scholar]
- 59.Hourvitz A, Shulman A, Madjar I, Levron J, Levran D, Mashiach S, et al. In vitro fertilization treatment for severe male factor: a comparative study of intracytoplasmic sperm injection with testicular sperm extraction and with spermatozoa from ejaculate. J Assist Reprod Genet. 1998;15:386–9. doi: 10.1023/A:1022537117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weissman A, Horowitz E, Ravhon A, Nahum H, Golan A, Levran D. Pregnancies and live births following ICSI with testicular spermatozoa after repeated implantation failure using ejaculated spermatozoa. Reprod Biomed Online. 2008;17:605–9. doi: 10.1016/S1472-6483(10)60306-9. [DOI] [PubMed] [Google Scholar]
- 61.Tsai CC, Huang FJ, Wang LJ, Lin YJ, Kung FT, Hsieh CH, et al. Clinical outcomes and development of children born after intracytoplasmic sperm injection (ICSI) using extracted testicular sperm or ejaculated extreme severe oligo-astheno-teratozoospermia sperm: a comparative study. Fertil Steril. 2011;96:567–71. doi: 10.1016/j.fertnstert.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 62.Amirjannati N, Heidari-Vala H, Akhondi MA, Hosseini Jadda SH, Kamali K, Sadeghi MR. Comparison of intracytoplasmic sperm injection outcomes between spermatozoa retrieved from testicular biopsy and from ejaculation in cryptozoospermic men. Andrologia. 2012;44(Suppl 1):704–9. doi: 10.1111/j.1439-0272.2011.01253.x. [DOI] [PubMed] [Google Scholar]
- 63.Esteves SC, Agarwal A. Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: case series and systematic review. Clinics (Sao Paulo) 2013;68(Suppl 1):141–50. doi: 10.6061/clinics/2013(Sup01)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104:1398–405. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 65.Mehta A, Bolyakov A, Schlegel PN, Paduch DA. Higher pregnancy rates using testicular sperm in men with severe oligospermia. Fertil Steril. 2015;104:1382–7. doi: 10.1016/j.fertnstert.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Bradley CK, McArthur SJ, Gee AJ, Weiss KA, Schmidt U, Toogood L. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology. 2016;4:903–10. doi: 10.1111/andr.12215. [DOI] [PubMed] [Google Scholar]
- 67.Cui X, Ding P, Gao G, Zhang Y. Comparison of the clinical outcomes of intracytoplasmic sperm injection between spermatozoa retrieved from testicular biopsy and from ejaculate in cryptozoospermia patients. Urology. 2017;102:106–10. doi: 10.1016/j.urology.2016.08.071. [DOI] [PubMed] [Google Scholar]
- 68.Ketabchi AA. Intracytoplasmic sperm injection outcomes with freshly ejaculated sperms and testicular or epididymal sperm extraction in patients with idiopathic cryptozoospermia. Nephrourol Mon. 2016;8:e41375. doi: 10.5812/numonthly.41375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu YT, Luo C, Li Y, Li H, Quan S, Deng YJ, et al. Differences and similarities between extremely severe oligozoospermia and cryptozoospermia in intracytoplasmic sperm injection. Asian J Androl. 2016;18:904–7. doi: 10.4103/1008-682X.165948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Malki AH, Alrabeeah K, Mondou E, Brochu-Lafontaine V, Phillips S, Zini A. Testicular sperm aspiration (TESA) for infertile couples with severe or complete asthenozoospermia. Andrology. 2017;5:226–31. doi: 10.1111/andr.12317. [DOI] [PubMed] [Google Scholar]
- 71.Pabuccu EG, Caglar GS, Tangal S, Haliloglu AH, Pabuccu R. Testicular versus ejaculated spermatozoa in ICSI cycles of normozoospermic men with high sperm DNA fragmentation and previous ART failures. Andrologia 2017;49. [DOI] [PubMed]
- 72.Kawwass JF, Chang J, Boulet SL, Nangia A, Mehta A, Kissin DM. Surgically acquired sperm use for assisted reproductive technology: trends and perinatal outcomes, USA, 2004–2015. J Assist Reprod Genet. 2018;35:1229–37. doi: 10.1007/s10815-018-1178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee SH, Park CW, Cheon YP, Lim CK. Potential of testicular sperm to support embryonic development to the blastocyst stage is comparable to that of ejaculated sperm. J Assist Reprod Genet. 2018;35:1103–11. doi: 10.1007/s10815-018-1191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Xue H, Qiu F, Zhong J, Su J. Testicular spermatozoon is superior to ejaculated spermatozoon for intracytoplasmic sperm injection to achieve pregnancy in infertile males with high sperm DNA damage. Andrologia. 2019;51:e13175. doi: 10.1111/and.13175. [DOI] [PubMed] [Google Scholar]
- 75.Alharbi M, Hamouche F, Phillips S, Kadoch JI, Zini A. Use of testicular sperm in couples with SCSA-defined high sperm DNA fragmentation and failed intracytoplasmic sperm injection using ejaculated sperm. Asian J Androl. 2020;22:348–53. doi: 10.4103/aja.aja_99_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamaguchi K, Ishikawa T, Mizuta S, Takeuchi T, Matsubayashi H, Kokeguchi S, et al. Clinical outcomes of microdissection testicular sperm extraction and intracytoplasmic sperm injection in Japanese men with Y chromosome microdeletions. Reprod Med Biol. 2020;19:158–63. doi: 10.1002/rmb2.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steele EK, McClure N, Maxwell RJ, Lewis SE. A comparison of DNA damage in testicular and proximal epididymal spermatozoa in obstructive azoospermia. Mol Hum Reprod. 1999;5:831–5. doi: 10.1093/molehr/5.9.831. [DOI] [PubMed] [Google Scholar]
- 78.Moskovtsev SI, Jarvi K, Mullen JB, Cadesky KI, Hannam T, Lo KC. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93:1142–6. doi: 10.1016/j.fertnstert.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Moskovtsev SI, Alladin N, Lo KC, Jarvi K, Mullen JB, Librach CL. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst Biol Reprod Med. 2012;58:142–8. doi: 10.3109/19396368.2012.667504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.