Abstract
Background
Fecal microbiota, live-jslm (RBL; REBYOTA™), the first microbiota-based live biotherapeutic approved by the US Food and Drug Administration to prevent recurrent Clostridioides difficile infection (rCDI) in adults, has been evaluated in 5 prospective clinical trials. A retrospective analysis considered the safety and efficacy of RBL administered under US Food and Drug Administration enforcement discretion to patients with rCDI and broad eligibility criteria mimicking real-world practice.
Methods
We retrospectively identified adults with rCDI treated with RBL under enforcement discretion between November 1, 2015, and September 30, 2019, across 5 study sites. CDI diagnosis was based on site-specific practice. The primary safety set (PSS) included all patients who were naïve to previous RBL treatment and had continuously comprehensive medical records for 6 months following treatment.
Results
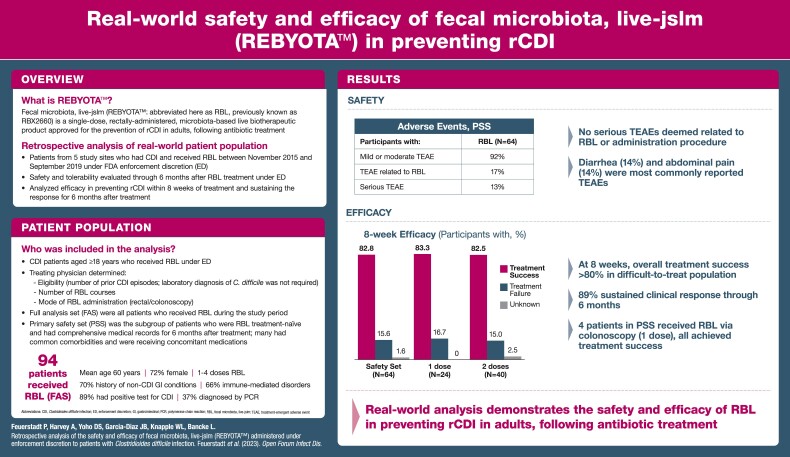
The primary treatment cohort had 94 patients; the PSS included 64 patients with common comorbidities receiving diverse chronic therapeutics. Most treatment-emergent adverse events were mild to moderate in severity and comparable between comorbidity subgroups and the overall population. There were no serious adverse events related to RBL or the administration procedure. In the PSS, 82.8% of RBL-treated patients responded at 8 weeks, of whom 88.7% had sustained response through 6 months. The number of RBL doses administered had no marked effect on outcome.
Conclusions
Together with prospective clinical trial outcomes, these findings support the efficacy and safety of RBL to prevent rCDI, with diagnostics and comorbidities representative of real-world clinical practice.
Keywords: Clostridioides difficile, general practice, immunosuppression, inflammatory bowel disease
Graphical Abstract
Graphical Abstract.
Clostridioides difficile is a leading cause of health care–associated infections in the United States [1] and has been identified as an urgent public health threat by the US Centers for Disease Control and Prevention [2]. C. difficile infection (CDI) and recurrent CDI (rCDI) are associated with substantial morbidity, mortality, and health care resource utilization [2–4].
Antibiotics, including vancomycin and fidaxomicin, are the standard-of-care (SOC) therapy for primary and recurrent episodes of CDI [5, 6]. Although antibiotic therapy is a known risk factor for rCDI [7], some SOC therapies are superior to others in reducing this risk [8]. Regardless of antibiotic choice, the likelihood of CDI recurrence increases exponentially after each episode [9–11], and there is a critical need for novel therapeutic strategies to prevent rCDI. Medical society guidelines now recommend fecal microbiota transplantation (FMT), a process of instilling normal microbiota via donor feces to correct gut microbiota disruption, for patients with rCDI who do not respond to SOC treatment [5, 6]. Quraishi et al. found an overall clinical response rate of 92% for FMT in patients with rCDI across 37 studies, the majority of which were retrospective case series [12]. However, across landmark efficacy trials for FMT in CDI, there have been no standardized study designs, methods, microbiota formulations, or study end points, leading to challenges in accurately interpreting the efficacy and safety of this therapy [13, 14]. Along with the emergence of transmissible pathogenic organisms, concerns over the safety of preliminary FMT practices have arisen. There is an acute need for standardized microbiome restoration therapies for rCDI, developed under rigorous manufacturing protocols and evaluated in a manner that is comparable across clinical trials [5, 15].
Fecal microbiota, live-jslm (REBYOTA™; abbreviated here as RBL, previously known as RBX2660) is the first single-dose, rectally administered, microbiota-based live biotherapeutic approved by the US Food and Drug Administration (FDA) for use to prevent recurrence of CDI in individuals 18 years and older following antibiotic treatment for rCDI [16]. RBL is manufactured from human fecal matter, which is sourced from qualified donors and screened for a panel of transmissible pathogens [16]. Throughout 5 prospective clinical trials, RBL has demonstrated consistent safety and efficacy in patients with rCDI [17, 18].
To best isolate the impact of an investigational drug on a specific disease state, clinical trials are designed to remove confounders, including patients with common comorbidities and medications encountered in general clinical practice [19]. During the clinical studies evaluating RBL, the sponsor acknowledged that patients who did not meet inclusion criteria might still require treatment to prevent rCDI. Hence, the “Assured Active Treatment” program was developed, whereby patients deemed ineligible for clinical trial participation or who needed additional treatment following study participation were offered RBL. Through this program, Rebiotix supplied RBL to clinical study sites upon request of the principal investigator and in compliance with the FDA 2013 enforcement discretion (ED) policy [20]. The current retrospective study details the safety and efficacy of RBL administered under ED in real-world patients with CDI.
METHODS
Study Design
This retrospective study reports on patients with CDI aged ≥18 years who received RBL under ED between November 1, 2015, and September 30, 2019, across 5 study sites. The treating physician determined a patient's eligibility to receive RBL under ED, in accordance with FDA guidance [20]. Each site obtained institutional review board (IRB) approval to conduct the study. Because this was a retrospective analysis of patient medical records, patients were not contacted; a waiver of informed consent/Health Insurance Portability and Accountability Act (HIPAA) authorization for the collection of the retrospective record review was obtained from each site's IRB before beginning the record review process. Laboratory diagnosis of C. difficile was not required, and there were no exclusion criteria. Patient data were retrospectively captured by site staff chart review and recorded using an electronic database. Although data were collected retrospectively, it was done using a prespecified protocol, and all data analyses were prespecified in a statistical analysis plan before database lock. Additional information pertaining to the study design is available in the Supplementary Methods.
The primary objective was to evaluate the safety and tolerability of RBL through 6 months after treatment in patients who received RBL under ED. The primary end point was the number of patients with RBL- and/or procedure-related treatment-emergent adverse events (TEAEs), defined as adverse events (AEs) occurring on or after the day of treatment with RBL. Other safety end points included the number of AEs per patient, number of TEAEs of special interest according to the study definition, relatedness of TEAEs, severity of TEAEs, and onset of new chronic conditions relative to treatment administration or worsening of preexisting conditions. Secondary objectives included an evaluation of the efficacy of RBL in preventing rCDI within 8 weeks of treatment and evaluation of the sustained response for 6 months after treatment.
Analysis Data Sets
Efficacy and safety results were analyzed for 3 different populations (Supplementary Figure 1). The full analysis set (FAS; n = 94) included all patients who met eligibility criteria and received RBL under ED during the study period. The primary safety set (PSS; n = 64) served as the analysis set for safety and efficacy end points, comprising patients who were treatment-naïve to RBL and met the study requirement for continuously comprehensive medical records (defined in the Supplementary Methods). The secondary safety set (SSS; n = 30) included patients who were enrolled but excluded from the PSS because they had received RBL during a prior clinical study, did not have continuous medical records for 6 months after administration of RBL, or exited the study early (on March 1, 2020).
Safety Analysis
The safety analysis reported the number of TEAEs and percentage of patients with a TEAE. A new chronic condition was defined as a TEAE lasting more than 3 months. TEAEs of special interest included gastrointestinal (GI) AEs, fever, chills, fatigue, possibility of disease transmission from donor to recipient, and AEs related to the administration procedure. Subgroup analysis of TEAEs was performed for comorbidity subgroups. Assignment to these subgroups was not mutually exclusive. These subgroups were determined by the Standardised MedDRA Queries of patient medical histories, namely GI and nonspecific inflammation and dysfunctional conditions and immune-mediated/autoimmune disorders.
Efficacy Analysis
An on-study episode of CDI was defined by a record of the use of medication (either microbiota therapy or anti-infective therapy) for the treatment of CDI or a record of suspected CDI diarrhea following the qualifying administration of RBL. Treatment success was defined as the absence of CDI recurrence within 8 weeks following the final dose of RBL administered for the qualifying CDI event. Sustained clinical response was defined as no documented on-study CDI event within 6 months after the final dose of RBL administered for the qualifying CDI event. An unknown outcome was defined as no documented on-study CDI event within 8 weeks/6 months after the final dose of RBL administered for the qualifying CDI event, but medical records were not continuously comprehensive. Unknown outcomes were recorded as recurrence to conservatively estimate efficacy for RBL. If an on-study CDI event occurred within 8 weeks of the last dose of RBL administered for the qualifying CDI event, then that participant was deemed a treatment failure.
RESULTS
Demographics and Baseline Characteristics
The FAS included 94 patients across 5 study sites in Arkansas, Connecticut, Idaho, Louisiana, and Virginia (Supplementary Table 1). Within the FAS, the mean age was 59.8 years, with 44.7% of patients being ≥65 years of age and 72.3% being female. The qualifying CDI event was detected by a positive laboratory test (enzyme immunoassay [EIA]/glutamate dehydrogenase assay, or polymerase chain reaction [PCR]) in 89.4% of patients. PCR was the most common test for a qualifying CDI episode (37.2%). Eight patients (8.5%) had a positive EIA alone, 5 patients (5.3%) had a positive C. DIFF QUIK CHEK COMPLETE test alone, 12 patients (12.8%) had multiple positive tests (all were PCR positive, with 6 EIA tests and 6 C. DIFF QUIK CHEK COMPLETE), and 24 patients (25.5%) had positive tests defined as “other,” which included stool culture assays for C. difficile and toxigenic C. difficile (Supplementary Table 2). These tests were ordered by the local provider using the typical diagnostic method for CDI at the respective study sites. Ten patients (10.6%) had no documented stool assay for C. difficile for the qualifying event and were diagnosed clinically.
Of the 94 FAS patients, 16 received doses in previous clinical studies, 15 had incomplete records, and 2 had their 6-month follow-up after March 1, 2020; these events were not mutually exclusive, and these patients were excluded from the PSS (n = 64) (Supplementary Figure 1). A single treatment course of RBL administered under ED in the PSS ranged between 1 and 2 doses. The decision to administer 1 or 2 doses was made by the treating physician before intervention and was completed regardless of the patient's clinical status. Patients who experienced rCDI after the first course may have received a second course of RBL under ED; 4 doses (2 treatment courses of 2 doses each) was the maximum number of doses that any patient received.
In the PSS, 70.3% of patients had GI and nonspecific inflammation and dysfunctional conditions, and 65.6% had immune-mediated/autoimmune disorders at baseline. Comorbid conditions included irritable bowel syndrome (21.9%), microscopic colitis (10.9%), Crohn's disease (7.8%), and ulcerative colitis (6.3%) (Supplementary Table 1); 17.2% of patients received concomitant medication capable of causing immunosuppression at the time of the qualifying administration of RBL, such as glucocorticoids (6.3%), tumor necrosis factor–α inhibitors (4.7%), and monoclonal antibodies (1.6%) (Supplementary Table 3).
PSS Safety Analysis
One hundred forty-four TEAEs were recorded in 62.5% of patients who received RBL under ED (Table 1). TEAEs were considered related to RBL in 17.2% of patients and related to the procedure in 4.7% of patients. The majority (93%) of TEAEs were mild to moderate in severity.
Table 1.
Overview of Treatment-Emergent Adverse Events (PSS)
| Adverse Event Category | PSS | |
|---|---|---|
| (n = 64) | ||
| Events | No. (%) | |
| Participants with any TEAE | 144 | 40 (62.5) |
| Related TEAEs | … | … |
| Related to RBL | 32 | 11 (17.2) |
| Related to procedure | 4 | 3 (4.7) |
| Severe and life-threatening TEAEs | 10 | 5 (7.8) |
| TEAEs leading to death | 1 | 1 (1.6) |
| Serious TEAEs | 11 | 8 (12.5) |
| TEAEs of special interest | 58 | 22 (34.4) |
| Onset of treatment-emergent new chronic conditions relative to treatment administration or worsening of preexisting conditions | 33 | 15 (23.4) |
Abbreviations: PSS, primary safety set; RBL, fecal microbiota, live-jslm; TEAE, treatment-emergent adverse event.
Serious TEAEs were experienced by 12.5% of patients. One patient in the PSS died during the study analysis period because of multi-organ failure with an onset 10 days after the first administration of RBL. Severe and life-threatening AEs were experienced by 7.8% of patients and included ileus, organ failure, failure to thrive, and major depression. None of these events were related to RBL or the procedure.
TEAEs of special interest were recorded in 34.4% of patients, the most common of which included worsened diarrhea and abdominal cramping or pain (14.1% of patients, each). Onset of treatment-emergent new chronic conditions relative to treatment administration was reported in 3.1% of patients; these were not considered serious AEs. Worsening of preexisting conditions was reported in 20.3% of patients, 3 of whom experienced serious AEs (1 patient experienced failure to thrive, 1 patient experienced major depression, and rectal hemorrhage was experienced twice by the same patient at 27 and 138 days after treatment, respectively). None of these events were considered related to RBL or the procedure. The most common TEAEs occurring in ≥5% of patients in the PSS were GI disorders (45.3%) (Supplementary Table 4).
TEAEs in the PSS Subgroups With Comorbidities
Patients with GI and nonspecific inflammation and dysfunctional conditions at baseline showed trends for more TEAEs and serious TEAEs, yet the incidence rates of TEAEs considered to be related to RBL and the procedure were comparable between comorbidity subgroups and the overall population (Supplementary Table 5). In the GI and nonspecific inflammation and dysfunctional conditions subgroup, 126 TEAEs were reported by 71.1% of patients, and 76 events by 59.5% of patients in the immune-mediated/autoimmune disorder subgroup.
When focusing on TEAEs related to RBL, 22 events were reported by 6 patients (13.3%) in the GI and nonspecific inflammation and dysfunctional conditions comorbidity subgroup, and 9 events were reported by 4 patients (9.5%) in the immune-mediated/autoimmune disorder subgroup. Subgroup percentages were less than the 17.2% overall TEAE rate. Three events related to procedure were reported in 2 patients (4.4%) in the GI and nonspecific inflammation and dysfunctional conditions comorbidity subgroup, and a single event related to procedure was reported in 1 patient (2.4%) in the immune-mediated/autoimmune disorder subgroup, which was similar to the 4.7% of TEAEs related to procedure reported in the overall PSS.
Efficacy Analyses
PSS
Overall, 82.8% of patients who received RBL under ED achieved treatment success (Table 2). The proportion of patients who remained recurrence-free 8 weeks following initial administration was similar for patients who received 1 dose of RBL (83.3%) vs 2 doses (82.5%). Four of the 24 patients who received 1 dose of RBL for the qualifying event received the product via colonoscopy; all 4 achieved treatment success.
Table 2.
Summary of Treatment Success by Number of RBL Doses Administered for the Qualifying CDI Event Under Enforcement Discretion (PSS)
| No. (%) of Participants | |||
|---|---|---|---|
| Efficacy outcome | PSS (n = 64) | 1 dose (n = 24)a | 2 doses (n = 40) |
| Treatment success | 53 (82.8) | 20 (83.3)a | 33 (82.5) |
| Treatment failure | 10 (15.6) | 4 (16.7) | 6 (15.0) |
| Unknown | 1 (1.6) | 0 | 1 (2.5) |
Abbreviations: CDI, Clostridioides difficile infection; PSS, primary safety set; RBL, fecal microbiota, live-jslm.
Four participants in the PSS received 1 dose of RBL via colonoscopy. Overall, 4 of 4 participants who received RBL via colonoscopy achieved treatment success.
Of the 53 patients in the PSS who demonstrated treatment success through 8 weeks, 47 (88.7%) experienced sustained clinical response through 6 months following treatment. The rates of sustained clinical response were similar in patients who received 1 (90.0%) or 2 (87.9%) doses of RBL.
To determine whether the method of detecting a CDI event affected efficacy outcomes, subgroup analyses of treatment success were performed for subgroups with and without a positive laboratory test for the qualifying and/or first on-study CDI events. In the PSS, the rates of treatment success were similar for participants with a positive laboratory test for the qualifying CDI event (46/55 [83.6%] participants) and for participants without a positive laboratory test (7/9 [77.8%] participants). The first on-study CDI event in the first 8 weeks was diagnosed with a positive laboratory test in half of the cases (5 participants) and without a positive laboratory test in the other half of the cases (5 participants).
FAS
Treatment outcomes for 8 patients in the FAS were unknown. Given this uncertainty, they were conservatively included as treatment failures in the efficacy analysis. Following this assumption, in the FAS, 70.2% of patients achieved treatment success. The number of RBL doses administered had no notable effect on treatment outcomes, with 74.4% and 66.7% of patients who received 1 and 2 doses experiencing treatment success, respectively. One patient received 3 doses, each 1 week apart, as set forth in the local providers’ plan as a single treatment course; this patient experienced treatment success. Ten of the 39 patients who received 1 dose of RBL for the qualifying event received the product via colonoscopy; 80% of these individuals achieved treatment success. Of the 66 patients in the FAS who experienced treatment success, 58 (87.9%) demonstrated a sustained clinical response through 6 months following treatment.
DISCUSSION
In this study, RBL administered under ED to a real-world population demonstrated high clinical efficacy to prevent rCDI during the first 8 weeks following treatment. Of the initial responders, a high percentage had sustained clinical response through 6 months. These efficacy findings are consistent with previous randomized controlled trials [17]. Similarly, the safety results are consistent with those recorded in RBL prospective trials [18].
Here, treatment success rates were similar to those in the prospective RBL phase 2 and 3 clinical studies. The phase 3 PUNCH CD3 study used a Bayesian analysis integrating data from the phase 2b PUNCH CD2 study for the primary efficacy end point, and the model-estimated treatment success rates were 70.6% and 57.5% for RBL and placebo, respectively [21]. Here, the FAS showed an efficacy rate of 70.2%, assuming that all 8 participants lost to follow-up recurred, and the PSS showed an efficacy of 82.8% over the 8-week period. Our results are consistent with the 75% of patients reported to be CDI recurrence-free through 8 weeks via interim results of the PUNCH CD3-OLS study evaluating RBL [22]. Furthermore, the sustained clinical response through 6 months of follow-up reported in our real-world population replicates that reported across the 5 prospective clinical trials [17]. Overall, in the clinical program, the majority of primary RBL responders remained CDI-free for 6 months and up to 24 months after treatment, with sustained response rates ranging from 82.0% to 92.1% [17]. Here, 88.7% of PSS patients who initially responded remained responsive 6 months following the initial treatment. Furthermore, a single dose of RBL in patients in the PSS was associated with an 83.3% response rate at 8 weeks, while those who received 2 doses had an 82.5% response rate. This response was sustained throughout 6 months in 90.0% and 87.5% of patients who received 1 and 2 RBL doses, respectively. Similar results were observed for the FAS population with both a single and second dose of RBL through week 8, sustained through 6 months. This highlights that a single dose of RBL is sufficient in preventing rCDI.
The efficacy observed in the current study is comparable to preliminary FMT studies. Tariq et al. reported an efficacy rate of 76.1% for FMT in trials that included a control group, 82.7% in open-label studies, and 67.7% in randomized controlled trials [23]. Here, 82.8% efficacy in patients in the PSS aligned with the 82.7% from the meta-analysis, showing consistent efficacy with other open-label studies [23]. This study also begins to inform the possibility of other routes of administration (ie, colonoscopy) for RBL, which occurred under ED because FMTs are commonly administered through this route [24].
In clinical trials, narrow eligibility criteria that isolate the disease of interest and remove confounding variables often result in the exclusion of patients with comorbidities and risk factors reflective of real-world populations [19]. The current retrospective analysis evaluated a patient population with broad eligibility criteria, inclusive of comorbidities and concomitant immunosuppressive medications. Data were collected retrospectively, but the collection process was monitored and source data verified by clinical research staff, and the analyses were prespecified before database lock. Another factor strengthening the simulation of real-world practice was that there were no specified stool assays to confirm CDI in this study, and the local investigator's clinical decision-making was unaltered when diagnosing and deciding on indications for SOC antimicrobial therapy and subsequent RBL treatment. However, future analyses may consider restricting the diagnostic eligibility to that of a nucleic acid amplification test—alone or in combination—to mitigate discrepancies in the accuracy of the different diagnostic strategies, particularly that of the symptomatic diagnosis by local providers.
The inclusion of patients with immune-mediated/autoimmune disorders and those receiving concomitant immunosuppressive medications is of clinical relevance to the safety analysis in the present study, as these populations are particularly susceptible to infections. This subgroup of patients had a favorable safety profile, with the incidence of TEAEs related to RBL and the procedure being comparable to that of the entire PSS. Although research exploring FMT in patients with rCDI who are immunosuppressed is limited, one study that included 80 immunosuppressed patients showed a favorable safety profile, similar to that reported here [25].
Although this study provides valuable real-world insights and data, some limitations exist. There are implicit limitations associated with retrospective studies, including potential site selection bias. Hence, the study was designed to include all patients who received RBL under ED at prespecified clinical sites. The selected sites were the highest users of RBL under ED, used an electronic medical record system, and were willing to participate in the study. By using a qualifying event defined by the first exposure to RBL under ED, possible treatment outcome selection bias was addressed, as all patients were included in the study irrespective of the RBL treatment effect. To counter any possible reporting bias, the quality of medical records was prospectively defined using the study definition of continuously comprehensive coverage of medical records. We addressed any additional reporting bias by providing transparency to data from all patients who received RBL under ED and their allocation into analysis populations. These approaches helped ensure that the quality of data for all patients could be assessed based on prospectively described objective criteria. Regarding the route of RBL administration, this study was designed as a retrospective observational safety study and was not powered to detect significant differences in efficacy based on administration route. Therefore, any conclusions regarding patients who received RBL via colonoscopy are limited by the small number of patients.
Overall, this retrospective analysis is representative of real-world diagnostics and a patient population typically encountered in everyday practice. The findings reinforce the potential efficacy and safety of RBL in real-world populations with common rCDI comorbidities representative of clinical practice. Future prospective studies with broad eligibility criteria across multiple centers may provide additional information on the efficacy and safety of RBL administration, as well as allow for comparative outcomes achieved with 1 and 2 doses, in the prevention of rCDI in real-world patient populations.
Supplementary Material
Acknowledgments
We thank all the patients who participated in the study. We thank Dr. Sho Ando, an employee of Ferring Pharmaceuticals Inc. at the time of this study, for assistance with statistical analysis. Medical writing assistance was provided by Shandré Pieterse, MD (ApotheCom, Yardley, PA, USA), and was funded by Ferring Pharmaceuticals Inc.
Contributor Information
Paul Feuerstadt, Yale University School of Medicine; PACT Gastroenterology Center, Hamden, Connecticut, USA.
Adam Harvey, Rebiotix (a Ferring Company), Roseville, Minnesota, USA.
David S Yoho, Department of Gastroenterology, Mid-Atlantic Permanente Medical Group, Springfield, Virginia, USA.
Julia B Garcia-Diaz, Department of Clinical Infectious Diseases Research and Medical Subspecialties, Ochsner Medical Center, New Orleans, Louisiana, USA.
Whitfield L Knapple, Department of Gastroenterology, Arkansas Gastroenterology, North Little Rock, Arkansas, USA.
Lindy Bancke, Rebiotix (a Ferring Company), Roseville, Minnesota, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Financial support. This work was supported by Ferring Pharmaceuticals Inc.
Author contributions. P.F.: conceptualization (lead), formal analysis (equal), investigation (lead), methodology (lead), project administration (supportive), writing (lead), review & editing (lead). A.H.: data curation (lead), formal analysis (lead), validation (lead), review & editing (lead). D.S.Y.: conceptualization (equal), formal analysis (equal), investigation (lead), project administration (lead), review & editing (equal). J.B.G.D.: conceptualization (equal), formal analysis (supportive), investigation (lead), project administration (supportive), review & editing (equal). W.L.K.: conceptualization (equal), formal analysis (supportive), investigation (lead), project administration (supportive), review & editing (equal). L.B.: data curation (lead), formal analysis (lead), validation (lead), review & editing (lead).
Patient consent. As per Good Clinical Practice, IRBs ensured the ethical, scientific, and medical appropriateness of the study before it was conducted and approved all relevant documentation. As this was a retrospective study of patient medical records, patients were not contacted; a waiver of informed consent/HIPAA authorization for the collection of the retrospective record review was obtained from each site's IRB before beginning the record review process.
References
- 1. Magill SS, O'Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018; 379:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Antibiotics resistance threats in the United States 2019. 2019. Available at:https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed October 5, 2021.
- 3. Rodrigues R, Barber GE, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol 2017; 38:196–202. [DOI] [PubMed] [Google Scholar]
- 4. Feuerstadt P, Stong L, Dahdal DN, Sacks N, Lang K, Nelson WW. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ 2020; 23:603–9. [DOI] [PubMed] [Google Scholar]
- 5. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:e1029–44. [DOI] [PubMed] [Google Scholar]
- 6. Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021; 116:1124–47. [DOI] [PubMed] [Google Scholar]
- 7. Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract 2013; 26:464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55(Suppl 2):S154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers 2016; 2:16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma GK, Brensinger CM, Wu Q, Lewis JD. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med 2017; 167:152–8. [DOI] [PubMed] [Google Scholar]
- 11. Rinaldi A, Reed EE, Stevenson KB, Coe K, Smith JM. Effectiveness of fidaxomicin versus oral vancomycin in the treatment of recurrent Clostridioides difficile. J Clin Pharm Ther 2021; 46:993–8. [DOI] [PubMed] [Google Scholar]
- 12. Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017; 46:479–93. [DOI] [PubMed] [Google Scholar]
- 13. Bafeta A, Yavchitz A, Riveros C, Batista R, Ravaud P. Methods and reporting studies assessing fecal microbiota transplantation: a systematic review. Ann Intern Med 2017; 167:34–9. [DOI] [PubMed] [Google Scholar]
- 14. Feuerstadt P, Aroniadis OC, Svedlund FL, et al. Heterogeneity of randomized controlled trials of fecal microbiota transplantation in recurrent Clostridioides difficile infection. Dig Dis Sci 2021;67:2763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khanna S. Microbiota restoration for recurrent Clostridioides difficile: getting one step closer every day! J Intern Med 2021; 290:294–309. [DOI] [PubMed] [Google Scholar]
- 16.REBYOTA™ (fecal microbiota, live-jslm). Prescribing information. Ferring Pharmaceuticals Inc. November 2022. Available at: https://ferringus2.corporate-us.ferring.tech/wp-content/uploads/sites/12/2022/12/9009000002_REBYOTA-PI_11-2022.pdf. Accessed April 28, 2023.
- 17. Bancke L, Su X. Efficacy of investigational microbiota-based live biotherapeutic RBX2660 in individuals with recurrent Clostridioides difficile infection: Data from five prospective clinical studies. Open Forum Infect Dis 2021; 8:S100–1. [Google Scholar]
- 18. Braun T, Guthmeuller B, Harvey A. Safety of investigational microbiota-based live biotherapeutic RBX2660 in individuals with recurrent Clostridioides difficile infection: Data from five prospective clinical studies. Open Forum Infect Dis 2021; 8:S611. [Google Scholar]
- 19. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol 2017; 35:3737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. US Department of Health and Human Services . Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. U.S. Food and Drug Administration website. Available at: https://www.federalregister.gov/documents/2013/07/18/2013-17223/guidance-for-industry-enforcement-policy-regarding-investigational-new-drug-requirements-for-use-of. Accessed April 28, 2023.
- 21. Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs 2022; 82:1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kraft C, Khanna S, Assi M, Feuerstadt P, Harvey A, Bancke L. Interim analysis of a phase 3 open-label study indicates safety and efficacy of RBX2660, an investigational live biotherapeutic, in a “real-world” population of patients with recurrent Clostridioides difficile infection. Gastroenterology 2021; 160:S573. [Google Scholar]
- 23. Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis 2019; 68:1351–8. [DOI] [PubMed] [Google Scholar]
- 24. Kelly CR, Yen EF, Grinspan AM, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT national registry. Gastroenterology 2021; 160:S573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterology 2014; 109:1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.