Abstract
Purpose
To investigate whether personalized embryo transfer (pET) protocol guided by an endometrial receptivity array (ERA) can improve clinical outcomes of assisted reproduction.
Methods
We searched PubMed, Embase, Web of Science, and the Cochrane library for studies in which analytical comparisons of outcomes of pET and standard embryo transfer (sET) groups were undertaken. The references to the included studies were also manually searched. The primary outcome was clinical pregnancy rate (CPR), and the secondary outcomes were live birth rate (LBR), human chorionic gonadotropin (HCG) positivity, biochemical pregnancy rate (BPR), miscarriage rate (MR), implantation rate (IR), and ongoing pregnancy rate (OPR).
Results
Ten studies were included in the meta-analysis, including one randomized controlled trial (RCT) and nine cohort studies. We observed no significant difference in the primary outcome of CPR between the pET and sET groups in unselected patients (RR = 1.07; 95% confidence interval [CI], 0.87–1.30; P = 0.53; I2 = 89%). In terms of secondary outcomes, we likewise noted no significant differences between the groups. Further subgroup analyses indicated that the pET protocol not only significantly reduced the MR for poor-prognosis patients, but it also reduced the CPR in donor cycles, elevated the BPR for good-prognosis patients, non-preimplantation genetic testing (PGT), and programmed cycles, and decreased the proportion of women showing HCG positivity in non-PGT cycles.
Conclusions
This meta-analysis revealed that ERA appears to possess limited guidance in embryo transfer. More high-quality RCTs are therefore needed to investigate the clinical validity and feasibility of ERA in the future.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02710-x.
Keywords: Endometrial receptivity array, Embryo transfer, Clinical pregnancy, Implantation failure
Introduction
A successful embryo transfer (ET) depends upon the following three factors: an embryo with implantation competency, an endometrium in a receptive state, and synchronized development between the embryo and the endometrium [1]. Optimizing ovarian stimulation protocols and developing preimplantation genetic testing (PGT) have dramatically improved the potential for embryo development [2]. Different endometrial preparation protocols can be implemented to transform the endometrium into an optimally receptive state. Although the aforementioned methods have greatly improved the clinical outcomes of ET, some patients still fail to become pregnant after transferring high-quality embryos. In recent years, investigators have found that the optimal endometrial window of implantation (WOI) actually exhibits a narrower duration than previously thought, and some patients may shift the WOI forward or backward due to individual differences or pathological factors [3, 4]. Thus, it is essential to evaluate endometrial receptivity (ER) so as to improve the efficiency of assisted reproduction.
The methods of evaluating ER are currently classified into four categories: ultrasonography, endometrial biopsy, endometrial fluid aspiration, and hysteroscopy [5]. Two-dimensional and three-dimensional ultrasonographic examinations of the endometrium constitute the most commonly employed methods in clinical practice, but can only provide approximate guidance with respect to the ET time. ER biomarkers evaluated in endometrial fluid aspirates are still under study, with few convincing clinical trials showing their effectiveness. Hysteroscopy is principally adopted to exclude physical abnormalities of the uterus and is rarely used to help determine the time for ET [6]. In 2011, however, an endometrial receptivity array (ERA) was developed to accurately evaluate the WOI of the endometrium [7].
The ERA is a novel method for diagnosing the temporal displacement of the WOI and is derived from the development of transcriptomics and bioinformatics. By measuring the expression of 238 genes from the endometrium with an ERA, the endometrium can be classified into receptive (R) and non-receptive (NR) categories, with the latter including pre-receptive and post-receptive [8]. Although ERAs only have limited use in the clinic due to their high cost, this novel technique offers hope to some infertile families, particularly for patients with a history of recurrent implantation failure (RIF). For patients with RIF, the value of ERAs is especially noteworthy due to the reduced numbers of embryos with this condition and the increasing costs associated with assisted reproduction; and the information on WOI derived from ERAs significantly exceeds the up-front costs. The current focus of reproductive clinical work is to adjust both the embryo and endometrium for the best fit, to reduce the number of ET cycles required, and to optimize the clinical outcomes to the maximum extent possible. ERAs appear to be able to guide the optimal time for ET, but it remains to be seen whether this method has any therapeutic advantages. Some studies have shown that clinical outcomes in patients undergoing a personalized embryo transfer (pET) protocol are not significantly different from those in patients undergoing a standard embryo transfer (sET) protocol, and they might even be worse [9–11]. In contradistinction, other studies have suggested that pET can significantly improve clinical outcomes [12, 13]. Given the small sample sizes and conflicting results of these studies, it is difficult to draw reliable conclusions. Therefore, in this study, we systematically analyzed the clinical outcomes between patients who used ERA to guide ET (the pET group) and those who did not (the sET group).
Materials and methods
The meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [14] and was written according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) proposal [15]. This study was also registered with PROSPERO (CRD 42,022,360,750).
Search strategy
We searched PubMed, Embase, Web of Science, and the Cochrane library from inception to September 15, 2022. The following terms and Boolean operators were used for searching: (“Endometrial Receptivity Array” OR “Endometrial Receptivity Analysis” OR “Endometrial Receptivity Testing” OR “Personalized Embryo Transfer”) AND (“Embryo Transfer” OR “In Vitro Fertilization” OR IVF OR “Intracytoplasmic Sperm Injection” OR ICSI OR “Assisted Reproduction”). The detailed search strategy is shown in Supplementary Table S1. The references to included studies were also carefully searched by hand, and no language restrictions were applied. Articles were screened using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia).
Eligibility criteria
We focused on randomized clinical trials (RCTs), prospective cohort studies, and retrospective cohort studies in which investigators compared the clinical outcomes of pET protocols with those of sET protocols. The studies we included reflected at least one of the following outcomes: clinical pregnancy rate (CPR), live birth rate (LBR), human chorionic gonadotropin (HCG) positivity, biochemical pregnancy rate (BPR), miscarriage rate (MR), implantation rate (IR), and ongoing pregnancy rate (OPR). Case reports, conference abstracts, and review articles were excluded.
The primary outcome was CPR per cycle, while the secondary outcomes were LBR per cycle, HCG positivity rate per cycle, BPR per pregnancy, MR per pregnancy, IR per embryo, and OPR per cycle. Clinical pregnancy was defined as the presence of one or more intrauterine gestational sacs as confirmed by transvaginal ultrasonography 28 to 35 days after frozen embryo transfer (FET); live birth was defined as a baby born with spontaneous breathing and heartbeat; positive HCG was defined as a positive serum level of beta-HCG; biochemical pregnancy was defined as a positive HCG without a gestational sac visualized upon ultrasonography; miscarriage was defined as clinical pregnancy loss; and implantation was defined as one or more gestational sacs observed on ultrasonography.
Study selection and data extraction
Two reviewers (R.L. and J.W.) independently performed an initial screening of the titles and abstracts for eligibility. If the title and abstract of an article met the inclusion criteria, we obtained the full text for further evaluation. A third senior reviewer (T.S.) resolved any disagreements raised by the previous two authors. If the same group of researchers published multiple reports that described outcomes from the same sample source, we included the most recent report or a complete report in this meta-analysis.
Information such as author, publication year, country of study origin, study design, study period, participants’ main characteristics, sample size, age, embryo characteristics, endometrial preparation protocols, and clinical outcomes were recorded by one of the reviewers (Y.L.). The other reviewer (X.Z.) evaluated the data to make sure that there were no transcription errors. For those studies containing questionable data or data without specific previous IFs, we attempted to contact the appropriate authors to confirm the authenticity of the data or for detailed data. For studies that included fresh ET cycles, we only extracted data from FET cycles since the pET protocol was principally used for FET.
Quality assessment
The revised Cochrane risk of bias tool (RoB2) [16] and the ROBINS-I tool [17] were used to assess the risk of bias of the RCTs and non-RCTs, respectively. In addition, we assessed the quality of the evidence by adopting the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [18]. The GRADE criteria include risk of bias, indirectness, imprecision, inconsistency, and publication bias. Two reviewers (R.L. and J.W.) completed the aforementioned quality assessment independently, and disagreements were resolved by a third reviewer (Y.L.).
Statistical analysis
We executed the software Review Manager program 5.4 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) and Stata Statistical Software (Release 16, TX, USA) to analyze our data. Effect sizes were described by relative risk (RR) and 95% confidence intervals (CIs). Owing to the limited quantity and quality of studies included in this meta-analysis, we applied a random effects model for all outcomes. Heterogeneity was assessed with the I-squared statistic (I2), with ≥ 50% indicating substantial heterogeneity.
In addition, we performed subgroup analyses to evaluate the outcomes in different patient or embryo subgroups: patient prognosis (good-prognosis [0 previous IFs after transferring a euploid embryo, or fewer than two IFs after transferring non-PGT embryos] and poor-prognosis [one or more previous IFs after transferring a euploid embryo, or two or more IFs after transferring non-PGT embryos]); embryo selection (PGT and non-PGT); the number of embryos transferred (single and one or more); oocyte source (autologous and donated); and the method of endometrial preparation (programmed protocol and unclear or both programmed and natural protocols). In order to explore the robustness of the results that comprised at least three studies, we performed sensitivity analysis using the leave-one-out approach. Analysis of publication bias was not conducted because the number of included studies for each outcome measure was less than 10 in this study. A P-value < 0.05 was considered to show statistical significance.
Results
Search results
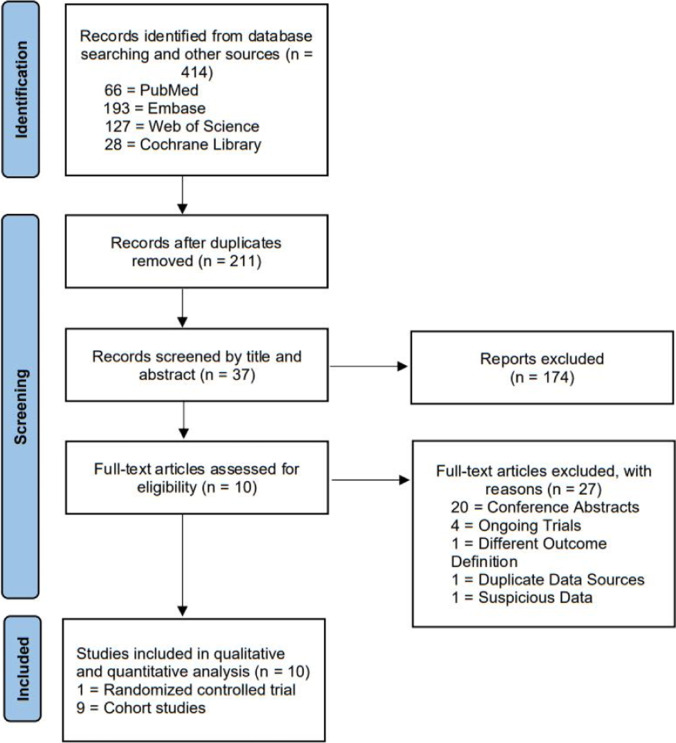
The detailed selection process of this study is shown in Fig. 1, which followed the recommendations of the PRISMA statement. A total of 414 articles were identified through our initial research, of which 203 were duplicates. After accessing titles and abstracts, 37 studies were potentially eligible for inclusion. Of these 37 studies, we excluded 27 studies after reviewing the full texts: 20 citations were conference abstracts [19–38], 4 citations were ongoing trials with no published data (register numbers: NCT03558399, NCT04497558, ChiCTR2100049841, and ChiCTR2100049041) [39–42], 1 citation contained a definition of OPR that was distinct from the conventional definition since its denominator was the number of embryos transferred [43], 1 citation [44] reflected a patient source that was the same as another eligible study in which more complete data were reported [13], and 1 citation showed questionable data, with a CPR of 23% (17/72) and IR of 4.1% (3/72), and the author could not be contacted via email [45]. Finally, 10 studies were identified as eligible for inclusion.
Fig. 1.
Flow diagram of study selection process
Characteristics of the eligible studies
The characteristics of the included studies are shown in Table 1. Of the 10 studies, 1 was an RCT [12], 1 was a prospective cohort study [10], and 8 were retrospective cohort studies [9, 11, 13, 46–50]. The studies were conducted in the USA, Spain, Canada, India, Brazil, China, and Latvia between October 2012 and March 2021. Data on the outcome of CPR were reported in eight studies [9–11, 13, 47–50], LBR in six [9–12, 47, 50], HCG positivity in seven [9–13, 47, 48], BPR in five [10–13, 48], MR in seven [9–13, 48, 50], IR in three [12, 13, 49], and OPR was reported in two articles [11, 46]. Since the IR in single embryo transfer cycles is equal to the CPR, two studies were also included in the meta-analysis of IR [10, 47].
Table 1.
Basic characteristics of the included studies
| Author, year, country | Study design | Period | Participants’ main characteristics | Sample size (pET/sET) | Age (pET/sET) | Embryo characteristics and endometrial preparation protocols | Outcomes |
|---|---|---|---|---|---|---|---|
|
Bassil et al., 2018 Canada [46] |
Single-center retrospective cohort | April 2016 to March 2017 |
pET group: patients who were < 42 years old, undergoing their first to third embryo transfer attempt; sET group: patients who underwent their first or second frozen embryo transfer at our clinic, < 42 years old; No patients with RIF in each group |
53/503 | (36.3 ± 0.40)a / (35.6 ± 4.00)a |
Oocyte source: Unclear; Developmental stage: blastocyst (inferring from the endometrial preparation protocol); PGT for aneuploidy: 34.1% and 14.7% for the pET and sET groups, respectively; No. of embryos transferred: (1.1 ± 0.4) and (1.1 ± 0.3) for the pET and sET groups, respectively; Endometrial preparation protocol: programmed or modified natural protocols |
OPR |
|
Bergin et al., 2021 USA [9] |
Single-center retrospective cohort | January 2014 to June 2019 | Patients with different prior numbers of embryo transfers | After PSM: 133/353 | After PSM: (36.25 ± 3.85)a / (36.05 ± 3.81)a |
Oocyte source: autologous; Developmental stage: blastocyst (inferring from the endometrial preparation protocol); PGT for aneuploidy: 75.19% and 72.80% for the pET and sET groups, respectively; No. of embryos transferred: (1.09 ± 0.42) and (1.10 ± 0.30) for the pET and sET groups, respectively; Endometrial preparation protocol: natural or programmed protocols |
CPR; LBR; MR; HCG positivity rate |
|
Cozzolino et al., 2022 Spain [11] |
Multicenter retrospective cohort | Unclear | Patients presenting with a previously failed embryo transfer |
Autologous cycles: 255/1862; Donor cycles: 319/639 |
Autologous cycles: (36.79 ± 3.50)a / (35.95 ± 3.82)a; Donor cycles: (41.13 ± 4.19)a / (42.16 ± 4.00)a |
Oocyte source: both autologous and donated; Developmental stage: both cleavage stage and blastocyst; PGT for aneuploidy: 31.9% and 36.5% for the pET and sET groups, respectively; No. of embryos transferred: ≥ 1; Endometrial preparation protocol: programmed protocol with GnRH downregulation |
CPR; LBR; MR; OPR; HCG positivity rate; BPR |
|
Doyle et al., 2022 USA [47] |
Single-center retrospective cohort | 2018 to 2019 | Patients with different prior numbers of embryo transfers | 307/2284 | (36.70 ± 4.10)a / (36.70 ± 4.30)a |
Oocyte source: autologous; Developmental stage: blastocyst; PGT for aneuploidy: all embryos transferred; No. of embryos transferred: 1; Endometrial preparation protocol: programmed protocol |
CPR; LBR; HCG positivity rate |
|
Fodina et al., 2021 Latvia [48] |
Single-center retrospective cohort | 2017 to 2020 | RIF: patients who failed to achieve a clinical pregnancy after transfers of at least three good-quality embryos in different single fresh or frozen embryo transfers |
PGT cycles: 72/87; Non-PGT cycles: 22/72 |
pET: (34.0 [38.0; 32.5])b; pET + PGT: (35.0 [37.0; 33.0])b; sET: (34.0 [37.0; 32.0])b; sET + PGT: (36.0 [38.0; 34.0])b |
Oocyte source: unclear; Developmental stage: blastocyst; PGT for aneuploidy: 76.6% and 54.7% for the pET and sET groups, respectively; No. of embryos transferred: unclear; Endometrial preparation protocol: unclear |
CPR; MR; HCG positivity rate; BPR |
|
Jia et al., 2022 China [13] |
Single-center retrospective cohort | November 2019 to March 2021 | Patients with multiple embryo implantation failures (at least 2 cycles of embryo transfer, or transfer of at least 3 good-quality blastocysts with a Gardner’s score of 4BB or higher) | 140/141 | (32.01 ± 2.99)a / (31.87 ± 3.21)a |
Oocyte source: unclear; Developmental stage: blastocyst; PGT for aneuploidy: unclear; No. of embryos transferred: ≥ 1; Endometrial preparation protocol: unclear |
CPR; MR; IR; HCG positivity rate; BPR |
|
Neves et al., 2019 Spain [49] |
Single-center retrospective cohort | October 2012 to December 2018 | Patients who had undergone one of the following: (a) ≥ 1 previous failed euploid embryo transfer (Euploid-ET) and (b) ≥ 2 previous failed ET from reception cycles (Donor-ET) without PGT-A | 24/119 | (39.25 ± 3.99)a / (39.18 ± 3.80)a |
Oocyte source: both autologous and donated; Developmental stage: blastocyst; PGT for aneuploidy: all autologous embryos; No. of embryos transferred: ≥ 1; Endometrial preparation protocol: programmed protocol |
CPR; IR |
|
Ohara et al., 2022 Japan [50] |
Two-center retrospective cohort | April 2019 to June 2020 | RIF: patients who failed to achieve clinical pregnancy with three or more IVF cycles in which one or two morphologically good-quality blastocysts were transferred | After PSM: 215/215 | After PSM: (38.50 ± 4.10)a / (38.20 ± 4.30)a |
Oocyte source: autologous; Developmental stage: blastocyst; PGT for aneuploidy: none; No. of embryos transferred: (1.39 ± 0.5) and (1.41 ± 0.5) for the pET and sET groups, respectively; Endometrial preparation protocol: programmed protocol |
CPR; MR; LBR |
|
Riestenberg et al., 2021 USA [10] |
Single-center prospective not randomized cohort | January 2018 to April 2019 | Patients who had their first single euploid FET in a programmed cycle | 147/81 | (36.90 ± 3.80)a / (34.90 ± 3.80)a |
Oocyte source: autologous; Developmental stage: blastocyst; PGT for aneuploidy: all embryos transferred; No. of embryos transferred: 1; Endometrial preparation protocol: programmed protocol |
CPR; LBR; MR; HCG positivity rate; BPR |
|
Simón et al., 2020 Spain [12] |
Multicentre RCT | November 2013 to April 2017 | Patients without recurrent miscarriage (more than two previous biochemical pregnancies or spontaneous abortions); implantation failure (more than three failed IVF cycles with good-quality embryos transferred) | Per-protocol analysis set: 80/92 | (33.00 ± 3.10)a / (32.80 ± 3.40)a |
Oocyte source: per-protocol analysis set: autologous; Developmental stage: both cleavage stage and blastocyst; PGT for aneuploidy: 4.3% and 2.7% for the pET and sET groups, respectively; No. of embryos transferred: ≥ 1; Endometrial preparation protocol: programmed protocol |
IR; LBR; MR; HCG positivity rate; BPR |
pET, personalized embryo transfer; sET, standard embryo transfer; No., number; FET, frozen-thawed embryo transfer; RIF, recurrent implantation failure; PGT, preimplantation genetic testing; CPR, clinical pregnancy rate; LBR, live birth rate; HCG, human chorionic gonadotropin; BPR, biochemical pregnancy rate; MR, miscarriage rate; IR, implantation rate; OPR, ongoing pregnancy rate; PSM, propensity score matching; RCT, randomized controlled trial; GnRH, gonadotropin-releasing hormone
aAges shown as mean age ± (standard deviation)
bAges shown as median age (interquartile range)
Two studies were conducted on good-prognosis patients [10, 11], four studies on poor-prognosis patients [13, 48–50], and the other four studies comprised both good- and poor-prognosis patients. We also contacted the authors of two papers that reported clinical outcomes but did not provide detailed data concerning the number of IFs for subgroup analysis; no authors responded [9, 47]. Four of the included studies only used autologous embryos for FET [9, 10, 47, 50], one RCT included autologous embryos in the per-protocol analysis set [12], and two studies individually reported autologous and donor cycles [11, 49]; however, the oocyte source was unclear in the remaining studies. In two studies, the authors only analyzed outcomes of cycles with euploid embryos [10, 47], one study only performed PGT for autologous embryos [49], in one study, FET cycles without PGT for embryo aneuploidy were analyzed [50], and the remaining studies included unspecified embryos. Most of the included reports (n = 8) were of cycles with transferred blastocysts, while two comprised the transfer of both cleavage-stage and blastocyst embryos [11, 12]. Patients in six studies underwent programmed FET with or without prior downregulation with gonadotropin-releasing hormone (GnRH) agonists [10–12, 47, 49, 50], patients in two studies used either a natural or programmed protocol for endometrial preparation [9, 46], and no relevant information reported in the remaining studies [13, 48]. Furthermore, two studies analyzed FET cycles in which a single embryo was transferred [10, 47], whereas the remaining publications consisted of cycles in which one or more embryos were transferred, or in which the number of transferred embryos was unclear.
Outcomes
CPR
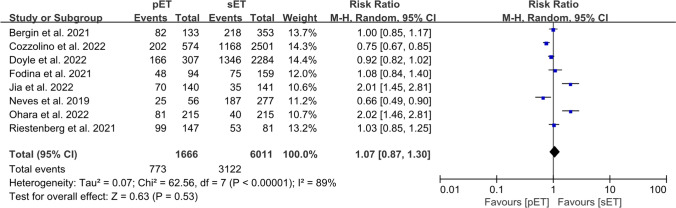
A total of eight studies reported CPR including 1666 cycles in the pET group and 6011 cycles in the sET group [9–11, 13, 47–50]. There was no significant difference in CPR between the pET and sET groups, with values of 46.4% and 51.9% (RR = 1.07; 95% CI, 0.87–1.30; P = 0.53; I2 = 89%; Fig. 2). The quality of evidence was “VERY LOW” according to GRADE (Supplementary Table S2).
Fig. 2.
Forest plots comparing clinical pregnancy rate after personalized versus standard frozen-thawed embryo transfer
When we conducted a subgroup analysis of the prognosis of patients, we noted no differences in the CPR between the pET and sET groups in patients with a poor prognosis (4 studies: n = 1297 cycles; RR = 1.30; 95% CI, 0.78–2.18; P = 0.32; I2 = 91%; with very low quality of evidence), or those exhibiting a good prognosis (2 studies: n = 3303 cycles; RR = 0.87; 95% CI, 0.64–1.20; P = 0.40; I2 = 87%; with very low quality of evidence) (Supplementary Fig. S1a; Table S2).
A subgroup analysis of the ploidy status of the embryos indicated that the CPR of the pET group was similar to that of the sET group, regardless of whether or not the embryos were screened for euploidy (PGT, 5 studies: n = 4218 cycles; RR = 0.92; 95% CI, 0.83–1.03; P = 0.15; I2 = 33%; with very low quality of evidence; non-PGT, 4 studies: n = 2692 cycles; RR = 0.91; 95% CI, 0.51–1.63; P = 0.75; I2 = 91%; with very low quality of evidence; Supplementary Fig. S1b; Table S2).
The number of embryos transferred did not affect the CPR between the two groups (single embryo, 3 studies: n = 2940 cycles; RR = 0.96; 95% CI, 0.87–1.05; P = 0.36; I2 = 43%; with very low quality of evidence; one or more embryos or unclear, 6 studies: n = 4737 cycles; RR = 0.93; 95% CI, 0.85–1.00; P = 0.06; I2 = 92% with very low quality of evidence; Supplementary Fig. S1c; Table S2).
With respect to autologous FET cycles, we observed no differences in the CPRs (6 studies: n = 5995 cycles; RR = 1.01; 95% CI, 0.84–1.21; P = 0.11; I2 = 82%; with very low quality of evidence). However, the sET protocol was associated with an increased CPR compared with the pET protocol in donor FET cycles (2 studies: n = 1148 cycles; RR = 0.68; 95% CI, 0.49–0.94; P = 0.02; I2 = 46%; with low quality of evidence; Supplementary Fig. S1d; Table S2).
We also performed a subgroup analysis concerning the methods of endometrial preparation. No differences were noted between the pET and sET groups when programmed protocols (5 studies: n = 6657 cycles; RR = 0.97; 95% CI, 0.76–1.23; P = 0.79; I2 = 89%; with very low quality of evidence) and unclear or both programmed and natural protocol (3 studies: n = 1020 cycles; RR = 1.27; 95% CI, 0.86–1.86; P = 0.22; I2 = 87%; with low quality of evidence; Supplementary Fig. S1e; Table S2) were administered to prepare endometrium.
LBR
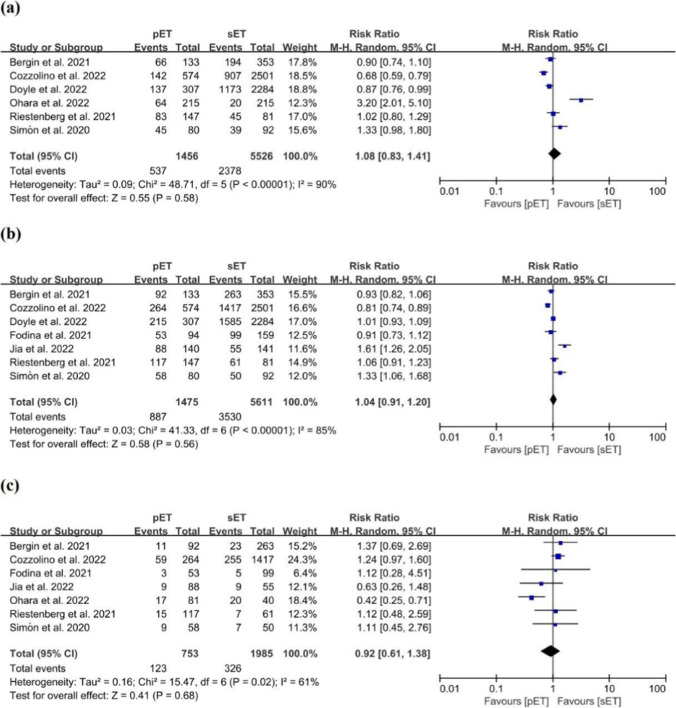
A meta-analysis of six studies that encompassed 6982 cycles in which LBR was reported showed no significant difference between the pET and sET groups, with values of 36.9% and 43.0% (RR = 1.08; 95% CI, 0.83–1.41; P = 0.58; I2 = 90%; with very low quality of evidence; Fig. 3a; Supplementary Table S3) [9–12, 47, 50].
Fig. 3.
Forest plots comparing outcomes after personalized versus standard frozen-thawed embryo transfer a live birth rate, b HCG positivity rate, c miscarriage rate
When we analyzed LBR in each subgroup according to patient prognosis, the ploidy status of embryos, the number of embryos transferred, oocyte source, and endometrial preparation protocols, we noted no significant difference in LBR between the two groups (Supplementary Fig. S2).
HCG positivity rate
A total of 7 studies revealed data on outcomes with respect to HCG positivity in 7086 cycles [9–13, 47, 48], and our analysis showed that the proportion of women showing HCG positivity in the pET group was similar to that for the sET group, with values of 60.1% vs. 62.9% (RR = 1.04; 95% CI, 0.91–1.20; P = 0.56; I2 = 85%; with very low quality of evidence; Fig. 3b; Supplementary Table S3).
Subgroup analysis in patients without PGT demonstrated a significant difference between the pET and sET groups (2 studies: n = 2072 cycles; RR = 0.83; 95% CI, 0.74–0.93; P = 0.001; I2 = 0%; Supplementary Fig. S3b). We discerned no significant difference between the two groups in the subgroup analyses concerning patient prognosis, the number of embryos transferred, oocyte source, or methods of endometrial preparation (Supplementary Figs. S3a, S3c–S3e).
MR
A meta-analysis of 7 studies comprising 2738 cycles in which MR was assessed indicated no significant difference between the pET and sET groups, with values of 16.3% and 16.4% (RR = 0.92; 95% CI, 0.61–1.38; P = 0.68; I2 = 61%; with low quality of evidence; Fig. 3c; Supplementary Table S3) [9–13, 48, 50].
A diminution in the MR favoring the pET group was observed for poor-prognosis patients (3 studies: n = 416 cycles; RR = 0.53; 95% CI, 0.35–0.82; P = 0.004; I2 = 1%; Supplementary Fig. S4a). The risk regarding the MR was similar between the two groups in the subgroup analyses according to embryonic ploidy status, as were the number of embryos transferred, the oocyte source, and endometrial preparation protocols (Supplementary Figs. S4b–S4e).
BPR
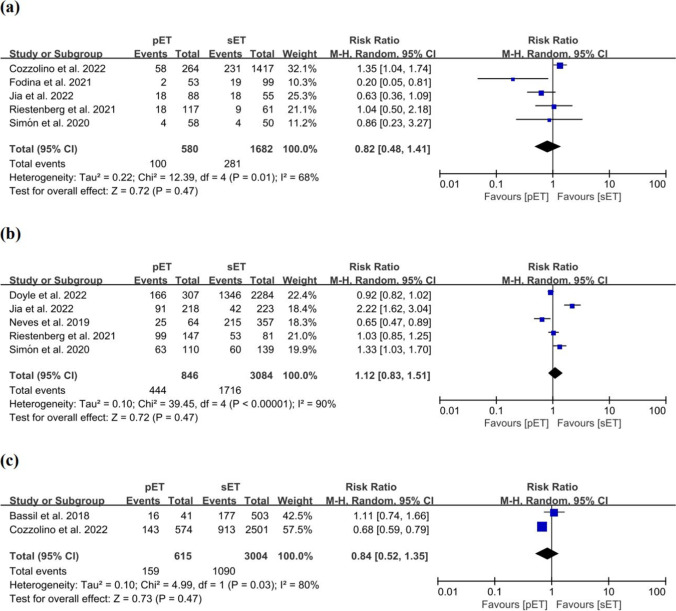
Five studies that comprised 2262 cycles were pooled in this meta-analysis, and we observed no difference in the BPR between the pET and sET groups, with values of 17.2% and 16.7%, respectively (RR = 0.82; 95% CI, 0.48–1.41; P = 0.47; I2 = 68%; with a very low quality of evidence; Fig. 4a; Supplementary Table S3) [10–13, 48].
Fig. 4.
Forest plots comparing outcomes after personalized versus standard frozen-thawed embryo transfer a biochemical pregnancy rate, b implantation rate, c ongoing pregnancy rate
Subgroup analyses indicated that the pET protocol increased the BPR for patients with a good prognosis (2 studies: n = 1859 cycles; RR = 1.31; 95% CI, 1.03–1.67; P = 0.03; I2 = 0%; Supplementary Fig. S5a) or without PGT (2 studies: n = 1104 cycles; RR = 1.39; 95% CI, 1.04–1.85; P = 0.03; I2 = 0%; Supplementary Fig. S5b). An increase in BPR for patients who underwent programmed cycles was also observed (3 studies: n = 1967 cycles; RR = 1.29; 95% CI, 1.02–1.64; P = 0.03; I2 = 0%; Supplementary Fig. S5e). No significant difference was uncovered between the two groups in the subgroup analyses concerning the number of embryos transferred or oocyte source (Supplementary Figs. S5c, S5d).
IR
In 5 studies consisting of 3930 embryos, the authors evaluated the IRs [10, 12, 13, 47, 49] and noted no differences between the pET and sET groups, with values of 52.5% and 55.6% (RR = 1.12; 95% CI, 0.83–1.51; P = 0.47; I2 = 90%; with very low quality of evidence; Fig. 4b; Supplementary Table S3).
Subgroup analyses concerning patient prognosis, the ploidy status of embryos, the number of embryos transferred, oocyte source, and programmed FET cycles showed no significant difference between the two groups (Supplementary Fig. S6).
OPR
In 2 studies encompassing 3619 cycles that provided data on the OPR [11, 46], the investigators did not discern any difference between the pET and sET groups (RR = 0.84; 95% CI, 0.52–1.35; P = 0.73; I2 = 80%; with very low quality of evidence; Fig. 4c).
Risk of bias and quality of the evidence
Of the nine non-RCTs, one study showed a critical risk of bias related to confounding factors, unbalanced co-intervention, and incomplete data [46]; four studies showed a serious risk of bias related to confounding factors [10, 13, 47, 48]; and the remaining four studies showed a moderate risk (Supplementary Table S4). One RCT also exhibited a moderate risk of bias [12] (Supplementary Table S5).
Based on the GRADE approach, we evaluated the quality of the evidence for each outcome. Since there was only one RCT, we upgraded or downgraded the study level according to the baseline score for the observational study criteria. The results of the outcome of CPR and its sub-analysis were all rated a score of “VERY LOW” according to the GRADE criteria, except for the subgroup of donor cycles that received a score of “LOW” (Supplementary Table S2). The results of the secondary outcomes were “VERY LOW” according to GRADE, except for the MR that achieved a score of “LOW” (Supplementary Table S3). The factors that led to study degradation were the risk of bias and inconsistency.
Sensitivity analysis
The sensitivity analyses for the primary and secondary outcomes showed that the pooled results culminating from our meta-analysis were robust (Supplementary Fig. S7).
Discussion
In this meta-analysis, we evaluated the clinical effects of an ERA in FET cycles and ascertained that the ERA did not significantly improve the clinical outcomes in IVF/ICSI cycles, including the CPR, LBR, the rate of HCG positivity, MR, BPR, IR, and OPR. However, in the analyses of subgroups, we found that the pET protocol significantly reduced the MR for poor-prognosis patients, and also that it led to a decrease in the CPR for patients using donated oocytes, an increase in the BPR for good-prognosis, non-PGT, and the programmed protocol-implemented patients, and a decrease in the rate of HCG positivity in non-PGT patients.
Using an ERA has recently become one of the alternative methods for solving the intractable problem of RIF in many reproductive centers, and while its application has been continuously promoted worldwide in the last 10 years, it also manifests the same disadvantages as PGT—including high cost, time, and equivocal effectiveness. To more fully evaluate the applicative value of ERA and to provide better treatment guidance for patients, it is important to conduct high-quality research on this technology. While the latest published meta-analysis showed that approximately one-third of infertile women experienced a displacement in their WOI and a pooled analysis of three studies suggested that the OPR/LBR of patients with a good prognosis did not benefit from ERA, the study also only analyzed a few studies, undertook no subgroup analysis, and only focused on comparing the OPR/LBR outcome between the ERA and non-ERA groups [51]. The primary purpose of this study, then, was to investigate whether ERA-guided ET could provide improved clinical outcomes after FET compared with standard ET protocols. This meta-analysis is the first-ever to comprise an amalgamation of the evidence of published studies that involved a relationship between all clinical outcomes in patients using an ERA and those without an ERA.
Multiple studies have revealed that the WOI displacement rate among patients with RIF was numerically higher than that among patients without RIF [52–54]. In 2013, Ruiz-Alonso et al. not only reported that patients with RIF had a higher WOI displacement rate but also introduced the concept of pET for the first time [52]. In 2014, the same team published a conference abstract that indicated that the IR (13% [12/90] vs. 45% [161/355]), pregnancy rate (23% [12/52] vs. 60% [123/205]), and OPR (0% [0/12] vs. 74% [91/123]) for their NR group with a standard transfer time were significantly lower than those for their R group, respectively [55]. These results suggested that pET was necessary for patients with an NR endometrium, and the authors’ meta-analyses revealed that the CPR and OPR/LBR of the pET group with an NR endometrium were similar to that of standard ET with an R endometrium [51]. We therefore conducted subgroup analyses concerning patient prognosis to reduce the influence of interference on clinical outcomes.
However, recent studies showed that using ERA to guide ET did not appear to significantly improve clinical outcomes in RIF patients. A study by Bergin et al. revealed that the LBR of the ERA group was not significantly higher than that of the control group with respect to both unselected patients (49.62% [66/133] vs. 54.96 [194/353], respectively; P = 0.292) and patients with ≥ 3 prior ETs (42.22% [19/45] vs. 44.17% [53/120], respectively) [9]. In contrast, some research findings supported the use of an ERA in RIF studies [13]. With the present meta-analysis, we demonstrated that an ERA did not improve clinical outcomes significantly for poor-prognosis patients, other than a statistical reduction in the MR.
Furthermore, the ERA was not conducive to a positive clinical outcome in patients with a favorable prognosis, and it may even have exerted a negative impact on clinical outcomes [10, 11, 46]. In our meta-analysis, we determined that there was no significant improvement in the CPR, LBR, positive HCG incidence, MR, or IR in patients with a good prognosis who underwent a pET protocol; and that pET may even increase the BPR for these patients.
Several studies have shown that the number of embryos transferred, use of donor vs. autologous embryos, methods of endometrial preparation, and whether PGT was used to screen embryos were related to clinical outcomes [56–59]. In this meta-analysis, the aforementioned four confounding factors that affected clinical outcomes were present, so relevant subgroup analyses based on the four factors were implemented. Our results suggested that pET may lead to a drop in the CPR for donor cycles, and can also lead to a reduction in the rate of HCG positivity and an elevation in the BPR rate in the non-PGT cycles. We noted that both blastocysts and cleavage-stage embryos were transferred in some studies, but no relevant subgroup analysis was performed due to the small number of studies involved [11, 12]. Embryonic implantation is a rigorous process, and in theory, once issues with embryonic quality are resolved by PGT, the issue of WOI displacement will be exposed to a greater extent and the advantages of pET will become evident. However, our results indicated that the CPR of euploid ET with the pET protocol was similar to that of the control group, and this phenomenon suggested that the WOI may be wider than that indicated by the ERA results which is consistent with previous studies that have shown the flexibility of the WOI lasting 1–3 days [60, 61]. Whether the results depicted above were caused by the interference of confounding factors or related to other unknown factors still requires further exploration.
The strength of this analysis was the inclusion of all studies involving ERAs in which the investigators reported clinical outcomes from more than 5000 patients, comprehensively analyzed the impact of the pET protocol on each outcome, and conducted multiple subgroup analyses that adjusted for potential confounding factors. We also note several limitations to this meta-analysis. First, the score for most clinical outcomes was “VERY LOW” according to the GRADE approach, and a few were rated as “LOW.” The principal reason for the low scores was the observational nature of the majority of the included studies, reducing the value of the present study. In addition, the substantial inter-study heterogeneity potentially caused by patient prognosis and embryo characteristics led to a deterioration of research quality. Second, while multiple subgroup analyses were adopted, subgroup analysis at each timepoint only eliminated the influence of one confounding factor on the clinical outcomes, and thus the results were still hampered by other confounding factors. Third, we were unable to exploit subgroup analyses for some indices such as cleavage-stage embryos and blastocysts due to the limited number of studies. Finally, since the cost of ERA was high, patients who chose to perform ERA may be older and had fewer retrieved oocytes or transplantable embryos. The use of unmatched data from most non-RCTs included in this meta-analysis would expose the analyses to some potentially important confounding factors and lead to results that favor the control group. This necessitates the careful consideration of our current results.
Conclusion
In summary, this study revealed comparable clinical outcomes after FET between the pET and sET groups. Evidence from our pooled results suggested that the use of ERA did not significantly increase the success rate of ET for unselected patients. Also in the subgroup analysis, our results suggested that ERA use reduced the MR of poor-prognosis patients, adversely affected the CPR of donor cycles and the rate of HCG positivity of non-PGT cycles, and led to an increase in the BPR in non-PGT patients, good-prognosis patients, and patients who underwent programmed FET protocol. Although these results showed that using an ERA might be an overtreatment and a potential waste of medical resources for patients, the aforementioned results should nonetheless be considered with caution considering the “VERY LOW” or “LOW” score observed for each outcome. In the future, more high-quality RCTs are therefore needed to investigate the clinical validity and feasibility of ERAs.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33(11):1419–1430. doi: 10.1007/s10815-016-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahdouh EM. Preimplantation genetic testing for aneuploidy: a review of the evidence. Obstet Gynecol. 2021;137(3):528–534. doi: 10.1097/aog.0000000000004295. [DOI] [PubMed] [Google Scholar]
- 3.Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611–617. doi: 10.1016/j.fertnstert.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Franasiak JM, Ruiz-Alonso M, Scott RT, Simón C. Both slowly developing embryos and a variable pace of luteal endometrial progression may conspire to prevent normal birth in spite of a capable embryo. Fertil Steril. 2016;105(4):861–866. doi: 10.1016/j.fertnstert.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(2):202–223. doi: 10.1093/humupd/dmy044. [DOI] [PubMed] [Google Scholar]
- 6.Bosteels J, van Wessel S, Weyers S, Broekmans FJ, D’Hooghe TM, Bongers MY, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev. 2018;12(12):Cd009461. doi: 10.1002/14651858.CD009461.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95(1):50–60, 60.e51–15. 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed]
- 8.Cozzolino M, Diaz-Gimeno P, Pellicer A, Garrido N. Evaluation of the endometrial receptivity assay and the preimplantation genetic test for aneuploidy in overcoming recurrent implantation failure. J Assist Reprod Genet. 2020;37(12):2989–2997. doi: 10.1007/s10815-020-01948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergin K, Eliner Y, Duvall DW, Jr, Roger S, Elguero S, Penzias AS, et al. The use of propensity score matching to assess the benefit of the endometrial receptivity analysis in frozen embryo transfers. Fertil Steril. 2021;116(2):396–403. doi: 10.1016/j.fertnstert.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Riestenberg C, Kroener L, Quinn M, Ching K, Ambartsumyan G. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2021;115(4):1001–1006. doi: 10.1016/j.fertnstert.2020.09.140. [DOI] [PubMed] [Google Scholar]
- 11.Cozzolino M, Diáz-Gimeno P, Pellicer A, Garrido N. Use of the endometrial receptivity array to guide personalized embryo transfer after a failed transfer attempt was associated with a lower cumulative and per transfer live birth rate during donor and autologous cycles. Fertil Steril. 2022;118(4):724–736. doi: 10.1016/j.fertnstert.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Simón C, Gómez C, Cabanillas S, Vladimirov I, Castillón G, Giles J, et al. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod Biomed Online. 2020;41(3):402–415. doi: 10.1016/j.rbmo.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Jia Y, Sha Y, Qiu Z, Guo Y, Tan A, Huang Y, et al. Comparison of the effectiveness of endometrial receptivity analysis (ERA) to guide personalized embryo transfer with conventional frozen embryo transfer in 281 Chinese women with recurrent implantation failure. Med Sci Monit. 2022;28:e935634. doi: 10.12659/msm.935634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Rose-Reneau Z, Riggs RN, Anderson DK. Endometrial receptivity testing and adjustment to window of implantation timing improve pregnancy rates with assisted reproductive technology (ART) Obstet Gynecol. 2022;139(SUPPL 1):96S. doi: 10.1097/01.AOG.0000825452.97976.94. [DOI] [Google Scholar]
- 20.Simon C, Gomez C, Cabanillas S, Vladimirov IK, Castillon G, Giles J, et al. In vitro fertilization with personalized blastocyst transfer versus frozen or fresh blastocyst transfer: a multicenter, randomized clinical trial. Fertil Steril. 2019;112(3):e56–e57. doi: 10.1016/j.fertnstert.2019.07.273. [DOI] [Google Scholar]
- 21.Combs JC, O’Brien JE, Devine K, Healy MW, Jahandideh S, DeCherney AH, et al. Endometrial receptivity analysis (ERA) for patients with PGT-A normal frozen embryo transfers (FET): a retrospective analysis. Fertil Steril. 2020;114(3):e424. doi: 10.1016/j.fertnstert.2020.08.1233. [DOI] [Google Scholar]
- 22.Selvaraj P, Selvaraj K, Sivakumar M, Pathy R, Balakrishnan S. Endometrial receptivity analysis in recurrent implantation failure: a prospective study comparing benefits in own versus donor cycles. Hum Reprod. 2019;34(SUPPL 1):i335. [Google Scholar]
- 23.Doyle N, Jahandideh S, Hill MJ, Widra EA, Levy M, Devine K. A randomized controlled trial comparing live birth from single euploid frozen blastocyst transfer using standardized timing versus timing by endometrial receptivity analysis. Fertil Steril. 2021;116(3):e101. doi: 10.1016/j.fertnstert.2021.07.283. [DOI] [Google Scholar]
- 24.Bamford T, Polson D, Lowe P, Easter C, Coomarasamy A. Endometrial receptivity analysis (ERA) and microbiome testing for recurrent implantation failure (RIF): a matched case control study. Hum Reprod. 2022;37:i94–i95. doi: 10.1093/humrep/deac106.P-350. [DOI] [Google Scholar]
- 25.Clemente-Císcar M, Ruiz-Alonso M, Blesa D, Jimenez-Almazan J, Bahceci M, Banker M, et al. Endometrial receptivity analysis (ERA) using a next generation sequencing (NGS) predictor improves reproductive outcome in recurrent implantation failure (RIF) patients when compared to ERA arrays. Hum Reprod. 2018;33:i8. doi: 10.1093/humrep/33.Supplement_1.1. [DOI] [Google Scholar]
- 26.Li Y. The role of the endometrial receptivity analysis (ERA) in patients with non-recurrent implantation failure in the Chinese population. Fertil Steril. 2021;116(3):e307. doi: 10.1016/j.fertnstert.2021.07.827. [DOI] [Google Scholar]
- 27.Bergin K, Eliner Y, Duvall DW, Jr, Elguero S, Penzias AS, Sakkas D, et al. The use of propensity score matching to evaluate the endometrial receptivity analysis (ERA) in euploid frozen embryo transfer cycles. Fertil Steril. 2021;116(3):e102–e103. doi: 10.1016/j.fertnstert.2021.07.286. [DOI] [PubMed] [Google Scholar]
- 28.Bergin K, Eliner Y, Vaughan DA, Sakkas D, Duvall DW, Elguero S, et al. Use of propensity score matching to assess the endometrial receptivity assay (ERA) in optimizing embryo transfer outcomes. Fertil Steril. 2020;114(3):e290–e291. doi: 10.1016/j.fertnstert.2020.08.799. [DOI] [PubMed] [Google Scholar]
- 29.Royster GD, Anderson R. A retrospective cohort study on the usefulness of endometrial receptivity analysis (ERA) prior to a gestational carriers’ (GC) first euploid frozen embryo transfer (FET) Hum Reprod. 2022;37:i391. doi: 10.1093/humrep/deac106.P-422. [DOI] [Google Scholar]
- 30.Teles G, Lacordia R, Bonetti T, Lorenzon-Ojea AR, Motta E. Is endometrial receptivity array (ERA) screening relevant to increase pregnancy rates in patients with failed IVF cycles? Hum Reprod. 2018;33:342–342. [Google Scholar]
- 31.Hombalegowda RB, Ziegler W. Evaluating the role of endometrial receptivity array (era) in patients with first frozen embryo transfers (FET) Fertil Steril. 2020;113(4 SUPPL):e39–e40. doi: 10.1016/j.fertnstert.2020.02.088. [DOI] [Google Scholar]
- 32.Riestenberg C, Kroener L, Ching K, Ambartsumyan G. Routine endometrial receptivity array in first embryo transfer cycles does not improve live birth rate. Fertil Steril. 2020;114(3 SUPPL):e281. doi: 10.1016/j.fertnstert.2020.08.777. [DOI] [PubMed] [Google Scholar]
- 33.Arikan G, Turan V, Yanik M, Kadi AK, Kafkasli A. Personalized embryo transfer pET after endometrial receptivity array (ERA) in patients with repeated implantation failure — personal experience. J Turkish German Gynecol Assoc. 2016;17:S181. [Google Scholar]
- 34.Martinez F, Raquel Neves A, Devesa M, Garcia-Martinez S, Rodriguez I, Coroleu B. What is the clinical impact of the endometrial receptivity array in PGT-A and oocyte donation cycles? Fertil Steril. 2019;112(3):e164–e164. doi: 10.1016/j.fertnstert.2019.07.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luna M, Alkon T, Hernandez-Nieto C, Cassis-Bendeck D, Sandler B. Evaluating the clinical utility of endometrial receptivity analysis test in women with recurrent pregnancy loss. Hum Reprod. 2020;35:I318–I318. [Google Scholar]
- 36.Simon C, Vladimirov IK, Castillon Cortes G, Ortega I, Cabanillas S, Vidal C, et al. Prospective, randomized study of the endometrial receptivity analysis (ERA) test in the infertility work-up to guide personalized embryo transfer versus fresh transfer or deferred embryo transfer. Fertil Steril. 2016;106(3):e46–e47. doi: 10.1016/j.fertnstert.2016.07.144. [DOI] [Google Scholar]
- 37.Jia Y, Sha YL, Qiu Z, Guo YH, Tan AX, Huang Y, et al. Endometrial receptivity analysis for personalized embryo transfer in patients with recurrent implantation failure: a retrospective analysis of a Chinese cohort. Hum Reprod. 2021;36:278–278. doi: 10.1093/humrep/deab130.312. [DOI] [Google Scholar]
- 38.Arikan G, Findikli N, Yagmur E, Karlikaya G, Gultomruk M, Bahceci M. Personolized embryo transfer (pET) after Endometrial receptivity array (ERA) in patients with recurrent implantation failure—an observational study. Hum Reprod. 2015;30:268–268. [Google Scholar]
- 39.Nct. Frozen blastocyst transfer using conventional timing versus timing by endometrial receptivity analysis. https://clinicaltrials.gov/ct2/show/NCT03558399.2018.
- 40.Nct. The clinical efficiency of ERA in Chinese RIF patients. https://clinicaltrials.gov/ct2/show/NCT04497558.2020.
- 41.ChiCtr. Effect of individualized embryo transfer based on endometrial receptivity test on clinical outcome of embryo implantation in patients with preimplantation genetic testing: a multicenter, randomized, controlled, open-label clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100049841.2021.
- 42.ChiCtr. Efficacy of the endometrial receptivity testing for recurrent implantation failure in patients with euploid embryo transfers. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100049041.2021. [DOI] [PMC free article] [PubMed]
- 43.Cozzolino M, Diaz-Gimeno P, Pellicer A, Garrido N. Evaluation of the endometrial receptivity assay and the preimplantation genetic test for aneuploidy in overcoming recurrent implantation failure. J Assist Reprod Genet. 2020;37(12):2989–2997. doi: 10.1007/s10815-020-01948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia Y, Dong YJ, Sha YL, Cai SC, Diao LH, Qiu Z, et al. Effectiveness comparison between endometrial receptivity array, immune profiling and the combination in treating patients with multiple implantation failure. Am J Reprod Immunol. 2022;87(3):e13513. doi: 10.1111/aji.13513. [DOI] [PubMed] [Google Scholar]
- 45.Amin J, Sr, Patel R, JayeshAmin G, Gomedhikam J, Surakala S, Kota M. Personalized embryo transfer outcomes in recurrent implantation failure patients following endometrial receptivity array with pre-implantation genetic testing. Cureus. 2022;14(6):e26248. doi: 10.7759/cureus.26248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bassil R, Casper R, Samara N, Hsieh TB, Barzilay E, Orvieto R, et al. Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet. 2018;35(7):1301–1305. doi: 10.1007/s10815-018-1190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle N, Combs JC, Jahandideh S, Wilkinson V, Devine K, O’Brien JE. Live birth after transfer of a single euploid vitrified-warmed blastocyst according to standard timing vs. timing as recommended by endometrial receptivity analysis. Fertil Steril. 2022;118(2):314–321. doi: 10.1016/j.fertnstert.2022.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fodina V, Dudorova A, Erenpreiss J. Evaluation of embryo aneuploidy (PGT-A) and endometrial receptivity (ERA) testing in patients with recurrent implantation failure in ICSI cycles. Gynecol Endocrinol. 2021;37(S1):17–20. doi: 10.1080/09513590.2021.2006466. [DOI] [PubMed] [Google Scholar]
- 49.Neves AR, Devesa M, Martinez F, Garcia-Martinez S, Rodriguez I, Polyzos NP, et al. What is the clinical impact of the endometrial receptivity array in PGT-A and oocyte donation cycles? J Assist Reprod Genet. 2019;36(9):1901–1908. doi: 10.1007/s10815-019-01535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohara Y, Matsubayashi H, Suzuki Y, Takaya Y, Yamaguchi K, Doshida M, et al. Clinical relevance of a newly developed endometrial receptivity test for patients with recurrent implantation failure in Japan. Reprod Med Biol. 2022;21(1):e12444. doi: 10.1002/rmb2.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, Liu X, Wang M, Zhao H, He S, Lai S, et al. The clinical efficacy of personalized embryo transfer guided by the endometrial receptivity array/analysis on IVF/ICSI outcomes: a systematic review and meta-analysis. Front Physiol. 2022;13:841437. doi: 10.3389/fphys.2022.841437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–824. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Tan J, Kan A, Hitkari J, Taylor B, Tallon N, Warraich G, et al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet. 2018;35(4):683–692. doi: 10.1007/s10815-017-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saxtorph MH, Hallager T, Persson G, Petersen KB, Eriksen JO, Larsen LG, et al. Assessing endometrial receptivity after recurrent implantation failure: a prospective controlled cohort study. Reprod Biomed Online. 2020;41(6):998–1006. doi: 10.1016/j.rbmo.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz Alonso M, Díaz-Gimeno P, Gómez E, Rincón-Bertolín A, Vladimirov Y, Garrido N, et al. Clinical efficiency of embryo transfer performed in receptive vs non-receptive endometrium diagnosed by the endometrial receptivity array (ERA) test. Fertil Steril. 2014;102(3):e292. doi: 10.1016/j.fertnstert.2014.07.994. [DOI] [Google Scholar]
- 56.Yeh JS, Steward RG, Dude AM, Shah AA, Goldfarb JM, Muasher SJ. Pregnancy rates in donor oocyte cycles compared to similar autologous in vitro fertilization cycles: an analysis of 26,457 fresh cycles from the Society for Assisted Reproductive Technology. Fertil Steril. 2014;102(2):399–404. doi: 10.1016/j.fertnstert.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Ma S, Peng Y, Hu L, Wang X, Xiong Y, Tang Y, et al. Comparisons of benefits and risks of single embryo transfer versus double embryo transfer: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2022;20(1):20. doi: 10.1186/s12958-022-00899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simopoulou M, Sfakianoudis K, Maziotis E, Tsioulou P, Grigoriadis S, Rapani A, et al. PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. J Assist Reprod Genet. 2021;38(8):1939–1957. doi: 10.1007/s10815-021-02227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Godiwala P, Makhijani R, Bartolucci A, Grow D, Nulsen J, Benadiva C, et al. Pregnancy outcomes after frozen-thawed embryo transfer using letrozole ovulation induction, natural, or programmed cycles. Fertil Steril. 2022;118(4):690–698. doi: 10.1016/j.fertnstert.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Erden M, Polat M, Mumusoglu S, Ozbek IY, Dere GO, Sokmensuer LK, et al. Vitrified–warmed blastocyst transfer timing related to LH surge in true natural cycle and its impact on ongoing pregnancy rates. Reprod Biomed Online. 2022;45(3):440–447. doi: 10.1016/j.rbmo.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 61.Bartels CB, Ditrio L, Grow DR, O’Sullivan DM, Benadiva CA, Engmann L, et al. The window is wide: flexible timing for vitrified–warmed embryo transfer in natural cycles. Reprod Biomed Online. 2019;39(2):241–248. doi: 10.1016/j.rbmo.2019.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.