Abstract
Fungal pathogens almost invariably trigger cell wall–associated defense responses, such as extracellular hydrogen peroxide generation and callose deposition, when they attempt to penetrate either resistant or susceptible plant cells. In the current study, we provide evidence that the expression of these defenses is dependent on adhesion between the plant cell wall and the plasma membrane. Peptides containing an Arg-Gly-Asp (RGD) motif, which interfered with plasma membrane–cell wall adhesion as shown by the loss of the thin plasma membrane–cell wall connections known as Hechtian strands, reduced the expression of cell wall–associated defense responses during the penetration of nonhost plants by biotrophic fungal pathogens. This reduction was associated with increased fungal penetration efficiency. Neither of these effects was seen after treatment with similar peptides lacking the RGD motif. Disruption of plant microfilaments had no effect on Hechtian strands but mimicked the effect of RGD peptides on wall defenses, suggesting that the expression of cell wall–associated defenses involves communication between the plant cell wall and the cytosol across the plasma membrane. To visualize the state of the plasma membrane–cell wall interaction during fungal penetration, we observed living cells during sucrose-induced plasmolysis. In interactions that were characterized by the early expression of cell wall–associated defenses, there was no change, or an increase, in plasma membrane–cell wall adhesion under the penetration point as the fungus grew through the plant cell wall. In contrast, for rust fungus interactions with host plants, there was a strong correlation between a lack of cell wall–associated defenses and a localized decrease in plasma membrane–cell wall adhesion under the penetration point. Abolition of this localized decreased adhesion by previous inoculation with a fungus that increased plasma membrane–cell wall adhesion resulted in reduced penetration by the rust fungus and induction of cell wall–associated defenses. These results suggest that rust fungi may induce a decrease in plasma membrane–cell wall adhesion as a means of disrupting the expression of nonspecific defense responses during penetration of host cells.
INTRODUCTION
Many fungal pathogens gain access to plant tissues by directly penetrating plant cell walls. Given that the cell wall is the first line of defense against the outside world, it is not surprising that plants have evolved means to perceive and respond defensively to the physical or chemical events associated with such penetration. For example, mechanical penetration of a cell with a needle will trigger the deposition of callose (containing β-1,3–linked glucan) around its tip (Russo and Bushnell, 1989), and localized pressure can elicit the translocation of the nucleus and cytoplasm, the intracellular generation of reactive oxygen species, and the transcription of defense-related genes (Gus-Mayer et al., 1998). Similarly, localized enzymatic degradation of the plant cell wall elicits the migration of the plant cell nucleus to the site (Heath et al., 1997) and the extracellular generation of H2O2 (D.G. Mellersh and M.C. Heath, unpublished data).
It has been suggested that numerous parallels exist between the plant cell wall and the mammalian extracellular matrix (ECM) (Reuzeau and Pont-Lezica, 1995). In mammalian cells, signaling across a dynamic continuum involving the ECM, the plasma membrane, and the cytoskeleton is maintained via interactions between plasma membrane–bound receptors known as integrins and protein ligands within the ECM that contain Arg-Gly-Asp (RGD) motifs (Giancotti and Ruoslahti, 1999). Although early efforts to find functional homologs of integrins in plants proved difficult, the recent demonstration of high-affinity RGD binding sites in the plasma membrane of Arabidopsis (Canut et al., 1998), the recent cloning of a gene from Arabidopsis with partial sequence similarity to integrins from fungi, insects, and humans (Nagpal and Quatrano, 1999), and the discovery of Arabidopsis genes that are predicted to contain β-integrin–like domains (Laval et al., 1999) all suggest that there may be at least some conservation between proteins involved in plasma membrane–ECM interactions in plant and animal systems. Further evidence of similarity between these systems is provided by the fact that treatment with RGD-containing peptides can influence several physiological events in plants (Schindler et al., 1989; Wayne et al., 1992; Barthou et al., 1999). RGD peptides also have been shown to cause a loss of plasma membrane–cell wall adhesion in plasmolyzed Arabidopsis cells and a loss of the thin plasma membrane–cell wall connections known as Hechtian strands that form during onion cell plasmolysis (Canut et al., 1998). A similar loss of localized plasma membrane–cell wall adhesion in plasmolyzed zygotes of the alga Pelvetia after RGD peptide treatments (Henry et al., 1996) indicates that this type of interaction may be widespread in the plant kingdom. Of particular interest is the fact that, recently, two bean proline-rich cell wall proteins that are upregulated during osmotic stress were shown to interact with the plasma membrane in a manner that was disrupted specifically by RGD-containing peptides even though these cell wall proteins apparently lack an RGD motif (García-Gómez et al., 2000). These data suggest that although the molecules involved in plasma membrane–ECM interactions in plants and animals may differ in their exact structures, plants may possess functionally analogous ECM proteins that contain an RGD-like configuration.
Some of the most economically important fungal plant pathogens are biotrophic, acquiring their nutrients from living plant cells. This acquisition commonly involves the formation of intracellular “feeding” structures, and although invaded cells may remain alive for considerable periods of time, fungal entry into the cell still usually elicits cell wall–associated defense responses (Heath and Škalamera, 1997), as would be expected if such responses are a nonspecific reaction to the mechanical and enzymatic effects of the penetration process. Powdery mildew fungi, for example, elicit callose deposition on, and the extracellular generation of reactive oxygen species in, the cell wall of both susceptible and resistant host plants (Thordal-Christensen et al., 1997; Hückelhoven and Kogel, 1998). However, rust fungi often cause no detectable plant defense responses to cell wall penetration in either susceptible or resistant genotypes of host species, and data suggest that this lack of plant reaction is due to active suppression by the fungus (Škalamera et al., 1997; Heath, 1998). The ability of rust fungi to negate plant defenses appears to be host specific, because they elicit a wide variety of defense responses in nonhost plants (Heath, 1997).
A role for plasma membrane–cell wall adhesion in plant–pathogen interactions was suggested recently by the observation that RGD-containing peptides interfere with the accumulation of an antimicrobial phytoalexin in pea epicotyls treated with an elicitor from spore germination fluids of Mycosphaerella pinodes (Kiba et al., 1998). In the current study, we provide several lines of evidence that the expression of cell wall–associated defense responses, triggered by biotrophic fungi as they penetrate the cell walls of host or nonhost plants, requires plasma membrane–cell wall adhesion. We also present evidence that rust fungi interfere specifically with the elicitation of penetration-induced defense responses in both susceptible and resistant host plants by causing a localized decrease in plasma membrane–cell wall adhesion directly under the penetration point.
RESULTS
Infection Process and Visualization of Defense Responses
Infection by the monokaryon of the cowpea rust fungus Uromyces vignae was as described previously (Heath, 1997; Heath et al., 1997). Briefly, basidiospores germinate on the surface of leaves to form short germ tubes. After the formation of appressoria, the fungus attempts to penetrate epidermal cells directly. If successful, the fungus forms an intracellular feeding structure known as an invasion hypha within the living epidermal cell. In susceptible host plants, invasion hyphae branch and the fungus invades adjacent epidermal cells and the underlying mesophyll. In resistant plants, either the fungus is stopped during penetration of the epidermal wall or, if the fungus successfully forms an invasion hypha, the invaded cell dies (a hypersensitive response) and fungal growth ceases.
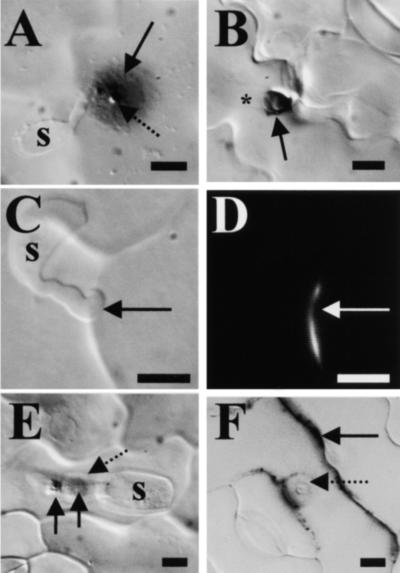
Previous studies have shown that there are few cytologically or cytochemically detectable responses to penetration by the cowpea rust fungus in most susceptible or resistant host cowpea plants (Heath et al., 1997; Škalamera et al., 1997; Heath, 1998). In contrast, we found in the current study that penetration of nonhost pea plants by the same fungus resulted in refractive deposits of callose between the plasma membrane and the cell wall. This callose could be in the form of hemispherical papillae beneath the sites of penetration attempts or in the form of collars surrounding the necks of invasion hyphae, as shown in Figures 1A and 1B, respectively. We also detected catalase-sensitive generation of H2O2, as visualized by 3,3′-diaminobenzidine-tetrahydrochloride (DAB) staining (Thordal-Christensen et al., 1997), in a circular (halo) pattern within the cell wall surrounding the fungal penetration peg (Figure 1A) and in callose papillae (Figure 1A) or collars (Figure 1B). H2O2 also was detected within anticlinal walls near the penetration point in cells containing invasion hyphae. This extracellular H2O2 generation was not associated with superoxide (O2−) production, because no nitroblue tetrazolium (NBT) staining (Doke, 1983) was seen at any penetration site. Attempted penetration of nonhost pea plants also was associated with the localized accumulation of autofluorescent phenolic compounds, as detected by blue light epifluorescence (Figures 1C and 1D).
Figure 1.
Light Micrographs of Wall-Associated Defenses in the Nonhost Interactions between the Cowpea Rust Fungus or the Plantain Powdery Mildew Fungus and Pea or Cowpea Cells, Respectively.
(A) and (B) Fixed, cleared pea leaf tissue 15 hr after inoculation with the rust fungus. (A) A fungal basidiospore (s) germinated to form an appressorium under which developed a DAB-stained circular halo (solid arrow) in the plant cell wall and a callose papilla (broken arrow). (B) DAB staining in a callose collar (arrow) around the neck of a fungal invasion hypha (*).
(C) and (D) A penetration attempt (arrows), 24 hr after inoculation, from a germinated rust fungus basidiospore (s) viewed under differential interference contrast microscopy (C) and blue light epifluorescence (D). A localized accumulation of autofluorescent phenolic compounds can be seen in the cell wall.
(E) and (F) Interaction between the plantain powdery mildew fungus and nonhost cowpea epidermal cells. (E) A powdery mildew conidium (s) formed an appressorium with two penetration attempts (solid arrows). The first attempt (closer to the spore) was associated with a halo of DAB staining, which is surrounded by a lighter DAB-stained ring (broken arrow), whereas the second attempt resulted in the formation of a DAB-stained callose papilla. (F) Diffuse NBT staining in anticlinal cell walls (solid arrow) and in a ring surrounding the penetration point (broken arrow) of the powdery mildew fungus.
 .
.
The nonhost interaction between cowpea plants and the plantain powdery mildew fungus Erysiphe cichoracearum also has been described (Meyer and Heath, 1988). Briefly, conidia germinate to form short germ tubes followed by the formation of elongate appressoria. In that study, each appressorium was found to be associated with two penetration attempts. The first penetration peg ceased growth as it became embedded in a callose papilla soon after it entered the cell lumen; the second penetration attempt was often more successful, and the fungus formed an intracellular haustorium. By 24 hr after inoculation, most haustorium-containing cells had died, and there was no further fungal growth. Infection of cowpea plants by the cowpea powdery mildew fungus E. polygoni (compatible interaction), examined in this study, was similar to that seen for E. cichoracearum except that appressoria were lobed in appearance and associated with just a single penetration attempt that frequently resulted in haustorium formation. In this case, invaded cells did not die, and the fungus continued to grow on the leaf surface, forming more haustoria in underlying epidermal cells.
As mentioned above, powdery mildew fungi, unlike many rust fungi, commonly trigger cell wall–associated defenses during penetration of both host (susceptible or resistant) and nonhost plants. In the current study, the attempted penetration of the plantain powdery mildew fungus into nonhost cowpea epidermal cells usually (>70%) was associated with DAB staining, as shown in Figure 1E, suggesting that extracellular H2O2 generation occurred at most interaction sites. Interestingly, DAB staining revealed circular haloes beneath appressoria in a region more proximal to the conidium than what was believed previously to be the site of the first penetration attempt, indicating that this fungus may attempt to penetrate nonhost tissues three times. Only one halo of DAB staining was seen beneath appressoria of the cowpea powdery mildew fungus on host cowpea leaves. As shown in Figure 1F for the nonhost interaction, in addition to H2O2 generation, a diffuse pattern of O2− generation was visualized by NBT staining in anticlinal walls and as circles within periclinal walls around penetration pegs in both host and nonhost interactions with the powdery mildew fungi. At least in the nonhost interaction, this response appeared to be transient, so that it was seen at only ∼20% of penetration sites at any time. These spatial and temporal differences in the localization of the two reactive oxygen species suggest that H2O2 generation was not a consequence of O2− dismutation. Attempted penetration by powdery mildew fungi also resulted in the localized accumulation of phenolic compounds in the cell wall, as detected by autofluorescence under blue light.
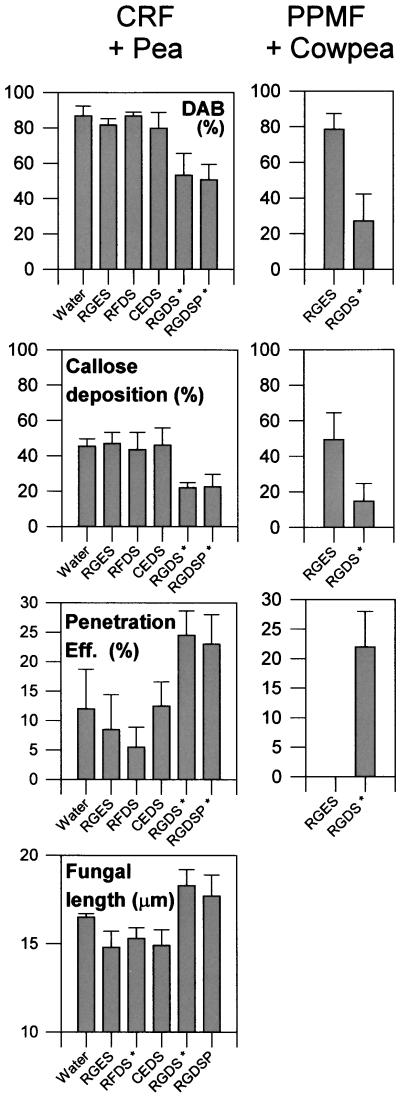
RGD Peptides Decrease the Expression of Cell Wall–Associated Defense Responses during Penetration and Increase Fungal Penetration Success and Intracellular Growth
As mentioned above, exogenous application of peptides containing the RGD sequence may specifically disrupt responses in animals involving integrin-mediated communication between the ECM and the cell interior (Ruoslahti, 1996). In the current study, we tested the effects of RGD-containing peptides on the two nonhost interactions described above. As Figure 2 demonstrates, two different RGD-containing peptides (but not three different peptides lacking this specific motif) caused a decrease in the percentage of infection sites with callose deposition and H2O2 generation in interactions between the cowpea rust fungus and nonhost pea plants and between the plantain powdery mildew fungus and nonhost cowpea plants. Reduction in the frequency of these responses after treatment with RGD peptides correlated with a significant increase in the penetration efficiency for both fungi and with increases in the intracellular growth of the rust fungus (Figure 2). The correlation between an increase in penetration efficiency and the RGD-mediated reduction of cell wall–associated phenolic compounds in host and nonhost interactions involving these fungi and the cowpea powdery mildew fungus is shown in Table 1. Significantly, treatment with these same peptides did not increase the penetration efficiency of the cowpea rust fungus on host plants that did not exhibit cell wall–associated defenses (Table 1). This observation strongly suggests that the effect of the RGD peptides on fungal penetration in the non-host interactions was related to the loss of cell wall defenses and was not due to some other effect on the plant or fungus.
Figure 2.
Effect of RGD and Non-RGD Peptides on the Nonhost Interactions between the Cowpea Rust Fungus (CRF) or the Plantain Powdery Mildew Fungus (PPMF) and Pea or Cowpea Cells, Respectively.
Peptide treatment labels (x axis) show only the first four to five amino acids of the sequences; full peptide sequences are listed in Methods. Fungal length was not applicable to the powdery mildew fungus because haustoria showed determinate growth. Asterisks indicate data that differ significantly from the water control values (P ⩽ 0.01). Eff., efficiency.
Table 1.
Correlation between Fungal-Induced Decreases in Plasma Membrane –Cell Wall Adhesion and/or the Effects of RGDS Peptides on Fungal Penetration and the Presence of Phenolic Compounds in the Cell Wall
| Fungus and Plant | Percentage of Cells with a Localized Decrease in Adhesion under the Penetration Point (n) |
Phenolics in the Cell Wall (%)a
|
Penetration Efficiency (%)c
|
||
|---|---|---|---|---|---|
| RGES | RGDS | RGES | RGDS | ||
| CRFb on CBc | 80.0 (10) | 3.5 | 2.0 | 78.0 | 81.5 |
| CRF on DC | 78.6 (14) | 8.5 | 1.0 | 79.5 | 87.5 |
| CRF on CC | 7.1 (14) | 33.5 | 16.2d | 36.0 | 72.0d |
| CRF on pea | 5.6 (18) | 24.9 | 7.4d | 4.5 | 13.5d |
| PPMF on CB | 5.9 (17) | 56.5 | 20.5d | 1.0 | 12.5d |
| CPMF on CB | 8.3 (12) | 48.5 | 9.5d | 93.0 | 99.0d |
Phenolic and penetration data represent means from 50 penetration attempts on each of four leaf pieces per treatment.
CRF, cowpea rust fungus; PPMF, plantain powdery mildew fungus; CPMF, cowpea powdery mildew fungus.
CB, DC, and CC represent cowpea cultivars California Blackeye, Dixie Cream, and Calico Crowder, respectively.
Significantly different from RGES-treated tissue. There was no significant difference between RGES-treated tissue and uninjected controls in any treatment.
RGD Peptides Decrease Plasma Membrane–Cell Wall Adhesion in Epidermal Cells
To demonstrate that the RGD-containing peptides decreased the expression of cell wall–associated defenses by interfering with plasma membrane –cell wall adhesion, we examined living epidermal cells after a stepwise plasmolysis procedure and subsequent application of one of the RGD peptides (RGDS). After plasmolysis of both pea and cowpea epidermal cells, protoplasts pulled away from the cell wall and became spherical. However, cytosol staining with fluorescein diacetate followed by confocal imaging revealed that large numbers of thin plasma membrane –cell wall connections (Hechtian strands) remained, as shown in Figure 3. These strands disappeared within 5 min of treatment with RGDS peptide (Figure 3A, left to right) but not after treatment with RGES peptide (Figure 3B, left to right). These observations demonstrate that peptides containing the RGD motif specifically disrupt plasma membrane –cell wall connections in epidermal cells.
Figure 3.
Effect of RGDS and RGES Peptides on Hechtian Strand Connections to the Plant Cell Wall in Pea.
(A) Confocal image of a fluorescein diacetate –treated, sucrose-plasmolyzed pea cell showing the gradual reduction with time in the numbers of Hechtian strand connections (arrows) to the plant cell wall after the addition of 1 mM RGDS peptide. Left to right: before (0 min) and 1, 3, and 5 min after treatment. The plant cell vacuole (V) did not stain with fluorescein diacetate.
(B) Two other plasmolyzed pea cells with multiple Hechtian strand connections (arrows) that remained intact after the addition of RGES peptide. Left to right: before (0 min) and 1, 3, and 5 min after treatment.
 .
.
Cytosolic Components Are Required for the Expression of Cell Wall–Associated Defenses
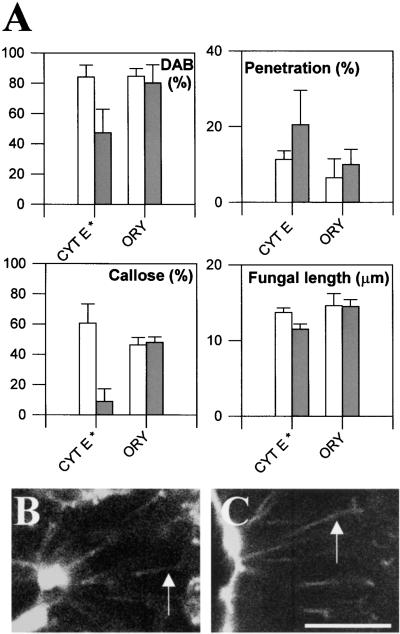
The hypothesis that RGD-mediated plasma membrane–cell wall adhesion is required for the fungus-induced expression of cell wall–associated defenses suggests that the induction of these defenses needs some form of communication between the cell wall and the cytosol. To investigate the requirement for cytosolic involvement in the expression of these defenses, we examined the need for a functional cytoskeleton. Cytochalasin E, an antimicrofilament agent, has been shown to stop cytoplasmic streaming and to disrupt the actin cytoskeleton in cowpea epidermal cells (Škalamera and Heath, 1998) and other plant tissues. In the current study, this drug was tested on the nonhost pea/cowpea rust fungus system because the single penetration peg produced by each appressorium facilitated the measurement of penetration efficiency. As shown in Figure 4A, this drug caused a significant reduction in H2O2 accumulation and callose deposition and allowed a slight increase in fungal penetration efficiency. Figure 4A also shows that cytochalasin E inhibited fungal intracellular growth, and this adverse effect on the fungus probably accounts for the fact that the effect on penetration efficiency was less than might be expected from the decrease in wall-associated defenses. The effect of cytochalasin E on these defenses is in agreement with recent evidence that actin microfilaments are important for the expression of penetration-related resistance in plants to a variety of fungal pathogens (Kobayashi et al., 1997). In contrast, the antimicrotubule agent oryzalin had no effect on defense responses or fungal penetration success (Figure 4A) at chemical concentrations that were otherwise sufficient to cause disruption of transvacuolar cytoplasmic strands and a reduction in the observed vigor of cortical cytoplasmic streaming in living pea and cowpea epidermal cells. These data indicate that microtubules do not play a significant role in the expression of cell wall–associated defense responses. Interestingly, although cytochalasin E mimicked the effect of RGD peptides on expression of wall-associated defenses, it did not mimic the peptides in its effect on Hechtian strand connections between the plasma membrane and cell wall; such strands were still visible in cells that were plasmolyzed after treatment with either cytochalasin E or oryzalin, as shown in Figures 4B and 4C.
Figure 4.
Effect of Anticytoskeletal Agents on the Interaction between the Cowpea Rust Fungus and Nonhost Pea Plants and on Hechtian Strand Formation.
(A) Effect of cytochalasin E (CYT E) and oryzalin (ORY) on expression of cell wall–associated defenses and on fungal penetration and intracellular fungal growth. White bars represent controls, and dark bars represent plants that underwent experimental treatments. Asterisks indicate data that are significantly different from control values (P ⩽ 0.01).
(B) and (C) Confocal images of fluorescein diacetate –treated, sucrose-plasmolyzed pea epidermal cells previously treated with either (B) cytochalasin E or (C) oryzalin at concentrations that had caused cessation of cytoplasmic streaming. Hechtian strand connections to the cell wall (arrows) are still visible after both treatments.  .
.
To determine if the RGDS peptide could directly affect the actin cytoskeleton, we examined cytoplasmic streaming in live cowpea epidermal cells after RGDS treatment. Unlike cytochalasin E, which stopped all cytoplasmic streaming at a concentration that reduced the expression of cell wall–associated defenses in the cowpea rust fungus–pea interaction, RGDS peptide at the equivalent concentration did not cause the disruption of transvacuolar strands or any appreciable slowing of cortical cytoplasmic streaming.
Rust Fungus–Induced Localized Decreases in Plasma Membrane–Cell Wall Adhesion Are Correlated with a Lack of Cell Wall–Associated Defenses
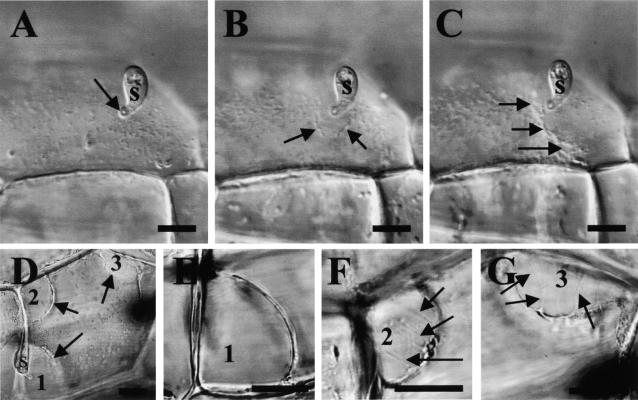
The form of protoplast plasmolysis often has been used to investigate plasma membrane–cell wall interactions in plants (Lee-Stadelmann et al., 1984; Oparka, 1994). Therefore, in the current work, we used video imaging and the stepwise application of sucrose solutions of increasing concentrations to examine the state of adhesion between the plant plasma membrane and the cell wall in the cowpea rust fungus/cowpea system in which cell wall–associated defenses rarely occur. In the early stages of cell wall penetration in susceptible (cv California Blackeye) and resistant (cv Dixie Cream) host cells, the plasma membrane near the penetration point was the first area to pull away from the plant cell wall during sucrose-induced plasmolysis. Such differential shrinkage of the plasma membrane from the cell wall, shown in Figure 5 (data shown in Table 1), indicates a localized decrease in plasma membrane–cell wall adhesion at the penetration site. This phenomenon was extremely difficult to detect because it occurred only at a specific stage of fungal penetration when cytoplasmic strands were directed toward the penetration peg and the plant nucleus had not migrated to the penetration point. This stage has been shown by electron microscopy to be initiated before the fungus fully breaches the epidermal wall (Mould and Heath, 1999). In stark contrast with what was seen in the two host cowpea cultivars, in nonhost pea cells and in a cowpea cultivar (Calico Crowder) that commonly expresses cell wall–associated defenses during cell wall penetration, the plasma membrane appeared to be uniformly attached to the cell wall at this same stage of rust fungus infection (Table 1). Similarly, there was no evidence of a localized decrease in plasma membrane–cell wall adhesion under penetration points of either of the two powdery mildew fungi in host or nonhost interactions (Table 1) that also are characterized by cell wall–associated defenses. In fact, in interactions involving powdery mildew fungi, many areas of the plasma membrane appeared to be more firmly attached to the cell wall than in uninfected cells, as demonstrated by a concave plasmolysis morphology. This concave plasmolysis was not influenced by pre- or postplasmolysis treatment with RGDS peptides. An increase in adhesion also has been reported for powdery mildew interactions with barley (Lee-Stadelmann et al., 1984).
Figure 5.
Early Stages of Sucrose-Induced Plasmolysis during the Interaction between the Cowpea Rust Fungus and a Host Cultivar of Cowpea.
(A) to (C) Light micrographs of a living cowpea vein epidermal cell (cv Dixie Cream) 7 hr after inoculation with a basidiospore (s).
(A) Cell before sucrose-induced plasmolysis showing a fungal appressorium (arrow) with its penetration peg just entering the periclinal epidermal wall. Note that the plant plasma membrane has a granular appearance in surface view.
(B) Same cell as in (A) at an early stage of sucrose-induced plasmolysis. The plasma membrane has pulled away (arrows) from the plant cell wall just underneath the penetration point to form a hemispherical bubble between the cell wall and the plasma membrane.
(C) The same cell a few moments later. More of the plasma membrane has pulled away from the cell wall (arrows).
(D) to (G) Another cowpea epidermal cell during fungal penetration from a basidiospore (s) at a slightly later stage of plasmolysis than that shown in (B) in which two additional plasma membrane –cell wall detachment areas (2 and 3) have developed after the first detachment area (1) under the fungal penetration point.
(D) Low-magnification image showing the three detachment areas.
(E) to (G) Areas 1, 2, and 3 at higher magnification. Note the absence of Hechtian strands in the membrane detachment region under the site of the fungal penetration attempt (E) and the presence of multiple plasma membrane –cell wall connections (arrows) in detachment areas 2 (F) and 3 (G) away from the penetration point.
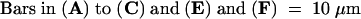 .
.
Rust Fungus–Induced Localized Decreases in Plasma Membrane–Cell Wall Adhesion Are Associated with a Localized Loss of Hechtian Strand Connections
Using stepwise plasmolysis, a detailed examination of the area of decreased plasma membrane–cell wall adhesion during penetration of host cells by the cowpea rust fungus was achieved at four infection sites. No Hechtian strand connections to the cell wall were seen. As shown in Figures 5D and 5E for a cell at a stage of plasmolysis (slightly after that shown in Figure 5B) when other parts of the plasma membrane were beginning to detach from the cell wall, there were no Hechtian strands in the space under the fungal penetration point. In contrast, other areas of plasma membrane–cell wall detachment shown in Figures 5F and 5G contained multiple Hechtian strand connections. These observations suggest that the decrease in plasma mem-brane –cell wall adhesion caused by rust fungi may be related to the disruption of Hechtian strand connections to the plant cell wall.
Increased Plasma Membrane–Cell Wall Adhesion Caused by Penetration by a Powdery Mildew Fungus Eliminates the Localized Decrease in Adhesion Caused by the Rust Fungus and Increases Cell Wall–Associated Defenses and Unsuccessful Penetration
To find further evidence that the absence of cell wall–associated defense responses in the cowpea rust fungus/cowpea system is due to the lack of plasma membrane–cell wall adhesion at the penetration site, we investigated whether the increased adhesion at unsuccessful penetration sites of the plantain powdery mildew fungus could overcome cowpea rust fungus–induced localized decreases in plasma membrane –cell wall adhesion and reinstate the capacity of cowpea cells to respond to the rust fungus in a defensive manner. Cowpea plants (cv California Blackeye, which is resistant to the powdery mildew fungus and susceptible to the cowpea rust fungus) were inoculated with conidia of the powdery mildew fungus, which were allowed to germinate and establish infection for 24 hr, before inoculation with rust fungal basidiospores. We concentrated on cells and their close neighbors that were associated with failed penetration attempts by the powdery mildew fungus because plasmolysis experiments had shown that they had the greatest degree of plasma membrane–cell wall adhesion. Table 2 shows that cells associated with failed powdery mildew fungus penetration attempts had increased plasma membrane–cell wall adhesion, as indicated by a shift from convex to concave plasmolysis morphology. These cells responded to attempted penetration by the cowpea rust fungus with localized generation of H2O2, which was correlated with a significant decrease in penetration efficiency compared with cells on the same leaves that were not in close proximity to powdery mildew fungus conidia. Video imaging of plasmolysis in double-inoculated cells indicated that during the early stages of cell wall penetration by the cowpea rust fungus, the latter was no longer able to cause a localized decrease in plasma membrane–cell wall adhesion beneath the penetration point. In fact, whereas large cytoplasmic aggregates rarely are found at interaction sites of the rust fungus with susceptible or resistant host plants under normal conditions, large cytoplasmic aggregates were associated with penetration attempts by this fungus in double-inoculated host cells.
Table 2.
Effect of 24-hr Preinoculation with the Plantain Powdery Mildew Fungus on the Percentage of Cells Exhibiting Concave Plasmolysis as well as DAB Response to, and Penetration Efficiency of, the Cowpea Rust Fungus on Cowpea Plants Normally Susceptible to this Fungusa
| Observed Features | Primed Cells | Unprimed Cells |
|---|---|---|
| Concave plasmolysis (%) | 73.0 ± 4.0b | 11.0 ± 7.0 |
| DAB response (%) | 47.0 ± 6.6b | 5.0 ± 5.0 |
| Penetration efficiency (%) | 8.5 ± 3.0b | 49.5 ± 8.1 |
Cowpea cv California Blackeye cells associated with unsuccessful powdery mildew penetration attempts, and cells directly touching these cells, were considered “primed” whereas cells on the same leaves at least 10 cells distant from powdery mildew conidia were considered “unprimed.”
These values (±sd) are significantly different from corresponding unprimed cells at P ⩽ 0.01.
DISCUSSION
In this study, we have provided evidence that the expression of cell wall–associated plant responses and the restriction of fungal growth during epidermal cell penetration are dependent on adhesion between the plant cell wall and the plasma membrane. Primary support for this hypothesis comes from the fact that RGD peptides, but not peptides lacking this specific motif, are capable of both disrupting plasma membrane connections to the plant cell wall (Hechtian strands) and interfering with the expression of cell wall–associated defenses. Although it could be argued that this latter effect of RDG peptides may be caused by interference with cellular processes other than adhesion, there is no precedence for this in the literature. Moreover, it is unlikely that these peptides cross the plasma membrane intact. In addition, their effect does not appear to be related to any direct interference with the cytoskeleton, because they did not eliminate cytoplasmic streaming, as did the antimicrofilament agent cytochalasin E, which also inhibited cell wall–associated defenses. Interestingly, as also shown in onion (Lang-Pauluzzi, 2000), destabilization of the actin cytoskeleton did not influence Hechtian strand attachment, suggesting that cytochalasin E does not exert its effect on cell wall–associated defenses by mimicking this effect of RGD peptides.
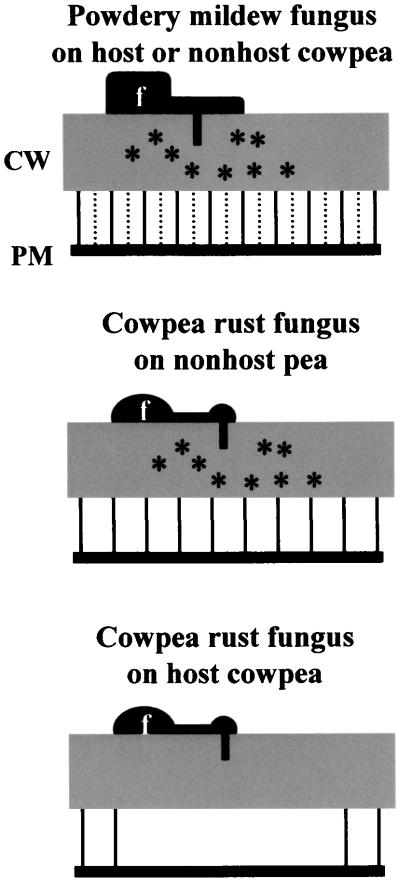
Supporting the RGD data are the results of the plasmolysis experiments that suggest that the lack of cell wall–associated defense responses to penetration by the rust fungus U. vignae is related to its ability to cause a localized, and host species–specific, disruption of plasma membrane–cell wall adhesion beneath the penetration point. Microscopic examination revealed that the mechanism by which the fungus achieves this decrease in adhesion involves a disruption of Hechtian strand connections to the cell wall, similar to that caused by RGD peptides. The rust fungus did not induce this localized decrease in adhesion under the penetration point in nonhost pea plants or in the cowpea cultivar Calico Crowder, both of which respond to attempted penetration by this fungus with cell wall–associated defenses. Similarly, species of powdery mildew fungi that triggered the expression of cell wall–associated defenses in both host and nonhost plants also did not cause localized decreases in plasma membrane –cell wall adhesion in either type of plant, instead causing an apparent overall increase in plasma membrane –cell wall adhesion in underlying cells. A summary of plasma membrane –cell wall adhesion states as related to the expression of wall-associated defenses during penetration of host and nonhost plants by rust and powdery mildew fungi can be found in Figure 6.
Figure 6.
Plasma Membrane –Cell Wall Adhesion State as it Relates to Expression of Cell Wall–Associated Defense Responses to Penetration of Host and Nonhost Plants by Biotrophic Fungi.
Solid vertical lines between the cell wall (CW) and plasma membrane (PM) represent RGD-disruptable plasma membrane –cell wall attachment sites. Broken vertical lines represent non-RGD-disruptable increases in plasma membrane –cell wall adhesion. Asterisks within the cell wall indicate presence of cell wall–associated defenses. f, fungus.
Unfortunately, there is no known way of experimentally increasing plasma membrane –cell wall adhesion in a direct manner that has no other effects on the cell. However, we did exploit the increased adhesion caused by powdery mildew infection to show that cowpea cells with this increased adhesion did not allow the cowpea rust fungus to induce a localized decrease in adhesion below the penetration site. In such situations, penetration frequency was reduced, and cell wall–associated defenses were observed when they usually were absent. Although open to other interpretations, these observations are entirely consistent with the thesis that plasma membrane –cell wall adhesion is necessary for the expression of cell wall–associated responses.
Defense responses are not always effective in restricting fungal growth (Perumalla and Heath, 1989, 1991), and the cowpea powdery mildew fungus penetrated host cowpea cells even in the presence of cell wall–associated defenses. Nevertheless, in nonhost plants, the elimination of cell wall–associated responses by the application of RGD peptides was correlated with a significant increase in penetration efficiency by both the cowpea rust fungus and the plantain powdery mildew fungus, and with a significant increase in the intracellular growth of the rust fungus. Although we do not know which cell wall–associated defense responses affect fungal growth, the data suggest that regardless of the cause of penetration-associated resistance, its expression requires plasma membrane –cell wall adhesion.
The dependence of cell wall–associated defense responses on the integrity of the actin cytoskeleton as shown in this study suggests that the induction or expression of these responses requires communication between the plant cell wall and the cytosol. Previous experiments have implicated components released as the fungus degrades its way through the plant cell wall as the elicitors of cell wall–associated defenses (Heath et al., 1997). Therefore, the results presented here suggest that the response to these elicitors may involve bidirectional communication between the plant cell wall and the cytoplasm that is dependent on adhesion between the plasma membrane and the cell wall. This is similar to mammalian systems, in which bidirectional communication between the extracellular and intracellular milieux is maintained via signaling across an ECM–plasma membrane –actin cytoskeleton continuum that is sensitive to RGD peptides (Giancotti and Ruoslahti, 1999). The unexpected ability of the rust fungus to locally disrupt this adhesion as a means of preventing cell wall–associated defenses in its host may be an important key to the development of new forms of resistance to these important plant pathogens.
The molecules involved in plasma membrane –cell wall adhesion in plants generally have not been identified. However, it is significant that both Wak1, a plant cell wall–associated receptor kinase that appears to link the cell wall to the plasma membrane, and the expression of AtELPs, which contain homology with mammalian integrins, are upregulated after bacterial infection (He et al., 1998; Laval et al., 1999). A multiplicity of molecules linking the plasma membrane to the cell wall may allow for different degrees or types of adhesion, leading to greater flexibility in signal transduction from the cell wall to the cell interior.
METHODS
Plant and Fungal Material
Plants were grown in lighted growth chambers at 150 μmol m−2 sec−1 with 16-hr days. The most recently opened leaves of 12-day-old pea (Pisum sativum cv Alaska) and 8- or 12-day-old cowpea (Vigna unguiculata cv California Blackeye, Dixie Cream, or Calico Crowder) plants were inoculated.
Basidiospores of the cowpea rust fungus (Uromyces vignae, race 1) were produced by incubating teliospores on 2% water agar in the dark for ∼52 hr. Squares of agar bearing germinating teliospores were laid spore side down on the upper surface of leaves. Plants were kept in a dark moist chamber overnight until further treatment.
Powdery mildew (Erysiphe cichoracearum and E. polygoni) conidia produced on susceptible plantain and cowpea plants, respectively, were transferred to the upper surface of cowpea leaves using a soft artist's paintbrush. Plants were then covered with clear plastic bags that had been sprayed lightly with distilled water and returned to the lighted growth chamber. For double inoculation experiments, E. cichoracearum conidia were inoculated on the surface of cowpea leaves 24 hr before inoculation with basidiospores of U. vignae, which were allowed to develop for another 15 hr.
Chemical Treatment of Leaves
Cytochalasin E (1 μg mL−1 in 0.01% DMSO) and RGD (Arg-Gly-Asp-Ser, Arg-Gly-Asp-Ser-Pro-Ala-Ser-Ser-Lys-Pro) and non-RGD (Arg-Gly-Glu-Ser, Arg-Phe-Asp-Ser, Cys-Gln-Asp-Ser-Glu-Thr-Arg-Thr-Phe-Tyr) peptides (500 μM in sterile deionized distilled water) were obtained from Sigma-Aldrich (Oakville, Ontario, Canada). Oryzalin (60 μg mL−1 in 0.6% DMSO) was a gift from I.B. Heath (York University, Toronto, Ontario, Canada). The appropriate concentrations of solvent or water were used as controls. The intercellular spaces of leaves were injected with chemical or control solutions by using a 1-mL syringe with a 30-gauge needle. Plants were inoculated with the fungal spores once signs of water soaking in the leaves had disappeared (typically 30 min).
Visualization of Defense Responses
H2O2 was visualized by injecting leaves with an aqueous solution of 2 mg mL−1 3,3′-diaminobenzidine-tetrahydrochloride (DAB; Thordal-Christensen et al., 1997; Heath, 1998) 1 hr before harvest or 2 hr before harvest in the case of tissues that had been injected previously with a chemical treatment. A positive response was recorded when DAB staining was as dark as, or darker than, U. vignae teliospores. In other experiments, leaves were injected with an aqueous solution of 0.05% nitroblue tetrazolium (NBT) 30 min before harvest; a blue color indicated superoxide (O2−) generation (Doke, 1983). In both cases, leaves were fixed and decolorized in boiling 95% (v/v) ethanol before being cleared in saturated chloral hydrate and mounted in modified Hoyer's medium (Stumpf and Heath, 1985). Leaves were examined using a Reichert-Jung (Reichert AG, Vienna, Austria) Polyvar microscope with differential interference contrast optics. Phenolic compounds were visualized as browning and/or autofluorescence under blue light epifluorescence (filter cube B1, excitation filter BP 330 to 380 nm, barrier filter LP 450 to 495 nm, and dichroic mirror DS 510 nm) in unstained, decolorized, cleared tissue. Callose was detected as refractive deposits on the plant wall that fluoresced in tissue stained with 0.005% (w/v) aniline blue in 0.07 M K2HPO4 and viewed using UV epifluorescence (filter cube U1, excitation filter BP 330 to 380 nm, barrier filter LP 418 nm, and dichroic mirror DS 420 nm).
Data Collection and Analysis
Data represent means of percentages from at least 50 fungal sites with appressoria on each of four leaf pieces per treatment. Penetration efficiency represents the percentage of U. vignae appressoria that formed invasion hyphae or the percentage of E. cichoracearum appressoria that formed haustoria on their first (papilla-inducing) penetration attempt. Where necessary, data were normalized using the arcsin square root transformation before the application of a t test. All experiments were performed at least twice with similar results; typical data from one experiment are shown in each figure.
Examination of Living Infected Epidermal Cells
In some experiments, the abaxial side of the midvein of a 1-cm2 piece of a cowpea leaf was removed with a razor blade, and the remainder of the leaf piece was mounted in distilled water, enabling living vein epidermal cells to be observed directly without interference from the underlying vascular tissues. For pea leaves, removal of the abaxial side of the midvein did not improve the optical resolution, so whole pieces of leaf were mounted directly in water on glass slides. Images were captured and enhanced using a Hamamatsu (Hamamatsu City, Japan) C2400-77 video camera and control unit and an Image-1 image processing and analysis system (Universal Imaging Corp., West Chester, PA) and/or a Northern Eclipse image processing and analysis system (Empix Imaging, Inc., Mississauga, Ontario, Canada).
To observe protoplast collapse, we added sucrose solutions (stepwise increase of 0.25, 0.50, 0.75, and 1.0 M at ∼2-min intervals) from the edge of the cover slip to previously mounted leaf tissue on the microscope stage. The solution was drawn across the tissue by applying absorbent paper to the opposite edge of the slide, which caused a gradual collapse of the epidermal protoplasts. In some cases, leaves were injected with or incubated on a drop of 50 μg mL−1 fluorescein diacetate in 1% (v/v) acetone before plasmolysis so that the cytoplasm could be seen under blue light epifluorescence.
Confocal Imaging of Living Epidermal Cells
Confocal microscopy was performed using an Olympus Fluoview confocal microscope (model BX50W1; excitation wavelength 488 nm, barrier filters LP 510 nm, and SP 550 nm) and the Fluoview FV300 software program (version 3.0.32; Olympus America, Inc., Melville, NY).
Acknowledgments
We thank Pui Tam and Rosemarie Christopher for excellent technical assistance. This research was supported in part by funds from the Natural Sciences and Engineering Research Council of Canada and by a joint Government of Ontario/University of Toronto scholarship (OGSST) to D.G.M.
References
- Barthou, H., Petitprez, M., Brière, C., Souvré, A., and Alibert, G. (1999). RGD-mediated membrane-matrix adhesion triggers agarose-induced embryoid formation in sunflower protoplasts. Protoplasma 206, 143–151. [Google Scholar]
- Canut, H., Carrasco, A., Galaud, J., Cassan, C., Bouyssou, H., Vita, N., Ferrara, P., and Pont-Lezica, R. (1998). High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana link the cell wall. Plant J. 16, 63–71. [DOI] [PubMed] [Google Scholar]
- Doke, N. (1983). Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 23, 345–357. [Google Scholar]
- García-Gómez, B.I., Campos, F., Hernandez, M., and Covarrubias, A.A. (2000). Two bean cell wall proteins more abundant during water deficit are high in proline and interact with a plasma membrane protein. Plant J. 22, 277–288. [DOI] [PubMed] [Google Scholar]
- Giancotti, F.G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Gus-Mayer, S., Naton, B., Hahlbrock, K., and Schmelzer, E. (1998). Local mechanical stimulation induces components of the pathogen defense response in parsley. Proc. Natl. Acad. Sci. USA 95, 8398–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z., He, D., and Kohorn, B.D. (1998). Requirement for the induced expression of a cell wall associated receptor kinase for survival during the pathogen response. Plant J. 14, 55–63. [DOI] [PubMed] [Google Scholar]
- Heath, M.C. (1997). Signalling between pathogenic rust fungi and resistant or susceptible host plants. Ann. Bot. 80, 713–720. [Google Scholar]
- Heath, M.C. (1998). Involvement of reactive oxygen species in the response of resistant (hypersensitive) or susceptible cowpeas to the cowpea rust fungus. New Phytol. 138, 251–263. [DOI] [PubMed] [Google Scholar]
- Heath, M.C., and Škalamera, D., (1997). Cellular interactions between plants and biotrophic fungal parasites. Adv. Bot. Res. 24, 195–225. [Google Scholar]
- Heath, M.C., Nimchuk, Z.L., and Xu, H. (1997). Plant nuclear migrations as indicators of critical interactions between resistant or susceptible cowpea epidermal cells and invasion hyphae of the cowpea rust fungus. New Phytol. 135, 689–700. [Google Scholar]
- Henry, C.A., Jordan, J.R., and Kropf, D.L. (1996). Localized membrane-wall adhesions in Pelvetia zygotes. Protoplasma 190, 39–52. [Google Scholar]
- Hückelhoven, R., and Kogel, K. (1998). Tissue-specific superoxide generation at interaction sites in resistant and susceptible near-isogenic barley lines attacked by the powdery mildew fungus (Erysiphe graminis f. sp. hordei). Mol. Plant-Microbe Interact. 11, 292–300. [Google Scholar]
- Kiba, A., Sugimoto, M., Toyoda, K., Ichinose, Y., Yamada, T., and Shiraishi, T. (1998). Interaction between cell wall and plasma membrane via RGD motif is implicated in plant defense responses. Plant Cell Physiol. 39, 1245–1249. [Google Scholar]
- Kobayashi, Y., Yamada, M., Kobayashi, I., and Kunoh, H. (1997). Actin microfilaments are required for the expression of nonhost resistance in higher plants. Plant Cell Physiol. 38, 725–733. [Google Scholar]
- Lang-Pauluzzi, I. (2000). The behaviour of the plasma membrane during plasmolysis: A study by UV microscopy. J. Microsc. 198, 188–198. [DOI] [PubMed] [Google Scholar]
- Laval, V., Chabannes, M., Carrière, M., Canut, H., Barre, A., Rougé, P., Pont-Lezica, R., and Galaud, J. (1999). A family of Arabidopsis plasma membrane receptors presenting animal β-integrin domains. Biochim. Biophys. Acta 1435, 61–70. [DOI] [PubMed] [Google Scholar]
- Lee-Stadelmann, O.Y., Bushnell, W.R., and Stadelmann, E.J. (1984). Changes of plasmolysis form in epidermal cells of Hordeum vulgare infected by Erysiphe graminis: Evidence for increased membrane-wall adhesion. Can. J. Bot. 62, 1714–1723. [Google Scholar]
- Meyer, S.L., and Heath, M.C. (1988). A comparison of the death induced by fungal invasion or toxic chemicals in cowpea epidermal cells. II. Responses induced by Erysiphe cichoracearum. Can. J. Bot. 66, 624–634. [Google Scholar]
- Mould, M.J.R., and Heath, M.C. (1999). Ultrastructural evidence of differential changes in transcription, translation, and cortical microtubules during in planta penetration of cells resistant or susceptible to rust infection. Physiol. Mol. Plant Pathol. 55, 225–236. [Google Scholar]
- Nagpal, P., and Quatrano, R.S. (1999). Isolation and characterization of a cDNA clone from Arabidopsis thaliana with partial sequence similarity to integrins. Gene 230, 33–40. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J. (1994). Tansley Review No. 67. Plasmolysis: New insights into an old process. New Phytol. 126, 571–591. [Google Scholar]
- Perumalla, C.J., and Heath, M.C. (1989). Effect of callose inhibition on haustorium formation by the cowpea rust fungus in the non-host, bean plant. Physiol. Mol. Plant Pathol. 35, 375–382. [Google Scholar]
- Perumalla, C.J., and Heath, M.C. (1991). The effect of inhibitors of various cellular processes on the wall modifications induced in bean leaves by the cowpea rust fungus. Physiol. Mol. Plant Pathol. 38, 293–300. [Google Scholar]
- Reuzeau, C., and Pont-Lezica, R.F. (1995). Comparing plant and animal extracellular matrix–cytoskeleton connections: Are they alike? Protoplasma 186, 113–121. [Google Scholar]
- Ruoslahti, E. (1996). RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715. [DOI] [PubMed] [Google Scholar]
- Russo, V.M., and Bushnell, W.R. (1989). Responses of barley cells to puncture by microneedles and to attempted penetration by Erysiphe graminis f. sp. hordei. Can. J. Bot. 67, 2912–2921. [Google Scholar]
- Schindler, M., Meiners, S., and Cheresh, D.A. (1989). RGD-dependent linkage between plant cell wall and plasma membrane: Consequences for growth. J. Cell Biol. 108, 1955–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škalamera, D., and Heath, M.C. (1998). Changes in the cytoskeleton accompanying infection-induced nuclear movements and the hypersensitive response in plant cells invaded by rust fungi. Plant J. 16, 191–200. [DOI] [PubMed] [Google Scholar]
- Škalamera, D., Jobodh, S., and Heath, M.C. (1997). Callose deposition during the interactions between cowpea (Vigna unguiculata) and the monokaryotic stage of the cowpea rust fungus (Uromyces vignae). New Phytol. 136, 511–524. [DOI] [PubMed] [Google Scholar]
- Stumpf, M.A., and Heath, M.C. (1985). Cytological studies of the interactions between the cowpea rust fungus and silicon-depleted French bean plants. Physiol. Plant Pathol. 27, 369–385. [Google Scholar]
- Thordal-Christensen, H., Zhang, Z., Wei, Y., and Collinge, D.B. (1997). Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Wayne, R., Staves, M.P., and Leopold, A.C. (1992). The contribution of the extracellular matrix to gravisensing in characean cells. J. Cell Sci. 101, 611–623. [DOI] [PubMed] [Google Scholar]