Abstract
Intracellular immune complexes known as inflammasomes sense breaches of cytosolic sanctity. Inflammasomes promote downstream proinflammatory events, including IL-1 family cytokine release and pyroptotic cell death. The NAIP/NLRC4 inflammasome is involved in a range of pathogenic and protective inflammatory processes in mammalian hosts. In particular, the NAIP/NLRC4 inflammasome responds to flagellin and components of the virulence-associated type III secretion apparatus in the host cytosol, thereby allowing it to be a critical mediator of host defense during bacterial infection. Notable species- and cell type-specific differences exist in NAIP/NLRC4 inflammasome responses to bacterial pathogens. With a focus on Salmonella enterica serovar Typhimurium as a model pathogen, we review differences between murine and human NAIP/NLRC4 inflammasome responses. Differences in NAIP/NLRC4 inflammasome responses across species and cell types may have arisen in part due to evolutionary pressures.
Keywords: NAIP, NLRC4, inflammasome, pyroptosis, Salmonella Typhimurium, Legionella pneumophila, macrophage, intestinal epithelial cell
Introduction
The mammalian innate immune system harbors pattern recognition receptors (PRRs) that sense and respond to pathogens by detecting pathogen-associated molecular patterns (PAMPs) [1,2]. A subset of cytosolic PRRs oligomerize into multiprotein structures termed inflammasomes upon sensing an insult within the cytosol, such as PAMPs or pathogenic disruption of host processes [3–7]. After assembly, inflammasomes recruit and activate inflammatory caspases, such as caspase-1 [3–7]. Active caspase-1 cleaves and activates downstream substrates, including interleukin-1 (IL-1) family cytokines and the pore-forming protein gasdermin D, resulting in the maturation and release of IL-1 family cytokines and other alarmins and an inflammatory form of cell death termed pyroptosis [8–13].
Many inflammasomes are composed of nucleotide-binding leucine-rich repeat (NLR) family proteins. One such inflammasome is the NLR family, apoptosis inhibitory protein (NAIP)/NLR family, CARD domain-containing protein 4 (NLRC4) inflammasome. The NAIP/NLRC4 inflammasome is a key mediator of host defense against several bacterial pathogens. This inflammasome has also been implicated in pathological inflammation resulting from gain-of-function NLRC4 mutations in humans and a sepsis-like disease triggered by pathobionts in mice [14–20]. NAIP senses the cytosolic presence of evolutionarily related virulence-associated bacterial proteins, including type III secretion system (T3SS) structural proteins and flagellin, during infection [21–29]. Upon ligand binding, NAIP recruits the adaptor protein, NLRC4, which oligomerizes to form the active NAIP/NLRC4 inflammasome [30–32].
There are species- and cell type-specific differences in NAIP/NLRC4 inflammasome responses. Mice express several different NAIPs (mNAIPs), which have arisen as a result of gene duplication events, and each mNAIP recognizes a specific bacterial ligand [22–29,33]. In contrast, humans express only one functional NAIP (hNAIP), recently demonstrated to promiscuously recognize the various bacterial ligands detected by the individual mNAIPs [23,26,27,34–39] (Figure 1). In this review, we will discuss recent findings on NAIP/NLRC4 inflammasome responses to bacterial pathogens in both murine and human cells, focusing on species- and cell type-specific differences in NAIP/NLRC4 inflammasome responses.
Figure 1: Species-specific NAIP/NLRC4 inflammasome responses to bacterial ligands.
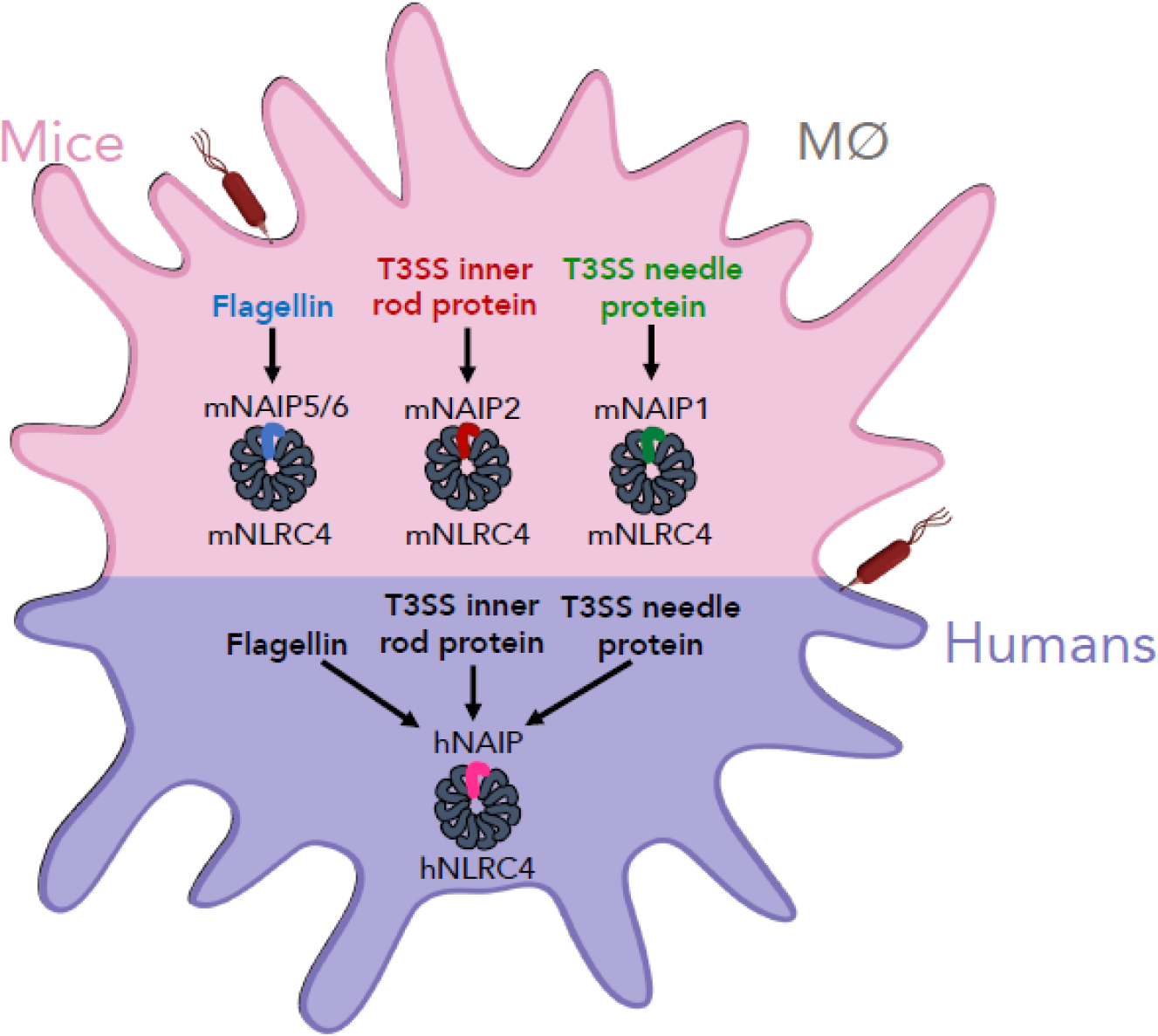
Mice and humans exhibit differences in NAIP/NLRC4 inflammasome responses to bacterial ligands. Mice express several different NAIPs (mNAIPs). Each mNAIP recognizes a distinct bacterial ligand: mNAIP5 and mNAIP6 detect flagellin, mNAIP2 detects T3SS inner rod proteins, and mNAIP1 detects T3SS needle proteins. Unlike mice, humans express a single NAIP (hNAIP), and this single hNAIP is capable of recognizing all three bacterial ligands that are individually recognized by the different mNAIPs. Upon ligand detection, NAIP recruits its adaptor, NLRC4 (murine NLRC4, mNRLC4; human NLRC4, hNLRC4), thereby forming the active NAIP/NLRC4 inflammasome.
Murine NAIP/NLRC4 inflammasome responses to bacterial ligands
In mice, NAIP/NLRC4 inflammasome responses to bacterial pathogens have been extensively characterized. The first findings focused on understanding the genetic basis for the susceptibility or resistance of inbred mouse strains to the intracellular Gram-negative bacterial pathogen Legionella pneumophila, causative agent of the severe pneumonia Legionnaires’ disease. mNAIP5 contributed to restricting Legionella replication in mice and murine macrophages [40,41], and this restriction was dependent on caspase-1 and the Legionella Dot/Icm type IV secretion system, which delivers bacterial effectors into the host cell cytosol [42]. Separate studies showed that NLRC4 was required for inflammasome responses to the intracellular enteric pathogen Salmonella enterica serovar Typhimurium (Salmonella) in murine macrophages [43]. Interestingly, inflammasome responses to Salmonella required its T3SS, which delivers virulence factors into the host cell cytosol [43]. These results suggested a potential role for specific T3SS- or T4SS-translocated bacterial ligands in NAIP or NLRC4 inflammasome activation.
When investigating bacterial components that potentially activate murine NAIP or NLRC4, multiple groups independently found that flagellin, the major subunit of flagella, from both Legionella (FlaA) and Salmonella (FliC) induced inflammasome activation in murine macrophages [21,24,25,44,45]. During infection, flagellin is thought to be injected into the host cell cytosol via bacterial secretion systems, as has been shown for Salmonella’s T3SS [46]. Still, a direct observation of endogenous flagellin being translocated into the host cell cytosol has yet to be reported and represents an important gap in knowledge. T3SS components themselves also induced inflammasome activation. T3SS inner rod proteins from several bacteria, including Salmonella (PrgJ) and Escherichia coli (EprJ and EscI), activated the NLRC4 inflammasome when delivered to the cytosol of murine bone marrow-derived macrophages (BMDMs) [21]. Salmonella’s T3SS needle protein (PrgI) was also detected, albeit poorly, in BMDMs in an NLRC4-dependent manner [21,26]. Non-immune cells can also detect bacterial ligands and mount inflammasome responses. Intestinal epithelial cells (IECs), the primary site of infection for enteric pathogens, undergo NAIP/NLRC4 inflammasome activation followed by pyroptosis and luminal expulsion in response to cytosolic delivery of Legionella’s flagellin using an anthrax-toxin based system (FlaTox) [47].
While NLRC4 was previously mischaracterized as the “sensor” of T3SS-related ligands, further studies found that NAIPs are generally the sensors that dictate ligand specificity, whereas NLRC4 is an adaptor protein [21–23,26–29,48,49]. mNAIP5 and mNAIP6 detect flagellin, while mNAIP2 detects T3SS inner rod proteins and mNAIP1 detects T3SS needle proteins [21–23,26–29,48] (Figure 2). Expression of the mNAIPs varies amongst different cell types, potentially yielding differential ligand detection. For example, expression of Naip1 is low in BMDMs, which poorly detect T3SS needle proteins, and is upregulated with IFN priming [26]. In contrast, peritoneal cavity (PerC) macrophages express higher Naip1 levels and readily detect cytosolic PrgI [26]. Intriguingly, a functional NLRC4 inflammasome can assemble in the absence of the NAIPs in response to Anaplasma phagocytophilum infection through a mechanism involving prostaglandin E2 signaling [50].
Figure 2: Murine cell type-specific inflammasome responses to Salmonella infection.
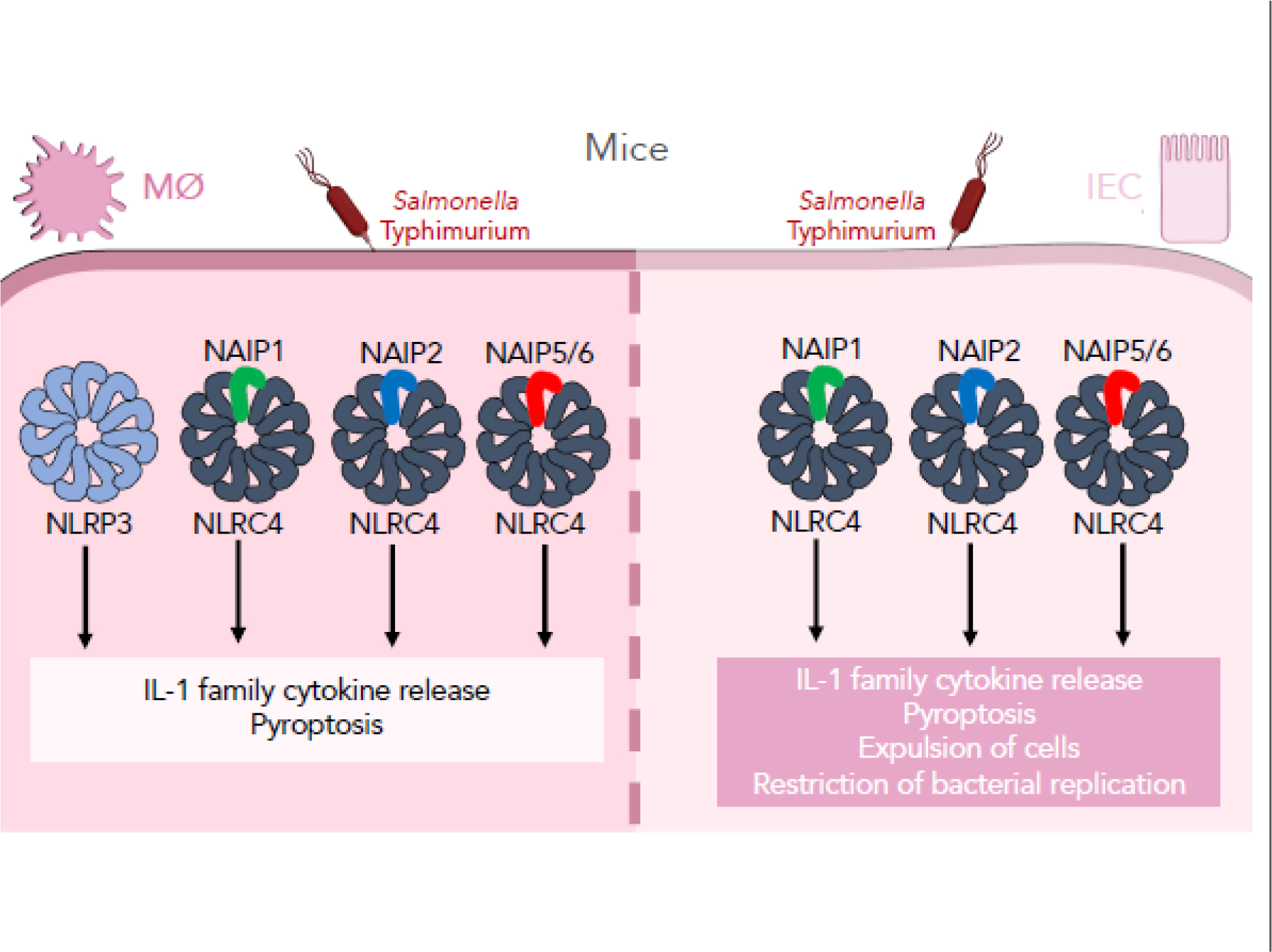
Murine macrophages and intestinal epithelial cells (IECs) display distinct inflammasome responses to Salmonella infection. In murine macrophages, Salmonella infection induces both NLRP3 and NAIP/NLRC4 inflammasome responses, leading to IL-1 cytokine release and an inflammatory form of cell death, pyroptosis. In murine IECs, the NAIP/NLRC4 inflammasome senses and responds to Salmonella infection, mediating IL-1 cytokine release, pyroptosis, and expulsion of cells, which ultimately leads to control of infection in vivo.
In agreement with in vitro findings, bacterial ligands activate the NAIP/NLRC4 inflammasome in vivo, with individual mNAIPs detecting their cognate ligands [15,28,29]. Intraperitoneal administration of FlaTox to mice resulted in NAIP/NLRC4-dependent diarrhea, circulatory collapse and ultimately mortality at high doses [15,28]. Furthermore, restricting Nlrc4 expression to either macrophages or IECs still resulted in mortality and circulatory collapse following FlaTox administration, due to caspase-1 activation and pathological release of eicosanoids [15,47]. These results highlight specific cell-intrinsic roles for NLRC4 in vivo (Figure 2).
Murine NAIP/NLRC4 inflammasome responses to Salmonella infection
While the findings highlighted above focused on NAIP/NLRC4 inflammasome activation by specific bacterial ligands, different bacterial components can activate distinct arms of the immune response. In turn, some bacterial pathogens downregulate expression of particular ligands to evade immune detection during infection. Thus, a number of studies have extensively characterized the role of the NAIP/NLRC4 inflammasome during bacterial infection. For this review, we will focus on Salmonella, which, as highlighted above, was recognized early on to activate the NLRC4 inflammasome in murine macrophages [43].
Salmonella harbors two distinct T3SSs, known as the Salmonella pathogenicity island (SPI)-1 and SPI-2 T3SSs. While the SPI-1 T3SS is expressed early during infection to mediate invasion into host cells, the SPI-2 T3SS is expressed later to promote intracellular replication [51–59]. Interestingly, Salmonella grown under SPI-1-inducing conditions activates robust inflammasome responses in BMDMs in an NLRC4-dependent manner; however, Salmonella grown under SPI-2 inducing conditions does not [21]. While these data suggest that the SPI-2 T3SS evades inflammasome detection, Salmonella can induce SPI-1-independent inflammasome responses at later time points, namely between 17 and 20 hours post-infection (hpi) [60]. Broz et al. characterized these late responses in BMDMs and found that Salmonella activates both the NAIP/NLRC4 inflammasome and the NLRP3 inflammasome [61] (Figure 2), which responds to perturbations of cell physiology, such as potassium efflux as a result of plasma membrane damage [62–66]. Distinct Salmonella signals appear to activate these inflammasomes [61]. Additional studies corroborated these findings, suggesting that NAIP/NLRC4 and NLRP3 coordinate the inflammasome response to Salmonella by associating in the same macromolecular complex [67,68].
During in vivo Salmonella infection, the NAIP/NLRC4 inflammasome promotes inflammation to ultimately clear infection. Wild type (WT) and Nlrc4−/− mice on a BALB/c or C57BL/6 background displayed differences in mortality during orogastric challenge with Salmonella [69,70], in part due to NLRC4-promoted clearance of infection via neutrophil recruitment [69]. In contrast, C57BL/6 mice lacking NLRC4 displayed no differences in mortality compared to their WT counterparts during intraperitoneal Salmonella infection [21,69]. This may be in part due to differential Salmonella ligand expression during gastrointestinal versus systemic infection. Salmonella expresses PAMPs that activate the NAIP/NLRC4 inflammasome, including flagellin and the SPI-1 T3SS, most highly in the cecal epithelium and downregulates expression as it invades deeper tissues [71]. Salmonella ectopically expressing flagellin or PrgJ during systemic infection were attenuated compared to WT bacteria, and this was due to NLRC4 inflammasome activation [29,72]. These studies indicate an important role for the NAIP/NLRC4 inflammasome in responding to these ligands to control Salmonella in vivo.
Murine IECs, the primary site of gastrointestinal Salmonella infection, express high levels of Naip and Nlrc4 [71,73–76]. The NAIP/NLRC4 inflammasome limits Salmonella accumulation within IECs, in part by facilitating expulsion of infected IECs into the lumen and limiting cellular spread of bacteria [47,71,76]. This restriction mechanism is independent of IL-1 release. Furthermore, IEC-intrinsic Naip or Nlrc4 expression was both necessary and sufficient to restrict intraepithelial Salmonella loads, suggesting an intestinal epithelium-intrinsic role for the NAIP/NLRC4 inflammasome in restricting Salmonella [47,76]. Subsequent studies using intestinal enteroid models further elucidated this epithelium-intrinsic mechanism of restriction. Activation of epithelial NAIP/NLRC4, either via Salmonella infection or treatment with FlaTox, initiated focal contractions at the site of activation [77]. In addition to restricting intraepithelial bacterial loads, IEC-specific NAIP/NLRC4 inflammasome activation prevents bacterial dissemination to systemic sites, supporting an important role for the inflammasome in intestinal barrier defense [71] (Figure 2). Collectively, these findings in the murine system elucidating NAIP/NLRC4 recognition of specific ligands and response to bacterial infection form a strong foundation for understanding NAIP/NLRC4-mediated immune amplification. Clear differences exist between different cell types and organ systems at large, begging the question of what drives the evolution of varying responses downstream of a common infectious or inflammatory signal. For example, it is possible that IEC responses differ from macrophage responses due to a necessity to maintain barrier integrity as a physical component of immune defense. Studying these questions in the murine system offers a powerful way to examine whole body consequences of NAIP/NLRC4 inflammasome responses and deepen understanding in the field.
Human NAIP/NLRC4 inflammasome responses to bacterial pathogens
In contrast to mice and rats, humans and other animals express a single functional NAIP (hNAIP) [34,35]. Early studies found that T3SS needle proteins from various bacteria, including Salmonella (PrgI) and enterohemorrhagic Escherichia coli (EHEC) (EprI), induced NAIP/NLRC4 inflammasome activation in the human monocytic cell lines U937 and THP-1 [23,26,27]. Strikingly, these same studies also found that U937 and THP-1 cells did not respond to cytosolic delivery of flagellin or certain T3SS inner rod proteins [23,26,27]. Thus, it appeared that hNAIP retained homologous function to mNAIP1, as it only detected the T3SS needle protein.
Subsequent studies found that hNAIP detects additional bacterial ligands. In primary human monocyte-derived macrophages (hMDMs), flagellin from Salmonella (FliC) or Legionella (FlaA) activated the NAIP inflammasome [36,37]. Subsequently, it was shown that hMDMs also undergo inflammasome responses to the T3SS inner rod protein from Salmonella (PrgJ) and other bacteria [37,39], and that this response was also dependent on hNAIP [37]. These apparent discrepancies compared to the earlier studies could be due to differences in ligand delivery systems and T3SS inner rod proteins used or lower expression of NAIP and NLRC4 in U937 and THP-1 cells compared to primary human macrophages [36]. Importantly, the single hNAIP is sufficient to mediate inflammasome responses to the T3SS needle, inner rod, and flagellin proteins [37]. Furthermore, both NAIP and NLRC4 are required for inflammasome responses to these bacterial ligands [38,78]. These studies show that in contrast to mice, which have evolved multiple specialist NAIPs that each recognize only one ligand, humans express a generalist NAIP which has evolved to promiscuously recognize three structurally related but distinct bacterial ligands. The structural basis for this difference in ligand recognition by mNAIPs and hNAIP is unclear. Interestingly, rats, like mice, also retain multiple NAIPs that have arisen as a result of gene duplication events, suggesting that pathogen-imposed selective pressure in the rodent lineage resulted in the emergence of specialist NAIPs. In contrast, other mammals express a single functional NAIP, including non-human primates, cows, horses, and bats [34,35]. Future studies are needed to investigate whether the single NAIP in other mammals also behaves as a generalist like hNAIP and broadly recognizes multiple ligands.
While Salmonella’s SPI-1 T3SS structural components activate mNAIPs and hNAIP, Salmonella’s SPI-2 T3SS inner rod protein (SsaI) is not sensed by murine NAIP2 or hNAIP [21,37]. In addition, Salmonella SPI-2 activity suppresses SPI-1-induced inflammasome responses in human macrophages [79]. Thus, a prevailing model posits that Salmonella’s SPI-2 T3SS evades inflammasome detection to promote Salmonella’s intracellular lifestyle. However, recent findings show that Salmonella’s SPI-2 T3SS needle protein (SsaG) is, in fact, sensed by hNAIP, leading to NAIP/NLRC4-dependent restriction of Salmonella replication within human macrophages [38] (Figure 3).
Figure 3: Human cell type-specific inflammasome responses to Salmonella infection.
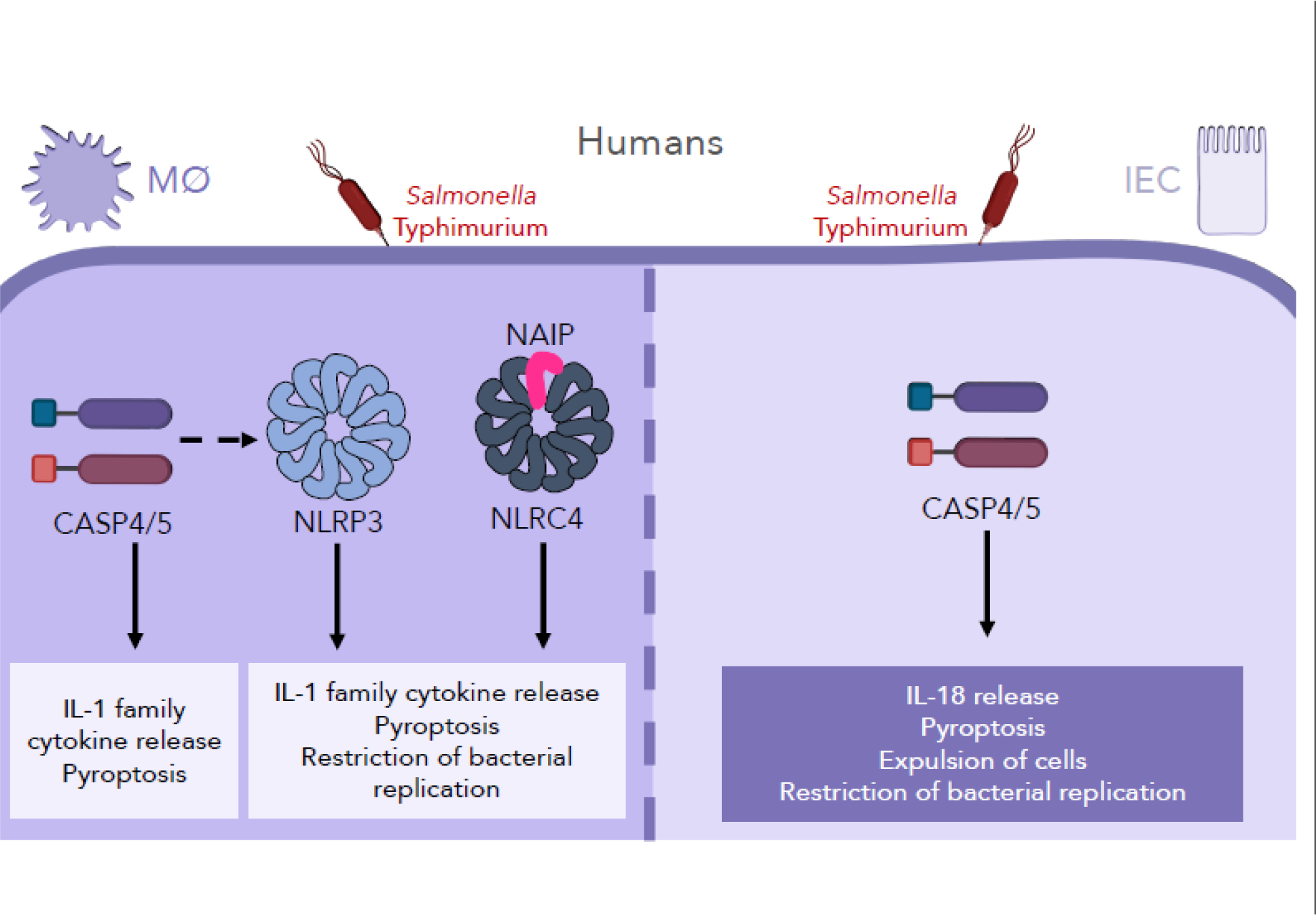
While human macrophages employ a multifactorial inflammasome response to Salmonella infection, human intestinal epithelial cells (IECs) rely on a single inflammasome, the caspase-4/5 inflammasome, during Salmonella infection. In human macrophages, Salmonella infection induces NAIP/NLRC4, NLRP3, and caspase-4/5 inflammasome responses, facilitating IL-1 cytokine release, pyroptosis, and ultimately restriction of bacterial replication. In contrast, in human IECs undergo a caspase-4-dependent, NAIP/NLRC4- and NLRP3-independent inflammasome response to Salmonella infection.
These critical differences between hNAIP and mNAIP recognition of bacterial ligands represent fascinating and fundamental distinctions between the two species, highlighting the importance of broadly conducting studies across different species. Much still remains unknown about hNAIP-mediated ligand detection, including the molecular determinants of hNAIP’s broad ligand detection, hNAIP’s binding affinity for each ligand, and the precise structure of ligand-bound hNAIP. These open questions present exciting opportunities for future expansion of the field of human inflammasome biology.
As noted previously, bacterial infections do not recapitulate purified ligand delivery, as they elicit more complex and nuanced inflammasome responses. As such, NAIP/NLRC4 inflammasome responses to bacterial infection have been studied in human cells. hNAIP is required to restrict intracellular Legionella replication in both THP-1 macrophages and A549 lung epithelial cells [80]. Legionella T4SS activity also activates the NLRP3 inflammasome and CASP4 inflammasome, which senses cytosolic LPS, in THP-1 cells and hMDMs [81]. Moreover, Salmonella infection induces robust inflammasome activation in human macrophages that requires SPI-1 T3SS or flagellin [27,36–38,78,79]. Salmonella elicits a multifactorial response in human macrophages, involving the NAIP/NLRC4, NLRP3, and CASP4/5 inflammasomes [38,78,79] (Figure 3). Furthermore, NLRC4 and NLRP3 co-localize to the same macromolecular complex upon in vitro Salmonella infection of human macrophages [68]. The recruitment of NLRC4 to punctate structures has also been reported in BMDMs during in vitro Legionella infection in an ASC-dependent manner [82]. Whether NLRC4 co-localizes with other inflammasome structures during in vivo infections remains unknown and requires further study. Finally, inflammasome activation mediates Salmonella control in human macrophages such that both the NAIP/NLRC4 and NLRP3 inflammasomes restrict Salmonella replication within human macrophages [38].
Like murine IECs, human IECs can mount cell-intrinsic inflammasome responses to bacterial pathogens, including Salmonella. In response to Salmonella, human IECs undergo caspase-4 inflammasome-dependent pyroptosis, IL-18 cytokine release, restriction of bacterial replication, and extrusion of infected cells [83–85] (Figure 3). However, the role of the NAIP/NLRC4 inflammasome in human IECs during infection remained unclear. Surprisingly, unlike in murine IECs where the NAIP/NLRC4 inflammasome is functional [28,29,47], delivery of bacterial ligands failed to induce NAIP/NLRC4 inflammasome activation in immortalized human IECs or human intestinal enteroids [86] (Figure 3). Furthermore, human NAIP−/− immortalized IECs had no defect in inflammasome responses to Salmonella. Immortalized human IECs and primary small intestinal enteroids express very low levels of NAIP and NLRC4 compared to human peripheral blood mononuclear cells [86], which potentially contributes to the lack of a functional NAIP/NLRC4 inflammasome in human IECs in these in vitro models. Whether the NAIP/NLRC4 inflammasome functions in human intestinal epithelium in vivo is unknown. It is possible that in vivo, host or microbial signals may upregulate NAIP/NLRC4 expression in human IECs, or NAIP/NLRC4 may be expressed in a rare IEC subset not represented in in vitro models. Intriguingly, human patients with gain-of-function NLRC4 mutations display enterocolitis early in infancy, although gastrointestinal disease no longer presents in patients that survive infancy [17–20]. Whether infantile enterocolitis is caused by NLRC4 activation in IECs or another cell type is unclear. Together, these findings in human macrophages and IECs underscore important cell type-specific and species-specific differences that exist between mice and humans and the importance of broadening these studies to include multiple cell types. Recent advances in ex vivo culture systems, including organoids, represent exciting new avenues with which to study these questions and deepen understanding of cellular roles
Concluding remarks and remaining questions
In summary, the NAIP/NLRC4 inflammasome senses bacterial components in the host cell cytosol and induces a cascade of inflammatory events to mediate host defense against bacterial pathogens. Recent studies have revealed species-specific NAIP/NLRC4 inflammasome responses. While mice express multiple NAIPs which each detect a specific ligand, humans harbor a single NAIP which broadly recognizes multiple ligands. We find this key difference between mice and humans compelling. It begs the question: is there a selective advantage or disadvantage to promiscuous ligand detection by human NAIP when compared to selective ligand detection by mouse NAIPs? Relative commensal and pathogen exposure by different species may have provided evolutionary pressures underlying selective versus promiscuous ligand detection. In addition, as previously highlighted, there are other non-human mammalian species that express a single NAIP; whether the single NAIP in these other species also promiscuously recognizes multiple ligands is unknown. Future studies more broadly comparing different species may help address these questions. Recent findings suggest cell type-specific differences in NAIP/NLRC4 inflammasome responses to bacteria that vary between mice and humans. For example, NAIP/NLRC4 inflammasome activation is both necessary and sufficient in IECs to control Salmonella infection in mice, whereas the NAIP/NLRC4 inflammasome appears to be nonfunctional in human IECs. In addition, there are also cell type-specific differences within a given species. While the NAIP/NLRC4 inflammasome senses and responds to Salmonella infection in human macrophages, the inflammasome appears dispensable in human IECs. Thus, there exists differential NAIP/NLRC4 inflammasome responses to pathogens that are species- and cell type-specific. We find the field of NAIP/NLRC4 inflammasome responses during bacterial infections to be an ever expanding and particularly fascinating one. While considerable work has been done to delineate these responses in mice and humans in various cell types, there are still many exciting unanswered questions in the realm of NAIP/NLRC4 biology that will be investigated for years to come.
Highlights.
Mice express several different NAIPs, each recognizing a specific bacterial ligand
Humans express one functional NAIP, which broadly detects multiple bacterial ligands
Salmonella activation of the NAIP inflammasome in murine IECs promotes control of infection
NAIP inflammasome is critical for controlling Salmonella in human macrophages but not in human IECs
Acknowledgment
This work was supported by National Institutes of Health grants AI123243 and AI118861 (to SS), 5T32AI141393 (to JZ), and National Science Foundation Graduate Research Fellowship DGE-1321851 (to MSE). SS holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Conflict of interest: none
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Janeway CA: Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 1989, 54 Pt 1:1–13. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Huang H, Liu B, Zhang Y, Pan X, Yu X-Y, Shen Z, Song Y-H: Inflammasomes as therapeutic targets in human diseases. Sig Transduct Target Ther 2021, 6:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes Fischer N, Naseer N, Shin S, Brodsky IE: Effector-triggered immunity and pathogen sensing in metazoans. Nat Microbiol 2019, 5:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan AH, Schroder K: Inflammasome signaling and regulation of interleukin-1 family cytokines. Journal of Experimental Medicine 2020, 217:e20190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathinam VAK, Fitzgerald KA: Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Man SM, Kanneganti T-D: Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 2016, 16:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broz P, Dixit VM: Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016, 16:407–420. [DOI] [PubMed] [Google Scholar]
- 8.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS-S, Flavell RA: Altered Cytokine Export and Apoptosis in Mice Deficient in Interleukin-1β Converting Enzyme. Science 1995, 267:2000–2003. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. : Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 1995, 80:401–411. [DOI] [PubMed] [Google Scholar]
- 10.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. : A novel heterodimeric cysteine protease is required for interleukin-1βprocessing in monocytes. Nature 1992, 356:768–774. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F: Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526:660–665. [DOI] [PubMed] [Google Scholar]
- 12.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. : Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526:666–671. [DOI] [PubMed] [Google Scholar]
- 13.Agard NJ, Maltby D, Wells JA: Inflammatory Stimuli Regulate Caspase Substrate Profiles. Molecular & Cellular Proteomics 2010, 9:880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayres JS, Trinidad NJ, Vance RE: Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med 2012, 18:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, et al. : Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 2012, 490:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K: An inherited mutation in NLRC4 causes autoinflammation in human and mice. Journal of Experimental Medicine 2014, 211:2385–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK, Huttner AJ, et al. : Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet 2014, 46:1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, et al. : Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. Journal of Allergy and Clinical Immunology 2017, 139:1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, DiMattia MA, Zaal KJM, Sanchez GAM, Kim H, et al. : An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 2014, 46:1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang J, Alfano DN, Squires JE, Riley MM, Parks WT, Kofler J, El-Gharbawy A, Madan-Kheterpal S, Acquaro R, Picarsic J: Novel NLRC4 Mutation Causes a Syndrome of Perinatal Autoinflammation With Hemophagocytic Lymphohistiocytosis, Hepatosplenomegaly, Fetal Thrombotic Vasculopathy, and Congenital Anemia and Ascites. Pediatr Dev Pathol 2017, 20:498–505. [DOI] [PubMed] [Google Scholar]
- 21.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A: Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences 2010, 107:3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kofoed EM, Vance RE: Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 2011, 477:592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, Liu L, Shao F: The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011, 477:596–600. [DOI] [PubMed] [Google Scholar]
- 24.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE: Flagellin-Deficient Legionella Mutants Evade Caspase-1- and Naip5-Mediated Macrophage Immunity. PLoS Pathogens 2006, 2:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS: Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. The Journal of Experimental Medicine 2006, 203:1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, Miao EA: Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein. J Immunol 2013, 191:3986–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Zhao Y, Shi J, Shao F: Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci USA 2013, 110:14408–14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauch I, Tenthorey JL, Nichols RD, Al Moussawi K, Kang JJ, Kang C, Kazmierczak BI, Vance RE: NAIP proteins are required for cytosolic detection of specific bacterial ligands in vivo. J Exp Med 2016, 213:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Shi J, Shi X, Wang Y, Wang F, Shao F: Genetic functions of the NAIP family of inflammasome receptors for bacterial ligands in mice. J Exp Med 2016, 213:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diebolder CA, Halff EF, Koster AJ, Huizinga EG, Koning RI: Cryoelectron Tomography of the NAIP5/NLRC4 Inflammasome: Implications for NLR Activation. Structure 2015, 23:2349–2357. [DOI] [PubMed] [Google Scholar]
- 31.Hu Z, Zhou Q, Zhang C, Fan S, Cheng W, Zhao Y, Shao F, Wang H-W, Sui S-F, Chai J: Structural and biochemical basis for induced self-propagation of NLRC4. Science 2015, 350:399–404. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Chen S, Ruan J, Wu J, Tong AB, Yin Q, Li Y, David L, Lu A, Wang WL, et al. : Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 2015, 350:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endrizzi MG: Genomic Sequence Analysis of the Mouse Naip Gene Array. Genome Research 2000, 10:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romanish MT, Lock WM, de Lagemaat LN van, Dunn CA, Mager DL: Repeated Recruitment of LTR Retrotransposons as Promoters by the Anti-Apoptotic Locus NAIP during Mammalian Evolution. PLoS Genet 2007, 3:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanish MT, Nakamura H, Lai CB, Wang Y, Mager DL: A novel protein isoform of the multicopy human NAIP gene derives from intragenic Alu SINE promoters. PLoS One 2009, 4:e5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kortmann J, Brubaker SW, Monack DM: Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. J Immunol 2015, 195:815–819. ** The authors show that human NAIP detects cytosolic flagellin within macrophages. These findings demonstrate that the human NAIP inflammasome is activated in response to both the T3SS needle and flagellin in macrophages.
- 37. Reyes Ruiz VM, Ramirez J, Naseer N, Palacio NM, Siddarthan IJ, Yan BM, Boyer MA, Pensinger DA, Sauer J-D, Shin S: Broad detection of bacterial type III secretion system and flagellin proteins by the human NAIP/NLRC4 inflammasome. Proceedings of the National Academy of Sciences 2017, 114:13242–13247. ** The authors find that a single human NAIP isoform senses multiple bacterial ligands, including flagellin, the T3SS needle protein, and the T3SS inner rod protein. This finding contrasts against ligand specificity conferred by individual murine NAIPs and identifies an important species-specific difference in NAIP inflammasome recognition and function.
- 38. Naseer N, Egan MS, Reyes Ruiz VM, Scott WP, Hunter EN, Demissie T, Rauch I, Brodsky IE, Shin S: Human NAIP/NLRC4 and NLRP3 inflammasomes detect Salmonella type III secretion system activities to restrict intracellular bacterial replication. PLoS Pathog 2022, 18:e1009718. ** The authors show that Salmonella activates the NAIP/NLRC4, CASP4/5, and NLRP3 inflammasomes in human macrophages. They also find that human NAIP detects the Salmonella SPI-2 T3SS needle protein SsaG. Furthermore, they show that the NAIP/NLRC4 and NLRP3 inflammasomes contribute to restricting intracellular Salmonella replication within macrophages.
- 39.Grandjean T, Boucher A, Thepaut M, Monlezun L, Guery B, Faudry E, Kipnis E, Dessein R: The human NAIP-NLRC4-inflammasome senses the Pseudomonas aeruginosa T3SS inner-rod protein. International Immunology 2017, 29:377–384. [DOI] [PubMed] [Google Scholar]
- 40.Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, Long EM, Sadigh K, Abney AL, Bernstein-Hanley I, Dietrich WF: Naip5 Affects Host Susceptibility to the Intracellular Pathogen Legionella pneumophila. Current Biology 2003, 13:27–36. [DOI] [PubMed] [Google Scholar]
- 41.Diez E, Lee S-H, Gauthier S, Yaraghi Z, Tremblay M, Vidal S, Gros P: Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat Genet 2003, 33:55–60. [DOI] [PubMed] [Google Scholar]
- 42.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, et al. : The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 2006, 7:318–325. [DOI] [PubMed] [Google Scholar]
- 43.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM: Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004, 430:213–218. [DOI] [PubMed] [Google Scholar]
- 44.Amer A, Franchi L, Kanneganti T-D, Body-Malapel M, Özören N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, et al. : Regulation of Legionella Phagosome Maturation and Infection through Flagellin and Host Ipaf. Journal of Biological Chemistry 2006, 281:35217–35223. [DOI] [PubMed] [Google Scholar]
- 45.Franchi L, Amer A, Body-Malapel M, Kanneganti T-D, Özören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. : Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nature Immunology 2006, 7:576–582. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y-H, Rolan HG, Tsolis RM: Injection of Flagellin into the Host Cell Cytosol by Salmonella enterica Serotype Typhimurium. Journal of Biological Chemistry 2007, 282:33897–33901. [DOI] [PubMed] [Google Scholar]
- 47. Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, et al. : NAIP-NLRC4 Inflammasomes Coordinate Intestinal Epithelial Cell Expulsion with Eicosanoid and IL-18 Release via Activation of Caspase-1 and −8. Immunity 2017, 46:649–659. * The authors find that IECs respond to cytosolic flagellin by activating the NAIP/NLRC4 inflammasome and undergoing subsequent IEC expulsion. This expulsion is IEC-intrinsic and involves both caspase-1 and caspase-8, highlighting a role for caspase-1 and caspase-8 inflammasomes in intestinal inflammation.
- 48.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun Y-H, Cado D, Dietrich WF, et al. : Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 2008, 9:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenthorey JL, Kofoed EM, Daugherty MD, Malik HS, Vance RE: Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol Cell 2014, 54:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Shaw DK, Hammond HL, Sutterwala FS, Rayamajhi M, Shirey KA, Perkins DJ, Bonventre JV, Velayutham TS, Evans SM, et al. : The Prostaglandin E2-EP3 Receptor Axis Regulates Anaplasma phagocytophilum-Mediated NLRC4 Inflammasome Activation. PLoS Pathog 2016, 12:e1005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mills DM, Bajaj V, Lee CA: A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Molecular Microbiology 1995, 15:749–759. [DOI] [PubMed] [Google Scholar]
- 52.Shea JE, Hensel M, Gleeson C, Holden DW: Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proceedings of the National Academy of Sciences 1996, 93:2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang FC, Holden DW: Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Molecular Microbiology 1998, 30:163–174. [DOI] [PubMed] [Google Scholar]
- 54.Galan JE, Zhou D: Striking a balance: Modulation of the actin cytoskeleton by Salmonella. Proceedings of the National Academy of Sciences 2000, 97:8754–8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galán JE: Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Current Opinion in Microbiology 1999, 2:46–50. [DOI] [PubMed] [Google Scholar]
- 56.Galan JE, Curtiss R: Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proceedings of the National Academy of Sciences 1989, 86:6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galán JE, Collmer A: Type III Secretion Machines: Bacterial Devices for Protein Delivery into Host Cells. Science 1999, 284:1322–1328. [DOI] [PubMed] [Google Scholar]
- 58.Cirillo DM, Valdivia RH, Monack DM, Falkow S: Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Molecular Microbiology 1998, 30:175–188. [DOI] [PubMed] [Google Scholar]
- 59.Ochman H, Soncini FC, Solomon F, Groisman EA: Identification of a pathogenicity island required for Salmonella survival in host cells. Proceedings of the National Academy of Sciences 1996, 93:7800–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monack DM, Detweiler CS, Falkow S: Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol 2001, 3:825–837. [DOI] [PubMed] [Google Scholar]
- 61.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM: Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. Journal of Experimental Medicine 2010, 207:1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G: K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38:1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E: Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008, 9:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franchi L, Kanneganti T-D, Dubyak GR, Núñez G: Differential Requirement of P2X7 Receptor and Intracellular K+ for Caspase-1 Activation Induced by Intracellular and Extracellular Bacteria. Journal of Biological Chemistry 2007, 282:18810–18818. [DOI] [PubMed] [Google Scholar]
- 65.Perregaux D, Gabel CA: Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 1994, 269:15195–15203. [PubMed] [Google Scholar]
- 66.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM: Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440:228–232. [DOI] [PubMed] [Google Scholar]
- 67.Qu Y, Misaghi S, Newton K, Maltzman A, Izrael-Tomasevic A, Arnott D, Dixit VM: NLRP3 recruitment by NLRC4 during Salmonella infection. Journal of Experimental Medicine 2016, 213:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP, Bryant CE: Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proceedings of the National Academy of Sciences 2014, 111:7403–7408. *The authors demonstrate that in murine and human macrophages infected with Salmonella, the NLRC4 and NLPR3 inflammasomes congregate into the same macromolecular complex. This study provides insight into how spatial localization of different components helps protein recruitment and coordination of inflammasome responses to infection.
- 69.Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S, Shaw MH, Kim Y-G, Núñez G: NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol 2012, 13:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carvalho FA, Nalbantoglu I, Aitken JD, Uchiyama R, Su Y, Doho GH, Vijay-Kumar M, Gewirtz AT: Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal Immunology 2012, 5:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hausmann A, Böck D, Geiser P, Berthold DL, Fattinger SA, Furter M, Bouman JA, Barthel-Scherrer M, Lang CM, Bakkeren E, et al. : Intestinal epithelial NAIP/NLRC4 restricts systemic dissemination of the adapted pathogen Salmonella Typhimurium due to site-specific bacterial PAMP expression. Mucosal Immunol 2020, 13:530–544. **The authors find that IEC-specific NAIP/NLRC4 inflammasome activation restricts Salmonella migration from the gut to systemic sites via the draining lymph nodes. These findings demonstrate a critical and specific role for IEC-specific NAIP/NLRC4 inflammasome activation in blocking bacterial dissemination in vivo.
- 72.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A: Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunology 2006, 7:569–575. [DOI] [PubMed] [Google Scholar]
- 73.Allam R, Maillard MH, Tardivel A, Chennupati V, Bega H, Yu CW, Velin D, Schneider P, Maslowski KM: Epithelial NAIPs protect against colonic tumorigenesis. Journal of Experimental Medicine 2015, 212:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nordlander S, Pott J, Maloy KJ: NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol 2014, 7:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, Flavell RA: Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA 2010, 107:21635–21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sellin ME, Müller AA, Felmy B, Dolowschiak T, Diard M, Tardivel A, Maslowski KM, Hardt W-D: Epithelium-Intrinsic NAIP/NLRC4 Inflammasome Drives Infected Enterocyte Expulsion to Restrict Salmonella Replication in the Intestinal Mucosa. Cell Host & Microbe 2014, 16:237–248. ** The authors demonstrate that epithelium-specific NAIP/NLRC4 inflammasome activation is critical for IEC expulsion to restrict intraepithelial bacterial burdens. These studies highlight Salmonella’s intraepithelial population and establish the importance of an epithelium-intrinsic inflammasome in restricting this population.
- 77. Samperio Ventayol P, Geiser P, Di Martino ML, Florbrant A, Fattinger SA, Walder N, Sima E, Shao F, Gekara NO, Sundbom M, et al. : Bacterial detection by NAIP/NLRC4 elicits prompt contractions of intestinal epithelial cell layers. Proc Natl Acad Sci USA 2021, 118:e2013963118. *The authors demonstrate that epithelial NAIP/NLRC4 activation triggers focal contractions preceding death and expulsion of Salmonella-infected IECs. These contractions allowed maintenance of monolayer integrity during epithelial expulsion in response to infection.
- 78. Gram AM, Wright JA, Pickering RJ, Lam NL, Booty LM, Webster SJ, Bryant CE: Salmonella Flagellin Activates NAIP/NLRC4 and Canonical NLRP3 Inflammasomes in Human Macrophages. J Immunol 2021, 206:631–640. ** The authors show that the NAIP/NLRC4 and NLRP3 inflammasomes both contribute to immune responses to Salmonella in human macrophages. They also identify flagellin as an activator of the NLRP3 inflammasome. These findings highlight human-specific inflammasome responses to Salmonella.
- 79. Bierschenk D, Monteleone M, Moghaddas F, Baker PJ, Masters SL, Boucher D, Schroder K: The Salmonella pathogenicity island-2 subverts human NLRP3 and NLRC4 inflammasome responses. J Leukoc Biol 2019, 105:401–410. * The authors find that both the NAIP/NLRC4 and NLPR3 inflammasomes contribute to human macrophage responses against Salmonella infection. Specifically, SPI-1 induced inflammasome activation that was suppressed by SPI-2, demonstrating opposing roles for Salmonella’s two type III secretion systems and a species-specific immune evasion mechanism.
- 80.Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, Slevogt H, N’guessan PD, Günther S, Schmeck B, Hippenstiel S, et al. : NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol 2008, 180:6808–6815. [DOI] [PubMed] [Google Scholar]
- 81.Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, Copenhaver AM, Nguyen HT, Collman RG, Shin S: Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci USA 2015, 112:6688–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Case CL, Roy CR: Asc Modulates the Function of NLRC4 in Response to Infection of Macrophages by Legionella pneumophila. mBio 2011, 2:e00117–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O: Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci USA 2010, 107:17733–17738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA: Noncanonical Inflammasome Activation of Caspase-4/Caspase-11 Mediates Epithelial Defenses against Enteric Bacterial Pathogens. Cell Host & Microbe 2014, 16:249–256. **The authors find that caspase-4 mediates IEC extrusion via induction of pyroptosis during Salmonella infection, which ultimately restricts Salmonella replication. These findings shed light on an important mechanism of epithelial cell noncanonical inflammasome activation in restricting Salmonella.
- 85. Holly MK, Han X, Zhao EJ, Crowley SM, Allaire JM, Knodler LA, Vallance BA, Smith JG: Salmonella enterica Infection of Murine and Human Enteroid-Derived Monolayers Elicits Differential Activation of Epithelium-Intrinsic Inflammasomes. Infect Immun 2020, 88:e00017–20. * The authors compare the importance of different caspases in murine and human IECs in response to Salmonella infection. They find that caspase-1 is critical for inflammasome activation and restriction of intracellular Salmonella in murine IECs whereas caspase-4 is critical in human IECs, highlighting important species-specific differences in inflammasome responses.
- 86. Naseer N, Zhang J, Bauer R, Constant DA, Nice TJ, Brodsky IE, Rauch I, Shin S: Salmonella enterica Serovar Typhimurium Induces NAIP/NLRC4- and NLRP3/ASC-Independent, Caspase-4-Dependent Inflammasome Activation in Human Intestinal Epithelial Cells. Infect Immun 2022, 90:e00663–21. ** The authors find that in contrast to human and murine macrophages and murine IECs, human IECs do not rely on NAIP/NLRC4 or NLRP3/ASC inflammasomes, but instead primarily relies on caspase-4 to sense and respond to Salmonella infection. This study demonstrates important species and cell type-specific inflammasome responses against infection


