Abstract
Background:
Rates of suicide and self-harm are elevated among people with opioid use disorder (OUD). This study examined incidence of self-harm and suicide among people who have entered OAT and assessed the impact of different OAT exposure periods on these events.
Method:
We conducted a retrospective population-based cohort study of all OAT recipients (N=45,664) in New South Wales, Australia (2002–2017), using linked administrative data. Incidence rates of self-harm hospitalisations and suicide deaths were estimated per 1000 person-years (PY). The first 28 days of an OAT episode, ≥29 days on OAT, the first 28 days off OAT, and ≥29 days off OAT (maximum four years post-OAT) were exposure periods. Poisson regression models with generalised estimating equations estimated the adjusted incidence rate ratios (ARR) of self-harm and suicide by OAT exposure periods, adjusting for covariates.
Results:
There were 7482 hospitalisations (4148 individuals) for self-harm and 556 suicides, equating to incidence rates of 19.2 (95% confidence intervals [CI]=18.8–19.7) and 1.0 (95%CI=0.9–1.1) per 1000 PY, respectively. Opioid overdose was implicated in 9.6% of suicides and 28% of self-harm hospitalisations. Compared to ≥29 days on OAT, the incidence rate of suicide was elevated in the 28 days following OAT cessation (ARR=17.4 [95%CI=11.7–25.9]), and the rate of self-harm hospitalisations was elevated during the first 28 days of OAT (ARR=2.2 [95%CI=1.9–2.6]) and the 28 days after leaving OAT (ARR=2.7 [95%CI=2.3–3.2]).
Conclusions:
OAT may reduce suicide and self-harm risk among people with OUD; however, OAT initiation and cessation are critical periods for targeting self-harm and suicide prevention interventions.
Keywords: Suicide, self-harm, opioid agonist treatment, opioid use disorder
1. Introduction
Globally, it is estimated that over 700,000 people die by suicide each year1. The estimated global rate of suicide is approximately 9.4 deaths per 100,000 people, and is higher among men (13.3 deaths per 100,000) than women (5.7 deaths per 100,000)2. The World Health Organization’s (WHO) Comprehensive Mental Health Action Plan aims to reduce the rate of suicide by one-third by 20303.
Non-fatal self-harm is the most well-established risk factor for suicide and, therefore, often a key outcome measure in suicide prevention intervention research4,5. Definitions of non-fatal self-harm vary in the literature, but often include non-suicidal self-injury and non-fatal suicide attempts. This is because suicidal intent can fluctuate within people who self-harm and is challenging to identify in clinical settings2,6. According to the Global Burden of Disease study, the incidence of non-fatal self-harm (regardless of suicidal intent) is 52.5 per 100,000 men and 75.9 per 100,000 women7,8. It is likely that the true burden of suicide and self-harm is higher due to under-reporting and miscoding2.
Suicide and self-harm are substantially elevated among people with opioid use disorder (OUD). A global systematic review found that people who use extra-medical opioids (i.e. heroin, illicitly manufactured opioids, or pharmaceutical opioids outside the bounds of a medical prescription) die by suicide at nearly eight times the expected rate, with suicide accounting for around one in 12 deaths among this group9. An Australian study found that people with OUD were over 23 times, (risk ratio [RR]=23.6; 95% confidence intervals [CI]=17.9–31.1), as likely to present to hospital with self-harm compared to matched controls without OUD10. A similar study conducted in Hong Kong observed that relative to other substance use disorders, OUD was associated with the highest risk of suicide and self-harm (hazards ratio [HR]=27.3; 95%CI=19.5–38.3) compared to matched controls11. Targeting self-harm and suicide prevention to people with OUD is therefore critical, yet, globally, national suicide prevention policies rarely include substance-use specific policies12,13.
Although there is evidence that some psychosocial (e.g. cognitive behavioural therapy and dialectical behavioural therapy) and pharmacological (e.g. anti-depressants and ketamine) interventions can reduce suicidal thoughts and behaviour in the general population, people with OUD are often excluded from these trials and few clinical interventions have been demonstrated to reduce fatal suicide risk5,14. There is, however, evidence that opioid agonist treatment (OAT) with methadone or buprenorphine is associated with a reduced risk of suicide among people with OUD15. Some form of OAT is available in 90 countries, however there is low coverage of access globally16. In Australia, OAT is administered within a framework including medical, social and psychological treatment and coverage is deemed high according to the WHO, with nearly 50,000 people accessing treatment in 202017.
The beneficial effects of OAT in reducing morbidity and mortality among people with OUD are well-documented 18,19. More recently, a systematic review found that time in OAT was associated with more than a 50% risk reduction in suicide compared to time out of OAT18. Although the review was able to look at specific risk periods in and out of OAT for all-cause and overdose mortality, there were insufficient data to examine the risk of suicide during specific time periods of OAT exposure20,21.
A recent UK study examining the impact of OAT on self-harm hospitalisations and suicide found the four weeks following treatment cessation were associated with an elevated risk for both outcomes, compared to more than four weeks on OAT21. The risk of self-harm hospitalisations was also elevated in the remainder of time off OAT; however, there was insufficient power to detect any effect of other OAT exposure periods on mortality due to the small number of suicide deaths. Hence, further work is needed to understand the risk of self-harm and suicide in relation to specific OAT exposure periods. Moreover, no study has yet examined the incidence of self-harm and suicide by the method used to self-harm, nor whether there are differential effects of OAT exposure across different self-harm methods.
This study aims to:
Estimate the incidence of self-harm and suicide among people with OUD, overall, and by sex and method.
Compare rates of self-harm and suicide during different OAT exposure time periods to stable periods on OAT.
2. Methods
We used data from the Opioid Agonist Treatment Safety (OATS) Study22. Full details of the study, setting, data sources, linkage approach, and prescribing practices in New South Wales (NSW) have been reported previously22. The findings of this study are reported in line with the Reporting of studies Conducted using Observational Routinely collected Data (RECORD) guidelines23.
2.1. Study design, setting, and participants
This was a retrospective cohort study using linked administrative data. The cohort comprised all people initiating or maintained on OAT between 1 August 2002 and 31 December 2017 in NSW, Australia, as recorded in the NSW Electronic Reporting and Recording of Controlled Drugs (ERRCD). A substantial proportion of people with a history of illicit opioid dependence in NSW enter OAT at some point in time24,25, therefore closely approximating the OUD population.
Methadone and buprenorphine (including buprenorphine-naloxone) are available in public, private, and carceral settings in NSW for the management of OUD. Prescribers are required to submit an “Authority to Prescribe” form to the NSW Ministry of Health for people entering treatment and are required to inform the regulatory body when OAT medication (e.g., methadone or buprenorphine), dispense setting, or treatment enrolment changes.
2.2. Data linkage
Four databases were linked to individuals in the ERRCD by the Centre for Health Record Linkage (CHeReL) using probabilistic linkage methods. Records were matched to an individual’s name, sex, date of birth, and state of residence. The Admitted Patients Data Collection (APDC) database includes all hospitalisations in NSW. Primary and secondary diagnoses were recorded using the International Statistical Classification of Diseases and Related Health Problems 10th edition (modified for use in Australia; ICD-10-AM).
Information on state-wide mental health treatment and incarceration were sourced from the Mental Health and Ambulatory Data Collection (MH-AMB) and the Re-offending database (ROD), respectively. Mortality and cause of death data were drawn from the National Death Index (NDI). Further detail on the data sources are profiled elsewhere22.
2.3. Variables
2.3.1. Exposure
OAT status was defined by four distinct time-periods: 1–28 days on OAT, ≥29 days on OAT, 1–28 days after OAT cessation, and ≥29 days after OAT cessation21. Consistent with previous work20,26–28, treatment episodes that recommenced within six days of an episode ceasing were considered a continuous episode, even if the OAT medication changed.
OAT exposure time commenced on 1 August 2002, or the date of a participant’s first OAT episode. Participants exited the study on 31 December 2017, or four years after treatment cessation, or their date of death29. The four-year cut-off was informed by the data, it was observed that 90% of re-entry treatment episodes started within 50 months of the cessation of the last treatment episode. All OAT episodes, including first and repeat episodes, were included. Participants who moved out of NSW during the study period were unable to be followed up and were considered out of treatment unless their death was otherwise captured in the NDI.
2.3.2. Outcomes
Self-harm hospitalisations and suicide deaths were the outcomes of interest. Coding from the ICD-10-AM was used to sub-categorise self-harm and underlying cause of suicide death into overdose (opioid [T40.0-T40.4] and other substances [T40.5-T40.9]; X60-X66 and X68-X69), violent (X67, X70-X75, and X80-X82), cutting or piercing (X78), and other (e.g. self-harm by smoke or blunt object; X76-X77, X79, X82-X84) methods, and were adapted from previously defined categories30 (Supplement; eTable 1).
2.3.3. Covariates and time-varying confounders
Several demographic and clinical variables were examined; justifications for the inclusion and ICD-10-AM codes for each of the variables are provided in eTable 2 in the Supplement.
Demographic:
Sex, Indigenous status, socio-economic disadvantage, and geographical remoteness were static variables. Participants who identified as Aboriginal or Torres Strait Islander in any dataset were considered Indigenous in the analyses. Postcode-level socio-economic disadvantage was categorised using the Socio-Economic Indexes for Areas (SEIFA) to postcode at last OAT enrolment. Geographical remoteness was determined based on the participant’s latest postcode of residence (drawn from the ERRCD)31. Age, calendar years of observation, and past 12-month incarceration were time-varying variables.
Clinical:
We derived five time-varying potential confounders to indicate receipt of drug-related or mental health treatment in the previous 12 months. Mental health treatment in an inpatient or outpatient setting that recorded anxiety or depression (without psychosis) or a severe mental disorder (i.e. schizophrenia, manic episode, bipolar affective disorder, or severe depressive episodes with psychotic symptoms) were derived. Hospital records in the previous 12 months for self-harm, overdose (unintentional), injecting-related injuries and diseases, or assault were also derived.
2.4. Data cleaning
The data cleaning process to derive the cohort is documented elsewhere32. A flowchart documenting participants excluded at each stage of the data cleaning process is in the Supplement (eFigure 1).
2.5. Data analysis
OAT treatment data for the cohort were transformed into discrete observation windows of defined exposure periods (see eFigure 2 for example). Within each observation period, the number of hospitalisations for self-harm and suicide were counted. The frequency of events and crude incidence rates with 95%CI for hospitalisations for any self-harm and suicide deaths were calculated and stratified by method of self-harm. Crude incidence rates of hospitalisations were calculated for each OAT exposure period, demographic characteristics, and recent hospitalisations per 1000 person-years (PY).
Poisson regression models with generalised estimating equations (GEE) were used to calculate the adjusted incidence rate ratios (ARR) of self-harm and suicide per 1000 PY with 95%CI. In addressing the second aim of the study, all variables were included as potential confounders in the adjusted model to compare the impact of different OAT exposure periods on self-harm hospitalisations and suicide mortality. GEE models adjust for multiple observations per individual by using a working correlation structure, providing robust parameter estimates. Due to a small number of events (n<5), the rates of suicide in the first 28 days of treatment were unable to be reported.
Tukey–Kramer adjustment was used to hold the experiment-wise error rate at 5% when comparing incidence rates by exposure level. The Score statistic was used to assess the importance of terms in the models. Analyses were conducted in SAS V9.4 (SAS Institute Inc. Cary, NC, USA).
2.6. Sensitivity analyses
As intent of some self-harm and suicide incidents may be unclear and therefore classified as undetermined33 (ICD-10-AM codes Y10-Y34; see eTable 1 in the Supplement), sensitivity analyses were conducted to include these events in the GEE models. Second, to account for deaths that occurred within six days of the last treatment episode (during which it is assumed that there may be some residual opioid exposure), an additional sensitivity analysis was conducted to extend the OAT exposure period by six days post-cessation in the GEE models. Finally, we tested whether adjusting the out of OAT exposure period from a maximum of four years to a cut-off of 12 months would change the interpretation of the results.
2.7. Ethics
Approval for this study was obtained from the NSW Population & Health Services Research Ethics Committee (2018/HRE0205), the NSW Corrective Services Ethics Committee and the Aboriginal Health and Medical Research Council Ethics Committee (1400/18).
3. Results
There were 45,664 individuals (67.7% male; median age 32 [IQR=26–39] years) in the cohort (Table 1). One in five were Indigenous (21.0%) and one in four were living in regional or remote communities (26.5%), and 23.6% of individuals ranked as the most socioeconomically disadvantaged.
Table 1.
Cohort demographic information
| Cohort characteristics | N | % | |
|---|---|---|---|
|
| |||
| Sex a | Female | 14,770 | 32.3% |
| Male | 30,892 | 67.7% | |
| Age at cohort entry | <=24 years | 9,471 | 20.7% |
| 25–34 years | 18,318 | 40.1% | |
| 35–44 years | 12,945 | 28.3% | |
| >=45 years | 4,930 | 10.8% | |
| Calendar year of entry | 2002–2005 | 26,251 | 57.5% |
| 2006–2009 | 6,755 | 14.8% | |
| 2010–2013 | 6,491 | 14.2% | |
| 2014–2017 | 6,167 | 13.5% | |
| Indigenous | 9,580 | 21.0% | |
| Geographical remoteness b | Major Cities of NSW | 33,345 | 73.0% |
| Regional/Remote NSW | 12,084 | 26.5% | |
| Socioeconomic status c | Most Disadvantaged | 10,769 | 23.6% |
| 2nd Quintile | 8,536 | 18.7% | |
| 3rd Quintile | 11,954 | 26.2% | |
| 4th Quintile | 8,358 | 18.3% | |
| Least Disadvantaged | 5,556 | 12.2% | |
| Hospitalisation | Self-harm | 4,148 | 9.1% |
| Deaths | Total | 4,491 | 9.8% |
| Suicide | 556 | 1.2% | |
Sex information was unavailable for 2 participants.
Geographical information was unavailable for 235 participants.
Socioeconomic status information was unavailable for 491 participants.
There were 4,148 people (9.1%) with a self-harm hospitalisation (Table 1 and Supplement eTable 3), equating to an overall crude rate of 19.2 per 1,000 PY (95%CI=18.8–19.7); the rate was higher among women than men (risk ratio [RR]=1.4; 95%CI=1.3–1.5; Table 2). The most common method of self-harm was non-opioid overdose (52.7% of all individuals with a self-harm hospitalisation), followed by opioid overdose (39.3%) and cutting or piercing (22.5%).
Table 2.
Crude incidence rates and risk ratios of intentional self-harm hospitalisations and suicide by method and sex.
| Crude incidence rate per 1000 person-years (95% CIa) | Risk ratio | |||
|---|---|---|---|---|
|
| ||||
| Outcome | All individuals | Females | Males | (ref b =Males) |
| Self-harm hospitalisations | 19.2 (18.8–19.7) | 23.7 (22.9–24.6) | 17.0 (16.5–17.5) | 1.40 (1.34–1.46) |
| Overdose | 14.4 (14.1–14.8) | 18.1 (17.4–18.8) | 12.6 (12.2–13.0) | 1.44 (1.37–1.51) |
| Opioid overdose | 5.3 (5.1–5.6) | 6.2 (5.8–6.6) | 4.9 (4.7–5.2) | 1.25 (1.15–1.37) |
| Non-opioid overdose | 9.1 (8.8–9.4) | 11.9 (11.3–12.5) | 7.6 (7.3–8.0) | 1.56 (1.46–1.66) |
| Violent self-harm method | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 1.01 (0.82–1.26) |
| Cutting or piercing | 3.7 (3.5–3.9) | 4.7 (4.3–5.1) | 3.0 (3.0–3.4) | 1.49 (1.34–1.64) |
| Other method | 0.9 (0.8–1.0) | 0.9 (0.7–1.0) | 0.9 (0.8–1.0) | 0.97 (0.78–1.21) |
| Suicide | 1.0 (0.9–1.1) | 0.7 (0.5–0.8) | 1.1 (1.0–1.2) | 0.61 (0.48–0.77) |
| Overdose | 0.3 (0.3–0.4) | 0.3 (0.2–0.4) | 0.4 (0.3–0.4) | 0.83 (0.58–1.19) |
| Opioid overdose | 0.1 (0.1–0.1) | 0.1 (0.0–0.1) | 0.1 (0.1–0.2) | 0.66 (0.33–1.30) |
| Non-opioid overdose | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.91 (0.59–1.40) |
| Violent suicide method | 0.6 (0.6–0.7) | 0.4 (0.3–0.5) | 0.7 (0.7–0.9) | 0.56 (0.42–0.75) |
CI=Confidence intervals
Referent category
Of the 4,491 deaths in the cohort, there were 556 suicide deaths (12.4%). The crude incidence rate of suicide was 1.0 per 1,000 PY (95%CI=0.9–1.1); this rate was lower among women than men (RR=0.6; 95%CI=0.5–0.8; Table 2). There was no gender disparity in rates of suicide by opioid overdose. Most suicide deaths did not involve opioids: they were predominantly classified as violent (66.0%), followed by non-opioid overdose (23.9%; eTable 3).
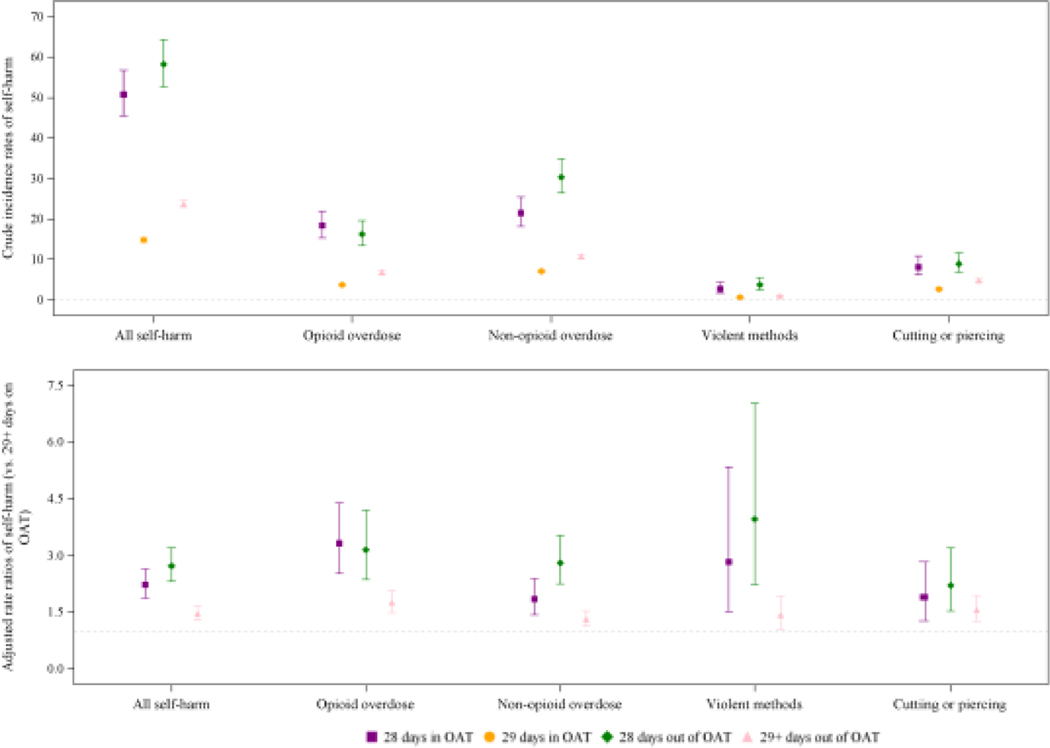
Table 3 presents the rates of self-harm hospitalisations by OAT exposure period; the crude incidence rate was 50.8 (95%CI=45.5–56.7) per 1000 PY in the first 28 days on OAT, 14.8 (95%CI=14.3–5.3) per 1000 PY in the remaining time on OAT, 58.2 (95%CI=52.7–64.3) per 1000 PY in the first 28 days off OAT, and 23.8 (95%CI=23.0–24.6) per 1000 PY in the remainder of time off OAT. This corresponded to similarly elevated risks of self-harm hospitalisation in the 28 days after OAT initiation and cessation (ARR=2.2; 95%CI=1.9–2.6; and ARR=2.7; 95%CI=2.3–3.2, respectively) compared to the remainder of time on OAT (Figure 1). The remainder of time off OAT was associated with a 46% increased risk of a self-harm hospitalisation (ARR 1.5; 95%CI=1.3–1.7). The impact of OAT exposure was similar across self-harm methods (eTable 4).
Table 3.
Crude incidence rates per 1000 person-years and crude and adjusted rate ratios of self-harm hospitalisations by opioid agonist treatment (OAT) exposure, demographic, and clinical information.
| Person-years | Self-harm hospitalisations n | Crude incidence rate (95%CI) | Crude rate ratio (95%CI) | Adjusted rate ratio (95%CI) | Score (p-value) | |
|---|---|---|---|---|---|---|
| Exposure | ||||||
| In OAT (1–28 days) | 50.8 (45.5–56.7) | 3.44 (3.07–3.85) | 2.23 (1.87–2.65) | 183.27 (<.001) | ||
| Out of OAT (1–28 days) | 58.2 (52.7–64.3) | 3.94 (3.55–4.37) | 2.73 (2.32–3.21) | |||
| In OAT (29+ days) | 14.8 (14.3–15.3) | Ref. | Ref. | |||
| Out of OAT (29+ days) | 23.8 (23.0–24.6) | 1.61 (1.54–1.68) | 1.46 (1.29–1.66) | |||
| Calendar year | ||||||
| 2014–2017 | 111399 | 2342 | 21.0 (20.1–21.8) | 1.18 (1.10–1.26) | 1.21 (1.10–1.32) | 16.92 (<.001) |
| 2010–2013 | 112339 | 1956 | 17.4 (16.6–18.2) | 0.98 (0.91–1.04) | 1.03 (0.94–1.13) | |
| 2006–2009 | 111117 | 2264 | 20.3 (19.5–21.2) | 1.14 (1.07–1.22) | 1.09 (0.97–1.23) | |
| 2002–2005 | 84948 | 1519 | 17.8 (16.9–18.7) | Ref. | Ref. | |
| Sex | ||||||
| Female | 138239 | 3288 | 23.7 (22.9–24.6) | 1.40 (1.34–1.46) | 1.23 (1.10–1.37) | 5.84 (0.016) |
| Male | 281563 | 4793 | 17.0 (16.5–17.5) | Ref. | Ref. | |
| Age | ||||||
| <=24 years | 27542 | 784 | 28.4 (26.5–30.5) | 2.06 (1.89–2.24) | 1.74 (1.50–2.01) | 16.66 (<.001) |
| 25–34 years | 132594 | 3063 | 23.0 (22.2–23.9) | 1.67 (1.57–1.78) | 1.49 (1.32–1.69) | |
| 35–44 years | 144993 | 2652 | 18.2 (17.5–18.9) | 1.32 (1.24–1.41) | 1.21 (1.11–1.33) | |
| >=45 years | 114672 | 1582 | 13.8 (13.1–14.5) | Ref. | Ref. | |
| Indigenous | ||||||
| Yes | 87097 | 2569 | 29.4 (28.3–30.6) | 1.78 (1.70–1.87) | 1.53 (1.34–1.74) | 8.77 (<.001) |
| No | 332704 | 5512 | 16.5 (16.1–17.0) | Ref. | Ref. | |
| Socio-economic status | ||||||
| Least Disadvantaged | 50661 | 1044 | 20.5 (19.3–21.8) | 1.27 (1.18–1.38) | 1.18 (1.04–1.33) | 5.78 (0.216) |
| 4th Quintile | 78991 | 1646 | 20.7 (19.8–21.8) | 1.29 (1.20–1.38) | 1.15 (1.03–1.29) | |
| 3rd Quintile | 109540 | 2214 | 20.2 (19.4–21.1) | 1.25 (1.18–1.34) | 1.14 (1.03–1.27) | |
| 2nd Quintile | 79899 | 1551 | 19.3 (18.4–20.3) | 1.20 (1.12–1.29) | 1.14 (0.93–1.40) | |
| Most Disadvantaged | 100711 | 1626 | 16.1 (15.3–16.9) | Ref. | Ref. | |
| Geographical remoteness | ||||||
| Major Cities of NSW | 316885 | 6267 | 19.7 (19.2–20.2) | 1.12 (1.06–1.18) | 1.12 (1.01–1.24) | 2.88 (0.089) |
| Regional/Rem ote NSW | 102916 | 1814 | 17.6 (16.8–18.4) | Ref. | Ref. | |
| Recenta incarceration | ||||||
| Yes | 52015 | 1886 | 36.2 (34.6–37.8) | 2.15 (2.04–2.27) | 1.61 (1.45–1.78) | 16.67 (<.001) |
| No | 367787 | 6195 | 16.8 (16.4–17.2) | Ref. | Ref. | |
| Received treatment in the past 12 months for... | ||||||
| Self-harm | ||||||
| Yes | 5960 | 2654 | 444.5 (427.9–461.8) | 34.02 (32.47–35.64) | 19.01 (16.69–21.67) | 111.06 (<.001) |
| No | 413841 | 5427 | 13.1 (12.7–13.4) | Ref. | Ref. | |
| Severe mental health | ||||||
| Yes | 23349 | 2846 | 121.2 (116.8–125.8) | 9.20 (8.78–9.63) | 4.62 (4.15–5.15) | 108.35 (<.001) |
| No | 396453 | 5235 | 13.2 (12.8–13.5) | Ref. | Ref. | |
| Depression/anxiety | ||||||
| Yes | 16281 | 4156 | 254.7 (247.1–262.6) | 26.29 (25.17–27.46) | 17.13 (15.88–18.46) | 694.23 (<.001) |
| No | 403521 | 3925 | 9.7 (9.4–10.0) | Ref. | Ref. | |
| Overdose | ||||||
| Yes | 7557 | 2295 | 303.0 (290.8–315.6) | 21.65 (20.63–22.73) | 9.08 (7.85–10.51) | 36.62 (<.001) |
| No | 412244 | 5786 | 14.0 (13.6–14.4) | Ref. | Ref. | |
| Injecting-related disease | ||||||
| Yes | 12662 | 1052 | 83.1 (78.2–88.2) | 4.83 (4.52–5.15) | 2.72 (2.28–3.25) | 19.72 (<.001) |
| No | 407140 | 7029 | 17.2 (16.8–17.6) | Ref. | Ref. | |
| Assault | ||||||
| Yes | 4803 | 523 | 108.7 (99.7–118.4) | 5.98 (5.48–6.54) | 2.71 (2.37–3.09) | 22.02 (<.001) |
| No | 414999 | 7558 | 18.2 (17.8–18.6) | Ref. | Ref. | |
Past 12 months.
CI: Confidence intervals
Figure 1. Crude incidence rates and adjusted rate ratios of self-harm hospitalisations by opioid agonist treatment (OAT) exposure and self-harm method.
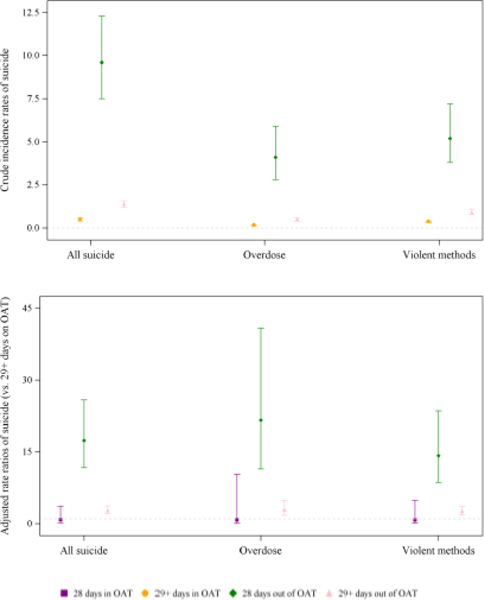
For the remainder of time on OAT (≥29 days), the crude incidence rate for suicide was 0.5 (95%CI=0.4–0.6) per 1000 PY, 9.6 (95%CI=7.5–12.3) per 1000 PY in the 28 days following OAT cessation, and 1.4 (95%CI=1.2–1.6) for the remainder of time off treatment (Table 4). Compared to ≥29 days on OAT, there was a markedly elevated risk of suicide mortality in the month following OAT cessation (ARR=17.4; 95%CI=11.7–25.8) and a 2.8-fold increased risk in the remainder of time off OAT (95%CI=2.1–3.7). There was no evidence of an increased risk of suicide mortality in the first 28 days on OAT compared to the rest of time on OAT. The increased risk of suicide in the 28 days following OAT cessation appeared to be higher for overdose (ARR=21.6 [95%CI=11.4–40.8]) and lower for violent methods (ARR=14.2 [95%CI=8.6–23.5]), compared to suicide overall; however, confidence intervals overlapped (Figure 2 and eTable 4).
Table 4.
Crude incidence rates per 1000 person-years and crude and adjusted rate ratios of suicide mortality by opioid agonist treatment (OAT) exposure, demographic, and clinical information.
| Person-years | Suicides n | Crude incidence rate (95%CI) | Crude rate ratio (95%CI) | Adjusted rate ratio (95%CI) | Score (p-value) | |
|---|---|---|---|---|---|---|
| Exposure | ||||||
| In OAT (1–28 days) | - | 0.5 (0.2–1.5) | 0.93 (0.30–2.92) | 0.82 (0.18–3.67) | 119.1 (<.001) | |
| Out of OAT (1–28 days) | - | 9.6 (7.5–12.3) | 18.83 (13.97–25.38) | 17.39 (11.69–25.85) | ||
| In OAT (29+ days) | - | 0.5 (0.4–0.6) | Ref. | Ref. | ||
| Out of OAT (29+ days) | - | 1.4 (1.2–1.6) | 2.71 (2.18–3.38) | 2.81 (2.10–3.75) | ||
| Calendar year | ||||||
| 2014–2017 | 8.3 | |||||
| 111399 | 131 | 1.2 (1.0–1.4) | 1.49 (1.11–2.00) | 1.73 (1.28–2.34) | (0.041) | |
| 2010–2013 | 112339 | 111 | 1.0 (0.8–1.2) | 1.25 (0.93–1.70) | 1.43 (1.05–1.94) | |
| 2006–2009 | 111117 | 96 | 0.9 (0.7–1.1) | 1.10 (0.80–1.50) | 1.15 (0.83–1.58) | |
| 2002–2005 | 84948 | 67 | 0.8 (0.6–1.0) | Ref. | Ref. | |
| Sex | ||||||
| Female | 138239 | 93 | 0.7 (0.5–0.8) | 0.61 (0.48–0.77) | 0.58 (0.46–0.74) | 20.7 (<.001) |
| Male | 281563 | 312 | 1.1 (1.0–1.2) | Ref. | Ref. | |
| Age | ||||||
| <=24 years | 27542 | 21 | 0.8 (0.5–1.2) | 0.75 (0.47–1.20) | 0.59 (0.37–0.95) | 7.0 (0.072) |
| 25–34 years | 132594 | 124 | 0.9 (0.8–1.1) | 0.92 (0.72–1.19) | 0.80 (0.62–1.05) | |
| 35–44 years | 144993 | 144 | 1.0 (0.8–1.2) | 0.98 (0.77–1.25) | 0.90 (0.70–1.15) | |
| >=45 years | 114672 | 116 | 1.0 (0.8–1.2) | Ref. | Ref. | |
| Indigenous | ||||||
| Yes | 87097 | 68 | 0.8 (0.6–1.0) | 0.77 (0.59–1.00) | 0.70 (0.54–0.92) | 8.5(0.003) |
| No | 332704 | 337 | 1.0 (0.9–1.1) | Ref. | Ref. | |
| Socio-economic status | ||||||
| Least Disadvantaged | 50661 | 58 | 1.1 (0.9–1.5) | 1.52 (1.08–2.14) | 1.52 (1.08–2.13) | 5.5(0.243) |
| 2nd Quintile | 79899 | 76 | 1.0 (0.8–1.2) | 1.26 (0.92–1.73) | 1.26 (0.90–1.75) | |
| 3rd Quintile | 109540 | 110 | 1.0 (0.8–1.2) | 1.33 (0.99–1.78) | 1.31 (0.97–1.75) | |
| 4th Quintile | 78991 | 85 | 1.1 (0.9–1.3) | 1.43 (1.05–1.94) | 1.40 (1.03–1.92) | |
| Most Disadvantaged | 100711 | 76 | 0.8 (0.6–0.9) | Ref. | Ref. | |
| Geographical remoteness | ||||||
| Major Cities of NSW | 316885 | 319 | 1.0 (0.9–1.1) | 1.20 (0.95–1.53) | 1.21 (0.95–1.54) | 0.7 (0.411) |
| Regional/Remote | 102916 | 86 | 0.8 (0.7–1.0) | Ref. | Ref. | |
| NSW | ||||||
| Recenta incarceration | ||||||
| Yes | 52015 | 67 | 1.3 (1.0–1.6) | 1.40 (1.08–1.82) | 1.13 (0.87–1.49) | 0.3 (0.584) |
| No | 367787 | 338 | 0.9 (0.8–1.0) | Ref. | Ref. | |
| Received treatment in the past 12 months for... | ||||||
| Self-harm | ||||||
| Yes | 5960 | 42 | 7.0 (5.2–9.5) | 8.03 (5.84–11.06) | 5.19 (3.56–7.57) | 1.3 (0.260) |
| No | 413841 | 363 | 0.9 (0.8–1.0) | Ref. | Ref. | |
| Severe mental health | ||||||
| Yes | 23349 | 66 | 2.8 (2.2–3.6) | 3.31 (2.54–4.30) | 2.45 (1.83–3.28) | 8.2 (0.004) |
| No | 396453 | 339 | 0.9 (0.8–1.0) | Ref. | Ref. | |
| Depression/anxiety | ||||||
| Yes | 16281 | 86 | 5.3 (4.3–6.5) | 6.68 (5.27–8.48) | 5.39 (4.09–7.09) | 34.4 (<.001) |
| No | 403521 | 319 | 0.8 (0.7–0.9) | Ref. | Ref. | |
| Overdose | ||||||
| Yes | 7557 | 49 | 6.5 (4.9–8.6) | 7.51 (5.57–10.12) | 5.12 (3.57–7.36) | 8.3 (0.004) |
| No | 412244 | 356 | 0.9 (0.8–1.0) | Ref. | Ref. | |
| Injecting-related disease | ||||||
| Yes | 12662 | 31 | 2.4 (1.7–3.5) | 2.66 (1.85–3.84) | 2.08 (1.43–3.03) | 3.9 (0.049) |
| No | 407140 | 374 | 0.9 (0.8–1.0) | Ref. | Ref. | |
| Assault | ||||||
| Yes | 4803 | 9 | 1.9 (1.0–3.6) | 1.96 (1.01–3.80) | 1.37 (0.69–2.69) | 0.0 (0.974) |
| No | 414999 | 396 | 1.0 (0.9–1.1) | Ref. | Ref. | |
Past 12 months.
CI: Confidence intervals
Figure 2. Crude incidence rates and adjusted rate ratios of suicide overall, by method, and by opioid agonist treatment (OAT) exposure.
3.1. Sensitivity analyses
There were 2,296 people who presented to hospital with self-harm of undetermined intent, and an additional 199 deaths of undetermined intent (eTable 5). Analyses that included these events did not generate any meaningful differences in the magnitude of risk for the first 28 days and ≥29 days off OAT, compared to ≥29 days in OAT (eTable 6). The crude incidence rate of suicide in the first 28 days of OAT was marginally higher than ≥29 days on OAT; however, the confidence intervals of the ARR point estimate (1.4) ranged from 0.5 to 3.8 and so no increased risk was interpreted. After extending the OAT exposure period to include the six days following treatment cessation, the number of suicides in the first 28 days of OAT increased to 6 and the crude incidence rate was estimated to be 0.9 (95%CI=0.4–2.1; eTable 7). In the first 28 days out of OAT, the crude incidence rate of suicide reduced to 3.9 (95%CI=2.7–5.7) and the ARR for this period reduced to 5.5 (95%CI=3.2–9.5) compared to ≥29 days in OAT (eTable 7). Finally, after censoring the ≥29 days out of OAT exposure period to 12 months, we found that the ARR reduced when compared to ≥29 days in OAT as we would expect; however, the overall interpretation did not change (eTable 8).
4. Discussion
4.1. Main findings
We observed that retention on OAT for ≥29 days had a protective effect against self-harm hospitalisations and suicide in our cohort. The period immediately following OAT cessation was associated with a substantially elevated risk of self-harm and suicide; compared to stable periods on OAT, the risk of suicide was 17 times higher in the month after leaving OAT, and more than 21 times higher for suicide by overdose. The risk of self-harm was two-three times higher during periods of OAT entry and exit compared to stable periods on OAT. Conversely, there was no increased risk of suicide in the first 28 days on OAT, but there was almost three times the risk for continuous periods off OAT.
Opioid poisoning is the leading cause of premature death among people with OUD in Australia and globally9,34. Despite likely access to large quantities of opioids among this cohort, opioids were implicated in a small minority of suicides. Consistent with patterns in the general population35, men were more likely to die by suicide and violent methods were the most common method used. Opioid overdose was implicated in only 9.6% of suicide deaths and 28% of self-harm hospitalisations. In contrast, overdoses on drugs other than opioids were recorded for 23.9% of deaths and over half of self-harm hospitalisations. This may reflect the significant challenge of discerning intent for opioid overdoses among people with OUD in clinical settings, particularly when suicidal ideation is common and can fluctuate within individuals36–38. However, this finding is consistent with self-report data from people with OUD, who rarely report using opioids in suicide attempts39,40.
4.2. Implications
Our findings emphasise the importance of OAT provision for people with OUD and support previous findings that longer-term OAT reduces a broad spectrum of mental, physical, and social health harms among this population18,19, 41. Engagement in OAT has important benefits in improving both physical and psychological quality of life, mood stabilisation, and facilitating access to structured physical and mental health service programs42–46.
Consistent with previous evidence18,20,21, our findings highlight important risk periods for self-harm and suicidal behaviour. The immediate period post-treatment cessation represents a significant risk period for people receiving OAT. The risk of overdose mortality is widely recognised18, but there is a need for greater attention on the risks of self-harm and suicide in this period. Further work developing interventions to prevent self-harm and suicide in people immediately following OAT cessation is warranted.
There was also an elevated risk for non-fatal self-harm in the period immediately following OAT initiation. On entry to OAT, individuals often require a period of treatment stabilisation, and will receive a full clinical assessment, including a clinical review of their mental health47. The factors underlying the increased risk of self-harm in the month following treatment initiation are not entirely clear. Health and social factors (e.g., housing instability, interpersonal violence, untreated physical and mental health comorbidities, etc.), which may be prevalent among people entering drug treatment48, can contribute to acute psychological crises49. As such, therapeutic suicide risk assessments and management, as well as safety planning, may be valuable during this period50.
4.3. Limitations
Our study is likely to have only captured more severe cases of non-fatal self-harm requiring urgent treatment (i.e., hospitalisation). Further work is needed to examine whether OAT engagement is associated with a reduced risk of self-harm in community settings, and whether similar risk periods for community-level self-harm exist.
Miscoding intentional suicide and self-harm as ‘undetermined intent’ may represent an underestimate in our results. Including these events in the sensitivity analyses did not change the interpretation of our findings; however, the ARR of suicide in the first month of OAT increased to 1.4 (95%CI=0.5–3.8). Although the confidence intervals are not considered statistically significant, it is impossible to ascertain which result most accurately reflects the risk of suicide. Further, miscoding may extend to diagnoses classified as accidental overdoses. There may be fear of stigma or involuntary detainment for people who are hospitalised with self-harm51; however, there is also reportedly ambivalence towards mortality for people who have a history of overdose52.
There may be some misclassification of the exposure groups due to administration errors that cannot be detected in the data available; however, this is likely non-differential and therefore may dilute the detected effects of OAT exposure periods. It was also not possible to stratify by planned and unplanned treatment cessation as the data were deemed unreliable. It is, therefore, not possible to determine the differential risk for those who planned to leave treatment and might have a lower risk of suicide and self-harm53.
Finally, a limitation of the design of this analysis when comparing treatment exposure periods and suicide is that a client is required to survive their OAT episode in order to reach the out of OAT period. By definition, a client cannot be in a state of “out of treatment” unless they have survived “in treatment”. This cohort is based on people who have had OAT, there is not a control group to compare those with opioid dependence who have not been in treatment with those in treatment. Therefore, when comparing out of treatment in this study it is conditional on them surviving their last treatment (and previous episodes).
5. Conclusion
OAT appears to have a protective effect against self-harm and suicide among people with OUD. Improving retention on OAT and reducing barriers to treatment access are critical components to minimise morbidity and mortality due to self-harm. Further work developing interventions to prevent self-harm and suicide immediately following treatment cessation is warranted.
Supplementary Material
Highlights: Incidence of suicide and self-harm among people with opioid use disorder and the impact of opioid agonist treatment: a retrospective data linkage study.
Opioid agonist treatment (OAT) is associated with a reduction in self-harm and suicide.
The month following OAT cessation is associated with an increase in both outcomes.
Self-harm increased during OAT initiation, compared to stable periods on OAT.
Opioid overdose was implicated in less than 10% of suicide deaths.
Non-opioid overdoses were recorded for almost a quarter of suicides.
6. Acknowledgements
We acknowledge the contribution of the OATS study team. Data was provided and linkage was conducted by the Australian Institute of Health and Welfare, NSW Ministry of Health, Centre for Health Record Linkage, and Bureau of Crime Statistics and Research. We also acknowledge the support and expertise of the OATS Study Aboriginal Advisory Group in reviewing this manuscript. The authors wish to acknowledge all data custodians for providing access to the datasets used in this study.
Funding:
The OATS study is funded by the National Institutes of Health (R01 DA144740 PI: Degenhardt). The National Drug Research Institute and the National Drug and Alcohol Research Centre are supported by funding from the Australian Government Department of Health under the Drug and Alcohol Program.
We acknowledge the contribution of the OATS study team. Data was provided and linkage was conducted by the Australian Institute of Health and Welfare, NSW Ministry of Health, Centre for Health Record Linkage, and Bureau of Crime Statistics and Research. We also acknowledge the support and expertise of the OATS Study Aboriginal Advisory Group in reviewing this manuscript. The authors wish to acknowledge all data custodians for providing access to the datasets used in this study. SCF is supported by the National Drug Research Institute. LD is supported by an Australian NHMRC Senior Principal Research Fellowship (#1135991) and a US National Institutes of Health (NIH) National Institute on Drug Abuse grant (R01DA1104470). PP is funded by the Medical Research Council Addiction Research Clinical Training programme (MR/N00616X/1). MH acknowledges funding from National Institute of Health Research (NIHR) Health Protection Research Unit in Behavioural Sciences and Evaluation, NIHR Bristol Biomedical Research Centre at Bristol, NIHR School for Public Health Research, and NIHR EPIToPe. NJ acknowledges funding from the ASCEND program grant (GNT 1150078).
In the past three years, LD and MF have received investigator-initiated untied educational grants for studies of opioid medications in Australia from Indivior and Seqirus.
Footnotes
Conflict of interest
All other authors have no conflicts of interest to declare.
Conflict of interest statement: Incidence of suicide and self-harm among people with opioid use disorder and the impact of opioid agonist treatment: a retrospective data linkage study
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosure statement: Incidence of suicide and self-harm among people with opioid use disorder and the impact of opioid agonist treatment: a retrospective data linkage study
Reference list
- 1.World Health Organization (WHO). Suicide worldwide in 2019: global health estimates. Geneva: World Health Organization, 2021. [Google Scholar]
- 2.Knipe D, Padmanathan P, Newton-Howes G, Chan LF, Kapur N. Suicide and self-harm. The Lancet 2022; 399(10338): 1903–16. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Comprehensive mental health action plan 2013–2030. Geneva: World Health Organization, 2021. [Google Scholar]
- 4.Carroll R, Metcalfe C, Gunnell D. Hospital Presenting Self-Harm and Risk of Fatal and Non-Fatal Repetition: Systematic Review and Meta-Analysis. PLoS ONE 2014; 9(2): e89944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witt KG, Hetrick SE, Rajaram G, et al. Psychosocial interventions for self-harm in adults. Cochrane Database of Systematic Reviews 2021; 2021(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen A, Slavova D, Cooper G, Zummer J, Costich J. An emergency department medical record review for adolescent intentional self-harm injuries. Injury Epidemiology 2021; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 2020; 396: 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Burden of Disease Collaborative Network, Institute for Health Metrics and Evaluation. GBD Compare: Global Self-harm. 2022. https://vizhub.healthdata.org/gbd-compare/ (accessed 28 December 2022). [Google Scholar]
- 9.Larney S, Tran L, Santo T, et al. All-cause and cause-specific mortality among people using extramedical opioids: a systematic review and meta-analysis. JAMA Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelty E, Hulse GK. Morbidity and mortality in opioid dependent patients after entering an opioid pharmacotherapy compared with a cohort of non-dependent controls. Journal of Public Health 2018; 40(2): 409–14. [DOI] [PubMed] [Google Scholar]
- 11.Chai Y, Luo H, Wei Y, et al. Risk of self‐harm or suicide associated with specific drug use disorders, 2004–2016: a population‐based cohort study. Addiction 2022; 117(7): 1940–9. [DOI] [PubMed] [Google Scholar]
- 12.Kalk NJ, Kelleher MJ, Curtis V, Morley KI. Addressing substance misuse: a missed opportunity in suicide prevention. Addiction 2019; 114(3): 387–8. [DOI] [PubMed] [Google Scholar]
- 13.Platt S, Arensman E, Rezaeian M. National Suicide Prevention Strategies – Progress and Challenges. Crisis 2019; 40(2): 75–82. [DOI] [PubMed] [Google Scholar]
- 14.Mann JJ, Michel CA, Auerbach RP. Improving Suicide Prevention Through Evidence-Based Strategies: A Systematic Review. American Journal of Psychiatry 2021; 178(7): 611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database of Systematic Reviews 2016. [DOI] [PubMed] [Google Scholar]
- 16.Colledge-Frisby S, Ottaviano S, Webb P, et al. Global coverage of interventions to prevent and manage drug-related harms among people who inject drugs: A systematic review. The Lancet Global Health under review. [DOI] [PubMed] [Google Scholar]
- 17.Australian Institute of Health and Welfare. National Opioid Pharmacotherapy Statistics Annual Data Collection. Canberra: Australian Institute of Health and Welfare, 2021. [Google Scholar]
- 18.Santo T Jr, Clark B, Hickman M, et al. Association of Opioid Agonist Treatment with All-Cause Mortality and Specific Causes of Death among People with Opioid Dependence: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. The Lancet 2019; 394(10208): 1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug and Alcohol Dependence 2009; 105(1): 9–15. [DOI] [PubMed] [Google Scholar]
- 21.Padmanathan P, Forbes HJ, Redaniel MT, et al. Self-harm and suicide during and after opioid agonist treatment among primary care patients in England: a cohort study. The Lancet Psychiatry 2021; 9(2): 151–9. [DOI] [PubMed] [Google Scholar]
- 22.Larney S, Jones N, Fiellin DA, et al. Data Resource Profile: The Opioid Agonist Treatment and Safety (OATS) Study, New South Wales, Australia. International Journal of Epidemiology 2021; 49(6): 1774–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLOS Medicine 2015; 12(10): e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peacock A, Uporova J, Karlsson A, et al. Australian Drug Trends 2019: Key Findings from the National Illicit Drug Reporting System (IDRS) Interviews. Sydney: National Drug and Alcohol Research Centre, UNSW Sydney, 2019. [Google Scholar]
- 25.Larance B, Degenhardt L, Grebely J, et al. Perceptions of extended‐release buprenorphine injections for opioid use disorder among people who regularly use opioids in Australia. Addiction 2020; 115(7): 1295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones NR, Hickman M, Larney S, et al. Hospitalisations for non-fatal overdose among people with a history of opioid dependence in New South Wales, Australia, 2001–2018: Findings from the OATS retrospective cohort study. Drug and Alcohol Dependence 2021; 218: 108354. [DOI] [PubMed] [Google Scholar]
- 27.Burns L, Randall D, Hall WD, et al. Opioid agonist pharmacotherapy in New South Wales from 1985 to 2006: patient characteristics and patterns and predictors of treatment retention. Addiction 2009; 104(8): 1363–72. [DOI] [PubMed] [Google Scholar]
- 28.Bharat C, Larney S, Barbieri S, et al. The effect of person, treatment and prescriber characteristics on retention in opioid agonist treatment: a 15‐year retrospective cohort study. Addiction 2021. [DOI] [PubMed] [Google Scholar]
- 29.Jones NR, Hickman M, Nielsen S, et al. The impact of opioid agonist treatment on fatal and non-fatal drug overdose among people with a history of opioid dependence in NSW, Australia, 2001–2018: Findings from the OATS retrospective linkage study. Drug and Alcohol Dependence 2022; 236: 109464. [DOI] [PubMed] [Google Scholar]
- 30.Beckman K, Mittendorfer-Rutz E, Waern M, Larsson H, Runeson B, Dahlin M. Method of self-harm in adolescents and young adults and risk of subsequent suicide. Journal of Child Psychology and Psychiatry 2018; 59(9): 948–56. [DOI] [PubMed] [Google Scholar]
- 31.Australian Bureau of Statistics. The Australian Standard Geographical Classification (ASGC) Remoteness Structure. 2020. https://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.005 (accessed August 2020. [Google Scholar]
- 32.Larney S, Hickman M, Fiellin DA, et al. Using routinely collected data to understand and predict adverse outcomes in opioid agonist treatment: Protocol for the Opioid Agonist Treatment Safety (OATS) Study. BMJ Open 2018; 8(8): e025204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohnert AS, McCarthy JF, Ignacio RV, Ilgen MA, Eisenberg A, Blow FC. Misclassification of suicide deaths: examining the psychiatric history of overdose decedents. Injury Prevalence 2013; 19: 326–30. [DOI] [PubMed] [Google Scholar]
- 34.Degenhardt L, Larney S, Randall D, Burns L, Hall W. Causes of death in a cohort treated for opioid dependence between 1985 and 2005. Addiction 2014; 109(1): 90–9. [DOI] [PubMed] [Google Scholar]
- 35.Australian Institute of Health and Welfare. Suicide & self-harm monitoring: Deaths by suicide over time. 2022. https://www.aihw.gov.au/suicide-self-harm-monitoring/data/deaths-by-suicide-in-australia/suicide-deaths-over-time (accessed 27 May 2022. [Google Scholar]
- 36.Connery HS, Taghian N, Kim J, et al. Suicidal motivations reported by opioid overdose survivors: A cross-sectional study of adults with opioid use disorder. Drug and Alcohol Dependence 2019; 205: 107612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maloney E, Degenhardt L, Darke S, Nelson EC. Are non-fatal opioid overdoses misclassified suicide attempts? Comparing the associated correlates. Addictive Behaviors 2009; 34(9): 723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darke S, Ross J, Marel C, et al. Patterns and correlates of attempted suicide amongst heroin users: 11-year follow-up of the Australian treatment outcome study cohort. Psychiatry Research 2015; 227(2): 166–70. [DOI] [PubMed] [Google Scholar]
- 39.Darke S, Ross J. The relationship between suicide and heroin overdose among methadone maintenance patients in Sydney, Australia. Addiction 2001; 96(10): 1443–53. [DOI] [PubMed] [Google Scholar]
- 40.Maloney E, Degenhardt L, Darke S, Mattick RP, Nelson EC. Suicidal behaviour and associated risk factors among opioid-dependent individuals: a case-control study. Addiction 2007; 102(12): 1933–41. [DOI] [PubMed] [Google Scholar]
- 41.Gisev N, Bharat C, Larney S, et al. The effect of entry and retention in opioid agonist treatment on contact with the criminal justice system among opioid-dependent people: a retrospective cohort study. The Lancet Public Health 2019; 4: e334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenore PL. Psychotherapeutic Benefits of Opioid Agonist Therapy. Journal of Addictive Diseases 2008; 27(3): 49–65. [DOI] [PubMed] [Google Scholar]
- 43.Nosyk B, Bray JW, Wittenberg E, et al. Short term health-related quality of life improvement during opioid agonist treatment. Drug and Alcohol Dependence 2015; 157: 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aas CF, Vold JH, Skurtveit S, et al. Health-related quality of life of long-term patients receiving opioid agonist therapy: a nested prospective cohort study in Norway. Substance abuse treatment, prevention, and policy 2020; 15(1): 68-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang K, Lee K, Lu T, et al. Opioid agonist treatment reduces losses in quality of life and quality-adjusted life expectancy in heroin users: Evidence from real world data. Drug and Alcohol Dependence 2019; 201: 197–204. [DOI] [PubMed] [Google Scholar]
- 46.Bråbäck M, Brådvik L, Troberg K, Isendahl P, Nilsson S, Håkansson A. Health Related Quality of Life in Individuals Transferred from a Needle Exchange Program and Starting Opioid Agonist Treatment. Journal of Addiction 2018; 2018: 3025683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gowing L, Ali R, Dunlop A, Farrell M, Lintzeris N. National Guidelines for Medication-Assisted Treatment of Opioid Dependence, 2014. [Google Scholar]
- 48.Yuodelis‐Flores C, Ries RK. Addiction and suicide: A review. The American Journal on Addictions 2015; 24(2): 98–104. [DOI] [PubMed] [Google Scholar]
- 49.Baxter LE, Sr., Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med 2013; 7(6): 377–86. [DOI] [PubMed] [Google Scholar]
- 50.Hawton K, Lascelles K, Pitman A, Gilbert S, Silverman M. Assessment of suicide risk in mental health practice: shifting from prediction to therapeutic assessment, formulation, and risk management. Lancet Psychiatry 2022; 9: 922–8. [DOI] [PubMed] [Google Scholar]
- 51.Mental Health Coordinating Council. Mental Health Rights Manual: Chapter 4 Section C: Admission to hospital under the Mental Health Act 2007 (NSW), 2020. [Google Scholar]
- 52.Gicquelais RE, Jannausch M, Bohnert AS, Thomas L, Sen S, Fernandez AC. Links between suicidal intent, polysubstance use, and medical treatment after non-fatal opioid overdose. Drug & Alcohol Dependence 2020; 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. General Hospital Psychiatry 2017; 47: 29–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.