Abstract
Aurora B is a protein kinase and a chromosomal passenger protein that undergoes dynamic redistribution during mitosis. We have probed the mechanism that regulates its localization with cells expressing green fluorescent protein (GFP)-tagged wild-type or mutant aurora B. Aurora B was found at centromeres at prophase and persisted until ∼0.5 min after anaphase onset, when it redistributed to the spindle midzone and became concentrated at the equator along midzone microtubules. Depolymerization of microtubules inhibited the dissociation of aurora B from centromeres at early anaphase and caused the dispersion of aurora B from the spindle midzone at late anaphase; however, centromeric association during prometaphase was unaffected. Inhibition of CDK1 deactivation similarly caused aurora B to remain associated with centromeres during anaphase. In contrast, inhibition of the kinase activity of aurora B appeared to have no effect on its interactions with centromeres or initial relocation onto midzone microtubules. Instead, kinase-inactive aurora B caused abnormal mitosis and deactivation of the spindle checkpoint. In addition, midzone microtubule bundles became destabilized and aurora B dispersed from the equator. Our results suggest that microtubules, CDK1, and the kinase activity each play a distinct role in the dynamics and functions of aurora B in dividing cells.
INTRODUCTION
Aurora/Ipl1p-related proteins are a family of well-conserved serine-threonine kinases involved in various stages of mitosis (Giet and Prigent, 1999). The founding members are Ipl1p from Saccharomyces cerevisiae (Francisco and Chan, 1994) and aurora from Drosophila melanogaster (Glover et al., 1995). Aurora A functions in centrosome separation and spindle bipolarity (Glover et al., 1995; Roghi et al., 1998; Giet and Prigent, 2000), whereas aurora B appears to function in both early and late mitotic events (Bischoff et al., 1998; Schumacher et al., 1998; Terada et al., 1998). Ablation of aurora B activity by RNA interference (Schumacher et al., 1998; Kaitna et al., 2000; Adams et al., 2001; Giet and Glover, 2001) or by deactivating a temperature-sensitive (ts) mutant (Severson et al., 2000) leads to a wide range of defects in prometaphase chromosomal congregation, anaphase chromosomal segregation, and cytokinesis. Aurora B also plays a role in chromosomal condensation by phosphorylating the H3 histone (Hsu et al., 2000; Adams et al., 2001; Giet and Glover, 2001).
Immunolocalization studies demonstrated that aurora B localizes at chromosomal centromeres during prometaphase, and subsequently relocates to midzone microtubules and midbodies during anaphase and telophase (Schumacher et al., 1998; Adams et al., 2001; Giet and Glover, 2001). This distribution pattern is similar to that of several chromosome passenger proteins, including inner centromere protein (INCENP; Cooke et al., 1987; Mackay et al., 1998), TD60 (Andreassen et al., 1991; Martineau-Thuillier et al., 1998), and survivin (Skoufias et al., 2000; Uren et al., 2000). Furthermore, as for aurora B, INCENP has been implicated in cytokinesis based on gene ablation and mutation experiments (Eckley et al., 1997; Mackay et al., 1998; Cutts et al., 1999; Adams et al., 2000, 2001; Kaitna et al., 2000). The similar behavior of these proteins raises the possibility that they form a complex that is transported by microtubule-based motors to the equator during cytokinesis. This notion is supported by the identification of a complex from Xenopus egg extracts and from HeLa cells extracts that contains both INCENP and aurora B (Adams et al., 2000; Kaitna et al., 2000).
From these studies, it is clear that the dynamics of aurora B and associated proteins plays a critical role in their functions. Changes in the localization of aurora B may serve as an effective mechanism for defining its substrates in a temporally and spatially dependent manner. Although there is still no direct evidence, it is possible that CDK1 serves as a master switch for regulating the localization of these proteins. An attractive possibility is that signals of CDK1 may be relayed through aurora B to other components of the chromosomal passenger complex and the associated motor molecule MKLP1/ZEN-4 (Terada et al., 1998; Severson et al., 2000). Phosphorylation of these proteins by aurora B may in turn effect the subsequent localization of aurora B at the spindle midzone.
So far, the localization of aurora B and other chromosomal passenger proteins has relied on immunofluorescence of fixed cells. Due to the highly dynamic nature of these proteins, detailed understanding of their function and regulation may benefit greatly from the direct observations and manipulations of living cells. In this study we have transfected rat aurora B as a green fluorescent protein (GFP) fusion protein into normal rat kidney (NRK) cells. To test the role of its kinase activity, we have also created a GFP fusion protein of kinase-inactive aurora B. We were able to follow the striking relocation of aurora B in living cells, and to test the effects of several factors that may affect aurora B distribution. Our results suggest that both microtubules and CDK1 play a major role in the relocation of aurora B from centromeres to the central spindle during anaphase. In contrast, the kinase activity of aurora B is required for its stable localization once aurora B is relocated along midzone microtubules.
MATERIALS AND METHODS
Cell Culture, Microscopy, and Image Processing
Normal rat kidney epithelial cells (NRK-52E; American Type Culture Collection, Rockville, MD) were cultured in Kaighn's modified F12 (F12K) medium supplemented with 10% fetal bovine serum (JRH Biosciences, Lenexa, KS), 50 U/ml penicillin, and 50 μg/ml streptomycin, on glass chamber dishes as previously described (Mckenna and Wang, 1989). The cells were maintained at 37°C in a stage incubator built on top of an Axiovert S100TV inverted microscope (Carl Zeiss, Thornwood, NY) and viewed with a 100×, numerical aperture 1.30 Fluar lens. All images were acquired with a cooled charge-coupled device camera (ST133 controller and CCD57 chip; Roper Scientific, Treton, NJ) and processed with custom software for background subtraction.
Plasmid Construction and Transfection
cDNA fragments encoding aurora B and its kinase-inactive mutant were amplified by polymerase chain reaction with FLAG-AIM-1 (WT) and FLAG-AIM-1 (K-R) as templates (Terada et al., 1998), respectively, and subcloned into pEGFP-N1 vector (CLONTECH, Palo Alto, CA). The enhanced green fluorescent protein (EGFP) sequence is located at the 3′ end of the aurora B sequence. For transient transfection, NRK cells were plated at a density of 5 × 104 cells/ml on a coverslip chamber dish and incubated for 18–24 h. Immediately before transfection, the cells were rinsed once in serum-free F12K or Opti-MEM I medium (Invitrogen, Carlsbad, CA). The cells were transfected with the DNA construct (2 μg) by using LipofectAMINE according to manufacturer's instructions (Invitrogen). After 4 h of incubation, the medium containing DNA-LipofectAMINE was replaced with the F12K medium containing 10% FBS, and the cells was cultured for an additional 14–16 h. To maintain the expression of aurora B-GFP, NRK cells on 60-mm culture dishes were transfected with 3 μg of aurora B-GFP as described above and cultured in the presence of 800 μg/ml G418 (Invitrogen).
In Vitro Transcription of Cyclin B Δ90 and Preparation of Rhodamine-labeled Tubulin
Messenger RNA for the nondegradable mutant of cyclin B (Δ90 cyclin B) was synthesized with the mMESSAGE mMACHINE kit (Ambion, Austin, TX) and prepared for microinjection as described previously with minor modifications (Wheatley et al., 1997): transcribed mRNA was separated from unincorporated nucleotides by a Spin Column 10 (Sigma Chemical, St. Louis, MO) and the yield was estimated by UV absorbance. Tubulin was prepared and labeled with 5- (and 6-) carboxytetramethylrhodamine (Molecular Probes, Eugene, OR) as described previously (Wheatley et al., 1997, 1998).
Microinjection, Drug Treatment, and Immunofluorescence
Microinjection was performed by low-speed continuous flow with custom-drawn glass needles and a custom-designed pressure control system (Wang, 1992). Nocodazole (Sigma Chemical) was stored at −20°C as 103 × stocks in dimethyl sulfoxide and diluted into prewarmed medium before application to cells.
For microtubule and aurora B immunofluorescence, cells were rinsed with warm cytoskeletal buffer and fixed with 4% paraformaldehyde (EM Scientific, Gibbstown, NJ) in warm cytoskeleton buffer for 10 min (Wheatley and Wang, 1996). They were then rinsed thoroughly in the cytoskeletal buffer and permeablized by incubation with 0.5% Triton X-100 for 5 min. Fixed cells were rinsed with the cytoskeletal buffer, blocked for 10 min with 1% bovine serum albumin (BSA) (Roche Applied Sciences, Indianapolis, IN) in PBS, and then incubated with anti-β-tubulin monoclonal antibodies (Amersham Biosciences, Piscataway, NJ) at a dilution of 1:10 in PBS with 1% BSA or anti-AIM-1 monoclonal antibodies (Murata-Hori et al., 2000) at a dilution of 1:50 for 45 min at 37°C. After washing with PBS/BSA thoroughly, the cells were incubated with Alexa 546-conjugated goat anti-mouse antibodies (Molecular Probes) at a dilution of 1:100 for 30 min at 37°C.
Mad2 staining was performed with a method modified from that of Waters et al. (1998). The cells were rinsed with PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, and 4 mM MgSO4, pH 6.9), lysed for 1 min in 0.1% Triton X-100 in PHEM buffer, and then fixed with 4% formaldehyde in PHEM buffer for 20 min. Fixed cells were rinsed with PBS with 0.05% Tween 20, blocked for 30 min with 1% BSA in PBS, and incubated with anti-Mad2 rabbit polyclonal antibodies (gift of Dr. E.D. Salmon, University of North Carolina, Chapel Hill, NC) at a dilution of 1:50 in PBS with 2.5% BSA for 45 min at 37°C. After washing with PBS with 2.5% BSA, cells were incubated with Alexa 546-conjugated goat anti-rabbit antibodies (Molecular Probes) at a dilution of 1:100 for 30 min at 37°C. Finally, cells were rinsed with PBS and mounted in an antibleaching medium (Wheatley and Wang, 1996).
RESULTS
Aurora B-GFP as a Probe of Aurora B Dynamics in Dividing NRK Cells
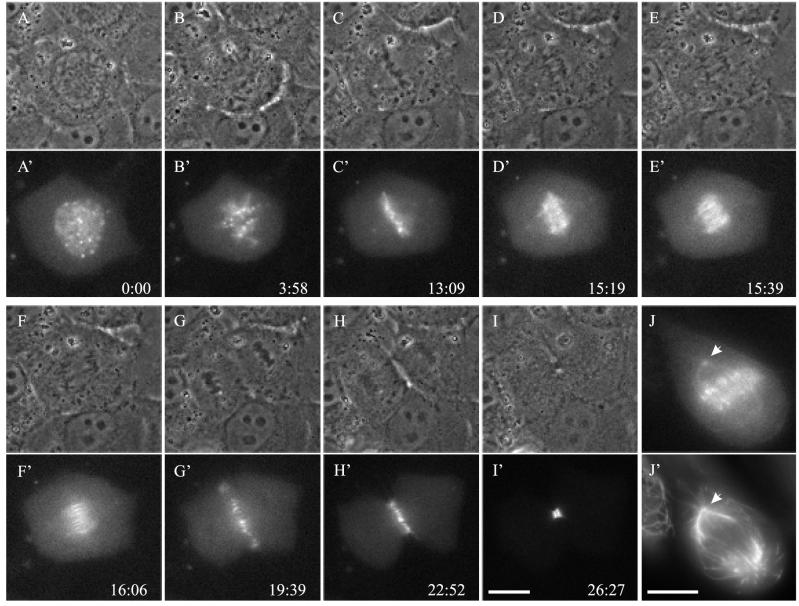
NRK cells were transfected with GFP-tagged aurora B and observed with fluorescence optics during mitosis and cytokinesis (Figure 1). Aurora B-GFP became localized as early as prophase (before nuclear envelope breakdown) at chromosomal centromeres (Figure 1A). Some aurora B-GFP was also found at the spindle pole (Figure 1J), as confirmed by counterstaining for β-tubulin (Figure 1J′). During anaphase, aurora B-GFP dissociated from centromeres and redistributed to midzone microtubules. However, the relocation occurred slightly after anaphase onset, because there was a period of 20–30 s when most aurora B-GFP was still associated with centromeres of separated chromosomes (Figure 1, D–F). Relocated aurora B initially spanned the region between separated chromosomes (Figure 1, E–F), but subsequently condensed into short segments on the equatorial plane that collapsed laterally during cytokinesis to form the midbody (Figure 1, G–I).
Figure 1.
Dynamics of aurora B in living mitotic NRK cells transfected with aurora B-GFP. Time-lapse phase-contrast (A–I) and corresponding fluorescence (A′–I′) images show the redistribution of aurora B during mitosis and cytokinesis. Time elapsed since prophase is shown in minutes:seconds. Aurora B was localized at centromeres at prophase (A and A′), and persisted through prometaphase (B and B′), metaphase (C and C′), and early anaphase (D and D′). It then started to relocate to midzone microtubules (E and E′), initially spanning the space between separating chromosomes (E and E′ and F and F′) but later condensing into short segments on the equatorial plane (G and G′ and H and H′). These segments then coalesced laterally to form the midbody (I and I′). (J and J′) Separate cell fixed at early anaphase and stained with anti-β-tubulin. Note that aurora B was localized at the spindle pole (arrows). Bars, 10 μm.
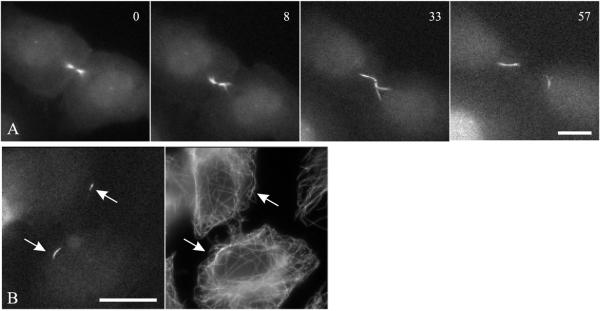
The distribution of aurora B-GFP was followed beyond the completion of cytokinesis, to determine the fate of aurora B-GFP concentrated at the midbody. The condensed structure extended into elongated segments and migrated toward the cell center (Figure 2A). Counterstaining for β-tubulin indicated that the movement took place along microtubules of the interphase network (Figure 2B). These aurora B-containing segments subsequently dispersed beyond detection, before they reached a defined destination.
Figure 2.
Dissociation of aurora B from the midbody after cytokinesis. Time-lapse images show the dissociation of aurora B-GFP from the midbody as one or more segments that migrate toward the cell center (A). Time elapsed in minutes is given at the upper right corner. Fixation and staining for β-tubulin in a separate cell showed that the condensed segments of aurora B (B, left), already well separated from the region occupied by the midbody, were localized along interphase microtubules (B, right). Bars, 10 μm.
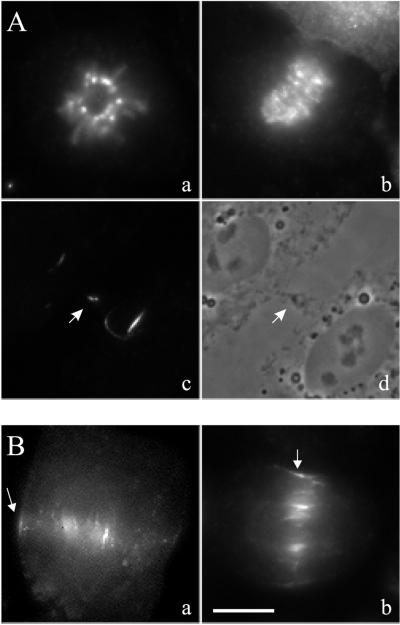
The distribution of aurora B-GFP was generally consistent with immunolocalization of aurora B in other systems (Schumacher et al., 1998; Adams et al., 2001; Giet and Glover, 2001). To confirm that aurora B-GFP mimics the behavior of endogenous aurora B, we fixed and stained nontransfected cells with antibodies against aurora B (Figure 3). As for aurora B-GFP, endogenous aurora B was localized at centromeres in prometaphase cells and remained associated with centromeres until shortly after anaphase onset (Figure 3A). Its subsequent relocation to midzone microtubules and the equatorial cortex (Figure 3B), and to bundle structures after the completion of cortical ingression, was also similar to what was observed with aurora B-GFP (Figure 3A). However, we were unable to detect the association of endogenous aurora B at spindle poles by immunofluorescence staining, possibly due to the limited amount of aurora B at these structures.
Figure 3.
Distribution of endogenous aurora B and aurora B-GFP during cell division. (A) Immunofluorescence localization of aurora B at prometaphase (a), anaphase onset (b), and after cytokinesis (c and d; arrows point to the midbody). The distribution is similar to that seen with aurora B-GFP in Figures 1 and 2. (B) Localization of aurora B during anaphase. Both aurora B-GFP (a) and endogenous aurora B (b) can be found on the equatorial cortex (arrows) and along midzone microtubules. Bar, 10 μm.
Based on the intensity of immunofluorescence staining, we were able to estimate the relative amount of aurora B in cells expressing aurora B-GFP and in nontransfected cells. Transfection under the present condition was found to increase the total amount of aurora B by an average of approximately twofold, which is likely lower than what was obtained previously when cells were transfected with a higher amount of DNA (Tatsuka et al., 1998).
Involvement of Microtubules and CDK1 in Dynamics of Aurora B
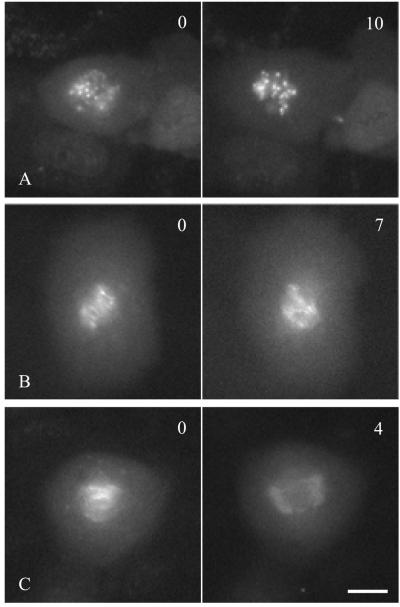
The ability to image aurora B in living cells allowed us to probe the mechanism that regulates its redistribution during cell division. We first treated aurora B-GFP–expressing cells with nocodazole to determine whether microtubules are required for its dynamic relocation. No obvious change was observed in the centromeric localization of aurora B-GFP when cells were treated at prophase or prometaphase (100% for n = 10; Figure 4A), suggesting that microtubules were not involved in maintaining the centromeric localization of aurora B during early mitosis. In contrast, microtubules were required for the dissociation of aurora B-GFP from centromeres and the relocation to the spindle midzone during anaphase, as indicated by the maintenance of aurora B-GFP on separated chromosomes when cells were treated with nocodazole shortly after anaphase onset (100% for n = 10; Figure 4B). Application of nocodazole during later stages of anaphase caused aurora B-GFP to disperse from the spindle midzone (100% for n = 10; Figure 4C), suggesting that microtubules were also involved in maintaining its midzone localization. Each of these experiments with nocodazole was repeated 10 times with consistent results.
Figure 4.
Effects of nocodazole on the dynamics of aurora B-GFP. NRK cells stably expressing aurora B-GFP were treated with 10–20 μM nocodazole at prometaphase (A), early anaphase (B) or midanaphase (C). Images of aurora B-GFP were recorded immediately before (left) and after nocodazole treatment (right). Time elapsed since the addition of nocodazole is given in minutes at the upper right corner. Treatment with nocodazole had no effect on centromeric association of aurora B at prometaphase (A); however, it inhibited the dissociation of aurora B from centromeres at early anaphase (B), and caused the dispersion of aurora B that was localized along midzone microtubules during late anaphase (C). Bar, 10 μm.
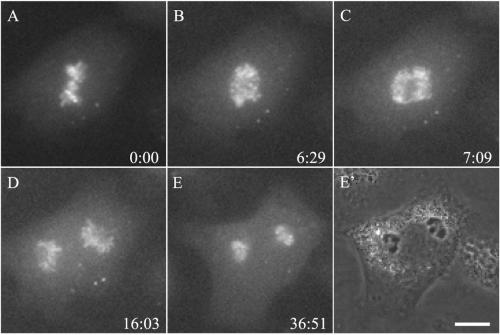
We speculate that the dissociation of aurora B-GFP from the centromeres may be regulated by cyclin B degradation/CDK1 deactivation. To address the involvement of CDK1, we microinjected the mRNA for Δ90 cyclin B, a nondegradable active domain of cyclin B, into dividing NRK cells transfected with aurora B-GFP. This treatment was previously shown to inhibit the degradation of cyclin B at anaphase onset, thus maintaining the CDK1 activity at a high level (Glotzer et al., 1991; Wheatley et al., 1997). The injection had no effect on metaphase chromosomal alignment or anaphase chromosomal separation (Figure 5, A–C; Wheatley et al., 1997). However, aurora B-GFP failed to dissociate from centromeres and cytokinesis failed as well (100% for n = 11; Figure 5, C–E). Thus, CDK1 inactivation is required for the redistribution of aurora B from chromosomes to the spindle midzone. To test whether CDK1 inactivation alone is sufficient for the release of aurora B, prometaphase cells were treated with a CDK1 inhibitor, purvalanol A (Chang et al., 1999; Chadebech et al., 2000). The treatment had no effect on the centromeric association (our unpublished data), suggesting that the release of centromeric aurora B requires additional events such as dephosphorylation by a phosphatase, proteolysis, or the assembly of midzone microtubules.
Figure 5.
Effect of Δ90 cyclin B on the dynamics of aurora B. An NRK cell stably expressing aurora B was microinjected with mRNA for Δ90 cyclin B at prometaphase. Neither chromosomal congregation nor anaphase onset appeared affected. However, aurora B remained associated with centromeres 30 min after anaphase onset (E), and cytokinesis was inhibited (E′). The phase image corresponding to E is shown in E′. Time elapsed since metaphase is given in minutes:seconds at the lower right corner. Bar, 10 μm.
Involvement of Kinase Activity of Aurora B in Mitosis and Cytokinesis
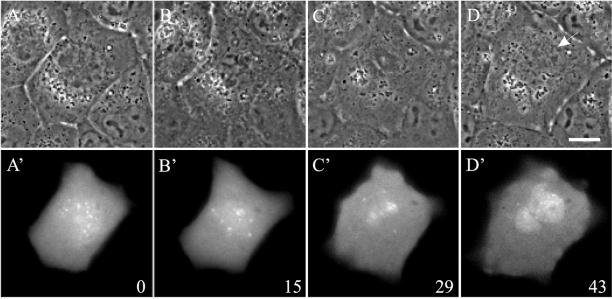
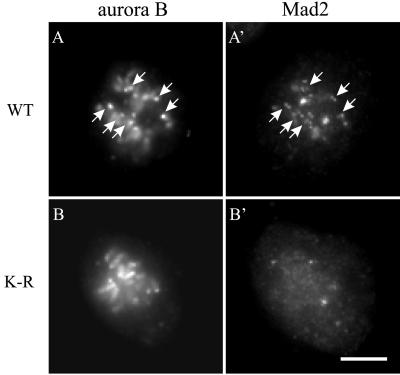
To address the possibility that the kinase activity of aurora B may regulate its localization, we constructed a GFP-tagged, kinase inactive mutant of aurora B, by using FLAG-AIM-1(K-R) as a template (Terada et al., 1998). NRK cells were transiently transfected with wild-type aurora B-GFP or aurora B(K-R)-GFP. No mitotic defect was observed in cells expressing wild-type aurora B-GFP (Table 1). Time-lapse imaging of aurora B(K-R)-GFP indicated an apparently normal localization at centromeres during prometaphase (Figure 6A′). However, expression of aurora B(K-R)-GFP caused strong dominant negative effects (Table 1). Defects in mitosis were observed in >80% of the cells (n = 30). A large fraction of cells (46.7%) failed to congregate their chromosomes into a metaphase plate (Figure 6, A–C). Interestingly, 66.7% of these cells exited mitosis directly within 1.5 h of nuclear envelope breakdown, by forming a nuclear envelope around chromosomes without going through anaphase (Figure 6D, arrow). Aurora B(K-R)-GFP gradually dispersed from the centromeres as the nuclear envelope reformed. Localization of a spindle checkpoint component, Mad2 (Li and Murray, 1991), indicated that Mad2 disappeared from the kinetochores of these prometaphase-arrested cells (Figure 7, B and B′), despite the absence of a metaphase plate. In control cells, Mad2 dissociates from kinetochores only as chromosomes become congregated into the metaphase plate (Figure 7, A and A′; Chen et al., 1996; Li and Benezra, 1996).
Table 1.
Effect of kinase inactive mutant of aurora B on mitosis
| Chromosome miscongregation | Chromosome missegregation | |
|---|---|---|
| aurora B (WT) | 0.0% (0/16) | 0.0% (0/16) |
| aurora B (K-R) | 46.7% (14/30) | 36.7% (11/30) |
NRK cells were transiently transfected with aurora B-GFP or aurora B (K-R)-GFP. Cells expressing GFP proteins in prophase or prometaphase were observed with phase optics to assess the behavior of chromosomes.
Figure 6.
Dynamics of aurora B(K-R)-GFP in a cell that failed to congregate the chromosomes at prometaphase. An NRK cell transiently expressing aurora B(K-R)-GFP was monitored by time-lapse phase contrast (A–D) and fluorescence optics (A′–D′). Time elapsed in minutes since nuclear envelope breakdown is shown at the lower right corner. Chromosomes in this cell failed to congregate (B and B′), and started to decondense ∼30 min after nuclear envelope breakdown (C and C′). A nuclear envelope then formed around the chromosomes (D, arrow). There was no sign of cytokinesis throughout the period of observation. Bar, 10 μm.
Figure 7.
Bypass of the spindle checkpoint in a prometaphase-arrested cells expressing aurora B(K-R)-GFP. Immunofluorescence indicates little Mad2 staining at kinetochores of a prometaphase-arrested cell transiently expressing aurora B(K-R)-GFP (B and B′), whereas a control prometaphase cell transiently expressing aurora B-GFP shows Mad2 staining on a number of kinetochores (A and A′, arrows). Bar, 10 μm.
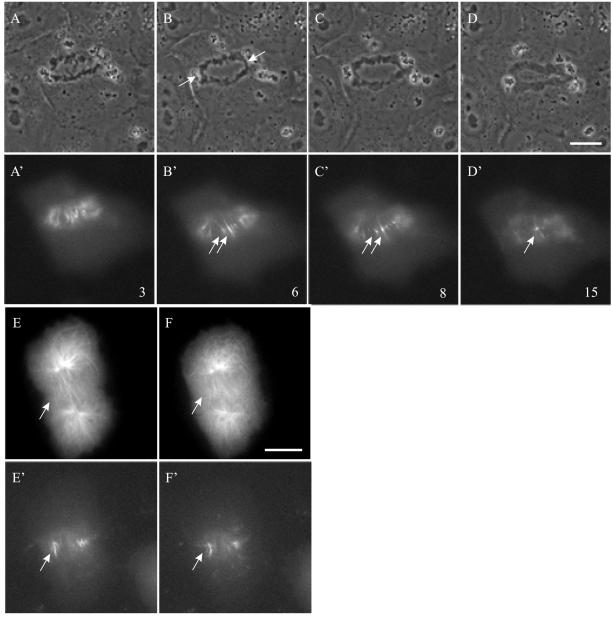
Other cells expressing aurora B(K-R)-GFP (36.7%) managed to enter anaphase, but with defects in chromosomal segregation similar to what was reported after the ablation or deactivation of aurora B (Figure 8B, arrows; Kaitna et al., 2000; Severson et al., 2000; Adams et al., 2001; Giet and Glover, 2001). All aurora B(K-R)-GFP–expressing cells showing mitotic defects also failed cytokinesis. In cells that failed to enter anaphase, there was no detectable initiation of cytokinesis (Figure 6D′). In cells that entered anaphase, cytokinesis failed after an initial ingression. Aurora B(K-R)-GFP was able to redistribute from centromeres to midzone microtubules (Figure 8A′), and to migrate into the equatorial plane to form discrete segments as in control cells (Figure 8, B′–C′). However, in contrast to the wild-type aurora B-GFP that became highly organized on the equatorial plane (Figure 1, F′–H′), aurora B(K-R)-GFP gradually dispersed from the spindle midzone (Figure 8, C′–D′). Microinjection of rhodamine-labeled tubulin into these aurora-B(K-R)-GFP–expressing cells indicated that, although midzone microtubules were present, they never became organized as in control cells (Figure 8, E and F; Wheatley et al., 1997). These observations suggest that the kinase activity of aurora B was not required for the initial localization to centromeres and the relocation to midzone microtubules, but was required for the organization of midzone microtubules and the stable localization of aurora B along the equator.
Figure 8.
Dynamics of aurora B(K-R) in a cell that entered anaphase. An NRK cell transiently expressing aurora B(K-R)-GFP was monitored by time-lapse phase (A–D) and fluorescence optics (A′–D′). Time elapsed in minutes after anaphase onset is shown at the lower right corner. Although anaphase onset took place normally, one or more chromosomes failed to segregate (B, arrows). Kinase-inactive aurora B (K-R)-GFP showed initial localization along midzone microtubules (A′), followed by concentration toward the equatorial plane to form segments (B′, arrows). However, the intensity of these segments decreased subsequently, reducing them to small aggregates (C′ and D′, arrows). Cytokinesis was also inhibited (D). Rhodamine-labeled tubulin was microinjected into a cell expressing aurora-B(K-R)-GFP and the images of tubulin (E and F) and aurora-B(K-R) (E′ and F′) were recorded over a period of 8 min. Arrows indicate the positions in the spindle midzone where aurora-B(K-R)-GFP was concentrated. Midzone microtubules remained disorganized as aurora B dissociated from the equatorial region. Bar, 10 μm.
DISCUSSION
Dynamics of Aurora B during Cell Division
Several significant aspects of aurora B distribution in living mammalian cells were noted in the present study. First, aurora B was localized not only at centromeres but also at spindle poles. The weakness of the polar signal might explain why this feature was not detected in immunofluorescence studies. Although the functional significance is unclear, a similar localization at spindle poles was observed with other kinases involved in cytokinesis such as polo (Arnaud et al., 1998). Second, aurora B-GFP remained associated with separated chromosomes for a brief period at anaphase before relocating to interzonal microtubules, suggesting that this event lies downstream of proteolysis involved in chromosomal separation. Third, the pattern of subsequent distribution, from broad localization between chromosomes to tight segments on the equatorial plane, suggests an active transport mechanism that brings aurora B and associated proteins toward the ends of microtubules. The kinesin-like motor that colocalizes with aurora B in the spindle midzone, known as CHO1/MKLP1, PAV-KLP, or ZEN-4 in different organisms, most likely plays a key role in this process (Nislow et al., 1992; Powers et al., 1998; Adams et al., 1998; Raich et al., 1998).
Taking advantage of the ability to observe aurora B in living cells, we have probed several factors that may affect the dynamics of aurora B. Our results indicated that neither microtubules nor the kinase activity of aurora B is required for the initial localization of aurora B at centromeres during prophase and prometaphase. A similar resistance to nocodazole was reported for the centromeric association of human survivin, a chromosome passenger protein with a similar localization behavior (Skoufias et al., 2000). These results argue against an active transport mechanism that delivers aurora B to the centromeres along kinetochore microtubules.
The subsequent relocation of aurora B to interzonal microtubules requires both microtubules and CDK1 deactivation, as indicated by the sensitivity to nocodazole and Δ90 cyclin B. However, this process appears to be independent of the kinase activity of aurora B as indicated with cells expressing aurora B(K-R), a kinase inactive mutant of aurora B. Because CDK1 deactivation causes inhibition of the formation of interzonal microtubules (Wheatley et al., 1997), the failure of aurora B relocation may reflect a consequence of the defect in central spindle organization, although it is also possible that the process involves additional steps regulated directly by CDK1. Treatment with nocodazole further suggested that microtubules were required for maintaining the localization of aurora B in the spindle midzone, similar to the behavior of INCENP that may function as an adapter protein for the association of aurora B with interzonal microtubules (Wheatley et al., 2001).
Functions of Aurora B Kinase in Cell Division
Transient transfection of aurora B(K-R) resulted in chromosome miscongregation and missegregation in NRK cells. The phenotypes are similar to those induced by the inhibition of aurora B expression in Drosophila cells (Adams et al., 2001; Giet and Glover, 2001), indicating that the kinase activity of aurora B is crucial to its functions in mitosis. Interestingly, Mad2 was released from the kinetochores despite the failure in chromosomal congregation, suggesting that the checkpoint was short-circuited upstream from the regulation of Mad2-kinetochore association. Because Mad2 release is controlled by the attachment of microtubules at kinetochores (Waters et al., 1998), aurora B may function both to promote interactions of kinetochores with microtubules and/or motors, and to relay such interactions for the regulation of the spindle checkpoint.
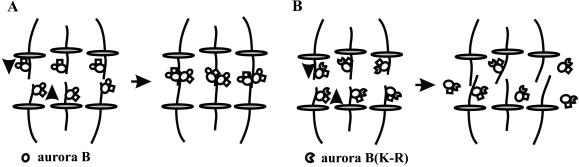
The most intriguing observation in the present study is that kinase-inactive aurora B(K-R)-GFP was able to redistribute from the chromosomes to the spindle midzone, but unable to localize stably along midzone microtubules or to organize into an array of concentrated segments on the equatorial plane. This defect was not simply due to the missegregation of chromosomes, because inhibition of topoisomerase II caused a total inhibition of chromosomal segregation while allowing normal cytokinesis in Caenorhabditis elegans (Severson et al., 2000), or causing only mislocalized cleavage furrows in mammalian cells (Wheatley et al., 1998). Instead, the results suggest that aurora B(K-R), and associated proteins, fell off the microtubules as they reached the ends. We propose that a substrate of aurora B is involved in maintaining stable microtubule associations of chromosomal passenger proteins as they are transported along midzone microtubules. The chromosomal passenger proteins in turn promote the organization of midzone microtubule bundles for the localization of cleavage signals (Figure 9). This model explains why cytokinesis fails after initiation in aurora B-ablated cells, and why the depletion of aurora B inhibits the interzonal localization of INCENP (Adams et al., 2001) and the ZEN-4/PAV-KLP motor (Schumacher et al., 1998; Severson et al., 2000; Adams et al., 2001; Giet and Glover, 2001).
Figure 9.
Model showing the possible effects of aurora B on midzone microtubules. Complexes of the chromosomal passenger proteins move toward ends of midzone microtubules (arrows). In cells expressing wild-type aurora B, complexes of chromosomal passenger proteins maintain their microtubule association and promote the formation of midzone microtubule bundles when they are transported to the ends of microtubules (A). In cells expressing kinase-inactive aurora B, the complex falls off microtubules as they reach the ends, and midzone microtubules remain disorganized (B).
To date the only confirmed substrate of aurora B is histone H3, which is known to promote the chromosomal condensation reaction (Hsu et al., 2000; Adams et al., 2001; Giet and Glover, 2001). However, the kinase activity of aurora B was found to reach its peak just after the inactivation of p34cdc2 (Bischoff et al., 1998), suggesting that the kinase activity is required primarily at prometaphase to anaphase. Therefore, an important task is to identify additional substrates of aurora B that regulate the movement of prometaphase chromosomes and the organization of midzone microtubules.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Ted Salmon (University of North Carolina, Chapel Hill, NC) for providing anti-Mad2 antibodies. This project was supported by National Institutes of Health grant GM-32476 (to Y.-L.W.).
Abbreviations used:
- GFP
green fluorescent protein
- INCENP
inner centromere protein
- NRK
normal rat kidney
Footnotes
Online version of this article contains video material for some figures. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–09–0467. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–09–0467.
REFERENCES
- Adams RR, Carmena M, Earnshaw WC. INCENP binds the aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10:1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetocore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–879. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RR, Tavares AA, Salzberg A, Bellen HJ, Glover DM. Pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 1998;12:1483–1494. doi: 10.1101/gad.12.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen PR, Palmer DK, Wener MH, Margolis RL. Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99:523–534. doi: 10.1242/jcs.99.3.523. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Pines J, Nigg EA. GFP tagging reveals human Polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma. 1998;107:424–429. doi: 10.1007/s004120050326. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadebech P, Truchet I, Brichese L, Valette A. Up-regulation of cdc2 protein during paclitaxel-induced apoptosis. Int J Cancer. 2000;87:779–786. [PubMed] [Google Scholar]
- Chang Y-T, Gray NS, Rosania GR, Sutherlin DP, Kwon S, Norman TC, Sarohia R, Leost M, Meijer L, Schults PG. Synthesis and application of functionally diverse 2, 6, 9-trisubstituted purine libraries as CDK inhibitors. Chem Biol. 1999;6:361–375. doi: 10.1016/S1074-5521(99)80048-9. [DOI] [PubMed] [Google Scholar]
- Chen R-H, Waters JC, Salmon ED, Murry AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- Cooke CA, Heck MMS, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutts MS, Fowler KJ, Kile BT, Hii LLP, O'Dowd RA, Hudson DF, Saffery R, Kalitsis P, Earle E, Choo KHA. Defective chromosome segregation, microtubule bundling and nuclear bridging in inner centromere protein gene (incenp)-disrupted mice. Hum Mol Genet. 1999;8:1145–1155. doi: 10.1093/hmg/8.7.1145. [DOI] [PubMed] [Google Scholar]
- Eckley DM, Ainsztein AM, Mackay AM, Goldberg IG, Earnshaw WC. Chromosomal proteins and cytokinsis: pattern of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J Cell Biol. 1997;136:1169–1183. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco L, Chan CS. Regulation of yeast chromosome segregation by Ipl1 protein kinase and type 1 protein phosphatase. Cell Mol Biol Res. 1994;40:207–213. [PubMed] [Google Scholar]
- Giet R, Glover DM. Drosophila Aurora B kinase is required for histone H3 phosphorylation and condensing recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol. 2001;152:669–681. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R, Prigent C. Aurora/Ipl1p-related protein kinase, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999;112:3591–3601. doi: 10.1242/jcs.112.21.3591. [DOI] [PubMed] [Google Scholar]
- Giet R, Prigent C. The Xenopus lavies aurora/Ipl1p-related kinase pEg2 participates in the stability of the bipolar mitotic spindle. Exp Cell Res. 2000;258:145–151. doi: 10.1006/excr.2000.4903. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirshner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Glover DM, Leibowits MH, Mclean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- Hsu J-Y, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an Aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Ainszrein AM, Eckley DM, Earnshaw WC. A dominant mutant of inner centromere protein (INCENP), a chromosomal passenger protein, disrupts prometaphase congression and cytokinesis. J Cell Biol. 1998;140:991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenna NM, Wang Y-L. Culturing cells on the microscope stage. Methods Cell Biol. 1989;29:195–205. doi: 10.1016/s0091-679x(08)60195-8. [DOI] [PubMed] [Google Scholar]
- Martineau-Thuillier S, Andreassen PA, Margolis RL. Colocalization of TD-60 and INCENP throughout G2 and mitosis: evidence for their possible interaction in signaling cytokinesis. Chromosoma. 1998;107:461–470. doi: 10.1007/s004120050330. [DOI] [PubMed] [Google Scholar]
- Murata-Hori M, Fumoto K, Fukuta Y, Kikuchi A, Tatsuka M, Hosoya H. Myosin II regulatory light chain as a novel substrate for AIM-1, an aurora/Ipl1p-related kinase from rat. J Biochem. 2000;128:903–907. doi: 10.1093/oxfordjournals.jbchem.a022840. [DOI] [PubMed] [Google Scholar]
- Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzymes that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Powers J, Bossinger O, Rose D, Strome S, Saxon W. A nematode kinesin required for cleavage furrow advancement. Curr Biol. 1998;8:1133–1136. doi: 10.1016/s0960-9822(98)70470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol Biol Cell. 1998;9:2037–2049. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghi C, Giet R, Uzbekov R, Morin N, Chartrain I, Le Guellec R, Anne Couturier A, Doree M, Philippe M, Prigent C. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J Cell Sci. 1998;111:557–572. doi: 10.1242/jcs.111.5.557. [DOI] [PubMed] [Google Scholar]
- Schumacher JM, Golden A, Donovan PJ. AIR-2: an Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J Cell Biol. 1998;143:1635–1646. doi: 10.1083/jcb.143.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson AF, Hamill DR, Carter JC, Schumacher J, Bowerman B. The aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr Biol. 2000;10:1162–1171. doi: 10.1016/s0960-9822(00)00715-6. [DOI] [PubMed] [Google Scholar]
- Skoufias DA, Mollinari C, Lacroix FB, Margolis R. Human survivin is a kinetochore-associated passenger protein. J Cell Biol. 2000;151:1575–1581. doi: 10.1083/jcb.151.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–4816. [PubMed] [Google Scholar]
- Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo AKH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- Wang Y-L. Fluorescence microscopic analysis of cytoskeletal organization and dynamics. In: Carraway KL, Carraway CAC, editors. The Cytoskeleton: A Practical Approach. Oxford, United Kingdom: IRL Press; 1992. pp. 1–22. [Google Scholar]
- Waters JC, Chen R-H, Murray AW, Salmon ED. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Hinchcliffe EH, Glotzer M, Hyman AA, Sluder G, Wang Y-L. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Kandels-Lewis SE, Adams RR, Ainsztein AM, Earnshow WC. Incenp binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow. Exp Cell Res. 2001;262:122–127. doi: 10.1006/excr.2000.5088. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, O'Connell CB, Wang Y-L. Inhibition of chromosomal separation provides insights into cleavage furrow stimulation in cultured epithelial cells. Mol Biol Cell. 1998;9:2173–2184. doi: 10.1091/mbc.9.8.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Wang Y-L. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol, 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.