Abstract
Purpose
To examine the effects of self-compassion training using videos (SCV) versus self-compassion training using digital stories (SC-DS) as compared to a control group (CG) on reducing anxiety and depression symptoms among women pursuing fertility treatment.
Methods
A three-armed, randomised controlled trial randomly assigned 200 eligible women to SCV(n = 65), SC-DS (n = 67), and CG (n = 68). All three randomised groups completed questionnaires immediately after randomisation (T1), after completing the interventions (T2), and 10 weeks after the interventions (T3). A generalised estimation equation was used with the intention-to-treat analysis. The primary outcomes were anxiety and depression, and secondary outcomes were self-compassion, infertility self-efficacy, and pregnancy rates.
Results
SCV and SC-DS participants experienced a significant reduction in anxiety and depression from T1 to T2 and from T1 to T3 (p < 0.001; d > 0.8). SCV and SC-DS participants experienced a significant increase in self-compassion and infertility self-efficacy from T1 to T2 and from T1 to T3 (p < 0.001; d > 0.8). SC-DS seemed to be superior to SCV and CG. No significant differences were found among the three groups in pregnancy rates. SCV and SC-DS participants rated self-compassion training programs positively and said they would highly recommend them to others.
Conclusion
These findings suggest that SCV and SC-DS were effective in reducing anxiety and depression and increasing self-compassion and infertility self-efficacy. Online flexible self-compassion interventions could make psychological support more accessible for women undergoing fertility treatment in resource-poor settings.
Trial registration
(ChiCTR2100046065) [12/04/2021].
Keywords: Infertility, Self-compassion, Randomised controlled trial, Digital storytelling, Mobile-based videos
Introduction
Infertility is clinically defined as the failure to establish a clinical pregnancy after 12 or more months of regular unprotected sexual intercourse, affecting 8–12% of couples aged 18–44 years worldwide [1]. Individuals with infertility undergo numerous medical interventions to aid in the conception of a biological child. Unfortunately, these procedures can lead to clinical exhaustion, often characterised by emotional and psychological challenges [2]. The most common psychological comorbidities of infertility and treatment include distress (anxiety and depression). Previous studies reported that these symptoms affect 25–53% of women before starting and 40–75% during reproductive medical treatment [3, 4]. The ambiguity of infertility diagnosis and associated treatment interventions can evoke and exaggerate the feeling of anxiety, worry, uncertainty, and even suicidality among affected individuals, particularly women. The use of medication to treat infertility can also exacerbate the risk of these psychological symptoms. In addition, some studies show that the risk for psychological symptoms increases with treatment duration, which can result in treatment termination, particularly for those experiencing numerous unsuccessful cycles [5, 6]. Furthermore, research has shown couples receiving fertility treatment, particularly women, may have difficulty overcoming emotional distress and report low levels of infertility self-efficacy and self-compassion [7, 8].
A growing body of research has pointed out self-compassion and digital storytelling (DST) as protective factors against negative outcomes of adverse life events such as infertility [9, 10]. For example, a quasi-experimental study that examined the effectiveness of self-compassion training among women pursuing fertility treatment found that participants in the experiment significantly had improved psychological well-being more than the control group [11]. Self-compassion embodies treating oneself with kindness during painful experiences suffering, or failure, being mindful of ones suffering rather than avoiding or overindulging with pain and recognising that experiences of suffering are a large part of the human experience rather than feeling isolated by them [12]. Digital stories (DS) are the first-person perspective of a personal experience told in a 3 to 5-min video using digital technologies such as photos, videos, music, and audio through the digital storytelling (DST) process [13]. Although DS has a deep root in teaching, advocacy, and community engagement, it has become increasingly popular in cancer care settings. It offers health professionals an innovative method to help patients increase their understanding of their experiences and the meaning attached to them through the reflective process [14, 15]. Documented benefits of DS include an improved sense of self, enhanced self-efficacy, well-being, quality of life, compassion, resilience, and empathy [10, 16]. However, we did not find any study on the use of DS among women receiving fertility treatment.
Despite this evidence that psychological interventions such as self-compassion-based training programs may benefit patients undergoing fertility treatment, very few patients take advantage of such support when offered to them [3, 17]. This low utilisation of psychological interventions may be attributed to the stigma attached to infertility, fear of being dismissed from reproductive treatment, cost, not knowing who to contact, travel difficulties, and scheduling difficulties [17]. Internet-based interventions, such as mobile-based, are promising alternative options to address these barriers and have been shown to effectively reduce emotional distress and improve pregnancy rates [18, 19]. Research has also shown that these interventions may appeal to women undergoing fertility treatment [19, 20]. Therefore, this modality of care may enable healthcare professionals to provide convenient, confidential, and effective psychological support and advice during the fertility treatment process.
Theoretical framework: stress and coping
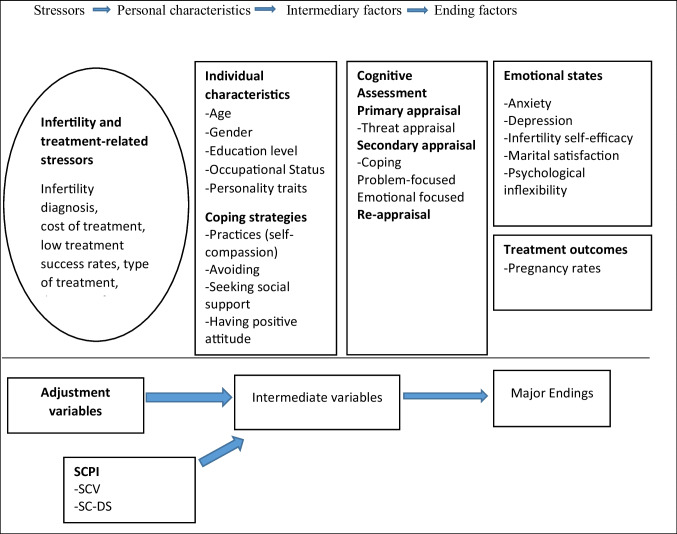
Stress results from an interaction between an individual and situations in their life. Lazarus and Folkman stated three aspects to consider in human response to stressful situations: how the person appraises the event, their coping strategies choices, and the resultant emotions [21] (Fig. 1). The appraisal of infertility and its treatment influences how women feel, react, and respond. Appraisals are a complex process that occurs in three phases—primary, secondary, and reappraisals and involves interactions of multiple domains such as individual characteristics, infertility-related characteristics, and coping strategies [22]. Primary appraisal is about the relevance of the situation to personal goals, values, and beliefs and asks what is at stake for me. Stress appraisal includes irrelevant, benign (positive), and stressful. Evidence indicates that most women are likely to primarily appraise fertility treatment as stressful rather than irrelevant or benign [23]. Given this likely appraisal of stress, secondary appraisal occurs, which asks what can I do? or how can I cope? The secondary appraisal includes harm and loss, threat, or challenge. Undergoing fertility treatment can lead to financial instability, social stigma, and treatment side effects, so a secondary appraisal of harm or loss is probable [2, 23], thus, requiring coping to manage the psychological stress of the situation.
Fig. 1.
Theoretical framework: stress and coping
Coping is of two types: emotion-focused and problem-focused. Emotion-focused coping aims to regulate emotions from stressful situations and may include distancing, humour, seeking support, and positive reappraisal. Problem-focused coping attempts to solve, reconceive, or minimise the effects of the stressor and includes strategies such as information gathering, planning, seeking advice, drawing on previous experience, negotiating, and problem-solving. According to Lazarus and Folkman, problem-focused coping is used more when situations can be controlled and are associated with more positive and few emotions. In contrast, emotion-focused coping is used more in circumstances that must be accepted and are associated with more negative and less positive emotions [21]. Terry and Hynes’s study found that women who use both problem-focused and emotional coping strategies were associated with better adjustment in the low-control context of failed fertility treatment [24]. The third appraisal phase is reappraisal, an ongoing assessment of the stressful event, and resource availability. Research shows that individuals can be instructed to re-appraise a stressful event to experience more adaptive outcomes [25].
The self-compassion-based programs for infertility (SCPI) were conceptualised using stress and coping theory (Fig. 1). The SCPI was intended to develop present-moment awareness and promote psychological flexibility and perception of self-efficacy to deal with fertility treatment constraints. Mindfulness involves being in touch with unfolding experiences in an open, non-judgemental, and curious way, which can be achieved through labelling emotions, reciting self-compassion phrases (this is a moment of suffering), and self-inquiry such as what do I need now? Another critical skill learnt during the SCPI is cognitive restructuring (refers to a process where an individual learns how to identify, evaluate, and modify the thoughts and beliefs that maintain psychological disturbances), recognising that women with fertility problems express feelings of inadequacy, failure, shame, blame themselves for their infertility, and tend to ruminate on their painful experiences or failures [26, 27]. Therefore, it is essential to help them be aware of their stories and reconstruct these narratives in a self-compassionate way. This skill can be achieved through DST [28]. In addition, women learnt about self-kindness, treating themselves with kindness, concern, and warmth, especially when suffering, instead of being self-critical. This skill can be achieved through both formal (sitting) and informal (during daily life) self-compassion practices and self-inquiry (what do I need right now?). Furthermore, other skills learnt during SCPI training included women learning to recognise their personal experiences as a part of large human experiences and adopting the attitude of gratitude, self-acceptance, and forgiveness of oneself and others [29].
Developing and evaluating the effectiveness of different delivery formats that can be used to train self-compassion, such as short videos and DS, is relevant in research. As presented, both DS and self-compassion overlap to some extent, including recognising, validating, understanding, and connecting to human experiences. It is, therefore, plausible that self-compassion training using videos (SCV) and self-compassion training using digital storytelling (SC-DS) will have partly similar effects. However, there are also differences between the two training protocols, which may lead to differential effects. For example, SVC involved training on self-compassion and infertility-related information via short videos, while DS only trained self-compassion while emphasising the DST process. These innovative methods of training self-compassion skills are needed to enhance access to evidence care and increase the opportunity to reach people living in remote locations, given the scarcity of professional therapists, particularly in low-middle-income countries.
The present study compared the effectiveness of SVC and SC-DS with a control group (CG) on anxiety and depression among women pursuing fertility treatment (Primary outcomes). The secondary outcomes were infertility self-efficacy, marital satisfaction, evaluation of interventions, and treatment outcome (pregnancy rates). We hypothesised that women in the SCV and SC-DS groups would report improved primary and secondary outcomes than those in the control group.
Methods
Study design and participants
This was a multi-centre, three-armed, randomised controlled trial conducted at three Kenyan government obstetrics and gynaecology outpatient clinics: Kenyatta National Hospital, Thika Level 5 Hospital, and Kiambu Level 5 hospital from November 2021 through March 2022. The study included volunteer women with the following characteristics: (1) aged 22–44 years, (2) diagnosed with infertility, (3) undergoing fertility treatment, (4) ability to follow the instructions, (5) ability to speak and read Kiswahili, and (6) daily access to the internet. We excluded women who (1) got pregnant during the study and (2) currently or previously participated in meditation, yoga, or psychosocial training.
The trial was conducted following the Declaration of Helsinki and the Consolidated Standard of Reporting Trials (CONSORT) guidelines. It was approved by the Institutional Review Board of Xiangya Nursing School of Central South University, the Kenyatta National Hospital-University of Nairobi Ethics Research Committee, and the National Commission for Science, Technology, and Innovation (NACOSTI). The women provided written informed consent after explaining the study’s purpose, the participant’s roles, the researcher’s role, potential benefits, and risks associated with the trial. Women were permitted to withdraw from the study at any time without their care being affected, and assured anonymity and confidentiality would be maintained. Furthermore, women in the SC-DS group were informed that they were the full custodians of their stories, and the stories would be used only for this study.
Randomisation and masking
An independent statistician randomly assigned participants (at an a1:1:1 ratio) to SCV, SC-DS, or CG using simple randomisation stratified according to research sites. The randomisation sequence was generated using a computer. Due to the nature of the intervention, participants, nurses, and researchers could not be fully masked. However, statisticians and data assessors were blinded to intervention groups. Participants remained blinded to the study hypothesis and other intervention content throughout the study.
Procedure
Three hundred twenty women were approached by trained nurses working in the three hospitals’ obstetrics and gynaecology outpatient clinics. Of the recruited participants, 120 were excluded because they did not meet the inclusion criteria. The reminded 200 women signed a written informed consent form and were randomly assigned to SCV (n = 65), SC-DS (n = 67), or CG (n = 68). Subsequently, all women were assigned to three WhatsApp groups based on their group allocation. Participants in the CG were told they were on the waiting list for SCV to start after the third assessment, and they did not receive the training that the intervention groups received.
The three groups completed self-reported questionnaires at three points: Time 1 assessment (T1: pre-intervention), which occurred immediately after randomisation. It comprised completing online demographic and clinical information and several self-reported questionnaires. Time 2 assessment (T2: post-intervention) occurred during the first week of completing the interventions. It comprised completing several online self-reported questionnaires, a medical review chart of pregnancy outcomes, and an evaluation of interventions. Time 3 assessment (T3: follow-up) occurred 10 weeks after the interventions ended. It comprised completing online several self-reported questionnaires and a medical review chart of clinical pregnancy outcomes (see Fig. 2 for complete details).
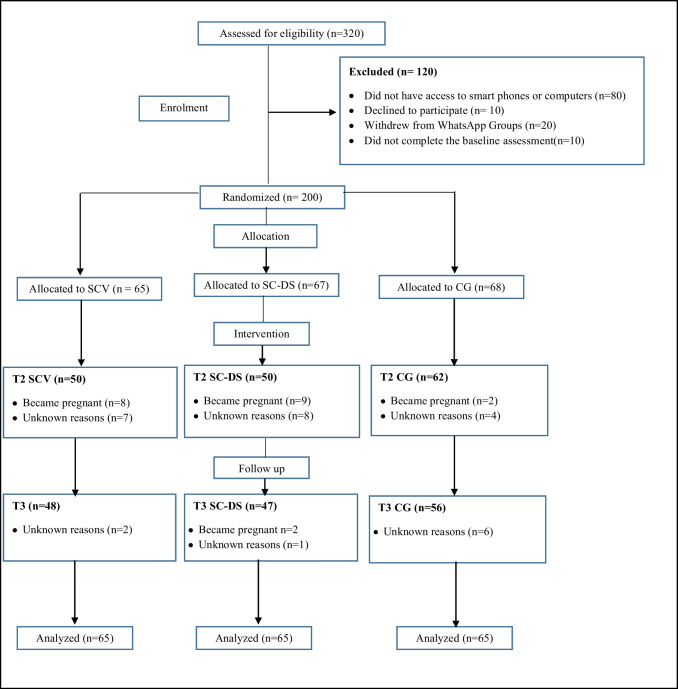
Fig. 2.
Participants’ flow chart. Note: SCV self-compassion training using videos, SC-DS self-compassion training using digital stories, CG control group
All surveys were distributed through the Google Form platform, and participants were informed in advance by e-mail or messaging on WhatsApp groups when the survey was due. Participants received as many as three automatic survey reminders at 3-day intervals. All women in the study received standard care, including routine consultation, treatments for infertility, general information about infertility, and counselling if deemed necessary as stipulated by the Kenya Ministry of Health clinical practice guidelines.
Interventions
The SCV and SC-DS programs
The development of the SCPI protocol was guided by the Medical Research Council (MRC) development framework for complex interventions [30]. The MRC framework recommends using theory, integrating research evidence and mixed-method data collection with experts and users, and testing the acceptability and feasibility of the interventions. Full details of the five phases of SCPI development are available from the corresponding author.
Two trained nurses in self-compassion and DS protocols led the training programs. The SVC involved sharing two daily videos (guided meditation and animated psychoeducation), each lasting 5 min for 8 weeks. Notably, the content of the videos aligned with each week’s session goals. Participants were requested to watch the videos daily at their convenience. The goals and content of each of the eight sessions are summarised in Table 1.
Table 1.
Content of SVC and SC-DS training programs
| Sessions | Goals | SVD content | DC-DS content | SVC and SC-DS assignment |
|---|---|---|---|---|
| 1 | -Overview of the programs and introduction of instructors and participants to each other |
-Discussion on self-compassion and it is essential to women pursuing fertility treatment -Discussion on backdraft and how to reduce its impacts |
-Discussion on self-compassion and it is importance to women pursuing fertility treatment -Discussion on backdraft and how to reduce its impacts |
-Observe and notice their self-conversations with themselves (type of language, tone, and content) |
| 11 | -Acquire self-compassion skills |
-Overview of previous sessions and assignment -Introduction of self-compassion components- self-kindness, mindfulness, and common humanity -Introduced self-compassion practices such as supportive touch -Ask questions and share learning and self-compassion practices experiences in the WhatsApp group |
-Overview of previous sessions and assignment -Introduction of self-compassion components- self-kindness, mindfulness, and common humanity -Introduced self-compassion practices such as supportive touch -Questions and answers -Group discussion |
-Practice loving-kindness meditation |
| 111 | -Learn to integrate Yin and Yang energy |
-Overview of previous sessions and assignment -Discussion on how to harness fierce self-compassion (Yin and Yang) to deal with infertility-related problems -Ask questions and share learning and self-compassion practices experiences in the WhatsApp group |
-Overview of previous sessions and assignment -Discussion on how to harness fierce self-compassion (Yin and Yang) to deal with infertility-related problems -Questions and answers -Group discussion |
-Practice a breathing Yin and Yang meditation |
| IV | -Learn to cope with difficult emotions |
-Overview of previous sessions and assignment -Discussion on how to deal with difficult emotions (anger, shame, and frustrations) through labelling emotions and soften-allow-soothe practices -Ask questions and share learning and self-compassion practices experiences in the WhatsApp group |
-Overview of previous sessions and assignment -Discussion on how to deal with difficult emotions (anger, shame, and frustrations) through labelling emotions and soften-allow-soothe practices -Questions and answers -Group discussion |
-Keep a self-compassion journal |
| V | -Learn to relate to the positive aspect of oneself |
-Self-acceptance, gratitude, and forgiveness -Ask questions and share their learning and self-compassion practices and experiences in the WhatsApp group |
-Self-acceptance, gratitude, and forgiveness -Questions and answer -Group discussion |
Keep a 1-week gratitude journal |
| VI |
-Learn about infertility and its causes (SVC) -Learn to create a digital story (DS-CS) |
-Overview of previous sessions and assignment -Discussion on infertility and its causes -Ask questions and share learning and self-compassion practices experiences in the WhatsApp group |
-Summary of prior sessions and assignment -Discussion on how to create a digital story -Questions and answers -Group discussion |
-Practice loving and kind phrases directed to themselves (may I be kind to me) - Write a self-compassion letter to themselves about their current fertility treatment challenge that they were experiencing from a kind, caring, and compassionate friend perspective (DS-CS) |
| VII |
-Learn about fertility treatment available in Kenya (SVC) -Create a 3 to 5-min video (DS-CS) |
-Overview of previous sessions and assignment -Discussion on fertility treatment available in Kenya -Ask questions and share their learning and self-compassion practices in the WhatsApp group |
-Overview of previous sessions and assignment -Create a 3 to 5-min video from a self-compassion letter with the help of the instructors -Questions and answers -Group discussion |
-Practice loving and kind phrases toward others (may I be kind to others) |
| VIII |
-Summarise all the key concepts learned -Sharing of digital stories (DS-CS) |
-Summarised all sessions content |
-Summarised all sessions content -For participants who wished to share their videos with others were given an opportunity |
-To continue with learned self-compassion practices |
The SC-DS program protocol was developed by adopting its first five sessions from the SCV program. The other three DST sessions were designed using Lang et al.’s methodology study [28]. The intervention comprised eight 1-h sessions on the zoom platform. The goals and content of each of the eight sessions are summarised in Table 1. Notably, participants in both programs were instructed to spend 10–45 min daily doing self-compassion exercises.
Outcomes
Primary outcomes were changes of scores anxiety and depression scores from T1 to T2 and T1 from T3, compared with intervention and control groups.
The Zung Self-Rating Anxiety Scale [Z-SAS]: was used to assess anxiety symptoms. The Z-SAS is a self-reported scale whose 20 items cover various anxiety symptoms, such as psychological and somatic. Responses are given on a 4-point scale which ranges from 1 (almost never) to 4 (almost always). Participants rated each item according to the frequency of symptoms over the past 7 days. The raw SAS scores range from 20 to 80. The raw scores are then standardised to the anxiety index scores by dividing the total raw score by 80 and multiplying by 100. The anxiety index scores range from 25 to 100. The higher index scores indicate higher anxiety levels. A Z-SAS raw score of 40 or an index score of 50 suggests clinically significant anxiety levels. The Z-SAS has demonstrated good internal and test–retest reliability in previous studies [31, 32]. In this sample, Cronbach alpha at pre-intervention, post-intervention, and follow-up was: 0.81, 0. 88, and 0.89.
The Zung Self-Rating Depression Scale [Z-SDS]: was used to screen for depression and depressive states among women pursuing fertility treatment. The Z-SDS is a self-reported scale with 20 items, and responses were given on a 4-point scale that ranged from 1 (almost never) to 4 (almost always). Participants rated each item according to the frequency of the symptoms during the past seven days. The raw score ranges from 20 to 80. The raw score can be converted to an SDS index score (which ranges between 25 and 100) by simply multiplying the raw score times 1.25. The higher index scores indicate higher depression levels. The Z-SDS has shown good psychometric properties across studies [33, 34]. In this sample, Cronbach alpha at pre-intervention, post-intervention, and follow-up was: 0.81, 0. 91, and 0.87.
Secondary outcomes
Demographic and clinical characteristics questionnaire: This form was used to obtain demographic information, including age, level of education, occupation, income, marital status, and the number of children and clinical characteristics, including the cause of infertility, type of infertility, duration of infertility, previous abortions (which included both spontaneous abortions and therapeutic terminations), and current treatment.
The Self-Compassion Scale Short-Form [SCS-SF]: is a 12-item self-reported questionnaire modified from 26 item self-compassion scale. The SCS-SF was used to measure self-compassion among women seeking fertility treatment. Responses are indicated using a 5-point Likert scale ranging from 1 (hardly ever) to 5 (almost always). The total scoring was done by reversing the negative statements and summing up all the responses. The higher the SCS scores, the higher the self-compassion. The SCS-SF has demonstrated adequate internal consistency, test and retest reliability, and high correlation with the original 26 items of SCS across studies [35]. In this sample, Cronbach alpha at pre-intervention, post-intervention, and follow-up was: 0.92, 0. 94, and 0.89.
Turkish version of the Infertility Self-Efficacy Scale–Short Form (TISE-SF): was used to measure women perceived self-efficacy for coping with infertility diagnosis and its treatment. TISE-SF is an eight-item questionnaire modified from the 16 items Infertility Self-Efficacy Scale (ISE). Responses are rated using a four-point Likert scale ranging from 1 (poor fit) to 4 (excellent fit). The total scores range from 8 to 32; the higher the score, the higher the self-efficacy. Construct validity (p = 0.07) and reliability (Cronbach alpha of 0.78) have been established [36, 37]. In this sample, Cronbach alpha at pre-intervention, post-intervention, and follow-up was: 0.88, 0. 91, 0.80; item-total correlations ranged from 0.72 to 0.86.
Pregnancy assessment
A medical chart review for three groups was examined at T2 and T3 to obtain data about pregnancy rates. Clinical pregnancy was confirmed through ultrasonography (visualisation of at least one gestation sac with the fetal heartbeat), performed 5 weeks from the last menstrual period or embryo transfer.
Interventions evaluations
Participants in intervention groups were asked to evaluate the interventions at T2. Participants were asked, “how would you rate your training program experience overall?” and “would you recommend this program to other women pursuing fertility treatment?”. The responses were given on a 10-point Likert scale from 1 (not at all) to 10 (extremely recommend/excellent experience).
The other study’s secondary outcomes, such as marital satisfaction, coping strategies, psychological inflexibility, and qualitative findings, will be reported in a future publication. All measures were administered in Kiswahili or English, and translation and adapting of the questionnaires followed Guillemin et al. and Beaton et al. recommendations [38, 39].
Sample size
The sample size was estimated using power calculations (one-way analysis of variance (ANOVA)) with G*Power 3.1.9.2 based on the mean treatment effects of a prior meta-analysis study (d = 0.59 or medium size) [40]. This analysis revealed that 144 participants were required to detect a significant difference in depression and anxiety scores between the three groups, with a power of 90%, α = 0.05, and medium effect size (f = 0.3). We anticipated a 20% attrition rate, making our total target 180 women at baseline.
Statistical analysis
The intention-to-treat (ITT) principle was applied to all analyses. Continuous variables were summarised as mean ± median, while categorical variables were expressed as frequencies and percentages. Descriptive statistics were used to describe baseline characteristics and study outcomes. Between-group differences at baseline were reported using the one-way analysis of variance (ANOVA) test, chi-square (χ2) test, or nonparametric test, as appropriate. Due to the prespecified sequence of the comparisons (SC-DS vs CG, SCV vs CG, SC-DS vs SCV, results were interpreted as if from three independent trials, where the interpretation for one independence was compared to each other). No adjustment for multiple comparisons was performed in the study.
The repeated measures generalised estimating equation (GEE) linear regression models were conducted for primary and secondary outcomes analysis to assess the effects of the interventions. The main effects of group, time, and their interaction effects were examined in the GEE using repeated measurements of the groups at pre-intervention and the two-time points. For example, at post-intervention and follow-up, the results were adjusted for age, income, marital status, education levels, cause of infertility, type of infertility, duration of infertility, number of children, previous abortions, current treatment, and the outcomes measured at baseline. The pregnancy rates and intervention evaluation were listed by group and compared by the χ2 test. All analyses were performed using the R package geepack (version 4.1.2). Cohen’s d for effect sizes was used to evaluate the effectiveness of the interventions, with 0.2 denoting a small effect, 0.2–0.5 a medium effect, and 0.8 a large effect [41]. p < 0.05 was considered statistically significant.
Results
Study flow
There were no significant differences between the intervention and control groups at baseline in terms of social demographics, clinical characteristics, and study variables (all ps > 0.5) (Tables 2 and 3). Figure 2 shows the study flow chart. Of 320 women approached by trained nurses, 80 did not have access to a computer or internet-enabled mobile device, ten declined to participate, 20 withdrew from WhatsApp groups, and ten did not complete the pre-intervention assignment. Two hundred women were randomly assigned to SCV, SC-DS, and CG. No adverse events were reported during the study.
Table 2.
Baseline characteristics of the three randomised groups
| CG | SC-DS | SCV | p.overall | |
|---|---|---|---|---|
| Variable | N = 68 | N = 67 | N = 65 | |
| Participants age: | 0.992 | |||
| 20–24 | 10 (14.71%) | 7 (10.45%) | 6 (9.23%) | |
| 25–29 | 15 (22.06%) | 16 (23.88%) | 15 (23.08%) | |
| 30–34 | 25 (36.76%) | 27 (40.30%) | 27 (41.54%) | |
| 35–39 | 10 (14.71%) | 10 (14.93%) | 11 (16.92%) | |
| 40–44 | 8 (11.76%) | 7 (10.45%) | 6 (9.23%) | |
| Educational level: | 0.998 | |||
| Primary | 7 (10.29%) | 7 (10.45%) | 6 (9.23%) | |
| Secondary | 19 (27.94%) | 21 (31.34%) | 21 (32.31%) | |
| Diploma | 31 (45.59%) | 29 (43.28%) | 29 (44.62%) | |
| Bachelor | 11 (16.18%) | 10 (14.93%) | 9 (13.85%) | |
| Occupation: | 0.985 | |||
| Employed | 25 (36.76%) | 24 (35.82%) | 26 (40.00%) | |
| Entrepreneur | 25 (36.76%) | 26 (38.81%) | 24 (36.92%) | |
| Unemployed | 18 (26.47%) | 17 (25.37%) | 15 (23.08%) | |
| Income per month (KSH): | 0.960 | |||
| Less than 10,000 | 23 (33.82%) | 23 (34.33%) | 23 (35.38%) | |
| 10,001–20,000 | 24 (35.29%) | 23 (34.33%) | 26 (40.00%) | |
| 20,001–30,000 | 12 (17.65%) | 13 (19.40%) | 8 (12.31%) | |
| Greater than 30,000 | 9 (13.24%) | 8 (11.94%) | 8 (12.31%) | |
| Marital status: | 0.991 | |||
| Married | 49 (72.06%) | 46 (68.66%) | 45 (69.23%) | |
| Living with a partner | 14 (20.59%) | 15 (22.39%) | 15 (23.08%) | |
| Others | 5 (7.35%) | 6 (8.96%) | 5 (7.69%) | |
| Cause of infertility: | 0.936 | |||
| Male | 13 (19.12%) | 12 (17.91%) | 10 (15.38%) | |
| Female | 41 (60.29%) | 37 (55.22%) | 36 (55.38%) | |
| Both male and female | 6 (8.82%) | 7 (10.45%) | 9 (13.85%) | |
| Unexplained | 8 (11.76%) | 11 (16.42%) | 10 (15.38%) | |
| Types of infertility: | 0.989 | |||
| Primary | 16 (23.53%) | 16 (23.88%) | 16 (24.62%) | |
| Secondary | 52 (76.47%) | 51 (76.12%) | 49 (75.38%) | |
| Duration of infertility (years): | 0.989 | |||
| 1 to 3 | 29 (42.65%) | 27 (40.30%) | 25 (38.46%) | |
| 4 to 6 | 27 (39.71%) | 27 (40.30%) | 28 (43.08%) | |
| 7 to 9 | 12 (17.65%) | 13 (19.40%) | 12 (18.46%) | |
| Duration of receiving treatment (years): | 0.992 | |||
| less than 1 | 18 (26.47%) | 17 (25.37%) | 15 (23.08%) | |
| 1 to 2 | 32 (47.06%) | 33 (49.25%) | 32 (49.23%) | |
| 3 to 4 | 18 (26.47%) | 17 (25.37%) | 18 (27.69%) | |
| Number of children: | 1.000 | |||
| One | 65 (95.59%) | 64 (95.52%) | 62 (95.38%) | |
| More than one | 3 (4.41%) | 3 (4.48%) | 3 (4.62%) | |
| Previous abortions: | 0.941 | |||
| None | 50 (73.53%) | 47 (70.15%) | 48 (73.85%) | |
| One | 15 (22.06%) | 18 (26.87%) | 14 (21.54%) | |
| More than one | 3 (4.41%) | 2 (2.99%) | 3 (4.62%) | |
| Current treatment: | 0.990 | |||
| Surgery | 25 (36.76%) | 26 (38.81%) | 25 (38.46%) | |
| Hormonal | 32 (47.06%) | 29 (43.28%) | 30 (46.15%) | |
| IVF | 6 (8.82%) | 7 (10.42%) | 6 (9.23%) | |
| ICSI | 5 (7.36%) | 5 (7.46%) | 4 (6.15%) | |
| History of previous treatment: | ||||
| No | 63 (92.65%) | 61 (91.04%) | 60 (92.31%) | 0.937 |
| Yes | 5 (7.35%) | 6 (8.96%) | 5 (7.69%) |
CG control group, SC-DS self-compassion training using digital stories, SCV self-compassion training using videos, IVF in vitro fertilisation, ICSI intracytoplasmic sperm injection
Table 3.
The comparison between primary and secondary outcomes of the three groups at per-intervention (T1)
| CG | SC-DS | SCV | p.overall | p.CG vs SC-DS | p.CG vs SCV | p.SC-DS vs SCV | |
|---|---|---|---|---|---|---|---|
| Variables | N = 68 | N = 67 | N = 65 | ||||
| Anxiety T1 | 49.06 ± 2.44 | 49.52 ± 2.81 | 48.68 ± 3.42 | 0.250 | 0.308 | 0.462 | 0.124 |
| Depression T1 | 50.09 ± 4.49 | 51.10 ± 5.47 | 49.08 ± 4.60 | 0.060 | 0.241 | 0.202 | 0.023 |
| Self-compassion T1 | 33.49 ± 7.90 | 32.87 ± 8.62 | 32.58 ± 5.61 | 0.778 | 0.664 | 0.448 | 0.824 |
| Infertility-self-efficacy T1 | 18.82 ± 2.25 | 18.63 ± 2.00 | 18.31 ± 3.02 | 0.476 | 0.592 | 0.268 | 0.477 |
T1 pre-intervention, CG control group, SC-DS self-compassion training using digital stories, SCV self-compassion training using videos
Anxiety
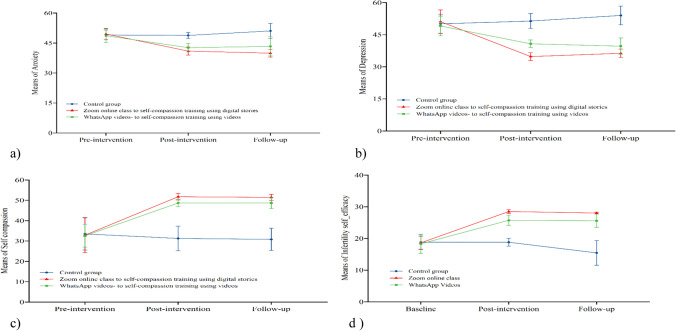
The GEE-adjusted models performed at baseline and post-interventions in different groups indicated that there was a significant mean difference in anxiety scores between SC-DS vs CG [− 8.38 (− 9.46 to − 7.30); d = 2.679, SCV vs CG [− 6.01 (− 7.26 to − 4.76); d = 1.668, and SC-DS vs SCV [− 2.55 (− 3.8 to − 1.3); d = 0.718 (Tables 4 and 5). In other words, SC-DS participants had the most reduced anxiety scores after the intervention, followed by the SCV and CG groups. The GEE analysis performed at baseline and follow-up in the different groups showed that there was a significant mean difference in anxiety scores in the SC-DS vs CG [− 11.63 (− 13.13 to − 10.14); d = 2.754, SCV vs CG [− 7.71 (− 9.62 to − 5.58); d = 1.418, and SC-DS vs SCV [− 4.25 (− 5.95 to − 2.54); d = 0.918 (Tables 4 and 5). This finding reveals that SC-DS participants had the most reduced anxiety scores at follow-up, followed by the SCV and CG groups. Figure 3a summarises the trends in anxiety means scores across the three groups. The SC-DS and SCV anxiety decreased significantly from baseline to post-intervention and from baseline to follow-up. The CG showed no significant decrease in anxiety scores at three-time points (Table 6 and Fig. 3a).
Table 4.
The mean differences between groups in the generalised estimating equations analysis
| Time | SC-DS vs CG | SCV vs CG | SC-DS vs SCV | |||
|---|---|---|---|---|---|---|
| Between-group difference (95% CI) ǂ | p value ǂ | Between-group difference (95% CI) ǂ | p value ǂ | Between-group difference (95% CI) ǂ | p value ǂ | |
| Anxiety | - | - | - | - | - | - |
| Baseline | - | - | - | - | - | - |
| Post-intervention | − 8.38 (− 9.46 to − 7.3) | < 0.001 | − 6.01 (− 7.26 to − 4.76) | < 0.001 | − 2.55 (− 3.8 to − 1.3) | < 0.001 |
| Follow-up | − 11.63 (− 13.13 to − 10.14) | < 0.001 | − 7.71 (− 9.62 to − 5.81) | < 0.001 | − 4.25 (− 5.95 to − 2.54) | < 0.001 |
| Depression | - | - | - | - | - | - |
| Baseline | - | - | - | - | - | - |
| Post-intervention | − 17.66 (− 19.64 to − 15.68) | < 0.001 | − 9.97 (− 11.79 to − 8.16) | < 0.001 | − 7.78 (− 9.62 to − 5.94) | < 0.001 |
| Follow-up | − 18.73 (− 20.85 to − 16.61) | < 0.001 | − 13.7 (− 16.06 to − 11.35) | < 0.001 | − 5.25 (− 7.44 to − 3.05) | < 0.001 |
| Self-compassion | - | - | - | - | - | - |
| Baseline | - | - | - | - | - | - |
| Post-intervention | 21.32 (18.06 to 24.58) | < 0.001 | 18.62 (15.8 to 21.44) | < 0.001 | 2.74 (0.08 to 5.4) | 0.043 |
| Follow-up | 21.41 (18.22 to 24.59) | < 0.001 | 19.14 (16.09 to 22.18) | < 0.001 | 2.45 (− 0.21 to 5.11) | 0.071 |
| Infertility-self-efficacy | - | - | - | - | - | - |
| Baseline | - | - | - | - | - | - |
| Post-intervention | 9.96 (9.14 to 10.79) | < 0.001 | 7.56 (6.55 to 8.57) | < 0.001 | 2.41 (1.46 to 3.35) | < 0.001 |
| Follow-up | 12.82 (11.52 to 14.12) | < 0.001 | 10.79 (9.14 to 12.43) | < 0.001 | 2.06 (0.87 to 3.25) | 0.001 |
CG control group, SC-DS self-compassion training using digital stories, SCV self-compassion training using video
ǂ Between-group difference for mean change from baseline, based on the Generalised estimating equations adjusted models, adjusted for age, income, marital status, education levels, cause of infertility, type of infertility, duration of infertility, number of children, previous abortions, and current treatment
Table 5.
The mean differences between groups in the generalised estimating equations analysis (Cohen’s effect sizes)
| CG vs SC-DS | CG vs SCV | SC-DS vs SCV | |
|---|---|---|---|
| Anxiety_d21 | 2.679 | 1.668 | 0.718 |
| Anxiety_d31 | 2.754 | 1.418 | 0.918 |
| Depression_d21 | 3.013 | 1.833 | 1.433 |
| Depression_d31 | 3.039 | 1.987 | 0.823 |
| Self_compassion_d21 | 2.192 | 2.174 | 0.388 |
| Self_compassion_d31 | 2.283 | 2.108 | 0.376 |
| Infertility_self_efficacy_d21 | 4.109 | 2.675 | 0.850 |
| Infertility_self_efficacy_d31 | 3.416 | 2.340 | 0.578 |
d21 post-intervention-pre-intervention, d31 follow-up-pre-intervention, CG Control group, SC-DS self-compassion training using digital stories, SCV self-compassion training using videos
Fig. 3.
Means differences for interventions and control groups across the three assessment points. (a) Anxiety differences for two interventions with the control group across the three assessment points. (b) Depression differences for two interventions with the control group across the three assessment points. (c) Self-compassion differences for two interventions with the control group across the three assessment points. (d) Infertility self-efficacy differences for two interventions with the control group across the three assessment points
Table 6.
The mean scores changes between primary and secondary outcomes within groups
| Within-group changes, mean (95% CI)a | p.overall | p.CG vs SC-DS | p.CG vs SCV | p.SC-DS vs SCV | |||
|---|---|---|---|---|---|---|---|
| CG(N = 68) | SC-DS(N = 67) | SCV(N = 65) | |||||
| Anxiety-d21 | − 0.22 (− 1.01 to 0.56) | − 8.64 (− 9.54 to − 7.74) | − 6.10 (− 7.20 to − 5.00) | < 0.001 | < 0.001 | < 0.001 | 0.001 |
| Anxiety-d31 | 2.04 (0.71 to 3.36) | − 9.68 (− 10.69 to − 8.68) | − 5.44 (− 7.05 to − 3.82) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Depression-d21 | 1.32 (− 0.17 to 2.81) | − 16.32 (− 17.96 to − 14.68) | − 8.64 (− 10.03 to − 7.25) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Depression-d31 | 3.88 (2.08 to 5.67) | − 14.89 (− 16.54 to − 13.24) | − 9.69 (− 11.71 to − 7.67) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Self-compassion-d21 | − 2.21 (− 4.78 to 0.37) | 19.16 (16.53 to 21.79) | 16.12 (14.38 to 17.86) | < 0.001 | < 0.001 | < 0.001 | 0.056 |
| Self-compassion-d31 | − 2.55 (− 5.31 to 0.21) | 19.02 (16.53 to 21.52) | 16.08 (14.03 to 18.14) | < 0.001 | < 0.001 | < 0.001 | 0.071 |
| Infertility-self-efficacy-d21 | − 0.10 (− 0.77 to 0.58) | 9.86 (9.26 to 10.46) | 7.62 (6.75 to 8.49) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Infertility-self-efficacy-d31 | − 3.46 (− 4.79 to − 2.14) | 9.36 (8.79 to 9.93) | 7.42 (6.16 to 8.68) | < 0.001 | < 0.001 | < 0.001 | 0.006 |
d21 post-intervention-pre-intervention, d31 follow-up-pre-intervention, CG control group, SC-DS self-compassion training using digital stories, SCV self-compassion training using videos
aIndicates mean change between baseline and follow-up
Depression
The GEE-adjusted models performed before and after intervention in different groups indicated that there was a significant mean difference in depression scores in the SC-DS vs CG [− 17.66 (− 19.64 to − 15.68); d = 3.013, SCV vs CG [− 9.97 (− 11.79 to − 8.16); d = 1.833, and SC-DS vs SCV [− 7.78 (− 9.62 to − 5.94); d = 1.433 (Tables 4 and 5). In other words, SC-DS participants had the most reduced depression scores after the intervention, followed by the SCV and CG groups. The GEE analysis performed at baseline and follow-up in the different groups showed that there was a significant mean difference in depression scores in the SC-DS vs CG [− 18.73 (− 20.85 to − 16.61); d = 3.039, SCV vs CG [− 13.71 (− 16.06 to − 11.35); d = 1.987, and SC-DS vs SCV [− 5.25 (− 7.44 to − 3.05); d = 0.823 (Tables 4 and 5). This finding reveals that SC-DS participants had the most reduced depression scores at follow-up, followed by the SCV and CG groups. Figure 3b summarises the trends in depression mean scores across the three groups. The SC-DS and SCV depression scores decreased significantly from baseline to post-intervention and from baseline to follow-up. The CG showed no significant decrease in depression scores between the three-time points (Table 6 and Fig. 3b).
Self-compassion
The results of GEE-adjusted models performed at baseline and post-interventions in different groups indicated that there was a significant mean difference in self-compassion scores in the SC-DS vs CG [21.32 (18.64 to 24.58); d = 2.192, SCV vs CG [18.62 (15.8 to 21.44); d = 2.174, and SC-DS vs SCV [2.74 (0.08 to 5.4); d = 0.388 (Tables 4 and 5). In other words, SC-DS participants had the most increased self-compassion scores after the intervention, followed by the SCV and CG groups. The GEE analysis performed at baseline and follow-up in the different groups showed that there was a significant mean difference in self-compassion scores in the SC-DS vs CG [21.41 (18.22 to − 24.59); d = 2.283 and SCV vs CG [19.14 (16.09 to 22.18); d = 2.108 but no significant difference in the SC-DS vs SCV [2.45 (− 0.21 to 5.11); d = 0.376 (Tables 4 and 5). This finding indicates that SC-DS participants had the most increased self-compassion scores at follow-up, followed by the SCV and CG groups. However, there was no statistically significant difference between the self-compassion scores of SC-DS and SCV (p > 0.05). Figure 3c summarises the trends in self-compassion means scores across the three groups. The SC-DS and SCV self-compassion increased significantly from baseline to post-intervention and from baseline to follow-up. The CG showed no significant increase in self-compassion scores between the three-time points (Table 6 and Fig. 3c).
Infertility-self-efficacy
The GEE-adjusted models performed before and after interventions in different groups indicated that there was a significant mean difference in infertility self-efficacy scores in the SC-DS vs CG [9.96 (9.14 to 10.79); d = 4.109, SCV vs CG [7.56 (6.55 to 8.57); d = 2.675, and SC-DS vs SCV [ 2.41 (1.46 to 3.35) d; 0.850 (Tables 4 and 5). In other words, SC-DS participants had the most increased self-efficacy scores after the intervention, followed by the SCV and CG groups. The GEE analysis performed at baseline and follow-up in the different groups showed that there was a significant mean difference in infertility self-efficacy scores in the SC-DS vs CG [12.82 (11.52 to − 14.12); d = 3.416, SCV vs CG [10.79 (9.14 to 12.43); d = 2.340, and SC-DS vs SCV [2.06 (0.87 to 3.25); d = 0.578 (Tables 4 and 5). This finding shows that SC-DS participants had the most increased infertility self-efficacy scores at follow-up, followed by the SCV and CG groups. Figure 3d summarises the trends in infertility self-efficacy means scores across the three groups. The SC-DS and SCV infertility self-efficacy scores increased significantly from baseline to post-intervention and from baseline to follow-up. The CG showed no substantial changes in infertility self-efficacy scores between the three-time assessments (Table 6 and Fig. 3d).
Pregnancy outcomes
Women in the intervention groups had no significant differences in the number of clinical pregnancies compared with women in the control group at post-intervention and follow-up (Tables 7 and 8).
Table 7.
Comparison between the groups regarding pregnancy rates at post-intervention (T2)
| CG | SC-DS | SCV | p.overall | p.CG vs SC-DS | p.CG vs SCV | p.SC-DS vs SCV | |
|---|---|---|---|---|---|---|---|
| N = 65 | N = 59 | N = 57 | |||||
| Pregnancy rates: | 0.050 | 0.039 | 0.044 | 1.000 | |||
| No | 63 (96.92%) | 50 (84.75%) | 49 (85.96%) | ||||
| Pregnant | 2 (3.08%) | 9 (15.25%) | 8 (14.04%) |
CG control group, SC-DS self-compassion training using digital stories, SCV self-compassion training using videos
Table 8.
Comparison between the groups regarding pregnancy rate at follow-up (T3)
| CG | SC-DS | SCV | p.overall | p.CG vs SC-DS | p.CG vs SCV | p.SC-DS vs SCV | |
|---|---|---|---|---|---|---|---|
| N = 56 | N = 49 | N = 48 | |||||
| Pregnancy rates: | 0.198 | 0.215 | 0.495 | ||||
| No | 56 (100.00%) | 47 (95.92%) | 48 (100.00%) | ||||
| Pregnant | 0 (0.00%) | 2 (4.08%) | 0 (0.00%) |
CG control group, SC-DS self-compassion training using digital stories, SCV self-compassion training using videos
Interventions evaluations
Participants in intervention groups rated the self-compassion interventions positively [SC-DS (8.06 ± 0.59) and SCV (8.04 ± 0.49)], with no significant mean difference between the two intervention groups (p > 0.847). Moreover, most women in both interventions reported that they would highly recommend self-compassion interventions to others [SC-DS 82% (41/50), SVD 84% (42/50)], with no significant statistical difference between the two intervention groups (p < 0.006).
Discussion
Our study shows that SCV and SC-DS programs produced beneficial results for women pursuing fertility treatment. Compared to a CG, both SCV and SC-DS reduced symptoms of anxiety and depression and increased scores of self-compassion and infertility self-efficacy. No significant result was observed in the pregnancy rates among the three groups. Women evaluated the SCPI positively, indicating they had a positive experience. Moreover, most women in both interventions said that they would highly recommend self-compassion training programs to others. The SC-DS appeared superior to SCV in reducing depression and anxiety and increasing self-compassion and infertility self-efficacy. However, no statistically significant self-compassion scores were found at follow-up between SC-DS and SCV. Effect sizes for intervention groups compared to the CG group from post to pre-intervention and follow-up to pre-intervention were large in anxiety, depression, self-compassion, and infertility self-efficacy. When SC-DS and SCV were compared, both training programs produced similar effects in anxiety, depression, self-compassion, and infertility self-efficacy, with the SC-DS effect sizes being somewhat larger.
Our main findings indicated that SC-DS and SCV significantly decreased anxiety and depression, which aligns with previous studies on self-compassion [11, 29, 42]. Anxiety and depressive symptoms are related to distorted thoughts about oneself, others, and the future, and the themes of worthlessness, shame, self-criticism, and rumination [43]. Adopting a compassionate stance was assumed to help women in the intervention groups disengage from unhelpful thoughts and negative feelings by allowing them to put their experiences into greater perspective and view their experiences with greater clarity and balance rather than over-identification with them [44]. Self-compassion practices such as loving-kindness meditation could have also contributed to activating a self-compassion mindset, thus, promoting positive feelings [12, 29].
Moreover, self-compassion practices such as soothing activities such as placing one hand over the heart could have deactivated the sympathetic system’s response while activating the parasympathetic system associated with releasing endorphins, opiates, and oxytocin. These hormones are related to the feeling of safety, calmness, secure attachment, and a sense of well-being [45]. These mechanisms may explain why self-compassion training interventions reduced anxiety and depression among women pursuing fertility treatment. Furthermore, positive evaluation of training interventions in terms of experience and recommendation, the high response, and completion rates suggest that online self-compassion interventions are appropriate psychological support for women undergoing fertility treatment. Therefore, this flexible online approach may be a practical intervention for women with fertility problems in poor resource settings such as Kenya.
In line with our expectations, we found that women in the SC-DS and SCV had increased self-compassion scores than the CG, suggesting that self-compassion training programs effectively taught the participants how to become more compassionate towards themselves. This result agrees with the previous studies [29, 46, 47]. Interestingly, they were no statistically significant difference in self-compassion scores between SC-DS and SCV at follow-up, meaning that the two programs were effective in training self-compassion. Similarly, women in SC-DS and SCV exhibited higher infertility self-efficacy scores than those in CG. Recitation of meaningful self-compassion phrases by intervention groups participants could have reduced fear of treatment failure and promoted self-acceptance and connection to others, thus increasing their perceptions of self-confidence in dealing with infertility and its treatment. The study findings align with non-pharmacological interventions [48, 49]. Evidence shows that high self-compassion and self-efficacy correlate significantly with less low psychological distress [46, 49].
Our study revealed no significant effect on the pregnancy rate between the groups, consistent with other studies [50–52]. However, our result contradicts findings reported in several other studies [53, 54] and the statistically significant pooled effect on pregnancy rates found in a recent meta-analysis of 10 studies (RR = 1.43; p < 0.01) (55). The SC-DS appeared superior to SCV in reducing depression and anxiety and increasing self-compassion and infertility self-efficacy. The possible explanation for the results could be that participating in the DST process could have offered women insights and understanding, causing them to change their beliefs, attitudes, and perceptions about infertility and its treatments. Moreover, it allowed them to redefine their story and adopt healthier coping strategies [28]. Notably, SC-DS and documentation of infertility stories on social media platforms such as Instagram, Facebook, and YouTube are slightly similar in that both modalities include expressing and processing infertility-related emotions [56]. However, the two methods differ because individuals documenting their infertility stories on social media do so to share their experiences, offer and receive social support, and exchange information [57].
Our study has several limitations. First, our inclusion criteria meant that women seeking treatment without internet access were excluded from the study. Second, women in this study had experienced infertility for 9 years and received fertility treatment for 4 years. Their exclusion and participant characteristics may limit the generalizability of our findings. Future studies on RCTs involving women seeking fertility treatment are needed in Kenya. A specific research recommendation is to conduct a similar study with men seeking fertility treatment. Another suggestion is to conduct a similar study with men and women with fertility problems but not seeking fertility treatment. Third, lack of software to monitor how participants interacted with study materials and assessments. We only relied on self-reported data, such as home works, checklists, and questionnaires, which may not always be accurate. It would have been helpful to know which shared videos participants found valuable and how many times they replayed them. This information can benefit future researchers in predicting which materials women undergoing fertility treatment find helpful [58]. Fourth, the instructors who led the interventions were qualified and experienced in self-compassion, more than many healthcare professionals in fertility clinics. Future studies conducted by trained fertility staff, such as nurses, are needed. Finally, a double-blind study was difficult to conduct due to the nature of the intervention, which could have led to performance biases.
Thus, despite the limitation mentioned, the study findings demonstrate the effectiveness of SC-DS and SCV. To the best knowledge, this was the first study in which short videos and DS were used to train self-compassion among women receiving fertility treatment. The sample size was large, and the study used robust methodology and statistical methods. Our study findings suggest that flexible online self-compassion interventions may be helpful for clinical practice. However, future large-size multi-centre trials are needed to confirm the effects of the interventions and explore how they may vary among different groups of participants.
Conclusions
This RCT found that SC-DS and SCV decreased anxiety and depression symptoms and increased self-compassion and infertility self-efficacy scores. Most SCV and SC-DS participants rated self-compassion training programs positively and said they would highly recommend them to others. These findings suggest that SCV and SC-DS may be effective psychological interventions for women undergoing fertility treatment and may suit women seeking fertility treatment in resource-poor settings. The SCV and SC-DS can be incorporated into a daily routine for women seeking fertility treatment. More research is needed to replicate this study’s findings in large-size multi-centre trials and explore how they may vary among different groups of participants. Moreover, healthcare professionals should develop policies that integrate psychological support services into fertility treatment to help women pursuing fertility treatment cope with psychological distress, low self-compassion, and infertility self-efficacy.
Acknowledgements
The authors would like to thank the women who participated in this study. We also want to thank the three hospitals’ obstetrics and gynaecology outpatient clinics’ medical staff for their cooperation and support.
Author contribution
AN, FK, and YL designed and conceptualised the study. JN, EB, AM, and FK assessed, collected, and conducted data analysis. AN, JN, EB, and FK drafted the manuscript, and LY and AM supervised the research process. All authors read and approved the final manuscript.
Funding
The first author provided funding for this study.
Data Availability
The datasets analysed during this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):293–308. doi: 10.1016/j.bpobgyn.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Pasch LA, Holley SR, Bleil ME, Shehab D, Katz PP, Adler NE. Addressing the needs of fertility treatment patients and their partners: are they informed of and do they receive mental health services? Fertil Steril. 2016;106(1):209–215.e2. doi: 10.1016/j.fertnstert.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Clifton J, Parent J, Seehuus M, Worrall G, Forehand R, Domar A. An internet-based mind/body intervention to mitigate distress in women experiencing infertility: a randomized pilot trial. Matsuoka YJ, editor. PLoS One. 2020;15(3):e0229379. doi: 10.1371/journal.pone.0229379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjaer TK, Jensen A, Dalton SO, Johansen C, Schmiedel S, Küjaer SK. Suicide in Danish women evaluated for fertility problems. Hum Reprod. 2011;26(9):2401–2407. doi: 10.1093/humrep/der188. [DOI] [PubMed] [Google Scholar]
- 6.Domar AD, Rooney K, Hacker MR, Sakkas D, Dodge LE. Burden of care is the primary reason why insured women terminate in vitro fertilization treatment. Fertil Steril. 2018;109(6):1121–1126. doi: 10.1016/j.fertnstert.2018.02.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malina A, Pooley JA. Psychological consequences of ivf fertilization—review of research. Ann Agric Environ Med. 2017;24(4):554–558. doi: 10.5604/12321966.1232085. [DOI] [PubMed] [Google Scholar]
- 8.Galhardo A, Cunha M, Pinto-Gouveia J, Matos M. The mediator role of emotion regulation processes on infertility-related stress. J Clin Psychol Med Settings. 2013;20(4):497–507. doi: 10.1007/s10880-013-9370-3. [DOI] [PubMed] [Google Scholar]
- 9.Raque-Bogdan TL, Hoffman MA. The relationship among infertility, self-compassion, and well-being for women with primary or secondary infertility. Psychol Women Q. 2015;39(4):484–496. doi: 10.1177/0361684315576208. [DOI] [Google Scholar]
- 10.Kim WS, Langer S, Todd M, et al. Feasibility of a Digital Storytelling Intervention for Hematopoietic Cell Transplant Patients. J Cancer Educ. 2022;37(5):1275–1285. doi: 10.1007/s13187-020-01948-2. [DOI] [PubMed] [Google Scholar]
- 11.Afshani SA, Abooei A, Fahadan AMA. Self-compassion training and psychological well-being of infertile female. Int J Reprod Biomed. 2019;17(10):757–762. doi: 10.18502/ijrm.v17i10.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neff K. Self-compassion: an alternative conceptualization of a healthyattitudetoward oneself. Self Identity. 2003;2:85–101. doi: 10.1080/15298860309032. [DOI] [Google Scholar]
- 13.Wang S, Zhan H. Enhancing teaching and learning with digital storytelling. Int J Inf Commun Technol Educ. 2010;6(2):76–87. doi: 10.4018/jicte.2010040107. [DOI] [Google Scholar]
- 14.Laing CM, Moules NJ, Estefan A, Lang M. “Stories take your role away from you”: understanding the impact on health care professionals of viewing digital stories of pediatric and adolescent/young adult oncology patients. J Pediatr Oncol Nurs. 2017;34(4):261–271. doi: 10.1177/1043454217697023. [DOI] [PubMed] [Google Scholar]
- 15.D’Cruz K, Douglas J, Serry T. Narrative storytelling as both an advocacy tool and a therapeutic process: perspectives of adult storytellers with acquired brain injury. Neuropsychol Rehabil. 2020;30(8):1409–1429. doi: 10.1080/09602011.2019.1586733. [DOI] [PubMed] [Google Scholar]
- 16.Laing CM, Moules NJ, Sinclair S, Estefan A. Digital storytelling as a psychosocial tool for adult cancer survivors. Oncol Nurs Forum. 2019;46(2):147–154. doi: 10.1188/19.ONF.147-154. [DOI] [PubMed] [Google Scholar]
- 17.Boivin J, Scanlan LC, Walker SM. Why are infertile patients not using psychosocial counselling? Hum Reprod. 1999;14(5):1384–1391. doi: 10.1093/humrep/14.5.1384. [DOI] [PubMed] [Google Scholar]
- 18.Meyers AJ, Domar AD. Research-supported mobile applications and internet-based technologies to mediate the psychological effects of infertility: a review. Reprod Biomed Online. 2021;42(3):679–685. doi: 10.1016/j.rbmo.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Van Dongen AJCM, Nelen WLDM, Inthout J, Kremer JAM, Verhaak CM. e-Therapy to reduce emotional distress in women undergoing assisted reproductive technology (ART): a feasibility randomized controlled trial. Hum Reprod. 2016;31(5):1046–1057. doi: 10.1093/humrep/dew040. [DOI] [PubMed] [Google Scholar]
- 20.Haemmerli K, Znoj H, Berger T. Internet-based support for infertile patients: a randomized controlled study. J Behav Med. 2010;33(2):135–146. doi: 10.1007/s10865-009-9243-2. [DOI] [PubMed] [Google Scholar]
- 21.Folkman S. Stress: Appraisal and coping. In: Gellman MD, Turner JR, editors. Encyclopedia of Behavioral Medicine. New York, NY: Springer; 2013. p. 1913–1915. https://doi.org/10.1007/978-1-4419-1005-9_215
- 22.Zurlo MC, Cattaneo Della Volta MF, Vallone F. Infertility-related stress and psychological health outcomes in infertile couples undergoing medical treatments: testing a multi-dimensional model. J Clin Psychol Med Settings. 2020;27(4):662–76. doi: 10.1007/s10880-019-09653-z. [DOI] [PubMed] [Google Scholar]
- 23.Swanson A, Braverman AM. Psychological components of infertility. Fam Court Rev. 2021;59(1):67–82. doi: 10.1111/fcre.12552. [DOI] [Google Scholar]
- 24.Terry DJ, Hynes GJ. Adjustment to a low-control situation: reexamining the role of coping responses. J Pers Soc Psychol. 1998;74(4):1078–1092. doi: 10.1037/0022-3514.74.4.1078. [DOI] [Google Scholar]
- 25.Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74(1):224–237. doi: 10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 26.Galhardo A, Pinto-Gouveia J, Cunha M, Matos M. The impact of shame and self-judgment on psychopathology in infertile patients. Hum Reprod. 2011;26(9):2408–2414. doi: 10.1093/humrep/der209. [DOI] [PubMed] [Google Scholar]
- 27.Cwikel J, Gidron Y, Sheiner E. Psychological interactions with infertility among women. Eur J Obstet Gynecol Reprod Biol. 2004;117(2):126–131. doi: 10.1016/j.ejogrb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Lang M, Laing C, Moules N, Estefan A. Words, Camera, Music, Action: A Methodology of digital storytelling in a health care setting. Int J Qual Methods. 2019;18:1–10. doi: 10.1177/1609406919863241. [DOI] [Google Scholar]
- 29.Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013;69(1):28–44. doi: 10.1002/jclp.21923. [DOI] [PubMed] [Google Scholar]
- 30.Craig P, Dieppe P, Macintyre S, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337(7676):979–983. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunstan DA, Scott N. Norms for Zung’s Self-rating Anxiety Scale. BMC Psychiatry. 2020;20(1):1–8. doi: 10.1186/s12888-019-2427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 33.Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 34.Dunstan DA, Scott N. Clarification of the cut-off score for Zung’s self-rating depression scale. BMC Psychiatry. 2019;19(1):1–7. doi: 10.1186/s12888-019-2161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neff KD. The development and validation of a scale to measure self-compassion. J Couns Psychol. 2003;2:85–102. [Google Scholar]
- 36.Cousineau TM, Green TC, Corsini EA, Barnard T, Seibring AR, Domar AD, et al. Development and validation of the Infertility Self-Efficacy scale. Fertil Steril. 2006;85(6):1684–1696. doi: 10.1016/j.fertnstert.2005.10.077. [DOI] [PubMed] [Google Scholar]
- 37.Arslan-Özkan İ, Okumuş H, Lash AA, Firat MZ. Cultural Validation of the Turkish Version of the Infertility Self-Efficacy Scale-Short Form (TISE-SF) J TranscultNurs. 2014;25(3):232–240. doi: 10.1177/1043659613508784. [DOI] [PubMed] [Google Scholar]
- 38.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25(24):3186–91. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 39.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417–1432. doi: 10.1016/0895-4356(93)90142-N. [DOI] [PubMed] [Google Scholar]
- 40.Frederiksen Y, Farver-Vestergaard I, Skovgård NG, Ingerslev HJ, Zachariae R. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ Open. 2015;5(1):e006592. doi: 10.1136/bmjopen-2014-006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioral sciences. Vol. 2, Lawrence Erlbaum Associates. New York; 1977. 1–41 p.
- 42.Finlay-Jones A, Kane R, Rees C. Self-compassion online: a pilot study of an internet-based self-compassion cultivation program for psychology trainees. J Clin Psychol. 2017;73(7):797–816. doi: 10.1002/jclp.22375. [DOI] [PubMed] [Google Scholar]
- 43.Galhardo A, Cunha M, Pinto-Gouveia J. Mindfulness-based program for infertility: efficacy study. Fertil Steril. 2013;100(4):1059–1067. doi: 10.1016/j.fertnstert.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 44.Neff KD, Germer C. Self-compassion and psychological well-being. In: Seppälä EM, Simon-Thomas E, Brown SL, Worline MC, Cameron CD, Doty JR, editors. The Oxford handbook of compassion science. Oxford University Press; 2017. pp. 371–385. [Google Scholar]
- 45.Gilbert P. Training our minds in, with and for compassion: An introduction to concepts and compassion-focused exercises. Interactions; 2010. p.1–82. https://www.getselfhelp.co.uk/docs/Gilbert-compassion-handout.pdf
- 46.Smeets E, Neff K, Alberts H, Peters M. Meeting suffering with kindness: effects of a brief self-compassion intervention for female college students. J Clin Psychol. 2014;70(9):794–807. doi: 10.1002/jclp.22076. [DOI] [PubMed] [Google Scholar]
- 47.Ferrari M, Hunt C, Harrysunker A, Abbott MJ, Beath AP, Einstein DA. Self-compassion interventions and psychosocial outcomes: a meta-analysis of RCTs. Mindfulness (N Y) 2019;10(8):1455–1473. doi: 10.1007/s12671-019-01134-6. [DOI] [Google Scholar]
- 48.Pasha H, Faramarzi M, Esmailzadeh S, Kheirkhah F, Salmalian H. Comparison of pharmacological and nonpharmacological treatment strategies in promotion of infertility self-efficacy scale in infertile women: a randomized controlled trial. Iran J Reprod Med. 2013;11(6):495–502. [PMC free article] [PubMed] [Google Scholar]
- 49.Cousineau TM, Green TC, Corsini E, Seibring A, Showstack MT, Applegarth L, et al. Online psychoeducational support for infertile women : a randomized controlled trial. Hum Reprod. 2008;23(3):554–566. doi: 10.1093/humrep/dem306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai CF, Cui NX, Xu X, Mi GL, Sun JW, Shao D, et al. Effectiveness of two guided self-administered interventions for psychological distress among women with infertility: a three-armed, randomized controlled trial. Hum Reprod. 2019;34(7):1235–1248. doi: 10.1093/humrep/dez066. [DOI] [PubMed] [Google Scholar]
- 51.Matthiesen S, Klonoff-Cohen H, Zachariae R, Jensen-Johansen MB, Nielsen BK, Frederiksen Y, et al. The effect of an expressive writing intervention (EWI) on stress in infertile couples undergoing assisted reproductive technology (ART) treatment: a randomized controlled pilot study. Br J Health Psychol. 2012;17(2):362–378. doi: 10.1111/j.2044-8287.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 52.Ockhuijsen H, Van Den Hoogen A, Eijkemans M, Macklon N, Boivin J. The impact of a self-administered coping intervention on emotional well-being in women awaiting the outcome of IVF treatment: a randomized controlled trial. Hum Reprod. 2014;29(7):1459–1470. doi: 10.1093/humrep/deu093. [DOI] [PubMed] [Google Scholar]
- 53.Boivin J. A review of psychosocial interventions in infertility. Soc Sci Med. 2003;57(12):2325–2341. doi: 10.1016/s0277-9536(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 54.Domar AD, Rooney KL, Wiegand B, Orav EJ, Alper MM, Berger BM, et al. Impact of a group mind/body intervention on pregnancy rates in IVF patients. Fertil Steril. 2011;95(7):2269–2273. doi: 10.1016/j.fertnstert.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 55.Zhou R, Cao YM, Liu D, Xiao JS. Pregnancy or psychological outcomes of psychotherapy interventions for infertility: a meta-analysis. Front Psychol. 2021;12(March):1–12. doi: 10.3389/fpsyg.2021.643395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sormunen T, Karlgren K, Aanesen A, Fossum B, Westerbotn M. The role of social media for persons affected by infertility. BMC Womens Health. 2020;20(1):1–8. doi: 10.1186/s12905-020-00964-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perone HR, Herweck AM, Stump HM, Levine HM, Wong AJ, Carugno J. The virtual infertility community: a qualitative analysis of patient experiences shared on Instagram. J Assist Reprod Genet. 2021;38(3):613–620. doi: 10.1007/s10815-020-02028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sexton MB, Byrd MR, O'Donohue WT, Jacobs NN. Web-based treatment for infertility-related psychological distress. Arch Womens Ment Health. 2010;13(4):347–358. doi: 10.1007/s00737-009-0142-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during this study are available from the corresponding author on reasonable request.