Abstract
Purpose
Essential metals may be crucial in obesity and type 2 diabetes (T2DM); diabesity pathogenesis and consequences. This study aimed to determine the metal levels in obese and non-obese patients with and without T2DM and their relationships with fetuin-A(Fet-A) levels, insulin sensitivity, and insulin resistance.
Methods
A total of 314 participants were enrolled, with 160 newly diagnosed T2DM patients and 154 non-T2DM subjects categorized into diabetic obese (n = 57), diabetic non-obese (n = 103), non-diabetic obese (n = 48), and non-diabetic non-obese (n = 106) subgroups. Fet-A, insulin sensitivity (QUCKI)/resistance (HOMA-IR), fasting glucose, and body mass index (BMI) were assessed. The essential metals were measured using inductively coupled plasma mass spectroscopy (ICP-MS).
Results
Fet-A levels were 3-fold higher (1391.4 ± 839.8 ng/ml) in T2DM patients than in non-T2DM (2165.6 ± 651.9 vs. 424.3 ± 219.1 ng/ml, p < 0.0001). Fet-A levels were 2.3-fold higher in the diabetic obese group than in the diabetic non-obese group (p < 0.0001). Fet-A levels were 2.0-fold higher in the diabetic non-obese group than in the non-diabetic obese group (p < 0.0001). Fet-A levels were positively correlated with insulin resistance (HOMA-IR) (r = 0.34, p < 0.0001) and negatively correlated with insulin sensitivity (QUIKI) (r = −0.41, p < 0.0001).
Cu, Se, Zn, and Fe levels were significantly lower in diabetic patients than in non-diabetic patients (p < 0.05). Se and Zn were significantly correlated with Fet-A (r = −0.41, p = 0.049 and r = −0.42, p = 0.001, respectively). Se and Zn were also correlated with insulin resistance (HOMA-IR) (r = −0.45, p = 0.049 and r = −0.36, p = 0.012, respectively) and insulin sensitivity (QUIKI) (r = 0.49, p = 0.042 and r = 0.30, p = 0.003, respectively). Similarly, Fe was negatively correlated with insulin levels (r = −0.33, p = 0.04) and insulin sensitivity (r = −0.34, p = 0.30). However, Mn was significantly correlated with Fet-A (r = 0.37, p = 0.001) and insulin resistance/sensitivity (r = 0.24, p = 0.026 and r = −0.24, p = 0.041) respectively in the diabetic obese group. Mg was an independent predictor of diabesity.
Conclusions
Mg play a significant role in obesity-related T2DM pathogenesis and complications via Fet-A, insulin sensitivity, and resistance modifications.
Keywords: Fetuin-A, Insulin resistance, Obesity, Obesity-related type 2 diabetes mellitus, Non-obese diabetes
Introduction
Obesity and Type-2 diabetes mellitus (T2DM) are lifestyle disorders that are affected by ethnicity and geographical diversity. Hyperglycemia, insulin resistance/sensitivity, and relative insulin insufficiency characterize T2DM [1, 2]. Obesity is a major epidemic in modern society and a link between obesity and diabetes (diabesity) is emerging. Data from the registry revealed that diabetes medication was less effective when body mass index was increased. Furthermore, altered adipokine secretion has been suggested to represent a basic link between obesity and T2DM [3].
Fet-A, an endogenous inhibitor of insulin receptor tyrosine kinase in the liver and muscles, effectively prevents obesity [4]. In humans, elevated Fet-A levels are an independent risk factor for T2DM [5] and are strongly related to insulin resistance and fat storage [6]. Body mass index (BMI) and waist-to-hip ratio (WHR) were significantly associated with Fet-A levels and increased in insulin-resistant obesity compared to insulin-sensitive obesity [7]. Moreover, relevant mediators such as fasting glucose, HbA1c, and C-peptide levels increase and low insulin levels are associated with the pathogenesis of T2DM in individuals with higher Fet-A levels [8]. Various essential metals have been implicated in the etiology of T2DM and their potential roles have been discussed for decades [9]. Essential metals may affect the onset and pathophysiology of diabetes in various ways. For example, metal imbalances may disrupt normal glucose and insulin metabolism, leading to insulin resistance/sensitivity and the development of diabetic complications [10, 11]. Previous studies on the possible functions of essential metals in diabetes have primarily focused on zinc and iron.
Zn is an integral component of insulin, and is involved in its production, storage, and secretion. Many enzymes involved in glucose metabolism require Zn as a cofactor [12, 13]. Similarly, Fe has been postulated to affect the development of diabetes through numerous pathways, including the generation of insulin insufficiency and resistance as well as the cause of hepatic dysfunction [14]. Most studies have concentrated on the relationship between a single essential element and T2DM, and have been confined to those who already have the disease. It is unclear whether these changes in essential element status occur before disease onset or as a result of disease. As a result, we intended to explore essential metal levels at the time of T2DM screening to probe variations in essential element levels in the early stages of the disease while controlling for possible confounders that were frequently overlooked in previous studies [15–17]. However, epidemiological data on the risk of metabolic dysfunction related to elementary metal levels in the North Indian population are scarce. Therefore, further studies are required to evaluate the reported associations and examine recent insights. Based on the above background context, the present study aimed to measure the levels of essential metals, including copper (Cu), magnesium (Mg), manganese (Mn), iron (Fe), selenium (Se), and zinc (Zn), as well as their relationships with Fet-A levels, insulin sensitivity, and insulin resistance among T2DM patients with and without obesity.
Material and methods
Selection of subjects and sample collection
This case-control study was conducted at the Department of Biochemistry, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India. Patients newly diagnosed with T2DM with or without obesity based on the American Diabetes Association (ADA) and World Health Organization (WHO) criteria (2019) [18] and non-diabetic (control) subjects were recruited for the study.
Subjects with diabetes combined with acute complications such as ketoacidosis and hyperosmolar coma, acute or chronic inflammation, severe hepatic and renal dysfunction, malignant tumors, and other endocrine and/or metabolic disorders were excluded from the study.
Anthropometric measurements
Age, sex, body weight, height, waist circumference, and hip circumference (WC) were recorded. Body mass index (BMI) was calculated as the weight (kg) divided by the square of height (m). Obesity was defined as BMI ≥ 30 kg/m2 based on the characteristics of the Asian population (ADA criteria 2019). WC was measured at the midpoint between the lower borders of the rib cage and iliac crest. WHR was calculated as waist circumference divided by hip circumference (WHO criteria 1997). Individuals with BMI ≥ 30 kg/m2 were considered obese, while those with BMI < 25 kg/m2 and WHR more than 0.90 for men and 0.85 for females were considered obese [19].
Experimental group
A total of 314 subjects were enrolled in the study, of whom 160 were naïve-diagnosed T2DM patients and 154 were non-T2DM subjects. According to BMI and fasting blood sugar (FBS) levels, the study participants were subdivided into
Group-1: Diabetic Obese (DO; n = 57)
Group-2: Diabetic Non-Obese (DNO; n = 103)
Group-3: Nondiabetic Obese (NDO; n = 48)
Group-4: Non-Diabetic Non-Obese (NDNO; n = 106)
Sampling
Venous blood samples from all study subjects were collected in the morning (after 12 h of fasting) in a fluoride, ethylenediaminetetraacetic acid (EDTA), and plain vacutainer. Serum samples were collected and stored at -80 °C until use. Plasma samples from fluoride vials were analyzed for fasting plasma glucose (FPG) levels, and serum samples from plain vials were analyzed for lipid and metal profiles.
Estimation of biochemical parameters
Fasting blood sugar (FBS) and lipid profiles were analyzed using commercial reagents on an automated analyzer (Beckman Coulter), and HbA1c was analyzed from samples collected in an EDTA vial on a BIORAD (Variant Turbo). Serum insulin concentrations were measured using electrochemiluminescence (ECL).
Insulin resistance (HOMA-IR) and insulin sensitivity (QUICKI) calculations
Insulin resistance was calculated by modified homeostasis model assessment of insulin resistance [20]:
The Quantitative Insulin Sensitivity Check Index (QUICKI) was calculated as follows [21]:
Estimation of fetuin-A levels
Fet-A levels were measured using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) method (Quantikine, R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s protocol. Briefly, serum samples were diluted to 1:10 in dilution buffer. The test was performed within 5 h. Absorbance was measured at a wavelength of 450 nm. The average detectable Fet-A level was 303–671 μg/ml. The intra-assay variability was 7.2%, and the inter-assay variability was 6.4%.
Estimation of essential metals
Essential metals (Cu, Mg, Mn, Fe, Se, Cr, and Zn) were measured in serum samples using inductively coupled plasma mass spectrometry (ICP-MS) (Perkin Elmer). Before analysis, 100 μL of serum samples were mixed with 100 μL of internal standard (IS), then made up to 5 mL by adding diluent; v/v 15 mL methanol +0.005% v/v Triton X solution +10 mL nitric acid, making it up to 1000 mL by adding double-distilled water. The metals were then analyzed by comparison with the standard using a linearity curve.
Statistical analysis
Quantitative variables are presented as Mean ± SD. Student’s t test was used to compare continuous data, while categorical data were analyzed using the χ 2 test to assess the difference in biochemical parameters between the two groups. One-way analysis of variance (ANOVA) was used to compare anthropometric and biochemical parameters between the diabetic and obese groups. Pearson’s correlation coefficients were calculated to determine the correlation between fetuin-A levels and FBG, HbA1c, lipid profile, insulin resistance (HOMA-IR), insulin sensitivity (QUICKI), metals, and C-peptide levels. Univariate and multivariate linear regression analyses were used to evaluate the independent serum essential metal predictors in the different diabetes groups. All analyses were conducted using SPSS 16.0 (Chicago, Inc. USA). Statistical significance was set at p < 0.05.
Ethics
This study was approved by the Ethics Committee (Ref. code: IEC-24/18) of the Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India. Written informed consent was obtained from all participants prior to enrollment. A structured pro forma was used to collect anthropometric and personal information.
Result
Anthropometric, biochemicals and elements levels in T2DM and non-T2DM
There were no significant differences in age and sex between patients (T2DM) and controls (non-T2DM), indicating adequate matching (p = 0.22 and p = 0.86, respectively). BMI and WHR were significantly higher in T2DM patients than in non-T2DM patients (P = 0.006 and P = 0.011, respectively). The T2DM group showed significantly higher levels of diabetes-associated markers such as FBS, HbA1c, insulin, insulin resistance (HOMA-IR), insulin sensitivity (QUICKI), and C-peptide (p < 0.05). The lipid profile (TC, TG, LDL-C, and VLDL-C) levels were significantly higher in the diabetic group than in the control group. Fet-A levels were 3-fold higher (1391.4 ± 839.8 ng/ml) in T2DM patients than in non-T2DM (444.9 ± 203.3 ng/ml). Cu, Se, Zn, and Fe levels were significantly lower in diabetic patients than in non-diabetic patients (p < 0.05) (Table 1).
Table 1.
Clinical characteristics, biochemical, and Essential metals of the Study Population
| Variables | T2DM (Patients) n = 160 (%) Mean ± SD | Non-T2DM (Controls) n = 154(%) Mean ± SD | p Value |
|---|---|---|---|
| Anthropometric Markers | |||
| Gender | |||
| Male N% | 71(44.37) | 58(37.66) | 0.227 |
| Female N% | 89(55.63) | 96(62.34) | |
| Age (Years) | |||
| Younger (<45 Years) | 93(58.13) | 91(59.09) | 0.862 |
| Older (≥45 Years) | 67(41.87) | 63(40.91) | |
| Mean ± SD | 52.68 ± 7.85 | 49.76 ± 17.56 | 0.056 |
| BMI (kg/m2) | 29.97 ± 5.73 | 28.23 ± 5.50 | 0.006* |
| WHR (cm) | 0.95 ± 0.06 | 0.94 ± 0.05 | 0.011* |
| TC (mg/dl) | 181.1 ± 45.42 | 163.0 ± 40.76 | <0.0001* |
| TG (mg/dl) | 174.7 ± 92.60 | 142.7 ± 68.73 | <0.0001* |
| HDL-C (mg/dl) | 43.98 ± 14.93 | 42.39 ± 9.94 | 0.284 |
| LDL-C (mg/dl) | 114.7 ± 40.30 | 100.5 ± 34.32 | <0.0001* |
| VLDL-C (mg/dl) | 34.98 ± 18.50 | 28.65 ± 13.73 | <0.0001* |
| Diabetic Markers | |||
| FBS (mg/dl) | 165.7 ± 65.56 | 99.7 ± 8.93 | <0.0001* |
| HbA1c (%) | 7.66 ± 2.31 | 5.22 ± 0.41 | <0.0001 |
| Insulin (μIU/ml) | 16.87 ± 13.14 | 11.97 ± 9.40 | <0.0001* |
| HOMA-IR | 7.04 ± 7.35 | 2.94 ± 2.23 | <0.0001* |
| QUICKI | 0.30 ± 0.02 | 0.34 ± 0.03 | <0.0001* |
| C-Peptide (ng/ml) | 2.88 ± 1.49 | 1.49 ± 1.10 | <0.0001* |
| Fetuin-A (ng/ml) | 1391.4 ± 839.8 | 444.9 ± 203.3 | <0.0001* |
| Mg 2+ (mg/dl) | 2.36 ± 0.43 | 2.41 ± 0.56 | 0.479 |
| Cu2+ (μg/dL) | 103.51 ± 43.90 | 115.30 ± 37.50 | 0.011* |
| Se2+ (μg/L) | 136.90 ± 33.39 | 157.07 ± 57.72 | 0.002* |
| Zn2+ (μg/dL) | 73.23 ± 23.28 | 92.04 ± 31.52 | <0.0001* |
| Cr2+ (μg/L) | 18.51 ± 12.76 | 28.82 ± 9.68 | 0.460 |
| Mn2+ (μg/L) | 2.58 ± 2.25 | 2.44 ± 2.05 | 0.166 |
| Fe2+ (μg/dl) | 138.24 ± 74.10 | 153.44 ± 108.30 | 0.049* |
Abbreviations: T2DM Type-2 Diabetes mellitus, BMI body mass index, WHR Waist-to-Hip Ratio, HOMA-IR Homeostasis model assessment-estimated insulin resistance, QUICKI Quantitative insulin sensitivity check index, FBS Fasting Blood Sugar, TC Total Cholesterol, TG Triglyceride, HDL High-density Lipoprotein, LDL Low-density Lipoprotein, VLDL Very low-density Lipoprotein. Student T-test was used to compare groups. * p value <0.05 is considered statistically significant
Fet-A and essential elements levels associated with diabesity
Among the subjects, 57 (18.16%) were obese-diabetic, 103 (32.80%) were non-obese-diabetic, while in the non-diabetic group, 48 (15.29%) were non-diabetic obese, and 106 (33.75%) were non-diabetic non-obese (Table 2).
Table 2.
Comparison of biochemical variables and essential metals within T2DM Obese, T2DM Non-Obese, Non-T2DM Obese, Non-Diabetic Obese, and Non-Diabetic Non-Obese
| Variables | Sub-groups | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| (A)DO (n = 57) | (B)DNO (n = 103) | (C)NDO (n = 48) | (D)NDNO (n = 106) | A vs. C | A vs D | A vs. B | C vs. D | |
| BMI (kg/m2) | 33.6 ± 3.5 | 24.7 ± 3.9 | 33.5 ± 2.8 | 24.3 ± 3.7 | 0.873 | <0.0001* | <0.0001* | <0.0001* |
| WHR (cm) | 0.96 ± 0.06 | 0.80 ± 0.03 | 0.95 ± 0.05 | 0.84 ± 0.02 | 0.361 | <0.0001* | <0.0001* | <0.0001* |
| TC (mg/dl) | 181.5 ± 43.6 | 180.9 ± 46.7 | 177.0 ± 39.3 | 156.5 ± 39.9 | 0.582 | 0.0003* | 0.936 | 0.003* |
| TG (mg/dl) | 181.6 ± 97.6 | 170.9 ± 89.8 | 158.4 ± 74.9 | 135.4 ± 64.7 | 0.183 | 0.0004* | 0.489 | 0.053 |
| HDL-C (mg/dl) | 45.4 ± 15.0 | 43.2 ± 14.9 | 42.5 ± 9.6 | 42.3 ± 10.1 | 0.250 | 0.118 | 0.373 | 0.908 |
| LDL-C (mg/dl) | 110.4 ± 36.3 | 117.1 ± 42.5 | 110.0 ± 32.4 | 96.1 ± 34.4 | 0.953 | 0.014* | 0.316 | 0.019* |
| VLDL-C (mg/dl) | 36.2 ± 19.6 | 34.3 ± 17.9 | 32.0 ± 14.8 | 27.1 ± 12.9 | 0.225 | 0.0005* | 0.535 | 0.038* |
| FBS (mg/dl) | 150.3 ± 43.8 | 174.7 ± 74.1 | 101.0 ± 8.1 | 98.9 ± 9.2 | <0.0001* | <0.0001* | 0.024* | 0.175 |
| HbA1c (%) | 6.9 ± 1.5 | 8.1 ± 2.6 | 5.3 ± 0.5 | 5.2 ± 0.4 | <0.0001* | <0.0001* | 0.001* | 0.159 |
| Insulin (μIU/ml) | 19.1 ± 13.7 | 15.6 ± 12.7 | 17.1 ± 12.5 | 9.6 ± 6.3 | 0.446 | <0.0001* | 0.109 | <0.0001* |
| C-Peptide (ng/ml) | 3.0 ± 1.3 | 2.5 ± 1.4 | 2.6 ± 1.2 | 2.3 ± 1.5 | 0.083 | 0.003* | 0.028* | 0.224 |
| Fetuin-A (ng/mL) | 2165.6 ± 651.9 | 941 ± 564.3 | 489.4 ± 157.1 | 424.3 ± 219.1 | <0.0001* | <0.0001* | <0.0001* | 0.065 |
| Mg2+ (mg/dL) | 2.4 ± 0.3 | 2.4 ± 0.5 | 2.7 ± 0.7 | 2.2 ± 0.3 | 0.004* | 0.0001* | 1.00 | <0.0001* |
| Cu2+ (μg/dL) | 99.24 ± 3.39 | 106.1 ± 47.5 | 103.6 ± 23.2 | 119.7 ± 40.3 | 0.163 | 0.0002* | 0.278 | 0.010* |
| Se2+ (μg/L) | 133.7 ± 36.7 | 137.81 ± 33.1 | 136.9 ± 40.9 | 150.9 ± 49.7 | 0.673 | 0.022* | 0.470 | 0.089 |
| Zn2+ (μg/dL) | 69.1 ± 18.2 | 75.73 ± 21.1 | 78.4 ± 20.4 | 83.4 ± 21.8 | 0.015* | <0.0001* | 0.047* | 0.180 |
| Cr2+ (μg/L) | 20.26 ± 13.45 | 17.50 ± 12.35 | 11.36 ± 6.64 | 34.29 ± 10.82 | 0.001* | <0.0001* | 0.191 | <0.001* |
| Mn2+ (μg/L) | 2.55 ± 1.92 | 2.61 ± 2.44 | 2.99 ± 2.10 | 2.24 ± 2.01 | 0.265 | 0.341 | 0.192 | 0.036* |
| Fe2+ (μg/dL) | 133.77 ± 89.58 | 140.93 ± 63.51 | 155.42 ± 110.36 | 146.38 ± 94.11 | 0.269 | 0.408 | 0.206 | 0.602 |
Abbreviations: DO Diabetic Obese, DNO Diabetic Non-Obese, DNO Diabetic Non-Obese, NDNO Non-Diabetic Non-Obese, BMI body mass index, WHR Waist-to-Hip Ratio, HOMA-IR Homeostasis model assessment-estimated insulin resistance, QUICKI Quantitative insulin sensitivity check index, FBS Fasting Blood Sugar, TC Total Cholesterol, TG Triglyceride, HDL High-density Lipoprotein, LDL Low-density Lipoprotein, VLDL Very low-density Lipoprotein. Student t-test was used to compare groups. The data has been represented in mean ± SD. * p value <0.05 is considered statistically significant
Fet-A levels were 2.3-fold higher in the diabetic obese group than in the non-diabetic obese group (p < 0.0001). Furthermore, Fet-A levels were 2.0-fold higher in the diabetic non-obese group than in the non-diabetic obese group (P < 0.0001). In contrast, the obese-non-diabetic and control groups (without diabetes or obesity) showed no significant difference (p = 0.065) in Fet-A levels (Table 2).
Zn was significantly decreased in the diabetic obese group compared to the non-diabetic non-obese group (69.1 ± 18.2 vs. 83.4 ± 21.8, p < 0.0001). The non-diabetic obese group Zn was significantly decreased compared to the non-diabetic non-obese (control) group (78.4 ± 20.4, vs. 83.4 ± 21.8, p = 0.015). Mg was significantly increased in the diabetic obese group as compared to the non-diabetic obese group (2.4 ± 0.3 vs. 2.2 ± 0.3, p < 0.0001).
In contrast, Cr was significantly increased in diabetic obese patients than in non-obese and non-diabetic patients (20.26 ± 13.45 vs.11.36 ± 6.64, p < 0.0001). Cu and Se were also significantly reduced in the diabetic obese group as compared to the non-diabetic non-obese group (Cu; 99.24 ± 3.39 vs. 119.7 ± 40.3, p = 0.0002; Se;133.7 ± 36.7 vs. 150.9 ± 49.7, p = 0.022).
Essential elements serum level correlated with obesity and diabetic-associated markers
Fet-A levels were negatively correlated with Cu, Se, Zn, and Mg (r = −0.31, p = 0.031; r = −0.41, p = 0.049; r = −0.22, p = 0.001; r = −0.42, p = 0.046, respectively), while HbA1c levels were negatively correlated with Cu and Zn (r = −0.30, p = 0.038; r = −0.32, p = 0.001, respectively). A significant positive correlation was found between QUICKI and Zn levels (r = 0.30, p = 0.003). Cu2+ also showed a negative correlation with insulin and C-peptide levels (r = −0.21, p = 0.04 for both) (Table 3).
Table 3.
Correlation of diabesity markers with essential metals and fetuin-A
| Variables | Fetuin-A (ng/mL) | Insulin (μIU/ml) | C-peptide (ng/dL) | HOMA-IR | QUICKI | HbA1c (%) | FBS (mg/dL) | BMI (kg/m2) | WHR (cm) |
|---|---|---|---|---|---|---|---|---|---|
| Cu2+ (μg/dL) | r = −0.31 | r = −0.21 | r = −0.21 | r = −0.11 | r = 0.12 | r = −0.30 | r = −0.05 | r = −0.20 | r = −0.10 |
| p = 0.031* | p = 0.040* | p = 0.045* | p = 0.127 | p = 0.136 | p = 0.038* | p = 0.478 | p = 0.047* | p = 0.160 | |
| Se2+ (μg/L) | r = −0.41 | r = −0.12 | r = −0.42 | r = −0.35 | r = 0.49 | r = −0.11 | r = −0.80 | r = −0.45 | r = −0.11 |
| p = 0.049* | p = 0.113 | p = 0.513 | p = 0.049* | p = 0.042* | p = 0.021 | p = 0.203 | p = 0.042* | p = 0.044 | |
| Zn2+ (μg/dL) | r = −0.42 | r = −0.14 | r = −0.13 | r = −0.36 | r = 0.30 | r = −0.32 | r = −0.13 | r = −0.16 | r = −0.17 |
| p = 0.001* | p = 0.033 | p = 0.047 | p = 0.012* | p = 0.003* | p = 0.001* | p = 0.038 | p = 0.011 | p = 0.043 | |
| Cr2+ (μg/L) | r = 0.10 | r = 0.03 | r = −0.02 | r = 0.04 | r = −0.05 | r = 0.05 | r = −0.01 | r = −0.03 | r = −0.02 |
| p = 0.425 | p = 0.653 | p = 0.734 | p = 0.475 | p = 0.447 | p = 0.393 | p = 0.879 | p = 0.685 | p = 0.753 | |
| Mn2+ (μg/L) | r = 0.06 | r = 0.01 | r = −0.01 | r = 0.01 | r = −0.01 | r = 0.01 | r = −0.11 | r = 0.16 | r = 0.11 |
| p = 0.314 | p = 0.943 | p = 0.864 | p = 0.813 | p = 0.942 | p = 0.924 | p = 0.074 | p = 0.040 | p = 0.074 | |
| Fe2+ (μg/dL) | r = −0.09 | r = −0.33 | r = −0.11 | r = 0.10 | r = −0.34 | r = −0.03 | r = −0.12 | r = −0.03 | r = −0.01 |
| p = 0.129 | p = 0.040* | p = 0.081 | p = 0.106 | p = 0.030* | p = 0.589 | p = 0.073 | p = 0.605 | p = 0.821 | |
| Mg2+ (mg/dL) | r = −0.42 | r = −0.15 | r = −0.02 | r = −0.06 | r = −0.06 | r = −0.12 | r = −0.12 | r = 0.16 | r = 0.11 |
| p = 0.046* | p = 0.820 | p = 0.820 | p = 0.371 | p = 0.373 | p = 0.560 | p = 0.042* | p = 0.010 | p = 0.074 | |
| Fetuin-A (ng/mL) | 1 | 0.23 | 0.27 | 0.33 | −0.40 | 0.40 | 0.38 | 0.33 | 0.19 |
| <0.0001* | <0.0001* | <0.0001* | <0.0001* | <0.0001* | <0.0001* | <0.0001* | <0.0001* |
Abbreviations: r Pearson correlation, BMI body mass index, WHR Waist-to-Hip Ratio, FBS Fasting Blood Sugar, HbA1c Glycosylated Hemoglobin, HOMA-IR Homeostasis model assessment-estimated insulin resistance, QUICKI Quantitative insulin sensitivity check index, r Correlation Coefficient. Pearson correlation was used to analyze correlation. Boldfaced *p value <0.05 is considered statistically significant
Fet-A serum levels correlated with essential elements in study subgroups
A significant positive correlation between Fet-A, HOMA-IR, and Cu in the diabetic obese group (r = 0.30, p = 0.021; r = 0.38, p = 0.010) and a negative correlation between QUICKI and Cu (r = −0.33, p = 0.026) was observed in the diabetic obese group. Fet-A and HOMA-IR were positively correlated with Mg (r = 0.37; p = 0.001; r = 0.25, p = 0.026) and a weak negative correlation was observed between QUICKI and Mn(r = −0.24, p = 0.041) in the diabetic obese group. However, in the non-diabetic group, Fet-A was positively correlated with Mg (r = 0.461, p = 0.004). In contrast, no correlation was found between Fet-A and the other essential metals (Table 4).
Table 4.
Correlation of fetuin-A with essential metals in study subgroups
| Biomarkers | Sub-Groups | Fetuin-A (ng/mL) | HOMA-IR | QUICKI | |||
|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | ||
| Cu2+ (μg/dL) | Diabetic Obese (DO) | 0.306 | 0.021* | 0.385 | 0.010* | −0.335 | 0.026* |
| Diabetic Non-Obese (DNO) | −0.103 | 0.384 | −0.122 | 0.304 | 0.035 | 0.768 | |
| Non-Diabetic. Obese (NDO) | −0.114 | 0.500 | −0.231 | 0.169 | 0.273 | 0.102 | |
| Mn2+ (μg/L) | Diabetic Obese (DO) | 0.133 | 0.349 | −0.191 | 0.175 | 0.118 | 0.404 |
| Diabetic Non-Obese (DNO) | −0.100 | 0.365 | −0.095 | 0.392 | 0.002 | 0.987 | |
| Non-Diabetic Obese (NDO) | −0.262 | 0.082 | −0.113 | 0.084 | 0.084 | 0.584 | |
| Mg2+ (mg/dL) | Diabetic Obese (DO) | 0.375 | 0.001* | 0.247 | 0.026* | −0.240 | 0.041* |
| Diabetic Non-Obese (DNO) | 0.461 | 0.004* | 0.170 | 0.271 | −0.191 | 0.213 | |
| Non-Diabetic Obese (NDO) | −0.248 | 0.052 | 0.045 | 0.789 | −0.068 | 0.688 | |
| Fe2+ (μg/dl) | Diabetic Obese (DO) | −0.119 | 0.440 | −0.186 | 0.228 | 0.127 | 0.411 |
| Diabetic Non-Obese (DNO) | −0.045 | 0.706 | −0.156 | 0.188 | 0.155 | 0.189 | |
| Non-Diabetic Obese (NDO) | −0.168 | 0.321 | −0.186 | 0.270 | 0.254 | 0.129 | |
| Se2+ (μg/L) | Diabetic Obese (DO) | −0.054 | 0.728 | −0.162 | 0.294 | 0.203 | 0.185 |
| Diabetic Non-Obese (DNO) | 0.091 | 0.445 | −0.121 | 0.307 | 0.001 | 0.992 | |
| Non-Diabetic Obese (NDO) | −0.116 | 0.495 | −0.226 | 0.179 | 0.133 | 0.434 | |
| Cr2+ (μg/L) | Diabetic Obese (DO) | 0.062 | 0.688 | 0.236 | 0.123 | −0.191 | 0.214 |
| Diabetic Non-Obese (DNO) | 0.068 | 0.565 | −0.152 | 0.199 | 0.200 | 0.090 | |
| Non-Diabetic Obese (NDO) | 0.103 | 0.543 | −0.024 | 0.888 | −0.061 | 0.720 | |
| Zn2+ (μg/dL) | Diabetic Obese (DO) | −0.004 | 0.979 | −0.152 | 0.326 | 0.185 | 0.229 |
| Diabetic Non-Obese (DNO) | −0.067 | 0.571 | −0.121 | 0.310 | 0.015 | 0.900 | |
| Non-Diabetic Obese (NDO) | −0.299 | 0.072 | −0.188 | 0.264 | 0.123 | 0.469 | |
Abbreviations: r Pearson correlation coefficient. Pearson correlation was analyzed for correlation. *p value <0.05 is considered statistically significant
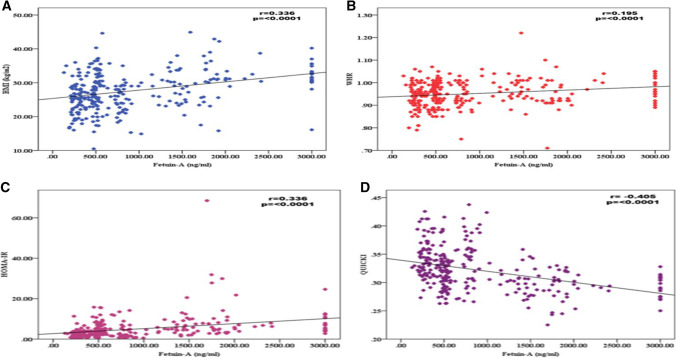
A significant positive correlation between Fet-A and BMI (r = 0.34, p < 0.0001) and a weak positive correlation between Fet-A and WHR (r = 0.2, p < 0.0001) was observed (Fig. 1A and B). Fet-A was significantly negatively correlated with insulin resistance (HOMA-IR) (r = 0.34, p < 0.0001) and positively correlated with insulin sensitivity (QUCKI) (r = −0.41, p < 0.001) (Fig. 1C and D).
Fig. 1.
Pearson Correlation between A. Fetuin-A and BMI, B. Fetuin-A and WHR, C. Between Fetuin-A and HOMA-IR, D. Between Fetuin-A and QUICKI in Diabetic Obese groups
Analysis using univariate and multivariate linear regression with Fet-A as the dependent variable
In univariate linear regression analysis, Cu showed a positive association with Fet-A (β = 0.266, p < 0.0001) in the diabetic obese group. Mg was negatively associated with diabetes (β = −262, p < 0.0001). Similarly, Mn was negatively associated with Fet-A in both diabetic obese groups (β = −0.248, p < 0.0001) and the non-diabetic obese group (β = −0.461, p < 0.0001) and was positively associated with diabetic non-obese (β = 0.375, p < 0.0001). Zn was negatively associated with Fet-A (β = −0.299, p < 0.0001) in the non-diabetic obese group.
In the Multivariate linear regression analysis, only Mn showed a positive association with Fet-A in the diabetic obese (β = 0.507, p < 0.0001) group. In contrast, a negative association was observed with Fet-A levels in the non-diabetic group (β = −0.521, p < 0.0001). The other essential metals were not associated with Fet-A (Table 5).
Table 5.
Univariate and multivariate linear regression model as fetuin-A dependent variables
| Biomarkers | Sub-Groups | Univariate Beta coefficient | p value | Multivariate Beta coefficient | p value |
|---|---|---|---|---|---|
| Cu2+ (μg/dL) | Diabetic Obese (DO) | 0.266 | <0.0001* | 0.315 | 0.103 |
| Diabetic Non-Obese (DNO) | −0.103 | <0.0001* | −0.144 | 0.272 | |
| Non-Diabetic Obese (NDO) | −0.114 | <0.0001* | 0.045 | 0.825 | |
| Mn2+ (μg/L) | Diabetic Obese (DO) | 0.133 | 0.059 | – | – |
| Diabetic Non-Obese (DNO) | −0.100 | <0.0001* | −0.078 | 0.505 | |
| Non-Diabetic Obese (NDO) | −0.262 | <0.0001* | −0.206 | 0.217 | |
| Mg2+ (mg/dL) | Diabetic Obese (DO) | −0.248 | <0.0001* | 0.507 | <0.0001* |
| Diabetic Non-Obese (DNO) | 0.375 | <0.0001* | −0.521 | 0.003* | |
| Non-Diabetic Obese (NDO) | −0.461 | <0.0001* | −0.212 | 0.281 | |
| Fe2+ (μg/dl) | Diabetic Obese (DO) | −0.119 | <0.0001* | 0.025 | 0.906 |
| Diabetic Non-Obese (DNO) | −0.045 | <0.0001* | −0.066 | 0.575 | |
| Non-Diabetic Obese (NDO) | −0.168 | <0.0001* | 0.139 | 0.484 | |
| Se2+ (μg/L) | Diabetic Obese (DO) | −0.054 | <0.0001* | −0.166 | 0.416 |
| Diabetic Non-Obese (DNO) | 0.091 | <0.0001* | 0.209 | 0.159 | |
| Non-Diabetic Obese (NDO) | −0.116 | <0.0001* | 0.261 | 0.237 | |
| Cr2+ (μg/L) | Diabetic Obese (DO) | 0.062 | <0.0001* | 0.109 | 0.557 |
| Diabetic Non-Obese (DNO) | −0.068 | <0.0001* | −0.075 | 0.563 | |
| Non-Diabetic Obese (NDO) | 0.103 | <0.0001* | 0.080 | 0.613 | |
| Zn2+ (μg/dL) | Diabetic Obese (DO) | −0.004 | <0.0001* | −0.038 | 0.865 |
| Diabetic Non-Obese (DNO) | −0.067 | <0.0001* | −0.229 | 0.127 | |
| Non-Diabetic Obese (NDO) | −0.299 | <0.0001* | −0.452 | 0.073 | |
| Insulin (μIU/ml) | Diabetic Obese (DO) | 0.034 | <0.0001* | −0.093 | 0.627 |
| Diabetic Non-Obese (DNO) | 0.078 | <0.0001* | 0.184 | 0.118 | |
| Non-Diabetic Obese (NDO) | 0.093 | <0.0001* | 0.162 | 0.338 |
Abbreviations: DO Diabetic Obese, DNO Diabetic Non-Obese, DNO Diabetic Non-Obese. Univariate and multivariate regression analysis was done to predict the independent marker. *p value <0.05 is considered statistically significant
Discussion
India’s culture, language and cuisine are diverse. Indian cuisine combines the tastes of subcontinents. It emphasizes sugar and fat as dietary items, and an integral Indian way of life. A strong association was observed between caloric intake and obesity. In India, obesity is rising rapidly due to increased energy intake resulting from greater purchasing power and the availability of high-fat, energy-dense foods, as well as a decrease in energy expenditure due to urbanization and mechanization [22, 23]. The incidence of T2DM has reached epidemic proportions in India (65 million), paralleling the increase in overweight and obesity (International Diabetes Federation, 2014) [18]. In the present study, we examined the relationship between essential metals (Cu, Mg, Mn, Fe, Se, Cr, and Zn) and insulin resistance (HOMA-IR, sensitivity (QUIKI], and Fet-A) among obese T2DM patients, non-obese T2DM patients and obese non T2DM.
Studies have demonstrated that Fet-A is directly involved in the concomitant development of insulin resistance and obesity [24, 25]. A recent meta-analysis of cohort studies showed that T2DM patients exhibit higher Fet-A levels than non-diabetic individuals [26, 27]. The results of the present study revealed that T2DM patients had significantly higher serum levels of Fet-A than non-obese patients with T2DM (controls). Fet-A levels were associated with diabetic markers (insulin, HbA1c, HOMA-IR, QUIKI, and BMI). Furthermore, Fet-A was significantly positively correlated with BMI, WHR, fasting blood sugar, HbA1c, insulin, C-peptide, HOMA-IR, and QUICKI, consistent with previous studies [5, 28]. Ismail et al. reported that Fet-A levels were elevated in obese adults and showed a significant correlation with obesity, consistent with our findings [29].
Magnesium (Mg) has a role in glucose metabolism and enhances insulin activity and secretion [30]. Insulin resistance and Mg reduction trigger the loss of the insulin resistance cycle and depletion of intracellular Mg, restricting its function in cellular processes [31]. Therefore, this deficiency might result in inadequate glucose regulation. A study demonstrated that patients with diabetes responded positively to orally administered Mn [32]. Parallel to the overhead lines, our data demonstrated that Mn levels moderately correlated with Fet-A levels, insulin resistance, and sensitivity in the diabetic obese group; however, obesity acts as a modulator.
In our study, Fe levels were decreased in patients with diabetes and had a significant but weak correlation with insulin sensitivity. Previous studies have shown increased insulin sensitivity and secretion with regular blood donation or iron chelation treatments [33, 34]. However, the specific mechanisms underlying iron-induced diabetes remain unclear. Similarly, Se was significantly lower in diabetic obese patients than in non-diabetic obese patients and was correlated with insulin resistance and sensitivity, respectively. Parrell’s findings also demonstrated insulin-mimetic effects on the expression of glucose-6-phosphate dehydrogenase (G6PD) in diabetic rats [35], which is noteworthy given that G6PD activity is considerably lower in diabetic rats than in controls [36].
Mg was significantly decreased in the diabetic group; in contrast, its levels were not influenced by obesity, which is consistent with previous studies that revealed that cellular and extracellular magnesium depletion is a hallmark of T2DM [37–39] and that insulin increases the movement of magnesium from the extracellular to the intracellular compartments of a cell, thus boosting the magnesium level available for energy production via magnesium-dependent adenosine triphosphate (ATP). However, in the present study, Mg was not correlated with insulin resistance; however, linear regression analysis revealed that Mg is responsible for obesity via Fet-A. This study demonstrated that Zn was decreased in the diabetic obese group; however, Zn was negatively associated with obesity. This finding demonstrates that zinc deficiency may alter the progression of T2DM. There is evidence of increased insulin secretion and, consequently, increased zinc levels in the early stages of the disease [40]. The pancreas can generate more insulin, but cannot synthesize more zinc, resulting in zinc depletion.
Similarly, copper insufficiency has been associated with decreased glucose tolerance and increased lipid peroxidation owing to increased HbA1c levels [41, 42]. This suggests that Cu decreased in the diabetic group, was correlated with Fet-A and insulin resistance/sensitivity, and was positively associated with diabesity. However, the cause of this increase and its repercussions have not been fully explained, nor is it understood whether aberrations in copper metabolism result from the disease or whether they are crucial for its progression. Linear regression analysis showed that Mn was an independent predictive marker and was positively associated with diabesity.
However, these findings have some limitations. First, it had a cross-sectional design and could not allow causal inferences. Second, because the number of subjects in this study was relatively small and eating habits and physical exercise were not recorded, the findings were probably influenced by other causative and genetic factors. Finally, as this study was conducted at a single center, and the study cohort was confined to one ethnic group, the data may not represent the whole population.
Conclusion
The present study suggests an association between Fet-A, diabetic markers and obesity, which may indicate its role in the pathogenesis of obesity-related T2DM. Cu, Mg, and Mn play significant roles in obesity-related T2DM, whereas Zn is only involved in obesity pathogenesis and complications via Fet-A, insulin sensitivity, and insulin resistance. Mg is an independent predictor for diabesity.
Authors’ contribution
Anumesh Pathak- Manuscript drafting and writing, data analysis, tables and graphs preparation.
Vandana Tiwari: conceptualization, funding acquisition, execution of the experiment, validation of data, and draft editing.
Manish Raj Kulshrestha- Manuscript preparation and editing.
Shivani Singh- Sample collection & data collection, help in tables preparation.
Shefali Singh- Sample collection & enrolled patients.
Vikram Singh- Clinical history & patient details.
Funding
The authors acknowledge an Intramural Research Grant (IEC-24/18) to Dr. Vandana Tiwari from Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, for their financial support.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ozougwu JC, Obimba KC, Belonwu CD, Unakalamba CB. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol. 2013;4:46–57. doi: 10.5897/JPAP2013.0001. [DOI] [Google Scholar]
- 2.Syed Ikmal SIQ, Zaman Huri H, Vethakkan SR, Wan Ahmad WA. Potential biomarkers of insulin resistance and atherosclerosis in type 2 diabetes mellitus patients with coronary artery disease. Int J Endocrinol. 2013:11. 10.1155/2013/698567. [DOI] [PMC free article] [PubMed]
- 3.Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 4.VJ, Leon MA, Goustin AS. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Mol Cell Endocrinol. 2000;164:87–98. doi: 10.1016/S0303-7207(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 5.Stefan N, Fritsche A, Weikert C, Boeing H, Joost H-G, Häring H-U, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–2767. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shidfar F, Zarrati M, Khamseh ME, Haghighat N, Rostami A, Zolfaghari H. Relationship between serum levels of fetuin-A with apo-A1, apo-B100, body composition and insulin resistance in patients with type 2 diabetes. Med J Islam Repub Iran. 2014;28:100. [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil H, Al-kuobaili F. Elevated fetuin - A level associated with an Atherogenic lipid profile in type 2 diabetes. Int J Pharm Sci Rev Res. 2013;21:266–269. [Google Scholar]
- 8.El-Deeb TS, Bakkar SM, Eltoony L, Zakhary MM, Kamel AA, Nafee AM, Hetta HF. The adipokine chemerin and fetuin-A serum levels in type 2 diabetes mellitus: relation to obesity and inflammatory markers. Egypt J Immunol. 2018;25(1):191–202. [PubMed] [Google Scholar]
- 9.Mooradian AD, Morley JE. Micronutrient status in diabetes mellitus. Am J Clin Nutr. 1987;45(5):877–895. doi: 10.1093/ajcn/45.5.877. [DOI] [PubMed] [Google Scholar]
- 10.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and -cell dysfunction? Diabetes. 2003;52(1):1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Matute P, Zulet MA, Martínez JA. Reactive species and diabetes: counteracting oxidative stress to improve health. Curr Opin Pharmacol. 2009;9(6):771–779. doi: 10.1016/j.coph.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Taylor C. Zinc, the pancreas, and diabetes: insights from rodent studies and future directions. Biometals. 2005;18(4):305–312. doi: 10.1007/s10534-005-3686-x. [DOI] [PubMed] [Google Scholar]
- 13.Wijesekara N, Chimienti F, Wheeler MB. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab. 2009;11:202–214. doi: 10.1111/j.1463-1326.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 14.Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab. 2013;17(3):329–341. doi: 10.1016/j.cmet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen AF, Simić A, Åsvold BO, Romundstad PR, Midthjell K, Syversen T et al. Trace elements in early phase type 2 diabetes mellitus—a population-based study. The HUNT study in Norway. J Trace Elem Med Biol. 2017;40:46–53. [DOI] [PubMed]
- 16.Chen H, Tan C. Prediction of type-2 diabetes based on several element levels in blood and chemometrics. Biol Trace Elem Res. 2012;147(1):67–74. doi: 10.1007/s12011-011-9306-4. [DOI] [PubMed] [Google Scholar]
- 17.Dubey P, Thakur V, Chattopadhyay M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients. 2020;12(6):1864. doi: 10.3390/nu12061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International diabetes federation. The IDF diabetes Atlas, 6th ed., 2013. Available online: http://www.idf.org/atlasmap/atlasmap. Accessed 5 May 2014.
- 19.Feller S, Boeing H, Pischon T. Body mass index, waist circumference, and the risk of type 2 diabetes mellitus: implications for routine clinical practice. Dtsch Arztebl Int. 2010;107(26):470. doi: 10.3238/arztebl.2010.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salgado AL, Carvalho L, Oliveira AC, Santos VN, Vieira JG, Parise ER. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47(2):165–169. doi: 10.1590/S0004-28032010000200009. [DOI] [PubMed] [Google Scholar]
- 21.Vanhala P, Vanhala M, Kumpusalo E, Keinanen-Kiukaanniemi S. The quantitative insulin sensitivity check index QUICKI predicts the onset of type 2 diabetes better than fasting plasma insulin in obese subjects: a 5-year follow-up study. J Clin Endocrinol & Metab. 2002;87(12):5834–5837. doi: 10.1210/jc.2002-020591. [DOI] [PubMed] [Google Scholar]
- 22.Misra A, Singhal N, Sivakumar B, Bhagat N, Jaiswal A, Khurana L. Nutrition transition in India: secular trends in dietary intake and their relationship to diet related non-communicable diseases. J Diabetes. 2011;3:278–292. doi: 10.1111/j.1753-0407.2011.00139.x. [DOI] [PubMed] [Google Scholar]
- 23.Misra A, Shrivastava U. Obesity and dyslipidemia in south Asians. Nutrients. 2013;5:2708–2733. doi: 10.3390/nu5072708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourebaba L, Marycz K. Pathophysiological implication of fetuin-A glycoprotein in the development of metabolic disorders: a concise review. J Clin Med. 2019;8(12):2033. doi: 10.3390/jcm8122033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boren J, Taskinen MR, Olofsson SO, Levin M. Ectopic lipid storage and insulin resistance: a harmful relationship. J Intern Med. 2013;274(1):25–40. doi: 10.1111/joim.12071. [DOI] [PubMed] [Google Scholar]
- 26.Cai MM, Smith ER, Holt SG. The role of fetuin-A in mineral trafficking and deposition. BoneKEy reports. 2015:4. [DOI] [PMC free article] [PubMed]
- 27.Kovářová M, Kalbacher H, Peter A, Häring HU, Didangelos T, Stefan N, Birkenfeld A, Schleicher E, Kantartzis K. Detection and characterization of phosphorylation, glycosylation, and fatty acid bound to fetuin A in human blood. J Clin Med. 2021;10(3):411. doi: 10.3390/jcm10030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q, Cornelis MC, Manson JE, Hu FB. Plasma levels of fetuin-A and hepatic enzymes and risk of type 2 diabetes in women in the US. Diabetes. 2013;62:49–55. doi: 10.2337/db12-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ismail NA, Ragab S, El Dayem SMA, ElBaky AA, Salah N, Hamed M. Fetuin-A levels in obesity: differences in relation to metabolic syndrome and correlation with clinical and laboratory variables. Arch Med Sci. 2012;8:826–833. doi: 10.5114/aoms.2012.31616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhary DP. Magnesium deficiency in type 2 diabetes. In Magnesium in Human Health and Disease 2013 pp. 119–126. Humana Press, Totowa. 10.1007/978-1-62703-044-1_7.
- 31.Hua H, Gonzales J, Rude RK. Magnesium transport induced ex vivo by a pharmacological dose of insulin is impaired in non-insulin-dependent diabetes mellitus. Magnes-Res. 1995;8(4):359–366. [PubMed] [Google Scholar]
- 32.Ekmekcioglu C, Prohaska C, Pomazal K, Steffan I, Schernthaner G, Marktl W. Concentrations of seven trace elements in different hematological matrices in patients with type 2 diabetes as compared to healthy controls. Biol Trace Elem Res. 2001;79(3):205–219. doi: 10.1385/BTER:79:3:205. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Real JM, Lopez-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem. 2005;51(7):1201–1205. doi: 10.1373/clinchem.2004.046847. [DOI] [PubMed] [Google Scholar]
- 34.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. Jama. 2004;291(6):711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 35.Al-Quraishy S, Dkhil MA, Moneim AE. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine. 2015;10:6741. doi: 10.2147/IJN.S91377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer JA, Spence DM. A perspective on the role of metals in diabetes: past findings and possible future directions. Metallomics. 2009;1(1):32–41. doi: 10.1039/b817203j. [DOI] [Google Scholar]
- 37.Dewitte K, Dhondt A, Giri M, StOckl D, Rottiers R, Lameire N, et al. Differences in serum ionized and total magnesium values during chronic renal failure between non-diabetic and diabetic patients: a cross-sectional study. Diabetes Care. 2004;27(10):2503–2505. doi: 10.2337/diacare.27.10.2503. [DOI] [PubMed] [Google Scholar]
- 38.De Valk HW, Verkaaik R, Van Rijn HJ, Geerdink RA, Struyvenberg A. Oral magnesium supplementation in insulin-requiring type 2 diabetic patients. Diabet Med. 1998;15(6):503–7. 10.1002/(SICI)1096-9136(199806)15:6<503::AID-DIA596>3.0.CO;2-M. [DOI] [PubMed]
- 39.Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care. 2003;26(4):1147–1152. doi: 10.2337/diacare.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 40.Sprietsma JE, Schuitemaker GE. Diabetes can be prevented by reducing insulin production. Med Hypotheses. 1994;42(1):15–23. doi: 10.1016/0306-9877(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 41.Dzięgielewska-Gęsiak S, Wysocka E, Michalak S, Nowakowska-Zajdel E, Kokot T, Muc-Wierzgoń M. Role of lipid peroxidation products, plasma total antioxidant status, and cu-, Zn-superoxide dismutase activity as biomarkers of oxidative stress in elderly prediabetics. Oxidative Med Cell Longev 2014; 2014. 10.1155/2014/987303. [DOI] [PMC free article] [PubMed]
- 42.Beshgetoor D, Hambidge M. Clinical conditions altering copper metabolism in humans. Am J Clin Nutr. 1998;67(5):1017S–1021S. doi: 10.1093/ajcn/67.5.1017S. [DOI] [PubMed] [Google Scholar]