Abstract
Introduction
The effectiveness and safety of immune checkpoint inhibitor (ICI) monotherapy in advanced upper tract urothelial carcinoma (UTUC) is less reported.
Methods
In total, 106 consecutive advanced UTUC patients receiving ICI monotherapy were collected from nine high volume centers. Clinical outcomes were analyzed according to multiple parameters (e.g., treatment line, metastatic sites). Objective response rate (ORR), overall survival (OS) and progression‐free survival (PFS) were captured after ICI initiation.
Results
With a median follow‐up of 12.0 months, 25 patients in the first‐line group and 15 patients in the second‐line group died of UTUC. We reported a median OS of 18.0 months, a median PFS of 5.0 months, and an ORR of 38.6% for patients in the first‐line group; a median OS of 10.0 months, a median OS of 4.0 months, and an ORR of 27.8% for patients in the second‐line group. Complete response was observed in two patients in the first‐line group and one patient in the second‐line group with a total complete response rate of 2.8%. In the univariate and multivariate analysis, visceral metastasis with a hazard ratio of 2.4 was associate with poor OS. The most common treatment‐related adverse events included fatigue (11.3%), pruritus (10.4%), and diarrhea (6.6%).
Conclusions
This real‐world study suggests that ICI monotherapy is active and has acceptable toxic effects for unresectable or metastatic UTUC as first‐line therapy in cisplatin‐ineligible patients or second‐line therapy in platinum‐refractory patients.
Keywords: advanced UTUC, cisplatin‐ineligible, immune checkpoint inhibitor
PD‐1 inhibitor is active and has acceptable toxic effects as a first‐line or second‐line treatment in unresectable or metastatic UTUC. PD‐1 inhibitors could be an alternative choice for UTUC patients who are ineligible for cisplatin..

1. INTRODUCTION
Upper tract urothelial carcinoma (UTUC), which originates from the upper tract, pyelocaliceal cavities and ureters, accounts for 5%–10% of urothelial carcinoma (UC) cases worldwide and 20%–30% of UC cases in China. 1 , 2 The 5‐year disease‐specific survival of UTUC is between 61% and 76%, but stands at 30% for patients with high‐risk tumors (>pT3 and/or N+M+). 3 , 4 , 5
The efficacy of platinum‐based chemotherapy is extrapolated from data collected in metastatic UC. 1 An analysis of three randomized controlled trials suggested potential clinical benefit of these regimens in UTUC. 6 There is a shift in the contemporary treatment paradigm for UTUC due to the breakthrough of immune checkpoint inhibitors (ICIs) in the treatment of UC. 7 , 8 , 9 , 10 Given that metastatic UTUC is characterized by impaired renal function, ICIs offer great promise for these patients. 11 Several monoclonal antibodies including pembrolizumab, atezolizumab, avelumab, and nivolumab have been approved by the USA Food and Drug Administration (FDA) in the first‐ and second‐line settings of locally advanced UC and metastatic UC. However, data on ICIs clinical activity in advanced UTUC are limited to an analysis of registered clinical trials, which included only a minority of UTUC patients, ranging from 14% to 27%. 12 This recent meta‐analysis showed that in the second‐line setting, the pooled objective response rate (ORR) from single‐arm studies was 21.2% (95% CI 12.5%–33.7%); a pooled analysis of two randomized controlled trials revealed no statistically significant difference in overall survival (OS) between patients treated with standard second‐line chemotherapy and those with ICIs despite a 24% reduction in the risk of death in patients treated with ICI. 12 Two trials IMvigor210 (cohort 1) and KEYNOTE‐052 in the cisplatin‐ineligible first‐line setting reported ORR of 39% and 22% in the UTUC subgroup, respectively. 8 , 9 However, no progression‐free survival (PFS) or OS was reported.
Recently, tislelizumab and toripalimab were conditionally approved in the second‐line setting by China National Medical Products Administration (NMPA). Single agent tislelizumab or toripalimab is generally well tolerated and has promising antitumor activities in patients who have received prior platinum‐based chemotherapy. 13 , 14 In the second‐line setting, tislelizumab resulted in a median PFS of 2.1 months and a median OS of 9.8 months, while toripalimab resulted in a median PFS of 2.3 months and a median OS of 14.4 months. 13 , 14 Although the results were encouraging, UTUC patients accounted for less than half of the participants in these trials, and clinical outcomes were still lacking for this subgroup.
Geographical factors were involved in various UTUC morbidities worldwide. A high proportion of UTUC patients were enrolled in clinical trials that were conducted in China. Given the inherent heterogeneity of UTUC, it is important to further explore the efficacy in the real‐world setting. Here, we focused on understanding the efficacy and safety of ICIs in UTUC patients.
2. PATIENTS AND METHODS
2.1. Patients
After the study protocol was approved by the independent institutional review board, we retrieved data from the electronic medical records of UTUC patients who visited nine participating centers and received first‐ or second‐line anti‐PD‐1 antibody monotherapy between 2018 and 2021. We included adult patients aged 18 or older who had histologically or cytologically confirmed unresectable or metastatic renal pelvis and ureter urothelial cancer (including pure urothelial and mixed urothelial cell histology). The eligibility criteria for first‐line patients included ineligibility for cisplatin‐based therapy 1 (defined as meeting at least one of the following criteria: Eastern Cooperative Oncology Group Performance Status 2 or 3, creatinine clearance <60 mL/min, grade ≥2 audiometric hearing loss, grade ≥2 peripheral neuropathy, or New York Heart Association Class III heart failure); second‐line was identified as follows: prior receipt of systemic chemotherapy for advanced disease (perioperative, platinum‐based chemotherapy with disease recurrence ≤12 months since completion was allowed); measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) 15 ; at least one imaging study of the target lesion after treatment. Exclusion criteria were treatment beyond second‐line, combination or sequential therapy; no imaging study was conducted after ICI initiation.
2.2. Treatment and procedures
ICIs used in this study were as follows: tislelizumab (n = 42, 40%), toripalimab (n = 31, 29%), pembrolizumab (n = 19, 18%), nivolumab (n = 5, 5%), sintilimab (n = 4, 4%), and camrelizumab (n = 5, 5%). Patients were treated with anti‐PD‐1 antibody by intravenous infusion once every 3 weeks with tislelizumab 200 mg, toripalimab 240 mg, pembrolizumab 200 mg, sintilimab 200 mg, or camrelizumab 200 mg and nivolumab 240 mg once every 2 weeks. Dose discontinuation was performed by the manufacturer's instructions to manage adverse events (AEs). Treatment continued until disease progressed, intolerable toxicities, or death.
2.3. Assessments
PD‐L1 expression was assessed in formalin‐fixed tumor samples at individual centers and re‐reviewed by a pathologist. Radiological evaluation was performed by computed tomography and/or magnetic resonance imaging of the abdomen, chest and brain as well as bone scintigraphy prior to initiation of treatment, and thereafter every 2–3 months by RECIST version 1.1, including complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). ORR and disease control rate (DCR) were defined as CR+PR and CR+PR+SD, respectively. OS was defined as the duration from the onset of ICI therapy to death from any cause. PFS was defined as the duration from the onset of ICI therapy to disease progression or death. Duration of confirmed response (DOR), defined as the time from the first documented complete or PR to disease progression or death.
All observed AEs and the severity of AEs were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
2.4. Statistical analysis
Statistical analysis was performed using SPSS version 26.0 and GraphPad Prism 8. The PFS and OS curves were plotted using Kaplan–Meier method and survival outcomes were compared across groups using the log‐rank test. The Pearson chi‐squared test or Fisher exact test was used to compare categorical variables. The Cox proportional hazard model was used to evaluate the predictors of PFS or OS. p < 0.05 was considered significant.
3. RESULTS
3.1. Patient population
Between 2018 and 2021, 188 consecutive UTUC patients were identified from nine sites around China. After excluding ineligible patients, 106 patients were included in this analysis (Figure S1). Table 1 summarizes the demographic and baseline clinicopathological characteristics of the study patients. Of these patients, 70 cisplatin‐ineligible patients (66.0%) received ICI monotherapy as first‐line treatment. The remaining 36 patients were identified as platinum pretreated and received ICI alone in the second‐line setting.
TABLE 1.
Baseline clinical characteristics and prior treatment of upper tract urothelial carcinoma patients.
| Characteristic | All patients (N = 106) |
|---|---|
| Age, years (median, range) | 67.5 (40–91) |
| Age ≥80 years | 15 (14.2%) |
| Male sex | 63 (59.4%) |
| ECOG | |
| 0–1 | 60 (56.6%) |
| ≥2 | 46 (43.4%) |
| Prior nephroureterectomy | 72 (67.9%) |
| Metastatic disease | |
| Visceral sites | 59 (55.7%) |
| Liver | 23 (21.7%) |
| Bone | 29 (27.4%) |
| Non‐visceral | 47 (44.3%) |
| Primary tumor site | |
| Renal pelvis | 54 (50.9%) |
| Ureter | 48 (45.3%) |
| Both | 4 (3.8%) |
| Treated as first line | 70 (66.0%) |
| Treated as second line after chemotherapy | 36 (34.0%) |
| Type of ICIs | |
| Tislelizumab | 42 (39.6%) |
| Toripalimab | 31 (29.2%) |
| Other agents | 33 (31.1%) |
| PD‐L1 expression | |
| <1% | 27 (25.5%) |
| ≥1% | 18 (17.0%) |
| Unknown | 61 (57.5%) |
| eGFR | |
| ≥60 | 39 (36.8%) |
| <60 | 67 (63.2.%) |
| Histology | |
| Pure urothelial | 88 (83.0%) |
| Mixed urothelial | 18 (17.0%) |
| Bajorin risk groups | |
| 0 | 25 (23.6%) |
| 1 | 56 (52.8%) |
| 2 | 25 (23.6%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitors; PD‐L1, programmed‐death ligand 1; UTUC, upper tract urothelial carcinoma.
3.2. Objective response rate
As shown in Table 2, confirmed ORR was 34.9% (37/106) of the total population. DCR was 58.5%. Despite limited enrollment, confirmed ORR (38.6%, 27/70) and DCR (61.4%, 43/70) in first‐line cisplatin‐ineligible patients were numerically higher than those in second‐line patients (ORR 27.8%, 10/36 and DCR 52.8%, 19/36). CR was observed in two patients in the first‐line group and one patient in the second‐line group with a total CR rate of 2.8%. The duration of response reached 28 months (95% CI NR–NR).
TABLE 2.
Objective response and duration of response in all treated patients.
| Subgroup | First line (n = 70) | Second line (n = 36) | Total (n = 106) |
|---|---|---|---|
| Confirmed objective response | 27/70 (38.6%) | 10/36 (27.8%) | 37 (34.9%) |
| Complete response | 2/70 (2.9%) | 1/36 (2.8%) | 3 (2.8%) |
| Partial response | 25/70 (35.7%) | 9/36 (25.0%) | 34 (32.1%) |
| Stable disease | 16/70 (22.9%) | 9/36 (25.0%) | 25 (23.6%) |
| Progressive disease | 27/70 (38.6%) | 17/36 (47.2%) | 44 (41.5%) |
| Duration of response, months | – | – | 28 (NR‐NR) |
| Age ≥80 years | – | – | 5/15 (33.3%) |
| eGFR | |||
| ≥60 | – | – | 14/39 (35.9%) |
| <60 | – | – | 23/67 (34.3%) |
| Metastatic disease | |||
| Visceral sites | – | – | 17/59 (28.8%) |
| Liver | – | – | 7/23 (30.4%) |
| Bone | – | – | 7/29 (24.1%) |
| Non‐visceral | – | – | 20/47 (42.6%) |
| PD‐L1 expression | |||
| <1% | – | – | 7/27 (25.9%) |
| ≥1% | – | – | 8/18 (44.4%) |
| Unknown | – | – | 22/61 (36.1%) |
| Histology | |||
| Pure urothelial | – | – | 30/88 (34.1%) |
| Mixed urothelial | – | – | 7/18 (38.9%) |
| Bajorin risk groups | |||
| 0 | – | – | 11/25 (44.0%) |
| 1 | – | – | 19/56 (33.9%) |
| 2 | – | – | 7/25 (28.0%) |
Subgroup analysis by age, metastasis, PD‐L1 expression, histology, and Bajorin risk showed similar ORR in patients ≥80 years of age and mixed urothelial histology (Table 2). In line with previous reports, ORR was higher in patients without visceral metastasis and PD‐L1 expression >1%, but lower in patients with visceral metastasis and PD‐L1 expression <1%. 16 In addition, the ORR was negatively associated with Bajorin risk score.
3.3. Survival outcomes
With a median follow‐up of 12 months, 25 patients in the first‐line group and 15 patients in the second‐line group died of UTUC. For cisplatin‐ineligible first‐line patients, the median OS was 18.0 months (95% CI 4.1–31.9), the 1‐year OS rate was 65.4%, and the median PFS was 5.0 months (95% CI 2.0–8.0; Figure 1A,B). For second‐line patients, the median OS was 10.0 months (95% CI 8.3–11.7), the 1‐year OS rate was 33.6%, and the median PFS was 4.0 months (95% CI 2.1–5.9; Figure 1A,B).
FIGURE 1.
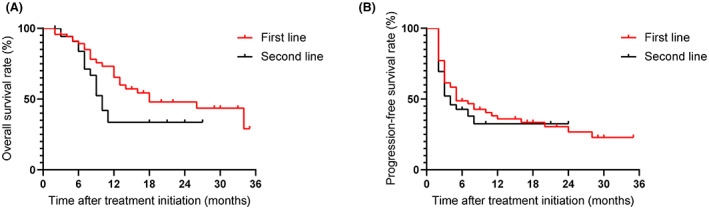
Overall survival (A) and progression‐free survival (B) of patients with advanced upper tract urothelial carcinoma stratified by treatment line of immune checkpoint inhibitors.
As shown in Table 3, the predictive factors of OS were analyzed by univariate and multivariate analysis. The OS in each subgroup was generally consistent with the overall population. Visceral metastasis, liver metastasis, and Bajorin risk were associated with significantly worse OS than those without (Figure 2A–C). Besides, OS with ICIs from different companies and PD‐L1 expression was comparable to the current sample size (Figure 2D,E).
TABLE 3.
Univariate and multivariate analysis of associations of various parameters with overall survival.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| Age (>65 vs. ≤65 years) | 0.828 (0.439–1.563) | 0.560 | ||
| Gender (male vs. female) | 0.857 (0.455–1.615) | 0.633 | ||
| ECOG (≥2 vs.<2) | 1.288 (0.692–2.399) | 0.425 | ||
| eGFR (<60 vs. ≥ 60) | 0.923 (0.486–1.753) | 0.807 | ||
| Prior nephroureterectomy (yes vs. no) | 0.750 (0.392–1.433) | 0.383 | ||
| Primary tumor site | ||||
| Renal pelvis | 1 | 0.399 | ||
| Ureter | 1.544 (0.808–2.950) | 0.189 | ||
| Both | 1.613 (0.372–7.003) | 0.523 | ||
|
Metastatic sites (Visceral vs. non‐visceral) |
2.300 (1.144–4.623) | 0.019 | 2.300 (1.144–4.623) | 0.019 |
| Liver metastasis (yes vs. no) | 1.973 (1.013–3.843) | 0.046 | ||
|
Histological subtype (Mixed urothelial vs. Pure urothelial) |
1.027 (0.453–2.328) | 0.949 | ||
| Type of PD‐1 antibody agent | ||||
| Tislelizumab | 1 | 0.614 | ||
| Toripalimab | 1.485 (0.678–3.254) | 0.323 | ||
| Other agents | 1.295 (0.558–3.006) | 0.548 | ||
| Bajorin risk groups | ||||
| 0 | 1 | 0.063 | ||
| 1 | 1.326 (0.531–3.307) | 0.546 | ||
| 2 | 2.661 (1.017–6.964) | 0.046 | ||
FIGURE 2.
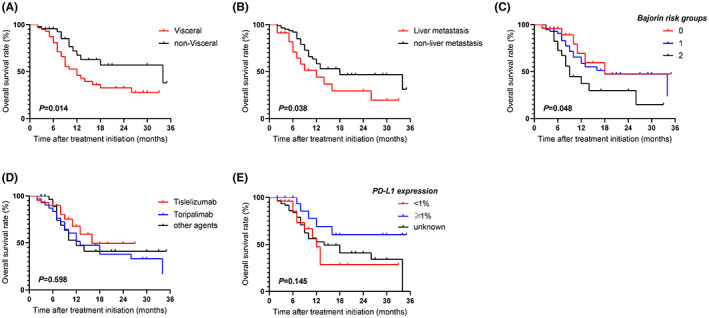
Subgroup analysis of upper tract urothelial carcinoma patients treated with immune checkpoint inhibitors (ICI) according to metastatic sites (A), the presence of liver metastasis (B), Bajorin risk groups (C), ICI agents (D), and PD‐L1 expression (E).
3.4. Safety prolife
As shown in Table 4, the most common AEs included fatigue (11.3%), pruritus (10.4%), diarrhea (6.6%), decreased appetite (7.5%), rash (10.4%), hypothyroidism (7.5%), and increased ALT (6.6%) (Table 4). Grade 3–4 AEs included diarrhea (1.9%), rash (1.9%), increased ALT (1.9%) and AST (1.9%), and myocarditis (0.9%). AEs resulted in five ICI discontinuations.
TABLE 4.
Treatment‐related adverse events.
| Adverse event | Any grade | Grades 3 and 4 |
|---|---|---|
| Any event | 64 (60.4%) | 11 (10.4%) |
| Event leading to discontinuation of treatment | 5 (4.7%) | 4 (3.8%) |
| Fatigue | 12 (11.3%) | 1 (0.9%) |
| Pruritus | 11 (10.4%) | 0 (0.0%) |
| Diarrhea | 7 (6.6%) | 2 (1.9%) |
| Decreased appetite | 8 (7.5%) | 0 (0.0%) |
| Rash | 11 (10.4%) | 2 (1.9%) |
| Hypothyroidism | 8 (7.5%) | 0 (0.0%) |
| Hyperthyroidism | 3 (2.8%) | 0 (0.0%) |
| Nausea | 5 (4.7%) | 0 (0.0%) |
| Pyrexia | 6 (5.7%) | 0 (0.0%) |
| ALT increased | 7 (6.6%) | 2 (1.9%) |
| AST increased | 4 (3.8%) | 2 (1.9%) |
| Constipation | 4 (3.8%) | 0 (0.0%) |
| Anemia | 7 (6.6%) | 2 (1.9%) |
| Interstitial pneumonia | 4 (3.8%) | 1 (0.9%) |
| Leucopenia | 6 (5.7%) | 0 (0.0%) |
| Neutrophil count decreased | 5 (4.7%) | 0 (0.0%) |
| lipase increase | 7 (6.7%) | 0 (0.0%) |
| Thrombocytopenia | 3 (2.8%) | 0 (0.0%) |
| Hypercholesteremia | 3 (2.8%) | 0 (0.0%) |
| Myocarditis | 2 (1.9%) | 1 (0.9%) |
| Bilirubin increase | 1 (0.9%) | 0 (0.0%) |
| Hyponatremia | 1 (0.9%) | 0 (0.0%) |
| Adrenal insufficiency | 1 (0.9%) | 0 (0.0%) |
| Arthralgia | 1 (0.9%) | 0 (0.0%) |
4. DISCUSSION
To date, this study is the first real‐world retrospective study designed to explore the efficacy and safety of immunotherapy in advanced UTUC in China. Here, we presented ORR, PFS and OS to UTUC patients receiving ICI monotherapy. Promising efficacy and a similar safety profile were observed independently of the treatment line, although ICI monotherapy appeared to be more effective in the first‐line than in the second‐line. Univariate and multivariate analysis of OS suggested that visceral metastases, a significant adverse predictor of OS, indicated the need for more effective treatment. Since prospective clinical trials designed for UC generally enroll a minority of UTUC, the data provide meaningful insight into the specific efficacy and safety of ICI monotherapy in UTUC.
The European Association of Urology (EAU) guidelines recommended two ICIs in first‐line UTUC patients unfit for cisplatin depending on PD‐L1 status. 17 The KEYNOTE‐052 trial enrolled 69 UTUC patients, accounting for 19% of the total population. The ORR was 22% for UTUC and 28% for lower tract UC. 9 In the IMvigor‐210 trial cohort 1, a numerically higher ORR 39% was observed in 33 UTUC patients than ORR 16% for lower tract UC. 8 No other data on UTUC was reported in these two trials. Three ICIs (pembrolizumab, avelumab and nivolumab) were recommended for the second‐line. Between 10% and 20% of UTUC patients were included in KETNOTE‐045, Javelin UC, and CheckMate‐275 studies. 10 , 16 , 18 A meta‐analysis showed an ORR of 21.2% in pooled analysis. 12 In the present study, a total of 70 first‐line and 36 second‐line patients were collected with ORR of 38.6% and ORR 27.8%, respectively. Interestingly, treated UTUC with ICI in the first‐line showed a higher ORR than in the second‐line.
The PFS of UTUC patients who received ICI monotherapy was rarely reported in clinical trials and retrospective studies. Our observations indicated that PFS after ICI initiation in first‐ and second‐line was 5.0 and 4.0 months, respectively. This is slightly higher than (2–3 months) in clinical trials enrolling first‐line cisplatin‐ineligible UC patients. 8 , 9 The median OS was 18.0 months in first‐line, which is comparable to (16.3 months) in IMvigor210 C1 and (11.3 months) KEYNOTE‐052. The median OS was 10.0 months in second‐line, which is also consistent with previous reports of UC. 10 , 16 , 19 Overall, the PFS and OS data of the UTUC subset were limited in the pivotal studies, except for a median OS of 7.9 months in IMvigor 210 cohort 2 and 10.9 months in IMvigor 211. 20 Esagian et al. retrospectively collected 746 UC patients involving a UTUC subset that received ICI monotherapy. UTUC patients were treated in the first‐line setting: ORR was 35% (95% CI 24%–48%), median PFS was 4.6 months (95% CI 2.5–8.3), and median OS was 13.4 months (95% CI 8.3–19.9); in the second‐line setting, ORR was 15% (95% CI 8%–26%), median PFS was 4.1 months (95% CI 2.8–5.9), and median OS 8.4 months (95% CI 5.3–14.0). 21 Overall, ICI monotherapy showed comparable efficacy between our and previous studies. 21
Since advanced UTUC patients usually present with renal impairment, they do not tolerate cisplatin combination therapy, which has promising efficacy in UC. 22 , 23 In addition, there is no specific prospective trial on the efficacy of platinum‐based chemotherapy in UTUC and chemotherapy is recommended based on extrapolated data from metastatic UC. 24 Retrospective comparison between UTUC and lower tract UC indicated that the location of the primary tumor had little impact on the response rate and had comparable efficacy. 6 , 24 A Phase III study compared the efficacy of pembrolizumab and the investigator's choice of chemotherapy in 542 UC patients. OS was significantly longer in the pembrolizumab group than in the chemotherapy group with HR 0.73 (95% CI 0.59–0.91, p = 0.02). For UTUC patients, the exploratory subset analysis of this trial demonstrated prolonged OS in the pembrolizumab group (HR 0.53, 95% CI 0.28–1.01). 10 This study shifted the paradigm of contemporary treatment strategy of UC in the post platinum‐based chemotherapy setting. ICI monotherapy showed two notable advantages in this group of patients: durable efficacy and a good safety profile. It was previously believed that advanced UC patients, especially those who had progressed from platinum‐based chemotherapy, had poor long‐term OS. 25 Patients rarely survived more than 24 months. 26 Administration of ICI increased ORR and provided meaningful OS benefits. The breakthrough of ICI is that one in five patients responding to ICI therapy shows prolonged DOR. 27 In this study, we found that the 24‐month PFS rate was about 30% in the first‐ and second‐line, and the DOR was over 28 months.
In the present study, we retrospectively collected AEs in electronic medical record systems. The most common treatment‐related AEs included fatigue (11.3%), pruritus (10.4%), and diarrhea (6.6%). Serious AEs causing ICI discontinuation were infrequent. However, due to the inherent disadvantage of retrospective analysis, treatment‐related AEs might be underestimated.
UTUC has been identified as a highly heterogeneous disease. 17 Genomic profile is inconsistent between UTUC and lower tract UC. 28 PD‐L1 is recognized as a classic predictive factor of response to ICI. However, its role in UC is contradictory due to limited efficacy and several conflicting results. 8 , 19 , 29 In our study, PD‐L1 status was available in 42.5% of patients, including 27 (25.5%) PD‐L1 < 1% and 18 (17.0%) PD‐L1 ≥ 1% patients. DACO 22C3 was used to identify PD‐L1 status. As shown in Table 2, a numerically higher ORR was observed in patients with PD‐L1 ≥ 1% than PD‐L1 < 1% (PD‐L1 ≥ 1% vs. PD‐L1 < 1%: 44.4% [8/18] vs 7/27 [25.9%]). Many issues remain to be explored in UTUC, such as the range of PD‐L1 ≥ 1% prevalence, consistency of different detection platforms, and the relationship between PD‐L1 status and prognosis. Lynch syndrome is prevalent in UTUC patients with a 4% positive rate, but is rarely found in lower tract UC. 30 Multiple studies proved that patients with tumors harbor germline mutations in mismatch repair (MMR) genes might respond to ICI treatment. Screening for such mutations is meaningful for deciding on further treatment strategies in UTUC patients. 17 Fibroblast growth factor receptor 3 (FGFR3) mutations are also more frequent in UTUC than lower tract UC. 31 Such mutations are associated with better survival and lower grade tumors. As the rising of FGFR inhibitors in mUC, combination of an FGFR3 inhibitor with an ICI may be a promising strategy. Despite debate about whether UTUC and bladder UC are two distinct diseases, results from our and previous studies suggested similar efficacy after ICI monotherapy. 12
Recently, many novel drugs, including erdafitinib, enfortumab vedotin, and sacituzumab govitecan, have emerged. Given the heterogeneity of UTUC, a combination strategy is promising in overcoming potential ICI resistance. Enfortumab vedotin plus pembrolizumab in first‐line cisplatin‐ineligible patients showed promising ORR PFS OS. 32 The combination strategy consists of ICI and novel drugs that may improve the prognosis of UTUC patients, especially those with visceral metastases. Prospective Phase III clinical trials (NCT04223856 and NCT05092958) are underway.
We previously compared the efficacy and safety of PD‐1 inhibitors and carboplatin combined with gemcitabine in the first‐line treatment of cisplatin‐unfit UTUC patients. 33 Our results support the role of PD‐1 inhibitors with comparable survival outcomes, longer DOR, and lower toxicity than carboplatin‐gemcitabine for cisplatin‐ineligible patients, although PD‐1 inhibitors may not surpass cisplatin dominance in the treatment of those eligible patients. In this study, we further explore the effect of ICIs in a second‐line setting and provide a full spectrum of efficacy and safety of ICI monotherapy for unresectable or metastatic UTUC as a first‐line therapy in cisplatin‐ineligible patients or second‐line therapy in platinum‐refractory patients. Due to the rarity of advanced UTUC, our studies have to integrate the results of patients receiving different ICIs. Future studies may focus on one specific agent, particularly a broadly applicable one such as pembrolizumab or atezolizumab, and prospectively investigate its role in the treatment of a large sample cohort of advanced UTUC.
The major limitation of this study included relatively short follow‐up, retrospective design, and potential selection bias. Short follow‐up may affect the interpretation of survival outcomes including OS and PFS. While the ORR and AEs observed were much more convincing. In addition, most of the ICIs used in our study were tislelizumab, toripalimab, sintilimab, and camrelizumab that were only available in China, which limits the broad application of our results. Nevertheless, the strength of this study is its provision of a large cohort of real‐world patients from nine participating high‐volume medical centers.
5. CONCLUSION
In conclusion, ICI monotherapy is active and has acceptable toxic effects for unresectable or metastatic UTUC as first‐line therapy in cisplatin‐ineligible patients or second‐line therapy in platinum‐refractory patients.
AUTHOR CONTRIBUTIONS
Ruopeng Su: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing – review and editing (equal). Zeyu Chen: Data curation (equal); formal analysis (equal); investigation (equal); visualization (equal). Daoping Hong: Data curation (equal); formal analysis (equal); investigation (equal); resources (equal). Shuai Jiang: Data curation (equal); formal analysis (equal); investigation (equal); resources (equal). Yichu Yuan: Data curation (equal); formal analysis (equal); investigation (equal). Xingyun Cai: Data curation (equal); formal analysis (equal); investigation (equal). Hai‐Long Hu: Data curation (equal); formal analysis (equal); investigation (equal); resources (equal); validation (equal). Changde Fu: Investigation (equal); validation (equal). Zhiyang Huang: Investigation (equal); validation (equal). Zhenyu Wang: Investigation (equal); validation (equal). Bing Zheng: Investigation (equal); validation (equal). Jian Huang: Investigation (equal); validation (equal). Zaoyu Wang: Investigation (equal); validation (equal). Yige Bao: Investigation (equal); resources (equal); supervision (equal); validation (equal). Ming Cai: Investigation (equal); supervision (equal). Jianming Guo: Investigation (equal); supervision (equal); validation (equal). Minfeng Chen: Investigation (equal); resources (equal); supervision (equal). Qiang Wei: Investigation (equal); resources (equal); supervision (equal). Jiwei Huang: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); supervision (equal); writing – original draft (equal). Wei Xue: Conceptualization (equal); investigation (equal); project administration (equal); resources (equal); supervision (equal).
FUNDING INFORMATION
This study was supported by the Natural Science Foundation of Shanghai (grant number 21ZR1438900), the Incubating Program for Clinical Research Innovation of Renji Hospital (grant number PYIII20‐07), and Basic Oncology Research Program from Bethune Charitable Foundation (BCF‐NH‐ZL‐20201119‐024).
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
PATIENT CONSENT FOR PUBLICATION
This retrospective, multicenter study has obtained informed consent from participating patients.
ETHICS APPROVAL
This retrospective, multicenter, real‐world study was conducted in accordance with the ethical standards of the Declaration of Helsinki and approved by the independent ethics committee at each participating center (ethics approval ID:KY2021‐102).
Supporting information
Figure S1:
Su R, Chen Z, Hong D, et al. Effectiveness and safety of immune checkpoint inhibitor monotherapy in advanced upper tract urothelial carcinoma: A multicenter, retrospective, real‐world study. Cancer Med. 2023;12:10587‐10596. doi: 10.1002/cam4.5796
Ruopeng Su, Zeyu Chen, Daoping Hong, Shuai Jiang, Yichu Yuan and Xingyun Cai have contributed equally to this work.
Contributor Information
Yige Bao, Email: baoygie@hotmail.com.
Minfeng Chen, Email: chenminfeng1999@csu.edu.cn.
Jiwei Huang, Email: huangjiwei@renji.com.
Wei Xue, Email: uroxuewei@163.com.
DATA AVAILABILITY STATEMENT
Further details that support the findings of this study are available from the corresponding authors upon request.
REFERENCES
- 1. Cathomas R, Lorch A, Bruins HM, et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2022;81(1):95‐103. doi: 10.1016/j.eururo.2021.09.026 [DOI] [PubMed] [Google Scholar]
- 2. Singla N, Fang D, Su X, et al. A multi‐institutional comparison of clinicopathological characteristics and oncologic outcomes of upper tract urothelial carcinoma in China and the United States. J Urol. 2017;197(5):1208‐1213. doi: 10.1016/j.juro.2016.11.094 [DOI] [PubMed] [Google Scholar]
- 3. Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. 2009;115(6):1224‐1233. doi: 10.1002/cncr.24135 [DOI] [PubMed] [Google Scholar]
- 4. Kaag MG. Perioperative chemotherapy in the management of high risk upper tract urothelial cancers. Transl Androl Urol. 2020;9(4):1881‐1890. doi: 10.21037/tau.2020.03.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall MC, Womack JS, Roehrborn CG, Carmody T, Sagalowsky AI. Advanced transitional cell carcinoma of the upper urinary tract: patterns of failure, survival and impact of postoperative adjuvant radiotherapy. J Urol. 1998;160(3 Pt 1):703‐706. doi: 10.1097/00005392-199809010-00019 [DOI] [PubMed] [Google Scholar]
- 6. Moschini M, Shariat SF, Roupret M, et al. Impact of primary tumor location on survival from the European Organization for the Research and Treatment of Cancer advanced urothelial cancer studies. J Urol. 2018;199(5):1149‐1157. doi: 10.1016/j.juro.2017.11.068 [DOI] [PubMed] [Google Scholar]
- 7. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218‐1230. doi: 10.1056/NEJMoa2002788 [DOI] [PubMed] [Google Scholar]
- 8. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first‐line treatment in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: a single‐arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67‐76. doi: 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balar AV, Castellano D, O'Donnell PH, et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): a multicentre, single‐arm, phase 2 study. Lancet Oncol. 2017;18(11):1483‐1492. doi: 10.1016/S1470-2045(17)30616-2 [DOI] [PubMed] [Google Scholar]
- 10. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015‐1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petros FG. Epidemiology, clinical presentation, and evaluation of upper‐tract urothelial carcinoma. Transl Androl Urol. 2020;9(4):1794‐1798. doi: 10.21037/tau.2019.11.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bersanelli M, Buti S, Giannatempo P, et al. Outcome of patients with advanced upper tract urothelial carcinoma treated with immune checkpoint inhibitors: a systematic review and meta‐analysis. Crit Rev Oncol Hematol. 2021;159:103241. doi: 10.1016/j.critrevonc.2021.103241 [DOI] [PubMed] [Google Scholar]
- 13. Ye D, Liu J, Zhou A, et al. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci. 2021;112(1):305‐313. doi: 10.1111/cas.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheng X, Chen H, Hu B, et al. Safety, efficacy, and biomarker analysis of toripalimab in patients with previously treated advanced urothelial carcinoma: results from a multicenter phase II trial POLARIS‐03. Clin Cancer Res. 2022;28(3):489‐497. doi: 10.1158/1078-0432.CCR-21-2210 [DOI] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer. 2009;45(2):228‐247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16. Sharma P, Retz M, Siefker‐Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2017;18(3):312‐322. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 17. Roupret M, Babjuk M, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2021;79(1):62‐79. doi: 10.1016/j.eururo.2020.05.042 [DOI] [PubMed] [Google Scholar]
- 18. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid tumor): pooled results from two expansion cohorts of an open‐label, phase 1 trial. Lancet Oncol. 2018;19(1):51‐64. doi: 10.1016/S1470-2045(17)30900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum‐treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open‐label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748‐757. doi: 10.1016/s0140-6736(17)33297-x [DOI] [PubMed] [Google Scholar]
- 20. Galsky MD, Banchereau R, Kadel EE, et al. 902P—biological features and clinical outcomes in atezolizumab (atezo)‐treated patients (pts) with metastatic urothelial cancer (mUC) of the upper vs lower urinary tract (UTUC vs LTUC). Ann Oncol. 2018;29:viii321. doi: 10.1093/annonc/mdy283.111 [DOI] [Google Scholar]
- 21. Esagian SM, Khaki AR, Diamantopoulos LN, et al. Immune checkpoint inhibitors in advanced upper and lower tract urothelial carcinoma: a comparison of outcomes. BJU Int. 2021;128(2):196‐205. doi: 10.1111/bju.15324 [DOI] [PubMed] [Google Scholar]
- 22. Lane BR, Smith AK, Larson BT, et al. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer. 2010;116(12):2967‐2973. doi: 10.1002/cncr.25043 [DOI] [PubMed] [Google Scholar]
- 23. Raman JD, Lin YK, Kaag M, et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol Oncol. 2014;32(1):e9‐e14. doi: 10.1016/j.urolonc.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 24. Hsieh MC, Chiang PH, Rau KM, Chen YY, Su YL, Huang CH. The comparison of oncologic outcomes between metastatic upper tract urothelial carcinoma and urothelial carcinoma of the bladder after cisplatin‐based chemotherapy. Urol Oncol. 2015;33(11):495 e9‐495 e14. doi: 10.1016/j.urolonc.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 25. Lobo N, Mount C, Omar K, Nair R, Thurairaja R, Khan MS. Landmarks in the treatment of muscle‐invasive bladder cancer. Nat Rev Urol. 2017;14(9):565‐574. doi: 10.1038/nrurol.2017.82 [DOI] [PubMed] [Google Scholar]
- 26. von der Maase H, Sengelov L, Roberts JT, et al. Long‐term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602‐4608. doi: 10.1200/jco.2005.07.757 [DOI] [PubMed] [Google Scholar]
- 27. Lopez‐Beltran A, Cimadamore A, Blanca A, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers (Basel). 2021;13(1):131. doi: 10.3390/cancers13010131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Lorenzis E, Albo G, Longo F, Bebi C, Boeri L, Montanari E. Current knowledge on genomic profiling of upper tract urothelial carcinoma. Genes (Basel). 2021;12(3):333. doi: 10.3390/genes12030333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tu MM, Ng TL, De Jong FC, Zuiverloon TCM, Fazzari FGT, Theodorescu D. Molecular biomarkers of response to PD‐1/ PD‐L1 immune checkpoint blockade in advanced bladder cancer. Bladder Cancer. 2019;5(2):131‐145. doi: 10.3233/BLC-190218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roupret M, Yates DR, Comperat E, Cussenot O. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (Lynch syndrome) tumor spectrum. Eur Urol. 2008;54(6):1226‐1236. doi: 10.1016/j.eururo.2008.08.008 [DOI] [PubMed] [Google Scholar]
- 31. van Oers JM, Zwarthoff EC, Rehman I, et al. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur Urol. 2009;55(3):650‐657. doi: 10.1016/j.eururo.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 32. Friedlander TW, Milowsky MI, Bilen MA, et al. Study EV‐103: update on durability results and long term outcome of enfortumab vedotin + pembrolizumab in first line locally advanced or metastatic urothelial carcinoma (la/mUC). J Clin Oncol. 2021;39(15_suppl):4528. doi: 10.1200/JCO.2021.39.15_suppl.4528 [DOI] [Google Scholar]
- 33. Huang J, Su R, Chen Z, et al. The efficacy and safety of first‐line treatment in cisplatin‐ineligible advanced upper tract urothelial carcinoma patients: a comparison of PD‐1 inhibitor and carboplatin plus gemcitabine chemotherapy. Onco Targets Ther. 2022;11(1):2124691. doi: 10.1080/2162402x.2022.2124691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Data Availability Statement
Further details that support the findings of this study are available from the corresponding authors upon request.


