Abstract
An altered metabolism of iron fuels cancer growth, invasion, metastasis, and recurrence. Ongoing research in cancer biology is delineating a complex iron-trafficking program involving both malignant cells and their support network of cancer stem cells, immune cells, and other stromal components in the tumor microenvironment. Iron-binding strategies in anticancer drug discovery are being pursued in clinical trials and in multiple programs at various levels of development. Polypharmacological mechanisms of action, combined with emerging iron-associated biomarkers and companion diagnostics, are poised to offer new therapeutic options. By targeting a fundamental player in cancer progression, iron-binding drug candidates (either alone or in combination therapy) have the potential to impact a broad range of cancer types and to address the major clinical problems of recurrence and resistance to therapy.
Keywords: iron, cancer, tumor microenvironment, macrophage, chelator, prochelator, stemness
Graphical Abstract
1. Introduction
The biochemistry of iron impacts large areas of human physiology and pathology. The most abundant transition metal in the human body, iron is key to the function of a multitude of proteins at the basis of fundamental processes such as oxygen transport, respiration, and DNA synthesis. The redox chemistry of this trace element, which can access several oxidation states, is associated not only to the activity of numerous enzymes but also to the Fenton reaction and the generation of reactive oxygen species as well as the iron-dependent cell death pathway known as ferroptosis [1]. This complex, multifaceted role requires a highly orchestrated regulation of the acquisition, storage, transport, and recycling iron in all cell types. In the case of malignant cells, the hallmarks of rapid proliferation and sustained progression are fueled by a reprogrammed iron metabolism [2–5] that has been described as an “iron addiction” [6].
Cancer cell walls are generally characterized by upregulated expression of the transferrin receptor TfR1, leading to increased cellular uptake of the iron-transport protein transferrin (Tf) (Fig. 1). Other components of the iron uptake system, namely transporter DMT1 and metalloreductase STEAP3, are also upregulated in malignant cells (Fig. 1). Conversely, ferroportin (FPN), which is the only iron export protein, is downregulated in cancer cells and its negative regulator hepcidin is abundant – two effects that inhibit iron efflux and favor its accumulation (Fig. 1). Although hepcidin is primarily a systemic liver-derived hormone, recent work on colorectal cancer showed that the tumor epithelium serves as a local source of hepcidin to downregulate ferroportin and promote cancer growth [7]. Critically, multiple analyses of the gene expression of iron regulators has revealed convincing prognostic value in predicting distant-metastasis-free survival particularly in breast [8], ovarian [6], and renal [9] cancer.
Figure 1.
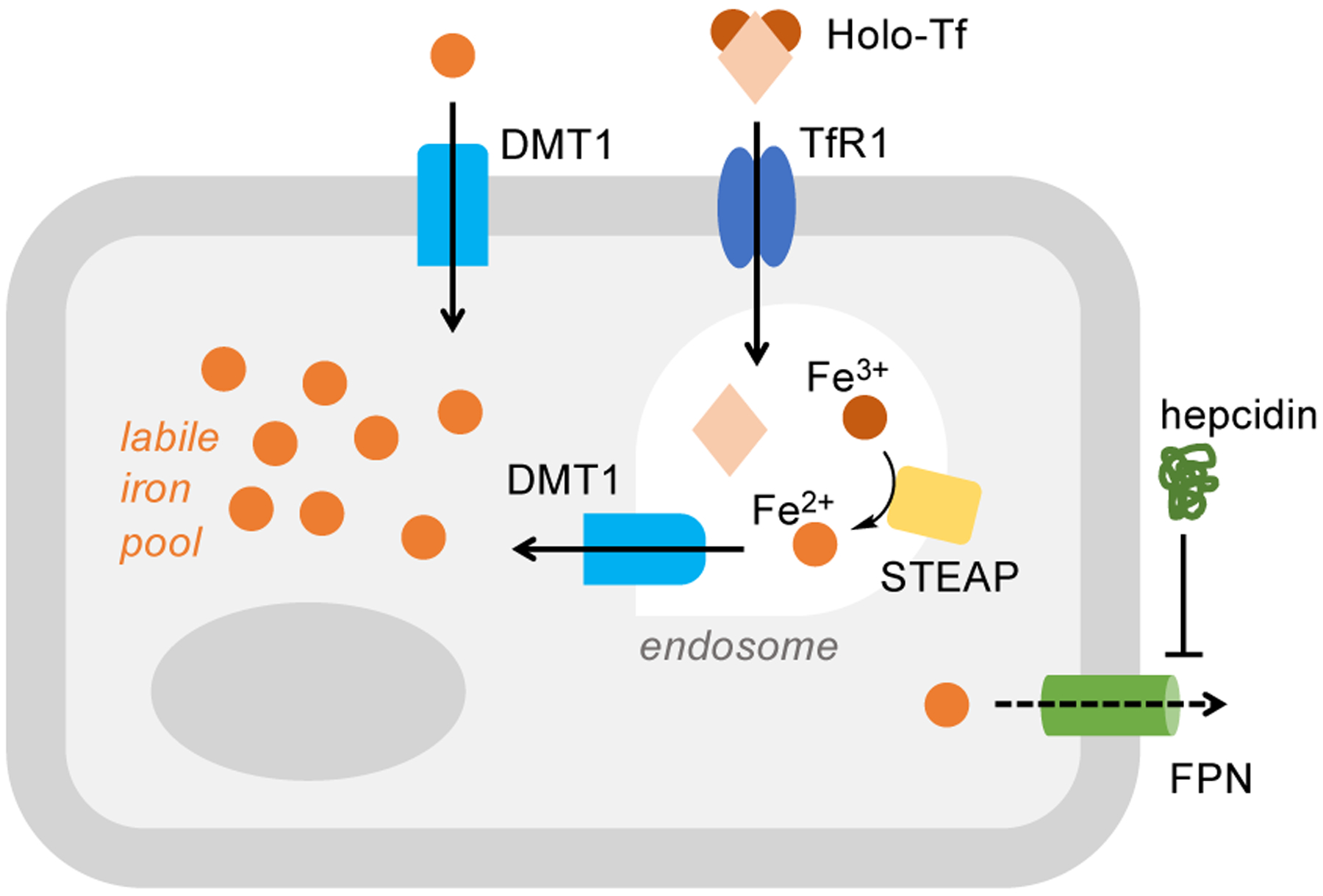
Simplified schematic showing the key components of the cellular iron import-export machinery. In cancer cells, a larger labile iron pool is maintained through the overexpression of the transferrin receptor TfR1, transporter DMT1, and STEAP metalloreductases, as well as through the downregulation of iron exporter FPN mediated by hormone hepcidin.
Recent advances in our understanding of the tumor microenvironment are highlighting a key role of iron in the mechanisms by which immune cells and stromal components promote tumor progression and metastasis. In this context, macrophages are important players because they process large quantities of iron through phagocytosis of red blood cells. These immune cells display remarkable functional plasticity and adopt the alternatively activated M2 polarization in the tumor microenvironment, which promotes tissue regeneration and anti-inflammatory response [4,10]. The tumor-associated macrophages (TAMs) exhibit an iron-release phenotype that relies on upregulation of ferroportin and/or release of lipocalin 2 (LCN2), an iron-transport protein that carries siderophore-bound iron to cancer cells [11]. Among the other immune cells associated to tumors, neutrophils were found to stimulate metastatic growth of breast cancer by secreting transferrin and hence contributing in a different way to the altered iron metabolism [12].
The crosstalk of neoplastic cells, immune cells, and other stromal components in the tumor microenvironment is not fully understood; however, iron has been repeatedly implicated in the complex program that supports growth, invasion, and metastasis [4,5,10]. Furthermore, the tumor microenvironment harbors cancer stem cells (CSCs), a minority population of cells that are characterized by their ability to both self-renew and differentiate, and are considered largely responsible for cancer recurrence. Notably, elevated iron content is emerging as critical for stemness [13] and, consistent with the tumorigenic nature of CSCs, this effect has been delineated as a negative prognostic factor [14].
As the role of iron continues to be an area of intense investigation in cancer biology, its relevance to treatment has been widely recognized and reviewed [3,4,15,16]. This Opinion focuses on recent findings within small-molecule strategies that involve the coordination of iron. Targeting the complexity of iron biochemistry is both a challenge and an opportunity to impact in multiple ways a fundamental aspect of cancer.
2. Chelation approaches
Iron-binding pharmaceuticals DFO (Desferrioxamine, Desferal, Fig. 2) and DFX (Deferasirox, Exjade, Fig. 2) are employed routinely to treat iron overload and were among the first chelators to be tested in clinical trials for cancer indications [15]. Although these first-generation compounds target primarily systemic iron, current studies are exploring strategies for cancer applications. For instance, DFX has been employed in conjugates to confer selectivity to specific organelles [17,18] and in transmetalation approaches to incorporate the antiproliferative activity of titanium(IV) species [19]. In addition, these FDA-approved chelators are increasingly tested in combination with standard chemotherapeutics because the role of iron in cancer stemness [13] could be exploited to overcome resistance to chemotherapy. Both DFO [20] and DFX [21] were found to impact stemness markers and to have synergistic effects in combination with cisplatin.
Figure 2.
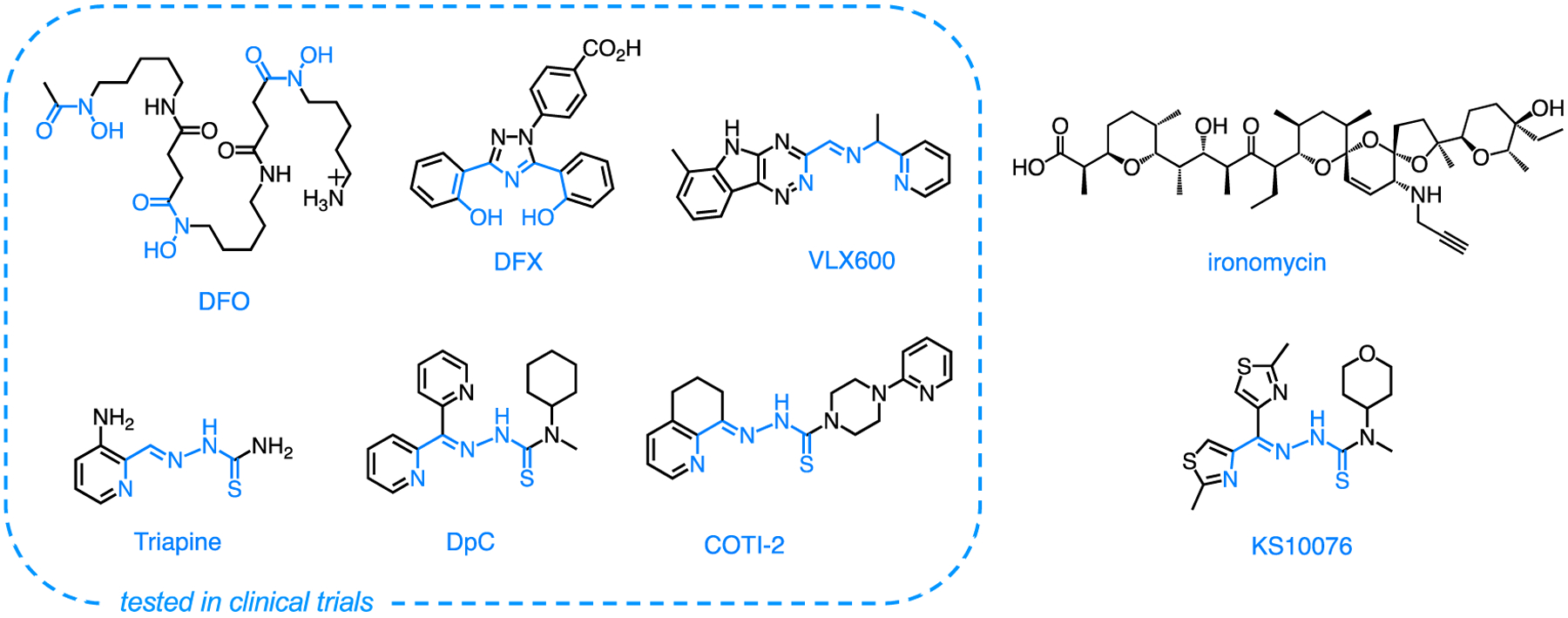
Examples of iron chelators studied for anticancer applications with known iron-binding units shown in blue.
Tridentate thiosemicarbazone Triapine (Fig. 2) is one of the best studied chelators for anticancer applications [22], and more recent analogs DpC [23,24] and COTI-2 [25,26] (Fig. 2) are currently under scrutiny for clinical development. Ongoing studies on these compounds and related analogs are revealing composite mechanisms of action that implicate not only iron sequestration and inhibition of iron-dependent enzyme ribonucleotide reductase but also coordination of other transition metals and oxidative damage [22,27]. A recent screen of ~80 thiosemicarbazones produced analog KS10076 (Fig. 2) of high cytotoxicity and good pharmacokinetic parameters, and genetic profiling on a large cancer cell panel indicated destabilization of the STAT3 pathway through the generation of reactive oxygen species (ROS) [28]. The formation of redox-active copper complexes is part of the mechanism of action of several antiproliferative thiosemicarbazones [29,30], and a ternary copper complex with COTI-2 and glutathione has been associated to drug resistance via efflux through the ATP-binding cassette (ABC) transporter ABCC1 [31]. Furthermore, the coordination of extracellular zinc has been associated with the anticancer activity of several thiosemicarbazones that serve as zinc metallochaperones to restore function of oncogenic p53 mutants [32].
Given the multifaceted roles of transition metals in cell biology, it is not surprising that metal-binding drugs present a polypharmacological profile. Indeed thiosemicarbazones are known to affect concurrently cancer growth, drug resistance, and metastasis [33]: for instance, through the upregulation of metastasis suppressor N-myc downstream-regulated gene-1 (NDRG1), these compounds inhibit multiple signaling pathways that affect both growth and metastasis [34]. For their ability to affect multiple tumor-supporting programs, polypharmacological compounds are particularly appealing for combination therapy with anticancer agents that are prone to resistance. In this context, Triapine was selected as a ribonucleotide reductase inhibitor and adjuvant to be tested in a recent clinical trial in combination with cisplatin-radiotherapy in advanced uterine cervix or vaginal cancers [35]. Initial studies on combination therapy of DpC with several standard anticancer agents have shown promising results [36].
Although tridentate thiosemicarbazones are arguably the most studied class of chelators in cancer applications, several programs are currently pursuing other frameworks. Interestingly, two new iron-binding scaffolds in cancer drug discovery were produced by high-throughput screens, which underscored the efficacy of iron-binding strategies in this research arena. A screen of 10,000 compounds in multicellular spheroids identified VXL600 (Fig. 2) as toxic towards quiescent cells under conditions of nutrient starvation [37]. This iron chelator has since been tested in a phase I clinical trial that documented good tolerability in patients with refractory advanced solid tumors [38]. More recently, its ability to disrupt homologous recombination DNA repair has shown potential for synergistic effects with cisplatin in ovarian cancer [39]. A high-throughput screen targeting activity against experimentally induced CSCs identified polyether ionophore antibiotic salinomycin as a lead compound [40], and then its mechanism of action was found to involve iron sequestration and ROS generation in lysosomes [41]. The analogous compound ironomycin (Fig. 2) recently showed promising antiproliferative and ferroptotic activity in primary cells from diffuse large B-cell lymphoma patients [42].
3. Prochelation strategies
The data accrued from multiple clinical trials have highlighted the promise as well as the challenges of chelators as anticancer agents. For example, methemoglobinemia (i.e., abnormal oxidation of hemoglobin in blood) is a well-documented consequence of the interaction of Triapine with systemic iron [27]. In addition, several antiproliferative thiosemicarbazones that form redox-active iron and copper complexes (e.g., Dp44mT) were found to present narrow therapeutic windows that prevented clinical development [22]. In this context, prochelation approaches are being pursued to release metal-binding species under specific conditions, thereby directing the coordination chemistry towards intracellular metals (relative to systemic metals) and/or preferentially targeting malignant cells (relative to normal tissue) [43,44].
The carboxylate donor of tridentate chelator deferitrin (Fig. 3) was masked as an ethyl ester to be activated through hydrolysis by intracellular esterases [45]. This prototype, which also included a cleavable polyamine to facilitate cellular uptake, showed improved activity relative to the parent chelator and showcased the advantages of the prochelation design.
Figure 3.
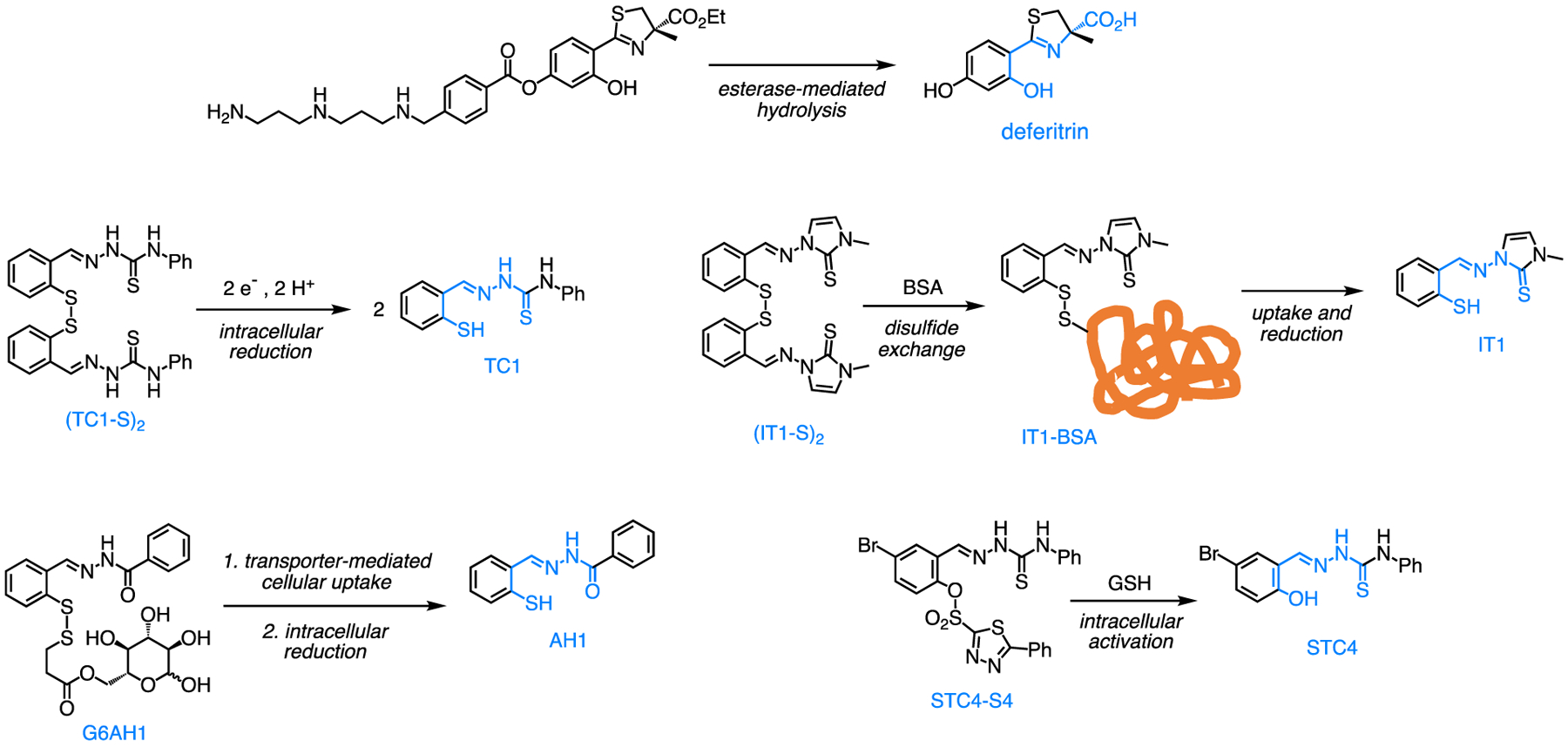
Examples of iron prochelation strategies showing the activation reactions that release antiproliferative chelators (with confirmed binding units in blue).
The tridentate binding units of thiosemicarbazones and aroylhydrazones have been masked with disulfide switches that release the metal-binding thiolate upon intracellular reduction (e.g., (TC1-S)2, Fig. 3). Indeed the high concentration of reduced glutathione (GSH) in the cytosol (1–10 mM) relative to that in blood plasma (2–20 μM) ensures the intracellular activation of these prochelators, which present antiproliferative activities at low micromolar levels in several cell lines [46]. The disulfide mask has also been employed to connect carbohydrate moieties (e.g., G6AH1, Fig. 3), which render the constructs rather hydrophilic and dependent on glucose transporters (e.g., GLUT1) for cellular uptake [47,48]. This prochelator design capitalizes on the overexpression of such transporters in various cancer cells and produces prochelators of improved therapeutic indexes. Two disulfide-based chelators featuring trimethyl thiosemicarbazone and imidazole-2-thione moieties (e.g., (IT1-S)2, Fig. 3) were found to form stable disulfide conjugates with serum albumin in the cell growth media [49]. The potential for albumin-mediated tumor accumulation and long lifetime in circulation makes these compounds attractive for preclinical development, particularly in light of their submicromolar antiproliferative activities in a panel of breast, ovarian, and colon cancer cell lines.
An alternative approach to intracellular activation employs the reactivity of arylsulfonates with GSH to release the phenolate donor of salicylaldehyde-based chelators (e.g., STC4, Fig. 3) [50]. In this approach, the rate of activation depends on the identity of the aryl group and could be tuned for specific conditions of high GSH levels characterizing a targeted cancer phenotype.
4. Effects within the tumor microenvironment
As new information emerges on oncogenic effects mediated by iron in the tumor microenvironment, interventions that modulate iron availability are revealing exciting opportunities to impact not only malignant cells but also their support network of immune cells, CSCs, and other stromal components.
The activation of metastasis suppressor NDRG1, which is a well-documented aspect of the activity of several thiosemicarbazones, has been recently implicated in the ability of DpC to inhibit the oncogenic crosstalk between pancreatic cancer cells and stromal stellate cells through the Wnt/β- catenin pathway [51].
The iron-releasing phenotype of TAMs, which correlate with poor prognosis, has been recognized as a potential target of chelators [52,53]. In a recent study aimed at examining iron distribution in renal cell carcinoma, iron secreted in extracellular fluids from primary tumors or in macrophage-conditioned media was found to promote the proliferation and migration of cancer cells. Notably, this effect was abolished in the presence of an extracellular chelator [54]. Overall, compounds that alter macrophage-mediated iron trafficking have the potential to impact cancer progression.
Iron is also critical to the survival of cancer cells in leptomeningeal metastases, which express LCN2 and its receptor SLC22A17 to grow in the nutrient-poor cerebrospinal fluid [55]. Consistent with a microenvironment that promotes cancer growth, the activation of this high-affinity system of iron acquisition is induced by inflammatory cytokines produced by macrophages. In a mouse model of leptomeningeal metastases from breast and lung cancers, treatment with DFO conferred a survival benefit and highlighted an opportunity for therapeutic intervention [55].
In a study that examined drug resistance in estrogen receptor-positive (ER+) breast cancer, [56] malignant cells were found to acquire cancer stem-like phenotypes when cocultured with bone marrow mesenchymal stem cells, which conferred reduced sensitivity to antiestrogenic therapy. Critically, the resistant phenotype was associated with increased labile iron and several iron chelators (i.e., DFO, DFX, and ironomycin) sensitized the malignant cells to antiestrogenic drugs.
5. Outlook
Because of the prominent role of iron in cancer biology, small-molecule scavengers (chelators), which affect iron availability and redox chemistry in biological settings, provide opportunities for new therapeutic interventions. Although no chelator has been approved to date for clinical use in cancer chemotherapy, ongoing investigations increasingly reveal a potential to impact a broad spectrum of cancer types by targeting a fundamental player of malignant behavior. Furthermore, accumulating evidence documents the ability of chelators to affect the tumor-supporting network of stromal cells, including CSCs, and hence to confront the major problems of cancer recurrence and drug resistance. Consistently, studies in combination with standard-of-care chemotherapy are producing promising results in refractory cancers.
Although the concentration of labile iron in cells is significantly higher than that of other transition metals, the coordination of copper and zinc has been recognized as part of the intracellular agenda of several metal-binding drug candidates. Strategies aimed at manipulating iron availability in cancer progression should therefore consider the potential involvement of other metals. These investigations are likely to enrich both our fundamental understanding of metals in cancer biology and the associated translational efforts. Achieving specific metal recognition is less likely (and perhaps less important) than understanding the relevance of other metals to the overall mechanism of action.
The design and development of prochelation strategies have demonstrated significant advantages of improved cellular uptake and/or selectivity towards malignant cells; however, the biological characterization of prochelator systems is still in its early stages in cell culture settings. The assessment of these strategies in vivo is expected to produce compounds of improved pharmacological profiles (and possibly reduced unwanted side effects) when compared to the original chelators that potentially interfere with systemic metals.
Contemporary methods in biomarker development and companion diagnostics are likely to enhance the efficacy of preclinical and clinical studies targeting iron in cancer treatment. Analyses of the iron-associated gene expression profile of large cohorts of patients (i.e., the “iron score” [42]) will allow identification of phenotypes that are more likely to benefit from iron-binding strategies. This information will continue to build the prognostic value of the iron signature gene (e.g., expression of TfR1, FPN) and the validation of biomarkers, which could be incorporated in the design of clinical trials. Furthermore, bioimaging methods could serve as valuable companion diagnostic tools: for instance, the detection of endogenous iron by magnetic resonance imaging in TAMs has shown promise to monitor immunotherapeutic response in breast cancer [57]. Overall, coupling the assessment of therapeutic regimens with iron-signature gene profiling and iron bioimaging techniques is likely provide precious information en route to new therapeutic options for cancer patients.
Highlights.
Iron plays a critical role in cancer growth, metastasis, recurrence, and resistance to therapy
Iron-modulating strategies affect not only cancer cells but also the tumor microenvironment
Prochelation approaches offer opportunities to improve therapeutic windows and reduce unwanted side effects
Acknowledgements
The author thankfully acknowledges support from the US National Institutes of Health (award R01 GM127646).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
Elisa Tomat has patent issued to Arizona Board of Regents of University of Arizona. Elisa Tomat has patent pending to Arizona Board of Regents of University of Arizona.
Data availability
No data was used for the research described in the article.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. : Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Q, Li L, Hou S, Yuan Z, Li C, Zhang W, Zheng L, Li X: The Role of Iron in Cancer Progression. Front Oncol 2021, 11:778492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torti SV, Torti FM: Iron: The cancer connection. Mol Asp Med 2020, 75:100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung M, Mertens C, Tomat E, Brüne B: Iron as a Central Player and Promising Target in Cancer Progression. Int J Mol Sci 2019, 20:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RAM, Richardson KL, Kabir TD, Trinder D, Ganss R, Leedman PJ: Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front Oncol 2020, 10:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G, Xian W, McKeon F, Lynch M, Crum CP, et al. : Iron addiction: A novel therapeutic target in ovarian cancer. Oncogene 2017, 36:4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz AJ, Goyert JW, Solanki S, Kerk SA, Chen B, Castillo C, Hsu PP, Do BT, Singhal R, Dame MK, et al. : Hepcidin sequesters iron to sustain nucleotide metabolism and mitochondrial function in colorectal cancer epithelial cells. Nat Metab 2021, 3:969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinnix ZK, Miller LD, Wang W, D’Agostino R, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, et al. : Ferroportin and Iron Regulation in Breast Cancer Progression and Prognosis. Sci Transl Med 2010, 2:43ra56–43ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene CJ, Attwood K, Sharma NJ, Gross KW, Smith GJ, Xu B, Kauffman EC: Transferrin receptor 1 upregulation in primary tumor and downregulation in benign kidney is associated with progression and mortality in renal cell carcinoma patients. Oncotarget 2017, 8:107052–107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRosa A, Leftin A: The Iron Curtain: Macrophages at the Interface of Systemic and Microenvironmental Iron Metabolism and Immune Response in Cancer. Front Immunol 2021, 12:614294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mertens C, Mora J, Ören B, Grein S, Winslow S, Scholich K, Weigert A, Malmström P, Forsare C, Fernö M, et al. : Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. OncoImmunology 2018, 7:e1408751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang W, Li Q, Ferrara N: Metastatic growth instructed by neutrophil-derived trasnferrin. Proc Natl Acad Sci USA 2018, 115:11060–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recalcati S, Gammella E, Cairo G: Dysregulation of iron metabolism in cancer stem cells. Free Radic Biol Med 2019, 133:216–220. [DOI] [PubMed] [Google Scholar]
- 14.Schonberg David L, Miller Tyler E, Wu Q, Flavahan William A, Das Nupur K, Hale James S, Hubert Christopher G, Mack Stephen C, Jarrar Awad M, Karl Robert T, et al. : Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell 2015, 28:441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalinowski DS, Richardson DR: The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev 2005, 57:547–583. [DOI] [PubMed] [Google Scholar]
- 16.Abdelaal G, Veuger S: Reversing oncogenic transformation with iron chelation. Oncotarget 2021, 12:106–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinbrueck A, Sedgwick AC, Han H-H, Zhao MY, Sen S, Huang D-Y, Zang Y, Li J, He X-P, Sessler JL: In vitro studies of deferasirox derivatives as potential organelle-targeting traceable anti-cancer therapeutics. Chem Commun 2021, 57:5678–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.pan Z-Y, Tan C-P, Rao L-S, Zhang H, Zheng Y, Hao L, Ji L-N, Mao Z-W: Recoding the Cancer Epigenome by Intervening in Metabolism and Iron Homeostasis with Mitochondria-Targeted Rhenium(I) Complexes. Angew Chem Int Ed 2020, 59:18755–18762. [DOI] [PubMed] [Google Scholar]
- 19.Gaur K, Pérez Otero SC, Benjamín-Rivera JA, Rodríguez I, Loza-Rosas SA, Vázquez Salgado AM, Akam EA, Hernández-Matias L, Sharma RK, Alicea N, et al. : Iron Chelator Transmetalative Approach to Inhibit Human Ribonucleotide Reductase. JACS Au 2021, 1:865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Li X, Mu Y, Lu C, Tang S, Lu K, Qiu X, Wei A, Cheng Y, Wei W: The iron chelator desferrioxamine synergizes with chemotherapy for cancer treatment. J Trace Elem Med Biol 2019, 56:131–138. [DOI] [PubMed] [Google Scholar]
- 21.Katsura Y, Ohara T, Noma K, Ninomiya T, Kashima H, Kato T, Sato H, Komoto S, Narusaka T, Tomono Y, et al. : A Novel Combination Cancer Therapy with Iron Chelator Targeting Cancer Stem Cells via Suppressing Stemness. Cancers 2019, 11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y, Kalinowski DS, Kovacevic Z, Siafakas AR, Jansson PJ, Stefani C, Lovejoy DB, Sharpe PC, Bernhardt PV, Richardson DR: Thiosemicarbazones from the Old to New: Iron Chelators That Are More Than Just Ribonucleotide Reductase Inhibitors. J Med Chem 2009, 52:5271–5294. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z-L, Richardson DR, Kalinowski DS, Kovacevic Z, Tan-Un KC, Chan GC-F: The novel thiosemicarbazone, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), inhibits neuroblastoma growth in vitro and in vivo via multiple mechanisms. J Hemat Oncol 2016, 9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovejoy DB, Sharp DM, Seebacher N, Obeidy P, Prichard T, Stefani C, Basha MT, Sharpe PC, Jansson PJ, Kalinowski DS, et al. : Novel Second-Generation Di-2-Pyridylketone Thiosemicarbazones Show Synergism with Standard Chemotherapeutics and Demonstrate Potent Activity against Lung Cancer Xenografts after Oral and Intravenous Administration in Vivo. J Med Chem 2012, 55:7230–7244. [DOI] [PubMed] [Google Scholar]
- 25.Lindemann A, Patel AA, Silver NL, Tang L, Liu Z, Wang L, Tanaka N, Rao X, Takahashi H, Maduka NK, et al. : COTI-2, A Novel Thiosemicarbazone Derivative, Exhibits Antitumor Activity in HNSCC through p53-dependent and -independent Mechanisms. Clin Cancer Res 2019, 25:5650–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salim KY, Maleki Vareki S, Danter WR, San-Marina S, Koropatnick J: COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget 2016, 7:41363–41379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffeter P, Pape VFS, Enyedy ÉA, Keppler BK, Szakacs G, Kowol CR: Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development. Antioxid Redox Signal 2018, 30:1062–1082. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Park A, Hwang J, Zhao X, Kwak J, Kim HW, Ku M, Yang J, Kim TI, Jeong K-S, et al. : KS10076, a chelator for redox-active metal ions, induces ROS-mediated STAT3 degradation in autophagic cell death and eliminates ALDH1+ stem cells. Cell Rep 2022, 40:111077. [DOI] [PubMed] [Google Scholar]
- 29.Lovejoy DB, Jansson PJ, Brunk UT, Wong J, Ponka P, Richardson DR: Antitumor Activity of Metal-Chelating Compound Dp44mT Is Mediated by Formation of a Redox-Active Copper Complex That Accumulates in Lysosomes. Cancer Res 2011, 71:5871. [DOI] [PubMed] [Google Scholar]
- 30.Falcone E, Ritacca AG, Hager S, Schueffl H, Vileno B, El Khoury Y, Hellwig P, Kowol CR, Heffeter P, Sicilia E, et al. : Copper-Catalyzed Glutathione Oxidation is Accelerated by the Anticancer Thiosemicarbazone Dp44mT and Further Boosted at Lower pH. J Am Chem Soc 2022, 144:14758–14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bormio Nunes JH, Hager S, Mathuber M, Pósa V, Roller A, Enyedy ÉA, Stefanelli A, Berger W, Keppler BK, Heffeter P, et al. : Cancer Cell Resistance Against the Clinically Investigated Thiosemicarbazone COTI-2 Is Based on Formation of Intracellular Copper Complex Glutathione Adducts and ABCC1-Mediated Efflux. J Med Chem 2020, 63:13719–13732. [DOI] [PMC free article] [PubMed] [Google Scholar]; * With a detailed analysis in resistant cell lines, this paper shows that the interactions of thiosemicarbazone COTI-2 with copper and glutathione are related to drug resistance through the efflux pump ABCC1.
- 32.Yu X, Vazquez A, Levine Arnold J, Carpizo Darren R: Allele-Specific p53 Mutant Reactivation. Cancer Cell 2012, 21:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansson PJ, Kalinowski DS, Lane DJR, Kovacevic Z, Seebacher NA, Fouani L, Sahni S, Merlot AM, Richardson DR: The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by Di-2pyridylketone thiosemicarbazones. Pharmacol Res 2015, 100:255–260. [DOI] [PubMed] [Google Scholar]
- 34.Chekmarev J, Azad MG, Richardson DR: The Oncogenic Signaling Disruptor, NDRG1: Molecular and Cellular Mechanisms of Activity. Cells 2021, 10:2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunos CA, Andrews SJ, Moore KN, Chon HS, Ivy SP: Randomized Phase II Trial of Triapine-Cisplatin-Radiotherapy for Locally Advanced Stage Uterine Cervix or Vaginal Cancers. Front Oncol 2019, 9:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dharmasivam M, Azad MG, Afroz R, Richardson V, Jansson PJ, Richardson DR: The thiosemicarbazone, DpC, broadly synergizes with multiple anti-cancer therapeutics and demonstrates temperature- and energy-dependent uptake by tumor cells. Biochim Biophys Acta Gen Subj 2022, 1866:130152. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Fryknäs M, Hernlund E, Fayad W, De Milito A, Olofsson MH, Gogvadze V, Dang L, Påhlman S, Schughart LAK, et al. : Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun 2014, 5:3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mody K, Mansfield AS, Vemireddy L, Nygren P, Gulbo J, Borad M: A phase I study of the safety and tolerability of VLX600, an Iron Chelator, in patients with refractory advanced solid tumors. Invest New Drugs 2019, 37:684–692. [DOI] [PubMed] [Google Scholar]
- 39.Ekstrom TL, Pathoulas NM, Huehls AM, Kanakkanthara A, Karnitz LM: VLX600 Disrupts Homologous Recombination and Synergizes with PARP Inhibitors and Cisplatin by Inhibiting Histone Lysine Demethylases. Mol Cancer Ther 2021, 20:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES: Identification of Selective Inhibitors of Cancer Stem Cells by High-Throughput Screening. Cell 2009, 138:645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mai TT, Hamaï A, Hienzsch A, Cañeque T, Müller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, et al. : Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem 2017, 9:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devin J, Cañeque T, Lin Y-L, Mondoulet L, Veyrune J-L, Abouladze M, Garcia De Paco E, Karmous Gadacha O, Cartron G, Pasero P, et al. : Targeting Cellular Iron Homeostasis with Ironomycin in Diffuse Large B-cell Lymphoma. Cancer Res 2022, 82:998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrates the use of an “iron score” based on gene expression profiling to identify phenotypes susceptible to ironomycin (and its combination with standard anticancer agents) in primary diffuse large B-cell lymphoma cells.
- 43.Wang Q, Franz KJ: Stimulus-Responsive Prochelators for Manipulating Cellular Metals. Acc Chem Res 2016, 49:2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinbrueck A, Sedgwick AC, Brewster JT, Yan K-C, Shang Y, Knoll DM, Vargas-Zúñiga GI, He X-P, Tian H, Sessler JL: Transition metal chelators, pro-chelators, and ionophores as small molecule cancer chemotherapeutic agents. Chem Soc Rev 2020, 49:3726–3747. [DOI] [PubMed] [Google Scholar]
- 45.Bergeron RJ, Singh S, Bharti N, Jiang Y: Design, Synthesis, and Testing of Polyamine Vectored Iron Chelators. Synthesis 2010, 21:3631–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akam EA, Utterback RD, Marcero JR, Dailey HA, Tomat E: Disulfide-masked iron prochelators: Effects on cell death, proliferation, and hemoglobin production. J Inorg Biochem 2018, 180:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akam EA, Tomat E: Targeting Iron in Colon Cancer via Glycoconjugation of Thiosemicarbazone Prochelators. Bioconj Chem 2016, 27:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sung Y-S, Kerimoglu B, Ooi A, Tomat E: Aroylhydrazone Glycoconjugate Prochelators Exploit Glucose Transporter 1 (GLUT1) to Target Iron in Cancer Cells. ACS Med Chem Lett 2022, 13:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This report on a prochelation approach showcases the advantage of glycoconjugation to direct the intracellular release of an iron chelator to malignant cells that overexpress the glucose transporter GLUT1.
- 49.Sung Y-S, Wu W, Ewbank MA, Utterback RD, Marty MT, Tomat E: Albumin Conjugates of Thiosemicarbazone and Imidazole-2-thione Prochelators: Iron Coordination and Antiproliferative Activity. ChemMedChem 2021, 16:2764–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu W, Sung Y-S, Tomat E: Thiol-Reactive Arylsulfonate Masks for Phenolate Donors in Antiproliferative Iron Prochelators. Inorg Chem 2022, 61:19974–19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geleta B, Tout FS, Lim SC, Sahni S, Jansson PJ, Apte MV, Richardson DR, Kovačević Ž: Targeting Wnt/tenascin C-mediated cross talk between pancreatic cancer cells and stellate cells via activation of the metastasis suppressor NDRG1. J Biol Chem 2022, 298:101608. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using an orthotopic mouse model of pancreatic cancer, this study shows that thiosemicarbazone chelator DpC upregulates metastasis suppressor NDRG1 and interferes with the tumor-promoting crosstalk between cancer cells and stromal stellate cells.
- 52.Mertens C, Akam EA, Rehwald C, Brüne B, Tomat E, Jung M: Intracellular Iron Chelation Modulates the Macrophage Iron Phenotype with Consequences on Tumor Progression. PLOS ONE 2016, 11:e0166164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Recalcati S, Locati M, Marini A, Santambrogio P, Zaninotto F, De Pizzol M, Zammataro L, Girelli D, Cairo G: Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol 2010, 40:824–835. [DOI] [PubMed] [Google Scholar]
- 54.Schnetz M, Meier JK, Rehwald C, Mertens C, Urbschat A, Tomat E, Akam EA, Baer P, Roos FC, Brüne B, et al. : The Disturbed Iron Phenotype of Tumor Cells and Macrophages in Renal Cell Carcinoma Influences Tumor Growth. Cancers 2020, 12:530. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Focused on the role of macrophage-secreted iron in renal cell carcinoma, this study shows that an extracellular iron chelator abolishes the positive effects of TAMs on the proliferation and migration rates of malignant cells.
- 55.Chi Y, Remsik J, Kiseliovas V, Derderian C, Sener U, Alghader M, Saadeh F, Nikishina K, Bale T, Iacobuzio-Donahue C, et al. : Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science 2020, 369:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buschhaus JM, Rajendran S, Humphries BA, Cutter AC, Muñiz AJ, Ciavattone NG, Buschhaus AM, Cañeque T, Nwosu ZC, Sahoo D, et al. : Effects of iron modulation on mesenchymal stem cell-induced drug resistance in estrogen receptor-positive breast cancer. Oncogene 2022, 41:3705–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using a co-culture model, this study demonstrates that iron chelators contrast resistance to antiestrogenic therapy induced by mesenchymal stem cells in breast cancer.
- 57.Leftin A, Ben-Chetrit N, Joyce JA, Koutcher JA: Imaging endogenous macrophage iron deposits reveals a metabolic biomarker of polarized tumor macrophage infiltration and response to CSF1R breast cancer immunotherapy. Sci Rep 2019, 9:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


