Abstract
Objective:
In the Mind Your Health Trial, acceptance-based behavioral treatment (ABT) for obesity outperformed standard behavioral treatment (SBT) at posttreatment. This trial compared effects over 2 years of follow-up.
Methods:
Participants with overweight or obesity (n = 190) were randomized to 25 sessions of SBT or ABT over 1 year and assessed at months 12 (i.e., posttreatment), 24 (1 year posttreatment), and 36 (2 years posttreatment).
Results:
Weight-loss differences previously observed at 12 months attenuated by follow-up, though a large difference was observed in the proportion of treatment completers who maintained 10% weight loss at 36 months (SBT = 17.1% vs. ABT = 31.6%; P = 0.04; intent-to-treat: SBT = 14.4% vs. ABT = 25.0%; P = 0.07). The amount of regain between posttreatment and follow-up did not differ between groups. ABT produced higher quality of life at 24 and 36 months. Autonomous motivation and psychological acceptance of food-related urges mediated the effect of condition on weight. No moderator effects were identified.
Conclusions:
Overall, results suggest that infusing SBT for weight loss with acceptance-based strategies enhances weight loss initially, but these effects fade in the years following the withdrawal of treatment. Even so, those receiving ABT were about twice as likely to maintain 10% weight loss at 36 months, and they reported considerably higher quality of life.
Introduction
Gold standard, high-intensity (“standard”) behavioral weight-loss treatment (SBT) produces 1-year weight losses of 5% to 8% (1). Yet a high proportion of participants do not achieve benchmarks (e.g., 10% weight loss). Moreover, most participants regain their lost weight in the months and years following the cessation of treatment. Acceptance-based behavioral treatment (ABT) is a variant of SBT premised on a model of self-regulation in which a failure to control weight stems from an inability to remain adherent to prescribed dietary intake and physical activity goals in the face of powerful internal (emotions, boredom, cravings, hunger, fatigue) and external cues (presence of palatable food, television, furniture designed for rest, labor-saving devices) (2–4). As such, ABT infuses SBT with self-regulation skills, such as an ability to tolerate uncomfortable internal states (e.g., urges, cravings, negative emotions) and a reduction of pleasure (e.g., choosing to exercise instead of watch television), behavioral commitment to clearly defined values (which is posited to increase motivation to persist in difficult weight-control behaviors), and metacognitive awareness of decision-making processes (4,5).
ABT and related treatments have shown promise in several randomized controlled trials (6–8). For example, one trial randomized participants (n = 162) to an SBT- or an ABT-based weight-loss program (which they called acceptance-based behavioral intervention) and reported that ABT and SBT demonstrated equivalent weight losses after treatment (12 months) but that ABT participants had better weight-loss maintenance at 24 months (1-year follow-up; 4.1% vs. 2.4% mean weight loss) (9). The Mind Your Health project (10) randomized 128 participants with overweight or obesity to receive 30 sessions of group-based ABT or SBT over the course of 40 weeks. At posttreatment and 6-month follow-up, the benefit of ABT over SBT was statistically significant only in the presence of particular vulnerabilities to internal and external cues for overeating (i.e., mood disturbance, elevated responsivity to food cues, and high disinhibition).
Mind Your Health II was a larger and longer follow-on study in which 190 participants with overweight or obesity were randomized to 25 group sessions of either SBT or ABT. As previously reported (11), those assigned to ABT demonstrated greater weight loss after treatment (12 months). Of note, separation between ACT and SBT became most apparent as sessions became less frequent, thus presumably providing reduced support and accountability. However, it is well known that the majority of behavioral weight-control participants regain much of the weight they lost once treatment ends completely. Presumably, regain occurs because utilization of weight-control behaviors and strategies fades over time. One possibility is that the more complex or abstract nature of ABT strategies leads to worse outcomes once treatment has ended and clinician support comes to an end. Alternatively, the benefits initially conferred by ABT could result in a prevention of regain (as happened in the Lillis et al. study (9)), or ABT may simply sustain its initial advantage. Also of interest is whether differences exist between the two treatments in the ability to sustain other desirable outcomes, including decreases in mood dysphoria and increases in quality of life, given that ABT focuses on methods of engaging in positive, adaptive, and valued behaviors.
Several findings have offered support for the postulated mechanisms of action of ABT, including the superiority of ABT at posttreatment being mediated by psychological acceptance and value-based motivation (11). The extent to which these mechanisms mediate the effect of ABT versus SBT on long-term outcomes has not been previously examined.
In addition, certain individual characteristics or predispositions, such as levels of depression, susceptibility to food cues, tendency toward disinhibited eating, and impulsivity, should theoretically make a person especially suitable for ABT given its emphasis on training the ability to experience aversive internal and external experiences while still acting consistently with long-term goals and values. Empirical support for these potential moderators has been mixed in terms of effects at posttreatment. Treatment effects on long-term outcomes and posttreatment maintenance may also vary by baseline characteristics.
In order to compare the long-term effects of ABT and SBT on weight, quality of life, and depression and to test moderation and mediation hypotheses, we followed participants in the Mind Your Health Trial for 2 years after their treatment concluded, with assessments at 1 year and 2 years after treatment (i.e., at 24 and 36 months after treatment started). Measures included depression, susceptibility to food cues, intrinsic (value-based) motivation, tendency toward disinhibited eating, weight, and quality of life.
Methods
Participants
The sample consisted of 190 adults with overweight or obesity (BMI 27-50 kg/m2) aged 18 to 70 years. Participants were recruited from the greater Philadelphia, Pennsylvania, area through primary care physician referrals and radio and newspaper advertisements. Exclusion criteria included severe medical or psychiatric conditions, conditions that precluded adherence to the exercise prescription of the program, recent (i.e., within the last 3 months) change in dosage of weight-influencing medications, pregnancy or plans to become pregnant within the study period, recent (i.e., within the last 6 months) weight loss of greater than 5% of one’s body weight, or a binge eating disorder diagnosis. For more information regarding the recruitment and enrollment procedure, see Forman et al. (11).
Procedure
Following informed consent and enrollment, participants were randomly assigned (stratified by gender and ethnicity) to one of the following two yearlong treatments: SBT (n = 90) or ABT (n = 100). Assessments were completed at months 0 (baseline), 6 (midpoint), 12 (posttreatment), 24 (1-year follow-up), and 36 (2-year follow-up). Procedures were approved by the Institutional Review Board of Drexel University and complied with all ethical standards for research.
Measurement
Outcomes.
Weight was measured at every assessment using a standardized Seca scale (Seca, Hamburg, Germany) accurate to 0.1 kg. Height was assessed via a stadiometer to calculate BMI (weight in kilograms divided by height in meters squared).
Mediators.
Psychological acceptance of food-related internal experiences was assessed via the Food Craving Acceptance and Action Questionnaire (FAAQ), a 10-item self-report measure that has adequate validity and reliability (α = 0.93; current sample α = 0.58-0.77) (12). Intrinsic motivation to enact health behaviors was measured with the 15-item Treatment Self-Regulation Questionnaire, which has good reliability (α = 0.76-0.93; current sample α = 0.71-0.74) and validity (13). Mediators were measured at baseline and midpoint.
Moderators.
Mood disturbance was measured via the 21-item Beck Depression Inventory-II, a self-report inventory that showed high internal consistency and validity in the current sample (α = 0.88). Quality of life was assessed via the Quality of Life Inventory, which measures satisfaction and meaning in various domains of life (14). This 32-item self-report measure has shown good internal consistency (α = 0.77-0.89), test-retest reliability (α = 0.80-0.91), and validity (14). Lastly, the Power of Food Scale, a 15-item self-report measure with good reliability (α = 0.91; current sample α = 0.79) and validity (15,16), was used to assess susceptibility to food cues. Tendency to eat in response to internal cues was measured via the Internal Disinhibition subscale of the Three-Factor Eating Questionnaire, a 20-item self-report measure that has shown adequate validity and reliability (α = 0.91; current sample α = 0.79) (17–19). All moderators were measured at baseline.
Intervention
Participants received one of two manualized treatments across 25 closed-group sessions, each lasting 75 minutes, with groups consisting of 10 to 14 participants. Groups were led by doctoral-level clinicians experienced in delivering behavioral weight-loss treatments, with interventionists leading an equal number of SBT and ABT groups. See Forman et al. (11) for more details regarding the composition and schedule of sessions.
Participants across conditions (SBT and ABT) were assigned the same balance-deficit diet and physical activity prescription. In addition, in both groups, behavioral skills such as stimulus control and problem solving were taught and emphasized. However, elements that were unique to SBT included content related to the cognitive behavioral model, cognitive restructuring, bolstering of self-efficacy and self-esteem, and distraction-based coping techniques. Alternatively, ABT-unique principles included selection of goals that align with personal values, acceptance of reduced pleasure (and, slightly less so, increased discomfort) when seeking weight loss in an obesogenic society, and recognition of the benefit inherent in understanding cues that influence eating and physical activity behavior. In addition, the ABT group stressed a “Control What You Can and Accept What You Can’t” framework to help participants identify aspects of their life that can and should be changed (e.g., their behaviors) versus those that cannot and those toward which direct attempts to control may be futile (e.g., involuntary urges).
Statistical analyses
Outcomes included percent of initial body weight lost at 24 months (1-year follow-up) and 36 months (2-year follow-up) and maintaining ≥ 10% weight loss at 24 and 36 months, a conventional standard to indicate success in behavioral weight-loss interventions (20). We also calculated a percent of 12-month weight loss regained at 24 and 36 months (for those who had lost at least 1% of their initial body weight at 12 months). Other outcomes included quality of life and depression. As reported in Forman et al. (11), no baseline differences in demographics, depression, age, or weight were detected, and as such, these variables were not included as covariates.
Analyses were conducted in SPSS Statistics version 25 (IBM Corp., Armonk, New York). Data were inspected visually for outliers. Maximum likelihood estimation based on the expectation maximization algorithm was used to impute missing data to account for the impact of missingness on other variables. Percent weight change and quality of life were normally distributed. Depression was positively skewed at all time points, and as such, we conducted a log transformation to normalize the data. Results were equivalent using transformed and actual values, and as such, analyses with original values are presented for interpretability. All analyses were conducted using imputed values (i.e., based on an intent-to-treat approach) (21) with the subset who attended all assessments points (i.e., without imputation) and with the subset of participants who “completed” the treatment, which we defined as having attended at least 80% of treatment sessions. Except where noted, results were equivalent, and thus the full intention-to-treat data set results are reported. Treatments were compared at each time point using a general linear model. Moderators were examined in separate (grand-mean centered) models via the addition of a main effect for moderator and a moderator × treatment condition interaction term. Baseline weight was included as a covariate in all analyses with percent weight loss as an outcome. Logistic regressions were used to examine odds of achieving 5% and 10% weight loss at each time point. To evaluate whether changes from baseline to end of treatment in psychological acceptance and autonomous motivation mediated the effect of treatment condition on 24- and 36-month weights as well as posttreatment regain, we used the Hayes bootstrapping simple mediation PROCESS macro for SPSS (https://www.processmacro.org/index.html).
Results
Baseline characteristics
The sample was 82.1% female and 70.5% Caucasian, 24.7% African American, 3.7% Hispanic, and 1.1% Asian. Age was 51.64 ± 0.73 years (mean ± SD), and starting BMI was 36.93 ± 0.42 kg/m2. The groups were equivalent in gender (χ2 = 0.002; df= 1; P = 0.97) and ethnicity (χ2 = 1.05; df = 3; P = 0.79), as well as on all outcome measures at baseline. Treatment completers (i.e., participants who attended ≥ 80% of treatment) made up 78.4% of the sample.
Attrition
There was no significant difference in assessment completion rates (Figure 1) by condition at 24 (SBT = 72.2%; ABT =78.0%; Wald χ2(1) = 0.85; P = 0.36) or 36 months (SBT = 67.8%; ABT = 74.0%; Wald χ2(1) = 0.85; P = 0.29).
Figure 1.
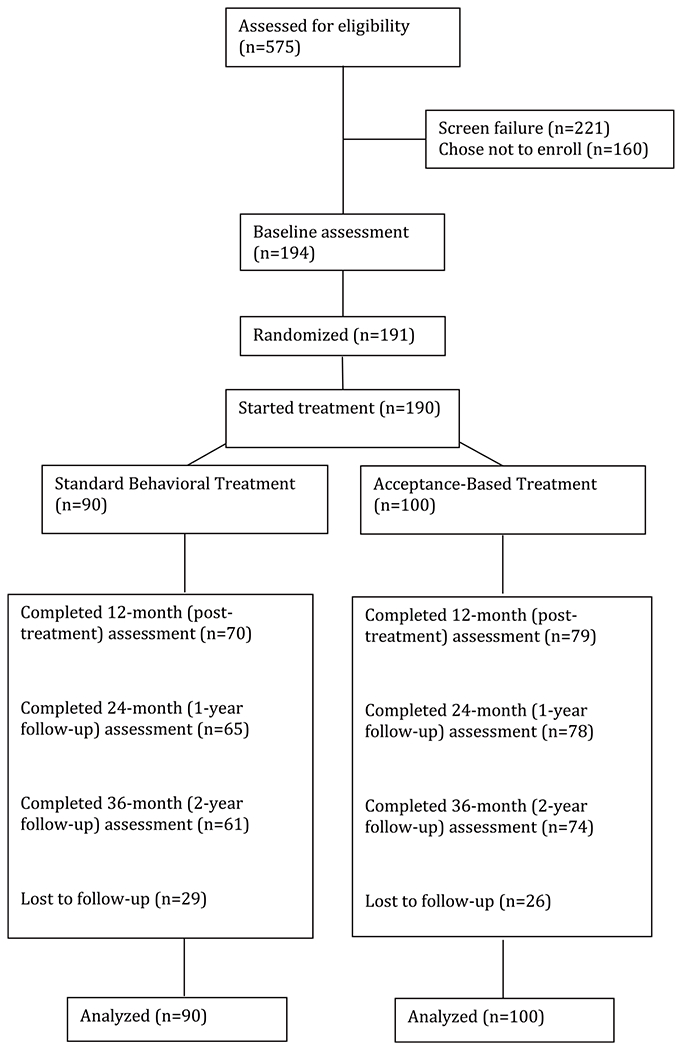
CONSORT diagram.
Weight loss
Overall, the effect of condition on weight loss at follow-up was modest. As depicted in Figure 2, percent weight losses at 24 months were 5.6% ± 8.2% for SBT and 7.5% ± 9.0% for ABT (F(2,189) = 2.21; P = 0.15). At 36 months, weight losses were 3.3% ± 8.2% for SBT and 4.7% ± 10.1% for ABT (F(2,189) = 1.05; P = 0.31). In terms of proportion reaching the 10% benchmark, differences were nonsignificant at 24 months (SBT = 31.1%; ABT = 34.0%; Wald χ2(1) = 0.18; P = 0.67; OR = 1.14). However, at 36 months, ABT participants were more likely to maintain 10% weight loss at trend level (SBT = 14.4%; ABT = 25.0%; Wald χ2(1) = 3.23; P = 0.07; OR = 1.97; Figure 3). The result was sharpened among treatment completers; those who received ABT achieved 10% weight loss at 36 months at higher rates than did those who received SBT (SBT = 17.1% vs. ABT = 31.6%; Wald χ2(1) = 4.08; OR = 2.24; P = 0.04).
Figure 2.
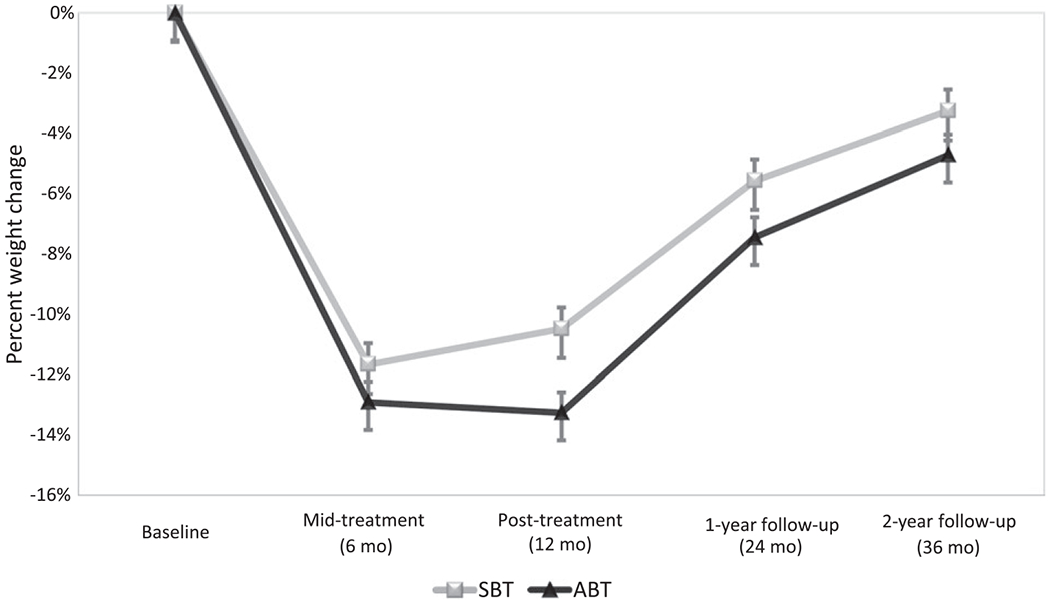
Percent weight change (intent-to-treat) by treatment condition over time.
Figure 3.
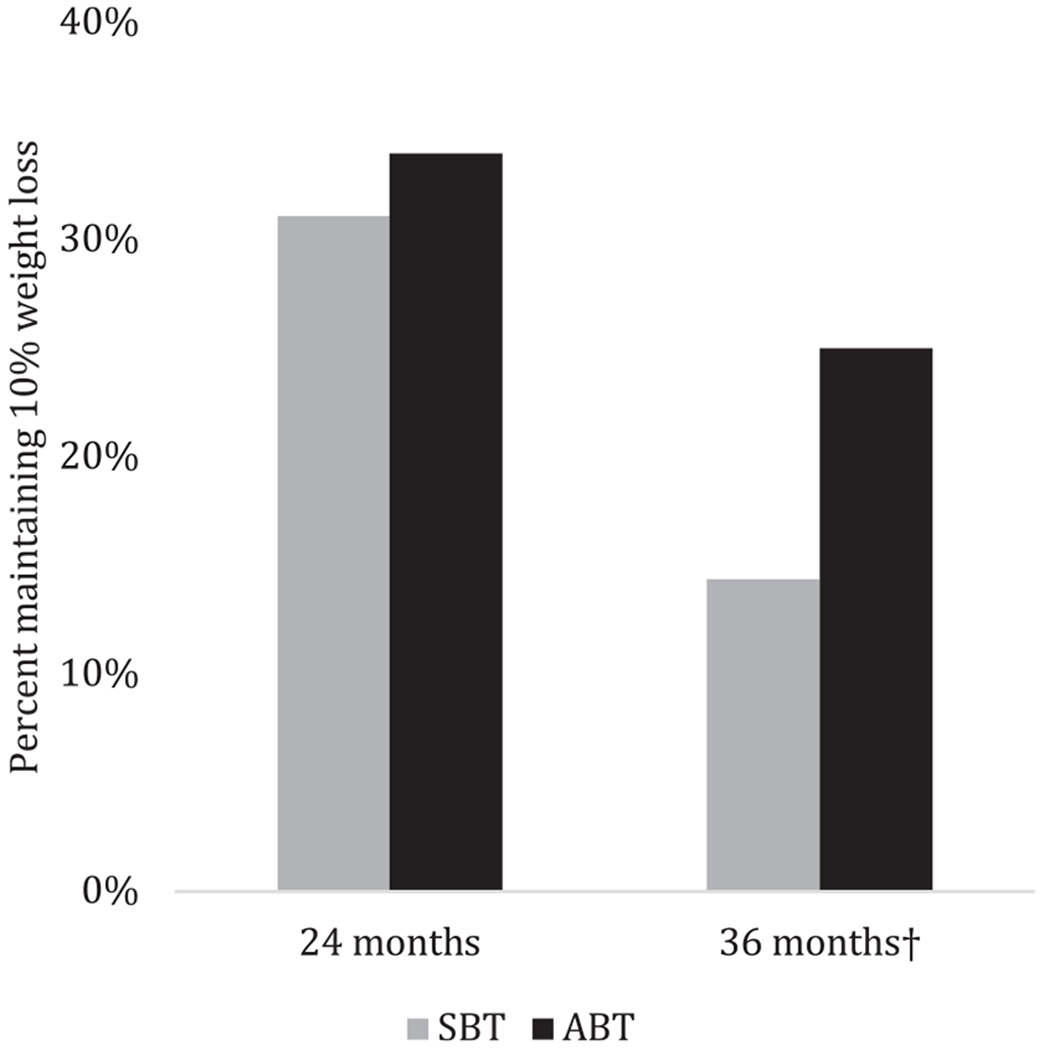
Percentage of participants (intent-to-treat sample) maintaining 10% weight loss at 24 months (1-year follow-up) and 36 months (2-year follow-up). †P = 0.07.
Weight regain (i.e., percent of 12-month weight loss regained) was equivalent between conditions at 24 months (SBT = 53.9%; ABT = 46.0%; F(2,173) = 0.75; P = 0.39) and 36 months (SBT = 74.0%; ABT = 72.7%; F(2,173) = 0.01, P = 0.99).
Mediation
Bootstrapping analyses revealed that changes during treatment (0-12 months) in autonomous motivation mediated the effect of treatment condition on percent weight loss at both 24 months (bindirect = −1.47; SE = 0.60; 95% CI: −2.74 to −0.42) and 36 months (bindirect = −1.47; SE = 0.60; 95% CI: −2.79 to −0.43). Change in food-based acceptance during treatment (0-12 months) mediated the effect of treatment on percent weight loss at 24 months (bindirect = −0.79; SE = 0.47; 95% CI: −1.86 to −0.07) but not at 36 months (bindirect = −0.32; SE = 0.40; 95% CI: −1.27 to 0.34). In terms of weight regain after treatment (12-36 months), results indicated that only food-based acceptance was a significant mediator (bindirect = 0.77; SE = 0.46; 95% CI: 0.07 to 1.86). The partially standardized indirect effect (22) of treatment condition on posttreatment weight regain through 12-month change in experiential acceptance was 0.10 (95% CI: 0.01 to 0.23), indicating that compared with participants in SBT, those in ABT had, on average, 0.10 SD less weight regain as a result of the indirect effect through FAAQ.
Moderation
No moderating effects of baseline depressive symptoms, susceptibility to food cues, or internal disinhibition were detected on the effect of treatment condition on weight loss at either 24 or 36 months (F = 0.03-1.24; P = 0.27-0.99).
Quality of life and depression
As depicted in Figure 4, participants in ABT reported significantly greater quality of life (controlling for baseline quality of life) than those in SBT at 24 months (MSBT = 2.82 ± 3.06; MABT = 3.70 ± 2.89; F(2,187) = 8.58; P < 0.01) and 36 months (MSBT = 2.76 ± 2.95; MABT=3.61 ± 3.05; F(2,187) = 6.05; P = 0.02). Effects remained when controlling for weight loss (24 months: MSBT = 2.82 ± 3.06; MABT = 3.70 ± 2.89; F(2,187) = 6.87; P=0.01; 36 months: MSBT = 2.76 ± 2.94; MABT = 3.61 ± 3.05; F(2,187) = 5.02; P = 0.03). In terms of clinical significance, 27.8% of SBT versus 50.5% of ABT participants made clinically significant improvement from baseline to 36 months (χ2(1) = 10.17; P = 0.001) according to the definition provided by Frisch et al. (23). No effects of condition were detected on depressive symptoms at either 24 months (MSBT = 8.19 ± 7.62; MABT = 6.85 ± 6.66; F(2,187) = 0.98, P = 0.32) or 36 months (MSBT = 8.78 ± 7.60; MABT = 7.18 ± 6.25; F(2,187) = 2.04; P = 0.15).
Figure 4.
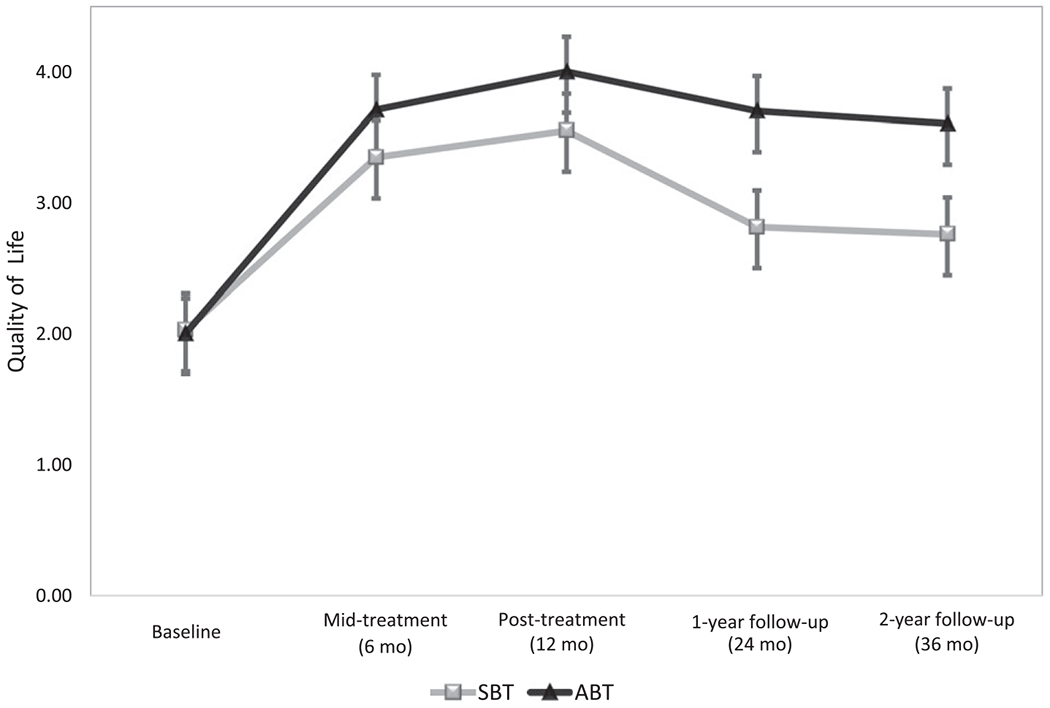
Quality of life (intent-to-treat) by condition over time.
Discussion
The Mind Your Health study randomized 190 participants with overweight or obesity to receive 25 group sessions of either gold standard SBT or ABT. As previously reported, participants receiving ABT had lost considerably more weight after treatment (i.e., 12 months) than those receiving SBT (11). Current results revealed that the separation between treatments attenuated by months 24 (i.e., 1-year follow-up) and 36 (2-year follow-up). Of note, ABT participants were almost twice as likely to have maintained 10% weight loss by the 2-year follow-up point (although this effect was significant only at the trend level). Also notable is that results held (or were strengthened) when examining only participants who completed a high percentage of the treatments and when examining only participants who attended all assessments. This study’s observation of participants for 3 years, and for 2 years after treatment, extends previous findings of ABT effects at 6 months and 12 months post treatment (9,10).
Notably, no evidence was obtained that ABT was protective against posttreatment regain (i.e., both groups regained about half of their lost weight by month 24, and about three-quarters by month 36). In contrast, Lillis et al. observed significantly less posttreatment regain in participants who had received acceptance-based behavioral intervention compared with those receiving SBT (9). One possible explanation for the differences in findings is that the Lillis study recruited a specific subgroup of participants with overweight (i.e., those showing strong tendencies toward disinhibited eating). Perhaps these participants regain largely because their restraint is disinhibited by internal and external cues, a tendency that is especially well addressed in ABT. Arguing against this idea is the fact that in our trial, internal disinhibition was not a moderator of follow-up weight loss and neither were related variables (e.g., susceptibility to food cues). Another possibility is that the difference in findings is attributable to differences in the treatment protocols. For instance, Lillis et al. (9) had a stronger emphasis on weight stigmatization and on clarifying life values beyond those related to health or weight loss. A future component analysis trial might be able to discern the independent efficacy of the specific components of ABT. A component-level understanding of efficacy would be extremely valuable in improving existing acceptance-based behavioral weight-loss interventions given that they (like most behavioral interventions) tend to be delivered in multifaceted packages (9–11,24).
We also examined the impact of treatment across the follow-up period on depression and quality of life. No effects were observed for depression. However, those randomized to ABT reported higher quality of life at months 24 and 36 (even when controlling for weight loss), and these differences were large. Moreover, twice as many ABT as SBT participants experienced clinically significant gains in quality of life between baseline and 36 months. Notably, differences existed above and beyond weight loss, suggesting that ABT had direct effects on quality of life in excess of those of SBT. Perhaps ABT’s focus on living out life values regardless of one’s internal experiences produced better quality of life relative to SBT.
The pattern of results evaluating postulated mediators was somewhat mixed but, overall, supported the hypotheses that gains over the course of treatment in both autonomous motivation and food/eating-related experiential acceptance mediated the impact of treatment condition on the persistence of weight loss across the 3 years. Intriguingly, although treatment condition had no main effect on regain after treatment, results revealed that changes in experiential acceptance mediated the effect of condition on regain and, specifically, that those in ABT had less weight regain (relative to SBT) as a result of the indirect effect through FAAQ. Taken together, mediation results offer partial support for the underlying theory of ABT and argue for the continued emphasis on identifying fundamental values that underpin motivation to lose weight. On the other hand, already discussed, a definitive answer on the independent efficacy of individual treatment components awaits an experimental component analysis.
Despite the intriguing finding regarding quality of life and the difference in participants maintaining 10% weight loss 2 years after treatment ended, an important conclusion to draw is that infusing SBT with acceptance-based strategies does not provide protection against weight regain after treatment. Yet the very strong effects at posttreatment underscore the potentially powerful impact of these novel strategies. A possible implication is that ABT must be continued in some form in order to sustain its superiority over SBT. While treatment beyond 1 year incurs additional costs and participant burden, it is consistent with the chronic care model increasingly accepted as necessary for sustaining weight loss (25–27). It is likely unreasonable to expect skill utilization and adherence to continue without degradation over long periods of time in the face of countervailing obesogenic forces, especially without any form of accountability. Of note, several relatively lower-cost and thus disseminable methods of sustaining treatment exist, such as booster sessions, brief phone coaching, and automated delivery of content through a smartphone application. Also possible is that no form of ABT would protect against longer-term regain and that an entirely different approach should be utilized in the years following weight loss. A future trial should compare SBT and ABT into the very long term, in which treatments are sustained using one or more of these methods.
Several limitations exist for the current trial. First, participants volunteered to be a part of this trial and thus were likely more motivated than is typically the case (e.g., in community or primary care settings); the impact of ABT relative to SBT could be weaker or greater with less-motivated individuals. The treatments were also delivered within a university research setting by expert clinicians with advanced degrees. As such, the efficacy of the intervention when delivered in community settings is not known. In addition, measures of moderators and secondary outcomes were dependent on self-report, which can be biased and subject to recall error. Strengths of the trial include stratified randomization, blinded assessors, fidelity assurance, imputation of missing data, and an assessment span of 3 years.
In conclusion, results suggest that ABT, which infuses behavioral weight-loss treatment with acceptance-based self-regulation skills (e.g., tolerating discomfort/reduction in pleasure, clarifying and deliberately holding in mind one’s life values, mindful awareness of key moments of decision-making), produced superior weight loss initially (at 1 year posttreatment), but this effect diminished in the years following the end of treatment. Moreover, receiving ABT offered no protection versus SBT against posttreatment regain. On the other hand, trend-level effects suggest that ABT participants were 1.9 times as likely to have maintained 10% weight loss at 36 months and also displayed considerably improved 36-month quality of life compared with SBT, even controlling for weight loss. A research priority should be determining ways to more powerfully sustain the initial effects of ABT, perhaps through continued contact or mobile health-based treatment delivery.
Acknowledgments
Deidentified participant data as well as the data dictionary will be provided upon request to other academic research groups for meta-analysis or to achieve aims in an approved written proposal. Other documents that will be available to such researchers include the Study Protocol and Informed Consent Form. Data will be available immediately following publication with no end date. Proposals should be directed to emf27@drexel.edu.
Funding agencies:
The Mind Your Health project was funded by grant from the National Institute for Diabetes and Digestive and Kidney Diseases (award R01 DK095069) to EMF.
Disclosure:
EMF and MLB report royalties from Oxford Press for two workbooks describing acceptance-based behavioral treatment. RDC reports consulting fees from Health Outcome Solutions outside the submitted work. The other authors declared no conflict of interest.
Footnotes
Clinical trial registration: ClinicalTrials.gov identifier NCT00746265.
References
- 1.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity Psychiatric Clin North Am 2011;34:841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr 2000;72:1088–1094. [DOI] [PubMed] [Google Scholar]
- 3.Lowe MR. Self-regulation of energy intake in the prevention and treatment of obesity: Is it feasible? Obes Res 2003;11(suppl):44S–59S. [DOI] [PubMed] [Google Scholar]
- 4.Forman EM, Butryn ML. A new look at the science of weight control: How acceptance and commitment strategies can address the challenge of self-regulation. Appetite 2015;84:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman EM, Butryn ML. Incorporating acceptance approaches into behavioral weight loss treatment. In: Haynos AF, Lillis J, Forman EM, Butryn ML, eds. Mindfulness and Acceptance Approaches to Treatment of Eating Disorders and Weight Concern. Oakland, CA: New Harbinger; 2016. [Google Scholar]
- 6.Lillis J, Hayes SC, Bunting K, Masuda A. Teaching acceptance and mindfulness to improve the lives of the obese: a preliminary test of a theoretical model. Ann Behav Med 2009;37:58–69. [DOI] [PubMed] [Google Scholar]
- 7.Tapper K, Shaw C, Ilsley J, Hill AJ, Bond FW, Moore L. Exploratory randomised controlled trial of a mindfulness-based weight loss intervention for women. Appetite 2009;52:396–404. [DOI] [PubMed] [Google Scholar]
- 8.Katterman SN, Goldstein SP, Butryn ML, Forman EM, Lowe MR. Efficacy of an acceptance-based behavioral intervention for weight gain prevention in young adult women. J Contextual Behav Sci 2014;3:45–50. [Google Scholar]
- 9.Lillis J, Niemeier HM, Thomas JG, et al. A randomized trial of an acceptance-based behavioral intervention for weight loss in people with high internal disinhibition. Obesity (Silver Spring) 2016;24:2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman EM, Butryn ML, Juarascio AS, et al. The Mind Your Health project: a randomized controlled trial of an innovative behavioral treatment for obesity. Obesity (Silver Spring) 2013;21:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman EM, Butryn ML, Manasse SM, et al. Acceptance-based versus standard behavioral treatment for obesity: Results from the Mind Your Health randomized controlled trial. Obesity (Silver Spring) 2016;24:2050–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juarascio A, Forman E, Timko CA, Butryn M, Goodwin C. The development and validation of the Food Craving Acceptance and Action Questionnaire (FAAQ). Eat Behav 2011;12:182–187. [DOI] [PubMed] [Google Scholar]
- 13.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ Res 2007;22: 691–702. [DOI] [PubMed] [Google Scholar]
- 14.Frisch MB, Cornell J, Villanueva M, Retzlaff PJ. Clinical validation of the Quality of Life Inventory. A measure of life satisfaction for use in treatment planning and outcome assessment. Psychol Assess 1992;4:92–101. [Google Scholar]
- 15.Cappelleri JC, Bushmakin AG, Gerber RA, et al. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes (Lond) 2009;33:913–922. [DOI] [PubMed] [Google Scholar]
- 16.Lowe MR, Butryn ML, Didie ER, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite 2009;53:114–118. [DOI] [PubMed] [Google Scholar]
- 17.Shearin EN, Russ MJ, Hull JW, Clarkin JF, Smith GP. Construct validity of the Three-Factor Eating Questionnaire: flexible and rigid control subscales. Int J Eat Disord 1994;16:187–198. [DOI] [PubMed] [Google Scholar]
- 18.Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83. [DOI] [PubMed] [Google Scholar]
- 19.Yeomans MR, Leitch M, Mobini S. Impulsivity is associated with the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Appetite 2008;50:469–476. [DOI] [PubMed] [Google Scholar]
- 20.Jensen M, Ryan D, Donato KA, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity (Silver Spring) 2014;22(S2):S1–S410. [DOI] [PubMed] [Google Scholar]
- 21.Little R, Yau L. Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics 1996;52:1324–1333. [PubMed] [Google Scholar]
- 22.MacKinnon DP. Introduction to Statistical Mediation Analysis. New York, NY: Lawrence Erlbaum Associates, Taylor & Francis Group; 2008. [Google Scholar]
- 23.Frisch MB, Clark MP, Rouse SV, et al. Predictive and treatment validity of life satisfaction and the quality of life inventory. Assessment 2005;12:66–78. [DOI] [PubMed] [Google Scholar]
- 24.Daubenmier J, Moran PJ, Kristeller J, et al. Effects of a mindfulness-based weight loss intervention in adults with obesity: a randomized clinical trial. Obesity (Silver Spring) 2016;24:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perri MG, Sears SF, Clark JE. Strategies for improving maintenance of weight loss: toward a continuous care model of obesity management. Diabetes Care 1993;16:200–209. [DOI] [PubMed] [Google Scholar]
- 26.Chen E, Bodenheimer T. Applying the chronic care model to the management of obesity. Obesity Manage 2008;4:227–231. [Google Scholar]
- 27.Wadden TA, Brownell KD, Foster GD. Obesity: responding to the global epidemic. J Consult Clin Psychol 2002;70:510–525. [DOI] [PubMed] [Google Scholar]


