Abstract
Aims:
Nerve growth factor (NGF) has been implicated as a key molecule of pathology-induced changes in C-fiber afferent nerve excitability, which contributes to the emergence of neurogenic detrusor overactivity due to spinal cord injury (SCI). It is also known that the second messenger signaling pathways activated by NGF utilize p38 Mitogen-Activated Protein Kinase (MAPK). We examined the roles of p38 MAPK on electrophysiological properties of capsaicin sensitive bladder afferent neurons with SCI mice.
Main Methods:
We used female C57BL/6 mice and transected their spinal cord at the Th8/9 level. Two weeks later, continuous administration of p38 MAPK inhibitor (0.51 μg/h, i.t. for two weeks) was started. Bladder afferent neurons were labelled with a fluorescent retrograde tracer, Fast-Blue (FB), injected into the bladder wall three weeks after SCI. Four weeks after SCI, freshly dissociated L6-S1 dorsal root ganglion neurons were prepared and whole cell patch clamp recordings were performed in FB-labelled neurons. After recording action potentials or voltage-gated K+ currents, the sensitivity of each neuron to capsaicin was evaluated.
Key findings:
In capsaicin-sensitive FB-labelled neurons, SCI significantly reduced the spike threshold and increased the number of action potentials during 800 ms membrane depolarization. Densities of slow-decaying A-type K+ (KA) and sustained delayed rectifier-type K+ (KDR) currents were significantly reduced by SCI. The reduction of KA, but not KDR, current density was reversed by the treatment with p38 MAPK inhibitor.
Significance:
P38 MAPK plays an important role in hyperexcitability of capsaicin-sensitive bladder afferent neurons due to the reduction in KA channel activity in SCI mice.
Keywords: P38 MAPK, potassium current, bladder afferent neuron, spinal cord injury, mice
1. Introduction
It has been well known that spinal cord injury (SCI) rostral to the lumbosacral level causes lower urinary tract dysfunctions (LUTD). SCI-induced LUTD develops, which is characterized by reduced voiding efficiency, detrusor sphincter dyssynergia (DSD) and detrusor overactivity (DO) mediated by spinal reflex pathways [1]. The recovery of reflex bladder activity after SCI is dependent on the reorganization of reflex pathways in the spinal cord as well as alterations in the properties of bladder afferent neurons [2].
Bladder afferent pathways consist of myelinated Aδ-fibers and unmyelinated C-fibers. Under the normal condition, C-fiber bladder afferents do not respond to bladder distention in the physiological range, therefore, are called silent C-fibers [3], whereas Aδ-fiber bladder afferents are involved in the control of micturition reflex. However, in the chronic SCI condition, excitability of C-fiber bladder afferent neurons is increased, thereby inducing neurogenic DO as evidenced by non-voiding bladder contraction (NVCs) before micturition [1]. Clinical and experimental studies demonstrated that DO after SCI is induced at least in part by phenotypic changes in capsaicin-sensitive C-fiber bladder afferent neurons [4, 5].
Nerve growth factor (NGF) expressed in the bladder and transported through bladder afferent pathways has been implicated as an important factor to induce DO and C-fiber afferent hyperexcitability after SCI [6, 7, 8]. Also, NGF upregulation in the bladder reportedly contributes to C-fiber afferent hyperexcitability to induce LUTD in rats with cerebral infarction [9]. In addition, previous studies showed that chronic NGF administration into the lumbosacral spinal cord or into the bladder induced DO and increased the firing rate of dissociated capsaicin sensitive bladder afferent neurons [10, 11, 12] and that immunoneutralization of NGF in the lumbosacral spinal cord after SCI decreased DO and DSD in rats [13]. Recent studies also demonstrated that NGF levels in the bladder mucosa was increased, and that systemic NGF antibody treatment decreased the number of NVCs in SCI mice [14]. It is also known that the second messenger signaling pathways activated by NGF utilize p38 Mitogen-Activated Protein Kinase (MAPK) [15]. It is a serine-threonine kinase that is activated by phosphorylation and mediates many cellular responses to a variety of chemical and physical insults [16]. A recent study showed that p38 MAPK inhibitor treatment improved the voiding dysfunction, such as urinary frequency and NVCs in SCI mice [17].
Patch clamp recordings from capsaicin sensitive bladder afferent neurons in L6-S1 dorsal root ganglion in SCI rats revealed enhanced excitability evident as increased firing rate during membrane depolarization [18]. Recent studies also showed that SCI induced hyperexcitability of capsaicin sensitive bladder afferent neurons in mice, which was reduced by treatment with anti-NGF antibody [19]. However, it has not been clarified whether the p38 MAPK signaling pathway is involved in the changes in electrophysiological properties of bladder afferent neurons following SCI. Therefore, in the present study, we examined the effects of a p38 MAPK inhibitor on electrophysiological properties of capsaicin-sensitive bladder afferent neurons using the same mouse model of SCI as used in our previous in-vivo studies [20, 21].
2. Material and Methods
2.1. Animals.
A total of 57 female C57BL/6 mice (8–10 weeks old, 18–22 g) (Harlan Laboratories Inc., Indianapolis, IN, USA) were used. They were housed in temperature-controlled conditions under a 12.12 h light/dark cycle (light on at 0700 hours) with libitum access to water and standard food. These mice were divided into three groups: spinal intact (SI, n=22), spinal cord injury (SCI, n=20), and SCI with p38 MAPK inhibitor groups (SCI + p38 MAPK, n=15). All animal experiments were conducted in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) and National Institutes of Health (NIH) guidelines, and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.2. Surgical procedures
1). Spinal cord transection
In SCI and SCI + p38 MAPK groups, the Th8/9 spinal cord was completely transected under isoflurane anesthesia as previously reported [19, 20, 22]. In the SI group, sham operation was performed without spinal cord transection.
2). Intrathecal catheter insertion and osmotic pump placement
Two weeks after spinal cord transection, a catheter (Mouse Intrathecal Catheter; Alzet osmotic pumps, Cupertino, CA, USA) was inserted into the intrathecal space at the level of the L6-S1 spinal cord via an incision in the dura at the L1 vertebra under isoflurane anesthesia. An osmotic pump (Alzet osmotic pumps) was connected to the intrathecal catheter and placed in subcutaneous space of the back. A p38 MAPK inhibitor, SB203580 (EMD Millipore Corp., Billerica, MA, USA) (1 mg/ml) was continuously supplied intrathecally at infusion rate of 0.51 μg/hr for two weeks. The dosage was determined according to previous studies [17, 23]. Instead of SB203580, artificial cerebrospinal fluid (Tocris Bioscience, Bristol, UK) was administered via implanted osmotic pumps in SI and SCI groups as vehicle treatment.
3). Fast Blue injection into the bladder wall
To label the population of L6-S1 DRG neurons innervating the bladder, a retrograde mono-synaptic fluorescent tracer, Fast-Blue (FB, 1.8% weight per volume, PolySciences Inc., Warrington, PA, USA), was injected into the bladder wall of 3 groups of animals under isoflurane anesthesia seven days before dissociation of DRG neurons, as previously reported [11, 19, 20]. The bladder was exposed by a middle lower abdominal incision and FB was injected with a 30-gauge needle at four sites (total volume of 20 μL) under isoflurane anesthesia. At each injection, the needle was kept in place for 15–20 seconds to prevent leak, and any leakage of FB was removed by application of cotton swab. The injection site was then rinsed with saline, and the abdominal incision was closed. We have previously demonstrated that dye labeling does not affect electrical properties of DRG neurons [24].
To prevent postoperative pain and surgical site infections, ketoprofen (5 mg/kg) and ampicillin (100 mg/kg) were administered subcutaneously for three days after each surgery. The bladder of SCI mice was emptied by abdominal compression once a day after spinal cord transection, because typical DO evident as NVCs and the overexpression of bladder NGF were observed using this once daily bladder compression method [21].
2.3. Dissociation of DRG neurons
Seven days after the FB injection and four weeks after the SCI surgery, isolated afferent neurons were obtained from DRG by enzymatic and mechanical dissociation methods as previously described [11, 19, 20] with slight modifications. We removed L6-S1 DRG, which contain cell bodies of bladder afferents carried through the pelvic nerve, under isoflurane anesthesia and incubated them in a water bath for 12 min at 35.4 °C in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO, USA) containing 0.4 mg/ml trypsin (Sigma-Aldrich), 1.0 mg/ml collagenase, and 0.1 mg/ml DNase (Sigma-Aldrich). Trypsin inhibitor (Sigma-Aldrich) was then added to neutralize the enzyme activity of trypsin. Individual DRG cell bodies were isolated by trituration and plated on poly-D-lysine-coated 35 mm glass bottom culture dishes (Mat Tek, Ashland, MA, USA) with minimal essential medium (Nacalai Tesque Inc., Kyoto, Japan), which was supplemented with 10% of fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and horse serum (Gibco) and 100 U/ml Penicillin-Streptomycin (Gibco) and 0.5 mg/ml DNase. Isolated DRG cells were incubated at 37.0 °C in 5% CO2 per 95% air overnight before patch-clamp experiments.
2.4. Whole-cell patch clamp recordings
Primary afferent neurons that innervate the bladder were identified as FB-labelled cells using an inverted phase contrast microscope (Nikon, Tokyo, Japan) with fluorescent attachments (UV-1A filter; excitation wavelength, 365 nm). We performed whole-cell patch clamp recordings to evaluate the characteristics of action potentials and isolated potassium (K+) currents. Cell membrane capacitances were obtained by reading the value for whole cell input capacitance neutralization directly from the amplifier. All recordings were performed at room temperature (20–22 °C) on freshly dissociated FB-labelled neurons in a culture dish using a MultiClamp 700B patch-clamp amplifier (Molecular Devices, Union City, CA, USA) within 24 hour after dissociation, and data were acquired and analyzed by PCLAMP 9.0 software (Molecular Devices).
1). Action potentials in the current clamp mode
The internal solution contained 140 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM EGTA, 10 mM HEPES and 2 mM Mg adenosine triphosphate, adjusted to pH 7.4 with KOH. The Osmolality was adjusted to 310 mOsm with sucrose. Patch glass electrodes had 2 to 4 MΩ resistance when filled with the internal solution. Neurons were superfused at a flow rate of 1.5 ml per minute with an external solution containing 150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM D-glucose, adjusted to pH 7.4 with NaOH. The Osmolality was adjusted to 340 mOsm with sucrose. In current-clamp recordings, data are presented from neurons that exhibited resting membrane potentials around −50 mV, and action potentials that overshot 0 mV. Durations of action potentials were measured at 50% of the spike amplitude. Thresholds for action potential activation were determined by injection of depolarizing current pulses in 10 or 20 pA steps from −40 pA. The data analyses were performed by investigators blinded to experimental conditions. When examining the number of action potentials during 800 ms membrane depolarization, current intensity was set to the value just above the threshold for inducing single spike activation with a 50 ms pulse.
2). K+ currents in the voltage clamp mode
To isolate K+ currents, as previously described [11], neurons were superfused at a flow rate of 1.5 ml per minute with a sodium (Na+) free external solution containing 110 mM choline-Cl, 5 mM KOH, 0.03 mM CaCl2, 3.4 mM Mg(OH)2, 10 mM HEPES and 10 mM D-glucose, adjusted to pH 7.4 with Trizma hydrochloride that suppresses Na+ and calcium (Ca2+) currents [25]. The osmolality was adjusted to 340 mOsm. In voltage-clamp recordings, the filter was set to −3 dB at 2,000 Hz. Leak currents were subtracted by P/4 pulse protocol, and the series resistance was compensated by 50–60%. The voltage error did not exceed 5 mV after compensation of the series resistance, and a charging time constant of the voltage clamp was < 300 μs, which was faster than gating properties of outward K+ currents in this study. Our previous reports indicated that slowly decaying A-type K+ (slow KA) currents in capsaicin-sensitive C-fiber bladder afferent neurons are activated by depolarizing voltage steps from hyperpolarized membrane potentials, but these currents are almost completely inactivated when membrane potentials are maintained at a depolarized level of greater than −40 mV [26]. Therefore, we estimated the density of slow KA currents by measuring the difference in the K+ currents evoked by depolarizing voltage pulses from holding potentials (HPs) of −120 and −40 mV. To correct the K+ current densities for cell size, the K+ current densities for each cell were normalized with respect to cell membrane capacitances that were obtained by reading the value for whole-cell input capacitance neutralization directly from the amplifier. Data were then analyzed by PCLAMP 9.0 software (Molecular Devices).
3). Sensitivity for capsaicin
After evaluation of action potential characteristics or isolated K+ currents, we evaluated the capsaicin sensitivity by direct application of capsaicin (2 μM) to the recording chamber containing neurons in a voltage-clamp mode with a HP at −60 mV. Inward shift of holding currents was observed in capsaicin-sensitive neurons. The stock solution of capsaicin (Sigma-Aldrich) at 5 mM containing 10% ethanol and 10% Tween 80 was prepared in the external solution described above for action potential evaluation, and then diluted to the final concentration in the same external solution before experiments.
2.5. Data analysis
All values are expressed as means ± SD. Statistical differences were determined using one-way ANOVA, followed by post hoc analysis with the Bonferroni’s method. Data analysis was performed using GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA), and statistical significance was defined as p < 0.05.
3. Results
The diameter and cell input capacitance of capsaicin-sensitive bladder afferent neurons from SCI mice were significantly greater than those from SI mice (Table). However, the p38 MAPK inhibitor treatment did not reverse SCI-induced changes in these cell size-related parameters (Table).
Table.
Electrophysiological properties of capsaicin sensitive bladder afferent neurons
| SI + vehicle | SCI + vehicle | SCI + p138 MAPK inhibitor | |
|---|---|---|---|
| Spikes: | |||
| Number of cells/mice | 19/13 | 24/11 | 20/8 |
| Diameter (μm) | 25.4 ± 4.8 | 29.3 ± 3.8* | 28.5 ± 3.8* |
| Input capacitance (pF) | 26.9 ± 11.6 | 36.6 ± 10.4* | 36.1 ± 10.6* |
| Resting membrane potentials (mV) | −50.0 ± 0.29 | −50.0 ± 0.68 | −50.0 ± 0.31 |
| Spike threshold (mV) | −21.5 ± 8.7 | −31.2 ± 5.2* | −19.3 ± 8.4# |
| Peak membrane potential (mV) | 39.7 ± 21.1 | 37.0 ± 15.6 | 48.5 ± 15.0# |
| Spike duration (ms) | 2.3 ± 1.0 | 3.7 ± 1.4* | 2.5 ± 1.8# |
| Number of spikes (800 ms depolarization) | 1.1 ± 0.3 | 5.3 ± 4.1* | 1.1 ± 0.3# |
| K+ current density: | |||
| Number of cells/mice | 24/9 | 24/9 | 18/7 |
| Slow decaying KA current density (pA/pF) | 48.4 ± 35.8 | 22.6 ± 14.6* | 49.4 ± 26.3# |
| Sustained KDR current density (pA/pF) | 120.7 ± 80.1 | 54.9 ± 35.4* | 63.2 ± 29.9* |
Values are means ± SD.
P < 0.05 and
P < 0.05, when compared with the Bonferroni’s method to the SI and SCI group, respectively. KA, A-type K+; KDR, delayed rectifier-type K+; SCI, mice with spinal cord injury; SI, spinal cord intact mice; SCI + p38 MAPK inhibitor, SCI mice treated with p38 Mitogen-Activated Protein Kinase inhibitor (0.51 μg per hour, i.t.) for 2 weeks.
1). Action potential properties
The representative recordings of action potentials in capsaicin-sensitive bladder afferent neurons were showed in Fig. 1. Before applying the depolarization current steps, resting membrane potentials in these neurons were set at around −50 mV. The mean threshold for eliciting action potentials was significantly lower in the SCI group compared to the SI group. The duration of action potential was significantly longer in the SCI group compared to the SI group. Also, the number of action potentials during 800 ms membrane depolarization in the SCI group was significantly increased compared to the SI group. These SCI-induced changes in action potential properties were significantly reversed by 2-weeks intrathecal treatment with a p38 MAPK inhibitor (0.51 μg/h, i.t.) that was started two weeks after SCI (Table 1).
Fig. 1.
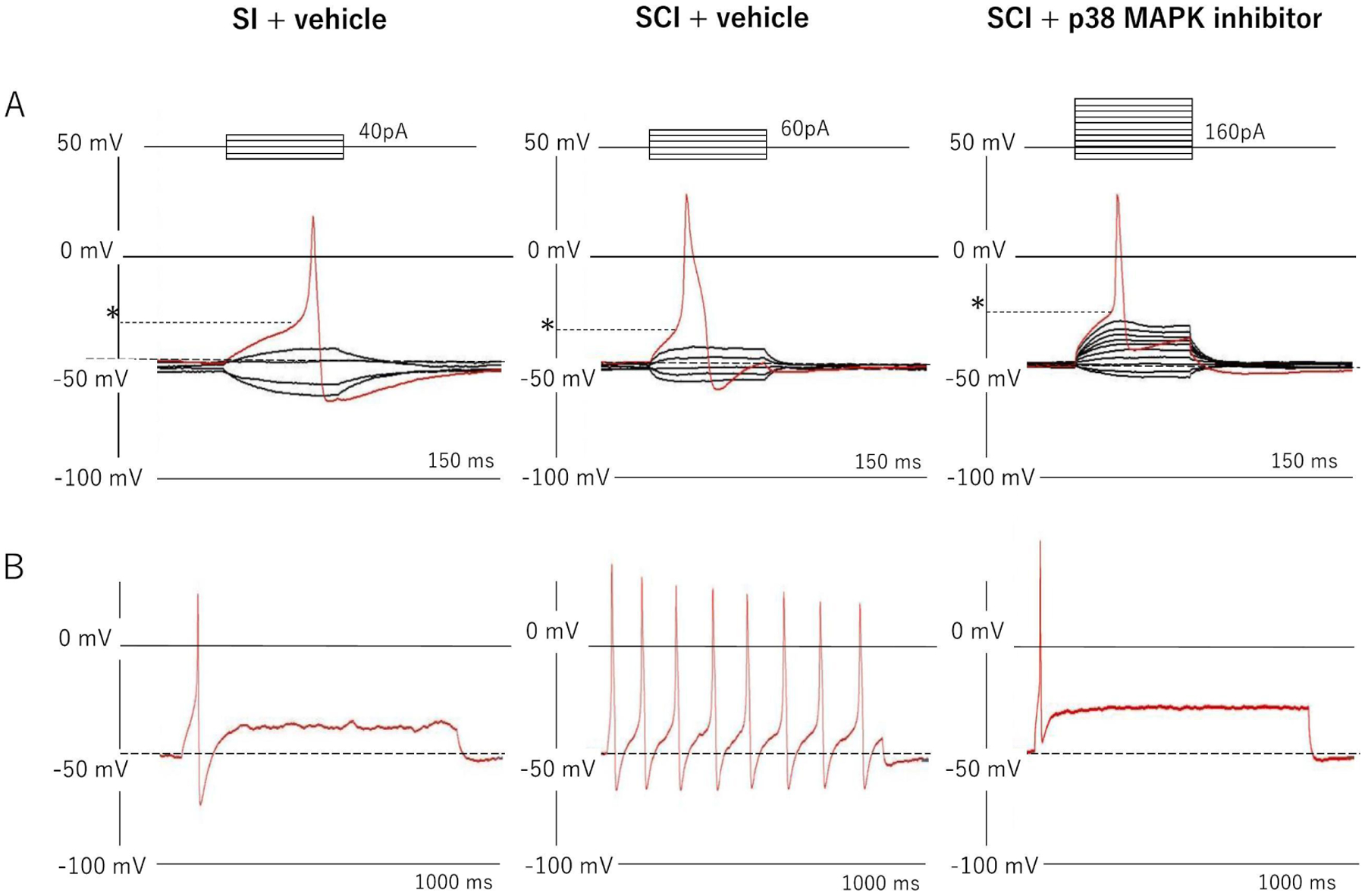
Representative recordings of action potentials and firing patterns in capsaicin-sensitive bladder afferent neurons from mice. A: Action potentials evoked by 50 ms depolarizing current pulses injected through glass patch pipettes during current clamp recordings. Asterisks with dashed lines indicate the thresholds for spike activation. B: Firing patterns of action potentials during 800 ms membrane depolarization. SI; spinal intact group, SCI; spinal cord injury group, SCI + p38 MAPK group; spinal cord injury treated with p38 MAPK inhibitor (0.51 μg/h, i.t.) for two weeks. The number of neurons/mice per group is 19/13; SI, 24/11; SCI and 20/8; SCI + p38 MAPK inhibitor, respectively.
2). K+ current properties
The representative recordings of isolated K+ currents in capsaicin-sensitive bladder afferent neurons were showed in Fig. 2. Densities of both slow KA and sustained delayed rectifier-type K+ (sustained KDR) currents were measured during membrane depolarization to 0 mV in capsaicin-sensitive bladder afferent neurons from three groups of mice. The peak density of slow KA currents was obtained by measuring the difference in outward currents evoked from HPs of −40 and −120 mV, and the peak density of sustained KDR currents was measured by evoking a depolarization to 0 mV from a HP of −40 mV. The densities of slow KA and sustained KDR currents were significantly lower in capsaicin-sensitive bladder afferent neurons in the SCI group than those in the SI group (Table and Figs. 2 and 3). In the voltage-current relationship, there were significant differences in the peak densities of slow KA and sustained KDR currents at depolarizing pulses greater than −20 mV and 0 mV, respectively, between SI and SCI groups (Fig. 3). The 2-weeks intrathecal treatment with a p38 MAPK inhibitor (0.51 μg/h, i.t.) that was started two weeks after SCI significantly reversed the SCI-induced reduction in the density of slow KA currents, but not of sustained KDR currents (Table and Figs. 2 and 3).
Fig. 2.
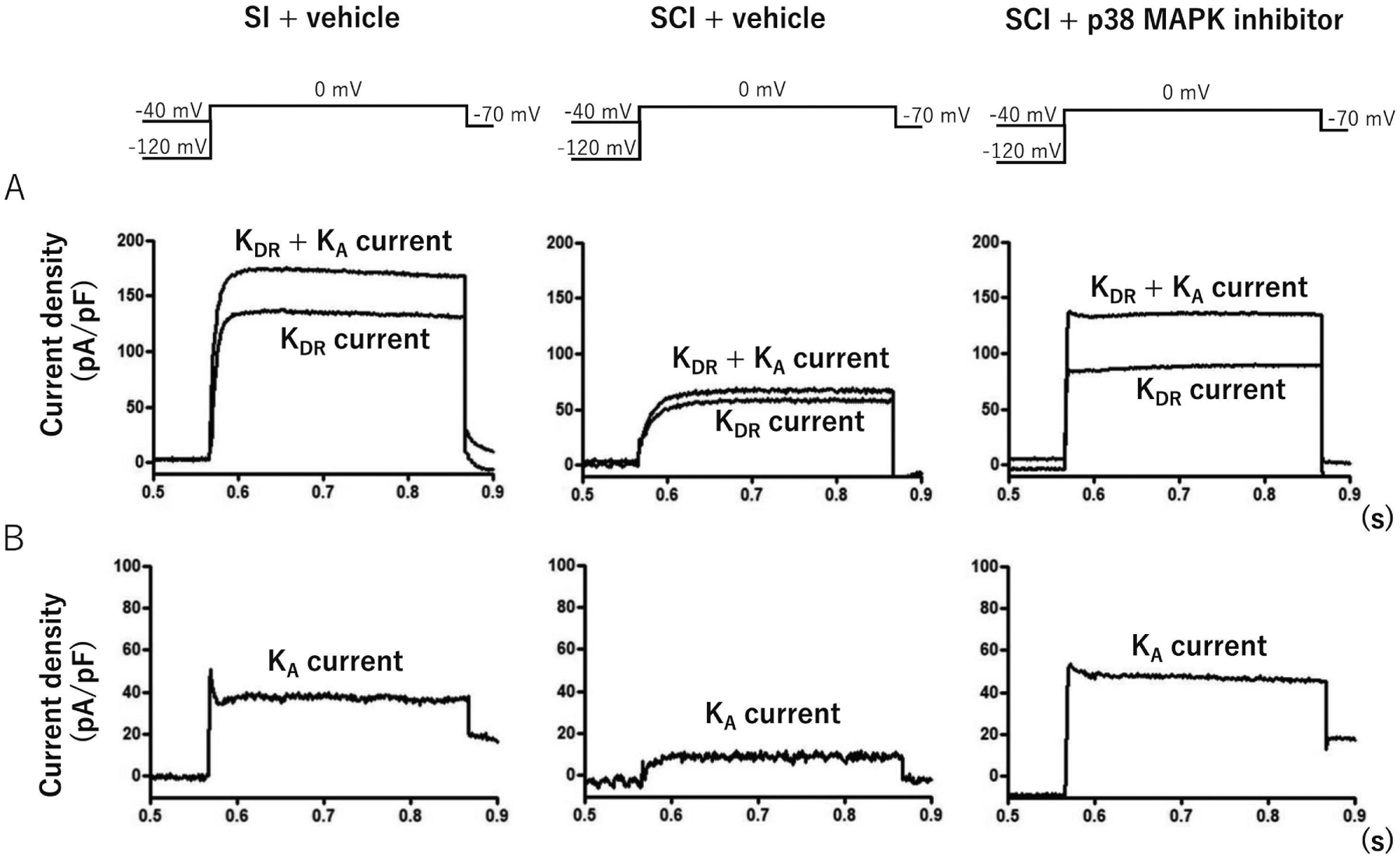
Representative recordings of K+ currents in capsaicin-sensitive bladder afferent neurons from mice. A: Recordings show superimposed outward K+ currents evoked by depolarizing voltage pulses to 0 mV from holding potentials (HPs) of −120 and −40 mV. Sustained delayed rectifier-type K+ (sustained KDR) currents were evoked by depolarization from −40 mV HP. B: Recordings slow decaying A-type K+ (slow KA) currents were obtained by subtracting K+ currents evoked by depolarization to 0 mV from HPs of −40 and −120 mV. SI; spinal intact group, SCI; spinal cord injury group, SCI + p38 MAPK group; spinal cord injury treated with p38 MAPK inhibitor (0.51 μg/h, i.t.) for two weeks. The number of neurons/mice per group is 24/9; SI, 24/9; SCI and 18/7; SCI + p38 MAPK inhibitor, respectively.
Fig. 3.
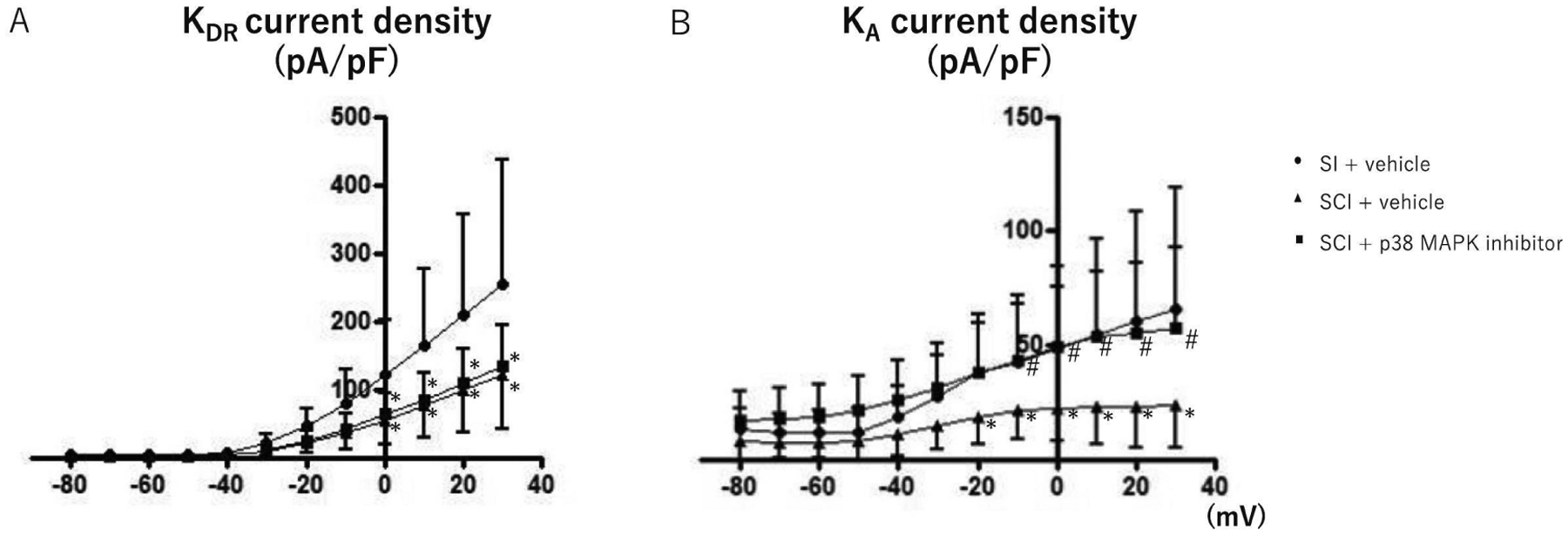
I-V relationships of K+ currents in capsaicin-sensitive bladder afferent neurons from mice. K+ currents evoked by depolarizing voltage pulses from −80 to +30 mV. Values present means ± SD. *p < 0.05, significant difference between SI and SCI groups or SI and SCI + p38 MAPK inhibitor groups, one-way ANOVA, followed by post hoc analysis with the Bonferroni’s method. #p < 0.05, significant difference between SCI and SCI + p38 MAPK inhibitor groups, one-way ANOVA, followed by post hoc analysis with the Bonferroni’s method. SI; spinal intact group, SCI; spinal cord injury group, SCI + p38 MAPK group; spinal cord injury treated with p38 MAPK inhibitor (0.51 μg/h, i.t.) for two weeks. The number of neurons/mice per group is 24/9; SI, 24/9; SCI and 18/7; SCI + p38 MAPK inhibitor, respectively.
4. Discussion
The results of this study indicated that; (1) capsaicin-sensitive bladder afferent neurons from SCI mice showed hyperexcitability, evident as decreased spike thresholds and increased firing rate of action potentials compared to SI mice, (2) slow KA and sustained KDR current densities of capsaicin-sensitive bladder afferent neurons from SCI mice were decreased compared to those from SI mice, and (3) p38 MAPK inhibitor treatment for two weeks significantly reversed SCI-induced changes in spike thresholds, firing frequencies and slow KA current density.
In the normal condition, Aδ-fibers bladder afferents are involved in the control of micturition whereas, in chronic SCI condition, excitability of C-fiber afferents, the majority of which express capsaicin-sensitive TRPV1 receptors, are increased to induce neurogenic DO as evidenced by non-voiding bladder contractions (NVC) during the storage phase [1]. Although the underlying mechanisms of SCI-induced DO and C-fiber hyperexcitability seem to be multifactorial, NGF has been proposed as an important mediator of afferent sensitization and DO after SCI. In previous studies, chronic administration of NGF induced DO and increased the firing rate of dissociated capsaicin-sensitive bladder afferent neurons in rats [10, 11, 12]. Additionally, we previously reported that NGF antibody treatment decreased DO in SCI rats [13] as well as in SCI mice [14]. Furthermore, in patch-clamp recording studies, the spike threshold for activation of action potentials was decreased and the firing frequency of action potentials during membrane depolarization were increased in capsaicin-sensitive bladder afferent neurons in SCI mice compared to SI mice, and these changes in electrophysiological properties were reversed by 2-weeks administration of NGF antibodies [19, 20]. Taken together, these data indicate that SCI-induced hyperexcitability of capsaicin-sensitive C-fiber bladder afferent pathway is involved in the emergence of neurogenic DO in rodents, and that NGF plays an important role in bladder afferent hyperexcitability and DO after SCI.
In this and previous studies, hyperexcitability of capsaicin-sensitive, C-fiber bladder afferent neurons was associated in the reduction in voltage-gated K+ channel (KV) activity in SCI rats and mice [18, 19]. In sensory neurons, KV currents are divided into two major categories, transient KA and sustained KDR currents. The slow KA current is preferentially expressed in small-sized DRG neurons that exhibit capsaicin sensitivity and tetrodotoxin-resistant action potentials [25, 26, 27], indicating that slow KA currents can be involved in modulating excitability in small-sized C-fiber afferent neurons. In this study, we confirmed that densities of both sustained KDR and slow KA currents are decreased in capsaicin-sensitive bladder afferent neurons from SCI mice. These results suggest that the reduction in these KV currents is one of the key events underlying the hyperexcitability of C-fiber afferent neurons innervating the bladder in SCI mice. Moreover, decreased slow KA current densities in capsaicin-sensitive bladder afferent neurons was almost completely reversed by2-weeks NGF antibody treatment started two weeks after SCI whereas the treatment had no effect on the reduced sustained KDR currents density in these neurons [19]. Thus, it is plausible that the NGF-dependent reduction of KA channel activity is a key factor for inducing C-fiber bladder afferent hyperexcitability that is responsible for DO after SCI.
In the downstream signaling pathways of NGF, p38 MAPK has been reported to be a major second messenger activated after NGF binding to high-affinity TrkA receptors [15, 23]. It has been documented that intrathecal administration of SB203580, a MAPK inhibitor, inhibits p38 protein phosphorylation and reduces mechanical allodynia and the upregulation of inflammatory mediators in lumber DRG [23]. It was also demonstrated that peripheral inflammation or axotomy induces p38 MAPK activation in DRG neurons, which participated in generating pain hypersensitivity [28] and that intrathecal inhibition of p38 MAPK reduces pain behavior induced by nerve injury [15]. In line with these reports, our previous study showed that p38 MAPK inhibitor treatment improved DO as evident as reductions in NVCs and urinary frequency in SCI mice [17]. In this study, we have used the same SCI mouse model as in our previous study with anti-NGF antibodies [19] and found that p38 MAPK inhibition reduced SCI-induced hyperexcitability in dissociated capsaicin-sensitive bladder afferent neurons in association with restored KA channel activity, which was decreased after SCI, as similarly found in our previous NGF suppression study [19]. Therefore, the current study suggests that the p38 MAPK second messenger system is involved as the downstream pathway of NGF in hyperexcitability of capsaicin-sensitive C-fiber bladder afferent neurons, which underlies SCI-induced neurogenic DO. In addition, because these neurons comprised a subset of afferent cells innervating the bladder, the results of our current study may not be applicable for the entire bladder afferent population.
This study has some limitations. First, we have focused on the changes in Kv channel activity after SCI. However, p38 MAPK is known to directly modulate the activity of tetrodotoxin-resistant sodium channels coded by the NaV1.8 sodium channel subunit [29]. One recent study using the same SCI model also showed that SCI induced decreases in tetrodotoxin-resistant sodium channels and NaV1.8 expression and increases in tetrodotoxin-sensitive sodium channels and NaV1.7 in small-sized bladder afferent neurons, both of which were reversed by anti-NGF antibody treatment [30]. Therefore, future studies are needed to clarify whether the p38 MAPK second messenger system is also involved in NGF-dependent plasticity of sodium channels, which are another determinant of afferent neuron excitability, in SCI. Secondly, our previous study showed that p38 MAPK inhibitor treatment improved not only DO during the storage phase, but also voiding problems evident as reduced post-void residual volume and increased micturition pressure and voiding efficiency, the latter of which were not observed after anti-NGF antibody treatment in SCI mice [17]. Thus, there is the possibility that NGF-independent p38 MAPK activation is involved in SCI-induced changes in voiding reflexes that may be controlled by Aδ-fiber bladder afferent pathways [31]. Also, the present study did not include a group of SI mice treated with p38 MAPK inhibitors. Because there may be the possibility that p38 MAPK inhibitor treatment may increase the current density of KA in SI mice, as shown in SCI mice, a further study using SI animals needs to be performed.
5. Conclusion
The results of this study suggest that p38 MAPK, which acts as an important second messenger of the NGF-TrkA pathway, has an essential pathophysiological role in SCI-induced hyperexcitability of capsaicin-sensitive C-fiber bladder afferent neurons, at least in part via the reduction of KA channel activity. Therefore, NGF and its p38 MAPK downstream signaling pathway could be effective targets for the treatment of LUTD such as DO after SCI.
Grant
This work was supported in part by a grant from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney (R01 DK129194).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
There is no conflict of interest for any of authors. This work was supported by a grant from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney (R01 DK129194).
References
- 1.de Groat WC, Yoshimura N, Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury, Prog. Brain Res 152 (2006) 59–84, 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 2.de Groat WC WC, Yoshimura N N, Afferent nerve regulation of bladder function in health and disease, Handb. Exp. Pharmacol 194 (2009) 91–138, 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Häbler HJ, Jänig W, Koltzenburg M, Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder in the cat, J. Physiol 425 (1990) 545–562, 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz F, Guimaraes M, Silva C, Rio ME, Coimbra A, Reis M, Desensitization of bladder sensory fibers by intravesical capsaicin has long lasting clinical and urodynamic effects in patients with hyperactive or hypersensitive bladder dysfunction, J. Urol 157 (1997) 585–589, 10.1016/S0022-5347(01)65211-X. [DOI] [PubMed] [Google Scholar]
- 5.de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, et al. , Mechanisms underlying the recovery of urinary bladder function following spinal cord injury, J. Auton. Nerv. Syst 30 (1990) S71–77, 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- 6.de Groat WC, Yoshimura N, Changes in afferent activity after spinal cord injury, Neurourol. Urodyn 29 (2010) 63–76, 10.1002/nau.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groat WC, Yoshimura N, Plasticity in reflex pathways to the lower urinary tract following spinal cord injury, Exp. Neurol 235 (2012) 123–132, 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochodnický P, Cruz CD, Yoshimura N, Michel MC, Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target, Neurourol. Urodyn 30 (2011) 1227–1241, 10.1002/nau.21022. [DOI] [PubMed] [Google Scholar]
- 9.Yokokawa R, Akino H, Ito H, Zha X, Yokoyama O, Nerve growth factor release from urothelium increases via activation of bladder C-fiber in rats with cerebral infarction, Neurourol. Urodyn 36(2017) 1448–1455, 10.1002/nau.23142. [DOI] [PubMed] [Google Scholar]
- 10.Lamb K, Gebhart GF, Bielefeldt K, Increased nerve growth factor expression triggers bladder overactivity, J. Pain 5 (2004) 150–156, 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, et al. , Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats, J. Neurosci 26 (2006) 10847–10855, 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zvara P, Vizzard MA, Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord, BMC Physiol. 7 (2007) 9, 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, et al. , Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats, J. Urol 171 (2002) 478–482, 10.1097/01.ju.0000088340.26588.74. [DOI] [PubMed] [Google Scholar]
- 14.Wada N, Shimizu T, Shimizu N, de Groat WC, Kanai AJ, Tyagi P, et al. , The effect of neutralization of nerve growth factor (NGF) on bladder and urethral dysfunction in mice with spinal cord injury, Neurourol. Urodyn 37 (2018) 1889–1896, 10.1002/nau.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ, p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia, Neuron 26 (2002) 57–69, 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 16.Cuenda A, Rousseau S, p38 MAP-kinases pathway regulation, function and role in human diseases, Biochim. Biophys. Acta Mol. Cell Res 1773 (2007) 1358–1375, 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu N, Wada N, Shimizu T, Suzuki T, Kurobe M, Kanai AJ, et al. , Role of p38 MAP kinase signaling pathways in storage and voiding dysfunction in mice with spinal cord injury, Neurourol. Urodyn 39 (2020) 108–115, 10.1002/nau.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, de Groat WC, et al. , Hyperexcitability of bladder afferent neurons associated with reduction of KV1.4 α-Subunit in rats with spinal cord injury, J. Urol 190 (2013) 2296–2304, 10.1016/j.juro.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu T, Majima T, Suzuki T, Shimizu N, Wada N, Kadekawa K, et al. , Nerve growth factor-dependent hyperexcitability of capsaicin sensitive bladder afferent neurones in mice with spinal cord injury, Exp. Physiol 103 (2018) 896–904, 10.1113/EP086951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadekawa K, Majima T, Shimizu T, Wada N, de Groat WC, Kanai AJ, et al. , The role of capsaicin-sensitive C-fiber afferent pathways in the control of micturition in spinal intact and spinal cord injured mice, Am. J. Physiol. Renal. Physiol 313 (2017) F796–F804, 10.1152/ajprenal.00097.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wada N, Shimizu T, Takai S, Shimizu N, Kanai AJ, Tyagi P, et al. , Post-injury bladder management strategy influences lower urinary tract dysfunction in the mouse model of spinal cord injury. Neurourol. Urodyn 36 (2017) 1301–1305, 10.1002/nau.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ, Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity, J. Urol 181 (2009) 1459–1466, 10.1016/j.juro.2008.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng HT, Dauch JR, Oh SS, Hayes JM, Hong Y, Feldman EL, p38 mediates mechanical allodynia in a mouse model of type 2 diabetes, Mol. Pain 6 (2010) 28, 10.1186/1744-8069-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura N, White G, Weight FF, de Groat WC, Patch clamp recordings from subpopulations of autonomic and afferent neurons identified by axonal tracing techniques, J. Auton. Nerv. Syst 49 (1994) 85–92, 10.1016/0165-1838(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura N, de Groat WC, Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation, J. Neurosci 19 (1999) 4644–4653, 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimura N, White G, Weight FF, de Groat WC, Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder, J. Physiol 494 (1996) 1–16, 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold MS, Shuster MJ, Levine JD, Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J. Neurophysiol 75(1996) 2629–2646, 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- 28.Obata K, Noguchi K, MAPK activation in nociceptive neurons and pain hypersensitivity, Life Sci. 74 (2004) 2643–2653, 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, et al. , Phosphorylation of sodium channel Nav 1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons, J. Neurosci 28 (2008) 3190–3201, 10.1523/JNEUROSCI.4403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni J, Suzuki T, Karnup SV, Gu B, Yoshimura N, Nerve growth factor-mediated Na+ channel plasticity of bladder afferent neurons in mice with spinal cord injury, Life Sci. 298 (2022) 120524, 10.1016/j.lfs.2022.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada N, Karnup SV, Kadekawa K, Shimizu N, Kwon J, Shimizu T, et al. , Current knowledge and novel frontiers in lower urinary tract dysfunction after spinal cord injury: Basic research perspectives, Urol. Sci 33 (2022) 101–113, 10.4103/uros.uros_31_22. [DOI] [PMC free article] [PubMed] [Google Scholar]


