Abstract
Inflammation is an essential immune response critical for responding to infection, injury and maintenance of tissue homeostasis. Upon injury, regenerative inflammation promotes tissue repair by a timed and coordinated infiltration of diverse cell types and the secretion of growth factors, cytokines and lipids mediators. Remarkably, throughout evolution as well as mammalian development, this type of physiological inflammation is highly associated with immunosuppression. For instance, regenerative inflammation is the consequence of an in situ macrophage polarization resulting in a transition from pro-inflammatory to anti-inflammatory/pro-regenerative response. Immune cells are the first responders upon injury, infiltrating the damaged tissue and initiating a pro-inflammatory response depleting cell debris and necrotic cells. After phagocytosis, macrophages undergo multiple coordinated metabolic and transcriptional changes allowing the transition and dictating the initiation of the regenerative phase. Differences between a highly efficient, complete ad integrum tissue repair, such as, acute skeletal muscle injury, and insufficient regenerative inflammation, as the one developing in Duchenne Muscular Dystrophy (DMD), highlight the importance of a coordinated response orchestrated by immune cells. During regenerative inflammation, these cells interact with others and alter the niche, affecting the character of inflammation itself and, therefore, the progression of tissue repair. Comparing acute muscle injury and chronic inflammation in DMD, we review how the same cells and molecules in different numbers, concentration and timing contribute to very different outcomes. Thus, it is important to understand and identify the distinct functions and secreted molecules of macrophages, and potentially other immune cells, during tissue repair, and the contributors to the macrophage switch leveraging this knowledge in treating diseases.
Keywords: acute injury, Duchenne muscular dystrophy, macrophage, regenerative inflammation, tissue repair
The crosstalk between inflammation and regeneration
Tissue repair and regeneration are conserved biological processes critical for survival. All species are able to regenerate [1], allowing the renewal and restoration of damaged cells, tissues, organs and even entire body parts. It is mediated by the differentiation and specification capacity of adult stem cells [2–4] and the contribution of cell proliferation of both, progenitors and fully differentiated cells. One example of this phenomenon is observed in amphibian limb regeneration after amputation [5,6]. Similarly, in mammals, upon injury or trauma liver repair depends on multipotent liver stem cells [7] and the induction of proliferation of hepatocytes that in basal conditions are low-proliferating cells [8]. However, these processes are late events responsible for the replacement of lost tissue. Prior to renewal, immune cells carry out functions essential for proper tissue repair, such as clearance of the injured area [9] and a subsequent inflammation, termed, regenerative inflammation [10–12]. Regenerative inflammation is associated with an immunosuppressive and pro-regenerative response generated by monocyte-derived macrophages that promote tissue repair. This unique type of inflammation is characterized by the secretion of growth factors such as platelet-derived growth factor [13], insulin-like growth factor 1 (IGF-1) [14], growth differentiation factor 3 (GDF3) [15] and GDF15 [16], vascular endothelial growth factor-α [17] and transforming growth factor beta (TGF-β) [18] supporting the remodelling of the tissue and the production of anti-inflammatory cytokines like interleukin-10 (IL-10) [19].
The targeted depletion of monocyte and macrophages during tissue repair from salamander [20] to mammals [21,22] highlights the essential role of these cells as orchestrators of regeneration and tissue repair. Upon damage, monocyte-derived macrophages together with neutrophils phagocytize necrotic, dead cells and debris. Additionally, macrophages are known to interact with other cells promoting the proliferation of adult stem cells at early stages post-trauma [10]. Importantly, macrophages undergo an in-situ specification into two subpopulations, first during an initial pro-inflammatory phase that converts into an anti-inflammatory/pro-regenerative one later [23,24]. This functional switch of macrophages tightly follows and most likely induces regenerative inflammation, a process that foments the growth and differentiation of adult stem cells allowing the replacement of the empty space in the injured area [10].
Key remaining questions in the field are what molecular mechanisms actively regulate the macrophage transitional switch and how different functional subtypes of macrophages affect regenerative processes. To answer both, several studies compared the two phases of the macrophage switch (pro-inflammatory and regenerative) with the well-known in vitro characterization of M1/M2 macrophages [25–27]. There are similarities in the cytokine production of M1 macrophages and the initial pro-inflammatory monocyte-derived macrophages, whereas the repair macrophages are more alike to M2 macrophages [28]. Nonetheless, single cell RNA sequencing (Sc.RNASeq) in lung [29,30], liver [31] or skeletal muscle [16,32–35] suggests that the molecular profile, the response and the functional heterogeneity found in vivo is much more complex as the broad classification of M1/M2. Therefore, the subclassification of macrophages, their distinct functions and secreted molecules and localization are actively pursued inquiries in the tissue repair field.
In this review, we will focus on how the immunosuppressive response is conserved throughout evolution and mammalian development and how it is highly correlated with an increasing regenerative capacity. In the same vein, we cover the differences during regenerative inflammation across an acute physiological condition and chronic pathological process using skeletal muscle as our example. Skeletal muscle is a tissue of great interest in regenerative medicine for its highly efficient regeneration and repair capacity upon acute injury. However, Duchenne Muscular Dystrophy (DMD) is characterized by progressive muscle loss and weakness due to the alterations of the protein dystrophin and an induced ongoing regenerative inflammation. The comparison of these two processes can be used effectively for the identification of targetable pathways and molecules playing roles in regenerative inflammation potentially answering why the acute and the chronic progression have entirely completely different impacts on muscle regeneration.
Immunosuppressive immune response correlates with a higher regenerative capacity: Examples from evolution to development
Regenerative inflammation and its immunosuppressive response are conserved processes present from metazoans and salamanders with a primitive immune system to organisms with a highly evolved immune systems like mammals. This is exemplified by the remarkable regenerative capacity of simple organisms [36,37] that possess an immune system with low immunocompetence during tissue repair [38,39]. It is further accentuated by the fact that organisms with a more complex immune system acquire a much broader role in self-defence while their regeneration capacity is gradually lost [40]. This suggests that the ability of immune cells to recognize and react to external agents contrasts with their support in tissue repair.
For instance, planarian or annelid worms are able to rebuild their entire body [41,42] with an immune system based on phagocytic cells and immunomodulators used as immunosuppressive therapy in humans [43]. Alike the immune system in humans, salamanders possess a complex network of innate and adaptive immune cells [44]. However, it still has low specificity against pathogens and the regulatory pathways are rather rudimentary compared to mammalians [45]. Interestingly, this translates into higher regenerative capacity compared to mammals but lower than metazoans being able to regenerate some body parts: lens, retina, heart, central nervous system and appendages after amputation [20]. The contribution of the adaptive immune system in regeneration has been described in Zebrafish as well. This animal model has been used to study tissue repair for the animals’ ability to repair spinal cord [46], heart [47], brain [48] and skin [49]. As it has been shown also in mammals, the role of T cells, in particular, regulatory T cells is to contribute to immunosuppression and to promote the macrophage switch [50,51]. Both events correlate with the regenerative phase, nonetheless, regeneration in this animal model relays on the initial infiltration of neutrophils [52,53] as well as the role of macrophages [54,55] during the entire process. In sharp contrast, mammals with highly efficient immune systems to combat pathogens can only regenerate efficiently injured skeletal muscles, peripheral nervous system and liver [56], and to a very limited capacity, other organs (Fig. 1). All these findings suggest that the immunosuppressive response of regenerative inflammation is linked with the regenerative ability and, at the same time, inversely correlates with immunocompetence.
Fig. 1.
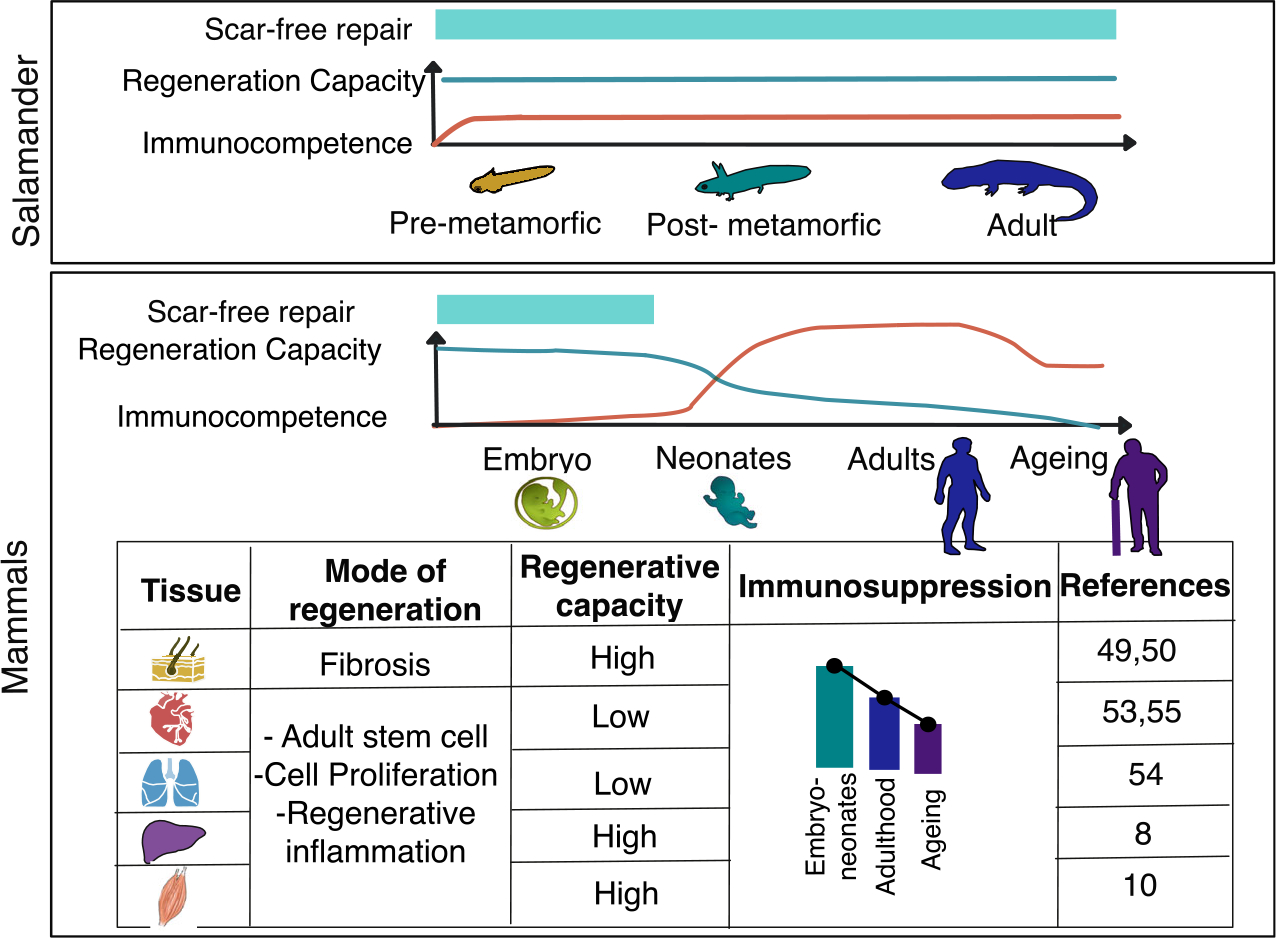
Regenerative inflammation across species (amphibians and mammals) and developmental stages. Comparing different organisms and their immune system as well as different stages during the lifespan in different organs suggests an inverse correlation between immunocompetence and tissue repair. Therefore, regenerative inflammation may be associated with an anti-inflammatory response besides the pro-regenerative capacity by the secretion of growth factors, cytokines, and lipid mediators from immune cells.
Similarly, during mammalian development, embryos, neonates and adult immune cells have different origins, functions and responses [57]. Embryos and neonates have a more immunosuppressive immune system in order to avoid an immunogenic response to maternal alloantigens [58], while the immune system is more specialized and has a potent immune response during childhood and adulthood to eliminate harmful substances. Through lifespan, the origin, function and immune response of immune cells change dramatically declining during ageing [57].
Strikingly, as in evolution, the lower immunocompetence in embryos and neonates correlates with higher regeneration capacity [38,39] as reported by the scar-free healing capacity in skin [59,60] or heart in the early stages of life (Fig. 1). Scarring is a consequence of a fibrotic process regulated by regenerative inflammation. In the last phase of regenerative inflammation macrophages [61] and other cell types like fibroblast or endothelial cells secrete TFG-β [62] promoting a controlled production of extracellular matrix to rebuild the normal tissue structure [51]. However, when regenerative inflammation fails the connective tissue replaces normal parenchymal tissue, a mechanism that is well tolerated during adult wound healing, but it may lead to loss of function and death in organs like heart [63] or lung [64]. Surprisingly, in mammals, scaring is not observed in embryos and neonates. For instance, in mice, wounds can repair scar-free until E18.5, and in humans up to 24 weeks [59].
Another example of scarless tissue repair is observed in heart [65]. Both neonatal and adult cardiac repair are highly dependent on the coordinated response in regenerative inflammation in which macrophages have a major role [66,67]. However, in neonates, cardiac repair is determined by resident macrophages derived from the yolk sac with self-renewal properties while in adults it is regulated by monocyte-derived macrophages [66]. These two types of macrophages are qualitatively and quantitatively different, explaining the correlation between the higher regenerative capacity and the immunosuppressive response in neonatal cardiac repair [66,67]. On the contrary, in adults, monocyte-derived macrophages promote differentiation of fibroblast and proliferation of stromal cells, increasing the production of extracellular matrix, and therefore, causing fibrosis [68]. Neonates can repair scar-free cardiac tissue until day 7 after birth [67], the difference between scar-free repair and the fibrotic process is an increasing concentration of proinflammatory cytokines such as Ccl2, Ccl3, Ccl4 and Cxcl2 [67]. As the result, regenerative inflammation is affected by the dynamic changes in gene regulation and adaptation of immune cells through lifespan as shown by the differences between resident macrophages in neonates and monocyte-derived macrophages in adults and the different outcomes in the progression of cardiac repair [66].
With ageing immune response goes through a series of transformations including immune senescence, maladaptation of tissues and a tendency to pro-inflammatory response with deviations from a normal inflammation [69]. This results in, for example, chronic low-grade inflammation in mice and human lungs, where prolonged inflammation hinders intrinsic cellular repair after injury and exacerbates organ damage. Pulmonary fibrosis is characterized by weakened anti-inflammatory activation, and aberrant resolution leading to excessive production and disorderly deposition of extracellular matrix proteins and collagen [70].
All these findings illustrate the strong correlation between highly efficient regeneration and an anti-inflammatory response. In the same manner, highly complex organisms pay the evolutionary price of having lower tissue repair capacity at the expense of extensive protection against infections. One wonders if the initial pro-inflammatory phase is necessary for tissue repair, or it is partly inhibiting regeneration. However, depletion of circulating or infiltrating monocytes in charge of this response results in impaired regeneration from salamanders [20] to mammals [21,71]. This highlights that tissue repair is a complex process orchestrated by the adaptation, polarization and secreted factors of immune cells highly coordinated regarding the amount in space and time.
Skeletal muscle, a highly regenerative tissue upon acute injury
Skeletal muscle has an astonishing regenerative capacity upon acute injury. In various sports, athletes are frequently exposed to different lesions (e.g. lacerations, strains, and contusions) [72]. However, in most cases, only time is needed to completely recover the function of the tissue with no further consequences. As previously mentioned, lung or heart regenerative inflammation undergoes a fibrotic process leading to clinical complications or even death, but upon acute injury, fibrosis is rarely observed in skeletal muscle (Fig. 1). One contributor to the highly efficient muscle repair are muscle satellite cells (MuSCs), adult stem cells that, upon injury, leave their quiescent state to form myoblasts that ultimately rinse to small centrally nucleated fibres replacing the lost tissue [73]. Although MuSCs commitment to myogenic lineage plays a crucial role in muscle regeneration, the interplay between these cells and the neighbouring ones, including immune cells [23], fibroblasts and vascular cells, like endothelial cells [74], is also necessary for proper tissue regeneration.
Regenerative inflammation is regulated by highly coordinated switches
Different murine acute injury models have been used to study muscle regeneration [75,76]. All of them can replicate the regeneration process common in humans, starting with necrosis of muscle fibres, followed by a pro-inflammatory response, and finishing with regenerative inflammation [77]. However, there are some differences in terms of kinetics of regeneration, loss of satellite cells and the effect on immune cells [75,76]. Specifically, the use of cardiotoxin (CTX) injection can induce a higher infiltration rate of immune cells [78] compared to other models. Thus, CTX is used in studies describing the ad integrum regeneration and its physiological inflammatory response.
Upon muscle injury, ‘damage-associated molecular patterns’ (DAMPs), molecules released by necrotic cells, are recognized by immune cells as an alarm signal. Some examples of DAMPs are proteins from the extracellular matrix such as biglycan, versican and heparan sulfate, free DNA product of netosis, intracellular proteins such as histones, high-mobility group box 1, S100 proteins and heat-shock proteins or plasma proteins such as β2-Glycoprotein I [79]. The recognition of DAMPs through ‘pattern recognition receptor’ promotes the recruitment and activation of circulating innate immune cells, being neutrophils and monocytes the first ones to infiltrate the damaged tissue [80]. From there the dynamic process of muscle repair can be distinguished into three stages: (a) pro-inflammatory (b) resolution and (c) repair (Fig. 2). The initial pro-inflammatory response is characterized by the clearance of necrotic, dead cells and debris. Resolution and remodelling are two stages of regenerative inflammation. Days post-injury, macrophage polarization and the subsequent in situ specification convert the inflammatory response to immunosuppressive starting with a series of metabolic, epigenetic and transcriptional changes characteristic of the resolution phase. During repairing, macrophages secrete cytokines such as IL-10 [81,82] or TFG-β [83], growth factors such as IGF-1 [14], GDF3 [15,84] and GDF15 [16] and lipids mediators such as Resolvin D2 [85] changing the niche and promoting regeneration.
Fig. 2.
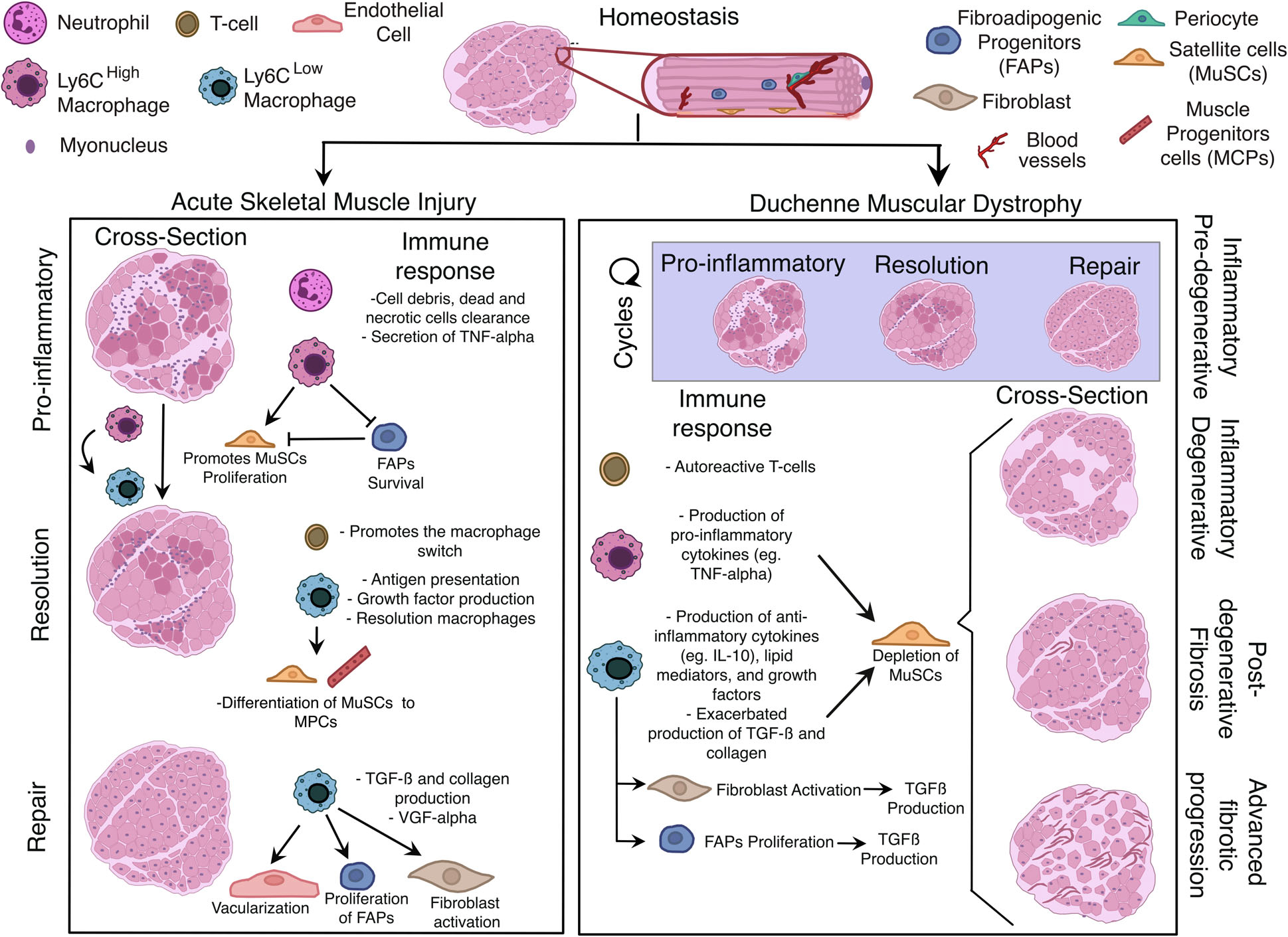
Regenerative inflammation changes after recurrent damage. Schematic representation of efficient physiological (acute muscle injury) and unresolved, chronic regenerative inflammation (DMD). While in acute muscle injury, regenerative inflammation consists of the coordinated response of immune cells, a timed macrophage polarization and the interaction of different macrophage subsets with other cell types, in DMD the response is completely disjointed and out of synchronization. After several cycles of regeneration and repair, skeletal muscle repair enters in a degenerative inflammation result of the continuous infiltration of monocyte and the exhaustion of MuSCs. The ongoing inflammation and the uncoordinated opposing signalling results in a fibrotic process and eventually muscle loss.
Specifically in the muscle, the pro-inflammatory stage starts few hours after injury, and it is kept until day 2–3 post-injury. It overlaps with the neutrophilic infiltration which reaches its peak at 12–24 h post-injury and is present until 3–4 days post-injury [86]. Depletion of neutrophils delays muscle regeneration [87] underpinning their role in phagocytosis of necrotic material and recruitment of monocytes. However, monocytes and macrophages have a large impact on tissue repair, depletion of monocytes by liposome-mediated monocyte/macrophage depletion [88,89] or Cd11b + monocytes/macrophages in CD11b-DTR (diphtheria toxin receptor) mice [59,90]. In the same manner, the inhibition of monocyte infiltration applying Ccr2 knock-out mice [91,92] mouse model or the neutralization of granulocyte/macrophage colony-stimulating factor receptor [93] results in impaired muscle repair showing the indispensable role of macrophages as orchestrators of muscle regeneration. Among all immune cells, monocytes and later macrophages are the major cell compartments present across the whole process.
Monocytes infiltrate the tissue after a few hours and differentiate towards macrophages. Bulk RNASeq from sorted Ly6Chi F4/80+ (pro-inflammatory monocyte-derived macrophages) show high expression of tumour necrosis factor alpha (TNFα) and interleukin 1 beta (IL-1β) [94,95]. However, regenerative inflammation is determined by the transient conversion into Ly6Clow F4/80+ (repair anti-inflammatory monocyte-derived macrophages) with high expressing levels of IL-10 [94,95]. This macrophage polarization, termed, macrophage switch starts at day 2 post-injury and it is completed at day 4 post-injury (Fig. 2) leading to the development of distinct effector functions on macrophages. The initiation of the immunosuppressive response correlates with the start of regenerative capacity, and it is maintained until the full recovery of the tissue at day 8 post-injury, according to histological analysis [95]. Although the macrophage switch has a direct effect on tissue repair it also has an indirect effect by changing the niche and cell–cell interactions. For instance, repair macrophages’ peak number overlaps with the highest number of T cells, mostly regulatory T cells (Tregs). Depletion of these cells delays muscle repair and prolongs inflammation [96–98]. This suggests that regenerative inflammation promotes an extensive immunosuppressive response which correlates with the regenerative ability.
Using skeletal muscle acute repair as a model of complete, ad integrum or physiological repair, we can conclude that successful repair depends on the coordinated response of immune cells. Especially important is the macrophage switch determining the transition from pro-inflammatory response to regenerative inflammation. Therefore, to understand regenerative inflammation it is crucial to identify the factors governing macrophage polarization. The macrophage switch is the result of rapid transcriptional and metabolic changes [99,100] (Fig. 3) modifying gene expression, and therefore the effector functions. Metabolic changes across macrophage switch are mostly studied in vitro by the comparison of M1/M2 macrophages leaving uncertainty on the processes taking place in vivo [101]. While pro-inflammatory M1 macrophages are associated with glycolytic metabolism and impaired mitochondrial oxidative phosphorylation (OXPHOS), M2s are characterized by an upregulation of genes involved in glutamine metabolism, associated with OXPHOS [102] and the secretion of iron [103]. All these changes meet the requirements and adaptation to the different functions carried out by the distinct subpopulations of macrophages (Fig. 3). For instance, some of these in vitro findings can be extrapolated to Ly6Chi and Ly6Clow F4/80+ macrophage populations in vivo [104,105]. In cancer, myeloid-derived suppressor cells (MDSCs) can differentiate into M1 and M2-like macrophages showing differences in the metabolic pathways between the two subpopulations [105]. During maturation and activation, these tumour-derived MDSCs exhibit an increase in central carbon metabolism, including glycolysis, the pentose-phosphate pathway, and the TCA cycle enhanced with the production of anti-bactericidal substances like ROS. Additionally, two breaks on the TCA cycle result in the accumulation of itaconate and succinate stabilizing the expression of HIF1-α and the subsequent production of IL1-β [104,105]. This population correlates with the Ly6ChiF4/80+ at days 1 and 2 post-injury when infiltrating monocytes differentiate into macrophages and the clearances of necrotic fibres take place [101,104]. However, more precise depletion models are needed to further characterize these changes in the muscle environment. On the contrary, during resolution, Ly6Clow F4/80+ macrophages are characterized by a strong upregulation of genes involved in glutamine metabolism, associated with oxidative metabolism increasing the production of ATP and affecting lipid metabolism. Correspondingly, the ratio of AMP/ATP via AMPKα1 pathway has a role during macrophage polarization [106].
Fig. 3.
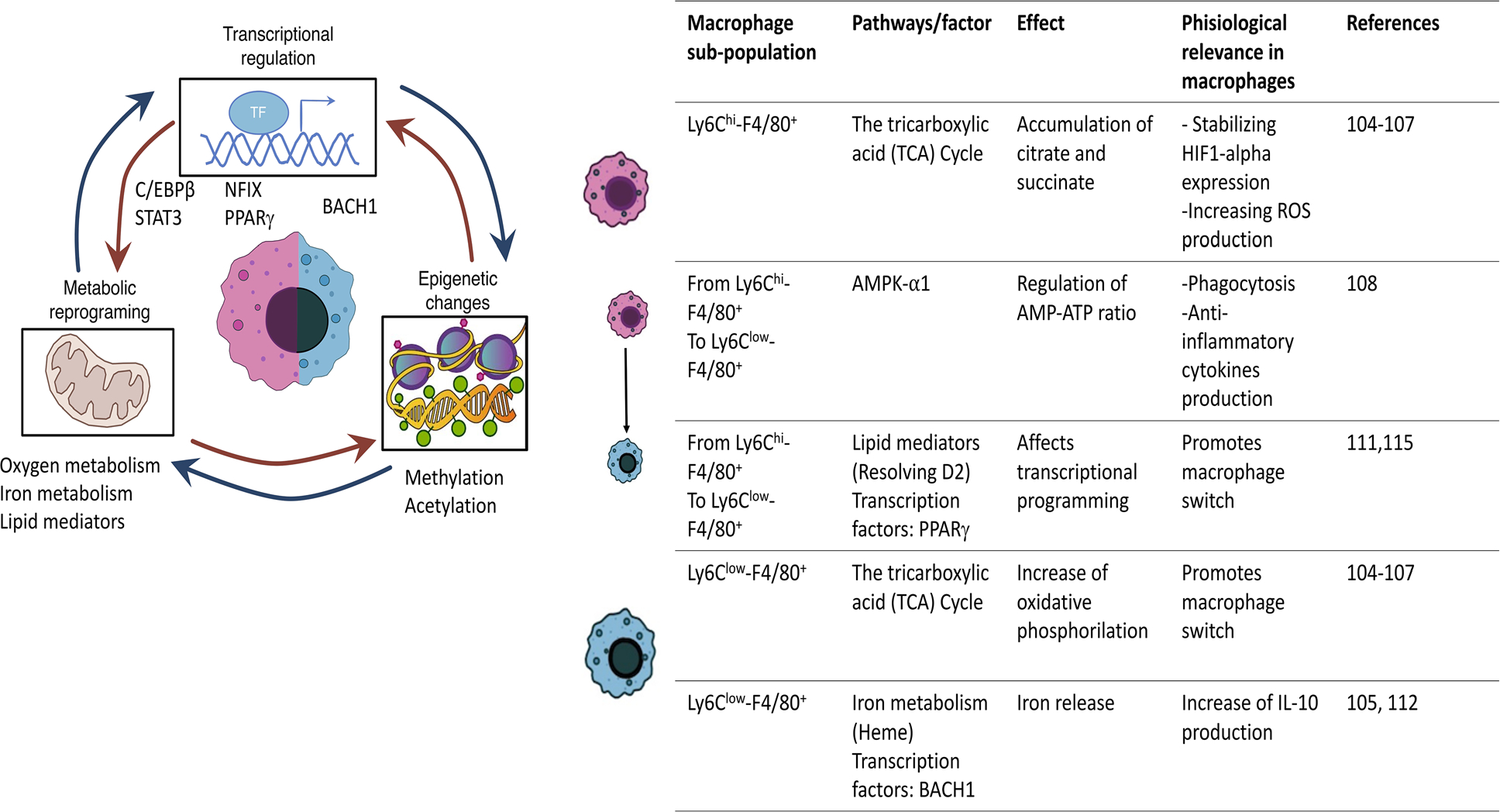
Mediators of the macrophage switch. A series of metabolic and transcriptional changes drive the conversion from pro-inflammatory monocyte-derived macrophages to anti-inflammatory pro-regenerative macrophages promoting tissue repair.
However, the metabolic variations alone cannot explain the magnitude of changes in gene expression observed by bulk RNASeq. Transcriptional regulation through transcription factors such as, C/EBPβ [107], STAT3 [108], NFIX [109], PPARγ [84] or BACH1 [110] and epigenetic changes [111–113] have been shown to contribute to the macrophage switch modifying macrophage gene expression, products and function. As a result of transcriptional changes, several signalling pathways involving cytokines [interleukin-6 (IL-6), IL-10], growth factors (IGF1) and lipid mediators (RvD1, RvD2, RvE1) [10,114] enhance further modifications necessary for the macrophage switch.
Macrophage switch translates into diverse cellular functions
Although gene expression changes between pro and repair macrophages have been identified by bulk RNASeq studies [94], FACS-sorted populations are not sufficient to characterize the different functions adopted by macrophages after specification. In this regard, Sc.RNASeq experiments have been able to deconvolute the cell types into populations classifying them by function and secreting molecules. These technologies have helped to elucidate the complexity of skeletal muscle repair, distinguishing cell types and their relative abundance [115]. In principle, uninjured and injured muscle is formed by the same cell types: satellite cells, fibroadipogenic (FAPs), pericytes, endothelial, immune and smooth muscle cells (Fig. 2). However, the number, the source of cells and their functions are constantly changing after injury until returning to ad integrum homeostasis.
The dynamic changes have been documented by several studies and the use of Sc.RNASeq on uninjured and injured mice at different time points posttreatment [16,32–35]. Focusing on the immune component, these studies clearly show the kinetics of regeneration, revealing the presence of immune cells from early onset injury until full recovery orchestrating the regeneration process. However, most of these studies have been carried out using the whole muscle which dramatically reduces the resolution of immune cells impeding the characterization of new populations. Our recent work [16] contributed to solve this issue by analysing exclusively CD45+ cell populations in Sc.RNASeq experiment and being able to identify four populations of macrophage subtypes at day 4 post-injury. These are, resolution macrophages, growth factor producing, pro-inflammatory and antigen presenting. Remarkably, these newly assigned functions can explain secondary effects observed in other studies. For instance, antigen-presenting capacity overlaps with T-cell infiltration peak while growth factors have been previously identified to induce and accelerate the growth of MCPs descendent cells of MuSC [71]. In the same manner, resolution macrophages highly express MerTK a gene associated with phagocytosis and Tgb1r the receptor of TFG-β influencing the polarization of the few remaining pro-inflammatory macrophages [116]. Given the limitation of single-cell approach other or more clusters can also be called, but this alignment between the features of the four clusters and the needed effector functions suggest that these are very likely the main macrophage populations.
Macrophages as conductors of tissue repair
Macrophage along with the onset of regenerative inflammation brings about changes between cell–cell interaction to the niche through changes in the microenvironment with great relevance to tissue repair [117]. Pro-inflammatory macrophages are known to induce adult stem cell proliferation before the macrophage switch while after they promote the growth and differentiation [10]. This fact highlights the importance of understanding not only the different functions of macrophages but how the secreted cytokines, growth factors and lipids affect other cells, especially, adult stem cells.
During acute injury, pro-inflammatory cytokines produced by neutrophils and Ly6Chi macrophages play an essential role in the clearance of debris and dead cells. TNFα is one of the first cytokines secreted activating the expression of pro-inflammatory genes in macrophages and correlates with ROS production [118]. The inhibition of TNFα cause impaired muscle regeneration [119]. Its signalling has a direct effect on MuSCs by epigenetically repressing Notch1 and Pax7 expression [120]. Additionally, Notch expression is also inhibited by ADAMTS1, a metalloproteinase secreted by Ly6Chi [121]. Notch signalling is required for the maintenance of MuSCs in a quiescent state, and its repression leads to the commitment of MuSCs to MPCs [122–124]. In conclusion, the expression of TNFα by monocyte-derived macrophages promotes MuSCs proliferation while inhibiting their differentiation. The secretion of TNFα at the early stages of the injury also contributes to regulate the number of FAPs inducing their apoptosis [125] (Fig. 2). Interestingly, IL-6 is highly expressed by infiltrating monocytes/macrophages from day 1 post-injury, and its expression continues up to day 7 [117,126]. Its depletion suppresses inflammation and impairs MPC proliferation and muscle regeneration [127,128]. This data suggests the important role of this cytokine throughout regeneration and transitional stages.
After the macrophage switch, the anti-inflammatory response associated with regenerative inflammation upregulates the expression IL-10 [129]. Interestingly, local delivery of this cytokine at early time points, when proinflammatory cytokine expression is predominant, reduced the size of newly forming fibres measured at day 7 post-injury. This indicates that the timely, sequential expression of pro- and anti-inflammatory cytokines produced by differentially activated macrophages is essential for proper tissue healing and regeneration. IL-10 production correlates with the deactivation of the pro-inflammatory macrophages and can the promote proliferation of non-myeloid cells. For instance, IL-10 cancelled the pro-proliferative effect of TNFα on SCs when the cells were simultaneously treated with the two cytokines [81]. Together with IL-10, during regenerative inflammation, growth factors are also up-regulated regulating the differentiation and proliferation MuSCs and non-myeloid cells. For example, FAPs’ proliferation is enhanced by the secretion of TGF-β secreted by repair macrophages [130]. However, the uncontrolled secretion of this growth factor leads to muscle fibrosis (Fig. 2).
These findings highlight the relevance of macrophages as conductors of tissue repair. Successful regeneration must undergo as sequential steps tightly controlled by regenerative inflammation. Dysregulation of the cell number or alterations in the macrophage switch can affect to the cell function and secretome leading to maladaptive and pathological processes such as chronic inflammation. One prime example is DMD.
A disjointed degeneration-regeneration cycle in DMD leads to chronic inflammation and fibrosis
Duchenne muscular dystrophy is an X-linked disease that affects 1 in 5000 males (20 000 cases per year) becoming the most commonly diagnosed dystrophy during childhood. The affected coding protein is dystrophin [131], in charge of connecting the interior of the cell to the extracellular matrix. The loss of this protein results in fragile muscle cells that are susceptible to contraction-induced injury. This process of cycled injury and regeneration ends in the incapacity of SCs to function properly and the continuum inflammation aggravates muscle loss promoting the replacement of the muscle fibres with fibrous tissue (Fig. 2).
Currently, the standard care to alleviate the constant inflammation is steroid treatment. Among the broad spectrum of corticosteroids, prednisone prescribed for children prevent the fast development of the disease prolonging the lifespan of patients [132–134]. A promising approach for treating this disease is the transfer of the dystrophin gene to restore its expression using a safe, non-pathogenic viral vector called adeno-associated viral vector [135]. However, recovery after the trials has been mild converting the fatal DMD into a milder phenotype similar to Becker Muscular Dystrophy [136]. The genetic therapy has to be administered together with immunosuppressors like corticosteroids [137]. This highlights the influence of chronic inflammation on the course of DMD and the necessity of understanding the mechanism controlling the different switches mentioned above. In this regard, applying the knowledge acquired from studying acute muscle regeneration can be of great help in order to identify new target pathways within the inflammation process.
When comparing acute muscle repair with DMD the progression and outcome are quite distinct. The first symptoms of DMD start in early childhood, around the age of 2–3 years old in humans, with skeletal muscle degeneration and weakness being the primary cause of dystrophin deficiency. Collectively, repeated cycles of necrosis and regeneration of muscle fibres trigger a strong immune response [138]. As a consequence, patients lose the ability to walk by the age of 12 having a life expectancy of 30–40 due to cardiac or respiratory dysfunction [139]. The same progression of the disease can be observed in mouse models where inflammation can be categorized into four stages: (a) inflammatory pre-degenerative, (b) inflammatory degenerative, (c) post-degenerative fibrosis and (d) advanced fibrotic progression (Fig. 2).
There are several animal models lacking functional dystrophin but not all of them can replicate exactly the symptoms observed in humans. For instance, mdx mice have minimal clinical symptoms and their lifespan is only reduced by ~ 25%, in contrast with the reduction of approximately 75% in humans [140,141]. The background of mice also has an impact on the phenotype, while the dystrophin-deficient mdx mouse on the C57BL/10 genetic background (B10.mdx) is mildly affected, a more severe muscle disease is observed when the mdx mutation is crossed onto the DBA/2 J genetic background (D2.mdx). Thus, the choice of model is critical to establish and study the desired mechanism. DBA/2-mdx mice are thought to better represent human disease because they display more fibrosis and less regeneration [142]. However, the DBA/2 strain carries mutations in at Tyrp1 (Tyrp1b), Gpnmb (GpnmbR150X), Klrd1 [143] and overexpression of TGF-β signalling [144], likely contributing to a changed immune milieu and more human-like disease progression.
Thus, DMD is characterized by an underlying chronic inflammation. Similar to acute muscle injury, the initial stages of DMD without damaging symptoms, is a necrotic injury controlled by a macrophage switch from pro-inflammatory macrophages that within them acquire anti-inflammatory/resolution phase. Both immunophenotypes are present in high numbers as shown by the higher number of macrophages marked as F4/80+ and a higher ratio of Cd11bhigh/Cd11blow population [145] compared to WT. Similar results were observed by single nuclei RNASeq (Sn.RNASeq) in mice and in Sc.RNAseq in rats [146] where the incremental number of macrophages is also shown [147]. In addition, there are substantial subpopulations of intramuscular macrophages exhibiting a mixed population of pro-inflammatory and anti-inflammatory/resolution macrophages [148–150]. The continuous infiltration and the presence of both signalling responses (pro and anti-inflammatory) end in an unbalanced number of cells. Additionally, the uncoordinated response causes multiple dysfunctions such as mitochondrial alterations, impairment in autophagy and angiogenesis. The alteration of these functions affects the metabolic and transcriptional reprogramming proper from the macrophage switch. As a result of the changes in macrophage polarization, the secreting molecules and how macrophages interact with the environment end in an aberrant regenerative inflammation influencing the outcome of the disease. Another major difference in inflammation comes from the adaptive immune response. Upon acute injury, the participation of the T-cell injury is limited to the infiltration of Tregs that enhance an immunosuppressive microenvironment and promote the macrophage switch. Recent studies based on depletion strategies also show the role of γδ T-cells during repair [151,152]. In ischaemic model, the depletion of these cells showed a higher number of pro-inflammatory macrophages and a reduction in endothelial cell proliferation, therefore, having an effect on angiogenesis [151] while in hindlimb CTX model, the depletion affected the proliferation of fibre prolonging the time of recovery [152]. However, the adaptive immune response in degenerating muscles like DMD, involves more subtypes of T cells incrementing the disturbances in the niche. Numerous observations have suggested that the presence of specific muscle autoantigens may drive the expansion of T lymphocytes and their activation [153].
The uncontrolled regenerative inflammation has qualitative and quantitative effects on cytokines and chemokines associated DMD pathology and disease progression. For instance, increased expression of TNFα, mainly produced by macrophages, was detected DMD muscle biopsies [154]. The high level of this cytokine correlates with histopathology damage observed in the diaphragm of mdx mice at the early stage (1 and 4 months of age) [155]. However, in dystrophic muscle the expression of TNFα is not inhibited differing from acute injury where TNFα is produced only in the first stage. Treatment with infliximab (a TNFα inhibitor) at late time-points shows a delayed appearance and improvement of muscle damage in DMD [156]. The opposite effect was observed after the complete depletion of TNFα in mdx [157]. These results show the important role of TNFα as it also has been proven in acute injury models, nonetheless, a high concentration of this cytokine in inadequate timing inhibits muscle regeneration. Other examples of exacerbated expression of pro-inflammatory cytokines by Ly6Chi macrophages in DMD are IL-1β and IL-6. Specifically, blockade of IL-6 with monoclonal antibody increase inflammation in mdx mice [158] while increased levels of IL-6 exacerbate the dystrophic muscle phenotype in mdx mice [159].
TGF-β plays important roles in inflammation, cell growth and tissue repair but it also contributes to the fibrotic process and accumulation of extracellular matrix which is a distinct process in muscular dystrophies but not acute injury. The elevated expressions of TGF-β, produced by CD206+ repair macrophages, are consistently reported in many studies [160,161], in an age-related manner increases of TGF-β causing increased fibrotic replacement of dystrophic tissue [162]. Interestingly, TGF-β also acts as a significant suppressor of the immune response in dystrophic muscles, as determined by antibody-mediated depletions of TGF-β which results in a dramatic increase in CD4+ T cells concentration in mdx diaphragm muscles. Thus, the elevated expression of TGF-β may suppress the inflammatory response in dystrophic muscle, but ultimately contribute to muscle fibrosis [163]. In addition, TGF-β secretion promotes the proliferation of FAPs during acute muscle injury. FAPs are also able to secrete high levels of TGF-β exacerbating the fibrotic progression (Fig. 2).
Regeneration and diseases beyond muscle
Although muscle is a great example to compare acute and chronic regenerative inflammation, it is not the only tissue where regenerative inflammation contributes to resolve regeneration. Every organ is susceptible to be impacted by damage, from infection to pathogen-free injury such as soft tissue damage affecting muscles, ligaments and tendons, by sprain, strain or contusion, ischemia or environmental conditions like ‘skin burn’ after high exposure to UVA and UVB ray. After these perturbances, successful regeneration requires a balanced immune cell response, with the recruitment of accurately polarized immune cells in an appropriate quantity. For instance, liver regeneration [164], heart repair after myocardial infraction [165] or wound repair [166–168] are also dependent on an initial pro-inflammatory response followed by a pro-regenerative one in a process orchestrated by macrophages and other immune cells. However, the immune system does not always perform a complementary role in regeneration and alterations in timing course can cause an unresolved or ongoing inflammation that could result in fibrosis or, in severe cases, chronic diseases [169]. For instance, resident macrophages are capable to recognize exogenous agents, such as iron oxide [170,171], silica dioxide or asbestos [172] generating an increasing amount of ROS. Although ROS is an effective way to eradicate pathogens, in sterile inflammation results in tissue destruction, fibroblast proliferation, aberrant collagen accumulation and finally, fibrosis [173].
Other compounds like calcium pyrophosphate or monosodium urate can crystalize inside the joins being recognized and external dangerous agents by neutrophils and monocytes derived-macrophages. This ends in inflammatory response, as it happens in other tissues and there is an abnormal tissue repair monocyte-derived macrophages can promote the differentiation of fibroblast in an uncontrolled manner which causes fibrosis and cartilage destruction, over time, the recurrent inflammatory response can damage the affected joint leading to chronic arthritis [174,175]. Endogenous molecules can be also recognized by the immune cells. For example, cholesterol crystals are phagocytized by macrophages, activating and recruiting immune cells, that, together with endothelial cell dysfunction and plaque formation end in atherosclerosis [176]. Ischaemia–reperfusion is considered an injury caused by the change in oxygen influx to cardiomyocytes endlessly affecting a normal mitochondria function therefore energy consumption which is key for the myofibril contraction of the heart. In response to the trauma, there is a neutrophil infiltration at the ischemic area, producing ROS, this excavates the injury causing microvascular obstruction and local and eventually systemic inflammation [177,178]. In other cases, the cause of the inflammation is an abnormal function of the tissue that results in an imbalance of one or several physiological properties and harms the homeostasis of the tissue. That is the case of diabetes type 2 [179] or DMD in which the inflammation could persist for months or years. This prolonged continuous inflammation has consequences systemically leading to impaired regeneration capacity. For instance, diseases like diabetes type II or unhealthy metabolic conditions like obesity are associated with impaired wound healing [180], and muscular atrophy or dystrophy [181]. As in DMD, the unbalanced inflammation may result in fibrosis and affects essential functions such as, hypoxia response [182,183] or macrophage polarization [184].
Obesity is also linked with sarcopenia, an age-related loss of muscle mass and function, cellular senescence being a common process during obesity and ageing. Accelerated cellular senescence may impact macrophages, fibroblasts or endothelial cells [185] resulting in multiple changes such as telomere shortening, accumulated DNA damage or oxidative stress. These cells secrete distinct factors that contribute to increase oxidative stress, multiplying their number and perpetuating inflammation. Although the immune system is responsible for eliminating senescent cells, but its elimination capacity also decreases with ageing and maladaptive metabolic conditions such as obesity, as a result, homeostasis becomes unbalanced leading to an increase in cells with senescent phenotype creating a vicious cycle [186,187]. Under these conditions, one potential therapeutic approach is to complement the missing key components of the innate immune system such as macrophages or their secreted products to revert the aged inflammatory environment and milieu to a healthy, physiological one. An example is provided by our work studying aged mice (24–28 months old ones). Ageing causes a decrease in the number of regenerative macrophages and their production of the growth factors such as GDF3, which in turn results in delayed regeneration of skeletal muscle of these mice after CTX injury. Recombinant GDF3 supplementation alone can restore muscle function, therefore, it constitutes a potentially new therapeutic approach [15]. Additionally, accumulating evidence suggests that reprogramming or elimination of senescent cells could delay or even prevent several age-related diseases [188].
Conclusions and future perspective
Inflammation is commonly known as the process of self-protection through the recognition and reaction to external agents like bacteria or viruses. However, little is known about regenerative inflammation which participates in tissue building and promotes tissue repair after trauma and injury. Interestingly, many of the mechanisms that link inflammation to damage repair and regeneration in mammals are also observed in lower organisms, suggesting that it is an evolutionarily conserved process. Surprisingly, the immunosuppressive inflammatory response in lower organism as well as in early developmental stages (embryos and neonates) is linked with a higher regenerative capacity, whereas in adults, regeneration is tightly controlled by an initial pro-inflammatory response followed by the conversion of macrophages into anti-inflammatory resolution phase.
In regenerative medicine, there is an increasing need to identify cells and regulators implicated in regenerative inflammation. Macrophages have a major role in this type of inflammation being candidates for therapy [189]. However, there are many uncertainties in how macrophages can coordinate the response and how it can be triggered. A priority in the field is the identification and characterization of subpopulations based on their function and the understanding of the biological niche regulated by the different subpopulations using various single-cell and in vivo imaging technologies. Nonetheless, newer technologies like spatial transcriptomics could bring new information about cell localization and their interaction. Another important question are the mediators responsible for the macrophage switch. Using muscle acute muscle injury as a model timed switched with two distinct macrophage populations (pro and anti-inflammatory/repair) can be used to understand aberrant regenerative inflammation leading to fibrosis and disease like, in DMD. In vivo studies at different timepoints using commonly used technologies in metabolomics like NMR, gas chromatography–mass spectrometry or capillary electrophoresis–mass spectrometry could reveal important metabolic changes throughout the macrophage switch. In the same manner, sc.ATACSeq in combination with sc.RNASeq could be used to identify transcriptional changes and their regulators during macrophage polarization. The new advances can lead to the finding of new biomarkers and target molecules for precise therapy instead of using general drugs like immunosuppressors that inhibit the necessary inflammation.
Acknowledgements
The authors acknowledge the contribution of members of the Nagy laboratory, for reading the manuscript and for their comments especially, Dr. Krisztian Bene, Dr. Zsolt Czimmerer and Petros Tzerpos as well as Dr. Andreas Patsalos, Dr. Lee Sweeney and Dr. David Hammers for discussions on this topic. European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860034 supported N.C-S. Work in the laboratory of LN is supported by grants from the National Institutes of Health – National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK115924, R01-DK124782). S.A-A’s visit to the University of Debrecen was supported by Hungarian Scientific Research Fund KKP 129909.
Abbreviations
- AAV
adeno-associated viral
- BACH1
BTB and CNC homology 1
- C/EBP
Ccaat-enhancer-binding proteins
- CCL
chemokine (C-C motif) ligand
- CTX
cardiotoxin
- CXCL2
Chemokine (C-X-C motif) ligand 2
- DAMPs
damage-associated molecular patterns
- DMD
Duchenne muscular dystrophy
- FAPs
fibroadipogenic
- GDF3/15
Growth Differentiation Factor 3/15
- IGF-1
Insulin-like growth factor 1
- IL-10
Interleukin-10
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin-6
- INF
Interferon
- MDSCs
myeloid-derived suppressor cells
- MuSCs
muscle satellite cells
- NFIX
Nuclear factor 1 X-type
- OXPHOS
oxidative phosphorylation
- PPARγ
Peroxisome Proliferator-Activated Receptor gamma
- STAT3
Signal Transducer and Activator of Transcription 3
- TGF-β
Transforming Growth Factor beta
- TNFα
Tumour Necrosis Factor alpha
- VEGF-α
Vascular endothelial growth factor
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Holstein TW, Hobmayer E, Technau U. Cnidarians: an evolutionarily conserved model system for regeneration? Dev Dyn. 2003;226:257–67. [DOI] [PubMed] [Google Scholar]
- 2.Dulak J, Szade K, Szade A, Nowak W, Józkowicz A. Adult stem cells: hopes and hypes of regenerative medicine. Acta Biochim Pol. 2015;62:329–37. [DOI] [PubMed] [Google Scholar]
- 3.Suman S, Domingues A, Ratajczak J, Ratajczak MZ. Potential clinical applications of stem cells in regenerative medicine. In: Ratajczak MZ, editor. Stem cells. Cham: Springer International Publishing; 2019. p. 1–22. [DOI] [PubMed] [Google Scholar]
- 4.De D, Karmakar P, Bhattacharya D. Stem cell aging and regenerative medicine. In: Turksen K, editor. Cell biology and translational medicine. Volume 12. Cham: Springer International Publishing; 2020. p. 11–37. [DOI] [PubMed] [Google Scholar]
- 5.Zielins ER, Ransom RC, Leavitt TE, Longaker MT, Wan DC. The role of stem cells in limb regeneration. Organogenesis. 2016;12:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egger B, Gschwentner R, Hess MW, Nimeth KT, Adamski Z, Willems M, et al. The caudal regeneration blastema is an accumulation of rapidly proliferating stem cells in the flatworm Macrostomum lignano. BMC Dev Biol. 2009;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruno S, Herrera Sanchez MB, Chiabotto G, Fonsato V, Navarro-Tableros V, Pasquino C, et al. Human liver stem cells: a liver-derived mesenchymal stromal cell-like population with pro-regenerative properties. Front Cell Dev Biol. 2021;9:644088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. [DOI] [PubMed] [Google Scholar]
- 9.Westman J, Grinstein S, Marques PE. Phagocytosis of necrotic debris at sites of injury and inflammation. Front Immunol. 2020;10:3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patsalos A, Tzerpos P, Wei X, Nagy L. Myeloid cell diversification during regenerative inflammation: lessons from skeletal muscle. Semin Cell Dev Biol. 2021;119:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–30. [DOI] [PubMed] [Google Scholar]
- 13.Shimokado K, Raines EW, Madtes DK, Barrett TB, Benditt EP, Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985;43:277–86. [DOI] [PubMed] [Google Scholar]
- 14.Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, et al. Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther. 2015;23:1189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patsalos A, Simandi Z, Hays TT, Peloquin M, Hajian M, Restrepo I, et al. In vivo GDF3 administration abrogates aging related muscle regeneration delay following acute sterile injury. Aging Cell. 2018;17:e12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek SJ, Eling T. Growth differentiation factor 15 (GDF15): a survival protein with therapeutic potential in metabolic diseases. Pharmacol Ther. 2019;198:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–25. [DOI] [PubMed] [Google Scholar]
- 18.Grande JP. Role of transforming growth factor- in tissue injury and repair. Exp Biol Med. 1997;214:27–40. [DOI] [PubMed] [Google Scholar]
- 19.King A, Balaji S, Le LD, Crombleholme TM, Keswani SG. Regenerative wound healing: the role of Interleukin-10. Adv Wound Care. 2014;3:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simkin J, Sammarco MC, Marrero L, Dawson LA, Yan M, Tucker C, et al. Macrophages are required to coordinate mouse digit tip regeneration. Development. 2017;144:3907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goren I, Allmann N, Yogev N, Schürmann C, Linke A, Holdener M, et al. A transgenic mouse model of inducible macrophage depletion. Am J Pathol. 2009;175:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inflammation Chazaud B. and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. 2020;41:481–92. [DOI] [PubMed] [Google Scholar]
- 24.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–7. [DOI] [PubMed] [Google Scholar]
- 25.Witherel CE, Sao K, Brisson BK, Han B, Volk SW, Petrie RJ, et al. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials. 2021;269:120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez MM, Liu JC, Trujillo-de Santiago G, Cha B-H, Vishwakarma A, Ghaemmaghami AM, et al. Delivery strategies to control inflammatory response: modulating M1–M2 polarization in tissue engineering applications. J Control Release. 2016;240:349–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan Z, Wang Y, Chen Y, Liu Y, Ma M, Ma Z, et al. The dynamic feature of macrophage M1/M2 imbalance facilitates the progression of non-traumatic osteonecrosis of the femoral head. Front Bioeng Biotechnol. 2022;10:912133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Qin X, Qin H, Jia C, Yuan Y, Sun T, et al. Characterization of the heterogeneity of endothelial cells in bleomycin-induced lung fibrosis using single-cell RNA sequencing. Angiogenesis. 2021;24:809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukui T, Sun K-H, Wetter JB, Wilson-Kanamori JR, Hazelwood LA, Henderson NC, et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 2020;11:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Hu W, Shen Z, Liu T, Dai W, Shen B, et al. Dissecting the single-cell transcriptome underlying chronic liver injury. Mol Ther Nucleic Acids. 2021;26:1364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oprescu SN, Yue F, Qiu J, Brito LF, Kuang S. Temporal dynamics and heterogeneity of cell populations during skeletal muscle regeneration. iScience. 2020;23:100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giordani L, He GJ, Negroni E, Sakai H, Law JYC, Siu MM, et al. High-dimensional single-cell cartography reveals novel skeletal muscle-resident cell populations. Mol Cell. 2019;74:609–21.e6. [DOI] [PubMed] [Google Scholar]
- 34.Dell’Orso S, Juan AH, Ko K-D, Naz F, Perovanovic J, Gutierrez-Cruz G, et al. Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions. Development. 2019;146:dev174177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Micheli AJ, Laurilliard EJ, Heinke CL, Ravichandran H, Fraczek P, Soueid-Baumgarten S, et al. Single-cell analysis of the muscle stem cell hierarchy identifies heterotypic communication signals involved in skeletal muscle regeneration. Cell Rep. 2020;30:3583–95.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Yang H, Zhong TP. Regeneration across metazoan phylogeny: lessons from model organisms. J Genet Genomics. 2015;42:57–70. [DOI] [PubMed] [Google Scholar]
- 37.Cebriá F Planarians are here to stay and to teach us a lot on regeneration. Semin Cell Dev Biol. 2019;87:1–2. [DOI] [PubMed] [Google Scholar]
- 38.Xia H, Li X, Gao W, Fu X, Fang RH, Zhang L, et al. Tissue repair and regeneration with endogenous stem cells. Nat Rev Mater. 2018;3:174–93. [Google Scholar]
- 39.Godwin J The promise of perfect adult tissue repair and regeneration in mammals: learning from regenerative amphibians and fish: prospects & overviews. Bioessays. 2014;36:861–71. [DOI] [PubMed] [Google Scholar]
- 40.Zhao A, Qin H, Fu X. What determines the regenerative capacity in animals? Bioscience. 2016;66:735–46. [Google Scholar]
- 41.Rink JC. Stem cell systems and regeneration in planaria. Dev Genes Evol. 2013;223:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez Acosta VG, Arellano-Carbajal F, Gillen K, Tweeten KA, Zattara EE. It cuts both ways: an annelid model system for the study of regeneration in the laboratory and in the classroom. Front Cell Dev Biol. 2021;9:780422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller WEG. Origin of the metazoan immune system: identification of the molecules and their functions in sponges. Integr Comp Biol. 2003;43:281–92. [DOI] [PubMed] [Google Scholar]
- 44.Bolaños-Castro LA, Walters HE, García Vázquez RO, Yun MH. Immunity in salamander regeneration: where are we standing and where are we headed? Dev Dyn. 2021;250:753–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai SL. The molecular interplay between progenitors and immune cells in tissue regeneration and homeostasis. J Immunol Regen Med. 2020;7:100024. [Google Scholar]
- 46.Möllmert S, Kharlamova MA, Hoche T, Taubenberger AV, Abuhattum S, Kuscha V, et al. Zebrafish spinal cord repair is accompanied by transient tissue stiffening. Biophys J. 2020;118:448–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klaourakis K, Vieira JM, Riley PR. The evolving cardiac lymphatic vasculature in development, repair and regeneration. Nat Rev Cardiol. 2021;18:368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Xiao J, Xu X, Li W, Zhong R, Qi L, et al. M-CSF, IL-6, and TGF-β promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci Adv. 2021;7:eabb6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grada A, Mervis J, Falanga V. Research techniques made simple: animal models of wound healing. J Invest Dermatol. 2018;138:2095–105.e1. [DOI] [PubMed] [Google Scholar]
- 50.Kikuchi K New function of zebrafish regulatory T cells in organ regeneration. Curr Opin Immunol. 2020;63:7–13. [DOI] [PubMed] [Google Scholar]
- 51.Hui SP, Sheng DZ, Sugimoto K, Gonzalez-Rajal A, Nakagawa S, Hesselson D, et al. Zebrafish regulatory T cells mediate organ-specific regenerative programs. Dev Cell. 2017;43:659–72.e5. [DOI] [PubMed] [Google Scholar]
- 52.Martínez-Navarro FJ, Martínez-Morcillo FJ, de Oliveira S, Candel S, Cabas I, García-Ayala A, et al. Hydrogen peroxide in neutrophil inflammation: lesson from the zebrafish. Dev Comp Immunol. 2020;105:103583. [DOI] [PubMed] [Google Scholar]
- 53.Houseright RA, Miskolci V, Mulvaney O, Bortnov V, Mosher DF, Rindy J, et al. Myeloid-derived growth factor regulates neutrophil motility in interstitial tissue damage. J Cell Biol. 2021;220:e202103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohaud C, Johansen MD, Jorgensen C, Ipseiz N, Kremer L, Djouad F. The role of macrophages during zebrafish injury and tissue regeneration under infectious and non-infectious conditions. Front Immunol. 2021;12:707824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denans N, Tran NTT, Swall ME, Diaz DC, Blanck J, Piotrowski T. An anti-inflammatory activation sequence governs macrophage transcriptional dynamics during tissue injury in zebrafish. Nat Commun. 2022;13:5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elchaninov A, Sukhikh G, Fatkhudinov T. Evolution of regeneration in animals: a tangled story. Front Ecol Evol. 2021;9:621686. [Google Scholar]
- 57.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B. 2015;282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. 2014;10:1171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larson BJ, Longaker MT, Lorenz HP. Scarless Fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monavarian M, Kader S, Moeinzadeh S, Jabbari E. Regenerative scar-free skin wound healing. Tissue Eng Part B Rev. 2019;25:294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lech M, Anders H-J. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832:989–97. [DOI] [PubMed] [Google Scholar]
- 62.Branton MH, Kopp JB. TGF-β and fibrosis. Microbes Infect. 1999;1:1349–65. [DOI] [PubMed] [Google Scholar]
- 63.Piek A, de Boer RA, Silljé HHW. The fibrosis-cell death axis in heart failure. Heart Fail Rev. 2016;21:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng P, Li S, Chen H. Macrophages in lung injury, repair, and fibrosis. Cell. 2021;10:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111:16029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sansonetti M, Waleczek FJG, Jung M, Thum T, Perbellini F. Resident cardiac macrophages: crucial modulators of cardiac (patho)physiology. Basic Res Cardiol. 2020;115:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mogilenko DA, Shchukina I, Artyomov MN. Immune ageing at single-cell resolution. Nat Rev Immunol. 2022;22:484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venosa A Senescence in pulmonary fibrosis: between aging and exposure. Front Med. 2020;7:606462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Järvinen TAH, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–64. [DOI] [PubMed] [Google Scholar]
- 73.Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomès D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen B, Shan T. The role of satellite and other functional cell types in muscle repair and regeneration. J Muscle Res Cell Motil. 2019;40:1–8. [DOI] [PubMed] [Google Scholar]
- 75.Gayraud-Morel B, Chrétien F, Tajbakhsh S. Skeletal muscle as a paradigm for regenerative biology and medicine. Regen Med. 2009;4:293–319. [DOI] [PubMed] [Google Scholar]
- 76.Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, et al. Comparative study of injury models for studying muscle regeneration in mice. PLoS One. 2016;11:e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernandes TL, Pedrinelli A, Hernandez AJ. Muscle injury – physiopathology, diagnosis, treatment and clinical presentation. Rev Bras Ortop. 2011;46:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol. 2017;17:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. [DOI] [PubMed] [Google Scholar]
- 80.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardí M, Caelles C, Serrano AL, et al. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195:307–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han H, Li M, Liu H, Li H. Electroacupuncture regulates inflammation, collagen deposition and macrophage function in skeletal muscle through the TGF-β1/Smad3/p38/ERK1/2 pathway. Exp Ther Med. 2021;22:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delaney K, Kasprzycka P, Ciemerych MA, Zimowska M. The role of TGF-β1 during skeletal muscle regeneration. Cell Biol Int. 2017;41:706–15. [DOI] [PubMed] [Google Scholar]
- 84.Varga T, Mounier R, Patsalos A, Gogolák P, Peloquin M, Horvath A, et al. Macrophage PPARγ, a lipid activated transcription factor controls the growth factor GDF3 and skeletal muscle regeneration. Immunity. 2016;45:1038–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giannakis N, Sansbury BE, Patsalos A, Hays TT, Riley CO, Han X, et al. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat Immunol. 2019;20:626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ciciliot S, Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des. 2010;16:906–14. [DOI] [PubMed] [Google Scholar]
- 87.Kawanishi N, Mizokami T, Niihara H, Yada K, Suzuki K. Neutrophil depletion attenuates muscle injury after exhaustive exercise. Med Sci Sports Exerc. 2016;48:1917–24. [DOI] [PubMed] [Google Scholar]
- 88.Tirone M, Giovenzana A, Vallone A, Zordan P, Sormani M, Nicolosi PA, et al. Severe heterotopic ossification in the skeletal muscle and endothelial cells recruitment to Chondrogenesis are enhanced by monocyte/macrophage depletion. Front Immunol. 2019;10:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–95. [DOI] [PubMed] [Google Scholar]
- 90.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol. 2014;184:1167–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011;25:3344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez CO, McHale MJ, Wells JT, Ochoa O, Michalek JE, McManus LM, et al. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am J Physiol Regul Integr Comp Physiol. 2010;299:R832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, et al. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res. 2008;314:3232–44. [DOI] [PubMed] [Google Scholar]
- 94.Varga T, Mounier R, Horvath A, Cuvellier S, Dumont F, Poliska S, et al. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J Immunol. 2016;196:4771–82. [DOI] [PubMed] [Google Scholar]
- 95.Patsalos A, Pap A, Varga T, Trencsenyi G, Contreras GA, Garai I, et al. In situ macrophage phenotypic transition is affected by altered cellular composition prior to acute sterile muscle injury. J Physiol. 2017;595:5815–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castiglioni A, Corna G, Rigamonti E, Basso V, Vezzoli M, Monno A, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One. 2015;10:e0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panduro M, Benoist C, Mathis D. Treg cells limit IFN-γ production to control macrophage accrual and phenotype during skeletal muscle regeneration. Proc Natl Acad Sci U S A. 2018;115:E2585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fanucchi S, Domínguez-Andrés J, Joosten LAB, Netea MG, Mhlanga MM. The intersection of epigenetics and metabolism in trained immunity. Immunity. 2021;54:32–43. [DOI] [PubMed] [Google Scholar]
- 100.Van den Bossche J, O’Neill LA, Menon D. Macrophage Immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 101.Guo C, Islam R, Zhang S, Fang J. Metabolic reprogramming of macrophages and its involvement in inflammatory diseases. EXCLI J. 2021;20:628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jha AK, Huang SC-C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–30. [DOI] [PubMed] [Google Scholar]
- 103.Corna G, Campana L, Pignatti E, Castiglioni A, Tagliafico E, Bosurgi L, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Juban G, Chazaud B. Metabolic regulation of macrophages during tissue repair: insights from skeletal muscle regeneration. FEBS Lett. 2017;591:3007–21. [DOI] [PubMed] [Google Scholar]
- 105.Hu C, Pang B, Lin G, Zhen Y, Yi H. Energy metabolism manipulates the fate and function of tumour myeloid-derived suppressor cells. Br J Cancer. 2020;122:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mounier R, Théret M, Arnold L, Cuvellier S, Bultot L, Göransson O, et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–64. [DOI] [PubMed] [Google Scholar]
- 107.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, et al. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106:17475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL, et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab. 2020;2:278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saclier M, Temponi G, Bonfanti C, Messina G. Selective ablation of Nfix in macrophages preserves muscular dystrophy by inhibiting FAPs-dependent fibrosis. Cell Biol. 2021. 10.1101/2021.05.12.443809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patsalos A, Tzerpos P, Halasz L, Nagy G, Pap A, Giannakis N, et al. The BACH1–HMOX1 regulatory axis is indispensable for proper macrophage subtype specification and skeletal muscle regeneration. J Immunol. 2019;203:1532–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gosselin D, Glass CK. Epigenomics of macrophages. Immunol Rev. 2014;262:96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davis FM, Gallagher KA. Epigenetic mechanisms in monocytes/macrophages regulate inflammation in cardiometabolic and vascular disease. Arterioscler Thromb Vasc Biol. 2019;39:623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Markworth JF, Maddipati KR, Cameron-Smith D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc Immunol Rev. 2016;22:110–34. [PubMed] [Google Scholar]
- 115.Hedlund E, Deng Q. Single-cell RNA sequencing: technical advancements and biological applications. Mol Aspects Med. 2018;59:36–46. [DOI] [PubMed] [Google Scholar]
- 116.Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7:52294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nguyen JH, Chung JD, Lynch GS, Ryall JG. The microenvironment is a critical regulator of muscle stem cell activation and proliferation. Front Cell Dev Biol. 2019;7:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Collins RA, Grounds MD. The role of tumor necrosis factor-alpha (TNF-alpha) in skeletal muscle regeneration. Studies in TNF-alpha(−/−) and TNF-alpha(−/−)/LT-alpha(−/−) mice. J Histochem Cytochem. 2001;49:989–1001. [DOI] [PubMed] [Google Scholar]
- 119.Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, et al. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J. 2002;16:1630–2. [DOI] [PubMed] [Google Scholar]
- 120.Acharyya S, Sharma SM, Cheng AS, Ladner KJ, He W, Kline W, et al. TNF inhibits Notch-1 in skeletal muscle cells by Ezh2 and DNA methylation mediated repression: implications in duchenne muscular dystrophy. PLoS One. 2010;5:e12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Du H, Shih C-H, Wosczyna MN, Mueller AA, Cho J, Aggarwal A, et al. Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat Commun. 2017;8:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Conboy IM, Rando TA. The regulation of notch Signaling controls satellite cell activation and cell fate determination in postnatal Myogenesis. Dev Cell. 2002;3:397–409. [DOI] [PubMed] [Google Scholar]
- 123.Bjornson CRR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–52. [DOI] [PubMed] [Google Scholar]
- 125.Lemos DR, Babaeijandaghi F, Low M, Chang C-K, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–94. [DOI] [PubMed] [Google Scholar]
- 126.Fujita R, Kawano F, Ohira T, Nakai N, Shibaguchi T, Nishimoto N, et al. Anti-interleukin-6 receptor antibody (MR16–1) promotes muscle regeneration via modulation of gene expressions in infiltrated macrophages. Biochim Biophys Acta. 2014;1840:3170–80. [DOI] [PubMed] [Google Scholar]
- 127.Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33–44. [DOI] [PubMed] [Google Scholar]
- 128.Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stepien DM, Hwang C, Marini S, Pagani CA, Sorkin M, Visser ND, et al. Tuning macrophage phenotype to mitigate skeletal muscle fibrosis. J Immunol. 2020;204:2203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aartsma-Rus A, Ginjaar IB, Bushby K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J Med Genet. 2016;53:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gloss D, Moxley RT, Ashwal S, Oskoui M. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: report of the guideline development Subcommittee of the American Academy of neurology. Neurology. 2016;86:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iannitti T, Capone S, Feder D, Palmieri B. Clinical use of immunosuppressants in Duchenne muscular dystrophy. J Clin Neuromuscul Dis. 2010;12:1–21. [DOI] [PubMed] [Google Scholar]
- 134.Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016;2016:CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elangkovan N, Dickson G. Gene therapy for Duchenne muscular dystrophy. J Neuromuscul Dis. 2021;8:S303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hashim HZ, Che Abdullah ST, Wan Sulaiman WA, Hoo FK, Basri H. Hunting for a cure: the therapeutic potential of gene therapy in Duchenne muscular dystrophy. Tzu Chi Med J. 2014;26:5–9. [Google Scholar]
- 137.Kourakis S, Timpani CA, Campelj DG, Hafner P, Gueven N, Fischer D, et al. Standard of care versus new-wave corticosteroids in the treatment of Duchenne muscular dystrophy: can we do better? Orphanet J Rare Dis. 2021;16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rosenberg AS, Puig M, Nagaraju K, Hoffman EP, Villalta SA, Rao VA, et al. Immune-mediated pathology in Duchenne muscular dystrophy. Sci Transl Med. 2015;7:299rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ferizovic N, Summers J, de Zárate IBO, Werner C, Jiang J, Landfeldt E, et al. Prognostic indicators of disease progression in Duchenne muscular dystrophy: a literature review and evidence synthesis. PLoS One. 2022;17:e0265879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007;21:2195–204. [DOI] [PubMed] [Google Scholar]
- 141.Li D, Long C, Yue Y, Duan D. Sub-physiological sarcoglycan expression contributes to compensatory muscle protection in mdx mice. Hum Mol Genet. 2009;18:1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fukada S, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yamaguchi M, et al. Genetic background affects properties of satellite cells and mdx phenotypes. Am J Pathol. 2010;176:2414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fletcher EL, Jobling AI, Vessey KA, Luu C, Guymer RH, Baird PN. Animal models of retinal disease. Prog Mol Biol Transl Sci. 2011;100:211–86. [DOI] [PubMed] [Google Scholar]
- 144.Heydemann A, Ceco E, Lim JE, Hadhazy M, Ryder P, Moran JL, et al. Latent TGF-β–binding protein 4 modifies muscular dystrophy in mice. J Clin Invest. 2009;119:3703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mojumdar K, Liang F, Giordano C, Lemaire C, Danialou G, Okazaki T, et al. Inflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR 2. EMBO Mol Med. 2014;6:1476–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taglietti V, Kefi K, Bronisz-Budzyńska I, Mirciloglu B, Rodrigues M, Cardone N, et al. Duchenne muscular dystrophy trajectory in R-DMDdel52 preclinical rat model identifies COMP as biomarker of fibrosis. Acta Neuropathol Commun. 2022;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chemello F, Wang Z, Li H, McAnally JR, Liu N, Bassel-Duby R, et al. Degenerative and regenerative pathways underlying Duchenne muscular dystrophy revealed by single-nucleus RNA sequencing. Proc Natl Acad Sci U S A. 2020;117:29691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2008;18:482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Juban G, Saclier M, Yacoub-Youssef H, Kernou A, Arnold L, Boisson C, et al. AMPK activation regulates LTBP4-dependent TGF-β1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep. 2018;25:2163–76.e6. [DOI] [PubMed] [Google Scholar]
- 150.Giordano C, Mojumdar K, Liang F, Lemaire C, Li T, Richardson J, et al. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum Mol Genet. 2015;24:2147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arnholdt C, Kumaraswami K, Götz P, Kübler M, Lasch M, Deindl E. Depletion of γδ T cells leads to reduced angiogenesis and increased infiltration of inflammatory M1-like macrophages in ischemic muscle tissue. Cell. 2022;11:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mann AO, Hanna BS, Muñoz-Rojas AR, Sandrock I, Prinz I, Benoist C, et al. IL-17A–producing γδT cells promote muscle regeneration in a microbiota-dependent manner. J Exp Med. 2022;219:e20211504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen Y-W, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies. J Cell Biol. 2000;151:1321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tews DS, Goebel HH. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol. 1996;55:342–7. [DOI] [PubMed] [Google Scholar]
- 155.de Senzi Moraes Pinto R, Ferretti R, Moraes LH, Neto HS, Marques MJ, Minatel E. N-Acetylcysteine treatment reduces TNF-α levels and myonecrosis in diaphragm muscle of mdx mice. Clin Nutr. 2013;32:472–5. [DOI] [PubMed] [Google Scholar]
- 156.Grounds MD, Torrisi J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18:676–82. [DOI] [PubMed] [Google Scholar]
- 157.Collins RA, Grounds MD. The role of tumor necrosis factor-alpha (TNF-α) in skeletal muscle regeneration: studies in TNF-α(−/−) and TNF-α(−/−)/LT-α(−/−) mice. J Histochem Cytochem. 2001;49:989–1001. [DOI] [PubMed] [Google Scholar]
- 158.Mammen AL, Sartorelli V. IL-6 blockade as a therapeutic approach for Duchenne muscular dystrophy. EBioMedicine. 2015;2:274–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pelosi L, Berardinelli MG, De Pasquale L, Nicoletti C, D’Amico A, Carvello F, et al. Functional and morphological improvement of dystrophic muscle by interleukin 6 receptor blockade. EBioMedicine. 2015;2:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Song Y, Yao S, Liu Y, Long L, Yang H, Li Q, et al. Expression levels of TGF-β1 and CTGF are associated with the severity of Duchenne muscular dystrophy. Exp Ther Med. 2017;13:1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kemaladewi DU, Pasteuning S, van der Meulen JW, van Heiningen SH, van Ommen G-J, ten Dijke P, et al. Targeting TGF-β Signaling by antisense oligonucleotide-mediated knockdown of TGF-β type I receptor. Mol Ther Nucleic Acids. 2014;3:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mázala DAG, Novak JS, Hogarth MW, Nearing M, Adusumalli P, Tully CB, et al. TGF-β–driven muscle degeneration and failed regeneration underlie disease onset in a DMD mouse model. JCI Insight. 2020;5:e135703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ceco E, McNally EM. Modifying muscular dystrophy through transforming growth factor-β. FEBS J. 2013;280:4198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li N, Hua J. Immune cells in liver regeneration. Oncotarget. 2017;8:3628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Simões FC, Riley PR. Immune cells in cardiac repair and regeneration. Development. 2022;149:dev199906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of acute and chronic wound healing. Biomolecules. 2021;11:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kloc M, Ghobrial RM, Wosik J, Lewicka A, Lewicki S, Kubiak JZ. Macrophage functions in wound healing. J Tissue Eng Regen Med. 2019;13:99–109. [DOI] [PubMed] [Google Scholar]
- 168.Smigiel KS, Parks WC. Macrophages, wound healing, and fibrosis: recent insights. Curr Rheumatol Rep. 2018;20:17. [DOI] [PubMed] [Google Scholar]
- 169.Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev. 2015;24:29–39. [DOI] [PubMed] [Google Scholar]
- 170.Billings CG, Howard P. Occupational siderosis and welders’ lung: a review. Monaldi Arch Chest Dis. 1993;48:304–14. [PubMed] [Google Scholar]
- 171.Mulens-Arias V, Rojas JM, Barber DF. The use of iron oxide nanoparticles to reprogram macrophage responses and the immunological tumor microenvironment. Front Immunol. 2021;12:693709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med. 1998;157:1666–80. [DOI] [PubMed] [Google Scholar]
- 173.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ea H-K, Lioté F Calcium pyrophosphate dehydrate and basic calcium phosphate crystal-induced arthropathies: update on pathogenesis, clinical features, and therapy. Curr Rheumatol Rep. 2004;6:221–7. [DOI] [PubMed] [Google Scholar]
- 175.Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther. 2006;8(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27:165–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang J, Wei X, Tang Z, Miao B, Luo Y, Hu X, et al. Elucidating the molecular pathways and immune system transcriptome during ischemia-reperfusion injury in renal transplantation. Int Immunopharmacol. 2020;81:106246. [DOI] [PubMed] [Google Scholar]
- 178.Erkens R, Totzeck M, Brum A, Duse D, Bøtker HE, Rassaf T, et al. Endothelium-dependent remote signaling in ischemia and reperfusion: alterations in the cardiometabolic continuum. Free Radic Biol Med. 2021;165:265–81. [DOI] [PubMed] [Google Scholar]
- 179.Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16:442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kimball A, Schaller M, Joshi A, Davis FM, den Dekker A, Boniakowski A, et al. Ly6CHi blood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2018;38:1102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gan Z, Fu T, Kelly DP, Vega RB. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018;28:969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of Immunometabolism in macrophage activation and inflammation. Circ Res. 2020;126:789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. 2017;8:1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. [DOI] [PubMed] [Google Scholar]
- 188.Roger L, Tomas F, Gire V. Mechanisms and regulation of cellular senescence. Int J Mol Sci. 2021;22:13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 2017;122:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


